Abstract
Accumulating evidence suggests that the kinetics of release from single secretory vesicles can be regulated and that quantal size can be modified during fast kiss-and-run fusion. Multiple pathways for vesicle retrieval have been identified involving clathrin and dynamin. It has been unclear whether dynamin could participate in a fast kiss-and-run process to reclose a transient fusion pore and thereby limit vesicle release. We have disrupted dynamin function in adrenal chromaffin cells by expression of the amphiphysin Src-homology domain 3 (SH3) or by application of guanosine 5′-[γ-thio]triphosphate (GTPγS), and have monitored single vesicle release events, evoked by digitonin and Ca2+, by using carbon-fiber amperometry. Under both conditions, there was an increase in mean quantal size accompanying an increase in the half-width of amperometric spikes and a slowing of the fall time. These data suggest the existence of a dynamin-dependent process that can terminate vesicle release under basal conditions. Protein kinase C activation changed release kinetics and decreased quantal size by shortening the release period. The effects of phorbol ester treatment were not prevented by expression of the amphiphysin SH3 domain or by GTPγS suggesting the existence of alternative dynamin-independent process underlying fast kiss-and-run exocytosis.
It has been debated over many years whether regulated exocytosis always occurs through a pathway involving full fusion of the secretory vesicle or whether it can occur through a transient kiss-and-run (1) mechanism as first proposed to occur at the neuromuscular junction (2). In the classical pathway (3), full fusion of the vesicle with the plasma membrane allows complete emptying of the vesicle contents, and vesicle recycling then occurs through a dynamin-dependent pathway (4) involving clathrin-coated vesicles (5). Exocytosis involves the formation of a transient fusion pore (6–8). Recycling of the vesicle in kiss-and-run exocytosis could occur by rapid reclosure of such a pore. This mechanism would allow for very rapid recycling and reuse of synaptic vesicles that could be important to maintain neurotransmission (9). Rapid endocytosis on a time scale of seconds has been directly observed in neurons (10, 11) and neuroendocrine cells (12–15), and a dynamin-dependent but clathrin-independent form of endocytosis has been described as potentially underlying kiss-and-run events (15, 16). It is clear, however, that a much faster type of kiss-and-run event can occur on a time scale of a few milliseconds (17). This third type of exo/endocytic event, which we will term “fast kiss-and-run,” could be important, if sufficiently rapid, as it could limit the amount released per vesicle and thereby modify quantal size.
Recent work has established the existence of kiss-and-run exocytosis of dense-core granules in adrenal chromaffin cells (17, 18), and various experimental data are consistent with regulation of quantal size in these cells by partial release of vesicle contents because of fast kiss-and-run events (19–23). In addition, morphological evidence for partial release has come from the demonstration of the presence of extracellular markers after exocytosis in intracellular vesicles similar in size to chromaffin granules that still retained an electron-dense core (24). The use of lipophilic FM dyes to follow vesicle cycling has also suggested the existence of fast kiss-and-run exocytosis in synaptosomes (25) and in synapses of hippocampal neurons (26) based on discrepancies between dye destaining and neurotransmitter release. The existence of kiss-and-run exocytosis in neurons is, however, controversial and not supported by other evidence (27, 28). The use of carbon-fiber amperometry to detect release of catecholamines from single granules of chromaffin cells (29) has shown more directly that the kinetics of release events and the extent of release can be modified by manipulation of the expression of proteins involved in the exocytotic machinery (19, 20) and by changes in second messenger pathways (21, 22, 30). Importantly, the continuous variation in amounts released per exocytotic event after acute changes in stimulation parameters (23) is consistent with control through fast kiss-and-run exocytosis rather than by recruitment of different sized granules.
It is unclear whether fast kiss-and-run, like other forms of vesicle recycling, proceeds via a dynamin-dependent pathway. The speed at which this process must occur to be able to limit release (within milliseconds, ref. 31) suggests the alternative explanation that fast kiss-and-run could be caused by rapid reversal of the fusion machinery (32). No direct data are available on this issue, and in the absence of dynamin function, synaptic fatigue appears within 20 ms, suggesting a rapid requirement for dynamin (33). We have investigated the effects of protein kinase C (PKC) activation, which leads to a reduction in quantal size, by an apparent switch to fast kiss-and-run (30) and the possible involvement in this effect of a dynamin-dependent retrieval process that could limit release. Our data suggest the existence of a rapid dynamin-dependent pathway in chromaffin cells, and also the operation of a dynamin-independent retrieval mechanism during PKC activation of fast kiss-and-run.
Materials and Methods
Chromaffin Cell Culture, Transfection, and Carbon-Fiber Amperometry.
Freshly isolated bovine adrenal chromaffin cells (34) were plated on nontissue culture-treated 10-cm dishes at a density of 1 × 106 cells per ml. The next day, nonattached cells were pelleted by centrifugation and resuspended in growth medium at a density of 1.5 × 107 per ml. The cells (1 ml) were mixed with 22.5 μg of pEGFP (enhanced green fluorescent protein plasmid) and 30 μg of the amphyphysin Src-homology domain 3 (SH3) plasmid (35) and electroporated at 250 V and 975 μF for 1 pulse, by using a Bio-Rad Gene Pulser II with 4-mm cuvettes. Cells were then diluted as rapidly as possible to 1 × 106 cells per ml with fresh growth media and 1 × 106 cells grown on 35-mm Petri dishes in a final volume of 3 ml of growth medium for a further 3–5 days. The cells were washed three times with Krebs–Ringer buffer (145 mM NaCl/5 mM KCl/1.3 mM MgCl2/1.2 mM NaH2PO4/10 mM glucose/20 mM Hepes, pH 7.4), incubated in bath buffer (139 mM potassium glutamate/20 mM Pipes/0.2 mM EGTA/2 mM ATP/2 mM MgCl2, pH 6.5), and viewed by using a Nikon TE300 inverted microscope. A 5-μm diameter carbon fiber electrode was placed in direct contact with the surface of a cell. For stimulation of the cells, a micropipette was filled with permeabilization buffer (139 mM potassium glutamate/20 mM Pipes/5 mM EGTA/2 mM ATP/2 mM MgCl2/20 μM digitonin/10 μM free Ca2+, pH 6.5) and positioned on the opposite side of the cell from the carbon fiber, and buffer pressure was ejected on to the cell for 20 s. A holding voltage of +700 mV was applied between the carbon fiber tip and the Ag/AgCl reference electrode in the bath. Amperometric responses were monitored with a VA-10 amplifier (NPI Electronic, Tamm, Germany), collected at 4 kHz, digitized with a Digidata 1200B acquisition system and monitored online with the AxoScope 7.0 program (Axon Instruments). Data were subsequently analyzed by using an automated peak detection and analysis protocol within the program origin (Microcal, Amherst, MA) (20, 30). The characteristics of the amperometric spikes after stimulation in permeabilized cells were closely similar to those found after stimulation of intact cells with nicotine in parallel experiments (unpublished observations).
Uptake of Biotin Transferrin.
Chromaffin cells transfected with the amphiphysin SH3 and EGFP plasmids were plated onto collagen-coated glass coverslips in 35-mm tissue culture dishes, at 1.5 × 106 cells per dish and transferrin uptake was assayed 3 days after transfection. Cells were washed twice with DMEM (GIBCO/BRL) then incubated for 1 h at 37°C. Cells were then incubated in fresh DMEM containing 25 μg/ml biotinylated human transferrin (Sigma) for 1 h at 37°C. After washing four times in PBS, cells were fixed in 4% formaldehyde in PBS for 1 h at room temperature. Coverslips were washed twice with PBS, incubated in PBT (PBS containing 0.1% Triton-X-100 and 0.3% BSA) for 1 h followed by a 1-h incubation with Streptavidin–Texas Red (Amersham Pharmacia) at 1/50 in PBT. After two washes in PBT, coverslips were mounted and examined by using the appropriate fluorescence filters.
Preparation of Adrenal Medulla Extract and Binding to Immobilized Glutathione S-Transferase (GST)–Amphiphysin SH3.
Adrenal medulla extract was prepared by homogenization in ice-cold buffer containing 150 mM NaCl, 20 mM Hepes, 4 mM DTT, 0.1 mM PMSF, 1 μg/ml pepstatin A, 10 μg/ml leupeptin, and 1 μg/ml aprotonin (pH 7.4), by using a Polytron homogenizer at 5 ml per gland. Triton X-100 was added to a final concentration of 1% and the homogenate was incubated on ice for 45 min. The supernatant, after centrifugation for 1 h at 100,000 × g, was retained and stored at −80°C. For the binding assay, glutathione-Sepharose beads (Amersham Pharmacia) were washed three times in adrenal medulla homogenization buffer. Twenty micrograms of either GST or GST–amphiphysin SH3, prepared as described (35), were immobilized on to 50 μl of the beads by incubation for 1 h at 4°C with mixing; 20 μl of adrenal medulla extract was added to the beads, and the mixture was incubated for 2 h at 4°C with mixing. The beads were washed five times with homogenization buffer containing 0.1% Triton X-100, by using CytoSignal filters (CytoSignal, Irvine, CA). Bound proteins were eluted by incubation in SDS/PAGE sample buffer for 15 min. Proteins were separated on a SDS/7% PAGE gel and transferred to nitrocellulose paper. Immunoblotting was performed with anti-dynamin 1 at 1/1,000 or anti-dynamin 2 (Transduction Labs, Lexington, KY) at 1/100 dilution overnight, followed by anti-mouse IgG-peroxidase at 1/400 for 1 h. We used enhanced chemiluminescence for the detection of bound antibodies.
Statistical Analyses.
As the data for the various spike parameters did not follow a normal distribution, all statistical comparisons were carried out by using the nonparametric Mann–Whitney U test.
Results
Changes in Single Granule Release Events Caused by PKC Activation.
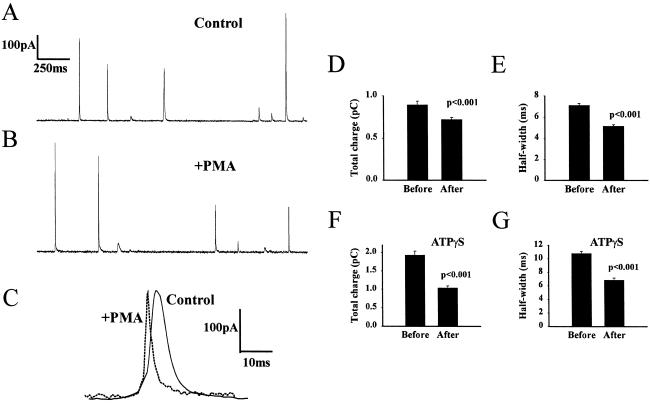
In these experiments, exocytotic events in adrenal chromaffin cells were monitored by using a carbon-fiber electrode placed in direct contact with the surface of the cells. The cells were stimulated by pressure ejection onto individual cells of a permeabilization buffer containing 20 μM digitonin and 10 μM free Ca2+. This approach allowed direct activation of exocytosis under defined Ca2+ concentrations; this was crucial, as changes in the concentration of intracellular Ca2+ modifies quantal size in these cells (23). In addition, this approach gave the possibility of controlling the intracellular composition of the cells. Single granule release events were measured as discrete amperometric spikes (Fig. 1 A and B), and these were very similar to those seen in intact cells after nicotinic stimulation (R. J. Fisher and R.D.B., unpublished results). Activation of protein kinase C by treatment with the phorbol ester PMA (phorbol 12-myristate 13-acetate) modified the kinetics of single granule release events detected as amperometric spikes. Major differences were not apparent in the overall patterns of the cell responses (Fig. 1 A and B), but detailed analysis revealed that PMA treatment reduced both the mean fall and rise times of the spikes (30) (see overlay of spikes in Fig. 1C). As a consequence the half-width of the spikes and also the total charge (amount released per secretory granule) were reduced on average (Fig. 1 C–E). It has recently been shown that rapid retrieval in retinal bipolar neurons is inhibited by adenosine 5′-[γ-thio]triphosphate (ATPγS) (10) indicating an acute requirement for ATP hydrolysis. Replacement of ATP in the bath and stimulation buffers with ATPγS did not prevent the changes in spike charge and half-width caused by pretreatment with PMA (Fig. 1 F and G).
Figure 1.
Amperometric recordings from adrenal chromaffin cells and the effect of treatment with phorbol ester. (A) Control cell. (B) Cell treated with PMA. (C) Spikes from control and PMA-treated cells at high-time resolution were overlaid to show the reduction in spike area and change in release kinetics in PMA-treated cells. (D) Mean values for total spike charge before (16 cells, 381 spikes) and after treatment with 100 nM PMA (15 cells, 609 spikes). (E) Mean values for spike half-width before and after treatment with PMA. (F) Mean values for total spike charge before (11 cells, 587 spikes) and after treatment with 100 nM PMA (10 cells, 541 spikes) for cells in the presence of ATPγS. (G) Mean values for spike half-width before and after treatment with PMA in the presence of ATPγS.
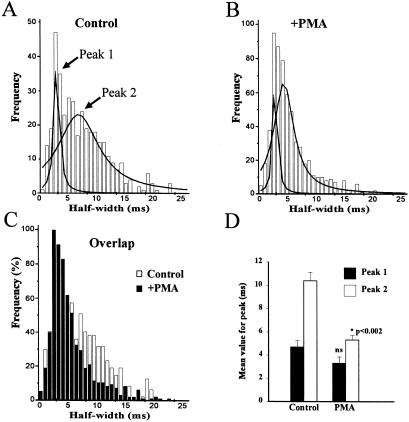
Data on spike half-widths were pooled from a series of cells to allow examination of the populations of the release events before and after PMA treatment. This frequency distribution analysis revealed an overall leftward shift in the distribution of half-widths to lower values (Fig. 2 A–C). The distribution data for both conditions could be best fitted, however, with two Gaussian curves (Fig. 2 A and B). The major effect of PMA treatment was not to switch the relative proportion between the two populations, but instead to shift the wider population of spikes (peak 2) to narrower half-widths. This shift was also demonstrated by analysis of the data from five individual experiments in which the mean value for peak 2 but not peak 1 was significantly reduced by PMA treatment (Fig. 2D). These data suggest that a population of smaller release events (around 20% of total) already exist in untreated chromaffin cells and that PMA results in the overall shift of a wider population to a new mean half-width. This finding is consistent with release time course from this population of granules being reduced (e.g., by earlier fusion-pore closure in a fast kiss-and-run type process) rather than a switch to the use of a smaller vesicle population.
Figure 2.
Characterization of spike populations before and after phorbol ester treatment. (A and B) Frequency distributions of spike half-widths from control (381 spikes) and PMA-treated (609 spikes) cells. The data were fitted to two Gaussian populations using nonlinear curve fitting. The two peak values are indicated as peak 1 and 2. (C) Overlap of the frequency distribution of spike half-widths for control and PMA-treated cells. (D) Mean values for the median half-widths from peak 1 and 2 for control and PMA-treated cells derived from five independent experiments.
Amphiphysin SH3 Domain (amph SH3) Interacts with Dynamin 1 and 2 in Chromaffin Cells and Inhibits Receptor-Mediated Endocytosis.
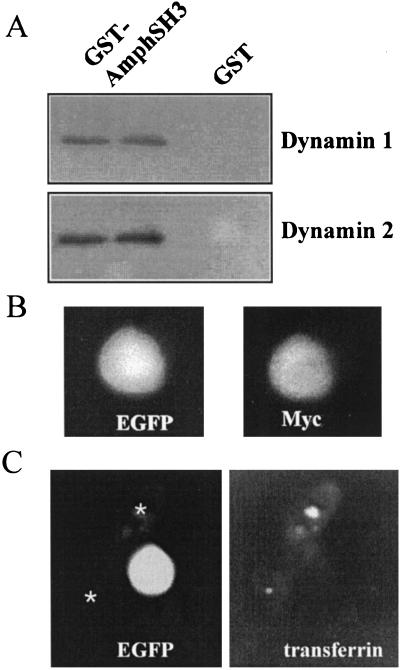
Dynamin interacts with the protein amphiphysin via its SH3 domain (36, 37). To determine the possible involvement of dynamin in the fast kiss-and-run exocytosis during PKC activation, we aimed to make use of expression of the amph SH3 that is known to sequester dynamin and inhibit endocytosis (35, 38). Chromaffin cells have been shown to express dynamin 1 and dynamin 2. It was initially assessed, therefore, whether the amph SH3 would interact with both dynamin isoforms expressed in chromaffin cells. We used immobilized GST-amphSH3 to specifically bind both dynamin 1 and 2 in a pull down assay by using an extract of adrenal medulla (Fig. 2A). No binding to control beads loaded with GST was observed.
To check that amph SH3 was expressed in transfected chromaffin cells, the cells were cotransfected with plasmids encoding amph SH3 and EGFP. The amph SH3 was myc-tagged, and dual expression of both EGFP and amph SH3 in virtually all transfected cells was demonstrated by using anti-myc immunofluorescence (Fig. 2B). To assess the effect of amph SH3 expression on receptor-mediated endocytosis, chromaffin cells were cotransfected and then incubated with biotin transferrin. After fixation, internalized biotin transferrin was detected by using streptavidin–Texas Red. In EGFP-negative, nontransfected cells (asterisks in Fig. 2C), biotin transferrin was taken up and became concentrated in a focal area near the nucleus. In contrast, adjacent EGFP-positive, transfected cells did not show any detectable biotin transferrin uptake in any of the cells examined, indicating an efficient inhibition of receptor-mediated endocytosis by amph SH3 in these cells.
Effect of amph SH3 Expression on the Characteristics of Single Granule Release Events and the Changes Caused by PKC Activation.
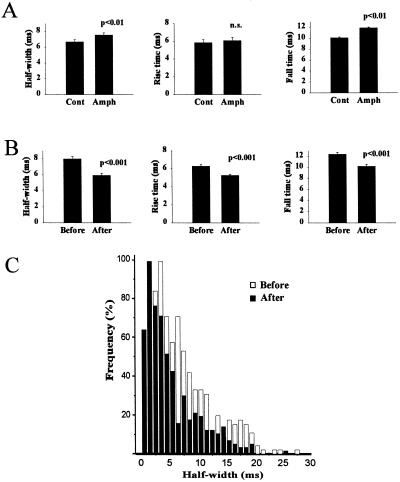
If the extent of release from chromaffin granules is limited during fast kiss-and-run type fusion events by reclosure of the fusion pore in a dynamin-dependent manner, then mean spike kinetics should be modified by amph SH3 expression with a specific effect on the falling phase of the spikes. Fig. 3A shows data from an experiment in which recordings were made from nontransfected control cells and amph SH3-transfected cells in the same dishes with the same carbon fibers. No gross changes in the overall responses of the cells were seen because of amph SH3 expression (not shown). Significant increases were observed, however, in the mean half-width and fall times but not the rise times of the amperometric spikes, as would be expected if dynamin-dependent closure of the fusion pore occurred in a proportion of the events. In a second completely independent experiment, significant changes specifically in the half-width and fall times but not the rise time were again observed indicating the reproducibility of these effects. These findings suggest that a dynamin-dependent process could contribute to changes in quantal size. Indeed, the mean charge per spike increased by 50% from 0.72 ± 0.037 pC in untransfected cells to 1.08 ± 0.02 pC in amph SH3-transfected cells.
Figure 3.
Binding of dynamin 1 and 2 to the GST–amph SH3 domain and inhibition of receptor-mediated endocytosis in chromaffin cells. (A) A GST pull down assay was carried out by using beads loaded with GST–amph SH3 (two left lanes) or GST (two right lanes). Both dynamin 1 and dynamin 2 were specifically recovered with GST–amph SH3. (B) Coexpression of EGFP and myc-amph SH3 in cotransfected chromaffin cells. (C) Uptake of biotin transferrin and concentration in a perinuclear region in untransfected chromaffin cells (asterisks) but not in EGFP/amph SH3-expressing cells.
The data in Fig. 4B are from an experiment in which recordings were taken from amph SH3-transfected cells before and after PMA treatment. The effects of PMA previously seen with control cells including a reduction in half-width, rise time and fall time all still occurred after PMA addition in amph SH3 expressing cells. The changes in the frequency distributions of the half-width (Fig. 4C) were similar to those of control cells with the median value for peak 2 being reduced from 10 to 5 ms. These data suggest that PMA-induced changes in release kinetics and switch to fast kiss-and-run fusion are independent of the function of dynamin 1 and 2.
Figure 4.
Amperometry on chromaffin cells transfected to express the amphiphysin SH3 domain. (A) Chromaffin cells were cotransfected with EGFP and amph SH3. Nontransfected control cells and transfected cells were assayed in the same dishes and mean values for spike half-width, rise time, and fall time determined. For control cells, the data are derived from 12 cells and 363 spikes. For transfected cells the data comes from 24 cells and 431 spikes. (B) Data from cells expressing EGFP and amph SH3 before and after phorbol ester treatment. The data are derived from 10 untreated (333 spikes) and 10 (330 spikes) PMA-treated cells. (C) Overlap of the frequency distributions of spike half-widths from amph SH3-expressing cells before and after PMA treatment.
Effect of Guanosine 5′-[γ-Thio]Triphosphate (GTPγS) on PMA-Induced Changes in Single Granule Release Events.
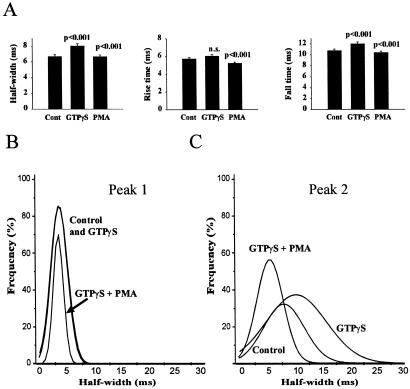
Dynamin is a GTPase (39), and its hydrolysis of GTP is essential to drive vesicle scission from the plasma membrane during endocytosis (40). In an independent approach to inhibit dynamin function, although in a less specific way, we aimed to examine the effects of PMA treatment in cells in the presence of GTPγS, a nonhydrolyzable analogue of GTP that would prevent dynamin function (41). The permeabilization protocol used for cell stimulation would allow rapid diffusion of GTPγS into the cells, particularly as a high concentration (1 mM) was maintained in the dish throughout the recording. This would prevent GTP hydrolysis by dynamin 1 or 2 and any other dynamin isoform (42) present in chromaffin cells. In a comparison of cells with or without GTPγS present (Fig. 5A) the effects of the GTP analogue were very similar to those caused by expression of amph SH3 with an increase in half-width and fall time, but no significant effect on the rise-time of the amperometric spikes. GTPγS increased the mean charge per spike (quantal size) by 30% from 1.04 ± 0.056 to 1.36 ± 0.093 pC. With GTPγS present, cells were also treated with PMA (Fig. 5A). PMA again reduced the half-width, rise time, and fall time of the spikes, even in the maintained presence of GTPγS, and produced a leftward shift in the frequency distribution of the half-widths of the spikes with the median value of peak 2 being reduced from 11.5 to 6.5 ms. These data indicate that the PMA-induced fast kiss-and-run was not prevented by the presence of GTPγS. Addition of GTPγS or PMA in its presence did not modify the peak 1 events (Fig. 5B), but in each case the major effects was a shift in the half-width of the peak 2 events (Fig. 5C).
Figure 5.
Amperometry on chromaffin cells and the effect of GTPγS and phorbol ester. (A) Chromaffin cells were stimulated under control conditions (20 cells, 617 spikes), in the presence of 1 mM GTPγS (18 cells, 472 spikes), and after 100 nM PMA treatment but with GTPγS present (20 cells, 740 spikes). The mean values for half-width, rise time, and fall time of the spikes are shown. (B) Comparison of the distribution of half-widths in the peak 1 population from control cells and cells treated with GTPγS or GTPγS plus PMA showing little effect of GTPγS or PMA. The best-fit curves from nonlinear curve fitting are shown. (C) Comparison of the distribution of half-widths in the peak 2 population from control cells and cells treated with GTPγS or GTPγS plus PMA showing the rightward shift in the presence of GTPγS and the leftward shift caused by PMA. The best-fit curves from nonlinear curve fitting are shown.
Discussion
Evidence has begun to accumulate from the use of patch-clamp capacitance measurement (17), ultrastructural studies (24), and carbon-fiber amperometry (19, 20, 22, 23) that fast kiss-and-run exocytosis of dense-core granules can occur in adrenal chromaffin cells. This form of exocytosis was first proposed for synaptic vesicle exocytosis (1, 2) but the evidence for its occurrence in synapses is less extensive and not supported in all studies (27, 28). Independent analysis of the effects of phorbol esters in both chromaffin cells (30) and in brain synaptosomes (25) has suggested, however, that PKC activation results in a switch to fast kiss-and-run exocytosis. Quantal analysis has shown a reduction in quantal size in some (43) but not all synapses (44) after PKC activation. The effect of PMA that we observed in chromaffin cells was caused by activation of PKC, as it was prevented by the inhibitor bis-indolylmaleimide (30). After prior treatment of cells with PMA, the changes in spike parameters were not acutely dependent on ATP hydrolysis, ruling out an involvement of N-ethylmaleimide-sensitive fusion protein (NSF), for example. This finding is in contrast with an inhibitory effect of ATPγS on rapid retrieval in retinal bipolar neurons (10), but is consistent with the maintenance of fast retrieval in chromaffin (45) and pituitary cells (13) after internal dialysis by buffer without MgATP. The decrease in rise time of the spikes after PMA treatment is consistent with studies showing that Ca2+ (46, 47) or PMA (48) can accelerate fusion pore expansion. Elevation of cAMP in intact chromaffin cells stimulated with high K+ resulted in an opposite increase in spike rise time and half-width (22). Although the data presented here are all derived from digitonin-permeabilized cells, we have observed a similar effect of cAMP in our assay (M.E.G. and R.D.B., unpublished observations) consistent with the data from permeabilized cells reflecting events in intact cells.
We have further explored the effect of PKC activation to examine whether fast kiss-and-run exocytosis in chromaffin cells involves the function of a dynamin-dependent pathway to close the fusion pore. It is clear that the retrieval of synaptic vesicles and secretory granules can occur through multiple pathways. The involvement of a clathrin- and dynamin-dependent (4) pathway for slow retrieval is well established (49). Various evidence support the existence of a faster clathrin-independent but dynamin-dependent pathway acting on a time course of seconds (15, 50). Evidence has also been accumulating for a fast kiss-and-run process of exo/endocytosis occurring on a millisecond time scale. It has not been known whether this involved the action of dynamin. Changes in a variety of conditions lead to a modification in the kinetics of single release events detected by using amperometry and the average amount released after each granule fusion (quantal size) in chromaffin cells (19, 20, 23, 30). These effects are most simply interpreted on the basis that release can be limited by rapid reclosure of the fusion pore in a fast kiss-and-run event and that the use of this pathway can be acutely regulated. Recent data showing graded release over varying stimulation parameters is consistent with this interpretation (23). In the present experiments, two populations of release events were observed. Those in peak 1 had a narrow half-width, and this population was unaffected by the manipulations used in this study. The nature of these distinct events is unclear. In contrast, the other population of events (peak 2) showed shifts to both narrow or wider mean half-widths indicating that this population was sensitive to alterations in the extent of kiss-and-run.
It appears that kiss-and-run exocytosis, under normal conditions, involves only a small proportion of the total exocytic events (17, 26) but the frequency can be increased by various treatments (17, 19, 23). The extent to which it regulated under physiological conditions is still to be resolved. An additional question is the physiological role of kiss-and-run exocytosis. For neurons, this could provide a more rapid mechanism for vesicle retrieval for reuse (9). In the case of chromaffin cells, it would have an additional function in maintaining the components of the protein core of the granules. This would allow them to be refilled with catecholamine and reused.
For the release of granule contents to be limited by kiss-and-run, this would have to be a rapid process. A recent paper in which a form of kiss-and-run was induced, because of use of nonphysiologically high external Ca2+, mean fusion pore open time was reduced to 40 ms, but this was still sufficient to allow complete vesicle emptying (17). Recent data from the use of FM dye measurements in hippocampal neurons suggest a maximal fusion pore open time of 6 ms. Based on the values for the time to peak of the amperometric spikes (≈6 ms), we can calculate that release would have to be ended by fusion pore closure on the same time scale during PKC activation. Here we have characterized the ability of PKC activation to change the kinetics of release events and reduce quantal size (reduced half-width and total charge of amperometric spikes) by apparently switching to fast kiss-and-run. The key question we aimed to address was whether fast kiss-and-run involved the activity of dynamin. The amph SH3 domain has been widely used as an inhibitor of dynamin function (35, 38). It was expressed in transfected chromaffin cells, where it inhibited receptor-mediated endocytosis as in other cell types (35) and also modified amperometric spike kinetics by increasing half-width and fall-time. This specific effect, without a modification of the rise-time of the spikes, is consistent with an involvement of a fast dynamin-dependent pathway that limits fusion pore open time under basal conditions. Previous work has suggested that chromaffin granules are not fully emptied during normal stimulation (51). It has generally been supposed that the fall time of the amperometric spikes is a parameter reflecting only the diffusion and consumption of catecholamine on the carbon fiber. We have previously shown, however, that this parameter is modified by disruption of the exocytotic machinery (19, 20). We now show that this is specifically modified by disruption of dynamin function indicating that the termination of the release time course measured by amperometry can involve a dynamin-dependent process.
The PMA-induced changes in amperometric spikes still occurred in amph SH3-expressing cells. As an independent method to inhibit the dynamin GTPase activity that would prevent function of any related GTPase, we examined the effect of GTPγS. This GTP analogue had exactly the same effects on spike parameters as amph SH3 expression, and again did not prevent the effects of PKC activation. These data suggest that during PKC activation, a dynamin-independent fusion-pore closure limits release. Other recent work has examined the effect of introducing anti-dynamin antibodies via a patch-pipette on amperometric spikes in chromaffin cells (23). The data from this study are consistent with our findings but are complex, as multiple effects were seen, including a progressive reduction in the numbers of exocytotic events. We did not see any evidence of a reduction in the extent of exocytosis after amph SH3 expression or because of the presence of GTPγS in the present study. The effects that we have observed were specifically confined to a modification of quantal size because of changes in spike duration. Overall, therefore, these findings suggest the existence of both dynamin-dependent and independent pathways for fast kiss-and-run that can contribute to the determination of quantal size.
Acknowledgments
We thank Alan Morgan for his comments on the manuscript. This work was supported by grants from The Wellcome Trust (to R.D.B.). D.W.O'C. was supported by a Wellcome Trust Prize Studentship.
Abbreviations
- EGFP
enhanced green fluorescent protein
- GST
glutathione S-transferase
- PKC
protein kinase C
- PMA
phorbol 12-myristate 13-acetate
- SH3
Src-homology domain 3
- GTPγS
guanosine 5′-[γ-thio]triphosphate
- ATPγS
adenosine 5′-[γ-thio]triphosphate
- amph SH3
amphiphysin SH3 domain
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Fesce R, Grohovaz F, Valtorta F, Meldolesi J. Trends Cell Biol. 1994;4:1–4. doi: 10.1016/0962-8924(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 2.Ceccarelli B, Hurlburt W P, Mauro A. J Cell Biol. 1973;57:499–524. doi: 10.1083/jcb.57.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heuser J E, Reese T S. J Cell Biol. 1973;57:315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koenig J H, Ikeda K. J Neurosci. 1989;11:3844–3860. doi: 10.1523/JNEUROSCI.09-11-03844.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cremona O, De Camilli P. Curr Opin Cell Biol. 1997;7:323–330. doi: 10.1016/s0959-4388(97)80059-1. [DOI] [PubMed] [Google Scholar]
- 6.Breckenridge L J, Almers W. Nature (London) 1987;328:814–817. doi: 10.1038/328814a0. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez J M, Neher E, Gomperts B D. Nature (London) 1984;312:453–455. doi: 10.1038/312453a0. [DOI] [PubMed] [Google Scholar]
- 8.Rosenboom H, Lindau M. Proc Natl Acad Sci USA. 1994;91:5267–5271. doi: 10.1073/pnas.91.12.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harata N, Pyle J L, Aravanis A M, Mozhayeva M, Kavali E T, Tsien R W. Trends Neurosci. 2001;24:637–643. doi: 10.1016/s0166-2236(00)02030-0. [DOI] [PubMed] [Google Scholar]
- 10.Heidelberger R. J Neurosci. 2001;21:6467–6474. doi: 10.1523/JNEUROSCI.21-17-06467.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gersdorff H V, Matthews G. Nature (London) 1994;367:735–739. doi: 10.1038/367735a0. [DOI] [PubMed] [Google Scholar]
- 12.Burgoyne R D. Pflügers Arch. 1995;430:213–219. doi: 10.1007/BF00374652. [DOI] [PubMed] [Google Scholar]
- 13.Thomas P, Lee A K, Wong J G, Almers W. J Cell Biol. 1994;124:667–675. doi: 10.1083/jcb.124.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith C, Neher E. J Cell Biol. 1997;139:885–894. doi: 10.1083/jcb.139.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Artalejo C R, Henley J R, M A, M, Palfery H C. Proc Natl Acad Sci USA. 1995;92:8328–8332. doi: 10.1073/pnas.92.18.8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palfrey H C, Artalejo C R. Neuroscience. 1998;83:969–989. doi: 10.1016/s0306-4522(97)00453-3. [DOI] [PubMed] [Google Scholar]
- 17.Ales E, Tabares L, Poyato J M, Valero V, Lindau M, Alvarez de Toledo G. Nat Cell Biol. 1999;1:40–44. doi: 10.1038/9012. [DOI] [PubMed] [Google Scholar]
- 18.Albillos A, Dernick G, Horstmann H, Almers W, Alvarez de Toledo G, Lindau M. Nature (London) 1997;389:509–512. doi: 10.1038/39081. [DOI] [PubMed] [Google Scholar]
- 19.Fisher R J, Pevsner J, Burgoyne R D. Science. 2001;291:875–878. doi: 10.1126/science.291.5505.875. [DOI] [PubMed] [Google Scholar]
- 20.Graham M E, Burgoyne R D. J Neurosci. 2000;20:1281–1289. doi: 10.1523/JNEUROSCI.20-04-01281.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Machado J D, Segura F, Brioso M A, Borges R. J Biol Chem. 2000;275:20274–20279. doi: 10.1074/jbc.M000930200. [DOI] [PubMed] [Google Scholar]
- 22.Machado J D, Morales A, Gomez J F, Borges R. Mol Pharmacol. 2001;60:514–520. [PubMed] [Google Scholar]
- 23.Elhamdani A, Palfrey H C, Artalejo C R. Neuron. 2001;31:819–830. doi: 10.1016/s0896-6273(01)00418-4. [DOI] [PubMed] [Google Scholar]
- 24.Henkel A W, Horstmann H, Henkel M K. FEBS Lett. 2001;505:414–418. doi: 10.1016/s0014-5793(01)02861-7. [DOI] [PubMed] [Google Scholar]
- 25.Cousin M A, Robinson P J. J Neurochem. 2000;75:1645–1653. doi: 10.1046/j.1471-4159.2000.0751645.x. [DOI] [PubMed] [Google Scholar]
- 26.Stevens C F, Williams J H. Proc Natl Acad Sci USA. 2000;97:12828–12833. doi: 10.1073/pnas.230438697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zenisek D, Steyer J A, Almers W. Nature (London) 2000;406:849–854. doi: 10.1038/35022500. [DOI] [PubMed] [Google Scholar]
- 28.Sankaranarayanan S, Ryan T A. Nat Neurosci. 2001;4:129–136. doi: 10.1038/83949. [DOI] [PubMed] [Google Scholar]
- 29.Wightman R M, Jankowski J A, Kennedy R T, Kawagoe K T, Schroeder T J, Leszczyszyn D J, Near J A, Diliberto E J, Jr, Viveros O H. Proc Natl Acad Sci USA. 1991;88:10754–10758. doi: 10.1073/pnas.88.23.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graham M E, Fisher R J, Burgoyne R D. Biochimie. 2000;82:469–479. doi: 10.1016/s0300-9084(00)00196-6. [DOI] [PubMed] [Google Scholar]
- 31.Rahamimoff R, Fernandez J M. Neuron. 1997;18:17–27. doi: 10.1016/s0896-6273(01)80043-x. [DOI] [PubMed] [Google Scholar]
- 32.Graham M E, Washbourne P, Wilson M C, Burgoyne R D. J Cell Sci. 2001;114:4397–4405. doi: 10.1242/jcs.114.24.4397. [DOI] [PubMed] [Google Scholar]
- 33.Kawasaki F, Hazen M, Ordway R W. Nat Neurosci. 2000;3:859–860. doi: 10.1038/78753. [DOI] [PubMed] [Google Scholar]
- 34.Burgoyne R D. In: Intracellular Messengers, Neuromethods. Boulton A, Baker G, Taylor C, editors. Vol. 20. Totowa, NJ: Humana; 1992. pp. 433–470. [Google Scholar]
- 35.Wigge P, Vallis Y, McMahon H T. Curr Biol. 1997;7:554–560. doi: 10.1016/s0960-9822(06)00254-5. [DOI] [PubMed] [Google Scholar]
- 36.Wigge P, Kohler K, Vallis Y, Doyle C A, Owen D, Hunt S P, McMahon H T. Mol Biol Cell. 1997;8:2003–2015. doi: 10.1091/mbc.8.10.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.David C, McPherson P S, Mundigl O, De Camilli P. Proc Natl Acad Sci USA. 1996;93:331–335. doi: 10.1073/pnas.93.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shupliakov O, Low P, Grabs D, Gad H, Chen H, David C, Takei K, De Camilli P, Brodin L. Science. 1997;276:259–263. doi: 10.1126/science.276.5310.259. [DOI] [PubMed] [Google Scholar]
- 39.Robinson P J, Sontag J-M, Liu J-P, Fykse E M, Slaughter C, McMahon H, Sudhof T C. Nature (London) 1993;365:163–166. doi: 10.1038/365163a0. [DOI] [PubMed] [Google Scholar]
- 40.Marks B, Stowell M H B, Vallis Y, Mills I G, Gibson A, Hopkins C R, McMahon H T. Nature (London) 2001;410:231–235. doi: 10.1038/35065645. [DOI] [PubMed] [Google Scholar]
- 41.Takei K, McPherson P S, Schmid S L, De Camilli P. Nature (London) 1995;374:186–190. doi: 10.1038/374186a0. [DOI] [PubMed] [Google Scholar]
- 42.McNiven M A, Cao H, Pitts K R, Yoon Y. Trends Biochem Sci. 2000;25:115–120. doi: 10.1016/s0968-0004(99)01538-8. [DOI] [PubMed] [Google Scholar]
- 43.Van der Kloot W. J Neurobiol. 1991;22:204–211. doi: 10.1002/neu.480220210. [DOI] [PubMed] [Google Scholar]
- 44.Yawo H. J Physiol. 1999;515:169–180. doi: 10.1111/j.1469-7793.1999.169ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neher E, Zucker S. Neuron. 1993;10:21–30. doi: 10.1016/0896-6273(93)90238-m. [DOI] [PubMed] [Google Scholar]
- 46.Fernandez-Chacon R, Alvarez de Toledo G. FEBS Lett. 1995;363:221–225. doi: 10.1016/0014-5793(95)00319-5. [DOI] [PubMed] [Google Scholar]
- 47.Hartmann J, Lindau M. FEBS Lett. 1995;363:217–220. doi: 10.1016/0014-5793(95)00318-4. [DOI] [PubMed] [Google Scholar]
- 48.Scepek S, Coorssen J R, Lindau M. EMBO J. 1998;17:4340–4345. doi: 10.1093/emboj/17.15.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jarousse N, Kelly R B. Curr Opin Cell Biol. 2001;13:461–469. doi: 10.1016/s0955-0674(00)00237-4. [DOI] [PubMed] [Google Scholar]
- 50.Daly C, Sugimori M, Moreira J E, Ziff E B, Llinas R. Proc Natl Acad Sci USA. 2000;97:6120–6125. doi: 10.1073/pnas.97.11.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schroeder T J, Borges R, Finnegan J M, Pihel J, Amatore C, Wightman R M. Biophys J. 1996;70:1061–1068. doi: 10.1016/S0006-3495(96)79652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]