Abstract
Galanin is a neuropeptide with a wide variety of biological functions, including that of a strong endogenous anticonvulsant. No nonpeptide ligands, capable of activating galanin receptors, are available today. Based on known pharmacophores of galanin, a combinatorial library was designed, synthesized, and screened at the rat hippocampal galanin receptor. A low molecular weight galanin receptor agonist, 7-((9-fluorenylmethoxycarbonyl)cyclohexylalanyllysyl)amino-4-methylcoumarin (galnon) was found to displace 125I-galanin with micromolar affinity at Bowes cellular and rat hippocampal membranes. Autoradiographic binding assay on rat spinal cord sections confirmed the ability of galnon to displace 125I-galanin from its binding sites. Galnon inhibited adenylate cyclase activity, suggesting an agonist action at galanin receptors. When injected i.p. galnon reduced the severity and increased the latency of pentylenetetrazole-induced seizures in mice and reversed the proconvulsant effects of the galanin receptor antagonist M35, injected into a lateral ventricle. Intrahippocampal injection of galnon also shortened the duration of self-sustaining status epilepticus in rats, confirming its agonist properties in vivo. Pretreatment of rats with antisense peptide nucleic acid targeted to galanin receptor type 1 mRNA abolished the effect of galnon, suggesting mediation of its anticonvulsant properties through this receptor subtype. These findings introduce a systemically active nonpeptide galanin agonist anticonvulsant.
Galanin, a neuropeptide of 29 aa (30 in humans), is widely distributed in the central and peripheral nervous systems and is involved in regulation of learning and memory (1), feeding (2), sexual behavior (3), pain threshold (4), and the release of insulin (5) and growth hormone (6).
Recent evidence suggests that galanin is also a potent endogenous anticonvulsant (7–9, 28). The importance of galanin receptor agonists for seizure control may arise from their ability to act at both presynaptic and postsynaptic sites to reduce excitability, to inhibit glutamate but not γ-aminobutyric acid release (10), and to hyperpolarize CA3 pyramidal cells by opening K+ channels, thus dampening seizure activity (11).
Nonpeptide ligands are available for receptors of cholecystokinin, angiotensin, bradykinin, and many other neuropeptides (12, 13). Most galanin receptor ligands available today, however, are peptides (14, 15), vulnerable to enzymatic degradation and unable to cross the blood–brain barrier.
Despite extensive random screening efforts, only two reports on nonpeptide antagonists for galanin receptors have been published: spirocoumaranon (16) (Sch 202596) and 2,3-dihydro-2-(4-methylphenyl)-1,4-dithiepin-1,1,4,4-tetroxide (17).
In this study, we report a nonpeptide galanin receptor ligand, galnon, discovered by application of a combinatorial library approach. We demonstrate its agonistic activity in adenylate cyclase assays and on seizure models.
Materials and Methods
Protected amino acids and diamines were from Neosystem Laboratoire (Strasbourg, France) or Senn Chemicals (Gentilly, France). 4-Methylbenzhydrylamine-polystyrene resin and activators were from Bachem. 7-Amino-4-methylcoumarin (AMC) was from Senn Chemicals. Trifluoroacetic acid, diisopropylethylamine, and dioxane were from Lancaster Synthesis, Morecambe, U.K. Dichloromethane, N,N-dimethylformamide (DMF), and acetonitrile were from Labscan, Dublin. All other chemicals were from Sigma-Aldrich.
Synthesis of Combinatorial Library.
Synthesis of the combinatorial library was started from the initial lead compound, Cbz-Phe-Arg-aromatic amine (A–B–C–D). The initial lead compound was found by screening a library of the analogues of Trp-Asn-Tyr (a tripeptide combining major pharmacophores in galanin, Trp2, Asn5, and Tyr9). We included modifications into all positions in the initial lead compound, resulting in the following lead structure: A–B–C–D, where A denoted a bulky hydrophobic group, B a hydrophobic amino acid, C an amino acid with protonated side chain (in physiological conditions), and D an aromatic amine (Table 1). We used a split synthesis approach to create a combinatorial library of compounds. All coupling steps were carried out separately for each compound; for deprotection the separate resins were pooled again. Fragment A–B–C was synthesized on 4-methylbenzhydrylamine-polystyrene resin and cleaved and, finally, the component D was coupled in the solution. Synthesized compounds were purified on Sep-Pak cartridges (Waters) by eluting with a stepwise gradient of acetonitrile in water. In total, 20 fractions were obtained, freeze-dried, and screened for activity in 125I-galanin displacement assay. The structure of galnon was deduced reiteratively by using the resin samples saved in each step (for details see Results).
Table 1.
The building blocks for the combinatorial library A-B-C-D, where A is a bulky hydrophobic group, B a hydrophobic amino acid, C an amino acid with protonated side chain (in physiological conditions) and D an aromatic amine coupled to the C terminus of amino acid C through an amide bond
| A | B | C | D |
|---|---|---|---|
| Fmoc- | Phe | Lys | 7-Amino-4-(methoxymethyl)- coumarin |
| Acetyl- | Trp | His | AMC |
| Benzyloxycarbonyl- | hPhe* | Orn | m-Anisidin |
| Adamantane-1-carbonyl- | Cha† | Dab‡ | Cyclohexylamine |
Homophenylalanine.
Cyclohexylalanine.
Diaminobutyric acid.
Synthesis of Galnon.
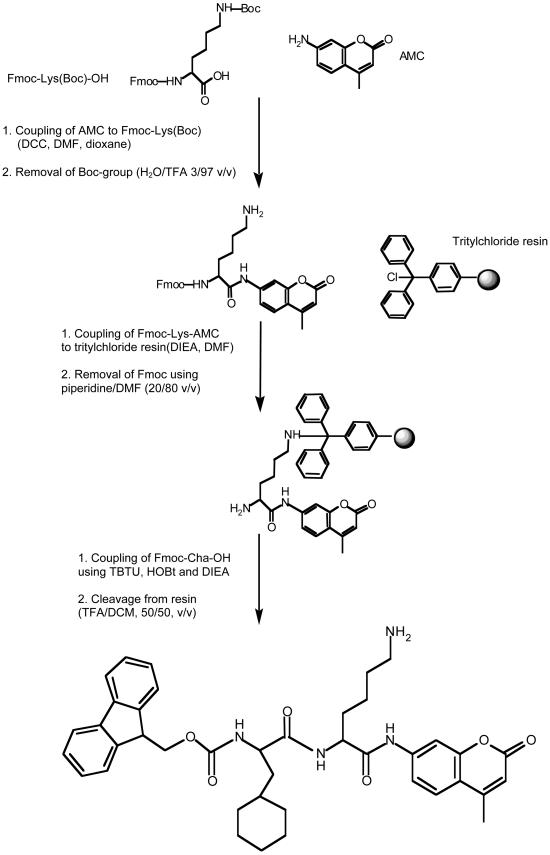
Galnon was synthesized following the scheme outlined in Fig. 1. The first step of the synthesis was the coupling of 9-fluorenylmethoxycarbonyl (Fmoc)-Lys(tert-butyloxycarbonyl, Boc)-OH to AMC. One millimole of Fmoc-Lys(Boc)-OH and 0.5 equivalents of dicyclohexylcarbodiimide were separately dissolved in dioxane, cooled on ice, and then pooled. Reaction mixture was stirred for 30 min at room temperature, then 0.5 equivalent of AMC dissolved in DMF was added to the symmetric anhydride solution and the mixture was stirred overnight. The solvents were evaporated, and Fmoc-Lys(Boc)-AMC was precipitated with petrol ether/ethyl acetate mixture (5/95 vol/vol) and dried under vacuum. The Boc group was removed with H2O/trifluoroacetic acid mixture (3/97 vol/vol) in ice bath for 5 min, followed by the evaporation of the solvents. The obtained Fmoc-Lys-AMC was coupled to chlorotrityl resin by incubation of 2–3 equivalents of Fmoc-Lys-AMC, 4–9 equivalents of diisopropylethylamine, and 1 equivalent of chlorotrityl resin for 2 h. Resin was washed and the Fmoc group was removed with piperidine/DMF (20/80 vol/vol). Coupling of Fmoc-cyclohexylalanine was performed by using 2 equivalents of amino acid, 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate, 1-hydroxybenzotriazole, and 4 equivalents of diisopropylethylamine. Galnon was cleaved from the resin by applying trifluoroacetic acid/dichloromethane mixture (50/50 vol/vol) in four aliquots. The filtrate was evaporated, and the obtained product was purified on Sep-Pak cartridges and analyzed by using a plasma desorption mass spectrometer (model Bioion 20, Bioion, Uppsala, Sweden).
Figure 1.
Synthesis route of galnon. DCC, dicyclohexylcarbodiimide; TBTU, 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate; HOBt, 1-hydroxybenzotriazole; DIEA, diisopropylethylamine; Cha, cyclohexylalanine; TFA, trifluoroacetic acid; DCM, dichloromethane.
Synthesis of Peptides and Peptide Nucleic Acid (PNA) Oligomers.
Human and rat galanin and M35 were synthesized on a model 431A peptide synthesizer (Perkin–Elmer/Applied Biosystems) as described (18). PNA oligomers with a general sequence biotin-Cys-PNA-Lys-amide were synthesized by using a Perkin–Elmer/Applied Biosystems stepwise peptide synthesizer model A433 as described (19). The antisense PNA sequence complementary to the position 18–38 of the coding region of galanin receptor type 1 (GalR1) mRNA was (PNA1): CCA-TTC-CCT-TCA-CTG-AGG-TTC and the scrambled sequence of it (PNAscr): GGC-ATG-GCT-GCT-CTC-CGT-CTG (20).
Ligand Binding to Galanin Receptors, Adenylate Cyclase Assay.
The mixtures of ligands and individual compounds were screened to displace 125I-galanin in equilibrium binding assay with membrane preparations from rat hippocampi and Bowes cells, rich in galanin binding sites as described in detail (21). Assays of the effect of galnon on basal and forskolin-stimulated cAMP production were performed essentially as described by Valkna et al. (22) in membranes from rat ventral hippocampi.
Autoradiography.
Rats were decapitated, and the spinal cord lumbar segments L4 and L5 were dissected out, rapidly frozen, cut in a cryostat, and processed for the autoradiographic ligand binding assay of Young and Kuhar (23), as described (20). Galnon was added at 1, 3, and 5 μM concentrations. The sections were processed in a BAS3000 Bio-Imagine Analyzer (Fuji) and quantified and then exposed to Hyperfilm-Max x-ray film (Amersham Biosciences).
Seizure Induction and Quantification.
All animal studies were approved by and carried out in accordance with the guidelines of the animal research committees of the University of California, Los Angeles and the West Los Angeles Veterans Administration Medical Center.
Effect of Galnon in Mice.
C57BL/6J male mice weighing 20–30 g (Simonsen Laboratories, Gilroy, CA) were anaesthetized with ketamine (100 mg/kg i.p.) and xylazine (15 mg/kg i.p.) and stereotaxically chronically implanted into a lateral ventricle (ICV) with guide cannulae. Galanin or M35 were injected ICV in freely moving mice (0.5 nmol, in 0.5 μl, over 5 min). Control animals were treated with saline.
Galnon was freshly dissolved in 50% DMSO in saline and administered i.p. in a dose of 2 mg/kg 15 min before pentylenetetrazole (PTZ, Research Biochemicals, Natick, MA), when the effect of galnon alone was studied, or 5 min after M35 injection, in the coadministration studies. Control animals were treated with 50% DMSO in saline.
Seizures were induced by i.p. injection of PTZ in a dose of 40 mg/kg (when studying the effects of galanin only or galnon only) or 30 mg/kg (when studying the effect of M35 and galnon + M35). Seizure latency and the highest behavioral seizure score were recorded (9). For statistical purposes, if the animal failed to show seizure of any particular score, a latency of 900 s was assigned to this score. No behavioral side effects of galnon were observed. Statistical methods used were t test (latency) and Mann–Whitney (seizure score).
Effect of Galnon in Rats.
Self-sustaining status epilepticus (SSSE) was induced as described (8). Briefly, male Wistar rats (Simonsen Laboratories), 10–12 weeks old, were anaesthetized with ketamine (60 mg/kg i.p.) and xylazine (10 mg/kg i.p.) and implanted with a bipolar stimulating electrode into the angular bundle of the perforant path. A recording electrode-cannula (Plastics One, Roanoke, VA) was implanted ipsilaterally into the granule cell layer of ventral dentate gyrus. After 7–10 days of recovery, SSSE was induced in free-running animals by 30 min of the perforant path stimulation (PPS, 20-Hz 10-s trains at 20 V delivered every min together with continuous 2-Hz stimulation). An electroencephalogram was recorded for 24 h and analyzed off-line with harmonie software (Stellate Systems, Montreal). Total time spent in seizures and the time of occurrence of the last seizure were calculated. Galanin (American Peptide, Sunnyvale, CA) and M35 were dissolved in saline, and galnon was dissolved in 50% DMSO. The compounds were injected into the dentate gyrus in a volume of 1 μl at a rate of 0.5 μl/min, 10 min after the end of PPS. Control animals were treated with vehicle.
Administration of PNA.
In addition to the electrode implantation described above, animals were implanted with the guide cannula ICV. Starting 4–6 h after surgery, rats received repeated bilateral ICV injections of PNA1. Control animals were injected with PNAscr. The PNA oligomers were dissolved in saline, at a concentration of 4 nmol/μl. Injections were delivered to free-moving rats through the injection cannula, placed inside the guide cannula, and connected to the 1 μl-Hamilton microsyringe placed into the SP 310i microsyringe pump (World Precision Instruments, Sarasota, FL). The injection volume was 1 μl/side and the duration was 5 min. PNA were delivered for 5 days, 10–14 h apart. Each rat received a total of 80 nmol of PNA. PPS was initiated 12–16 h after the last PNA injection.
Results
Reiterative Evaluation of the Combinatorial Library.
After the completion of synthesis of the combinatorial library, we had four resins bearing (theoretically) 64 compounds each. The synthesized compounds in these four mixtures were separated and the fractions obtained were tested for the ability to displace 125I-galanin from galanin receptors in rat hypothalamic membranes. The structure of galnon was deduced reiteratively in the synthesis, step by step. First, we were able to define that AMC-containing compounds were most active in 125I-galanin displacement assay. Therefore, we coupled AMC to four types of A-B-C resins (A-B-Lys, A-B-His, A-B-Orn, and A-B-Dab) and tested them again in binding assay. We found that the mixtures containing Lys at position C were most active in 125I-galanin displacement assay. In the same way, the most active components of A and B were identified, resulting in galnon, Fmoc-cyclohexylalanine-Lys-amidomethyl coumarin (according to International Union of Pure and Applied Chemistry systematic nomenclature: 1-[5-amino-1-(4-methyl-2-oxo-2H-chromen-7-ylcarbamoyl)-pentylcarbamoyl]-2-cyclohexyl-ethyl-carbamic acid 9H-fluoren-9-ylmethyl ester). The synthesis of galnon is shown in Fig. 1. The iterative deduction indicated that two more compounds from our library could serve as candidates for peptidomimetic galanin receptor ligands, however, with lower affinities than galnon at galanin receptors.
Binding Studies and Inhibition of Adenylate Cyclase Activity.
125I-galanin was displaced by galnon in membranes from Bowes cells (Ki value 2.9 μM) and rat ventral hippocampus with a Ki value of 4.8 μM (Table 2). The ability of galnon to displace 125I-galanin from its binding sites in a concentration-dependent manner was confirmed in an autoradiographic binding assay on rat spinal cord sections, where the autoradiographic signal of 125I-galanin and its dose-dependent displacement by galnon could be studied best. Attenuation of 125I-galanin binding was detected at 1, 3, and 5 μM concentration of galnon by 23 ± 6%, 21 ± 10%, and 42 ± 11%, respectively.
Table 2.
Comparison of galanin and galnon in inhibition of adenylate cyclase activity (basal and forskolin stimulated) in membranes from rat ventral hippocampi and displacement of 125I-galanin from its specific binding sites
| EC50, M of inhibition of adenylate cyclase activity in rat hippocampal membranes
|
KD, M
|
|||
|---|---|---|---|---|
| Basal | Forskolin stimulated | Rat hippocampus | Bowes cells (GalR1) | |
| Galanin | 1.1 × 10−9 | 1.1 × 10−9 | 0.8 × 10−9 | 0.4 × 10−9 |
| Galnon | 8 × 10−6 | 10 × 10−6 | 4.8 × 10−6 | 2.9 × 10−6 |
The activity of galnon on adenylate cyclase activity was studied. Like galanin, galnon inhibited both basal and forskolin-stimulated adenylate cyclase activity (Table 2). Galnon (10 μM) inhibited basal cAMP production by 25%, whereas the inhibitory effect of 10 μM galanin was 36%. Inhibition of adenylate cyclase activity suggested that galnon exhibited agonist-like properties at galanin receptors.
Anticonvulsant Properties of Galnon.
Effects of systemic galnon in mice.
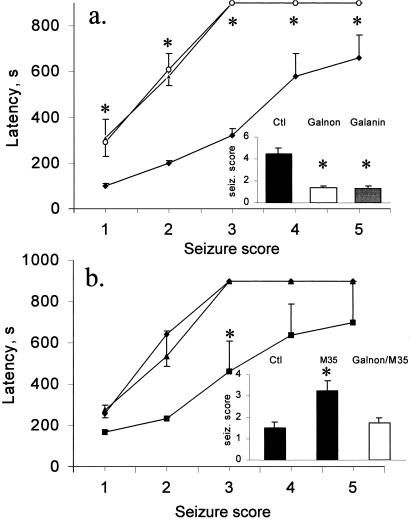
In the first set of experiments, we induced seizures with 40 mg/kg of PTZ (i.p.), and injected 2 mg/kg (3 μmol/kg) of galnon (i.p.) 15 min before PTZ injection. Galnon lowered the maximal seizure score from 4.5 (control mice) to 1.45 (galnon-treated mice), and increased seizure latency 3-fold compared with controls (P < 0.01, Fig. 2a). The anticonvulsant effect of galnon was comparable to that of galanin administered ICV in a dose 0.5 nmol (9).
Figure 2.
Effects of galnon (2 mg/kg i.p.) and/or M35 (0.5 nmol ICV) on PTZ-induced seizures on mice. Data are presented as mean ± SEM. (Insets) Maximal seizure scores (mean ± SEM). (a) Effect of galnon on PTZ-induced seizures. Galanin (0.5 nmol ICV, ○), galnon (2 mg/kg i.p., ▴) treated animals, and controls (DMSO i.p., ⧫). *, P < 0.01 vs. control. (b) Effects of coadministration of M35 and galnon on PTZ-induced seizures. Animals treated with M35 (■), with M35 and galnon (▴); controls (⧫). *, P < 0.05 versus control.
Next, we examined the pattern of PTZ-induced convulsions (30 mg/kg i.p.) in mice treated ICV with the galanin receptor antagonist M35 and galnon (i.p.). In accordance with our previous finding (9) M35 increased the severity of PTZ-induced convulsions. Galnon (Fig. 2b) completely abolished the seizure-facilitating effect of M35.
Effects of intrahippocampal galnon in rats.
SSSE consisted of recurrent limbic seizures of varying intensity (stages 1–5), which lasted for 12–18 h after PPS was discontinued. Behavioral seizures were accompanied by high-frequency (13+ Hz) and amplitude (1+ mV) discharges with the duration of individual events between 30 s and 3 min. Between the seizures, ictal spikes with the amplitude of 0.8 mV and more and frequency of 3 Hz and less were continuously generated (spike frequency of 3 Hz and more was recognized as a seizure) (8).
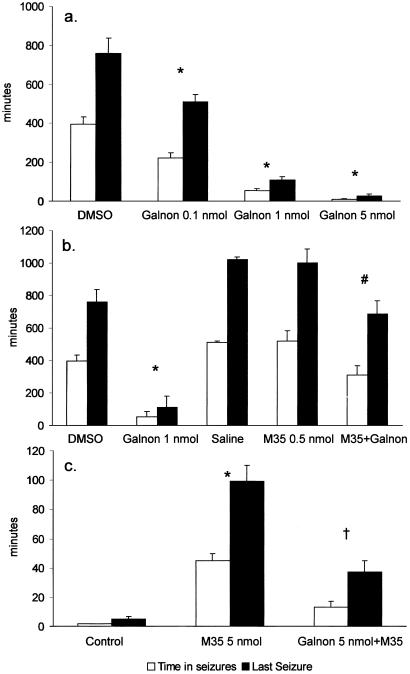
Intrahippocampal administration of galnon shortened the duration of SSSE and decreased the time spent in seizures in a dose-dependent manner (Fig. 3a). In the maximal dose used (5 nmol), galnon shortened SSSE duration to 28 ± 8 min, from 760 ± 77 min in controls (P < 0.05). The anticonvulsant effects of galnon were abolished by pretreatment with the galanin receptor antagonist M35, when the latter was administered in a dose that alone, as it has been previously reported (8), did not alter the course of SSSE (0.5 nmol, Fig. 3b). When galnon was injected immediately after M35, both time spent in seizures and the duration of SSSE were significantly higher than in galnon-treated rats without M35, and these parameters did not differ from those in control animals (Fig. 3b).
Figure 3.
Effects of galnon on SSSE induced by 30 min of PPS. (a) Intrahippocampal injection of galnon 10 min after the end of PPS attenuated SSSE in a dose-dependent manner. P < 0.05: * vs. control (50% DMSO). (b) Anticonvulsant effects of galnon in animal model of SSSE were M35 sensitive. M35 was injected 10 min after PPS, followed 5 min later by galanin or galnon. The amount of galnon, which attenuated SSSE, failed to do so in M35-pretreated rats. P < 0.05: *, vs. DMSO (control for galnon); #, vs. both galnon and saline (control for M35). There were no significant differences between the two control groups (in DMSO group time in seizures 395 ± 38 min and the time of the last seizure was 760 ± 77 min; in the saline-treated group these parameters were 510 ± 65 and 1,000 ± 87 min, respectively). (c) Galnon inhibited M35-induced facilitation of SSSE. Intrahippocampal injection of M35 (5 nmol) enabled the establishment of SSSE after 7 min of PPS, which is not sufficient to induce seizures in naïve animals (control). When galnon (5 nmol) was injected 5 min before M35 administration, 7 min of PPS induced seizures, which lasted significantly less, than in M35-only treated rats. P < 0.05: *, vs. control; †, vs. M35. DMSO and saline-treated groups were pooled in one group, because there were no differences among them. Open bars: time in seizures; filled bars; time of the last seizure.
In a separate set of experiments, equimolar galnon blocked the seizure-facilitating effect of intrahippocampal injections of M35 in a amount of 5 nmol, a dose that was previously shown to potentiate seizures (9): under these conditions PPS as short as 7 min is sufficient to induce self-sustaining seizures (9). When an equimolar amount of galnon was administered into the hippocampus before M35, total seizure time and duration of SSSE were reduced from 45 ± 5 to 13 ± 4 min and from 99 ± 11 to 37 ± 8 min, respectively (Fig. 3c).
Anticonvulsant effect of galnon in antisense PNA-pretreated rats.
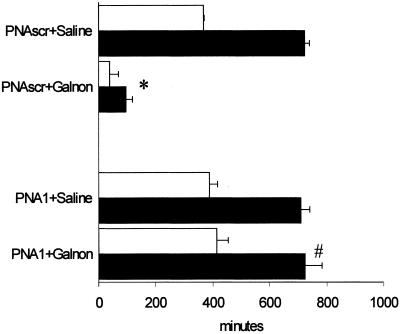
We examined the anticonvulsant effects of galnon in SSSE. As shown previously, down-regulation of GalR1 by PNA1 did not affect the parameters of SSSE (7). However, in PNA1-pretreated animals, galnon (1 nmol) had no effect on self-sustaining seizures, whereas in control rats injected with scrambled PNA, galnon reduced total seizure time 9-fold and duration of SSSE 7-fold (Fig. 4).
Figure 4.
Effect of galnon in the rats pretreated with antisense PNA targeted to the GalR1 mRNA (PNA1) or with scrambled PNA (PNAscr). *, P < 0.05 vs. PNAscr + saline; #, vs. PNAscr + galnon. Open bars: time in seizures; filled bars: time of the last seizure.
Discussion
Recent massive screening efforts, often in combination with classical medicinal chemistry optimization, have led to the identification of nonpeptide ligands for several neuropeptide receptors (12). Intriguingly, such approaches have been largely unsuccessful in the case of galanin. At the present time, galanin itself is still the best agonist for the three cloned galanin receptor subtypes. Several chimeric galanin-based peptides (M35, M40) have been shown to act as high affinity, selective antagonists at galanin receptors in tissues and/or in vivo assays (24).
The first nonpeptide galanin receptor ligand, Sch202596 (spirocoumaranon), discovered by random screening of fungal metabolites, displaces 125I-galanin in Bowes cell membranes with IC50 value of 1.7 μM (16) but does not appear ideal for optimization because of its complex chemical structure. For dithiepin-1,1,4,4-tetroxide, the second low molecular weight galanin receptor ligand described, an IC50 value of 0.17 μM in Bowes cell membranes was reported (17). This compound behaves as a galanin receptor antagonist in several functional assays, but its chemically reactive nature precludes any therapeutic use.
We report in this study on the design and synthesis of galnon, Fmoc-cyclohexylalanine-Lys-amidomethylcoumarin, a low molecular weight, blood–brain barrier-penetrating galanin receptor ligand with agonist properties, both in vitro and in vivo. We synthesized a combinatorial library of 256 compounds composed of analogues of galanin pharmacophores. Reiterative screening of binding activities of these compounds resulted in galnon, with binding affinities to galanin receptors comparable to those of spirocoumaranon and dithiepin-1,1,4,4-tetroxide in Bowes cellular membranes. Galnon was also tested in an autoradiographic binding assay on rat spinal cord sections, to confirm the binding results obtained in membranes from Bowes cells and rat hippocampus, using a different source of galanin receptors as well as different methodology. Attenuation of 125I-galanin binding was detected at different concentrations of galnon, confirming its ability to displace 125I-galanin from its binding sites in yet another system. We cannot rule out the possibility that our library contained compounds with 125I-galanin-displacing activity superior to galnon, but we did not discover them because of their low abundance (caused by inefficient coupling between their building blocks and losses in cleavage and separation steps).
Galnon was further studied in the adenylate cyclase assay to elucidate whether it has agonist- or antagonist-like properties at galanin receptor(s). It inhibited basal and forskolin-stimulated adenylate cyclase activity with a pattern similar to galanin, indicating that it acts as an agonist at galanin receptors.
To test the effects of galnon in vivo, we used the pentylenetetrazole model of epileptic seizure in mice. Galnon applied i.p lowered the maximal seizure score, increased seizure latency, and blocked the proconvulsant effect of ICV M35, hence we can assume that it penetrated the blood–brain barrier to act at galanin receptors located centrally. It must be noted that PTZ-induced convulsions represent a model for generalized absence seizures (25), whereas a model of status epilepticus used in this study is a model of focal limbic seizures. From this perspective, galnon shows a broad-spectrum anticonvulsant activity, which makes it a promising agent for managing multiple forms of epilepsy.
Experiments in rats showed that galnon also possessed strong anticonvulsant effect against SSSE, a particularly severe form of epileptic seizures that is resistant to conventional antiepileptic drugs (26). No currently available anticonvulsant in the United States pharmacopoeia acts through peptide receptors, and one major drawback of current agents is that they all work through a few, very similar mechanisms. The potency of galnon in the present studies provides evidence that a systemically active galanin agonist can be an effective anticonvulsant in rodents and deserves evaluation in a broad spectrum of seizure and epilepsy models.
We have shown previously that intrathecal administration of 21-mer PNA targeting the region 18–38 of the coding sequence of rat GalR1 mRNA (PNA1) decreased 125I-galanin binding in spinal cord (27), suggesting that PNA1 may have caused down-regulation of GalR1 expression. It was also demonstrated by immunoprecipitation analysis that the same PNA oligomer down-regulates the expression of GalR1 in vitro (20). In line with these results, we studied the effect of galnon on SSSE in PNA1-pretreated rats. Galnon completely failed to alter SSSE in the rats with GalR1 down-regulation but successfully blocked SSSE in rats treated with PNAscr. This finding suggests that galnon's anticonvulsant effects may be mediated by means of GalR1.
In conclusion, we report that galnon, 7-((9-fluorenylmethoxycarbonyl)cyclohexylalanyllysyl)amino-4-methylcoumarin, a nonpeptide ligand for galanin receptors, possesses agonist properties in vitro and in vivo and strong anticonvulsant properties in vivo.
Acknowledgments
We thank Dr. Anders Undén, Dr. Anders Juréus, Dr. Kalle Kaljuste, Dr. Ello Karelson, and Dr. Khadijeh Rezaei for advice and help. This work was supported by a grant from AstraZeneca and The Swedish Natural and Medical Research Councils and Marianne and Marcus Wallenberg's Foundation, the Research Service of the Veterans Health Administration, and Grant NS 13515 from the National Institute of Neurological Disorders and Stroke.
Abbreviations
- AMC
7-amino-4-methylcoumarin
- Boc
tert-butyloxycarbonyl
- DMF
N,N-dimethylformamide
- Fmoc
9-fluorenylmethoxycarbonyl
- PTZ
pentylenetetrazole
- GalR1
galanin receptor type 1
- PNA
peptide nucleic acid
- SSSE
self-sustaining status epilepticus
- PPS
perforant path stimulation
References
- 1.Wrenn C C, Crawley J N. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:283–299. doi: 10.1016/s0278-5846(00)00156-1. [DOI] [PubMed] [Google Scholar]
- 2.Crawley J N. Neuropeptides. 1999;33:369–375. doi: 10.1054/npep.1999.0049. [DOI] [PubMed] [Google Scholar]
- 3.Bloch G J, Butler P C, Eckersell C B, Mills R H. Ann N Y Acad Sci. 1998;863:188–205. doi: 10.1111/j.1749-6632.1998.tb10695.x. [DOI] [PubMed] [Google Scholar]
- 4.Xu X J, Hökfelt T, Bartfai T, Wiesenfeld-Hallin Z. Neuropeptides. 2000;34:137–147. doi: 10.1054/npep.2000.0820. [DOI] [PubMed] [Google Scholar]
- 5.Sjöholm A A, Efendic S. Exp Clin Endocrinol Diabetes. 2001;109:S109–S121. doi: 10.1055/s-2001-18574. [DOI] [PubMed] [Google Scholar]
- 6.Todd J F, Edwards C M, Ghatei M A, Bloom S R. Am J Physiol. 2000;278:E1060–E1066. doi: 10.1152/ajpendo.2000.278.6.E1060. [DOI] [PubMed] [Google Scholar]
- 7.Mazarati A, Langel Ü, Bartfai T. Neuroscientist. 2001;7:506–517. doi: 10.1177/107385840100700607. [DOI] [PubMed] [Google Scholar]
- 8.Mazarati A M, Liu H, Soomets U, Sankar R, Shin D, Katsumori H, Langel Ü, Wasterlain C G. J Neurosci. 1998;18:10070–10077. doi: 10.1523/JNEUROSCI.18-23-10070.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazarati A M, Hohmann J G, Bacon A, Liu H, Sankar R, Steiner R A, Wynick D, Wasterlain C G. J Neurosci. 2000;20:6276–6281. doi: 10.1523/JNEUROSCI.20-16-06276.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zini S, Roisin M P, Langel Ü, Bartfai T, Ben-Ari Y. Eur J Pharmacol Mol Pharm. 1993;245:1–7. doi: 10.1016/0922-4106(93)90162-3. [DOI] [PubMed] [Google Scholar]
- 11.Xu Z Q, Ma X, Soomets U, Langel Ü, Hökfelt T. Proc Natl Acad Sci USA. 1999;96:14583–14587. doi: 10.1073/pnas.96.25.14583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freidinger R M. Curr Opin Chem Biol. 1999;3:395–406. doi: 10.1016/S1367-5931(99)80060-X. [DOI] [PubMed] [Google Scholar]
- 13.Beeley N R A. Drug Discovery Today. 2000;5:354–363. doi: 10.1016/s1359-6446(00)01528-2. [DOI] [PubMed] [Google Scholar]
- 14.Branchek T A, Smith K E, Gerald C, Walker M W. Trends Pharmacol Sci. 2000;21:109–117. doi: 10.1016/s0165-6147(00)01446-2. [DOI] [PubMed] [Google Scholar]
- 15.Floren A, Land T, Langel Ü. Neuropeptides. 2000;34:331–337. doi: 10.1054/npep.2000.0808. [DOI] [PubMed] [Google Scholar]
- 16.Chu M, Mierzwa R, Truumees I, King A, Sapidou E, Barrabee E, Terracciano J, Patel M G, Gullo V P, Burrier R, et al. Tetrahedron Lett. 1997;38:6111–6114. [Google Scholar]
- 17.Scott M K, Ross T M, Lee D H, Wang H Y, Shank R P, Wild K D, Davis C B, Crooke J J, Potocki A C, Reitz A B. Bioorg Med Chem. 2000;8:1383–1391. doi: 10.1016/s0968-0896(00)00062-6. [DOI] [PubMed] [Google Scholar]
- 18.Langel Ü, Land T, Bartfai T. Int J Pept Protein Res. 1992;39:516–522. doi: 10.1111/j.1399-3011.1992.tb00282.x. [DOI] [PubMed] [Google Scholar]
- 19.Koch T. In: Peptide Nucleic Acids: Protocols and Applications. Nielsen P E, Egholm M, editors. Norfolk, VA: Horizon Scientific; 1999. pp. 21–39. [Google Scholar]
- 20.Pooga M, Soomets U, Hällbrink M, Valkna A, Saar K, Rezaei K, Kahl U, Hao J-X, Xu X-J, Wiesenfeld-Hallin Z, et al. Nat Biotechnol. 1998;16:857–861. doi: 10.1038/nbt0998-857. [DOI] [PubMed] [Google Scholar]
- 21.Land T, Langel Ü, Fisone G, Bedecs K, Bartfai T. Methods Neurosci. 1991;5:225–234. [Google Scholar]
- 22.Valkna A, Juréus A, Karelson E, Zilmer M, Bartfai T, Langel Ü. Neurosci Lett. 1995;187:75–78. doi: 10.1016/0304-3940(95)11340-1. [DOI] [PubMed] [Google Scholar]
- 23.Young W S, 3rd, Kuhar M J. Brain Res. 1979;179:255–270. doi: 10.1016/0006-8993(79)90442-6. [DOI] [PubMed] [Google Scholar]
- 24.Langel Ü, Bartfai T. Ann N Y Acad Sci. 1998;863:86–93. doi: 10.1111/j.1749-6632.1998.tb10686.x. [DOI] [PubMed] [Google Scholar]
- 25.Snead O C., 3rd J Neural Transm Suppl. 1992;35:7–19. doi: 10.1007/978-3-7091-9206-1_2. [DOI] [PubMed] [Google Scholar]
- 26.Mazarati A M, Baldwin R A, Sankar R, Wasterlain C G. Brain Res. 1998;814:179–185. doi: 10.1016/s0006-8993(98)01080-4. [DOI] [PubMed] [Google Scholar]
- 27.Rezaei K, Xu I S, Wu W P, Shi T J, Soomets U, Land T, Xu X J, Wiesenfeld-Hallin Z, Hökfelt T, Bartfai T, Langel Ü. NeuroReport. 2001;12:317–320. doi: 10.1097/00001756-200102120-00027. [DOI] [PubMed] [Google Scholar]
- 28.Kokaia M, Holmberg K, Nanobashvili A, Xu Z-QD, Kokaia Z, Lendahl U, Hilke S, Theodorsson E, Kahl U, Bartfai T, et al. Proc Natl Acad Sci USA. 2001;98:14006–14011. doi: 10.1073/pnas.231496298. [DOI] [PMC free article] [PubMed] [Google Scholar]