Abstract
Atrial natriuretic peptide (ANP) is an important regulator of arterial blood pressure. The mechanisms mediating its hypotensive effects are complex and involve the inhibition of the sympathetic and renin-angiotensin-aldosterone (RAA) systems, increased diuresis/natriuresis, vasodilation, and enhanced vascular permeability. In particular, the contribution of the direct vasodilating effect of ANP to the hypotensive actions remains controversial, because variable levels of the ANP receptor, guanylyl cyclase A (GC-A), are expressed in different vascular beds. The objective of our study was to determine whether a selective deletion of GC-A in vascular smooth muscle would affect the hypotensive actions of ANP. We first created a mutant allele of mouse GC-A by flanking a required exon with loxP sequences. Crossing floxed GC-A with SM22-Cre transgene mice expressing Cre recombinase in smooth muscle cells (SMC) resulted in mice in which vascular GC-A mRNA expression was reduced by ≈80%. Accordingly, the relaxing effects of ANP on isolated vessels from these mice were abolished; despite this fact, chronic arterial blood pressure of awake SMC GC-A KO mice was normal. Infusion of ANP caused immediate decreases in blood pressure in floxed GC-A but not in SMC GC-A knockout mice. Furthermore, acute vascular volume expansion, which causes release of cardiac ANP, did not affect resting blood pressure of floxed GC-A mice, but rapidly and significantly increased blood pressure of SMC GC-A knockout mice. We conclude that vascular GC-A is dispensable in the chronic and critical in the acute moderation of arterial blood pressure by ANP.
The heart is involved in cardiovascular homeostasis by the secretion of two natriuretic peptides (NPs), atrial (ANP) and B-type natriuretic peptides (BNP) (1–3). These NPs activate a common guanylyl cyclase A (GC-A) receptor expressed in a wide variety of tissues, thereby increasing intracellular cGMP concentrations (4). NPs are secreted from atrial granules into the circulation in response to acute or chronic atrial stretch to act as antihypertensive and antihypervolemic factors through GC-A in distant organs (1–4). In addition, in chronic hemodynamic overload there is a significant increase in ANP and, in particular, BNP expression in cardiac ventricles (3). It is postulated that in this situation, NPs not only act as endocrine factors but also exert local antihypertrophic and antifibrotic actions through the GC-A that is expressed in the heart (5–7). A second specific receptor subtype that also triggers the effects of NPs is the C receptor (NPR-C) (1), a clearance receptor that is devoid of guanylyl cyclase activity and mediates the cellular internalization and degradation of NPs. It may also participate in mediating some of their cellular actions by means of coupling to Gi proteins and negative modulation of adenylyl cyclase activity (8). However, within the cardiovascular system, the primary action of the NPR-C seems to be the modulation of circulating and local NP concentrations (9).
The important physiological role of the ANP/GC-A system in arterial blood pressure (ABP) and blood volume homeostasis has been emphasized in different genetic mouse models. Targeted deletion of the peptide (ANP−/−) or its receptor (GC-A−/−), leads to severe, chronic arterial hypertension (10–13). In contrast, overexpression of ANP or GC-A elicits a “dose-dependent” fall in ABP (14, 15). The main known GC-A-mediated hypotensive actions of ANP include relaxation of vascular smooth muscle, resulting in vasodilation (16, 17); increased vascular permeability, facilitating plasma extravasation (18); inhibition of the renin–angiotensin–aldosterone (RAA) system by direct actions on juxtaglomerular cells and the adrenal glomerulosa (19, 20); inhibition of sympathetic tone (21); and stimulation of renal function, leading to increased natriuresis and diuresis (22–24). In particular, the reduction of peripheral vascular resistance is a main component of the hypotensive actions of ANP. However, the mechanisms by which ANP exerts this effect remain controversial. Many studies characterized the direct vasodilating effects of ANP on large arteries such as the aorta, and also in the pulmonary, renal, and coronary vascular bed (13, 16, 17, 23). Other studies indicated that the resistance vasculature, especially the mesenteric vessels, has a scarcity of GC-A receptors (25) and is insensitive to direct relaxation by ANP (26, 27).
The objective of our study was to determine whether a selective inhibition of the direct vasodilating effects of ANP (preserving all other cardiovascular effects of the peptide) would affect its hypotensive actions. Using Cre-lox technology to achieve a smooth muscle-restricted knockout of GC-A, we now demonstrate that the direct vasodilating effect of ANP is dispensable in chronic blood pressure homeostasis, but has a critical role in the acute regulation of arterial blood pressure by ANP.
Materials and Methods
All experimental protocols included in this manuscript were approved by the local animal care committee and conform with the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH Publication No. 85–23, revised 1996).
Generation of Floxed GC-A Mice.
The whole murine GC-A gene (exons 1–22) and additional upstream sequences were isolated from a 129/SvJ mouse genomic library (Genome Systems, St. Louis). The targeting vector contained exons 1–13 (with introns) and additional 3.8 kb of 5′ sequence of the GC-A gene, the Herpes simplex virus thymidine kinase gene (at −3.8 kb of exon 1), a loxP-flanked neomycin resistance cassette (Neo, at −2.6 kb of exon 1), and a third loxP site in the middle of intron 1 (Fig. 1). The construct was 18.5 kb in length, and the 5′ and 3′ homologous sequences for targeting were 1.2 kb and 6.3 kb, respectively. After linearization with NotI, the targeting vector was electroporated into R1 ES cells (kindly provided by Andras Nagy; Samuel Lunenfeld Research Institute, Toronto) (28). Geneticin (G418) and FIAU [1-(2-deoxy-2-fluoro-β-d-arabinofuranosyl)-5-iodouracil]-resistant clones were screened by PCR for homologous recombination of the short arm. Positive embryonic stem (ES)-cell clones were expanded, and genomic DNAs were isolated for Southern blot analysis to verify the third loxP site through the additional BamHI site in intron 1 with probe A (Fig. 1 A and B). Five independent positive clones containing all three loxP sites (a loxP-flanked Neo cassette upstream of the promoter; a third loxP site in the middle of intron 1) were injected into C57/Bl6 blastocysts. Chimeric animals from two independent ES cell clones were used to establish homozygous floxed GC-A mice on a C57Bl6/129Sv mixed background.
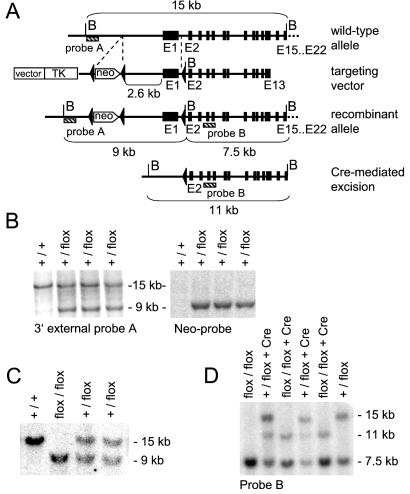
Figure 1.
Generation and Southern Blot characterization of flox GC-A (GC-A +/flox) ES cells, “flox/flox GC-A” mice (F2) and “flox/flox GC-A × SM22-Cretg” mice (F4). (A) Restriction map of a part of the mouse GC-A gene locus, the targeting construct, the homologous recombinant allele and the SM22-Cre-generated deletion allele (B, BamHI site). (B) Southern blot analysis of homologous recombination in ES cells. DNAs from ES cells were digested with BamHI and probed with the external probe A and with a neo probe; the 15-kb and 9-kb bands represent the wild-type and targeted alleles, respectively. Hybridization with the neo probe indicates that a single recombination has occurred in ES cell clones. (C) Southern blot analyses of genomic tail DNA obtained from wild-type (GC-A+/+), heterozygous (+/flox) and homozygous (flox/flox) floxed-GC-A mice (F2). DNAs were digested with BamHI and probed with probe A. (D) Southern blot analysis of genomic tail DNA obtained from the flox/flox GC-A F4-offspring, demonstrating the deletion event in SMC within the vascular bed of the tail from mice harboring the SM22-Cre-transgene (SMC GC-A KO mice). The Cre-mediated deletion event abolishes the additional BamHI site inserted in intron 1 by the recombination event. BamHI-digested DNA was probed with probe B; 15-kb, 11-kb, and 7.5-kb bands represent the wild-type, deletion allele, and targeted allele, respectively.
Generation of SM22-Cre Transgenic Mice (SM22-Cretg).
The smooth muscle-specific SM22 promoter was excised from the plasmid SM2736 (generous gift from Eric Olson, Univ. of Texas Southwestern, Dallas; ref. 29) as a 2.8-kb fragment and then ligated to the plasmid NukCre, which contains the modified bacteriophage P1 Cre recombinase gene that includes translational consensus and nuclear localization signals (generously provided by Jamie Marth, Univ. of California at San Diego, La Jolla). The resulting construct was cut with NotI to remove the vector backbone and used to express the nuclear localized Cre recombinase under control of the SM22 promoter in vascular smooth muscle cells (SMC). The transgenic mice (SM22-Cretg) were generated from C57BL6xSJLF2 oocytes as described (30), and recombinase activity by 8 weeks of age was confirmed through breeding with the ROSA-26 β-galactosidase (β-gal) reporter mice available from Jackson Laboratories (31). SM22-Cretg animals were genotyped by PCR. The Cre transgene was detected by using primers within the coding region (MG-Cre800, GCTGCCACGACCAAGTGACAGCAATG; and MG-cre1200, GTAGTTATTCGGATCATCAGCTACAC) or primers localized in the SM22 promoter and Cre coding region (MG-gSM22, CAGACACCGAAGCTACTCTCCTTCC; and MG-gCre, CGCATAACCAGTGAAACAGCATTGC). To detect the LacZ transgene, we used the primers MG-lacZ 1080, CCTCTGCATGGTCAGGTCATGGATG; and MG-lacZ 1620 reverse, GTGGGCGTATTCGCAAAGGATCAGC.
LacZ Staining of Tissue Sections.
Double transgenic mice (SM22-Cre and ROSA26 β-gal) and ROSA β-gal transgenic controls were perfused with 3% paraformaldehyde, and tissues were harvested and immersed in 20% sucrose in phosphate buffer for 3 h. Tissues were frozen in tissue-tek, sectioned at 25 μm, and stained by using the β-gal staining kit from Specialty Media (www.specialtymedia.com).
Generation of Mice with a Selective Deletion of GC-A in SMC (SMC GC-A Knockout Mice).
SMC-specific GC-A knockout mice were derived from breedings of floxed GC-A mice with heterozygous SM22-Cretg mice (flox/flox GC-A × SM22-Cretg) (Fig. 1D). All mice used in the present study were on a mixed C57Bl6/129Sv background. Chronic blood pressure measurements were in male and female mice, 2–5 months old. All other experiments were performed with 2- to 4-month-old male mice.
Quantitation of GC-A mRNA Expression by Real-Time Reverse Transcription (RT)-PCR.
A quantitative analysis of vascular GC-A mRNA expression in isolated arteries from wild-type (GC-A+/+), floxed GC-A, and SMC GC-A KO mice was performed by RT-PCR using the LightCycler Detection System (Roche Diagnostics) (32). The following oligonucleotide primers and fluorogenic probes were used (Tib Molbiol, Berlin): for GC-A, sense primer (located in exon 1) 5′-CTCAACATCACAGTAAATCACC; antisense primer (located in exon 5) 3′-CCTGAAGGCACCTGTCTCG; detection probe 5′-LC-Red640 ACATAGTCCTCCCCAGTCAGGCCAGCATCC-p-3′; anchor probe 5′-GCTTTGCCCAAACACATCCAGGTGGAAGAA-fluorescein (both probes located in exon 2). Quantitative PCR analysis was performed by using the lightcycler software (Roche Diagnostics). Data were calculated from the kinetic curve of the PCR by interpolation with a standard curve generated by using known amounts of the target DNA. GC-A transcripts were normalized to GAPDH (glyceraldehyde-3-phosphate dehydrogenase; ref. 32).
Guanylyl Cyclase Assay.
ANP-dependent guanylyl cyclase activity in membranes prepared from the adrenal glands, lungs, kidneys, dissected renal hilus, and thoracic aorta was determined according to Goy et al. (33). For adrenal gland and aorta, tissues were pooled from three mice to obtain sufficient material. To initiate cyclase activity, 100 μg of membrane protein was incubated in assay buffer [50 mM Hepes/8 mM MgCl2/2 mM 3-isobutyl-1-methylxanthine/4 mM ATP/4 mM GTP/60 mM phosphocreatine/800 μg/ml creatine phosphokinase (185 units/mg)/1 mg/ml BSA] at 37°C, with or without 0.1 μM ANP (33). After 10 min of incubation, the reaction was stopped by addition of ice-cold 100% (vol/vol) ethanol (final concentration, 70%). After centrifugation (3,000 × g, 5 min, 4°C), the supernatants were dried in a speed vacuum concentrator, resuspended in sodium acetate buffer (50 mM, pH 6.0), and acetylated, and the cGMP content was determined by RIA (32).
In Vitro Studies of Vascular Tone.
Ring segments of the descending thoracic aorta (luminal diameter, Ø, 2,000–2,200 μm), pulmonary artery (Ø, 1,000–1,200 μm), distal femoral artery (Ø, 200–400 μm), and third-order side branches of the renal artery (Ø, 150–200 μm) were mounted in a myograph (model 410A; J.P. Trading, Aarhus, Denmark) for recording of isometric wall tension (34). After a 15-min equilibration in temperated (37°C), oxygenated (95% O2/5% CO2) Krebs–Ringer bicarbonate (KRB) buffer, rings were contracted with phenylephrine (PE, 10 μM; Sigma), and cumulative concentrations of ANP (Calbiochem-Novabiochem, Bad Soden, Germany) and sodium nitroprusside (Sigma) were tested.
Measurements of Arterial Blood Pressure.
Systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate were measured in conscious mice by a tail cuff method (Softron, Tokyo) (13).
Acute Effects of Exogenous ANP or Volume Expansion on ABP.
To study the contribution of vascular GC-A to the acute moderation of ABP by ANP, we tested the effects of exogenous ANP administration and of acute vascular volume expansion. Mice were previously trained for tail cuff measurements for at least 4 days, and were then anesthetized with xylazine [1 μg per g body weight (BW)] and ketamine (100 μg per g BW). The right jugular vein was cannulated with PE-tubing (inside diameter 0.8 mm), and the tubing was exteriorized between the scapulae (13). The infusion studies were carried out in conscious mice after 2 days of recovery by connecting the jugular catheter to a microinfusion pump (Harvard Apparatus, South Natick, MA). ABP was measured continuously by tail cuff before and during the infusions (13). ANP was infused with 500 ng per kg BW per min (2.5 μl per kg BW per h) for 45 min (13). Before volume expansion, basal ABP was determined during infusion with lactated Ringer's solution containing 4.5% BSA at a rate of 4.3 μl per g BW per h. After 15 min, the rate was increased to 114 μl per g BW per h for 15 min to give a bolus of ≈3% of BW. The infusion was continued at 4.3 μl per g BW per h for another 15 min (24). This protocol has been shown to increase cardiac ANP release and plasma ANP (24).
Data Analysis.
Results are expressed as means ± SE. Statistical comparison of wild-type and mutant mice was performed by unpaired Student's t test. The serial changes in ABP and heart rate before (control period) and during/after infusions were analyzed by a repeated-measures ANOVA followed by Bonferroni and Student-Newman-Keuls post hoc test for multiple comparisons (graphpad instat software). P values of less than 0.05 were considered statistically significant.
Results
Generation of Floxed GC-A Mice.
To obtain a Cre recombinase-mediated, loxP-directed deletion of the GC-A gene, a targeting construct was designed in which a Neo cassette flanked by loxP sites was introduced upstream from the promoter region (at −2.6 kb from exon 1), and a third loxP site was introduced into the first intron of the murine GC-A gene. Thereby the first exon, which contains the ATG start codon encoding the signal peptide and is essential for GC-A transcription and function, was flanked by loxP sites (Fig. 1A). Because the loxP sequences and the neomycin-expression cassette lie within noncoding segments of the gene, the floxed allele was expected to function normally. Indeed, homozygous animals carrying two floxed GC-A alleles (floxed GC-A mice, Fig. 1C) are viable, have normal body weights, breed normally, and are indistinguishable from wild-type littermates. The mRNA expression of GC-A (in isolated vessels, heart, and kidney), resting ABP and effects of ANP on isolated vessels (in vitro) and ABP (in vivo) of homozygous floxed GC-A mice is identical to wild-type GC-A+/+ mice (see below and Figs. 3–7, refs. 5–7).
Figure 3.
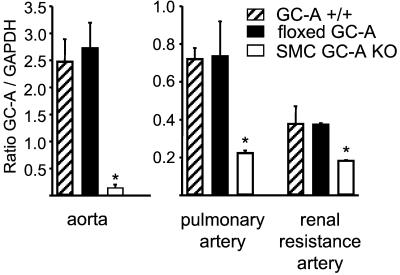
Quantitative RT-PCR analyses (lightcycler) of GC-A mRNA in aortic, renal (third-order side branches), and pulmonary arteries from wild-type (GC-A+/+), floxed GC-A, and SMC GC-A KO mice. Signal intensities were normalized to GAPDH. Expression is significantly decreased in SMC GC-A KO as compared with GC-A+/+ and floxed GC-A mice (n = 4 of each vessel and genotype; *, P < 0.05 compared with floxed GC-A).
Figure 7.
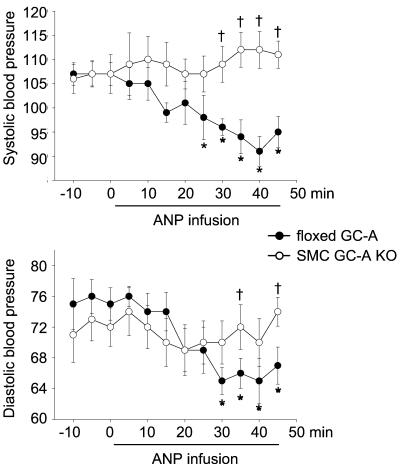
Effect of i.v. ANP (500 ng per kg BW per min, as infusion) on arterial blood pressure of floxed GC-A and SMC GC-A KO mice. Measurements were made by tail cuff in awake mice. (Upper) Systolic blood pressure. (Lower) Diastolic blood pressure. (n = 9 per genotype; *, P < 0.05 vs. pretreatment at 0 min; †, P < 0.05 vs. floxed GC-A).
Expression Pattern of the SM22-Cre Transgene.
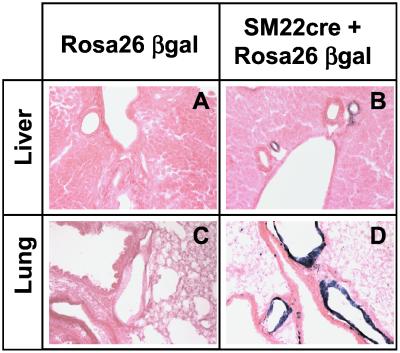
The expression pattern of the Cre mRNA mimicked that of the SM22 mRNA, with the highest levels in the aorta, intestine, and uterus of SM22-Cretg mice, all of which have a large smooth muscle compartment (data not shown). Lower levels of Cre mRNA were detected in all other organs, most likely reflecting the presence of vascular smooth muscle in these tissues. Recombination was analyzed by breeding the SM22-Cretg mice with ROSA-26 β-gal reporter mice (31) and then analyzing X-Gal (5-bromo-4-chloro-3-indolyl β-d-galactoside) staining in tissue sections. As shown in Fig. 2, vascular SMC of hepatic and pulmonary arteries show efficient recombination and accordingly intensive staining, whereas nonmuscle cells do not undergo recombination.
Figure 2.
Transverse sections of X-gal-stained liver and lung tissue, derived from ROSA26 β-gal animals with and without the SM22-Cre transgene. Control animals show no staining (A and C), whereas in double transgenics (B and D) hepatic arteries (B) as well as pulmonary arteries (D) show strong staining. No expression outside the vascular walls is detected. Data are representative of three experiments per genotype.
High Efficiency, SMC-Selective Deletion of the Floxed GC-A Gene.
Homozygous floxed GC-A mice (F2) were mated with SM22-Cretg mice. As shown in Fig. 1D, Southern blot analysis of tail DNA obtained from the resulting “flox/flox GC-A × SM22-Cretg” offspring (F4) demonstrated the deletion event, which occurs in SMC within the vascular bed of the tail. Quantitative RT-PCR analysis demonstrated that the expression of GC-A mRNA in isolated blood vessels of homozygous floxed GC-A offspring not harboring the SM22-Cre transgene is identical to wild-type GC-A+/+ mice (Fig. 3). In contrast, in floxed GC-A mice harboring the SM22-Cre transgene (SMC GC-A KO mice), vascular GC-A mRNA expression is reduced by 50–80% (Fig. 3).
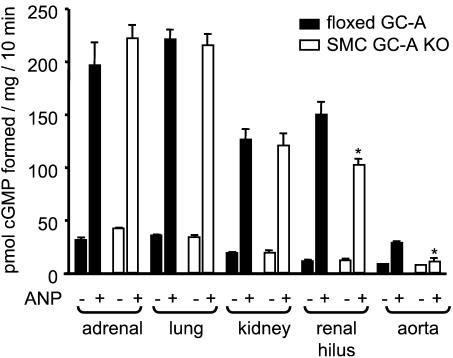
To further demonstrate the selective deletion of GC-A in SMC, membrane-bound guanylyl cyclase activity was assayed in different organs obtained from floxed GC-A and SMC GC-A KO mice. Fig. 4 shows that ANP-stimulated enzyme activities in plasma membranes from adrenal glands, whole kidneys, and lungs did not differ between genotypes. In contrast, in the renal hilus from SMC GC-A KO mice (the portion of the kidney containing the renal artery and its main branches), ANP-stimulated guanylyl cyclase activity was significantly lower. In agreement with previous studies in wild-type mice (33), the ANP-stimulated enzyme activity in the aortas of floxed GC-A mice was rather low (3.2-fold vs. basal, see Fig. 4), which is probably related to the difficult preparation of SMC membranes. In isolated aortas of SMC GC-A KO mice, ANP-dependent activity was almost abolished (Fig. 4).
Figure 4.
Basal- and ANP (0.1 μM)-stimulated guanylyl cyclase activity of plasma membranes isolated from different organs obtained from floxed GC-A and SMC GC-A KO mice. Enzymatic activity is expressed as picomoles of cGMP formed per milligram of protein over 10 min (n = 4 for each tissue and genotype; *, P < 0.05 compared with floxed GC-A).
Vasorelaxing Responses to ANP Are Abolished in Isolated Arteries from SMC GC-A KO mice.
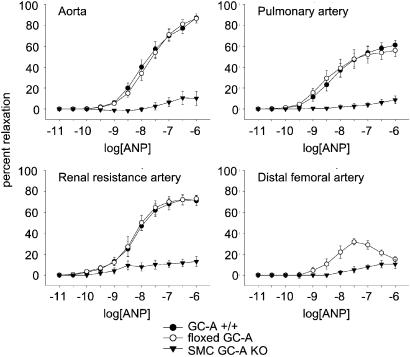
Contractile responses to 10 μM PE were stable in all four types of arteries (thoracic aorta, pulmonary artery, distal femoral artery, and third-order side branches of the renal artery), with a slight reduction of the initial maximum of contraction by about 5–10% over 30 min. These α1-adrenergic contractions were not different between respective arteries obtained from wild-type (GC-A+/+), floxed GC-A and SMC GC-A KO mice. As shown in Fig. 5, in preconstricted arteries from GC-A+/+ and floxed GC-A mice, ANP induced similar rapid and concentration-dependent relaxations. In the femoral arteries, the relaxing responses to ANP were clearly less pronounced (Fig. 5). In all preconstricted arteries from SMC GC-A KO mice, the vasorelaxing responses to ANP were abolished. There was a small relaxation in response to high ANP concentrations, which was also observed in PE-contracted arteries in the absence of ANP, indicating a spontaneous (ANP-independent) loss of tone.
Figure 5.
Effects of ANP on preconstricted arteries from GC-A+/+, floxed GC-A, and SMC GC-A KO mice. Concentration–response curves. The vasorelaxing effects of ANP are presented as a percentage of the phenylephrine (PE, 10 μM)-induced contraction (n = 6 determinations, corresponding to 6 mice; except n = 2 for femoral artery).
After repeated washout with KRB buffer, a second PE contraction was obtained in all vessels and the relaxing response to sodium nitroprusside (0.1–100 μM) was tested. The nitric oxide donor-induced concentration-dependent relaxations of all four tested types of arteries, up-to a 80–90% inhibition of the PE contraction in isolated vessels from GC-A+/+, floxed GC-A, and SMC GC-A KO mice (data not shown).
SMC GC-A KO Mice Exhibit Normal Blood Pressure Under Resting Conditions.
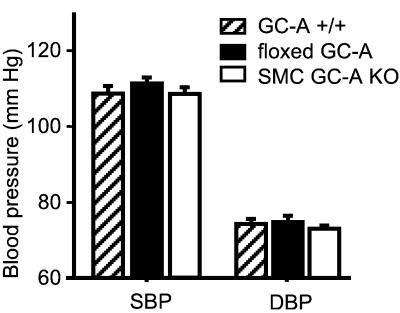
To ascertain whether deletion of vascular smooth muscle GC-A affects chronic ABP, conscious homozygous floxed GC-A and SMC GC-A KO mice (n = 20 per genotype) were subjected to ABP measurements by tail cuff. All experiments were carried out in a blinded fashion on 2- to 5-month-old male and female mice eating a standard rodent chow (0.7% NaCl). Remarkably, under resting conditions, both homozygous floxed GC-A and SMC GC-A KO mice displayed normal systolic and diastolic blood pressure values as compared with wild-type (GC-A+/+) mice (Fig. 6). Also, heart rates did not differ significantly between the groups (in beats per min: floxed GC-A, 650 ± 12; SMC GC-A KO mice, 680 ± 17; GC-A+/+, 630 ± 14; n = 20).
Figure 6.
Effect of disruption of the vascular GC-A gene on arterial blood pressure of conscious mice. Meaurements were in wild-type (GC-A+/+), floxed GC-A, and SMC GC-A KO mice, by tail cuff. (n = 20 per genotype.)
Acute Effects of Exogenous ANP on Blood Pressure Are Abolished in SMC GC-A KO Mice.
Infusion of ANP at a dose of 500 ng per kg BW per min to floxed GC-A mice caused a significant decrease in SBP and DBP (Fig. 7). The delay in the hypotensive response is very likely caused by the delayed passage of ANP through the indwelling jugular catheter. Similar effects of ANP have been reported in wild-type, GC-A+/+ mice (13). In contrast, ANP-infusion failed to lower blood pressure in SMC GC-A KO mice (Fig. 7). There were no significant changes in heart rate during ANP infusion in either genotype.
Acute Vascular Volume Expansion Increases Blood Pressure in SMC GC-A KO but Not in Floxed GC-A Mice.
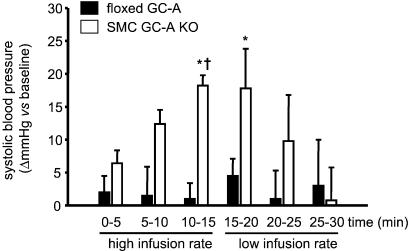
Volume expansion did not affect ABP in conscious floxed GC-A mice, but significantly raised ABP in the SMC GC-A KO mice. A rapid, significant increase in SBP (Fig. 8) and slight increase in DBP (from 74 ± 1.5 to 83 ± 4.8 mmHg; 1 mmHG = 133 Pa) was observed during the 15 min of high-volume infusion in SMC GC-A KO mice. These changes were fully reversible 10 min after volume overload. A small but not significant decrease in heart rate was observed in both groups of mice (in beats per min; floxed GC-A from basal 568 ± 35 to 516 ± 37 at 15 min of volume expansion; SMC GC-A KO from 546 ± 23 to 522 ± 13).
Figure 8.
Effect of acute vascular volume expansion on systolic blood pressure of floxed GC-A and SMC GC-A KO mice. Changes in SBP (Δ mmHg) during and immediately after volume expansion as compared with baseline (before volume expansion) (n = 5 per genotype; *, P < 0.05 vs. baseline; †, P < 0.05 vs. floxed GC-A).
Discussion
The present study provides a report of a tissue-specific inactivation of the GC-A gene in mice by using the loxP/Cre system. Selective inactivation of GC-A in SMC abolishes the direct vasorelaxing effects of ANP on isolated arteries. Surprisingly, contrasting with the hypertensive phenotype of mice with a generalized deletion of GC-A (GC-A−/−) (11, 12), chronic ABP of SMC GC-A KO mice is completely normal. However, the acute hypotensive response to exogenous ANP is totally prevented. Moreover, acute vascular volume expansion, which stimulates cardiac ANP release, does not affect ABP in mice with normal GC-A levels, but markedly and rapidly increases ABP in SMC GC-A KO mice. We conclude that vascular GC-A is dispensable in the chronic and critical in the acute regulation of blood pressure by ANP.
To achieve a selective deletion of GC-A, we created floxed GC-A mice, which then were mated with transgene mice carrying a SM22-directed overexpression of Cre recombinase in SMCs. Based on quantitative mRNA analyses of isolated blood vessels obtained from the resulting SMC GC-A KO offspring, we estimate the efficacy of the vascular GC-A deletion to be 50–80%. Furthermore, ANP-stimulated guanylyl cyclase activity was nearly abolished in dissected aortas from SMC GC-A KO mice and markedly reduced in renal hilus, a portion of the kidney that contains the renal artery and its main branches, and therefore a high densitiy of vascular SMC. The GC-A mRNA and enzyme activity that remains in the isolated vessel preparations might reflect several sources. It is possible that Cre was not adequatelly expressed in a subset of SMC. Alternatively, Cre might delete only a single copy of the floxed GC-A gene because of low levels of expression. Finally, GC-A mRNA might derive from vascular endothelium as well as from contaminating neighboring cells of the adventitia and surrounding tissue. Indeed, functional analyses with isolated blood vessels clearly showed that the vasorelaxing responses to ANP are abolished, demonstrating that SM22-Cre-mediated excision of the GC-A gene in vascular SMCs is highly efficient. Thus, the resting GC-A mRNA and enzyme activity detected in isolated vessels very likely derives from the endothelium and from surrounding nonvascular tissue.
A concern with any tissue-specific knockout approach is the selectivity of the deletion, especially because the expression of Cre recombinase, even during a very brief period at any stage of life, has the potential to permanently delete a floxed gene (35). In particular, SM22α expression in adult is restricted to arterial, venous, and visceral SMCs (29), whereas in the early stages of development, it is also present in skeletal and cardiac muscle tissues (36). However, recent studies have shown the usefulness of this promoter to target the overexpression of pressor systems specifically to vascular SMCs to study their effect on vascular tone and systemic ABP (37). In the present study, the heart weights and heart-to-body weight ratios of the SMC GC-A KO mice were not different to wild-types; the mice are viable, have normal body weights, breath and breed normally, and under resting conditions are indistinguishable from wild-type littermates. In addition, we have evaluated the specificity of Cre-expression by using the ROSA-26 β-gal reporter mouse, which ubiquitously expresses β-gal after Cre-mediated excision of a stop sequence (31). SM22-Cretg/ROSA-26 β-gal double transgenic mice showed strong staining in the vascular SMC and no staining in surrounding tissues. Accordingly, in floxed GC-A mice, the SM22-Cre transgene will lead to specific deletion of GC-A in SMCs. Indeed, further experiments demonstrated that organs containing various types of cells expressing GC-A, such as the adrenal gland, kidney, and lung, retain normal levels of ANP-dependent guanylyl cyclase activity. Thus, cellular GC-A expression sites other than SMC, for example the pulmonary and renal epithelium, renal mesangial, and adrenal glomerulosa cells, are clearly not affected by the conditional gene deletion approach used in the current study.
Intriguingly, despite the clear abolition of the direct vasorelaxing effects of ANP, the resting ABP of conscious SMC GC-A KO mice was completely normal. This finding is unexpected because the decisive role of the ANP/GC-A system in the chronic regulation of arterial blood pressure has been clearly shown by the hypertensive phenotype of mice with generalized GC-A gene deletion (11, 12). However, in this model with global GC-A abolition, it is impossible to distinguish which of the known endocrine actions of ANP is mainly involved in ABP homeostasis. Our observations in a model with tissue-specific GC-A gene deletion add an important piece of information to the understanding of ANP/GC-A physiology, demonstrating that the direct vasodilating effect of ANP is dispensable in chronic ABP regulation. However, NPs regulate arterial blood pressure not only chronically, but also in an acute fashion, for instance in situations of acute volume expansion or sudden increases in blood pressure which cause a release of atrial granules from the heart (1, 3, 24). To study the role of vascular GC-A in the acute regulation of ABP, first we mimicked the sudden cardiac release of NPs by i.v. application of synthetic ANP. As shown, i.v. ANP caused an immediate hypotension in floxed GC-A mice, which was completely prevented in SMC GC-A KO mice. In a second series of experiments, mice were subjected to acute vascular volume expansion to provoke a rapid endogenous release of cardiac NPs (24). In agreement with studies in wild-type mice (38), floxed GC-A mice were totally able to compensate this volume overload and to maintain normal ABP. In contrast, SMC GC-A KO mice reacted to volume expansion with prompt and marked increases in ABP. Taken together, these observations indicate that vascular GC-A is crucial in the acute moderation of blood pressure by ANP. In this context, our observation that SMC GC-A KO mice are normotensive under resting conditions is even more surprising. One possibility is that in the long-term setting other vasodilating systems, such as the NO/soluble guanylyl cyclase system, compensate for the missing vasodilating effects of ANP. Alternatively, the chronic reduction of blood pressure by ANP might be mainly mediated by other actions of the hormone such as the inhibition of the sympathetic and RAA systems as well as the stimulation of renal function. This later hypothesis is corroborated by pharmacological and biochemical studies in ANP transgenic and knockout mice showing that tonic cardiovascular sympathetic tone varies inversely with the chronic level of endogenous ANP activity and that its increase has a major role in the hypertensive phenotype of ANP deficient mice (21).
In summary, our study shows that the direct vasodilating effect of ANP is dispensable for chronic blood pressure homeostasis and crucial for the acute moderation of blood pressure by ANP. Future studies must try to elucidate the role of vascular GC-A in the renal effects of NPs and how this specific cellular expression site contributes to the known actions of ANP on vascular permeability and, overall, to the regulatory effects of this peptide on blood volume homeostasis.
The GC-A gene is widely distributed in many different cell types and mediates the effects of ANP and BNP in many biological functions. In addition to its role in blood pressure control, recent reports indicated that GC-A might be critical in the regulation of other completely different physiological processes such as cellular growth in the heart, brain, and kidney (1, 5, 6), or even lipolysis in adipose tissue (39). The floxed GC-A gene mouse can be used with any transgenic animal possessing a cell-specific promoter driving the production of Cre recombinase. The availability of this genetic model, combined with a large number of tissue-specific Cre mice, provides a unique opportunity to dissect the physiological functions of the ANP/BNP/GC-A system.
Acknowledgments
We gratefully acknowledge the excellent technical assistance of Karim Sabrane and Alberto Vargas. M.K. would like to thank Dr. David L. Garbers (Howard Hughes Medical Institute, Univ. of Texas Southwestern Medical Center, Dallas) for critical review of the manuscript and also for many good discussions that greatly stimulated the initiation of this project. This work was supported by the Bundesministerium für Bildung und Forschung (BMBF 01EC9801), the University of Münster (Interdisziplinäre Klinische Forschung, IZKF B12), and the Deutsche Forschungsgemeinschaft (SFB 556) (all to M.K.).
Abbreviations
- ANP
atrial natriuretic peptide
- BNP
B-type natriuretic peptide
- GC-A
guanylyl cyclase A
- DBP
diastolic blood pressure
- SBP
systolic blood pressure
- SMC
smooth muscle cells
- ABP
arterial blood pressure
- RAA
renin–angiotensin–aldosteron
- β-gal
β galactosidase
- X-Gal
5-bromo-4-chloro-3-indolyl β-d-galactoside
- PE
phenylephrine
- RT
reverse transcription
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Brenner B M, Ballermann B J, Gunning M E, Zeidel M L. Pharmacol Rev. 1990;70:665–699. doi: 10.1152/physrev.1990.70.3.665. [DOI] [PubMed] [Google Scholar]
- 2.Drewett J G, Garbers D L. Endocrine Rev. 1994;13:135–162. doi: 10.1210/edrv-15-2-135. [DOI] [PubMed] [Google Scholar]
- 3.De Bold A J, Ma K K, Zhang Y, de Bold M L, Bensimon M, Khoshbaten A. Can J Physiol Pharmacol. 2001;79:705–714. [PubMed] [Google Scholar]
- 4.Garbers D L. Methods. 1999;19:477–484. doi: 10.1006/meth.1999.0890. [DOI] [PubMed] [Google Scholar]
- 5.Kishimoto I, Rossi K, Garbers D L. Proc Natl Acad Sci USA. 2001;98:2703–2706. doi: 10.1073/pnas.051625598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knowles J W, Esposito G, Mao L, Hagaman J R, Fox J E, Smithies O, Rockman H A, Maeda N. J Clin Invest. 2001;107:975–984. doi: 10.1172/JCI11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamura N, Ogawa Y, Chusho H, Nakamura K, Nakao K, Suda M, Kasahara M, Hashimoto R, Katsuura G, Mukoyama M, et al. Proc Natl Acad Sci USA. 2000;97:4239–4244. doi: 10.1073/pnas.070371497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murthy K S, Teng B Q, Zhou H, Jin J G, Grider J R, Makhlouf G M. Am J Physiol. 2000;278:G974–G980. doi: 10.1152/ajpgi.2000.278.6.G974. [DOI] [PubMed] [Google Scholar]
- 9.Matsukawa N, Grzesik W J, Takahashi N, Pandey K N, Pang S, Yamauchi M, Smithies O. Proc Natl Acad Sci USA. 1999;96:7403–7408. doi: 10.1073/pnas.96.13.7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.John S W M, Veress A T, Honrath U, Chong C K, Peng L, Smithies O, Sonnenberg H. Am J Physiol. 1996;271:R109–R114. doi: 10.1152/ajpregu.1996.271.1.R109. [DOI] [PubMed] [Google Scholar]
- 11.Lopez M J, Wong S K, Kishimoto I, Dubois S, Mach V, Friesen J, Garbers D L, Beuve A. Nature (London) 1995;378:65–68. doi: 10.1038/378065a0. [DOI] [PubMed] [Google Scholar]
- 12.Oliver P M, Fox J E, Kim R, Rockman H A, Kim H S, Reddick R L, Pandey K N, Milgram S L, Smithies O, Maeda N. Proc Natl Acad Sci USA. 1997;94:14730–14735. doi: 10.1073/pnas.94.26.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez M J, Garbers D L, Kuhn M. J Biol Chem. 1997;272:23064–23068. doi: 10.1074/jbc.272.37.23064. [DOI] [PubMed] [Google Scholar]
- 14.Steinhelper M E, Cochrane K L, Field L J. Hypertension. 1990;16:301–307. doi: 10.1161/01.hyp.16.3.301. [DOI] [PubMed] [Google Scholar]
- 15.Oliver P M, John S W M, Purdy K E, Kim R, Maeda N, Goy M F, Smithies O. Proc Natl Acad Sci USA. 1998;95:2547–2551. doi: 10.1073/pnas.95.5.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winquist R J, Faison E P, Waldman S A, Schwartz K, Murad F, Rapoport R M. Proc Natl Acad Sci USA. 1984;81:7661–7664. doi: 10.1073/pnas.81.23.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melo L G, Veress A T, Ackermann U, Pang S C, Flynn T G, Sonnenberg H. Reg Pept. 1999;79:109–115. doi: 10.1016/s0167-0115(98)00149-9. [DOI] [PubMed] [Google Scholar]
- 18.Almeida F A, Suzuki M, Maack T. Life Sci. 1986;39:1193–1199. doi: 10.1016/0024-3205(86)90351-6. [DOI] [PubMed] [Google Scholar]
- 19.Johnston C I, Hodsman P G, Kohzuki M, Casley D J, Fabris B, Phillips P A. Am J Med. 1989;87,Suppl. 6B:24S–28S. doi: 10.1016/0002-9343(89)90087-9. [DOI] [PubMed] [Google Scholar]
- 20.Kudo T, Baird A. Nature (London) 1984;312:756–757. doi: 10.1038/312756a0. [DOI] [PubMed] [Google Scholar]
- 21.Melo L G, Veress A T, Ackermann U, Steinhelper M E, Pang S C, Tse Y, Sonnenberg H. Cardiovasc Res. 1999;43:437–444. doi: 10.1016/s0008-6363(99)00104-2. [DOI] [PubMed] [Google Scholar]
- 22.Martin-Grez M, Fleming J T, Steinhausen M. Nature (London) 1986;324:473–476. doi: 10.1038/324473a0. [DOI] [PubMed] [Google Scholar]
- 23.Sonnenberg H, Honrath U, Chong C K, Wilson D R. Am J Physiol. 1986;250:F963–F966. doi: 10.1152/ajprenal.1986.250.6.F963. [DOI] [PubMed] [Google Scholar]
- 24.Kishimoto I, Dubois S K, Garbers D L. Proc Natl Acad Sci USA. 1996;93:6215–6219. doi: 10.1073/pnas.93.12.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Lean H, Bonhomme M C, Thibault G, Garcia R. Circ Res. 1995;77:64–72. doi: 10.1161/01.res.77.1.64. [DOI] [PubMed] [Google Scholar]
- 26.Osol G, Halpern W, Tesfamarian B, Nakayama K, Weinberg D. Hypertension. 1986;8:606–610. doi: 10.1161/01.hyp.8.7.606. [DOI] [PubMed] [Google Scholar]
- 27.Barbee R W, Harrison-Bernard L M, Carmines P K. Peptides. 1992;13:1181–1185. doi: 10.1016/0196-9781(92)90026-y. [DOI] [PubMed] [Google Scholar]
- 28.Jung M-Y, Skryabin B V, Arai M, Abbondanzo S, Fu D, Brosius J, Robakis N K, Polites H G, Pintar J E, Schmauss C. Neuroscience. 1999;91:911–924. doi: 10.1016/s0306-4522(98)00705-2. [DOI] [PubMed] [Google Scholar]
- 29.Li L, Miano J M, Mercer B, Olson E N. J Cell Biol. 1996;132:849–859. doi: 10.1083/jcb.132.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hofmann S L, Russell D W, Brown M S, Goldstein J L, Hammer R E. Science. 1988;239:1277–1281. doi: 10.1126/science.3344433. [DOI] [PubMed] [Google Scholar]
- 31.Mao X, Fujiwara Y, Orkin S H. Proc Natl Acad Sci USA. 1999;96:5037–5042. doi: 10.1073/pnas.96.9.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Potthast R, Ehler E, Scheving L A, Sindic A, Schlatter E, Kuhn M. Endocrinology. 2001;142:3087–3097. doi: 10.1210/endo.142.7.8274. [DOI] [PubMed] [Google Scholar]
- 33.Goy M F, Oliver P M, Purdy K E, Knowles J W, Fox J E, Mohler P J, Qian X, Smithies O, Maeda N. Biochem J. 2001;358:379–387. doi: 10.1042/0264-6021:3580379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinmetz M, Schlatter E, Boudier H A J S, Rahn K H, De Mey J G R. J Pharmacol Exp Ther. 2000;294:1175–1181. [PubMed] [Google Scholar]
- 35.Regan C P, Manabe I, Owens G K. Circ Res. 2000;87:363–369. doi: 10.1161/01.res.87.5.363. [DOI] [PubMed] [Google Scholar]
- 36.Miano L L J M, Cserjesi P, Olson E N. Circ Res. 1996;78:188–195. doi: 10.1161/01.res.78.2.188. [DOI] [PubMed] [Google Scholar]
- 37.Imai T, Morita T, Shindo T, Nagai R, Yazki Y, Kurihara H, Suematsu M, Katayama S. Circ Res. 2001;89:55–62. doi: 10.1161/hh1301.092679. [DOI] [PubMed] [Google Scholar]
- 38.Cervenka L, Mitchell K D, Oliverio M I, Coffman T M, Navar L G. Kidney Int. 1999;56:1855–1862. doi: 10.1046/j.1523-1755.1999.00757.x. [DOI] [PubMed] [Google Scholar]
- 39.Singenés C, Berlan M, De Glisezinski I, Lafontan M, Galitzky J. FASEB J. 2000;14:1345–1351. [PubMed] [Google Scholar]