Abstract
Background & aims
Traditional stool-based colorectal cancer (CRC) screening tests require patients to collect and swab their own stool, which can reduce adherence due to aversion and introduce variability from user error. A novel multitarget stool RNA test (mt-sRNA, ColoSense) eliminates the need for scraping or swabbing stool. Instead, patients merely deposit and ship a sample to the lab. Once recieved, laboratory technologists swab the sample and quantify hemoglobin concentrations using the fecal immunochemical test (FIT). This study evaluates the reliability and reproducibility of this in-laboratory fecal sampling method.
Methods
Analytical validation was performed by generating stool pools with known hemoglobin concentrations and exposing pools to various conditions prior to testing with the in-lab FIT. Analytical validation assessed freeze thaw stability, interfering substances, stool input volume, precision, and in-transit stability. Clinical equivalency was also evaluated.
Results
All studies met predefined acceptance criteria. The assay demonstrated stability for up to three freeze-thaw cycles. Interference testing with nine dietary substances showed no impact on assay performance. The in-lab FIT maintained accuracy across five different stool input volumes and demonstrated high precision. In-transit stability was confirmed for up to 120 hours, supporting sample robustness during shipping and handling. Clinical equivalency demonstrated in-lab FIT sensitivity of 78 % for CRC and 33 % for advanced adenomas, aligning with previously reported performance of the at-home FIT method.
Conclusions
Analytical validation of the in-lab FIT demonstrates the reliability and robustness of this streamlined, single-sample collection method. This improvement could enhance adherence and patient ease-of-use in stool-based CRC screening.
Keywords: mt-sRNA, ColoSense, CRC screening, FIT, screening adherence, multitarget stool RNA, early detection, colorectal cancer, cancer prevention
1. Introduction
Colorectal cancer (CRC) screening has evolved substantially over the past five decades [1]. Colonoscopy emerged in the 1970s as the gold standard due to direct visualization with the opportunity for immediate polypectomy, thereby enabling both detection and prevention [2]. In parallel, the fecal immunochemical test (FIT), a guaiac-free, antibody-based assay for human hemoglobin, was introduced as a low-cost, noninvasive alternative that could be deployed at population scale [3]. Guideline groups now recommend multiple options, including colonoscopy every 10 years, stool testing every 3 years, or annual FIT, reflecting the complementary strengths of endoscopic and stool-based strategies with the need to maximize screening uptake [4].
FIT for CRC screening relies on at-home sample collection [5]. A patient passes a stool into the toilet, uses a plastic wand to swab the stool surface at several locations, and places the wand in a buffered tube that stabilizes hemoglobin during shipment to the laboratory (at-home FIT) [6]. Sensitivity depends on uniform sampling of occult blood within a heterogeneous specimen [7]. Further, inadequate swabbing can dilute signal and generate false-negative results, while environmental contamination can produce false positive results [8]. Despite simplified instructions and prepaid mailers, real-world studies show that of kits returned, 10–20 % are unused or unsatisfactory, underscoring the friction introduced by patient-performed fecal handling [[9], [10],[9], [10]].
To improve performance, molecular diagnostics have combined genomic and transcriptomic targets with FIT. Multi-target stool DNA (mt-sDNA) assays combine hemoglobin concentrations with mutant KRAS, aberrant methylation markers, and β-actin, achieving higher sensitivity for advanced adenomas and stage I–II CRC [11,12]. However, these tests still require a patient-collected FIT swab to quantify hemoglobin, perpetuating the same adherence barriers and pre-analytical variability that limit stand-alone FIT [[13], [14],[13], [14]].
More recently, a multi-target stool RNA test (mt-sRNA, ColoSense) was FDA-approved for CRC screening [15]. This assay demonstrated 94 % sensitivity for CRC, 46 % sensitivity for advanced adenomas (AA), and 88 % specificity for no findings on a colonoscopy. Importantly, the mt-sRNA test does not require patients to complete a fecal swab as part of screening. Instead, fecal scraping is completed by technologists in the laboratory after a full bowel movement is collected and shipped to the laboratory (in-lab FIT). As such, this collection process eliminates the most commonly cited barrier to adherence with stool-based testing [[16], [17], [16], [17]].
Eliminating at-home stool scraping represents a pragmatic opportunity to streamline sample collection, enhance user acceptability, and reduce analytical noise [18]. Here we present the analytical validation of the in-lab FIT method. Studies performed to assess the in-lab FIT include freeze thaw stability, interfering substances, stool input volume, precision, in-transit stability, and clinical equivalency [19].
2. Methods
2.1. Study design
The aim of this study was to evaluate the analytical performance of the in-lab FIT method. Stool samples leveraged for analytical validation were obtained from subjects who consented to participate in the approved study (Advarra institution review board). Subjects were instructed to complete a stool sample collection and ship the sample to a centralized laboratory.
2.2. Sample collection
Stool samples were collected using previously defined methods [20,21]. Mt-sRNA collection kits were shipped at ambient temperature to the subject's residence. Subjects collected stool samples according to the provided instructions for use (IFU) with modifications, as needed, to complete required studies. Instructions included the requirement to complete an at-home FIT to obtain a baseline hemoglobin concentration for stool samples. Completed collection kits were shipped back to the laboratory at ambient temperature, with up to 96 hours permitted for transit.
2.3. Stool sample collection, pool generation, and characterization
Human stool samples were collected in bulk and mixed to create sample pools targeting specific hemoglobin concentrations or ranges of hemoglobin concentrations. When needed, human peripheral blood was used to adjust hemoglobin concentrations. To simulate the at-home FIT collection method, sample pools were characterized with FIT swabs prior to aliquoting. Each sample pool was then divided into aliquots. Aliquots were prepared to generate eukaryotic cell fractions.
2.4. Analytical validation
Analytical validation was performed by generating stool pools with known hemoglobin concentration values and exposing stool to various conditions prior to testing with the in-lab FIT method [18]. Freeze-thaw stability was assessed by subjecting stool pools to ≤3 freeze thaw cycles. Interfering substances were evaluated by incubating stool in mt-sRNA buffer with common dietary components, including red radish, broccoli, cauliflower, horseradish, turnip, cantaloupe, beef, pork, and parsnip. Stool input volume was tested by combining 50–250 grams of stool with 250 mL of buffer prior to in-lab FIT analysis. Precision was measured as the coefficient of variation (%CV) or concordance across three FIT lots, two laboratory users, three days, and two instruments. In-transit stability was evaluated by incubating stool in buffer across six timepoints (0–96 hours) prior to evaluation with the in-lab FIT method.
2.5. Clinical equivalency
Clinical equivalency was evaluated by providing subjects with stool collection kits and comparing the at-home FIT results to in-lab FIT results. Additional stool samples were obtained from subjects with a confirmed diagnosis of CRC or AA prior to surgical ressection or treatment. In-lab FIT was performed on samples subjected to at least one freeze–thaw cycle and results were compared with published literature.
2.6. In-lab FIT processing
All FIT swabs were assessed using the OC Auto Micro 80 Analyzer (510(k), k041408). For all studies, the same FIT cutoff threshold (positive versus negative) was used [18].
2.7. Statistical and computational analyses
Whenever possible, statistical analysis approaches aligned with appropriate Clinical and Laboratory Standards Institute (CLSI) guidance documentation. Concordance was calculated by comparing the expected results with the observed results (i.e., replicates from a high positive cohort should generate a positive result). %CV was calculated by dividing the standard deviation by the mean and multiplying by 100. The Clopper-Pearson exact method or the Wilson Score method was used to determine 2-sided 95 % confidence intervals (CI). 95 % CIs were compared using Z scores and associated P values.
3. Results
3.1. Study population and baseline characteristics
Analytical studies evaluated a total of 1368 stool replicates using the in-lab FIT method. No invalid results were observed. Freeze thaw stability, interfering substances, stool input volume, and in-transit stability studies were conducted using two stool pool types (positive and negative), which were compared to in-lab FIT performance after exposure to each condition. Precision was assessed using four stool pool types (low negative, high negative, low positive, and high positive). Clinical equivalency evaluated an additional 111 samples.
3.2. Freeze thaw stability
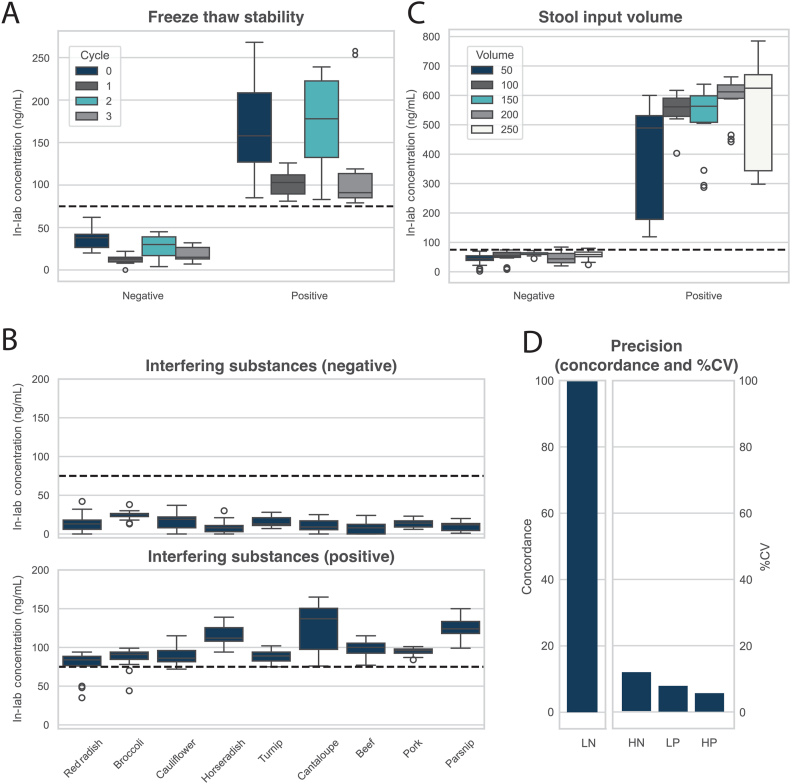
To assess freeze thaw stability, negative and positive stool pools were assessed with the at-home FIT, aliquoted into replicates, and then exposed to ≤3 freeze thaw cycles prior to assessment with the in-lab FIT. Each freeze thaw cycle required 24 hours at −60 degrees Celsius and two hours at ambient temperature. When comparing at-home FIT results to in-lab FIT results, all replicates (n = 30 replicates per cycle) demonstrated 100 % concordance with the original sample classification (positive or negative) (Table 1, Fig. 1A).
Table 1.
Summary of analytical validationand clinical equivalencyresults for the in-lab FIT. Acceptance criteria required ≥85 % concordance or <15 % coefficient of variation (%CV) across all samples within a given experiment. Clinical equivalency results were compared to previously reported sensitivity metrics.
| Study | Sample Size | Result |
|---|---|---|
| Freeze thawstability | ||
| 0 freeze thaw cycles | n = 30 | 100 % concordance |
| 1 freeze thaw cycle | n = 30 | 100 % concordance |
| 2 freeze thaw cycles | n = 30 | 100 % concordance |
| 3 freeze thaw cycles | n = 30 | 100 % concordance |
| Interferingsubstances | ||
| Red radish | n = 30 | 90 % concordance |
| Broccoli | n = 30 | 93 % concordance |
| Cauliflower | n = 30 | 97 % concordance |
| Horseradish | n = 30 | 100 % concordance |
| Turnip | n = 30 | 100 % concordance |
| Cantaloupe | n = 30 | 100 % concordance |
| Beef | n = 30 | 100 % concordance |
| Pork | n = 30 | 100 % concordance |
| Parsnip | n = 30 | 100 % concordance |
| Stoolinputvolume | ||
| 50 grams stool / 250 ml buffer | n = 48 | 100 % concordance |
| 100 grams stool / 250 ml buffer | n = 30 | 100 % concordance |
| 150 grams stool / 250 ml buffer | n = 30 | 97 % concordance |
| 200 grams stool / 250 ml buffer | n = 30 | 97 % concordance |
| 250 grams stool / 250 ml buffer | n = 48 | 96 % concordance |
| Precision | ||
| Low Negative (concordance) | n = 108 | 100 % concordance |
| High Negative (%CV) | n = 108 | 14 %CV |
| Low Positive (%CV) | n = 108 | 9.5 %CV |
| High Positive (%CV) | n = 108 | 6.8 %CV |
| In-transit stability | ||
| 0 hours | n = 60 | 100 % concordance |
| 24 hours | n = 60 | 97 % concordance |
| 48 hours | n = 60 | 100 % concordance |
| 72 hours | n = 60 | 100 % concordance |
| 96 hours | n = 60 | 100 % concordance |
| 120 hours | n = 60 | 98 % concordance |
| Clinical equivalency | ||
| Negative findings | n = 70 | 94 % concordance |
| Colorectal cancer | n = 35 | 77 % sensitivity |
| Advanced adenomas | n = 6 | 33 % sensitivity |
Fig. 1.
A) Box plot showing in-lab FIT results for two stool pools (negative and positive) across zero to three freeze thaw cycles. B) Box plot showing in-lab FIT results for two stool pools (negative and positive) across nine interfering substances. C) Box plot showing in-lab FIT results for two stool pools (negative and positive) across five stool input volumes. D) Bar plot showing concordance for low negative (LN) pool and percent coefficient of variation (%CV) for high negative (HN), low positive (LP), and high positive (HP) stool pools for the in-lab FIT when evaluated across multiple lots, days, users, and instruments.
3.3. Interfering substances
To assess interfering substances, negative and positive stool pools were assessed with the at-home FIT, aliquoted into replicates, and then exposed to various dietary substances (red radish, broccoli, cauliflower, horseradish, turnip, cantaloupe, beef, pork, and parsnip). Across all nine dietary substances (n = 30 replicates per substance), ≥90 % concordance was observed with the original sample classification (positive or negative) (Table 1, Fig. 1B).
3.4. Stool input volume
To ensure that change in stool input volumes does not impact the in-lab FIT, varied volumes of stool (50 , 100, 150, 200, and 250 grams) were assessed with the at-home FIT prior to adding 250 mL buffer, and subsequently assessing with the in-lab FIT. Across all replicates (n = 30 or 48 replicates per stool input volume), ≥96 % concordance was observed with the original sample classification (positive or negative) (Table 1, Fig. 1C).
3.5. Precision
Precision was used to assess total variance within the test system as it relates to days, operators, reagent lots, and instruments. Assessment was performed for four FIT concentration levels (low negative, high negative, low positive, and high positive). Stool pools were generated to target the following concentrations: 10 ng/mL, 50 ng/mL, 100 ng/mL, and 140 ng/mL as determined using the at-home FIT. Subsequently, replicates were assessed using the in-lab FIT across three unique days, two unique operators, three reagent lots, and two unique instruments. For high negative, low positive, and high positive stool pools (n = 108 replicates per pool), the %CV was 14 %, 9.5 %, and 6.8 %, respectively. The low negative pool showed 100 % concordance with the original sample classification (negative) (Table 1, Fig. 1D).
3.6. In-transit stability
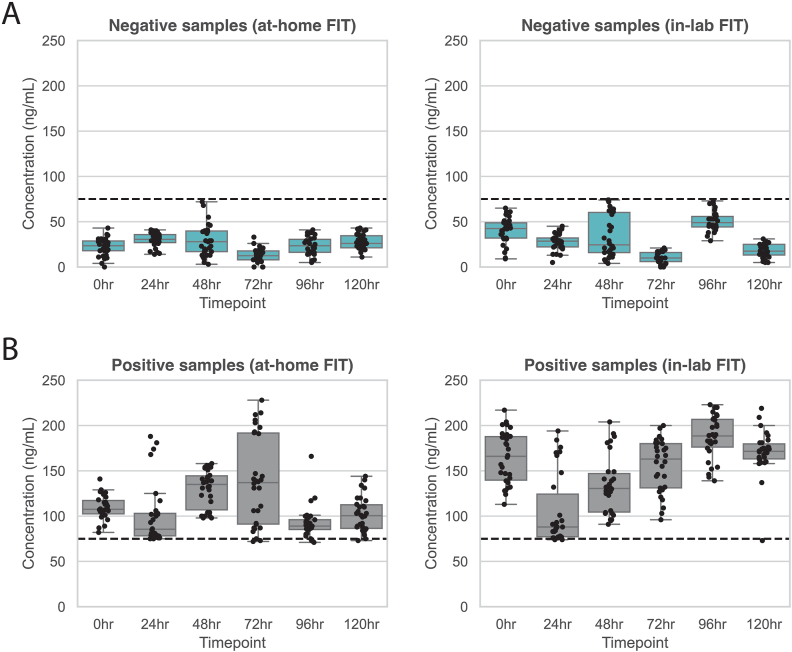
The in-lab FIT is performed after sample collection, buffer stabilization, and sample transit at ambient temperature for up to 96 hours. As such, stability was assessed at six incubation timepoints (<6, 24, 48, 72, 96, and 120 hours). Stool pools were assessed with the at-home FIT prior to aliquoting and exposure to buffer for the required timepoint. Subsequently, replicates were assessed using the in-lab FIT. Across all replicates (n = 60 replicates per timepoint), ≥97 % concordance was observed with the original sample classification (positive or negative) (Table 1, Fig. 2).
Fig. 2.
FIT scores for theat-home and thein-lab method were generated at six [] timepoints (<6hours, 24hours, 48hours, 72hours, 96hours, and 120hours). FITs tested at various timepoints were assessed for concordance with the original sample type, which was characterized using theat-home FIT method prior to aliquoting. A) Results for at-home and in-lab results for aliquots in the negative pool. B) Results for at-home and in-lab results for aliquots in the positive pool.
3.7. Clinical equivalency
A total of 70 healthy subjects were enrolled in the prospective, clinical equivalency analysis, of whom 50 were aged 45 or older. At-home FIT swabs were returned using collection kits and accessioned within 96 hours of collection. Concordance between at-home and in-lab FIT results was 94 % (n = 66/70). The positive predictive value (PPV) was 100 % (n = 7/7; CI95 %, 59–100 %) and the negative predictive value (NPV) was 94 % (n = 59/63; CI95 %, 85–98 %).
To further assess performance, a retrospective cohort of stool samples from subjects with known CRC (n = 35) or AA (n = 6) was evaluated using the in-lab FIT. The in-lab FIT demonstrated 77 % sensitivity for CRC (n = 27/35) and 33 % sensitivity for AA (n = 2/6). Findings were consistent with previously reported at-home FIT performance (78 % and 29 %, respectively) [14].
4. Discussion
The multitarget stool RNA test (mt-sRNA, ColoSense) is currently the only FDA-approved, stool-based CRC screening test that does not require patients to perform a fecal swab. Eliminating at-home FIT swabbing represents a transformative approach to CRC screening by mitigating barriers to adherence, while maintaining diagnostic integrity through standardized processing.
The in-lab FIT showed strong analytical performance. It maintained 100 % concordance through three freeze-thaw cycles and ≥97 % concordance for up to 120 hours of stability at ambient temperature during simulated transit. Interfering substances had minimal effect, with ≥90 % concordance across all assessed interfering substances. The assay tolerated variable stool inputs (50–250 grams) with ≥96 % concordance across all replicates. Precision demonstrated low variation (%CV: 6.8–14 %) for positive and high negative replicates, and 100 % concordance for low negative replicates. These findings support the test's robustness under real-world conditions.
Clinical equivalency studies demonstrated high concordance between at-home and in-lab FIT results, supporting the validity of laboratory-based assessments as a reliable surrogate for patient-collected swabs. Importantly, retrospective testing of CRC and AA samples demonstrated sensitivity consistent with prior reports, confirming that the in-lab method preserves diagnostic accuracy.
The in-lab FIT is a novel approach for measuring fecal hemoglobin that leverages a full bowel movement already collected for molecular analysis, eliminating the need for patients to separate stool samples. This streamlined process simplifies collection, and may increase participation in stool-based screening programs, particularly as stool-based molecular tests become more widely adopted.
Laboratory-based testing by trained personnel ensures greater consistency in sample handling, reducing the likelihood of user error that can compromise at-home FIT performance. The high precision and reproducibility observed in this analytical validation support the enhanced standardization and quality control achievable through in-lab processing.
This study has several limitations. First, stool pools were used to evaluate analytical performance, which may not reflect the heterogeneity observed across multiple bowel movements or different stool regions. Temperature-related variability was not reassessed in this study, as prior literature demonstrated no impact on test accuracy. Further testing may be needed to evaluate the effect of in-lab FIT at different time points or in different settings, such as the time of collection. Further, most testing was conducted under controlled laboratory conditions, which may not fully reflect operational variability in real-world settings. While clinical equivalency studies were performed, subjects with disease were collected retrospectively, which might not reflect samples from subjects who are undergoing average-risk screening.
In summary, in-lab FIT offers a promising and analytically robust alternative to at-home collection. By addressing key adherence barriers and minimizing pre-analytical variability, this approach may enhance the effectiveness of stool-based CRC screening and improve early detection at the population level. Future studies should evaluate clinical validity, real-world feasibility, and integration into existing screening paradigms.
CRediT authorship contribution statement
Erica K. Barnell: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Resources, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Kimberly Kruse: Writing – review & editing, Formal analysis, Data curation, Conceptualization. Elizabeth M. Wurtzler: Writing – review & editing, Validation, Supervision, Formal analysis, Conceptualization. Maya Crowder Scott: Writing – review & editing, Data curation. Andrew R. Barnell: Writing – review & editing, Writing – original draft, Resources, Conceptualization. Eric J. Duncavage: Writing – review & editing, Supervision, Conceptualization.
Funding statement
All research was funded by Geneoscopy Inc.
Declaration of competing interest
These authors disclose the following: Eric Duncavage is a paid consultant for Geneoscopy Inc. Erica Barnell, Kimberly Kruse, Elizabeth Wurtzler, and Andrew Barnell are inventors of the intellectual property owned by Geneoscopy Inc. Erica Barnell, Kimberly Kruse, Elizabeth Wurtzler, Maya Crowder Scott, and Andrew Barnell are employees and owners of Geneoscopy Inc.
Data availability
Data may be made available upon request.
References
- 1.Helsingen L.M., Kalager M. Colorectal cancer screening - approach, evidence, and future directions. NEJM Evid. 2022;1 doi: 10.1056/EVIDra2100035. [DOI] [PubMed] [Google Scholar]
- 2.Wolff W.I., Shinya H. Earlier diagnosis of cancer of the colon through colonic endoscopy (colonoscopy) Cancer. 1974;34(suppl):912–931. doi: 10.1002/1097-0142(197409)34:3+<912::aid-cncr2820340720>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 3.Issaka RB, Avila P, Whitaker E, Bent S, Somsouk M. Population health interventions to improve colorectal cancer screening by fecal immunochemical tests: A systematic review. Prev Med. 2019 Jan;118:113–121. doi: 10.1016/j.ypmed.2018.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Preventive Services Task Force Screening for colorectal cancer: US Preventive services task force recommendation statement: US preventive services task force recommendation statement. JAMA. 2021;325:1965–1977. doi: 10.1001/jama.2021.6238. [DOI] [PubMed] [Google Scholar]
- 5.Doubeni C.A., et al. Fecal immunochemical test screening and risk of colorectal cancer death. JAMA Netw. Open. 2024;7 doi: 10.1001/jamanetworkopen.2024.23671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gies A., Gruner L.F., Schrotz-King P., Brenner H. Effect of imperfect compliance with instructions for fecal sample collection on diagnostic performance of 9 fecal immunochemical tests. Clin. Gastroenterol. Hepatol. 2019;17:1829–1839.e4. doi: 10.1016/j.cgh.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Symonds EL, Fraser CG, Bastin D, Berwald G, Young GP. The effect of the Variability in Fecal Immunochemical Test Sample Collection Technique on Clinical Performance. Cancer Epidemiol Biomarkers Prev. 2021 Jan;30(1):175–181. doi: 10.1158/1055-9965.EPI-20-0984. [DOI] [PubMed] [Google Scholar]
- 8.de Klerk CM, Vendrig LM, Bossuyt PM, Dekker E. Participant-Related Risk Factors for False-Positive and False-Negative Fecal Immunochemical Tests in Colorectal Cancer Screening: Systematic Review and Meta-Analysis. Am J Gastroenterol. 2018 Dec;113(12):1778–1787. doi: 10.1038/s41395-018-0212-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu P.-H., et al. Unsatisfactory fecal immunochemical tests for colorectal cancer screening: prevalence, reasons, and subsequent testing. Cancer Epidemiol. Biomarkers Prev. 2024;33:215–223. doi: 10.1158/1055-9965.EPI-23-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rohan E.A., Boehm J.E., DeGroff A., Glover-Kudon R., Preissle J. Implementing the CDC's colorectal cancer screening demonstration program: wisdom from the field. Cancer. 2013;119(Suppl 15):2870–2883. doi: 10.1002/cncr.28162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imperiale T.F., et al. Next-generation multitarget stool DNA test for colorectal cancer screening. N. Engl. J. Med. 2024;390:984–993. doi: 10.1056/NEJMoa2310336. [DOI] [PubMed] [Google Scholar]
- 12.Imperiale T.F., et al. Multitarget stool DNA testing for colorectal-cancer screening. N. Engl. J. Med. 2014;370:1287–1297. doi: 10.1056/NEJMoa1311194. [DOI] [PubMed] [Google Scholar]
- 13.Miller-Wilson L.-A., et al. Cross-sectional adherence with the multi-target stool DNA test for colorectal cancer screening in a medicaid population. Prev. Med. Rep. 2022;30 doi: 10.1016/j.pmedr.2022.102032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiser E., et al. Cross-sectional adherence with the multi-target stool DNA test for colorectal cancer screening: real-world data from a large cohort of older adults. J. Med. Screen. 2021;28:18–24. doi: 10.1177/0969141320903756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnell E.K., et al. Multitarget stool RNA test for colorectal cancer screening. JAMA. 2023;330:1760–1768. doi: 10.1001/jama.2023.22231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chambers J.A., Callander A.S., Grangeret R., O'Carroll R.E. Attitudes towards the faecal occult blood test (FOBT) versus the faecal immunochemical test (FIT) for colorectal cancer screening: perceived ease of completion and disgust. BMC Cancer. 2016;16:96. doi: 10.1186/s12885-016-2133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luque J.S., et al. Formative research on knowledge and preferences for stool-based tests compared to colonoscopy: what patients and providers think. J. Community Health. 2018;43:1085–1092. doi: 10.1007/s10900-018-0525-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis M.M., et al. Mailed FIT (fecal immunochemical test), navigation or patient reminders? Using microsimulation to inform selection of interventions to increase colorectal cancer screening in medicaid enrollees. Prev. Med. 2019;129S:105836. doi: 10.1016/j.ypmed.2019.105836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnell E.K., et al. Analytical validation of the multitarget stool RNA test for colorectal cancer screening. J. Mol. Diagn. 2024;26:700–707. doi: 10.1016/j.jmoldx.2024.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Barnell E.K., et al. Multitarget stool RNA test for noninvasive detection of colorectal neoplasias in a multicenter, prospective, and retrospective cohort. Clin. Transl. Gastroenterol. 2021;12 doi: 10.14309/ctg.0000000000000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnell E.K., et al. Noninvasive detection of high-risk adenomas using stool-derived eukaryotic RNA sequences as biomarkers. Gastroenterology. 2019;157 doi: 10.1053/j.gastro.2019.05.058. 884–887.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data may be made available upon request.