Abstract
Sebaceous glands may be involved in a pathway conceptually similar to that of the hypothalamic-pituitary-adrenal (HPA) axis. Such a pathway has been described and may occur in human skin and lately in the sebaceous glands because they express neuropeptide receptors. Corticotropin-releasing hormone (CRH) is the most proximal element of the HPA axis, and it acts as central coordinator for neuroendocrine and behavioral responses to stress. To further examine the probability of an HPA equivalent pathway, we investigated the expression of CRH, CRH-binding protein (CRH-BP), and CRH receptors (CRH-R) in SZ95 sebocytes in vitro and their regulation by CRH and several other hormones. CRH, CRH-BP, CRH-R1, and CRH-R2 were detectable in SZ95 sebocytes at the mRNA and protein levels: CRH-R1 was the predominant type (CRH-R1/CRH-R2 = 2). CRH was biologically active on human sebocytes: it induced biphasic increase in synthesis of sebaceous lipids with a maximum stimulation at 10−7 M and up-regulated mRNA levels of 3β- hydroxysteroid dehydrogenase/Δ5–4 isomerase, although it did not affect cell viability, cell proliferation, or IL-1β-induced IL-8 release. CRH, dehydroepiandrosterone, and 17β-estradiol did not modulate CRH-R expression, whereas testosterone at 10−7 M down-regulated CRH-R1 and CRH-R2 mRNA expression at 6 to 24 h, and growth hormone (GH) switched CRH-R1 mRNA expression to CRH-R2 at 24 h. Based on these findings, CRH may be an autocrine hormone for human sebocytes that exerts homeostatic lipogenic activity, whereas testosterone and growth hormone induce CRH negative feedback. The findings implicate CRH in the clinical development of acne, seborrhea, androgenetic alopecia, skin aging, xerosis, and other skin disorders associated with alterations in lipid formation of sebaceous origin.
Communication and reciprocal regulation among nervous, endocrine, and immune systems is essential for biological stability and responses to external and internal challenges (1). In particular, neuropeptides, hormones and cytokines act as signaling molecules that mediate communication among the three interacting systems. Neuropeptides, originally described in central nervous tissue (2), are also expressed in target organs in which they exhibit a number of immunomodulatory influences on cell differentiation.
The skin is exposed continuously to an environment with potentially noxious stimuli and represents a barrier maintaining internal homeostasis (3, 4). In analogy with central responses to stress, which involve predominantly the hypothalamic-pituitary-adrenal (HPA) axis, it has been recently proposed that the skin could share similar mediators (3–5). This postulate was based on the capability of the mammalian skin to produce propiomelanocortin-derived peptides and to be a target of actions through locally expressed melanocortin and opioid receptors (3, 6).
The corticotropin-releasing hormone (CRH), its binding protein (CRH-BP), and receptors (CRH-R, types 1 and 2) act as a central regulatory system of the HPA axis (4, 7). Pro-CRH processing into CRH appears to be similar at the central and peripheral levels, including the skin (4). So far, CRH has been detected in epidermal and follicular keratinocytes, melanocytes, endothelial cells, and dermal nerves but not in fibroblasts (4, 6). CRH-R1 has been detected in epidermal and follicular keratinocytes, melanocytes, and dermal fibroblasts. CRH and CRH-R have been recently detected in human sebaceous glands by in situ experiments (8); therefore, we engaged a currently developed reproducible human sebocyte culture system (9) to perform a comprehensive analysis of CRH, CRH-BP, and CRH-R1/CRH-R2 expression and function on human sebocytes in vitro. The expression of CRH/CRH-R system was analyzed by reverse transcription (RT)-PCR, real time quantitative RT-PCR, and immunocytochemistry under basic conditions and after stimulation by several hormones. Furthermore, the role of CRH on its own receptor, cell proliferation, immune function, lipogenesis, and expression of 3β-hydroxysteroid dehydrogenase/Δ5–4 isomerase (Δ5-3β-HSD) mRNA was investigated.
Materials and Methods
Reagents, Active Compounds, and Antibodies.
If not otherwise stated, chemicals (analytical grade), hormones, and assay reagents or kits were from Sigma. CRH was from Calbiochem, IL-1β and the IL-8 ELISA kit from R & D Systems. The affinity-purified goat polyclonal antibodies directed against human CRH (sc-1759), human CRH-BP (sc-1822), and human CRH-R (sc-1757) were from Santa Cruz Biotechnology; the purified rabbit monoclonal antibody directed against human CRH (C5348) was from Sigma. All secondary antibodies used and the APAAP complex were from Dako.
Cell Cultures.
Immortalized human facial SZ95 sebocytes, which have been shown to conserve the major characteristics of normal sebocytes (9), and a dysplastic oral keratinocyte cell line (European Collection of Cell Cultures ref. no. 94122104), used as positive control, were maintained in Sebomed Basal Medium (Biochrom, Berlin) supplemented with 5 ng/ml human epidermal growth factor (Sigma), 10% heat-inactivated FCS (Biochrom), 50 μg/ml gentamicin (GIBCO/BRL), and 1 mM Ca2+, in a humidified atmosphere of 5% CO2 at 37°C. Shortly before the experiments were performed, BSA-medium [constituted of Sebomed Basal Medium supplemented with 1 mg/ml fatty acid-free BSA (Boehringer Mannheim), 1 mM Ca2+, and 10−6 M linoleic acid (Sigma)] or Sebomed Complete Medium (Biochrom) was added to the cells.
RT-PCR Analysis.
Total RNA was extracted from SZ95 sebocytes and dysplastic oral keratinocytes by using a commercial kit (miniprep; Stratagene). An aliquot of total RNA (1 μg) was reverse transcribed into single-stranded cDNA with oligo(dT)15 (1 μg) and Maloney murine leukemia virus reverse transcriptase (200 units; Promega) in a final volume of 25 μl as indicated by the manufacturer's instruction. One microliter of reverse transcription mixture was used for PCR reaction. PCR was carried out in a total volume of 25 μl with a primer pair at 0.5 μM in 50 mM KCl, 10 mM Tris⋅HCl (pH 8.3), 1.5 mM MgCl2, 200 μM each of dATP, dCTP, dTTP, and dGTP, and 1 unit Ampli Tag DNA polymerase (GIBCO/BRL) for 3 min of initial denaturation at 94°C, then 35 cycles of 94°C denaturation (30 s), annealing for 1 min at 60°C, and extension for 3 min at 72°C in a Perkin–Elmer 9700 automated thermal cycler. The following oligonucleotides were found in the GenBank data bank, selected from different gene exons to avoid amplification of genomic DNA and were used for mRNA detection by RT-PCR. The accession number of the sequences and the length of the expected products are given in parentheses: CRH, sense primer 5′-TCC GAG GAG CCT CCC ATC-3′, antisense primer 5′-AAT CTC CAT GAG TTT CCT GTT GC-3′ (no. V00571, 122 bp; ref. 10); CRH-BP, sense primer 5′-GTT CAT TAC CAT CCA CTA CGA-3′, antisense primer 5′-CTC CAG CTG ACG ATA CTC AAA-3′ (634 bp; ref. 11). Primers for β-actin, which was used as control, were purchased from CLONTECH. PCR products were separated electrophoretically on 1.5–2% Tris-acetate EDTA-agarose gels, stained with ethidium bromide and photographed under UV light.
Real Time Quantitative RT-PCR Analysis.
One microgram of total RNA from SZ95 sebocytes treated with Dnase I (GenHunter, Nashville, TN) were reverse transcribed to cDNA by using the Thermoscript reverse transcriptase (GIBCO/BRL) with random hexamers as primers in a 20–μl reaction solution. To quantify expression of CRH-R1, CRH-R2, and Δ5-3β-HSD mRNA, real time quantitative RT-PCR (TaqMan PCR; PE Applied Biosystems) using a Perkin-Elmer 7700 sequence detector was performed (12). The reaction contained 1× TaqMan Universal PCR Master Mix (PE Applied Biosystems), 900 nM of the forward and reverse primers, and 200 nM of TaqMan probes. Primers and probes were designed by using the primer design software primer express (PE Applied Biosystems). The accession number of the sequences and the length of the expected products are given in parentheses: CRH-R1, forward primer 5′-CGC ATC CTC ATG ACC AAG CT-3′, reverse primer 5′-TCA CAG CCT TCC TGT ACT GAA TG-3′ (no. NM_004382, 66 bp); TaqMan probe, 5′-CGG GCA TCC ACC ACG TCT GAG A-3′; CRH-R2, forward primer 5′-CCC GGG CCA TGT CCA T-3′, reverse primer 5′-ACA GCG GCC GTC TGC TT-3′ (no. NM_001883, 69 pb); TaqMan probe, 5′-CTA CAT CAC CCA CAC GGC TCA GCT TC-3′; Δ5-3β-HSD, forward primer 5′-CCA CTC TAC AGC TGG GAG GAA-3′, reverse primer CGG TCC ACA AGG GAA CCA-3′ (no. M38180, 65 bp); TaqMan probe, CCA AGC AGA AAA CGG TGG AGT GGG-3′. 18S rRNA primers and probe (PE Applied Biosystems), serving as internal control, were added at 50 nM. Thermal cycling proceeded with 50 cycles of 95°C for 15 s and 60°C for 1 min. Input RNA amounts were calculated with a multiplex comparative method for the mRNAs of interest and 18S rRNA. Experiments were performed in triplicate for each data point.
Immunocytochemistry.
SZ95 sebocytes (25,000 cells) were cultured in two-chamber culture slides or cytocentrifuged in a Shandon Cytospin 2 cytocentrifuge (Frankfurt, Germany) on microscopic slides. The preparations were fixed in methanol at −20°C for 5 min and incubated with the goat antibodies against human CRH (diluted 1:25), human CRH-BP (diluted 1:100), human CRH-R (diluted 1:50), or rabbit antibody against human CRH (diluted 1:100) for 30 min. Respectively, the secondary antibodies used were alkaline phosphatase complex-conjugated goat anti-human IgG (H+L) (diluted 1:500) or mouse anti-rabbit (diluted 1:50), rabbit anti-mouse (diluted 1:20), and APAAP complex according to the instructions for the alkaline phosphatase anti-alkaline phosphatase labeling method (all incubation steps at room temperature for 30 min). The preparations were developed with new fuchsin, counterstained with hematoxylin, and mounted. Triplicate slides were stained for each data point.
Proliferation Analysis.
Cells were cultured in 96-well tissue culture plates at a density of 2,000 cells per well for 2 days. The wells were then washed with PBS, and BSA-medium with or without active compounds was added. Cell proliferation was assessed by the 4-methylumbelliferyl heptanoate fluorescence assay and measured automatically, as previously described (13). Briefly, on the day of evaluation the medium was removed, the cells were washed twice with PBS, and 100 μl of a 100 μg/ml 4-methylumbelliferyl heptanoate solution in PBS was added to each well. The plates were then incubated at 37°C for 30 min, and the released fluorescence, which is representative for cell numbers, was read on a Molecular Devices SPECTRAmax Gemini spectrofluorometer using 355 nm excitation and 460 nm emission filters. Experiments were performed in quadruplicate, with 10 wells evaluated for each data point in each experiment.
Detection of Cytotoxicity.
Cells were cultured in 96-well tissue culture plates at a density of 20,000 cells per well for 24 h. The wells were then washed with PBS, and BSA-medium was added. After 2 days, the medium was harvested, and fresh BSA-medium without or with active compounds was given to the cells. The supernatants were collected 24 h later and were centrifuged to remove cell detritus, and 100 μl was proceeded to measurement of lactate dehydrogenase (LDH) release with the Boehringer LDH Assay kit according to the manufacturer's instructions. Experiments were performed in triplicate, with 10 wells evaluated for each data point in each experiment.
Detection of Lipids.
The cells were cultured in 96-well tissue culture plates at a density of 2,500 cells per well for 2 days. The wells were then washed with PBS, and Sebomed Complete Medium was added. After 2 days, the medium was harvested, and fresh medium without or with CRH 10−8–10−6 M was given to the cells. The supernatants were harvested 72 h later, the wells were washed twice with PBS, and 100 μl of a 10 μg/ml nile red solution in PBS was added to each well. The plates were then incubated at 37°C for 30 min, and the released fluorescence was read on a Molecular Devices SPECTRAmax Gemini spectrofluorometer. The results are presented as percentages of the absolute fluorescence units in comparison with the controls, using 485 nm excitation and 565 nm emission filters for neutral lipids and 540 nm excitation and 620 nm emission filters for polar lipids. Experiments were performed in triplicate, with 10 wells evaluated for each data point in each experiment.
Determination of IL-8 Release.
Cells were cultured in six-well tissue culture plates at a density of 150,000 per well for 24 h. The wells were then washed with PBS, and Sebomed Complete Medium including 1 mM Ca2+ was added. After 3 days, the medium was removed, and the cells were gently washed with PBS and were incubated with 2 ml of fresh Sebomed Complete Medium supplemented with CRH 10−10–10−7 M, 50 ng/ml IL-1β, or their combinations for 24 h. The culture supernatants were then collected and centrifuged to remove cell detritus. Aliquots were stored at −70°C until use. IL-8 release in the culture supernatants was measured by ELISA in triplicate wells for all individual treatments, with duplicate wells being used for each individual assessment. The detection limit of IL-8 was less than 10 pg/ml. The results are presented as absolute optical density units at 450 nm or as a ratio compared with the results of the untreated controls, whereas the ratio 1 represents the IL-8 release of the controls.
Statistical Analysis.
Values represent the mean values ± SE. Statistical significance was calculated by the two-sample independent-groups t test. Mean differences were considered to be significant when P < 0.05.
Results
Human Sebocytes Express CRH, CRH-BP, and CRH-R.
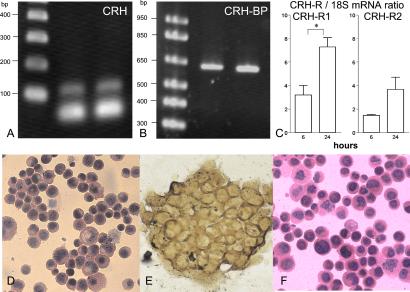
We examined the expression of CRH and CRH-BP in SZ95 sebocytes at the mRNA level by RT-PCR and of CRH, CRH-R1, and CRH-R2 by real time quantitative RT-PCR and studied their protein expression and cellular distribution by immunocytochemical labeling with specific antibodies. Specific RT-PCR signals were detected for CRH, CRH-BP, CRH-R1, and CRH-R2 (Fig. 1 A–C). CRH-R1 was the predominant receptor type (CRH-R1/CRH-R2 mRNA ratio = ≈2 at 6–24 h in culture). CRH-R mRNA levels increased with time. CRH, examined by two different antibodies, and CRH-BP were distributed diffusely in the cytoplasm, whereas the positive labeling with the CRH-R antibody could not be clearly attributed to the cell membrane, the cytoplasm, or both (Fig. 1 D–F).
Figure 1.
Detection of CRH, CRH-BP and CRH-R1 and CRH-R2 at the mRNA (A–C) and the protein (D–F) levels. In A and B, RT-PCR-specific signals of 122 and 634 bp for CRH and CRH-BP were found in SZ95 sebocytes, respectively (lane 2), and in cells of the control dysplastic oral keratinocyte cell line (lane 3). (Lane 1) Marker ladder. CRH-R1 was the predominant receptor type (CRH-R1/CRH-R2 ratio = ≈2), and its mRNA levels significantly increased with time as detected by real time quantitative RT-PCR (C; P < 0.05). CRH (shown are the results with the rabbit anti-human antibody; D), and CRH-BP (E) were distributed diffusely in the cytoplasm, whereas the positive labeling with the CRH-R antibody (F) could not be clearly attributed to the cell membrane or the cytoplasm.
CRH Does Not Influence Either Sebocyte Proliferation or Their Viability.
To examine the intracrine biological effects of CRH on SZ95 sebocytes, its effects on cell proliferation and cell viability were studied by the 4-methylumbelliferyl heptanoate fluorescence assay and the LDH assay, respectively. Neither altered CRH at concentrations of 10−11–10−4 M the proliferation of SZ95 sebocytes at time points between 24 and 72 h nor affected cell viability at concentrations of 10−10 and 10−7 M at 24 h (data not shown).
CRH Does Not Affect the IL-1β-Induced IL-8 Release from Human Sebocytes.
As previously described,** IL-1β strongly stimulated IL-8 release from SZ95 sebocytes at 24 h (4.6-fold, P < 0.001). CRH did not affect either the spontaneous IL-8 release or the IL-1β-induced IL-8 release from SZ95 sebocytes at 24 h (Fig. 2).
Figure 2.
Effect of IL-1β on IL-8 release from human sebocytes to their supernatants and its regulation by CRH. (A) IL-1β strongly stimulated IL-8 release from SZ95 sebocytes at 24 h in a dose-dependent manner (4.6-fold increase at 50 ng/ml; ***, P < 0.001). CRH did not affect either the spontaneous IL-8 release (B) or the IL-1β-induced IL-8 release from SZ95 sebocytes at 24 h (C). IL-8 release in culture supernatants was measured by ELISA and is presented as absolute optical density units at 450 nm in A and as ratio compared with the results of the untreated controls in B and C, whereas ratio = 1 represents the IL-8 release of the controls.
CRH Up-Regulates Lipid Synthesis in Human Sebocytes.
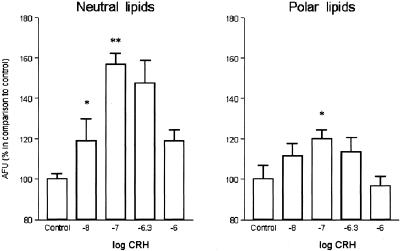
CRH exhibited a biphasic effect on sebaceous lipids, up-regulating the lipid content of SZ95 sebocytes at concentrations up to 10−7 M and inducing a decrease toward the basal levels at higher concentrations (Fig. 3). A stronger CRH effect was assessed on neutral than on polar sebaceous lipids at the optimum concentration of 10−7 M (P < 0.01 and P < 0.05, respectively).
Figure 3.
CRH influence on sebaceous lipogenesis. CRH exhibited a biphasic effect on sebaceous lipids, up-regulating the lipid content of SZ95 sebocytes at concentrations up to 10−7 M (*, P < 0.05; **, P < 0.01) and inducing a decrease toward the basal levels at higher concentrations. A stronger CRH effect was assessed on neutral than on polar sebaceous lipids at the optimum concentration of 10−7 M. Intracellular lipids were measured after cell labeling with nile red and detection of the released fluorescence in a spectrofluorometer. The results are presented as percentages of the absolute fluorescence units in comparison with the controls using 485 nm excitation and 565 nm emission filters for neutral lipids and 540 nm excitation and 620 nm emission filters for polar lipids.
CRH Up-Regulates mRNA Levels of Δ5-3β-HSD.
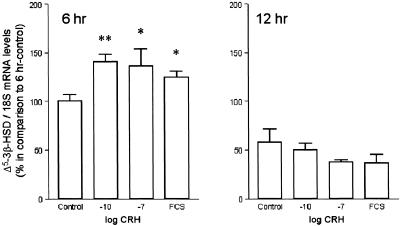
To detect possible mechanisms of stimulation of sebaceous lipid synthesis by CRH, we investigated the hypothesis that CRH may regulate Δ5-3β-HSD, which is the key enzyme for testosterone synthesis in human sebocytes (14). By using real time quantitative RT-PCR of Δ5-3β-HSD mRNA levels, CRH was found to exhibit an early up-regulation of Δ5-3β-HSD mRNA levels at 6 h (P < 0.01), which was abolished later (at 12 h) (Fig. 4).
Figure 4.
Detection of Δ5-3β-HSD mRNA level regulation by CRH. By using real time quantitative RT-PCR, Δ5-3β-HSD mRNA levels were found to increase early (at 6 h) under treatment with CRH (*, P < 0.05; **, P < 0.01) at a similar magnitude to those detected under treatment with 10% heat-inactivated FCS; FCS includes several hormones and growth factors. The CRH effect on Δ5-3β-HSD mRNA levels was annulled at 12 h. The results are presented as percentages of Δ5-3β-HSD/18S mRNA levels in comparison with the 6 h control (100%).
CRH-R mRNA Levels Are Regulated by Growth Hormone (GH) and Testosterone.
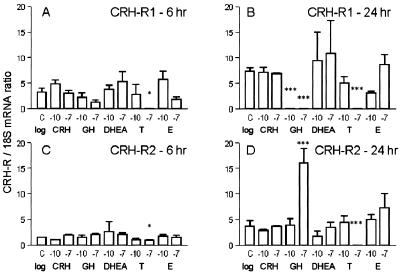
CRH synthesis in human sebocytes and its control of lipogenesis led us to assess the presence of a feedback mechanism that may involve CRH-R. To investigate this hypothesis, real time quantitative RT-PCR of CRH-R1 and CRH-R2 mRNA levels was performed after treatment of SZ95 sebocytes with CRH, GH, and testosterone, also shown to up-regulate sebaceous lipogenesis (15, 16), DHEA and 17β-estradiol, up-stream and down-stream metabolites of testosterone, respectively (14). CRH was found not to modify mRNA levels of its receptors (Fig. 5). In contrast, GH and testosterone acted as feedback mechanism inducers. GH down-regulated CRH-R1 mRNA levels and up-regulated those of CRH-R2 at 24 h (P < 0.001; Fig. 5 B and D). Testosterone, at 10−7 M, markedly down-regulated CRH-R1 and CRH-R2 mRNA levels at 6 (P < 0.05) and 24 h (P < 0.001). DHEA and 17β-estradiol did not affect CRH-R mRNA levels. All hormones tested were not cytotoxic for SZ95 sebocytes, as detected by the LDH release assay (data not shown).
Figure 5.
Detection of CRH-R mRNA levels under treatment with CRH, GH, DHEA, testosterone (T), and 17β-estradiol (E) by using real time quantitative RT-PCR. CRH, DHEA, and 17β-estradiol did not modify mRNA levels of CRH-R1 (A and B) or CRH-R2 (C and D). In contrast, GH down-regulated CRH-R1 mRNA levels (***, P < 0.001) and up-regulated those of CRH-R2 at 24 h (10−7 M). Testosterone, at 10−7 M, markedly down-regulated CRH-R1 and CRH-R2 mRNA levels at 6 (*, P < 0.05) and 24 h. The results are presented as percentages CRH-R/18S mRNA ratio.
Discussion
The CRH-propiomelanocortin system is highly organized, entrained, and of crucial importance for cutaneous responses to stress (4, 17–19). CRH activity on skin seems to depend on the cellular target. CRH can modify vascular reactions, act as a local growth factor, or induce cell differentiation and lipogenesis. It has been implicated in angiogenesis, and in neurogenic and neuroinflammatory skin disorders, including atopic dermatitis and psoriasis. In addition, the CRH/CRH-R1 pathway has been associated with the hair cycle in the mouse (20).
The sebaceous gland is the organ conferring on the skin an independent peripheral endocrine function (6). It also seems to be involved in responses to stress, expressing receptors for α-melanocyte-stimulating hormone (α-MSH; ref. 21), β-endorphin,‡‡ vasoactive intestinal polypeptide, neuropeptide Y, and calcitonin gene-related peptide.†† α-MSH and calcitonin gene-related peptide down-regulate the enhanced IL-8 synthesis in IL-1β-challenged human sebocytes, but are not produced in human sebocytes themselves (21).†† β-Endorphin is also a paracrine neuropeptide for human sebocytes, which suppresses their proliferation induced by epidermal growth factor in Ca2+-rich medium.‡‡
In the current study, we investigated the presence of the CRH/CRH-BP/CRH-R loop in human sebocytes and its regulation and biological activity in these cells in vitro. As currently detected in human sebaceous glands by in situ experiments (8), CRH and CRH-R were also present in our human sebocyte cultures. CRH is probably an autocrine hormone for human skin cells of epithelial origin, such as keratinocytes and sebocytes, and for melanocytes, whereas it exhibits a paracrine activity on human fibroblasts (4, 6). Whereas the hypothalamic CRH stimulates the pituitary CRH-R leading to the production and secretion of propiomelanocortin-derived peptides, sebocyte CRH did not regulate its receptor at the mRNA level in our study. In addition, α-MSH and β-endorphin are not produced by human sebocytes (21, ††); therefore, CRH may take over functions in human sebocytes other than inducing an intrinsic HPA-like axis. Indeed, whereas the release of glucocorticoids is regulated via the HPA axis with CRH and adrenocorticotropic hormone as the respective secretagogues (22), data from different experimental models have verified that CRH influences steroidogenesis independently from the HPA axis (23, 24).
According to our findings, CRH induces sebaceous lipogenesis in a biphasic manner which is characteristic for nuclear receptor ligands (25). However, CRH is not a ligand for nuclear receptors; therefore, CRH may directly enhance availability or activity of nuclear receptor ligands, such as androgens (15), and indirectly influence sebaceous lipogenesis through the latter. Indeed, our experiments revealed that CRH induces an early up-regulation of Δ5-3β-HSD mRNA expression. Sebocytes are the only human skin cells that express Δ5-3β-HSD and, therefore, are able to convert dehydroepiandrosterone to testosterone (14). Androgens play an important role in sebaceous gland growth and differentiation (26), whereas sebaceous gland function is regulated not only by testosterone and 5α-dihydrotestosterone, but also by dehydroepiandrosterone sulfate, especially in fetal and neonatal life and with the adrenarche (26, 27). The skin is capable of synthesizing cholesterol used in cell membranes, in formation of the epidermal barrier, and in sebum, and is also used as substrate for sex steroid hormone synthesis.§§ The local formation of sex steroids provides autonomous control to human skin, which is thus able to adjust their formation and metabolism according to local needs. Sebaceous glands and sweat glands account for the vast majority of androgen metabolism in skin (14, 26). The early regulation of Δ5-3β-HSD by CRH in human sebocytes is in accordance with the HPA-independent CRH influence on steroidogenesis reported above (23, 24). The discontinuation of Δ5-3β-HSD mRNA regulation by CRH and the finding that testosterone and GH, which also enhances sebaceous lipogenesis (16), down-regulate or modify CRH-R expression indicate that these are associated physiological mechanisms. In addition, the abundant expression of CRH in sebocytes (ref. 8 and this study) makes likely that sebocyte CRH may be the source of a paracrine regulation of the HPA-like axis in human skin.
CRH-Rs represent a family with at least two distinct members in humans, CRH-R1 and CRH-R2, encoded by separate genes, sharing high sequence homology (≈70%), and differing markedly in their pharmacological profiles and anatomical distribution (17). These receptors are seven transmembrane receptor proteins coupled to the Gs signaling system. Human skin cells have been shown to express functional CRH-R1 (5, 17). The CRH-R1 human gene contains 14 exons (28); eight alternatively spliced CRH-R1 transcripts have been identified (29). For CRH-R2, three splice variants have been detected (30). The present investigation indicates that at least one human skin cell type, the sebocyte, expresses mRNA for both members of the CRH-R family, with CRH-R1 being the predominant receptor type. The biological significance of CRH-R regulation by testosterone in our experiments with human sebocytes as a CRH feedback mechanism is corroborated by (i) the strong inhibitory effect of testosterone on the mRNA levels of 3-hydroxy 3-methylglutaryl CoA reductase in human sebocytes, an enzyme that catalyzes the first step of steroidogenesis (unpublished observation), (ii) the stimulation of testosterone production in mouse Leydig cells by CRH agonists through binding to CRH-R1 (31), and (iii) the significant attenuation of behavioral, neuroendocrine, and autonomic responses to stress in primates by oral administration of a CRH-R antagonist (32).
CRH-BP, shown here to be expressed in skin cells, is a glycoprotein with a molecular mass of 37 kDa predominantly produced by the liver (7). CRH-BP binds CRH and antagonizes CRH-induced release of adrenocorticotropic hormone in vitro. Most CRH in plasma is bound to CRH-BP, being, therefore, inactive and unable to bind its receptor. It has been shown that pituitary CRH-BP mRNA levels increase in response to acute restraint stress and that glucocorticoids play a significant role in this positive regulation (33). By expressing CRH-BP, human sebocytes probably possess another regulatory mechanism for both their own and for paracrine CRH secretion and activity.
GH is widely expressed in human skin (6) and is likely to be involved in sebaceous gland development, stimulates sebocyte differentiation, and also augments the effect of 5α-dihydrotestosterone on sebaceous lipid synthesis (16). We investigated the effect of GH on feedback regulation of CRH-R expression and found that GH switched completely the predominant CRH-R1 in human sebocytes to CRH-R2. Interestingly, CRH-R1 and CRH-R2 expression is hair cycle dependent; CRH-R1 is mainly expressed in hair compartments of murine anagen follicles and is absent in telogen ones, whereas CRH-R2 expression is higher in telogen and decreases during anagen progression (20). Therefore, testosterone, by down-regulating CRH-R1 mRNA levels, and, especially, GH, by down-regulating CRH-R1 and up-regulating CRH-R2 mRNA levels, may represent signals for induction of hair loss.
In summary, our findings include (i) the expression of CRH, CRH-BP, and CRH-R at the mRNA and protein levels, (ii) the presence of both CRH-R1 and CRH-R2 with predominance of CRH-R1 at the mRNA level, (iii) the confirmation of a biological activity of CRH on human sebocytes, namely a biphasic regulation of sebaceous lipids with a maximum up-regulation at 10−7 M, and an early up-regulation of Δ5-3β-HSD mRNA (at 6 h), but no effect on cell viability, cell proliferation, or IL-1β-induced IL-8 release, (iv) the down-regulation of CRH-R mRNA levels by testosterone, the complete switch of CRH-R1 mRNA expression to CRH-R2 by GH, and the missing regulation of CRH-R mRNA levels by CRH itself. The data confirmed the presence of a complete CRH/CRH-R system in human sebocytes (Fig. 6). CRH is likely to serve as an important autocrine hormone in this cell type with a homeostatic pro-differentiation activity. It directly induces lipid synthesis and enhances mRNA expression of Δ5-3β-HSD, the enzyme that converts dehydroepiandrosterone to testosterone in human sebocytes (14). Testosterone and GH, which also enhance sebaceous lipid synthesis (15, 16), were found to antagonize CRH by down-regulating or modifying CRH-R expression, respectively. These findings implicate an involvement of CRH in the clinical development of acne, seborrhea, androgenetic alopecia, skin aging, xerosis, and any other skin disease associated with alterations in the formation of sebaceous lipids.
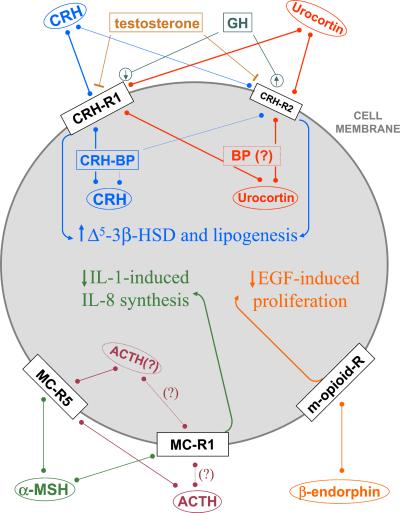
Figure 6.
The sebocyte HPA-like axis may contribute to sebocyte differentiation (decreased proliferation and enhanced lipogenesis) and anti-inflammatory signaling (reduced IL-8 secretion). Data from refs. 8, 21, 34–36; this manuscript; and ††. ACTH, adrenocorticotropin; BP, binding protein; EGF, epidermal growth factor; MC-R, melanocortin receptor; R, receptor
Acknowledgments
We acknowledge Birgit Bogdanoff for skillful technical assistance. This work was supported in part by research grants of the Bundesinstitut für gesundheitlichen Verbraucherschutz und Veterinärmedizin (BgVV Z 5.1-1328-156), the Research Committee of the Medical Faculty, The Free University of Berlin, the Sonnenfeld Stiftung (to Ch.C.Z.), and the Deutsche Forschungsgemeinschaft (FOR 441/1-1; to S.R.B.). W.C. was supported by a grant from the National Science Council Taiwan (Grant 89-2314-B-006-186).
Abbreviations
- Δ5-3β-HSD
3β-hydroxysteroid dehydrogenase/Δ5–4 isomerase
- CRH
corticotropin releasing hormone
- CRH-BP
corticotropin releasing hormone-binding protein
- CRH-R
corticotropin releasing hormone receptor(s)
- GH
growth hormone
- HPA
hypothalamic-pituitary-adrenal
- LDH
lactate dehydrogenase
- α-MSH
α-melanocyte-stimulating hormone
- RT
reverse transcription
Footnotes
Seltmann, H., Rudawski, I. M., Holland, K. T., Orfanos, C. E. & Zouboulis, Ch. C (2000). J. Invest. Dermatol. 114, 816 (abstr.).
Böhm, M., Li, Z., Zouboulis, Ch. C. & Luger, T. A. (2002) J. Invest. Dermatol., in press (abstr.).
Seiffert, K., Zouboulis, Ch. C., Seltmann, H. & Granstein, R. D (2000). Horm. Res. 53, 102 (abstr.).
Thiboutot, D. M., Sivarajah, A., Gilliland, K., Cong, Z. & Clawson, G (2001). J. Invest. Dermatol. 117, 410 (abstr.).
References
- 1.Bornstein S R, Böttner A, Chrousos G P. Mol Psychiatry. 1999;4:403–407. doi: 10.1038/sj.mp.4000602. [DOI] [PubMed] [Google Scholar]
- 2.Chrousos G P. Ann N Y Acad Sci. 1998;30:311–335. doi: 10.1111/j.1749-6632.1998.tb09006.x. [DOI] [PubMed] [Google Scholar]
- 3.Slominski A T, Wortsman J. Endocr Rev. 2000;21:457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- 4.Slominski A, Wortman J, Luger T, Paus R, Solomon S. Physiol Rev. 2000;80:979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- 5.Slominski A T, Botchkarev V, Choudhry M, Fazal N, Fechner K, Furkert J, Krause E, Roloff B, Sayeed M, Wei E, et al. Ann NY Acad Sci. 1999;885:287–311. doi: 10.1111/j.1749-6632.1999.tb08686.x. [DOI] [PubMed] [Google Scholar]
- 6.Zouboulis Ch C. Horm Res. 2000;54:230–242. doi: 10.1159/000053265. [DOI] [PubMed] [Google Scholar]
- 7.Zhao X J, Hoheisel G, Schauer J, Bornstein S R. Horm Metab Res. 1997;29:373–378. doi: 10.1055/s-2007-979058. [DOI] [PubMed] [Google Scholar]
- 8.Kono M, Nagata H, Umemura S, Kawana S, Yoshiyuki R. FASEB J. 2001;15:2297–2299. doi: 10.1096/fj.01-0254fje. [DOI] [PubMed] [Google Scholar]
- 9.Zouboulis Ch C, Seltmann H, Neitzel H, Orfanos C E. J Invest Dermatol. 1999;113:1011–1020. doi: 10.1046/j.1523-1747.1999.00771.x. [DOI] [PubMed] [Google Scholar]
- 10.Simoncini T, Apa R, Reis F M, Miceli F, Stomati M, Driul L, Lanzone A, Genazzani A R, Petraglia F. J Clin Endocrinol Metab. 1999;84:2802–2806. doi: 10.1210/jcem.84.8.5875. [DOI] [PubMed] [Google Scholar]
- 11.Asakura H, Zwain I H, Yen S S. J Clin Endocrinol Metab. 1997;82:2720–2725. doi: 10.1210/jcem.82.8.4119. [DOI] [PubMed] [Google Scholar]
- 12.Hiroi N, Wong M L, Licinio J, Park C, Young M, Gold P W, Chrousos G P, Bornstein S R. Mol Psychiatry. 2001;6:540–546. doi: 10.1038/sj.mp.4000908. [DOI] [PubMed] [Google Scholar]
- 13.Zouboulis Ch C, Garbe C, Krasagakis K, Krüger S, Schröder K, Orfanos C E. Melanoma Res. 1991;1:91–95. doi: 10.1097/00008390-199106000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Fritsch M, Orfanos C E, Zouboulis Ch C. J Invest Dermatol. 2001;116:793–800. doi: 10.1046/j.1523-1747.2001.01312.x. [DOI] [PubMed] [Google Scholar]
- 15.Rosenfield R L, Deplewski D, Kentsis A, Ciletti N. Dermatology. 1998;196:43–46. doi: 10.1159/000017864. [DOI] [PubMed] [Google Scholar]
- 16.Deplewski D, Rosenfield R L. Endocrinology. 1999;140:4089–4094. doi: 10.1210/endo.140.9.6957. [DOI] [PubMed] [Google Scholar]
- 17.Slominski A T, Wortsman J, Pisarchik A, Zbytek B, Linton E A, Mazurkiewicz J E, Wei E T. FASEB J. 2001;15:1678–1693. doi: 10.1096/fj.00-0850rev. [DOI] [PubMed] [Google Scholar]
- 18.Böhm M, Luger T A. Horm Res. 2000;54:287–293. doi: 10.1159/000053273. [DOI] [PubMed] [Google Scholar]
- 19.McCann S M, Antunes-Rodrigues J, Franci C R, Anselmo-Franci J A, Karanth S, Rettori V. Braz J Med Biol Res. 2000;33:1121–1131. doi: 10.1590/s0100-879x2000001000001. [DOI] [PubMed] [Google Scholar]
- 20.Roloff B, Fechner K, Slominski A T, Furkert J, Botchkarev V A, Bulfone-Paus S, Zipper J, Krause E, Paus R. FASEB J. 1998;12:287–297. doi: 10.1096/fasebj.12.3.287. [DOI] [PubMed] [Google Scholar]
- 21.Böhm M, Schiller M, Ständer S, Seltmann H, Li Z, Brzoska T, Metze D, Schiöth H B, Skottner A, Seiffert K, et al. J Invest Dermatol. 2002;118:533–539. doi: 10.1046/j.0022-202x.2001.01704.x. [DOI] [PubMed] [Google Scholar]
- 22.Ehrhart-Bornstein M, Hinson J P, Bornstein S R, Scherbaum W A, Vinson GP. Endocr Rev. 1998;19:101–143. doi: 10.1210/edrv.19.2.0326. [DOI] [PubMed] [Google Scholar]
- 23.Bornstein S R, Ehrhart M, Scherbaum W A, Pfeiffer E F. Cell Tissue Res. 1990;260:161–166. doi: 10.1007/BF00297501. [DOI] [PubMed] [Google Scholar]
- 24.Smith R, Mesiano S, Chan E C, Brown S, Jaffe R B. J Clin Endocrinol Metab. 1998;83:2916–2920. doi: 10.1210/jcem.83.8.5020. [DOI] [PubMed] [Google Scholar]
- 25.Zouboulis Ch C, Korge B P, Mischke D, Orfanos C E. J Invest Dermatol. 1993;101:628–633. doi: 10.1111/1523-1747.ep12366092. [DOI] [PubMed] [Google Scholar]
- 26.Deplewski D, Rosenfield R L. Endocr Rev. 2000;21:363–392. doi: 10.1210/edrv.21.4.0404. [DOI] [PubMed] [Google Scholar]
- 27. Zouboulis, Ch. C., Fimmel, S., Ortmann, J., Turnbull, J. R., Boschnakow, A. & Pochi, P. E. Neonatal Skin: Structure and Function. eds. Hoath, S. B. & Maibach, H. (Dekker, New York), 2nd Ed., in press.
- 28.Sakai K, Yamada M, Horiba N, Wakui M, Demura H, Suda T. Gene. 1998;219:125–130. doi: 10.1016/s0378-1119(98)00322-9. [DOI] [PubMed] [Google Scholar]
- 29.Pisarchik A, Slominski A T. FASEB J. 2001;15:2754–2756. doi: 10.1096/fj.01-0487fje. [DOI] [PubMed] [Google Scholar]
- 30.Dautzenberg F M, Kilpatrick G J, Hauger R L, Moreau J. Peptides. 2001;22:753–760. doi: 10.1016/s0196-9781(01)00388-6. [DOI] [PubMed] [Google Scholar]
- 31.Heinrich N, Meyer M R, Furkert J, Sasse A, Beyermann M, Bonigk W, Berger H. Endocrinology. 1998;139:651–658. doi: 10.1210/endo.139.2.5754. [DOI] [PubMed] [Google Scholar]
- 32.Habib K E, Weld K P, Rice K C, Pushkas J, Champoux M, Listwak S, Webster E L, Atkinson A J, Schulkin J, Contoreggi C, et al. Proc Natl Acad Sci USA. 2000;97:6079–6084. doi: 10.1073/pnas.97.11.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McClennen S J, Cortright D N, Seaholtz A F. Endocrinology. 1998;139:4435–4441. doi: 10.1210/endo.139.11.6311. [DOI] [PubMed] [Google Scholar]
- 34.Slominski A, Roloff B, Curry J, Dahiya M, Szczesniewski A, Wortsman J. J Clin Endocrinol Metab. 2000;85:815–823. doi: 10.1210/jcem.85.2.6381. [DOI] [PubMed] [Google Scholar]
- 35.Thiboutot D, Sivarajah A, Gilliland K, Cong Z, Clawson G. J Invest Dermatol. 2000;115:614–619. doi: 10.1046/j.1523-1747.2000.00094.x. [DOI] [PubMed] [Google Scholar]
- 36.Hoogduijn M J, McGurk S, Smit N P, Nibbering P H, Ancans J, van der Laarse A, Thody A J. Biochem Biophys Res Commun. 2002;290:844–850. doi: 10.1006/bbrc.2001.6283. [DOI] [PubMed] [Google Scholar]