Abstract
As another part continue for our previous study, variable substituted pyrazoles bearing sulfamoylphenyl moiety were synthesized and screened against two cancer related human carbonic anhydrase (hCA) isoforms and acetazolamide (AAZ) used as a reference standard. Some compounds as 4e and 6c manifested a promising inhibitory activity against both isoforms (KI = 0.072, 0.081 and 0.073, 0.095 µM), respectively. While others as 4a and 5e showed inhibitory activity against hCA IX only (KI = 0.062, 0.04 µM) or against hCA XII only as compound 5b (KI = 0.106 µM) compared to AAZ (KI = 0.065, 0.046 µM), respectively. Also, the anticancer efficacy against 60 cancer cell lines for the target compounds was assessed, and the most promising ones were 4d and 5a-d. Further investigation of the anticancer activity of 5b on MCF-7 cell line explored (IC50 = 5.21 µM) compared to doxorubicin (IC50 = 11.58 µM). Moreover, compound 5b was exposed to cell cycle analysis and apoptotic assay on MCF-7 breast cancer cell line under both normal and hypoxic conditions at its IC50 concentration with elevation of total apoptotic cells % in MCF-7 relative to the control cells; respectively. Finally, molecular modelling simulations rationalized the in vitro testing results.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11030-024-11023-3.
Keywords: Sulfamoylphenyl, Acetazolamide, Carbonic anhydrase inhibition, Molecular modelling
Introduction
Carbonic anhydrases (CAs, EC 4.2.1.1), the prevalent zinc-containing metalloprotein that catalyze effectively the reversible transformation of carbon dioxide into bicarbonate with the release of a proton, are vital enzymes that perform a number of crucial physiological and pathological roles in the biological system [1–4]. All human CAs (hCAs) are members of the α-class and exist in 16 diverse isoforms of which only 12 are catalytically active [4, 5]. The majority of powerful CAIs have been linked to the presence of an appropriate zinc binding group (ZBG) to create the necessary interactions inside the hCAs active sites [6]. Membrane-bound CA IX is an enzyme whose expression is highly stimulated by hypoxia, a state linked to low oxygen levels in various solid tumor forms, including colon, glioma, and breast cancer [7, 8], while CA XII was identified as tumor related isoform in 1998 [9]. It’s expression is several times higher in cancer and tumor tissues, it is highly expressed in a number of tissues, including the kidney, intestine, reproductive epithelia, and ocular tumors [2, 10]. Regulation of CA XII expression is dependent on estrogen receptors and hypoxia. Low-grade breast cancer is indicated by higher expression of CA XII. CA XII is essential for a number of physiological processes [10].
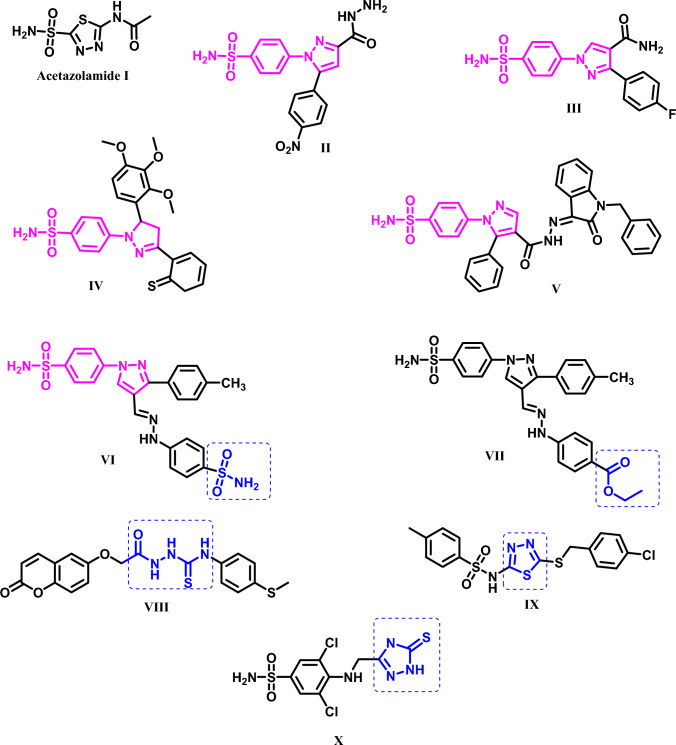
Sulfamoyl moiety is regarded as a cardinal carbonic anhydrase inhibitor pharmacophore as in acetazolamide (AAZ) I; it is accountable for its coordination with the enzyme metal ion (Zn2+) in all isoforms with KI = 25 nM and 5.7 nM against hCA IX and hCA XII; respectively [11, 12]. Accordingly, sulfamoylphenyl scaffold is the most common core in most CAIs [7, 13, 14]. In addition, different pyrazoles bearing sulfamoylphenyl scaffold displayed noteworthy CA inhibition against, hCA IX only as compound II with KI value = 2.3 nM relative to the reference drug AAZ [15], against hCA XII only such as compound III which is a powerful CA XII inhibitor with KI value of 1.9 nM compared to AAZ (KI value 5.7 nM) on hCA XII, [16] or against both isoforms as compounds IV [17] and compound V [18] with KI values: (0.044 µM and 13.6 nM) and (0.009 µM and 6.5 nM); respectively, compared to AAZ [19] (Fig. 1).
Fig. 1.
Structures of some reported CAIs
It is important to specify that the existence of certain functional groups had favourable effect on CA inhibition such as AAZ I; the well-known CAI [11, 12], sulfamoyl group or its ester congener [20], acylthiosemicarbazide [21], 1,3,4-thiadiazole [22] or 1,2,4-triazoles [23, 24] 5-memebered rings as shown in compounds I, VI-X, individually (Fig. 1).
In the current research, we are keeping our former attempts [20] to explore novel anticancer sulfamoylphenyl pyrazoles adopted the required features to inhibit the cancer related CA isoforms; hCA IX and hCA XII.
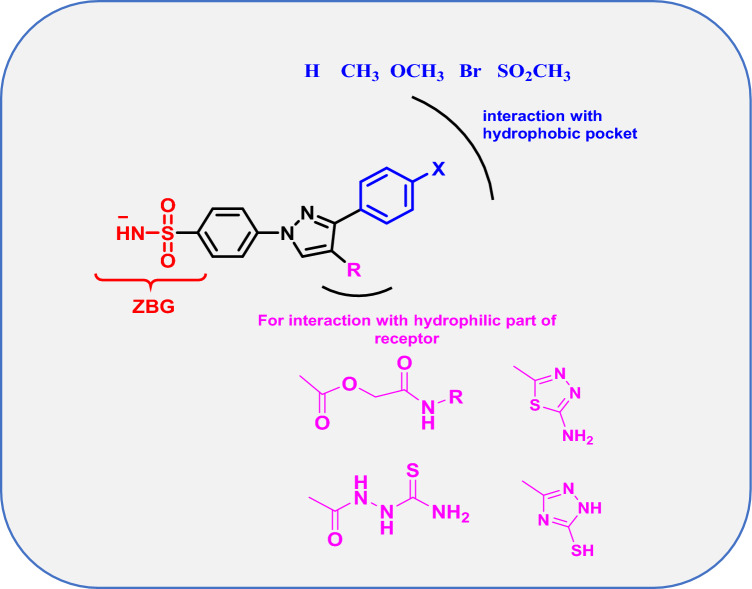
We focused on attaching different moieties at position 4 of pyrazole of sulfamoylphenyl pyrazole scaffold to perform the required interactions with the hydrophilic half of the receptor using either 5 or 6- membered aromatic ring directly attached or through a five-atom spacer. On the other hand, the receptor is accommodated with p-substituted phenyl moiety at position 3 of the pyrazole ring to accommodate the hydrophobic half of the receptor (Fig. 2).
Fig. 2.
General structure of the target compounds
Moreover, molecular modelling was performed to explore types of interaction of the most active compounds in the vicinity of CA IX and CA XII active sites for better understanding of their biological results.
Results and discussion
Chemistry
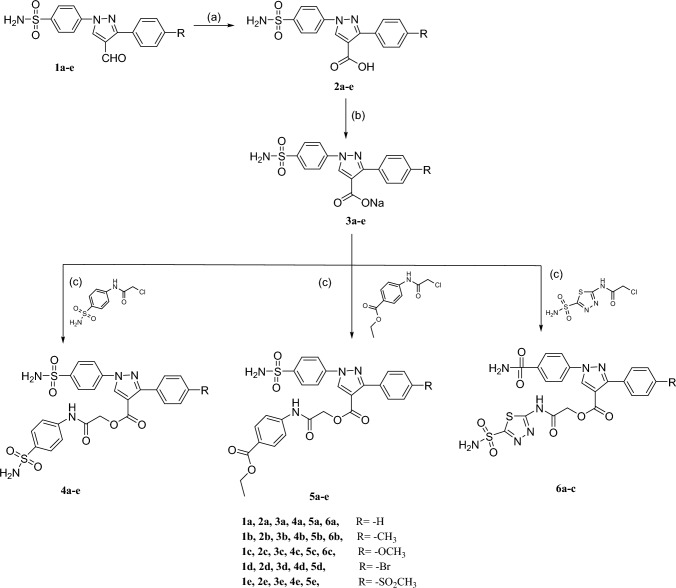
The synthetic routes for the final compounds are depicted in schemes 1 and 2. Initially, the 4-substituted pyrazole carboxylic acid derivatives 2a-e were prepared via the oxidation of the corresponding 4-formyl pyrazole derivatives 1a-e [25, 26] with potassium permanganate to give 2a-e in good yields [16, 20, 27, 28]. Final target compounds were achieved through the condensation of the sodium carboxylate salts 3a-e with different chloroacetamido derivatives namely 2-chloro-N-(4-sulfamoylphenyl) acetamide, ethyl 4-(2-chloroacetamido) benzoate and 2-chloro-N-(5-sulfamoyl-1,3,4-thiadiazol-2-yl) acetamide to furnish 4a-e, 5a-e and 6a-c; respectively (Scheme 1).
Scheme 1.
Reagents and reaction conditions: a KMnO4, pyridine, 0 °C, b Sod. metal, absolute methanol, reflux 25–30 h, c Different acetamido derivatives, DMF, reflux
Scheme 2.
Reagents and reaction conditions: a Absolute ethanol, conc. H2SO4, reflux b NH2NH2, reflux, c Ammonium thiocyanate, 10% HCl, absolute ethanol, reflux, d 9b, H2SO4, 90 °C, 2h, e 9b, 1% NaOH, ethanol, reflux
In scheme 2, the hydrazine carbothioamides 9a-e were achieved via the reaction of the corresponding acid hydrazide derivatives 8a-e with ammonium thiocyanate in absolute ethanol containing 10% HCl. Then, the acylthiosemicarbazide derivatives 9a-e were cyclized under acidic or basic conditions to give the cyclized derivatives 10 and 11; respectively.
All the newly prepared derivatives were confirmed through spectral and elemental analyses as illustrated in details in the experimental part.
Biological assays
CA inhibitory activity
The CA inhibitory test conducted in vitro was used to assess the activity of the synthesized target compounds 4a-e, 5a-e, 6a-c, 9a-e, 10 and 11 as well as the reference standard AAZ against the cancer-related isoforms CA IX and CA XII in order to explore their potentiality as anticancer derivatives. The IC50 (µM) values of the examined compounds and AAZ against hCA IX and hCA XII are listed in Table 1.
Table 1.
IC50 (µM) of the final compounds and AAZ against hCA IX and hCA XII
| Compound | IC50 (µM) ± SD | Compound | IC50 (µM) ± SD | ||
|---|---|---|---|---|---|
| hCA IX | hCA XII | hCA IX | hCA XII | ||
| 4a | 0.062 ± 0.003* | 0.226 ± 0.009 | 6a | 0.591 ± 0.028 | 0.358 ± 0.013 |
| 4b | 0.331 ± 0.015 | 0.17 ± 0.006 | 6b | 0.339 ± 0.016 | 1.7 ± 0.064 |
| 4c | 0.175 ± 0.008 | 0.415 ± 0.016 | 6c | 0.073 ± 0.003* | 0.095 ± 0.004* |
| 4d | 0.509 ± 0.024 | 0.814 ± 0.031 | 9a | 0.215 ± 0.01 | 0.605 ± 0.023 |
| 4e | 0.072 ± 0.003* | 0.081 ± 0.003* | 9b | 0.339 ± 0.019 | 0.335 ± 0.013 |
| 5a | 0.945 ± 0.044 | 0.395 ± 0.015 | 9c | 0.365 ± 0.017 | 0.168 ± 0.006 |
| 5b | 0.148 ± 0.007 | 0.106 ± 0.004* | 9d | 1.108 ± 0.052 | 1.417 ± 0.053 |
| 5c | 0.22 ± 0.01 | 0.272 ± 0.01 | 9e | 1.77 ± 0.083 | 0.929 ± 0.035 |
| 5d | 0.631 ± 0.029 | 0.99 ± 0.037 | 10 | 0.663 ± 0.031 | 1.148 ± 0.043 |
| 5e | 0.04 ± 0.002* | 0.167 ± 0.006 | 11 | 0.24 ± 0.011 | 0.332 ± 0.013 |
| AAZ | 0.065 ± 0.003 | 0.046 ± 0.002 | |||
*IC50 results that are considered not significantly different from that of AAZ at (P < 0.05)
Five of the tested compounds were non-significantly different from AAZ in inhibiting either hCA IX only as compounds 4a and 5e (0.06 and 0.04 µM) or hCA XII only as compound 5b (0.10 µM) or both isoforms like compounds 4e and 6c (0.07 µM), (0.08 and 0.09 µM); respectively, compared to AAZ (0.065 and 0.046 µM), respectively.
The proposed compounds showed inhibitory action against hCA IX, with an IC50 ranging from 0.04 to 1.77 µM. The ester derivatives 4a, 4e, 5b, 5e and 6c showed high potency on hCA IX with IC50 (0.04 to 0.07 µM) and compound 5e elicited the most effective inhibitory activity with IC50 = 0.04 µM relative to AAZ (IC50 = 0.06 µM). The unsubstituted derivatives 4a and the derivatives with electron withdrawing substitutions, e.g., SO2CH3 or Br 5e and 6c; respectively, showed the best inhibitory activity with (IC50 = 0.06, 0.04 and 0.07 µM; respectively).
Compounds 4a-e with IC50 (0.06–0.50 μM) showed good inhibitory activity, where the unsubstituted derivative 4a become the most active one in this series with IC50 (0.06 μM) and the activity of p-methyl derivative 4b is slightly decreased with IC50 (0.33 μM), by replacing the methyl group with methoxy one in 4c, the activity is enhanced with IC50 (0.17 μM). While the activity in the derivatives with electron withdrawing group 4d and 4e is enhanced with IC50 (0.50 and 0.07 μM, respectively). Moreover, in compounds 5a-e, the tested compounds exhibited inhibitory activity with IC50 ranging from 0.04 to 3.55 µM. Compound 5e elicited the most potent inhibitory activity with IC50 of 0.04 µM comparable to that of AAZ (IC50 = 0.05 µM). In compounds 6a-c showed good inhibitory activity with IC50 (0.07–0.59 μM), where the p-methoxy derivative 6c was the most active compound. Furthermore, the acylthiosemicarbazide derivatives 9a-e with IC50 (0.21–1.77 µM), the unsubstituted derivative 9a showed good inhibitory activity with IC50 (0.21 µM) and in compounds with electron donating groups 9b and 9c with IC50 (0.33 and 0.36 µM, respectively) the activity is slightly decreased, while the activity is decreased in derivatives with electron withdrawing groups 9d and 9e with IC50 (1.18 and 1.77 µM, respectively). Cyclization of the acylthiosemicarbazide derivative 9b IC50 (0.33 µM) into thiadiazol one 10 led to decrease in activity with IC50 (0.66 µM), while cyclization to triazol congener 11 led to increase in the activity with IC50 (0.24 µM).
On the other hand, concerning inhibition of hCA XII, the tested compounds showed suppressive effect with IC50 values range from 0.08 to 1.70 µM. The ester derivative 4e revealed the best activity with IC50 of 0.08 µM compared to that of AAZ (IC50 = 0.04 µM). Compounds 4e, 5b and 6c demonstrated the best inhibitory activity compared to most of the series.
In compounds 4a-e IC50 (0.08–0.81 µM), showed good inhibitory activity, where the CH3SO2 substituted derivative 4e become the most active derivative in this series with IC50 (0.08 µM) and the activity of the p-methyl derivative 4b is slightly decreased with IC50 (0.17 µM), the activity increased in the unsubstituted derivative 4a, the p-methoxy derivative 4c and the bromo derivative 4d with IC50 (0.22, 0.41 and 0.81, respectively µM).
Furthermore, in the ester derivatives 5a-e IC50 (0.10–0.99 µM), the activity of the p-methoxy derivative 5c and of the CH3SO2 derivative 5e is decreased with IC50 (0.27 and 0.16 µM, respectively). The p-methyl derivative 5b was the most active among this series with IC50 (0.10 µM) and by removing this methyl group as in compound 5a or replacing it with a bromo group in 5d, the activity is decreased with IC50 (0.39 and 0.99 µM, respectively).
In 6a-c, the unsubstituted derivative 6a and the p-methyl derivative 6b elicited inhibitory activity with IC50 (0.35 and 1.70 µM, respectively). While the methoxy derivative 6c was the most potent in this series with IC50 (0.09 µM).
In acylthiosemicarbazide derivatives 9a-e showed good inhibitory activity with IC50 (0.16–1.41 µM). Cyclization of the acylthiosemicarbazide derivative 9b IC50 (0.33 µM) into thiadiazole ring 10 led to decrease in activity with IC50 (1.14 µM), while cyclization to the triazole one 11 showed the same activity as 9b with IC50 (0.33 µM).
Anticancer activity
Preliminary anticancer screening at a single dose (10 µM)
The National Cancer Institute (NCI) Developmental Therapeutic Program (www.dtp.nci.nih.gov) selected the 20 novel synthesized compounds 4a-e, 5a-e, 6a-c, 9a-e, 10 and 11 to test for their anticancer activity at a single dose of 10 µM using SRB assay. Results are demonstrated as percentage growth inhibition (GI %) as well as mean graph of the growth percentage. A closer look on the in vitro preliminary anticancer screening revealed that best GI % against the tested cancer panels were for the ester compounds 4d and 5a-d with obvious clustered anti-cancer activity for compounds 5a-d. Compound 4d exhibited good activity over the panels under investigation with growth inhibition ranging from 8.46 to 86.55%. Interestingly, compounds 5a-d revealed significant anticancer activity against all cancer types with GI % ranges (5.67–92.08%), (12.78–83.81%), (5.25–65.90%) and (13.33–93.98%), respectively. Supplementary Data S3; Table S1 presents the detailed screening results of the most promising compounds. The preliminary screening’s detailed results for the remaining compounds are elucidated in the (Supplementary Data S3; Table S2).
In vitro cytotoxic activity against MCF-7
In an attempt to find a suitable correlation between the effect on certain tumor type and its mechanism of action, compound 5b as one of the most promising derivatives in the NCI screening along with its significant inhibitory results on hCA XII was selected for further biological evaluation. MCF-7 breast cancer cell line was chosen being one of the most cancer types where hCA XII is overexpressed from one side and from the other side compound 5b exhibited excellent inhibitory activity on it. Compound 5b was tested for its in vitro cytotoxicity against MCF-7 breast cancer cell line by using MTT assay under normal and hypoxic conditions. The results shown in Table 2 and 3 under both normal and hypoxic conditions, respectively, displayed significant inhibitory activity compared to doxorubicin as a reference (IC50 = 5.21 and 11.58 µM); respectively under normal conditions and under hypoxic conditions (IC50 = 3.50 and 2.72 µM); respectively indicating its significant anticancer activity.
Table 2.
Cytotoxic activity of compound 5b and doxorubicin against MCF-7 breast cancer cell line under normal conditions
| Compound | IC50 (µM) ± SD |
|---|---|
| 5b | 5.21 ± 0.21 |
| Doxorubicin | 11.58 ± 0.46 |
Table 3.
Cytotoxic activity of compound 5b and doxorubicin against MCF-7 breast cancer cell line under hypoxia
| Compound | IC50 (µM) ± SD |
|---|---|
| 5b | 3.50 ± 0.14 |
| Doxorubicin | 2.72 ± 0.11 |
Cell cycle analysis and apoptotic assay
The promising inhibitory results of compound 5b on hCA XII along with its significant effect on MCF-7 breast cell line was a determined factor for further investigation of its biological activity via cell cycle analysis and apoptotic activity under normal and hypoxic conditions.
Cell cycle analysis
In this part, a flow cytometric analysis of compound 5b was performed on MCF-7 under both normal and hypoxic conditions at the specified IC50 (5.21 and 3.50 µM); respectively, to investigate its impact on the cell cycle development. The outcomes under normal conditions depicted in Table 4 and Fig. 3, showed that derivative 5b affected mainly cell growth arrest at G1/S phase by increasing the cellular population from 58.41 to 67.39% (0.87 folds) compared to the control along with significant reduction at the G2/M phase from 16.6 to 3.05% (5.44 folds). While the results under hypoxic conditions depicted in Table 5 and Fig. 4, showed that derivative 5b affected mainly cell growth arrest at G2/M phase by increasing the cellular population from 11.61 to 32.28% (0.36 folds) compared to the control along with significant reduction at the S phase from 27.02 to 19.59% (1.38 folds).
Table 4.
Cell cycle analysis results of 5b against MCF-7 cell line under normal conditions
| DNA content | Cell growth arrest at | |||
|---|---|---|---|---|
| % G0-G1 | % S | % G2/M | ||
| 5b/MCF-7 | 67.39 | 29.56 | 3.05 | G1/S |
| Control/MCF-7 | 58.41 | 24.99 | 16.6 | – |
Fig. 3.
Influence of compound 5b on the cell cycle of MCF-7 cells after 24 h compared to the control under normal conditions
Table 5.
Cell cycle analysis results of 5b against MCF-7 cell line under hypoxic conditions
| DNA content | Cell growth arrest at | |||
|---|---|---|---|---|
| % G0-G1 | % S | % G2/M | ||
| 5b/MCF-7 | 48.13 | 19.59 | 32.28 | G2/M |
| Control/MCF- | 61.37 | 27.02 | 11.61 | – |
Fig. 4.
Influence of compound 5b on the cell cycle of MCF-7 cells after 24 h compared to the control under hypoxic conditions
Apoptotic effect
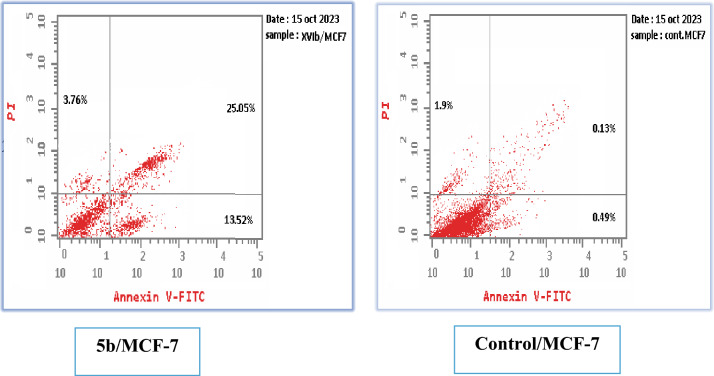
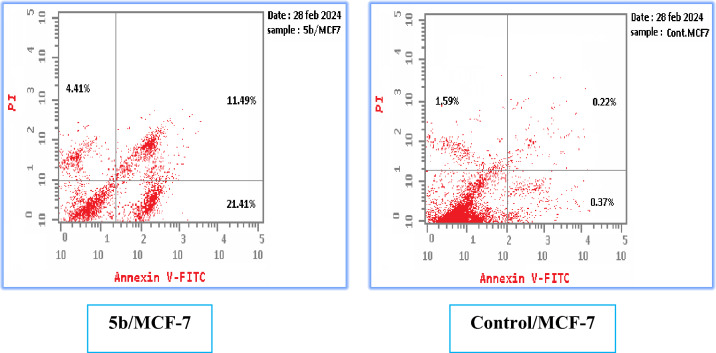
The apoptotic assay was conducted utilizing the Annexin V-FITC/propidium iodide technique to explore the cell apoptosis caused under the effect of the promising compound 5b on MCF-7 breast cancer cells in accordance with the reported method [29, 30]. The effect of compound 5b on the apoptotic liability in the selected cell line under normal condition is demonstrated in Table 6 and Fig. 5. These results showed an increase in the total of the apoptotic cells after the inclusion of compound 5b in MCF-7 cell line (42.33%) as well as the necrotic cells (3.76%) relative to the control cells (2.52 and 1.9%, respectively). Added to that, investigating compound 5b under hypoxic conditions, it showed an elevation in the total % of the apoptotic cells in MCF-7 cell line (37.31%) as well as the necrotic cells (4.41%) relative to the control cells (2.18 and 1.59%; respectively) as shown in Table 7 and Fig. 6. These findings may suggest compound 5b as a potential inducer of apoptosis.
Table 6.
Impact of compound 5b on apoptotic creation on MCF-7 relative to the control cell under normal conditions
| Compound | Apoptosis % | Necrosis % | ||
|---|---|---|---|---|
| Total % | Early % | Late % | ||
| 5b/MCF-7 | 42.33 | 13.52 | 25.05 | 3.76 |
| Cont.MCF-7 | 2.52 | 0.49 | 0.13 | 1.9 |
Fig. 5.
Results of the apoptotic and necrotic effects of derivative 5b compared to the control cells under normal conditions
Table 7.
Impact of compound 5b on apoptotic creation on MCF-7 relative to the control cell under hypoxic conditions
| Compound | Apoptosis % | Necrosis % | ||
|---|---|---|---|---|
| Total % | Early % | Late % | ||
| 5b/MCF-7 | 37.31 | 21.41 | 11.49 | 4.41 |
| Cont.MCF-7 | 2.18 | 0.37 | 0.22 | 1.59 |
Fig. 6.
Results of the apoptotic and necrotic effects of derivative 5b compared to the control cells under hypoxic conditions
In silico studies
Molecular modelling
Among the synthesized series, compounds 4a, 4e, 5e and 6c showed the highest hCA IX inhibitors, based on the in vitro carbonic anhydrase IC50 values. While compounds 4e, 5b and 6c elicited the best hCA XII inhibition compared to AAZ (IC50 = 0.04 µM). Consequently, molecular docking study on the specified compounds in the hCA IX and hCA XII active sites was done.
In the present study, the thiophene sulfamoyl inhibitor (9FK) [31] and AAZ [32] co-crystallized with hCA IX and hCA XII (PDB IDs: 5FL4 and 1JD0, respectively) were employed. Validating the molecular docking approach using self-docking was demonstrated by the minimal RMSD values between the re-docked poses in hCA XII (1.0507 Å) and hCA IX (1.0810 Å) and the native co-crystallized ligands. Additionally, the appropriateness of the suggested protocol was validated by the ability of the naturally co-crystallized ligands (9FK) and the experimentally employed reference AZZ) to replicate every significant interaction it possessed with the hot areas in CA IX and CA XII active sites; respectively.
The most important docking parameters; energy scores (Kcal/mol), binding interaction, hydrogen bond length compared to their IC50 values compared to the co-crystallized ligands in the hot spot of hCA IX and hCA XII isozymes were displayed in (Supplementary data: S.6.2, Table: S3 and S4), respectively.
Regarding hCA IX, the newly designed compounds showed comparable binding modes in the hot spot of hCA IX, which entail the interaction of the sulfamoyl moiety as the ZBG with the Zn+2 ion along with the creation of hydrogen bond with the crucial amino acid Thr200. The pyrazole ring and the hydrophobic side chain of the amino acid Gln71 make another hydrophobic interaction in compound 4a as depicted in the (Supplementary data: S.6.3, Figure: S66).
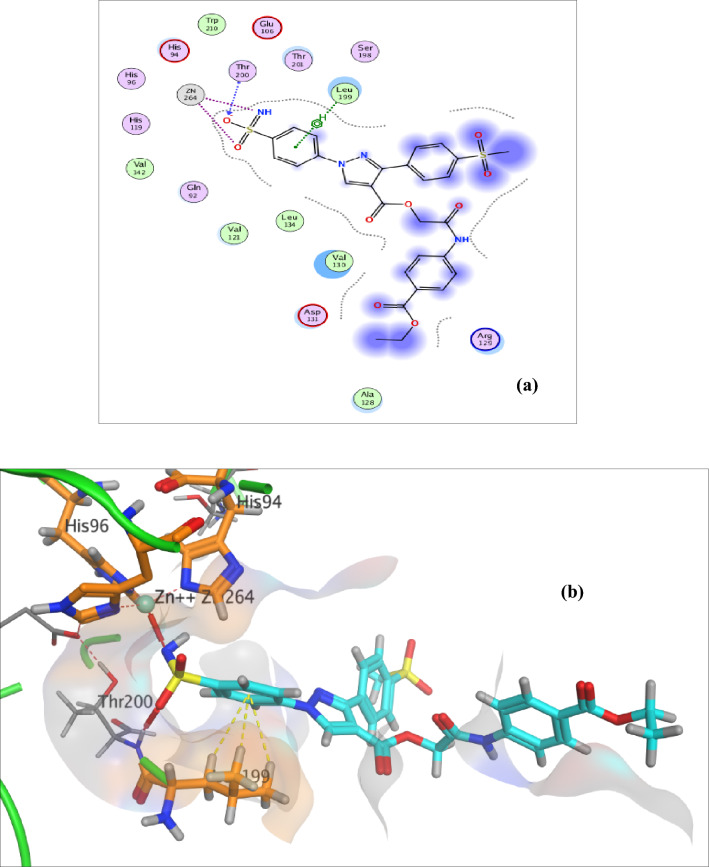
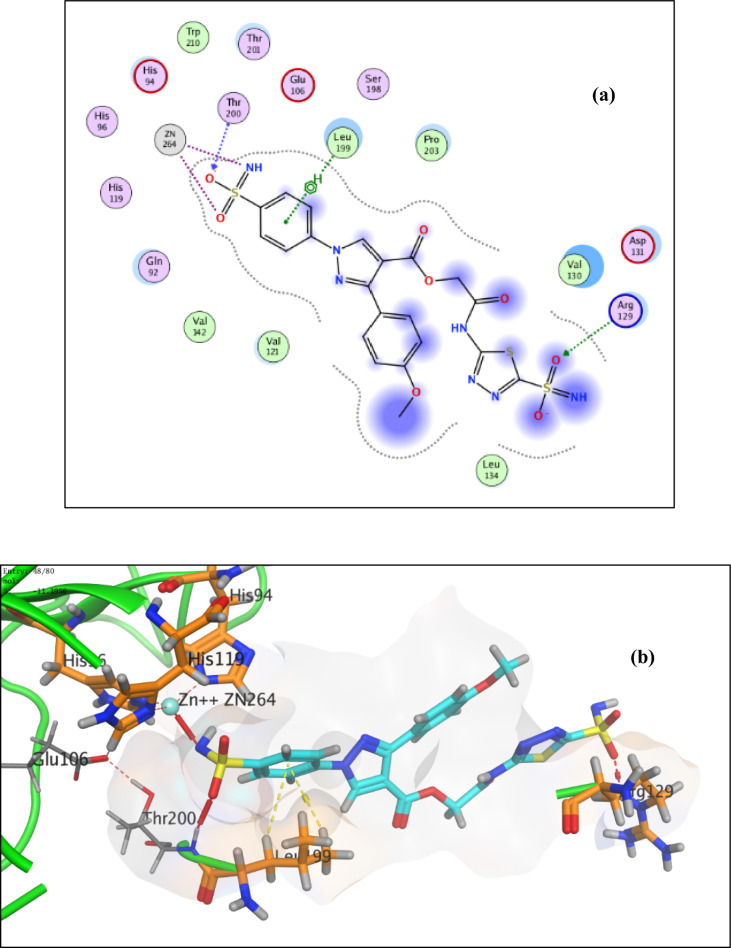
Regarding the ester derivatives 4e, 5e and 6c, the sulfamoyl phenyl ring establish hydrophobic contact with the hydrophobic side chain of the amino acid Leu199 (Supplementary data: S.6.3, Figure S67), Fig. 7 and 8, respectively. Also, extra two additional hydrogen bonds between the nitrogen of the pyrazole ring and the side chain amino group of His68 and between NHCO and the carbonyl side chain of the amino acid Gln71 are well characterized as in compound 4a (Supplementary data: S.6.3, Figure S66). Also, it is obvious that an extra hydrogen bond between the oxygen of the sulfamoyl of acetazolamide moiety and sidechain amino group of the amino acid Arg129 was accomplished as in compound 6c (Fig. 8). Furthermore, a hydrophobic interaction is formed between the hydrophobic side chain of the amino acid Leu91 and the benzene ring as in compounds 4e (Supplementary data: S.6.3, Figure: S67).
Fig. 7.
2D a and 3D b interaction of compound 5e in the hCA IX binding site
Fig. 8.
2D a and 3D b interaction of compound 6c in the hCA IX binding site
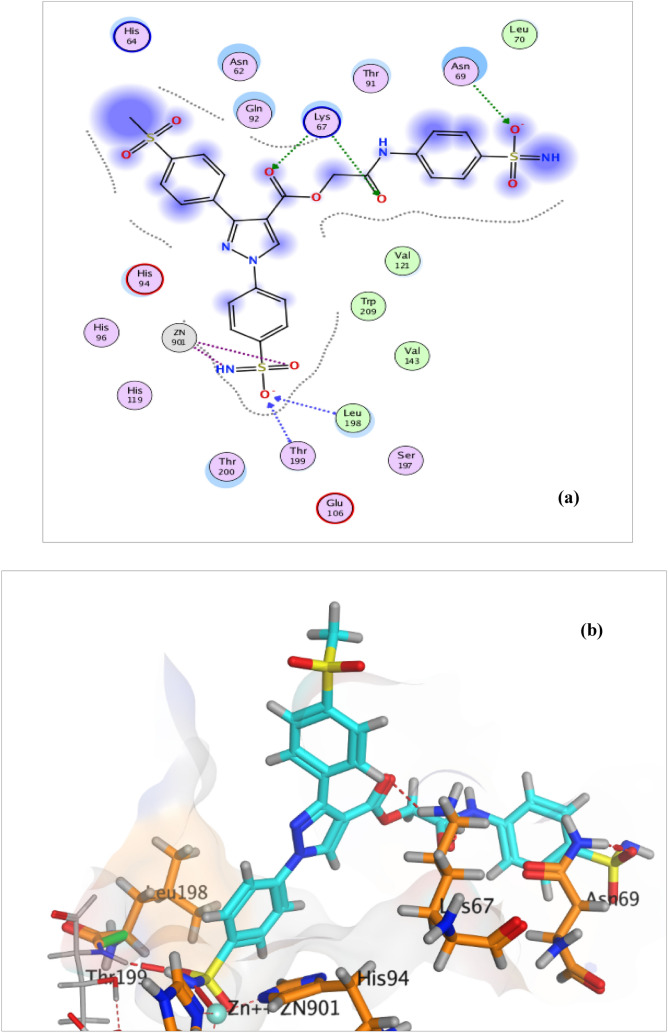
Concerning hCA XII, all the synthesized candidates elicited three major interactions: with Zn+2 ion at the active site, Leu198 and Thr199. An additional hydrogen bond is formed between compounds 4e, 5b and 6c and the side chain amino group of the amino acid Lys67 (Fig. 9, 10)asis> and (Supplementary data: S.6.3, Figure S68), respectively. Furthermore, an extra hydrogen bond was achieved in compounds 4e and 5b between the amino acids Asn69 and Gln 92 side chain carbonyl group with oxygen of the added sulfamoyl moiety and NH group; respectively. Also, compound 6c forms two extra hydrogen bonds between the NH and oxygen of sulfamoyl of acetazolamide with the amino acid His64 and Lys170, respectively, as depicted in the (Supplementary data: S.6.3, Figure S68); respectively.
Fig. 9.
2D a and 3D b interaction of compound 4e in the hCA XII binding site
Fig. 10.
2D a and 3D b interaction of compound 5b in the hCA XII binding site
Toxicity prediction
The newly synthesized compounds 4a, 4e, 5b, 5e and 6c were examined by the web server; Osiris Property Explorer (http://www.organic-chemistry.org/prog/peo/) to investigate their toxicities. This web server’ prediction is relied on how well the tested compound’s functional group match the precomputed set of structural fragments that are included in its database. Prediction results are demonstrated as colors; red, green, and yellow. Whereby, red color assumes a high risk of toxicity, yellow represents mild toxicity and the green color suggests low toxic effect [33]. The findings elucidated that all the tested candidates are anticipated to be safe and show no toxicity in terms of mutagenicity, tumorigenicity, irritating effect, or influence on the reproductive system except compound 6c which has toxic effect on the reproductive system.
Conclusion
Twenty pyrazole derivatives bearing sulfamoylphenyl moiety were designed via tail approach strategy where the sulfamoylphenyl pyrazole scaffold as ZBG was 3,4-disubstituted with suitable hydrophobic and hydrophilic moieties; respectively to accommodate the diverse nature of the enzyme active site halves. The IC50 of compound 5b (0.10 µM) were non-significantly different from that of AAZ as hCA XII inhibitors. The results of the anticancer screening showed that derivatives 4d and 5a-d had a promising anticancer activity against all the tumor cell lines in the panel. Also, compound 5b elicited an increment of the total apoptotic cells % in MCF-7 under both normal and hypoxic conditions (42.33 and 37.31%) compared to the control cells (2.52 and 2.18%); respectively. The in silico molecular modelling simulation indicated critical interaction pattern with the active site of carbonic anhydrase which might explain the obtained activity. On the other hand, the toxicity prediction revealed that the synthesized compounds are anticipated to be safe exhibiting no toxicity in terms of mutagenicity, tumorigenicity, irritability, or reproductive system effects. These encouraging results open the door for further investigation of the synthesized derivatives and introduce compound 5b as potential anticancer compound via hCA XII inhibition.
Experimental
Chemistry
The intense steps of the methodology used for the chemical syntheses of the given compounds are presented in (Supplementary data, S1.1.)
Based on the published literature, the following starting and intermediate compounds were synthesized: 1a-e [26], 2a-e [16, 20, 27, 28], 7a-e [20] and 8a-e.[16, 20, 34]
General procedure for the synthesis of compounds (3a-e)
Sodium methoxide solution (1 mol Na metal dissolved in 15 mL absolute methanol) was added to the appropriate acid 2a-e (1 mol) dissolved in 10 mL absolute methanol. The reaction mixture was refluxed for 25–30 h. The formed precipitate was washed by diethyl ether and then filtered to give the target compounds.
Sodium 3-phenyl-1-(4-sulfamoylphenyl)-1H-pyrazole-4-carboxylate (3a)
Yield: 85%, m.p. 145–150 °C. Rf = 0.17 (CHCl3, methanol). IR (KBr) υmax/cm−1: 3363, 3300 (NH2), 3062 (CH Ar), 1690 (C=O), 1589 (NH bending), 1535, 1504 (C=C), 1365, 1165 (SO2). 1H NMR (400 MHz, DMSO-d6) δ ppm: 7.36- 7.47 (m, 5H, Ar H), 7.94 (s, 2H, NH2, D2O exchangeable), 8.09–8.17 (m, 4H, Ar H), 8.81 (s, 1H, CH pyrazole). 13C NMR (100 MHz, DMSO-d6) δ ppm: 118.4, 127.4, 127.7, 127.9, 128.1, 129.2, 129.5, 133.9, 141.6, 142.0, 152.5 (C Ar), 167.8 (C=O).
Sodium 1-(4-sulfamoylphenyl)-3-(p-tolyl)-1H-pyrazole-4-carboxylate (3b)
Yield: 88%, m.p. 279–280 °C. Rf = 0.20 (CHCl3, methanol). IR (KBr) υmax/cm−1: 3300, 3294 (NH2), 3066 (CH Ar), 2924, 2850 (CH aliph.), 1690 (C=O), 1597 (NH bending), 1558, 1508 (C=C), 1365, 1161 (SO2). 1H NMR (400 MHz, DMSO-d6) δ ppm: 2.34 (s, 3H, CH3), 7.16 (d, J = 7.96 Hz, 2H, Ar H), 7.38 (s, 2H, NH2, D2O exchangeable), 7.90 (d, J = 8.68 Hz, 2H, Ar H), 8.08–8.10 (m, 4H, Ar H), 8.56 (s, 1H, CH pyrazole). 13C NMR (100 MHz, DMSO-d6) δ ppm: 21.3 (CH3), 118.3, 125.7, 127.7, 128.4, 129.3, 131.2, 131.6, 137.1, 141.4, 142.0, 151.8 (C Ar), 167.2 (C=O).
Sodium 3-(4-methoxyphenyl)-1-(4-sulfamoylphenyl)-1H-pyrazole-4-carboxylate (3c)
Yield: 90%, m.p. > 300 °C. Rf = 0.18 (CHCl3, methanol). IR (KBr) υmax/cm−1: 3441, 3383 (NH2), 3082 (CH Ar), 2935, 2835 (CH aliph.), 1704 (C=O), 1597 (NH bending), 1577, 1527 (C=C), 1346, 1165 (SO2). 1H NMR (400 MHz, DMSO-d6) δ ppm: 3.79 (s, 3H, OCH3), 6.91 (d, J = 8.84 Hz, 2H, Ar H), 7.78 (d, J = 8.68 Hz, 2H, Ar H), 7.85 (d, J = 8.60 Hz, 2H, Ar H), 7.92 (s, 2H, NH2, D2O exchangeable), 8.18 (d, J = 8.84 Hz, 2H, Ar H), 8.48 (s, 1H, CH pyrazole). 13C NMR (100 MHz, DMSO-d6) δ ppm: 55.5 (OCH3), 113.2, 117.6, 124.2, 126.7, 127.0, 130.7, 131.8, 139.9, 148.3, 151.1, 159.2 (C Ar), 168.0 (C=O).
Sodium 3-(4-bromophenyl)-1-(4-sulfamoylphenyl)-1H-pyrazole-4-carboxylate (3d)
Yield: 78%, m.p. 289–291 °C. Rf = 0.22 (CHCl3, methanol). IR (KBr) υmax/cm−1: 3383, 3313 (NH2), 3070 (CH Ar), 1690 (C=O), 1597 (NH bending), 1558, 1527 (C=C), 1365, 1161 (SO2). 1H NMR (400 MHz, DMSO-d6) δ ppm: 7.48 (s, 2H, NH2, D2O exchangeable), 7.58 (d, J = 8.28 Hz, 2H, Ar H), 7.94 (d, J = 8.40 Hz, 2H, Ar H), 8.09–8.16 (m, 4H, Ar H), 8.84 (s, 1H, CH pyrazole). 13C NMR (100 MHz, DMSO-d6) δ ppm: 118.8, 121.8, 130.2, 130.9, 131.1, 131.5, 132.0, 133.0, 141.7, 142.1, 151.2 (C Ar), 166.5 (C=O).
Sodium 3-(4-(methylsulfonyl) phenyl)-1-(4-sulfamoylphenyl)-1H-pyrazole-4-carboxylate (3e)
Yield: 85%, m.p. 125–129 °C. Rf = 0.18 (CHCl3, methanol). IR (KBr) υmax/cm−1: 3402, 3250 (NH2), 3020 (CH Ar), 2924, 2831 (CH aliph.), 1690 (C=O), 1593 (NH bending), 1527, 1504 (C=C), 1361, 1149 (SO2). 1H NMR (400 MHz, DMSO-d6) δ ppm: 3.23 (s, 3H, CH3SO2), 7.92 (d, J = 8.24 Hz, 6H, Ar H and NH2, D2O exchangeable), 8.07 (d, J = 8.60 Hz, 2H, Ar H), 8.46 (d, J = 8.46 Hz, 2H, Ar H), 8.77 (s, 1H, CH pyrazole). 13C NMR (100 MHz, DMSO-d6) δ ppm: 44.1 (CH3SO2), 118.7, 125.0, 126.7, 127.7, 130.2, 132.7, 138.8, 139.8, 141.4, 142.9, 150.4 (C Ar), 167.5 (C=O).
General procedure for the synthesis of compounds (4a-e), (5a-e) and (6a-e)
Equimolar amounts of 3a-e (1 mmol) and 2-chloro-N-(4-sulfamoylphenyl) acetamide (0.24 gm, 1 mmol), ethyl 4-(2-chloroacetamido) benzoate (1 mmol, 0.23 gm) or 2-chloro-N-(5-sulfamoyl-1,3,4-thiadiazol-2-yl) acetamide (0.25 gm, 1 mmol) were dissolved in DMF and refluxed. After reaction completion as monitored by TLC, the mixture was poured on ice-water, the solid formed was filtered off, washed with cold water and crystallized from ethanol to give target compounds (4a-e), (5a-e) and (6a-e), respectively.
2-Oxo-2-[(4-sulfamoylphenyl) amino] ethyl 3-phenyl-1-(4-sulfamoylphenyl)-1H-pyrazole-4-carboxylate (4a)
Yield: 73%, m.p. 160–162 °C. Rf = 0.54 (CHCl3, methanol). IR (KBr) υmax/cm−1: 3302, 3271, 3128 (NH2, NH), 3066 (CH Ar), 1705, 1690 (2 C=O), 1597 (NH bending), 1535, 1504 (C=C), 1334, 1157 (SO2). 1H NMR (400 MHz, DMSO-d6) δ ppm: 4.90 (s, 2H, CH2), 7.28 (s, 2H, NH2, D2O exchangeable), 7.46–7.49 (m, 4H, Ar H), 7.75–7.81 (m, 3H, Ar H), 7.86 (d, J = 5.00 Hz, 2H, Ar H), 7.95 (s, 2H, NH2, D2O exchangeable), 8.00 (d, J = 8.44 Hz, 2H, Ar H), 8.25 (d, J = 8.32 Hz, 2H, Ar H), 9.39 (s, 1H, CH pyrazole), 10.54 (s, 1H, NH, D2O exchangeable). 13C NMR (100 MHz, DMSO-d6) δ ppm: 63.1 (CH2), 113.3, 119.4, 119.7, 127.2, 127.8, 128.4, 129.4, 129.6, 131.8, 135.0, 139.2, 141.1, 141.8, 143.1, 154.0 (C Ar), 162.1 (COO), 166.5 (CO–NH). Anal. Calcd. for C24H21N5O7S2 (555.58): C, 51.89; H, 3.81; N, 12.61. Found: C, 52.17; H, 3.98; N, 12.86.
2-Oxo-2-[(4-sulfamoylphenyl) amino] ethyl 1-(4-sulfamoylphenyl)-3-(p-tolyl)-1H-pyrazole-4-carboxylate (4b)
Yield: 80%, m.p. 169–172 °C. Rf = 0.57 (CHCl3, methanol). IR (KBr) υmax/cm−1: 3300, 3271, 3132 (NH2, NH), 3070 (CH Ar), 2931, 2877 (CH aliph.),1716, 1680 (2 C=O), 1597 (NH bending), 1527, 1504 (C=C), 1334, 1157 (SO2). 1H NMR (400 MHz, DMSO-d6) δ ppm: 2.36 (s, 3H, CH3), 4.89 (s, 2H, CH2), 7.27 (d, J = 7.88 Hz, 4H, Ar H), 7.48 (s, 2H, NH2, D2O exchangeable), 7.74–7.80 (m, 4H, Ar H), 7.95 (s, 2H, NH2, D2O exchangeable), 7.99 (d, J = 8.68 Hz, 2H, Ar H), 8.23 (d, J = 8.68 Hz, 2H, Ar H), 9.37 (s, 1H, CH pyrazole), 10.52 (s, 1H, NH, D2O exchangeable). 13C NMR (100 MHz, DMSO-d6) δ ppm: 21.3 (CH3), 63.1 (CH2), 113.2 (C4, pyrazole), 119.4, 119.7, 127.2, 127.8, 128.9, 129.0, 129.4, 134.9, 138.9, 139.1, 141.1, 141.7, 143.0, 154.0 (C Ar), 162.1 (COO), 166.5 (CO–NH). Anal. Calcd. for C25H23N5O7S2 (569.61): C, 52.72; H, 4.07; N, 12.30. Found: C, 53.01; H, 4.29; N, 12.47.
2-Oxo-2-[(4-sulfamoylphenyl) amino] ethyl 3-(4-methoxyphenyl)-1-(4-sulfamoylphenyl)-1H-pyrazole-4-carboxylate (4c)
Yield: 88%, m.p. 138–140 °C. Rf = 0.51 (CHCl3, methanol). IR (KBr) υmax/cm−1: 3325, 3263, 3109 (NH2, NH), 3070 (CH Ar), 2939, 2839 (CH aliph.),1720, 1701 (2 C=O), 1593 (NH bending), 1527, 1500 (C=C), 1330, 1157 (SO2). 1H NMR (400 MHz, DMSO-d6) δ ppm: 3.80 (s, 3H, OCH3), 4.89 (s, 2H, CH2), 7.01 (d, J = 8.80 Hz, 2H, Ar H), 7.27 (s, 2H, NH2, D2O exchangeable), 7.47 (s, 2H, NH2, D2O exchangeable), 7.76–7.80 (m, 4H, Ar H), 7.83 (d, J = 8.68 Hz, 2H, Ar H), 7.98 (d, J = 8.72 Hz, 2H, Ar H), 8.23 (d, J = 8.72 Hz, 2H, Ar H), 9.35 (s, 1H, CH pyrazole), 10.53 (s, 1H, NH, D2O exchangeable). 13C NMR (100 MHz, DMSO-d6) δ ppm: 55.6 (OCH3), 63.1 (CH2), 113.0, 113.8, 119.4, 119.6, 124.1, 127.2, 127.8, 130.9, 134.9, 139.1, 141.1, 141.7, 142.9, 153.8, 160.3 (C Ar), 162.2 (COO), 166.5 (CO–NH). Anal. Calcd. for C25H23N5O8S2 (585.61): C, 51.28; H, 3.96; N, 11.96. Found: C, 51.55; H, 3.85; N, 12.07.
2-Oxo-2-[(4-sulfamoylphenyl) amino] ethyl 3-(4-bromophenyl)-1-(4-sulfamoylphenyl)-1H-pyrazole-4-carboxylate (4d)
Yield: 75%, m.p. 178–180 °C. Rf = 0.75 (CHCl3, methanol). IR (KBr) υmax/cm−1: 3329, 3259, 3113 (NH2, NH), 3074 (CH Ar), 1720, 1681 (2 C=O), 1597 (NH bending), 1531, 1504 (C=C), 1334, 1157 (SO2). 1H NMR (400 MHz, DMSO-d6) δ ppm: 4.90 (s, 2H, CH2), 7.28 (s, 2H, NH2, D2O exchangeable), 7.49 (s, 2H, NH2, D2O exchangeable), 7.67 (d, J = 8.40 Hz, 2H, Ar H), 7.74–7.80 (m, 4H, Ar H), 7.84 (d, J = 8.48 Hz, 2H, Ar H), 7.99 (d, J = 8.72 Hz, 2H, Ar H), 8.24 (d, J = 8.72 Hz, 2H, Ar H), 9.41 (s, 1H, CH pyrazole), 10.55 (s, 1H, NH, D2O exchangeable). 13C NMR (100 MHz, DMSO-d6) δ ppm: 63.2 (CH2), 113.3, 119.4, 119.8, 123.0, 127.2, 127.8, 131.0, 131.4, 131.6, 135.2, 139.2, 141.0, 141.7, 143.2, 152.8 (C Ar), 162.0 (COO), 166.4 (CO–NH). Anal. Calcd. for C24H20BrN5O7S2 (634.48): C, 45.43; H, 3.18; N, 11.04. Found: C, 45.70; H, 3.40; N, 11.27.
2-Oxo-2-[(4-sulfamoylphenyl) amino] ethyl 3-(4-(methylsulfonyl) phenyl)-1-(4-sulfamoylphenyl)-1H-pyrazole-4-carboxylate (4e)
Yield: 80%, m.p. 132–135 °C. Rf = 0.59 (CHCl3, methanol). IR (KBr) υmax/cm−1: 3300, 3263, 3180 (NH2, NH), 3066 (CH Ar), 2927, 2808 (CH aliph.), 1715, 1698 (2 C=O), 1597 (NH bending), 1535, 1500 (C=C), 1311, 1153 (SO2). 1H NMR (400 MHz, DMSO-d6) δ ppm: 3.26 (s, 3H, CH3), 4.91 (s, 2H, CH2), 7.28 (s, 2H, NH2, D2O exchangeable), 7.50 (s, 2H, NH2, D2O exchangeable), 7.73–7.80 (m, 4H, Ar H), 7.95–8.03 (m, 4H, Ar H), 8.14 (d, J = 8.28 Hz, 2H, Ar H), 8.25 (d, J = 8.52 Hz, 2H, Ar H), 9.45 (s, 1H, CH pyrazole), 10.56 (s, 1H, NH, D2O exchangeable). 13C NMR (100 MHz, DMSO-d6) δ ppm: 43.9 (CH3SO2), 63.2 (CH2), 113.7, 119.4, 119.9, 127.1, 127.2, 127.8, 130.5, 135.3, 136.7, 139.1, 141.0, 141.3, 141.7, 143.3, 152.4 (C Ar), 161.9 (COO), 166.4 (CO–NH). MS, m/Z (%): 633.7 (M+), 17.34%. Anal. Calcd. for C25H23N5O9S3 (633.67): C, 47.39; H, 3.66; N, 11.05. Found: C, 47.61; H, 3.80; N, 11.31.
2-[(4-(Ethoxycarbonyl) phenyl) amino]-2-oxoethyl 3-phenyl-1-(4-sulfamoylphenyl)-1H-pyrazole-4-carboxylate (5a)
Yield: 80%, m.p. 128–131 °C. Rf = 0.35 (CHCl3, methanol). IR (KBr) υmax/cm−1: 3332, 3259, 3132 (NH2, NH), 3066 (CH Ar), 2939, 2904 (CH aliph.), br. 1701 (3 C=O), 1600 (NH bending), 1535, 1504 (C=C), 1338, 1280 (SO2). 1H NMR (400 MHz, DMSO-d6) δ ppm: 1.31 (t, J = 6.92 Hz, 3H, CH3CH2), 4.28 (q, J = 7.08 Hz, 2H, CH3CH2), 4.89 (s, 2H, CH2), 7.45–7.48 (m, 4H, Ar H and NH2, D2O exchangeable), 7.74 (d, J = 8.36 Hz, 2H, Ar H), 7.86 (d, 2H, J = 8.36 Hz, Ar H), 7.94 (d, J = 8.48 Hz, 3H, Ar H), 7.99 (d, J = 8.44 Hz, 2H, Ar H), 8.24 (d, J = 8.32 Hz, 2H, Ar H), 9.39 (s, 1H, CH pyrazole), 10.53 (s, 1H, NH, D2O exchangeable). 13C NMR (100 MHz, DMSO-d6) δ ppm: 14.6 (CH3CH2), 60.9 (CH3CH2), 63.1 (CH2), 113.2, 119.1, 119.7, 125.0, 127.8, 128.4, 130.7, 131.8, 135.0, 141.1, 143.0, 143.1, 154.0 (C Ar), 162.1, 165.7 (2 COO), 166.5 (CO–NH). Anal. Calcd. for C27H24N4O7S (548.57): C, 59.12; H, 4.41; N, 10.21. Found: C, 59.39; H, 4.52; N, 10.47.
2-[(4-(Ethoxycarbonyl) phenyl) amino]-2-oxoethyl 1-(4-sulfamoylphenyl)-3-(p-tolyl)-1H-pyrazole-4-carboxylate (5b)
Yield: 85%, m.p. 125–128 °C. Rf = 0.55 (CHCl3, methanol). IR (KBr) υmax/cm−1: 3332, 3271, 3124 (NH2, NH), 3070 (CH Ar), 2939, 2904 (CH aliph.), br. 1701 (3 C=O), 1600 (NH bending), 1535, 1504 (C=C), 1311, 1161 (SO2). 1H NMR (400 MHz, DMSO-d6) δ ppm: 1.31 (t, J = 7.08 Hz, 3H, CH3CH2), 2.35 (s, 3H, CH3), 4.29 (q, J = 7.08 Hz, 2H, CH3CH2), 4.88 (s, 2H, CH2), 7.26 (d, J = 7.96 Hz, 2H, Ar H), 7.48 (s, 2H, NH2, D2O exchangeable), 7.43–7.78 (m, 4H, Ar H), 7.94 (d, J = 8.76 Hz, 2H, Ar H), 7.99 (d, J = 8.72 Hz, 2H, Ar H), 8.23 (d, J = 8.76 Hz, 2H, Ar H), 9.37 (s, 1H, CH pyrazole), 10.53 (s, 1H, NH, D2O exchangeable). 13C NMR (100 MHz, DMSO-d6) δ ppm: 14.6 (CH3CH2), 21.3 (CH3), 60.9 (CH3CH2), 63.1 (CH2), 113.2, 119.1, 119.7, 125.0, 127.8, 129.0, 129.4, 130.7, 134.9, 138.9, 141.1, 143.2, 154.0 (C Ar), 162.1, 165.7 (2 COO), 166.5 (CO–NH). Anal. Calcd. for C28H26N4O7S (562.60): C, 59.78; H, 4.66; N, 9.96. Found: C, 60.04; H, 4.79; N, 9.84.
2-[(4-(Ethoxycarbonyl) phenyl) amino]-2-oxoethyl 3-(4-methoxyphenyl)-1-(4-sulfamoylphenyl)-1H-pyrazole-4-carboxylate (5c)
Yield: 88%, m.p. 121–124 °C. Rf = 0.30 (CHCl3, methanol). IR (KBr) υmax/cm−1: 3321, 3294, 3120 (NH2, NH), 3070 (CH Ar), 2939, 2904 (CH aliph.), br. 1701 (3 C=O), 1600 (NH bending), 1527, 1503 (C=C), 1311, 1161 (SO2). 1H NMR (400 MHz, DMSO-d6) δ ppm: 1.31 (t, J = 7.32 Hz, 3H, CH3CH2), 3.80 (s, 3H, OCH3), 4.28 (q, J = 7.96 Hz, 2H, CH3CH2), 4.89 (s, 2H, CH2), 7.01 (d, J = 8.32 Hz, 2H, Ar H), 7.48 (s, 2H, NH2, D2O exchangeable), 7.74 (d, J = 8.80 Hz, 2H, Ar H), 7.84 (d, J = 8.32 Hz, 2H, Ar H), 7.93–8.00 (m, 4H, Ar H), 8.23 (d, J = 8.40 Hz, 2H, Ar H), 9.35 (s, 1H, CH pyrazole), 10.54 (s, 1H, NH, D2O exchangeable). 13C NMR (100 MHz, DMSO-d6) δ ppm: 14.6 (CH3CH2), 55.6 (OCH3), 60.9 (CH3CH2), 63.1 (CH2), 113.8, 119.1, 119.6, 124.1, 125.0, 127.8, 130.5, 130.7, 130.9, 134.9, 141.1, 142.9, 143.2, 153.8, 160.3 (C Ar), 162.2, 165.7 (2 COO), 166.5 (CO–NH). Anal. Calcd. for C28H26N4O8S (578.60): C, 58.12; H, 4.53; N, 9.68. Found: C, 58.43; H, 4.70; N, 9.85.
2-[(4-(Ethoxycarbonyl) phenyl) amino]-2-oxoethyl 3-(4-bromophenyl)-1-(4-sulfamoylphenyl)-1H-pyrazole-4-carboxylate (5d)
Yield: 80%, m.p. 96–99 °C. Rf = 0.70 (CHCl3, methanol). IR (KBr) υmax/cm−1: 3310, 3263, 3190 (NH2, NH), 3070 (CH Ar), 2935, 2904 (CH aliph.), br. 1697 (3 C=O), 1597 (NH bending), 1531, 1504 (C=C), 1311, 1161 (SO2). 1H NMR (400 MHz, DMSO-d6) δ ppm: 1.31 (t, J = 7.04 Hz, 3H, CH3CH2), 4.28 (q, J = 7.21 Hz, 2H, CH3CH2), 4.90 (s, 2H, CH2), 7.50 (s, 2H, NH2, D2O exchangeable), 7.66 (d, J = 8.32 Hz, 2H, Ar H), 7.74 (d, J = 8.56 Hz, 2H, Ar H), 7.93–8.01 (m, 4H, Ar H), 8.19 (d, J = 8.44 Hz, 2H, Ar H), 8.24 (d, J = 8.40 Hz, 2H, Ar H), 9.40 (s, 1H, CH pyrazole), 10.55 (s, 1H, NH, D2O exchangeable). 13C NMR (100 MHz, DMSO-d6) δ ppm: 14.6 (CH3CH2), 60.9 (CH3CH2), 63.2 (CH2), 113.3, 119.1, 119.8, 122.9, 125.0, 127.8, 130.7, 131.3, 131.4, 131.6, 134.8, 135.2, 141.0, 141.2, 143.2, 152.8 (C Ar), 162.0, 165.7 (2 COO), 166.4 (CO–NH). Anal. Calcd. for C27H23BrN4O7S (627.47): C, 51.68; H, 3.69; N, 8.93. Found: C, 51.82; H, 3.85; N, 9.17.
2-[(4-(Ethoxycarbonyl) phenyl) amino]-2-oxoethyl 3-(4-(methylsulfonyl) phenyl)-1-(4-sulfamoylphenyl)-1H-pyrazole-4-carboxylate (5e)
Yield: 82%, m.p. 127–130 °C. Rf = 0.41 (CHCl3, methanol). IR (KBr) υmax/cm−1: 3329, 3271, 3120 (NH2, NH), 3070 (CH Ar), 2927, 2873 (CH aliph.), br. 1712 (3 C=O), 1600 (NH bending), 1531, 1504 (C=C), 1311, 1149 (SO2). 1H NMR (400 MHz, DMSO-d6) δ ppm: 1.30 (t, J = 8.00 Hz, 3H, CH3CH2), 3.27 (s, 3H, CH3), 4.27–4.28 (m, J = 8.44 Hz, 2H, CH3CH2), 4.92 (s, 2H, CH2), 7.50 (s, 2H, NH2, D2O exchangeable), 7.74 (d, J = 8.16 Hz, 2H, Ar H), 7.93–8.01 (m, 6H, Ar H), 8.14 (d, J = 8.00 Hz, 2H, Ar H), 8.26 (d, J = 8.48 Hz, 2H, Ar H), 9.46 (s, 1H, CH pyrazole), 10.61 (s, 1H, NH, D2O exchangeable). 13C NMR (100 MHz, DMSO-d6) δ ppm: 14.6 (CH3CH2), 43.9 (CH3SO2), 60.9 (CH3CH2), 63.3 (CH2), 113.7, 119.1, 119.9, 125.0, 127.1, 127.8, 130.4, 130.7, 135.3, 136.7, 141.0, 141.3, 143.2, 143.3, 152.3 (C Ar), 161.9, 165.7 (2 COO), 166.4 (CO–NH). MS, m/Z (%): 626.1 (M+), 11.57%. Anal. Calcd. for C28H26N4O9S2 (626.66): Calculated: C, 53.67; H, 4.18; N, 8.94. Found: C, 53.81; H, 4.40; N, 9.15.
2-Oxo-2-[(5-sulfamoyl-1,3,4-thiadiazol-2-yl) amino] ethyl 3-phenyl-1-(4-sulfamoylphenyl)-1H-pyrazole-4-carboxylate (6a)
Yield: 70%, m.p. 135–137 °C. Rf = 0.53 (CHCl3, methanol). IR (KBr) υmax/cm−1: 3310, 3248, 3140 (NH2, NH), 3066 (CH Ar), 2935, 2820 (CH aliph.), 1708, 1639 (2 C=O), 1597 (NH bending), 1531, 1508 (C=C), 1311, 1161 (SO2). 1H NMR (400 MHz, DMSO-d6) δ ppm: 4.97 (s, 2H, CH2), 7.43 (s, 4H, 2 NH2, D2O exchangeable), 7.91 (d, J = 7.56 Hz, 2H, Ar H), 7.96–8.00 (m, 3H, Ar H), 8.11 (d, J = 8.68 Hz, 2H, Ar H), 8.24 (d, J = 8.56 Hz, 2H, Ar H), 8.67 (s, 1H, CH pyrazole), 9.38 (s, 1H, NH, D2O exchangeable). 13C NMR (100 MHz, DMSO-d6) δ ppm: 52.8 (CH2), 118.5, 119.7, 127.8, 128.4, 129.1, 129.6, 131.7, 134.9, 138.8, 141.8, 143.0, 152.7, 155.5 (C Ar), 167.5 (COO), 173.8 (CO–NH). Anal. Calcd. for C20H17N7O7S3 (563.58): C, 42.62; H, 3.04; N, 17.07. Found: C, 42.93; H, 3.21; N, 17.28.
2-Oxo-2-[(5-sulfamoyl-1,3,4-thiadiazol-2-yl) amino] ethyl 1-(4-sulfamoylphenyl)-3-(p-tolyl)-1H-pyrazole-4-carboxylate (6b)
Yield: 80%, m.p. 115–120 °C. Rf = 0.78 (CHCl3, methanol). IR (KBr) υmax/cm−1: 3310, 3251, 3136 (NH2, NH), 3070 (CH Ar), 2924, 2850 (CH aliph.), 1712, 1651 (2 C=O), 1597 (NH bending), 1527, 1504 (C=C), 1311, 1161 (SO2). 1H NMR (400 MHz, DMSO-d6) δ ppm: 2.37 (s, 3H, CH3), 5.05 (s, 2H, CH2), 7.26 (d, J = 7.96 Hz, 2H, Ar H), 7.47 (s, 4H, 2 NH2, D2O exchangeable), 7.74 (d, J = 7.32 Hz, 2H, Ar H), 7.97 (d, J = 9.04 Hz, 2H, Ar H), 8.18 (d, J = 8.20 Hz, 2H, Ar H), 9.17 (s, 1H, CH pyrazole), 9.36 (s, 1H, NH, D2O exchangeable). 13C NMR (100 MHz, DMSO-d6) δ ppm: 21.3 (CH3), 62.8 (CH2), 119.5, 119.7, 127.8, 128.9, 129.0, 129.4, 134.5, 135.0, 138.6, 141.3, 142.7, 153.9, 154.1 (C Ar), 164.1 (COO), 168.2 (CO–NH). Anal. Calcd. for C21H19N7O7S3 (577.61): C, 43.67; H, 4.32; N, 16.98. Found: C, 43.93; H, 4.50; N, 16.84.
2-Oxo-2-[(5-sulfamoyl-1,3,4-thiadiazol-2-yl) amino] ethyl 3-(4-methoxyphenyl)-1-(4-sulfamoylphenyl)-1H-pyrazole-4-carboxylate (6c)
Yield: 75%, m.p. 240–241 °C. Rf = 0.40 (CHCl3, methanol). IR (KBr) υmax/cm−1: 3371, 3251, 3140 (NH2, NH), 3074 (CH Ar), 2935, 2839 (CH aliph.), 1697, 1666 (2 C=O), 1597 (NH bending), 1527, 1508 (C=C), 1338, 1161 (SO2). 1H NMR (400 MHz, DMSO-d6) δ ppm: 3.82 (s, 3H, OCH3), 4.94 (s, 2H, CH2), 7.02 (d, J = 8.32 Hz, 2H, Ar H), 7.47 (s, 4H, 2 NH2, D2O exchangeable), 7.83 (d, J = 6.84 Hz, 2H, Ar H), 7.97 (d, J = 8.56 Hz, 2H, Ar H), 8.19 (d, J = 8.44 Hz, 2H, Ar H), 9.17 (s, 1H, CH pyrazole), 12.64 (s, 1H, NH, D2O exchangeable). 13C NMR (100 MHz, DMSO-d6) δ ppm: 55.6 (OCH3), 61.9 (CH2), 113.7, 114.7, 119.4, 124.5, 127.7, 130.9, 134.6, 141.3, 142.7, 153.6, 160.1, 162.2, 162.7, (C Ar), 164.2 (COO), 166.4 (CO–NH). MS, m/Z (%): 593.7 (M+), 42.62%. Anal. Calcd. for C21H19N7O8S3 (593.60): C, 42.49; H, 3.23; N, 16.52. Found: C, 42.71; H, 3.48; N, 16.70.
General procedure for the synthesis of compounds (9a-e)
A mixture of the acid hydrazide compounds 8a-e (0.01 mol) and ammonium thiocyanate (0.95 g, 0.01 mol) was dissolved in ethanol (10 mL). 10% HCl (3.6 mL) was then added, and refluxing the reaction mixture for 22–25 h. The solution was poured onto ice. The resultant precipitate was filtered, dried, and recrystallized from ethanol.
2-[3-Phenyl-1-(4-sulfamoylphenyl)-1H-pyrazole-4-carbonyl] hydrazine-1-carbothioamide (9a)
Yield: 85%, m.p. 180–181 °C. Rf = 0.34 (CHCl3, methanol). IR (KBr) υmax/cm−1: 3452, 3340, 3182 (NH2, NH), 3090 (CH Ar), 1678 (C=O), 1600 (NH bending), 1543, 1504 (C=C), 1311, 1161 (SO2), 1273 (C=S). 1H NMR (400 MHz, DMSO-d6) δ ppm: 7.43–7.45 (m, 3H, Ar H), 7.48 (s, 2H, NH2, D2O exchangeable), 7.59 (s, 1H, NH, D2O exchangeable), 7.89–7.91 (m, 2H, Ar H), 8.05 (d, J = 7.84 Hz, 4H, Ar H), 9.18 (s, 1H, CH pyrazole), 9.45 (s, 1H, NH, D2O exchangeable), 10.25 (s, 2H, NH2, D2O exchangeable). 13C NMR (100 MHz, DMSO-d6) δ ppm: 116.1, 119.0, 128.1, 128.5, 128.9, 129.1, 132.0, 141.3, 142.7, 152.6 (C Ar), 162.5 (C=O), 182.5 (C=S). MS, m/Z (%): 416.4 (M+), 13.87%. Anal. Calcd. for C17H16N6O3S2 (416.47): C, 49.03; H, 3.87; N, 20.18. Found: C, 49.21; H, 4.06; N, 20.41.
2-[1-(4-Sulfamoylphenyl)-3-(p-tolyl)-1H-pyrazole-4-carbonyl] hydrazine-1-carbothioamide (9b)
Yield: 85%, m.p. 178–180 °C. Rf = 0.41 (CHCl3, methanol). IR (KBr) υmax/cm−1: 3429, 3305, 3182 (NH2, NH), 3080 (CH Ar), 2974, 2920 (CH aliph.), 1678 (C=O), 1597 (NH bending), 1539, 1504 (C=C), 1311, 1161 (SO2), 1273 (C=S). 1H NMR (400 MHz, DMSO-d6) δ ppm: 2.36 (s, 3H, CH3), 7.25 (d, J = 8.04 Hz, 2H, Ar H), 7.47 (s, 2H, NH2, D2O exchangeable), 7.57 (s, 1H, NH, D2O exchangeable), 7.80 (d, J = 7.84 Hz, 2H, Ar H), 8.03–8.10 (m, 4H, Ar H), 9.16 (s, 1H, CH pyrazole), 9.44 (s, 1H, NH, D2O exchangeable), 10.23 (s, 2H, NH2, D2O exchangeable). 13C NMR (100 MHz, DMSO-d6) δ ppm: 21.3 (CH3), 115.9, 118.8, 128.1, 128.7, 129.1, 129.2, 132.0, 138.6, 141.4, 142.6, 152.6 (C Ar), 162.6 (C=O), 182.5 (C=S). Anal. Calcd. for C18H18N6O3S2 (430.50): C, 50.22; H, 4.21; N, 19.52. Found: C, 50.46; H, 4.37; N, 19.80.
2-[3-(4-Methoxyphenyl)-1-(4-sulfamoylphenyl)-1H-pyrazole-4-carbonyl] hydrazine-1-carbothioamide (9c)
Yield: 90%, m.p. 205–207 °C. Rf = 0.37 (CHCl3, methanol). IR (KBr) υmax/cm−1: 3433, 3332, 3248 (NH2, NH), 3090 (CH Ar), 2974, 2839 (CH aliph.), 1670 (C=O), 1604 (NH bending), 1550, 1504 (C=C), 1338, 1161 (SO2), 1280 (C=S). 1H NMR (400 MHz, DMSO-d6) δ ppm: 3.81 (s, 3H, OCH3), 7.00 (d, J = 6.88 Hz, 2H, Ar H), 7.47 (s, 2H, NH2, D2O exchangeable), 7.58 (s, 1H, NH, D2O exchangeable), 7.87 (d, J = 8.64 Hz, 2H, Ar H), 8.02–8.10 (m, 4H, Ar H), 9.15 (s, 1H, CH pyrazole), 9.43 (s, 1H, NH, D2O exchangeable), 10.22 (s, 2H, NH2, D2O exchangeable). 13C NMR (100 MHz, DMSO-d6) δ ppm: 55.6 (OCH3), 113.9, 115.7, 118.8, 124.4, 128.1, 130.2, 132.0, 141.4, 142.5, 152.4, 160.1 (C Ar), 162.7 (C=O), 182.5 (C=S). Anal. Calcd. for C18H18N6O4S2 (446.50): C, 48.42; H, 4.06; N, 18.82. Found: C, 48.68; H, 4.20; N, 19.03.
2-[3-(4-Bromophenyl)-1-(4-sulfamoylphenyl)-1H-pyrazole-4-carbonyl] hydrazine-1-carbothioamide (9d)
Yield: 85%, m.p. 238–241 °C. Rf = 0.52 (CHCl3, methanol). IR (KBr) υmax/cm−1: 3348, 3271, 3186 (NH2, NH), 3062 (CH Ar), 1662 (C=O), 1597 (NH bending), 1527, 1496 (C=C), 1330, 1157 (SO2), 1284 (C=S). 1H NMR (400 MHz, DMSO-d6) δ ppm: 7.48 (s, 2H, NH2, D2O exchangeable), 7.65 (d, J = 8.56 Hz, 2H, Ar H), 7.88 (d, J = 8.40 Hz, 3H, Ar H and NH, D2O exchangeable), 8.03–8.10 (m, 4H, Ar H), 9.17 (s, 1H, CH pyrazole), 9.44 (s, 1H, NH, D2O exchangeable), 10.28 (s, 2H, NH2, D2O exchangeable). 13C NMR (100 MHz, DMSO-d6) δ ppm: 116.1, 119.1, 122.6, 128.1, 130.9, 131.3, 131.5, 132.3, 141.2, 142.8, 151.5 (C Ar), 162.3 (C=O), 182.6 (C=S). Anal. Calcd. for C17H15BrN6O3S2 (495.37): C, 41.22; H, 3.05; N, 16.97. Found: C, 41.39; H, 3.24; N, 17.15.
2-[3-(4-(Methylsulfonyl) phenyl)-1-(4-sulfamoylphenyl)-1H-pyrazole-4-carbonyl] hydrazine-1-carbothioamide (9e)
Yield: 90%, m.p. 261–262 °C. Rf = 0.10 (CHCl3, methanol). IR (KBr) υmax/cm−1: 3309, 3236 (NH2, NH), 3016 (CH Ar), 2989, 2904 (CH aliph.), 1701 (C=O), 1612 (NH bending), 1597, 1508 (C=C), 1307, 1153 (SO2), 1284 (C=S). 1H NMR (400 MHz, DMSO-d6) δ ppm: 3.28 (s, 3H, CH3), 7.50 (s, 2H, NH2, D2O exchangeable), 7.65 (s, 1H, NH, D2O exchangeable), 8.00 (d, J = 8.48 Hz, 2H, Ar H), 8.06–8.10 (m, 4H, Ar H), 8.17 (d, J = 8.20 Hz, 2H, Ar H), 9.21 (s, 1H, CH pyrazole), 9.46 (s, 1H, NH, D2O exchangeable), 10.34 (s, 2H, NH2, D2O exchangeable). 13C NMR (100 MHz, DMSO-d6) δ ppm: 43.9 (CH3SO2), 116.6, 119.3, 127.2, 128.1, 129.7, 132.4, 136.9, 141.0, 141.2, 143.1, 151.0 (C Ar), 162.2 (C=O), 182.6 (C=S). Anal. Calcd. for C18H18N6O5S3 (494.56): C, 43.72; H, 3.67; N, 16.99. Found: C, 43.90; H, 3.81; N, 17.15.
General procedure for the synthesis of compound (10)
A solution of the acylthiosemicarbazide compound 9b (2.1 g, 0.005 mol) and sulfuric acid (10 mL) was heated in a water bath at 90 °C for 8 h. After that, the reaction mixture was added to ice-water and neutralized with ammonium hydroxide while cooling. After being filtered, dried, and crystalized from ethanol, the precipitate was obtained.
4-[4-(5-Amino-1,3,4-thiadiazol-2-yl)-3-(p-tolyl)-1H-pyrazol-1-yl] benzenesulfonamide (10)
Yield: 85%, m.p. 230–234 °C. Rf = 0.35 (CHCl3, methanol). IR (KBr) υmax/cm−1: 3271, 3194 (NH2), 3062 (CH Ar), 2850, 2773 (CH aliph.), 1635 (NH bending), 1593, 1516 (C=C), 1315, 1161 (SO2). 1H NMR (400 MHz, DMSO-d6) δ ppm: 2.38 (s, 3H, CH3), 7.30 (d, J = 7.92 Hz, 2H, Ar H), 7.46 (s, 4H, 2 NH2, D2O exchangeable), 7.60 (d, J = 8.20 Hz, 2H, Ar H), 7.98 (d, J = 8.80 Hz, 2H, Ar H), 8.17 (d, J = 8.80 Hz, 2H, Ar H), 9.20 (s, 1H, CH pyrazole). 13C NMR (100 MHz, DMSO-d6) δ ppm: 21.3 (CH3), 113.0, 119.3, 127.8, 129.3, 129.5, 130.6, 139.2, 141.3, 142.7, 148.3, 151.6, 164.0, 169.2 (C Ar). MS, m/Z (%): 412.2 (M+), 10.97%. Anal. Calcd. for C18H16N6O2S2 (412.49): C, 52.41; H, 3.91; N, 20.37. Found: C, 52.69; H, 4.07; N, 20.52.
General procedure for the synthesis of compound (11)
To a solution of acylthiosemicarbazide compound 9b (2.1 g, 0.005 mol) in ethanol, sodium hydroxide 1% (15 mL) was added. Then, the reaction mixture was refluxed for 6 h. Then, acidify the reaction mixture with HCl (10%) while cooling. The resultant precipitate was filtered, dried, and crystallized from ethanol.
4-[4-(5-Mercapto-1H-1,2,4-triazol-3-yl)-3-(p-tolyl)-1H-pyrazol-1-yl] benzenesulfonamide (11)
Yield: 80%, m.p. 255–257 °C. Rf = 0.40 (CHCl3, methanol). IR (KBr) υmax/cm−1: 3421, 3229 br. (NH2, NH), 3070 (CH Ar), 2920, 2881 (CH aliph.), 1631 (NH bending), 1597, 1512 (C=C), 1315, 1161 (SO2), 1226 (C=S). 1H NMR (400 MHz, DMSO-d6) δ ppm: 2.37 (s, 3H, CH3), 7.28 (d, J = 7.92 Hz, 2H, Ar H), 7.47 (s, 2H, NH2, D2O exchangeable), 7.66 (d, J = 8.20 Hz, 2H, Ar H), 8.01 (d, J = 8.92 Hz, 2H, Ar H), 8.07 (d, J = 8.92 Hz, 2H, Ar H), 9.11 (s, 1H, CH pyrazole), 13.55 (s, 1H, NH, D2O exchangeable), 13.59 (s, 1H, SH, D2O exchangeable). 13C NMR (100 MHz, DMSO-d6) δ ppm: 21.3 (CH3), 108.5, 119.3, 128.0, 128.6, 129.0, 129.3, 131.4, 138.8, 141.3, 142.7, 144.9, 151.5 (C Ar), 166.7 (C-SH). Anal. Calcd. for C18H16N6O2S2 (412.49): C, 52.41; H, 3.91; N, 20.37. Found: C, 52.58; H, 4.12; N, 20.49.
Biological assays
Carbonic anhydrase inhibitory activity
Biological investigation of the target compounds as hCA IX and hCA XII inhibitors was carried out at the laboratory of the Egyptian company for the production of vaccines, sera, and medications (VACSERA, Giza, Egypt), using the spectrophotometric method described by Pocker and Meany [35, 36] (Supplementary data, S.1.2).
Anticancer activity
The guidelines of the Drug Evaluation Branch’s protocol at the NCI in Bethesda served as the basis for the in vitro anticancer screening assays [37, 38]. at one dose 10 µM against a panel of 60 cancer cell lines. The human tumor cell lines used belonged to nine panels: leukemia, melanoma, lung, colon, CNS, ovarian, renal, prostate and breast cancers using the sulforhodamine B (SRB) assay in a 48 h drug exposure protocol [39].
In vitro cytotoxic activity
Compound 5b was selected for further investigation of its in vitro cytotoxicity against MCF-7 breast cancer cell line using MTT assay [40]. Additional data were depicted in the Supplementary data, S.1.3.
Cell cycle analysis
Following the manufacturer’s instructions, the Annexin V-FITC Apoptosis Detection Kit (Bio Vision) was used to assess the impact of compound 5b on the MCF-7 cell line’s cell cycle stages [41–43]. and the experimental procedure was shown in the Supplementary data, S.1.4.1.
Apoptotic assay
Studying the apoptotic induction liability of compound 5b on MCF-7 cancer cell line was performed using annexin V-FITC/propidium iodide double staining protocol. [41–44] More information was presented in the Supplementary data, S.1.4.2.
In silico studies
Molecular modelling
Using two X-ray crystallographic structures of CA IX (PDB ID: 5FL4) and CA XII (PDB ID: 1JD0) co-crystallized with thiophene sulfamoyl inhibitor (9FK) [31] and (AAZ) inhibitor [32], respectively, molecular docking studies were carried out using Molecular Operating Environment (MOE) software version 2022.02. The methodology is described in detail in the Supplementary data, S.6.1.
Toxicity prediction
Compounds were drawn using ChemDraw, then the smiles were copied into another virtual filter (Osiris Property Explorer (https://www.organic-chemistry.org/prog/peo/) [45].
ANOVA statistical analysis and CA IC50 calculations
The software used to calculate CA IC50 along with statistical analysis of the results is GraphPad Prism 9.00. Results were estimated by using one-way ANOVA and the Bonferroni’s multiple comparisons posthoc test was performed (P < 0.05) [46] (Supplementary data, S4 and S5).
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We acknowledge the Taif University, Saudi Arabia members for their coworking in this research.
Author contributions
Rehab F. Ahmed wrote the initial draft of manuscript and synthesized the compounds Nagwa M. abdelgawad suggesting the research idea Walaa R. Mahmoud and Mona F. Said work supervision and Manuscript reviewing Amany Belal and Reem I. Alsantali reviewing the manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Imtaiyaz Hassan M, Shajee B, Waheed A, Ahmad F, Sly WS (2013) Structure, function and applications of carbonic anhydrase isozymes. Bioorg Med Chem 21:1570–1582. 10.1016/j.bmc.2012.04.044 [DOI] [PubMed] [Google Scholar]
- 2.Alterio V, Di Fiore A, D’Ambrosio K, Supuran CT, De Simone G (2012) Multiple binding modes of inhibitors to carbonic anhydrases: How to design specific drugs targeting 15 different isoforms? Chem Rev 112:4421–4468. 10.1021/cr200176r [DOI] [PubMed] [Google Scholar]
- 3.Monti SM, Supuran CT, De Simone G (2013) Anticancer carbonic anhydrase inhibitors: a patent review (2008–2013). Expert Opin Ther Pat 23:737–749. 10.1517/13543776.2013.798648 [DOI] [PubMed] [Google Scholar]
- 4.Akocak S, Supuran CT (2019) Activation of α-, β-, γ-δ-, ζ-and η-class of carbonic anhydrases with amines and amino acids: a review. J Enzyme Inhib Med Chem 34:1652–1659. 10.1080/14756366.2019.1664501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirakawa Y, Senda M, Fukuda K, Yu HY, Ishida M, Taira M, Kinbara K, Senda T (2021) Characterization of a novel type of carbonic anhydrase that acts without metal cofactors. BMC Biol 19:1–15. 10.1186/s12915-021-01039-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Supuran CT (2008) Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 7:168–181. 10.1038/nrd2467 [DOI] [PubMed] [Google Scholar]
- 7.Ibrahim HS, Allam HA, Mahmoud WR, Bonardi A, Nocentini A, Gratteri P, Ibrahim ES, Abdel-Aziz HA, Supuran CT (2018) Dual-tail arylsulfone-based benzenesulfonamides differently match the hydrophobic and hydrophilic halves of human carbonic anhydrases active sites: selective inhibitors for the tumor-associated hCA IX isoform. Eur J Med Chem 152:1–9. 10.1016/j.ejmech.2018.04.016 [DOI] [PubMed] [Google Scholar]
- 8.Carradori S, Secci D, De Monte C, Mollica A, Ceruso M, Akdemir A, Sobolev AP, Codispoti R, De Cosmi F, Guglielmi P (2016) A novel library of saccharin and acesulfame derivatives as potent and selective inhibitors of carbonic anhydrase IX and XII isoforms. Bioorg Med Chem 24:1095–1105 [DOI] [PubMed] [Google Scholar]
- 9.Türeci Ö, Sahin U, Vollmar E, Siemer S, Göttert E, Seitz G, Parkkila AK, Shah GN, Grubb JH, Pfreundschuh M, Sly WS (1998) Human carbonic anhydrase XII: cDNA cloning, expression, and chromosomal localization of a carbonic anhydrase gene that is overexpressed in some renal cell cancers. Proc Natl Acad Sci USA 95:7608–7613. 10.1073/pnas.95.13.7608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waheed A, Sly WS, Doisy EA (2017) Carbonic anhydrase XII functions in health and disease. Gene 623:33–40. 10.1016/j.gene.2017.04.027.Carbonic [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George RF, Said MF, Bua S, Supuran CT (2020) Synthesis and selective inhibitory effects of some 2-oxindole benzenesulfonamide conjugates on human carbonic anhydrase isoforms CA I, CA II, CA IX and CAXII. Bioorg Chem 95:103514. 10.1016/j.bioorg.2019.103514 [DOI] [PubMed] [Google Scholar]
- 12.Winum J-Y, Scozzafava A, Montero J-L, Supuran C (2006) New zinc binding motifs in the design of selective carbonic anhydrase inhibitors. Mini-Rev Med Chem 6:921–936. 10.2174/138955706777934946 [DOI] [PubMed] [Google Scholar]
- 13.Park DK, Lee MS (2019) Kinetic study of catalytic CO2 hydration by metal-substituted biomimetic carbonic anhydrase model complexes. R Soc Open Sci 6:190407–190416. 10.1098/rsos.190407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindskog S (1997) Structure and mechanism of carbonic Anhydrase. Pharmacol Ther 74:1–20. 10.1016/s0163-7258(96)00198-2 [DOI] [PubMed] [Google Scholar]
- 15.Yamali C, Gul HI, Ece A, Bua S, Angeli A, Sakagami H, Sahin E, Supuran CT (2019) Synthesis, biological evaluation and in silico modelling studies of 1,3,5-trisubstituted pyrazoles carrying benzenesulfonamide as potential anticancer agents and selective cancer-associated hCA IX isoenzyme inhibitors. Bioorg Chem 92:103222. 10.1016/j.bioorg.2019.103222 [DOI] [PubMed] [Google Scholar]
- 16.Khloya P, Celik G, Sitaram, Vullo D, Supuran CT, Sharma PK (2014) 4-Functionalized 1,3-diarylpyrazoles bearing benzenesulfonamide moiety as selective potent inhibitors of the tumor associated carbonic anhydrase isoforms IX and XII. Eur J Med Chem 76:284–290. 10.1016/j.ejmech.2014.02.023 [DOI] [PubMed] [Google Scholar]
- 17.Gul HI, Yamali C, Sakagami H, Angeli A, Leitans J, Kazaks A, Tars K, Ozgun DO, Supuran CT (2018) New anticancer drug candidates sulfonamides as selective hCA IX or hCA XII inhibitors. Bioorg Chem 77:411–419. 10.1016/j.bioorg.2018.01.021 [DOI] [PubMed] [Google Scholar]
- 18.Ibrahim HS, Abou-Seri SM, Tanc M, Elaasser MM, Abdel-Aziz HA, Supuran CT (2015) Isatin-pyrazole benzenesulfonamide hybrids potently inhibit tumor-associated carbonic anhydrase isoforms IX and XII. Eur J Med Chem 103:583–593. 10.1016/j.ejmech.2015.09.021 [DOI] [PubMed] [Google Scholar]
- 19.Chandak N, Ceruso M, Supuran CT, Sharma PK (2016) Novel sulfonamide bearing coumarin scaffolds as selective inhibitors of tumor associated carbonic anhydrase isoforms IX and XII. Bioorg Med Chem 24:2882–2886. 10.1016/j.bmc.2016.04.052 [DOI] [PubMed] [Google Scholar]
- 20.Ahmed RF, Mahmoud WR, Abdelgawad NM, Fouad MA, Said MF (2023) Exploring novel anticancer pyrazole benzenesulfonamides featuring tail approach strategy as carbonic anhydrase inhibitors. Eur J Med Chem 261:115805. 10.1016/j.ejmech.2023.115805 [DOI] [PubMed] [Google Scholar]
- 21.Gumus A, Bozdag M, Akdemir A, Angeli A, Selleri S, Carta F, Supuran CT (2022) Thiosemicarbazide-substituted coumarins as selective inhibitors of the tumor associated human carbonic anhydrases IX and XII. Molecules 27:1–14. 10.3390/molecules27144610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abas M, Bahadur A, Ashraf Z, Iqbal S, Rajoka MSR, Rashid SG, Jabeen E, Iqbal Z, Abbas Q, Bais A, Hassan M, Liu G, Feng K, Lee SH, Nawaz M, Qayyum MA (2021) Designing novel anticancer sulfonamide based 2,5-disubstituted-1,3,4-thiadiazole derivatives as potential carbonic anhydrase inhibitor. J Mol Struct 1246:131145. 10.1016/j.molstruc.2021.131145 [Google Scholar]
- 23.Fahmy AMF, Abdel-Hamid HA, Megally Abdo NY (2015) Uses of isothiocyanate as building block in syntheses of triazole, thiadiazole, quinazoline, and pyrimidine systems of agrochemical and biological activities. Egypt J Chem 58:645–657 [Google Scholar]
- 24.Urbelytė L, Bagdonas M, Grybaitė B, Vaickelionienė R, Mickevičiūtė A, Michailovienė V, Matulis D, Mickevičius V, Zubrienė A (2021) Design and synthesis of hydrazone-bearing benzenesulfonamides as carbonic anhydrase VB inhibitors. ChemistrySelect 6:13506–13513. 10.1002/slct.202103636 [Google Scholar]
- 25.Vilsmeier A, Haack A (1927) A Vilsmeier und A Haack: aber die Einwirkung von Halogenphosphor auf Alkyl-formanilide. Eine neue Methode zur Darstellung sekundarer und tertiarer p-Alkylamino-benzaldehyde. Ber Dtsch Chem Ges 60:119–122. 10.1002/cber.19270600118 [Google Scholar]
- 26.Assali M, Abualhasan M, Sawaftah H, Hawash M, Mousa A (2020) Synthesis, biological activity, and molecular modeling studies of pyrazole and triazole derivatives as selective COX-2 inhibitors. J Chem 2020:1–14. 10.1155/2020/6393428 [Google Scholar]
- 27.Khalifah RG (1971) The carbon dioxide hydration activity of carbonic anhydrase. J Biol Chem 246:2561–2573. 10.1016/s0021-9258(18)62326-9 [PubMed] [Google Scholar]
- 28.Sharma PK, Chandak N, Kumar P, Sharma C, Aneja KR (2011) Synthesis and biological evaluation of some 4-functionalized-pyrazoles as antimicrobial agents. Eur J Med Chem 46:1425–1432. 10.1016/J.EJMECH.2011.01.060 [DOI] [PubMed] [Google Scholar]
- 29.Rieger AM, Nelson KL, Konowalchuk JD, Barreda DR (2011) Modified annexin V/propidium iodide apoptosis assay for accurate assessment of cell death. J Vis Exp 50:1–4. 10.3791/2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gorczyca W (1999) Cytometric analyses to distinguish death processes. Endocr Relat Cancer 6:17–19. 10.1677/erc.0.0060017 [DOI] [PubMed] [Google Scholar]
- 31.Leitans J, Kazaks A, Balode A, Ivanova J, Zalubovskis R, Supuran CT, Tars K (2015) Efficient expression and crystallization system of cancer-associated carbonic anhydrase isoform IX. J Med Chem 58:9004–9009. 10.1021/acs.jmedchem.5b01343 [DOI] [PubMed] [Google Scholar]
- 32.Whittington DA, Waheed A, Ulmasov B, Shah GN, Grubb JH, Sly WS, Christianson DW (2001) Crystal structure of the dimeric extracellular domain of human carbonic anhydrase XII, a bitopic membrane protein overexpressed in certain cancer tumor cells. PNAS 98:9545–9550. 10.1073/pnas.161301298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sander T, Freyss J, Von Korff M, Reich JR, Rufener C (2009) OSIRIS, an entirely in-house developed drug discovery informatics system. J Chem Inf Model 49:232–246. 10.1021/ci800305f [DOI] [PubMed] [Google Scholar]
- 34.Entezari Heravi Y, Sereshti H, Saboury AA, Ghasemi J, Amirmostofian M, Supuran CT (2017) 3D QSAR studies, pharmacophore modeling, and virtual screening of diarylpyrazole–benzenesulfonamide derivatives as a template to obtain new inhibitors, using human carbonic anhydrase II as a model protein. J Enzyme Inhib Med Chem 32:688–700. 10.1080/14756366.2016.1241781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pocker Y, Meany JE (1967) The catalytic versatility of erythrocyte carbonic anhydrase: II—Kinetic studies of the enzyme-catalyzed hydration of pyridine aldehydes. Biochemistry 6:239–246. 10.1021/bi00853a037 [DOI] [PubMed] [Google Scholar]
- 36.Ur Rehman N, Ahsan Halim S, Khan M, Hussain H, Yar Khan H, Khan A, Abbas G, Rafiq K, Al-Harrasi A (2020) Antiproliferative and carbonic anhydrase II inhibitory potential of chemical constituents from lycium shawii and aloe vera: evidence from in silico target fishing and in vitro testing. Pharmaceuticals 13:1–18. 10.3390/ph13050094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langley J, Cronise P, Vaigro-Wolff A, Gray-Goodrich M, Campbell H, Mayo J, Boyd M (1991) Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst 83:757–766. 10.1093/jnci/83.11.757 [DOI] [PubMed] [Google Scholar]
- 38.Boyd MR, Paull KD (1995) Some practical considerations and applications of the national cancer institute in vitro anticancer drug discovery screen. Drug Dev Res 34:91–109. 10.1002/ddr.430340203 [Google Scholar]
- 39.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR (1990) New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 82:1107–1112. 10.1093/jnci/82.13.1107 [DOI] [PubMed] [Google Scholar]
- 40.Boncler M, Rózalski M, Krajewska U, Podswdek A, Watala C (2014) Comparison of PrestoBlue and MTT assays of cellular viability in the assessment of anti-proliferative effects of plant extracts on human endothelial cells. J Pharmacol Toxicol Methods 69:9–16. 10.1016/j.vascn.2013.09.003 [DOI] [PubMed] [Google Scholar]
- 41.Tao D, Wu J, Feng Y, Qin J, Hu J, Gong J (2004) New method for the analysis of cell cycle-specific apoptosis. Cytometry A 57:70–74. 10.1002/cyto.a.10117 [DOI] [PubMed] [Google Scholar]
- 42.El-Hussieny M, El-Sayed NF, Fouad MA, Ewies EF (2021) Synthesis, biological evaluation and molecular docking of new sulfonamide-based indolinone derivatives as multitargeted kinase inhibitors against leukemia. Bioorg Chem 117:105421. 10.1016/j.bioorg.2021.105421 [DOI] [PubMed] [Google Scholar]
- 43.Fouad MA, Zaki MY, Lotfy RA, Mahmoud WR (2021) Insight on a new indolinone derivative as an orally bioavailable lead compound against renal cell carcinoma. Bioorg Chem 112:104985. 10.1016/j.bioorg.2021.104985 [DOI] [PubMed] [Google Scholar]
- 44.Ewies EF, El-Hussieny M, El-Sayed NF, Fouad MA (2019) Design, synthesis and biological evaluation of novel α-aminophosphonate oxadiazoles via optimized iron triflate catalyzed reaction as apoptotic inducers. Eur J Med Chem 180:310–320. 10.1016/j.ejmech.2019.07.029 [DOI] [PubMed] [Google Scholar]
- 45.Hassan GS, Georgey HH, Mohammed EZ, George RF, Mahmoud WR, Omar FA (2021) Mechanistic selectivity investigation and 2D-QSAR study of some new antiproliferative pyrazoles and pyrazolopyridines as potential CDK2 inhibitors. Eur J Med Chem 218:113389. 10.1016/j.ejmech.2021.113389 [DOI] [PubMed] [Google Scholar]
- 46.Kim H-Y (2015) Statistical notes for clinical researchers: post-hoc multiple comparisons. Restor Dent Endod 40:172–176. 10.5395/rde.2015.40.2.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.