Abstract
Introduction
Alopecia areata (AA) is an immune-mediated inflammatory skin disease that targets hair follicles. Current research yields varied lists of differentially expressed genes (DEGs). A meta-analytic approach is essential to consolidate these findings into a consistent tissue signature. This study aimed to perform a meta-analysis of gene expression datasets to establish a comprehensive molecular signature of AA lesional scalp.
Methods
We conducted a meta-analysis of transcriptomic data from human studies on lesional skin gene expression in AA. Reanalyzing 132 samples (82 patients with AA and 50 controls) from five Gene Expression Omnibus (GEO) datasets (GSE68801, GSE45512, GSE80342, GSE58573, GSE74761), we employed an effect size approach within a random-effects model to identify unique and shared DEGs and enriched biological pathways. The protocol is registered in PROSPERO (CRD42024559847).
Results
The meta-analysis identified 5109 DEGs, with 2710 upregulated and 2399 downregulated genes, significantly more than the 120 DEGs shared across the five studies. Consistently expressed genes included CXCL9, CCL18, CXCL10, CD8A, and GZMB (FDR < 0.05). The analysis highlighted chemokines/receptors (CCL13, CCR1, XCL1) and markers of cytotoxic T lymphocytes (GZMA, GZMH, GZMK) and NK cells (NKG2A, NKG2D). Downregulated genes involved type I (KRT31–35, KRT38) and type II (KRT81-86) keratins and proteins crucial for hair follicle structure and function (PADI3, GPRC5D, DSG4, FGF18). Functional analysis showed enrichment in Th1, Th2, and Th17 pathways, particularly through JAK-STAT signaling (p < 0.01).
Conclusion
This core transcriptome of AA lesions provides new insights into the disease’s pathogenesis and identifies potential targets for treatment.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-025-01471-6.
Keywords: Alopecia areata, Meta-analysis, Transcriptome analysis, Chemokines, Gene expression profiling, Microarrays analysis, JAK-STAT signaling, Cytotoxic T cells, Therapeutic targets, Computational molecular biology
Key Summary Points
| Why carry out this study? |
| Alopecia areata (AA) is an autoimmune disease affecting hair follicles, with inconsistent gene expression findings across studies. |
| A unified transcriptomic signature is needed to clarify disease mechanisms and guide therapy development. |
| What was learned from the study? |
| This meta-analysis integrates five datasets to define a robust core transcriptome of AA. |
| A total of 5109 differentially expressed genes were identified, surpassing overlaps seen in individual studies. |
| This meta-analysis confirmed known immune pathways (JAK-STAT, Th1/Th2/Th17, interferon) and uncovered novel genes linked to stress, metabolism, and immune regulation. |
| This study provides a reliable framework for biomarker discovery and therapeutic innovation. |
Introduction
Alopecia areata (AA) is an immune-mediated inflammatory disease of the skin that affects the hair follicle, leading to sudden hair loss and consequent significant impairment of quality of life [1]. With a prevalence of 0.5–2% over a lifetime, an estimated 6.6 million individuals are affected in the USA and 147 million worldwide [2]. Most patients experience scalp involvement in the form of patches of hair loss, with a good prognosis of complete hair regrowth. However, 10% of these patients progress to severe forms such as alopecia totalis (AT), where all scalp hair is lost, or alopecia universalis (AU), where hair loss occurs over the entire body surface.
Currently, it is widely accepted that the pathogenesis of AA is driven by complex immune-mediated processes, prominently involving the Th1/interferon-γ (IFNγ) axis [3]. This axis, primarily activated by cytotoxic T cells, disrupts the immune privilege of hair follicles, triggering the production of interleukin (IL)-15 and IL-2 cytokines that enhance the Th1 response, creating a self-perpetuating feedback loop. Recent advances in genomic technologies have facilitated the exploration of gene expression profiles associated with AA and expanded this understanding, identifying significant roles for other pathways including Th2 (characterized by IL-4, IL-13), Th17 (IL-17A), and the involvement of IL-23 produced by dendritic cells [4].
However, studies often yield inconsistent results due to differences in experimental design, sample size, and analytical methods. This variability underscores the need for an integrated approach to reconcile these findings and derive a coherent understanding of AA at the molecular level. Meta-analysis of gene expression data presents a robust method to synthesize findings across multiple studies, offering a more comprehensive view of the disease’s genetic underpinnings [5]. By aggregating and analyzing disparate datasets, researchers can identify a core set of differentially expressed genes (DEGs) that are consistently implicated in AA, thus providing insights into potential therapeutic targets and biomarkers [6]. This approach has been effectively applied to reveal the core transcriptomes of skin lesions in various dermatological conditions, such as in atopic dermatitis [7], psoriasis [8], and, recently, epidermoid carcinoma [9].
The aim of this study was to perform a meta-analysis of gene expression datasets from lesional scalp skin in alopecia areata to define a core transcriptomic signature of disease. This approach is designed to resolve inconsistencies across studies, identify robustly differentially expressed genes and pathways, and pinpoint potential molecular targets for future therapeutic interventions.
Methods
The protocol for this meta-analysis was registered in PROSPERO with the identifier CRD42024559847.
Literature Search
For the systematic study search, we utilized gene expression data hosted in the Gene Expression Omnibus (GEO) [10], a public repository of gene expression and RNA methylation data managed by the National Center for Biotechnology Information (NCBI), and ArrayExpress [11], a free public database storing and providing access to gene expression data derived from microarrays and RNA sequencing, maintained by the European Bioinformatics Institute. Both databases were searched on March 23, 2024, using specific search strategies tailored to each database. For GEO, we searched using (“alopecia”[MeSH Terms] OR alopecia[All Fields]) AND (“skin”[MeSH Terms] OR skin[All Fields]) AND (“pathology”[Subheading] OR “biopsy”[MeSH Terms] OR biopsy[All Fields]). For ArrayExpress search included “alopecia areata”, “alopecia totalis”, “alopecia universalis”.
Inclusion and Exclusion Criteria
The inclusion criteria for this meta-analysis were strictly defined to ensure relevance and consistency across studies analyzing gene expression in AA. Key inclusion criteria were (1) studies must use microarray technology to analyze gene expression; (2) only human studies were considered, specifically examining lesional scalp skin samples from patients with AA compared to healthy control samples; (3) both observational studies and intervention studies providing baseline (pre-treatment or placebo) samples were included. Exclusion criteria were applied to (1) studies without control samples; (2) those using RNA sequencing technology; (3) studies on non-lesional skin, or (4) those involving other species, cell cultures, or biological materials not directly collected from lesional scalp areas. This approach was designed to focus on active disease states in AA and avoid biases from different technological platforms or biological materials.
Meta-Analysis
In this meta-analysis, normalized gene expression data was processed using the Integrative Meta-Analysis of GEO Data (ImaGEO) tool [12]. After categorizing genes from the GEO datasets into AA or control groups, a random-effects model incorporating an effect-size approach was used to manage batch effects and harmonize data across studies. Z-scores derived from p values were crucial for evaluating the consistency and scale of gene expression variations. The false discovery rate (FDR) was controlled via the Benjamini/Hochberg method, setting a significance threshold at FDR < 0.05.
Functional Analysis
Functional analysis of gene expression signatures was conducted using the bioinformatics application GeneCodis [13]. Predefined gene sets in different gene ontology databases (GO) such as Kyoto Encyclopedia of Genes and Genomes (KEGG), Reactome, Biological Process, Celullar Component, and Molecular Functions were used. A standard hypergeometric method with p values corrected by FDR was employed for analysis. Significant findings were identified by applying a stringent threshold of FDR < 0.05.
Unique and Shared DEG and Enriched Pathways
We compared the DEG signatures from each independent gene set and those derived from the meta-analysis. To generate the list of DEGs from the RNAseq study, we employed DESeq2 via GEO2R to analyze gene expression data. This involved comparing six pre-treatment samples from patients with AA against four samples from healthy controls, applying a strict FDR of less than 0.05 and a log fold change (FC) cutoff of 1.5.
Additionally, we conducted a principal component analysis (PCA) to explore the variability and relationships in pathway enrichment and adjusted p values among various data sources. This analysis focused on two key variables, “relative enrichment” and “log10(pval_adj)”, which represent the pathway enrichment values and the log-transformed adjusted p values, respectively.
Ethics Statement
This meta-analysis was conducted in accordance with established ethical research guidelines. No new data were collected from human or animal participants. Instead, the study relied exclusively on previously published datasets, all of which had received appropriate ethical approval from their respective institutional review boards or ethics committees. As such, no additional ethical approval or informed consent was required for this analysis.
Results
Search Results
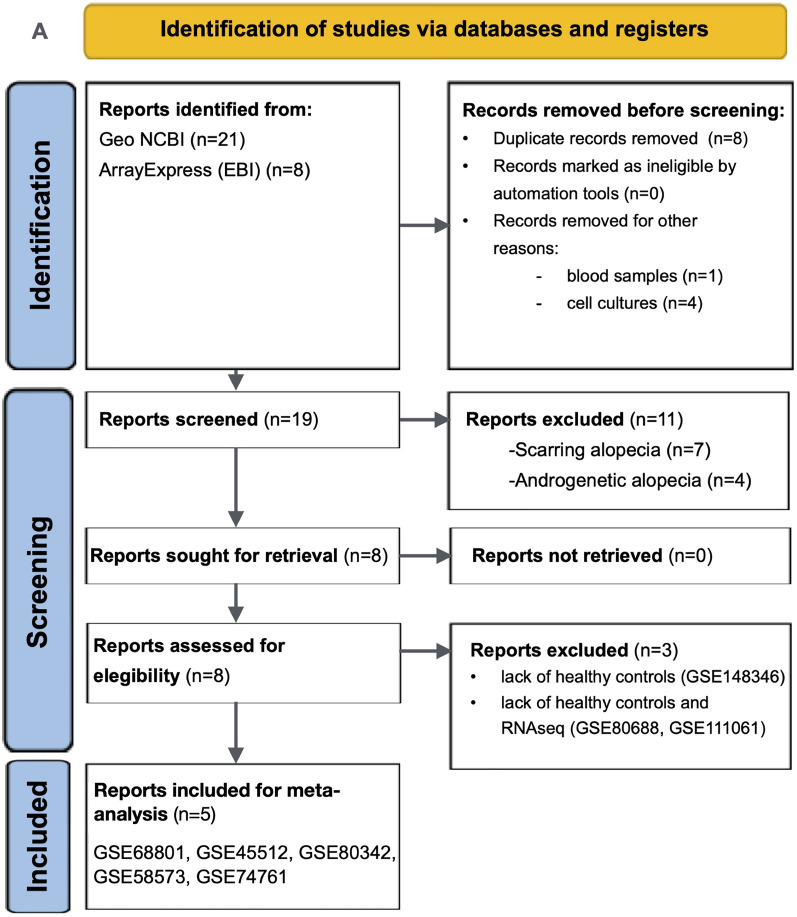
This review is reported according to the PRISMA for systematic reviews (Supplementary Methods). Initially, 23 non-duplicated results were identified (Fig. 1). After evaluating the data and metadata of each study, a total of five microarray datasets deposited in the NCBI GEO database were selected for analysis. Between 2014 and 2017 were finally included (GSE68801, GSE45512, GSE80342, GSE58573, GSE74761) [14–17]. They involved skin scalp tissue RNA data of a total of 82 patients with varying degrees of AA severity and 50 heathy controls derived from various cohorts and interventional studies. All studies employed the Affymetrix Human Genome U133 Plus 2.0 Array platform (GPL 570). The main characteristics of the studies included are summarized in Table 1. Table S1 in the Supplementary Material also reports the studies that did not meet the inclusion criteria, along with the reasons for their exclusion [18–20]. This provides a transparent overview of the study selection process for the meta-analysis. The only study conducted using RNA-seq (GSE111061) was excluded from the meta-analysis because of the analytical complexities and technological disparities involved [21]. However, a comparative analysis was performed to identify both shared and unique markers between the results of this RNAseq study and those obtained from the meta-analysis.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 flow diagram for systematic reviews and meta-analysis and pathway enrichment analysis workflow. a The PRISMA 2020 flow diagram, detailing the process of identifying, screening, and including studies in the systematic review and meta-analysis; b this panel demonstrates the workflow for meta-analysis and pathway enrichment analysis using bioinformatic tools: (1) Gene expression data from Gene Expression Omnibus (GEO) and custom datasets are loaded; (2) Quality control is performed to ensure data reliability and integrity; (3) Meta-analysis is conducted using the Integrative Meta-Analysis of GEO Data (ImaGEO) tool. A random-effects model is used to correct for batch effects and variability across studies. Z-scores from p values are used to calculate overall effect sizes and assess consistency in gene expression changes; (4) Functional enrichment analysis was performed using the GeneCodis platform. Pathway enrichment analysis identifies significant biological pathways associated with the gene expression changes
Table 1.
Main characteristics of the datasets included in the meta-analysis
| Gene set Id | Disease | Organism | Design | Drugs | Summary | Source | Analysis | Platform | Cases vs controls |
|---|---|---|---|---|---|---|---|---|---|
| GSE68801 | AA | Human | Observational | – | Gene expression profiling of scalp skin biopsies from patients with AA or normal healthy controls | Scalp | mRNA array | [HG-U133_Plus_2] Affymetrix Human Genome U133 Plus 2.0 Array | AA (n = 60): AAP (patchy type disease, n = 22 patients; lesional samples n = 22, nonlesional samples n = 20), AAP.T (transient patchy-type disease, disease duration less than 1 year, n = 6; lesional samples n = 6, nonlesional samples n = 6), AU (n = 23 patients), AT (n = 9 patients); controls (n = 36) |
| GSE45512 | AA | Human | Observational | – | Human AA skin profiling | Scalp | mRNA array | [HG-U133_Plus_2] Affymetrix Human Genome U133 Plus 2.0 Array | AA (n = 5); controls (n = 5) |
| GSE80342 | AA | Human | Drug intervention | Ruxolitinib | Pilot open label clinical trial of orally administered ruxolitinib in patients with AA | Scalp | mRNA array | [HG-U133_Plus_2] Affymetrix Human Genome U133 Plus 2.0 Array | AA (n = 12); controls (n = 3) |
| GSE58573 | AA | Human | Drug intervention | Ruxolitinib | First ruxolitinib treatment of human patients with AA | Scalp | mRNA array | [HG-U133_Plus_2] Affymetrix Human Genome U133 Plus 2.0 Array | AA (n = 3); controls (n = 2) |
| GSE74761 | AA | Human | Drug intervention | Ustekinumab | Extensive AA is reversed by IL-23 cytokine antagonism | Scalp | mRNA array | [HG-U133_Plus_2] Affymetrix Human Genome U133 Plus 2.0 Array | AA (n = 3); controls (n = 1) |
AA alopecia areata, AU alopecia universalis, AT alopecia totalis, AAP patchy type disease, AAP.T transient patchy-type disease
The Meta-Analysis-Derived Transcriptome in AA
Given the wide range in the number of DEGs for individual datasets, we conducted a random effects meta-analysis to identify similarities and differences in DEGs across the five datasets. Quality analysis of each gene set showed homogeneity among samples with low rate for missing data (Fig. S1 of Supplementary Results). Figure 2a displays a comprehensive heatmap of 2710 DEGs (2710 upregulated and 2399 downregulated), detailing the expression profiles across 50 healthy controls and 82 patients with AA. Additionally, a focused heatmap showcases the expression patterns of the top 100 most significantly overexpressed and underexpressed genes in a set of samples of each dataset (Fig. 2b).
Fig. 2.
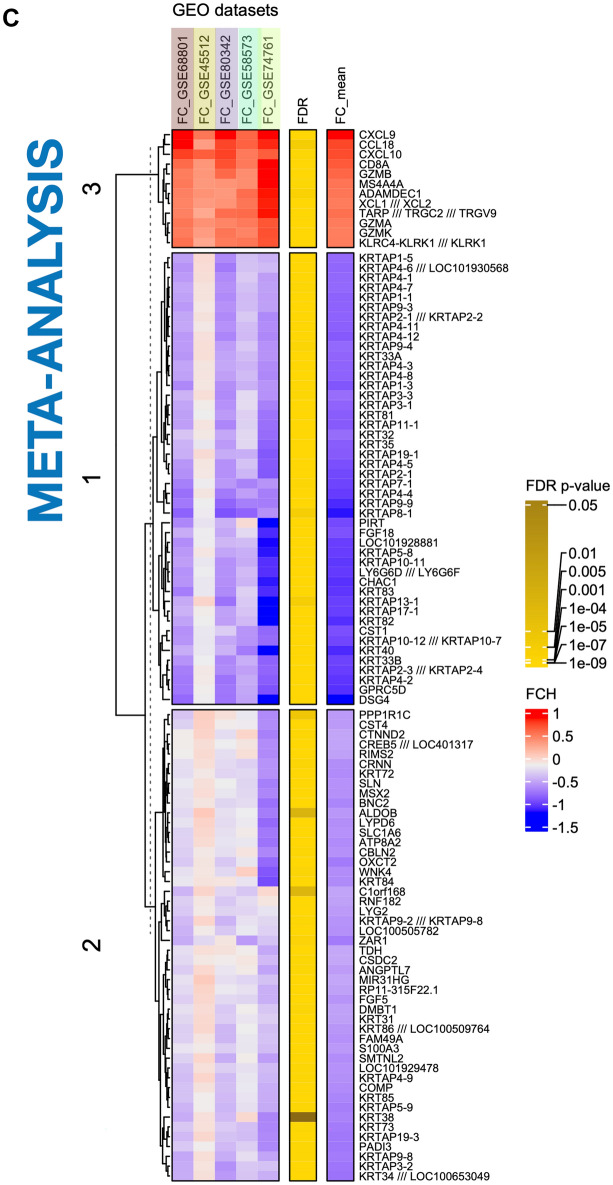
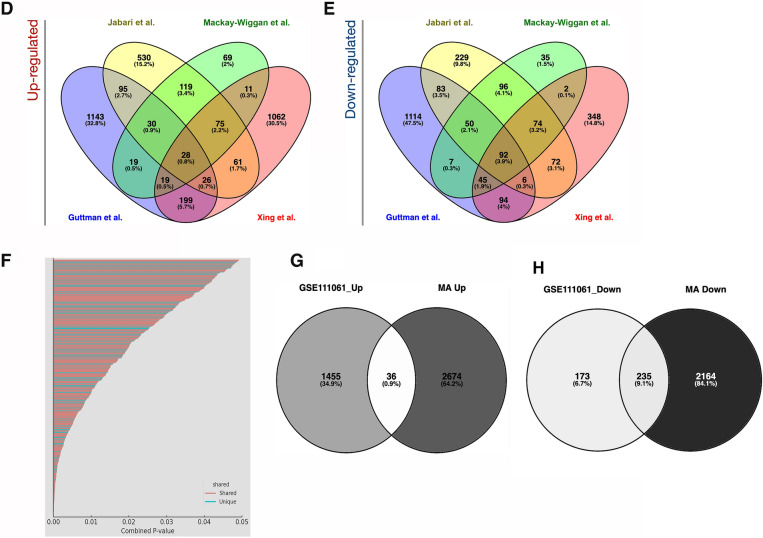
Comprehensive analysis of differentially expressed genes (DEGs) in AA and control groups. a Heatmap of all DEGs. This heatmap displays the expression levels of all DEGs across control (n = 50, green) and AA (n = 82, purple) samples. Each row represents a gene, and each column represents a sample. The color scale indicates gene expression levels, with red representing upregulation and green representing downregulation. b Heatmap of the top 100 DEGs. This heatmap highlights the top 100 most significantly overexpressed and underexpressed genes identified in the meta-analysis in a set of samples of each dataset. c Meta-analysis results. The heatmap summarizes the meta-analysis results for different Gene Expression Omnibus (GEO) datasets. The color scale indicates the effect size, with red representing a higher effect size and blue a lower effect size. The genes are grouped into clusters based on their expression patterns across the datasets. d Venn diagram of upregulated DEGs across studies. This Venn diagram shows the overlap of upregulated DEGs among different studies (Guttman et al., Jabari et al., Mackay-Wiggan et al., Xing et al.). Each oval represents a study, with the numbers indicating shared and unique DEGs. e Venn diagram of downregulated DEGs across studies. Similar to panel d, this Venn diagram illustrates the overlap of downregulated DEGs among the same studies. Each oval represents a study, with the numbers indicating shared and unique DEGs. f Comparison of DEGs between meta-analysis dataset and RNA-seq data. This plot compares DEGs identified in the meta-analysis dataset with those found in GSE111061 RNA-seq data. Genes are plotted based on their combined p values, with shared genes in red, and unique genes in blue. g Venn diagram of upregulated genes comparison. This Venn diagram compares the upregulated genes identified in the meta-analysis dataset with those found in the GSE111061 RNA-seq data. h Venn diagram of downregulated genes comparison. Similar to panel g, this Venn diagram compares the downregulated genes identified in the meta-analysis dataset with those found in the GSE111061 RNASeq data. FDR, false discovery rate; FC, fold change
Figure 2c presents a dendrogram clustering the top 100 significant genes derived from the meta-analysis. Cluster #3 consists of upregulated genes related to immunity, primarily cytokines and chemokines (CXCL9, CCL18, CXCL10, XCL1/XCL2), cytotoxic T lymphocyte markers (GZMA, GZMB, GZMK), and NK cells (NKG2A, NKG2D), cytotoxic and regulatory proteins (CD8A, GZMB, GZMA, GZMK, ADAMDEC1), natural killer (NK) cell markers (KLRC4, KLRK1/NKG2D), and T cell receptors (TCR) and related proteins (TARP, TRGC2, TRGV9, MS4A4A).
Cluster #1 predominantly consists of genes that are downregulated and primarily associated with structural components of the hair follicle. This includes type I keratins (specifically KRT31–35, KRT38) and type II keratins (notably KRT81-86), which are integral to the hair follicle’s structural integrity. Additionally, this cluster encompasses genes coding for keratin-associated proteins (KRTAP) that contribute to the cohesive properties and resilience of hair fibers. The cluster also contains genes such as FGF18 and DSG4, which are essential for the functional regulation and developmental processes of hair follicles, further underscoring the biological significance of these genes in maintaining follicular health and pathology.
Cluster #2 contains downregulated genes with intermediate expression profiles. These genes are involved in various aspects of hair follicle biology, including development and differentiation (ZAR1, BNC2, MSX2, GPRC5D), structural integrity (COMP), post-translational modification during hair shaft formation (PADI3), and cuticle structure (S100A3). Additionally, some genes in this cluster are associated with immune responses and immune-related pathways (ANGPTL7, DMBT1, LYPD6) or play roles in more general cellular functions (ALDOB, CSDC2, RNF182).
To further explore immune activation patterns, we examined canonical interferon-stimulated genes (ISGs) such as CXCL9, CXCL10, IFI44, and CCL5, which showed marked upregulation in AA samples (Fig. 3a). Unsupervised hierarchical clustering of these ISGs stratified patients by molecular profile and highlighted potential differences between AA subtypes (Fig. 3b).
Fig. 3.
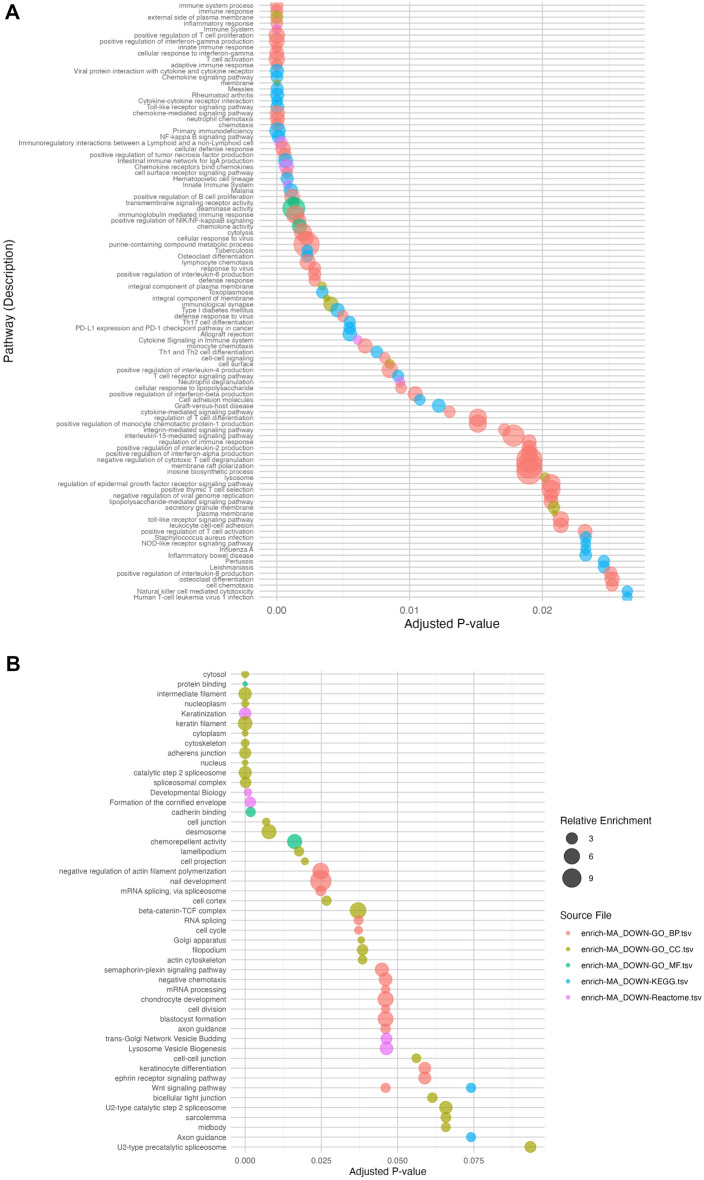
Pathway enrichment analysis of gene expression studies in alopecia areata. a Top 50 Pathways Enrichment UP-REGULATED. This plot represents the top 50 pathways that are significantly upregulated in skin samples of patients with alopecia areata. Each dot corresponds to a specific pathway, with the size of the dot indicating the relative enrichment score. Pathways are ordered by adjusted p value (x-axis), with lower p values indicating higher statistical significance. Color coding represents different source files used in the meta-analysis, as indicated in the legend. b Top 50 Downregulated Pathways Enrichment. This plot illustrates the top 50 pathways that are significantly downregulated in the same samples. Similar to panel a, each dot signifies a pathway, sized by relative enrichment. The pathways are arranged by adjusted p value on the x-axis, and color-coded by source file. c PCA- Enrichment and Adjusted p-values (Normalized and Scaled). Principal component analysis (PCA) plot showing the enrichment and adjusted p values of pathways across different source files. Each point represents a pathway, and the direction of the arrow indicates the contribution of each pathway to the principal components. The plot helps to visualize the variance in pathway enrichment between different studies and sources. d PCA - Enrichment and Adjusted p-values by Source Groups. This PCA plot highlights the clustering of pathways based on the source groups. The pathways enriched by the meta-analysis and the Guttman gene set form a distinct cluster, indicating a unique pathway enrichment profile. In contrast, the pathways from other source groups (Xing, Jabbari, Mackay) are more dispersed, reflecting variability in their pathway enrichment profiles among the different studies
Analysis of Unique and Shared Genes
The analysis explored both unique and shared genes across independent datasets and the meta-analysis, employing Venn diagrams to visually represent the intersections and exclusivities in gene expression profiles. Figure 2d and e showcase Venn diagrams that underscore the overlap and uniqueness among the DEG analyzed across these independent datasets (Table S2).
The convergence point in the studies highlighted 28 upregulated genes, including CCL13, CD1B, and CD8A, and 92 downregulated genes, which are mainly keratin and keratin-associated proteins, consistently identified across all datasets. Notably, genes such as CXCL9, CXCL10, MMP12, and GZMA were identified in all datasets except for those from Guttman’s study. Furthermore, the gene CCL18 was absent in the dataset from Xing. Despite these specific overlaps, the comprehensive gene transcriptome profile from the meta-analysis encompasses a significantly broader array of these genes, successfully cataloging all 43 mentioned genes.
Our meta-analysis successfully identified a substantial number of keratin-related genes, capturing 75 in total. This is followed by a notable convergence in the studies by Guttman, Jabbari, and Mackay-Wiggan, which collectively reported 23 shared keratin-related genes. In contrast, the study by Xing showed a more limited capture, identifying only seven of these genes.
Interestingly, the meta-analysis uniquely identified a significant number of non-keratin genes from cluster #3, which are implicated in various aspects of hair follicle biology. These genes were not detected in any independent gene set. Notable examples include ZAR1, CSDC2, GPRC5D, LYPD6, TARP, and PADI3. This underscores the unique insights provided by the meta-analysis, highlighting specific biological processes that may not be evident in smaller, independent datasets.
Comparative Analysis with RNA-Seq Study
The only study using RNA sequencing (seq) (GSE111061) was excluded from our meta-analysis as a result of its analytical complexities and technological disparities [18]. However, we conducted a comparative analysis to identify both shared and unique markers between this RNAseq study and the meta-analysis findings. This analysis identified 593 DEGs, with 185 upregulated and 408 downregulated (Fig. 2f–h). Remarkably, half of the DEG from the RNA-seq overlapped with those identified in the meta-analysis, with six upregulated genes—CXCL9, CXCL10, CD1B, CCL5, CCL13, and GZMA—featuring among the top 100 DEGs in our meta-analysis results (Table S2).
Core Biological Pathways in Alopecia Areata
The pathway enrichment analysis of meta-analysis-derived DEG associated with AA revealed significant enrichment in Th1, Th2, and Th17 cell differentiation pathways, illustrating the complex interaction of T cell lineages in the inflammatory dynamics of AA. This was complemented by notable enhancements in cytokine-regulated pathways involving IFNγ, IL-2, IL-4, IL-6, IL-8, and IL-15, which are pivotal in modulating immune responses in AA. Additionally, the analysis highlighted active involvement of innate immunity through Toll-like receptor and NF-κB signaling pathways in recognizing and responding to autoimmune triggers in AA.
The study also identified a significant downregulation of genes linked to structural pathways like intermediate filament formation and keratinization, impacting cellular structure and interactions essential for maintaining tissue integrity. This downregulation extended to critical signaling pathways such as the Wnt, ephrin receptor, and semaphorin-plexin pathways, suggesting disruptions in cellular communication and developmental processes. Moreover, significant downregulation in pathways related to mRNA processing and splicing indicates broader implications for gene expression regulation, potentially affecting various cellular functions.
Insights from Comparative Pathway Analysis in Alopecia Areata
The comparative analysis between different datasets and the meta-analysis gene list offers significant insights into the biological pathways involved in AA (Fig. 3c, d). Notably, the Guttman dataset closely aligns with the meta-analysis gene list, indicating a strong overlap in gene expression profiles, particularly in pathways like IFNγ signaling, cytokine-cytokine receptor interaction, chemokine signaling, Toll-like receptor signaling. This overlap underscores a consistent immune and structural response across studies. In contrast, Jabbari and Mackay-Wiggan datasets emphasize pathways linked to chronic inflammation and T cell differentiation, while the Xing dataset uniquely highlights early-stage immune activation pathways, such as Toll-like receptor and NF-κB signaling, offering a distinct perspective from other datasets.
Deviation From Protocol
There were no deviations from the protocol registered in PROSPERO (CRD42024559847). All analyses and procedures were conducted as outlined, ensuring adherence to the original plan. This consistency reinforces the reliability and transparency of our findings.
Discussion
To the best of our knowledge, this is the first comprehensive meta-analysis delineating the core gene expression signature of AA lesions. Our analysis highlights prominently upregulated chemokines and immune-related genes, which improves our understanding of disease pathology and points to potential therapeutic targets. At the same time, the consistent downregulation of hair keratins and structural follicular proteins reflects the disruption of follicular integrity and loss of immune privilege.
The prominent upregulation of IFNγ-inducible chemokines (CXCL9, CXCL10, and CXCL11) supports the central role of Th1-mediated immune responses. It reinforces the rationale for targeting the JAK-STAT pathway with agents like ruxolitinib, baricitinib, and ritlecitinib, all of which are now approved for treating patients with moderate to severe AA. Concurrently, the strong expression of cytotoxic markers such as GZMB and PRF1 emphasizes the involvement of CD8+ T cells, suggesting a benefit from complementary approaches targeting their recruitment or function, including anti-CXCR3 agents or strategies that modulate T cell exhaustion.
Interestingly, our findings also reveal increased expression of Th2-associated chemokines (CCL5, CCL13, and CCL18), suggesting overlapping Th1 and Th2 activity in AA. This could be particularly relevant in subsets with atopic features or pediatric cases, where therapies like dupilumab or other IL-4/IL-13 blockers may be beneficial [21]. Similarly, the upregulation of Th17-related genes, including IL23A, supports the potential utility of IL-23/IL-17 inhibitors (e.g., ustekinumab, guselkumab) in selected patients [22, 23].
Downregulated genes such as KRT31–35, PADI3, and HOXC13 reflect follicular collapse and loss of immune privilege. While these targets are not currently druggable, their expression levels may serve as biomarkers of follicular health and response to therapy. Their restoration with JAK inhibition further supports their potential as surrogate markers of disease reversal [24]. The utility of non-invasive approaches such as tape strips to monitor early molecular responses to treatment remains to be established and may provide a valuable tool for patient stratification and therapeutic monitoring.
Beyond canonical immune pathways, we identified novel biological processes not previously emphasized in AA. These include cellular stress and innate immune sensing, with genes such as XBP1, DDIT3, IFI16, and HSPA5, suggesting roles for ER stress, mitochondrial dysfunction, and inflammasome activation. These may offer novel therapeutic angles targeting stress adaptation, proteostasis, or immune surveillance.
Emerging tolerogenic strategies may complement immune suppression by promoting immune tolerance. The CD200–CD200R axis has shown promise in preclinical models of inflammation [25]. Likewise, fusion cytokines such as IL-10 and IL-4 have demonstrated synergistic anti-inflammatory effects [26]. Transforming growth factor-β (TGFβ) mimetics, such as synthetic peptides that avoid fibrotic effects, have also shown efficacy in dampening immune responses [27]. These findings open up new translational directions for promoting immune tolerance in autoimmune skin diseases such as AA.
Our meta-analysis demonstrated greater statistical power than individual studies, revealing associations that were otherwise undetectable and reducing inconsistencies across datasets. This reinforces the strength of integrative transcriptomic analyses in identifying shared and novel elements of disease biology [14–17, 28, 29]. These insights emphasize the meta-analysis’s ability to identify new candidate genes that may play roles in the pathology of AA, providing a broader spectrum of biological insights and paving the way for targeted therapies.
A literature review identified no comparable gene expression meta-analyses in AA beyond one genome-wide association study (GWAS) [30]. Other reanalyses were limited by dataset size or scope, further underscoring the uniqueness of our approach. Shi et al. identified 185 DEGs in skin of AA, highlighting immune-related upregulation and keratin-related downregulation [31]. Zhang et al. analyzed scalp biopsy samples using the GSE dataset and Gene-Cloud of Biotechnology Information, an online comprehensive bioinformatics analysis platform, finding 111 genes altered in AA lesions, mostly linked to epidermis development and inflammatory responses [32]. However, the limited scope and diversity of the datasets used in these studies may restrict the generalizability of their findings.
Our study offers several key strengths. We integrated multiple independent datasets, all generated using the same microarray platform, which enhanced data consistency, statistical power, and the generalizability of results. Using comprehensive bioinformatics pipelines and curated gene ontology databases, we identified robust enrichment of biological pathways central to AA pathogenesis—including type I and II interferon signaling, cytotoxic effector functions, Th1/Th2/Th17 polarization, and antigen presentation. Our methodology is thoroughly documented and transparent, facilitating reproducibility and future comparative analysis.
Nonetheless, some limitations remain. Our meta-analysis was confined to microarray gene expression data, excluding one study with RNA-seq data, which presents significant analytical challenges due to differences in data characteristics and analysis techniques. We also limited dataset selection to those available on GEO or ArrayExpress, which may overlook a growing body of RNA-seq data and datasets from other repositories. Additionally, our analysis did not account for varying disease severities or pediatric cases, possibly limiting insights into disease progression and response variability. Further, our reliance on bulk tissue gene expression analysis may mask cellular heterogeneity, a gap that could be addressed using single-cell analysis techniques in future studies.
Another limitation of our study was the exclusion of RNA-seq data, which poses analytical challenges distinct from those of microarray data due to fundamental differences in data characteristics and analysis requirements [33]. Meta-analysis techniques developed for microarray data, which typically involve t tests that assume Gaussian distributions after log transformation, are not directly applicable to RNA-seq data. RNA-seq data are typically modeled using distributions like overdispersed Poisson or negative binomial because of their nature as count data, complicating the calculation and interpretation of effect sizes.
Our study did not differentiate gene expression among different severity grades of AA since most primary studies did not include this type of metadata. This fact may limit understanding of molecular differences associated with disease progression and severity. Additionally, our study was based on bulk tissue gene expression analysis, which may obscure cellular heterogeneity and specific interactions among different cell types involved in AA pathogenesis. Future studies should leverage single-cell RNA-seq and spatial transcriptomics to dissect the heterogeneity of immune infiltrates and identify actionable subpopulation-specific targets [34].
Despite these challenges, our findings hold considerable reliability owing to the comprehensive and rigorous methodological and analytical approach. However, they still require further validation to confirm their clinical relevance.
Conclusions
This study reinforces the autoimmune and inflammatory nature of AA while defining its core immune and structural transcriptomic features. In addition to validating previously implicated mechanisms, it uncovers novel molecular pathways and therapeutic targets. These findings lay the groundwork for future translational research and the development of precision medicine strategies.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Medical Writing/Editorial Assistance
The authors acknowledge the assistance of ChatGPT-4 (OpenAI, USA) in language editing, generating ideas, and providing support with R and Python for data analysis and visualization, under the guidance and responsibility of the authors.
Author Contributions
Irene Rivera-Ruiz contributed equally to the conceptualization, investigation, and resource acquisition. She led the visualization efforts and was the lead author in drafting the original version of the manuscript. Jesús Gay-Mimbrera was equally involved in the conceptualization and investigation. He led the data curation and contributed equally to resource acquisition and original manuscript drafting. Pedro J. Gómez-Arias contributed to the review and editing of the manuscript in a supporting role. Macarena Aguilar-Luque led the project administration and contributed equally to data curation. Miguel Juan-Cencerrado contributed equally to data curation. Carmen Mochón-Jiménez also participated equally in data curation. Esmeralda Parra-Peralbo contributed equally to project administration, resource acquisition, and drafting of the original manuscript. Beatriz Isla-Tejera was equally involved in the conceptualization and supervision of the study. She led the resource acquisition and the review and editing process of the manuscript. Francisco Gómez-García contributed equally to the conceptualization and investigation. He led the methodological design and shared supervision and manuscript revision duties. Juan Ruano led the conceptualization, funding acquisition, methodological development, and software implementation. He also contributed equally to supervision, visualization, original manuscript drafting, and critical revision. All authors had full access to the study data, participated in the critical review and editing of the manuscript, and approved the final version prior to submission.
Funding
This article forms part of the PhD thesis of Irene Rivera-Ruiz, conducted within the framework of the Official Doctoral Programme in Biomedicine at the University of Córdoba, Spain. The research was supported by the Plan Propio de Investigación of the Instituto Maimónides de Investigación Clínica de Córdoba (IMIBIC), with awards granted to JG-M and MA-L. PG-A received additional support from the International Eczema Council’s 2022 Research Fellowship Program. EP-P acknowledges funding from Universidad Europea de Madrid. This study was exclusively supported by public institutions; no private funding was involved. Partial financial support was provided by Project PI23/01590 (awarded to JR), funded by the Instituto de Salud Carlos III (ISCIII) and co-financed by the European Union. The funding bodies had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. Importantly, no financial support was received from pharmaceutical companies. No funding or sponsorship was received for the publication of this article.
Data Availability
The datasets analyzed during the current study are available in the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) repository, and can be accessed through the following accession numbers: GSE68801, GSE45512, GSE80342, GSE58573, GSE74761, GSE111061. These public datasets were used under open access terms and can be freely accessed for further research. Additional data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Conflict of Interest
Juan Ruano is an Editorial Board member of Dermatology and Therapy. He was not involved in the selection of peer reviewers nor in any editorial decisions related to this manuscript. Irene Rivera-Ruiz, Jesús Gay-Mimbrera, Pedro J. Gómez-Arias, Macarena Aguilar-Luque, Miguel Juan-Cencerrado, Carmen Mochón-Jiménez, Esmeralda Parra-Peralbo, Beatriz Isla-Tejera, and Francisco Gómez-García have nothing to disclose.
Ethical Approval
This meta-analysis was conducted in accordance with established guidelines for ethical research. This meta-analysis did not involve the collection of new data from human or animal participants. Instead, it utilized data from previously published studies, all of which had obtained appropriate ethical approvals from their respective institutional review boards or ethics committees. Therefore, ethical approval and informed consent were not required.
Footnotes
Prior Publication: Preliminary data from this manuscript were presented as an oral communication at the Annual Congress of the Spanish Academy of Dermatology (AEDV), May 2024, Valencia, Spain.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Irene Rivera-Ruiz, Jesús Gay-Mimbrera, Francisco Gómez-García, and Juan Ruano contributed equally to this work.
Contributor Information
Beatriz Isla-Tejera, Email: beatrizislatj@gmail.com.
Juan Ruano, Email: juanruanoruiz@mac.com.
References
- 1.Rencz F, Gulácsi L, Péntek M, Wikonkál N, Baji P, Brodszky V. Alopecia areata and health-related quality of life: a systematic review and meta-analysis. Br J Dermatol. 2016;175(3):561–71. 10.1111/bjd.14497. [DOI] [PubMed] [Google Scholar]
- 2.Harries M, Macbeth AE, Holmes S, et al. The epidemiology of alopecia areata: a population-based cohort study in UK primary care. Br J Dermatol. 2022;186:257–65. 10.1111/bjd.20628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajabi F, Drake LA, Senna MM, Rezaei N. Alopecia areata: a review of disease pathogenesis. Br J Dermatol. 2018;179(5):1033–48. 10.1111/bjd.16808. [DOI] [PubMed] [Google Scholar]
- 4.Renert-Yuval Y, Pavel AB, Del Duca E, et al. Scalp biomarkers during dupilumab treatment support Th2 pathway pathogenicity in alopecia areata. Allergy. 2023;78(4):1047–59. 10.1111/all.15561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waldron L, Riester M. Meta-analysis in gene expression studies. Methods Mol Biol. 2016;1418:161–76. 10.1007/978-1-4939-3578-9_8. [DOI] [PubMed] [Google Scholar]
- 6.Sharov AA, Schlessinger D, Ko MS. ExAtlas: an interactive online tool for meta-analysis of gene expression data. J Bioinform Comput Biol. 2015;13(6):1550019. 10.1142/S0219720015500195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ewald DA, Malajian D, Krueger JG, et al. Meta-analysis derived atopic dermatitis transcriptome defines a robust AD signature. BMC Med Genom. 2015;8:60. 10.1186/s12920-015-0133-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian S, Krueger JG, Li K, et al. Meta-analysis derived transcriptome of psoriasis defines the “core” pathogenesis of disease. PLoS ONE. 2012;7(9):e44274. 10.1371/journal.pone.0044274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bencomo T, Lee CS. Gene expression landscape of cutaneous squamous cell carcinoma progression. Br J Dermatol. 2024. 10.1093/bjd/ljae249. [DOI] [PubMed] [Google Scholar]
- 10.Gene Expression Omnibus. https://www.ncbi.nlm.nih.gov/geo/. Accessed 23 Mar 2024
- 11.ArrayExpress. https://www.ebi.ac.uk/biostudies/arrayexpress. Accessed 23 Mar 2024
- 12.Toro-Domínguez D, Martorell-Marugán J, López-Domínguez R, et al. ImaGEO: integrative gene expression meta-analysis from GEO database. Bioinformatics. 2019;35:880–2. 10.1093/bioinformatics/bty721. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Moreno A, López-Domínguez R, Villatoro-García JA, et al. Functional enrichment analysis of regulatory elements. Biomedicines. 2022;10(3):590. 10.3390/biomedicines10030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jabbari A, Cerise JE, Chen JC, et al. Molecular signatures define alopecia areata subtypes and transcriptional biomarkers. EBioMedicine. 2016;7:240–7. 10.1016/j.ebiom.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xing L, Dai Z, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20:1043–9. 10.1038/nm.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackay-Wiggan J, Jabbari A, Nguyen N, et al. Oral ruxolitinib induces hair regrowth in patients with moderate-to-severe alopecia areata. JCI Insight. 2016;1:e89790. 10.1172/jci.insight.89790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guttman-Yassky E, Ungar B, Noda S, et al. Extensive alopecia areata is reversed by IL-12/IL-23p40 cytokine antagonism. J Allergy Clin Immunol. 2016;137:301–4. 10.1016/j.jaci.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Jabbari A, Sansaricq F, Cerise J, et al. An open-label pilot study to evaluate the efficacy of tofacitinib in moderate to severe patch-type alopecia areata, totalis, and universalis. J Invest Dermatol. 2018;138:1539–45. 10.1016/j.jid.2018.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guttman-Yassky E, Pavel AB, Diaz A, et al. Ritlecitinib and brepocitinib demonstrate significant improvement in scalp alopecia areata biomarkers. J Allergy Clin Immunol. 2022;149:1318–28. 10.1016/j.jaci.2021.10.036. [DOI] [PubMed] [Google Scholar]
- 20.Crispin MK, Ko JM, Craiglow BG, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1:e89776. 10.1172/jci.insight.89776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.David E, Shokrian N, Del Duca E, et al. Dupilumab induces hair regrowth in pediatric alopecia areata: a real-world, single-center observational study. Arch Dermatol Res. 2024;316(7):487. 10.1007/s00403-024-03225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guttman-Yassky E, Ungar B, Noda S, et al. Extensive alopecia areata is reversed by IL-12/IL-23p40 cytokine antagonism. J Allergy Clin Immunol. 2016;137(1):301–4. 10.1016/j.jaci.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Aleisa A, Lim Y, Gordon S, et al. Response to ustekinumab in three pediatric patients with alopecia areata. Pediatr Dermatol. 2019;36(1):e44-45. 10.1111/pde.13699. [DOI] [PubMed] [Google Scholar]
- 24.Kim JE, Lee YJ, Park HR, et al. The effect of JAK inhibitor on the survival, anagen re-entry, and hair follicle immune privilege restoration in human dermal papilla cells. Int J Mol Sci. 2020;21(14):5137. 10.3390/ijms21145137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorczynski RM, Chen Z, Kai Y, et al. CD200 is a ligand for all members of the CD200R family of immunoregulatory molecules. J Immunol. 2004;172(12):7744–9. 10.4049/jimmunol.172.12.7744. [DOI] [PubMed] [Google Scholar]
- 26.Steen-Louws C, Hartgring SAY, Popov-Celeketic J, et al. IL4-10 fusion protein: a novel immunoregulatory drug combining activities of interleukin 4 and interleukin 10. Clin Exp Immunol. 2019;195(1):1–9. 10.1111/cei.13224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Araujo GR, Aglas L, Vaz ER, et al. TGFβ1 mimetic peptide modulates immune response to grass pollen allergens in mice. Allergy. 2020;75(4):882–91. 10.1111/all.14108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jabbari A, Sansaricq F, Cerise J, et al. An open-label pilot study to evaluate the efficacy of tofacitinib in moderate to severe patch-type alopecia areata, totalis, and universalis. J Invest Dermatol. 2018;138(7):1539–45. 10.1016/j.jid.2018.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toro-Domínguez D, Villatoro-García JA, Martorell-Marugán J, et al. A survey of gene expression meta-analysis: methods and applications. Brief Bioinform. 2021;22(2):1694–705. 10.1093/bib/bbaa019. [DOI] [PubMed] [Google Scholar]
- 30.Betz RC, Petukhova L, Ripke S, et al. Genome-wide meta-analysis in alopecia areata resolves HLA associations and reveals two new susceptibility loci. Nat Commun. 2015;6:5966. 10.1038/ncomms6966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi J, Peng P, Liu W, et al. Bioinformatics analysis of genes associated with the patchy-type alopecia areata: CD2 may be a new therapeutic target. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2020;164:380–6. 10.5507/bp.2019.049. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Z, Wang X, Zhang R. Pathogenesis of alopecia areata based on bioinformatics analysis. Indian J Dermatol. 2019;64:1–6. 10.4103/IJD.IJD_68_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rau A, Marot G, Jaffrézic F. Differential meta-analysis of RNA-seq data from multiple studies. BMC Bioinform. 2014;15:91. 10.1186/1471-2105-15-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van de Sande B, Lee JS, Mutasa-Gottgens E, et al. Applications of single-cell RNA sequencing in drug discovery and development. Nat Rev Drug Discov. 2023;22:1–14. 10.1038/s41573-023-00688-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are available in the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) repository, and can be accessed through the following accession numbers: GSE68801, GSE45512, GSE80342, GSE58573, GSE74761, GSE111061. These public datasets were used under open access terms and can be freely accessed for further research. Additional data that support the findings of this study are available from the corresponding author upon reasonable request.