Abstract
Obesity refers to the excessive accumulation of fat caused by a long-term imbalance between energy intake (EI) and energy expenditure (EE). Over recent years, obesity has become a major public health challenge. Caffeine is a natural product that has been demonstrated to exert anti-obesity effects; however, the mechanisms responsible for the effect of caffeine on weight loss have yet to be fully elucidated. Most obesity-related deaths are due to cardiovascular disease. Recent research has demonstrated that caffeine can reduce the risk of death from cardiovascular disease; thus, it can be hypothesized that caffeine may represent a new therapeutic agent for weight loss. In this review, we synthesize data arising from clinical and animal studies over the last decade and discuss the potential mechanisms by which caffeine may induce weight loss, focusing particularly on increasing energy consumption, suppressing appetite, altering lipid metabolism, and influencing the gut microbiota. Finally, we summarize the major challenges associated with caffeine and anti-obesity research and highlight possible directions for future research and development.
Keywords: Obesity, Caffeine, Energy consumption, Appetite, Lipid metabolism
Introduction
Obesity is a serious health problem that is strongly associated with an increased overall risk of death and is defined as a body mass index greater than or equal to 30 [1]. The World Health Organization defines obesity as the excessive accumulation of body fat that may be hazardous to health; the main cause of this condition is an imbalance between the body’s energy intake (EI) and energy expenditure (EE) [2]. Excessive body weight and obesity are becoming increasingly prevalent in some countries and showing a clear trend towards a global epidemic [3, 4]; consequently, obesity represents one of the most significant medical problems of the twenty-first century. Given the excessive mortality, high morbidity, and consequential economic burdens associated with obesity, this disease represents a major concern to the medical community. There are many factors that contribute to obesity, including genetics, poor dietary habits, and the lack of adequate physical activity [5, 6]. Obesity can lead to potentially life-threatening comorbidities. Excessive body weight and obesity increase the risk of an individual developing a variety of diseases, including hypertension, type 2 diabetes (T2D), cardiovascular disease (CVD), metabolic syndrome, degenerative joint disease, and certain cancers [7], thus complicating the assessment of drug safety. Obesity and obesity-related diseases cause a reduction in an individual’s quality-of-life and life expectancy, and the incidence of these diseases has risen dramatically over the last few years, thus becoming a major public health problem that places a significant burden on global health and the world economy [8–11]. Addressing this multifaceted issue necessitates a comprehensive and interdisciplinary approach that emphasizes preventive strategies, lifestyle modifications, and innovative interventions to mitigate the pervasive impact of obesity on global health.
Current medical treatment for obesity includes medication and surgery. The main medications approved by the Food and Drug Administration (FDA) for the treatment of obesity are orlistat, lorcaserin, phentermine, and topiramate [12]. With regard to the specific mechanism of action, orlistat is a reversible pancreatic and gastric lipase inhibitor that reduces fat digestion and absorption [13], lorcaserin is a selective 5-hydroxytryptamine 2 C (5-HT2C) receptor agonist that acts on 5-HT2C receptors in the brain and reduces body weight by reducing appetite and increasing satiety, and phentermine reduces appetite by increasing the level of hypothalamic norepinephrine, whereas topiramate may reduce appetite by acting on GABA receptors [12]. Significant progress has been achieved over recent years in the use of glucagon-like peptide-1 (GLP-1) receptor agonists to treat obesity, including exenatide and semaglutide. These drugs promote weight loss by mimicking the effects of GLP-1, increasing satiety, and by reducing appetite. In particular, semaglutide is being evaluated as a weight loss drug in obese subjects without diabetes [14]. However, there are many side effects associated with the therapeutic options available for the treatment of obesity at present, including paresthesia, dizziness, dysgeusia, insomnia, nausea, constipation, dry mouth, allergic reactions, and liver problems [15–18]. Bariatric surgery is the most effective method for inducing weight loss and can result in a significant reduction in mortality from CVD [19] or cancer [20]. However, due to economic, technological, and individual differences, this type of surgery is unlikely to be able to meet global medical needs. Most obesity-related deaths are caused by CVD [21] while stroke is the second leading cause of global death; furthermore, statistics show that obesity increases the risk of stroke [22]. In addition, obesity leads to increased risk factors for CVD, including high blood pressure, high cholesterol, and diabetes, and can directly influence the health and functionality of blood vessels while increasing the risk of thrombosis [23], all of which increase the incidence of stroke. Therefore, improving cardiovascular health is the main goal of weight loss therapy. Unfortunately, most of the clinical drugs designed to reduce food intake ultimately failed in clinical trials due to insufficient cardiovascular safety [24]. Therefore, there is a growing need to identify and apply safe and effective natural alternatives to prevent or suppress obesity in order to reduce the impact and cost of the metabolic consequences of this disease [25]. This underscores the importance of advancing research and development in pursuit of holistic and globally applicable solutions to address the intricate challenges posed by obesity.
Previous research suggested that weight maintenance or loss may be associated with increased EE, thermogenesis, and the oxidation of fat [26–28]. Furthermore, researchers have previously demonstrated that caffeine may reduce body weight by maintaining or enhancing EE, improving exercise capacity, increasing fat oxidation, and stimulating the thermogenic response [29–33]. Caffeine has also been shown to suppress a variety of obesity-related abnormalities, including hypometabolism, dyslipidemia, systemic/tissue inflammation, and insulin resistance [34]. Interestingly, individuals who successfully maintain weight tend to consume higher volumes of coffee and caffeinated beverages [35]. Regular coffee consumption is associated with moderate weight loss [36] and caffeine has been shown to exert effect on energy balance by increasing EE and decreasing EI [37]. Given the side effects of the drugs that are currently on the market that can be used to treat obesity, efficacy and safety are two major concerns for the development of new anti-obesity drugs. Some studies have shown that caffeine, a natural component of coffee, tea, guaraná, and mate, can all exert therapeutic and anti-obesity effects but without adverse effects [38–40]. While 400 mg/day of caffeine is considered safe for the average person [41], previous research demonstrated that the long-term consumption of 400–600 mg/day of caffeine can prevent CVD and significantly reduce the risk of cardiovascular mortality [42–44]. It is evident that caffeine represents a potential new target for the clinical management of body weight; however, there is a significant lack of knowledge relating to the specific mechanisms involved in the effects of caffeine on body weight [45]. In this paper, we review the literature relating to the anti-obesity effects of caffeine and provide an overview of the basic mechanisms involved, discuss the advantages of caffeine with regard to anti-obesity effects, highlight the challenges that still need to be addressed, and discuss the potential direction of future research.
Caffeine
Caffeine is an alkaloid extracted from tea and coffee and represents the most common psychoactive substance in the world [46]. Tea and coffee beans are also the main sources of dietary caffeine globally and the highest consumption rates of caffeine are associated with beverages, including coffee (71%), soft drinks (16%), and tea (12%) [47]. Caffeine is a methylxanthine substance that stimulates the central nervous system, promotes metabolism, and enhances blood circulation [48, 49]. The half-life of caffeine in adults is usually 4 to 6 h, although this varies from person to person [50]. Caffeine can exert a range of physiological effects, including the improvement of endurance, physical performance, cognition, resting EE, and the improvement of mood [48]. Furthermore, previous research has investigated the potential therapeutic effects of caffeine on various health issues, including neurodegenerative diseases [51–54], diabetes [55], kidney stones [56], aging [57], and obesity [34, 58], while improving exercise and relieving fatigue [33]. Four basic mechanisms have been associated with the pharmacological effects of caffeine. The pharmacological actions of caffeine involve multiple targets, including adenosine receptors, phosphodiesterase, intracellular calcium, and γ-aminobutyric acid (GABA) receptors [59–62]. However, the physiological impacts of caffeine are primarily attributed to its antagonistic action on adenosine receptors. Previous research showed that caffeine can block the activation of sterol regulatory element-binding protein 2 (SREBP2), thus inhibiting the expression of proprotein convertase subtilisin/kexin type 9 (PCSK9) to effectively reduce the levels of low-density lipoprotein cholesterol (LDLc) in the blood [61]. This research highlighted a potential mechanism for the cardiovascular protective effects of caffeine. Although a large number of studies have been conducted on the efficacy of caffeine, these have predominantly focused on caffeine refreshing, anti-depressant, and the treatment of neurodegenerative diseases. Consequently, we know relatively little about the specific mechanisms by which caffeine can promote weight loss. It is vital that we gain such knowledge if we are to develop caffeine as a potential clinical therapeutic.
Mechanisms
Increasing energy consumption in the body
Mendelian randomization studies have indicated that higher plasma levels of caffeine may reduce the risk of obesity and the development of T2D. The increased EE induced by caffeine intake may be modulated by increasing thermogenesis in the brown fat. Further clinical studies are now necessary to investigate the translational potential of caffeine with regard to reducing the burden of metabolic disease [63].
Caffeine is a xanthine alkaloid that is found in coffee, tea, soft drinks, and chocolate. The main mechanism of action involves the antagonism of adenosine receptors [64, 65]. Adenosine receptors are members of the G protein-coupled receptor family, which includes four isoforms in mammals: A1, A2A, A2B, and A3. These isoforms are widely expressed and play a wide range of physiological and pathobiological functions [65–67]. Of these, the A1 receptor (A1R) is expressed at higher levels in the brain, A2AR is predominantly expressed in the striatum at high levels, and A2BR is widely expressed, but has a lower overall abundance. A3R is expressed at lower levels and exhibits only low affinity for caffeine [68]. Caffeine antagonizes A1R, A2AR, and A2BR in a non-selective manner [69]. In vitro, A2BR has been shown to activate only at high pathological concentrations of adenosine. Therefore, it is generally accepted that A1R and A2AR are the main targets of caffeine action in the central nervous system (CNS) [66]. While A1R is known to play an important role in analgesia [70] and sleep disorders [71], attempts to develop adenosine/A1R as a potential analgesic have failed due to lack of sufficient on-target selectivity and off-tissue adverse reactions [72]. A2AR plays an important role in cancer [73], Parkinson’s disease [74], Alzheimer’s disease [75], sleep regulation [76], and depression [77, 78]. Moreover, animal studies have demonstrated that adenosine receptors, especially A2AR, represent the main pharmacological targets of caffeine [68, 79], and that caffeine can block approximately 50% of A2AR sin the coronal striatum [74]. Istradefylline, a selective A2AR antagonist, is used as an adjunct to levodopa to alleviate episodes of Parkinson’s disease and first received FDA approval in 2019 [80, 81]. Collectively, these lines of evidence suggest that adenosine receptors are potential drug targets for several conditions, and a number of clinical development studies are already underway.
Previous research has also demonstrated that caffeine can exert thermic effects; for example, the intake of caffeinated coffee has been shown to increase EE [82]. Since brown adipose tissue (BAT) is dedicated to EE, increasing the activity of BAT represents a potential target for the development of anti-obesity strategies. A2AR signaling has been shown to activate BAT and induce the browning of white adipose tissue, leading to increased thermogenesis, thereby protecting experimental mice from diet-induced obesity [83]. A2ARs are the most abundant form of adenosine receptor in the BAT of humans and mice. In addition, A2ARs are known to be associated with the activation of human BAT; therefore, targeting A2ARs in BAT provides a potential pathway to enhance the functionality of BAT and improve human obesity [84]. Experiments have also shown that caffeine can promote the functionality of BAT at doses that are compatible with human consumption and mobilize the brown form of fat that is actively involved in metabolism; consequently, caffeine can exert influence on metabolism in humans [85]. Both the central and peripheral administration of caffeine has been shown to inhibit appetite and increase thermogenesis in BAT and EE in dietary obese (DIO) mice. These effects have been shown to be related to the antagonistic effects of caffeine on A1Rs in the hypothalamus, which suppressed appetite and promoted EE, thereby reducing diet-induced obesity [58]. Caffeine-induced BAT thermogenesis in rodents is a consequence of central activation. However, whether caffeine-induced BAT thermogenesis in humans occurs as a result of systemic or central activation remains unclear; it is possible that caffeine may influence human BAT by acting on metabolic neural pathways [86]. It is considered that this process involves the activation of orexinergic neurons in the perifornical lateral hypothalamus and lateral hypothalamus, and the broader antagonism of the adenosine receptors [86]. The activation of lateral hypothalamic neurons has been shown to significantly increase sympathetic activity in the BAT of rodents. Furthermore, neurons in the lateral hypothalamic region can influence sympathetic activity and thermogenesis in the BAT, and heart rate via pathways that are dependent of the activation of the dorsal hypothalamus and pallidocyte neurons [87]. The neural pathways controlling the regulation of thermogenesis in the BAT have been studied and described previously [88].
Collectively, these findings indicate that adenosine increases thermogenesis in BAT and that A2ARs antagonists can block these effects. A1Rs appear to exert opposite effects to A2ARs and A2BRs. Previous research demonstrated that a selective A2ARs antagonist, KD-64-A, exerts anti-inflammatory activity but does not reduce diet-induced obesity in mice when compared to caffeine, a non-selective antagonist [89]. Thus, selective A2ARs antagonists are very different from non-selective adenosine receptor antagonists in terms of weight loss. The precise mechanisms responsible for these differences have yet to be elucidated. Many studies have demonstrated that caffeine can play a significant role in weight loss [46–48, 58]; consequently, the studies described in this section may provide evidence that A1Rs are a preferred target for caffeine, an adenosine receptor antagonist, and play a key role in its anti-obesity effects [90]. In addition, caffeine is a non-specific antagonist of adenosine receptors, and may also exert effects on a range of other peripheral mechanisms. Therefore, it is necessary to investigate the effects of caffeine combined with selective adenosine A2A or A2B agonists on thermogenic anti-obesity. Such combination therapies may increase the efficacy of caffeine [86].
The activation of BAT thermogenesis is regulated by the sympathetic nervous system via β-adrenergic receptors (β-ARs) in rodents and is thought to occur in a similar manner in humans. The pathway by which caffeine stimulates a thermogenic response may be similar to the pathway by which an organism reacts to cold stimuli. Caffeine can increase excitability in the sympathetic nervous system (SNS) to exert effects on the regulation of energy balance and lipolysis [37, 48]. The effect of low-dose caffeine on tissue thermogenesis has been shown to be entirely dependent on intact sympathetic innervation [89]. Norepinephrine (NE) is a major regulator of the SNS and can increase the utilization of adenosine triphosphate (ATP) via ion pumps and substrate cycling, and increase the oxidation rate of mitochondria in a manner that is only weakly coupled with ATP synthesis, thus resulting in an increase in thermogenesis at the organismal level. The BAT is responsible for consuming energy to generate heat via the mitochondrial uncoupling protein 1 (UCP1) to resist obesity [91, 92]. UCP1, a thermogenic protein located on the inner mitochondrial membrane, orchestrates mitochondrial respiratory chain uncoupling, ultimately increasing metabolism and heat production [93]. NE is also known to activate the β-ARs on BAT to induce lipolysis [94]. The β3-adrenergic receptors (β3-ARs) are the major regulators of thermogenesis in the BAT of rodents [95]. Furthermore, NE interacts with β3-ARs to promote thermogenesis [96]; the activation of β3-ARs promotes the increased expression of cyclic adenosine monophosphate (cAMP) which activates protein kinase A (PKA), stimulates lipolysis, releases free fatty acids, and induces the expression of UCP1 [91, 93, 94, 97, 98].
Research has also shown that the β-ARs can also activate mitogen-activated protein kinase (MAPK) in adipocytes. The activation of p38 mitogen-activated protein kinase (p38 MAPK) is indirectly dependent on PKA. p38 MAPK directly targets peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) and activating transcription factor-2 (ATF-2) to induce expression of the UCP1 gene and enhance mitochondrial oxidative activity [99]. PGC-1α is a transcriptional coactivator involved in the control of energy metabolism and is expressed at high levels in BAT. The expression levels of the PGC-1α gene are up-regulated by cAMP-mediated pathways in BAT [100]; this process is crucial for the cAMP-dependent activation of BAT thermogenesis and plays a key role in inducing UCP1 gene expression and enhancing overall mitochondrial oxidative activity [101]. Therefore, increasing the levels of PGC-1α may represent a reasonable strategy with which to treat obesity. Caffeine induces PGC-1α expression and mitochondrial biosynthesis [102, 103], thus, increasing the levels of cellular cAMP may represent the mechanism by which caffeine increases UCP1 activity and thus thermogenesis. BAT generates heat rapidly and metabolizes glucose and lipids by activating UCP1. Therefore, caffeine may induce fat thermogenesis via the PGC-1α-UCP1 signaling pathway, thus increasing EE and ultimately exerting anti-obesity effects.
The suppression of appetite
Caffeine can reduce food intake by stimulating the CNS and by suppressing appetite. Previous studies have established that peripheral and central injections of adenosine can inhibit food intake in rats, and that mechanisms by which the peripheral and central nervous systems inhibit food intake are different [104]. One study found that the increased levels of adenosine in the cerebrospinal fluid and the overexpression of A1Rs in neurons of the hypothalamus paraventricular nucleus (PVN) in experimental mice were related to the development of obesity. Previously, researchers investigated the mechanism of action of caffeine by intracerebroventricular injection and by feeding caffeine, and found that both the central and peripheral administration of caffeine inhibited appetite and increased thermogenesis and EE in the BAT of DIO mice, thus reducing body weight. These studies in DIO mice showed that the anti-obesity effect of caffeine was predominantly mediated by antagonizing the expression of A1Rs in PVN neurons, inhibiting the expression of A1Rs in PVN oxytocin (Oxt) neurons, and negatively regulating the energy balance. A1Rs are expressed in adipose tissue and their activation can inhibit lipolysis. Thus, peripherally administered caffeine may promote lipolysis primarily by directly antagonizing the A1Rs. The central administration of caffeine has been shown to negatively regulate energy balance by facilitating the release of Oxt. Research has shown that the caffeine-A1R signaling system utilizes Oxt to suppress appetite and reduce weight, thus suggesting that Oxt acts as a key mediator of the caffeine-induced negative regulation of energy balance in DIO mice. Targeting the A1Rs in the PVN via caffeine or its derivatives may represent a relevant strategy with which to combat obesity and related comorbidities [58]. The downregulation of striatal dopamine D2 receptors (D2Rs) is required in addiction-like reward dysfunction and compulsive eating in obese rats [105], and A2ARs expressed in the striatum may be involved in the pathogenesis of DIO. The antagonistic effect of caffeine on A2ARs may increase the activity of D2Rs, thereby attenuating food addiction in DIO animals [58]. However, studies have found that A2ARs play an important role in binge eating behavior, and their activation may help reduce food intake [106, 107], thus suggesting that A2ARs may have a complex and dual role in regulating food intake behavior. Therefore, there is a clear need for in-depth research to investigate the mechanism of its action in the nervous system.
Studies have demonstrated that the intake of coffee can reduce body fat, increase the plasma levels of serotonin, reduce the plasma levels of ghrelin, and protect DNA integrity [108]. Increased serotonin secretion by intestinal neuronal cells in response to luminal stimulation has been reported to reduce food intake [109], whereas increased ghrelin secretion by gastric cells has been shown to stimulate appetite and food intake in both rodents and humans [110]. Thus, the intake of coffee can reduce food intake. Caffeine can increase the excitability of the SNS [48], suppress hunger, and enhance satiety [111]. Corticotropin-releasing factor (CRF) mediates the anorexic effects of caffeine in a manner which does not necessarily involve the sympathetic-adrenal system [112]. Therefore, caffeine may cause the effect of CRF on food intake to be independent of the sympathetic-adrenal system. One study has revealed that the blockade of A2ARs requires the activation of CRF2R [113]. This interaction between adenosine and CRF receptors could explain the possible mechanism of action of how caffeine could be used to combat obesity. Furthermore, the effects of caffeine on food intake are known to be mediated by multiple pathways. Research has shown that the stimulation of astrocytes can reduce ghrelin-induced food intake via the inactivation of A1Rs-mediated agouti-related peptide neurons [114]. Therefore, caffeine and astrocytes may co-regulate appetite through different but related pathways, although the specific interactions and mechanisms of action still need to be determined by further in-depth investigation.
Leptin is a hormone secreted by adipose tissue that has been reported to suppress appetite, and regulate both body weight and energy balance [115]. In obesity, elevated blood levels of leptin result in leptin resistance, which may be one of the main factors underlying obesity [116]. Several studies have investigated the impact of caffeine intake on leptin levels. Research indicates that caffeine may alleviate endoplasmic reticulum stress and improve leptin resistance in neurons [117]. In addition, an experimental study demonstrated that a combination of choline, carnitine, and caffeine reduced the serum levels of leptin in a rat model [118]. Although coffee consumption has been associated with lower leptin levels [119], observational studies have failed to establish a significant association between leptin and coffee intake [120]. Overall, caffeine has the potential to modulate the effects of leptin by influencing the activities of the nervous system and metabolic pathways that regulate appetite and energy metabolism. Nevertheless, further research is still needed to fully comprehend the relationship between caffeine and leptin, which may be influenced by individual variances and environmental factors.
Previous research demonstrated that the typical consumption of caffeinated coffee has no short-term effects on appetite, EI, glucose metabolism, or inflammatory markers, thus requiring long-term intake [121]. It has also been shown that moderate coffee intake can effectively reduce EI at the next meal and throughout the day, but has no significant effect on appetite [122]. Drinking decaffeinated coffee can significantly reduce hunger; however, caffeine does not produce this effect alone [123]. Furthermore, while many studies have shown that caffeine can suppress appetite and reduce weight, some other studies have demonstrated that caffeine can increase appetite [124]. Consequently, there is clear ambiguity in the literature relating to the effects of caffeine on appetite. Therefore, further research is needed to identify the precise mechanisms involved, as well as to further consider the potential of incorporating caffeine-containing substances into weight loss diets as a dietary behavior.
The alteration of lipid metabolism in the body
Previous studies have reported that caffeine can inhibit adipogenesis [125] and the differentiation of adipocytes [126], inhibit the intracellular accumulation of lipid [127, 128], and promote lipolysis, fat oxidation [129], and fat thermogenesis [130].
Caffeine may affect weight loss by inhibiting adipogenesis and adipocyte differentiation, thereby inhibiting lipid deposition. Obesity is a disease characterized by the excessive accumulation of triglycerides (TG). Adipose tissue is predominantly composed of adipocytes and 3T3-L1 adipocytes are widely considered as a classical model of fat metabolism [131]. The inhibition of TG accumulation in 3T3-L1 cells may be crucial for the clinical treatment of obesity. Caffeine is an anti-adipogenic and bioactive compound that plays a role in the regulation of mitotic clonal expansion during adipocyte differentiation via the AKT/GSK3 pathway, thereby inhibiting the expression of two major adipogenic transcription factors: CCAAT/enhancer-binding protein (C/EBPα) and peroxisome proliferator-activated receptor γ (PPARγ) [125]. However, it is worth noting that the concentrations of caffeine used in these particular experiments were well outside the physiological range. Therefore, it is important to investigate the effects of caffeine in vivo with full consideration of both dose and exposure time. Similarly, a treatment combining catechins and caffeine was shown to inhibit the mRNA expression of transcription factors and adipogenesis-related enzymes in 3T3-L1 cells and increased the protein expression of lipolysis-related enzymes in the presence of NE, thereby inhibiting fat deposition [132]. In addition, the combined action of chlorogenic acid (CGA) and caffeine was shown to retard adipogenesis by modulating lipid metabolism and by inhibiting the differentiation of adipocytes in 3T3-L1 cells. In summary, the combination of CGA and caffeine inhibited the middle to late stages of differentiation in 3T3-L1 cells and attenuated adipogenesis by regulating the expression of adipose metabolism-associated enzymes such as adipose triglyceride lipase and hormone-sensitive lipase (HSL) in 3T3-L1 cells, thereby reducing the accumulation of adipose via the AMP-activated protein kinase (AMPK) pathway [133].
Previous research involving high-fat diet (HFD)–induced mice showed that caffeine reduced serum levels of lipid, promoted lipolysis, and inhibited the deposition of fat [127]. AMPK is an important therapeutic target for the treatment of obesity, and in mature adipocytes, AMPK was shown to exert significant anti-lipolytic effects [134]. SREBP-1c is a major transcriptional regulator of lipogenic enzymes and can be inhibited by AMPK via the phosphorylation of serine 365 [135]. Caffeine has also been reported to reduce the mRNA levels of genes related to adipogenesis, such as SREBP-1c and FAS by phosphorylating AMPK in human hepatocellular carcinoma HepG2 cells [136]. The mRNA expression levels of AMPK, CAT, and ACO in the combined CGA and caffeine group were significantly up-regulated [137], thus suggesting that the increased expression of AMPK may promote the expression of ACO and CAT, the major contributors to β-oxidation, while the expression level of PPARg2 mRNA was down-regulated. These researchers demonstrated that an 8% dose of caffeine reduced the accumulation of lipid in the livers of zebrafish larvae by upregulating the expression of the lipid β-oxidation gene ACO and by down-regulating the lipogenesis-related genes SREBP1, acetyl-CoA carboxylase 1 (ACC1), and fatty acid translocase [138]. Caffeine inhibits lipogenesis and stimulates lipolysis by modulating the AMPK-SREBP signaling pathway, effectively depleting triglyceride and cholesterol levels.
Caffeine may reduce weight by promoting lipolysis and fat oxidation. Research indicates that caffeine promotes lipolysis [139]. Furthermore, studies comparing the effects of exercise and caffeine intake on serum fatty acid profiles have demonstrated that the combination of exercise and caffeine can lead to a heightened lipolytic response [140]. A1Rs and A2ARs are expressed abundantly in adipose tissue and participate in lipolysis [141]. A1Rs are expressed in adipose tissue and their activation inhibits lipolysis. Thus, the peripheral administration of caffeine may promote lipolysis by directly inhibiting the expression of A1Rs in adipocytes while the central administration of caffeine may negatively regulate energy balance by promoting the release of Oxt [58]. The antagonistic effect of caffeine on A1Rs leads to the activation of adenylate cyclase (AC), followed by the increased activity of cAMP and PKA [142]. The caffeine-A1R signaling pathway may contribute to weight loss by promoting lipolysis. Extracellular adenosine is known to increase the intracellular levels of cAMP by activating AC to bind to the A2ARs and A2BRs [65, 143], while an increase of intracellular cAMP can promote lipolysis. Agonists of A2ARs and A2BRs can increase lipolysis and BAT thermogenesis, and in brown adipocytes, the expression of A2ARs has been shown to increase with increased levels of NE or intracellular cAMP [83].
Obesity is associated with the overgrowth and expansion of adipose tissue mass; thus, the number of adipocytes is a major determinant of fat mass in adults [144]. White adipose tissue (WAT) is known to be innervated by the SNS and needs to be activated for lipolysis. PKA can activate HSL in the adipocytes of WAT, promote the hydrolysis of stored triglycerides into free fatty acids, and play a key role in lipolysis [145]. This is the main pathway by which fat cells release stored energy. In long-term studies of rodents, the intake of caffeine was shown to reduce the size of fat pads and the number of fat cells [146], and has also been shown to enhance anti-inflammatory responses in human exercise trials [147]. In addition, caffeine increases the excitability of the SNS [48], and SNS-NE-mediated WAT lipolysis is completely dependent on the stimulation of β-ARs, ultimately depending on HSL and the phosphorylation of perilipin A [148, 149]; therefore, the intake of caffeine may promote the catabolism of white fat via the SNS-NE pathway.
Previous research showed that consumers of high amounts of caffeine lost more weight, fat mass, and waist circumference than those who consumed low amounts of caffeine; this was associated with relatively large heat production and fat oxidation [150]. Additionally, a randomized controlled trial revealed that caffeine consumption alone increased fat oxidation compared to a placebo [151]. In addition, caffeine may enhance athletic performance by promoting the oxidation of fat. The combination of moderate caffeine intake before exercise and moderate intensity exercise in the afternoon was shown to significantly increase the so-called maximal rate of fat oxidation, which appears to be optimal for individuals seeking to increase fat utilization during continuous aerobic exercise [152, 153]. Therefore, the combination of moderate caffeine consumption and exercise may be more conducive to weight loss.
In addition to acting as an antagonist of the adenosine receptor, caffeine also acts as a non-selective phosphodiesterase (PDE) inhibitor [60]. Caffeine is metabolized in the liver and the most abundant primary metabolite is paraxanthine. Methylxanthines (theophylline and/or caffeine) have been reported to act as thermogenic and lipolytic stimulators, inducing fat oxidation in adipocytes and the release of glycerol and fatty acids into the blood [130]. Methylxanthines increase fat oxidation by elevating the serum levels of catecholamine and prolonging the half-life of cAMP. Caffeine regulates cAMP levels by inhibiting PDE [154], thereby inhibiting the breakdown of cAMP in adipocytes to reduce the threshold of lipid solubility [155]. Pilot human intervention studies have clearly demonstrated that normal levels of daily coffee consumption have a significant effect on PDE activity in the platelets [156]. Caffeine acts by inhibiting PDE, leading to a subsequent increase in intracellular cAMP concentration [157]; this is responsible for the catabolism of cAMP and can rapidly hydrolyze intracellular cAMP into adenosine monophosphate (AMP). Increases in cAMP usually promote NE secretion [158]. Caffeine inhibits the ability of PDE to induce the degradation of cAMP, thus maintaining NE at a certain level [154] can stimulate lipolysis and induce the expression of UCP1. Caffeine promotes lipid metabolism through multiple mechanisms. It activates AMPK to attenuate lipogenesis and enhance lipolysis, while also inhibiting the differentiation and proliferation of preadipocytes to reduce lipid accumulation [159].
Additionally, caffeine may contribute to weight reduction by promoting fat thermogenesis. A previous study investigated the impact of caffeine on the thermogenesis of BAT in vivo, assessing thermographic temperature in the supraclavicular region. The results revealed an increase in BAT region temperature following caffeine ingestion, indicating enhanced BAT activity after the intake of a relatively low dose of caffeine [85]. These findings suggest that the rise in metabolic rate subsequent to caffeine consumption may be attributed to the augmentation of BAT function preceding changes in skin temperature [160].
Both beige and brown adipocytes have the ability to burn glucose and fat to produce heat [161]. Beige adipocytes are unique thermogenic adipocytes with extremely low basal expression levels of UCP1 [92]. UCP1 plays a central role in the non-shivering thermogenesis of brown fat but is dispensable for thermogenesis in beige adipocytes in vivo [162]. Research has shown that mechanisms exist in organisms that do not rely on UCP1 for thermogenesis [163]. The extracellular clearance of calcium has been shown to have no effect on the EE of NE-stimulated beige fat, while the intracellular clearance of calcium significantly inhibited the EE of NE-stimulated beige fat. Collectively, these results suggested that intracellular calcium is essential for the non-UCP1-dependent thermogenesis of beige fat, and also confirmed the involvement of sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA) in regulating intracellular calcium cycling [162]. The sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA2) calcium pump is one of several Ca2+-ATPase enzymes in the sarcoplasmic reticulum (SR) and endoplasmic reticulum (ER) that are responsible for regulating the transmembrane transport of calcium [164]. There are two major isoforms: sarcoplasmic/endoplasmic reticulum calcium ATPase 2 A (SERCA2A), which is primarily localized in the SR of muscle cells, and sarcoplasmic/endoplasmic reticulum calcium ATPase 2B (SERCA2B), which is primarily localized in the ER of most cell types [165]. By experimenting with cAMP agonists, researchers found that cAMP can specifically regulate SERCA2B. The application of SERCA2B inhibitors, knockouts, and the overexpression of SERCA2B further showed that SERCA2B is essential for the thermogenesis of beige fat [162]. The SERCA-ryanodine receptor (RyR) null-cycling thermogenesis mechanism is known to occur in the ER of thermogenic fats. This promotes thermogenesis through the null-cycling process of Ca2+ in and out of the ER; in other words, SERCA2B transports Ca2+ into the ER while ryanodine receptor 2 (RyR2) releases Ca2+. This process is coupled with the hydrolysis of ATP by SERCA2B, which mediates thermogenesis. Research has suggested that non-UCP1-dependent thermogenesis in beige fat acts through muscle/ER calcium cycle 2+-SERCA2B and the RYR2. Increased levels of Ca2+ act by activating the α1-ARs and β3-ARs or SERCA2B-RyR2 pathways to circulate and stimulate UCP1-independent thermogenesis in beige adipocytes [162]. These results suggest that enhanced calcium cycling promotes non-UCP1-dependent thermogenesis in beige adipose tissue. Cytoplasmic Ca2+ signaling is usually amplified by the significant release of calcium from the ER. Research has demonstrated that coffee, and the components of coffee, mobilizes intracellular calcium, causing PC-12 cells to release dopamine, which increases the levels of Ca2+ in hepatic ER [61, 166]. Caffeine acts as an agonist for the ryanodine receptor and can trigger the RyR-sensitive calcium pool at basal cytoplasmic levels of Ca2+ to promote Ca2+ release [167], thus increasing the levels of intracellular Ca2+ while enhancing the sensitivity of muscle fibers to Ca2+ [168]. Therefore, caffeine may promote non-UCP1-dependent thermogenesis in beige fat via the Ca2+-SERCA2b-RyR pathway, thereby increasing EE. In addition, cytoplasmic calcium has been shown to directly stimulate AC activity during adrenergic stimulation in brown adipocytes, thereby increasing cAMP production and PKA activation to induce lipolysis and thermogenesis [169]. Second, Ca2+ is able to achieve transcriptional control of metabolism through its dependent kinases and phosphatases, such as Ca2+/calmodulin-dependent protein kinase (CAMK) and calcineurin [170], thus initiating the increased phosphorylation of p38 MAPK and ATF-2. The kinases and phosphatases acting within this pathway increase PGC-1α expression and mitochondrial biogenesis in muscle [171], thus increasing thermogenesis and exerting anti-obesity effects.
In addition, elevated levels of GABA in the brain may contribute to increased thermogenesis in rats [172]. Caffeine can significantly increase the levels of GABA [62], which is known to promote the production of UCP1 and PGC-1α, facilitate lipid metabolism, promote fatty acid oxidation, and inhibit hepatic triglyceride levels [173].
Effects on the gut microbiota
The gut microbiota is now recognized as one of the key factors regulating host health. Deviations in the gut microbiota have been associated with many diseases, including obesity, T2D, hepatic steatosis, inflammatory bowel disease, and several cancers [174]. Recent studies have also demonstrated that the gut microbiota can influence energy metabolism [175, 176]. Tea and coffee have been shown to alter the gastrointestinal microbiota in both animals and humans [45, 177]. In addition, caffeine may play a role in anti-obesity by regulating the gut microbiota.
Coffee is initially absorbed in the stomach and small intestine, but further fermented in the colon by the gut microbiota [177]. The proportions of Firmicutes and Bacteroidetes were found to be increased in the ileum of rats fed with high-calorie diets, while the proportions of Enterobacteriaceae or Clostridium decreased [45]. Furthermore, the consumption of coffee altered the gut microbiota of rats fed with a HFD, and attenuated the ratio of Firmicutes to Bacteroides [177]. The combination of epigallocatechin-3-gallate (EGCG) and caffeine in low doses led to alterations in the gut microbiota, reducing the levels of Firmicutes and increasing the levels of Bifidobacterium [178]. Similarly, the in vitro colonic metabolism of coffee and CGA led to selective changes in the growth of human fecal microbiota, thus inducing an increase in the proportion of Bifidobacterium [179]. Researchers have hypothesized that individuals who possess the relevant gut microbiota had lower levels of cholesterol in their blood [180]. Drinking coffee may alter the gastrointestinal tract and reduce the absorption of lipids and glucose [181, 182]. High-doses of caffeine were shown to reduce body weight and liver weight in mice suffering from obesity induced by a HFD, while improving hyperlipidemia and insulin resistance. It is possible that the underlying mechanism may be related to changes in the intestinal flora, along with the metabolism of bile acids, lipids, and energy [183]. The synthesis and excretion of bile acids (BA) represent major pathways for the metabolism of cholesterol and lipids. The combination of low doses of EGCG and caffeine produced synergistic anti-obesity effects that were comparable to those of high-doses of EGCG; analysis suggested that these synergistic effects may be attributed to modulation of the gut microbiota and the metabolism of BA [178].
Nuclear receptor peroxisome proliferator-activated receptor α (PPARα) and liver X receptors (LXRα and LXRβ) are major regulators of cholesterol homeostasis; the activation of these receptors leads to reduced intestinal cholesterol absorption [184]. Niemann-Pick C1-like 1 (NPC1L1) is a protein that plays a critical role in the absorption of intestinal cholesterol. Previous in vivo studies have shown that the activation of LXRα reduces the intestinal expression of NPC1L1, thus leading to reduced cholesterol absorption [184, 185]. The effective hypolipidemic effect of Coffea arabica pulp aqueous extract (CPE) on hyperlipidemia was clearly demonstrated in a rat model of hypercholesterolemia. CPE reduced the expression of intestinal NPC1L1 while activating LXRα and reducing the intestinal absorption of cholesterol. When considering the two main components of CPE, CGA, and caffeine, only the latter was able to exert an inhibitory effect on cholesterol uptake in Caco-2 cells, thus suggesting that caffeine may be involved in NPC1L1 alterations, at least in part [186].
Drinking coffee is known to change the composition of the gut microbiota [177] and can exert beneficial effects [187]; however, the specific effects of coffee have yet to be clarified [188]. The gut microbiota can directly or indirectly influence obesity via microbiome-gut-brain-liver interactions. In future, research needs to investigate the specific effects of caffeine from the perspective of microbiome-gut-brain-liver interactions.
Challenges remaining
Low doses of caffeine can induce behavioral stimulation and achieve weak benefits [69]; however, at higher doses (> 400–500 mg/day), caffeine may lead to anxiety, aversion, irritability, and malaise, although these effects appear to be subject to large individual differences [68] (Table 1). An important issue when investigating the effect of caffeine on weight loss is determining the effective and optimal dose of caffeine.
Table 1.
Dosage of caffeine in animal models or human clinical studies
| Species | Dosage |
|---|---|
| Human | 600 mg [26] |
| Human | 100 mg, 200 mg, 400 mg [30] |
| Rats | 20 mg [34] |
| Mice | 80 mg [38] |
| Human | 100 mg, 200 mg [52] |
| Mice | 1.5 mg [53] |
| Mice | 30 mg/kg [54] |
| Human | 200 mg [55] |
| Mice | 60 mg/kg [58] |
| Rats | 50 mg/kg, 100 mg/kg [62] |
| Human | 129.5 mg, 259 mg [74] |
| Human | 100 mg, 200 mg, 400 mg [111] |
| Rats | 50 mg/kg [112] |
| Rats | 0.1 g/kg [118] |
| Human | 3 mg/kg [121] |
| Human | 3 mg/kg, 6 mg/kg [122] |
| Human | 6 mg/kg [123] |
| Mice | 6 mg/kg, 12 mg/kg, 24 mg/kg [124] |
| Human | 8 mg/kg, 4 mg/kg [129] |
| Human | 2.5 mg/kg, 4 mg/kg [139] |
| Human | 5 mg/kg [140] |
| Human | 6 mg/kg [147] |
| Human | 25 mg [150] |
| Human | 100 mg [151] |
| Human | 3 mg/kg [152] |
| Mice | 10 mg/kg, 20 mg/kg [159] |
| Rats | 20 mg/kg [178] |
| Mice | 0.3 g/kg, 0.6 g/kg [189] |
| Human | 100–400mg [191] |
| Human | 25 mg, 50 mg, 75 mg [192] |
| Human | 77 mg [193] |
| Human | 5 mg/kg [195] |
| Human | 6 mg/kg [197] |
| Human | 100 mg [198] |
| Human | 100 mg, 200 mg, 300 mg, 400mg [199] |
Astilbin and CGA were previously shown to enhance the anti-obesity effect of caffeine in an HFD-induced mouse model of obesity, thereby reducing the minimum effective dose of caffeine in vivo [189, 190]. Caffeine can amplify the anorectic effects of other substances, particularly when combined with soluble fiber, catechin, or glucosyl hesperidin (G-hesperidin), resulting in a heightened synergistic effect compared to their individual actions [191, 192]. Moreover, when caffeine is mixed with tea catechin, known as tea polyphenols, the oxidizing and fat-activating effect was even stronger than for caffeine alone [31, 193].
Caffeine may play a role in weight loss via the many mechanisms described herein. However, this does not mean that individuals need to drink a lot of coffee and other caffeinated beverages each day to lose weight. The dose of caffeine ingested by experimental mice in previous research was equivalent to the dose of caffeine that would be ingested by individuals who drink 24 to 36 cups of coffee per day; this is far higher than the dose that is generally considered to be safe [58]. The chronic overconsumption of caffeine can also lead to toxicity, as characterized by an increase in certain symptoms, such as anxiety or depression. Therefore, reducing the effective dose of caffeine is important for the long-term use of caffeine in the body. The anti-obesity effect of caffeine is not effective in the long-term and large differences can occur between individuals. However, chronic or excessive caffeine consumption can result in addiction, insomnia, migraines, and other side effects. Moreover, high intake of caffeine may adversely affect the growth and development of children and could lead to cognitive deficits in the offspring of pregnant women, thereby increasing their susceptibility to diseases in adulthood. Hence, it is advisable for special populations and individuals sensitive to caffeine to restrict or reduce their caffeine intake to mitigate potential negative consequences [194]. When using caffeine as an aid for weight loss, it should be taken in moderation and combined with a healthy diet and exercise habits. The combination of caffeine therapy with calorie shifting diets may also represent an effective alternative for the loss of both weight and fat [195]. Therefore, caffeine and its derivatives, or combinations, with other products may represent an effective candidate for the development of therapeutic strategies to combat obesity.
The effects of caffeine on exercise performance indicate that acute caffeine intake may exert a greater effect on anaerobic performance in males than in females [196]; thus, there are notable gender differences in terms of caffeine metabolism [197, 198]. The consumption of caffeine caused a greater increase in plasma caffeine concentration (mg/L) in females, and was reduced in stress myocardial blood flow and myocardial blood flow reserve assessments in males; in contrast, no changes were reported in females [199]. Therefore, gender difference is an important factor to be considered in future studies related to caffeine.
Regular coffee consumption is associated with moderate weight loss [36]. Furthermore, the caffeine content of coffee is known to enhance satiety [150]; there is also evidence that caffeine-free coffee can also reduce hunger [123]. Caffeine has not been shown to reduce body fat, and its effect on fat metabolism is generally considered to be negligible [200]. The effect of caffeine on appetite remains unclear; therefore, future research should investigate the specific mechanisms involved.
Conclusions
Obesity and obesity-related diseases pose a serious threat to human health and have become a major public health problem. Globally, obesity has become a major epidemic, placing an enormous burden on the world economy and global health. Both human and animal studies have shown that caffeine may play a role in weight loss by increasing energy consumption, suppressing appetite, altering lipid metabolism, and impacting the gut microbiota (Fig. 1). Most of the biological effects of caffeine are mediated via antagonistic effects on adenosine receptors, although its ability to enhance sympathetic stimulation of the metabolic rate and thermogenesis occurs mainly by inhibiting the activity of phosphodiesterase inhibitors. Although the specific mechanism of action remains largely unknown, its low cost and widespread availability make caffeine a potentially promising candidate for the development of a new weight management strategy. Considering our ability to regulate energy balance and the dosage of caffeine, it is now necessary to conduct further animal and clinical studies to identify the optimal intake dose of caffeine to achieve weight loss, as well as to investigate the method of intake, combination treatments, the duration of treatment, and gender differences. The results of such studies will be of great significance for the development of caffeine-containing functional foods and their applications in health care, as well as for the development of complementary medicines.
Fig. 1.
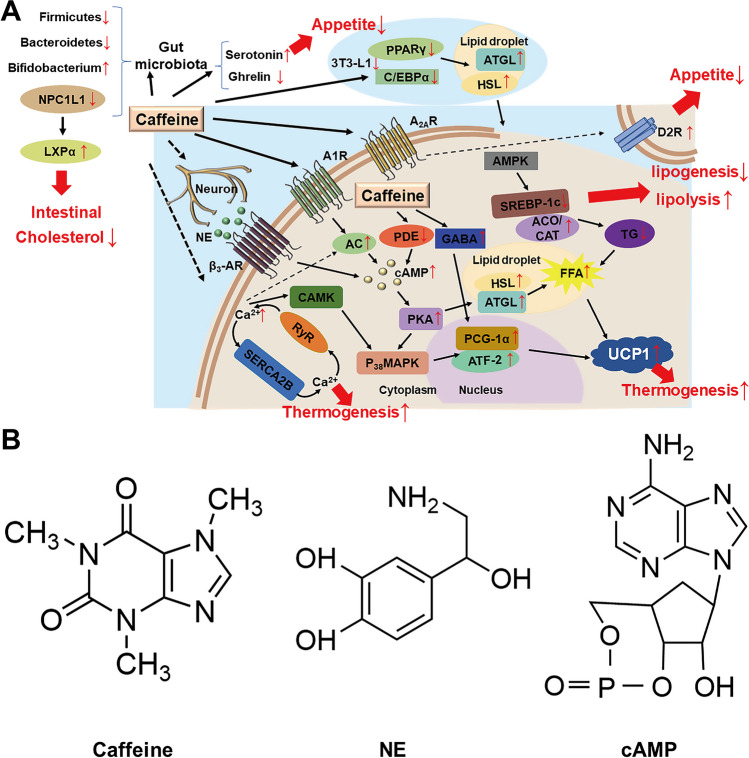
Possible mechanisms of the anti-obesity of caffeine. A This picture depicts the possible anti-obesity effects of caffeine through pathways that increase body energy expenditure, suppress appetite, alter body lipid metabolism, and influence the gut microbiota. Caffeine acts as an adenosine receptor blocker, and its antagonistic effect on A1Rs leads to activation of AC, followed by an increase in the activity of cAMP and PKA, which phosphorylate lipid droplet proteins, including HSL and periplasmic proteins, thereby activating ATGL, and ultimately hydrolysis of the triglycerides stored in lipid droplets to FFAs; as antagonism of A2AR may increase D2R activity, thereby suppressing appetite, caffeine intake increases plasma serotonin levels and decreases plasma gastric hunger hormone levels, thereby reducing food intake. Caffeine acts on the SNS, and NE, which is released from SNS endings, binds to β-ARs. The latter couples with G proteins that activate AC, increasing the cellular concentration of cAMP, which in turn activates PKA. Caffeine is an inhibitor of PDE, which prevents catabolism of cAMP; caffeine regulates the expression of adipose-metabolism-related enzymes such as HSL in 3T3-L1 cells, inhibits the expression of lipogenic transcription factor (C/EBPα and PPARγ), and inhibits 3T3-L1 cell differentiation, and reduces fat formation, thereby reducing fat accumulation through the AMPK pathway. Caffeine can increase the levels of GABA, which is known to promote the production of PGC-1α and UCP1, thereby inhibiting hepatic triglyceride levels. FFAs released from lipolysis activate existing UCP1 in mitochondria and their oxidation generates heat. As a calcium mobilizer, caffeine mobilizes intracellular Ca2+, elevated Ca2+ levels circulate and stimulate UCP1-independent thermogenesis in beige adipocytes through activation of the SERCA2B-RyR pathway, and secondly, Ca2+ is able to cause an increase in p38 MAPK and ATF-2 through its dependent kinases and phosphatases, such as CAMK increased phosphorylation, leading to PGC-1α expression and thus increased thermogenesis. Caffeine leads to alterations in the gut microbiota, decreasing the levels of Firmicutes and Bacteroidetes and increasing the levels of Bifidobacterium; caffeine may activate LXRα thereby decreasing the expression of NPC1L1 in the gut, leading to a decrease in cholesterol absorption. B The chemical structure of caffeine, NE, and cAMP. A1R and A2AR, adenosine receptors; cAMP, cyclic adenosine phosphate; AC, adenylate cyclase; PKA, protein kinase A; HSL, hormone-sensitive lipase; ATGL, adipose triglyceride lipase; FFAs, free fatty acids; D2R, dopamine D2 receptors; SNS, sympathetic nerves; NE, norepinephrine; β-ARs, β-adrenergic receptors; PDE, phosphodiesterase; CAMK, calcineurin-regulated kinase; C/EBPα, CCAAT/enhancer-binding protein; PPARγ, peroxisome proliferator-activated receptor γ; AMPK, AMP-activated protein kinase; GABA, γ-aminobutyric acid; PGC-1α, peroxisome proliferator-activated receptor γ coactivator-1α; UCP1, uncoupling protein 1; SERCA2B, sarcoplasmic/endoplasmic reticulum calcium ATPase 2B; RyR, ryanodine receptor 2; p38 MAPK, p38 mitogen-activated protein kinase; ATF-2, activating transcription factor-2; CAMK, Ca2+/calmodulin-dependent protein kinase; LXRα, liver X receptor α; NPC1L1, Niemann-Pick C1-like 1
Meng Wang
is a master’s student at Chengdu University of Traditional Chinese Medicine. She currently focuses on the study of neuro-endocrine-immune mechanisms regulated by acupuncture.

Author contribution
Meng Wang conceived and wrote the article. Wei Guo reviewed the content and edited the manuscript. Jiang-Fan Chen provided supervision on the article. All authors critically read and commented on the final manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (Grant No. 82151308 to J.-F.C.); the Research Fund for International Senior Scientists (Grant No. 82150710558 to J.-F.C); Pro-gram Project from the State Key Laboratory of Ophthalmology, Optometry and Vision Science, Wenzhou Medical University (Grant No. J01-20190101 to J.-F.C); and Key Research Project (Grant No. 2023C03079 to J.-F.C) from Zhejiang Provincial Administration of Science & Technology.
Data availability
No new data were created or analyzed in this study. All data referenced in this article are obtained from previously published studies, which are cited in the References section.
Compliance with ethical standards
Competing interests
The authors declare no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Flegal KM, Kit BK, Orpana H, Graubard BI (2013) Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA 309(1):71–82. 10.1001/jama.2012.113905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blüher M (2019) Obesity: global epidemiology and pathogenesis. Nat Reviews Endocrinol 15(5):288–298. 10.1038/s41574-019-0176-8 [DOI] [PubMed] [Google Scholar]
- 3.Nguyen DM, El-Serag HB (2010) The epidemiology of obesity. Gastroenterol Clin N Am 39(1):1–7. 10.1016/j.gtc.2009.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jia W (2015) Obesity in China: its characteristics, diagnostic criteria, and implications. Front Med 9(2):129–133. 10.1007/s11684-015-0387-x [DOI] [PubMed] [Google Scholar]
- 5.Allom V, Mullan B, Smith E, Hay P, Raman J (2018) Breaking bad habits by improving executive function in individuals with obesity. BMC Public Health 18(1). 10.1186/s12889-018-5392-y [DOI] [PMC free article] [PubMed]
- 6.Booth FW, Roberts CK, Laye MJ (2012) Lack of exercise is a major cause of chronic diseases. Compr Physiol 1143–1211 [DOI] [PMC free article] [PubMed]
- 7.Zeng Q, Li N, Pan X-F, Chen L, Pan A (2021) Clinical management and treatment of obesity in China. Lancet Diabetes Endocrinol 9(6):393–405. 10.1016/s2213-8587(21)00047-4 [DOI] [PubMed] [Google Scholar]
- 8.Aranceta Bartrina J (2013) Public health and the prevention of obesity: failure or success? Nutr Hosp 28(5):128–137. 10.3305/nh.2013.28.sup5.6928 [DOI] [PubMed] [Google Scholar]
- 9.Deen D (2004) Metabolic syndrome: time for action. Am Fam Physician Am Fam Physician 69(12):2875–2882 [PubMed] [Google Scholar]
- 10.Engin A (2017) The definition and prevalence of obesity and metabolic syndrome. Obesity and Lipotoxicity. Adv Exp Med Biol, p. 1–17 [DOI] [PubMed]
- 11.Kulkarni K, Karssiens T, Kumar V, Pandit H (2016) Obesity and osteoarthritis. Maturitas. 10.1016/j.maturitas.2016.04.006. 89:22 – 8 [DOI] [PubMed] [Google Scholar]
- 12.Bray GA (2014) Medical treatment of obesity: the past, the present and the future. Best Pract Res Clin Gastroenterol 28(4):665–684. 10.1016/j.bpg.2014.07.015 [DOI] [PubMed] [Google Scholar]
- 13.Kwon Y-J, Kwon GE, Lee HS, Choi MH, Lee J-W (2022) The effect of orlistat on sterol metabolism in obese patients. Front Endocrinol 13. 10.3389/fendo.2022.824269 [DOI] [PMC free article] [PubMed]
- 14.Nauck MA, Quast DR, Wefers J, Meier JJ (2021) GLP-1 receptor agonists in the treatment of type 2 diabetes – state-of-the-art. Mol Metabolism 46. 10.1016/j.molmet.2020.101102 [DOI] [PMC free article] [PubMed]
- 15.Mannucci E, Dicembrini I, Rotella F, Rotella CM (2008) Orlistat and sibutramine beyond weight loss. Nutrition, Metabolism and Cardiovascular diseases. 18(5):342–348. 10.1016/j.numecd.2007.03.010 [DOI] [PubMed]
- 16.Oberholzer HM, van der Schoor C, Bester MJ (2015) Sibutramine, a serotonin–norepinephrine reuptake inhibitor, causes fibrosis in rats. Environ Toxicol Pharmacol 40(1):71–76. 10.1016/j.etap.2015.05.011 [DOI] [PubMed] [Google Scholar]
- 17.Gadde KM, Allison DB, Ryan DH, Peterson CA, Troupin B, Schwiers ML et al (2011) Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet 377(9774):1341–1352. 10.1016/s0140-6736(11)60205-5 [DOI] [PubMed] [Google Scholar]
- 18.Connolly HM, Crary JL, McGoon MD, Hensrud DD, Edwards BS, Edwards WD et al (1997) Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med 337(9):581–588. 10.1056/NEJM199708283370901 [DOI] [PubMed] [Google Scholar]
- 19.Elsaid MI, Li Y, Bridges JFP, Brock G, Minacapelli CD, Rustgi VK (2022) Association of bariatric surgery with cardiovascular outcomes in adults with severe obesity and nonalcoholic fatty liver disease. JAMA Netw Open 5(10). 10.1001/jamanetworkopen.2022.35003 [DOI] [PMC free article] [PubMed]
- 20.Aminian A, Wilson R, Al-Kurd A, Tu C, Milinovich A, Kroh M et al (2022) Association of bariatric surgery with cancer risk and mortality in adults with obesity. JAMA 327(24). 10.1001/jama.2022.9009 [DOI] [PMC free article] [PubMed]
- 21.Collaborators GO, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K et al (2017) Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 377(1):13–27. 10.1056/NEJMoa1614362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collaborators GBDS (2021) Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol 20(10):795–820. 10.1016/S1474-4422(21)00252-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim MS, Kim WJ, Khera AV, Kim JY, Yon DK, Lee SW et al (2021) Association between adiposity and cardiovascular outcomes: an umbrella review and meta-analysis of observational and mendelian randomization studies. Eur Heart J 42(34):3388–3403. 10.1093/eurheartj/ehab454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Müller TD, Blüher M, Tschöp MH, DiMarchi RD (2021) Anti-obesity drug discovery: advances and challenges. Nat Rev Drug Discovery 21(3):201–223. 10.1038/s41573-021-00337-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qi X, Liu Y, Guo J, Zhu R, Chen W, Zheng X et al (2013) Dietary supplementation with purified mulberry (Morus Australis Poir) anthocyanins suppresses body weight gain in high-fat diet fed C57BL/6 mice. Food Chem 141(1):482–487. 10.1016/j.foodchem.2013.03.046 [DOI] [PubMed] [Google Scholar]
- 26.Gosselin C, Haman F (2012) Effects of green tea extracts on non-shivering thermogenesis during mild cold exposure in young men. Br J Nutr 110(2):282–288. 10.1017/s0007114512005089 [DOI] [PubMed] [Google Scholar]
- 27.Yoneshiro T, Aita S, Kawai Y, Iwanaga T, Saito M (2012) Nonpungent capsaicin analogs (capsinoids) increase energy expenditure through the activation of brown adipose tissue in humans. Am J Clin Nutr 95(4):845–850. 10.3945/ajcn.111.018606 [DOI] [PubMed] [Google Scholar]
- 28.Janssens PLHR, Hursel R, Westerterp-Plantenga MS (2016) Nutraceuticals for body-weight management: the role of green tea catechins. Physiol Behav. 10.1016/j.physbeh.2016.01.044. 162:83 – 7 [DOI] [PubMed] [Google Scholar]
- 29.Hursel R, Westerterp-Plantenga MS (2013) Catechin- and caffeine-rich teas for control of body weight in humans. Am J Clin Nutr 98(6):1682S–93S. 10.3945/ajcn.113.058396 [DOI] [PubMed] [Google Scholar]
- 30.Astrup A, Toubro S, Cannon S, Hein P, Breum L, Madsen J (1990) Caffeine: a double-blind, placebo-controlled study of its thermogenic, metabolic, and cardiovascular effects in healthy volunteers. Am J Clin Nutr 51(5):759–767. 10.1093/ajcn/51.5.759 [DOI] [PubMed] [Google Scholar]
- 31.Hursel R, Viechtbauer W, Dulloo AG, Tremblay A, Tappy L, Rumpler W et al (2011) The effects of catechin rich teas and caffeine on energy expenditure and fat oxidation: a meta-analysis. Obes Rev 12(7):e573–e81. 10.1111/j.1467-789X.2011.00862.x [DOI] [PubMed] [Google Scholar]
- 32.Riedel A, Pignitter M, Hochkogler CM, Rohm B, Walker J, Bytof G et al (2012) Caffeine dose-dependently induces thermogenesis but restores ATP in HepG2 cells in culture. Food Funct 3(9). 10.1039/c2fo30053b [DOI] [PubMed]
- 33.Grgic J, Grgic I, Pickering C, Schoenfeld BJ, Bishop DJ, Pedisic Z (2020) Wake up and smell the coffee: caffeine supplementation and exercise performance—an umbrella review of 21 published meta-analyses. Br J Sports Med 54(11):681–688. 10.1136/bjsports-2018-100278 [DOI] [PubMed] [Google Scholar]
- 34.Liu C-W, Tsai H-C, Huang C-C, Tsai C-Y, Su Y-B, Lin M-W et al (2018) Effects and mechanisms of caffeine to improve immunological and metabolic abnormalities in diet-induced obese rats. Am J Physiology-Endocrinology Metabolism 314(5):E433–E47. 10.1152/ajpendo.00094.2017 [DOI] [PubMed] [Google Scholar]
- 35.lcken D, Feller S, Engeli S, Mayr A, Müller A, Hilbert A et al (2015) Caffeine intake is related to successful weight loss maintenance. Eur J Clin Nutr 70(4):532–534. 10.1038/ejcn.2015.183 [DOI] [PubMed] [Google Scholar]
- 36.Thom E (2007) The effect of chlorogenic acid enriched coffee on glucose absorption in healthy volunteers and its effect on body mass when used long-term in overweight and obese people. J Int Med Res 35(6):900–908. 10.1177/147323000703500620 [DOI] [PubMed] [Google Scholar]
- 37.Harpaz E, Tamir S, Weinstein A, Weinstein Y (2017) The effect of caffeine on energy balance. J Basic Clin Physiol Pharmacol 28(1):1–10. 10.1515/jbcpp-2016-0090 [DOI] [PubMed] [Google Scholar]
- 38.Lee L-S, Choi JH, Sung MJ, hur J-Y, Hur HJ, Park J-D et al (2015) Green tea changes serum and liver metabolomic profiles in mice with high-fat diet-induced obesity. Mol Nutr Food Res 59(4):784–794. 10.1002/mnfr.201400470 [DOI] [PubMed] [Google Scholar]
- 39.Dangol M, Kim S, Li CG, Fakhraei Lahiji S, Jang M, Ma Y et al (2017) Anti-obesity effect of a novel caffeine-loaded dissolving microneedle patch in high-fat diet-induced obese C57BL/6J mice. J Controlled Release 265:41–47. 10.1016/j.jconrel.2017.03.400 [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Kujawinski DM, Federherr E, Schmidt TC, Jochmann MA (2012) Caffeine in your drink: natural or synthetic? Anal Chem 84(6):2805–2810. 10.1021/ac203197d [DOI] [PubMed] [Google Scholar]
- 41.McGuire S (2014) Institute of Medicine. 2014. Caffeine in food and dietary supplements: examining safety—workshop summary. Washington, DC: The National Academies Press, 2014. Advances in Nutrition. 5(5):585-6. 10.3945/an.114.006692 [DOI] [PMC free article] [PubMed]
- 42.Simon J, Fung K, Raisi-Estabragh Z, Aung N, Khanji MY, Kolossváry M et al (2022) Light to moderate coffee consumption is associated with lower risk of death: a UK Biobank study. Eur J Prev Cardiol 29(6):982–991. 10.1093/eurjpc/zwac008/6512055 [DOI] [PubMed] [Google Scholar]
- 43.Chieng D, Kistler PM (2022) Coffee and tea on cardiovascular disease (CVD) prevention. Trends Cardiovasc Med 32(7):399–405. 10.1016/j.tcm.2021.08.004 [DOI] [PubMed] [Google Scholar]
- 44.Ding M, Bhupathiraju SN, Satija A, van Dam RM, Hu FB (2014) Long-term coffee consumption and risk of cardiovascular disease: a systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation 129(6):643–659. 10.1161/circulationaha.113.005925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pan M-H, Tung Y-C, Yang G, Li S, Ho C-T (2016) Molecular mechanisms of the anti-obesity effect of bioactive compounds in tea and coffee. Food Funct 7(11):4481–4491. 10.1039/c6fo01168c [DOI] [PubMed] [Google Scholar]
- 46.Samoggia A, Rezzaghi T (2021) The consumption of caffeine-containing products to enhance sports performance: an application of an extended model of the theory of planned behavior. Nutrients 13(2). 10.3390/nu13020344 [DOI] [PMC free article] [PubMed]
- 47.Heckman MA, Weil J, De Gonzalez E (2010) Caffeine (1, 3, 7-trimethylxanthine) in foods: a comprehensive review on consumption, functionality, safety, and regulatory matters. J Food Sci 75(3):R77–R87. 10.1111/j.1750-3841.2010.01561.x [DOI] [PubMed] [Google Scholar]
- 48.Glade MJ (2010) Caffeine—not just a stimulant. Nutrition 26(10):932–938. 10.1016/j.nut.2010.08.004 [DOI] [PubMed] [Google Scholar]
- 49.Herman A, Herman AP (2013) Caffeine’s mechanisms of action and its cosmetic use. Skin Pharmacol Physiol 26(1):8–14. 10.1159/000343174 [DOI] [PubMed] [Google Scholar]
- 50.Grant SS, Magruder KP, Friedman BH (2018) Controlling for caffeine in cardiovascular research: a critical review. Int J Psychophysiol 133:193–201. 10.1016/j.ijpsycho.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 51.Kolahdouzan M, Hamadeh MJ (2017) The neuroprotective effects of caffeine in neurodegenerative diseases. CNS Neurosci Ther 23(4):272–290. 10.1111/cns.12684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Postuma RB, Lang AE, Munhoz RP, Charland K, Pelletier A, Moscovich M et al (2012) Caffeine for treatment of Parkinson disease: a randomized controlled trial. Neurology 79(7):651–658. 10.1212/WNL.0b013e318263570d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arendash GW, Schleif W, Rezai-Zadeh K, Jackson EK, Zacharia LC, Cracchiolo JR et al (2006) Caffeine protects Alzheimer’s mice against cognitive impairment and reduces brain beta-amyloid production. Neuroscience 142(4):941–952. 10.1016/j.neuroscience.2006.07.021 [DOI] [PubMed] [Google Scholar]
- 54.Dall’Igna OP, Fett P, Gomes MW, Souza DO, Cunha RA, Lara DR (2007) Caffeine and adenosine A(2a) receptor antagonists prevent beta-amyloid (25–35)-induced cognitive deficits in mice. Exp Neurol 203(1):241–245. 10.1016/j.expneurol.2006.08.008 [DOI] [PubMed] [Google Scholar]
- 55.MacKenzie T, Comi R, Sluss P, Keisari R, Manwar S, Kim J et al (2007) Metabolic and hormonal effects of caffeine: randomized, double-blind, placebo-controlled crossover trial. Metabolism 56(12):1694–1698. 10.1016/j.metabol.2007.07.013 [DOI] [PubMed] [Google Scholar]
- 56.Ferraro PM, Taylor EN, Gambaro G, Curhan GC (2013) Soda and other beverages and the risk of kidney stones. Clin J Am Soc Nephrol 8(8):1389–1395. 10.2215/CJN.11661112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Furman D, Chang J, Lartigue L, Bolen CR, Haddad F, Gaudilliere B et al (2017) Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nat Med 23(2):174–184. 10.1038/nm.4267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu L, Meng J, Shen Q, Zhang Y, Pan S, Chen Z et al (2017) Caffeine inhibits hypothalamic A1R to excite oxytocin neuron and ameliorate dietary obesity in mice. Nat Commun 8(1). 10.1038/ncomms15904 [DOI] [PMC free article] [PubMed]
- 59.Lopes JP, Pliassova A, Cunha RA (2019) The physiological effects of caffeine on synaptic transmission and plasticity in the mouse hippocampus selectively depend on adenosine A(1) and A(2A) receptors. Biochem Pharmacol 166:313–321. 10.1016/j.bcp.2019.06.008 [DOI] [PubMed] [Google Scholar]
- 60.Moustafa F, Feldman SR (2014) A review of phosphodiesterase-inhibition and the potential role for phosphodiesterase 4-inhibitors in clinical dermatology. Dermatol Online J 20(5). 10.5070/d3205022608 [PubMed]
- 61.Lebeau PF, Byun JH, Platko K, Saliba P, Sguazzin M, MacDonald ME et al (2022) Caffeine blocks SREBP2-induced hepatic PCSK9 expression to enhance LDLR-mediated cholesterol clearance. Nat Commun 13(1). 10.1038/s41467-022-28240-9 [DOI] [PMC free article] [PubMed]
- 62.Owolabi J, Olatunji S, Olanrewaju A (2017) Caffeine and cannabis effects on vital neurotransmitters and enzymes in the brain tissue of juvenile experimental rats. Ann Neurosci 24(2):65–73. 10.1159/000475895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Larsson SC, Woolf B, Gill D (2023) Appraisal of the causal effect of plasma caffeine on adiposity, type 2 diabetes, and cardiovascular disease: two sample mendelian randomisation study. BMJ Med 2(1). 10.1136/bmjmed-2022-000335 [DOI] [PMC free article] [PubMed]
- 64.Ribeiro JA, Sebastião AM (2010) Caffeine and adenosine. J Alzheimers Dis 20(s1). 10.3233/jad-2010-1379 [DOI] [PubMed]
- 65.Chen J-F, Eltzschig HK, Fredholm BB (2013) Adenosine receptors as drug targets — what are the challenges? Nat Rev Drug Discovery 12(4):265–286. 10.1038/nrd3955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fredholm BB, IJzerman AP, Jacobson KA, Klotz K-N, Linden J (2001) International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev 53(4):527–552 [PMC free article] [PubMed] [Google Scholar]
- 67.Fredholm BB, IJzerman AP, Jacobson KA, Linden J, Müller CE (2011) International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors—an update. Pharmacol Rev 63(1):1–34. 10.1124/pr.110.003285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen J-F, Yu L, Shen H-Y, He J-C, Wang X, Zheng R (2010) What knock-out animals tell us about the effects of caffeine. J Alzheimers Dis 20(s1):S17–S24. 10.3233/jad-2010-1403 [DOI] [PubMed] [Google Scholar]
- 69.Fredholm BB, Yang J, Wang Y (2017) Low, but not high, dose caffeine is a readily available probe for adenosine actions. Mol Aspects Med 55:20–25. 10.1016/j.mam.2016.11.011 [DOI] [PubMed] [Google Scholar]
- 70.Johansson B, Dunwiddie LHTV, Masino SA, Poelchen W, Giménez-Llort L, Escorihuela RM, Fernández-Teruel A, Wiesenfeld-Hallin Z, Xu XJ, Hårdemark A, Betsholtz C, Herlenius E, Fredholm BB (2001) Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc Natl Acad Sci U S A 98(16):9407–9412. 10.1073/pnas.161292398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oishi Y, Huang Z-L, Fredholm BB, Urade Y, Hayaishi O (2008) Adenosine in the tuberomammillary nucleus inhibits the histaminergic system via A1 receptors and promotes non-rapid eye movement sleep. Proc Natl Acad Sci U S A 105(50):19992–19997. 10.1073/pnas.0810926105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Draper-Joyce CJ, Bhola R, Wang J, Bhattarai A, Nguyen ATN, Cowie-Kent I et al (2021) Positive allosteric mechanisms of adenosine A1 receptor-mediated analgesia. Nature 597(7877):571–576. 10.1038/s41586-021-03897-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Allard B, Jacoberger-Foissac C, Cousineau I, Bareche Y, Buisseret L, Chrobak P et al (2023) Adenosine A2A receptor is a tumor suppressor of NASH-associated hepatocellular carcinoma. Cell Rep Med 4(9). 10.1016/j.xcrm.2023.101188 [DOI] [PMC free article] [PubMed]
- 74.Ishibashi K, Miura Y, Wagatsuma K, Toyohara J, Ishiwata K, Ishii K (2022) Adenosine A2A receptor occupancy by caffeine after coffee intake in Parkinson’s disease. Mov Disord 37(4):853–857. 10.1002/mds.28897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Canas PM, Porciuncula LO, Cunha GM, Silva CG, Machado NJ, Oliveira JM et al (2009) Adenosine A2A receptor blockade prevents synaptotoxicity and memory dysfunction caused by beta-amyloid peptides via p38 mitogen-activated protein kinase pathway. J Neurosci 29(47):14741–14751. 10.1523/JNEUROSCI.3728-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang Z-L, Zhang Z, Qu W-M (2014) Roles of adenosine and its receptors in sleep–wake regulation. Adenosine receptors in neurology and psychiatry. Int Rev Neurobiol 349–371 [DOI] [PubMed]
- 77.El Yacoubi MLC, Parmentier M, Bertorelli R, Ongini E, Costentin J, Vaugeois JM (2001) Adenosine A2A receptor antagonists are potential antidepressants: evidence based on pharmacology and A2A receptor knockout mice. Br J Pharmacol 134(1):68–77. 10.1038/sj.bjp.0704240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaster MP, Machado NJ, Silva HB, Nunes A, Ardais AP, Santana M et al (2015) Caffeine acts through neuronal adenosine A2A receptors to prevent mood and memory dysfunction triggered by chronic stress. Proc Nat Acad Sci 112(25):7833–8. 10.1073/pnas.1423088112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fredholm BB, Chen J-F, Masino SA, Vaugeois J-M (2005) Actions of adenosine at its receptors in the CNS: insights from knockouts and drugs. Annu Rev Pharmacol Toxicol 45(1):385–412. 10.1146/annurev.pharmtox.45.120403.095731 [DOI] [PubMed] [Google Scholar]
- 80.Mizuno Y, Kondo T (2013) Adenosine A2A receptor antagonist istradefylline reduces daily OFF time in Parkinson’s disease. Mov Disord 28(8):1138–1141. 10.1002/mds.25418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen J-F, Cunha RA (2020) The belated US FDA approval of the adenosine A2A receptor antagonist istradefylline for treatment of Parkinson’s disease. Purinergic Signalling 16(2):167–174. 10.1007/s11302-020-09694-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hursel R, Viechtbauer W, Dulloo A, Tremblay A, Tappy L, Rumpler W et al (2011) The effects of catechin rich teas and caffeine on energy expenditure and fat oxidation: a meta-analysis. Obes Rev 12(7). 10.1111/j.1467-789X.2011.00862.x [DOI] [PubMed]
- 83.Gnad T, Scheibler S, von Kügelgen I, Scheele C, Kilić A, Glöde A et al (2014) Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature 516(7531):395–399. 10.1038/nature13816 [DOI] [PubMed] [Google Scholar]
- 84.Cai Y, Chen X, Yi B, Li J, Wen Z (2022) Pathophysiology roles for adenosine 2A receptor in obesity and related diseases. Obes Rev 23(10). 10.1111/obr.13490 [DOI] [PubMed]
- 85.Velickovic K, Wayne D, Leija HAL, Bloor I, Morris DE, Law J et al (2019) Caffeine exposure induces browning features in adipose tissue in vitro and in vivo. Sci Rep 9(1). 10.1038/s41598-019-45540-1 [DOI] [PMC free article] [PubMed]
- 86.Van Schaik L, Kettle C, Green R, Irving HR, Rathner JA (2021) Effects of caffeine on brown adipose tissue thermogenesis and metabolic homeostasis: a review. Front NeuroSci 15. 10.3389/fnins.2021.621356 [DOI] [PMC free article] [PubMed]
- 87.Cerri M, Morrison S (2005) Activation of lateral hypothalamic neurons stimulates brown adipose tissue thermogenesis. Neuroscience 135(2):627–638. 10.1016/j.neuroscience.2005.06.039 [DOI] [PubMed] [Google Scholar]
- 88.Morrison SF, Nakamura K (2011) Central neural pathways for thermoregulation. Front Biosci (Landmark Ed) 16(1):74–104. 10.2741/3677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dulloo A, Seydoux J, Girardier L (1991) Peripheral mechanisms of thermogenesis induced by ephedrine and caffeine in brown adipose tissue. Int J Obes 15(5):317–326 [PubMed] [Google Scholar]
- 90.Sacramento JF, Martins FO, Rodrigues T, Matafome P, Ribeiro MJ, Olea E et al (2020) A (2) adenosine receptors mediate whole-body insulin sensitivity in a prediabetes animal model: primary effects on skeletal muscle. Front Endocrinol (Lausanne) 11:262. 10.3389/fendo.2020.00262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cannon B, Nedergaard J (2004) Brown adipose tissue: function and physiological significance. Physiol Rev 84(1):277–359. 10.1152/physrev.00015.2003 [DOI] [PubMed] [Google Scholar]
- 92.Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang A-H et al (2012) Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150(2):366–376. 10.1016/j.cell.2012.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fedorenko A, Lishko PV, Kirichok Y (2012) Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 151(2):400–413. 10.1016/j.cell.2012.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zeng X, Ye M, Resch JM, Jedrychowski MP, Hu B, Lowell BB et al (2019) Innervation of thermogenic adipose tissue via a calsyntenin 3β–S100b axis. Nature 569(7755):229–235. 10.1038/s41586-019-1156-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cero C, Lea HJ, Zhu KY, Shamsi F, Tseng Y-H, Cypess AM (2021) β3-Adrenergic receptors regulate human brown/beige adipocyte lipolysis and thermogenesis. JCI Insight 6(11). 10.1172/jci.insight.139160 [DOI] [PMC free article] [PubMed]
- 96.Mund RA, Frishman WH (2013) Brown adipose tissue thermogenesis: β3-adrenoreceptors as a potential target for the treatment of obesity in humans. Cardiol Rev 21(6):265–269. 10.1097/CRD.0b013e31829cabff [DOI] [PubMed] [Google Scholar]
- 97.Bartness T, Vaughan C, Song C (2010) Sympathetic and sensory innervation of brown adipose tissue. Int J Obes 34(S1):S36–S42. 10.1038/ijo.2010.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oelkrug R, Polymeropoulos ET, Jastroch M (2015) Brown adipose tissue: physiological function and evolutionary significance. J Comp Physiol B 185(6):587–606. 10.1007/s00360-015-0907-7 [DOI] [PubMed] [Google Scholar]
- 99.Collins S (2012) β-Adrenoceptor signaling networks in adipocytes for recruiting stored fat and energy expenditure. Front Endocrinol 2. 10.3389/fendo.2011.00102 [DOI] [PMC free article] [PubMed]
- 100.Cao W, Daniel KW, Robidoux J, Puigserver P, Medvedev AV, Bai X et al (2004) p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol Cell Biol 24(7):3057–3067. 10.1128/mcb.24.7.3057-3067.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM (1998) A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92(6):829–839. 10.1016/s0092-8674(00)81410-5 [DOI] [PubMed] [Google Scholar]
- 102.Schnuck JK, Gould LM, Parry HA, Johnson MA, Gannon NP, Sunderland KL et al (2017) Metabolic effects of physiological levels of caffeine in myotubes. J Physiol Biochem 74(1):35–45. 10.1007/s13105-017-0601-1 [DOI] [PubMed] [Google Scholar]
- 103.Vaughan RA, Garcia-Smith R, Bisoffi M, Trujillo KA, Conn CA (2012) Effects of caffeine on metabolism and mitochondria biogenesis in rhabdomyosarcoma cells compared with 2,4-dinitrophenol. Nutrition and Metabolic Insights 5. 10.4137/nmi.S10233 [DOI] [PMC free article] [PubMed]
- 104.Levine A, Morley J (1983) Effect of intraventricular adenosine on food intake in rats. Pharmacol Biochem Behav 19(1):23–26. 10.1016/0091-3057(83)90305-2 [DOI] [PubMed] [Google Scholar]
- 105.Johnson PM, Kenny PJ (2010) Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci 13(5):635–641. 10.1038/nn.2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Di Micioni MV, Pucci M, Giusepponi ME, Romano A, Lambertucci C, Volpini R et al (2019) Regulation of adenosine A(2A) receptor gene expression in a model of binge eating in the amygdaloid complex of female rats. J Psychopharmacol 33(12):1550–1561. 10.1177/0269881119845798 [DOI] [PubMed] [Google Scholar]
- 107.Di Micioni MV, Cifani C, Lambertucci C, Volpini R, Cristalli G, Massi M (2012) A2A adenosine receptor agonists reduce both high-palatability and low-palatability food intake in female rats. Behav Pharmacol 23(5–6):567–574. 10.1097/FBP.0b013e3283566a60 [DOI] [PubMed] [Google Scholar]
- 108.Bakuradze T, Montoya Parra GA, Riedel A, Somoza V, Lang R, Dieminger N et al (2014) Four-week coffee consumption affects energy intake, satiety regulation, body fat, and protects DNA integrity. Food Res Int 63:420–427. 10.1016/j.foodres.2014.05.032 [Google Scholar]
- 109.van Galen KA, ter Horst KW, Serlie MJ (2021) Serotonin, food intake, and obesity. Obes Rev 22(7). 10.1111/obr.13210 [DOI] [PMC free article] [PubMed]
- 110.Wren A, Small C, Ward H, Murphy K, Dakin C, Taheri S et al (2000) The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 141(11):4325–4328. 10.1210/endo.141.11.7873 [DOI] [PubMed] [Google Scholar]
- 111.Astrup A, Toubro S, Cannon S, Hein P, Madsen J (1991) Thermogenic synergism between ephedrine and caffeine in healthy volunteers: a double-blind, placebo-controlled study. Metabolism 40(3):323–329. 10.1016/0026-0495(91)90117-f [DOI] [PubMed] [Google Scholar]
- 112.Racotta IS, Leblanc J, Richard D (1994) The effect of caffeine on food intake in rats: involvement of corticotropin-releasing factor and the sympatho-adrenal system. Pharmacol Biochem Behav 48(4):887–892. 10.1016/0091-3057(94)90196-1 [DOI] [PubMed] [Google Scholar]
- 113.Valadas JS, Batalha VL, Ferreira DG, Gomes R, Coelho JE, Sebastiao AM et al (2012) Neuroprotection afforded by adenosine A2A receptor blockade is modulated by corticotrophin-releasing factor (CRF) in glutamate injured cortical neurons. J Neurochem 123(6):1030–1040. 10.1111/jnc.12050 [DOI] [PubMed] [Google Scholar]
- 114.Yang L, Qi Y, Yang Y (2015) Astrocytes control food intake by inhibiting AGRP neuron activity via adenosine A1 receptors. Cell Rep 11(5):798–807. 10.1016/j.celrep.2015.04.002 [DOI] [PubMed] [Google Scholar]
- 115.Kratz M, von Eckardstein A, Fobker M, Buyken A, Posny N, Schulte H et al (2002) The impact of dietary fat composition on serum leptin concentrations in healthy nonobese men and women. J Clin Endocrinol Metab 87(11):5008–5014. 10.1210/jc.2002-020496 [DOI] [PubMed] [Google Scholar]
- 116.JM. F, (2003) A war on obesity, not the obese. Science. 299(5608):856–858. 10.1126/science.1079856 [DOI] [PubMed] [Google Scholar]
- 117.Hosoi T, Toyoda K, Nakatsu K, Ozawa K (2014) Caffeine attenuated ER stress-induced leptin resistance in neurons. Neurosci Lett. 10.1016/j.neulet.2014.03.053. 569:23 – 6 [DOI] [PubMed] [Google Scholar]
- 118.Hongu N, DS. S (2000) Caffeine, carnitine and choline supplementation of rats decreases body fat and serum leptin concentration as does exercise. J Nutr 130(2):152–157. 10.1093/jn/130.2.152 [DOI] [PubMed] [Google Scholar]
- 119.Yamashita K, Yatsuya H, Muramatsu T, Toyoshima H, Murohara T, Tamakoshi K (2012) Association of coffee consumption with serum adiponectin, leptin, inflammation and metabolic markers in Japanese workers: a cross-sectional study. Nutr Diabetes 2(4):e33. 10.1038/nutd.2012.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lagiou PSL, Mantzoros CS, Trichopoulos D, Hsieh CC, Trichopoulou A (1999) Hormonal, lifestyle, and dietary factors in relation to leptin among elderly men. Ann Nutr Metab 43(1):23–29. 10.1159/000012763 [DOI] [PubMed] [Google Scholar]
- 121.Gavrieli A, Yannakoulia M, Fragopoulou E, Margaritopoulos D, Chamberland JP, Kaisari P et al (2011) Caffeinated coffee does not acutely affect energy intake, appetite, or inflammation but prevents serum cortisol concentrations from falling in healthy men1–4. J Nutr 141(4):703–707. 10.3945/jn.110.137323 [DOI] [PubMed] [Google Scholar]
- 122.Gavrieli A, Karfopoulou E, Kardatou E, Spyreli E, Fragopoulou E, Mantzoros C et al (2013) Effect of different amounts of coffee on dietary intake and appetite of normal-weight and overweight/obese individuals. Obesity 21(6):1127–1132. 10.1002/oby.20190 [DOI] [PubMed] [Google Scholar]
- 123.Greenberg JA, Geliebter A (2012) Coffee, hunger, and peptide YY. J Am College Nutrit 31(3):160–6. 10.1080/07315724.2012.10720023 [DOI] [PubMed] [Google Scholar]
- 124.Sweeney P, Levack R, Watters J, Xu Z, Yang Y (2016) Caffeine increases food intake while reducing anxiety-related behaviors. Appetite 101:171–177. 10.1016/j.appet.2016.03.013 [DOI] [PubMed] [Google Scholar]
- 125.Kim HJ, Yoon BK, Park H, Seok JW, Choi H, Yu JH et al (2016) Caffeine inhibits adipogenesis through modulation of mitotic clonal expansion and the AKT/GSK3 pathway in 3T3-L1 adipocytes. BMB Rep 49(2):111–115. 10.5483/BMBRep.2016.49.2.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Su S-H, Shyu H-W, Yeh Y-T, Chen K-M, Yeh H, Su S-J (2013) Caffeine inhibits adipogenic differentiation of primary adipose-derived stem cells and bone marrow stromal cells. Toxicol in Vitro 27(6):1830–1837. 10.1016/j.tiv.2013.05.011 [DOI] [PubMed] [Google Scholar]
- 127.Kim HY, Lee MY, Park HM, Park YK, Shon JC, Liu K-H et al (2015) Urine and serum metabolite profiling of rats fed a high-fat diet and the anti-obesity effects of caffeine consumption. Molecules 20(2):3107–3128. 10.3390/molecules20023107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nakabayashi H, Hashimoto T, Ashida H, Nishiumi S, Kanazawa K (2008) Inhibitory effects of caffeine and its metabolites on intracellular lipid accumulation in murine 3T3-L1 adipocytes. BioFactors 34(4):293–302. 10.3233/BIO-2009-1083 [DOI] [PubMed] [Google Scholar]
- 129.Acheson K, Zahorska-Markiewicz B, Pittet P, Anantharaman K, Jéquier E (1980) Caffeine and coffee: their influence on metabolic rate and substrate utilization in normal weight and obese individuals. Am J Clin Nutr 33(5):989–997. 10.1093/ajcn/33.5.989 [DOI] [PubMed] [Google Scholar]
- 130.Dulloo AG (2011) The search for compounds that stimulate thermogenesis in obesity management: from pharmaceuticals to functional food ingredients. Obes Rev 12(10):866–883. 10.1111/j.1467-789X.2011.00909.x [DOI] [PubMed] [Google Scholar]
- 131.Kim EY, Kim WK, Oh K-J, Han BS, Lee SC, Bae K-H (2015) Recent advances in proteomic studies of adipose tissues and adipocytes. Int J Mol Sci 16(3):4581–4599. 10.3390/ijms16034581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhu X, Yang L, Xu F, Lin L, Zheng G (2017) Combination therapy with catechins and caffeine inhibits fat accumulation in 3T3-L1 cells. Experimental Therapeutic Med 13(2):688–694. 10.3892/etm.2016.3975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kong L, Xu M, Qiu Y, Liao M, Zhang Q, Yang L et al (2021) Chlorogenic acid and caffeine combination attenuates adipogenesis by regulating fat metabolism and inhibiting adipocyte differentiation in 3T3-L1 cells. J Food Biochem 45(7). 10.1111/jfbc.13795 [DOI] [PubMed]
- 134.Daval M, Diot-Dupuy F, Bazin R, Hainault I, Viollet Bt, Vaulont S et al (2005) Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. J Biol Chem 280(26):25250–25257. 10.1074/jbc.M414222200 [DOI] [PubMed] [Google Scholar]
- 135.Deng X, Dong Q, Bridges D, Raghow R, Park EA, Elam MB (2015) Docosahexaenoic acid inhibits proteolytic processing of sterol regulatory element-binding protein-1c (SREBP-1c) via activation of AMP-activated kinase. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of lipids. 1851(12):1521–1529. 10.1016/j.bbalip.2015.08.007 [DOI] [PubMed]
- 136.Quan HY, Kim DY, Chung SH (2013) Caffeine attenuates lipid accumulation via activation of AMP-activated protein kinase signaling pathway in HepG2 cells. BMB Rep 46(4):207–212. 10.5483/BMBRep.2013.46.4.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zheng G, Qiu Y, Zhang Q-F, Li D (2014) Chlorogenic acid and caffeine in combination inhibit fat accumulation by regulating hepatic lipid metabolism-related enzymes in mice. Br J Nutr 112(6):1034–1040. 10.1017/s0007114514001652 [DOI] [PubMed] [Google Scholar]
- 138.Zheng X, Dai W, Chen X, Wang K, Zhang W, Liu L et al (2015) Caffeine reduces hepatic lipid accumulation through regulation of lipogenesis and ER stress in zebrafish larvae. J Biomed Sci 22(1). 10.1186/s12929-015-0206-3 [DOI] [PMC free article] [PubMed]
- 139.Vandenberghe CS-PV, Courchesne-Loyer A, Hennebelle M, Castellano CA, Cunnane SC (2017) Caffeine intake increases plasma ketones: an acute metabolic study in humans. Can J Physiol Pharmacol 95(4):455–458. 10.1139/cjpp-2016-0338 [DOI] [PubMed] [Google Scholar]
- 140.Mougios VRS, Petridou A, Nikolaidis MG (2003) Duration of coffee- and exercise-induced changes in the fatty acid profile of human serum. J Appl Physiol 94(2):476–484. 10.1152/japplphysiol.00624.2002 [DOI] [PubMed] [Google Scholar]
- 141.DeOliveira CC, Paiva Caria CRe, Ferreira Gotardo EM, Ribeiro ML, Gambero A (2017) Role of A1 and A2A adenosine receptor agonists in adipose tissue inflammation induced by obesity in mice. Eur J Pharmacol 799:154–159. 10.1016/j.ejphar.2017.02.017 [DOI] [PubMed] [Google Scholar]
- 142.Stohs SJ, Badmaev V (2016) A review of natural stimulant and non-stimulant thermogenic agents. Phytother Res 30(5):732–740. 10.1002/ptr.5583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Preti D, Baraldi PG, Moorman AR, Borea PA, Varani K (2015) History and perspectives of A2AAdenosine receptor antagonists as potential therapeutic agents. Med Res Rev 35(4):790–848. 10.1002/med.21344 [DOI] [PubMed] [Google Scholar]
- 144.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O et al (2008) Dynamics of fat cell turnover in humans. Nature 453(7196):783–787. 10.1038/nature06902 [DOI] [PubMed] [Google Scholar]
- 145.Ding L, Zhang F, Zhao M-X, Ren X-S, Chen Q, Li Y-H et al (2016) Reduced lipolysis response to adipose afferent reflex involved in impaired activation of adrenoceptor-cAMP-PKA-hormone sensitive lipase pathway in obesity. Sci Rep 6(1). 10.1038/srep34374 [DOI] [PMC free article] [PubMed]
- 146.Greenberg JA, Boozer CN, Geliebter A (2006) Coffee, diabetes, and weight control. Am J Clin Nutr 84(4):682–693. 10.1093/ajcn/84.4.682 [DOI] [PubMed] [Google Scholar]
- 147.Tauler P, Martínez S, Moreno C, Monjo M, Martínez P, Aguiló A (2013) Effects of caffeine on the inflammatory response induced by a 15-km run competition. Med Sci Sports Exerc 45(7):1269–1276. 10.1249/MSS.0b013e3182857c8a [DOI] [PubMed] [Google Scholar]
- 148.Bartness TJ, Liu Y, Shrestha YB, Ryu V (2014) Neural innervation of white adipose tissue and the control of lipolysis. Front Neuroendocr 35(4):473–493. 10.1016/j.yfrne.2014.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kolditz C-I, Langin D (2010) Adipose tissue lipolysis. Current opinion in Clinical Nutrition and Metabolic Care. 13(4):377–381. 10.1097/MCO.0b013e32833bed6a [DOI] [PubMed] [Google Scholar]
- 150.Margriet S, Westerterp-Plantenga MPGML, Eva MR, Kovacs (2005) Body weight loss and weight maintenance in relation to habitual caffeine intake and green tea supplementation. Obes Res 13(7):1195–1204. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 151.Zhang S, Takano J, Murayama N, Tominaga M, Abe T, Park I et al (2020) Subacute ingestion of caffeine and oolong tea increases fat oxidation without affecting energy expenditure and sleep architecture: a randomized, placebo-controlled, double-blinded cross-over trial. Nutrients 12(12). 10.3390/nu12123671 [DOI] [PMC free article] [PubMed]
- 152.Ramírez-Maldonado M, Jurado-Fasoli L, del Coso J, Ruiz R, Amaro-Gahete J FJ (2021) Caffeine increases maximal fat oxidation during a graded exercise test: is there a diurnal variation? J Int Soc Sports Nutr 18(1). 10.1186/s12970-020-00400-6 [DOI] [PMC free article] [PubMed]
- 153.Collado-Mateo D, Lavín-Pérez AM, Merellano-Navarro E, Coso JD (2020) Effect of acute caffeine intake on the fat oxidation rate during exercise: a systematic review and meta-analysis. Nutrients 12(12). 10.3390/nu12123603 [DOI] [PMC free article] [PubMed]
- 154.Boswell-Smith V, Spina D, Page CP (2006) Phosphodiesterase inhibitors. Br J Pharmacol 147(S1):S252–S7. 10.1038/sj.bjp.0706495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Escalante G, Bryan P, Rodriguez J (2018) Effects of a topical lotion containing aminophylline, caffeine, yohimbe, l-carnitine, and gotu kola on thigh circumference, skinfold thickness, and fat mass in sedentary females. J Cosmet Dermatol 18(4):1037–1043. 10.1111/jocd.12801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Montoya GA, Bakuradze T, Eirich M, Erk T, Baum M, Habermeyer M et al (2014) Modulation of 3′,5′-cyclic AMP homeostasis in human platelets by coffee and individual coffee constituents. Br J Nutr 112(9):1427–1437. 10.1017/s0007114514002232 [DOI] [PubMed] [Google Scholar]
- 157.Horrigan LA, Kelly JP, Connor TJ (2006) Immunomodulatory effects of caffeine: friend or foe? Pharmacol Ther 111(3):877–892. 10.1016/j.pharmthera.2006.02.002 [DOI] [PubMed] [Google Scholar]
- 158.Martin PT, Koshland DEJ (1992) Regulation of neurosecretory habituation in PC12 cells: parallel pathways used by cAMP and calcium. Proc Natl Acad Sci USA 89(21):10257–10261. 10.1073/pnas.89.21.10257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang SJ, Li YF, Wang GE, Tan RR, Tsoi B, Mao GW et al (2015) Caffeine ameliorates high energy diet-induced hepatic steatosis: sirtuin 3 acts as a bridge in the lipid metabolism pathway. Food Funct 6(8):2578–2587. 10.1039/c5fo00247h [DOI] [PubMed] [Google Scholar]
- 160.Koot P, Deurenberg P (1995) Comparison of changes in energy expenditure and body temperatures after caffeine consumption. Ann Nutr Metab 39(3):135–142. 10.1159/000177854 [DOI] [PubMed] [Google Scholar]
- 161.Kajimura S, Spiegelman BM, Seale P (2015) Brown and beige fat: physiological roles beyond heat generation. Cell Metabol 22(4):546–559. 10.1016/j.cmet.2015.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ikeda K, Kang Q, Yoneshiro T, Camporez JP, Maki H, Homma M et al (2017) UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat Med 23(12):1454–1465. 10.1038/nm.4429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kazak L, Chouchani ET, Jedrychowski MP, Erickson B, Shinoda K, Cohen P et al (2015) A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 163(3):643–655. 10.1016/j.cell.2015.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Vangheluwe P, Raeymaekers L, Dode L, Wuytack F (2005) Modulating sarco(endo)plasmic reticulum Ca2 + ATPase 2 (SERCA2) activity: cell biological implications. Cell Calcium 38(3–4):291–302. 10.1016/j.ceca.2005.06.033 [DOI] [PubMed] [Google Scholar]
- 165.de Smedt H, Eggermont JA, Wuytack F, Parys JB, Van den Bosch L, Missiaen L et al (1991) Isoform switching of the sarco(endo)plasmic reticulum Ca2 + pump during differentiation of BC3H1 myoblasts. J Biol Chem 266(11):7092–7095. 10.1016/s0021-9258(20)89614-8 [PubMed] [Google Scholar]
- 166.Walker J, Rohm B, Lang R, Pariza M, Hofmann T, Somoza V (2012) Identification of coffee components that stimulate dopamine release from pheochromocytoma cells (PC-12). Food Chem Toxicol 50(2):390–398. 10.1016/j.fct.2011.09.041 [DOI] [PubMed] [Google Scholar]
- 167.Herrmann-Frank ALH, Stephenson DG (1999) Caffeine and excitation-contraction coupling in skeletal muscle: a stimulating story. J Muscle Res Cell Motil 20(2):223–237. 10.1023/a:1005496708505 [DOI] [PubMed] [Google Scholar]
- 168.Ehrlich BE, Kaftan E, Bezprozvannaya S, Bezprozvanny I (1994) The pharmacology of intracellular ca(2+)-release channels. Trends Pharmacol Sci 15(5):145–149. 10.1016/0165-6147(94)90074-4 [DOI] [PubMed] [Google Scholar]
- 169.Chen Y, Zeng X, Huang X, Serag S, Woolf CJ, Spiegelman BM (2017) Crosstalk between KCNK3-mediated ion current and adrenergic signaling regulates adipose thermogenesis and obesity. Cell 171(4):836-48e13. 10.1016/j.cell.2017.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Maus M, Cuk M, Patel B, Lian J, Ouimet M, Kaufmann U et al (2017) Store-operated Ca2+ entry controls induction of lipolysis and the transcriptional reprogramming to lipid metabolism. Cell Metabol 25(3):698–712. 10.1016/j.cmet.2016.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wright DC, Geiger PC, Han D-H, Jones TE, Holloszy JO (2007) Calcium induces increases in peroxisome proliferator-activated receptor γ coactivator-1α and mitochondrial biogenesis by a pathway leading to p38 mitogen-activated protein kinase activation. J Biol Chem 282(26):18793–18799. 10.1074/jbc.M611252200 [DOI] [PubMed] [Google Scholar]
- 172.Horton R, Rothwell N, Stock M (1988) Chronic inhibition of GABA transaminase results in activation of thermogenesis and brown fat in the rat. Gen Pharmacol 19(3):403–405. 10.1016/0306-3623(88)90037-7 [DOI] [PubMed] [Google Scholar]
- 173.Weerawatanakorn M, He S, Chang C-H, Koh Y-C, Yang M-J, Pan M-H (2023) High gamma-aminobutyric acid (GABA) oolong tea alleviates high-fat diet-induced metabolic disorders in mice. ACS Omega 8(37):33997–34007. 10.1021/acsomega.3c04874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.de Vos WM, Tilg H, Van Hul M, Cani PD (2022) Gut microbiome and health: mechanistic insights. Gut 71(5):1020–1032. 10.1136/gutjnl-2021-326789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chevalier C, Stojanović O, Colin DJ, Suarez-Zamorano N, Tarallo V, Veyrat-Durebex C et al (2015) Gut microbiota orchestrates energy homeostasis during cold. Cell 163(6):1360–1374. 10.1016/j.cell.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 176.Kang Y, Ren P, Shen X, Kuang X, Yang X, Liu H et al (2023) A newly synbiotic combination alleviates obesity by modulating the gut microbiota–fat axis and inhibiting the hepatic TLR4/NF-κB signaling pathway. Mole Nutrit Food Res 10.1002/mnfr.202300141 [DOI] [PubMed]
- 177.Cowana TE, Palmnäs MSA, Yang J, Bomhof MR, Ardell KL, Reimer RA et al (2014) Chronic coffee consumption in the diet-induced obese rat: impact on gut microbiota and serum metabolomics. J Nutr Biochem 25(4):489–495. 10.1016/j.jnutbio.2013.12.009 [DOI] [PubMed] [Google Scholar]
- 178.Zhu M-z, Zhou F, Ouyang J, Wang Q-y, Li Y-l, Wu J-l et al (2021) Combined use of epigallocatechin-3-gallate (EGCG) and caffeine in low doses exhibits marked anti-obesity synergy through regulation of gut microbiota and bile acid metabolism. Food Funct 12(9):4105–4116. 10.1039/d0fo01768j [DOI] [PubMed] [Google Scholar]
- 179.Mills CE, Tzounis X, Oruna-Concha M-J, Mottram DS, Gibson GR, Spencer JPE (2015) In vitro colonic metabolism of coffee and chlorogenic acid results in selective changes in human faecal microbiota growth. Br J Nutr 113(8):1220–1227. 10.1017/s0007114514003948 [DOI] [PubMed] [Google Scholar]
- 180.Kenny DJ, Plichta DR, Shungin D, Koppel N, Hall AB, Fu B et al (2020) Cholesterol metabolism by uncultured human gut bacteria influences host cholesterol level. Cell Host Microbe 28(2):245–257. 10.1016/j.chom.2020.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Johnston KL, Clifford MN, Morgan LM (2003) Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: glycemic effects of chlorogenic acid and caffeine. Am J Clin Nutr 78(4):728–733. 10.1093/ajcn/78.4.728 [DOI] [PubMed] [Google Scholar]
- 182.Cha KH, Song D-G, Kim SM, Pan C-H (2012) Inhibition of gastrointestinal lipolysis by green tea, coffee, and gomchui (Ligularia fischeri) tea polyphenols during simulated digestion. J Agric Food Chem 60(29):7152–7157. 10.1021/jf301047f [DOI] [PubMed] [Google Scholar]
- 183.Chen L, Wang X-j, Chen J-x, Yang J-c, Ling L, Cai X-B et al (2023) Caffeine ameliorates the metabolic syndrome in diet-induced obese mice through regulating the gut microbiota and serum metabolism. Diabetol Metab Syndr 15(1). 10.1186/s13098-023-00993-3 [DOI] [PMC free article] [PubMed]
- 184.Duval C, Touche V, Tailleux A, Fruchart J-C, Fievet C, Clavey Vr et al (2006) Niemann–pick C1 like 1 gene expression is down-regulated by LXR activators in the intestine. Biochem Biophys Res Commun 340(4):1259–1263. 10.1016/j.bbrc.2005.12.137 [DOI] [PubMed] [Google Scholar]
- 185.Lo Sasso G, Murzilli S, Salvatore L, D’Errico I, Petruzzelli M, Conca P et al (2010) Intestinal specific LXR activation stimulates reverse cholesterol transport and protects from atherosclerosis. Cell Metabol 12(2):187–193. 10.1016/j.cmet.2010.07.002 [DOI] [PubMed] [Google Scholar]
- 186.Ontawong A, Duangjai A, Muanprasat C, Pasachan T, Pongchaidecha A, Amornlerdpison D et al (2019) Lipid-lowering effects of Coffea arabica pulp aqueous extract in Caco-2 cells and hypercholesterolemic rats. Phytomedicine 52:187–197. 10.1016/j.phymed.2018.06.021 [DOI] [PubMed] [Google Scholar]
- 187.Motta EVS, Arnott RLW, Moran NA (2023) Caffeine consumption helps honey bees fight a bacterial pathogen. Microbiol Spectr 11(3). 10.1128/spectrum.00520-23 [DOI] [PMC free article] [PubMed]
- 188.McConnell MN, Bakermans C (2023) Nutrients mediate caffeine inhibition of Escherichia coli. Environ Microbiol Rep 15(5):422–425. 10.1111/1758-2229.13165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yang L, Zhu Y, Zhong S, Zheng G (2020) Astilbin lowers the effective caffeine dose for decreasing lipid accumulation via activating AMPK in high-fat diet‐induced obese mice. J Sci Food Agric 101(2):573–581. 10.1002/jsfa.10669 [DOI] [PubMed] [Google Scholar]
- 190.Xu M, Yang L, Zhu Y, Liao M, Chu L, Li X et al (2019) Collaborative effects of chlorogenic acid and caffeine on lipid metabolism via the AMPKα-LXRα/SREBP-1c pathway in high-fat diet-induced obese mice. Food Funct 10(11):7489–7497. 10.1039/c9fo00502a [DOI] [PubMed] [Google Scholar]
- 191.Carter BE, Drewnowski A (2012) Beverages containing soluble fiber, caffeine, and green tea catechins suppress hunger and lead to less energy consumption at the next meal. Appetite 59(3):755–761. 10.1016/j.appet.2012.08.015 [DOI] [PubMed] [Google Scholar]
- 192.Ohara T, Muroyama K, Yamamoto Y, Murosaki S (2016) Oral intake of a combination of glucosyl hesperidin and caffeine elicits an anti-obesity effect in healthy, moderately obese subjects: a randomized double-blind placebo-controlled trial. Nutr J 15(1). 10.1186/s12937-016-0123-7 [DOI] [PMC free article] [PubMed]
- 193.Yoneshiro T, Matsushita M, Hibi M, Tone H, Takeshita M, Yasunaga K et al (2017) Tea catechin and caffeine activate brown adipose tissue and increase cold-induced thermogenic capacity in humans. Am J Clin Nutr 105(4):873–881. 10.3945/ajcn.116.144972 [DOI] [PubMed] [Google Scholar]
- 194.Saimaiti A, Zhou DD, Li J, Xiong RG, Gan RY, Huang SY et al (2023) Dietary sources, health benefits, and risks of caffeine. Crit Rev Food Sci Nutr 63(29):9648–9666. 10.1080/10408398.2022.2074362 [DOI] [PubMed] [Google Scholar]
- 195.Davoodi SH, Hajimiresmaiel SJ, Ajami M, Mohseni-Bandpei A, Ayatollahi SA, Dowlatshahi K et al (2014) Caffeine treatment prevented from weight regain after calorie shifting diet induced weight loss. Iran J Pharm Res 13(2):707–718 [PMC free article] [PubMed] [Google Scholar]
- 196.Mielgo-Ayuso J, Marques-Jiménez D, Refoyo I, Del Coso J, León-Guereño P, Calleja-González J (2019) Effect of caffeine supplementation on sports performance based on differences between sexes: a systematic review. Nutrients 11(10). 10.3390/nu11102313 [DOI] [PMC free article] [PubMed]
- 197.Domaszewski P (2023) Gender differences in the frequency of positive and negative effects after acute caffeine consumption. Nutrients 15(6). 10.3390/nu15061318 [DOI] [PMC free article] [PubMed]
- 198.Adan A, Prat G, Fabbri M, Sànchez-Turet M (2008) Early effects of caffeinated and decaffeinated coffee on subjective state and gender differences. Prog Neuropsychopharmacol Biol Psychiatry 32(7):1698–1703. 10.1016/j.pnpbp.2008.07.005 [DOI] [PubMed] [Google Scholar]
- 199.Lassen ML, Byrne C, Sheykhzade M, Wissenberg M, Hurry PK, Schmedes AV et al (2022) Sex differences and caffeine impact in adenosine-induced hyperemia. J Nucl Med 63(3):431–437. 10.2967/jnumed.121.261970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Jeukendrup AE, Randell R (2011) Fat burners: nutrition supplements that increase fat metabolism. Obes Rev 12(10):841–851. 10.1111/j.1467-789X.2011.00908.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. All data referenced in this article are obtained from previously published studies, which are cited in the References section.