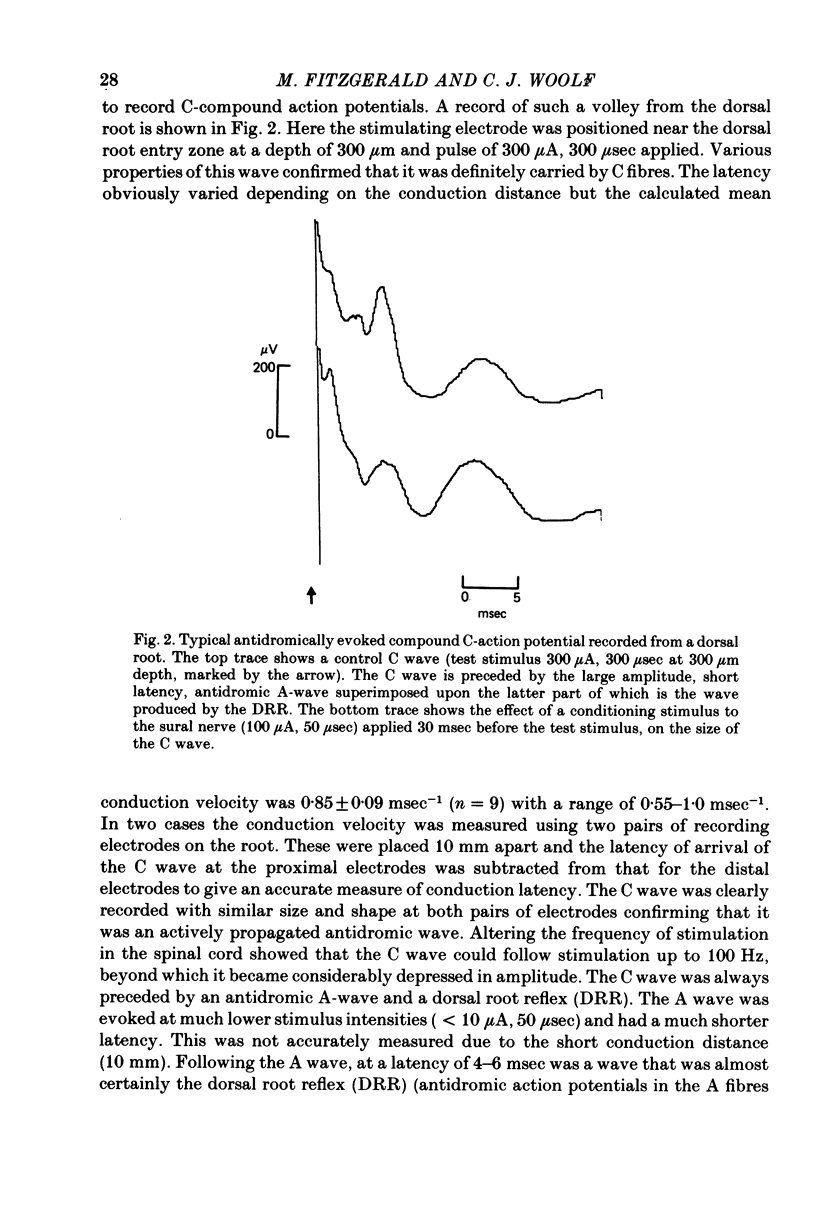
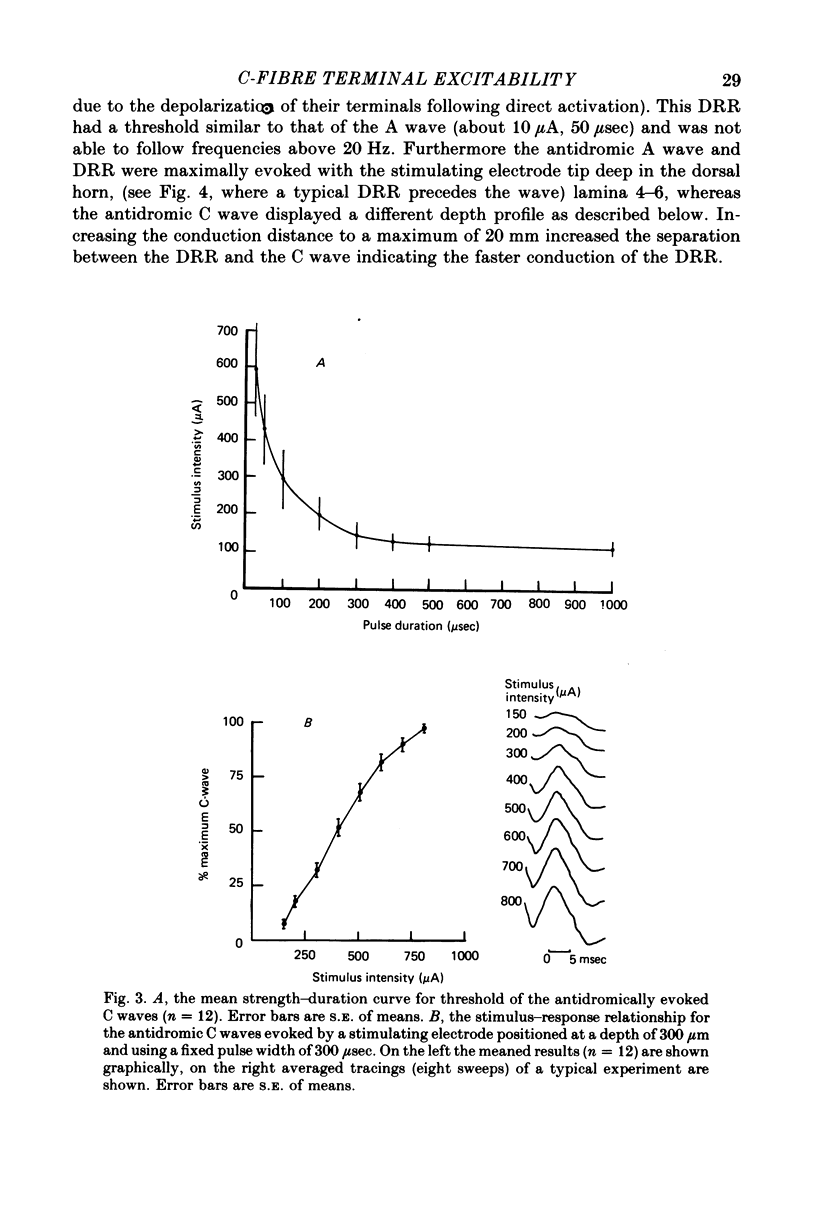
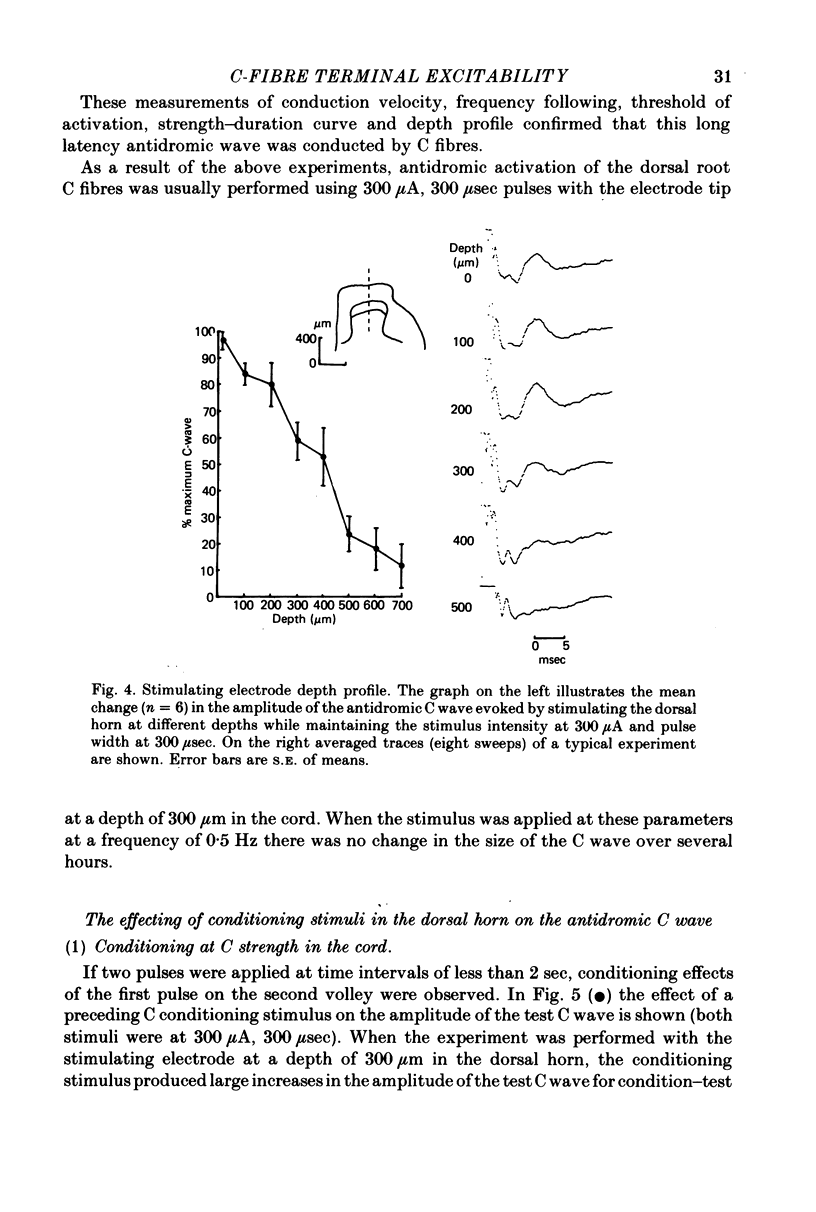
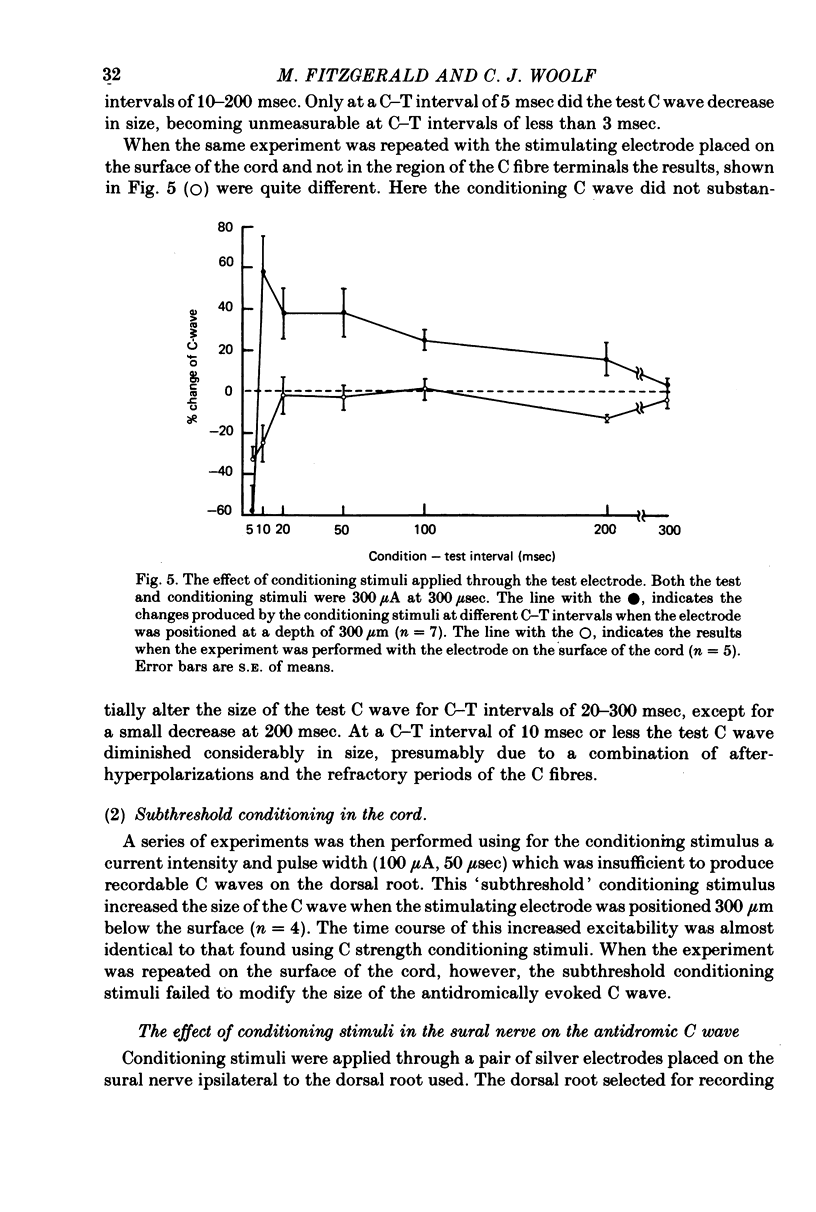
Abstract
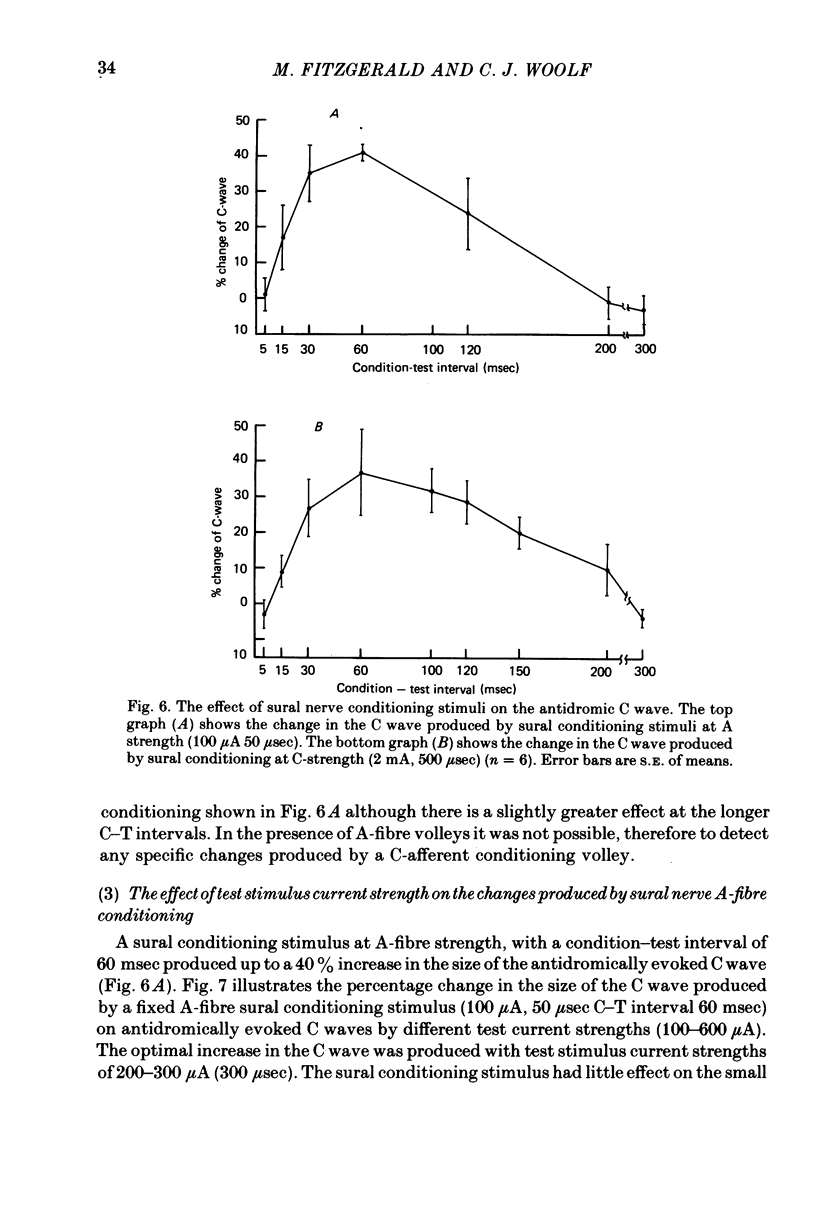
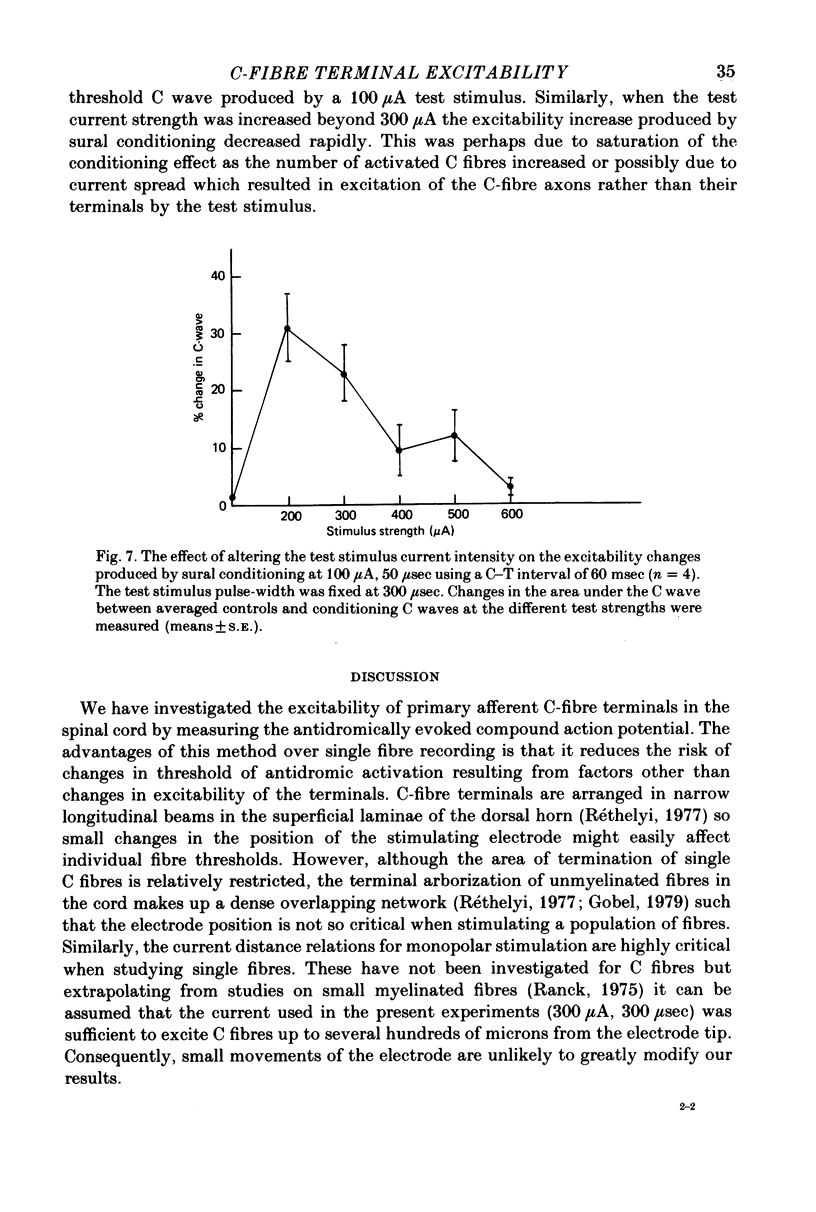
1. Changes in the threshold for antidromic activation of C-fibre afferent terminals in the lumbar spinal cord of the decerebrate-spinalized rat have been examined. 2. The antidromic compound action potential elicited by stimulation in the dorsal horn was recorded in a sectioned dorsal root. 3. The antidromic C wave had conduction velocities and strength-duration properties similar to that described for other unmyelinated fibres. 4. The optimal position of the stimulating electrode within the spinal cord for eliciting the antidromic C wave was found to correlate with the site of entry and termination of C-afferent fibres. 5. Local stimulation within the dorsal grey of the spinal cord was shown to produce prolonged increased excitability of the C-afferent terminals in that segment. This effect was restricted to the terminals and could not be demonstrated in the stem axons of the C fibres. 6. Cutaneous afferent conditioning volleys from the sural nerve produced marked increases in the excitability of the C-afferent terminals. This effect was present at A-fibre strength sural stimulation, with no significant alteration when C-fibre strength stimulation was used. The alteration in the threshold for antidromic stimulation produced by the sural conditioning stimuli only occurred at the C-afferent terminals and not at their axons. 7. The results are discussed in terms of presynaptic inhibition of C-fibre input at a segmental level.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown A. G., Kirk E. J., Martin H. F., 3rd Descending and segmental inhibition of transmission through the spinocervical tract. J Physiol. 1973 May;230(3):689–705. doi: 10.1113/jphysiol.1973.sp010212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervero F., Iggo A., Ogawa H. Nociceptor-driven dorsal horn neurones in the lumbar spinal cord of the cat. Pain. 1976 Mar;2(1):5–24. doi: 10.1016/0304-3959(76)90042-7. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., RITCHIE J. M. Mammalian nonmyelinated nerve fibers. Physiol Rev. 1962 Apr;42:297–334. doi: 10.1152/physrev.1962.42.2.297. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., MAGNI F. Central inhibitory action attributable to presynaptic depolarization produced by muscle afferent volleys. J Physiol. 1961 Nov;159:147–166. doi: 10.1113/jphysiol.1961.sp006798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields H. L., Basbaum A. I. Brainstem control of spinal pain-transmission neurons. Annu Rev Physiol. 1978;40:217–248. doi: 10.1146/annurev.ph.40.030178.001245. [DOI] [PubMed] [Google Scholar]
- GASSER H. S. Properties of dorsal root unmedullated fibers on the two sides of the ganglion. J Gen Physiol. 1955 May 20;38(5):709–728. doi: 10.1085/jgp.38.5.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GASSER H. S. The postspike positivity of unmedullated fibers of dorsal root origin. J Gen Physiol. 1958 Mar 20;41(4):613–632. doi: 10.1085/jgp.41.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWLAND B., LETTVIN J. Y., McCULLOCH W. S., PITTS W., WALL P. D. Reflex inhibition by dorsal root interaction. J Neurophysiol. 1955 Jan;18(1):1–17. doi: 10.1152/jn.1955.18.1.1. [DOI] [PubMed] [Google Scholar]
- Handwerker H. O., Iggo A., Zimmermann M. Segmental and supraspinal actions on dorsal horn neurons responding to noxious and non-noxious skin stimuli. Pain. 1975 Jun;1(2):147–165. doi: 10.1016/0304-3959(75)90099-8. [DOI] [PubMed] [Google Scholar]
- Hentall I. D., Fields H. L. Segmental and descending influences on intraspinal thresholds of single C-fibers. J Neurophysiol. 1979 Nov;42(6):1527–1537. doi: 10.1152/jn.1979.42.6.1527. [DOI] [PubMed] [Google Scholar]
- Hodge C. J., Jr Potential changes inside central afferent terminals secondary to stimulation of large- and small-diameter peripheral nerve fibers. J Neurophysiol. 1972 Jan;35(1):30–43. doi: 10.1152/jn.1972.35.1.30. [DOI] [PubMed] [Google Scholar]
- Kennedy D., McVittie J., Calabrese R., Fricke R. A., Craelius W., Chiapella P. Inhibition of mechanosensory interneurons in the crayfish. I. Presynaptic inhibition from giant fibers. J Neurophysiol. 1980 Jun;43(6):1495–1509. doi: 10.1152/jn.1980.43.6.1495. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Morris M. E. Input-output relation of transmission through cuneate nucleus. J Physiol. 1976 Jun;257(3):791–815. doi: 10.1113/jphysiol.1976.sp011398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisney S. J. Evidence for primary afferent depolarization of single tooth-pulp afferents in the cat. J Physiol. 1979 Mar;288:437–447. [PMC free article] [PubMed] [Google Scholar]
- Mendell L. M. Physiological properties of unmyelinated fiber projection to the spinal cord. Exp Neurol. 1966 Nov;16(3):316–332. doi: 10.1016/0014-4886(66)90068-9. [DOI] [PubMed] [Google Scholar]
- Merrill E. G., Wall P. D., Yaksh T. L. Properties of two unmyelinated fibre tracts of the central nervous system: lateral Lissauer tract, and parallel fibres of the cerebellum. J Physiol. 1978 Nov;284:127–145. doi: 10.1113/jphysiol.1978.sp012531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranck J. B., Jr Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res. 1975 Nov 21;98(3):417–440. doi: 10.1016/0006-8993(75)90364-9. [DOI] [PubMed] [Google Scholar]
- Réthelyi M. Preterminal and terminal axon arborizations in the substantia gelatinosa of cat's spinal cord. J Comp Neurol. 1977 Apr 1;172(3):511–521. doi: 10.1002/cne.901720307. [DOI] [PubMed] [Google Scholar]
- Shapiro E., Castellucci V. F., Kandel E. R. Presynaptic inhibition in Aplysia involves a decrease in the Ca2+ current of the presynaptic neuron. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1185–1189. doi: 10.1073/pnas.77.2.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALL P. D. Excitability changes in afferent fibre terminations and their relation to slow potentials. J Physiol. 1958 Jun 18;142(1):1–21. doi: 10.1113/jphysiol.1958.sp005997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall P. D., Sweet W. H. Temporary abolition of pain in man. Science. 1967 Jan 6;155(3758):108–109. doi: 10.1126/science.155.3758.108. [DOI] [PubMed] [Google Scholar]
- Wall P. D. The role of substantia gelatinosa as a gate control. Res Publ Assoc Res Nerv Ment Dis. 1980;58:205–231. [PubMed] [Google Scholar]
- Woolf C. J., Mitchell D., Barrett G. D. Antinociceptive effect of peripheral segmental electrical stimulation in the rat. Pain. 1980 Apr;8(2):237–252. doi: 10.1016/0304-3959(88)90011-5. [DOI] [PubMed] [Google Scholar]


