Abstract
Numerous studies have revealed that the ATP-gated ion channel purinergic 2X7 receptor (P2X7R) plays an important role in tumor progression and the pathogenesis of cancer pain. P2X7R requires activation by extracellular ATP to perform its regulatory role functions. During tumor development or cancer-induced pain, ATP is released from tumor cells or other cells in the tumor microenvironment (such as tumor-associated immune cells), which activates P2X7R, opens ion channels on the cell membrane, affects intracellular molecular metabolism, and regulates the activity of tumor cells. Furthermore, peripheral organs and receptors can be damaged during tumor progression, and P2X7R expression in nerve cells (such as microglia) is significantly upregulated, enhancing sensory afferent information, sensitizing the central nervous system, and inducing or exacerbating pain. These findings reveal that the ATP-P2X7R signaling axis plays a key regulatory role in the pathogenesis of tumors and cancer pain and also has a therapeutic role. Accordingly, in this study, we explored the role of P2X7R in tumors and cancer pain, discussed the pharmacological properties of inhibiting P2X7R activity (such as the use of antagonists) or blocking its expression in the treatment of tumor and cancer pain, and provided an important evidence for the treatment of both in the future.
Keywords: Tumors, P2X7 receptor (P2X7R), Cancer pain, Antagonists
Introduction
Several molecular factors are involved in tumor pathogenesis, which is a complicated pathological process [1]. Tumor recurrence and metastasis are the leading causes of mortalities [2]. Tumor cells can modify their growth and metastasis rates by modifying their tumor microenvironment. During metastasis (especially in advanced tumors), tumor cells can invade surrounding tissues and organs, including distant organs, erode peripheral receptors, increase sensory afferent information, and induce pain [3–5]. Therefore, elucidating common molecular targets that restrain tumor progression, relieve cancer-induced pain, and improve the survival rate and quality of life of patients is urgently required, and is a research topic of important concern among many researchers. ATP is an important energy source in the body which participates in cellular activities, such as cell movement and cell adhesion, and plays an important regulatory role [6–8], including regulating cellular activity by mediating other molecular substances, such as membrane receptors [9, 10]. During tumor progression, ATP in the tumor microenvironment can affect the fate of tumor cells through interactions with factors, such as tumor-related immune cells [11, 12], and can also directly act on the tumor cells themselves via interaction with tumor cell membrane receptors, such as P2X or P2Y receptors, which play a role in regulating tumor progression [13, 14].
ATP is a natural activator of P2X purinergic receptors in the body [15]. P2X receptors are activated in combination with ATP to enhance their functional characteristics [16]. Although P2X purinergic receptors are currently divided into seven subtypes, the purinergic 2X7 receptor (P2X7R) is most researched owing to its unique biological characteristics and has become an important research topic on many diseases, including inflammation [17], cardiovascular disease [18], pain [19], neurological diseases [20], and tumors. Several studies have confirmed the role of P2X7R in tumor growth and metastasis [21]. P2X7R regulates the growth, apoptosis, migration, and invasion of tumor cells; however, P2X7R has dual functions in regulating tumor cell activities [22, 23]. Furthermore, several studies have shown that P2X7R plays an indispensable role in cancer-induced pain [24]. Increased P2X7R expression induces or aggravates cancer pain and inhibiting the activation of P2X7R or reducing its expression can alleviate cancer pain [25]. Increased P2X7R expression can activate microglia; activated microglia can release various inflammatory mediators, including Tumour Necrosis Factor alpha (TNFα), interleukin (IL) 1β, damage nerve tissues, sensitive central nervous system, and increase pain production [26]. Moreover, studies have revealed that P2X7R plays a regulatory role in tumor progression and a key role in the pathogenesis of cancer pain. Therefore, in this study, we elucidated the potential role of P2X7R in cancer and cancer pain and the potential value of using P2X7R antagonists in treating both.
Structure and functional relationships of P2X7R
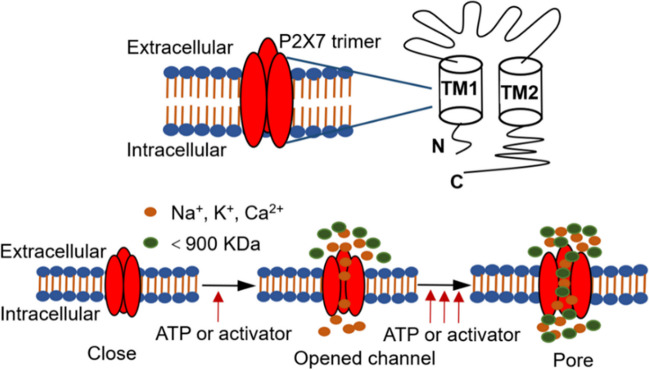
P2X7R activity depends on the ATP ion channel-type trimer structure [27]. However, P2X purinergic receptors contain all the three basic domains, including intracellular domain, transmembrane domain, and extracellular domain [28]. P2X7R does not form heterotrimeric structures with other P2X subtypes, including P2X1, P2X2, P2X3, P2X5, and P2X6; however, heterotrimers can be formed with the P2X4 receptor. The genetic characteristics and biological functions of P2X7R differ from the others in that the long intracellular C-terminal contains approximately 200 more amino acid residues than the other six subtypes. It is the longest watershed in the P2X family, and its unique molecular function is based on its ability to bind to other proteins and lack of homology with other known proteins [26, 29]. The C-terminus of P2X7R plays a key role in regulating the stability of receptor functions, including signal pathway activation, cell localization, protein interactions, and post-translational modifications [30, 31]. Studies have shown that HSP90 may play a vital role in the stability and function of P2X7R by acting on the cysteine-rich domain at the C-terminus of the cytoplasm [32]. The intracellular N-terminus is short, has the characteristic of regulating calcium ion influx, and has 35–40% homology with other P2X subtypes. Recent studies have found that the intracellular N-terminal gene sequence regulates the total calcium current, and a change in the N-terminal structure changes the ability of calcium ions to selectively penetrate the pore [33]. Another important feature is the extracellular domain containing three ATP-binding sites. However, compared with other P2X subtypes (for example, P2X4 receptor is more sensitive to low concentration ATP), P2X7R has a lower affinity to ATP (EC50 > 100 µM is required for initial activation) [34]. Therefore, an ATP concentration exceeding 100 μM can activate the P2X7R, opening the cation channel. Notably, another feature of P2X7R that distinguishes it from other P2X subtypes is its open channel. The opening of pores in the membrane differed from that of the other P2X subtypes. Under the stimulation of short-term ATP or other agonists (such as BzATP), the transmembrane domain of P2X7R opens to form cation channels (influx of sodium and calcium ions and outflow of potassium ions). However, notably, when ATP or an agonist continues to act, the molecular conformation of P2X7R changes and the membrane pore becomes larger, allowing some biomolecules up to 900 Da to pass through, increasing cell permeability and eventually leading to cell edema and apoptosis [35–37]. Studies have found that continuous exogenous ATP or agonist stimulation can rapidly alter the diameter of a membrane pore from 0.8–1.1 nm to 3–5 nm [38, 39] (Fig. 1). However, the flow of macromolecules mediated by P2X7R has not yet been fully elucidated.
Fig. 1.
Schematic diagram of P2X7R structure and its activation-mediated channel opening. P2X7R contains three subunits: an intracellular domain, an extracellular domain, and two transmembrane domains (TM1 and TM2). The two transmembrane domains control the opening of the ion channels. The N-terminus is more conserved, and the C-terminus is longer, containing over 200 amino acids, which can interact with other proteins. P2X7R activity and ion channel/macropore formation causes changes in its functional configuration. Under normal circumstances, P2X7R is in a state of low activity or inactivity. With low dose agonist (ATP) or short time treatment, P2X7R is activated by opening the ion channels on the membrane (mainly mediated calcium and sodium ion influx, potassium ion efflux), regulates intracellular molecular metabolism and signal transduction, and displays its functional characteristics. However, under continuous or high concentrations of ATP or other activators, the configuration of P2X7R changes, and the ion channel further increases the formation of membrane pores, allowing some molecular substances up to 900 Da to pass through, resulting in an imbalance in intracellular molecular metabolism and an increase in membrane permeability, which can eventually lead to cell edema, apoptosis, and death
Previously, studies on the biological functions of P2X7R focused on inflammation and immune-related diseases [40, 41]. P2X7R plays a role in inflammatory and immune responses by regulating monocytes, lymphocytes, and dendritic cells [42–44]. Later, it was discovered that P2X7R was expressed in almost all tissues of the body and was involved in the pathogenesis of many physiological diseases [45]. Generally, under normal physiological conditions, the concentration of ATP released from cells is relatively low and P2X7R is in a low or inactive state. However, when the body is stressed due to pathological conditions, such as tumor stimulation, ATP is released in large quantities, which activates P2X7R, changes the state of membrane pores, allows movement of molecules, changes intracellular molecular metabolism, regulates intracellular signal transduction, and ultimately affects the biological behavior of cells [26, 46, 47].
The role of P2X7R in tumors and signaling pathways
Recently, studies on the involvement of P2X7R in tumors have been conducted [48]. Progress has been made regarding understanding the role of P2X7R in tumor progression [49]. P2X7R activation regulates the growth, proliferation, apoptosis, migration, and invasion of tumor cells [50, 51]. Different studies have shown that P2X7R is expressed in most tumor tissues and cells, including lung cancer [52], endometrial cancer [53], lung cancer [54], colorectal cancer [55], liver cancer [56], stomach cancer [57], breast cancer [58], pancreatic cancer [59], prostate cancer, [60] and Glioma [61], and plays an important regulatory role in tumor development (Fig. 2). Different studies have revealed that P2X7R is highly expressed in tumor tissues and is significantly associated with clinicopathological characteristics (such as lymph node metastasis, poor survival prognosis, and vascular invasion) [62–64]. P2X7R is highly expressed in patients with colorectal cancer tissues, and its overall survival time is shortened. Its expression of P2X7R is also found to increase significantly in metastatic colorectal cancer, suggesting that P2X7R may be a potential biomarker for determining the prognosis and metastasis of colorectal cancer [65]. P2X7R is highly expressed in gastric cancer tissues and is significantly associated with vascular invasion, lymph node metastasis, distant metastasis, tumor stage, and impacts overall survival [66].
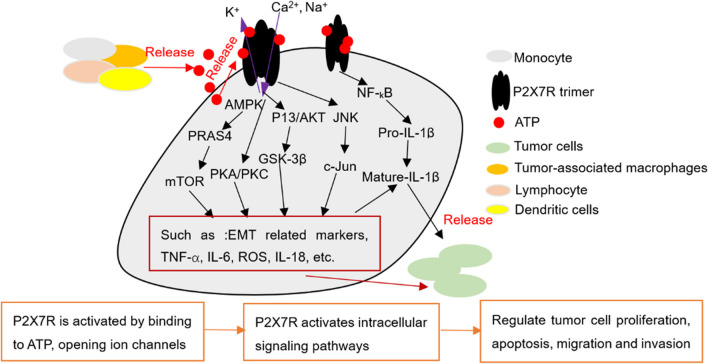
Fig. 2.
P2X7R is involved in tumor progression as an important mediator of tumor cell activity. After tumorigenesis, tumor cells and tumor-related immune cells (such as macrophages and lymphocytes) in the tumor microenvironment release large amounts of ATP. The concentration of extracellular ATP increased sharply, activated P2X7R on the membrane, changed its own structure, opened ion channels, activated different intracellular signal pathways, released damage factors (such as TNF-a, IL-6, and ROS) or regulated the expression of EMT-related genes (such as snail, E-cadherin and N-cadherin), and affected the biological activities of tumor cells (proliferation, apoptosis, migration, invasion, and the formation of EMT)
P2X7R is activated by binding to ATP to change its molecular conformation, open pores in the membrane, and regulate intracellular signal channels, thereby affecting the biological behavior of the cell [67, 68]. Moreover, the activation of P2X7R can activate multiple intracellular signal axes, such as MAPK, JNK, AKT, and NF-κB, and induce the release of damaging factors, such as TNF-α, IL-18, reactive oxygen species (ROS), and IL-1β [69–71], and play an important regulatory role in the pathogenesis of most diseases, such as inflammation [72], nerve damage [73], pain [74], hemorrhagic stroke [75], alcoholic fatty liver disease, [76] and tumor [72]. For example, studies have found that BzATP increased microglia activation, induced phosphorylation of ERK, AKT, and JNK, upregulated the levels of caspase-1 and IL-1β, and increased neuronal damage. Application of the P2X7R antagonist brilliant blue G (BBG) reverses this phenomenon [77]. Correspondingly, P2X7R can also mediate different intracellular signal transduction pathways and regulate the biological behavior of tumor cells. Studies have found that ATP and BzATP activate P2X7R, increase the phosphorylation levels of ERK and AKT in human ovarian cancer cells, and promote cancer cell activity [78]. In non-small cell lung cancer A549 cells, P2X7R functions as an ion channel for macropore formation [54]. Activation of P2X7R by ATP or BzATP can promote epithelial-mesenchymal transition (EMT) and invasion of A549 cells and increase the level of phosphorylated protein kinase B (p-Akt). Activation of P2X7R can also promote the expression of EMT and PI3K/Akt in transplanted tumors [54]. Studies have shown that P2X7R activation promotes the proliferation of human pancreatic cancer cells via ERK1/2 and JNK [79]. It was further discovered that P2X7R activation activated PI3K/Akt/GSK-3beta signaling and promoted the growth and formation of EMT of colorectal cancer cells [80]. Moreover, some in vivo studies have revealed the role of P2X7R in tumor progression [81, 82]. Studies have shown that P2X7R activation promotes tumor growth in vivo, while the use of a P2X7R inhibitor (oxidized ATP) significantly reduces tumor growth and angiogenesis [83]. Studies show that tumor growth and metastasis were significantly accelerated in mice lacking the P2X7R gene [84]. These results indicate that P2X7R is a potential biological indicator of tumors (Fig. 3).
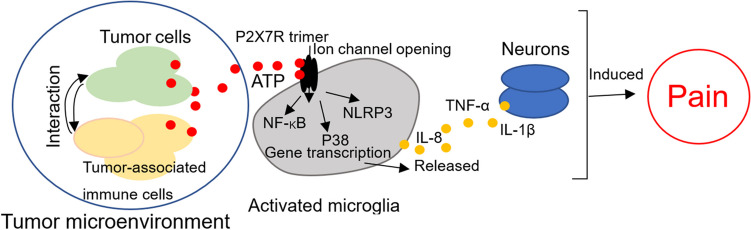
Fig. 3.
P2X7R contributes to the development of cancer pain. The tumor microenvironment contains many cell types of cells (including tumor and immune cells). Tumors can invade surrounding or distant tissues and induce immune cell invasion. In the microenvironment, cells can release a large amount of ATP, activate the P2X7R, open ion channels on the membrane, and release inflammatory cytokines (such as TNF-α and IL-1β) by activating intracellular signals such as NF-κB and NLRP3. These released inflammatory cytokines further damage the nerve tissue and neurons, enhance sensory information transmission, and induce pain
However, some studies have revealed the opposite phenomenon. Continuous stimulation or high concentrations of activators (such as ATP and BzATP) can have toxic effects on tumor cells and inhibit their growth and metastasis [85–87]. P2X7R activation can also inhibit the proliferation and metastasis of tumor cells and promote their apoptosis [85, 88]. Tumor cells are exposed to high concentrations of ATP and activation of P2X7R regulates PI3K/AKT and AMPK-PRAS40-mTOR signaling and promote tumor cell death [89]. Studies have shown that low concentrations of ATP and BzATP promote the invasion of pancreatic ductal cell carcinoma, whereas at high concentrations of ATP (5 mM) and BzATP (1 mM), P2X7R mediates tumor cell death. Furthermore, P2X7R antagonists, A438079 and AZ10606120, antagonize ATP-induced pore formation and cell death [90]. These studies have revealed that P2X7R plays an important role in regulating the biological behavior of tumor cells; however, the results of various studies are contradictory. This may be related to the characteristics of P2X7R, in particular, regarding the opening of membrane pores, and its interaction with immune and inflammatory cells in the tumor microenvironment.
Another notable finding is that P2X7R activation mediates tumor angiogenesis and provides an environment for tumor growth [21, 81, 83]. For example, ATP (> 20 μM) strongly inhibits the migration of human breast cancer vascular endothelial cells and reduces the permeability of endothelial cells [91]. P2X7R antagonist BBG or knocked-down P2X7R promotes tumor vascular growth and the proliferation of glioma cells, which may be related to the upregulation of the expression of epidermal growth factor receptor, hypoxia-inducible factor 1-alpha and vascular endothelial growth factor [92]. Additionally, P2X7R affects the effects of immunotherapy, radiotherapy, and chemotherapy on tumor cells, mediates immune cells in the tumor microenvironment (such as tumor-associated macrophages and T lymphocytes), and increases the drug resistance of tumor cells [93–95]. P2X7R is highly expressed in tumor-associated macrophages and immunosuppressive cells. Moreover, P2X7R inhibitors (O-ATP, A438079, and A740003) can overcome drug resistance to immunotherapy (anti-PD-1 antibody) and chemotherapy (cisplatin) during lung cancer treatment [96]. Studies have shown that, after γ-ray (2GY) treatment for 24 h, both P2X7R antagonists and P2X7R silencing can block the cytotoxicity caused by irradiation [97]. Taken together, P2X7R plays a dual role in regulating tumor activity; it has pro-proliferative and pro-apoptotic effects. Therefore, the potential of P2X7R as a tumor treatment must consider the activity characteristics of P2X7R, the use of extracellular activator concentrations, and the influence of immune-related cells in the tumor microenvironment on the function of P2X7R, and much remains in the treatment of tumors regarding P2X7R.
Targeted P2X7 receptor as a potential therapy for tumor
Currently, the treatment of tumors involves diverse methods, including surgery, chemotherapy, immunotherapy, radiotherapy, gene therapy, and targeted therapy, which improves the overall survival rate and quality of life of patients [98, 99]. However, tumor recurrence, metastasis, and chemotherapy resistance significantly increase patient mortality [100]. Numerous factors are involved in the pathogenesis of tumors, including genetic changes and immune escape. Therefore, exploring new factors involved in tumor pathogenesis and developing better targeted therapies is urgently required to improve tumor treatment outcomes. The role of P2X7R in tumors has been confirmed by several studies, which provide a theoretical basis and data support for anti-tumor therapy and a promise for future targeted therapy of tumors.
As P2X7R activation or increased expression plays a key role in the proliferation, apoptosis, migration, and invasion of tumor cells, [49] inhibiting P2X7R activation or reducing its expression may be a potential target for antitumor therapy. Furthermore, numerous studies have shown that reducing the expression of P2X7R has anti-tumor effects [101, 102]. For example, short hairpin RNA silencing of P2X7R expression or blocking its expression using a P2X7R antibody can significantly reduce lymph node metastasis in lymphoma [103]. Knocking out P2X7R promotes apoptosis in breast cancer cells by downregulating Bcl-2 and increasing caspase-3 levels [104].
Recently, with the exploration of the biological functions of P2X7R, great progress has been made in the development and application of P2X7R antagonists. Initially, the use of P2X7R antagonists was limited to broad-spectrum P2X receptor antagonists (such as TNP-ATP and oxidized ATP). Given the unique biological characteristics of P2X7R, its effect on P2X7R antagonists is relatively weak [105, 106]. These broad-spectrum antagonists exert therapeutic effects on diseases mediated by P2X7R (such as tumors [107, 108]. Broad-spectrum antagonists also have a certain inhibitory effect on tumors, but their application is limited. With the development and application of P2X7R selective or competitive antagonists, it has become a new pharmacological target for antitumor therapy.
Presently, P2X7R antagonists such as BBG have shown some anti-tumor activity, including commonly used P2X7R antagonists BBG [109], A438079 [96], A804598 [110], AZD9056 [80], A740003 [111], and AZ10606120 [112] (Fig. 4). Studies have shown that P2X7R antagonists (AZ10606120 or A740003) can inhibit the growth of neuroblastomas [113]. The P2X7R antagonist A438079 can prevent ATP-induced pore formation and tumor cell death, whereas the P2X7R allosteric inhibitor AZ10606120 can inhibit the proliferation of pancreatic ductal cell carcinoma [90]. Further research confirmed that AZ10606120 and A438079 inhibit the activation of P2X7R, reduce ERK and AKT phosphorylation levels, and inhibit the growth and proliferation of human ovarian cell carcinoma [78, 114]. Studies have also shown that the P2X7R antagonist AZ10606120 can inhibit the growth of gliomas by inactivating microglial cells, and it has been found that its inhibitory effect on the proliferation of tumor cells is better than that of conventional chemotherapeutics. However, the P2X7R antagonist BBG did not have a significant inhibitory effect [115]. Recent studies have shown that P2X7R activation promotes the proliferation of colon cancer cells, whereas P2X7R antagonists (A438079 and AZD9056) or siRNA knockdown of P2X7R inhibit the proliferation of colon cancer cells [80]. Similarly, further studies found that A438079 inhibits the proliferation, migration, and invasion of colon cancer cells [116]. Recently, a new P2X7R antagonist (1-piperidimidazole) was found to significantly inhibit the migration and invasion of breast cancer cells [117]. However, some studies have shown that the P2X7R antagonists A438079 and AZ10606120 have no apparent chemopreventive effect on pancreatic cancer; instead, it increases the metastasis of pancreatic cancer cells [118, 119]. These differences may be related to the application of P2X7R antagonists under different activation conditions, tumor types, and individual differences. Moreover, other antagonists (such as KN62) inhibit the activation of P2X7R and have antitumor effects [79]. Furthermore, P2X7R is involved in the activity of cancer stem cells, and its antagonists may antagonize the activity of cancer stem cells [197, 120]. For example, the P2X7R activators ATP and BzATP can upregulate the expression of markers related to EMT and increase the migration and invasiveness of glial stem cells, while the P2X7R antagonist A438079 can inhibit this activity [121, 122]. Additionally, the application of P2X7R antagonists may impact the effects of tumor radiotherapy and chemotherapy [123]. The P2X7R antagonist BBG can enhance the cytotoxicity of B16 melanoma cells induced by radiation in vitro and in vivo, and has a radiosensitizing effect [124]. In summary, significant progress has been made regarding understanding the involvement of P2X7R in tumor progression, and that P2X7R antagonists can antagonize the activity of tumor cells. Various studies have confirmed that P2X7R antagonists can inhibit tumor progression, including tumor growth and metastasis. However, P2X7R can produce toxic effects on cells in the presence of high-dose agonists or continuous stimulation. Therefore, P2X7R antagonists should consider and control the dosage and action time of P2X7R antagonists should be considered and controlled in tumor therapy. Antagonists should be used effectively with P2X7R activation to promote tumor growth; however, it should not lead to the use of agonists that continuously activate P2X7R to mediate membrane pore opening and lead to cytotoxicity, which may increase the risk of promoting tumor growth. Accordingly, an in-depth development and utilization of P2X7R antagonists can potentially become another pharmacological target for anti-tumor therapy.
Fig. 4.
P2X7R mediates tumor progression and serves as a potential target for tumor therapy. The TME contains many molecular substances, such as ECM, fibroblasts, macrophages, lymphocytes, and tumor cells. These cells release large amounts of ATP into the extracellular space to act on P2X7R. Finally, ATP is further cleaved into ADP and Ado (CD73 and CD39). P2X7R changes its molecular configuration after activation, opens ion channels, regulates intracellular signal transduction, promotes gene transcription, and promotes the growth, metastasis, and apoptosis of tumor cells. Importantly, P2X7R antagonists (such as A438079, AZD9056, and A740003) antagonize its activation and inhibit the growth, apoptosis, and EMT of tumor cells
The role of P2X7R in cancer pain
Cancer pain
Cancer pain is a serious complication in patients with metastatic or advanced cancer and is more complicated than other types of pain (inflammatory and neuropathic pain). Currently, cancer pain is mainly attributed to the following three aspects: (1) pain caused by the tumor itself; in the process of tumor proliferation and metastasis, the tumor compresses or damages peripheral nerves, leading to sensitization of peripheral sensory organs and enhanced pain information transmission, which can lead to nociceptive pain or neuropathic pain [125, 126]. Additionally, carcinogenic sites can induce the infiltration of immune cells (such as lymphocytes and tumor-associated macrophages), release inflammatory factors, and enhance pain [4]. Cancer pain is divided into the nociceptive and inflammatory types. (2) Pain induced during tumor treatment (such as surgery, radiotherapy, and chemotherapy), including radiation neuritis, dermatitis, and tissue necrosis caused by the leakage of chemotherapy drugs outside the blood vessels, are mainly nociceptive and neuropathic pain syndromes [127, 128]. Opioids are effective in preventing this type of pain [136,137]. (3) Another cause of pain in patients with cancer is chronic non-cancer pain, which is caused by persistent stimulation of the nervous system and pathological changes [129, 130]. The duration of chronic noncancerous pain may be longer than the healing period of the tumor and may persist even after the noxious stimulus is eliminated [131]. Therefore, there is no effective treatment for this type of pain because the type and degree of pain in patients are related to genetic differences. Therefore, this type of pain requires individualized treatment, making cancer pain more complicated to resolve than other type of pain and seriously affecting the quality of life [132].
The role and mechanism of P2X7R in cancer pain
P2X7R is widely expressed in the central and peripheral systems, mainly in the microglia. However, the expression of P2X7R in neurons and astrocytes remains controversial [133–135]. ATP activates P2X7R, which induces the activation of inflammatory and nerve cells, enhances peripheral receptors and pain information transmission, and triggers pain production. This has been confirmed in inflammatory and neuropathic pain [28, 136]. Correspondingly, progress has been made in understanding the contribution of P2X7R to cancer pain [4, 137, 138] (Fig. 3). Studies have shown that the P2X7R antagonist BBG or small interfering RNA targeting P2X7R inhibit the expression of P2X7R in the rostral ventromedial nucleus (RVM), significantly reducing the expression levels of 5-HT and Fos in the spinal cord, and reduce the pain behavior in rats with tumors [139].
Different animal models have revealed that P2X7R is involved in the occurrence of cancer pain, and it has been determined that P2X7R is an important mediator of the development of cancer pain [19]. Cancer bone pain, such as that in breast and prostate cancers with bone metastases, is the most widely studied [140, 141]. Microglia are resident immune cells in the central nervous system and play a key role in maintaining the stability of nervous system functions. Microglia are involved in the development of bone cancer. The transition from the pro-inflammatory M1 phenotype to the anti-inflammatory M2 phenotype provides a new treatment strategy for cancer pain [142, 143]. A rat model of bone cancer-related pain was established by injecting AT-3.1 prostate cancer cells into the tibia. It can cause progressive bone destruction in the proximal tibia, progressive thermal hyperalgesia, mechanical hyperalgesia, and spontaneous retraction. Furthermore, it was found that astrocytes and microglia were significantly activated in the ipsilateral spinal cord, characterized by enhanced immunostaining of glial fibrillary acidic protein (GFAP) and OX-42 (microglial marker) [144]. P2X7R participates in the development of cancer pain by regulating the activity of microglia and is a key mechanism of cancer pain mediated by P2X7R. Expression of P2X7R and M1 microglial markers is upregulated during the occurrence and development of bone cancer. BBG (P2X7R specific antagonist) can relieve pain, promote the polarization of microglia on the M2 phenotype, and inhibit the M1 phenotype in vivo and in vitro [143].
Walker-256 breast cancer cells were inoculated into the tibias of rats to establish an animal model of bone cancer. BBG could effectively relieve bone cancer pain, and its inhibitory effect on spinal cord P2X7R may be by inhibiting the formation of microglia NF-κB p-p65, NLRP3 and the expression of downstream pain factor IL-1β [144]. Moreover, P2X7R siRNA reduced the activity of BV2 microglia, nuclear translocation of NF-κB and synthesis of NLRP3 and IL-1β, resulting in an analgesic effect [144]. Studies have shown that bone cancer increases the ATP levels in the cerebrospinal fluid and upregulates P2X7R, phosphorylated p38, and IL-18 in spinal microglia. Inhibition of the P2X7R/p-38/IL-18 pathway can relieve pain in advanced bone cancer and inhibit overactivity of spinal cord neurons [70]. Studies have shown that P2X7R knockout mice exhibit earlier bone cancer pain behavior, and treatment with the P2X7R antagonist A-438079 fails to alleviate the pain behavior induced by astrocyte activation. This indicates that P2X7R in astrocytes plays a lesser role in bone cancer pain [145, 146].
Although P2X7R is involved in the occurrence and development of cancer pain, different contradictory results have been reported regarding its role in cancer bone pain. P2X7R gene knockout studies have shown nociceptive effects, whereas studies on the pharmacological effects of P2X7R antagonists have shown nociceptive or no effects [147]. Studies have shown that in a bone cancer pain model, the expression of P2X7R in the ventrolateral area of the midbrain periaqueductal gray (VlPAG) is increased, and the P2X7R antagonist A740003 can antagonize the analgesic effect of tramadol in rats with bone cancer pain [148]. In a chronic bone cancer pain treatment study, the administration of the P2X7R antagonist AFC5261 (300 mg/kg) aggravated pain-related behavior but did not affect overall bone degeneration or tumor progression. In contrast, 50 and 100 mg/kg AFC5261 had no effect on pain-related behaviors in mice [149]. This suggests that chronic high-dose P2X7R inhibitors may aggravate bone pain caused by cancer.
Another notable finding was that P2X7R played an important role in mediating advanced cancer pain [150]. Studies have shown that microglia in the spinal dorsal horn continue to be activated in bone cancer on days 14 and 21, upregulating the expression of P2X7R, promoting the release of the inflammatory cytokine IL-18, enhance the synaptic transmission of spinal cord nociceptive neurons, and inducing hyperalgesia, which play a role in maintaining advanced cancer pain in women. However, it did not activate microglia on day 7 [151]. There were differences in sensitivity to P2X7R-mediated pain at different stages of cancer. This difference may be related to the characteristics of the P2X7R gene, and may also be related to the degree of glial cell activation and inflammatory factor stimulation. Some studies have revealed that P2X7R in the tumor area can mediate immune cell infiltration and the release of damaging factors [145, 152, 153]. Other studies have also shown that P2X7R is involved in the development of chronic postoperative pain [154, 155]. For example, P2X7R expression is upregulated in chronic postoperative pain, microglia are activated, serotonin (5-HT) is released, and pain is induced, whereas the use of the P2X7R antagonist BBG reverses this [156].
In summary, P2X7R is an important mediator of cancer pain and plays various roles in pain regulation. These studies also suggest that inhibiting P2X7R activation or reducing its expression may provide greater value and broader scope for the treatment of cancer pain. Therefore, P2X7R antagonists are potential pharmacological targets for the treatment of cancer pain.
Discussion and conclusion
The role of P2X7R in the pathogenesis of tumors and cancer pain has been significantly elucidated. Many studies have confirmed that P2X7R has a regulatory effect on both tumors and cancer pain. P2X7R is activated by ATP in the tumor microenvironment and plays an important role in regulating the biological behavior of tumor cells. However, P2X7R can also promote cell survival and induce cytotoxicity. The mechanism by which these two opposing effects are controlled is not fully understood. However, this may be related to the concentration of activators and the degree of activation of P2X7R. Under temporary stimulation by ATP or activators, the activation of P2X7R opens ion channels and promotes the growth and metastasis of tumor cells. However, under continuous or high activator concentrations, the ion channels enlarge the membrane pores, which mediates cell apoptosis and death. Therefore, it is very important to explore the optimal concentration and time of ATP or activators and the degree of activation of P2X7R to regulate tumor progression. In addition, immune escape or stimulation in the tumor microenvironment plays a decisive role in the development and fate of tumor cells, and P2X7R mediates these immune cell activities to regulate tumor cell progression. Therefore, improvement of the tumor microenvironment (reducing immunosuppression, increasing immune cell activation, and proliferation) can be the basis of anti-tumor therapy. Thus, the effects of these factors should be considered before P2X7R can be used as an antitumor therapy.
Moreover, tumors can injure and invade the nerves, causing pain. P2X7R activation or increased expression can induce cancer pain and play an important regulatory role in the development of pain. Importantly, the use of P2X7R antagonists or knockdown of their expression has antitumor efficacy, which may have a certain relieving effect on cancer pain. Furthermore, inhibiting P2X7R activation or reducing its expression can inhibit the activity of microglia, reduce the release of inflammatory injury factors, and decrease the synaptic plasticity of neurons. P2X7R has a protective effect on neurons, and analgesic and pharmacological effects on cancer pain. Therefore, the development and use of P2X7R antagonists to inhibit cancer pain and antitumor therapy have great prospects, and are expected to become new pharmacological targets for the treatment of both.
Acknowledgements
Thanks to Si-jian Lin for designing, completing and revising this article, getting fund support, and thanks to Yong-sheng Xu and Jun Xiang for providing valuable help.
Yong-sheng Xu
master degree student, mainly engaged in gastrointestinal cancer research, have a certain understanding of tumor.

Author contributions
Yong-sheng Xu: wrote and revised this article. Jun Xiang: written, revised and graphed. Si-jian Lin: completed the revision of this article and obtained the fund.
Funding
These studies were supported by grants from the Science and Technology Plan Project of Jiangxi Health Commission (202310504). The funding body played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Data availability
All data generated or analyzed during this study are included in this article. And we have not used other data that has already been published. All the data presented in this article are original results derived from this study.
Declarations
Ethics approval and consent to participate
None applicable.
Consent for publication
None applicable.
Competing interests
The authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mair F, Erickson JR, Frutoso M, Konecny AJ, Greene E, Voillet V, Maurice NJ, Rongvaux A, Dixon D, Barber B, Gottardo R, Prlic M (2022) Extricating human tumour immune alterations from tissue inflammation. Nature 605(7911):728–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D’Alterio C, Scala S, Sozzi G, Roz L, Bertolini G (2020) Paradoxical effects of chemotherapy on tumor relapse and metastasis promotion. Semin Cancer Biol 60:351–361 [DOI] [PubMed] [Google Scholar]
- 3.Lei G, Zhuang L, Gan B (2024) The roles of ferroptosis in cancer: Tumor suppression, tumor microenvironment, and therapeutic interventions. Cancer Cell 42(4):513–534 [DOI] [PubMed] [Google Scholar]
- 4.Zhang WJ, Luo C, Pu FQ, Zhu JF, Zhu Z (2020) The role and pharmacological characteristics of ATP-gated ionotropic receptor P2X in cancer pain. Pharmacol Res 161:105106 [DOI] [PubMed] [Google Scholar]
- 5.Ding Z, Liang X, Wang J, Song Z, Guo Q, Schäfer MKE, Huang C (2023) Inhibition of spinal ferroptosis-like cell death alleviates hyperalgesia and spontaneous pain in a mouse model of bone cancer pain. Redox Biol 62:102700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma Y, Han C, Xie C, Dang Q, Yang L, Li Y, Zhang M, Cheng J, Yang Y, Xu Q, Li P (2023) ATP promotes resident CD34+ cell migration mainly through P2Y2-Stim1-ERK/p38 pathway. Am J Physiol Cell Physiol 325(5):C1228–C1243 [DOI] [PubMed] [Google Scholar]
- 7.Koizumi S, Fujishita K (2007) Gliotransmitter ATP-mediated cell-to-cell communication. Brain Nerve 59(7):707–715 [PubMed] [Google Scholar]
- 8.Zhao GL, Zhou H, Guo YH, Zhong SM, Zhou H, Li F, Lei B, Wang Z, Miao Y (2023) Modulation of Rac1/PAK1/connexin43-mediated ATP release from astrocytes contributes to retinal ganglion cell survival in experimental glaucoma. Glia 71(6):1502–1521 [DOI] [PubMed] [Google Scholar]
- 9.Schumacher D, Strilic B, Sivaraj KK, Wettschureck N, Offermanns S (2013) Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell 24(1):130–137 [DOI] [PubMed] [Google Scholar]
- 10.Lalo U, Pankratov Y (2023) ATP-mediated signalling in the central synapses. Neuropharmacology 229:109477 [DOI] [PubMed] [Google Scholar]
- 11.Huang KC, Chiang SF, Lin PC, Hong WZ, Yang PC, Chang HP, Peng SL, Chen TW, Ke TW, Liang JA, Chen WT, Chao KSC (2024) TNFα modulates PANX1 activation to promote ATP release and enhance P2RX7-mediated antitumor immune responses after chemotherapy in colorectal cancer. Cell Death Dis 15(1):24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Marchi E, Orioli E, Pegoraro A, Adinolfi E, Di Virgilio F (2020) Detection of extracellular ATP in the tumor microenvironment, using the pmeLUC biosensor. Methods Mol Biol 2041:183–195 [DOI] [PubMed] [Google Scholar]
- 13.Di Virgilio F, Sarti AC, Falzoni S, De Marchi E, Adinolfi E (2018) Extracellular ATP and P2 purinergic signalling in the tumour microenvironment. Nat Rev Cancer 18(10):601–618 [DOI] [PubMed] [Google Scholar]
- 14.Zou FL, Liu JP, Zuo C, He PF, Ye JX, Zhang WJ (2024) The functional role of P2 purinergic receptors in the progression of gastric cancer. Purinergic Signal. 10.1007/s11302-024-10000-7 [DOI] [PMC free article] [PubMed]
- 15.Schmid R, Evans RJ (2019) ATP-Gated P2X receptor channels: molecular insights into functional roles. Annu Rev Physiol 81:43–62 [DOI] [PubMed] [Google Scholar]
- 16.Hattori M, Gouaux E (2012) Molecular mechanism of ATP binding and ion channel activation in P2X receptors. Nature 485(7397):207–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith J, Menéndez Méndez A, Alves M, Parras A, Conte G, Bhattacharya A, Ceusters M, Nicke A, Henshall DC, Jimenez-Mateos EM, Engel T (2023) The P2X7 receptor contributes to seizures and inflammation-driven long-lasting brain hyperexcitability following hypoxia in neonatal mice. Br J Pharmacol 180(13):1710–1729 [DOI] [PubMed] [Google Scholar]
- 18.Genetzakis E, Gilchrist J, Kassiou M, Figtree GA (2022) Development and clinical translation of P2X7 receptor antagonists: A potential therapeutic target in coronary artery disease? Pharmacol Ther 237:108228 [DOI] [PubMed] [Google Scholar]
- 19.Bernier LP, Ase AR, Séguéla P (2018) P2X receptor channels in chronic pain pathways. Br J Pharmacol 175(12):2219–2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thawkar BS, Kaur G (2019) Inhibitors of NF-κB and P2X7/NLRP3/Caspase 1 pathway in microglia: Novel therapeutic opportunities in neuroinflammation induced early-stage Alzheimer’s disease. J Neuroimmunol 326:62–74 [DOI] [PubMed] [Google Scholar]
- 21.Rabelo ILA, Arnaud-Sampaio VF, Adinolfi E, Ulrich H, Lameu C (2021) Cancer metabostemness and metabolic reprogramming via P2X7 receptor. Cells 10(7):1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Virgilio F, Vultaggio-Poma V, Sarti AC (2021) P2X receptors in cancer growth and progression. Biochem Pharmacol 187:114350 [DOI] [PubMed] [Google Scholar]
- 23.Di Virgilio F, Ferrari D, Adinolfi E (2009) P2X(7): a growth-promoting receptor-implications for cancer. Purinergic Signal 5(2):251–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franceschini A, Adinolfi E (2014) P2X receptors: New players in cancer pain. World J Biol Chem 5(4):429–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chessell I, Hatcher J, Billinton A (2011) Mechanistic differentiation of cancer pain: a pivotal role of P2X7 is absent. Pain 152(8):1703–1704 [DOI] [PubMed] [Google Scholar]
- 26.Zhang WJ, Zhu ZM, Liu ZX (2020) The role and pharmacological properties of the P2X7 receptor in neuropathic pain. Brain Res Bull 155:19–28 [DOI] [PubMed] [Google Scholar]
- 27.Ronning KE, Déchelle-Marquet PA, Che Y, Guillonneau X, Sennlaub F, Delarasse C (2023) The P2X7 receptor, a multifaceted receptor in alzheimer’s disease. Int J Mol Sci 24(14):11747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu SQ, Hu JL, Zou FL, Liu JP, Luo HL, Hu DX, Wu LD, Zhang WJ (2022) P2X7 receptor in inflammation and pain. Brain Res Bull 187:199–209 [DOI] [PubMed] [Google Scholar]
- 29.Martínez-Cuesta MÁ, Blanch-Ruiz MA, Ortega-Luna R, Sánchez-López A, Álvarez Á (2020) Structural and functional basis for understanding the biological significance of P2X7 receptor. Int J Mol Sci 21(22):8454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuo C, Xu YS, He PF, Zhang WJ (2023) ATP ion channel P2X7 receptor as a regulatory molecule in the progression of colorectal cancer. Eur J Med Chem 261:115877 [DOI] [PubMed] [Google Scholar]
- 31.Costa-Junior HM, Sarmento Vieira F, Coutinho-Silva R (2011) C terminus of the P2X7 receptor: treasure hunting. Purinergic Signal 7(1):7–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Migita K, Ozaki T, Shimoyama S, Yamada J, Nikaido Y, Furukawa T, Shiba Y, Egan TM, Ueno S (2016) HSP90 regulation of P2X7 receptor function requires an intact cytoplasmic C-Terminus. Mol Pharmacol 90(2):116–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang X, Samways DSK, Cox J et al (2019) Ca2+ flux through splice variants of the ATP-gated ionotropic receptor P2X7 is regulated by its cytoplasmic N terminus. J Biol Chem 294:12521–12533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong Y, Chen Y, Zhang L, Tian Z, Dong S (2020) P2X7 receptor acts as an efficient drug target in regulating bone metabolism system. Biomed Pharmacother 125:110010 [DOI] [PubMed] [Google Scholar]
- 35.Sperlágh B, Illes P (2014) P2X7 receptor: an emerging target in central nervous system diseases. Trends Pharmacol Sci 35(10):537–547 [DOI] [PubMed] [Google Scholar]
- 36.Bartlett R, Stokes L, Sluyter R (2014) The P2X7 receptor channel: recent developments and the use of P2X7 antagonists in models of disease. Pharmacol Rev 66(3):638–675 [DOI] [PubMed] [Google Scholar]
- 37.De Marchi E, Orioli E, Dal Ben D, Adinolfi E (2016) P2X7 Receptor as a therapeutic target. Adv Protein Chem Struct Biol 104:39–79 [DOI] [PubMed] [Google Scholar]
- 38.Michel AD, Chambers LJ, Walter DS (2008) Negative and positive allosteric modulators of the P2X(7) receptor. Br J Pharmacol 153:737–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hattori M, Gouaux E (2012) Molecular mechanism of ATP binding and ion channel activation in P2X receptors. Nature 485:207–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alves LA, Bezerra RJ, Faria RX, Ferreira LG, da Silva FV (2013) Physiological roles and potential therapeutic applications of the P2X7 receptor in inflammation and pain. Molecules 18(9):10953–10972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wiley JS, Sluyter R, Gu BJ, Stokes L, Fuller SJ (2011) The human P2X7 receptor and its role in innate immunity. Tissue Antigens 78(5):321–332 [DOI] [PubMed] [Google Scholar]
- 42.Alarcón-Vila C, Baroja-Mazo A, de Torre-Minguela C, Martínez CM, Martínez-García JJ, Martínez-Banaclocha H, García-Palenciano C, Pelegrin P (2020) CD14 release induced by P2X7 receptor restricts inflammation and increases survival during sepsis. Elife 9:e60849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rivas-Yáñez E, Barrera-Avalos C, Parra-Tello B, Briceño P, Rosemblatt MV, Saavedra-Almarza J, Rosemblatt M, Acuña-Castillo C, Bono MR, Sauma D (2020) P2X7 receptor at the crossroads of T cell fate. Int J Mol Sci 21(14):4937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vultaggio-Poma V, Di Virgilio F (2022) P2 receptors: novel disease markers and metabolic checkpoints in immune cells. Biomolecules 12(7):983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Francistiová L, Bianchi C, Di Lauro C, Sebastián-Serrano Á, de Diego-García L, Kobolák J, Dinnyés A, Díaz-Hernández M (2020) The role of P2X7 receptor in Alzheimer’s disease. Front Mol Neurosci 13:94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kasuya G, Yamaura T, Ma XB, Nakamura R, Takemoto M, Nagumo H, Tanaka E, Dohmae N, Nakane T, Yu Y, Ishitani R, Matsuzaki O, Hattori M, Nureki O (2017) Structural insights into the competitive inhibition of the ATP-gated P2X receptor channel. Nat Commun 8(1):876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jindrichová M, Zemková H (2013) Purinergní P2X rodina a specifické vlastnosti P2X7 podtypu [Purinergic P2X family and specific features of the P2X7 subtype]. Cesk Fysiol 62(2):40–46 [PubMed] [Google Scholar]
- 48.Sharma S, Kalra H, Akundi RS (2021) Extracellular ATP mediates cancer cell migration and invasion through increased expression of cyclooxygenase 2. Front Pharmacol 11:617211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pegoraro A, De Marchi E, Adinolfi E (2021) P2X7 variants in oncogenesis. Cells 10(1):189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burnstock G, Knight GE (2018) The potential of P2X7 receptors as a therapeutic target, including inflammation and tumour progression. Purinergic Signal 14(1):1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Di Virgilio F, Adinolfi E (2017) Extracellular purines, purinergic receptors and tumor growth. Oncogene 36(3):293–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Q, Zhu X, Song W, Peng X, Zhao R (2020) The P2X7 purinergic receptor: a potential therapeutic target for lung cancer. J Cancer Res Clin Oncol 146(11):2731–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang Y, Qiao C, Li Q, Zhu X, Zhao R, Peng X (2023) Research progress in the relationship between P2X7R and cervical cancer. Reprod Sci 30(3):823–834 [DOI] [PubMed] [Google Scholar]
- 54.Bai X, Li Q, Peng X, Li X, Qiao C, Tang Y, Zhao R (2023) P2X7 receptor promotes migration and invasion of non-small cell lung cancer A549 cells through the PI3K/Akt pathways. Purinergic Signal 19(4):685–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roliano GG, Azambuja JH, Brunetto VT, Butterfield HE, Kalil AN, Braganhol E (2022) Colorectal cancer and purinergic signalling: an overview. Cancers (Basel) 14(19):4887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Künzli BM, Bernlochner MI, Rath S, Käser S, Csizmadia E, Enjyoji K, Cowan P, d’Apice A, Dwyer K, Rosenberg R, Perren A, Friess H, Maurer CA, Robson SC (2011) Impact of CD39 and purinergic signalling on the growth and metastasis of colorectal cancer. Purinergic Signal 7(2):231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lili W, Yun L, Tingran W, Xia W, Yanlei S (2019) P2RX7 functions as a putative biomarker of gastric cancer and contributes to worse prognosis. Exp Biol Med (Maywood) 244(9):734–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu X, Li Q, Song W, Peng X, Zhao R (2021) P2X7 receptor: a critical regulator and potential target for breast cancer. J Mol Med (Berl) 99(3):349–358 [DOI] [PubMed] [Google Scholar]
- 59.Magni L, Bouazzi R, Heredero Olmedilla H, Petersen PSS, Tozzi M, Novak I (2021) The P2X7 receptor stimulates IL-6 release from pancreatic stellate cells and tocilizumab prevents activation of STAT3 in pancreatic cancer cells. Cells 10(8):1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song H, Arredondo Carrera HM, Sprules A, Ji Y, Zhang T, He J, Lawrence E, Gartland A, Luo J, Wang N (2023) C-terminal variants of the P2X7 receptor are associated with prostate cancer progression and bone metastasis - evidence from clinical and pre-clinical data. Cancer Commun (Lond) 43(3):400–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matyśniak D, Nowak N, Chumak V, Pomorski P (2020) P2X7 receptor activity landscape in rat and human glioma cell lines. Acta Biochim Pol 67(1):7–14 [DOI] [PubMed] [Google Scholar]
- 62.Benzaquen J, Dit Hreich SJ, Heeke S, Juhel T, Lalvee S, Bauwens S, Saccani S, Lenormand P, Hofman V, Butori M, Leroy S, Berthet JP, Marquette CH, Hofman P, Vouret-Craviari V (2020) P2RX7B is a new theranostic marker for lung adenocarcinoma patients. Theranostics 10(24):10849–10860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Calik I, Calik M, Turken G, Ozercan IH (2020) A promising independent prognostic biomarker in colorectal cancer: P2X7 receptor. Int J Clin Exp Pathol 13(2):107–121 [PMC free article] [PubMed] [Google Scholar]
- 64.Qian F, Xiao J, Hu B, Sun N, Yin W, Zhu J (2017) High expression of P2X7R is an independent postoperative indicator of poor prognosis in colorectal cancer. Hum Pathol 64:61–68 [DOI] [PubMed] [Google Scholar]
- 65.Zhang Y, Ding J, Wang L (2019) The role of P2X7 receptor in prognosis and metastasis of colorectal cancer. Adv Med Sci 64(2):388–394 [DOI] [PubMed] [Google Scholar]
- 66.Calik I, Calik M, Sarikaya B, Ozercan IH, Arslan R, Artas G, Dagli AF (2020) P2X7 receptor as an independent prognostic indicator in gastric cancer. Bosn J Basic Med Sci 20(2):188–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Markwardt F (2021) Human P2X7 receptors - Properties of single ATP-gated ion channels. Biochem Pharmacol 187:114307 [DOI] [PubMed] [Google Scholar]
- 68.Matyśniak D, Chumak V, Nowak N, Kukla A, Lehka L, Oslislok M, Pomorski P (2022) P2X7 receptor: the regulator of glioma tumor development and survival. Purinergic Signal 18(1):135–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S (2017) The P2X7 receptor in infection and inflammation. Immunity 47(1):15–31 [DOI] [PubMed] [Google Scholar]
- 70.Wu P, Wu X, Zhou G, Wang Y, Liu X, Lv R, Liu Y, Wen Q (2022) P2X7 Receptor-induced bone cancer pain by regulating microglial activity via NLRP3/IL-1beta signaling. Pain Physician 25(8):E1199–E1210 [PubMed] [Google Scholar]
- 71.Stachon P, Heidenreich A, Merz J, Hilgendorf I, Wolf D, Willecke F, von Garlen S, Albrecht P, Härdtner C, Ehrat N, Hoppe N, Reinöhl J, von Zur MC, Bode C, Idzko M, Zirlik A (2017) P2X7 Deficiency blocks lesional inflammasome activity and ameliorates atherosclerosis in mice. Circulation 135(25):2524–2533 [DOI] [PubMed] [Google Scholar]
- 72.Adinolfi E, De Marchi E, Orioli E, Pegoraro A, Di Virgilio F (2019) Role of the P2X7 receptor in tumor-associated inflammation. Curr Opin Pharmacol 47:59–64 [DOI] [PubMed] [Google Scholar]
- 73.Magnaghi V, Martin S, Smith P, Allen L, Conte V, Reid AJ, Faroni A (2021) Peripheral nerve regeneration following injury is altered in mice lacking P2X7 receptor. Eur J Neurosci 54(5):5798–5814 [DOI] [PubMed] [Google Scholar]
- 74.Bravo D, Maturana CJ, Pelissier T, Hernández A, Constandil L (2015) Interactions of pannexin 1 with NMDA and P2X7 receptors in central nervous system pathologies: Possible role on chronic pain. Pharmacol Res 101:86–93 [DOI] [PubMed] [Google Scholar]
- 75.Liu C, Tian Q, Wang J, He P, Han S, Guo Y, Yang C, Wang G, Wei H, Li M (2023) Blocking P2RX7 attenuates ferroptosis in endothelium and reduces HG-induced hemorrhagic transformation after MCAO by inhibiting ERK1/2 and P53 signaling pathways. Mol Neurobiol 60(2):460–479 [DOI] [PubMed] [Google Scholar]
- 76.Rossato M, Di Vincenzo A, Pagano C, El Hadi H, Vettor R (2020) The P2X7 receptor and NLRP3 Axis in non-alcoholic fatty liver disease: a brief review. Cells 9(4):1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Caglayan B, Caglayan AB, Beker MC, Yalcin E, Beker M, Kelestemur T, Sertel E, Ozturk G, Kilic U, Sahin F, Kilic E (2017) Evidence that activation of P2X7R does not exacerbate neuronal death after optic nerve transection and focal cerebral ischemia in mice. Exp Neurol 296:23–31 [DOI] [PubMed] [Google Scholar]
- 78.Vázquez-Cuevas FG, Martínez-Ramírez AS, Robles-Martínez L, Garay E, García-Carrancá A, Pérez-Montiel D, Castañeda-García C, Arellano RO (2014) Paracrine stimulation of P2X7 receptor by ATP activates a proliferative pathway in ovarian carcinoma cells. J Cell Biochem 115(11):1955–1966 [DOI] [PubMed] [Google Scholar]
- 79.Choi JH, Ji YG, Ko JJ, Cho HJ, Lee DH (2018) Activating P2X7 receptors increases proliferation of human pancreatic cancer cells via ERK1/2 and JNK. Pancreas 47(5):643–651 [DOI] [PubMed] [Google Scholar]
- 80.Zhang WJ, Luo C, Huang C, Pu FQ, Zhu JF, Zhu ZM (2021) PI3K/Akt/GSK-3β signal pathway is involved in P2X7 receptor-induced proliferation and EMT of colorectal cancer cells. Eur J Pharmacol 899:174041 [DOI] [PubMed] [Google Scholar]
- 81.Yang C, Shi S, Su Y, Tong JS, Li L (2020) P2X7R promotes angiogenesis and tumour-associated macrophage recruitment by regulating the NF-κB signalling pathway in colorectal cancer cells. J Cell Mol Med 24(18):10830–10841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ryu JK, Jantaratnotai N, Serrano-Perez MC, McGeer PL, McLarnon JG (2011) Block of purinergic P2X7R inhibits tumor growth in a C6 glioma brain tumor animal model. J Neuropathol Exp Neurol 70(1):13–22 [DOI] [PubMed] [Google Scholar]
- 83.Adinolfi E, Raffaghello L, Giuliani AL, Cavazzini L, Capece M, Chiozzi P, Bianchi G, Kroemer G, Pistoia V, Di Virgilio F (2012) Expression of P2X7 receptor increases in vivo tumor growth. Cancer Res 72(12):2957–2969 [DOI] [PubMed] [Google Scholar]
- 84.Adinolfi E, Capece M, Franceschini A, Falzoni S, Giuliani AL, Rotondo A, Sarti AC, Bonora M, Syberg S, Corigliano D, Pinton P, Jorgensen NR, Abelli L, Emionite L, Raffaghello L, Pistoia V, Di Virgilio F (2015) Accelerated tumor progression in mice lacking the ATP receptor P2X7. Cancer Res 75(4):635–644 [DOI] [PubMed] [Google Scholar]
- 85.Draganov D, Gopalakrishna-Pillai S, Chen YR, Zuckerman N, Moeller S, Wang C, Ann D, Lee PP (2015) Modulation of P2X4/P2X7/Pannexin-1 sensitivity to extracellular ATP via Ivermectin induces a non-apoptotic and inflammatory form of cancer cell death. Sci Rep 5:16222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Garg AD, Krysko DV, Vandenabeele P, Agostinis P (2016) Extracellular ATP and P2X7 receptor exert context-specific immunogenic effects after immunogenic cancer cell death. Cell Death Dis 7(2):e2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dreisig K, Sund L, Dommer MW, Kristensen NP, Boddum K, Viste R, Fredholm S, Odum N, Jäättelä M, Skov S, Kornum BR (2018) Human p2y11expression level affects human P2X7 receptor-mediated cell death. Front Immunol 9:1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Andrade MP, Bian S, Savio LEB, Zhang H, Zhang J, Junger W, Wink MR, Lenz G, Buffon A, Wu Y, Robson SC (2017) Hyperthermia and associated changes in membrane fluidity potentiate P2X7 activation to promote tumor cell death. Oncotarget 8(40):67254–67268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bian S, Sun X, Bai A, Zhang C, Li L, Enjyoji K, Junger WG, Robson SC, Wu Y (2013) P2X7 integrates PI3K/AKT and AMPK-PRAS40-mTOR signaling pathways to mediate tumor cell death. PLoS ONE 8(4):e60184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Giannuzzo A, Pedersen SF, Novak I (2015) The P2X7 receptor regulates cell survival, migration and invasion of pancreatic ductal adenocarcinoma cells. Mol Cancer 14:203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Avanzato D, Genova T, Fiorio Pla A, Bernardini M, Bianco S, Bussolati B, Mancardi D, Giraudo E, Maione F, Cassoni P, Castellano I, Munaron L (2016) Activation of P2X7 and P2Y11 purinergic receptors inhibits migration and normalizes tumor-derived endothelial cells via cAMP signaling. Sci Rep 6:32602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fang J, Chen X, Zhang L, Chen J, Liang Y, Li X, Xiang J, Wang L, Guo G, Zhang B, Zhang W (2013) P2X7R suppression promotes glioma growth through epidermal growth factor receptor signal pathway. Int J Biochem Cell Biol 45(6):1109–1120 [DOI] [PubMed] [Google Scholar]
- 93.Zhou Y, Fei M, Zhang G, Liang WC, Lin W, Wu Y, Piskol R, Ridgway J, McNamara E, Huang H, Zhang J, Oh J, Patel JM, Jakubiak D, Lau J, Blackwood B, Bravo DD, Shi Y, Wang J, Hu HM, Lee WP, Jesudason R, Sangaraju D, Modrusan Z, Anderson KR, Warming S, Roose-Girma M, Yan M (2020) Blockade of the phagocytic Receptor MerTK on tumor-associated macrophages enhances P2X7R-dependent STING activation by tumor-derived cGAMP. Immunity 52(2):357-373.e9 [DOI] [PubMed] [Google Scholar]
- 94.Arnaud-Sampaio VF, Rabelo ILA, Ulrich H, Lameu C (2020) The P2X7 receptor in the maintenance of cancer stem cells, chemoresistance and metastasis. Stem Cell Rev Rep 16(2):288–300 [DOI] [PubMed] [Google Scholar]
- 95.De Marchi E, Orioli E, Pegoraro A, Sangaletti S, Portararo P, Curti A, Colombo MP, Di Virgilio F, Adinolfi E (2019) The P2X7 receptor modulates immune cells infiltration, ectonucleotidases expression and extracellular ATP levels in the tumor microenvironment. Oncogene 38(19):3636–3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qin J, Zhang X, Tan B, Zhang S, Yin C, Xue Q, Zhang Z, Ren H, Chen J, Liu M, Qian M, Du B (2020) Blocking P2X7-mediated macrophage polarization overcomes treatment resistance in lung cancer. Cancer Immunol Res 8(11):1426–1439 [DOI] [PubMed] [Google Scholar]
- 97.Gehring MP, Kipper F, Nicoletti NF, Sperotto ND, Zanin R, Tamajusuku AS, Flores DG, Meurer L, Roesler R, Filho AB, Lenz G, Campos MM, Morrone FB (2015) P2X7 receptor as predictor gene for glioma radiosensitivity and median survival. Int J Biochem Cell Biol 68:92–100 [DOI] [PubMed] [Google Scholar]
- 98.van den Bent MJ, Geurts M, French PJ, Smits M, Capper D, Bromberg JEC, Chang SM (2023) Primary brain tumours in adults. Lancet 402(10412):1564–1579 [DOI] [PubMed] [Google Scholar]
- 99.Lee YT, Tan YJ, Oon CE (2018) Molecular targeted therapy: Treating cancer with specificity. Eur J Pharmacol 834:188–196 [DOI] [PubMed] [Google Scholar]
- 100.Vessoni AT, Filippi-Chiela EC, Lenz G, Batista LFZ (2020) Tumor propagating cells: drivers of tumor plasticity, heterogeneity, and recurrence. Oncogene 39(10):2055–2068 [DOI] [PubMed] [Google Scholar]
- 101.Vultaggio-Poma V, Sarti AC, Di Virgilio F (2020) Extracellular ATP: a feasible target for cancer therapy. Cells 9(11):2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morrone FB, Gehring MP, Nicoletti NF (2016) Calcium channels and associated receptors in malignant brain tumor therapy. Mol Pharmacol 90(3):403–409 [DOI] [PubMed] [Google Scholar]
- 103.Ren S, Zhang Y, Wang Y, Lui Y, Wei W, Huang X, Mao W, Zuo Y (2010) Targeting P2X7 receptor inhibits the metastasis of murine P388D1 lymphoid neoplasm cells to lymph nodes. Cell Biol Int 34(12):1205–1211 [DOI] [PubMed] [Google Scholar]
- 104.Zheng L, Zhang X, Yang F, Zhu J, Zhou P, Yu F, Hou L, Xiao L, He Q, Wang B (2014) Regulation of the P2X7R by microRNA-216b in human breast cancer. Biochem Biophys Res Commun 452(1):197–204 [DOI] [PubMed] [Google Scholar]
- 105.Tsuzuki K, Ase A, Séguéla P et al (2003) TNP-ATP-resistant P2X ionic current on the central terminals and somata of rat primary sensory neurons. J Neurophysiol 89:3235–3242 [DOI] [PubMed] [Google Scholar]
- 106.Cho JH, Jung KY, Jung Y et al (2013) Design and synthesis of potent and selective P2X3 receptor antagonists derived from PPADS as potential pain modulators. Eur J Med Chem 70:811–830 [DOI] [PubMed] [Google Scholar]
- 107.Chen K, Zhang J, Zhang W et al (2013) ATP-P2X4 signaling mediates NLRP3 inflammasome activation: a novel pathway of diabetic nephropathy. Int J Biochem Cell Biol 45:932–943 [DOI] [PubMed] [Google Scholar]
- 108.Mousawi F, Peng H, Li J, Ponnambalam S, Roger S, Zhao H, Yang X, Jiang LH (2020) Chemical activation of the Piezo1 channel drives mesenchymal stem cell migration via inducing ATP release and activation of P2 receptor purinergic signaling. Stem Cells 38(3):410–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Drill M, Jones NC, Hunn M, O’Brien TJ, Monif M (2021) Antagonism of the ATP-gated P2X7 receptor: a potential therapeutic strategy for cancer. Purinergic Signal 17(2):215–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Donnelly-Roberts DL, Namovic MT, Surber B, Vaidyanathan SX, Perez-Medrano A, Wang Y, Carroll WA, Jarvis MF (2009) [3H]A-804598 ([3H]2-cyano-1-[(1S)-1-phenylethyl]-3-quinolin-5-ylguanidine) is a novel, potent, and selective antagonist radioligand for P2X7 receptors. Neuropharmacology 56(1):223–229 [DOI] [PubMed] [Google Scholar]
- 111.Santos AA Jr, Cappellari AR, de Marchi FO, Gehring MP, Zaparte A, Brandão CA, Lopes TG, da Silva VD, Pinto LFR, Savio LEB, Moreira-Souza ACA, Coutinho-Silva R, Paccez JD, Zerbini LF, Morrone FB (2017) Potential role of P2X7R in esophageal squamous cell carcinoma proliferation. Purinergic Signal 13(3):279–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kan LK, Drill M, Jayakrishnan PC, Sequeira RP, Galea E, Todaro M, Sanfilippo PG, Hunn M, Williams DA, O’Brien TJ, Drummond KJ, Monif M (2023) P2X7 receptor antagonism by AZ10606120 significantly reduced in vitro tumour growth in human glioblastoma. Sci Rep 13(1):8435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Amoroso F, Capece M, Rotondo A, Cangelosi D, Ferracin M, Franceschini A, Raffaghello L, Pistoia V, Varesio L, Adinolfi E (2015) The P2X7 receptor is a key modulator of the PI3K/GSK3β/VEGF signaling network: evidence in experimental neuroblastoma. Oncogene 34(41):5240–5251 [DOI] [PubMed] [Google Scholar]
- 114.Vázquez-Cuevas FG, Cruz-Rico A, Garay E, García-Carrancá A, Pérez-Montiel D, Juárez B, Arellano RO (2013) Differential expression of the P2X7 receptor in ovarian surface epithelium during the oestrous cycle in the mouse. Reprod Fertil Dev 25(7):971–984 [DOI] [PubMed] [Google Scholar]
- 115.Kan LK, Seneviratne S, Drummond KJ, Williams DA, O’Brien TJ, Monif M (2020) P2X7 receptor antagonism inhibits tumour growth in human high-grade gliomas. Purinergic Signal 16(3):327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang Y, Li F, Wang L, Lou Y (2021) A438079 affects colorectal cancer cell proliferation, migration, apoptosis, and pyroptosis by inhibiting the P2X7 receptor. Biochem Biophys Res Commun 558:147–153 [DOI] [PubMed] [Google Scholar]
- 117.Park JH, Williams DR, Lee JH, Lee SD, Lee JH, Ko H, Lee GE, Kim S, Lee JM, Abdelrahman A, Müller CE, Jung DW, Kim YC (2016) Potent suppressive effects of 1-Piperidinylimidazole based novel P2X7 receptor antagonists on cancer cell migration and invasion. J Med Chem 59(16):7410–7430 [DOI] [PubMed] [Google Scholar]
- 118.Mohammed A, Janakiram NB, Madka V, Pathuri G, Li Q, Ritchie R, Biddick L, Kutche H, Zhang Y, Singh A, Gali H, Lightfoot S, Steele VE, Suen CS, Rao CV (2017) Lack of chemopreventive effects of P2X7R inhibitors against pancreatic cancer. Oncotarget 8(58):97822–97834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sainz B Jr, Alcala S, Garcia E, Sanchez-Ripoll Y, Azevedo MM, Cioffi M, Tatari M, Miranda-Lorenzo I, Hidalgo M, Gomez-Lopez G, Cañamero M, Erkan M, Kleeff J, García-Silva S, Sancho P, Hermann PC, Heeschen C (2015) Microenvironmental hCAP-18/LL-37 promotes pancreatic ductal adenocarcinoma by activating its cancer stem cell compartment. Gut 64(12):1921–1935 [DOI] [PubMed] [Google Scholar]
- 120.Ganguly A, Michael M, Goschin S, Harris K, McFarland DC (2022) Cancer pain and opioid use disorder. Oncology (Williston Park) 36(9):535–541 [DOI] [PubMed] [Google Scholar]
- 121.Ziberi S, Zuccarini M, Carluccio M, Giuliani P, Ricci-Vitiani L, Pallini R, Caciagli F, Di Iorio P, Ciccarelli R (2019) Upregulation of epithelial-to-mesenchymal transition markers and P2X7 receptors is associated to increased invasiveness caused by P2X7 receptor stimulation in human glioblastoma stem cells. Cells 9(1):85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jeddi HM, Busse JW, Sadeghirad B, Levine M, Zoratti MJ, Wang L, Noori A, Couban RJ, Tarride JE (2024) Cannabis for medical use versus opioids for chronic non-cancer pain: a systematic review and network meta-analysis of randomised clinical trials. BMJ Open 14(1):e068182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gehring MP, Pereira TC, Zanin RF, Borges MC, Braga Filho A, Battastini AM, Bogo MR, Lenz G, Campos MM, Morrone FB (2012) P2X7 receptor activation leads to increased cell death in a radiosensitive human glioma cell line. Purinergic Signal 8(4):729–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tanamachi K, Nishino K, Mori N, Suzuki T, Tanuma SI, Abe R, Tsukimoto M (2017) Radiosensitizing Effect of P2X7 receptor antagonist on melanoma in Vitro and in Vivo. Biol Pharm Bull 40(6):878–887 [DOI] [PubMed] [Google Scholar]
- 125.Landini L, Marini M, Souza Monteiro de Araujo D, Romitelli A, Montini M, Albanese V, Titiz M, Innocenti A, Bianchini F, Geppetti P, Nassini R, De Logu F (2023) Schwann cell insulin-like growth factor receptor type-1 mediates metastatic bone cancer pain in mice. Brain Behav Immun 110:348–364 [DOI] [PubMed] [Google Scholar]
- 126.Wang K, Donnelly CR, Jiang C, Liao Y, Luo X, Tao X, Bang S, McGinnis A, Lee M, Hilton MJ, Ji RR (2021) STING suppresses bone cancer pain via immune and neuronal modulation. Nat Commun 12(1):4558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dantigny R, Tanty A, Fourneret P et al (2018) Prevalence of pain in radiotherapy and improvement of its management. Bull Cancer 105:1183–1192 [DOI] [PubMed] [Google Scholar]
- 128.Navia-Pelaez JM, Borgespaes Lemes J, Gonzalez L, Delay L, Dos Santos Aggum Capettini L, Lu JW, Gonçalves Dos Santos G, Gregus AM, Dougherty PM, Yaksh TL, Miller YI (2023) AIBP regulates TRPV1 activation in chemotherapy-induced peripheral neuropathy by controlling lipid raft dynamics and proximity to TLR4 in dorsal root ganglion neurons. Pain 164(6):e274–e285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cheatle MD, Falcone M, Dhingra L et al (2020) Independent association of tobacco use with opioid use disorder in patients of European ancestry with chronic non-cancer pain. Drug Alcohol Depend 209:107901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Diasso PDK, Abou-Kassem D, Nielsen SD, Main KM, Sjøgren P, Kurita GP (2023) Long-term opioid treatment and endocrine measures in chronic non-cancer pain patients. Eur J Pain 27(8):940–951 [DOI] [PubMed] [Google Scholar]
- 131.Hu JL, Zhang WJ (2023) The role and pharmacological properties of P2Y12 receptor in cancer and cancer pain. Biomed Pharmacother 157:113927 [DOI] [PubMed] [Google Scholar]
- 132.Turk DC, Wilson HD, Cahana A (2011) Treatment of chronic noncancer pain. Lancet 377:2226–2235 [DOI] [PubMed] [Google Scholar]
- 133.Huang S, Dong W, Lin X, Xu K, Li K, Xiong S, Wang Z, Nie X, Bian JS (2024) Disruption of the Na+/K+-ATPase-purinergic P2X7 receptor complex in microglia promotes stress-induced anxiety. Immunity 57(3):495-512.e11 [DOI] [PubMed] [Google Scholar]
- 134.Miras-Portugal MT, Sebastián-Serrano Á, de Diego GL, Díaz-Hernández M (2017) Neuronal P2X7 receptor: involvement in neuronal physiology and pathology. J Neurosci 37(30):7063–7072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Queipo MJ, Gil-Redondo JC, Morente V, Ortega F, Miras-Portugal MT, Delicado EG, Pérez-Sen R (2018) P2X7 Nucleotide and EGF receptors exert dual modulation of the dual-specificity phosphatase 6 (MKP-3) in granule neurons and astrocytes, contributing to negative feedback on ERK signaling. Front Mol Neurosci 10:448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang J, Gao L, Zhang Y, Wang H, Sun S, Wu LA (2024) Involvement of microglial P2X7 receptor in pain modulation. CNS Neurosci Ther 30(1):e14496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yan J, Sun J, Zeng Z (2018) Teniposide ameliorates bone cancer nociception in rats via the P2X7 receptor. Inflammopharmacology 26(2):395–402 [DOI] [PubMed] [Google Scholar]
- 138.Wu P, Wang Y, Liu Y, Liu Y, Zhou G, Wu X, Wen Q (2023) Emerging roles of the P2X7 receptor in cancer pain. Purinergic Signal 19(2):441–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Huang ZX, Lu ZJ, Ma WQ, Wu FX, Zhang YQ, Yu WF, Zhao ZQ (2014) Involvement of RVM-expressed P2X7 receptor in bone cancer pain: mechanism of descending facilitation. Pain 155(4):783–791 [DOI] [PubMed] [Google Scholar]
- 140.Yuan X, Qian N, Ling S, Li Y, Sun W, Li J, Du R, Zhong G, Liu C, Yu G, Cao D, Liu Z, Wang Y, Qi Z, Yao Y, Wang F, Liu J, Hao S, Jin X, Zhao Y, Xue J, Zhao D, Gao X, Liang S, Li Y, Song J, Yu S, Li Y (2021) Breast cancer exosomes contribute to pre-metastatic niche formation and promote bone metastasis of tumor cells. Theranostics 11(3):1429–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jin X, Cheng J, Zhang Q, Ji H, Zhu C, Yang Y, Zhou Y, Yu G, Wang C, Tang Z (2023) Aconitine - A promising candidate for treating cold and mechanical allodynia in cancer induced bone pain. Biomed Pharmacother 161:114284 [DOI] [PubMed] [Google Scholar]
- 142.McNamara NB, Munro DAD, Bestard-Cuche N, Uyeda A, Bogie JFJ, Hoffmann A, Holloway RK, Molina-Gonzalez I, Askew KE, Mitchell S, Mungall W, Dodds M, Dittmayer C, Moss J, Rose J, Szymkowiak S, Amann L, McColl BW, Prinz M, Spires-Jones TL, Stenzel W, Horsburgh K, Hendriks JJA, Pridans C, Muramatsu R, Williams A, Priller J, Miron VE (2023) Microglia regulate central nervous system myelin growth and integrity. Nature 613(7942):120–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wu P, Zhou G, Wu X, Lv R, Yao J, Wen Q (2022) P2X7 receptor induces microglia polarization to the M1 phenotype in cancer-induced bone pain rat models. Mol Pain 18:17448069211060962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang RX, Liu B, Wang L, Ren K, Qiao JT, Berman BM, Lao L (2005) Spinal glial activation in a new rat model of bone cancer pain produced by prostate cancer cell inoculation of the tibia. Pain 118(1–2):125–136 [DOI] [PubMed] [Google Scholar]
- 145.Yang Y, Li H, Li TT, Luo H, Gu XY, Lü N, Ji RR, Zhang YQ (2015) Delayed activation of spinal microglia contributes to the maintenance of bone cancer pain in female Wistar rats via P2X7 receptor and IL-18. J Neurosci 35(20):7950–7963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang W, Zhong X, Li Y, Guo R, Du S, Wen L, Ying Y, Yang T, Wei XH (2019) Rostral ventromedial medulla-mediated descending facilitation following P2X7 receptor activation is involved in the development of chronic post-operative pain. J Neurochem 149(6):760–780 [DOI] [PubMed] [Google Scholar]
- 147.Hansen RR, Nielsen CK, Nasser A, Thomsen SIM, Eghorn LF, Pham Y, Schulenburg C, Syberg S, Ding M, Stojilkovic SS, Jorgensen NR, Heegaard AM (2011) P2X7 receptor-deficient mice are susceptible to bone cancer pain. Pain 152(8):1766–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Falk S, Schwab SD, Frøsig-Jørgensen M, Clausen RP, Dickenson AH, Heegaard AM (2015) P2X7 receptor-mediated analgesia in cancer-induced bone pain. Neuroscience 291:93–105 [DOI] [PubMed] [Google Scholar]
- 149.Li P, Zhang Q, Xiao Z, Yu S, Yan Y, Qin Y (2018) Activation of the P2X7 receptor in midbrain periaqueductal gray participates in the analgesic effect of tramadol in bone cancer pain rats. Mol Pain 14:1744806918803039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Falk S, Appel CK, Bennedbæk HB, Al-Dihaissy T, Unger A, Dinkel K, Heegaard AM (2019) Chronic high dose P2X7 receptor inhibition exacerbates cancer-induced bone pain. Eur J Pharmacol 845:48–55 [DOI] [PubMed] [Google Scholar]
- 151.Zhao X, Liu HZ, Zhang YQ (2016) Effect of P2X7 receptor knock-out on bone cancer pain in mice. Sheng Li Xue Bao 68(3):224–232 [PubMed] [Google Scholar]
- 152.Demeules M, Scarpitta A, Abad C, Gondé H, Hardet R, Pinto-Espinoza C, Eichhoff AM, Schäfer W, Haag F, Koch-Nolte F, Adriouch S (2020) Evaluation of P2X7 receptor function in tumor contexts using rAAV vector and nanobodies (AAVnano). Front Oncol 10:1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Barberà-Cremades M, Gómez AI, Baroja-Mazo A, Martínez-Alarcón L, Martínez CM, de Torre-Minguela C, Pelegrín P (2017) P2X7 Receptor induces tumor necrosis factor-α converting enzyme activation and release to boost TNF-α production. Front Immunol 8:862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hang RX, Liu B, Wang L, Ren K, Qiao JT, Berman BM, Lao L (2005) Spinal glial activation in a new rat model of bone cancer pain produced by prostate cancer cell inoculation of the tibia. Pain 118(1–2):125–136 [DOI] [PubMed] [Google Scholar]
- 155.Ying YL, Wei XH, Xu XB, She SZ, Zhou LJ, Lv J, Li D, Zheng B, Liu XG (2014) Over-expression of P2X7 receptors in spinal glial cells contributes to the development of chronic postsurgical pain induced by skin/muscle incision and retraction (SMIR) in rats. Exp Neurol 261:836–843 [DOI] [PubMed] [Google Scholar]
- 156.Song J, Ying Y, Wang W, Liu X, Xu X, Wei X, Ruan X (2018) The role of P2X7R/ERK signaling in dorsal root ganglia satellite glial cells in the development of chronic postsurgical pain induced by skin/muscle incision and retraction (SMIR). Brain Behav Immun 69:180–189 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. And we have not used other data that has already been published. All the data presented in this article are original results derived from this study.