Abstract
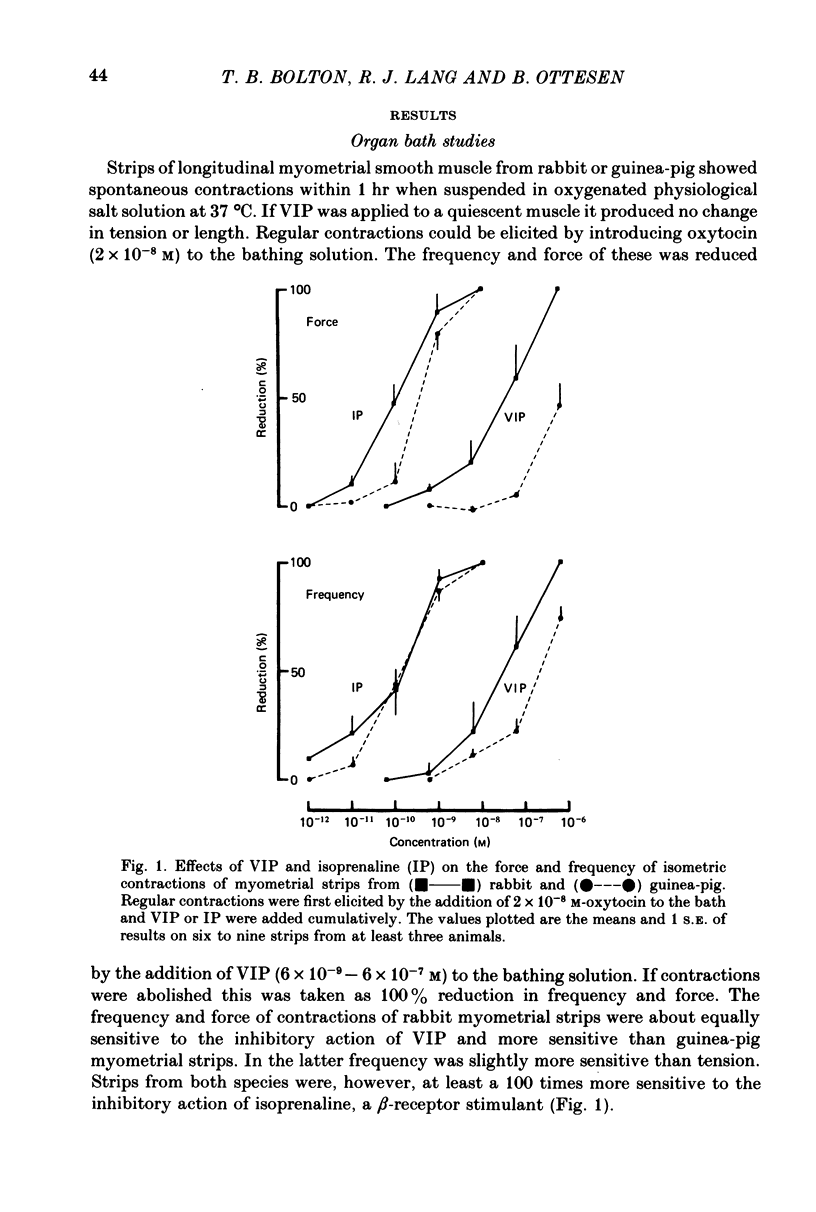
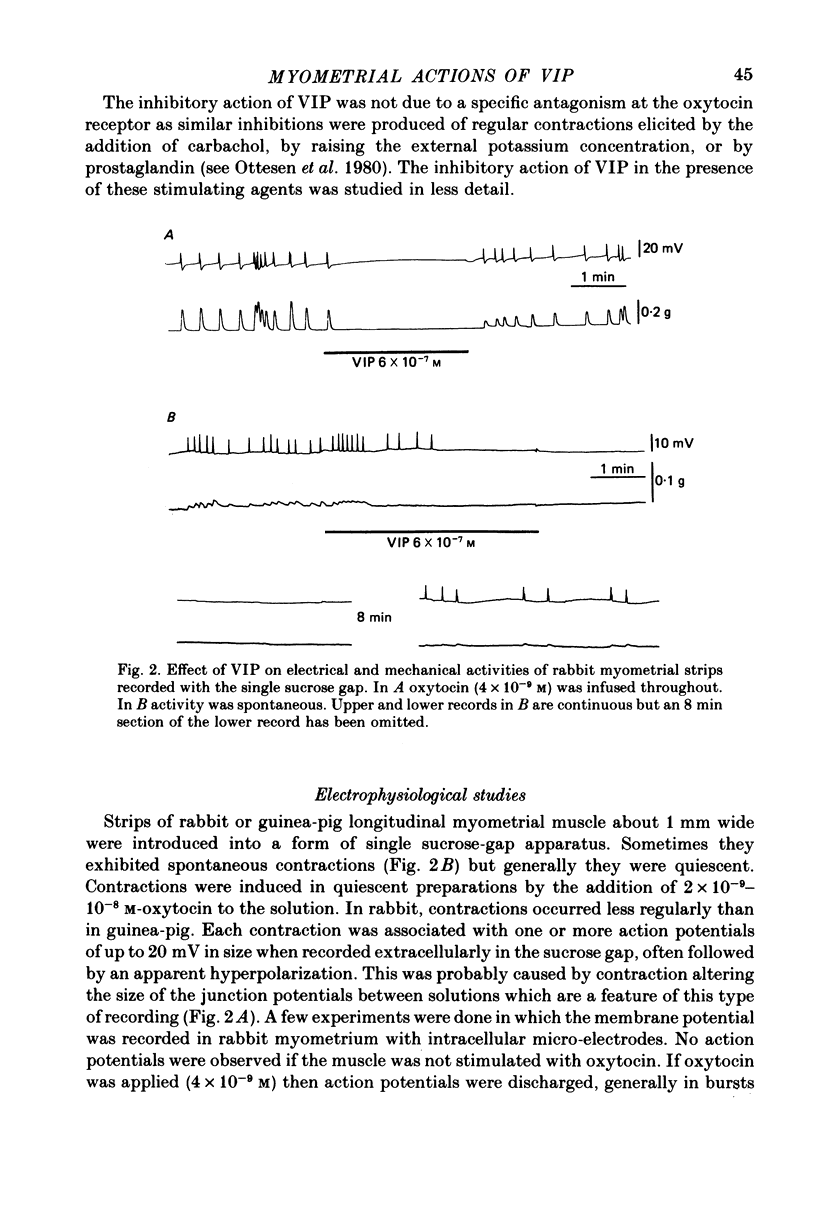
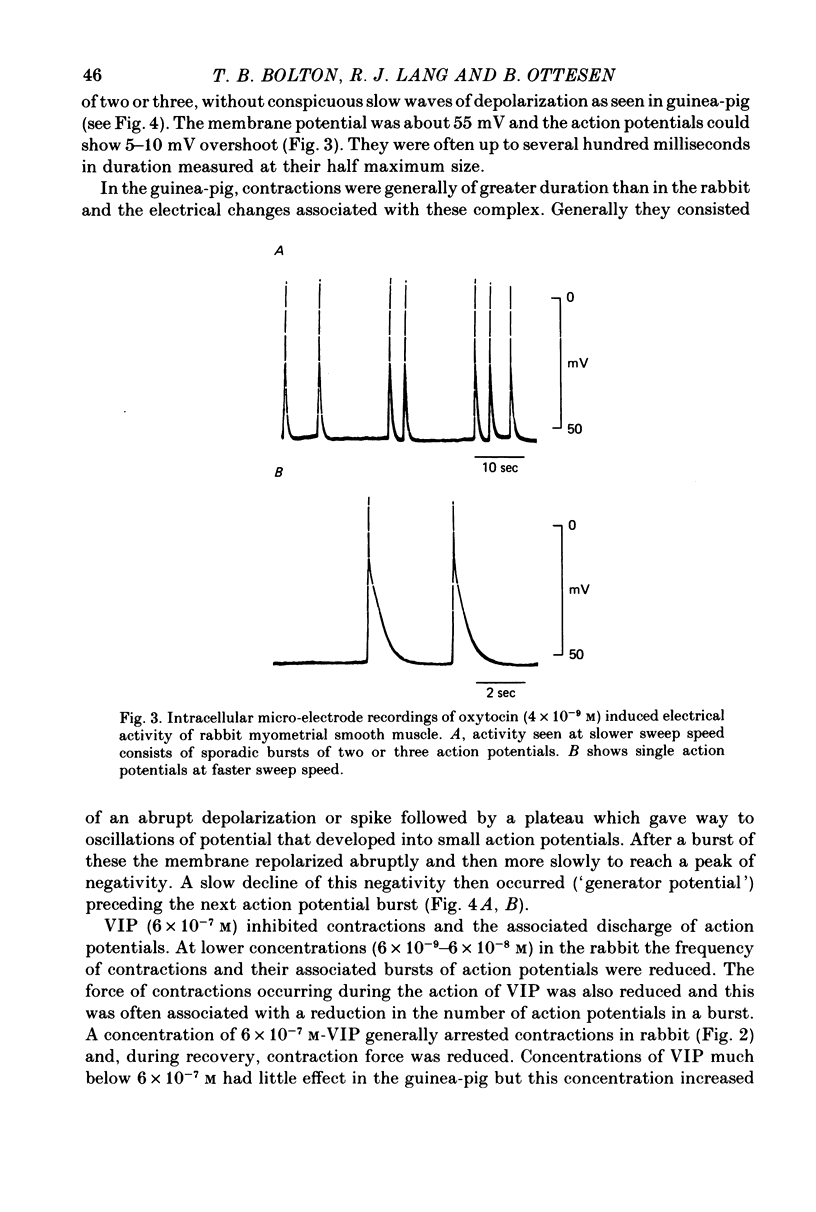
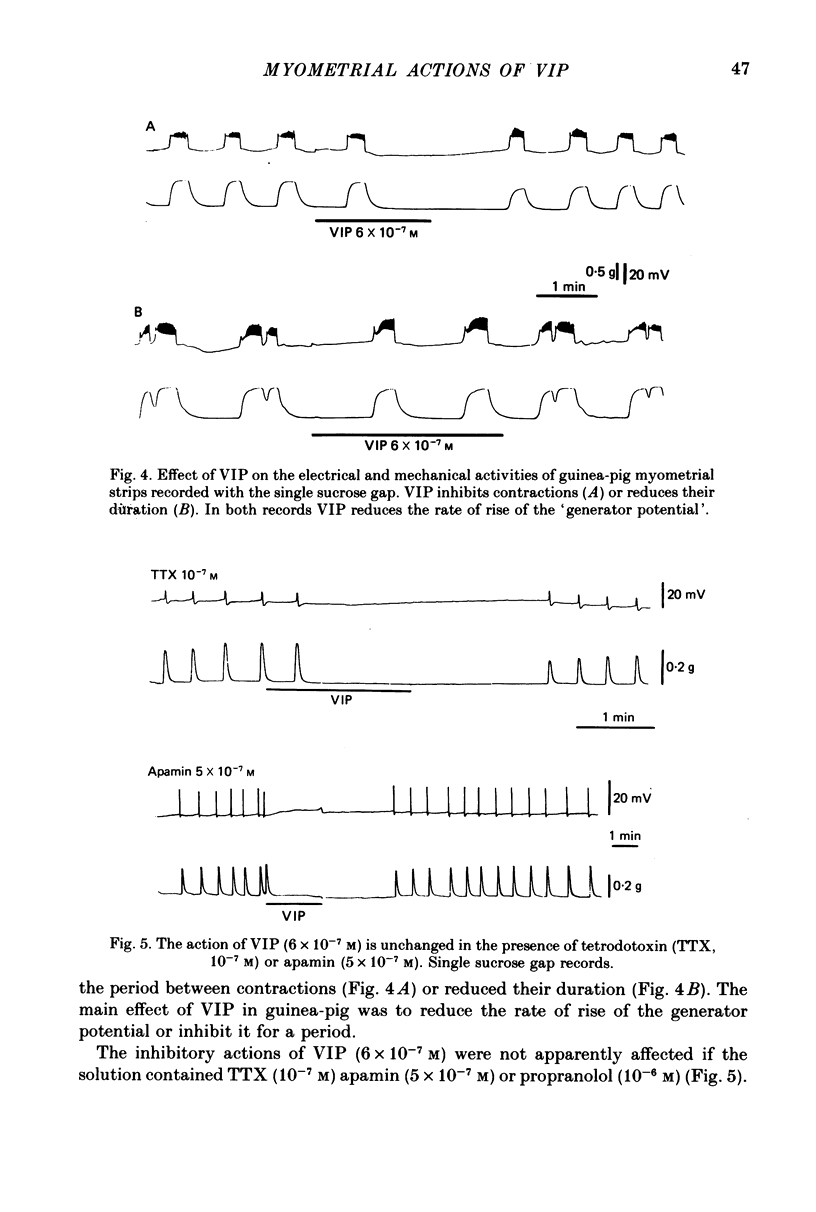
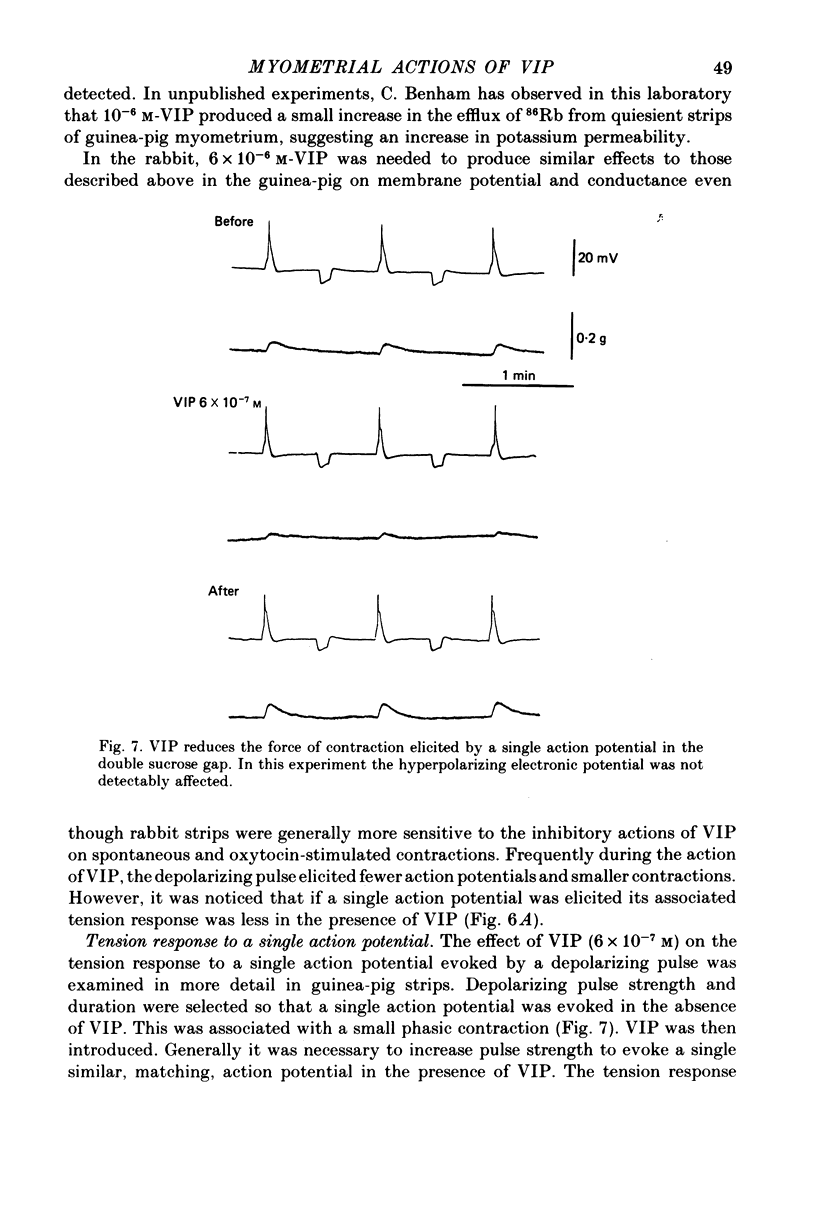
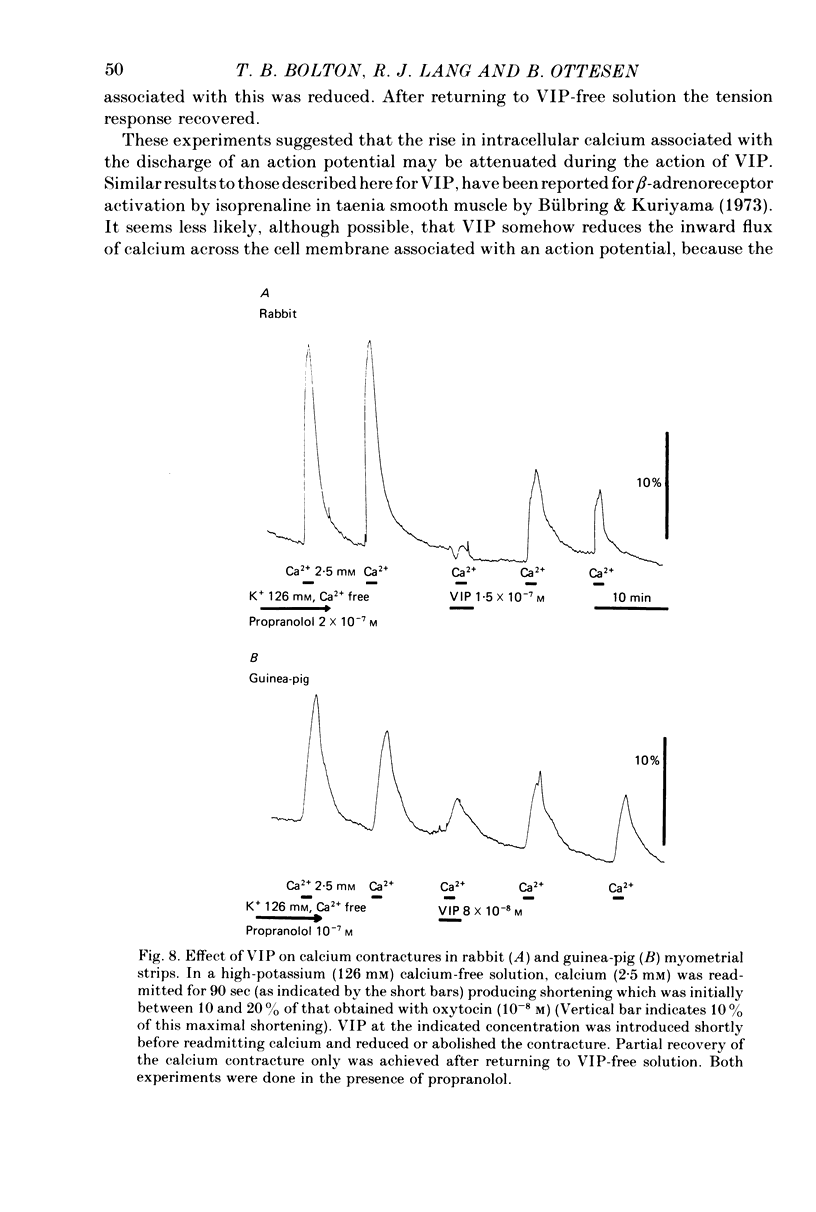
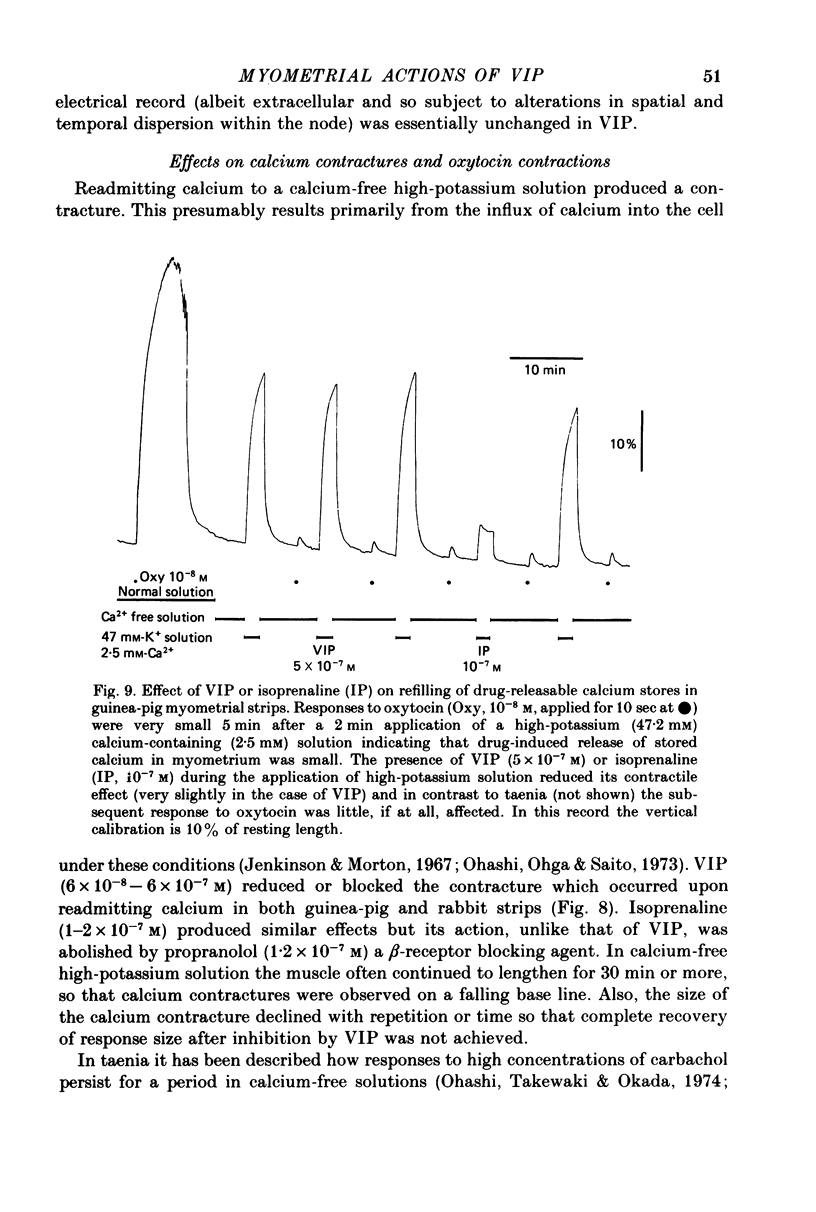
1. The action of vasoactive intestinal peptide (VIP) on the electrical and mechanical activity of strips of longitudinal myometrial smooth muscle from rabbits and guinea-pigs treated with oestradiol was studied in the sucrose-gap apparatus. 2. In myometrial strips which spontaneously exhibited regular contractions, or which were induced to contract rhythmically to the application of oxytocin, VIP reduced both the frequency and the force of contraction. 3. Contractions were associated with bursts of action potential discharge. In guinea-pig, the membrane potential reached its most negative value shortly following a burst and a slow decay of negativity followed ("generator potential'). VIP inhibited the decay of this negativity and increased the duration of the period between bursts. In rabbit myometrical strips, electrical discharges occurred less regularly but VIP also had an inhibitory action. The inhibitory action of VIP was not affected by the beta-adrenoreceptor blocker propranolol, by tetrodotoxin, or by apamin. 4. Using the double sucrose-gap apparatus, bursts of action potentials and contractions were elicited with depolarizing electrical pulses in the absence of oxytocin. Changes in membrane resistance were also estimated by eliciting hyperpolarizing electrotronic potentials. VIP hyperpolarized the membrane and inhibited contractions as depolarizing pulses now failed to reach threshold for action potential discharge or fewer action potentials were discharged. A small (about 10%) reduction in membrane resistance was freqeuently observed during the hyperpolarization. 5. If a single action potential was elicited in the presence of VIP, the tension generated by the muscle was less than in its absence. 6. In a calcium-free high-potassium (126 mM) solution, readmitting calcium produced contraction; VIP inhibited this contraction. Activation of beta-receptors by means of isoprenaline had a similar effect but unlike isoprenaline the action of VIP was not blocked by propranolol. 7. It is suggested that the primary action of VIP is on the calcium economy of the myometrial smooth muscle cell, possibly to accelerate sequestration and/or extrusion of calcium from the cell. In some way this is associated with inhibition of the generator potential, hyperpolarization, and with a small increase in permeability of the membrane to potassium.
Full text
PDF


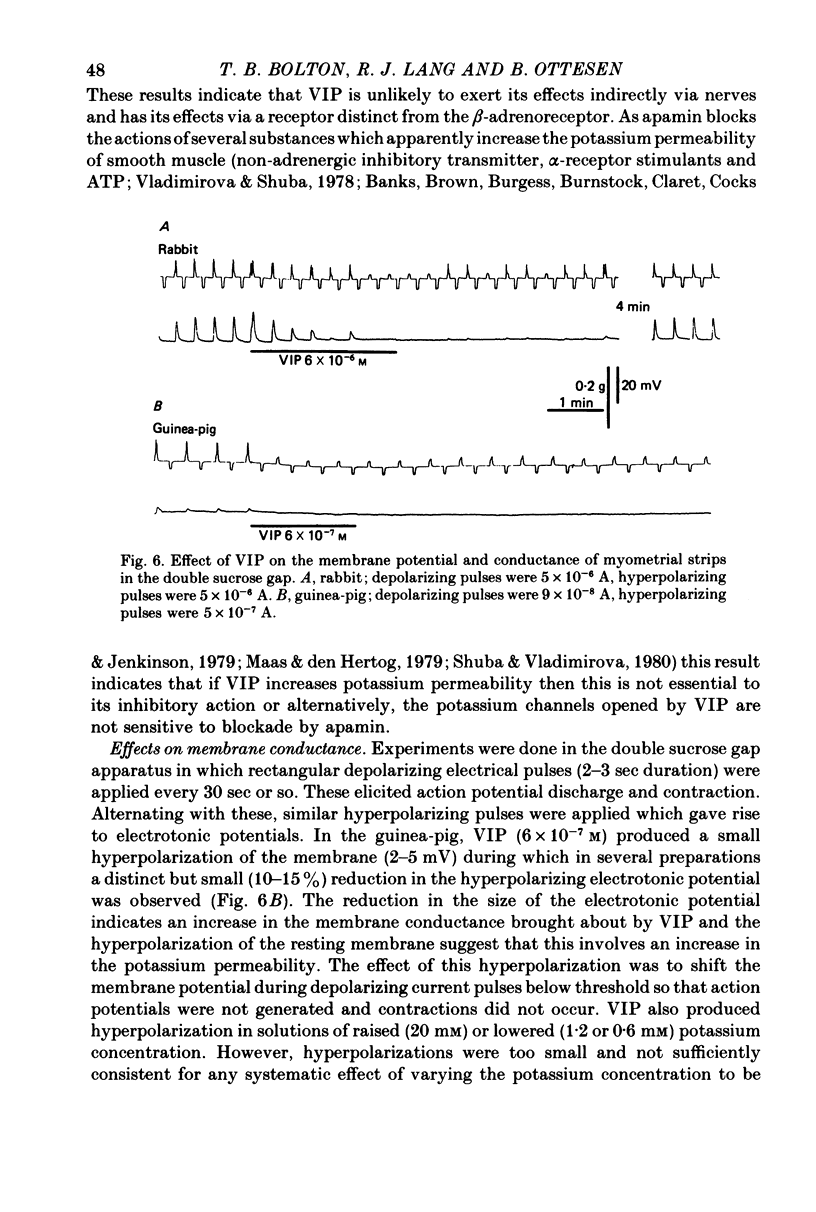




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alm P., Alumets J., Håkanson R., Owman O., Sjöberg N. O., Sundler F., Walles B. Origin and distribution of VIP (vasoactive intestinal polypeptide)-nerves in the genito-urinary tract. Cell Tissue Res. 1980;205(3):337–347. doi: 10.1007/BF00232276. [DOI] [PubMed] [Google Scholar]
- Banks B. E., Brown C., Burgess G. M., Burnstock G., Claret M., Cocks T. M., Jenkinson D. H. Apamin blocks certain neurotransmitter-induced increases in potassium permeability. Nature. 1979 Nov 22;282(5737):415–417. doi: 10.1038/282415a0. [DOI] [PubMed] [Google Scholar]
- Bolton T. B., Clark J. P. Actions of various muscarinic agonists on membrane potential, potassium efflux, and contraction of longitudinal muscle of guinea-pig intestine. Br J Pharmacol. 1981 Feb;72(2):319–334. doi: 10.1111/j.1476-5381.1981.tb09131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. The depolarizing action of acetylcholine or carbachol in intestinal smooth muscle. J Physiol. 1972 Feb;220(3):647–671. doi: 10.1113/jphysiol.1972.sp009728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F., Sneddon P. Evidence for multiple sources of calcium for activation of the contractile mechanism of guinea-pig taenia coli on stimulation with carbachol. Br J Pharmacol. 1980 Oct;70(2):229–240. doi: 10.1111/j.1476-5381.1980.tb07928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E., Kuriyama H. The action of catecholamines on guinea-pig taenia coli. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):115–121. doi: 10.1098/rstb.1973.0014. [DOI] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. Suppression of spontaneous spike generation by catecholamines in the smooth muscle of the guinea-pig taenia coli. Proc R Soc Lond B Biol Sci. 1969 Mar 11;172(1027):103–119. doi: 10.1098/rspb.1969.0014. [DOI] [PubMed] [Google Scholar]
- Bülbring E., den Hertog A. The action of isoprenaline on the smooth muscle of the guinea-pig taenia coli. J Physiol. 1980 Jul;304:277–296. doi: 10.1113/jphysiol.1980.sp013324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Raeymaekers L. The action of acetylcholine and catecholamines on an intracellular calcium store in the smooth muscle cells of the guinea-pig taenia coli. J Physiol. 1979 Sep;294:51–68. doi: 10.1113/jphysiol.1979.sp012914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. L., Landry A. S. Vasoactive intestinal polypeptide: increased tone, enhancement of acetylcholine release, and stimulation of adenylate cyclase in intestinal smooth muscle. Life Sci. 1980 Mar 10;26(10):811–822. doi: 10.1016/0024-3205(80)90288-x. [DOI] [PubMed] [Google Scholar]
- Diamond J., Marshall J. M. Smooth muscle relaxants: dissociation between resting membrane potential and resting tension in rat myometrium. J Pharmacol Exp Ther. 1969 Jul;168(1):13–20. [PubMed] [Google Scholar]
- Edvinsson L., Fahrenkrug J., Hanko J., Owman C., Sundler F., Uddman R. VIP (vasoactive intestinal polypeptide)-containing nerves of intracranial arteries in mammals. Cell Tissue Res. 1980;208(1):135–142. doi: 10.1007/BF00234179. [DOI] [PubMed] [Google Scholar]
- Edwards A. V., Birchman P. M., Mitchell S. J., Bloom S. R. Changes in the concentration of vasoactive intestinal peptide in intestinal lymph in response to vagal stimulation in the calf. Experientia. 1978 Sep 15;34(9):1186–1187. doi: 10.1007/BF01922948. [DOI] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J. Inotropic and arrhythmogenic effects of potassium-depleted solutions on mammalian cardiac muscle. J Physiol. 1979 Sep;294:255–277. doi: 10.1113/jphysiol.1979.sp012929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenkrug J., Haglund U., Jodal M., Lundgren O., Olbe L., de Muckadell O. B. Nervous release of vasoactive intestinal polypeptide in the gastrointestinal tract of cats: possible physiological implications. J Physiol. 1978 Nov;284:291–305. doi: 10.1113/jphysiol.1978.sp012541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenkrug J. Vasoactive intestinal polypeptide: measurement, distribution and putative neurotransmitter function. Digestion. 1979;19(3):149–169. doi: 10.1159/000198339. [DOI] [PubMed] [Google Scholar]
- Frandsen E. K., Krishna G. A., Said S. I. Vasoactive intestinal polypeptide promotes cyclic adenosine 3',5'-monophosphate accumulation in guinea-pig trachea. Br J Pharmacol. 1978 Mar;62(3):367–369. doi: 10.1111/j.1476-5381.1978.tb08471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal R. K., Rattan S., Said S. I. VIP as a possible neurotransmitter of non-cholinergic non-adrenergic inhibitory neurones. Nature. 1980 Nov 27;288(5789):378–380. doi: 10.1038/288378a0. [DOI] [PubMed] [Google Scholar]
- Jenkinson D. H., Morton I. K. The role of alpha- and beta- adrenergic receptors in some actions of catecholamines on intestinal smooth muscle. J Physiol. 1967 Feb;188(3):387–402. doi: 10.1113/jphysiol.1967.sp008145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen K. R., Polak J. M., Van Noorden S., Bloom S. R., Burnstock G. Peptide-containing neurones connect the two ganglionated plexuses of the enteric nervous system. Nature. 1980 Jan 24;283(5745):391–393. doi: 10.1038/283391a0. [DOI] [PubMed] [Google Scholar]
- Kroeger E. A., Marshall J. M. Beta-adrenergic effects on rat myometrium: mechanisms of membrane hyperpolarization. Am J Physiol. 1973 Dec;225(6):1339–1345. doi: 10.1152/ajplegacy.1973.225.6.1339. [DOI] [PubMed] [Google Scholar]
- Larsson L. I., Fahrenkrug J., Schaffalitzky De Muckadell O., Sundler F., Håkanson R., Rehfeld J. R. Localization of vasoactive intestinal polypeptide (VIP) to central and peripheral neurons. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3197–3200. doi: 10.1073/pnas.73.9.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas A. J., Den Hertog A. The effect of apamin on the smooth muscle cells of the guinea-pig taenia coli. Eur J Pharmacol. 1979 Sep 15;58(2):151–156. doi: 10.1016/0014-2999(79)90006-2. [DOI] [PubMed] [Google Scholar]
- Marshall J. M. Modulation of smooth muscle activity by catecholamines. Fed Proc. 1977 Sep;36(10):2450–2455. [PubMed] [Google Scholar]
- Morgan K. G., Schmalz P. F., Szurszewski J. H. The inhibitory effects of vasoactive intestinal polypeptide on the mechanical and electrical activity of canine antral smooth muscle. J Physiol. 1978 Sep;282:437–450. doi: 10.1113/jphysiol.1978.sp012474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutt V., Said S. I. Structure of the porcine vasoactive intestinal octacosapeptide. The amino-acid sequence. Use of kallikrein in its determination. Eur J Biochem. 1974 Mar 1;42(2):581–589. doi: 10.1111/j.1432-1033.1974.tb03373.x. [DOI] [PubMed] [Google Scholar]
- Oashi H., Oga A., Saito K. Enhancement of Ca-contractions by catecholamines and temperature dependency in the depolarized teania coli of the guinea-pig. Jpn J Pharmacol. 1973 Aug;23(4):467–477. doi: 10.1254/jjp.23.467. [DOI] [PubMed] [Google Scholar]
- Oashi H., Takewaki T., Okada T. Calcium and the contractile effect of carbachol in the depolarized guinea-pig taenia caecum. Jpn J Pharmacol. 1974 Aug;24(4):601–611. doi: 10.1254/jjp.24.601. [DOI] [PubMed] [Google Scholar]
- Ottesen B., Larsen J. J., Fahrenkrug J., Stjernquist M., Sundler F. Distribution and motor effect of VIP in female genital tract. Am J Physiol. 1981 Jan;240(1):E32–E36. doi: 10.1152/ajpendo.1981.240.1.E32. [DOI] [PubMed] [Google Scholar]
- Ottesen B., Ulrichsen H., Wagner G., Fahrenkrug J. Vasoactive intestinal polypeptide (VIP) inhibits oxytocin induced activity of the rabbit myometrium. Acta Physiol Scand. 1979 Nov;107(3):285–287. doi: 10.1111/j.1748-1716.1979.tb06477.x. [DOI] [PubMed] [Google Scholar]
- Ottesen B., Wagner G., Fahrenkrug J. Vasoactive intestinal polypeptide (VIP) inhibits prostaglandin-F2 alpha-induced activity of the rabbit myometrium. Prostaglandins. 1980 Mar;19(3):427–435. doi: 10.1016/0090-6980(80)90076-3. [DOI] [PubMed] [Google Scholar]
- Piper P. J., Said S. I., Vane J. R. Effects on smooth muscle preparations of unidentified vasoactiv peptides from intestine and lung. Nature. 1970 Mar 21;225(5238):1144–1146. doi: 10.1038/2251144a0. [DOI] [PubMed] [Google Scholar]


