Abstract
Introduction
Vitiligo, a chronic depigmenting disorder affecting 1–2% of the global population, is caused by immune-mediated melanocyte destruction. While its pathogenesis is multifactorial, the relationship between vitiligo and malignancy risk remains controversial. Some studies suggest an increased cancer risk, while others propose a potential protective effect, particularly against skin cancers. This study provides a comprehensive evaluation of malignancy risks in patients with vitiligo.
Methods
This systematic review and meta-analysis followed PRISMA 2020 guidelines and was registered with PROSPERO (CRD42023483130). A comprehensive search was conducted across Medline, EMBASE, and Cochrane databases. Studies reporting hazard ratios for malignancy incidence in patients with vitiligo were included. Data extraction and risk of bias assessment were performed using the Cochrane ROBINS-E tool.
Results
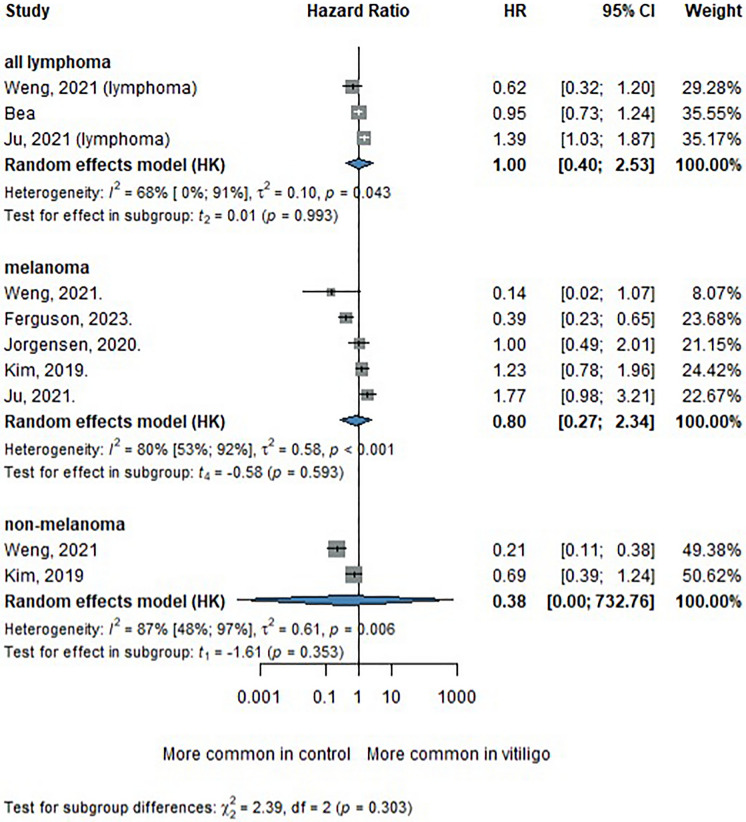
Of 7753 records identified, 6378 remained after duplicates were removed, and 12 studies were included in the final review. Quantitative analysis was performed on six studies. The combined sample comprised 3,267,951 participants, including 289,322 patients with vitiligo. Three meta-analyses were conducted for melanoma, non-melanoma skin cancer, and lymphoma. The pooled hazard ratio (HR) for lymphoma was 1.00 (95% CI 0.40–2.53), for melanoma 0.80 (95% CI 0.27–2.34), and for non-melanoma skin cancer 0.38 (95% CI 0.00–732.76), suggesting no consistent associations.
Conclusion
This meta-analysis did not identify significant differences in cancer risk across the examined subgroups. While a protective effect of vitiligo against some malignancies cannot be excluded, substantial heterogeneity among studies warrants cautious interpretation. Further high-quality research is needed to clarify these associations.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-025-01520-0.
Keywords: Vitiligo, Skin cancer, Melanoma, Non-melanoma skin cancer, Lymphoma, Cancer risk, Systematic review, Meta-analysis
Key Summary Points
| Why carry out this study? |
| Vitiligo is an autoimmune condition in which the risk of skin and non-skin cancers remains unclear, with conflicting data in the literature. |
| Previous reviews have focused mainly on skin cancer; there is a need to assess broader malignancy risks, including lymphoma. |
| This study aimed to evaluate the association between vitiligo and the risk of melanoma, non-melanoma skin cancer (NMSC), and lymphoma. |
| What was learned from the study? |
| Pooled hazard ratios showed no statistically significant association between vitiligo and the risk of melanoma (HR 0.80), NMSC (HR 0.38), or lymphoma (HR 1.00). |
| These findings suggest that vitiligo is not associated with increased cancer risk and may potentially confer a protective effect against some skin cancers in certain populations. |
| However, as a result of study heterogeneity and limited data on non-cutaneous malignancies, further large-scale and standardised research is warranted. |
Introduction
Vitiligo is a chronic depigmenting disorder affecting 1–2% of the global population, characterised by the selective destruction of melanocytes, which results in the appearance of well-demarcated, non-scaly, amelanotic macules and patches [1, 2]. Its pathogenesis is multifactorial, involving a complex interplay of genetic predisposition, environmental triggers, abnormalities in metabolic processes, oxidative stress, and cell adhesion, culminating in an autoimmune response targeting melanocytes [3, 4]. Despite being frequently misperceived as a cosmetic condition, vitiligo imposes a profound psychological burden, markedly reducing the quality of life of the patients [5].
Interestingly, idiopathic vitiligo and melanoma-associated vitiligo-like depigmentation have been linked to improved 5-year survival rates in patients with melanoma [6]. Therapies for metastatic melanoma such as BRAF inhibitor vemurafenib and immune checkpoint inhibitors targeting CTLA-4 and PD-1 often induce vitiligo, suggesting a shared immune mechanism [7, 8]. Both involve heightened immune activity, with melanoma clearance sometimes triggering vitiligo-like depigmentation [7]. This has led to speculation that vitiligo may reflect enhanced immune surveillance, potentially lowering melanoma risk. Thus, although vitiligo and melanoma lie at opposite ends of a spectrum, one marked by melanocyte destruction, the other by unchecked proliferation, they share immune and genetic pathways that merit further investigation [7, 8].
Narrow-band ultraviolet B (NB-UVB) phototherapy remains a cornerstone in treating vitiligo, with its immunomodulatory effects facilitating melanocyte proliferation and migration, ultimately promoting repigmentation. However, achieving sustained repigmentation typically requires prolonged treatment courses with ongoing maintenance [7]. The carcinogenic risk of phototherapy in patients with vitiligo, particularly in amelanotic skin, has been an area of significant inquiry. While one small study has reported an increased incidence of skin cancer in patients with vitiligo, its findings are limited by the absence of a general population comparator and a small sample size [9]. Conversely, other studies suggest a reduced risk of skin malignancies in patients with vitiligo, contributing to ongoing inconsistencies in the literature [10–12].
Sun-seeking behaviour in patients with vitiligo may differ, as sun exposure can accentuate the contrast between lesional and non-lesional skin in certain individuals [13]. This adds further complexity to the relationship between vitiligo and skin cancer risk, underscoring the need for larger-scale studies to explore additional potential cancer risks beyond the skin.
Although previous meta-analyses have explored the risk of skin cancer in vitiligo, our study expands on this by encompassing a broader range of malignancies. By synthesising a comprehensive body of literature, this analysis aims to provide a clearer and more conclusive assessment of malignancy risks associated with vitiligo, addressing existing gaps in knowledge.
Methods
The study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines [14] and the latest version of the Cochrane Handbook [15]. The protocol was registered with PROSPERO (CRD42023483130).
Eligibility Criteria
Eligible studies were evaluated using the CoCoPop framework: Condition—malignancy risk, Context—vitiligo, Population—patients documented in healthcare databases or hospital registries.
Information Sources
A systematic search was performed on November 11, 2023 in Medline, EMBASE, and Cochrane databases without language restrictions or filters, using search terms related to malignancies and vitiligo. Search keys and hit counts are included in the supplementary material.
Search Strategy
Search results were exported to EndNote 20 (Clarivate Analytics, Philadelphia, PA, USA) for duplicate removal, completed both automatically and manually by AAM.
Selection Process
Articles were screened using the Rayyan Intelligent Systematic Review software. Two independent reviewers (AAM, IS) conducted the selection based on titles, abstracts, and full texts, following inclusion criteria. Discrepancies were resolved by a third reviewer (FAM). Cohen’s kappa coefficient was calculated at each step to assess inter-rater reliability.
Data Collection Process
Data extraction was performed independently by three reviewers (AAM, BN, ZC) using a preconstructed data collection sheet. Any disagreements were resolved by a fourth reviewer (IS).
Data Items
Extracted data included the first author, publication year, study population, study period, population characteristics, and malignancy incidence reported as hazard ratios.
Risk of Bias Assessment and Quality of Evidence
The risk of bias was independently assessed by two reviewers (AAM, IS) using the Cochrane ROBINS-E tool. Outcomes were categorized as “low,” “some concerns,” or “high” risk of bias, with disagreements resolved by a third reviewer (FAM). The certainty of evidence for primary analysis outcomes was evaluated using the GRADE framework via the GRADEPro software tool [16].
Synthesis Method
Hazard ratios (HR) were utilized as the effect size measure, accompanied by 95% confidence intervals (CI). Pooled HRs were calculated directly from the HRs and their corresponding standard errors as reported in the included studies. Results were deemed statistically significant if the pooled CI did not include the null effect value. The meta-analysis findings were summarized in forest plots. Between-study heterogeneity was assessed using Thompson’s I2 statistic [17]. Small study publication bias was evaluated through visual inspection of funnel plots. All statistical analyses were performed in R [18] using the meta package [19] for core meta-analysis calculations and plot generation.
Analysis of Subgroups
A fixed-effects plural model (also called a mixed-effects model) was employed for subgroup analyses. This approach assumed different τ2 values across subgroups while positing that all subgroups shared a common τ2 value, as variations in between-study heterogeneity were not anticipated, and some subgroups contained relatively few studies [20]. Differences between subgroups were assessed using an omnibus test, Cochrane Q test [21]. Statistical significance was determined at the 5% level, with the null hypothesis rejected if significant variation between subgroups was observed.
Results
Search and Selection
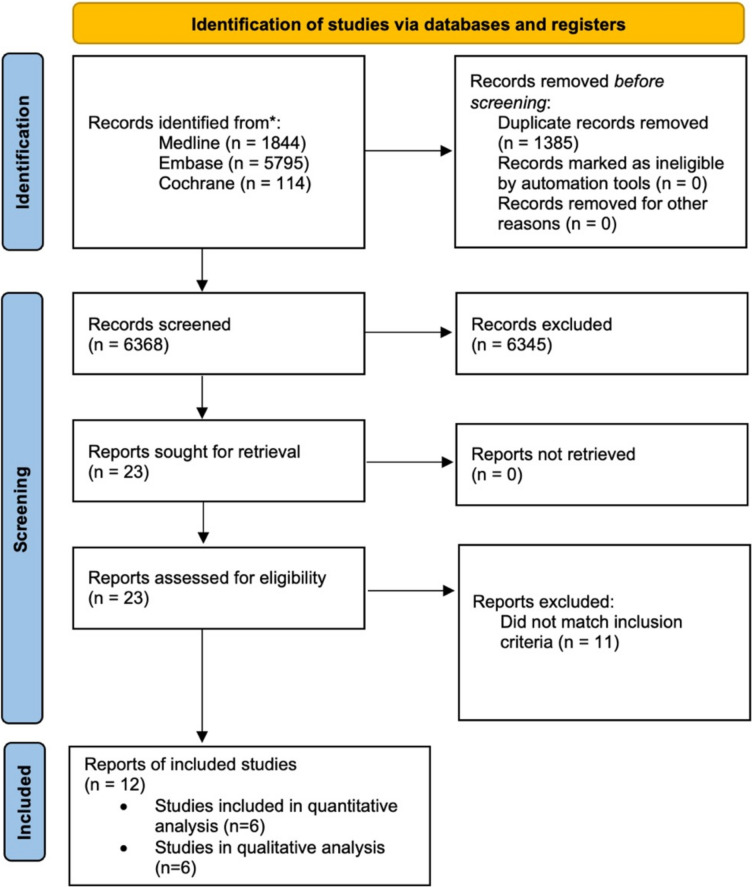
The systematic search yielded 7753 studies across Medline (1844), Embase (5795), and the Cochrane Library (114). Following the removal of duplicates, 6378 articles were screened, ultimately resulting in the inclusion of 12 studies ([9–11, 22–30], six of which were included in the quantitative study and the remaining six could only be included in the qualitative analysis as a result of difficulties in synthesising the findings because of heterogeneity in data reporting. The selection process is summarised in Fig. 1.
Fig. 1.
PRISMA 2020 flow diagram for the selection process
Basic Characteristics
The basic characteristics of the included studies are detailed in Table 1. The meta-analysis assessing the incidence of non-melanoma skin cancers incorporated two retrospective cohort studies [10, 27]. The meta-analysis evaluating melanoma incidence included five retrospective cohort studies [10, 11, 23, 26, 27]. The meta-analysis examining lymphoma incidence was based on three retrospective cohort studies [10, 22, 23]. All selected studies consistently reported their findings using hazard ratios.
Table 1.
Basic characteristics of included studies
| Author | Study site | Study type | Study period | Study setting | Total number of patients analysed | Type of malignancies investigated | Vitiligo population | Comparison group | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | Number of analysed patients | Male (n) | Mean age (SD) | Characteristics | Number of participants | Male (n) | Mean age (SD) | |||||||
| Weng | Taiwan | Retrospective cohort study | 1997–2013 | Taiwan’s National Health Insurance Research Database (NHIRD) | 69,120 | All internal malignancies and skin cancers | All patients with a diagnosis of vitiligo | 13,824 | 5799 | 43.5 (20.5) | Each subject in the vitiligo group was matched to four reference subjects matched by age, gender and propensity score to reduce confounders | 55,296 | 23,196 | 43.5 (20.5) |
| Bae | South Korea | Retrospective cohort study | 2007–2016 | Korean National Healthcare Insurance (NHI) | 303,234 | All internal malignancies (excluding haematological malignancies) and skin cancer | Patients who had at least two documented contacts with a physician with a diagnosis of vitiligo and who had not been diagnosed with any form of cancer before the diagnosis of vitiligo | 101,078 | 41,707 | 48.7 (15.4) | Matched controls selected randomly with two controls per patient after frequency matching with vitiligo group for age and sex | 202,156 | 83,414 | 48.7 (15.4) |
| Ju | South Korea | Retrospective cohort study | 2001–2009 | Hospital databases | 25,694 | Skin cancers and lymphoma | Patients with vitiligo who received calcineurin inhibitors, phototherapy or both | 25,694 | 11,544 | NA | Patients with vitiligo who did not receive treatment | NA | NA | NA |
| Ferguson | UK | Cohort study | 2010–2020 | National Healthcare database | 75,771 | Melanoma and non-melanoma skin cancer | Any adults diagnosed with vitiligo | 15,156 | 6900 | 47* (35–61) | Matched unaffected controls 4:1 not diagnosed with vitiligo at the date of matching | 60,615 | 27,599 | 47 (34–61) |
| Jorgensen | Denmark | Cohort study | 1994–2017 | Danish National patient register | 37,987 | Melanoma and non-melanoma skin cancer | All Danish patient alive and residing in Denmark between 1994 and 2017 with a first-time diagnosis of vitiligo | 2325 | 959 | 38 (21) | Randomly matched 10 control subjects for each patients with vitiligo with same sex and birth date | 23,293 | 9639 | 38 (21) |
| Kim | South Korea | Cohort study | 2005–2017 | National Healthcare Database | 2,756,145 | Melanoma and non-melanoma skin cancer | Any patient with vitiligo as the main diagnosis | 131,245 | 52,609 | NA | Randomly selected non-vitiligo controls matched 20:1 by age and sex on date of vitiligo diagnosis | 2,624,900 | 1,052,180 | NA |
*Median (IQR)
Quantitative Analysis
Three separate meta-analyses were performed, focusing respectively on the incidence of non-melanoma skin cancers, melanoma, and lymphomas.
Risk of Melanoma in Patients with Vitiligo
Five studies [10, 11, 23, 26, 27] were included in the melanoma subgroup analysis. The pooled HR for melanoma incidence in patients with vitiligo was 0.80 (95% CI 0.27–2.34), showing no statistically significant association (see Fig. 2). Despite a trend suggesting a potentially reduced risk, the substantial heterogeneity (I2 = 80%; p < 0.001) indicates variability in study results, requiring further research to clarify this relationship.
Fig. 2 .
Forest plot of the meta-analysis assessing the association between vitiligo and the risk of lymphoma, melanoma, and non-melanoma skin cancer
Risk of Non-melanoma Skin Cancers in Patients with Vitiligo
The association between vitiligo and non-melanoma skin cancer was evaluated in two studies [10, 27]. The pooled HR was 0.38 (95% CI 0.00–732.76) (see Fig. 2). Although that suggests a potential reduction in non-melanoma skin cancer risk among patients with vitiligo, the wide confidence interval undermines the precision and reliability of this finding.
Risk of Lymphoma in Patients with Vitiligo
The meta-analysis for lymphoma included three studies [5, 10, 23], yielding a pooled hazard ratio (HR) of 1.00 (95% CI 0.40–2.53) for lymphoma in patients with vitiligo compared to non-vitiligo controls (see Fig. 2). This result indicates no significant difference in lymphoma risk, suggesting no association between vitiligo and lymphoma incidence.
Overall Subgroup Comparison
A test for subgroup differences revealed no statistically significant variation in cancer risk among the lymphoma, melanoma, and non-melanoma groups (χ2 = 3.47, df = 2, p = 0.176) (see Fig. 2). Nonetheless, the considerable heterogeneity observed across studies and subgroups highlights the variability in the findings and calls for careful interpretation of the pooled estimates.
Risk of Bias Assessment and Certainty of Evidence
The risk of bias assessment revealed a low risk in four studies, while the remaining studies demonstrated a moderate risk of bias. Detailed evaluations are presented in Supplementary Table 2. A critical appraisal of the study methodologies led to the exclusion of four studies because of inappropriate study designs. The funnel plot generated to evaluate publication bias appeared largely symmetrical, indicating a low likelihood of significant publication bias (Supplementary Fig. S1).
Qualitative Analysis
Six studies were not eligible for quantitative analysis from the meta-analysis because of the lack of hazard ratio reporting. Hadi et al. [30] conducted a decade-long investigation into comorbid conditions among patients with vitiligo, identifying a markedly elevated incidence of lymphoma (3.33-fold increase, p < 0.0001) compared to the general population. Hexsel et al. [9] assessed the incidence of non-melanoma skin cancers in a cohort of 477 patients with vitiligo, observing higher rates across all subtypes, except for basal cell carcinoma in women, relative to US population rates; however, these differences did not achieve statistical significance. Lindelöf et al. [28] examined a cohort of 1052 patients with vitiligo for melanoma and squamous cell carcinoma, reporting three cases of melanoma and no instances of squamous cell carcinoma. Similarly, Schallreuter et al. [29] performed a retrospective cohort analysis of 136 Caucasian patients with vitiligo with extensive sun exposure, correlating clinical records and histological findings. Their study revealed significantly lower levels of photodamage and a reduced incidence of non-melanoma skin cancers in patients with vitiligo compared to the general population. Furthermore, Paradisi et al. [24] investigated the incidence of melanoma and non-melanoma skin cancers (NMSC) in patients with vitiligo compared to controls in a vascular surgery setting. They found that, despite increased UV exposure due to therapy, patients with vitiligo have a reduced risk of developing skin neoplasms. Teulings et al. [25] conducted a retrospective cohort study to evaluate the lifetime prevalence of melanoma and NMSC in patients with vitiligo compared to non-vitiligo controls. After adjusting for confounders, they observed that patients with vitiligo had a significantly lower likelihood of developing melanoma (adjusted OR 0.32; 95% CI 0.12–0.88) and NMSC (adjusted OR 0.28; 95% CI 0.16–0.50). Subgroup analyses of patients undergoing UV-B and psoralen plus UVA (PUVA) therapy showed no dose-related increase in age-adjusted lifetime prevalence of melanoma or NMSC.
Discussion
Our systematic review and meta-analysis aimed to comprehensively evaluate the association between vitiligo and the risk of malignancies, including melanoma, NMSC, and lymphoma. While previous studies have largely focused on the connection between vitiligo and skin cancers, such as melanoma and NMSC [12, 31], our study is the first to simultaneously investigate the incidence of lymphoma, a non-skin-related malignancy, alongside skin malignancies. By employing rigorous statistical analyses, we address a critical gap in the existing literature.
Melanoma Risk
Our analysis found no statistically significant association between vitiligo and the risk of melanoma, although trends toward a reduced risk were observed. The pooled hazard ratio (HR 0.80; 95% CI 0.27–2.34) highlights the necessity of cautious interpretation because of substantial heterogeneity (I2 = 80%). Immune-mediated destruction of melanocytes, a hallmark of vitiligo, may explain these observed trends. Enhanced immune surveillance, particularly through heightened T cell activity, is proposed as a protective mechanism that targets aberrant melanocytes, thereby reducing the likelihood of malignant transformation [32, 33]. This hypothesis aligns with prior studies, such as those by Teulings et al., who reported a threefold reduction in melanoma incidence among patients with vitiligo [25] and Lindelöf et al., who documented minimal melanoma incidence in vitiligo populations over long-term follow-up periods [28].
However, these results must be interpreted in the context of broader epidemiological trends. For instance, declining melanoma incidence in regions like Sweden and Hungary may reflect improvements in preventive measures and early detection efforts, which could confound the observed associations [34, 35].
Non-melanoma Skin Cancer Risk
For NMSC, the pooled HR of 0.38 (95% CI 0.00–732.76) suggests a potential reduction in risk, but the wide confidence intervals limit definitive conclusions. It is noteworthy that both studies included in the NMSC meta-analysis were based on Asian populations [10, 27]. Prior studies, such as those by Schallreuter et al. [29] and Paradisi et al. [24], have reported reduced photodamage and lower NMSC incidence in patients with vitiligo despite comparable UV exposure. This paradox may be explained by the protective effects of immune hyperactivity, which targets atypical keratinocytes in addition to melanocytes [36]. However, conflicting evidence exists, as Hexsel et al. [9] reported statistically non-significant elevated NMSC rates in patients with vitiligo.
Lymphoma Risk
Unlike autoimmune diseases such as rheumatoid arthritis, Sjögren’s syndrome, and celiac disease—which exhibit increased lymphoma risk due to chronic B cell and T cell activation [37–40]—our analysis found no significant association between vitiligo and lymphoma risk. Nevertheless, these findings must be interpreted with caution, as statistical insignificance may be influenced by the limited number of studies included in the analysis.
It is plausible that vitiligo operates through distinct immune pathways compared to other autoimmune disorders. However, isolated studies, such as Hadi et al. [30], have reported a 3.33-fold increased risk of lymphoma in patients with vitiligo, underscoring the potential heterogeneity in outcomes that warrants further exploration; therefore, further studies reporting lymphoma risk in such populations are required.
Proposed Mechanisms Linking Vitiligo to Reduced Cancer Risk
Several hypotheses have been proposed to explain a potentially reduced risk of skin cancer in vitiligo. A key mechanism involves enhanced immune surveillance, particularly elevated levels of circulating natural killer (NK) cells in patients with vitiligo [31, 41]. NK cells play a critical role in defending against cutaneous squamous cell carcinoma, and their increased presence in both lesional and non-lesional skin may contribute to antitumour activity [31].
In melanoma, genome-wide association studies have identified protective polymorphisms in the TYR gene, which encodes tyrosinase, an essential enzyme in melanin synthesis [42]. These variants may reduce tyrosinase activity or alter melanocyte behaviour, decreasing the likelihood of malignant transformation [11, 42]. Tyrosinase, along with other melanogenic proteins such as gp100 and MART-1, are key antigens targeted by cytotoxic CD8+ T cells, which are central to tumour surveillance and control [43]. In vitiligo, autoreactive CD8+ T cells specific to melanocyte antigens are both necessary and sufficient for melanocyte destruction, with their numbers correlating with disease activity [44]. A similar response occurs in melanoma, where CD8+ T cells recognise shared antigens, contributing to autoimmune manifestations such as melanoma-associated leukoderma or halo phenomena [43]. This shared immune pathway suggests that melanocyte-targeted autoimmunity in vitiligo may also promote anti-melanoma immunity and tumour regression [43]. Supporting this, prior research demonstrated that melanocytic antigens, including both UV-induced neoantigens and differentiation antigens expressed by healthy melanocytes, can enhance responses to immune checkpoint blockade [45]. Notably, mice that had previously cleared melanoma showed improved immune control of pancreatic cancer cells expressing melanocytic antigens, whereas this effect was absent in pancreatic tumours with no melanocytic antigen expression [45]. These findings highlight a potential cross-protective role of melanocyte-specific immune memory, warranting further research into how vitiligo-associated immune mechanisms could inform cancer immunotherapy.
Furthermore, behavioural factors may contribute to the reported lower incidence of melanoma in patients with vitiligo. Sunlight exposure, known to exacerbate vitiligo lesions, often leads to increased sensitivity and avoidance of UV radiation by patients with vitiligo [46]. This behaviour, while protective against further depigmentation, may also reduce UV-induced DNA damage, a key factor in melanoma development [47].
Risk of Non-Skin Malignancies
Interestingly, some studies have reported an increased risk of certain non-skin-related cancers in patients with vitiligo compared to healthy controls. A comprehensive nationwide study in Taiwan by Li et al. [48] revealed that the overall incidence of malignancies in individuals with vitiligo was 0.71 per 100 person-years, notably exceeding the 0.28 per 100 person-years observed in the general population. The study also found that the standardised incidence ratio for post-vitiligo malignancies was significantly elevated, with no discernible gender disparity. Furthermore, sex-specific analyses revealed that male patients were more likely to develop prostate cancer, while female patients exhibited a significantly higher propensity for thyroid and breast cancers, as reported in other studies [49, 50]. These findings suggest that the correlation between vitiligo and malignancy remains elusive, potentially because of the different pathomechanisms underlying various cancers and their unique relationship to vitiligo.
One plausible explanation for these observations lies in the role of CD8+cytotoxic T lymphocytes (CTLs), a specialised subset of immune cells, which play a pivotal role in the pathogenesis of vitiligo by targeting and destroying melanocytes, resulting in the characteristic depigmentation [48, 51]. Beyond their role in autoimmunity, these CTLs are also integral to the immune system’s defence against tumours, orchestrating the recognition and elimination of malignant cells [48, 52, 53]. However, aberrant activation of CD8+ CTLs can lead to their infiltration into tumour sites, potentially compromising the integrity of the tumour capsule and influencing tumour progression and metastasis [48, 52]. In the case of thyroid cancer, this aberrant CTL activation may contribute to the increased risk of thyroid malignancies in patients with vitiligo, although further research is needed to clarify the exact mechanisms involved [48]. Similarly, while vitiligo has been associated with breast cancer, the relationship remains poorly understood and is primarily based on isolated case studies [49, 48]. Furthermore, in prostate cancer, studies indicated that both ultraviolet exposure and vitamin D levels could influence prostate cancer risk [54].
Strengths and Limitations
Our study possesses strengths and limitations. We rigorously adhered to the Cochrane Collaboration guidelines to ensure the highest standards of quality, transparency, and replicability in our methodology and findings. Notably, to the best of our knowledge, this is the first meta-analysis to include non-skin cancers, specifically lymphoma, in the context of vitiligo, which represents a novel contribution to the existing literature.
Nevertheless, several limitations must be considered. There was substantial heterogeneity across the included studies, particularly in the melanoma subgroup, where the I2 statistic reached 80%, indicating significant variability in the data. Additionally, only 6 out of 12 eligible studies could be included in the quantitative synthesis because of the absence of hazard ratio reporting, which limits the comprehensiveness of the meta-analysis. The small number of included studies, combined with the wide confidence intervals observed in several pooled estimates, undermines statistical power and reduces the precision and reliability of the findings. As a result, the ability to draw definitive conclusions is limited. Furthermore, key confounding factors such as exposure to phototherapy, behavioural tendencies toward sun avoidance, and regional variations in melanoma incidence were not consistently accounted for across studies, potentially influencing the robustness of our conclusions. Finally, for rare malignancies such as CTCL, the low number of events limits the ability to detect meaningful differences in incidence, and results should therefore be interpreted with caution in these contexts.
Implications for Practice and Research
The integration of scientific advancements into clinical practice is fundamental to enhancing patient care [55, 56]. Interestingly, patients with vitiligo do not appear to have a higher risk of skin cancers, despite the absence of melanocyte-mediated UV protection.
Our findings show that patients with vitiligo may exhibit a non-significant trend of reduced risk of melanoma and NMSC, potentially as a result of enhanced immune surveillance and melanocyte depletion. Clinicians should recognise these protective trends; however, they should also consider the heterogeneity of outcomes across studies and possible population differences. Although sun avoidance behaviours in patients with vitiligo may further reduce cumulative UV exposure, healthcare providers should continue to emphasise routine skin examinations, especially for patients undergoing treatments that may influence immune pathways, such as phototherapy or systemic immunosuppressants.
While our analysis did not identify an increased lymphoma risk in patients with vitiligo, clinicians should remain cautious, as evidence remains limited, and isolated studies have suggested variable outcomes. This underscores the need for tailored surveillance strategies and patient counselling, particularly for individuals with additional risk factors or long-standing immune dysregulation.
Future research must focus on addressing the current gaps and limitations in the literature. Large, multicentre cohort studies with robust methodologies are needed to further clarify the relationship between vitiligo and malignancies. Standardised reporting of outcomes, including hazard ratios, will be essential for improving comparability across studies. Additionally, prospective studies should evaluate the long-term impact of phototherapy and immunomodulatory treatments on cancer risk in patients with vitiligo. Research exploring non-skin-related malignancies, such as prostate, thyroid, and breast cancers, is warranted to better understand their association with vitiligo and identify potential underlying immunological or genetic mechanisms. Clinicians should always recommend regular follow-up. Given that patients with vitiligo often avoid sun exposure, it is important for clinicians to reinforce the need for adequate sun protection while also safely recommending UV-based therapies when appropriate. By addressing these knowledge gaps, future studies can guide evidence-based surveillance strategies, optimise clinical management, and enhance our understanding of vitiligo’s broader implications for cancer risk.
Conclusion
This meta-analysis found no statistically significant association between vitiligo and the risk of melanoma, NMSC, or lymphoma. While some included studies suggested trends toward a reduced risk of skin cancers, these findings should be interpreted with caution because of the limited number of studies, wide confidence intervals, and substantial heterogeneity. Any potential protective effect remains speculative and may be population-specific or influenced by unmeasured confounding factors. Further research in diverse populations is needed to clarify these associations and to better understand the possible role of immune mechanisms in vitiligo. In the meantime, clinicians should adopt an individualised approach to patient counselling, considering each patient’s baseline skin cancer risk and treatment history, including exposure to phototherapy.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We extend our gratitude to our colleagues for their valuable insights and to the editorial team for their support throughout the review process. We would also like to express our sincere gratitude to the original participants of the studies included in our analysis, whose contributions made this research possible.
Author Contributions
Alzahra Ahmed Mohammed: conceptualisation, project administration, data curation, visualisation and writing—original draft, Anna Sára Lengyel: conceptualisation, methodology, validation, project administration, writing—review and editing, Fanni Adél Meznerics: conceptualisation, methodology, validation, project administration, writing—review and editing, István Szondy: investigation, data curation, writing—review and editing, Anna Walter: formal analysis, visualisation, writing—review and editing, Benedek Nagy: data curation, writing—review and editing, Zsófia Csábi: data curation, writing—review and editing, András Bánvölgyi: conceptualisation, writing—review and editing, Norbert Kiss: conceptualisation, writing—review and editing, Péter Hegyi: conceptualisation, methodology, project administration, writing—review and editing, Zsuzsanna Kurgyis: conceptualisation, methodology, validation, writing—review and editing, supervision, Lajos Vince Kemény: conceptualisation, methodology, validation, writing—review and editing, supervision.
Funding
L.V.K. is a recipient of the János Bolyai Research Scholarship from the Hungarian Academy of Sciences and is supported by the Hungarian National Research, Development and Innovation Office (OTKA FK138696) grant. The Rapid Service Fee is funded by the corresponding authors. L.V.K. and the MTA-SE Lendület “Momentum” Dermatooncology Research Group are the recipients of the Lendület “Momentum” grant from the Hungarian Academy of Sciences (LP2024-12/2024). This project was funded by grant agreement No. 739593 of the EU Horizon 2020 research and innovation program.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Declarations
Conflict of Interest
Alzahra A. Mohammed, Anna S. Lengyel, Fanni A. Meznerics, István Szondy, Anna Walter, Benedek Nagy, Zsófia Csábi, András Bánvölgyi, Norbert Kiss, Péter Hegyi, Lajos V. Kemény, and Zsuzsanna Kurgyis have nothing to disclose.
Ethics Statement
No ethical approval was required for this study as all data were sourced from peer-reviewed journals. No patients were involved in the design, conduct or interpretation of the study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zsuzsanna Kurgyis and Lajos V. Kemény contributed equally to this work.
Contributor Information
Zsuzsanna Kurgyis, Email: zsuzsanna.kurgyis@tum.de.
Lajos V. Kemény, Email: kemeny.lajos@semmelweis.hu
References
- 1.Kruger C, Schallreuter KU. A review of the worldwide prevalence of vitiligo in children/adolescents and adults. Int J Dermatol. 2012. 10.1111/j.1365-4632.2011.05377.x. [DOI] [PubMed] [Google Scholar]
- 2.Bergqvist C, Ezzedine K. Vitiligo: a review. Dermatology. 2020;236(6):571–92. [DOI] [PubMed] [Google Scholar]
- 3.Picardo M, Dell’Anna ML, Ezzedine K, et al. Vitiligo. Nat Rev Dis Primers. 2015. 10.1038/nrdp.2015.11. [DOI] [PubMed] [Google Scholar]
- 4.Spritz RA, Andersen GH. Genetics of vitiligo. Dermatol Clin. 2017. 10.1016/j.det.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ezzedine K, Eleftheriadou V, Jones H, et al. Psychosocial effects of vitiligo: a systematic literature review. Am J Clin Dermatol. 2021;22:757–74. [DOI] [PMC free article] [PubMed]
- 6.Naveh HP, Rao UN, Butterfield LH. Melanoma-associated leukoderma - immunology in black and white? Pigment Cell Melanoma Res. 2013. 10.1111/pcmr.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodrigues M. Skin cancer risk (nonmelanoma skin cancers/melanoma) in vitiligo patients. Dermatol Clin. 2017;35(2):129–34. [DOI] [PubMed] [Google Scholar]
- 8.Alonso-Castro L, Rios-Buceta L, Vano-Galvan S, Moreno C, Soria-Rivas A, Jaen P. Vitiligo in 2 patients receiving vemurafenib for metastatic melanoma. J Am Acad Dermatol. 2013. 10.1016/j.jaad.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Hexsel CL, Eide MJ, Johnson CC, et al. Incidence of nonmelanoma skin cancer in a cohort of patients with vitiligo. J Am Acad Dermatol. 2009. 10.1016/j.jaad.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weng YC, Ho HJ, Chang YL, Chang YT, Wu CY, Chen YJ. Reduced risk of skin cancer and internal malignancies in vitiligo patients: a retrospective population-based cohort study in Taiwan. Sci Rep. 2021. 10.1038/s41598-021-99786-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferguson J, Eleftheriadou V, Nesnas J. Risk of melanoma and nonmelanoma skin cancer in people with vitiligo: United Kingdom population-based cohort study. J Invest Dermatol. 2023. 10.1016/j.jid.2023.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Ban L, Labbouz S, Grindlay D, Batchelor JM, Ratib S. Risk of skin cancer in people with vitiligo: a systematic review and meta-analysis. Br J Dermatol. 2018. 10.1111/bjd.16703. [DOI] [PubMed] [Google Scholar]
- 13.Nicolaidou E, Katsambas AD, Lotti TM. Vitiligo. In: Katsambas AD, Lotti TM, Dessinioti C, D’Erme AM, editors. European handbook of dermatological treatments. Berlin: Springer; 2015.
- 14.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed]
- 15.Higgins JPT, Thomas J, Chandler J, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.5 (updated August 2024): Cochrane; 2024. https://www.cochrane.org/handbook. Accessed 29 Aug 2024.
- 16.GRADEpro. http://gradepro.org/. Accessed 15 Sept 2024.
- 17.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002. 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 18.Team RC. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2021.
- 19.Schwarzer G. Meta: General Package for Meta-Analysis. 2022.
- 20.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. Wiley; 2009. [Google Scholar]
- 21.Harrer M, Cuijpers P, Furukawa Toshi A, Ebert DD. Doing meta-analysis with R: a hands-on guide. Boca Raton: Chapman & Hall/CRC; 2021. [Google Scholar]
- 22.Bae JM, Chung KY, Yun SJ, et al. Markedly reduced risk of internal malignancies in patients with vitiligo: a nationwide population-based cohort study. J Clin Oncol. 2019. 10.1200/JCO.18.01223. [DOI] [PubMed] [Google Scholar]
- 23.Ju HJ, Han JH, Kim MS, et al. The long-term risk of lymphoma and skin cancer did not increase after topical calcineurin inhibitor use and phototherapy in a cohort of 25,694 patients with vitiligo. J Am Acad Dermatol. 2021. 10.1016/j.jaad.2021.01.067. [DOI] [PubMed] [Google Scholar]
- 24.Paradisi A, Tabolli S, Didona B, Sobrino L, Russo N, Abeni D. Markedly reduced incidence of melanoma and nonmelanoma skin cancer in a nonconcurrent cohort of 10,040 patients with vitiligo. J Am Acad Dermatol. 2014. 10.1016/j.jaad.2014.07.050. [DOI] [PubMed] [Google Scholar]
- 25.Teulings HE, Overkamp M, Ceylan E, et al. Decreased risk of melanoma and nonmelanoma skin cancer in patients with vitiligo: a survey among 1307 patients and their partners. Br J Dermatol. 2013;168(1):162-71. [DOI] [PubMed]
- 26.Jorgensen MG, Toyserkani NM, Egeberg A, Sorensen JA. Risk of skin cancer in patients with vitiligo in Denmark: a nationwide cohort study. JAAD Int. 2020;1(1):31–38. [DOI] [PMC free article] [PubMed]
- 27.Kim HS, Kim HJ, Hong ES, et al. The incidence and survival of melanoma and nonmelanoma skin cancer in patients with vitiligo: a nationwide population-based matched cohort study in Korea. Br J Dermatol. 2020. 10.1111/bjd.18247. [DOI] [PubMed] [Google Scholar]
- 28.Lindelöf B, Hedblad MA, Sigurgeirsson B. On the association between vitiligo and malignant melanoma. Acta Derm Venereol. 1998;78(6):483–4. [DOI] [PubMed]
- 29.Schallreuter KU, Tobin DJ, Panske A. Decreased photodamage and low incidence of non-melanoma skin cancer in 136 sun-exposed caucasian patients with vitiligo. Dermatology. 2002. 10.1159/000057881. [DOI] [PubMed] [Google Scholar]
- 30.Hadi A, Wang JF, Uppal P, Penn LA, Elbuluk N. Comorbid diseases of vitiligo: a 10-year cross-sectional retrospective study of an urban US population. J Am Acad Dermatol. 2020. 10.1016/j.jaad.2019.07.036. [DOI] [PubMed] [Google Scholar]
- 31.Rooker A, Ouwerkerk W, Bekkenk MW, Luiten RM, Bakker WJ. The risk of keratinocyte cancer in vitiligo and the potential mechanisms involved. J Invest Dermatol. 2024. 10.1016/j.jid.2023.08.012. [DOI] [PubMed] [Google Scholar]
- 32.Faraj S, Kemp EH, Gawkrodger DJ. Patho-immunological mechanisms of vitiligo: the role of the innate and adaptive immunities and environmental stress factors. Clin Exp Immunol. 2022. 10.1093/cei/uxab002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teulings HE, Limpens J, Jansen SN, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015. 10.1200/JCO.2014.57.4756. [DOI] [PubMed] [Google Scholar]
- 34.Liszkay G, Kiss Z, Gyulai R, et al. Changing trends in melanoma incidence and decreasing melanoma mortality in hungary between 2011 and 2019: a Nationwide Epidemiological Study. Front Oncol. 2021;10:612459. [DOI] [PMC free article] [PubMed]
- 35.Helgadottir H, Mikiver R, Schultz K, et al. Melanoma incidence and mortality trends among patients aged 59 years or younger in Sweden. JAMA Dermatol. 2024. 10.1001/jamadermatol.2024.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu W, 23andMe Research Team, Amos CI, et al. Inverse relationship between vitiligo-related genes and skin cancer risk. J Invest Dermatol. 2018;138(9):2072–75. [DOI] [PubMed]
- 37.Kleinstern G, Maurer MJ, Liebow M, et al. History of autoimmune conditions and lymphoma prognosis. Blood Cancer J. 2018. 10.1038/s41408-018-0105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang SS, Vajdic CM, Linet MS, et al. Associations of non-Hodgkin lymphoma (NHL) risk with autoimmune conditions according to putative NHL loci. Am J Epidemiol. 2015;181(6):406–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zintzaras E, Voulgarelis M, Moutsopoulos HM. The risk of lymphoma development in autoimmune diseases: a meta-analysis. Arch Intern Med. 2005;165(20):2337–44. [DOI] [PubMed]
- 40.Hakulinen T, Isomaki H, Knekt P. Rheumatoid arthritis and cancer studies based on linking nationwide registries in Finland. Am J Med. 1985. 10.1016/0002-9343(85)90242-6. [DOI] [PubMed] [Google Scholar]
- 41.Tulic MK, Cavazza E, Cheli Y, N, et al. Innate lymphocyte-induced CXCR3B-mediated melanocyte apoptosis is a potential initiator of T-cell autoreactivity in vitiligo. Nat Commun. 2019. 10.1038/s41467-019-09963-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bishop DT, Demenais F, Iles MM, et al. Genome-wide association study identifies three loci associated with melanoma risk. Nat Genet. 2009. 10.1038/ng.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Failla CM, Carbone ML, Fortes C, Pagnanelli G, D’Atri S. Melanoma and vitiligo. In good company. Int J Mol Sci. 2019. 10.3390/ijms20225731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodrigues M, Ezzedine K, Hamzavi I, Pandya AG, Harris JE, Vitiligo Working Group. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol. 2017. 10.1016/j.jaad.2016.10.048. [DOI] [PubMed] [Google Scholar]
- 45.Lo JA, Kawakubo M, Juneja VR, et al. Epitope spreading toward wild-type melanocyte-lineage antigens rescues suboptimal immune checkpoint blockade responses. Sci Transl Med. 2021. 10.1126/scitranslmed.abd8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhatia B, Kechichian E, Eleftheriadou V, et al. Habits and risk perception associated with sun exposure in vitiligo patients according to their participation in a patients’ organization. J Eur Acad Dermatol Venereol. 2019;33(3):e100–e103. [DOI] [PubMed]
- 47.Schadendorf D, Fisher DE, Garbe C, et al. Melanoma. Nat Rev Dis Primers. 2015. 10.1038/nrdp.2015.3. [DOI] [PubMed] [Google Scholar]
- 48.Li CY, Dai YX, Chen YJ, et al. Cancer risks in vitiligo patients: a nationwide population-based study in Taiwan. Int J Environ Res Public Health. 2018. 10.3390/ijerph15091847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jin Y, Birlea SA, Fain PR, et al. Variant of TYR and autoimmunity susceptibility loci in generalized vitiligo. N Engl J Med. 2010;362(18):1686–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rao MV, Shayne M. Vitiligo in the affected breast during neo-adjuvant chemotherapy for breast cancer. Breast Cancer Res Treat. 2009. 10.1007/s10549-008-0074-6. [DOI] [PubMed] [Google Scholar]
- 51.Lili Y, Yi W, Ji Y, Yue S, Weimin S, Ming L. Global activation of CD8+ cytotoxic T lymphocytes correlates with an impairment in regulatory T cells in patients with generalized vitiligo. PLoS ONE. 2012. 10.1371/journal.pone.0037513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Man YG, Stojadinovic A, Mason J, et al. Tumor-infiltrating immune cells promoting tumor invasion and metastasis: existing theories. J Cancer. 2013. 10.7150/jca.5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yuan H, Hsiao YH, Zhang Y, et al. Destructive impact of T-lymphocytes, NK and mast cells on basal cell layers: implications for tumor invasion. BMC Cancer. 2013. 10.1186/1471-2407-13-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khan MA, Partin AW. Vitamin D for the management of prostate cancer. Rev Urol. 2004;6(2):95–7. [PMC free article] [PubMed]
- 55.Hegyi P, Petersen OH, Holgate S, et al. Academia Europaea position paper on translational medicine: the cycle model for translating scientific results into community benefits. J Clin Med. 2020;9(5):1532. [DOI] [PMC free article] [PubMed]
- 56.Hegyi P, Eross B, Izbeki F, Parniczky A, Szentesi A. Accelerating the translational medicine cycle: the Academia European pilot. Nat Med. 2021;27(8):1317–19. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.