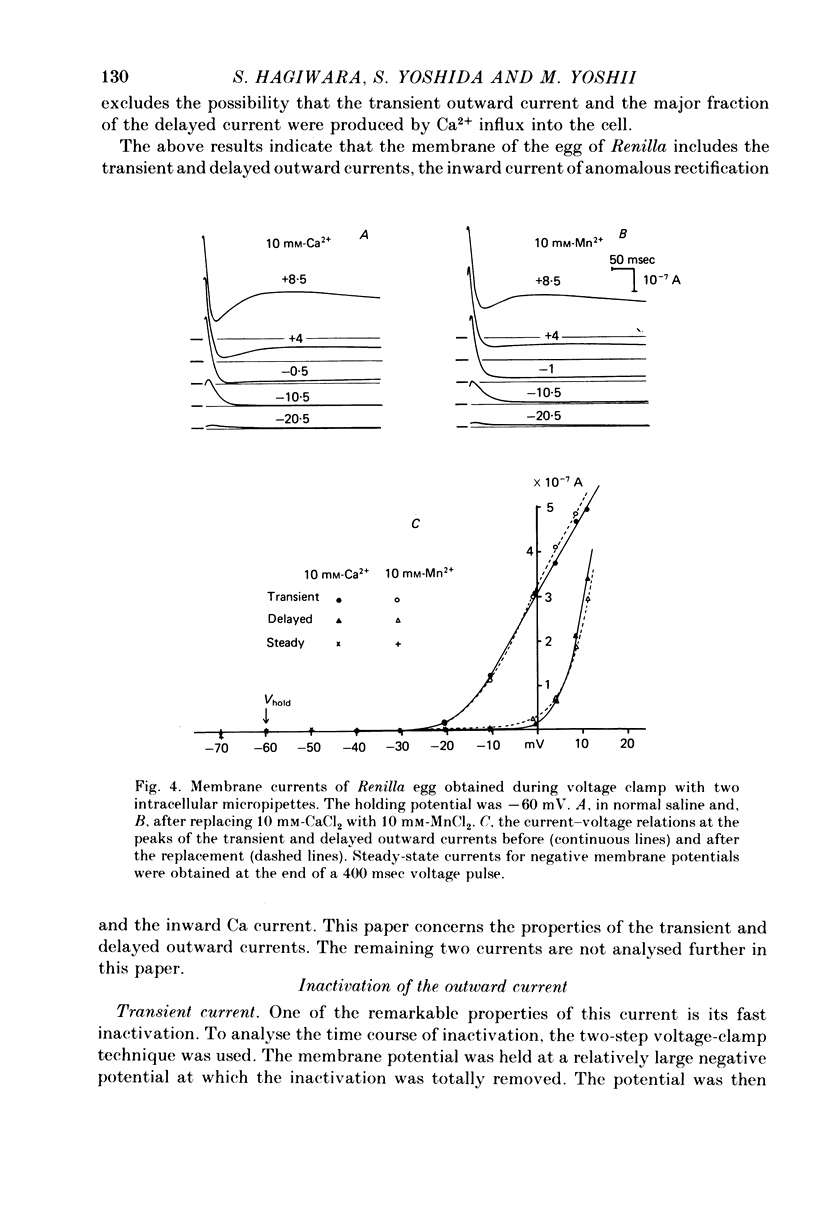
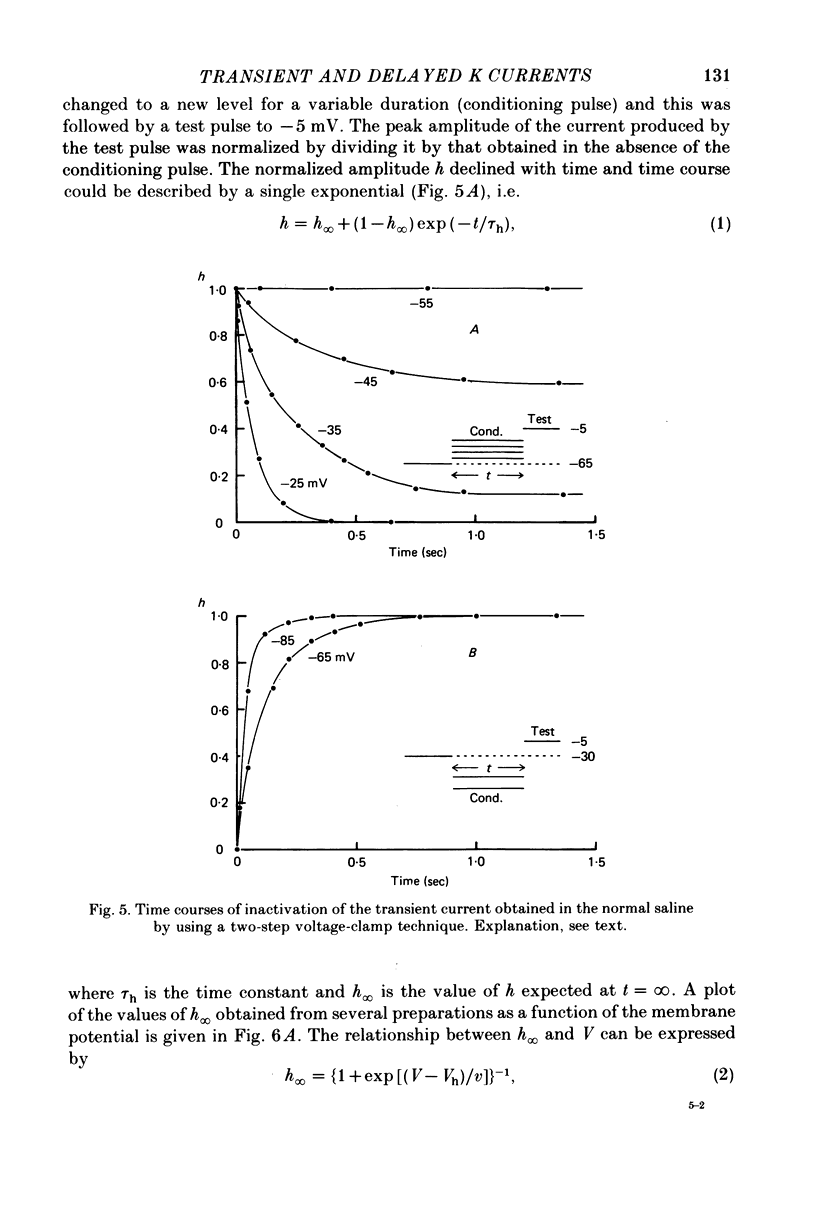
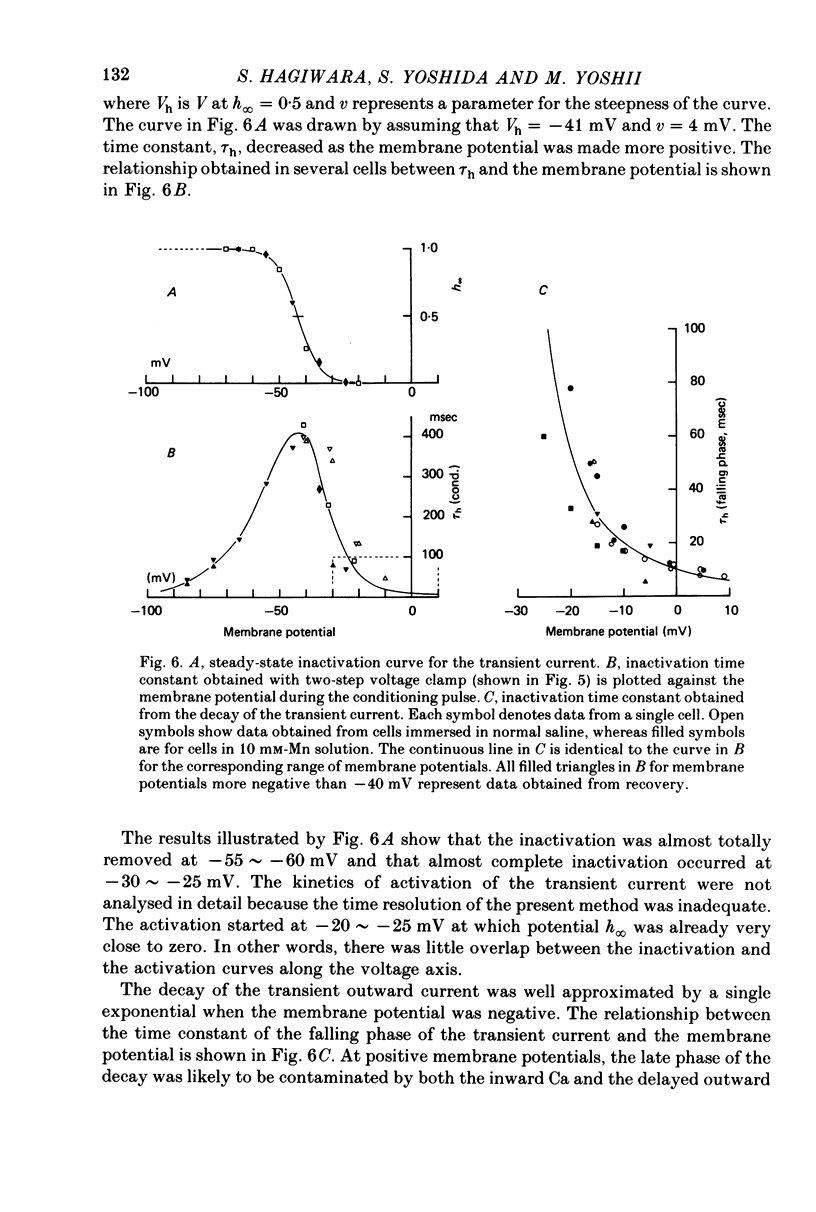
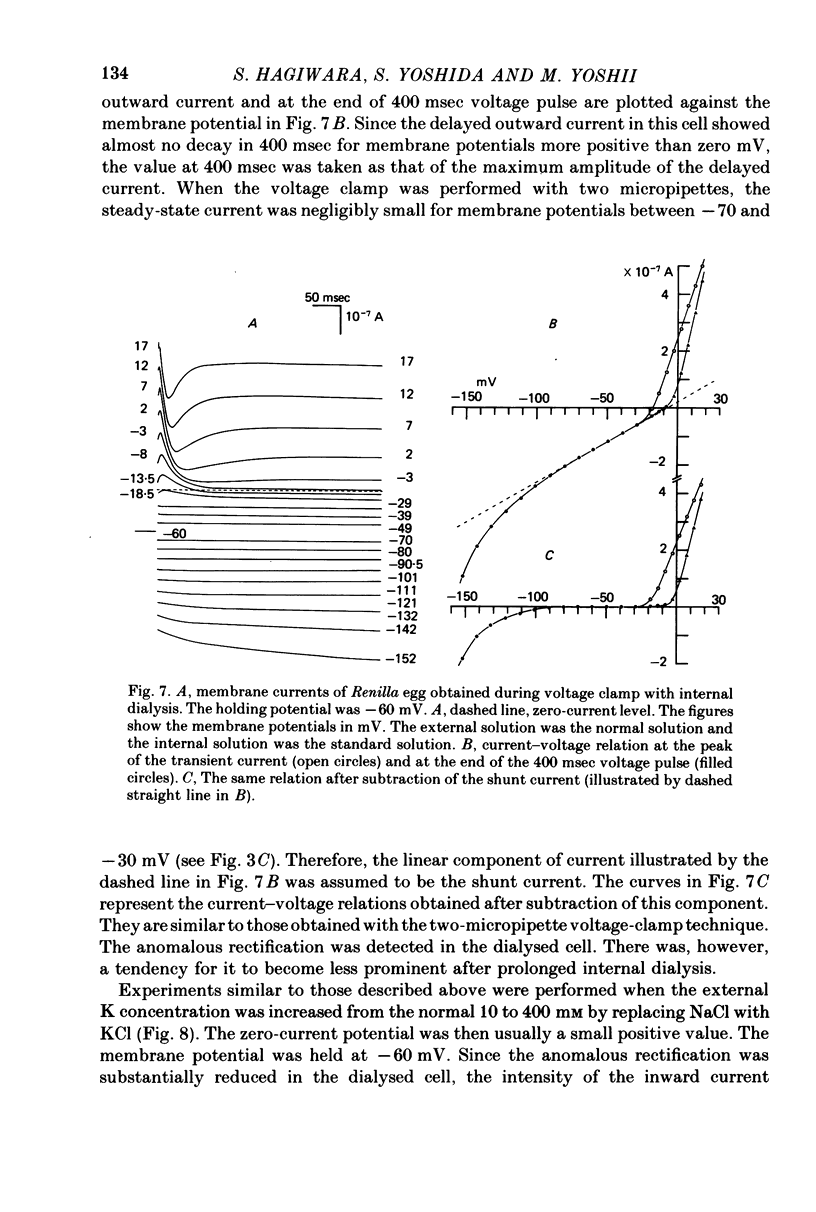
Abstract
1. The properties of the fast-inactivating or transient K current and the slowly inactivating or delayed K current of the membrane of immature eggs of the clonial marine coelenterate, Renilla Koellikeri, were studied by using voltage clamp and intracellular dialysis techniques. 2. The transient current is activated when the membrane potential becomes more positive than -25 approximately -20 mV (resting potential, -72 +/- 5 mV) whereas the activation potential of the delayed current is -10 approximately OmV. These potentials are independent of either [k+]o or [K+]i. 3. The inactivation of the transient current is rapid and is almost complete for membrane potentials more negative than the activation potential while it is slow for the delayed current and incomplete within a few seconds. 4. Both currents shows similar reversal potentials which are predominantly determined by the K concentration gradient across the membrane. 5. The sensitivities of the conductance upon the internal K concentration differ between the two currents, suggesting that the interaction between the site and ions in the membrane channels differ between them. 6. Neither current is a Ca-activated K current. 7. 4-AP suppresses the transient current at concentrations substantially smaller than those that suppress the delayed current while TEA shows no effect on either current. 8. Intracellular application of pronase or tannic acid at relatively high concentrations does not alter the inactivation of either current. 9. The membrane includes a voltage-dependent Ca permeability which results in action potentials under current-clamp conditions.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Bezanilla F., Rojas E. Destruction of sodium conductance inactivation in squid axons perfused with pronase. J Gen Physiol. 1973 Oct;62(4):375–391. doi: 10.1085/jgp.62.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Negative conductance caused by entry of sodium and cesium ions into the potassium channels of squid axons. J Gen Physiol. 1972 Nov;60(5):588–608. doi: 10.1085/jgp.60.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne J. H. Analysis of ionic conductance mechanisms in motor cells mediating inking behavior in Aplysia californica. J Neurophysiol. 1980 Mar;43(3):630–650. doi: 10.1152/jn.1980.43.3.630. [DOI] [PubMed] [Google Scholar]
- Connor J. A. Neural repetitive firing: a comparative study of membrane properties of crustacean walking leg axons. J Neurophysiol. 1975 Jul;38(4):922–932. doi: 10.1152/jn.1975.38.4.922. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. The electrical properties of crustacean muscle fibres. J Physiol. 1953 Apr 28;120(1-2):171–204. doi: 10.1113/jphysiol.1953.sp004884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., KUSANO K., SAITO N. Membrane changes of Onchidium nerve cell in potassium-rich media. J Physiol. 1961 Mar;155:470–489. doi: 10.1113/jphysiol.1961.sp006640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., SAITO N. Voltage-current relations in nerve cell membrane of Onchidium verruculatum. J Physiol. 1959 Oct;148:161–179. doi: 10.1113/jphysiol.1959.sp006279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Jaffe L. A. Electrical properties of egg cell membranes. Annu Rev Biophys Bioeng. 1979;8:385–416. doi: 10.1146/annurev.bb.08.060179.002125. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Rosenthal N. P. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. J Gen Physiol. 1976 Jun;67(6):621–638. doi: 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Ozawa S., Sand O. Voltage clamp analysis of two inward current mechanisms in the egg cell membrane of a starfish. J Gen Physiol. 1975 May;65(5):617–644. doi: 10.1085/jgp.65.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Yoshii M. Effect of temperature on the anomalous rectification of the membrane of the egg of the starfish, Mediaster aequalis. J Physiol. 1980 Oct;307:517–527. doi: 10.1113/jphysiol.1980.sp013451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Yoshii M. Effects of internal potassium and sodium on the anomalous rectification of the starfish egg as examined by internal perfusion. J Physiol. 1979 Jul;292:251–265. doi: 10.1113/jphysiol.1979.sp012849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Schwarz W. Potassium channels as multi-ion single-file pores. J Gen Physiol. 1978 Oct;72(4):409–442. doi: 10.1085/jgp.72.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon J. L., Gibbons W. R. 4-Aminopyridine and the early outward current of sheep cardiac Purkinje fibers. J Gen Physiol. 1979 Feb;73(2):139–157. doi: 10.1085/jgp.73.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon J. L., Gibbons W. R. Influence of chloride, potassium, and tetraethylammonium on the early outward current of sheep cardiac Purkinje fibers. J Gen Physiol. 1979 Feb;73(2):117–138. doi: 10.1085/jgp.73.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S. I., Ohmori H., Sasaki S. Potassium rectifications of the starfish oocyte membrane and their changes during oocyte maturation. J Physiol. 1975 Mar;246(1):55–78. doi: 10.1113/jphysiol.1975.sp010880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Two fast transient current components during voltage clamp on snail neurons. J Gen Physiol. 1971 Jul;58(1):36–53. doi: 10.1085/jgp.58.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas E., Armstrong C. Sodium conductance activation without inactivation in pronase-perfused axons. Nat New Biol. 1971 Feb 10;229(6):177–178. doi: 10.1038/newbio229177a0. [DOI] [PubMed] [Google Scholar]
- Shrager P. G., Macey R. I., Strickholm A. Internal perfusion of crayfish, giant axons: action of tannic acid, DDT, and TEA. J Cell Physiol. 1969 Aug;74(1):77–90. doi: 10.1002/jcp.1040740111. [DOI] [PubMed] [Google Scholar]
- Siegelbaum S. A., Tsien R. W. Calcium-activated transient outward current in calf cardiac Purkinje fibres. J Physiol. 1980 Feb;299:485–506. doi: 10.1113/jphysiol.1980.sp013138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Yoshii M. Effects of internal free calcium upon the sodium and calcium channels in the tunicate egg analysed by the internal perfusion technique. J Physiol. 1978 Jun;279:519–549. doi: 10.1113/jphysiol.1978.sp012360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]



