Abstract
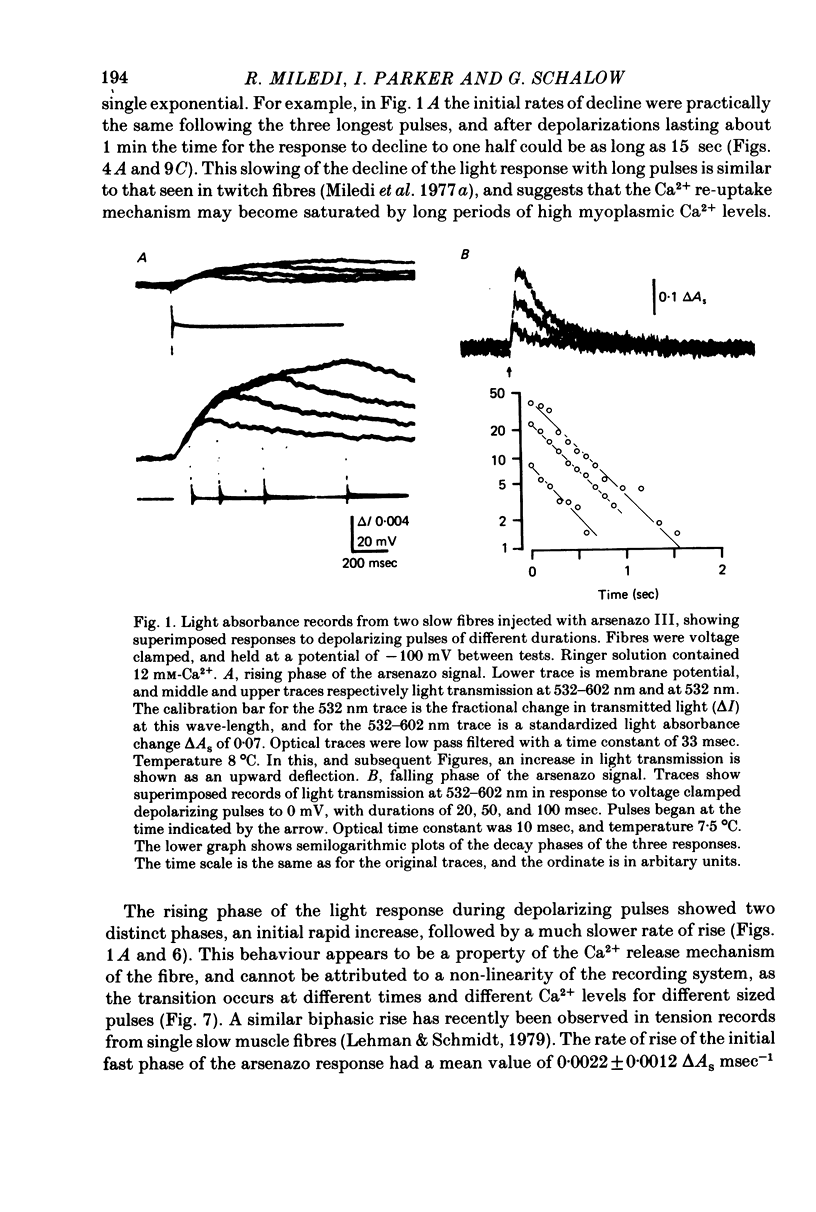
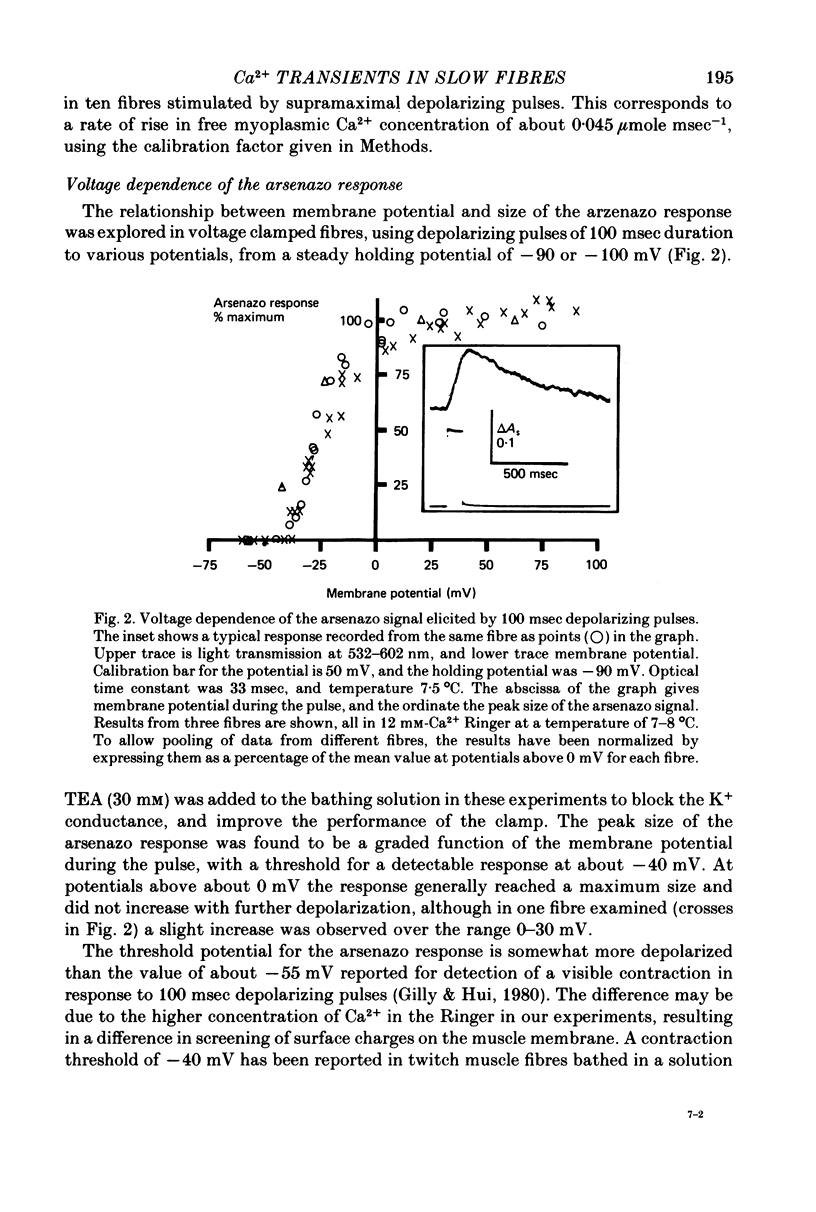
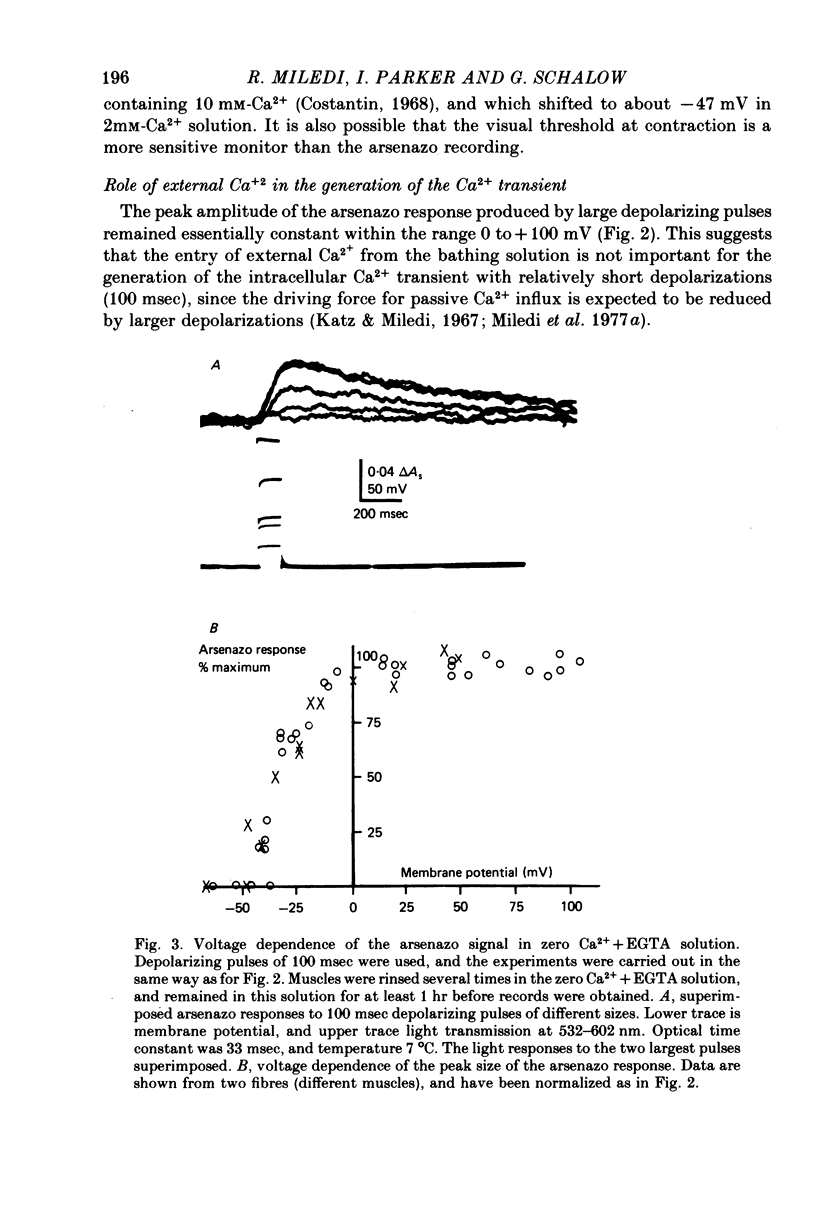
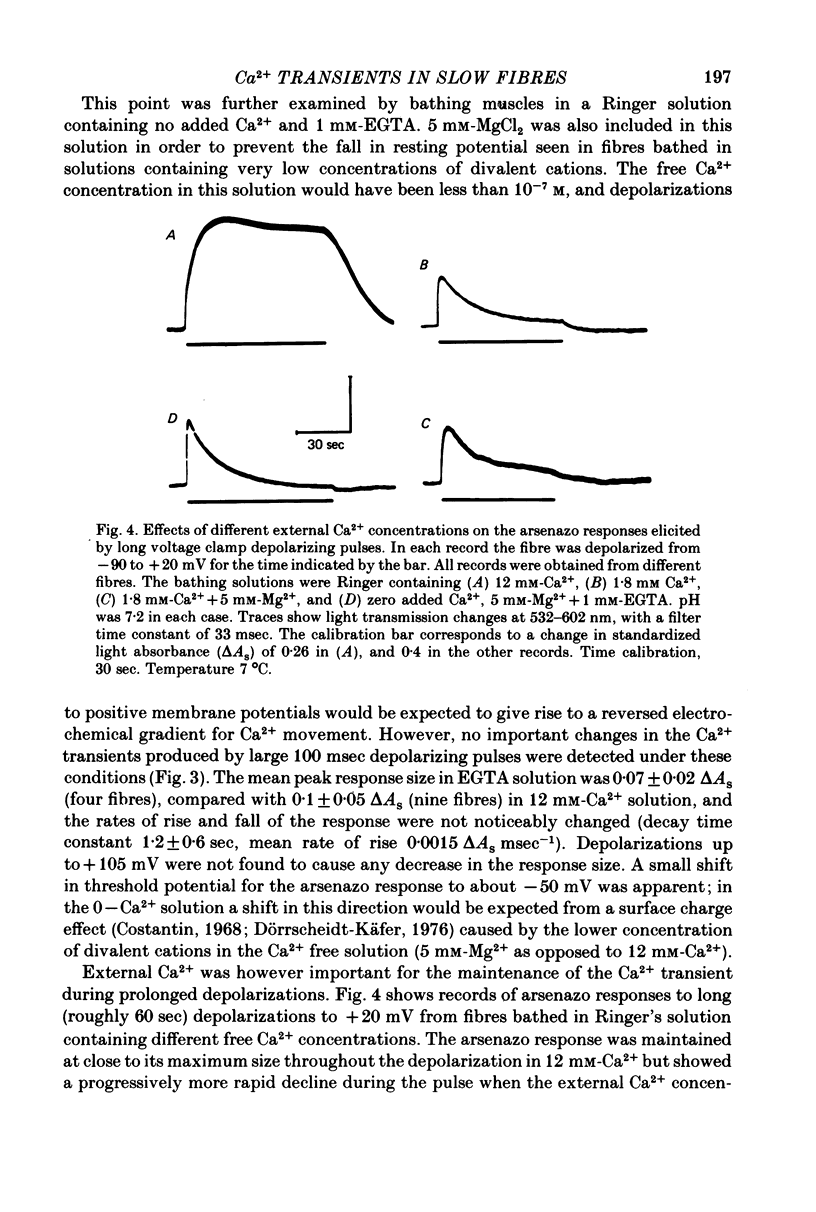
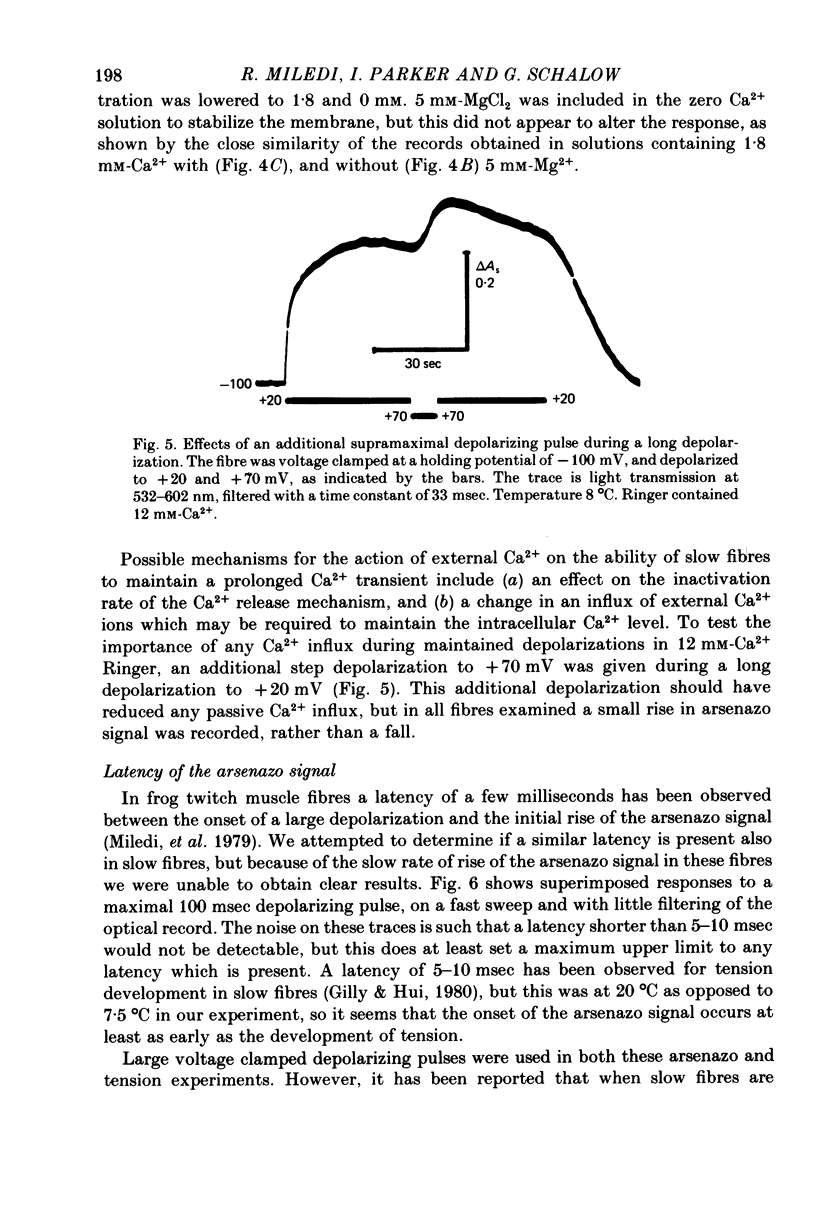
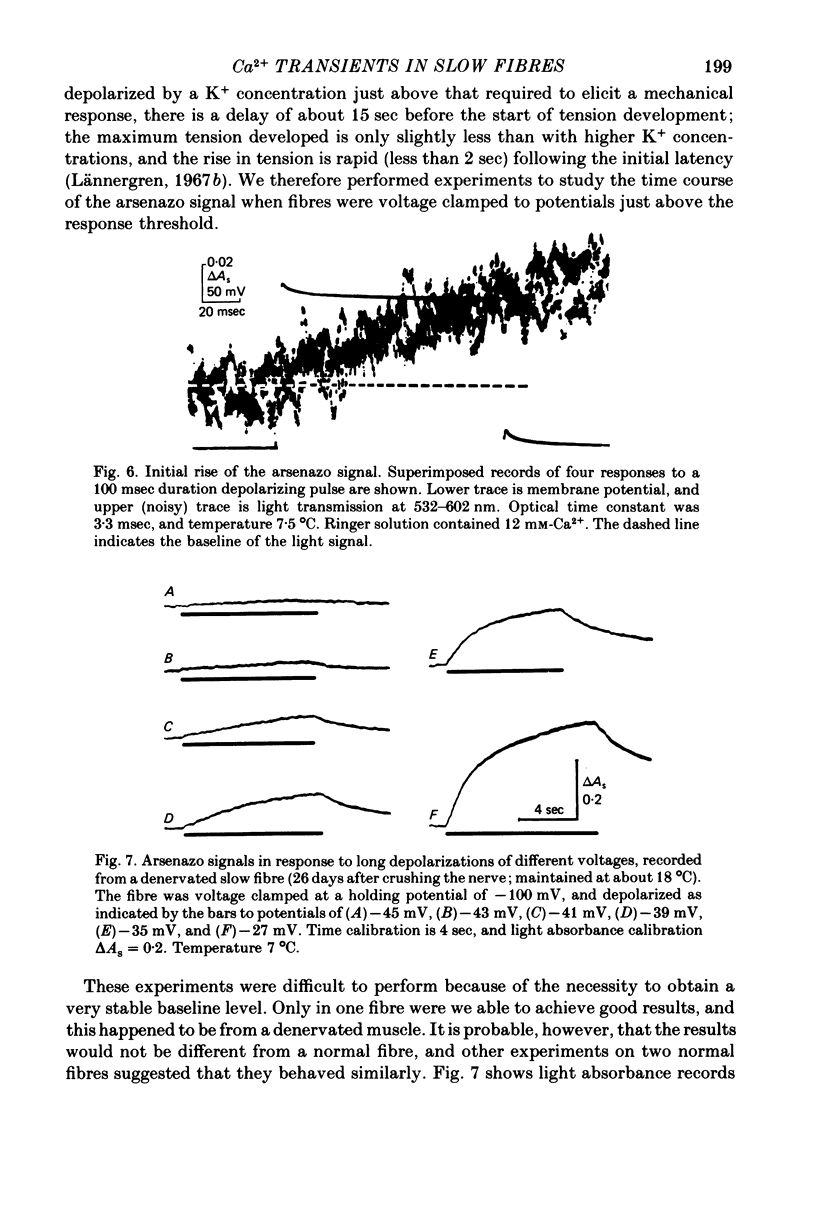
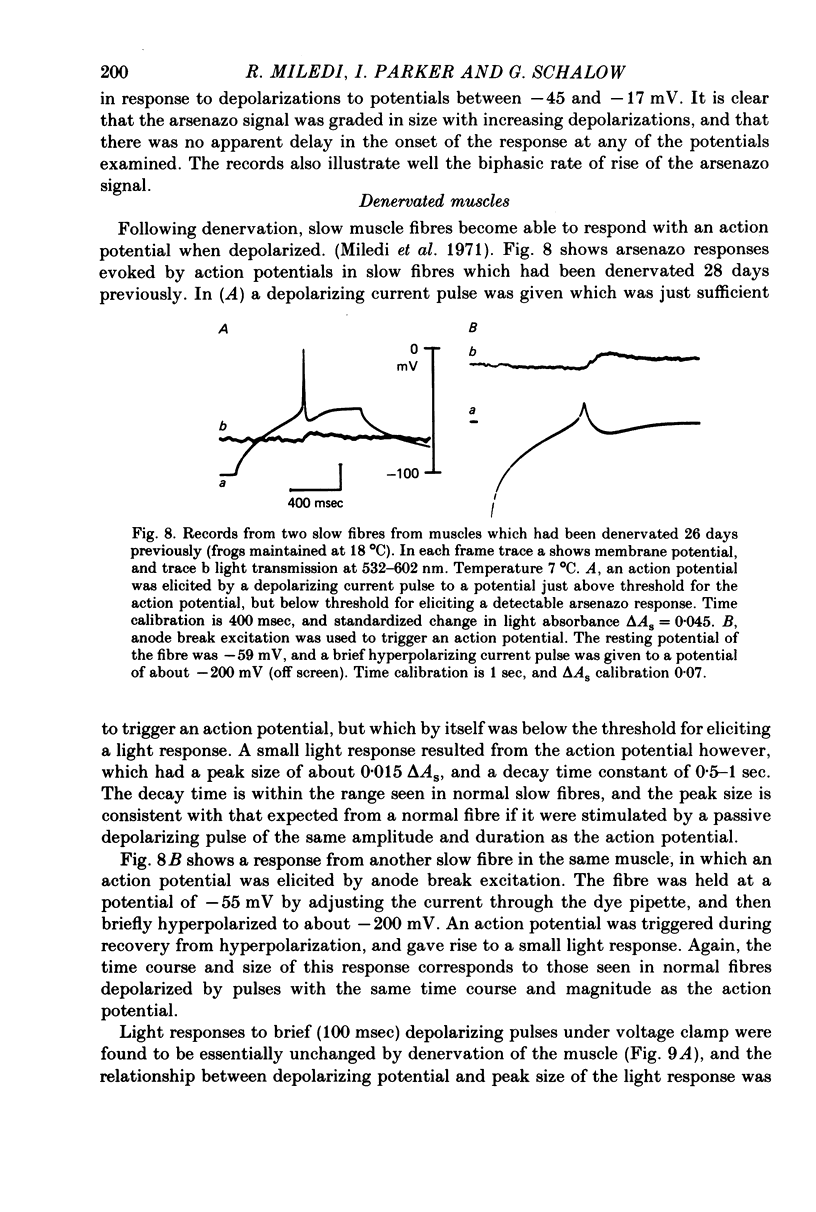
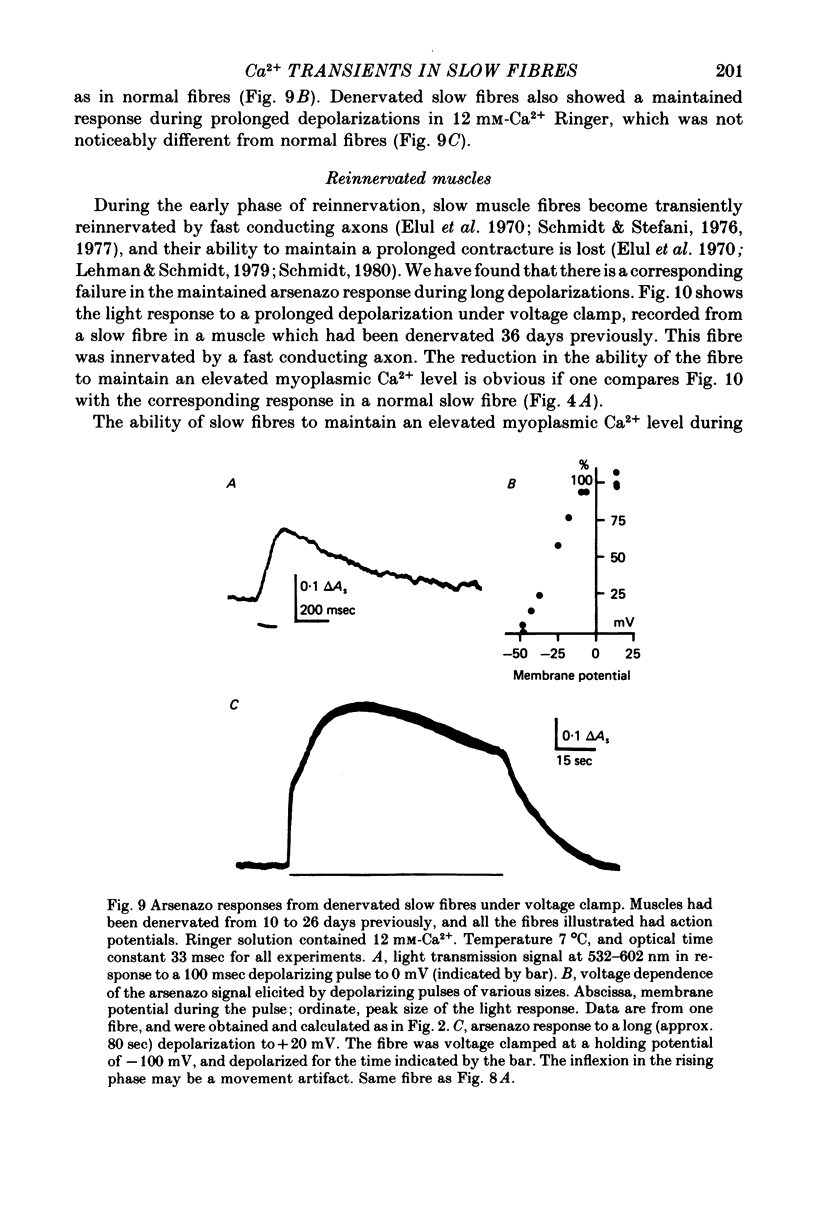
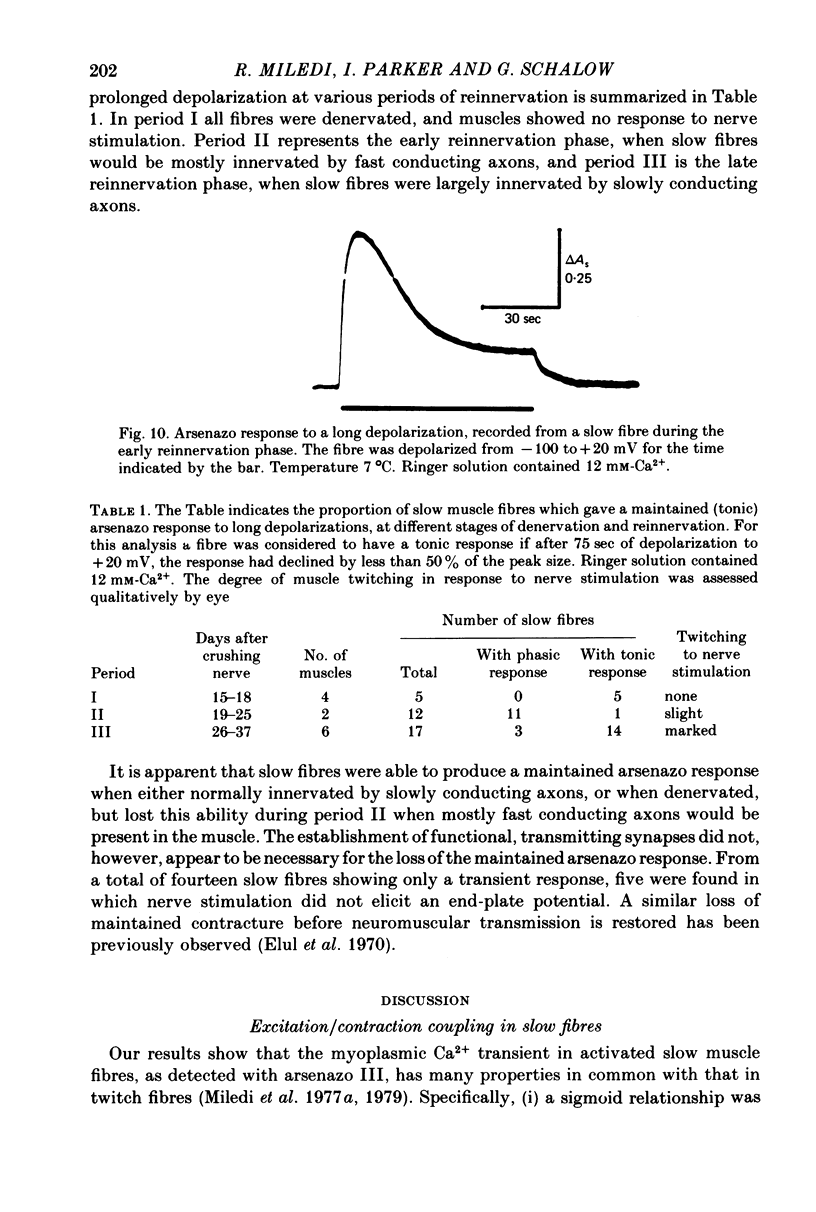
1. Intracellular changes in free Ca2+ concentration were recorded from slow muscle fibres in the pyriformis muscle of Rana temporaria, using the dye arsenazo III. Fibres were voltage clamped, and arsenazo signals were recorded in response to depolarizing pulses. 2. The size of the arsenazo response to depolarizing pulses of 100 msec duration was a sigmoid function of membrane potential over the range -45 to 0 mV, and remained constant with further depolarizations up to +100 mV. 3. The peak size of the arsenazo signal to supramaximal depolarizations increased with increasing pulse length. The initial rising phase during a pulse was much slower than in twitch fibres, and this phase was followed by an even slower rise. Following short pulses the decay of the response was exponential, with a time constant of about 1.4 sec, while after long pulses the decline became much slower. 4. Decreasing free Ca2+ concentration in the bathing medium to very low levels, using EGTA , did not affect the responses to short (100 msec) depolarizations. 5. Slow fibres bathed in Ringer's solution containing 12 mM-Ca2+ showed a well maintained arsenazo response to supramaximal depolarizations lasting over 1 min. Reduction of external Ca2+ to 1.8 and (nominally) 0 mM caused the response to become progressively more transient. 6. After denervation, slow fibres developed action potentials, but non of the parameters of the arsenazo response was significantly changed. During the early phase of reinnervation by a mixed nerve, when fast conduction axons begin to innervate slow fibres, the ability to give a maintained response during long depolarizations was reduced. 7. It is concluded that intracellular Ca2+ transients in slow muscle fibres are probably generated by a similar mechanism as in twitch fibres and entry of external Ca2+ is not an appreciable factor. The slow time course of the transients may be important in determining the time courses of tension development and relaxation.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURKE W., GINSBORG B. L. The electrical properties of the slow muscle fibre membrane. J Physiol. 1956 Jun 28;132(3):586–598. doi: 10.1113/jphysiol.1956.sp005551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C., Fernandez de Bolaños P. Membrane potential, contractile activation and relaxation rates in voltage clamped short muscle fibres of the frog. J Physiol. 1979 Apr;289:175–189. doi: 10.1113/jphysiol.1979.sp012731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantin L. L. Contractile activation in skeletal muscle. Prog Biophys Mol Biol. 1975;29(2):197–224. doi: 10.1016/0079-6107(76)90023-7. [DOI] [PubMed] [Google Scholar]
- Costantin L. L., Podolsky R. J., Tice L. W. Calcium activation of frog slow muscle fibres. J Physiol. 1967 Jan;188(2):261–271. doi: 10.1113/jphysiol.1967.sp008137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantin L. L. The effect o f calcium on contraction and conductance thresholds in frog skeletal muscle. J Physiol. 1968 Mar;195(1):119–132. doi: 10.1113/jphysiol.1968.sp008450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elul R., Miledi R., Stefani E. Neural control of contracture in slow muscle fibres of the frog. Acta Physiol Lat Am. 1970;20(3):194–226. [PubMed] [Google Scholar]
- Flitney F. W. The volume of the T-system and its association with the sarcoplasmic reticulum in slow muscle fibres of the frog. J Physiol. 1971 Aug;217(1):243–257. doi: 10.1113/jphysiol.1971.sp009569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini-Armstrong C. Studies of the triad. IV. Structure of the junction in frog slow fibers. J Cell Biol. 1973 Jan;56(1):120–128. doi: 10.1083/jcb.56.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilly W. F., Hui C. S. Mechanical activation in slow and twitch skeletal muscle fibres of the frog. J Physiol. 1980 Apr;301:137–156. doi: 10.1113/jphysiol.1980.sp013195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess A. Vertebrate slow muscle fibers. Physiol Rev. 1970 Jan;50(1):40–62. doi: 10.1152/physrev.1970.50.1.40. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W., VAUGHAN WILLIAMS E. M. Small-nerve junctional potentials; the distribution of small motor nerves to frog skeletal muscle, and the membrane characteristics of the fibres they innervate. J Physiol. 1953 Aug;121(2):289–317. doi: 10.1113/jphysiol.1953.sp004948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann N., Schmidt H. Contractile responses to direct stimulation of frog slow muscle fibres before and after denervation. Pflugers Arch. 1979 Oct;382(1):43–50. doi: 10.1007/BF00585902. [DOI] [PubMed] [Google Scholar]
- Lännergren J. Contractures of single slow muscle fibres of Xenopus laevis elicited by potassium, acetylcholine or choline. Acta Physiol Scand. 1967 Apr;69(4):362–372. doi: 10.1111/j.1748-1716.1967.tb03533.x. [DOI] [PubMed] [Google Scholar]
- Lännergren J. The force-velocity relation of isolated twitch and slow muscle fibres of Xenopus laevis. J Physiol. 1978 Oct;283:501–521. doi: 10.1113/jphysiol.1978.sp012516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. The acetylcholine sensitivity of frog muscle fibres after complete or partial devervation. J Physiol. 1960 Apr;151:1–23. [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Orkand P. Effect of a "fast" nerve on "slow" muscle fibres in the frog. Nature. 1966 Feb 12;209(5024):717–718. doi: 10.1038/209717a0. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I., Schalow G. Calcium transients in frog slow muscle fibres. Nature. 1977 Aug 25;268(5622):750–752. doi: 10.1038/268750a0. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I., Schalow G. Measurement of calcium transients in frog muscle by the use of arsenazo III. Proc R Soc Lond B Biol Sci. 1977 Aug 22;198(1131):201–210. doi: 10.1098/rspb.1977.0094. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I., Schalow G. Transition temperature of excitation-contraction coupling in frog twitch muscle fibres. Nature. 1979 Jul 26;280(5720):326–328. doi: 10.1038/280326a0. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I., Schalow G. Transmitter induced calcium entry across the post-synaptic membrane at frog end-plates measured using arsenazo III. J Physiol. 1980 Mar;300:197–212. doi: 10.1113/jphysiol.1980.sp013158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Stefani E., Steinbach A. B. Induction of the action potential mechanism in slow muscle fibres of the frog. J Physiol. 1971 Sep;217(3):737–754. doi: 10.1113/jphysiol.1971.sp009597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasledov G. A., Zachar J., Zacharová D. The ionic requirements for the development of contracture in isolated slow muscle fibres of the frog. Physiol Bohemoslov. 1966;15(4):293–306. [PubMed] [Google Scholar]
- Peachey L. D. Muscle. Annu Rev Physiol. 1968;30:401–440. doi: 10.1146/annurev.ph.30.030168.002153. [DOI] [PubMed] [Google Scholar]
- Schmidt H., Stefani E. Action potentials in slow muscle fibres of the frog during regeneration of motor nerves. J Physiol. 1977 Sep;270(2):507–517. doi: 10.1113/jphysiol.1977.sp011965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H., Stefani E. Re-innervation of twitch and slow muscle fibres of the frog after crushing the motor nerves. J Physiol. 1976 Jun;258(1):99–123. doi: 10.1113/jphysiol.1976.sp011409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani E., Uchitel O. D. Potassium and calcium conductance in slow muscle fibres of the toad. J Physiol. 1976 Feb;255(2):435–448. doi: 10.1113/jphysiol.1976.sp011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. V. Arsenazo III forms 2:1 complexes with Ca and 1:1 complexes with Mg under physiological conditions. Estimates of the apparent dissociation constants. Biophys J. 1979 Mar;25(3):541–548. doi: 10.1016/S0006-3495(79)85322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


