Abstract
1. Plasma proteins have been demonstrated to be present in early fetal sheep brain in amounts which cannot be accounted for by blood contamination. 2. The distribution of alpha-fetoprotein, albumin, fetuin, alpha 1-antitrypsin and transferrin has been studied by immunoassay of extracts from brain homogenates and by immunoperoxidase histochemistry of fetal brains between 37 and 125 days gestation (term is 150 days). 3. At 35 days gestation fetuin and albumin were quantitatively the most important proteins in fetal brain, both as estimated by extraction and by immunohistochemistry. Both of these proteins, and also alpha-fetoprotein and alpha 1-antitrypsin, declined considerably in amount by 60 days gestation. After 60 days the concentrations of albumin and alpha-fetoprotein were not significantly different from that due to blood contamination and only occasional cells could be demonstrated by immunohistochemistry. Fetuin and alpha 1-antitrypsin were present in reduced but significant amounts at least up to 125 days gestation. 4. The immunohistochemical results showed that considerable numbers of immature neurones were stained for some plasma proteins. Fetuin positive cells predominated both in terms of the larger number of cells which stained at 35-40 days gestation and in the persistence of positive cells up to 125 days gestation. Numerous cells in the neuroependymal layer and in several layers of the developing cortical plate were positive, especially early in gestation. Only a few transferrin or alpha 1-antitrypsin positive cells were observed. 5. Permeability of the blood-brain barrier to sheep or human serum albumin (labelled with 125I) was tested in 60 day fetal sheep by intravenous injection and estimation of brain radioactivity at 3 or 6 hr with allowance for blood contamination. Only a very small (but significant) penetration of protein was detected. Unlike penetration of protein into c.s.f. at the same age, it did not reach its natural steady state in brain. 6. It is concluded that the blood-brain barrier to protein is well developed in the immature fetal sheep but that developing neurones probably acquire certain plasma proteins directly from the c.s.f. when they are differentiating in the neuroependyma. The subsequent distribution of plasma protein positive cells in different brain regions is suggested to be due to the migration of developing neurones for the neuroependyma although the possibility of local synthesis of plasma proteins has not been excluded.
Full text
PDF














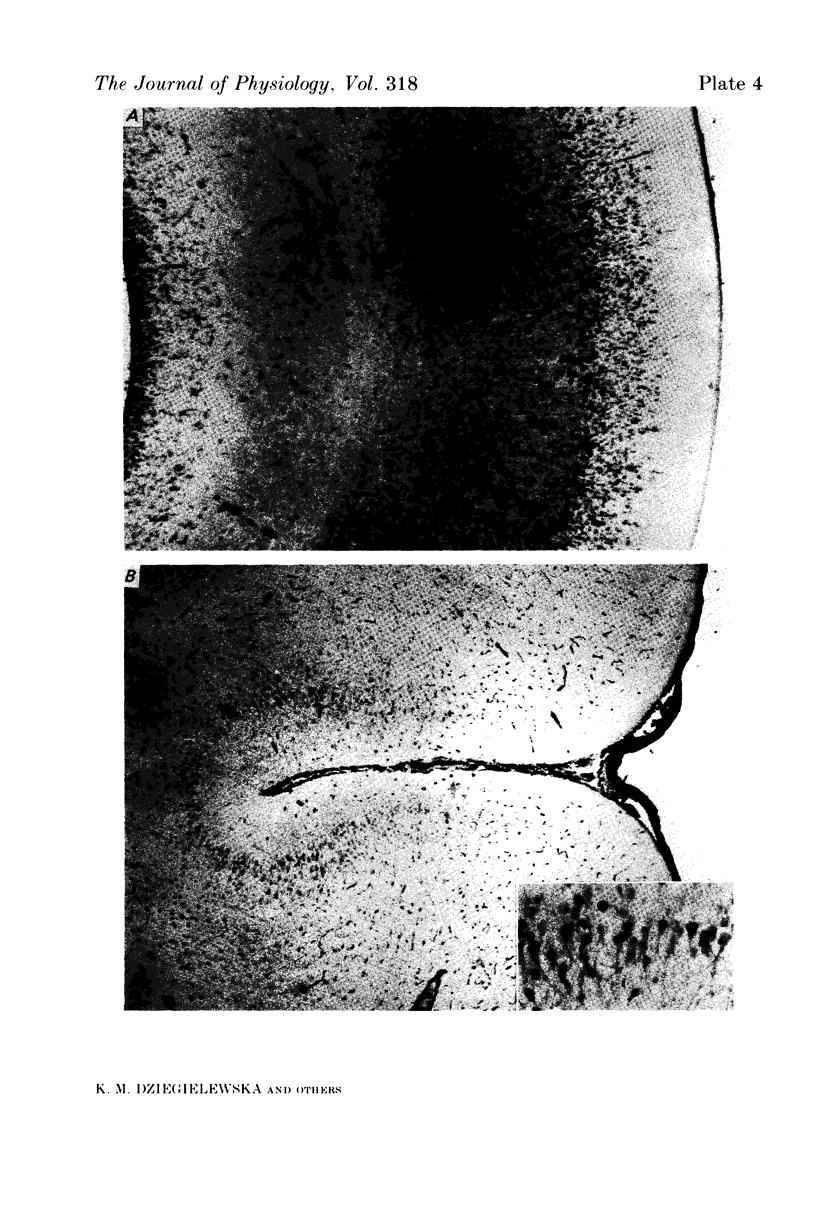
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amtorp O. Transfer of I125-albumin from blood into brain and cerebrospinal fluid in newborn and juvenile rats. Acta Physiol Scand. 1976 Mar;96(3):399–406. doi: 10.1111/j.1748-1716.1976.tb10208.x. [DOI] [PubMed] [Google Scholar]
- Benno R. W., Williams T. H. Evidence for intracellular localization of alpha-fetoprotein in the developing rat brain. Brain Res. 1978 Feb 17;142(1):182–186. doi: 10.1016/0006-8993(78)90189-0. [DOI] [PubMed] [Google Scholar]
- Bock E. Identification and characterization of water soluble rat brain antigens. I. Brain and species specificity. J Neurochem. 1972 Jul;19(7):1731–1736. doi: 10.1111/j.1471-4159.1972.tb06217.x. [DOI] [PubMed] [Google Scholar]
- Brightman M. W., Reese T. S. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969 Mar;40(3):648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen P. P., Thomsen P. Demonstration of hepatitis B-surface antigen in liver biopsies. A comparative investigation of immunoperoxidase and orcein staining on identical sections of formalin fixed, paraffin embedded tissue. Acta Pathol Microbiol Scand A. 1978 Sep;86A(5):383–388. [PubMed] [Google Scholar]
- Dziegielewska K. M., Evans C. A., Fossan G., Lorscheider F. L., Malinowska D. H., Møllgård K., Reynolds M. L., Saunders N. R., Wilkinson S. Proteins in cerebrospinal fluid and plasma of fetal sheep during development. J Physiol. 1980 Mar;300:441–455. doi: 10.1113/jphysiol.1980.sp013171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziegielewska K. M., Evans C. A., Malinowska D. H., Møllgård K., Reynolds J. M., Reynolds M. L., Saunders N. R. Studies of the development of brain barrier systems to lipid insoluble molecules in fetal sheep. J Physiol. 1979 Jul;292:207–231. doi: 10.1113/jphysiol.1979.sp012847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziegielewska K. M., Evans C. A., Malinowska D. H., Møllgård K., Reynolds M. L., Saunders N. R. Blood-cerebrospinal fluid transfer of plasma proteins during fetal development in the sheep. J Physiol. 1980 Mar;300:457–465. doi: 10.1113/jphysiol.1980.sp013172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C. A., Reynolds J. M., Reynolds M. L., Saunders N. R., Segal M. B. The development of a blood-brain barrier mechanism in foetal sheep. J Physiol. 1974 Apr;238(2):371–386. doi: 10.1113/jphysiol.1974.sp010530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosain F., McIntyre P. A., Poulose K., Stern H. S., Wagner H. N., Jr Binding of trace amounts of ionic indium-113m to plasma transferrin. Clin Chim Acta. 1969 Apr;24(1):69–75. doi: 10.1016/0009-8981(69)90142-9. [DOI] [PubMed] [Google Scholar]
- Lai P. C., Hay D. M., Lorscheider F. L. Radioimmunoassay of ovine alpha-fetoprotein. J Immunol Methods. 1978;20:1–10. doi: 10.1016/0022-1759(78)90238-7. [DOI] [PubMed] [Google Scholar]
- Lai P. C., Hay D. M., Peters E. H., Lorscheider F. L. Immunochemical purification and characterization of ovine alpha-fetoprotein. Biochim Biophys Acta. 1977 Jul 22;493(1):201–209. doi: 10.1016/0005-2795(77)90273-2. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Mollgård K., Saunders N. R. A possible transepithelial pathway via endoplasmic reticulum in foetal sheep choroid plexus. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):321–326. doi: 10.1098/rspb.1977.0142. [DOI] [PubMed] [Google Scholar]
- Møllgård K., Jacobsen M., Jacobsen G. K., Clausen P. P., Saunders N. R. Immunohistochemical evidence for an intracellular localization of plasma proteins in human foetal choroid plexus and brain. Neurosci Lett. 1979 Sep;14(1):85–90. doi: 10.1016/0304-3940(79)95349-7. [DOI] [PubMed] [Google Scholar]
- Olsson Y., Klatzo I., Sourander P., Steinwall O. Blood-brain barrier to albumin in embryonic new born and adult rats. Acta Neuropathol. 1968 Mar 4;10(2):117–122. doi: 10.1007/BF00691305. [DOI] [PubMed] [Google Scholar]
- Sidman R. L., Rakic P. Neuronal migration, with special reference to developing human brain: a review. Brain Res. 1973 Nov 9;62(1):1–35. doi: 10.1016/0006-8993(73)90617-3. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand C. D. Coexistence of alpha-fetoprotein, albumin and transferrin immunoreactivity in neurones of the developing mouse brain. Nature. 1980 Aug 14;286(5774):733–735. doi: 10.1038/286733a0. [DOI] [PubMed] [Google Scholar]
- Trojan J., Uriel J. Localisation intracellulaire de l'alpha-foetoprotéine et de la sérumalbumine dans le système nerveux central du Rat au cours du développement foetal et postnatal. C R Seances Acad Sci D. 1979 Dec 10;289(15):1157–1160. [PubMed] [Google Scholar]