Abstract
Objective
To investigate the effects of preoperative neuropathic-like pain and central sensitisation on clinical outcomes after total knee arthroplasty (TKA).
Design
This systematic review and meta-analysis followed PRISMA guidelines and was prospectively registered. Studies were included if they assessed preoperative neuropathic-like pain or central sensitisation before TKA and clinical outcomes after surgery, such as pain, function, or psychological status. Cross-sectional studies and case reports were excluded. Two authors independently screened, extracted data and rated articles' quality using a quality assessment tool. A meta-analysis was performed for studies reporting comparable methods and outcomes.
Results
From 6061 identified records, 15 studies met the inclusion criteria (total sample: 2385 individuals; follow-up periods: less than 1 year to 5 years). Most studies exhibited low/moderate risk of bias, primarily due to small sample sizes. The risk ratio of chronic pain (VAS ≥3 after at least 3 months) was 2.75 (CI: 1.78; 4.26) for patients with neuropathic-like pain (PainDETECT score ≥13). Seven out of eleven studies identified neuropathic-like pain and central sensitisation as risk factors for clinical outcomes such as decreased self-reported function, satisfaction, and anxiety (p < 0.05). Studies that adjusted for covariates showed mixed results, with some losing statistical significance.
Conclusion
The presence of neuropathic-like pain and central sensitisation in candidates for TKA is a risk factor for postoperative chronic pain. Evidence for decreased function, satisfaction, and psychological conditions is inconsistent. Screening and managing neuropathic-like pain and central sensitisation preoperatively could possibly improve clinical outcomes. Further research with standardized methods is needed.
Prospero id
CRD42024622693.
Keywords: Total knee arthroplasty, Neuropathic pain, Central sensitisation, Knee osteoarthritis, Function
1. Introduction
Knee osteoarthritis (OA) is a degenerative joint disease marked by progressive loss of function and chronic pain [1]. Individuals with this condition often endure pain for many years before resorting to surgical treatment [2]. Total knee arthroplasty (TKA) is the most effective treatment for late-stage disease and is typically performed when other interventions have failed [3]. However, approximately 10 % of patients continue to experience chronic pain even after surgery, and the reasons for this are still under debate [4,5].
Recently, the presence of neuropathic-like pain and central sensitisation has gained interest in the context of TKA and chronic pain. Evidence has demonstrated that a significant proportion of patients present signs and symptoms of neuropathic-like pain and central sensitisation before surgery [6]. Moreover, a systematic review has shown that neuropathic-like pain and central sensitisation are risk factors for poor outcomes after surgery [7]. Neuropathic pain is defined by the International Association for the Study of Pain as pain caused by a lesion or disease of the somatosensory nervous system [8]. Its pathophysiology can involve both the peripheral and central pathways, and peripheral and central sensitisation, through a range of molecular mechanisms. Neuropathic pain manifests as a burning sensation, tingling, numbness, and pricking and is frequently accompanied by touch-evoked allodynia, while central sensitisation is referred to as a syndrome with similar symptoms to neuropathic pain, characterized by “increased responsiveness of nociceptive neurons in the central nervous system to their normal or subthreshold afferent input” according to International Association for the Study of Pain and can occur when no organic cause can be found [8,9]. In knee OA, studies have reported plausible mechanisms for both conditions. However, because there is no investigation of the organic cause for neuropathic pain, within these studies, the terms central sensitisation and neuropathic-like pain are often used interchangeably [[10], [11], [12]].
Frequently, studies employ self-reported questionnaires to assess both conditions. These questionnaires are easier to administer than quantitative sensory tests, which require specific equipment and training. PainDETECT is used to assess neuropathic-like pain and is considered a reliable tool that has been used in over 300,000 individuals [13]. Likewise, the Central Sensitisation Inventory (CSI) has been compared against quantitative sensory testing (QST), demonstrating better predictive ability for clinical outcomes [14] and significant correlation to QST in all 5 QST modalities [9].
A recent systematic review selected only studies employing self-reported questionnaires to investigate the relationship between preoperative neuropathic-like pain and central sensitisation on postoperative outcomes [7]. The review demonstrated a two-fold increased risk for individuals with these conditions. Since the previous review, the number of published studies has tripled, providing additional insights into these conditions’ effect on postoperative outcomes in TKA. The objective of this systematic review is to update the previous review and investigate if preoperative neuropathic-like pain and central sensitisation are risk factors for persistent pain and poor clinical outcomes.
2. Methods
2.1. Protocol and registration
This systematic review was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [15] and was prospectively registered in the PROSPERO database (CRD42024622693).
2.2. Eligibility
Studies were eligible for inclusion if they investigated clinical outcomes following TKA in relation to the presence or absence of preoperative neuropathic-like pain or central sensitisation. Studies were excluded if they enrolled patients undergoing TKA for indications other than osteoarthritis (E.g., rheumatoid arthritis) or revision surgery. Cross-sectional studies, case series and case reports were excluded from this review.
2.3. Information sources
Information sources included medical databases and reference lists from screened articles. The search string was used in five databases: PubMed, Scopus, Embase, CINAHL and LILACS (Supplementary Material).
2.4. Study selection
Two expert reviewers, in a first stage, independently evaluated the titles and abstracts of the identified studies and, in a second stage of review, evaluated the full-text articles for eligibility using the Covidence platform after removing duplicates. Any disagreements between the reviewers were resolved through discussion, and if consensus could not be reached, a third reviewer was consulted to make the final decision.
2.5. Data collection process and data items
A standardized template was employed to collect data on the characteristics and outcomes of the included studies. Two authors independently extracted data, including the country of origin, number of participants, average age, recruitment source, method of pain sensitisation measurement, study duration, and follow-up percentage. Additionally, data regarding the relationship between pre-operative neuropathic-like pain and clinical outcomes were extracted. This included the proportion of participants with preoperative sensitisation, study completion rates, descriptions of preoperative neuropathic-like pain, pain metrics (such as mean pain intensity), functionality, psychological condition (such as the Hospital Anxiety and Depression Scale) and patient satisfaction after TKA.
2.6. Risk of bias in individual studies
Studies’ quality was assessed using National Institutes of Health Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. This tool consists of 14 questions, including the following domains: study population, uniform eligibility criteria, sample size justification, exposure measures and assessment, repeat exposure assessment, outcome measures, blinding of outcome assessment, follow-up rate, and statistical analyses.
Two investigators independently evaluated each study through a detailed analysis of the full text. They then reconciled their assessments to agree on a final consensus rating for each criterion. Additionally, they independently determined the overall quality rating for each study, in accordance with the tool's guidelines (available at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tool).
2.7. Summary measures and synthesis of results
The primary outcome was the risk of developing chronic pain after TKA in patients with preoperative neuropathic-like pain or central sensitisation. Differences in postoperative pain scores were also investigated between groups. Secondary outcomes included the risk of poor clinical results based on preoperative neuropathic-like pain scores and mean differences between groups with or without neuropathic-like pain or central sensitisation. Chronic pain was defined in most of the studies as a Visual Analog Scale of Pain (VAS) or Numeric Rating Scale for pain (NRS) score ≥3 at three months postoperatively (Table 1). These values also represent well-established thresholds within the literature for central sensitisation, chronic and neuropathic-like pain [7,13,16,17].
Table 1.
Study characteristics.
| Author | Study design | Questionnaire | Groups | n (Baseline) | n (last follow-up) | % Loss of follow-up | Females (%) | Age | Follow-up | BMI (kg/m2) |
|---|---|---|---|---|---|---|---|---|---|---|
| Phillips 2014 | Prospective cohort |
PainDETECT Score No categorization (linear modeling) |
No categorization (linear modeling) | 96 | 86 | 10.4 | 56 | 71 (range 48–89) | 3 days, 6 weeks, 3,6, 9, and 12 months | NR |
| Kurien 2018 | Prospective cohort |
PainDETECT Score Neuropathic pain: ≥19 Unclear/Unlikely: <19 |
Neuropathic pain (PainDETECT score ≥19) | 15 | 13 | 13.3 | 66.6 | 65.1 (SD: 8.9) | 6 months post-op | 30 (IQR: 27–33.4) |
| Unclear/Unlikely (PainDETECT score <19) | 35 | 33 | 5.7 | 57.6 | 66.9 (SD: 8.0) | 6 months post-op | 30 (IQR: 27–39) | |||
| Soni 2018 | Prospective cohort |
PainDETECT Score Neuropathic pain: ≥19 Unclear: 13–18 Unlikely: <13 |
Nociceptive pain group (EPIONE) | 63 | 42 | 33.3 | 43 | 72 (SD: 8) | 2–12 Months | 29.5 (SD: 5.1) |
| Nociceptive pain group (COASt) | 233 | 219 | 6.0 | 50 | 70 (SD: 9) | 29.6 (SD: 4.9) | ||||
| Unclear pain (EPIONE) | 32 | 16 | 50.0 | 63 | 68 (SD: 8) | 30.2 (SD: 5.2) | ||||
| Unclear pain (COASt) | 112 | 107 | 4.5 | 55 | 67 (SD: 9) | 31.2 (SD: 5.6) | ||||
| Neuropathic pain (EPIONE) | 25 | 14 | 44.0 | 56 | 70 (SD: 10) | 31.8 (SD: 4.9) | ||||
| Neuropathic pain (COASt) | 59 | 58 | 1.7 | 63 | 66 (SD: 9) | 31.5 (SD: 5.5) | ||||
| Soni 2019 | Prospective cohort study |
PainDETECT Score Neuropathic pain: ≥19 Unclear: 13–18 Unlikely: <13 |
Nociceptive pain group | 10 | 10 | 0.0 | 30 | 70 (SD: 7) | 12 months | NR |
| Neuropathic-like + unclear pain group | 14 | 9 | 35.7 | 57 | 67 (SD:10) | |||||
| Hasegawa 2019 | Retrospective cohort |
PainDETECT Score Neuropathic pain: ≥19 Unclear: 13–18 Unlikely: <13 |
Unclear pain (PainDETECT score 13–18) | 21 | N/A | N/A | 81 | 77.4 (Dispersion NR) | 5.1 years | 26.4 (Dispersion NR) |
| Nociceptive pain (PainDETECT score <13) | 201 | N/A | N/A | 86.1 | 77.9 (Dispersion NR) | 4.7 years | 26.5 (Dispersion NR) | |||
| Larsen 2021 | Prospective cohort |
PainDETECT Score No categorization (linear modeling) |
No categorization (linear modeling) | 185 | 131 | 29.2 | 55.7 | 67.73 (SD: 8.98) | 12 months | NR |
| Hasegawa 2021 | Prospective cohort |
PainDETECT score Neuropathic pain: ≥19 Possible neuropathic pain: >13 Unclear: 13–18 Nociceptive: <13 |
Possible neuropathic pain (PainDETECT score >13) | 40 | 40 | 0.0 | 82.5 | 74.5 (Dispersion NR) | 6 months | 27.2 (Dispersion NR) |
| Nociceptive pain group (painDETECT score <13) | 140 | 140 | 0.0 | 80 | 73.5 (Dispersion NR) | 25.8 (Dispersion NR) | ||||
| Shemesh 2023 | Prospective cohort |
PainDETECT score Positive neuropathic: ≥19 Negative neuropathic: <19 |
PainDETECT <19 | 74 | 74 | 0.0 | 50 | 68 (SD: 10) | 4 weeks | 31.1 (Dispersion NR) |
| painDETECT≥ 19 | 19 | 19 | 0.0 | 52.6 | 66 (SD: 12) | 29.7 (Dispersion NR) | ||||
| Vigotsky 2024 | Prospective cohort |
PainDETECT Score No categorization (linear modeling) |
NRS modeled as a function of PainDETECT scores | 77 | 77 | 0.0 | 57 | 64.2 (SD: 6.9) | 3, 6, 12 months | NR |
| Kim 2024 | Retrospective cohort |
PainDETECT Score Neuropathic pain: ≥19 Unclear: 13–18 Unlikely: <13 CSI score Central sensitisation: ≥40 Unlikely: <40 |
Group 1: Central sensitisation and neuropathic pain | 55 | 55 | 0.0 | 94.5 | 70.3 (SD: 6.9) | >24 months | 24.5 (SD: 5.5) |
| Group 2: Central sensitisation only | 68 | 68 | 0.0 | 85.3 | 72.7 (SD: 5.9) | >24 months | 24.2 (SD: 6.3) | |||
| Group 3: Neuropathic pain only | 35 | 35 | 0.0 | 91.4 | 72.7 (SD: 5.6) | >24 months | 25.4 (SD: 4.9) | |||
| Group 4: Neither central sensitisation nor neuropathic pain | 158 | 158 | 0.0 | 86.7 | 72.9 (SD: 6.6) | >24 months | 25.2 (SD: 4.8) | |||
| Fitzsimmons 2018 | Prospective cohort |
S-LANSS Suspected neuropathic pain: ≥12 Unlikely: <12 |
Unsuspected neuropathic pain (S-LANS <12) | 99 | 74 | 25.3 | 71.6 | 65.6 (SD: 8.7) | 6 months | NR |
| Suspected neuropathic pain (S-LANSS ≥12) | ||||||||||
| Lee 2022 | Prospective cohort |
Douleur Neuropathique 4 (DN4) Neuropathic pain: ≥4/10 No neuropathic Pain:< 4/10 |
Non-neuropathic pain | 126 | 126 | 0.0 | 87.3 | 71.26(SD: 4.92) | 1 year | 27.24 (SD: 3.5) |
| Neuropathic pain | 22 | 22 | 0.0 | 100 | 67.64 (SD: 6.56) | 1 year | 27.09 (SD: 4.66) | |||
| Kim 2015 | Prospective cohort |
CSI Score Central sensitivity syndrome: ≥40 points Unlikely: <40 |
Low CSI score group (<40) | 47 | 47 | 0.0 | 100 | 71.1 (SD: 5.5) | 1 and 3 months | 26.5 (Dispersion NR) |
| High CSI score group (≥40) | 44 | 44 | 0.0 | 100 | 69.2 (SD: 5.9) | 26.8 (Dispersion NR) | ||||
| Koh 2020 | Retrospective cohort |
CSI Score Central sensitivity syndrome: ≥40 points Unlikely: <40 |
CS | 55 | 55 | 0.0 | 91 | 71 (Dispersion NR) | 2 years | 25.4 (Dispersion NR) |
| Non-CS | 167 | 167 | 0.0 | 90 | 69 (Dispersion NR) | 26.7 (Dispersion NR) | ||||
| Hasegawa 2022 | Prospective cohort |
CSI Score Central sensitivity syndrome: ≥14 points Unlikely: <14 |
CSI ≥14 | 58 (Overall. NR for subgrups) | NR | NR | 82.8 | 73 (Dispersion NR) | 6 months | 25.4 (Dispersion NR) |
| CSI≤14 | ||||||||||
BMI: Body Mass Index; CSI: Central Sensitisation Inventory; CS: Central Sensitisation; DN4: Douleur Neuropathique 4 (Neuropathic Pain Diagnostic Questionnaire); S-LANSS: Self-reported Leeds Assessment of Neuropathic Symptoms and Signs Scale; EPIONE: Efficacy of Pregabalin in the Management of Osteoarthritis-related Neuropathic Pain Study; NRS: Numerical Rating Scale; IQR: Interquartile Range; n: Number of participants; NR: Not Reported; SD: Standard Deviation; % Loss of follow-up: Percentage of participants who did not complete the study during the follow-up period; Follow-up (months): Duration of follow-up reported in months.
This systematic review also included studies utilizing correlation metrics to evaluate the association between preoperative neuropathic-like pain or central sensitisation questionnaire scores and postoperative clinical outcomes. These studies were analyzed to determine the predictive value of the pain-DETECT, S-LANSS, and CSI questionnaires for chronic pain and other recovery metrics after TKA.
Quantitative synthesis was performed for studies reporting common outcomes using similar methodologies and comparable populations, while qualitative synthesis summarized trends for studies employing varied methodologies or reporting insufficient data for pooling. A quantitative analysis was performed on three included studies (four cohorts) [[18], [19], [20]]. The remaining studies, which did not meet criteria for quantitative analysis, were subjected to qualitative analysis.
The Mantel-Haenszel method was applied to combine study-level effect sizes, with studies weighted by the inverse of their variance. Under the random-effects framework, these weights were further adjusted to incorporate heterogeneity across studies by accounting for the between-study variance (Tau-squared). To further assess heterogeneity, Cochran's Q test was performed, and the I-squared statistic was calculated to quantify the proportion of variability attributable to heterogeneity rather than sampling error.
Chi-squared test was interpreted as statistically significant if < 0.05. I-squared statistics followed Cochrane manual for interpretation: 0 %–40 % might not be important; 30 %–60 %, may represent moderate heterogeneity; 50 %–90 %, may represent substantial heterogeneity; 75 %–100 %, considerable heterogeneity [21]. Although not precise and with overlapping intervals, these thresholds offer an opportunity for interpretation based on the included studies. P-value and 95 % confidence intervals were calculated. P-value was considered statistically significant when p ≤ 0.05. All data analysis was performed using R software (version 4.1.2, R Foundation for Statistical Analysis, Vienna, Austria).
2.8. Additional analysis
Subgroup analyses were planned to explore variations in outcomes based on the categorization of pain-DETECT scores. Studies often used differing thresholds to define neuropathic-like pain (e.g., PainDETECT ≥13 vs PainDETEC ≥19). To address this variability, subgroup analyses were planned according to the pain-DETECT score categorizations as reported in the included studies.
3. Results
3.1. Study selection
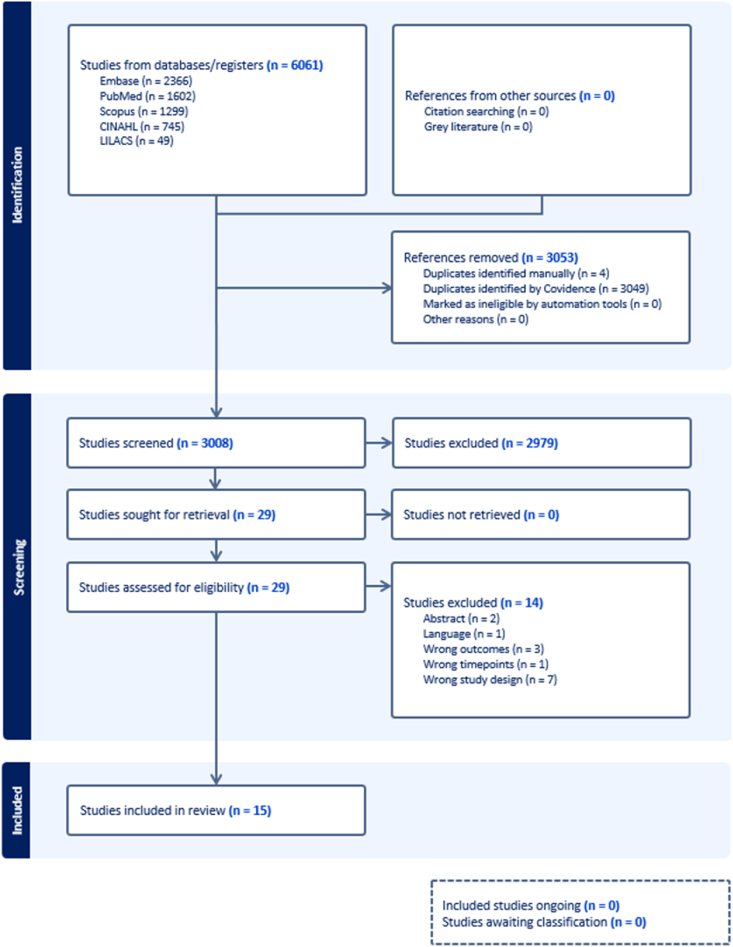
We identified 6061 records through database searching from inception to January 9, 2025 (Embase, PubMed, Scopus, CINAHL, and LILIACS). No additional articles were identified through the reference lists of identified articles. After the removal of duplicates using a systematic review-specific software (Covidence, Veritas Health Innovation, 2023, USA), 3008 articles had their titles and/or abstracts screened. The eligibility criteria were used to screen title and abstracts, after which 2979 articles were excluded. Twenty-nine papers underwent full-text screening, and 14 were excluded due to irrelevant outcomes, incorrect time points, inappropriate study design, non-English language, or being abstracts only. Fig. 1 shows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram, which describes the complete process of searching and selection of the studies.
Fig. 1.
Flowchart diagram of the study. 3008 studies were screened, and 15 studies were included in the review.
3.2. Study characteristics
3.2.1. Design
Fifteen studies were ultimately eligible in the present review, with a total of 16 cohorts and a sample of 2385 individuals. The included studies followed a longitudinal cohort design, with 12 being prospective cohort studies and 3 being retrospective cohort studies (Table 1).
3.2.2. Demographics
The mean age of participants across the studies ranged from 64.2 to 77.9 years, the proportion of females ranged from 30 % to 100 %, and the mean BMI ranged from 24.2 to 31.8.
3.2.3. Follow-up
A total of 6 studies reported a maximum follow-up of less than 1 year [18,[22], [23], [24], [25], [26]], six studies ranging from 1 to less than 2 years [11,19,[27], [28], [29], [30]], and three studies had follow-ups exceeding 2 years [20,31,32]. The longest follow-up, with a mean duration of 5.1 years, was reported by Hasegawa et al. [20].
Loss of follow-up at the last timepoint reported for each study ranged from 0 % to 35.7 %.
3.2.4. Countries
Three studies are from the United Kingdom [11,18,19,27], 3 from Korea [25,30,31], 3 from Japan [20,22,26], 1 from Canada [24], 1 from the USA [29,32], 1 from Denmark [28] and 1 from Switzerland [23].
3.2.5. Outcome data
Neuropathic-like pain and central sensitisation were defined in most of the included studies by a pain-DETECT score: ≥19, or S-LANSS score ≥12 (Table 1) and CSI score ≥40.
In terms of postoperative pain, 6 studies reported postoperative outcome data on the VAS [18,23,27,28,31,32], 1 on The Verbal Numerical Rating scale [27], four on the NRS [19,20,22,26,29], 1 on the Intermittent and Constant Osteoarthritis Pain questionnaire [24] and 1 in pain duration [18], 3 on the proportion of patients with long-term pain [11,19,32], 2 on Oxford Knee Score pain subscale [11,19] and 1 study on the proportion of patients achieving MCID for the VAS [32].
In terms of functionality, postoperative outcome data on the Knee Society Score (KSS) were reported by 2 studies [20,32], 2 studies reported data on the Oxford Knee Score [11,19], and 3 studies reported data on the Western Ontario and McMaster Universities Arthritis Index (WOMAC) [[30], [31], [32]]. Four studies reported outcome data on knee flexion angle [20,22,25,32].
Psychological outcomes were reported in 3 studies: one using the Patient Health Questionnaire-9 (PHQ-9) [24], 1 using the Hospital Anxiety and Depression Scale (HADS) [11], and 1 using the State-Trait Anxiety Inventory [11].
In terms of pain perception, 2 studies reported outcome data on the Pain Catastrophizing Scale [11,24].
3.2.6. Neuropathic-like pain and central sensitisation categorization preoperatively
Ten studies used the PainDETECT score preoperatively [11,[18], [19], [20],22,23,[27], [28], [29],31], 1 study used the short version of the Leeds Assessment of Neuropathic Symptoms and Signs pain scale (S-LANSS) [24] and 1 study used the Douleur Neuropathique 4 score (DN4) [30].
Among the studies that used the PainDETECT score preoperatively, 7 categorized patients into different groups of neuropathic-like pain status based on the total score [11,[18], [19], [20],22,23,31]. Five of these studies adopted the thresholds of unlikely (<13), unclear (13–18), and neuropathic-like pain (>18). Two used a slightly different approach by combining the unlikely and unclear groups into a single category, categorizing patients as having neuropathic-like pain if the score was ≥13 and no neuropathic-like pain if the score was <13 [18,23]. Three studies did not categorize patients into neuropathic-like pain groups but rather used linear models to investigate the relationship between preoperative neuropathic-like pain and central sensitisation with clinical outcomes [[27], [28], [29]].
Three studies used the CSI score to categorize patients based on central sensitisation, with a score of ≥40 indicating central sensitisation and a score of <40 suggesting it is unlikely [25,26,32].
3.3. Risk of bias within studies
The assessment of methodological quality and risk of bias revealed that the majority of the studies exhibited a good or fair quality and therefore, exhibit a low/moderate risk of bias. It is noteworthy that, despite these findings, the studies have limitations regarding their design and the generalizability of the results (Table 2).
Table 2.
Study quality assessment.
| Criteria | Lee 2022 | Vigotsky 2024 | Kim 2024 | Phillips 2014 | Kim 2015 | Larsen 2021 | Soni 2019 | Hasegawa 2022 | Hasegawa 2021 | Shamesh 2023 | Koh 2020 | Fitzsimmons 2018 | Kurien 2018 | Hasegawa 2019 | Soni 2018 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 2 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 3 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | NR | Yes | Yes | Yes | Yes | Yes | Yes |
| 4 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 5 | Yes | Yes | No | No | No | No | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes |
| 6 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 7 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 8 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 9 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 10 | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| 11 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 12 | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| 13 | Yes | No | Yes | Yes | Yes | Yes | Yes | NR | NR | Yes | Yes | No | Yes | Yes | Yes |
| 14 | No | Yes | No | No | No | Yes | No | Yes | No | No | No | Yes | No | Yes | Yes |
| Quality rating AB (good, fair, or poor): | Good | Fair | Fair | Fair | Fair | Fair | Fair | Poor | Poor | Fair | Good | Good | Fair | Fair | Good |
| Quality rating RP (good, fair, or poor): | Good | Fair | Good | Fair | Fair | Fair | Fair | Fair | Fair | Good | Good | Good | Fair | Fair | Good |
The following criteria were assessed using responses of Yes, No, or “cannot determine (CD)/not reported (NR)/not applicable (NA)” for each item. (1) Was the research question or objective in this paper clearly stated? (2) Was the study population clearly specified and defined? (3) Was the participation rate of eligible persons at least 50 %? (4) Were all the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study prespecified and applied uniformly to all participants? (5) Was a sample size justification, power description, or variance and effect estimates provided? (6) For the analyses in this paper, were the exposure(s) of interest measured prior to the outcome(s) being measured? (7) Was the timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed? (8) For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (e.g., categories of exposure, or exposure measured as continuous variable)? (9) Were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? (10) Was the exposure(s) assessed more than once over time? (11) Were the outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? (12) Were the outcome assessors blinded to the exposure status of participants? (13) Was loss to follow-up after baseline 20 % or less? (14) Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)?.
3.4. Results of individual studies
Results of individual studies are presented in Table 3.
Table 3.
Study results.
| Author | Groups | Post-op Pain | Post-op Clinical Outcomes | Results |
|---|---|---|---|---|
| Phillips 2014 | No categorization (linear modeling) | VAS-pain | NR |
Linear correlation (pre-op painDETECT and post op-VAS) VAS post-op not reported according to neuropathic pain status. No correlation between pre-op painDETEC and post-op VAS-pain (r < 0.5) PainDETECT scores did not predict dissatisfaction postoperatively (p > 0.05). |
| Kurien 2018 | Neuropathic pain (PainDETECT score ≥19) | VAS (6 months): 4 cm (range: 0.75–7) | NR |
Group comparison (VAS scores of neuropathic vs unclear/unlikely pain) The neuropathic pain group reported higher postoperative VAS pain scores at 6 months after TKR surgery compared with the unclear/unlikely group (p = 0.0003) |
| Unclear/Unlikely (PainDETECT score <19) | VAS (6 months): 0 cm (range: 0–1) | |||
|
Linear Regression (PainDETECT vs post-op pain) A linear stepwise regression, including preoperative significant associated parameters, found that preoperative PainDETECT was the only independent factor associated with postoperative pain (p = 0.006) | ||||
| Soni 2018 | Nociceptive pain group (EPIONE) | Epione Study Long term pain (VAS≥ 3 at 12 months post-op) = 6/42 (14 %) |
OKS score (2 months post-op): 39 (IQR: 29–4) OKS score (12 months post-op): 43 (IQR: 38–46) |
Logistic Regression (PainDETECT vs post-op pain) (Univariable & multivariable model) EPIONE Unclear pain = OR: 3.7 & 3.4 (p = 0.05 & 0.08) neuropathic pain = OR: 3.4 & 2.6 (p = 0.08 & 0.23) COASt Unclear pain = OR: 2.3 & 2 (p = 0.001 & 0.006) neuropathic pain = OR: 3.6 & 3 (p = 0.001 & 0.001) Group comparison (unclear vs nociceptive vs neuropathic pain post-op pain severity) Epione Cohort: The unclear pain group prior to surgery were significantly (p = 0.05) more likely to report moderate to severe long-term pain after arthroplasty at 12-months post-operatively, compared to the nociceptive group. No significant difference after adjusting for confounding factors No significant difference when a higher cut-off value was used to define moderate to severe long-term pain after arthroplasty COASt Cohort: Patients in the unclear and neuropathic groups were significantly more likely to report moderate to severe long-term pain after arthroplasty, when compared to the nociceptive group (p < 0.05). This relationship remained significant after adjusting for confounding factors and when a higher threshold for moderate to severe long-term pain after arthroplasty was used |
| Nociceptive pain group (COASt) | COAst Cohort Long term pain (NRS≥ 4 at 12 months post-op) = 53/219 (24 %) |
OKS score (12 months post-op): 42 (IQR: 35–46) | ||
| Unclear pain (EPIONE) | Epione Cohort Loong term pain (VAS≥ 3 at 12 months post-op) = 6/16 (38 %) |
OKS score (2 months post-op): 35 (IQR: 29–40) OKS score (12 months post-op): 43.5 (IQR: 35–44) |
||
| Unclear pain (COASt) | COAst Cohort (NRS≥ 4 at 12 months post-op) = 44/107 (41 %) | OKS score (12 months post-op): 39 (IQR: 29.5–44) | ||
| Neuropathic pain (EPIONE) | Epione Cohort ong term pain (VAS≥ 3 at 12 months post-op) = 5/14 (36 %) | OKS score (2 months post-op): 32 (IQR: 18–41) OKS score (12 months post-op): 39 (IQR: 32–43) |
||
| Neuropathic pain (COASt) | COAst Cohort Long term pain (NRS≥ 4 at 12 months post-op) = 29/58 (50 %) |
OKS score (12 months post-op): 37 (IQR: 25–43) | ||
| Soni 2019 | Nociceptive pain group | OKS pain subscale (12 months): 26.0 (IQR: 24.0–32.0) Moderate-to-severe long-term pain (12 months): 0 (0 %) |
OKS function (12 months): 20 (IQR: 20.0–28.6) OKS (12 months): 46 (IQR: 40.0–47.0) Patient-acceptable symptom state (12 months): 9 (90 %) HADS anxiety (12 months): 0.5 (IQR: 0.0–2.0) HADS depression (12 months): 1.0 (IQR: 0.0–3.0) STAI state anxiety (12 months): 24.0 (SD: 10.2) STAI trait anxiety (12 months): 28.0 (SD: 5.5) PCS (12 months): 5 (IQR: 0–6) PSQI (12 months): 7.8 (SD: 2.9) |
Group comparison (neuropathic vs nociceptive pain severity) The neuropathic-like pain group presented a higher proportion of patients with moderate-to-severe long-term pain after arthroplasty, compared to the nociceptive pain group (p = 0.0356). The neuropathic-like pain group presented a higher HAD anxiety and PCS score after arthroplasty, compared to the nociceptive pain group (p < 0.05). |
| Neuropathic-like + unclear pain group | OKS pain subscale (12 months): 36.0 (IQR: 20.0–52.0) Moderate-to-severe long-term pain (12 months): 4 (44 %) |
OKS function (12 months): 31.7 (IQR: 21.5–37.2) OKS (12 months): 40 (IQR: 33.0–48.0) Patient-acceptable symptom state (12 months): 5 (56 %) HADS anxiety (12 months): 3.0 (IQR: 1.0–7.0) HADS depression (12 months): 1.0 (IQR: 0.0–7.0) STAI state anxiety (12 months): 33.0 (SD: 15.8) STAI trait anxiety (12 months): 33.9 (SD: 12.7) PCS (12 months): 14 (IQR: 2–17) † PSQI (12 months): 8.7 (SD: 4.3) |
||
| Hasegawa 2019 | Unclear pain (PainDETECT score 13–18) | NRS (4.7 years): 5 Dispersion NR |
(4.7 years) Moderate to severe pain %: 85.7 Flexion angle: 121 KSS symptoms: 13.5 KSS satisfaction: 20.7 KSS functional: 46 KSS alignment: 20 KSS mediolateral/anterolateral instability: 12.4/10 Dispersion NR |
Group comparison (unclear vs nociceptive NRS and KSS scores) Mean NRS scores were higher among patients with unclear pain than with nociceptive pain. Patients with unclear pain had lower symptom scores, patient satisfaction, patient expectations, And functional activities. NRS score: p < 0.5 KSS symptoms: p < 0.5 KSS satisfaction: p < 0.5 KSS functional: p < 0.5 |
| Nociceptive pain (PainDETECT score <13) | NRS (4.7 years): 1.9 Dispersion NR |
(4.7 years) Flexion angle: 124 KSS symptoms: 20.1 KSS satisfaction: 26.8 KSS functional: 62.5 KSS alignment: 24.3 KSS mediolateral/anterolateral instability: 12.4/9.9 Dispersion NR |
||
| Larsen 2021 | No categorization (linear modeling) | VAS-pain | NR |
Linear modeling (PainDETECT vs post-op pain intensity) Positive correlation between preoperative PainDETECT and postoperative pain intensity (VAS-pain) (r = 0.298)(p = 0.001)(R2 = 0.0889) Multiple regression analysis including PainDETECT score was significant (p < 0.001) but PainDETECT score itself was not a significant independent predictor (p = 0.58) in the model. |
| Hasegawa 2021 | Possible neuropathic pain (PainDETECT score >13) | NRS (6 months): 1.5 (NR) | Flexion angle: 113 (NR) |
Group comparison (neuropathic vs nociceptive pain) No differecence in post-op NRS scores and knee flexion angle between the possbile neuropathic paiin group and nociceptive pain group (p = 0.15 and 0.94) There were no differences in preoperative pain in relation to sex, age, BMI, KL grade, and preoperative Flexion angle The preoperative presence of possible neuropathic pain might be associated with the development of persistent postoperative pain following TKA. |
| Nociceptive pain group (painDETECT score <13) | NRS (6 months): 1.2 (NR) | Flexion angle: 117 (NR) | ||
| Shemesh 2023 | PainDETECT <19 | VAS-pain (4 weeks): 7.68 (SD: 2.4) | Distance walked POD1: 82.3 (SD: 70.6) Maximal distance walked before discharge: 168.5 (SD: 101.5) Length of stay (hours): 73.6 (SD: 45.4) Discharge destination (Home): 57 (77 %) Discharge destination (Acute rehabilitation): 4 (5.4 %) Discharge destination (Rehabilitation): 13 (17.5 %) Early post-operative complications: 6 (12 %) 30-days readmission rate: 1 (1.35 %) 90-days readmission rate: 1 (1.35 %) |
Group comparison (Paindetect ≥ 19 vs < 19 clinical outcomes) The group with PainDETECT scores ≥19 showed statistically higher VAS (4 weeks) compared the <19 PainDETECT scores. (p = 0.006) The existence of neuropathic pain did affect the discharge destination, with a significantly higher proportion of patients necessitating referral to rehabilitation than patients with PainDETECT score <19 (47.3 % vs. 17.5 %, p = 0.02). Linear modeling (PainDETECT vs post-op outcomes PainDETECT was not a predictor of length of stay, discharge destination, and early postoperative complications (p > 0.05) |
| PainDETECT≥ 19 | VAS-pain (4 weeks): 9.3 (SD: 8) | Distance walked POD1: 65 (SD: 72) Maximal distance walked before discharge: 120 (SD: 83.3) Length of stay (hours): 70.8 (SD: 28.7) Discharge destination (Home): 10 (52.6 %) Discharge destination (Acute rehabilitation): 0 (0 %) Discharge destination (Rehabilitation): 9 (47.3 %) Early post-operative complications: 6 (12 %) 30-days readmission rate: 0 (0 %) 90-days readmission rate: 0 (0 %) |
||
| Vigotsky 2024 | NRS modeled as a function of PainDETECT scores | NRS (0-10) 3 months(β = 0.012, p = 0.052, pmvt = 0.162) 6 months (β = 0.024,p < 0.001,pmvt = 0.002) 12 months (β = 0.021,p = 0.004, pmvt = 0.014) |
NR |
Linear modeling (PainDETECT vs post-op pain) PainDETECT scores were significantly predictive of pain at 6 and 12 months after TKA. Preoperative neuropathic pain scores captured 30 % and 20 % of the variance in postoperative pain at 6 and 12 months, respectively. |
| Kim 2024 | Group 1: Central sensitisation and neuropathic pain | VAS pain (2 year post-op): 2.9 (SD: 1.0) | WOMAC (2 yr post-op): 42.4(SD: 14.2) WOMAC pain (2 yr post-op): 7.4 (SD: 4.1) Satisfaction = 63.6 % |
Group comparison (CS, NP, CS + NP clinical outcomes) Group 1 (central sensitisation and neuropathic pain) showed inferior WOMAC pain, function and total scores compared with the other groups (p < 0.05 for all). Groups 2 (central sensitisation) and 3 (neuropathic pain) had worse WOMAC pain, function and total scores at 2 years after surgery compared with group 4 (no CS nor neuropathic pain) (p < 0.05 for all) |
| Group 2: Central sensitisation only | VAS pain (2 year post-op): 2.8 (SD: 1.1) | WOMAC (2 yr post-op): 33.3 (SD: 14.6) WOMAC pain (2 yr post-op): 4.9 (SD: 3.5) Satisfaction = 73.5 % |
||
| Group 3: Neuropathic pain only | VAS pain (2 year post-op): 2.3 (SD: 1.6) | WOMAC (2 yr post-op): 30.1 (SD: 18.6) WOMAC pain (2 yr post-op): 4.3 (SD: 4.4) Satisfaction = 74.3 % |
||
| Group 4: Neither central sensitisation nor neuropathic pain | VAS pain (2 year post-op): 1.6 (SD: 0.9) | WOMAC (2 yr post-op): 16.4 (SD: 11.1) WOMAC pain (2 yr post-op): 2.1 (SD: 2.1) Satisfaction 88 % |
||
| Fitzsimmons 2018 | Unsuspected neuropathic pain (S-LANS <12) | ICOAP (1 month): 11.7 (SD: 8.5) ICOAP (6 months): 5 (SD: 6.1) |
PCS (1 month): 5.3 (SD: 6.2) PCS (6 months): 2.3 (SD: 4.3) PHQ-9 (1 month): 4.4 (SD: 4.1) PHQ-9 (6 months): 1.9 (SD: 4) |
Group comparison of neuropathic pain and non-neuropathic pain ICOAP, pain catostrophizing and depression scores Those with suspected neuropathic pain had higher scores for ICOAP total pain (p = 0.05), pain catastrophizing (p < 0.01), and depression (p < 0.01) at each assessment. Linear Regression (neuropathic pain identification vs ICOAP scores) After adjusting for potential confounding, pre-TKA suspected neuropathic pain did not predict ICOAP total pain or PHQ-9 depression scores at 6 months. |
| Suspected neuropathic pain (S-LANSS ≥12) | ICOAP (1 month): 22.7 (SD: 7.4) ICOAP (6 months): 19 (SD: 12.5) |
PCS (1 month): 15.4 (SD: 11.6) PCS (6 months): 13.6 (SD: 13.2) PHQ-9 (1 month): 10 (SD: 7.4) PHQ-9 (6 months): 8.1 (SD: 7.3) |
||
| Lee 2022 | Nonneuropathic pain | NR | WOMAC (1 year): 11.82 (SD: 11.28) EQ-5D health (1 year): 75 (SD: 13.86) EQ-5D (1 year): 6.37 (SD: 1.6) |
Group comparison (neuropathic vs non-neuropathic WOMAC, EQ-5D scores) There was no difference between the two groups in WOMAC, EQ-5D and EQ-5D health scores 1 year after surgery |
| Neuropathic pain | NR | WOMAC (1 year): 8.36 (SD: 9.24) EQ-5D health (1 year): 74.77 (SD: 10.74) EQ-5D (1 year): 6.18 (SD: 1.18) |
||
| Kim 2015 | Low CSI score group (<40) | VNRS (1 month): 3 (IQR: 2–5) VNRS (3 month): 2 (IQR: 2–4) VNRS≥ 5 (n%) (1 month) = 25 % VNRS≥ 5 (n%) (3 month) = 6 % Pain relief (poor/fair/good/excellent) = 1(2 %)/8(17 %)/28(59 %)/10(21 %) |
Functional improvement (poor/fair/good/excellent) 1(2 %)/6(12 %)/29(61 %)/11(23 %) Knee flexion (1 month): 90 (IQR: 80–100) Knee flexion (3 month): 120 (IQR: 110–120) |
Group comparison (low CSI pain scores post op vs high CSI pain post op) High CSI score group presented statistically higher pain scores, greater proportion of patients presenting moderate-to-severe pain (≥5 VNRS) at 1 and 3 months of follow-up, and less pain relief rates at 3 months, compared to low CSI score group (p < 0.05) Other outcomes were not statistically significant (p > 0.05) Logistic Regression (CSI vs post-op pain) In multivariate analysis, a preoperative CSI score ≥40 was the strongest determinant with 5.091 of the highest odds ratio (95 % CI 1.324 to 19.523, p = 0.016) for predicting a persistent pain 3 months after surgery among demographic and pain-related variables. |
| High CSI score group (≥40) | VNRS (1 month): 4 (IQR: 4–6) VNRS (3 month): 4 (IQR: 3–5) VNRS≥ 5 (n%) (1 month) = 47 % VNRS≥ 5 (n%) (3 month) = 27 % Pain relief (poor/fair/good/excellent) = 4(9 %)/17(38 %)/19(43 %)/5(11 %) |
Functional improvement (poor/fair/good/excellent) = 2(4 %)/7(15 %)/25(56 %)/10(22 %) Knee flexion (1 month): 90 (IQR: 80–100) Knee flexion (3 month): 120 (IQR: 100–120) |
||
| Koh 2020 | CS |
2 years post-op VAS: 2.3 Changes from preoperative status (pain VAS): 3.5 Proportion of MCID (pain VAS) (%): 47 (86 %) Proportion of persistent pain (%): 21 (38 %) Dispersion NR |
2 years post-op Flexion contracture (°): 0.3 Further flexion (°): 128.7 Hip-knee-ankle axis (°): 0.7 KSS: 165.3 Changes from preoperative status (knee Society score): 58.6 Proportion of MCID (knee Society score) (%): 45 (89 %) WOMAC score: 25.2 Changes from preoperative status (WOMAC): 29.5 Proportion of MCID (WOMAC) (%): 44 (80 %) Dispersion NR |
Group comparison (CS vs Non-CS VAS, WOMAC, KSS, satisfaction scores) The CS group presented statistically higher pain VAS, WOMAC scores, and proportion of persistent pain compared to the Non-CS group (p < 0.05). Non-CS group had statistically higher knee Society scores, changes from preoperative WOMAC status, and the proportion of patients achieving MCID for WOMAC compared to the CS group (p < 0.05). Non-CS patients were more satisfied with all kinds of activities (sitting, lying in bed, getting out of bed, light household duties, leisure recreational activities, and total overall satisfaction) than CS patients (p < 0.05) (data not extracted due to absence of table). Logistic Regression (CSI vs post-op dissatisfaction) Multivariate regression analyses revealed that preoperative CSI score, postoperative pain VAS, and WOMAC score were risk factors for dissatisfaction following TKA (P < 00.01) |
| Non-CS |
2 years post-op VAS: 1 Changes from preoperative status (pain VAS): 4.2 Proportion of MCID (pain VAS) (%): 154 (92 %) Proportion of persistent pain (%): 19 (11 %) Dispersion NR |
2 years post-op Flexion contracture (°): 0.1 Further flexion (°): 128.1 Hip-knee-ankle axis (°): 0.8 KSS: 177.6 Changes from preoperative status (knee Society score): 65.2 Proportion of MCID (knee Society score) (%): 157 (94 %) WOMAC score: 15.4 Changes from preoperative status (WOMAC): 42.1 Proportion of MCID (WOMAC) (%): 150 (90 %) Dispersion NR |
||
| Hasegawa 2022 | CSI ≥14 | NRS (6 months post-op): 1.8 (p = 0.025) |
Multivariate regression analysis of post-op knee pain Pre-op CSI ≥14 - OR: 2.5 (p = 0.438) Correlation between preop CSI and walking and standing score - (r = −0.422, p= <0.001) Correlation between pre-op CSI and Post-op NRS (r-0.311,p = 0.018) |
Group Comparison The CSI ≥14 group presented higher post-op NRS than the group with <14 in CSI (p = 0.025). Linear modeling (pre-op CSI score with pain) There was a weak positive correlation with pre-op CSI and post-op NRS (r = 0.311)(p = 0.018). Preoperative CSI correlated negatively with postoperative walking and standing score (r = −0.442)(p < 0.001). Using a multivariate analysis, CSI was not a significant factor in postoperative pain (p = 0.438). |
| CSI <14 | NRS (6 months post-op): 0.9 (p = 0.025) |
BMI: Body Mass Index; CSI: Central Sensitisation Inventory; CS: Central Sensitisation; DN4: Douleur Neuropathique 4; EQ-5D: EuroQol 5-Dimensions Questionnaire; HADS: Hospital Anxiety and Depression Scale; ICOAP: Intermittent and Constant Osteoarthritis Pain Questionnaire; IQR: Interquartile Range; KL: Kellgren-Lawrence Grade; KSS: Knee Society Score; LOS: Length of Stay; MCID: Minimal Clinically Important Difference; n: Number of Participants; NR: Not Reported; NRS: Numeric Rating Scale; OKS: Oxford Knee Score; PCS: Pain Catastrophizing Scale; PHQ-9: Patient Health Questionnaire-9; POD1: Postoperative Day 1; PSQI: Pittsburgh Sleep Quality Index; SD: Standard Deviation; S-LANSS: Self-reported Leeds Assessment of Neuropathic Symptoms and Signs Scale; TKA: Total Knee Arthroplasty; VAS: Visual Analog Scale; VNRS: Verbal Numeric Rating Scale; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index; OR: Odds Ratio.
3.5. Synthesis of results
3.5.1. Primary outcomes: are preoperative neuropathic-like pain and central sensitisation risk factors for postoperative pain?
Twelve out of 14 studies identified that neuropathic-like pain or central sensitisation were risk factors for increased postoperative pain [11,[18], [19], [20],[23], [24], [25], [26],28,29,31,32]. Among the ten studies that used the PainDETECT questionnaire [11,[18], [19], [20],22,23,[27], [28], [29],31], two studies did not find an association between neuropathic-like pain and pain-related patient-reported outcome measures [22,27]. One of the 15 included studies did not investigate pain, only functionality and quality of life [30]. Among the studies that applied the PainDETECT score, only 2 studies adopted the threshold of ≥13 to define neuropathic-like pain [11,22], instead of ≥19.
Among the studies that applied the CSI score to investigate central sensitisation, 4 out of 4 studies reported that preoperative CSI scores were associated with increased postoperative pain. Three studies adopted the threshold of ≥40 to define central sensitisation [25,31,32], and one study ≥14 26.
The only study that used the S-LANSS score to detect neuropathic-like pain, reported significant associations between higher scores before surgery and postoperative pain [24].
Seven studies (8 cohorts) that detected positive associations between neuropathic-like pain or central sensitisation and postoperative outcomes assessed the effect of confounders such as including age, sex, BMI, pain severity at baseline, PCS score, PHQ-9 score, among others. Four studies reported that the PainDETECT or CSI scores remained independently associated with postoperative pain [18,19,25,29], while the other 4 studies did not find an association between preoperative neuropathic-like pain or central sensitisation and postoperative pain [19,24,26,28].
3.5.2. Meta-analysis
A meta-analysis was performed by pooling the data from three studies (4 cohorts) that examined the prevalence of individuals with chronic pain after TKA in patients presenting neuropathic-like pain (Fig. 2). The neuropathic-like pain group was defined in this analysis by a PainDETECT score ≥13 and the no neuropathic-like pain group by a PainDETECT score<13. Their follow-up periods were 6 months [18], 12 months [19] and an average of 4.7 years [20].
Fig. 2.
Forest plot demonstrating that individuals with neuropathic-like pain (PainDETECT score ≥13) before TKA exhibit a 2.75-fold increased risk (95 % CI: 1.78; 4.26) of developing chronic postoperative pain (VAS or NRS ≥3 at least 3 months post-surgery) compared to individuals without neuropathic-like pain (PainDETECT score <13).
The risk ratio of chronic pain (VAS or NRS ≥3 after at least 3 months) was 2.75 (95 % CI: 1.78; 4.26) for patients with neuropathic-like pain (PainDETECT score ≥13) compared with patients without neuropathic-like pain (PainDETECT score <13). The heterogeneity across studies was substantial and statistically significant (I2 = 76 %, τ2 = 0.121 p = 0.005), indicating a high level of variability in outcomes between the studies.
3.5.3. Secondary outcomes: are preoperative neuropathic-like pain and central sensitisation risk factors for poor postoperative clinical outcomes?
Seven out of 11 studies found an association between preoperative neuropathic-like pain or central sensitisation and clinical outcomes after surgery [11,19,20,23,24,31,32]. The authors reported worse outcomes in the presence of preoperative neuropathic-like pain or central sensitisation when investigating psychological status, quality of life, functionality, satisfaction and discharge-related variables.
Among the 3 studies that investigated the association of preoperative central sensitisation alone and poor clinical outcomes [25,26,32], 1 did not find an association between these variables [25]. Koh et al. reported worse KSS and lower satisfaction with daily activities such as sitting, lying in bed, getting out of bed, light household duties, leisure recreational activities, and total overall satisfaction [32] and Hasegawa et al. reported that preoperative CSI correlated negatively with postoperative walking and standing score (r = −0.442) (p < 0.001) [26]. However, when using a multivariate analysis, CSI was not a significant factor in postoperative pain (p = 0.438).
Four studies [22,25,27,30] did not find an association between preoperative neuropathic-like pain and clinical outcomes after surgery when analyzing PCS, OKS, EQ-5D score, patient satisfaction, patient interpretation of success, knee flexion angle, complications, and further surgery.
3.5.4. Risk of bias across studies
Publication date, sample size, and effect size variation in individual studies were reported in Table 1, Table 3 Due to the limited number of studies, and methodological differences between studies, funnel plots were deemed inappropriate for assessing publication bias and, therefore, were not included in this analysis. Evidence of publication bias among the included studies remains inconclusive.
3.5.5. Additional analysis
Meta-analyses with other PainDETECT scores was not possible because Hasegawa et al. reported no patients with PainDETECT score ≥19 [20]. Analysis of mean differences between pain scores between Kurien et al. [18] and Kim et al. [31] was not possible because VAS pain was obtained in different conditions.
A sensitivity analysis assessing the impact of excluding the study by Hasegawa et al. [20] was conducted in a prior meta-analysis [7], demonstrating no effect on the overall results; thus, it was not replicated in the present work.
Qualitatively, differences in the thresholds adopted for the CSI score, seemed not to affect the results. Out of 4 studies exploring the association between preoperative CSI score and postoperative pain, three studies demonstrated a positive association with a threshold of ≥40 and one with a threshold of ≥14. PainDETECT thresholds also did not appear to affect the results. Two studies that used a more liberal cut-off (≥13) showed similar results to those that used the more conservative cut-off (≥19).
4. Discussion
This systematic review investigated the effects of preoperative neuropathic-like pain and central sensitisation on clinical outcomes in patients who underwent TKA surgery. The main finding is that there is consistent evidence supporting an association between preoperative factors and chronic postoperative pain, whereas evidence regarding associations with other clinical outcomes, such as function, quality of life, and mental health, remains inconsistent. Patients with neuropathic-like pain (PainDETECT score ≥13) had a 2.75-times higher risk of developing chronic pain (VAS or NRS ≥3 after at least 3 months) compared to those without neuropathic-like pain (PainDETECT score <13), with a confidence interval of 1.78–4.26. The present research updated and expanded a previous systematic review and meta-analysis with four studies [18,24,25,27] conducted in 2020 7, incorporating eleven more recent studies. The present findings confirmed the previous results, reinforcing the influence of preoperative neuropathic-like pain and central sensitisation on chronic postoperative pain after TKA.
The majority of the included studies identified an association between the presence of neuropathic-like pain or central sensitisation before surgery and postoperative pain, function, and psychological status. Regarding postoperative pain, 85.7 % (12 out of 14) of the studies found that neuropathic-like pain or central sensitisation were risk factors for pain after surgery, with follow-up periods ranging from 4 weeks to 5 years. Eight out of ten studies assessing neuropathic pain, as well as all three studies on central sensitisation, support these findings. One of the two studies that did not find this association included only one patient with preoperative neuropathic-like pain [27]. The other [22], reported a 5.6 % prevalence of neuropathic-like pain, which is lower than previous studies 5 %–32 % [[33], [34], [35]]. To address the low prevalence, the latter study dichotomized participants using a lower PainDETECT threshold (≥13 vs < 13), which indicates unclear neuropathic-like pain, while the former study applied linear modeling without categorizing patients. These differences may account for the lack of association found in the two studies between preoperative neuropathic-like pain and postoperative pain.
Some studies investigated the influence of covariates on postoperative pain, with conflicting results. Four studies reported that the PainDETECT or CSI scores remained independently associated with postoperative pain [18,19,25,29], while another four studies reported no significant association [19,24,26,28]. Small sample sizes [19,24], and methodological differences, such as modeling multiple variables [28], may have limited statistical power and obscured true associations. However, it may also indicate that other variables associated with neuropathic-like pain also play a role in the poor outcomes after TKA surgery in patients with neuropathic-like pain. Future studies should be adequately powered and designed to disentangle the effects of neuropathic-like pain from related confounding variables, thereby clarifying its independent role in influencing postoperative outcomes.
Regarding clinical outcomes, the included articles presented a variety of outcomes, making between-studies comparisons difficult. Overall, 63 % (7 out of 11) of the studies demonstrated that neuropathic-like pain or central sensitisation are risk factors for poor clinical outcomes. The studies that analyzed functionality, measured with validated questionnaires, yielded the most conflicting results, with five demonstrating significant associations [11,19,22,31,32] and 4 showing no significant differences [[25], [27], [28], [30]]. Intriguingly, Koh et al. reported conflicting findings, showing differences favoring only one of the two functional questionnaires assessed [32]. They found significantly higher WOMAC scores in the central sensitisation group, but lower KSS scores compared to the no central sensitisation group. This highlights the ongoing uncertainty regarding how functional outcomes are affected after TKA surgery in patients with central sensitisation. The rationale that neuropathic-like pain impairs function is supported by the literature, as neuropathic-like pain can affect daily activities and quality of life differently, compared to nociceptive pain [12,33]. Unlike nociceptive pain, neuropathic-like pain does not necessarily worsen with increased activities and can present variably between individuals [12,33]. This variability in presentation, along with the fact that mechanical triggers may or may not be present, could explain the inconsistent findings regarding functional loss across studies, as some patients may experience worsening symptoms with increased physical activity, whereas others may not.
Similarly, outcomes related to psychological aspects were not consistent across studies. Fitzsimmons et al. identified postoperative depression in patients who presented with neuropathic-like pain before surgery [24]; however, after adjusting for baseline confounding factors—specifically PCS, PHQ-9, comorbidities, and Intermittent and Constant Osteoarthritis Pain total pain—the association was no longer statistically significant. Instead, preoperative PHQ-9 scores emerged as the primary predictor of postoperative depression. Moreover, Soni et al. did not find statistically significant differences in a similar study [19]. A significant limitation of the analysis of psychological aspects was the lack of standardization of the instruments and psychological features being assessed. There were numerous outcome variables with substantial differences among the studies. Improved standardization is recommended to facilitate between-study comparisons. Preference should be given to validated questionnaires that are widely applicable and easy to administer to ensure broader implementation and comparability.
Anxiety appears to have a more robust biological link to neuropathic-like pain and central sensitisation and may be worth further exploration in additional studies [11]. Anxiety was investigated only by Soni et al. [11], and their results suggest that preoperative neuropathic-like pain was a risk factor in patients undergoing TKA. These findings were further compared with functional magnetic resonance imaging by the authors, revealing that brain activity in regions related to pain modulation was altered after surgery in patients with preoperative neuropathic-like pain.
This study presents limitations inherent to the included studies. First, it was not possible to meta-analyze data from all the included studies due to divergence in the statistical approaches and outcome measurements. The pooled analysis included slightly different measurement instruments, such as the NRS [20] and VAS-pain [18,19]. However, the NRS and VAS pain scales are comparable and have been combined in previous studies [36]. Moreover, categorizing pain scores (chronic pain = VAS ≥3 or NRS ≥3) rather than analyzing them as continuous variables may have masked subtle differences between the two measurement tools [36]. Loss of follow-up and missing data were significant in different studies, ranging from 0 % to 35.7 %. Recovering missing data could contribute to confirming the findings of this review. Additionally, the previous systematic review's limitations included not accounting for covariates such as catastrophizing and fear avoidance. While some of the new studies in this review addressed this weakness, it was not a consistent approach across all studies. Lastly, recent studies have examined alternative thresholds for both the PainDETECT and CSI scores, highlighting that while general guidelines assist with interpretation, they should not be considered definitive [9]. Adjusting the thresholds, whether to a higher or lower value, can potentially influence the classification outcomes and overall findings. Although variations in PainDETECT thresholds have been investigated across different studies in this review, often yielding similar results, these alternative cut-offs were not examined within individual studies to allow for more definitive conclusions. Future research should address this gap to clarify the impact of threshold selection on classification outcomes.
5. Conclusion
This study provides evidence that the presence of preoperative neuropathic-like pain or central sensitisation is associated with an increased risk of chronic postoperative pain. However, the evidence for decreased function and psychological aspects remains conflicting and requires further investigation. Preoperative screening for neuropathic-like pain and central sensitisation is advised to identify patients at risk for developing chronic postoperative pain. Future steps involve controlling confounders and standardizing the methods to enable quantitative comparisons.
Authors Contributions
Felipe F. Gonzalez: Manuscript writing, analysis and interpretation of the data, critical revision of the article for important intellectual content, final approval of the article, collection and assembly of data.
Alessandro Barone: Critical revision of the article for important intellectual content, collection and assembly of data, final approval of the article.
Rithik Pallaniappan: Critical revision of the article for important intellectual content, collection and assembly of data, final approval of the article.
Raffaella Russo: Manuscript writing, collection and assembly of data, critical revision of the article for important intellectual content, final approval of the article.
Giorgio Gasparini: Manuscript writing, collection and assembly of data, final approval of the article.
Leonardo Metsavaht: Analysis and interpretation of the data, critical revision of the article for important intellectual content, final approval of the article.
Jorge Chahla: Critical revision of the article for important intellectual content, final approval of the article, obtaining of funding, administrative, technical, or logistic support.
Filippo Familiari: Conception and design, analysis and interpretation of the data, critical revision of the article for important intellectual content, final approval of the article, obtaining of funding, administrative, technical, or logistic support.
Role of the funding source
The Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil – Finance Code 001 provided a PhD scholarship to one of the authors (Felipe F. Gonzalez).
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgments
We would like to thank Jennifer C. Westrick from the Library of Rush University Medical Center for her work on the literature search strategy for this review. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001.
Handling Editor: Professor H Madry
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ocarto.2025.100674.
Contributor Information
Felipe F. Gonzalez, Email: felipe_gonzalez@rush.edu.
Alessandro Barone, Email: alessandro.barone28@gmail.com.
Rithik Palaniappan, Email: rithik_palaniappan@rush.edu.
Raffaella Russo, Email: raffaella.russo.md@gmail.com.
Giorgio Gasparini, Email: gasparini@unicz.it.
Leonardo Metsavaht, Email: leo@metsavaht.com.br.
Jorge Chahla, Email: jorge.chahla@rushortho.com.
Filippo Familiari, Email: filippofamiliari@unicz.it.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Sharma L. Osteoarthritis of the knee. Solomon C.G., editor. N. Engl. J. Med. 2021;384(1):51–59. doi: 10.1056/NEJMCP1903768. [DOI] [PubMed] [Google Scholar]
- 2.Steinmetz J.D., Culbreth G.T., Haile L.M., et al. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: a systematic analysis for the global burden of disease study 2021. Lancet Rheumatol. 2023;5(9) doi: 10.1016/S2665-9913(23)00163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hussain S.M., Neilly D.W., Baliga S., Patil S., Meek R.M.D. Knee osteoarthritis: a review of management options. Scott. Med. J. 2016;61(1) doi: 10.1177/0036933015619588. [DOI] [PubMed] [Google Scholar]
- 4.Beswick A.D., Wylde V., Gooberman-Hill R., Blom A., Dieppe P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open. 2012;2(1) doi: 10.1136/bmjopen-2011-000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeFrance M.J., Scuderi G.R. Are 20% of patients actually dissatisfied following total knee arthroplasty? A systematic review of the literature. J. Arthroplast. 2023;38(3) doi: 10.1016/j.arth.2022.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Fernández-de-las-Peñas C., Florencio L.L., de-la-Llave-Rincón A.I., et al. Prognostic factors for postoperative chronic pain after knee or hip replacement in patients with knee or hip osteoarthritis: an umbrella review. J. Clin. Med. 2023;12(20) doi: 10.3390/jcm12206624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wluka A.E., Yan M.K., Lim K.Y., Hussain S.M., Cicuttini F.M. Does preoperative neuropathic-like pain and central sensitisation affect the post-operative outcome of knee joint replacement for osteoarthritis? A systematic review and meta analysis. Osteoarthr. Cartil. 2020;28(11) doi: 10.1016/j.joca.2020.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Merskey H., Bogduk N. second ed. Classification of Chronic Pain; 2012. IASP Taxonomy. Updated from Pain Terms, A Current List with Definitions and Notes on Usage; pp. 209–214. IASP Task Force on Taxonomy. Published online. [Google Scholar]
- 9.Neblett R., Cohen H., Choi Y., et al. The central sensitization inventory (CSI): establishing clinically significant values for identifying central sensitivity syndromes in an outpatient chronic pain sample. J. Pain. 2013;14(5) doi: 10.1016/j.jpain.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dainese P., De Mits S., Wittoek R., et al. Neuropathic-like pain in knee osteoarthritis: exploring differences in knee loading and inflammation Across-sectional study. Eur. J. Phys. Rehabil. Med. 2024;60(1) doi: 10.23736/S1973-9087.23.07877-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soni A., Wanigasekera V., Mezue M., et al. Central sensitization in knee osteoarthritis: relating presurgical brainstem neuroimaging and PainDETECT-Based patient stratification to arthroplasty outcome. Arthritis Rheumatol. 2019;71(4) doi: 10.1002/art.40749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finnerup N.B., Kuner R., Jensen T.S. Neuropathic pain: from mechanisms to treatment. Physiol. Rev. 2021;101(1):259–301. doi: 10.1152/PHYSREV.00045.2019. [DOI] [PubMed] [Google Scholar]
- 13.Freynhagen R., Tölle T.R., Gockel U., Baron R. The painDETECT project - far more than a screening tool on neuropathic pain. Curr. Med. Res. Opin. 2016;32(6) doi: 10.1185/03007995.2016.1157460. [DOI] [PubMed] [Google Scholar]
- 14.Fingleton C., Smart K., Moloney N., Fullen B.M., Doody C. Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthr. Cartil. 2015;23(7) doi: 10.1016/j.joca.2015.02.163. [DOI] [PubMed] [Google Scholar]
- 15.Liberati A., Altman D.G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weingarten T.N., Watson J.C., Hooten W.M., et al. Validation of the S-LANSS in the community setting. Pain. 2007;132(1–2) doi: 10.1016/j.pain.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 17.Thong I.S.K., Jensen M.P., Miró J., Tan G. The validity of pain intensity measures: what do the NRS, VAS, VRS, and FPS-R measure? Scand J Pain. 2018;18(1) doi: 10.1515/sjpain-2018-0012. [DOI] [PubMed] [Google Scholar]
- 18.Kurien T., Arendt-Nielsen L., Petersen K.K., Graven-Nielsen T., Scammell B.E. Preoperative neuropathic pain-like symptoms and central pain mechanisms in knee osteoarthritis predicts poor outcome 6 months after total knee replacement surgery. J. Pain. 2018;19(11) doi: 10.1016/j.jpain.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Soni A, Leyland KM, Kiran A, et al. Can neuropathic pain predict response to arthroplasty in knee osteoarthritis? A prospective observational cohort study. bioRxiv. Published online July 5, 2018:360412. doi:10.1101/360412.
- 20.Hasegawa M., Tone S., Naito Y., Wakabayashi H., Sudo A. Prevalence of persistent pain after total knee arthroplasty and the impact of neuropathic pain. J. Knee Surg. 2019;32(10) doi: 10.1055/s-0038-1675415. [DOI] [PubMed] [Google Scholar]
- 21.Higgins J.P.T., Thomas J., Chandler J., et al. second ed. Wiley-Blackwell; 2019. Cochrane Handbook for Systematic Reviews of Interventions. [Google Scholar]
- 22.Hasegawa M., Tone S., Naito Y., Sudo A. Possible neuropathic pain in patients with osteoarthritis of the knee before and after total knee arthroplasty. J. Pain Res. 2021;14 doi: 10.2147/JPR.S330091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shemesh S.S., Dieterich J.D., Chen D., et al. Preoperative pain catastrophizing and neuropathic pain do not predict length of stay and early post-operative complications following total joint arthroplasty. J. Personalized Med. 2023;13(2) doi: 10.3390/jpm13020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fitzsimmons M., Carr E., Woodhouse L., Bostick G.P. Development and persistence of suspected neuropathic pain after total knee arthroplasty in individuals with osteoarthritis. PM and R. 2018;10(9) doi: 10.1016/j.pmrj.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Kim S.H., Yoon K.B., Yoon D.M., Yoo J.H., Ahn K.R. Influence of centrally mediated symptoms on postoperative pain in osteoarthritis patients undergoing total knee arthroplasty: a prospective observational evaluation. Pain Pract. 2015;15(6) doi: 10.1111/papr.12311. [DOI] [PubMed] [Google Scholar]
- 26.Hasegawa M., Tone S., Naito Y., Sudo A. Preoperative pain catastrophizing affects pain outcome after total knee arthroplasty. J. Orthop. Sci. 2022;27(5) doi: 10.1016/j.jos.2021.05.011. [DOI] [PubMed] [Google Scholar]
- 27.Phillips J.R.A., Hopwood B., Arthur C., Stroud R., Toms A.D. The natural history of pain and neuropathic pain after knee replacement: a prospective cohort study of the point prevalence of pain and neuropathic pain to a minimum three-year follow-up. Bone and Joint Journal. 2014;69B(9) doi: 10.1302/0301-620X.96B9.33756. [DOI] [PubMed] [Google Scholar]
- 28.Larsen D.B., Laursen M., Edwards R.R., Simonsen O., Arendt-Nielsen L., Petersen K.K. The combination of preoperative pain, conditioned pain modulation, and pain catastrophizing predicts postoperative pain 12 months after total knee arthroplasty. Pain Med. 2021;22(7):1583–1590. doi: 10.1093/PM/PNAA402. [DOI] [PubMed] [Google Scholar]
- 29.Vigotsky A.D., Cong O., Pinto C.B., et al. Prognostic value of preoperative mechanical hyperalgesia and neuropathic pain qualities for postoperative pain after total knee replacement. Eur. J. Pain. 2024;28(8):1387–1401. doi: 10.1002/EJP.2295. [DOI] [PubMed] [Google Scholar]
- 30.Lee N.K., Won S.J., Lee J.Y., Kang S.B., Yoo S.Y., Chang C.B. Presence of night pain, neuropathic pain, or depressive disorder does not adversely affect outcomes after total knee arthroplasty: a prospective cohort study. J. Kor. Med. Sci. 2022;37(43) doi: 10.3346/jkms.2022.37.e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim M.S., Kim J.J., Kang K.H., Lee J.H. In Y. Central sensitization and neuropathic pain cumulatively affect patients reporting inferior outcomes following total knee arthroplasty. J. Bone Joint Surg. 2024;106(2) doi: 10.2106/JBJS.23.00399. [DOI] [PubMed] [Google Scholar]
- 32.Koh I.J., Kang B.M., Kim M.S., Choi K.Y., Sohn S. In Y. How does preoperative central sensitization affect quality of life following total knee arthroplasty? J. Arthroplast. 2020;35(8) doi: 10.1016/j.arth.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Blikman T., Rienstra W., Van Raay J.J.A.M., et al. Neuropathic-like symptoms and the association with joint-specific function and quality of life in patients with hip and knee osteoarthritis. PLoS One. 2018;13(6) doi: 10.1371/journal.pone.0199165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valdes A.M., Suokas A.K., Doherty S.A., Jenkins W., Doherty M. History of knee surgery is associated with higher prevalence of neuropathic pain-like symptoms in patients with severe osteoarthritis of the knee. Semin. Arthritis Rheum. 2014;43(5) doi: 10.1016/j.semarthrit.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Hochman J.R., Gagliese L., Davis A.M., Hawker G.A. Neuropathic pain symptoms in a community knee OA cohort. Osteoarthr. Cartil. 2011;19(6) doi: 10.1016/j.joca.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 36.Hjermstad M.J., Fayers P.M., Haugen D.F., et al. Studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: a systematic literature review. J. Pain Symptom Manag. 2011;41(6) doi: 10.1016/j.jpainsymman.2010.08.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.