Abstract
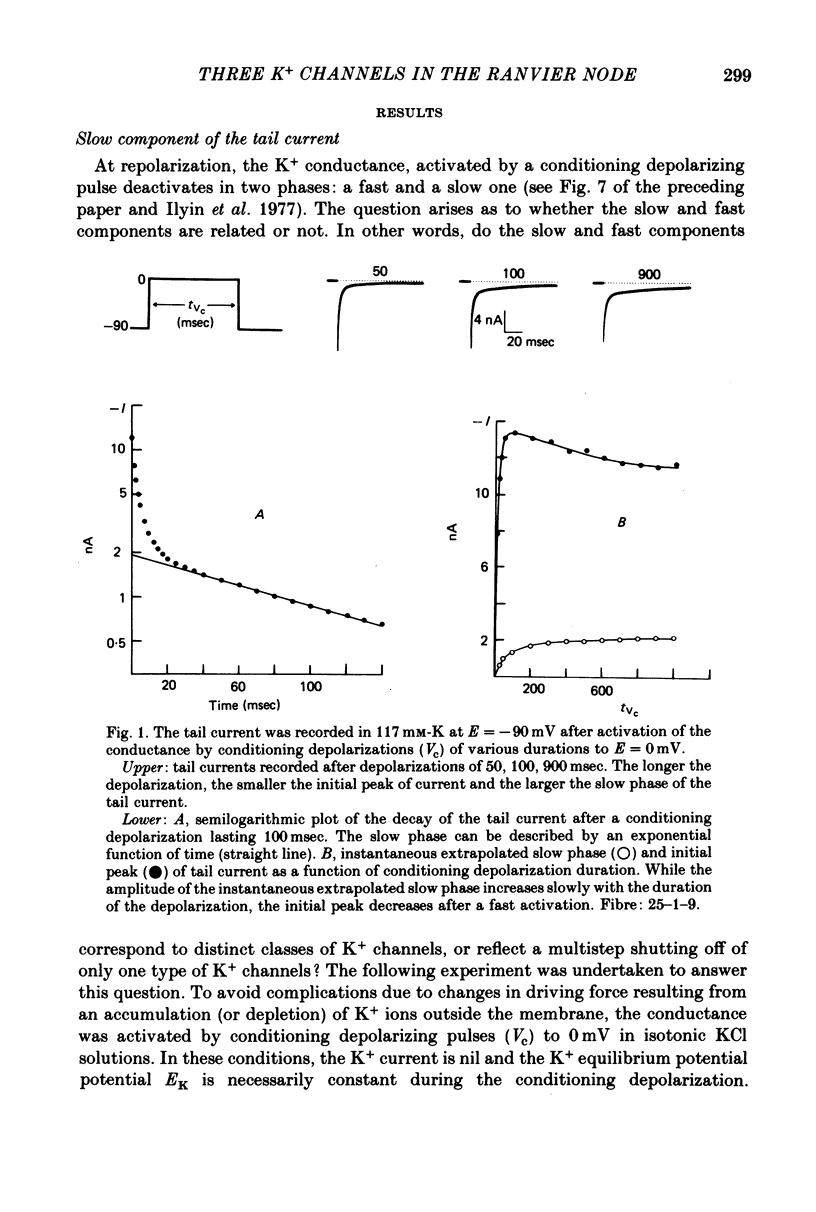
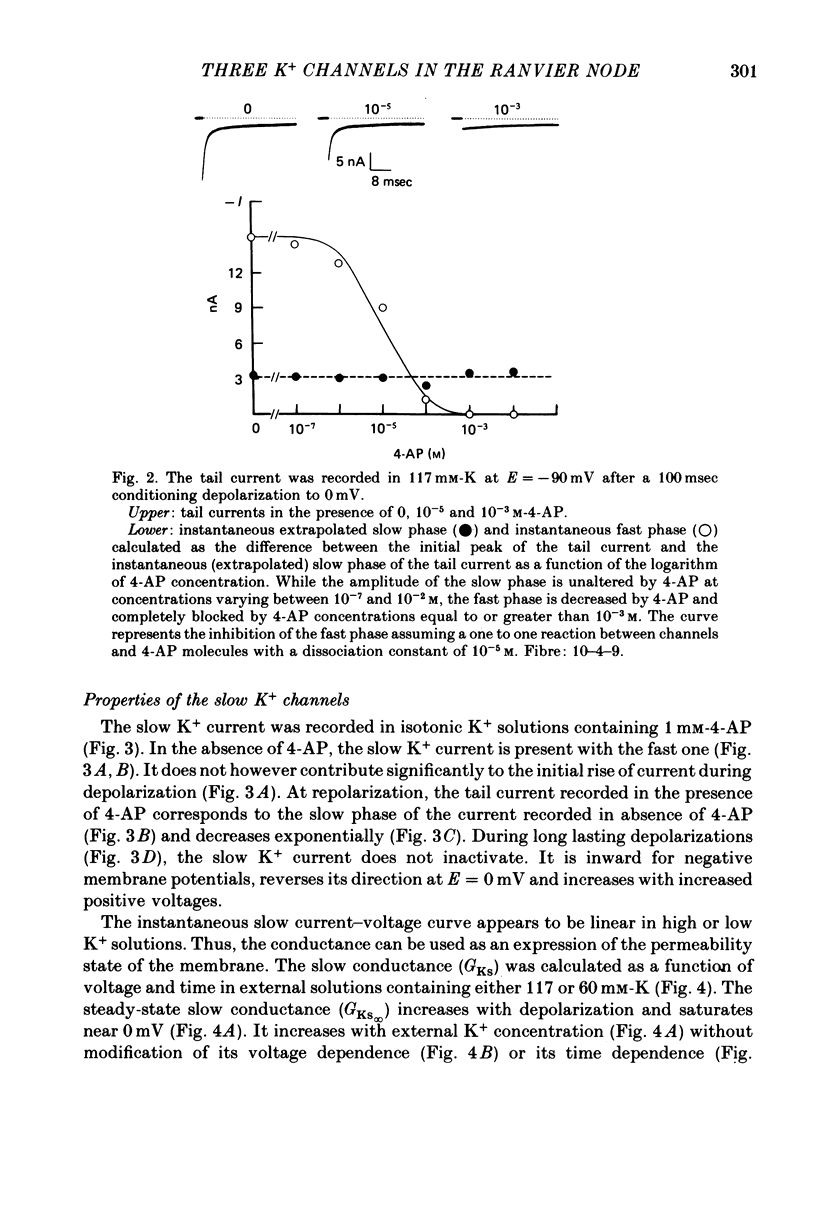
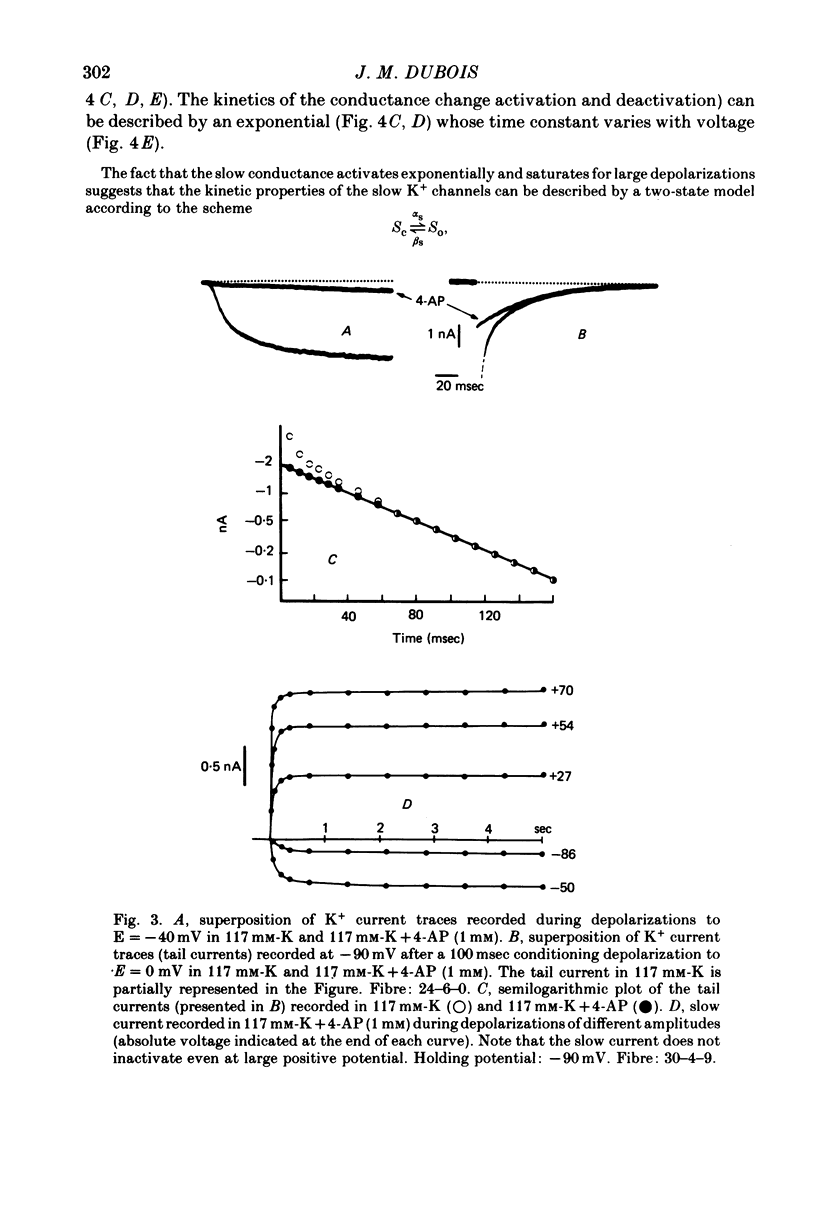
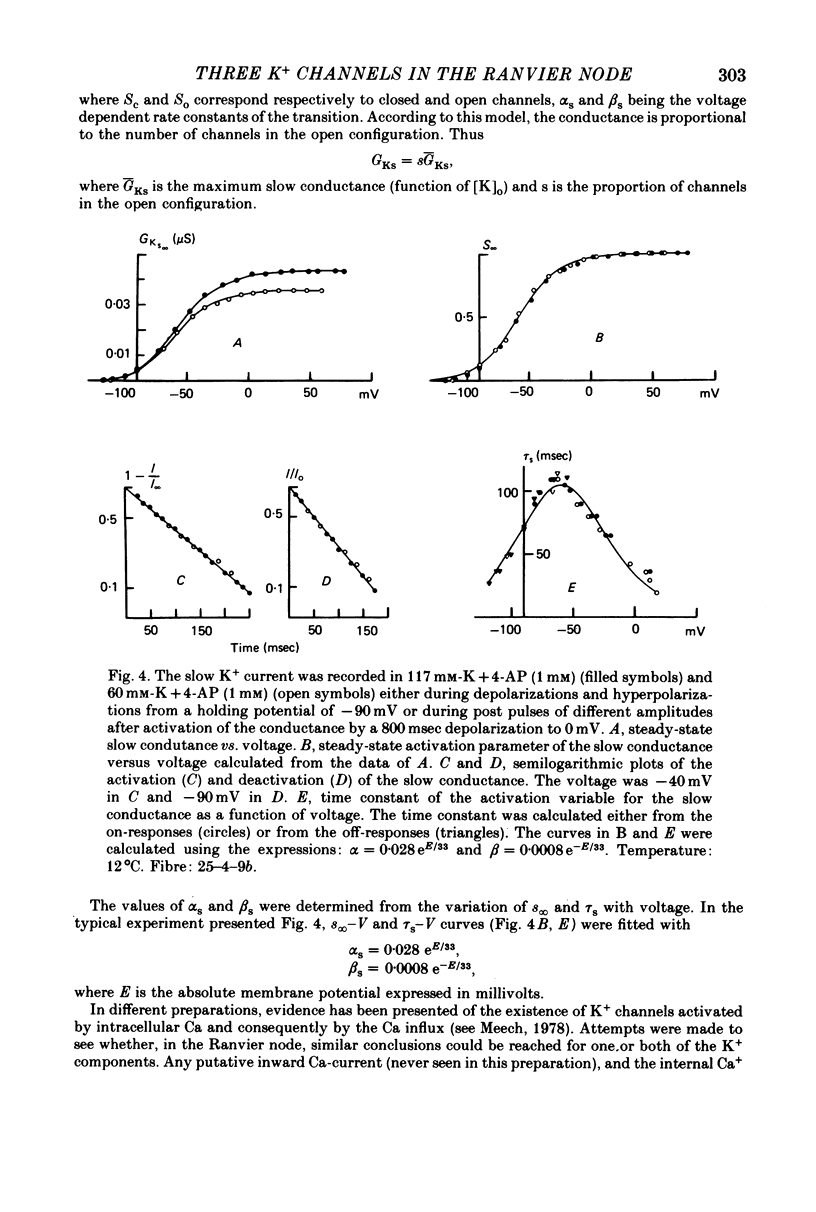
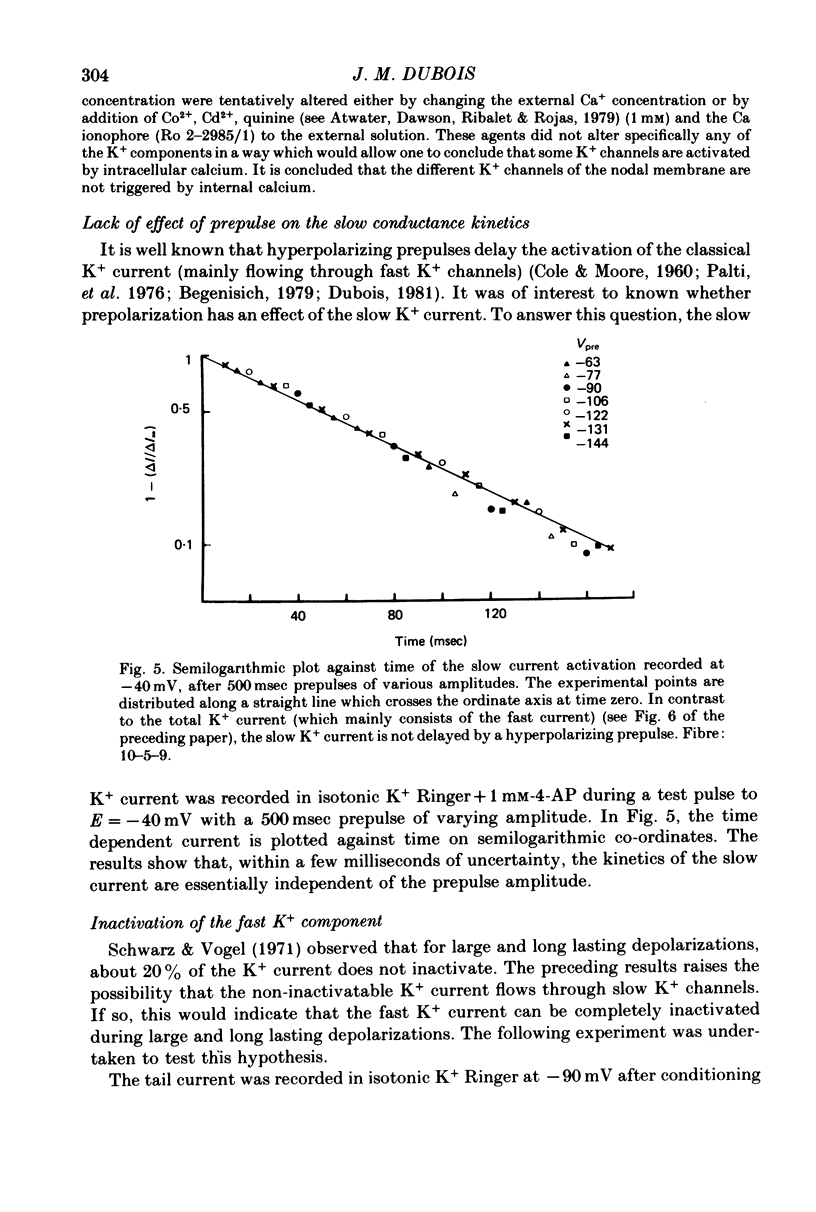
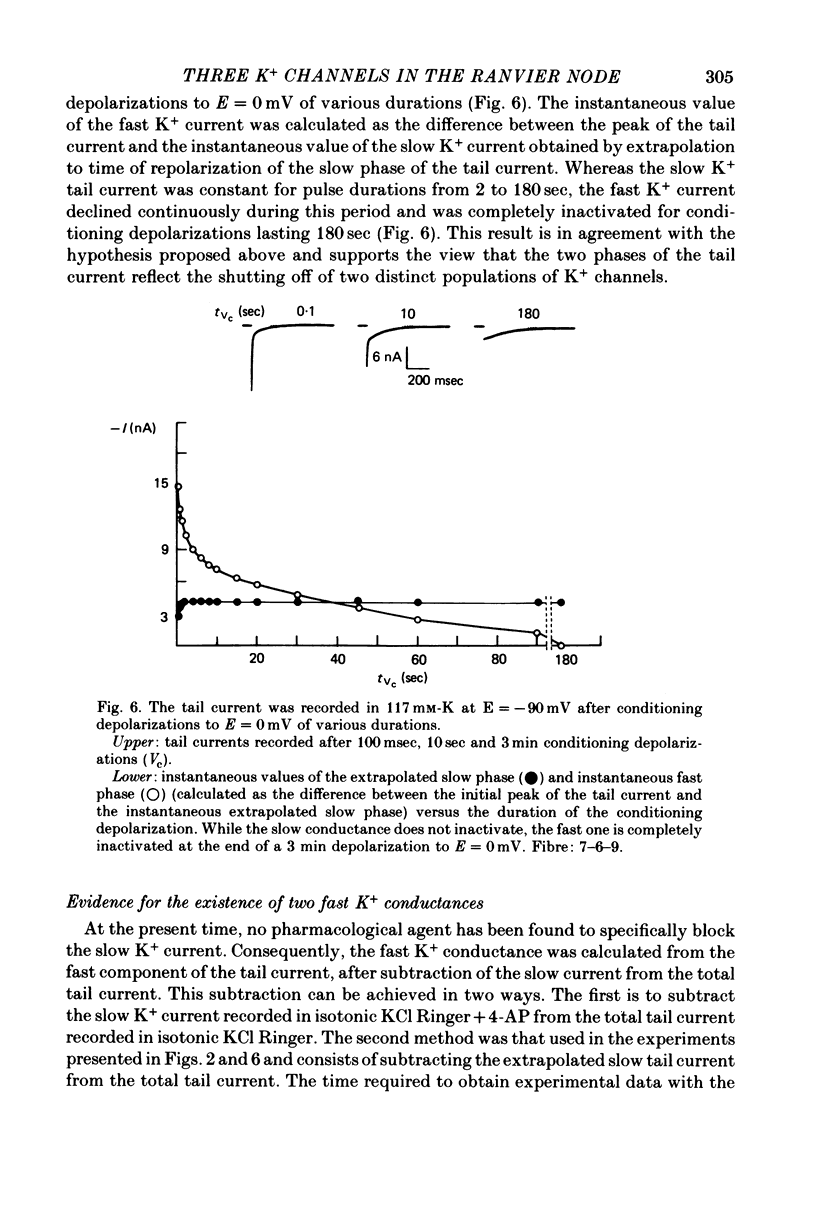
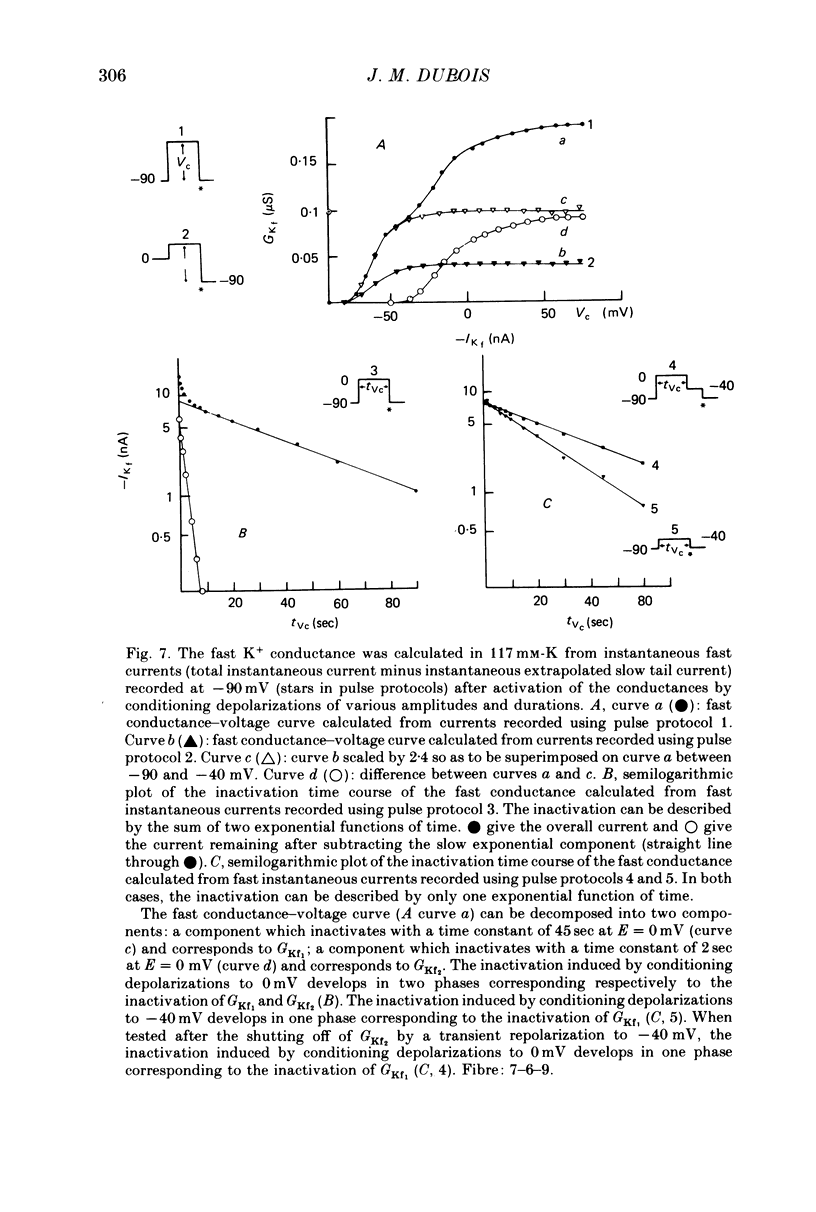
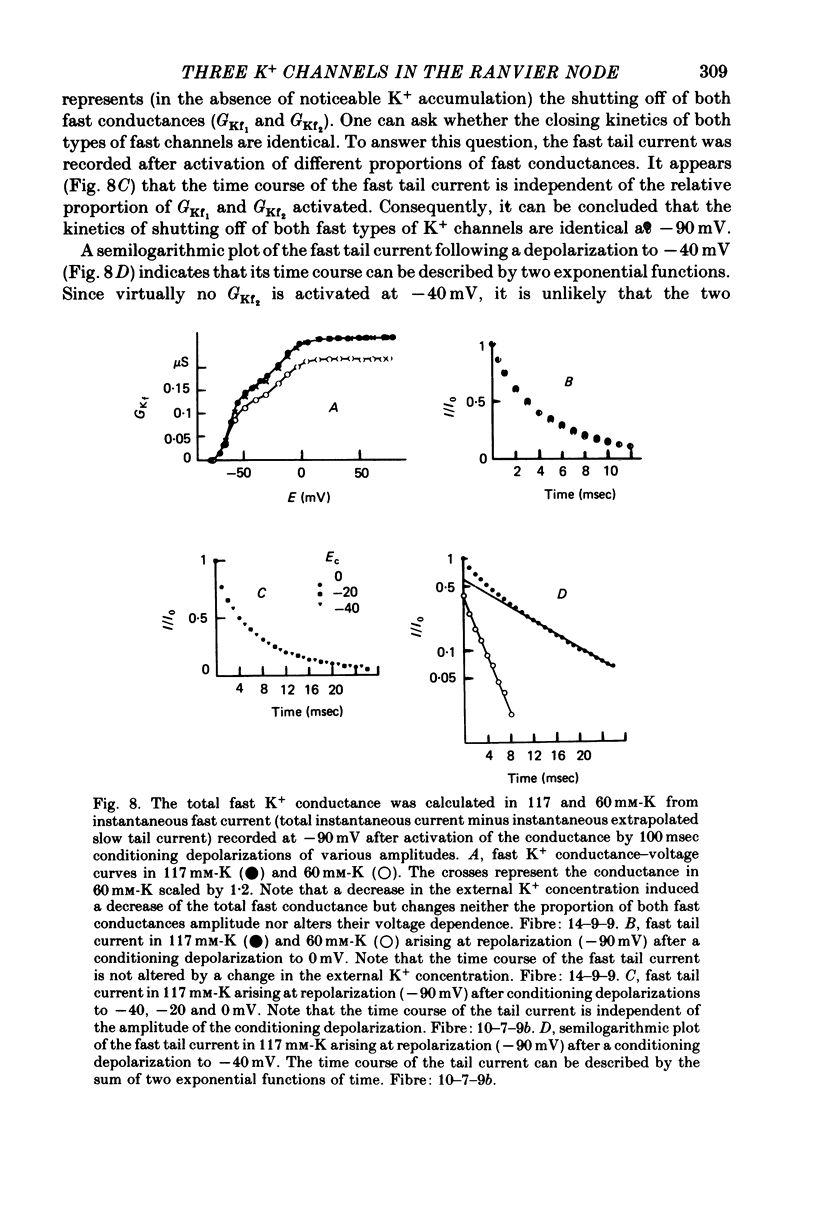
1. In voltage clamped myelinated fibres, the K+ current was recorded in high-K+ media to allow analysis without complications due to K+ accumulation. 2. After a depolarization, the tail of K+ current following repolarization decreases in two phases: a fast phase lasting about 20 msec and a slow exponential phase lasting several hundred milliseconds. When the duration of the depolarization is increased, the amplitude at time zero of the fast phase increases (activation of the conductance) and then decreases slowly (inactivation of the conductance). Simultaneously, the amplitude of the slow phase, extrapolated to time zero of repolarization, increases slowly and reaches a steady-state level (about 20% of the maximum instantaneous current) after about 600 msec of depolarization. 3. The fast phase of the tail current is blocked by external application of 4-aminopyridine (4-AP) (KD = 10(-5)M). The slow phase is unaltered by 4-AP (10(-7)-10(-2)M). 4. In the presence of 4-AP (10-3M), the remaining slow K+ current, activated by depolarizations, does not inactivate. 5. During depolarizations and repolarizations, the conductance of the slow current (GKs) varies exponentially. The steady-state value of the slow conductance and its time constant of activation vary with voltage. The variation of the slow conductance with time and voltage can be described by a closed-open mode, assuming that each channel is gated by one particle. The activation kinetics of the slow current is unaltered by long lasting (500 msec) prepolarizations. 6. The fast K+ conductance, calculated from the fast tail current, is fully inactivated at the end of a 3 min depolarization to 0 mV. 7. The fast K+ conductance can be decomposed into two components: one component (GKf1) activating between -80 and -30 mV and inactivating very slowly (tau = 45 sec at E = 0 mV); one component (GKf2) activating between -40 mV and +30 mV and inactivating slowly (tau = 2 sec at E = 0 mV). t = 12 degrees C. 8. The maximum slow and fast conductances increase with [K]0. While the maximum fast conductance tends to saturate at high external K+ concentrations, the maximum slow conductance shows no sign of saturation. 9. A comparison between motor and sensory fibres shows that, while the amplitude of maximum slow and fast conductances are identical for both types of fibres, the amplitude of fast-1 conductance is larger and consequently the amplitude of fast-2 is smaller in motor than in sensory fibres. The different spike frequency adaptations observed on both types of fibres are discussed in relation to these different relative fast conductances amplitudes. 10. It is concluded that the K+ conductance of the nodal membrane is composed of three components (GKS, GKf1 and GKf2) corresponding to three different and distinct types of K+ channels.
Full text
PDF




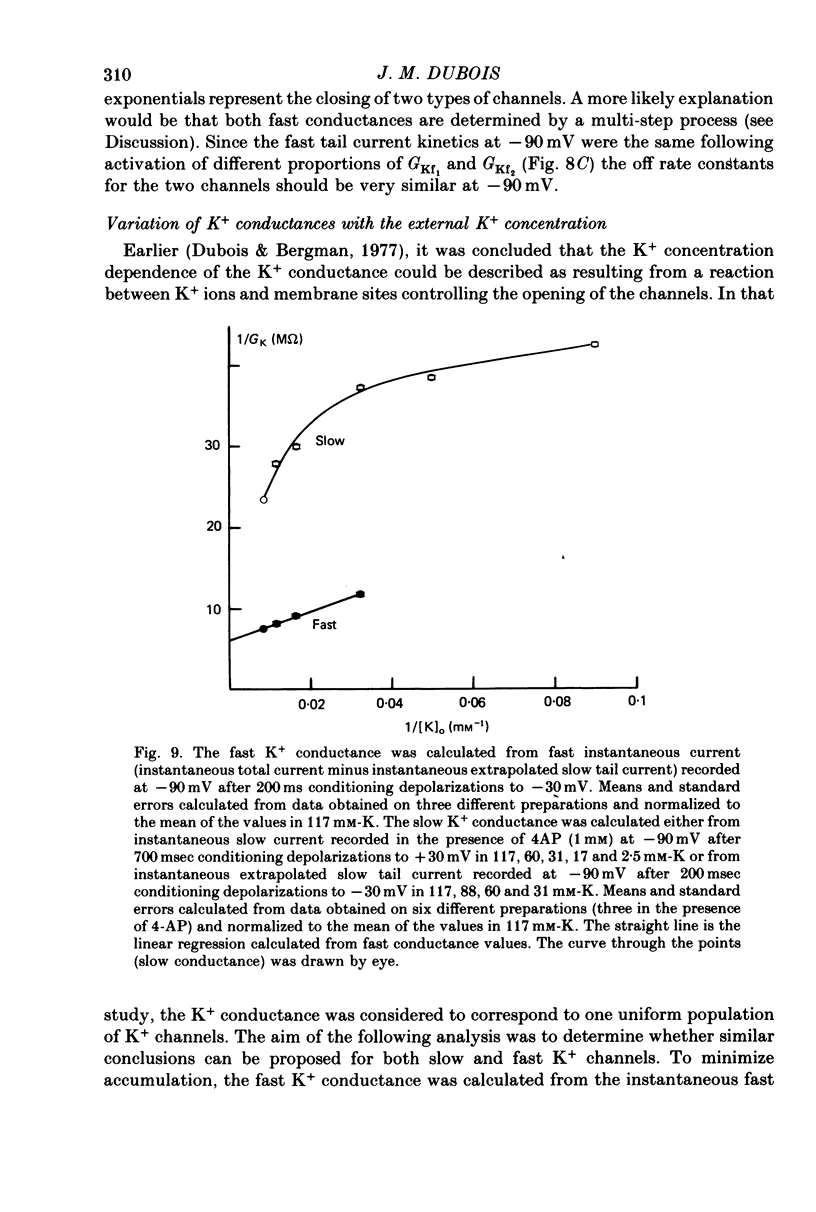

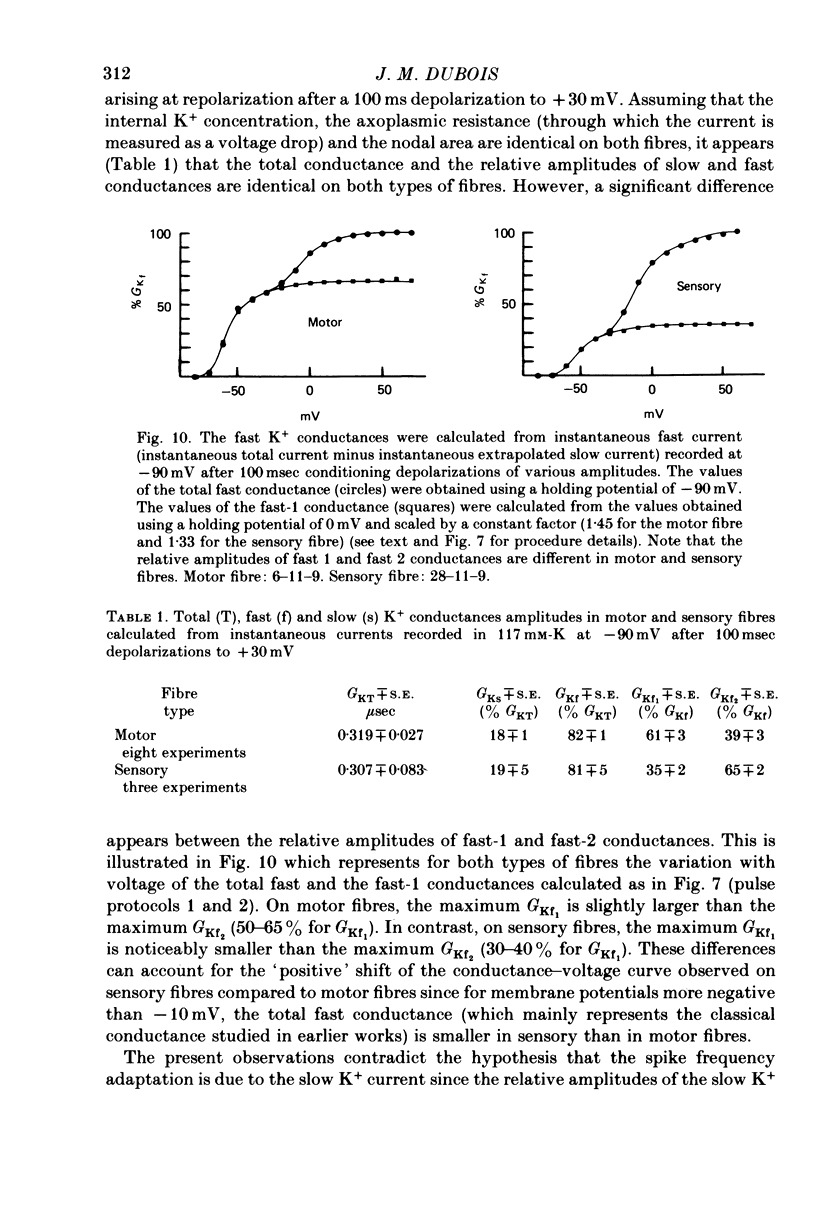




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Chandler W. K., Hodgkin A. L. Slow changes in potassium permeability in skeletal muscle. J Physiol. 1970 Jul;208(3):645–668. doi: 10.1113/jphysiol.1970.sp009140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D., Dubois J. M., Ojeda C. Fully activated potassium current-voltage relationship in the Ranvier node: discrepancy between the results of two methods of analysis. Pflugers Arch. 1980 Mar;384(1):49–56. doi: 10.1007/BF00589513. [DOI] [PubMed] [Google Scholar]
- Atwater I., Dawson C. M., Ribalet B., Rojas E. Potassium permeability activated by intracellular calcium ion concentration in the pancreatic beta-cell. J Physiol. 1979 Mar;288:575–588. [PMC free article] [PubMed] [Google Scholar]
- Begenisich T. Conditioning hyperpolarization-induced delays in the potassium channels of myelinated nerve. Biophys J. 1979 Aug;27(2):257–265. doi: 10.1016/S0006-3495(79)85215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretag A. H., Stämpfli R. Differences in action potentials and accommodation of sensory and motor myelinated nerve fibres as computed on the basis of voltage clamp data. Pflugers Arch. 1975;354(3):257–271. doi: 10.1007/BF00584649. [DOI] [PubMed] [Google Scholar]
- COLE K. S., MOORE J. W. Potassium ion current in the squid giant axon: dynamic characteristic. Biophys J. 1960 Sep;1:1–14. doi: 10.1016/s0006-3495(60)86871-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J. M., Bergman C. Late sodium current in the node of Ranvier. Pflugers Arch. 1975;357(1-2):145–148. doi: 10.1007/BF00584552. [DOI] [PubMed] [Google Scholar]
- Dubois J. M., Bergman C. The steady-state potassium conductance of the Ranvier node at various external K-concentrations. Pflugers Arch. 1977 Aug 29;370(2):185–194. doi: 10.1007/BF00581693. [DOI] [PubMed] [Google Scholar]
- Dubois J. M. Simultaneous changes in the equilibrium potential and potassium conductance in voltage clamped Ranvier node in the frog. J Physiol. 1981 Sep;318:279–295. doi: 10.1113/jphysiol.1981.sp013864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Il'in V. I., Katina I. E., Lonskii A. V., Makovskii V. S., Polishchuk E. V. Dannye o sushchestvovanii dvukh nezavisimykh komponent kalievogo toka v membrane perekhvata Ranv'e liagushki Rana ridibunda. Dokl Akad Nauk SSSR. 1977;234(6):1467–1470. [PubMed] [Google Scholar]
- Krylov B. V., Makovsky V. S. Spike frequency adaptation in amphibian sensory fibres is probably due to slow K channels. Nature. 1978 Oct 12;275(5680):549–551. doi: 10.1038/275549a0. [DOI] [PubMed] [Google Scholar]
- Meech R. W. Calcium-dependent potassium activation in nervous tissues. Annu Rev Biophys Bioeng. 1978;7:1–18. doi: 10.1146/annurev.bb.07.060178.000245. [DOI] [PubMed] [Google Scholar]
- Meves H., Pichon Y. The effect of internal and external 4-aminopyridine on the potassium currents in intracellularly perfused squid giant axons. J Physiol. 1977 Jun;268(2):511–532. doi: 10.1113/jphysiol.1977.sp011869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Two fast transient current components during voltage clamp on snail neurons. J Gen Physiol. 1971 Jul;58(1):36–53. doi: 10.1085/jgp.58.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D., Tsien R. W. Outward membrane currents activated in the plateau range of potentials in cardiac Purkinje fibres. J Physiol. 1969 Jan;200(1):205–231. doi: 10.1113/jphysiol.1969.sp008689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palti Y., Ganot G., Stämpfli R. Effect of conditioning potential on potassium current kinetics in the frog node. Biophys J. 1976 Mar;16(3):261–273. doi: 10.1016/S0006-3495(76)85686-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J. R., Vogel W. Potassium inactivation in single myelinated nerve fibres of Xenopus laevis. Pflugers Arch. 1971;330(1):61–73. doi: 10.1007/BF00588735. [DOI] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbricht W., Wagner H. H. Block of potassium channels of the nodal membrane by 4-aminopyridine and its partial removal on depolarization. Pflugers Arch. 1976 Nov 30;367(1):77–87. doi: 10.1007/BF00583659. [DOI] [PubMed] [Google Scholar]
- Yeh J. Z., Oxford G. S., Wu C. H., Narahashi T. Dynamics of aminopyridine block of potassium channels in squid axon membrane. J Gen Physiol. 1976 Nov;68(5):519–535. doi: 10.1085/jgp.68.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg R. J., Siebenga E., de Bruin G. Potassium ion noise currents and inactivation in voltage-clamped node of Ranvier. Nature. 1977 Jan 13;265(5590):177–179. doi: 10.1038/265177a0. [DOI] [PubMed] [Google Scholar]


