Abstract
Freshwater bivalves are vital to aquatic ecosystems but face severe global threats. Understanding their genomic traits and evolution is crucial for effective conservation. This study conducted whole-genome sequencing on 8 Lamprotula species. These 8 species exhibited high genomic complexity, characterized by large genomes (1.89 to 2.65 Gb), high heterozygosity (>0.8), and high repeat content (>60%), estimated by k-mer analysis. Genome assemblies showed that L. caveata had the largest genome, while L. polysticta had the smallest. Furthermore, the assembled genome sizes of these 8 species exhibited an average increase of 22.58% compared to k-mer analysis estimates, largely due to their high heterozygosity. The mitochondrial genomes of these 8 species ranged in size from 15.69 kb to 17.13 kb, with GC contents varying from 36.36% to 40.77%. Phylogenetic analysis indicated early divergence of L. leai and L. caveata from the other 6 species. Pairwise Sequentially Markovian Coalescent analysis revealed population bottlenecks over the past million years, with L. rochechouarti showing more significant population size fluctuations during the Pleistocene Glacial Epoch. In summary, this study provides comprehensive genomic insights into 8 Lamprotula species, highlighting their high genomic complexity and evolutionary divergence, thereby establishing a crucial foundation for future conservation and genetic research efforts.
Keywords: Lamprotula, K-mer analysis, mitochondrial genome, phylogenetic analysis, PSMC
1. Introduction
Freshwater bivalves serve as vital components of aquatic biodiversity and play indispensable roles in freshwater ecosystems.1,2 Functioning as key mediators in nutrient cycling and energy flow, they provide essential ecological services, including water purification, habitat engineering, and biological indicators for environmental monitoring.3–8 Furthermore, these molluscs represent crucial trophic resources for benthic carnivorous fishes, crustaceans, and rare avian species. Particularly noteworthy is the obligate symbiotic relationship between unionid mussels (Unionidae) and bitterling fishes (Acheilognathinae), where the gills of freshwater mussels serve as exclusive incubation chambers for bitterling embryos during their reproductive phase.9 As a global biodiversity hotspot for freshwater molluscs, China harbours exceptional species richness. However, escalating anthropogenic pressures, including aquatic habitat degradation, water pollution intensification, and destructive harvesting practices, have precipitated severe declines in native mussel populations.6,10 Numerous endemic species now face critical endangerment, with some taxa approaching functional extinction in their natural habitats. Freshwater bivalves (order Unionoida) are one of the most threatened animal groups in the world.11 This alarming trajectory of biodiversity erosion urgently necessitates the implementation of comprehensive conservation strategies, encompassing habitat restoration, germplasm resource preservation, and ecosystem-based management approaches to safeguard these ecologically invaluable organisms.
Lamprotula species belongs to the phylum Mollusca, class Bivalvia, order Unionoida, and family Unionidae.12 These freshwater bivalves are primarily distributed across East Asia, including China, Korea, Vietnam, and Japan, with approximately 26 species recorded worldwide. This taxon exhibits both ecological vulnerability and evolutionary distinctiveness.10,13 Most of these species are found in China, many of which are endemic and possess significant economic and ecological value.14 Due to environmental changes, river pollution, and overfishing, these species face the threat of population extinction.15 Notably, the International Union for Conservation of Nature Red List identifies 17 Lamprotula species as critically endangered, vulnerable or data deficient, yet their evolutionary relationships and adaptive genetic mechanisms remain poorly characterized, highlighting the urgent need for integrated conservation strategies and scientific population assessments.10,16 Persistent taxonomic controversies significantly impede conservation efforts for Lamprotula species. While Wu et al. proposed the division of Lamprotula into 2 genera (Lamprotula and Aculamprotula) based on morphological and anatomical characteristics,13,17 subsequent molecular phylogenetic analyses have corroborated the polyphyletic nature of the genus while suggesting alternative subfamilial placements for these clades.12 Additionally, the taxonomic status of L. rochechouarti requires clarification, as evidenced by conflicting morphological and molecular biological data.18 These unresolved issues regarding interspecific divergence, taxonomic delineation, and phylogenetic reconstruction highlight critical knowledge gaps. However, genomic research on these species is limited, posing significant challenges for understanding their genetic characteristics, evolutionary history, and population dynamics.
While genomic resources for marine molluscs have expanded rapidly, freshwater bivalve genomics lags significantly, primarily focussed on biomineralization and pearl formation mechanisms.19–21 For Lamprotula species, existing studies predominantly centres on resource assessments,14 morphology,22,23 taxonomy24 and phylogeny.12 This lack of data hinders our understanding of its evolutionary history and impedes efforts to mine genetic information for broader phylogenetic studies of the sinipercids. Therefore, it is imperative to conduct genome-level analyses of the germplasm resources, genetic background, and evolutionary history of Lamprotula to resolve its taxonomic status and support future domestication, breeding, and conservation biology research.
Currently, the advancement of high-throughput sequencing technologies and the continuous reduction in sequencing costs have significantly improved our ability to study the genetic characteristics of aquatic animals.25 Genomic survey analysis has proven to be a highly effective and cost-efficient method for rapidly obtaining key genomic characteristics, such as genome size, heterozygosity rate, repeat sequence proportion, and ploidy level, particularly when no reference genome is available.26,27 The high-depth clean data generated from these surveys provides an essential foundation for de novo genome assembly. Tools like SOAPdenovo2 have shown their effectiveness in generating high-quality draft genomes using paired-end short reads, offering crucial genomic resources for studying genetic traits.28 A wide range of well-established and robust bioinformatics tools allow for the accurate characterization of the mitochondrial genome,29,30 the inference of evolutionary relationships,31 and the estimation of effective population sizes.32 Importantly, the genomic characteristics of Lamprotula species obtained through genome survey provide a robust foundation for constructing high-quality reference genomes in future studies.
In this study, a comprehensive whole-genome sequencing analysis was conducted on 8 Lamprotula species. Based on high-depth paired-end data, genome characteristics such as genome size, heterozygosity, and repetitive sequence proportion were estimated using K-mer analysis. Draft genomes were obtained through genome assembly, and the mitochondrial genomes were characterized. Simple sequence repeats (SSRs) were also identified, and phylogenetic relationships with other species were analysed. Finally, demographic analysis using Pairwise Sequentially Markovian Coalescent (PSMC) revealed significant phases in the population history of these Lamprotula species. In summary, our findings provide critical genomic resources that are essential for the conservation of Lamprotula species in China and significantly contribute to our knowledge of the evolutionary history within the family Unionidae.
2. Materials and methods
2.1. Sample collection and DNA extraction
Eight species of Lamprotula were included in this study, specifically L. rochechouarti, L. tortuosa, L. leai, L. caveata, L. scripta, L. zonata, L. fibrosa, and L. polysticta, which were supplied by Wuhu Yangtze River Mollusk Conservation Base of the Ministry of Agriculture and Rural Affairs (Table 1). The specimens included L. rochechouarti, L. scripta, L. zonata, and L. fibrosa from Poyang Lake, Jiangxi Province; L. leai and L. caveata from Jingshan River, Anhui Province; L. tortuosa from Leishui River, Hunan Province; and L. polysticta from Xihe River, Guangxi Zhuang Autonomous Region. Among these, L. caveata had the lowest individual weight at 54.7 g, while L. polysticta had the highest at 297.66 g. Detailed measurements of their shell length, shell width, and shell height were provided in Table 1.
Table 1.
Sample information for the 8 Lamprotula species used in this study.
| Species | Collection date | Shell length (mm) | Shell width (mm) | Shell height (mm) | Weight (g) | Sample source |
|---|---|---|---|---|---|---|
| Lamprotula rochechouarti | 2024-4-11 | 77.44 | 29.77 | 57.71 | 108.4 | Poyang Lake, Jiangxi Province, China |
| Lamprotula tortuosa | 2024-4-11 | 106.16 | 50.6 | 52.54 | 264.74 | Leishui River, Hengyang, Hunan Province, China |
| Lamprotula leai | 2024-4-11 | 106.83 | 35.16 | 55.39 | 175.93 | Jing Shan River, Wuhu, Anhui Province, China |
| Lamprotula caveata | 2024-4-11 | 65.45 | 26.98 | 34.27 | 54.7 | Jing Shan River, Wuhu, Anhui Province, China |
| Lamprotula scripta | 2024-4-11 | 86.08 | 45.71 | 60.82 | 202.33 | Poyang Lake, Jiangxi Province, China |
| Lamprotula zonata | 2024-4-11 | 85.96 | 42.46 | 59.84 | 180.47 | Poyang Lake, Jiangxi Province, China |
| Lamprotula fibrosa | 2024-4-11 | 96.24 | 42.57 | 43.23 | 226.37 | Poyang Lake, Jiangxi Province, China |
| Lamprotula polysticta | 2024-4-11 | 97.08 | 52.73 | 40.85 | 297.66 | Xihe River, Guilin, Guangxi Zhuang Autonomous Region, China |
For genome sequencing, an individual was selected based on its morphological characteristics, and muscle tissue samples were preserved in 95% alcohol to prepare for DNA extraction. The DNA extraction process followed the traditional phenol/chloroform method with RNase A treatment for DNA template purification. Initially, adductor muscle tissues were ground into a fine powder under liquid nitrogen conditions. Subsequently, SDS buffer and proteinase K were added to lyse cells and eliminate contaminants. DNA extraction was performed using a mixture of phenol:chloroform:isoamyl alcohol (25:24:1), and DNA was precipitated by isopropanol. The resulting DNA pellet was washed with chilled 70% ethanol. After removing residual ethanol through evaporation, the DNA was resuspended in sterile water.
2.2. Whole-genome sequencing and quality control
Genomic DNA extracted from each Lamprotula species was utilized to construct sequencing libraries with fragment lengths ranging from 300 to 400 base pairs (bp). Sequencing was conducted on MGI’s DNBseq-T7 platform using paired-end reads of 2 × 150 bp, in accordance with the manufacturer’s protocols. The raw sequencing data were processed using Fastp (v0.23.2) for quality control.33 This involved adapter trimming, filtering out low-quality reads (reads where more than 40% of bases had a quality score below Q15 or contained over 5 ambiguous ‘N’ bases), and removing duplicate reads. Following these steps, clean data for each species were obtained (Table 2).
Table 2.
High-throughput sequencing data statistics for the 8 Lamprotula species.
| Species | Library Size (bp) | Sequencing type | Raw data (Gb) | Total clean reads | Total clean bases (Gb) | Q20 (%) | Q30 (%) | GC content (%) |
|---|---|---|---|---|---|---|---|---|
| Lamprotula rochechouarti | 300–400 | Paired-end | 195.57 | 1,249,280,958 | 186.63 | 98.97 | 97.45 | 37.03 |
| Lamprotula tortuosa | 300–400 | Paired-end | 248.26 | 1,558,836,150 | 232.79 | 98.88 | 97.25 | 35.77 |
| Lamprotula leai | 300–400 | Paired-end | 194.70 | 1,242,405,262 | 185.73 | 98.91 | 97.25 | 36.08 |
| Lamprotula caveata | 300–400 | Paired-end | 232.91 | 1,476,561,200 | 220.53 | 98.85 | 97.13 | 36.09 |
| Lamprotula scripta | 300–400 | Paired-end | 251.78 | 1,585,295,636 | 236.57 | 98.85 | 97.18 | 35.73 |
| Lamprotula zonata | 300–400 | Paired-end | 217.41 | 1,379,786,284 | 206.00 | 98.88 | 97.25 | 35.62 |
| Lamprotula fibrosa | 300–400 | Paired-end | 222.36 | 1,405,719,326 | 208.77 | 99.24 | 97.80 | 35.78 |
| Lamprotula polysticta | 300–400 | Paired-end | 253.43 | 1,591,112,558 | 237.51 | 99.21 | 97.60 | 35.75 |
To assess potential contamination in the clean data, a random selection of 10,000 paired-end clean reads per species was subjected to BLAST analysis against the NCBI Nucleotide (NT) database (Blastn v2.11.0).34 Species with an identity score exceeding 80% were recorded. The number of reads aligning to any species in the NT database, as well as those specifically aligning to the corresponding genus, were counted and their proportions calculated to determine the extent of contamination and to filter out the contaminated reads accordingly. Ultimately, 8 high-quality, contamination-free datasets were obtained, suitable for downstream analyses.
2.3. K-mer analysis and ploidy determination of 8 Lamprotula species
To investigate the genomic characteristics of 8 Lamprotula species, K-mer analysis was conducted to estimate genome size, heterozygosity, and repeat content. The K-mer size for all 8 species was set to 17, and the 17-mers depth distribution was calculated using Jellyfish (v2.2.4).35 The 17-mers frequency distribution was found to be consistent with a Poisson distribution, and the peak depth value was determined, representing the average and variance of the associated Poisson distribution. The genome size of each species was estimated using the following equation: G = K-mer-num/K-mer-depth, where K-mer-num was the total number of 17-mers, K-mer-depth was the K-mer depth, and G represents the estimated genome size. This process was facilitated using GCE (v1.0.0) software.27 Furthermore, 17-mers with a depth of 1 were considered to be errors, and the error rate was calculated to revise the estimated genome size. During this analysis, the heterozygosity ratio and proportions of repeat sequences for the 8 species were also obtained. To further explore the ploidy of these 8 species, Smudgeplot (v0.2.3.dev) was applied to analyse their genome structures, with parameters set to -k 17 -m100 -ci 1 -cs 10000.36
2.4. Genome assembly and mitochondrial genome characterization
To assembly the draft genomes of the 8 species, SOAPdenovo (v2.04) was used for genome assembly with the parameters set as: -d 1 -R -K 127 -p 60.28 During the assembly process, clean reads were first assembled into contigs. Subsequently, all clean reads were realigned to these contigs, and scaffolds were constructed step by step using paired-end reads with fixed insert sizes, resulting in a scaffold-level genome assembly. Assembly metrics, including N50, N90, and the length of the longest sequence, were obtained using the Seqkit (v2.8.2) tool.37
Meanwhile, to obtain their mitochondrial genomes, this study utilized NOVOPlasty (v4.3.1) with the following parameters: Genome Range = 13,000 to 19,500, K-mer = 33, Read Length = 150, Insert size = 300, Single/Paired = PE.29 The raw data (with only adapters removed) from the 8 species were assembled. The assembled mitochondrial genomes were manually verified to ensure they formed complete circular sequences.
To assess the consistency of the mitochondrial genome sequences, the cleaned data were aligned to the mitochondrial genomes using bwa (v0.7.12-r1039),38 facilitating the calculation of alignment rates and coverage at different depths. Annotation of mitochondrial genomes was performed using mitoZ (v3.6) with the parameters set as mitoz annotate --clade Mollusca --genetic_code 5.39 Finally, visualization of the mitochondrial genomes was achieved using both mitoZ (v3.6)39 and OGDRAW (v1.3.1).40
2.5. Identification of SSRs
To identify the types and numbers of SSRs in the 8 Lamprotula species, the Perl script ‘misa.pl’ from the MISA (v2.1) software was employed to analyse potential microsatellite motifs within their genomes.41 The search parameters were configured to detect di-, tri-, tetra-, penta-, and hexa-nucleotide repeats with minimum repeat lengths of 6, 5, 5, 5, and 5 repeats, respectively. Following the identification of SSRs, their distribution characteristics were visualized using the ggplot2 package in R,42 providing a comprehensive overview of SSR patterns across the species.
2.7 Construction of phylogenetic tree based on 8 mitochondrial genomes
Mitochondrial genomes (mitogenomes) play a crucial role in evolutionary studies.43 Consequently, the utilization of complete mitogenomes for phylogenetic investigations has become standard practice.39 To elucidate the evolutionary relationships among Lamprotula species, this study employed a strategy based on their entire mitogenome sequences. Mitochondrial genome sequences were aligned using MAFFT (v7.526) with the L-INS-i algorithm (--localpair), implementing 1,000 iterative refinements for maximum accuracy (--maxiterate 1000).44 Phylogenetic inference was performed under the generalized time-reversible nucleotide substitution model using FastTree (v2.1.11).45 Node support was assessed with 1,000 bootstrap replicates (-boot 1000), and branch lengths were optimized with maximum-likelihood approximation.
2.8 Inference of population size dynamics for 8 Lamprotula species
To infer the population size history of these Lamprotula species, the PSMC method (v0.6.5) was utilized.32 First, clean reads were aligned to the assembled genome using BWA-MEM2,38 generating BAM files. Following this, the ‘fq2psmcfa’ and ‘splitfa’ tools from the PSMC software suite were employed to prepare the input files necessary for PSMC modelling.
The PSMC analysis was conducted with specific parameters: -N25 to define the number of algorithm cycles and -t15 to establish the upper limit for the most recent common ancestor. The resulting population history was visualized using the ‘psmc_plot.pl’ script, applying a substitution rate of 1.16e-8 and assuming a generation time of 2 years.
3. Results
3.1. High-throughput sequencing and K-mer analysis of 8 Lamprotula species genomes
This study included 8 Lamprotula species collected in 2024 from 4 provinces in China, with their weights ranging from 54.7 to 297.66 g, for high-throughput paired-end sequencing (Table 1). A library with a size range of 300 to 400 base pairs was constructed for each species and sequenced using DNBSeq technology. The raw data obtained for L. rochechouarti, L. tortuosa, L. leai, L. caveata, L. scripta, L. zonata, L. fibrosa, and L. polysticta were 195.57, 248.26, 194.70, 232.91, 251.78, 217.41, 222.36, and 253.43 Gb, respectively (Table 2). Subsequently, the data were quality-controlled using fastp, resulting in clean data with Q30 scores greater than 97% and Q20 scores greater than 98% for all 8 species. Among these, the GC content in the clean data of L. zonata was the lowest at 35.62%, while that of L. rochechouarti was the highest at 37.03%. Additionally, the NT database comparison results indicated that the top 3 species identified through BLAST searches were predominantly members of the Family Unionidae (Table S1). This finding confirmed that there was no significant exogenous contamination during the library construction process. Ultimately, the clean data sizes for L. rochechouarti, L. tortuosa, L. leai, L. caveata, L. scripta, L. zonata, L. fibrosa, and L. polysticta were 186.63, 232.79, 185.73, 220.53, 236.57, 206.00, 208.77, and 237.51 Gb, respectively (Table 2).
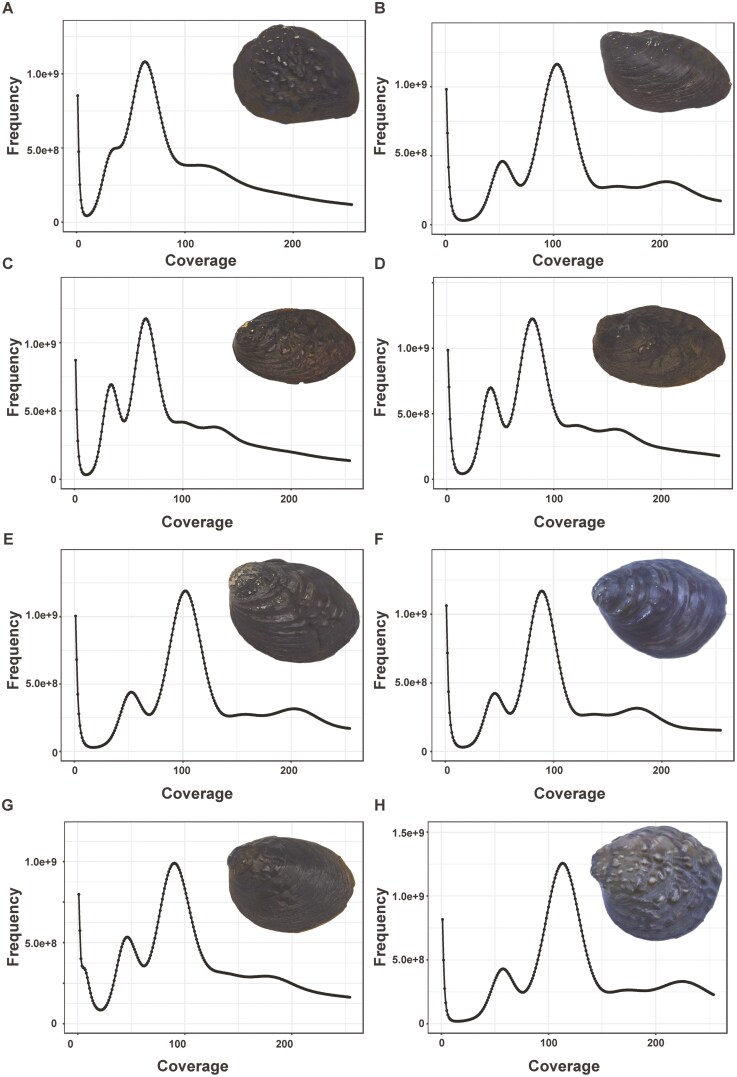
K-mer analysis was employed to estimate the genome size, heterozygosity ratio, and repeat content of 8 Lamprotula species genomes, with a uniform K-value of 17 set for all analyses (Table 3). The expected K-mer depth was calculated as 60, 101, 64, 77, 100, 87, 87, and 111 for the respective Lamprotula species (Fig. 1). Using the formula genome size = (total number of k-mers)/(peak depth), the initial estimated genome sizes for L. rochechouarti, L. tortuosa, L. leai, L. caveata, L. scripta, L. zonata, L. fibrosa, and L. polysticta were determined to be 2.66, 2.03, 2.59, 2.56, 2.07, 2.05, 2.14, and 1.89 Gb, respectively. After correction using Genome Character Estimator, the final estimated genome sizes ranged from 1.89 to 2.65 Gb for these species. All 8 species exhibited high heterozygosity rates (greater than 0.8) and high repeat content (exceeding 60%), posing significant challenges for generating high-quality reference genomes in subsequent studies. According to the Smudgeplot analysis results, the proportions of AB-type k-mers for the 8 species were 0.61, 0.56, 0.62, 0.60, 0.54, 0.56, 0.66, and 0.52 (Fig. 2 and Table S2). When combined with the previous findings, this suggested that the genomes of these 8 species were all large, highly heterozygous, and highly repetitive, typical of complex diploid genomes.
Table 3.
Results of K-mer analysis for 8 Lamprotula species.
| Species | K-mer size | Total K-mer | K-mer depth | Esitimated genome size (bp) | Revised genome size (bp) | Heterozygous ratio (%) | Repeat (%) |
|---|---|---|---|---|---|---|---|
| Lamprotula rochechouarti | 17 | 166,567,551,905 | 60 | 2,662,230,000 | 2,648,606,184 | 0.99 | 65.78 |
| Lamprotula tortuosa | 17 | 207,744,257,664 | 101 | 2,028,720,000 | 2,019,138,943 | 0.87 | 63.47 |
| Lamprotula leai | 17 | 165,777,903,081 | 64 | 2,590,279,736 | 2,576,668,228 | 1.42 | 63.46 |
| Lamprotula caveata | 17 | 196,808,135,862 | 77 | 2,555,949,816 | 2,543,155,844 | 1.35 | 62.70 |
| Lamprotula scripta | 17 | 211,106,855,124 | 100 | 2,067,440,000 | 2,057,609,126 | 0.84 | 64.52 |
| Lamprotula zonata | 17 | 183,837,950,251 | 87 | 2,052,000,000 | 2,040,140,119 | 0.86 | 64.35 |
| Lamprotula fibrosa | 17 | 186,246,494,094 | 87 | 2,140,764,300 | 2,131,595,254 | 1.63 | 62.25 |
| Lamprotula polysticta | 17 | 212,018,477,813 | 111 | 1,894,800,000 | 1,887,507,685 | 0.82 | 61.67 |
Fig. 1.
K-mer (K = 17) analysis for genomic characterization of 8 Lamprotula freshwater bivalve species. a–h) L. rochechouarti, L. tortuosa, L. leai, L. caveata, L. scripta, L. zonata, L. fibrosa, and L. polysticta, respectively. The x axis indicates K-mer depth, and the y axis indicates K-mer frequency.
Fig. 2.
Smudgeplot estimation of ploidy for 8 Lamprotula freshwater bivalve species. a–h) L. rochechouarti, L. tortuosa, L. leai, L. caveata, L. scripta, L. zonata, L. fibrosa, and L. polysticta, respectively. The x axis represents relative coverage (CovB/(CovA + CovB)), the y axis represents total coverage (CovA + CovB), and colour indicates k-mer pair frequency.
3.2. Genome assembly and mitochondrial genome assembly of 8 Lamprotula species
To explore further genomic characteristics of these 8 Lamprotula species, SOAPDenovo2 was utilized for assembling their draft genomes. The results showed that L. caveata had both the largest genome size and the longest sequence among these 8 species. Conversely, L. polysticta had the smallest genome size at the contig level, measuring 2.26 Gb compared to L. caveata’s 3.33 Gb. At the scaffold level, the sizes ranged from 2.35 Gb to 3.47 Gb (Table 4). Despite having the smallest draft genome size, L. polysticta exhibited the largest scaffold N50 of 2.28 kb, while L. fibrosa had shown the smallest at 0.95 kb. Compared to the estimated sizes from K-mer analysis, the assembled genome sizes of these 8 species were on average 22.58% larger than expected, a discrepancy that was closely related to the high heterozygosity characteristic of their genomes. The GC content across the draft genomes of these 8 species had been largely consistent, fluctuating between 35.08% and 35.82%, with an average of 35.29%. Overall, this information had provided a solid foundation for constructing high-quality reference genomes in future studies.
Table 4.
Statistics of genome assembly for 8 Lamprotula species.
| Species | Total contig length (bp) | Total scaffolds | Total scaffold length (bp) | Scaffold N50 (bp) | Largest scaffold (bp) | GC (%) |
|---|---|---|---|---|---|---|
| Lamprotula rochechouarti | 3,065,532,295 | 4,038,592 | 3,209,067,863 | 1,681 | 85,765 | 35.82 |
| Lamprotula tortuosa | 2,325,850,842 | 2,911,977 | 2,411,778,601 | 1,730 | 88,999 | 35.14 |
| Lamprotula leai | 2,926,540,197 | 3,281,778 | 3,061,408,379 | 1,687 | 66,635 | 35.50 |
| Lamprotula caveata | 3,327,384,479 | 4,036,935 | 3,465,891,362 | 2,008 | 105,433 | 35.48 |
| Lamprotula scripta | 2,322,900,503 | 3,098,426 | 2,424,923,496 | 1,754 | 99,338 | 35.08 |
| Lamprotula zonata | 2,283,197,578 | 2,902,651 | 2,382,349,442 | 1,787 | 79,547 | 35.10 |
| Lamprotula fibrosa | 2,568,870,412 | 4,567,553 | 2,685,642,119 | 946 | 48,928 | 35.12 |
| Lamprotula polysticta | 2,261,352,812 | 2,520,167 | 2,347,051,140 | 2,278 | 89,097 | 35.09 |
Mitochondria played multiple crucial roles in mussel species, significantly contributing to the maintenance of normal physiological functions and adaptation to environmental changes.43,46 To obtain complete mitochondrial genome sequences, high-quality sequencing data were generated, with clean data sizes ranging from 9.70 Gb to 12.60 Gb, and Q20 values reaching approximately 99% (Table S3). The assembly results indicated that all 8 species obtained high-quality circular mitochondrial genomes, with sizes ranging from 15.69 kb to 17.13 kb, and GC contents fluctuating between 36.36% and 40.77% (Table 5).
Table 5.
Assembly and annotation of mitochondrial genomes for 8 Lamprotula species.
| Lamprotula rochechouarti | Lamprotula tortuosa | Lamprotula leai | Lamprotula caveata | Lamprotula scripta | Lamprotula zonata | Lamprotula fibrosa | Lamprotula polysticta | |
|---|---|---|---|---|---|---|---|---|
| Genome length (bp) | 16,111 | 15,724 | 16,118 | 17,132 | 15,689 | 15,699 | 15,700 | 15,693 |
| Contig Num | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| GC content (%) | 39.07 | 36.36 | 39.83 | 40.77 | 37.1 | 37.01 | 36.62 | 36.65 |
| Circularized | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Number of protein-coding genes | 13 | 13 | 13 | 13 | 13 | 13 | 13 | 13 |
| Number of tRNA genes | 22 | 22 | 22 | 22 | 22 | 22 | 22 | 22 |
| Number of rRNA genes | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Total genes | 37 | 37 | 37 | 37 | 37 | 37 | 37 | 37 |
To assess the sequence consistency of these mitochondrial genomes, the quality-controlled data were aligned to the mitochondrial genomes using bwa.38 The results showed an average read mapping rate of 96.54%, with the mitochondrial genomes achieving 100% average coverage. Regions with coverage greater than 20x accounted for an average of 98.98%, demonstrating excellent sequence consistency among them (Fig. S1 and Table S4). Additionally, after annotating the protein-coding genes in their mitochondrial genomes, it was found that all 8 species contained 37 genes, including 13 protein-coding genes, 22 tRNAs, and 2 rRNAs (Table 5). Visualization of their mitochondrial genomes revealed that the gene structures and positions were highly conserved (Fig. 3 and Figs. S2–S8).
Fig. 3.
Mitochondrial genome structure of L. rochechouarti. Yellow blocks represent NADH dehydrogenase, pink blocks represent cytochrome c oxidase, green blocks represent ATP synthase, blue blocks represent tRNA, red blocks represent rRNA, and purple-red blocks represent other genes.
3.3. Characterization of potential microsatellite markers
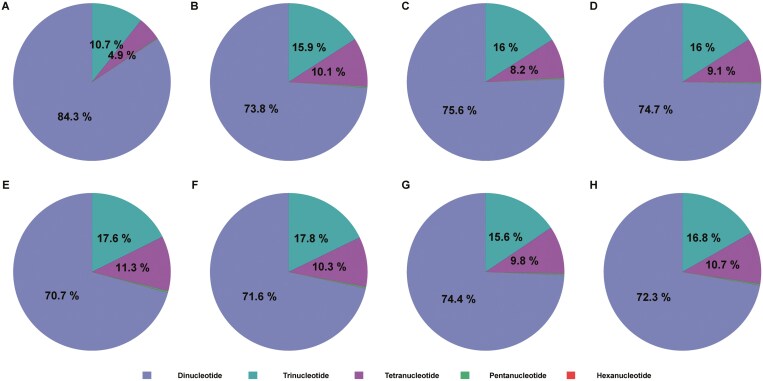
Based on the draft genomes of 8 Lamprotula species, a total of 1,282,789, 826,136, 983,887, 1,263,379, 964,217, 845,103, 892,958, and 901,611 SSRs were identified, respectively (Table 6). Among the microsatellite motif types observed in these species, dinucleotide repeats were predominant (ranging from 70.73% to 84.29%), followed by trinucleotide repeats (10.72% to 17.80%), tetranucleotide repeats (4.85% to 11.30%), pentanucleotide repeats (0.12% to 0.30%), and hexa-nucleotide repeats (0.01% to 0.02%) (Fig. 4 and Table S5). The proportion of microsatellite motifs decreased as the repeat unit length increased. The SSR density across these species varied from 321 per Mb in L. leai to 400 per Mb in L. rochechouarti (Table 6). These findings facilitated the development of molecular markers for species identification and contributed further to the population genetics studies of Lamprotula species.47,48
Table 6.
Microsatellite motif statistics of 8 Lamprotula species.
| Lamprotula rochechouarti | Lamprotula tortuosa | Lamprotula leai | Lamprotula caveata | Lamprotula scripta | Lamprotula zonata | Lamprotula fibrosa | Lamprotula polysticta | |
|---|---|---|---|---|---|---|---|---|
| Total size of examined sequences (bp) | 3,209,067,863 | 2,411,778,601 | 3,061,408,379 | 3,465,891,362 | 2,424,923,496 | 2,382,349,442 | 2,685,642,119 | 2,347,051,140 |
| Total number of SSRs | 1,282,789 | 826,136 | 983,887 | 1,263,379 | 964,217 | 845,103 | 892,958 | 901,611 |
| SSR density (SSR number/genome size) | 399.74 | 342.54 | 321.38 | 364.52 | 397.63 | 354.74 | 332.49 | 384.15 |
| Number of dinucleotide repeats | 1,081,367 | 609,589 | 743,565 | 943,620 | 682,004 | 605,425 | 664,033 | 651,442 |
| Number of trinucleotide repeats | 137,560 | 131,147 | 157,416 | 201,592 | 170,159 | 150,428 | 139,029 | 151,305 |
| Number of tetranucleotide repeats | 62,200 | 83,220 | 80,796 | 115,182 | 108,965 | 86,920 | 87,574 | 96,299 |
| Number of pentanucleotide repeats | 1,531 | 2,012 | 1,939 | 2,716 | 2,859 | 2,167 | 2,150 | 2,388 |
| Number of hexa-nucleotide repeats | 131 | 168 | 171 | 269 | 230 | 163 | 172 | 177 |
Fig. 4.
The distribution of different repeat motif types within 8 Lamprotula freshwater bivalve species. a–h) L. rochechouarti, L. tortuosa, L. leai, L. caveata, L. scripta, L. zonata, L. fibrosa, and L. polysticta, respectively.
3.4. Phylogenetic relationship of 8 Lamprotula species based on complete mitochondrial genome sequences
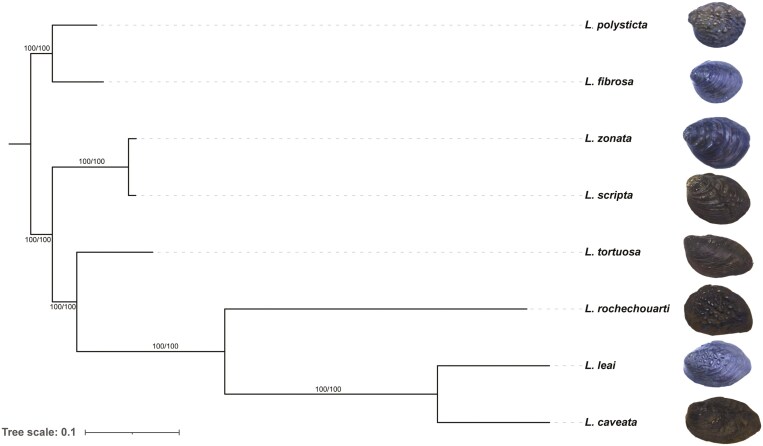
To resolve the evolutionary relationships within the genus Lamprotula, a phylogenetic tree was constructed based on complete mitochondrial genome sequences. The resulting maximum-likelihood phylogenetic tree revealed 2 major clades within Lamprotula (Fig. 5). One clade included L. polysticta and L. fibrosa, indicating close genetic relationships of these 2 species. The second major clade comprised L. zonata, L. scripta, L. tortuosa, L. rochechouarti, L. leai, and L. caveata, with L. leai and L. caveata forming a well-supported sister group. All nodes were strongly supported with bootstrap values of 100, underscoring the robustness of the phylogenetic relationships inferred. Representative shell morphologies of each species are shown alongside the tree, illustrating morphological variation in correspondence with phylogenetic divergence.
Fig. 5.
The phylogenetic tree was inferred from the complete mitochondrial genomes of 8 Lamprotula freshwater bivalve species.
3.5. The population size dynamics of 8 Lamprotula species
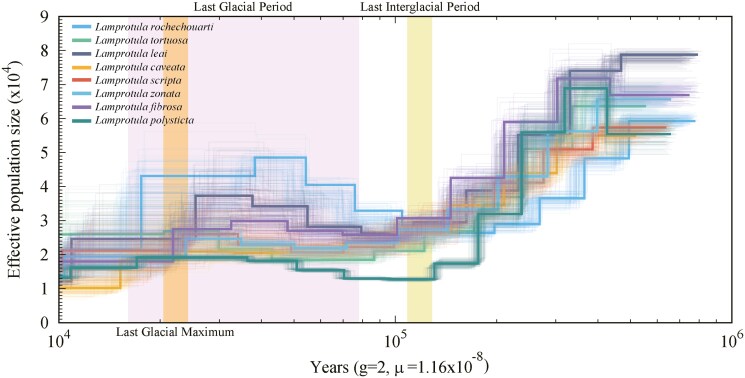
PSMC analysis revealed that the 8 Lamprotula species experienced population bottlenecks during the past million years (Fig. 6). During the Last Interglacial Period (~130 to 116 Kya), the effective population size of all 8 Lamprotula species decreased at a constant rate from its peak. As global temperatures rose, sea levels increased, and glaciers melted during this period, the sustained decline in population size may be associated with habitat changes, such as the loss of shallow coastal areas and alterations in ecosystem structure, making it difficult for the species to maintain large populations.49,50 During the Last Glacial Period (~70 to 15 Kya), the effective population sizes of these 8 species began to increase at a relatively stable rate. Subsequently, their population sizes showed a significant decline again during the Last Glacial Maximum, reaching their lowest points. Among the 8 Lamprotula species, L. tortuosa exhibited notably larger effective population sizes compared to the other 7 species. Throughout the Pleistocene Glacial Epoch, L. rochechouarti’s population size fluctuated more significantly than the other species.
Fig. 6.
Effective population size estimates using PSMC for 8 Lamprotula freshwater bivalve species. The x axis represents time before present (Kya), ranging from 10 to 1,000 thousand years (left to right). The y axis represents Ne. Parameter ‘g’ denotes generation time (years), and ‘μ’ represents mutation rate.
4. Discussion
This study represented the first comprehensive genome sequencing analysis of 8 Lamprotula species. Utilizing high-depth clean data, K-mer analysis had confirmed that these species possessed complex diploid genomes characterized by high heterozygosity and high proportions of repetitive sequences. Concurrently, the mitochondrial genome sizes of these 8 species had shown modest variation, ranging from 15.69 kb to 17.13 kb, with GC content fluctuating between 36.36% and 40.77%. Upon annotating the protein-coding genes within their mitochondrial genomes, it was found that all 8 species contained a total of 37 genes, comprising 13 protein-coding genes, 22 tRNAs, and 2 rRNAs. Among the observed microsatellite motif types in these species, dinucleotide repeats had been predominant, accounting for 70.73% to 84.29% of the motifs. In the phylogenetic tree constructed, L. leai and L. caveata had diverged early from the other 6 Lamprotula species, forming a distinct clade indicative of greater evolutionary divergence, likely influenced by their unique environmental conditions and characteristics.13,51 Within the clade containing the remaining 6 species, L. scripta and L. zonata had exhibited closer genetic relationships. Furthermore, PSMC analysis had revealed that these 8 Lamprotula species had experienced population bottlenecks over the past million years.
Considering the genomic complexity of these 8 species, the short-read sequencing strategy employed in this study presents inherent limitations. Firstly, substantial discrepancies exist between the draft assemblies and k-mer-based size estimates. Specifically, k-mer analysis predicted genome sizes of 1.89-2.65 Gb, approximately 22% smaller on average than the SOAPdenovo2 assemblies (2.35 to 3.47 Gb). This pattern aligns with observations in other aquatic genomes and stems from fundamental challenges in resolving repetitive and heterozygous regions with short reads.52–54 High genomic repetitiveness and heterozygosity frequently cause inflated assembly sizes and fragmentation. When short-read assemblies fail to resolve these complex regions correctly, these sequences may be artificially duplicated, increasing assembly size, or cause contig fragmentation. Second, fragmented assemblies affect downstream analyses. For SSR marker identification, longer SSR motifs spanning fragment boundaries become difficult to identify. For PSMC inference, fragmentation introduces biases in heterozygous SNP detection through haplotype breakpoints where short contigs disrupt linkage disequilibrium blocks, impairing phasing accuracy. Boundary artefacts causing elevated spurious heterozygosity near contig ends and coverage bias from uneven mapping in repetitive regions that inflates false heterozygosity. These artefacts consequently distort PSMC-inferred population size events, promote overestimation of recent effective population size and reduce resolution of recent demographic events, particularly for recent bottlenecks. Future work could leverage the integration of short-read sequencing, long-read sequencing, and Hi-C mapping technologies to produce a high-quality, chromosome-scale reference genome for these freshwater mussels.19,55,56 Combined with comprehensive whole-genome annotation and comparative genomics analyses, this will play a crucial role in deepening our understanding of the evolutionary mechanisms and environmental adaptations of these species. Moreover, it will furnish materials for analysing the endangered mechanisms of Lamprotula populations. Additionally, this will also offer crucial genetic resources for enhancing their significant traits, conducting selective breeding, and facilitating aquaculture practices.
Furthermore, while this study revealed the demographic dynamics of Lamprotula species using PSMC analyses based on single samples from 8 species, these inferred population size changes require validation with larger datasets. Crucially, cryptic diversity or significant population structure might exist within Lamprotula species, complexities that a single sample cannot capture.13 For instance, if the sampled individual belonged to a genetically distinct subpopulation, the PSMC-inferred effective population size might only reflect the history of that subpopulation, not the entire species.57 Moreover, PSMC assumes a randomly mating population; the presence of cryptic diversity or population structure could violate this assumption, potentially leading to misinterpretation of bottleneck or expansion events.58 Future studies should validate these findings and enhance understanding by incorporating broader sampling per species and integrating complementary population genetics methods to provide a more comprehensive picture of Lamprotula’s evolutionary history.
Mitochondria play a crucial role in the biological functions of freshwater mussels, involving multiple aspects such as energy metabolism, cell apoptosis, genetic diversity, and evolutionary studies.43,46 Therefore, characterizing the mitochondrial genome of Lamprotula was essential. In this study, NOVOPlasty was employed for the de novo assembly of their mitochondrial genomes,29 a tool that has proven to be highly effective for this purpose. The mitochondrial genomes of these 8 species were found to be similar in size and circular in structure, with high sequence identity, confirming the reliability of these mitochondrial genomes. When compared with publicly available mitochondrial data from NCBI, the mitochondrial genome size of L. tortuosa assembled in this study is 15,724 bp, while the previously reported sequence (NC_021404.1) was 15,722 bp.59 Their GC contents are 36.36% and 36.25%, respectively. The consistency not only validated the accuracy of the assembly process but also underscored the utility of NOVOPlasty in generating reliable mitochondrial genome. These mitochondrial datasets provided significant contributions to the genetic resources of Lamprotula, enhancing our understanding of their genetic makeup and evolutionary history.
Overall, the natural resources of the Lamprotula species had drastically diminished, pushing global populations to the brink of depletion.8,10,16,60 The urgency to adopt effective measures for the protection and proliferation of these species could not have been overstated. Our research provided genomic data resources for 8 Lamprotula species, which held significant implications for the ecology and conservation of Lamprotula species. Additionally, our findings contributed to uncovering critical genetic traits essential for artificial breeding and seedling cultivation of Lamprotula, thus facilitating initiatives focussed on the preservation and enhancement of Lamprotula resources.
Supplementary Material
Contributor Information
Min Jiang, Shanghai Universities Key Laboratory of Marine Animal Taxonomy and Evolution, Shanghai Ocean University, Shanghai 201306, China; Key Laboratory of Freshwater Fisheries and Germplasm Resources Utilization, Ministry of Agriculture and Rural Affairs, Freshwater Fisheries Research Center, Chinese Academy of Fishery Sciences, Wuxi 214081, China.
Qi Liu, College of Fisheries, Southwest University, Chongqing 400715, China.
Chao Jiang, Wuxi Fisheries College, Nanjing Agricultural University, Wuxi 214081, China.
Mingfei Zhan, Anhui Shuiyun Environmental Protection Co., Ltd., Wuhu 241000, China.
Haibo Wen, Key Laboratory of Freshwater Fisheries and Germplasm Resources Utilization, Ministry of Agriculture and Rural Affairs, Freshwater Fisheries Research Center, Chinese Academy of Fishery Sciences, Wuxi 214081, China; Wuxi Fisheries College, Nanjing Agricultural University, Wuxi 214081, China.
Fengyue Shu, Qufu Normal University, Qufu 273165, China.
Lingli Xie, Key Laboratory of Freshwater Fisheries and Germplasm Resources Utilization, Ministry of Agriculture and Rural Affairs, Freshwater Fisheries Research Center, Chinese Academy of Fishery Sciences, Wuxi 214081, China.
Tengteng Liu, Key Laboratory of Freshwater Fisheries and Germplasm Resources Utilization, Ministry of Agriculture and Rural Affairs, Freshwater Fisheries Research Center, Chinese Academy of Fishery Sciences, Wuxi 214081, China.
Chenliang Ren, School of Ecology and Environment, Anhui Normal University, Wuhu 241000, China.
Wenqiao Tang, Shanghai Universities Key Laboratory of Marine Animal Taxonomy and Evolution, Shanghai Ocean University, Shanghai 201306, China.
Kai Liu, Shanghai Universities Key Laboratory of Marine Animal Taxonomy and Evolution, Shanghai Ocean University, Shanghai 201306, China; Key Laboratory of Freshwater Fisheries and Germplasm Resources Utilization, Ministry of Agriculture and Rural Affairs, Freshwater Fisheries Research Center, Chinese Academy of Fishery Sciences, Wuxi 214081, China; Wuxi Fisheries College, Nanjing Agricultural University, Wuxi 214081, China; School of Ecology and Environment, Anhui Normal University, Wuhu 241000, China.
Author contributions
M.J., K.L. and W.T. conceived and designed the study. C.J., M.Z., H.W., F.S., L.X. and C.R. prepared the samples. Q.L. and K.L. performed analyses. M.J. and Q.L. wrote the paper with input from co-authors. T.L. is responsible for figures production. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Chinese Academy of Fishery Sciences, Central Public-interest Scientific Institution Basal Research Fund, CAFS (NO. 2023TD11) and the Key Waters Aquatic Biological lonitoring Project in Anhui Province (2023AHNYC016XQ).
Conflict of interest
None declared.
Data availability
Illumina paired-end reads have been deposited in NCBI/BioProject PRJNA1301049. The whole-genome sequence data reported in this paper have been deposited in NCBI, under accession numbers (SAMN50434169, SAMN50434170, SAMN50434171, SAMN50434172, SAMN50434173, SAMN50434174, SAMN50434175, SAMN50434176). The mitochondrial genomes of these 8 species are available in Figshare (https://doi.org/10.6084/m9.figshare.29498255).
References
- 1. Vaughn CC, Hakenkamp CC.. The functional role of burrowing bivalves in freshwater ecosystems. Freshw Biol. 2001:46:1431–1446. https://doi.org/ 10.1046/j.1365-2427.2001.00771.x [DOI] [Google Scholar]
- 2. Howard JK, Cuffey KM.. The functional role of native freshwater mussels in the fluvial benthic environment. Freshw Biol. 2006:51:460–474. https://doi.org/ 10.1111/j.1365-2427.2005.01507.x [DOI] [Google Scholar]
- 3. Vaughn CC, Nichols SJ, Spooner DE.. Community and foodweb ecology of freshwater mussels. J North Am Benthol Soc. 2008:27:409–423. https://doi.org/ 10.1899/07-058.1 [DOI] [Google Scholar]
- 4. Vaughn CC. Ecosystem services provided by freshwater mussels. Hydrobiologia 2018:810:15–27. https://doi.org/ 10.1007/s10750-017-3139-x [DOI] [Google Scholar]
- 5. Strayer, D. L. Freshwater mussel ecology: a multifactor approach to distribution and abundance. University of California Press; 2008. https://doi.org/ 10.1111/j.1365-2427.2008.02151.x [DOI] [Google Scholar]
- 6. Chen X, Yang J, Liu H, Jiang T.. Freshwater Mussel Watch: An innovative approach for interpretations of aquatic pollution and toxicology. J. Lake Sci 2021:33:11–27. https://doi.org/ 10.18307/2021.0104 [DOI] [Google Scholar]
- 7. Bogan AE. Global diversity of freshwater mussels (Mollusca, Bivalvia) in freshwater. Hydrobiologia 2008:595:139–147. https://doi.org/ 10.1007/s10750-007-9011-7 [DOI] [Google Scholar]
- 8. Haag, W. R. North American freshwater mussels: natural history, ecology, and conservation. Cambridge University Press; 2012. [Google Scholar]
- 9. Lopes-Lima M, et al. Biology and conservation of freshwater bivalves: past, present and future perspectives. Hydrobiologia 2014:735:1–13. https://doi.org/ 10.1007/s10750-014-1902-9 [DOI] [Google Scholar]
- 10. Liu X, et al. Systematics, distribution, biology, and conservation of freshwater mussels (Bivalvia: Unionida) in China. Aquat Conserv Mar Freshwater Ecosyst. 2022:32:859–895. https://doi.org/ 10.1002/aqc.3799 [DOI] [Google Scholar]
- 11. Lydeard C, et al. The global decline of nonmarine mollusks. BioScience 2004:54:321–330. https://doi.org/ 10.1641/0006-3568(2004)054[0321:tgdonm]2.0.co;2 [DOI] [Google Scholar]
- 12. Pfeiffer JM, Graf DL.. Re-analysis confirms the polyphyly of Lamprotula Simpson, 1900 (Bivalvia: Unionidae). J Molluscan Stud. 2013:79:249–256. https://doi.org/ 10.1093/mollus/eyt022 [DOI] [Google Scholar]
- 13. Huang X-C, et al. Towards a global phylogeny of freshwater mussels (Bivalvia: Unionida): species delimitation of Chinese taxa, mitochondrial phylogenomics, and diversification patterns. Mol Phylogenet Evol. 2019:130:45–59. https://doi.org/ 10.1016/j.ympev.2018.09.019 [DOI] [PubMed] [Google Scholar]
- 14. Liu X, et al. Conservation status assessment and a new method for establishing conservation priorities for freshwater mussels (Bivalvia: Unionida) in the middle and lower reaches of the Yangtze River drainage. Aquat Conserv Mar Freshwater Ecosyst. 2020:30:1000–1011. https://doi.org/ 10.1002/aqc.3298 [DOI] [Google Scholar]
- 15. Liu X, et al. Assessment of the genetic diversity of Chinese freshwater mussels and refuge areas in the Yangtze River floodplain. Aquat Conserv Mar Freshwater Ecosyst. 2023:33:488–501. https://doi.org/ 10.1002/aqc.3940 [DOI] [Google Scholar]
- 16. Bogan AE. Freshwater mussels (Bivalvia: Unionida) of Vietnam: diversity, distribution, and conservation status. Freshwater Mollusk Biol Conserv 2018:21:1–18. https://doi.org/ 10.31931/fmbc.v21i1.2018.1-18 [DOI] [Google Scholar]
- 17. Wu R-W, et al. Analysis of mitochondrial genomes resolves the phylogenetic position of Chinese freshwater mussels (Bivalvia, Unionidae). ZooKeys 2019:812:23–46. https://doi.org/ 10.3897/zookeys.812.29908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang X-C, et al. Reclassification of Lamprotula rochechouartii as Margaritifera rochechouartii comb. nov. (Bivalvia: Margaritiferidae) revealed by time-calibrated multi-locus phylogenetic analyses and mitochondrial phylogenomics of Unionoida. Mol Phylogenet Evol. 2018:120:297–306. https://doi.org/ 10.1016/j.ympev.2017.12.017 [DOI] [PubMed] [Google Scholar]
- 19. Bai Z, et al. The first high-quality genome assembly of freshwater pearl mussel Sinohyriopsis cumingii: new insights into pearl biomineralization. Int J Mol Sci . 2024:25:3146. https://doi.org/ 10.3390/ijms25063146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ma X, et al. Chromosome-level genome assembly of the freshwater mussel Sinosolenaia oleivora (Heude, 1877). Sci Data. 2024:11:606. https://doi.org/ 10.1038/s41597-024-03451-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang T, et al. Dissecting the chromosome-level genome of the Asian Clam (Corbicula fluminea). Sci Rep. 2021:11:15021. https://doi.org/ 10.1038/s41598-021-94545-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xiao-ping W, Yan-ling L, Hong-zhu W, Yang-na O.. A comparative study on glochidial morphology of Unionidae (Bivalvia) II. Lanceolaria, Lamprotula, Hyriopsis and Cristaria. Acta Hydrobiol Sin. 2000:24:252–256. [Google Scholar]
- 23. Lopes-Lima M, Bolotov IN, Aldridge DC, et al. Expansion and systematics redefinition of the most threatened freshwater mussel family, the Margaritiferidae. Mol Phylogenet Evol. 2018:127:98–118. https://doi.org/ 10.1016/j.ympev.2018.04.041 [DOI] [PubMed] [Google Scholar]
- 24. Wu R, et al. DNA barcoding, multilocus phylogeny, and morphometry reveal phenotypic plasticity in the Chinese freshwater mussel Lamprotula caveata (Bivalvia: Unionidae). Ecol Evol. 2022:12:e9035. https://doi.org/ 10.1002/ece3.9035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yue G, Wang L.. Current status of genome sequencing and its applications in aquaculture. Aquaculture 2017:468:337–347. https://doi.org/ 10.1016/j.aquaculture.2016.10.036 [DOI] [Google Scholar]
- 26. Manekar SC, Sathe SR.. A benchmark study of k-mer counting methods for high-throughput sequencing. GigaScience. 2018:7:giy125. https://doi.org/ 10.1093/gigascience/giy125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu B, Shi Y, Yuan J, et al. Estimation of genomic characteristics by analyzing k-mer frequency in de novo genome projects. arXiv:1308.2012. 2013. https://doi.org/ 10.48550/arXiv.1308.2012 [DOI] [Google Scholar]
- 28. Luo R, et al. SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. GigaScience. 2012:1:2047-2217X-2041-2018. https://doi.org/ 10.1186/2047-217x-1-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dierckxsens N, Mardulyn P, Smits G.. NOVOPlasty: de novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 2017:45:e18–e18. https://doi.org/ 10.1093/nar/gkw955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Allio R, et al. MitoFinder: Efficient automated large‐scale extraction of mitogenomic data in target enrichment phylogenomics. Mol Ecol Resour. 2020:20:892–905. https://doi.org/ 10.1111/1755-0998.13160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nguyen L-T, Schmidt HA, Von Haeseler A, Minh BQ.. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2015:32:268–274. https://doi.org/ 10.1093/molbev/msu300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li H, Durbin R.. Inference of human population history from individual whole-genome sequences. Nature. 2011:475:493–496. https://doi.org/ 10.1038/nature10231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen S, Zhou Y, Chen Y, Gu J.. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018:34:i884–i890. https://doi.org/ 10.1093/bioinformatics/bty560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Johnson M, et al. NCBI BLAST: a better web interface. Nucleic Acids Res. 2008:36:W5–W9. https://doi.org/ 10.1093/nar/gkn201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marçais G, Kingsford C.. A fast, lock-free approach for efficient parallel counting of occurrences of k-mers. Bioinformatics. 2011:27:764–770. https://doi.org/ 10.1093/bioinformatics/btr011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ranallo-Benavidez TR, Jaron KS, Schatz MC.. GenomeScope 2.0 and Smudgeplot for reference-free profiling of polyploid genomes. Nat Commun. 2020:11:1432. https://doi.org/ 10.1038/s41467-020-14998-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shen W, Le S, Li Y, Hu F.. SeqKit: a cross-platform and ultrafast toolkit for FASTA/Q file manipulation. PLoS One. 2016:11:e0163962. https://doi.org/ 10.1371/journal.pone.0163962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li H, Durbin R.. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics. 2009:25:1754–1760. https://doi.org/ 10.1093/bioinformatics/btp324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Meng G, Li Y, Yang C, Liu S.. MitoZ: a toolkit for animal mitochondrial genome assembly, annotation and visualization. Nucleic Acids Res. 2019:47:e63–e63. https://doi.org/ 10.1093/nar/gkz173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Greiner S, Lehwark P, Bock R.. OrganellarGenomeDRAW (OGDRAW) version 1.3. 1: expanded toolkit for the graphical visualization of organellar genomes. Nucleic Acids Res. 2019:47:W59–W64. https://doi.org/ 10.1093/nar/gkz238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Beier S, et al. MISA-web: a web server for microsatellite prediction. Bioinformatics. 2017:33:2583–2585. https://doi.org/ 10.1093/bioinformatics/btx198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wickham H. ggplot2. WIREs Comput Stat. 2011:3:180–185. https://doi.org/ 10.1002/wics.147 [DOI] [Google Scholar]
- 43. Sokolova I. Mitochondrial adaptations to variable environments and their role in animals’ stress tolerance. Integr Comp Biol. 2018:58:519–531. https://doi.org/ 10.1093/icb/icy017 [DOI] [PubMed] [Google Scholar]
- 44. Katoh K, Misawa K, Kuma K, Miyata T.. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002:30:3059–3066. https://doi.org/ 10.1093/nar/gkf436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Price MN, Dehal PS, Arkin AP.. FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evol. 2009:26:1641–1650. https://doi.org/ 10.1093/molbev/msp077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Breton S, et al. Novel protein genes in animal mtDNA: a new sex determination system in freshwater mussels (Bivalvia: Unionoida)? Mol Biol Evol. 2011:28:1645–1659. https://doi.org/ 10.1093/molbev/msq345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Abdelkrim J, Robertson BC, Stanton J-AL, Gemmell NJ.. Fast, cost-effective development of species-specific microsatellite markers by genomic sequencing. Biotechniques. 2009:46:185–192. https://doi.org/ 10.2144/000113084 [DOI] [PubMed] [Google Scholar]
- 48. Selkoe KA, Toonen RJ.. Microsatellites for ecologists: a practical guide to using and evaluating microsatellite markers. Ecology Lett. 2006:9:615–629. https://doi.org/ 10.1111/j.1461-0248.2006.00889.x [DOI] [PubMed] [Google Scholar]
- 49. Selwood KE, McGeoch MA, Mac Nally R.. The effects of climate change and land‐use change on demographic rates and population viability. Biol Rev. 2015:90:837–853. https://doi.org/ 10.1111/brv.12136 [DOI] [PubMed] [Google Scholar]
- 50. Sousa R, et al. The role of anthropogenic habitats in freshwater mussel conservation. Global Change Biol. 2021:27:2298–2314. https://doi.org/ 10.1111/gcb.15549 [DOI] [PubMed] [Google Scholar]
- 51. West-Eberhard MJ. Developmental plasticity and the origin of species differences. Proc Natl Acad Sci USA. 2005:102:6543–6549. https://doi.org/ 10.1073/pnas.0501844102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang Z, Guo X, Allen SK, Wang R.. Heterozygosity and body size in triploid Pacific oysters, Crassostrea gigas Thunberg, produced from meiosis II inhibition and tetraploids. Aquaculture 2002:204:337–348. https://doi.org/ 10.1016/s0044-8486(01)00845-6 [DOI] [Google Scholar]
- 53. Takeuchi T, et al. Draft genome of the pearl oyster Pinctada fucata: a platform for understanding bivalve biology. DNA Res 2012:19:117–130. https://doi.org/ 10.1093/dnares/dss005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Li R, et al. The whole-genome sequencing and hybrid assembly of Mytilus coruscus. Front Genet. 2020:11:440. https://doi.org/ 10.3389/fgene.2020.00440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Penaloza C, et al. A chromosome-level genome assembly for the Pacific oyster Crassostrea gigas. GigaScience. 2021:10:giab020. https://doi.org/ 10.1093/gigascience/giab020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yang J-L, et al. Chromosome-level genome assembly of the hard-shelled mussel Mytilus coruscus, a widely distributed species from the temperate areas of East Asia. GigaScience. 2021:10:giab024. https://doi.org/ 10.1093/gigascience/giab024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cousins T, et al. Accurate inference of population history in the presence of background selection. bioRxiv. 2024, 2024.01.18.576291. https://doi.org/ 10.1101/2024.01.18.576291 [DOI] [Google Scholar]
- 58. Hilgers L, et al. Avoidable false PSMC population size peaks occur across numerous studies. Curr Biol. 2025:35:927–930.e3. e923. https://doi.org/ 10.1016/j.cub.2024.09.028 [DOI] [PubMed] [Google Scholar]
- 59. Wang G, Cao X, Li J.. Complete F-type mitochondrial genome of Chinese freshwater mussel Lamprotula tortuosa. Mitochondrial DNA 2013:24:513–515. https://doi.org/ 10.3109/19401736.2013.770508 [DOI] [PubMed] [Google Scholar]
- 60. Rodrigues AS, et al. The value of the IUCN Red List for conservation. Trends Ecol Evol 2006:21:71–76. https://doi.org/ 10.1016/j.tree.2005.10.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Illumina paired-end reads have been deposited in NCBI/BioProject PRJNA1301049. The whole-genome sequence data reported in this paper have been deposited in NCBI, under accession numbers (SAMN50434169, SAMN50434170, SAMN50434171, SAMN50434172, SAMN50434173, SAMN50434174, SAMN50434175, SAMN50434176). The mitochondrial genomes of these 8 species are available in Figshare (https://doi.org/10.6084/m9.figshare.29498255).