Abstract
In 2024, the Food and Drug Administration approved 47 new molecular entities (NMEs), including 15 therapeutic antibody-based molecules, marking the 30th anniversary of the first approved recombinant antibody. Ten of these were recombinant immunoglobulin G antibodies, while the rest comprised three bispecific antibodies, one immunocytokine, and one Fc-fusion protein. Seven antibodies targeted previously approved targets like programmed cell death receptor-1, programmed cell death receptor ligand-1, complement factor C5, interleukin (IL)-13, human epidermal growth factor receptor 2 (HER2) (biparatopic), and a novel form of amyloid-beta for conditions like esophageal squamous cell carcinoma, cutaneous squamous cell carcinoma, paroxysmal nocturnal hemoglobinuria, atopic dermatitis, biliary tract cancer, and Alzheimer’s disease, respectively. The other seven recognized novel targets such as activin for pulmonary arterial hypertension, IL-15Rβγ agonist for bladder cancer, delta-like ligand-3 × cluster of differentiation-3 for small cell lung cancer (SCLC), IL-31 receptor for prurigo nodularis, colony stimulating factor-1 receptor for graft-versus-host disease, tissue factor pathway inhibitor for Hemophilia A and B, and claudin 18.2 for gastric or gastroesophageal junction cancers. Additionally, a HER2–HER3 bispecific antibody was approved for non-SCLC and pancreatic adenocarcinoma. Three reformulated antibodies with hyaluronidase HP20 for subcutaneous administration were also approved, although not as New Molecular Entities (NME)s.
Keywords: therapeutic antibodies, bispecific antibodies, biparatopic antibody, new molecular entities, subcutaneous administration, US FDA
Statement of Significance Fifteen novel therapeutic antibodies were approved by the US FDA in 2024.
Introduction—antibodies approved by US FDA in 2024
The year 2024 marks the 30th anniversary of the approval of the first recombinant monoclonal antibody (mAb), abciximab (Reopro®), by the United States Food and Drug Administration (FDA). From 1986 to the end of 2024, the FDA has approved a total of 159 antibody-based biologics, with an average of 5.6 approvals per year starting in 1997 (Strohl, unpublished work [1]). This total includes 106 immunoglobulin G (IgG)-based antibodies, 6 antibody fragments, 15 Fc fusions or Fc-based proteins, 13 antibody drug conjugates (ADCs), 14 bispecific antibodies (BisAbs, of which nine are T-cell engagers, or TCEs), two antibody mixtures, and two radioimmunoconjugates. This total does not include the emergency use authorization (EUAs) for four anti-severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) antibodies (bebtelovimab, sotrovimab, casirivimab/imdevimab, and tixagevimab/cilgavimab) [2], which have since been rescinded, or pemivibart (Pemgarda™), which was approved under an EUA on 22 March 2024, and is still authorized under the EUA for prevention of infection in high-risk coronavirus disease-19 patients [3].
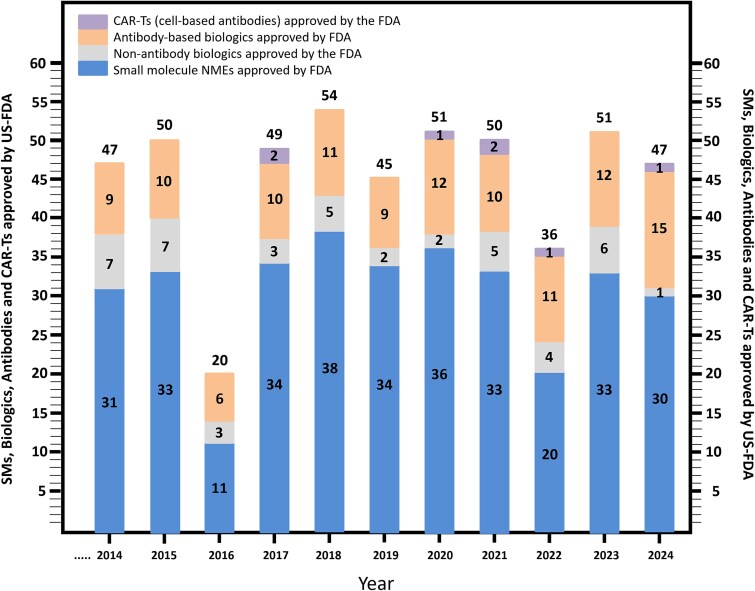
In 2024, the FDA approved 47 new molecular entities (NMEs), which included 29 small-molecule (SM) drugs, one peptide, one non-antibody biologic, one chimeric antigen receptor-T (CAR-T) cell based drug, and 15 antibody-based biologics [4–6] (Fig. 1). Additionally, five gene therapy or gene expression modulation-based molecules, two vaccines, and two other cell-based entities were approved by the FDA in 2024 [4–6]. A comparison of these 2024 FDA approvals with the ten previous years is shown in Fig. 1, indicating that 2024 was the strongest year ever for FDA approval of antibody-based drugs. Not only was the group of 15 antibody-based drugs (plus one antibody-targeted CAR-T [6]) approved in 2024 the new record for the highest number of antibodies approved by the FDA in a calendar year ever, but 15/47 total NMEs is the highest percentage (ca. 32%) of antibodies/total novel FDA-approved NMEs in a single calendar year (Fig. 1). Many of the antibodies approved by the FDA in 2024 had previously been identified as “Antibodies to Watch in 2024” [7]. Further information on antibodies approved in 2024, including antibodies approved by regulatory authorities outside the USA, can be found in Crescioli et al. [8].
Figure 1.
US FDA approvals for 2024 and the previous 1 year. Number of SMs, antibody-based biologics, non-antibody recombinant biologics (both CDER and CBER), and CAR-Ts approved by the US FDA on an annual basis from 2014 to 2024, was derived from references [1, 4–6]. Note that for the purposes of this figure, peptides were included with SMs, and gene modulation, gene therapy, vaccines, and diagnostics were not included, making this number slightly different than the official US FDA count [5].
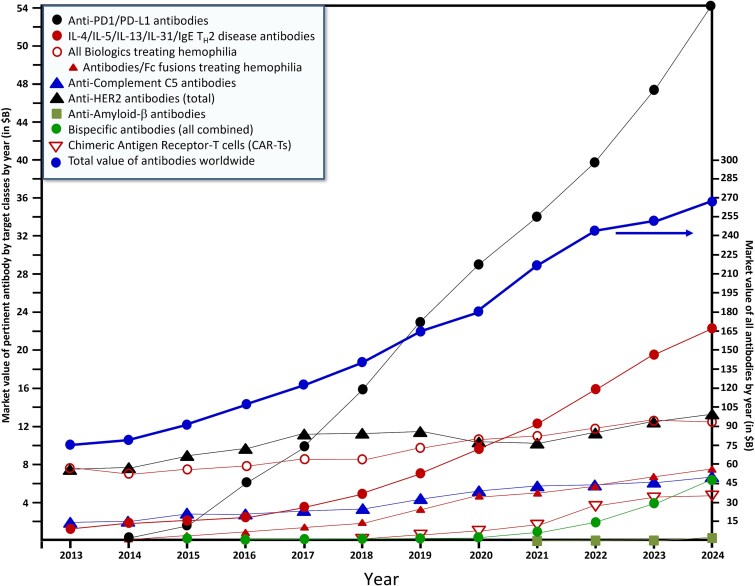
As noted above, Fig. 1 gives a snapshot of the number of FDA antibody approvals per year since 2014. From 1997 to 2013, the average number of novel antibodies approved by the FDA was 2.35 per year [9]. Over the past 11 years (2014–24), however, that number has increased to more than 10.4 novel antibody-based drugs approved per year. In the same period, the worldwide value of antibodies has increased from $65 billion (USD) to $267 billion (USD) [10–21] (Fig. 2), more than quadrupling in value, representing a nearly linear average increase in sales of roughly $18 billion (USD)/year (Fig. 2). Note that antibody sales figures represent a multi-year lagging indicator that cannot be used to accurately forecast the future. Nevertheless, models based on the current pipeline predict that the value of marketed antibodies worldwide will increase to ca. $581 billion (USD) by 2034, representing an ~8% forward compound annual growth rate (CAGR) [22].
Figure 2.
The historical value of markets from 2013–24 for antibodies approved in 2024. These include anti-PD-1/PD-L1 mAbs, mAbs with targets in the TH2 pathway (e.g. IL-4, IL-5, IL-13, IL-31, plus IgE, which acts in the TH2 pathway), all biologics approved for treatment of Hemophilia A and/or B, with a subset of antibody-based biologics approved for treatment of Hemophilia A and/or B, anti-C5 mAbs, anti-HER2 antibodies, anti-Aβ mAbs, BisAbs, and all approved CAR-Ts (all on left side numbers). The total market value of antibody-like biologics worldwide is also included (right side numbers). Markets for IL-2βγ agonists (e.g. IL-2, IL-15, or mutants thereof), antibodies used for treatment of graft versus host disease (GVHD), and antibodies used to treat SCLC were not included because they are historically of little market value. These data are derived from references [10–21].
Overview of 2024 FDA-approved antibody-based biologics
Targets and indications
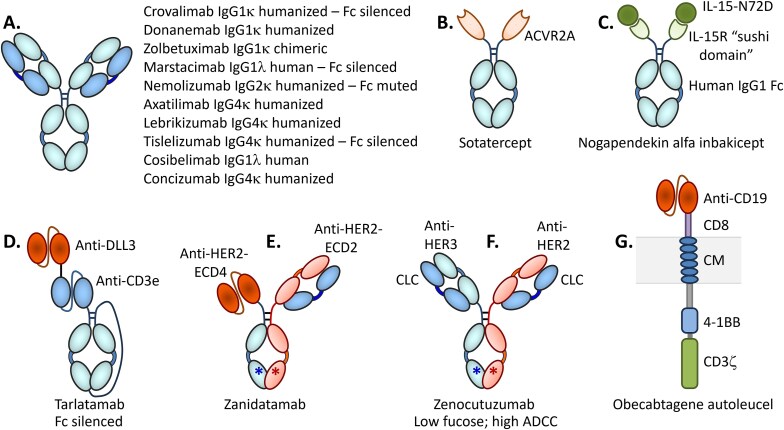
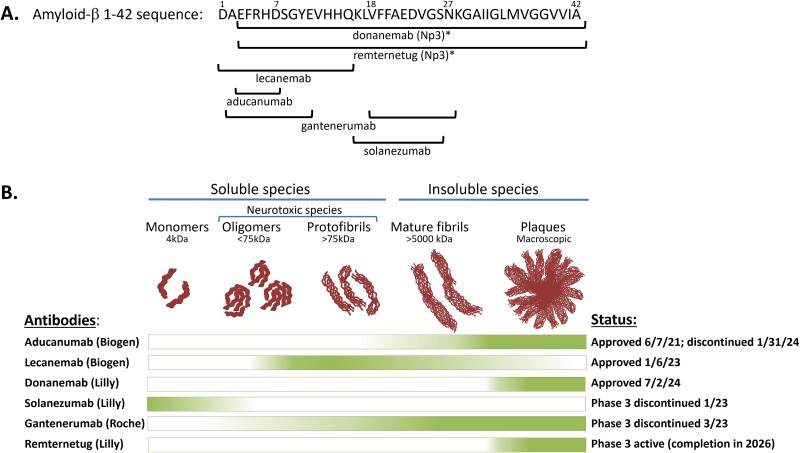
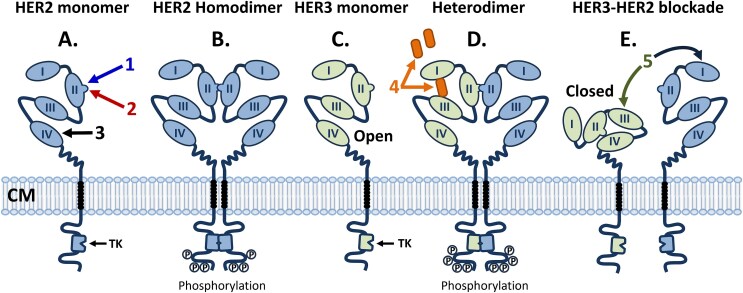
The 15 antibody-like drugs approved by the FDA in 2024 include molecules targeting both well-known pathways for which other molecules have been approved and novel targets. The known pathways include programmed cell death receptor-1 (PD-1), programmed cell death receptor ligand-1 (PD-L1), interleukin (IL)-13, amyloid-beta (Aβ), complement factor C5, and human epidermal growth factor receptor (HER2, aka erbB2; [erbB, “erythroblastic leukemia viral oncogene homologue”]). On the other hand, several of the new antibody-like biologics were directed against novel targets such as activin, IL-15 receptor subunits beta/gamma (IL-15Rβγ), delta-like ligand-3 (DLL3), IL-31 receptor, tissue factor pathway inhibitor (TFPI), colony stimulating factor-1 receptor (CSF-1R) and HER3 (aka erbB3) (Table 1, Fig. 3). The newly approved molecules include three BisAbs, including the TCE BisAb tarlatamab, which targets DLL3 and cluster of differentiation-3 (CD3) [23]; zenocutuzumab, which binds both HER2 and HER3 [24], and zanidatamab, which is a biparatopic antibody targeting two distinct epitopes on HER2 [25] (Table 1, Fig. 3). Zanidatamab is the first biparatopic antibody to be approved by the FDA, marking a new targeting format that will surely become more prevalent in the future.
Table 1.
Antibodies and antibody-based molecules approved by the US FDA in 2024
| Trade name (generic name) | Sponsor/ partner(s) | Date FDA approved | Molecular target | Approved Indication |
Potential marketa | Structure descriptionb | Refs |
|---|---|---|---|---|---|---|---|
| Tevimbra® (tislelizumab-jsgr) | BeiGene | 13 March 2024 Full approval |
PD-1 | ESCC, GAC, GEJ-AC |
$1.6B (2030) |
Mouse/human humanized IgG4κ; HC is 445aa with Fc silencing modifications: M113T, S226P, E231P, F232V, L233A, D263A, R407K. Humanized kappa LC is 214aa. | [4, 5, 33–35] |
| Winrevair® (sotatercept-csrk) | Merck (Acceleron) |
26 March 2024 Full approval |
Activin | PAH | $4.0B (“peak”) | Fc fusion protein (344 aa) with human activin receptor type 2A (ACVR2A) residues 21–135 fused to human IgG1 Fc hinge-CH2-CH3 domains via “TGGGT” linker; A227V modification. See Fig. 3B. | [4, 5, 36–38] |
| Anktiva® (nogapendekin alfa inbakicept-pmln) | ImmunityBio | 22 April 2024 Full approval |
IL-15Rβγ | NMIBC | $900 M (2028) | Two proteins: Protein 1 (“inbakicept”): human IL-15Rα residues 1-65aa “sushi domain” fused to 232aa C-terminal residues of human IgG1-Fc fusion protein (297aa total); Protein 2 (“nogapendikin alfa”): human IL-15 N72D mutant (114aa) with ca. 9.2 nM 1:1 affinity for Protein 1 (inbakicept). See Fig. 3C. | [4, 5, 39–42] |
| Imdelltra® (tarlatamab-dlle) | Amgen | 16 May 2024 Accelerated approvalc |
DLL3 × CD3 | SCLC | $2.0B (“peak”) |
Half-life Fc-extended BiTE (scFv-scFv-scFc) single-chain construct of 982aa: anti-DLL3 human scFv (κ LC) fused via linker to anti-CD3 scFv (λ LC), which is fused via linker to sc-Fc as shown in Fig. 3D. Fc is silenced and non-glycosylated. Fc modifications: R572C, N577G, V582C, R827C, N832G, and V837C. | [4, 5, 43–45] |
| PiaSky® (crovalimab-akkz) | Chugai (Roche) | 20 June 2024 Full approval |
C5 | PNH | $462 M (2030) | Rabbit/human humanized IgG1κ; HC is 451aa and engineered to be “recycling antibody” and Fc-silenced; R220K, L241R, G242R, S245K, A333G, A336S, P337S, M434L, N440A, Q444R, S446E, C-terminal DGK; Human kappa LC is 217aa. | [4, 5, 46–49] |
| Kisunla® (donanemab-abzt) | Eli Lilly | 2 July 2024 Full approval |
Ab 3–42, N3pG | ALZD | $5.0B (“peak”) |
Mouse/human humanized IgG1κ; HC is 444aa and C-terminal DK. Humanized kappa LC is 219aa. | [4, 5, 50–52] |
| Nemluvio® (nemolizumab-ilto) | Galderma (from Roche/ Chugai [53]) | 13 August 2024 Full approval |
IL-31 receptor | PN, AD | $2.1B (“peak”) |
Mouse/human humanized IgG2κ; HC is 445aa with modifications at C135S, R137K, E141G, S142G, C223S, H268Q, R355Q, Q419E; C-terminal DGK; Humanized kappa LC is 214aa. Reduced FcγR binding due to IgG2-H268Q. | [4, 5, 53–56] |
| Niktimvo® (axatilimab-csfr) | Incyte, Syndax | 14 August 2024 Full approval |
CSF-1R | GVHD | $1.0B (“peak”) |
Mouse/human humanized IgG4κ; HC is 453aa with S234P modification; Humanized kappa LC is 214aa. | [4, 5, 57–59] |
| Ebglyss® (lebrikizumab-lbkz) | Eli Lilly | 13 September 2024 Full approval |
IL-13 | AD | $2.8B (2032) |
Mouse/human humanized IgG4κ; HC is 445aa with S226P modification. Humanized kappa LC is 218aa. | [4, 5, 60–62] |
| Hympavzi® (marstacimab-hncq) | Pfizer | 11 October 2024 Full approval |
TFPI | Hemophilia A and B with inhibitors | $300 M (2030) |
Human IgG1λ; HC is 449aa with Fc silencing modifications at R217K, L237A, L238A, G240A; C-terminal DK. Human lambda LC is 218aa. | [4, 5, 63–65] |
| Vyloy® (zolbetuximab-cizb) | Astellas Pharma | 18 October 2024 Full approval |
Claudin 18.2 | HER2− GAC, HER2− GEJ AC | $750 M (2030) |
Mouse/human chimeric IgG1κ; HC is 448aa; Chimeric kappa LC is 220aa. | [4, 5, 66–68] |
| Ziihera® (zanidatamab-hrii) | Jazz Pharma-ceuticals (Zymeworks) |
20 October 2024 Accelerated approvalc | HER2-HER2 biparatopic, bispecific | Her2+ BTC | $120 M (2030) |
Mouse/human humanized bispecific, biparatopic IgG1κ targeting two different, non-overlapping epitopes on HER2 exodomain; HC1 is 481aa in Vκ-20-mer linker-VH-CH2-CH3 configuration with modifications at: C255S, T385V, T401L, K427L, T429W, C-terminal DK. HC2 is 449aa with modifications at: T353V, L354Y, F408A, Y410V, C-terminal DK. Humanized kappa LC is 215aa. See Fig. 3E. | [4, 5, 24, 69–71] |
| Bizengri® (zenocutuzumab-zbco) |
Merus | 4 December 2024 Accelerated approvalc |
HER2-HER3 bispecific | NRG-1+ NSCLC, PAC | $462 M (“peak”) |
Mouse/human humanized bispecific IgG1κ; HC1, targeting HER3, is 453aa with modifications at: L358K, T373K, C-terminal DK. HC2, targeting HER2, is 450aa with modifications at: L355D, L372E, C-terminal DK. Human kappa common LC is 214aa. See Fig. 3F. | [4, 5, 25, 72–74] |
| Unloxcyt® (cosibelimab-ipdl) | Checkpoint Therapeutics, Inc | FDA approved 13 December 2024 |
PD-L1 | laCSCC, mCSCC | $1.6B (“peak”) |
Human IgG1λ; HC is 450aa; Human lambda LC is 218aa. No Fc modifications. | [4, 5, 75–77] |
| Alhemo® (concizumab-mtci) | Novo Nordisk | FDA approved 20 December 2024 |
TFPI | Hemophilia A and B with inhibitors | NE | Mouse/human humanized IgG4κ; HC is 448aa with modifications at: M116T, S229P. Humanized kappa LC is 219aa. | [4, 5, 78–80] |
| Aucatzyl® (obecabtagene autoleucel) | Autolus | FDA approved 8 November 2024 | CD19 | R/R B-ALL | NE | CAR-T (anti-CD19 scFv, CD8 stalk, transmembrane domain, 4-1BB, CD3ε) See Fig. 3G. |
[6, 81–83] |
Abbreviations: aa, amino acid residues. Aβ, amyloid beta. AC, adenocarcinoma. AD, atopic dermatitis. ADC, antibody-drug conjugate. ALZD, Alzheimer’s disease. ANPC, advanced nasopharyngeal carcinoma. B-ALL, B-cell acute lymphoblastic leukemia. BiTE, bispecific T-cell engager. BTC, biliary tract cancer. CAR-T, chimeric antigen receptor-T cell. CH, constant heavy (domain). CSF-1R, colony-stimulating factor-1 receptor. DLL3, delta-like ligand-3. ESCC, unresectable or metastatic esophageal squamous cell carcinoma. GAC, gastric adenocarcinoma. GEJ, gastroesophageal junction. GVHD, graft-versus-host disease. HER2, human epidermal growth factor receptor 2. HC, heavy chain. HER, human epidermal growth factor receptor. IL, interleukin. laCSCC, locally advanced cutaneous squamous cell carcinoma. LC, light chain. mAb, monoclonal antibody. mCSCC, metastatic cutaneous squamous cell carcinoma. N3pG, N-terminal pyro-glutamate. NE, no estimate given. NMIBC, non-muscle-invasive bladder cancer. NRG-1, neuregulin-1. NSCLC, non-small cell lung cancer. PAC, pancreatic adenocarcinoma. PAH, pulmonary arterial hypertension. PD, programmed cell death protein. PN, Prurigo nodularis. PNH, Paroxysmal nocturnal hemoglobinuria. Refs, references. R/R, relapsing/refractory. SC, subcutaneous. sc-Fc, single chain fragment, crystallizable. scFv, single chain, fragment variable. SCLC, small cell lung cancer. TFPI, tissue factor pathway inhibitor.
afrom Fierce [84].
bUnless otherwise specified, amino acid residue numbers cited in this table are those found in the International Nonproprietary Names (INN) sequence and are specific for each antibody (rather than using standard Eu numbering).
c[5].
Figure 3.
Cartoon showing the generalized structures of the 15 new antibody-based biologics and one CAR-T-based antibody approved by the US FDA in 2024. A. Ten of the antibodies are canonical IgGs, three of which have been engineered to significantly reduce binding to FcγRs and C1q (i.e. “silenced”) and one engineered to reduce already minimal FcγRIIα binding. B. Sotatercept is a homodimeric human activin receptor-2A (ACVR2A)-fc fusion protein of ca. 78 kDa [36, 93]; C. Nogapendekin alfa inbakicept is a human IL-15Rα “sushi domain” Fc fusion protein complexed non-covalently with a human IL-15N72D mutant [39, 40, 94]; D. Tarlatamab is a TCE single chain scFv-ScFv-scFc format BisAb in which one scFv binds DLL3 and the other binds CD3ε [28, 43]; E. Zanidatamab is a BisAb constructed with a heterodimeric Fc with one arm occupied by a single chain and the other arm occupied by a Fab domain, each targeting different, non-overlapping epitopes (E1 and E2) on the HER2 exodomain [24, 69]; F. Zenocutuzumab is a BisAb constructed with a heterodimeric Fc with one Fab arm binding HER2 and the other binding HER3, each Fab possessing a CLC [72, 95]; G. Obecabtagene autoleucel is an autologous CAR-T cell containing an anti-CD19 scFv fused to a CD8 stalk, a cytoplasmic membrane (CM)-traversing domain, a 4-1BB activation module, and a CD3ζ module [81, 82, 96].
Nine out of the 15 antibody-based biologics approved by the FDA in 2024 recognized eight new targets for which antibody-based biologics have not been previously approved, including activin, IL-15Rβγ, DLL3, IL-31 receptor, CSF-1R, HER3, claudin 18.2, and two antibodies targeting TFPI (Table 1). Thus, significant new biology was added in 2024 to FDA-approved indications. The first of these is Winrevair® (sotatercept-csrk), a human activin receptor type 2A (ACVR2A)-Fc fusion protein approved for the treatment of pulmonary arterial hypertension (PAH), the first biologic of any kind to be approved for that indication [26]. The second was Anktiva® (nogapendekin alfa inbakicept-pmln), a complex of two proteins, including a mutated form of IL-15 complexed with an IL-15 receptor sushi domain-Fc fusion protein, designed for the targeting of IL-15Rβγ without interaction with IL-15Rα (CD25) [27]. Anktiva® was approved for the treatment of bladder cancer, a significant unmet medical need. The next novel target was addressed with Imdelltra®, a DLL3 × CD3 half-life extended bispecific TCE (BiTE) antibody, constructed entirely in a single chain protein format [28], for the novel treatment of small cell lung cancer (SCLC), another significant medical need [29]. Vyloy® (zolbetuximab-cizb), which targets the cell surface protein, claudin 18.2, was approved for the treatment of HER2-negative gastric cancer (GC) and gastroesophageal junction adenocarcinoma (GEJ-AC) [30]. This is noteworthy as zolbetuximab is the first antibody approved for targeting any of the claudin family of receptors, and one of the relatively few new antibodies against solid tumor targets [31]. Finally, two new antibodies were approved by the FDA in 2024 that target the protein TFPI for prevention of bleeding episodes in patients with Hemophilia A and B. Hympavzi® (marstacimab-hncq) is an Fc-silenced IgG1λ antibody approved for once-weekly (QW) dosing for prevention of bleeding from Hemophilia A and B without inhibitors [32], whereas Alhemo® (concizumab-mtci) was approved for subcutaneous (SC) daily dosing for prevention of bleeding episodes in patients with Hemophilia A and B, even in the presence of inhibitors (anti-factor VIII [FVIII] or IX [FIX] antibodies) [32]. The eight new targets noted above equals the year 2014 (in which antibody-based biologics for the then-new eight targets PD-1, CD19, α4β7, FVIII, Factor Xa (FXa), VEGFR2, glucagon-like peptide (GLP)-1R, and IL-6 were FDA approved) for the greatest number and diversity of new antibody targets in any given year thus far. On the other hand, six of the 15 newly approved antibody-based biologics targeted proteins for which there are already approved drugs, including PD-1, PD-L1, IL-13, HER2, Aβ, and C5 [1].
Structural features of the newly approved antibody-based biologics
Of the 15 novel antibody-based proteins approved by the FDA in 2024, five are IgG1 antibodies (Table 1, Fig. 3), one was an IgG2 isotype, and four were IgG4 isotype antibodies. All of the IgG4 antibodies approved in 2024 possess the S228P mutation (Eu numbering) [85–87] to prevent half-antibody formation [86]. Two of the antibodies were derived from human sources, seven were humanized, and one was a chimeric mouse-human-derived antibody. One of the humanized antibodies, crovalimab, was derived from a rabbit [48].
Five of the antibody-like biologics approved by the FDA in 2024 were engineered to be “silenced” or “muted” for FcγR/C1q binding to limit interaction of the Fc with immune cells (Table 1). These include the anti-PD-1 IgG tislelizumab, the anti-C5 mAb crovalimab, the DLL3 × CD3 bispecific TCE tarlatamab, the anti-IL-31R antibody nemolizumab, and the anti-TFPI antibody, marstacimab (Table 1, Fig. 3). As noted previously [88, 89]), several forms of IgG Fc silencing have been introduced into therapeutic candidates since 2007 to reduce the potential for undesired interactions with Fcγ receptors (FcγRs) and/or C1q [88–92]. The total number of Fc-muted antibody-based proteins has reached 29 out of the 159 total approved by the FDA by the end of 2024 [1]. Thus, nearly 18% of all FDA-approved antibodies and Fc fusion proteins have modified hinge/Fc regions to reduce Fc/complement interactions, almost triple the number of 10 FDA-approved Fc-enhanced mAbs [1], or more than the 13 FDA-approved ADCs and 14 FDA-approved BisAbs (Table 2) combined, indicating the importance of Fc-silencing technologies to the field [88, 89].
Table 2.
Fourteen bispecific antibodies approved by the US FDA, half of which have been approved over the past 2 years
| Antibody | Sponsor | FDA approval | Targets | Primary indication |
Format | Comment and reference |
|---|---|---|---|---|---|---|
| Blincyto® (blinatumomab) | Amgen (Micromet) | 3 December 2014 | CD19 × CD3 TCE | B-ALL | Mouse BiTE – scFv-linker-scFv bispecific fragment | First FDA approval of TCE; Very short half-life [97] |
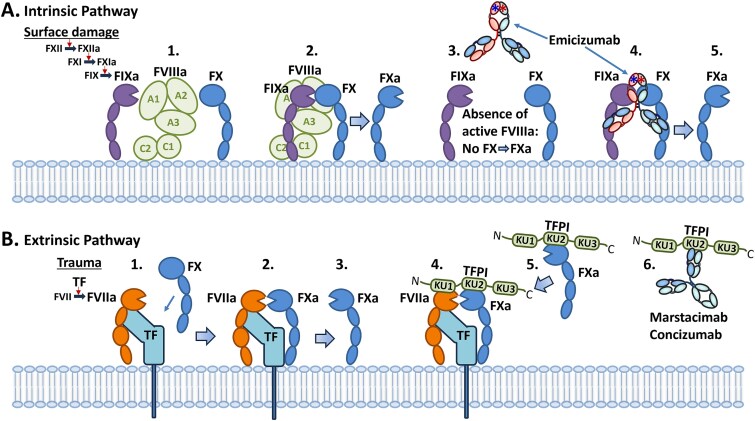
| Hemlibra® (emicizumab) | Chugai (Roche) | 16 November 2017 | Factor IXa × FXa | HA/FI | Humanized heterodimeric IgG4κ; CLC | Mimics Factor VIII activity [98] |
| Rybrevant® (amivantamab) | Janssen R&D (JNJ) | 21 May 2021 | EGFR × cMET | NSCLC | Human heterodimeric IgG1κ Duobody | Low fucose for improved ADCC activity [99] |
| Kimmtrak® (tebentafusp)a | Immunocore | 25 January 2022 | Gp100 × CD3 TCE | MMEL | Gp100-specific TCR fused to anti-CD3 scFv | HLA-A2 restricted [100] |
| Vabysmo® (faricimab) | Genentech/ Roche | 28 January 2022 | Ang2 × VEGF-A | Wet AMD | Humanized Bispecific IgG1 “Crossmab antibody”; KIH and Fab domain exchange | Silenced isotype IgG1 LALA-P329G [101] |
| Tecvayli® (teclistamab) | Janssen R&D (JNJ) | 25 October 2022 | BCMA × CD3 TCE | MM | Humanized heterodimeric IgG4PAA Duobody | Silenced isotype [102] |
| Lunsumio® (mosunetuzumab) | Genentech (Roche) | 22 December 2022 | CD20 × CD3 TCE | R/R FL | Humanized heterodimeric IgG1κ | Asymmetric IgG assembled post-production [103] |
| Epkinly® (epcoritamab) | Genmab/AbbVie | 19 May 2023 | CD20 × CD3 TCE | R/R DLBCL | Human heterodimeric IgG1κ Duobody | Human IgG1k (CD20 side) x chimeric IgGl (CD3 side) [104] |
| Columvi® (glofitamab) | Roche | 15 June 2023 | CD20 × CD3 TCE | DLBCL | Trimeric bispecific with 2 arms binding CD20 and 1 arm binding CD3 (2:1) | Uses crossmab technology [105] |
| Talvey® (talquetamab) | Janssen R&D (JNJ) | 9 August 2023 | GPRC5D × CD3 TCE | MM | Humanized heterodimeric IgG4PAA Duobody | Silenced isotype [106] |
| Elrexfio® (elranatamab) | Pfizer | 14 August 2023 | BCMA × CD3 TCE | MM | Human heterodimeric IgG2 | [107] |
| Imdelltra® (tarlatamab) | Amgen | 16 May 2024 | DLL3 × CD3 TCE | SCLC | Bispecific half-life-extended sc-Fc-BiTE | See Fig. 3 [28] |
| Ziihera® (zanidatamab) | Jazz Pharmaceuticals | 20 November 2024 | HER2 × HER2 | NBC | Bispecific, biparatopic Ab; Fab arm x scFv arm on asymmetric Fc | See Fig. 3 [24] |
| Bizengri® (zenocutuzumab) | Merus | 4 December 2024 | HER2 × HER3 | NRG1+ NSCLC, PAC | Human biclonics heterodimeric IgG1κ | CLC, low fucose for improved ADCC; See Fig. 3 [108] |
Abbreviations: ADCC, antibody-dependent cellular cytotoxicity. aka, also known as. AMD, age-related macular degeneration. Ang-2, angiopoietin-2. B-ALL, B-cell acute lymphoblastic leukemia. BCMA, B-cell maturation antigen. BiTE, bispecific T-cell engager (scFv-linker-scFv design). CD, cluster of differentiation. CLC, common light chain. cMet, receptor tyrosine kinase mesenchymal-epithelial transition factor; DLBCL, Diffuse large B-cell lymphoma. DLL3, delta-like ligand-3. EGFR, epidermal growth factor receptor. FL, follicular lymphoma. GPRC5D, G-coupled protein receptor class C, group 5, member D. HA/FI, treatment for Hemophilia A in patients with or without factor VIII inhibitors. HER, human epidermal growth factor receptor. HLA-A2, human leukocyte antigen-A2. IgG4-PAA, IgG4 isotype with mutations of S228P, F234A, L235A (Eu numbering) [109] to reduce Fc effector function. JNJ, Johnson & Johnson. KIH, knobs-into-holes bispecific antibody technology [110]. LALA, L234A, L235A (Eu numbering) [109]. MM, multiple myeloma. MMEL, malignant melanoma. NBC, nonresectable biliary cancer. NRG-1, neuregulin-1. NSCLC, non-small cell lung cancer. PAC, pancreatic adenocarcinoma. R&D, research and development. R/R, relapsing/refractory. sc-Fc, single chain fragment, crystallizable. scFv, single chain, fragment variable. SCLC, small cell lung cancer. TCE, T-cell engager. TCR, T-cell receptor. VEGF-A, vascular endothelial growth factor-A.
aKimmtrak® is not officially a bispecific antibody, but rather a TCR × anti-CD3 T-cell engaging antibody. Considering the similarity in structure and function between antibodies and TCR α- and β- subunits, and that it is paired with an anti-CD3 scFv, it is being included with bispecific antibodies here.
Three of the antibodies approved by the FDA in 2024 were BisAbs, one of which was a TCE (Table 1, Table 2). Prior to 2022, the FDA had approved only three therapeutic BisAbs, only one of which was a TCE, in which the T-cell receptor (TCR) subunit CD3ε was bound by a BisAb also targeting a tumor surface antigen to promote synapse-dependent, T-cell-mediated killing (Table 2) (31,64). With the 11 new BisAbs approved over the past 3 years, i.e. 2022 [111], 2023 [9] and 2024 (Table 1), 14 BisAbs have now been approved, nine of which are TCEs and another five that are non-TCE BisAbs (Table 2). Moreover, the number of BisAbs approved by the FDA has doubled from seven to 14 in just the past 2 years, indicating that this antibody format has finally come of age [112–117].
Finally, three additional BisAbs have been approved in countries outside the USA, including ivonescimab (PD-1 × VEGF) and candonilimab (PD-1 × CTLA-4), both of which have been approved in China, and odronextamab (CD20 × CD3), which has been approved in Europe. Of these, ivonescimab has made the greatest stir when it provided significantly greater progression-free survival (PFS) than pembrolizumab in the treatment of patients with PD-1-positive non-small cell lung cancer (NSCLC) [118].
Potential valuations of the newcomers
As shown in Fig. 2, the total market for antibody-like biologics in 2024 was estimated to be about $270 billion [21]. The combined class of 2024 antibody-based biologics is projected to add about $23 billion to the total market value of biologics by around 2030 (Table 1). By far, the largest fractional value is represented by the PD-1/PD-L1 target class of antibodies, which in 2024 totaled about $54 billion in sales value (Fig. 2) [21] and includes 11 FDA-approved members (Table 3). No other target class of antibodies has ever come close to this valuation, with the closest being the total target class of anti-TNF-α antibodies in 2017, led by Humira®, which reached combined peak sales of $39.8 billion [14]. At peak sales (typically around the fifth year after launch [119]), both anti-PD-1 tislelizumab and anti-PD-L1 cosibelimab are projected to reach around $1.6 billion [84] (Table 1).
Table 3.
FDA-approved anti-PD-1/PD-L1 antibodies
| Antibody | Sponsor | FDA approval | Format | Target/ Epitope | Affinity KD (nM) |
Indications approved | Refs |
|---|---|---|---|---|---|---|---|
| Keytruda® (pembrolizumab) | Merck | 4 September 2014 | Humanized IgG4κ, S > P; functional Fc | PD-1; primarily C’D loop (D85 critical) | 0.027 | BTC, CC, CRC, CSCC, dMMR, EC, EMC, HCC, HL, GC, HNSCC, MCC, MM, MPM, NSCLC, PMBCL, RCC, TMB-HC, TNBC, UC | [120–124] |
| Opdivo® (nivolumab) | BMS | 22 December 2014 | Human IgG4κ, S > P; functional Fc | PD-1; N-loop (unique), FG, BC loops | 4.1 | CRC, EC, GC, GEC, HCC, HL, HNSCC, MM, MPM, NSCLC, RCC, UC | [120, 122,123, 125] |
| Tecentriq® (atezolizumab) | Roche/ Genentech | 18 May 2016 | Humanized IgG1κ; N298A (non-glycosylated Fc); Fc function muted |
PD-L1; BC, CC’, C’C″, FG loops | 0.4 | ASPS, HCC, MM, NSCLC, SCLC | [120, 126,127] |
| Bavencio® (avelumab) | Merck KGaA (EMD Serono) | 23 March 2017 | Human IgG1λ; functional Fc | PD-L1; CC’ loop | 0.04 | MCC, RCC, UC | [126, 128] |
| Imfinzi® (durvalumab) | AstraZeneca | 1 May 2017 | Human IgG1κ; L238F, L239E, P335S; Fc function muted | PD-L1; N-terminal region, CC’ loop | 0.67 | BTC, dMMR-EMC, HCC, NSCLC, SCLC | [120, 126,129] |
| Libtayo® (cemiplimab) | Regeneron/ Sanofi | 28 September 2018 | Human IgG4κ, S > P; functional Fc | PD-1; primarily BC loop; also C’D, FG loops | 0.60 | BCC, CSCC, NSCLC | [123, 130] |
| Jemperli® (dostarlimab) | GSK | 22 April 2021 | Human IgG4κ, S > P; functional Fc | PD-1; primarily C’D loop; also BC and FG loops | 0.30 | dMMR-EC, dMMR tumors | [123, 131] |
| Zynyz® (retifanlimab) | Incyte/ Macrogenics | 22 March 2023 | Humanized IgG4κ, S > P; functional Fc | PD-1; FG, C’D, and BC loops | 0.60 | MCC | [132, 133] |
| Loqtorzi® (toripalimab) | Coherus BioSciences | 27 October 2023 | Humanized IgG4κ, S > P; functional Fc | PD-1; FG loop | 0.32 | NPC | [134, 135] |
| Tevimbra® (tislelizumab) | BeiGene | 13 March 2024 | Humanized IgG4κ, S > P; E231P, F232V, L233A, D263A, R407K; Fc function muted | PD-1; CC‘loop (unique), FG, C’D loops | 0.13 | ESCC, HER2-negative GC, GEC | [34, 35, 136] |
| Unloxcyt® (cosibelimab) | Checkpoint Therapeutics | 13 December 2024 | Human IgG1λ; functional Fc | PD-L1; unknown (blocks both PD-1 and B7.1 binding) | 0.85 | CSCC | [76, 137] |
Notes: S > P, denotes S228P (Eu numbering) hinge stabilizing modification [85, 109].
Abbreviations: ASPS, Alveolar soft part sarcoma. BCC, basal cell carcinoma. BMS, Bristol Myers Squibb. BTC, biliary tract cancer. CC, cervical cancer. CRC, colorectal cancer (mismatch repair deficient cancer [microsatellite instability-high]). CSCC, cutaneous squamous cell carcinoma. dMMR, mismatch repair deficient cancer (microsatellite instability-high). EC, esophageal cancer. EMC, endometrial carcinoma. ESCC, esophageal squamous cell carcinoma. GC, gastric cancer. GEC, gastroesophageal junction cancer. GSK, GlaxoSmithKline. HCC, hepatocellular carcinoma. HER2, human epidermal growth factor receptor 2. HL, Hodgkin lymphoma (classical). HNSCC, head and neck squamous cell carcinoma. MCC, Merkel cell carcinoma. MM, metastatic melanoma. MPM, malignant pleural mesothelioma. NPC, nasopharyngeal carcinoma (advanced). NSCLC, non-small cell lung cancer. PMBCL, primary mediastinal large B-cell lymphoma. RCC, renal cell carcinoma. Refs, references. SCLC, small cell lung cancer. TMB-HC, tumor mutational burden-high cancer. TNBC, triple-negative breast cancer. UC, urothelial cancer.
The antibody of class 2024 with the greatest projected value is the anti-Aβ N3pG antibody, donanemab-abzt (Kisunla®), which is projected to have annual peak sales of ca. $5 billion for the treatment of Alzheimer’s disease (AD) (Table 1) [84]. This is significant because, as of the end of 2024, the total sales of anti-Aβ antibodies were a fledgling $225 million [21]. The next highest projected valuation comes from Merck’s sotatercept, which is expected to reach peak annual sales of $4 billion for the treatment of PAH (Table 1) [84]. Merck acquired Acceleron, the small biotech originator of sotatercept, for $11.5 billion in 2021 [138], which potentially makes that a very good investment if the projection holds true.
A recent analysis projects that the current cost of discovering and developing any type of drug in today’s market averages ca. $2.23 billion [139], typically spent over an 8–12 year period from discovery to launch. Excluding the “runaway” GLP-1 market (which significantly skews the data), the average peak annual sales for newly approved drugs is $370 million [84]. Of the 14 antibodies and antibody-based biologics approved by the FDA in 2024 for which projected peak sales are provided (Table 1) [84], all but two of the antibody-like biologics with projected estimates (anti-TFPI marstacimab-hncq [projected $300 million peak sales for Hemophilia A and B] and anti-HER2 × HER2 BisAb zanidatamab-hrii [projected $120 million peak sales for HER2+ biliary tract cancer {BTC}]) are projected to exceed the calculated average [84].
TEVIMBRA® (TISLELIZUMAB-JSGR) (anti-PD-1) and UNIOXCYT® (COSIBELIMAB-IPDL) (anti-PD-L1)
PD-1 and PD-L1 checkpoint targets
PD-1 (aka PCD1, CD279) was first described in detail by Tasuku Honjo and his colleagues in 1992 [140]. PD-L1 (aka B7-H1, CD274) was first discovered and named B7-H1 in 1999 [141], and soon after, it was demonstrated to be a binding partner for PD-1 [142]. PD-1 is found on activated T- and B-lymphocytes, whereas its ligands, PD-L1 and PD-L2 (B7-DC) [143], are typically expressed on antigen-presenting cells (APCs) [144]. Under normal circumstances, PD-L1 or PD-L2 binding to PD-1 constrains T-cell-mediated immune responses, such as T-cell proliferation and cytokine production, to avoid over-stimulation of the immune system, leading to autoimmune reactions [144]. PD-L1 is also overexpressed on many tumor cell types, where it plays a role in suppression of anti-tumor T-cell responses [145].
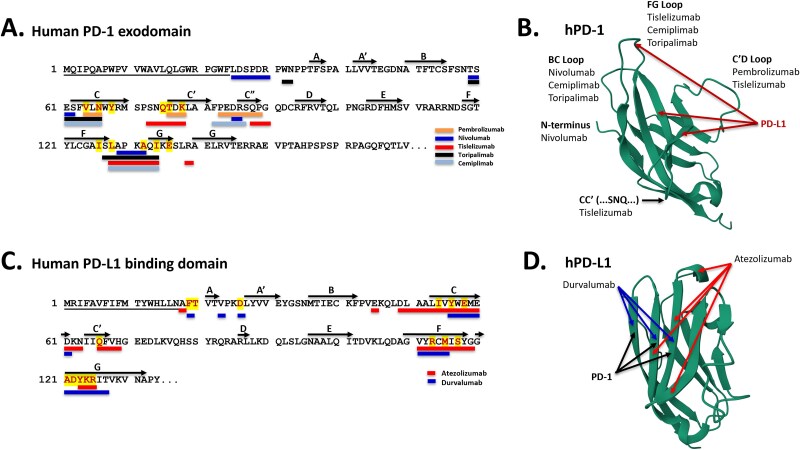
PD-1 binds to the N-terminal domain of PD-L1 with a native 1:1 affinity of 8.2 mM, and the buried surface area in that interaction is 1970 Å2 [120]. Most of the anti-PD-1 and anti-PD-L1 antibodies developed and approved for the treatment of various forms of cancer are sub- to low-nanomolar binders to PD-1 or PD-L1 [120] (Table 3), and all of the FDA-approved anti-PD-1 and anti-PD-L1 antibodies possess epitopes that overlap with the binding of the ligand, PD-L1, and the receptor, PD-1, respectively (Fig. 4).
Figure 4.
Sequences and structures of the binding domains of PD-1 and PD-L1. A. Sequence of the binding domain of human PD-1 (Genbank UMM61402.1) showing the residues to which PD-L1 binds (differentially colored) as well as the epitopes to which five anti-PD-1 antibodies (pembrolizumab, nivolumab, tislelizumab, toripalimab, ceplimimab) bind (color coded bars) [120–123]. The major sheets are represented by capital letters, and the N-terminal signal sequence is underlined. B. Structure of human PD-1 (protein DataBank 2M2D) [146, 147], in which the major significant loops are noted and both PD-L1 and antibody binding sites are shown [120–123]. C. Sequence of the binding domain of human PD-L1 (Genbank AAH69381.1) showing the residues to which PD-1 binds (differentially colored) as well as the epitopes to which two anti-PD-L1 antibodies (atezolizumab, durvalumab) bind (color-coded bars) [126]. The major sheets are represented by capital letters and the N-terminal signal sequence is underlined. D. Structure of human PD-L1 binding domain (protein DataBank 5X8M) [146, 148], in which the major binding sites for PD-1, durvalumab, and atezolizumab are noted by arrows [126].
The first FDA-approved anti-PD-1 mAb was Merck’s pembrolizumab (Keytruda®), which was approved in September 2014 for the treatment of metastatic melanoma [124]. Pembrolizumab is now approved in the USA for 20 different indications (Table 3), with worldwide sales of nearly $30 billion [21]. Nivolumab (Opdivo®), the second FDA-approved anti-PD-1 mAb, was approved by the FDA in December 2014 for the treatment of metastatic melanoma [125]. The first anti-PD-L1 antibody to be approved by the FDA was atezolizumab (Tecentriq®), which was approved in May 2016 for the treatment of bladder cancer [126].
Including the anti-PD-1 and anti-PD-L1 antibodies described here, a total of seven anti-PD-1 antibodies and three anti-PD-L1 antibodies have now been approved by the FDA for several oncology indications (Table 3). As of the end of 2023, the combined PD-1/PD-L1 worldwide market was valued at about $48 billion/year, which comprised about 16% of all antibody sales worldwide [21]. This market is expected to continue its dominance since at least 18 mono- or bispecific anti-PD-1 antibodies are being studied in late-stage (Phase IIb, Phase III) clinical trials [1]. The projected peak sales for tislelizumab and cosibelimab are estimated to each be in the $1.6B annual gross income range (Table 1).
Esophageal squamous cell carcinoma
As of 2020, esophageal cancer (EC) is the sixth most common cause of death by cancer worldwide, with over 544 000 deaths [149]. EC is comprised of two subtypes, esophageal squamous cell carcinoma (ESCC), strongly linked with smoking and alcohol consumption, and esophageal adenocarcinoma (EAC), linked with Barrett’s esophagus, gastroesophageal reflux disease, obesity, and smoking [149]. Of these two forms, ESCC comprises ~85%, or over 500 000 cases per year, of all EC [149]. Beyond surgical intervention, first-line treatment of ESCC has historically included platinum/fluoropyrimidine-based systemic chemotherapy, with or without immunotherapy with checkpoint inhibitors [150]. Both pembrolizumab and nivolumab have been approved with chemotherapy as a first-line treatment for ESCC. In the EU, the treatment is specifically for high PD-L1 expressing ESCC, whereas in the USA, the anti-PD-1 therapies are used with chemotherapy regardless of PD-L1 expression [151].
Tevimbra (tislelizumab-jsgr) approval for treatment of ESCC
Tislelizumab-jsgr (Tevimbra®), sponsored by BeiGene, is the most recent anti-PD-1 mAb to be approved for use to treat cancer. Tislelizumab (aka BGB-A317) is a humanized IgG4κ mAb engineered to minimize binding to FcγRs [152] (Table 1). Engagement of tislelizumab to the high-affinity receptor, FcγRI, was shown to induce crosslinking of PD-1-positive T-cells and FcγRI-positive macrophages, significantly reducing the anti-PD-1 activity of the antibody [152]. As a result, the antibody Fc was silenced to reduce FcγR binding and resulting crosslinking activity [152]. Even though both pembrolizumab and nivolumab are human IgG4s that bind FcγRI, it is currently believed that binding to FcγRs is not required for anti-PD-1 activity, and may be a liability with certain anti-PD-1 mAbs [9, 152–154]. Tislelizumab binds to the CC’, C’D, and FG loops as well as CC’FG b-sheets of PD-1. Binding to the CC’ loop is unique amongst anti-PD-1 antibodies and competes directly with PD-L1 binding (Fig. 4, Table 3). The 1:1 affinity of tislelizumab for PD-1 is 0.114 nM, and the buried surface area upon binding PD-1 is 2014 Å2 [155]. Importantly, the 1:1 dissociation rate (KD) of tislelizumab from PD-1 is 4.82 x 10−5 s−1, which is about 30-fold and 80-fold slower than the 1:1 dissociation rates of nivolumab and pembrolizumab, respectively, resulting in a >30–80-fold higher off-rate half-life compared with the other two antibodies [155]. The threshold for optimal activity of an anti-PD-1 mAb was recently demonstrated to be ca. 300 pM, with greater improvements in affinity not resulting in greater efficacy [156]. Additionally, single-arm binders were shown to be just as efficacious as bivalent binders in vitro, even though bivalent binders should have a pharmacological advantage in real-life dosing [156].
Tislelizumab was first approved in December 2019 in China by the National Medical Products Administration (NMPA) [157] and is now approved in China for at least 10 separate indications [158]. Additionally, tislelizumab was approved by the European Commission in 2023 for the treatment of advanced or metastatic ESCC. Tislelizumab was given full approval by the FDA in March 2024, as monotherapy for the treatment of unresectable or metastatic ESCC in patients who had been previously treated with chemotherapy but had not received prior treatment with immune checkpoint inhibitors. The approval was based on the Phase III clinical trial, RATIONALE-502 (NCT03430843) [159], in which 512 patients across 132 research sites and 11 countries with unresectable or metastatic ESCC were treated [160]. Treatment with tislelizumab resulted in a median overall survival (mOS) of 8.6 mo compared to 6.3 mo in the chemotherapy arm [160].
Additionally, in late December 2024, tislelizumab was granted approval, in conjunction with chemotherapy, for the treatment of HER2-negative GC or GEC AC [161], after showing significant survival benefits [162]. This first line approval was given based on the RATIONALE-305 study demonstrated a 20% reduction in death risk with tislelizumab, achieving a mOS of 15.0 mo versus 12.9 mo for placebo controls [162].
Tislelizumab has a clearance at steady state of 0.153 L/d, which is lower than each of the six other FDA-approved (pembrolizumab [124], nivolumab [125], cemiplimab [130], dostarlimab [131], retifanlimab [133], toripalimab [135]) anti-PD-1 mAbs (range of 0.17–0.31 L/d) and a terminal steady state half-life (TSST1/2) of 24 days (d), which is at the higher end of the range for half-life values of the other six anti-PD-1 mAbs (range of 18–25 d). This long half-life supports a dosing regimen of 200 mg Q3W [34]. The incidence of anti-tislelizumab antibodies was around 23% with about 6% of them categorized as neutralizing, but no effect was observed on drug efficacy or pharmacokinetics [34]. Adverse events (AEs) in trials with tislelizumab largely tracked with the other FDA-approved anti-PD-1 antibodies.
Cutaneous squamous cell carcinoma
Cutaneous squamous cell carcinoma (CSCC), or squamous cell carcinoma of the skin, is a skin cancer that begins in the squamous cells. CSCC is one of the more common cancers in humans, accounting for over 2.2 million cases globally in 2021 [163]. CSCC, which is the second leading skin cancer behind melanoma, is generally a slower-growing cancer that rarely metastasizes. Thus, when diagnosed early and resected as a localized tumor, the 5-year survival rate is very high [163]. On the other hand, late diagnosis can lead to advanced disease, metastasis, and a poor prognosis. In the USA, it has been estimated that 40 000 cases of CSCC advance to a more serious state, and 15 000 patients die annually [163]. Both number of cases and severity of CSCC have increased annually for many years, especially in Caucasian populations, mostly associated with the increase in exposure to ultraviolet irradiation [163].
Unloxcyt® (cosibelimab-ipdl) approval for treatment of CSCC
Unloxcyt® (cosibelimab-ipdl) (aka CK-301) is an anti-PD-L1 antibody approved by the FDA on 13 December 2024, for the treatment of CSCC. While two anti-PD-1 antibodies, pembrolizumab and cemiplimab, have previously been approved for the treatment of CSCC, cosibelimab is the first anti-PD-L1 antibody to be approved for that indication (Table 3).
Cosibelimab, sponsored by Checkpoint Therapeutics [164], is a human IgG1λ isolated from the Adimab yeast display library [137]. It binds to PD-L1 with an affinity of 0.85 nM and blocks both the binding of PD-1 and B7.1 to PD-L1 [137]. The specific epitope for cosibelimab binding to PD-L1 is not yet known. Cosibelimab has a functional Fc and possesses antibody-dependent cellular cytotoxicity (ADCC) activity, similar to the anti-PD-L1 antibody, avelumab [137, 165], but different from atezolizumab and durvalumab, both of which possess muted Fc functions [165, 166] (Table 3). In addition to blocking PD-L1 binding to PD-1, cosibelimab kills tumor cell lines in vitro via both ADCC and complement-dependent cytotoxicity (CDC) mechanisms of action [137].
A Phase 1, multicenter, open-label clinical trial (CK-301-101; NCT03212404) of cosibelimab was conducted on 109 adult patients with metastatic or locally advanced CSCC (mCSCC and laCSCC, respectively). Cosibelimab treatment resulted in a confirmed objective response rate (ORR) (ICR and RECIST v1.1 criteria) of 47% of 78 mCSCC patients, with 40% partial responses (PRs) and 8% complete responses (CRs) [76, 167]. In 31 laCSCC patients, cosibelimab treatment resulted in a confirmed ORR of 48%, including a CR rate of 10% (n = 31) [76]. Based on these results, cosibelimab was granted full approval as “an alternative therapy” if other therapeutic approaches were unsuccessful [76, 164]. No other anti-PD-L1 antibody has been approved for these indications, although the anti-PD-1 mAb, nivolumab, is also an “other recommended” systemic therapy for mCSCC.
Cosibelimab is dosed intravenously at 1200 mg Q3W, resulting in steady-state concentrations in circulation of 120–453 mg/l. Clearance of cosibelimab was 0.256 L/d and the TSST1/2 was 17.8 d, which allowed for the Q3W dosing. Antidrug antibodies were observed in about 50% of patients, although neutralizing antibodies were found in only about 3% of patients tested, and in those patients, cosibelimab serum concentrations were only reduced by about 20%, resulting in no clinically significant effect of the ADAs [76].
WINREVAIR® (sotatercept-csrk)
Activin
The transforming growth factor (TGF)-β/bone morphogenic protein (BMP) family of regulatory cytokines is comprised of up to 33 human proteins, including TGF-β1–3, multiple BMPs, activins, growth differentiating factors (GDFs), nodal, inhibins, and other similar cytokines [168]. These cytokines bind to receptors that signal via various Mothers against Decapentaplegic (SMAD) signal transduction pathways to control a wide variety of different physiological processes, including cellular differentiation and proliferation, regulation of follicle-stimulating hormone (FSH) secretion, embryogenesis, osteogenesis, chondrogenesis, homeostasis, and extracellular matrix remodeling, amongst other processes [168, 169].
In addition to the complex family of TGF-β/BMP-like regulatory cytokines, numerous receptors and receptor combinations bind them differentially. These receptors fall into three families: Type I, of which there are seven, Type II (five members), and one Type III receptor. Two Type I and two Type II receptors form a heterotetrameric complex to which specific TGF-β/BMP cytokines will bind, resulting in signaling through a specific SMAD signaling cascade [169]. There are two major pathways for these interactions: the BMP pathway, for which more than 20 ligands exist, and the activin/TGFβ pathway, for which more than 15 ligands exist [170].
Activins are comprised of two dimeric β subunits of either bA or bB, held together by a disulfide bond [171]. Three different forms of activins can be formed: the homodimeric βAβA and βBβB activins, or the heterodimeric βAβB activin [171]. These activins can bind to two Type II receptors, ActRII(A) and ActRIIB, both of which are single-pass, serine/threonine kinase receptors [168, 171]. Once an activin has bound to either Type II receptor, a Type I receptor, ActRIB (aka ALK4), is recruited to the receptor-activin complex, and signaling occurs [168]. Activins are involved in regulation of FSH synthesis, osteogenesis, immune responses, and hematopoiesis, amongst other functions. Between the presence of multiple ligands, mechanisms of pro-ligand maturation, the presence of inhibitory ligands, the use of differential receptors, and complex signaling paradigms, the TGF-β/BMP-controlled processes represent some of the most complex regulatory mechanisms in human biology.
Pulmonary arterial hypertension
Pulmonary hypertension (PH) is a vascular disease characterized by high blood pressure in the lungs. PAH is one form of PH that is triggered by obstructions in the small arteries in the lung, resulting in restricted flow and high pressure in the pulmonary arteries [172, 173]. These obstructions cause high blood pressure in the pulmonary arteries that carry oxygenated blood from the right side of the heart to the lungs. Key hallmarks of PAH include pulmonary arterial pressure exceeding 20 mm Hg and pulmonary vascular resistance (PVR) above 3 Wood Units (WU) [173]. PAH can lead to progressive right-sided heart failure and, ultimately, death [172]. Symptoms of PAH include tightness in the chest, shortness of breath, and dizziness. PAH typically progresses with time and can shorten life span if not treated.
The World Health Organization (WHO) has classified PAH into five categories based on etiology of the disease [173, 174]. In Type I PAH, which includes inheritable (genetic) causes amongst other etiologies, some of the pathophysiology includes dysregulation of TGF-β, activin, and GDF biology [174].
Winrevair® (sotatercept-csrk) approval for treatment of PAH
Winrevair® (sotatercept-csrk; aka ACE-011), which is a homodimeric human activin receptor type 2A (ACVR2A)-IgG1 Fc fusion protein (See Table 1, Fig. 3), has the ability to trap activin A and GDF ligands, which results in a recalibration of pro-proliferative (ActRIIA/SMAD2/3-mediated) and anti-proliferative (BMPRII/SMAD1/5/8-mediated) signaling pathways, helping to modulate vascular remodeling [174].
Sotatercept, which has been in the public literature since 2008 [175], is now projected to be ca. $4 billion annually at peak (Table 1). In the early days of its development, sotatercept and a murine version of it (ACVR2A-mouse IgG2a-Fc fusion) [176], were studied for the ability to increase bone mass [176], to promote rapid erythropoiesis [175, 177], as well as improve hemoglobin and hematocrit levels in postmenopausal women [175, 178].
Sotatercept was approved by the FDA on 26 March 2024 based on the results of the STELLAR clinical trial (NCT04576988) of 323 patients with PAH (WHO group I) [37, 38]. STELLAR was a double-blinded, placebo-controlled, multicenter, clinical trial in which 323 patients with PAH were randomized 1:1 between active drug and placebo. Sotatercept treatment resulted in an improvement in at least one WHO functional class of 29% as compared with 14% for those in the placebo arm, as well as an 84% reduction in death by any cause compared with the placebo arm. Sotatercept is indicated for the treatment of adults with PAH (WHO group 1) with the goal of increasing exercise capacity, improving function, as well as reducing clinical worsening [37].
Additionally, pooled data from PULSAR (n = 106; NCT03496207 [179]) and STELLAR (n = 323; NCT04576988 [180]) clinical trials have been used to analyze both efficacy and safety of sotatercept in patients. Efficacy was assessed at 24 weeks of dosing improved exercise capacity (6-min walk distance), PVR, WHO functional class, and delayed time to first occurrence of death or clinical worsening event. Of the pooled 429 participants, sotatercept treatment significantly (all tests, P < .001) improved median 6-min walk distance over placebo (33.9 vs 63.7 m), PVR (−202.8 vs −395.4 dyn·s·cm−5), and the change in concentration of N-terminal pro-B-type natriuretic peptide (∆ NT-proBNP) of −317.3 vs −1041.2 pg/ml, a strong predictor of ongoing mortality [181].
Sotatercept is administered SC with bioavailability of ca. 66% and a Tmax of about 7 d. The TSST1/2 of sotatercept is 24 d, allowing for Q3W administration [37]. Overall, AEs with the treatment of sotatercept were acceptable, with headaches, epistaxis, and rash as the most significant in terms of numbers [37, 182]. In the Phase III study (NCT04576988), the incidence of anti-drug antibodies (ADAs) against sotatercept in 162 patients receiving treatment was found to be 26%–27%, with an overall incidence of neutralizing antibodies of ca. 7% [37, 183]. Nevertheless, the ADAs were not found to have an overall effect on efficacy, PK, or safety of sotatercept [37, 183].
Anktiva® (nogapendekin alfa inbakicept-pmln)
IL-2/IL-15 receptor complex
IL-2 and IL-15 are two related pro-inflammatory cytokines in the type I 4-α-helix bundle family that share two subunits of their receptors, IL-2/IL-15Rβ (CD122) and the common gamma chain (γc; CD132), each with a third α-subunit (IL-2Rα, aka CD25 vs IL-15Rα, aka CD215) unique to its own receptor biology [184–186]. The IL-2/IL-15Rβ and γc subunits are responsible for signal transduction from Janus kinase (JAK) to STAT5, whereas the α-subunits of both receptors do not appear to have a signaling domain [184–186]. For IL-2R, the IL-2Rα subunit is an integral part, with IL-2/IL-15Rβ and γc, of the high-affinity receptor as shown in Fig. 5. IL-2 can bind IL-2Rα with low affinity (10−8 M KD), and it can bind IL-2Rβγ in the absence of IL-2Rα with intermediate affinity (10−9 M KD) [187]. The heterotrimeric receptor IL-2Rαβγ, however, binds IL-2 with high affinity (10−11 M KD), which translates into strong signaling [187]. Thus, IL-2 production or exogenous IL-2-based therapy provides a signal to cells possessing all three IL-2R subunits, which include both effector and regulatory T cell subsets [187].
Figure 5.
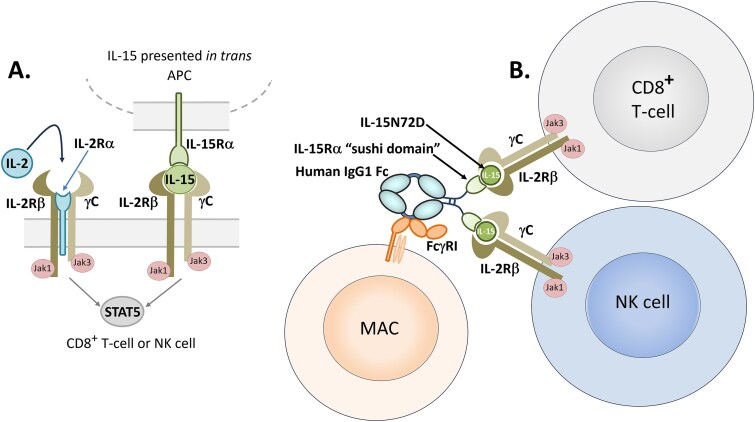
Cartoons of IL-2 and IL-15 and their respective receptors. A. IL-2 cytokine and it’s receptor complex which includes IL-2/IL-15Rβ (CD122), common gamma chain (IL-2Rγ; CD132), and the IL-2R-specific subunit, IL-2Rα (CD25) all in cis configuration, versus the IL-15 receptor complex, comprised of IL-2/IL-15Rβ, IL-2Rγ, and the IL-15 specific IL-15Rα (CD215), which presents IL-15 to the IL-2/IL-15Rβγ complex in trans [184–186]. The IL-15Rα subunit is typically expressed by APCs as a mechanism to increase activation and proliferation of NK and T cells [184–186]. B. The general mechanism by which nogapendekin alfa inbakicept-pmln functions. Nogapendekin alfa (IL-15N72D) is presented in trans to IL-2/IL-15Rβγ-expressing cells such as CD8+ T cells or NK cells by inbakicept (IL-15Rα sushi domain fused to IgG1-Fc), which has an active Fc region that can cross-link with FcγRI on monocyte-derived dendritic cells and macrophages [188].
The IL-15 receptor-α (IL-15Rα) and IL-15 are together expressed by APCs and “presented” in trans to the IL-2/IL-15Rβγ-positive, CD25-negative CD8+ T-cells and natural killer (NK) cells as shown in Fig. 5. Because the affinity of IL-15Rα for IL-15 is so high (100 pM) [189], it appears that IL-15 exists in situ mostly as an IL-15Rα/IL-15 complex on the surface of APCs [185]. Moreover, the IL-15Rα/IL-15 complex has a 150-fold higher affinity for IL-2/IL-15Rβγ than IL-15 alone, demonstrating the biological relevance of the trans presentation of IL-15 as a complex with its IL-15Rα carrier [185]. It has been demonstrated that once the IL-15-IL-15Rα-IL-15Rβγ cell–cell complex is formed, the IL-15Rα-IL-15 complex is cleaved from the surface of APCs and endocytosed by the recipient CD8+ T-cells or NK cells [190]. Once endocytosed, the IL-15Rα-IL-15 can be processed and reunited in cis with the IL-2/IL-15Rβγ complex to form a high-affinity IL-15/IL-15R signaling complex [190].
The pharmacological importance of this difference between IL-2 receptor biology and IL-15 receptor biology is that IL-2 alone can be given as a therapeutic protein. The product Proleukin® (aldesleukin), which is naked recombinant human IL-2, was approved in 1992 and again in 1998 for the treatment of metastatic renal cell carcinoma (mRCC) and metastatic melanoma, respectively [191]. While high-dose aldesleukin (IL-2) therapy yielded a 15%–20% ORR in mRCC and melanoma [190–192], it suffered from three significant issues: (i) it is too broadly potent, which results in significant inflammatory side effects, including capillary leak syndrome, cytokine storm, liver toxicity, severe nausea, fever, and chills [193], (ii) treatment with wild-type IL-2 not only activates CD8+ T cells and NK cells, but also expands potently suppressive CD25+ Foxp3+ Treg cells, which could significantly limit its anti-cancer efficacy [194], and (iii) it had a very short half-life. IL-15, on the other hand, cannot be provided as a naked cytokine, but rather needs to be provided as an IL-15-IL-15Rα complex so that it is properly presented, in trans, similar to its normal biology, to IL-2/IL-15Rβγ-possessing cells [185–187]. It has been shown that, minimally, only the IL-15 binding domain of IL-15Rα, the N-terminal 65-amino acid residue domain called the “sushi domain”, is required for in trans presentation of IL-15 to CD8+ T-cells and NK cells [185–187]. Because IL-2Rα (CD25) is not part of this mechanism of action (MOA), Tregs are not affected by IL-15-IL-15Rα presentation.
The ability to specifically stimulate CD8+ T cells and NK cells while not activating CD25+ Treg cells [185–187], or vice versa [195] has been a holy grail of sorts for several decades, essentially ever since the approval of aldesleukin in 1992 [191]. Over the years, several attempts have been made to generate mutant forms of IL-2 or identify formats to present IL-15, that would bind IL-2/IL-15Rβ/γc chains without binding IL-2Rα (CD25). The raison d’être for generation of such receptor-biased molecules has been to mimic, as closely as possible, the activity of the IL-15/IL-5Rα complex in activation, via IL-2/IL-15Rβγ, of CD8+ T cells and NK cells without binding to or activating IL-2Rα (CD25)-positive Treg cells. These efforts have included generation of IL-2 muteins that bind IL-2/IL-15Rβγ but not IL-2Rα, IL-2 molecules in which the IL-2Rα binding site is obscured via polyethylene glycol, antibodies, or other means, and a variety of IL-15/IL-15Rα types of molecules [196–198]. Table 4 provides a representation of some of these IL-2 and/or IL-15-based immune stimulatory molecules studied in clinical trials.
Table 4.
Examples of modified (mutein) IL-2 or IL-15/IL-15Rα IL-2Rβγ (but IL-2Rα non-binding) agonists for activation of CD8+ T cells and NK cells in absence of CD25+ Treg activationa
| Molecule | Sponsor | Current status | Example NCT | Structure | Ref |
|---|---|---|---|---|---|
| Anktiva® (nogapendekin alfa inbakicept-pmln) | ImmunityBio | FDA approved | NCT03022825 | IL-15N72D noncovalently bound to IL-15Rα-sushi domain-IgG1 Fc fusion protein | [199] |
| Bempegaldesleukin | Nektar/ BMS | Phase 3 studies terminated | NCT03635983 | PEG-conjugated IL-2 for elongated half-life, with releasable PEG moieties conjugated to lysine residues near CD25 binding site that are released in vivo resulting in in situ activation | [200, 201] |
| AU-007 | Aulos | Phase 1/2 recruiting | NCT05267626 | IL-2 noncovalently bound with an anti-IL-2-CD25-binding site mAb. | [202] |
| SLC-3010 | Selecxine | Phase 1/2 recruiting | NCT05525247 | IL-2 noncovalently bound with an anti-IL-2-CD25-binding site mAb. | [203] |
| MDNA11 | Medicenna | Phase 1/2 recruiting | NCT05086692 | IL-2 mutein fused to HSA to extend half-life. Mutations include L80F, R81D, L85V, I86V and I92F for increased CD122 affinity and F42A and E62A to abolish CD25 binding. | [204] |
| Pegenzileukin | Sanofi (Synthorx) |
Phase 2 trials terminated; Phase 1/2 trial ANR |
NCT05179603 (NCT04009681) |
Site-specific (P65) PEG-conjugated (via Click chemistry) mutated CD122-biased IL-2 mutein | [205] |
| NIZ-985 | Novartis | Phase 1/1b trials terminated | NCT04261439 | Soluble shed form of IL-15Rα and IL-15 in complex | [206, 207] |
| NL-201 | Neurogene (Neoleukin) |
Phase 1 completed, molecule dropped | NCT04659629 | PEG-conjugated half-life extended form of Neo-2/15, a computationally-designed de novo IL-2 analog lacking CD25 binding. | [208, 209] |
| SHR-1916 | Jiangsu Hengrui Pharma |
Phase 1 terminated | NCT04842630 | PEG-conjugated IL-2 mutein lacking CD25 binding that promotes proliferation of CD8+ T cells and NK cells, but not Treg cells | [210] |
Abbreviations: ANR, active, not recruiting. BMS, Bristol-Myers Squibb. CD, cluster of differentiation. FDA, (US) Food and Drug Administration. HSA, human serum albumin. IL, interleukin. mAb, monoclonal antibody. NCT, National Clinical Trial (clinical trial register). PEG, polyethylene glycol. Ref, reference(s).
aOnly IL-2/IL-15 and IL-2/IL-15R agonists are included for brevity. Note that there are several PD-1 and PD-L1-targeted IL-2/IL-15 “bispecific” agonists also in clinical trials.
Non-muscle invasive bladder cancer
Non-muscle invasive bladder cancer (NMIBC) is a relatively common form of cancer that is found in the tissue that lines the inner surface of the bladder, but which has not penetrated into the muscle wall surrounding the bladder. The term carcinoma in situ (CIS) refers to a higher grade of NMIBC in which the tumor lies flat against the bladder lining [211]. Approximately 10% of NMIBC cases also involve CIS. NMIBC is usually treated by transurethral resection of the bladder tumor (TURBT) from the lining of the bladder [211]. Usually, after surgery, intravesical (direct administration into the bladder via catheter) treatment in combination with Bacillus Calmette-Guérin (BCG) vaccination is prescribed to activate the immune system to decrease the risk of the cancer recurring [211]. Alternatively, intravesical chemotherapy using mitomycin C, epirubicin, or gemcitabine may be used, especially in more serious cases. NMIBC typically recurs within a year in about 50% of the patients, requiring further treatment.
Anktiva® (nogapendekin alfa inbakicept-pmln) for treatment of bladder cancer
Nogapendekin alfa inbakicept-pmln (trade name Anktiva®) was discovered by Altor BioScience and called ALT-803 (later, called N-803 by ImmunityBio, who acquired Altor in 2017). N-803 is a first-in-class IL-2βγ receptor “super-agonist” that promotes activation and proliferation of natural killer (NK) cells and CD8+ T cells without inducing regulatory T cells (Tregs) [94] (Fig. 5). The activated NK and T-cells attack the tumor cells directly, while memory T cells are stimulated, which can provide a CR for up to 4 years in some patients [212].
N-803 is a protein complex of two proteins, nogapendekin alfa, which is a 12 770 Da N72D variant of human IL-15, in a non-covalent combination with inbakicept, which is comprised of the 65 amino acid residue N-terminal region of the IL-15 receptor-alpha (IL-15Rα) (sushi domain), fused to the C-terminal 232 amino acid residues of each IgG1 Fc dimer [94]. There are two molecules of the IL-15N72D complexed with one Fc fusion protein, one bound non-covalently to each arm (Figs 3 and 5) to form a three-protein stable complex of ca. 58 kDa (Fig. 3). The IL-15N72D/Sushi domain-Fc has a higher affinity for IL-2Rβγ than wild-type, with no ability to bind IL-2Rα, providing the desired target cell specificity [94]. The Fc fusion provides a longer half-life to the complex due to its natural interaction with neonatal fragment crystallizable receptor (FcRn) [94].
Anktiva® was approved by the FDA on 22 April 2024, in combination with BCG, for the treatment of adult patients with NMIBC who had undergone resection, with CIS with or without papillary tumors, that was non-responsive to BCG alone [41]. N-803 is administered intravesically to provide direct access to the tumor. Induction dosing is 400 mg with BCG dosed QW for up to six weeks, followed by maintenance dosing of weekly dosing for 3 weeks at months 4, 7, 10, 13, and 19, with the potential for additional rounds of dosing as needed [41].
FDA approval of Anktiva® was based on the QUILT-3.032 trial (NCT03022825) [213], a multi-center, single-arm, open-label Phase II/III trial. Patients who had high-risk NMIBC with CIS were treated with an induction regimen of 400 mg N-803 with BCG QW for six weeks, followed by a maintenance therapy consisting of treatment for three consecutive weeks at 4, 7, 10, 13, and 19 mo. Of 77 patients treated, 62% had a CR to treatment with N-803, with 58% of the patients having a duration of response greater than 12 mo and 40% >24 mo [41]. The FDA granted expedited approval of N-803 via the breakthrough therapy designation.
Because N-803 is administered directly into the bladder at the site of the tumor, systemic exposure is expected to be low. Indeed, systemic exposure of the drug is <100 pg/ml, which is below the lower limit of quantitation (LLOQ) [41]. In part due to the very low systemic exposure, the side effects were mostly tolerable, with only up to 3% Grade 3/4 AEs [41].
IMDELLTRA® (TARLATAMAB-DLLE)
DLL3 and neuroendocrine tumors
DLL3 is one of three members of the delta-like ligand family, a group of single-pass membrane proteins that are homologs of the Drosophila Notch ligand [214]. All three members of the DLL family, along with two other proteins, Jagged-1 (JAG1) and Jagged-2 (JAG2), are involved in the regulation of the Notch signaling pathways, which also include four Notch receptors [214]. Notch pathway dysregulation is a critical factor in the development of certain tumors [214]. DLL3, which is normally a Notch pathway inhibitor, is expressed at low levels in normal tissues, but is highly overexpressed in about 80%–85% of SCLC cells, as well as cells of other neuroendocrine tumors (NETs) [215]. In general, NETs are highly aggressive, heterogeneous tumors that arise from endocrine (hormone-producing cells) or nerve cells [216]. These tumors, which are found in the gastrointestinal tract, lungs, and pancreas, are relatively rare, and if caught early, are associated with a good prognosis [216]. One of the most aggressive and fast-growing NETs, however, is SCLC.
The overexpression of DLL3 in SCLC, and very low expression in normal tissues, has made DLL3 an attractive target for the treatment of SCLC, which has promulgated several approaches to target DLL3 for the treatment of SCLC [215, 217–219]. The most interesting of these was rovalpituzumab tesirine (Rova-T), an anti-DLL3 IgG1 antibody conjugated to pyrrolobenzodiazepine (PBD) by a protease-cleavable linker [217, 220]. This anti-DLL3 ADC initially offered promise for treating SCLC [215, 219–221]. Unfortunately, in multiple late-stage trials, Rova-T did not demonstrate significant efficacy over standard of care, resulting in the sponsor, AbbVie, dropping the ADC candidate [221]. The failure of Rova-T was ultimately attributed to the PDB payload, which provided too small of an efficacy/toxicity window [215, 221].
Small cell lung cancer
Lung cancer is the second most widely diagnosed cancer in the USA, behind only breast cancer. When taking into account all forms of lung cancer, it is the leading cause of death from cancer in the USA, causing up to a quarter of all US cancer deaths. There are two major forms of lung cancer, SCLC, which accounts for about 15% of all lung cancer diagnoses, and NSCLC, the major form of lung cancer that accounts for about 85% of all cases [222]. While SCLC is a relatively smaller population of patients, it is the sixth-ranked cancer for cancer-related mortality [217, 222].
There are two forms of SCLC: SCLC, also known as oat cell cancer, and combined-SCLC, which is SCLC with certain aspects of NSCLC, such as squamous cell or adenocarcinoma. In both cases, SCLC is a fast, aggressive NET associated with poor prognosis. One of the significant issues with SCLC is that it disseminates quickly, with up to 70% of all cases already disseminated prior to diagnosis [217, 222].
Imdelltra™ (tarlatamab-dlle) for treatment of SCLC
Micromet was founded in Germany in 1993 to make small scFv-based BisAbs that they termed BiTEs® (for bispecific TCEs). The first BiTE®, a 17-1A (now known as EpCAM [epithelial cell adhesion molecule]) × CD3ε, was published in 1995 [223]. Another BiTE®, a CD19 × CD3 construct dubbed MT-103, was made public in 2006 [224], with the generalized structure of: N-scFv(CD19)-GGGGS-scFv(CD3ε)-C [225]. MT-103 eventually became blinatumomab, ultimately resulting in the acquisition of Micromet by Amgen in 2008 [226]. In 2014, blinatumomab was the first BisAb to be approved by the FDA as Blincyto®, for the treatment of B-cell acute lymphoblastic leukemia (B-ALL) [227] (Table 2). In the ten years since that time, 13 additional BisAbs have been approved, one of them being another BiTE®, tarlatamab-dlle (Imdelltra™) [23, 28].
Tarlatamab (aka AMG 757) is a half-life Fc-extended bispecific TCE BiTE with a single-chain construct structure [43]: N-[VH(DLL3)-(GGGGS)3-Vκ(DLL3)]-GGGGS-[VH(CD3ε)-(GGGGS)3-Vλ(CD3ε)]-GGGG-[hingeDTK...-CH2-CH3ΔCH]-(GGGGS)6-[hingeDTK...-CH2-CH3] (1–982) of about 105 kDa as shown in cartoon format in Fig. 3D [43]. The Fc is silenced using the mutations R572C, N577G, V582C, R827C, N832G, and V837C, and the protein is non-glycosylated, owing to the N-glycosylation site N577G and N832G mutations (all in 1–982 numbering, not Eu numbering) [43, 91, 228].
Tarlatamab acts as a TCE [218, 229], whereby the target cell is bound by the antibody by one combining site, and the other combining site is used to bind CD3ε of the TCR, which results in the formation of a synapse between the target cell and T cell, triggering release of perforins and granzymes by the T cell, which lyse DLL3-expressing target cells [44].
Tarlatamab was granted accelerated approval by the FDA on 16 May 2024, for the treatment of extensive-stage SCLC with disease progression for patients either previously treated or currently on platinum therapy [44, 45]. The accelerated approval was based on the results of the open-label, multicenter, multi-cohort Phase 2 clinical trial, DeLLphi-301 (NCT05060016) [230], for the treatment of SCLC patients who had failed two or more prior treatments. Of the 99 patients studied in DeLLphi-301, 40% of patients dosed with 10 mg Q2W achieved an ORR of 40%, a median duration of response (mDOR) of 9.7 mo, and a mOS of 14.3 mo [44, 231, 232]. Patients dosed with 100 mg Q2W only achieved a 32% ORR [44, 231, 232], indicating a likely bell-shaped dose response that has been observed previously in both computer simulations and experimental results with TCE BisAbs [233].
As expected with a TCE like tarlatamab, the most common AEs were cytokine-release syndrome (CRS), which occurred in 51% and 61% of patients in the 10 mg and 100 mg Imdelltra™ treatment groups, respectively [44, 231, 232]. In a long-range follow-up using pooled data from DeLLphi-300 Phase I and DeLLphi-301 Phase II trials across all dose levels ≥10 mg, the ORR was 25%, mDOR was 11.2 mo, and mOS was 17.5 mo [234].
Grade 3 CRS was observed in 1% and 6% of patients treated with 10 mg and 100 mg Imdelltra™, respectively [44, 231, 232]. Most of the CRS AEs occurred in the first treatment cycle, and most were Grades 1 and 2 [44, 231, 232]. Additionally, 9% of patients treated with Imdelltra™ experienced immune effector cell-associated neurotoxicity syndrome (ICANS) [44]. In pooled data from multiple trials, neurological sequelae, a subset of which included ICANS, occurred in 47% of patients, 10% of which were Grade 3 [44, 231, 232]. Both CRS and ICANS are typical AEs, and often times severe AEs, associated with the treatment of tumors with TCEs, as well as with CAR-T cells [235]. For Imdelltra™, step-up dosing and/or treatment with tosilizumab have been recommended to mitigate CRS [44].
Tarlatamab was designed with a fusion of the scFv/scFv BiTE® portion to an active Fc domain, which confers upon it, via recycling through FcRn [236], a longer half-life than would be possessed by the BiTE® modality alone. Thus, the mean half-life of tarlatamab is 5.8 d [44, 237], instead of ca. 2.1 h, which would be expected of a BiTE® like blinatumomab alone [238]. Clearance was 0.65 L/d, and serum exposures were dose-proportional [237], suggesting an absence of target-mediated clearance. The half-life and clearance allowed for dosing on a Q2W schedule [44, 231, 232]. In the DeLLphi-300 and 301 studies, the incidence of ADAs against tarlatamab of only ca. 6.6% and 3%, respectively, was observed [44, 237]. None of those patients with positive anti-tarlatamab ADAs had neutralizing antibodies [44].
PIASKY® (CROVALIMAB-AKKZ)
Complement-mediated diseases and treatments
The complement system, or complement cascade, is a significant part of the innate immune system that provides a front-line innate immune response to both sterile and infective immune insults [239]. This system, which contains >30 protein components, results in a robust, fast-acting innate immune response that also bridges to the adaptive immune response. Activation of the complement cascade can occur via three different routes: the classical pathway, the alternative pathway, and the lectin pathway [239]. These complement activation pathways converge at C5, which is cleaved by C5 convertase (C4bC2aC3b) into C5a and C5b. This reaction initiates the terminal complement pathway, which results in opsonization and lysis of pathogens via interaction of C5b with C6, C7, C8, and C9, to form the cylinder-shaped membrane attack complex (MAC) [239].
Under healthy conditions, the complement cascade is tightly regulated at multiple steps in the pathway to provide a homeostatic environment. When homeostasis is disregulated, however, individual proteins of the complement cascade can become pathogenic and damage normal cells and tissue. The second cleavage product of C5, C5a, is a potent inflammatory mediator and strong chemoattractant that recruits immune cells such as neutrophils, eosinophils, monocytes, and T lymphocytes to the site of inflammation [240]. When C5a is dysregulated, it induces, via its receptor C5aR1, cellular activation that is associated with a wide range of inflammatory disorders, including sepsis, acute respiratory distress syndrome, acute antibody-mediated rejection, lupus nephritis, age-related macular degeneration, C3 glomerulonephritis, atypical hemolytic uremic syndrome (aHUS), and paroxysmal nocturnal hemoglobinuria (PNH) [240].
Paroxysmal nocturnal hemoglobinuria
C5a also induces neutrophils to produce tissue factor (TF), which initiates the clotting cascade and activates platelets, especially those with reduced expression of the complement regulators, CD55 and CD59 [241]. PNH is a relatively rare blood disorder caused by the loss of the protective proteins CD55 and CD59 on platelets and red blood cells (RBCs), resulting in complement-mediated attack of the RBCs and platelets. PNH results in intravascular hemolysis, leading to autoimmune hemolytic anemia, chronic kidney disease, and thrombosis, which can cause life-threatening blood clots [242].
PiaSky® (crovalimab-akkz) approval for treatment of PNH
PiaSky® (crovalimab-akkz), sponsored by Chugai [48], is the fourth anti-C5 antibody to be approved by the FDA. The first three include eculizumab (Soliris®), approved by the FDA in 2007, ravulizumab (Ultomiris®), an extended half-life version of Soliris® approved in 2018, and pozelimab-bbfg (Veopoz™), approved in 2023 [9]. Both Soliris® and Ultomiris® are currently approved for the treatment of PNH, aHUS, generalized myasthenia gravis, and neuromyelitis optica spectrum disorder (NMOSD) [243, 244]. Veopoz™, on the other hand, is approved only for the treatment of adult and pediatric patients for the ultra-rare CHAPLE (“CD55 deficiency with Hyper-activation of complement, Angiopathic thrombosis, and severe Protein-Losing Enteropathy”) disease [245, 246].
For the treatment of PNH, both eculizumab and ravulizumab have some significant limitations. First, plasma concentrations of C5 can reach 80 mg/mL, which is one of the highest soluble ligand concentrations to be targeted by therapeutic antibodies [247]. This results in the need for very high doses of eculizumab, which has a maintenance dosing at 900 mg Q2W [243]. Ravulizumab, which has a longer half-life, has a maintenance dose of 3000–3600 mg Q8W [47]. Both antibodies require IV dosing for both loading and maintenance dosing.
The second challenge for the treatment of PNH by both eculizumab and ravulizumab is that they are not efficacious in patients carrying the C5a subunit polymorphism, R885H, which is part of the epitope for these antibodies [248]. The R885H polymorphism is found in over 3% of Asian patients and may also be present in some non-Asian patients as well [248].
PiaSky® (crovalimab-akkz) is a rabbit/human humanized IgG1κ that binds C5 and blocks its cleavage into C5a (anaphylatoxin) and C5b [48, 249], thereby inhibiting terminal complement activation and blocking the formation of the C5b-C9 membrane-attack complex (MAC) (C5b-C9, a structure mediating cell lysis) [250]. Crovalimab binds to an epitope unique from the epitope of eculizumab and ravulizumab and one for which binding and activity are not affected by the C5a R885H polymorphism or other known polymorphisms [48, 248]. This provides a significant advantage for crovalimab over the previously approved anti-C5 antibodies.
Another advantage is that crovalimab is engineered as a “recycling antibody,” [48, 249] a technology invented at Chugai and first used in satralizumab-mwge (Enspryng®), an anti-IL-6 receptor antibody approved by the FDA in 2020 for treatment of Aquaporin-4 (AQP4) positive NMOSD [251]. The recycling antibody technology utilizes the incorporation of histidine residues in the CDRs, which confers a pH-dependent binding of the antibody to the antigen [48]. Upon binding FcRn and entering the endosome, the IgG dissociates from the antigen at acidic pH within the endosome, resulting in recycling of the IgG while the antigen is degraded via the lysosomal protein degradation pathway. This permits a single IgG molecule to bind antigen through multiple cycles of FcRn recycling, as compared with normal antibodies that bind only once. This results in significantly lower requirements for dosed levels of drug, which allows for SC delivery (SCD) of the maintenance doses [47], a distinct advantage over eculizumab and ravulizumab.
Crovalimab was approved by the FDA based on the results of the COMMODORE 2 clinical trial (NCT04434092) [252], in which it was found to be non-inferior to eculizumab in the co-primary endpoints of hemolysis control (79.3% vs 79.0%, respectively) and transfusion avoidance (65.7% vs 68.1%, respectively), as well as in the secondary efficacy endpoints, breakthrough hemolysis (10.4% vs 14.5%, respectively) and hemoglobin stabilization (63.4% vs 60.9%, respectively) [47, 253]. In the COMMODORE 3 trial (NCT04654468) [254], a single-arm, multi-center study, efficacy for the treatment of PNH was confirmed in a larger population [255], and pooled results from three Phase 3 COMMODORE clinical trials demonstrated a safety profile similar to that of eculizumab [253].
For treatment of PNH, crovalimab is given as an IV weight-based loading dose of 1000–1500 mg, followed by 340 mg IV doses QW for four weeks, and then followed with maintenance weight-based doses of 680–1020 mg, administered SC Q4W thereafter [47]. Bioavailability after SC dosing is 83% and mean clearance is very low at 0.079 L/d, resulting in a TSST1/2 of about 53 d [47], which allows for the Q4W SC maintenance dosing schedule. These values for “recycling engineered” crovalimab [47] are essentially the same as the extended-half-life-engineered ravulizumab [256], which has a clearance of 0.08 L/d and a TSST1/2 of 49 d [244].
PiaSky® (crovalimab-akkz) was demonstrated in clinical trials to have a very low incidence (0.5%) of ADAs, and of those that were observed, there was no apparent effect on PK or PD [47].
As with all anti-C5 antibodies that block the terminal pathway for complement activation, treatment of PNH patients with PiaSky® (crovalimab-akkz) presents a significant risk for infection by encapsulated bacteria such as Neisseria meningitidis, Streptococcus pneumoniae, type b Haemophilus influenzae, and Neisseria gonorrhoeae [47, 257]. Thus, similar to other approved anti-C5 antibodies, it is recommended that all patients taking crovalimab should be vaccinated against meningococcal infection at least 2 weeks prior to dosing [47].
KISUNLA® (DONANEMAB-ABZT)
Alzheimer’s disease
Approximately 55 million people worldwide are living with some form of dementia, 60%–80% of which is due to AD. In the USA alone, about 6.9 million people have dementia caused by AD, with half a million new cases diagnosed annually, a number expected to double by 2060 [258].
AD, which mostly affects elderly individuals over 65 years of age [259], results in progressive cognitive decline, memory loss, and behavioral impairment. For almost 35 years now, the “amyloid hypothesis”, which posits that deposition of Aβ peptides in neurotoxic plaques in brain tissue is a central mediator of AD pathology, has been a widely recognized explanation for the pathogenesis of AD [260, 261]. Cleavage of amyloid precursor protein (APP) by beta and gamma secretases generates extracellular Aβ peptides of 40 and 42 amino acids in length [259, 260]. These peptides do not accumulate in healthy subjects, but in individuals with mutations in APP [262], or in elderly individuals who can no longer degrade the peptides, Aβ40 and Aβ42 accumulate and form plaques in the brain, contributing to AD [259, 260]. These Aβ-driven plaques also promote tau aggregation and pathology [259], and the combination of Aβ plaques, tau tangles, and toxic oligomers of both species results in neuronal cell death and neurodegeneration [260, 263].
Aduhelm® (aducanumab), which received accelerated approval by the FDA on 7 June 2021, was the first mAb to be approved for the treatment of AD [264]. Aducanumab targets residues 3–7 of Aβ [265] and binds Aβ plaques, resulting in the reduction of plaque volume (Fig. 6) [266]. The approval of aducanumab was controversial because the risk/benefit ratio had not been well established, and approval was granted even though the advisory committee had recommended against it [267, 268]. As a result, after Leqembi® was approved in 2023, Biogen discontinued Aduhelm® on 31 January 2024, so they could focus resources on their more efficacious antibody, Leqembi® [268].
Figure 6.
Amyloid sequences and structures targeted by anti-Aβ antibodies. A. The primary amino acid sequence of Aβ1–42 with the epitopes to which the leading anti-Aβ mAbs bind. B. Amyloid quaternary structures to which the leading anti-Aβ mAbs bind. Adapted from Loeffler [265], Lannfelt et al. [269], and Söderberg et al. 2022 [270]. Current status of anti-Aβ antibodies was partly informed by Noorda et al. [271]. Redrawn and modified from Strohl [9].
Kisunla® (donanemab-abzt) approval for treatment of Alzheimer’s disease
Kisunla® (donanemab, aka LY3002813) is a humanized IgG1 mAb derived from the mouse mE8 IgG2a antibody that recognizes an N-terminal pyroglutamate Aβ3–42 (N3pG) form of Aβ that is found primarily in amyloid plaques, so donanemab attacks fully formed plaques instead of precursor molecules, intermediate aggregates, or fibrils (Fig. 6) [272]. The success of donanemab is tied directly to its ability to reduce plaque in early-stage AD. Different from Biogen’s lecanemab (Leqembi®) [9], treatment of AD with donanemab is based on measurement of amyloid plaque removal [51].
Donanemab, sponsored by Eli Lilly, was granted full approval by the FDA on 2 July 2024, specifically for the treatment of early symptomatic AD [51, 52, 273]. In clinical trials, the patient population was largely focused on those patients in the early stages of AD with mild cognitive impairment or mild dementia [274]. Donanemab was approved based on results from the Phase 3 TRAILBLAZER-ALZ 2 (NCT04437511) [275] placebo-controlled, randomized (1:1) clinical trial of 1736 patients with AD [51, 276]. The primary endpoint was based on the differential results from 0 to 76 weeks of treatment of the integrated AD Rating Scale (iADRS) score, which is comprised of data from the Alzheimer’s Disease Assessment Scale–Cognitive subscale (ADAS-Cog13) and the AD Cooperative Study–Instrumental Activities of Daily Living (ADCS-iADL) [51, 276]. Additionally, the Clinical Dementia Rating Scale–Sum of Boxes (CDR-SB) and data from the independent iADRS and ADAS-Cog13 analyses provided secondary endpoints. In the treatment arm of 860 patients entering the study with mild AD, treatment with donanemab significantly slowed cognitive decline by ca. 40% over those in the placebo arm, as measured by iADRS [51, 276].
Additionally, major secondary clinical endpoints (CDR-SB, ADAS-Cog13, ADCS-iADL) showed statistical improvement in patients or placebo controls as well [51, 276]. The amyloid plaque burden in patients was also analyzed using positron emission tomography (PET), and patients who had a substantial measured loss of amyloid plaque were removed from treatment. Those patients who were eligible to be removed from donanemab treatment due to loss of amyloid plaque increased over treatment time, with 17%, 47%, and 69% being eligible after 24, 52, and 76 weeks of study, respectively [51, 276]. One of the key takeaways from the TRAILBLAZER-ALZ 2 trial (NCT04437511) [275] was that a reduction in cognitive decline in mild AD patients was associated with a significant decrease in amyloid burden [277]. How this plays out in the long run could determine the future of amyloid-based therapies for AD. Based on the results of the Phase 3 TRAILBLAZER-ALZ 2 trial (NCT04437511) [275], a subsequent pharmacoeconomic study demonstrated that limited plaque-burden based treatment with donanemab was cost-effective for the health care system [278].
Treatment with donanemab can cause AEs, with the most common of which are amyloid-related imaging abnormalities (ARIA), headaches, and infusion-related reactions (IRRs) [51]. Split out, temporary swelling in areas of the brain (ARIA-E) and small spots of bleeding in/on the brain surface (ARIA-H) affected ca. 24 and 31% of patients treated with donanemab, respectively [51, 279]. Serious ARIA occurred in 1.6% of treated patients and was linked with treatment-related deaths. Patients who were homozygotic for ApoE ε4 were shown to have a higher incidence of ARIA, resulting in a recommendation that ApoE ε4 status be determined before treatment to inform the risk level to patients [51, 279].
Donanemab is dosed IV on a Q4W schedule with 700 mg infused for the first three doses, followed by 1400 mg thereafter. PET imaging is used to determine the effect of dosing on amyloid plaque burden. Donanemab dosing is continued until amyloid plaques are reduced to minimal levels, at which time the protocol calls for dosing to be discontinued [51]. The circulating half-life of donanemab is 12.1 d, over twice as long as lecanemab [9], allowing for the Q4W dosing schedule [51].
Eli Lilly already has a late-stage clinical back-up in development for donanemab [9, 271, 272]. Remternetug (LY3372993) also targets Aβ (N3pG-42); it is formulated for SCD for both patient and caregiver convenience, and it has demonstrated encouraging results in earlier clinical trials [280]. Remternetug is currently being investigated for the treatment of AD in Phase 3 TRAILRUNNER-ALZ 1 (NCT05463731) [281] clinical trials. The next few years will be significant in the development of anti-Aβ antibodies, especially those like donanemab and remternetug that target Aβ (N3pG-42), which is strictly resident in amyloid plaques, to determine how Aβ plaque burden-related treatment will fare in the long term.
NEMLUVIO® (NEMOLIZUMAB-ILTO) and EBGLYSS® (LEBRIKIZUMAB-LBKZ)
Introduction
In 2024, two new antibodies, Nemluvio® (nemolizumab-ilto) and Ebglyss® (lebrikizumab-lbkz), were approved by the FDA for the treatment of atopic dermatitis (ADM). Additionally, nemolizumab was approved for the treatment of prurigo nodularis (PN), a skin disease characterized by the presence of crusty, itchy, raised bumps (or nodules, per the name), usually on the extremities [282]. Both diseases are T helper-2 (TH2)-associated skin diseases [283], although ADM is significantly more prevalent, with more than 26 million people in the US living with ADM [284], as compared with ~170 000 US residents afflicted with PN [285].
TH2 cells produce cytokines such as IL-4, IL-5, and IL-13, which play significant roles in humoral immunity and protection from helminth infection. These cytokines, often referred to as TH2 cytokines, also play a significant role in the pathogenesis of allergic inflammatory diseases, including skin diseases [283, 284, 286]. While both IL-13 [286] and IL-31 [287] are cytokines in the TH2 family, IL-31 is somewhat less widely recognized as such in part because it was only discovered more recently, in 2004 [288].
Atopic dermatitis
Eczema, which affects about 32 million Americans, is a term typically used to describe skin rashes, of which there are seven known types (ADM, contact dermatitis, dyshidrotic eczema, neurodermatitis, nummular eczema, seborrheic dermatitis, and stasis dermatitis) [289]. ADM is by far the most common form of eczema, accounting for about 80% of eczema cases in the US Eczema, and by association, AD, are about 3.5–4 times more common in the USA than is psoriasis, another major, significant disease of the skin [284, 289].
ADM, which is characterized by rough, dry skin and rashes, is caused by both genetic and environmental factors. Typical insults that can trigger AD include irritants such as washing detergents or allergens, such as pollen and dust mites. Low systemic vitamin D levels are also associated with ADM flares [284, 286].
Over the years, topical creams and ointments such as hydrocortisone, over-the-counter antihistamines, topical JAK inhibitors, and aryl hydrocarbon receptor agonists (e.g. tapinarof) have been used traditionally to treat ADM. Prior to 2024 two mAbs, the anti-IL-4 receptor-α (IL-4RA) antibody dupilumab (Dupixent®, approved 2017) [285] and the anti-IL-13 antibody tralokinumab (Adbry®, FDA approved in 2021) [286] have been approved for treating ADM. Both of these antibodies affect the IL-4/IL-13 cytokine pathways to treat diseases caused primarily by disregulated TH2-related pathways [283].
Prurigo nodularis
PN is a chronic skin disease characterized by high-itchy, small nodules on the skin. The nodules are thought to be caused by abnormalities in nerve endings in the skin that cause the itchiness, which are exacerbated by a variety of irritants [282]. Inflammatory skin conditions such as eczema, ADM, and chronic pruritis (itching), and imbalances in neurotransmitters such as histamine and substance P also contribute to the condition, resulting in neuroimmunological dysregulation [290, 291]. Cell-based analysis of PN versus ADM showed the distinct presence of IL-31, oncostatin M, and neuromedin B (associated with nerve endings) in PN lesions [290].
While PN itself is not life-threatening, it can significantly reduce the quality of life and increase the risk of infection due to constant itching, which can result in excoriation of the nodules. Patients with PN often experience poor sleep, anxiety, and depression that accompany the constant itchiness of the condition. Historical treatments for PN have notably been challenging and have been limited to a few approaches, such as narrow band ultraviolet phototherapy, systemic steroids, and topical antihistamines such as calamine lotion [282]. Anti-itch creams containing menthol or camphor have also been used to reduce pruritus from PN.
IL-31 and IL-31Rα
First discovered in 2004 [288], IL-31 is a four-helix bundle cytokine and member of the IL-6 family of cytokines [287, 288]. IL-31, which signals through the heterodimeric receptor IL-31 receptor-α (IL-31Rα) and oncostatin M receptor-β (OSMRβ) [292], is associated with TH2-shifted inflammation [283]. IL-31 is produced by TH2 lymphocytes, and its expression has been correlated with the expression of the TH2 cytokines IL-4 and IL-13 [283]. In vivo, IL-31 production is rapidly induced by the presence of staphylococcal superantigen, providing a link between skin-resident staphylococci and ADM [293]. Epithelial cells, keratinocytes, skin-associated monocytes, and sensory nerves associated with itch express both the IL-31Rα and OSMRβ, and significantly higher levels of IL-31 mRNA were found in human skin biopsies in PN and lesional ADM than in psoriatic or normal tissues [293]. Thus, the IL-31 cytokine pathway causes inflammation, itch, fibroblast activation, and keratinocyte differentiation, and has been associated with a variety of chronic pruritic skin diseases such as ADM, atopic eczema, and PN [288, 291]. Accordingly, IL-31 is sometimes referred to as a “neuroimmune cytokine” or “itchy cytokine” [291, 293–295].
Nemluvio® (nemolizumab-ilto) approval for treatment of PN and AD
Nemolizumab (aka CIM331) is a humanized IgG2κ antibody that binds the IL-31 receptor A (IL-31RA) and blocks binding of the cytokine IL-31 to its receptor. Nemolizumab, which was first discovered and engineered at Chugai [296], was licensed to Galderma in 2016 [53]. Nemolizumab was given full approval for the treatment of PN by the FDA on 13 August 2024 [56]. This was followed on 13 December 2024, by full approval for the treatment of adolescents and adults with moderate to severe ADM [297]. Nemolizumab was first approved on 28 March 2022, in Japan for the treatment of itch associated with ADM [298]. More recently, nemolizumab was approved by the European Commission for the treatment of both ADM and PN in February 2025 [299].
Nemolizumab was developed as a human IgG2 isotype rather than an IgG1 to reduce binding to FcγRs [296]. Additionally, the nemolizumab IgG2 was mutated at H268Q [54] in the CH2 B-C loop (residues 265–273), which has been shown to alter the conformation of the loop [300], resulting in reduced binding to FcγRIIα [301–303]. Another key feature in the modifications in the nemolizumab Fc is the mutation Q419E [54], which was introduced to give the Fc a slightly stronger negative charge to reduce the rate of internalization across membranes [304].
Nemolizumab was approved by the FDA for treatment of PN based on the results of two randomized, double-blind, placebo-controlled, multicenter Phase 3 clinical trials, OLYMPIA 1 [305] and OLYMPIA 2 [306], which combined to treat 370 patients with nemolizumab, with another 186 subjects given a placebo [51]. Results of disease severity and nemolizumab efficacy were evaluated using the Investigator’s Global Assessment (IGA) of PN nodules as well as the peak pruritus numeric rating scale (PP-NRS) score, which is 0–4 with 4 being most severe [51]. Using both scoring systems, patients treated with nemolizumab were significantly improved over those in the placebo group [51, 305, 306]. For example, over a 16-week treatment period, 49%–56% of patients treated with nemolizumab in the combined trials had a change of ≥4 in their PP-NRS as compared with 16% in the placebo group [51, 305, 306].
AEs associated with treatment with nemolizumab were headaches (6% vs 3% in the treatment group and placebo group, respectively), ADM (4% vs 0.5%), and eczema (4% vs 2%). Very few serious AEs were observed [51, 306].
Nemolizumab was dosed at 60 mg SC by a dual chamber pen with a Tmax at about 6 d post dose [51]. The half-life of nemolizumab was 18.9 d with a clearance rate of 0.263 L/d, allowing for SC dosing Q4W, which would allow for patient self-administration. In the combined Phase 3 trials, the incidence of ADAs against nemolizumab was about 7%, with only 3% neutralizing antibodies. It was reported that there was no significant effect on PK or efficacy due to the ADAs [51].
IL-13
IL-13 is a four helical bundle cytokine (comprised of α-helices A, B, C, and D), structurally related to IL-4, that is typically produced by TH2 cells, mast cells, eosinophils, basophils, and other cell types at sites of inflammation [307, 308]. IL-13 can be produced by many cells of the immune system and has been found to contribute to the inflammatory processes and decreased barrier functions in ADM [308, 309]. IL-13 acts primarily on myeloid cells and non-hematopoietic cells, promoting mucus production, smooth muscle contraction, and epithelium permeabilization, all of which contribute to allergic asthma [307]. Dysregulated IL-13 in the skin [308] and lung [307] are primary mediators of ADM and allergic asthma, respectively.
IL-13 binds two different receptors, the Type II heterodimeric receptor, comprised of IL-13Rα1 and IL-4Rα [310], and IL-13Rα2 [311]. IL-13 binds IL-13Rα1 at a relatively low affinity of ca. 30 nM [311, 312], and this complex then recruits IL-4Rα to form the high-affinity, ternary signaling complex [310]. IL-13 can bind IL-4Rα directly, but the affinity is very low and not physiologically significant [310, 313]. IL-13 also binds the IL-13Rα2 receptor, which is thought to act as a decoy as part of the regulation of the TH2 pathway, at high affinity (<100 fM) [311, 312]. The binding sites on IL-13 for IL-13Rα1 and IL-13Rα2, which are mostly associated with helices A and D as well as the D1 and CD loops, are highly overlapping, making it almost impossible to find an antibody that could block one and not the other [311]. The binding site on IL-13 for IL-4Rα, as part of the tripartite complex, comprised of helices A and C, is on the opposite side of IL-13 from the binding sites on the IL-13R receptors [313].
IL-4 also binds the Type II receptor, similar to IL-13, but in the opposite sequence: it binds IL-4Rα at high affinity (ca. 20 pM), and the IL-4:IL-4Rα complex recruits IL-13Rα1 to form the ternary signaling receptor [312]. IL-4, however, also binds to the Type I receptor comprised of IL-4Rα and γc chain, which is primarily expressed in hematopoietic immune cells, where it regulates lymphocytes by inducing TH2 polarization, mast cell activation, and by inducing IgG1/IgE class switching in B cells [309, 312].
Besides lebrikizumab, there are two significant anti-IL-13 antibodies, tralokinumab and cendakimab. Tralokinumab was approved in 2021 as Adbry® (Leo Pharma), whereas cendakimab is currently being investigated by Celgene in several late-stage clinical trials [308]. Both of these antibodies compete with and block the ability of IL-13 to bind both IL-13Rα1 and IL-13Rα2 receptors [308].
Ebglyss® (lebrikizumab-lbkz) approval for treatment of AD
Eblgyss® (lebrikizumab-lbkz, aka RG3637, MILR-1444A, and TNX-659) is a humanized IgG4κ (S228P, Eu numbering) antibody that binds IL-13 at high affinity (<10 pM) [313] at a site completely different from tralokinumab and cendakimab [314]. Instead, lebrikizumab binds IL-13 on the “back side” at residues R65-G69, which are required for IL-13, in complex with IL-13Rα1, to bind IL-4Rα, thereby disrupting the Type II receptor signaling complex [313]. While blocking the ability of IL-4Rα to bind the IL-13/IL-13Rα1 complex, lebrikizumab still allows binding of IL-13 to both IL-13Rα1 and IL-13Rα2 [311]. So, from a biology standpoint, lebrikizumab would block the Type 2 receptor biology while not inhibiting binding to the high-affinity “decoy” IL-13Rα2. Moreover, lebrikizumab blocks the formation of the heterodimeric receptor, which results in significant internalization of the incomplete lebrikizumab/IL-13/IL-13Rα1 complex, whereas the antibodies tralokinumab and cendakimab, which block IL-13 binding to its primary receptors, do not promote internalization [314].
Lebrikizumab was approved by the FDA based on the results of three multicenter, double-blind, placebo-controlled randomized Phase 3 clinical trials, ADvocate 1 (NCT04146363) [315], ADvocate 2 (NCT04178967) [316], and Adhere (NCT04250337) [317]. These three trials included over 1000 children over 12 and adults with moderate-to-severe ADM. Results of disease severity and lebrikizumab efficacy were evaluated using the IGA as well as and Eczema Area and Severity Index (EASI) score [61]. In the combined ADvocate 1 and 2 trials, 38% of patients had clear skin at 16 weeks versus 12% in the placebo arm [318]. Additionally, patients treated with lebrikizumab had reductions in EASI at 16 weeks of 72%–75% versus 35%–43% for the placebo-controlled group [318]. Including several secondary endpoints, all data showed a significant difference between the lebrikizumab-treated versus placebo groups [318].
Lebrikizumab is dosed SC Q2W for the first 16 weeks as an induction dosing period, followed by maintenance dosing at Q4W. Tmax is achieved ~7–8 d after dosing with a bioavailability of 86%, and the TSST1/2 is 24.5 d [61], which allows for the maintenance dosing of Q4W. ADAs against lebrikizumab were observed in only 2.8% of patients dosed, and the ADAs did not interfere with PK, efficacy, or safety [61].
Summary of TH2 pathway antibodies
The treatment of TH2-related diseases, including ADM, PN, asthma, and similar diseases, is a very significant therapeutic area for mAbs. With the two new approvals described here, the total number of antibodies treating TH2-related diseases has now reached eight FDA-approved antibodies. Previously approved antibodies in this area include Xolair® (omalizumab, anti-IgE, FDA approved 2003), Cinqair® (reslizumab, anti-IL-5, FDA approved 2016), Dupixent® (dupilumab, anti-IL-4Rα, FDA approved 2017), Fasenra® (benralizumab, anti-IL-5R, FDA approved 2017), and Adbry® (tralokinumab, anti-IL-13, FDA approved 2021) [1]. As of the end of 2024, the combined market for these therapeutic antibodies has hit the $22.2 billion mark, trailing only the PD-(L)-1 market ($54 billion), the anti-TNF market ($39.4 billion in 2024), and the anti-IL-17/23 market ($33.9 billion in 2024) [21]. Nemluvio® and Ebglyss® are both expected to reach blockbuster status at $2.1 billion and $2.8 billion at peak sales, respectively [84] (Table 1).
NIKTIMVO® (AXATILIMAB-CSFR)
Colony-stimulating factor-1 receptor
CSF-1R, encoded by the protooncogene c-fms, is a receptor tyrosine kinase (TK) expressed on the surface of myeloid cells, including macrophages and microglia, for which two ligands are known, colony-stimulating factor-1 (CSF-1) and IL-34 [319, 320]. CSF-1, also known as macrophage colony-stimulating factor (M-CSF), is a dimeric four helical bundle cytokine that controls the survival, function, activation, proliferation, and development of myeloid cells, including macrophages, microglia, osteoclasts, other myeloid cell types, and their progenitors [320, 321]. The only known receptor for CSF-1 is CSF-1R [320]. IL-34, a second ligand for CSF-1R, is a dimeric four-helix bundle cytokine distantly related to CSF-1 that is known to bind four different receptors, CSF-1R, syndecan-1, protein-tyrosine-phosphatase ζ (PTP-ζ), and triggering receptor on myeloid cells 2 (TREM2) [322]. In binding to CSF-1R, IL-34 plays a role in homeostasis and development. Dysregulation of IL-34, which is induced by proinflammatory cytokines, has been linked recently to a variety of inflammatory diseases, including rheumatoid arthritis [322].
Upon binding to CSF-1R, both CSF-1 and IL-34 cause the formation of an active CSF-1R dimer that results in phosphorylation and downstream signaling through multiple pathways depending on cell type, ligand concentration, and other factors [320]. As an antagonist against CSF-1R, an antibody could either block CSF-1R ligand binding as a direct antagonist or it could block the ability of CSF-1R to dimerize, which would inhibit downstream signaling but not necessarily inhibit ligand binding [323].
Graft-versus-host disease
Graft-versus-host disease (GVHD) is a multisystem disorder in which transplanted donor allogeneic hematopoietic cells recognize the host cells or tissue as foreign and begin attacking them. GVHD is typically associated with allogeneic stem cell transplantation and often begins as those cells begin to engraft in the bone marrow [324]. Acute GVHD (aGVHD) occurs within days or so of engraftment, or may be delayed as much as 6 mo after engraftment [324]. Chronic GVHD (cGVHD) usually begins at least 3 mo after transplantation and can be a lifelong event. [324]. cGVHD is a major cause of morbidity and mortality amongst the 17 000 US patients receiving allogeneic stem cell transplantation, with almost 50% of those patients requiring three or more lines of therapy [59].
Niktimvo™ (axatilimab-csfr) approval for treatment of cGVHD
Niktimvo™ (axatilimab-csfr, aka SNDX-6352) is a humanized IgG4κ mAb that binds the ligand-binding domain of CSF-1R and blocks its activation by either of its cytokines, CSF-1 or IL-34 [325, 326]. Axatilimab binds CSF-1R at a high affinity of 4–8 pM and inhibits ligand-induced activation, as measured by monocyte chemoattractant protein-1 (MCP-1; aka CCL2) release, with IC50s of 270 pM and 100 pM for CSF-1 and IL-34, respectively [325, 326]. Axatalimab also has been shown to bind known CSF-1R polymorphs, including mutants V32G, A245S, P247H, and V279M [325, 326]. Importantly, no antibody-induced agonism or internalization was observed with axatilimab [325, 326].
Axatilimab, sponsored by Incyte and Syndax Pharmaceuticals, was approved by the FDA on 14 August 2024 for the treatment of cGVHD (Table 1) [59]. Axatilimab is also under development for other indications, such as idiopathic pulmonary fibrosis (IPF) [59]. Approval by the FDA for axatilimab treatment of cGVHD was based on the results from the AGAVE-201 clinical trial (NCT04710576) [58, 327], an open-label, randomized, multicenter Phase 2 trial studying the efficacy in 241 patients of three different doses of axatilimab on disease outcomes. Axatilimab was administered IV at 0.3 mg/kg Q2W or other doses until either 9 mo passed with no efficacy, evidence of disease progression, or unacceptable toxicity [58]. PRs or CRs were graded according to the 2014 NIH Consensus Development Project on Response Criteria [58]. PRs were observed in 75% of the 79 patients dosed at 0.3 mg/kg Q2W, but no CRs were recorded [58]. The mean time to response was 1.5 mo [58, 327].
Both the clearance and resulting half-life of axatilimib were non-linear, ranging from 2.32 to 0.21 mL/h/kg and 10.7 to 108 h, respectively. Therapeutic levels of exposure, however, were achieved during repeat Q2W dosing at the approved dose of 0.3 mg/kg [58]. The most common AEs included injection site reactions and liver enzyme elevation [58]. ADAs were found in ca. 34% of patients tested, about half of which were neutralizing. Nevertheless, it was reported that the ADAs had no meaningful effect on efficacy, PK, PD, or safety [58].
Novel antibodies for treatment of hemophilia
Tissue factor pathway inhibitor
The coagulation cascade, also known as secondary homeostasis, is a pathway to ensure homeostasis in blood so that neither excessive bleeding nor unwanted clotting occurs under normal conditions. The cascade is carried out as a series of enzymatic steps that regulate the clotting process [328]. These are separated into two initiation pathways known as the intrinsic and extrinsic pathways, which converge at coagulation FXa to form the third, or common, pathway [328].
The intrinsic coagulation pathway is initiated by activation of Factor XII by damage to the vascular endothelium (Fig. 7) [328, 329]. A key step in the intrinsic pathway is the formation of the tenase complex, consisting of FIXa, FVIIIa, and FX, on the cell membrane surface [329]. The tenase complex activates FX to FXa, which then complexes with Factor Va (FVa) to form prothrombinase, which converts prothrombin to thrombin, which in turn converts fibrinogen to fibrin, which can cross-link to stabilize platelet aggregates at the site of vascular injury to form a clot [328, 329].
Figure 7.
Cartoons of major reactions of intrinsic (A) and extrinsic (B) pathways of coagulation. A. Key steps in the intrinsic pathway, initiated by surface damage to endothelial tissue [328, 329]. Formation of the “tenase” complex (1.), including factor IXa (FIXa), factor VIIIa (FVIIIa), and factor X (FX), results in activation of FX to FXa (2.), which can then enter the common coagulation pathway [328, 329]. In the absence of active FVIIIa due to either genetic loss or presence of inhibitors, the BisAb emicizumab (3.), which binds FIXa and FX (4.), can act as a FVIIIa mimetic, which confers the conformation of FIXa required to activate FX to FXa (5.) [330], which can enter the common coagulation pathway. B. Key steps in extrinsic pathway, initiated by TF in response to external trauma [328, 329]. TF recruits factor VII and activates it to FVIIa (1.). The TF/FVIIa complex recruits FX and immediately converts it to FXa (2.), which can enter the common coagulation pathway (3.) [328, 331]. TFPI recognizes, binds and blocks the TF/FVIIa/FXa ternary complex through binding of Kunitz domain 1 (KU1) to FVIIa/TF and Kunitz domain 2 (KU2) to FXa (4.). Alternatively, TFPI can bind free FXa (5.) which then complexes with TF/FVIIa to form the inhibited ternary complex (4.). Both marstacimab and concizumab bind to the KU2 domain of TFPI (6.) to block the inhibition of TFPI on FXa and the TF/VIIa/FXa ternary complex [332, 333]. The antibody action allows for production of FXa which can enter the common coagulation pathway [328, 329].
The extrinsic coagulation pathway, on the other hand, is activated by TF in response to external trauma to tissues (Fig. 7) [328, 331]. TF, on the surface of TF-producing cells, complexes with and activates FVII to FVIIa, and the TF-FVIIa complex attracts and activates FX to FXa, which then enters the common pathway as above [328, 331].
TFPI is a natural inhibitor of the procoagulant activity of the extrinsic pathway that helps to maintain homeostasis [332, 333]. TFPI, which is primarily synthesized by microvascular endothelium, circulates in very low concentrations in plasma, where it exerts its regulatory effects on the extrinsic pathway [332, 333]. TFPI is comprised of three Kunitz domains: K1, which is involved in FVIIa inhibition, K2, which binds FXa and inhibits its protease activity, and K3, which binds protein S [333]. TFPI inhibits the extrinsic pathway in multiple ways. First, TF-FVIIa is recognized by K1 of TFPI, which can bind and inhibit, albeit poorly, the TF-VIIa complex [332, 333]. This complex, however, recruits FX and converts it to FXa in the ternary complex. TFPI, which binds FXa via its K2 domain, is a strong inhibitor of the TF-FVIIa-FXa ternary complex as soon as FX is activated to FXa [333], blocking the release of free FXa. TFPI can also inhibit the ternary complex by binding FXa directly [333], which can then form the ternary complex with TF-FVIIa (Fig. 7). Once FXa is released by either the intrinsic or extrinsic pathway and bound with FVa to form the prothrombinase complex, however, it is protected from TFPI [332, 333]. While FXa can activate prothrombin by itself, the FVa-FXa prothrombinase complex is 3 × 105-fold more efficient at doing so [332]. In the presence of the TFPI-inhibited extrinsic pathway, the intrinsic pathway, via the tenase complex, can still generate enough FXa, so the coagulation is not completely inhibited [332, 333].
Hemophilia
Hemophilia A and B are bleeding disorders caused by the deficiency or dysfunction of FVIII and FIX, respectively, caused by mutations in the genes that encode [334]. These genetic disorders are rare and linked to the X-chromosome, so they are much more prevalent in (but not exclusive to) males over females [334]. Hemophilia A occurs about four-fold more prevalent than Hemophilia B, with an incidence of about 1/5000 male births [335] as compared to ca. 1/20 000 male births for Hemophilia B [336]. The end result is that at least 200 000 males worldwide, and maybe as many as 1000 000, have hemophilia, with 80% of them having Hemophilia A [334–336]. Hemophilia C (aka Rosenthal syndrome), which is much rarer (at 1/100 000 births), is caused by a deficiency or dysfunction of FXI and is milder than either Hemophilia A or B [337].
The severity of hemophilia is measured by the amount of FVIII (for Hemophilia A) in blood [335]: 0 (not measurable) to <0.01 IU/ml or <1% of normal FVIII levels defines severe hemophilia, resulting in spontaneous bleeding. Levels of 0.02–0.05 IU/ml (2%–5%) or 0.06–0.40 IU/ml (6%–40%) result in moderate or mild hemophilia, respectively, both of which can cause excessive bleeding after even mild trauma [335]. Similarly, severe Hemophilia B is defined by less than 1% of normal FIX levels in blood, moderate is 1%–5%, and mild is 6%–49% [336]. Patients with hemophilia can experience potentially life-threatening spontaneous bleeding in muscles and organs. These bleeding incidents can be managed by on-demand, periodic treatment or by prophylaxis using FVIII or FIX mimetic biologics [334–336].
Acquired hemophilia is a rare autoimmune disease in which the body develops a humoral immune response against either FVIII or FIX, usually the former [338]. Hemophilia in which anti-FVIII antibodies are present is known as “Hemophilia A with inhibitors” [338, 339]. While the incidence of acquired hemophilia is rare at 1.5 cases/million people, it can be very serious. Importantly, patients who have Hemophilia A or B may also develop autoantibodies against FVIII or FIX, respectively [339, 340]. As many as 30% and 20% of all patients with Hemophilia A and Hemophilia B, respectively, can develop autoantibodies (i.e. “inhibitors”), and up to 50% of patients with severe Hemophilia A can develop autoantibody inhibitors [340].
Acquired hemophilia, or Hemophilia A or B with inhibitors, cannot be treated with FVIII or FIX replacements/substitutes (recombinant, PEGylated recombinant, Fc-fused, or albumin-fused FVIII or FIX proteins), because they also will be attacked by autoantibodies to the natural blood factors [341]. Thus, there is a significant need for new, alternative treatments and prophylactics for both Hemophilia A and B “with inhibitors” that do not include FVIII or FIX proteins. Similar to the FVIII mimetic, Hemlibra® (emicizumab) [342], both of the new anti-TFPI antibodies approved in 2024, Hympavzi® (marstacimab-hncq) and Alhemo® (concizumab-mtci), help to fill that void (Table 5).
Table 5.
Comparison of antibodies used in treatment and/or prophylaxis of Hemophilia A and B
| Parameter | Antibody | ||
|---|---|---|---|
| Hympavzi® (marstasimab) | Alhemo® (concizumab) | Hemlibra® (emicizumab) | |
| Antibody | Human IgG1λ | Humanized IgG4κ | Humanized bispecific, bivalent IgG; common LC |
| Status | FDA approved 10/11/24 | FDA approved 12/20/24 | FDA approved 10/4/18 |
| Sponsor | Pfizer | Novo Nordisk | Genentech/Roche |
| Target(s) | TFPI | TFPI | FIXa/FX |
| Hemophilia treated | A and B | A and B | A |
| Inhibitors | Without inhibitorsb | With inhibitorsb | With or without inhibitorsb |
| MOA | Binds K2 domain of TFPI and inhibits | Binds K2 domain of TFPI and inhibits | Mimetic of FVIIIa |
| Pathway affected | Extrinsic | Extrinsic | Intrinsic |
| Nominal half-life | 16–18 d | Ca. 1 d | 27 d |
| Dose | Prophylactic or prevention QW 150 mg SCa |
Prophylactic or prevention QD 0.2 mg/kga (14 mg/70 kg person) |
6 mg/kg SC Q4Wa after loading dose (420 mg/70 kg person) |
| Cost | 1 prefilled pen of 150 mg/mL is $14.7-16 K × 4 aliquots/mo = $58-62 K per mo | 60 mg/1.5 ml solution is $10,783 × 7.5 aliquots/mo = ca. $81 K/mo | 60 mg/0.4 mL is $7583 × 7 aliquots/mo; projects to ca. $53 K/mo |
| References | [64, 65, 343] | [78, 79, 344] | [330, 342, 345, 346] |
Abbreviations: FIXa, Factor IXa. FVIIIa, Factor VIIIa. FX, Factor X. QD, QW, Q4W, once daily dosing, once weekly dosing, once every four-week dosing, respectively. MOA, mechanism of action. SC, subcutaneous. TFPI, tissue factor pathway inhibitor.
aAfter loading dose.
b“Without inhibitors” refers to the absence of anti-FVIIIa or anti-FIXa autoantibodies in patients with hemophilia A and B, respectively. “With inhibitors” refers to the presence of anti-FVIIIa or anti-FIXa autoantibodies in patients with Hemophilia A and B, respectively.
Hympavzi® (marstacimab-hncq) for treatment of Hemophilia A and B with inhibitors
Hympavzi® (marstacimab-hncq, aka PF-06741086) is a human IgG1λ mAb that binds to the Kunitz-2 domain of TFPI to block its ability to bind and inhibit the procoagulant TF/VIIa/FXa ternary complex in the extrinsic pathway of the coagulation cascade (Fig. 7), the activity of which increases the ability of the extrinsic pathway to contribute to coagulation. Marstacimab is approved as a prophylactic antibody to prevent or reduce the frequency of bleeding episodes in patients over the age of 12 with Hemophilia A (congenital FVIII deficiency) without FVIII inhibitors, or with Hemophilia B (congenital FIX deficiency) without FIX inhibitors [64, 65, 347].
Marstacimab, sponsored by Pfizer, was approved by the FDA on October 11, 2024, based on the open-label, multi-center Phase 3 clinical trial, BASIS (NCT03938792) [348], which studied 116 adult and pediatric (age 12 and up) patients with severe Hemophilia A without FVIII inhibitors or severe Hemophilia B without FIX inhibitors [64, 348] (Table 5).
In the BASIS study, patients received replacement factors either prophylactically (83 patients) or on-demand (33 patients) for 6 mo, followed by 12 mo of prophylactic dosing (300 mg loading dose, followed by QW 150 mg SC) of marstacimab [348]. The primary endpoint for efficacy was the annualized bleeding rate, which was reduced from an annualized bleeding rate of 38 in the first 6 mo to 3.2 with marstacimab, demonstrating that it was superior to the on-demand factor replacement [64, 348]. Additionally, the estimated annualized bleeding rate was 7.85 for the first 6 mo of factor replacement therapy versus 5.08 for the 12-mo period with marstacimab prophylaxis, indicating that treatment resulted in similar bleeding rates [64].
Marstacimab was dosed SC with a bioavailability of 71% and a Tmax ranging from 23 to 59 h, with a TSST1/2 of 7-to-10 d. It was reported that some marstacimab was eliminated by target-mediated clearance due to complexation with TFPI [64]. Approximately 20% of patients dosed with marstacimab developed ADAs, which decreased the steady-state concentration of the drug by 24%–50%. Nevertheless, it was reported that ADAs did not affect the efficacy or safety of marstacimab. This profile supported the QW dosing regimen [64].
Alhemo® (concizumab-mtci) for treatment of Hemophilia A and B with inhibitors
Alhemo® (concizumab-mtci, aka mab 2021) is a humanized IgG4κ mAb that binds to the Kunitz-2 domain of TFPI to block its ability to bind and inhibit the procoagulant TF/VIIa/FXa ternary complex in the extrinsic pathway of the coagulation cascade (Fig. 7), the activity of which increases the ability of the extrinsic pathway to contribute to coagulation. Concizumab was approved by the FDA on 20 December 2024 as a prophylactic drug to prevent or reduce the frequency of bleeding episodes in patients over the age of 12 with Hemophilia A (congenital factor VIII deficiency) with FVIII inhibitors, or with Hemophilia B (congenital FIX deficiency) with FIX inhibitors [79, 80, 349].
Concizumab was approved based on the open-label, multi-center Phase 3 clinical trial, explorer7 (NCT04083781) [349–351], which studied 133 adult and pediatric (age 12 and up) patients with severe Hemophilia A with FVIII inhibitors or severe Hemophilia B with FIX inhibitors [79, 351] (Table 5). The explorer7 trial consisted of four arms, two of which were randomized and two, which were not. In arms 1 and 2, 27 and 25 patients with Hemophilia A and B with inhibitors, respectively, were randomized 1:2, with arms 1 and 2 receiving on-demand treatment with bypassing agents, and those receiving daily treatment with concizumab, respectively. In arms 3 and 4, 53 Hemophilia A patients with inhibitors and 28 Hemophilia B patients with inhibitors were dosed SC with concizumab QD [79, 351]. The primary endpoint was the number of treated spontaneous and traumatic bleeding episodes (measured by annual bleeding rate (ABR)). Those patients treated with concizumab showed an 86% reduction in ABR as compared with those receiving no prophylaxis [79, 351]. The mean and median ABR for those treated with concizumab vs untreated were 1.7 and 0 versus 11.8 and 9.8, respectively, indicating significant efficacy of concizumab as a prophylactic [79, 351]. Additionally, 11% of untreated patients in the randomized arm experienced spontaneous or trauma-induced bleeds during the first 24 weeks of treatment as compared with 0% in those patients receiving prophylactic concizumab [79, 351].
While concizumab is currently only approved by the FDA for use in patients with Hemophilia A or B with inhibitors, the explorer8 Phase 3 clinical trial (NCT04082429) [352, 353] also tested the efficacy of concizumab as a prophylactic in patients without autoantibodies against either FVIII (hemophilia A) or FIX (Hemophilia B), confirming the efficacy and acceptable safety for concizumab prophylaxis in both Hemophilia A and B patients without inhibitors [353]. Thus, the ability of concizumab to act as a prophylactic against spontaneous or trauma-induced bleeding episodes is not affected by either the presence or absence of autoantibodies targeting FVIII or FIX. This potential expansion of indication has not yet been added to the label.
Concizumab is dosed SC with a Tmax of 8–99 h following a single dose, and is cleared very quickly with a TSST1/2 in the range of 1 d, requiring a daily dosing regimen [79]. Elimination at sub-saturating concentrations was non-linear due to TFPI target-mediated clearance [79]. The half-life of concizumab is striking, since concizumab is not specifically engineered to have a short half-life (Table 1), and marstacimab, which also binds KU2 of TFPI and also has some level of target-mediated clearance, still has a TSST1/2 of a week or so [64].
About 25% of those patients receiving concizumab generated ADAs against the drug, of which about 25% were neutralizing [79]. One out of the 12 patients who had neutralizing antibodies demonstrated loss of efficacy, whereas there was no effect on PK, PD, efficacy, or safety in the remainder of the patients exhibiting ADAs.
Summary of antibodies for Hemophilia A and B
Traditionally, FVIII or FIX-based biologics (recombinant, PEGylated recombinant, Fc-fused, or albumin-fused forms of FVIII or FIX proteins) have served to treat and prevent bleeding in both hemophilia A and B patients. There are currently more than a dozen of these clotting factor substitutes and, combined, they make up a market of about $7 billion (not including Hemlibra®) [21]. Most of these types of drugs are subject to autoantibody inhibitors of the native proteins [339, 341], and many are administered IV, which is not convenient. Thus, there is a significant need for new, efficacious drugs for the prevention of Hemophilia A and B, with or without inhibitors [339, 341]. The approval of Hemlibra® 2018 for Hemophilia A was an enormous step in that direction (Table 5). This has also borne out from a market standpoint in that Hemlibra® sales alone were nearly $5 billion in 2024 [21]. Combined, the market in 2024 for biologics addressing the needs of patients with hemophilia A and B was ca. $12 billion (Fig. 2). Marstacimab and concizumab are two significant additions to the armamentarium for helping patients with hemophilia, particularly those with inhibitors.
VYLOY® (ZOLBETUXIMAB-CIZB)
Claudin 18.2
The claudin family of proteins comprises 26 human tetraspan transmembrane proteins typically found in cells of either epithelial or endothelial tissues. The claudins possess four transmembrane domains, two extracellular loops, and both N- and C-termini reside on the intracellular side of the cell membrane [354]. A major role for claudins is the formation of tight junctions, contributing to barrier functions in epithelial cell–cell interactions, helping to control the flow of molecules between cells [354, 355]. Claudin 18. 2 is an isoform of claudin-18 expressed in gastric mucosal epithelial cells that plays a role in helping to maintain cell polarity, barrier function, and acid resistance [354].
Claudin 18.2 has been reported to be expressed in some gastric and organ adenocarcinomas such as gastric adenocarcinoma (GAC) (27%–56% positive), GEJC-AC (50%), pancreatic adenocarcinoma (PAC) (30%–60%, and cervical adenocarcinoma (20% positive), but less so in colorectal adenocarcinoma (4% positive), and not expressed in breast or appendix adenocarcinoma [356, 357]. Additionally, claudin 18.2 was overexpressed in 77% of primary ovarian mucinous tumors, with 100% expression in mucinous borderline tumors [356]. As such, Claudin 18.2 has been considered to be a good target for gastric tumors for the last several years [354, 357].
HER2-negative gastric and gastroesophageal junction adenocarcinoma
GC is the fifth most common cancer globally, at 1 million cases/year, and also the fifth most common cause of global cancer-related deaths, with 660 000 deaths/year. GEJC-AC, which occurs at the junction of the esophagus and stomach, adds another 500 000–600 000 cases and as many as 445 000 deaths per year globally [358, 359]. The 5-year survival rates for GC and GEJ-AC are in the range of 35% and 20%, respectively. Historically, there has been a significant need for new therapies for these very serious cancers. As noted earlier in this paper, the checkpoint inhibitor Tevimbra® (tislelizumab-jsgr) was approved for both GAC and GEJ-AC [358] (Table 1). Additionally, two anti-HER2 mAbs, Herceptin® and Enhertu®, have been approved in past years for treatment of HER2-overexpressing tumors (Table 6). Until recently, however, there was a need for the treatment of GAC and GEJ-AC not expressing HER2. Vyloy® (zolbetuximab-cizb), an antibody targeting claudin 18.2, has now gained approval in the USA for treatment of HER2-negative G-AC and GEJ-AC [358]. Thus, significant gains were made in 2024 for new treatment options for various forms of GAC and GEJ-AC.
Table 6.
FDA-approved anti-HER2 antibodies
| Antibody | Sponsor | FDA approval date | Format and MOA | Epitopea | Affinity KD (nM) |
Indications approved | Refs |
|---|---|---|---|---|---|---|---|
| Herceptin® (Trastuzumab) |
Genentech | 25 September 1998 | IgG1κ, humanized | HER2 IVb | 0.9–1.8 | HER2-OE BC, HER2-OE MGC, HER2-OE GEJAC | [360–362] |
| Perjeta® (Pertuzumab) |
Genentech | 8 June 2012 | Blocks HER2 dimerization Humanized IgG1κ |
HER2 IIb | 1.1 | HER2+ MBC | [360, 363] |
| Kadcyla® (trastuzumab emtansine) | Roche/Genentech | 23 February 2013 | humanized IgG1κ ADC conjugated with maytansanoid | HER2 IV | 2.7 | HER2+ MBC | [362, 364] |
| Enhertu® ([fam]-Trastuzumab deruxtecan-nxki) | Daiichi-Sankyo / AstraZeneca | 20 December 2019 | Humanized IgG1κ ADC with novel topoisomerase I inhibitor (exatecan; DXd) | HER2 IV | c | HER2+ MBC, MNSCLC, HER2-OE MGC, HER2-OE GEJAC, HER2-OE ST |
[365, 366] |
| Margenza® (Margetuximab-cmkb) |
TerSera Therapeutics | 16 December 2020 | Fc-modified chimeric IgG1κ (K214R, L235V, P243L, R292P, Y300L, P396L) (high ADCC) | HER2 IV | d | HER2+ MBC | [367–369] |
| Ziihera® (Zanidatamab-hrii) |
Jazz Pharmaceuticals Zymeworks / BeiGene |
20 November 2024 Accelerated approval | Bispecific, biparatopic Ab | HER2 II, HER2 IV | ECD-IV-scFv = 1.1 nM ECD-II Fab = 1.7 nM |
NR-BTC | [24, 70, 370] |
| Bizengri® (zenocutuzumab-zbco) |
Merus | 4 December 2024 Accelerated approval |
HER2-HER3 bispecific | HER2, I HER3, III |
HER2 = 2.2–3.9 nM HER3 = 0.23–0.99 nM |
NRG-1+ NSCLC, PAC | [73, 371, 372] |
Abbreviations: BC, breast cancer. BTC, biliary tract cancer. GEJAC, gastroesophageal junction adenocarcinoma. HER2, human epidermal growth factor receptor 2. MBC, metastatic breast cancer. MGC, metastatic gastric cancer. MNSCLC, metastatic small cell lung cancer. NR, non-resectable. NRG-1, neuregulin-1. NSCLC, non-small cell lung cancer. OE, over-expressing. PAC, pancreatic adenocarcinoma. ST, solid tumors.
aSee Fig. 8.
bPertuzumab IgG preferentially binds “inter-receptor” to two HER2 proteins, one Fab arm binding each receptor, whereas trastuzumab primarily forms a monovalent complex [373].
cAssumed to be similar to trastuzumab, the targeting antibody used in the ADC.
dStated to be “similar to trastuzumab.”
Vyloy® (zolbetuximab-cizb) approval for treatment of G-AC and GEJ-AC
Zolbetuximab, a chimeric anti-claudin 18.2 IgG1κ antibody with no Fc modification, was approved by the FDA on 18 October 2024 [68, 374]. Zolbetuximab, previously known as IMAB362 (“Ideal Monoclonal AntiBody” platform) was initially discovered by Ganymed, which was acquired by Astellas in November 2016. Zolbetuximab depletes claudin 18.2-positive cells using ADCC and CDC, which are associated with a normal human IgG1 Fc [375, 376].
Zolbetuximab, in combination with mFOLFOX6, was approved by the FDA based on the results of the SPOTLIGHT Phase 3 double-blind, randomized, multicenter study (NCT03504397) [377]. The SPOTLIGHT trial studied 565 patients with claudin 18.2-positive [378], HER2-negative, locally advanced unresectable or metastatic GC or GEJ-AC [67]. Across the SPOTLIGHT and additional clinical trials, the measured strong positivity rate for claudin 18.2 in patient samples (i.e. ≥75% of cells were claudin 18.2 positive) was ca. 38% [379]. Those patients receiving zolbetuximab plus chemotherapy lived on average 2.2–2.7 mo longer than those on chemotherapy plus placebo [378]. Across two Phase 3 trials, SPOTLIGHT and GLOW (NCT03653507) [380], treatment of 537 patients with zolbetuximab plus chemotherapy led to significantly longer median PFS (9.2 mo vs 8.2 mo) and mOS (16.4 mo vs 13.7 mo) than the 535 patients treated with chemotherapy plus placebo [381].
Zolbetuximab, which is dosed on a Q3W schedule, is infused IV over 2 h, and has a very low clearance rate of 0.013 L/h, leading to an estimated TSST1/2 of 41 d [67], which is one of the longer TSST1/2 values for any non-Fc-modified IgG [1].
New anti-HER2 and HER-3 bispecific antibodies for cancer treatments
HER2 and HER3 cancer targets
HER2 is a member of the EGFR TK receptor family [382]. This family consists of four single-pass (Type I) receptors, EGFR (erbB1), HER2 (erbB2, neu), HER3 (erbB3), and HER4 (erbB4) [382]. The erbB receptors are found to be overexpressed in a variety of cancers, making them attractive cancer targets [382]. In fact, as of now, there are four approved antibodies targeting EGFR, six antibodies targeting HER2, and one new antibody targeting HER3 [1].
HER2 and HER3 each have four domains, I, II, III, and IV (N-terminus to membrane direction, respectively). HER2 does not have a ligand, but can spontaneously form a HER2-HER2 homodimer, which triggers phosphorylation of its kinase domain [383] (Fig. 8). HER3, on the other hand, has a ligand, neuregulin 1 (NRG-1; aka heregulin), which is overexpressed in some cancerous tissues such as NSCLC [384]. When HER3 binds NRG-1, it converts it from a non-dimerizing conformation to a dimerizing conformation, which can then heterodimerize with HER2 to trigger receptor kinase phosphorylation [385] (Fig. 8). Trastuzumab binds domain IV nearest the membrane and causes allosteric modifications to the receptor that alter HER2 signaling [383], but it has little effect on the ability of HER2 to dimerize with either itself or HER3 [383]. Pertuzumab, on the other hand, binds the dimerization domain, domain II, and while it does not block homodimerization, it alters the signaling of the HER2-HER2 dimer [386]. Pertuzumab does appear to inhibit heterodimerization with HER3 [386].
Figure 8.
Cartoons of HER2 and HER3. A. HER2 monomer showing extracellular domains I through IV, the CM spanning region, and the intracellular TK domain. Domain II (arrow 1) is the dimerization domain. The binding site on the dimerization domain II for pertuzumab and the Fab arm of zanidatamab are noted with arrow 2. The binding sites on domain IV for trastuzumab, margetuximab, and the scFv arm of zanidatamab are noted with arrow 3. B. HER2 homodimer, which becomes phosphorylated, setting off downstream signaling. C. HER3 monomer in open conformation. D. NRG 1 (aka heregulin), the HER3 ligand (arrow 4), binds HER3 via domains 1 and 3, which then allows for heterodimerization with HER2, setting off phosphorylation and downstream signaling. E. The HER2 × HER3 BisAb, zenocutuzumab, binds HER2 domain I with one Fab arm and HER3 domain III in closed conformation with the other Fab arm (arrow 5), blocking both HER2 and HER3 from forming dimers. This figure was inspired by figures in references [382, 387].
The erbB family, and HER2 (erbB2) itself, has been around as far back as any other major antibody target, having been first described in 1986 [388] and was already associated with breast cancer by 1987 [389, 390]. The anti-HER2 antibody, Herceptin (trastuzumab), was approved by the FDA in 1988 as the seventh antibody-based biologic to be approved by the FDA [391]. Including the two new anti-HER2 antibodies approved in 2024 (Tables 1 and 6), six additional antibodies and ADCs targeting HER2 have been approved by the FDA (Table 6). Besides HER2-overexpressing breast cancer or metastatic breast cancer, anti-HER2 antibodies have now been approved for six additional indications, including BTC, GEJ-AC, metastatic GC, metastatic SCLC, PAC, as well as HER-2 overexpressing solid tumors. These additional approvals and broadened indications have led to a total HER2-targeted market size of $13.5 billion [21] (Fig. 2).
Biliary tract cancer
Bile ducts are slender tubes that connect the liver to the gallbladder and small intestine. BTC, also known as cholangiocarcinoma and bile duct cancer, is a rare cancer (incidence of 0.3–6 per 100 000 people) that forms in the bile ducts [392]. BTC often progresses to later-stage disease before detection, causing the 5-year survival rate to be in the range of 15%, depending on the stage when it is detected [392].
Ziihera® (zanidatamab-hrii) approval for treatment of BTC
Ziihera® (zanidatamab-hrii, aka ZW25) is a humanized bispecific, biparatopic antibody that binds two separate, non-overlapping epitopes on HER2 [370]. Zanidatamab is comprised of an Fc-hinge in which one arm is a Fab and the other is an scFv (Fig. 3). The two asymmetric Fc halves are held together by mutations in the CH3 domains of each heavy chain (HC): HC1 has C255S, T385V, T401L, K427L, T429W, and HC2 has T353V, L354Y, F408A, Y410V [370]. The Fab arm binds domain II of HER2 with a 1:1 affinity of 1.7 nM, and the scFv arm binds to domain IV of HER2 with an affinity of 1.1 nM [370] (Table 6, Fig. 8). By binding two sites on HER2, zanidatamab effectively blocks both homo- and hetero-dimerization, which disrupts phosphorylation and signaling through the HER kinase domains [370]. Additionally, zanidatamab induces clustering of HER2 receptors and kills HER23+ cells via CDC and promotes receptor internalization and degradation [370]. Zanidatamab is the 13th BisAbs to be approved by the FDA, but it is the first therapeutic biparatopic antibody approved.
Zanidatamab, discovered by Zymeworks and licensed in 2022 to the commercial sponsor, Jazz Pharmaceuticals [393], was granted accelerated approval by the FDA on 20 November 2024 for the treatment of previously-treated, unresectable or metastatic HER2-positive (immunohistochemistry [IHC]3+) BTC [70, 71]. The accelerated approval was based mostly on results from Cohort 1 of the HERIZON-BTC-01 multicenter, open-label, single-arm, Phase 2b clinical trial (NCT04466891) [70, 394–396]. Patients were dosed IV with 20 mg/kg Q2W of zanidatamab as a single agent. Cohort 1 patients (n = 80), who entered the trial with high HER2+ tumors, achieved a 41% ORR in which tumors shrank by at least 30%, and an mDOR of 12.4 mo, which was reviewed by independent central review (ICR) [70]. The most common AEs included diarrhea, which occurred in 48% of patients, as well as abdominal pain and fatigue [70, 395, 396]. Additionally, IRRs were also observed in some patients, but most AEs were considered manageable [70].
Neuregulin-1-positive NSCLC and pancreatic adenocarcinoma
As noted above, NRG-1 is the ligand for HER3 that not only activates HER3 but also confers conformation changes in HER3 that allow it to heterodimerize with HER2 and other erbB receptors, increasing downstream signaling and tumorigenesis [397]. Over the past several years, aberrant gene rearrangements leading to NRG-1 fusions have been associated with certain types of cancer, such as pancreatic cancer [398, 399], NSCLC [400, 401], and other solid tumors [402]. One of the most prevalent fusions is the CD74-NRG-1 [403]. NRG-1 fusions with other proteins, such as ATPase Na+/K+ Transporting Subunit Beta 1 (ATP1B1), syndecan-4, and RNA-binding protein with multiple splicing (RBPMS), have also been found. The CD74-NRG-1 fusion appears to be present in as much as 10%–30% of invasive mucinous adenocarcinomas of the lung, a subset of NSCLC [403]. These tumors were originally treated with SM pan-erbB kinase inhibitors such as afatinib, but newer, more targeted treatments also have been sought.
One hypothesis that has been forwarded is that fusions of NRG-1 to membrane proteins such as CD74 may result in higher concentrations of NRG-1 in the proximity of HER3, driving oncogenesis [404]. Initial experiments showing that the HER2 × HER3 BisAb, zenocutuzumab (MCLA-128), which disrupted NRG-1 binding to HER3 as well as disrupting the HER3-HER2 heterodimerization, resulted in shrinkage of NRG-1-fusion-positive tumors (NRG1+ tumors), supported the concept of the NRG-1-protein fusions have been a driver of oncogenesis [404].
The concept of using the BisAb, zenocutuzumab, targeting both HER3 and HER2 simultaneously to treat patients with NRG-1-protein fusions was tested early with a few patients who had developed chemotherapy-resistant NRG1-fusion-positive metastatic cancer [397]. Two patients with ATP1B1-NRG-1-positive pancreatic cancer achieved rapid responses that lasted at least 12 mo, and one patient with CD74-NRG1-positive NSCLC who had progressed on six prior lines of treatment, which included afatinib, had a PR [397]. These types of studies suggested that targeting HER2 and HER3 simultaneously with a single, BisAb could potentially help patients with NRG1 fusion-positive cancers [405].
Bizengri® (zenocutuzumab-zbco) approval for treatment of NRG-1-positive NSCLC and PAC
Bizengri® (zenocutuzumab-zbco, aka MCLA-128) is a humanized, low-fucose, heterodimeric IgG-like BisAb (Fig. 3), simultaneously targeting the domain I of HER2 with a KD of 2.2–3.9 nM with one Fab arm and the domain III of HER3 with a KD of 0.23–0.99 nM with the other Fab arm [371, 372] (Figs 3 and 8, Table 6). The asymmetric Fc is formed by mutations in the CH3 domains of each Fc half. In HC1, which targets HER3, the modifications are at residues L351K and T366K (Eu numbering), and in HC2, targeting HER2, the modifications are at L351D and L368E (Eu numbering) [372]. The positively charged HC1 and negatively charged HC2 (dubbed the “DEKK” mutations) form electrostatic interactions that favor the formation and stability of the asymmetric heterodimer over homodimers [372]. Zenocutuzumab utilizes the common light chain (CLC) approach to deal with the issue of HC/light chain (LC) reassortment [372].
Zenocutuzumab kills cancer cells by three MOAs, the first of which is by blocking the dimerization between HER2 and HER3, and the second is by blocking the ability of the NRG1-fusions to activate HER3 [371]. Interestingly, even though the Fc of zenocutuzumab is fully functional, preclinical experiments showed that the precursor of this antibody did not function by ADCC [371]. Thus, GlymaxX® technology was used to make MCLA-128 [406] a low fucosylated IgG1 [72, 406], enhancing its ability to kill cancer cells via ADCC, the third MOA [406].
Zenocutuzumab, sponsored by Merus, was granted accelerated approval by the FDA on 4 December 2024, for the treatment of patients with NRG-1-fusion-positive NSCLC and NRG-1-fusion-positive pancreatic ductal adenocarcinoma (PDAC), based on the results of the open-label, multi-center, single-agent Phase 1/2 clinical trial, eNRGy trial (NCT02912949). Overall, the trial studied 204 patients with 12 different NRG-1-fusion positive tumor types [407]. A total of 158 patients were evaluated across all enrolled tumor types with an ORR of 30% and an mDOR of 11 mo [407].
Of 64 patients with NSCLC studied in eNRGy, 37/64 (58%) had NRG-1-CD74 fusions, 14/64 (22%) had NRD-1-SLC3A2 (Solute Carrier Family 3 Member 2) fusions, 7/64 (11%) had NRG-1-syndecan-4 fusions, and other patients had rarer fusions [73]. The 64 NRG1-fusion-positive NSCLC patients treated with zenocutuzumab had an ORR of 33%, with 31% PRs and ca. 2% CRs [73]. In a larger population (93 NSCLC patients), 29% had an ORR [407]. mDOR was 7.4 mo, and 43% of patients had an mDOR >6 mo [73].
Amongst the 30 patients with advanced or metastatic NRG1-fusion-positive PAC studied in eNRGy, 14/30 (47%) had NRG-1-ATP1B1 fusions, and the rest had rarer fusions [72]. Of a total of 36 patients with advanced or metastatic NRG1-fusion-positive PAC treated with zenocutuzumab in the eNRGy trial, ORR was 42% [407]. mDOR was 6.8 mo [407], and 67% of patients had an mDOR >6 mo [73].
The most serious side effects associated with zenocutuzumab treatment were diarrhea, which 18% of all patients experienced (Schram), IRRs (14%), and nausea (11%), as well as fatigue, dyspnea, pain, and liver enzyme elevation, amongst other AEs [73]. The TSST1/2 of zenocutuzumab was 8 d, supporting the 750 mg Q2W dosing schedule, and only 4.6% of patients treated had ADAs, none of which affected PK, efficacy, or safety [73].
AUCATZYL® (OBECABTAGENE AUTOLEUCEL)
Acute lymphoblastic leukemia
ALL is an aggressive, fast-growing form of leukemia characterized by the bone marrow overproducing one form or another of poorly differentiated, abnormal lymphoid progenitor cells [408]. ALL is typically a disease of children and young adults, with ~4000 new cases/year in the USA, making it nearly one-third of all childhood cancers [408]. Of ALL, B-cell ALL (B-ALL) comprises about 80% of all cases. B-ALL appears to be linked to a series of mutations at the pluripotent stem cell stage, which ultimately differentiate and proliferate into abnormal B-cells.
Treatment of B-ALL can require up to 2 years and includes three phases: induction, consolidation, and maintenance [409]. Chemotherapy is typically used for the induction phase, intensive chemotherapy with or without Blincyto® is often used for the consolidation phase, and methotrexate with or without chemotherapy agents may be used for maintenance. Generally, 80%–90% have complete remissions, of which about half relapse, resulting in a ca. 40% cure rate. Those who relapse, or those who do not respond to treatment (refractory), are treated with either an mAb or CAR-T targeting B-cells [409].
Aucatzyl® (obecabtagene autoleucel; Obe-cel) approval for treatment of B-ALL
Aucatzyl® (obecabtagene autoleucel, aka “obe-cel” or AUTO1) is an autologous CAR-T cell therapy, sponsored by Autolus, that was approved by the FDA on 8 November 2024, for treatment of adults with relapsing or refractory (R/R) B-cell precursor ALL [419]. Obe-cell is the 7th CAR-T cell product to be approved by the FDA, and the 5th targeting CD19. The general structure of obe-cel CAR-T, shown in Fig. 3, is, from N- to C-terminus, anti-CD19 scFv-CD8 stalk-transmembrane domain-linker-4-1BB domain-CD3ζ domain [420, 421].
One of the key factors differentiating obe-cel from other CD19 CAR-T constructs is that the scFv is a low-affinity binder called CAT [421, 422]. It has an off-rate from CD19 of 9.8 s, as compared with the off-rate of the anti-CD19 scFv FMC63, used in Kymriah®, of 21 min [423]. This significantly faster off-rate is considered by the developers to result in avoidance of overstimulation and T-cell exhaustion, increased CAR-T persistence, and improved safety profile [421–423].
The efficacy of obe-cel in patients with relapsed or refractory B-ALL was tested in an open-label, multi-center, single-arm Phase 1b/2 clinical trial, FELIX (NCT04404660) [424]. Patients received a target dose of 410 × 106 CAR T cells, split between two doses, one on day 1 and the other on day 10 (±2 d) [425]. Of the 127 evaluable patients 77% had ORRs, with 55% CRs, a mOS of 15.6 mo, and a 12-mo OS rate of 61% [425, 426]. As with all CAR-T therapeutics to date, significant AEs included Grade 3 or higher CRS (2.4% of patients) or ICANS (7.1% of patients) [425, 426].
Newly approved antibody-hyaluronidase coformulations for subcutaneous administration
While not NMEs, there was a group of antibodies co-formulated with recombinant human hyaluronidase (rHuPH20) that were approved in 2024, which deserves special recognition. The enzyme hyaluronidase has been used for a decade now to enhance SCD of specific biologics due to its ability to degrade, locally and transiently, hyaluronan in the SC space [427] (Table 7).
Table 7.
FDA-approved antibody-based biologics combined with rHuPH20
| US Trade Name | Sponsor | FDA approval date | Molecular target | Major indication | Protein format | Delivery information | Bioavailabilitya | Refs |
|---|---|---|---|---|---|---|---|---|
| Hyqvia® | CSL Behring (now Takeda) | 12 September 2014 | Multiple | PID | 10% HIg/ rHuPH20 | SCD of 100 mg/ml at 1–2 ml/min; 24GN; peristaltic or syringe pump | 93% | [423. 425] |
| Rituxan Hylecta® | Genentech | 22 June 2017 | CD20 | NHL | Rituximab/ rHuPH20 | SCD of 120 mg/ml at ca. 2 ml/min; 25GN; syringe | 63–64% | [410, 411] |
| Herceptin Hylecta® | Genentech | 28 February 2019 | HER2 | BC | Trastuzumab/ rHuPH20 | SCD of 120 mg/ml at ca. 2 ml/min; 25GN; syringe | 77% | [410, 412] |
| Darzalex Faspro® | Janssen (JNJ) | 1 May 2020 | CD38 | MM | Daratumumab/ rHuPH20 | SCD of 120 mg/ml at ca. 3–5 ml/min; 23GN; syringe | 70% | [410, 413] |
| Phesgo® | Genentech | 29 June 2020 | HER2 | BC | Pertuzumab/ trastuzumab/ rHuPH20 | SCD of 120 mg/ml at ca. 2 ml/min; 25GN; syringe | 70% Pertuzumab; 80% trastuzumab |
[410, 414] |
| Vyvgart Hytrulo® | Argenx | 20 June 2023 | FcRn | AchR-Ab+ gMG | Efgartigimod/ rHuPH20 | SCD of 180 mg/ml at ca. 3.7 ml/min; 25GN; syringe | ~50% | [415] |
| Tecentriq Hybreza® | Genentech | 12 September 2024 | PD-L1 | NSCLC, SCLC, HCC, MMLN, ASPS | Atezolizumab/ rHuPH20 | 1875 mg atezolizumab and 30 000 units’ hyaluronidase per 15 ml (125 mg/2000 units per ml) solution; single-dose vial; 23-25GN; syringe | 72% | [416] |
| Ocrevus zunovo® | Genentech | 13 September 2024 | CD20 | RMS, PPMS | Ocrelizumab/ rHuPH20 | 920 mg ocrelizumab and 23 000 units’ hyaluronidase per 23 ml (40 mg and 1000 units per ml) solution; single-dose vial; 24-26GN; syringe | 81% | [417] |
| Opdivo Qvantig® | BMS | 27 December 2024 | PD-1 | >10 cancer indications | Nivolumab/ rHuPH20 | 600 mg/10 000 units every 2 weeks or 1200 mg/20 000 units every 4 weeks; single-dose vial; 23-25GN; syringe | 74% | [418] |
Abbreviations: AChR-Ab+ gMG, anti-acetylcholine receptor (AChR) antibody-positive generalized myasthenia gravis (gMG). ASPS, alveolar soft part sarcoma. BMS, Bristol Myers-Squibb. CD, cluster of differentiation. FcRn, neonatal fragment crystallizable receptor. GN, gauge needle. HCC, hepatocarcinoma. HER2, human epidermal growth factor 2. HIg, human immunoglobulin. JNJ, Johnson & Johnson. MM, multiple myeloma. MMLN, metastatic melanoma. NHL, non-Hodgkin lymphoma (multiple forms). NSCLC, non-small cell lung cancer. PD-1, programmed death-receptor 1. PD-L1, programmed death-receptor-ligand 1. PID, primary immunodeficiency. PPMS, primary progressive multiple sclerosis. Refs, references. rHuPH20, recombinant human hyaluronidase 20 developed as Enhanze® by Halozyme. RMS, remitting multiple sclerosis. SCD, subcutaneous delivery. SCLC, small cell lung cancer.
aAs compared with IV administration.
Hyaluronic acid (aka hyaluronan or hyaluronate; HA) is a lubricating, highly aquaphilic, high molecular weight glycosaminoglycan that is widely distributed in the extracellular matrix of the SC tissue, which tends to restrict the movement of molecules in that region. It plays a wide range of roles as a homeostatic factor in cell integrity, cell renewal, and cell death [428]. HA has a half-life of ca. 12 h, the depolymerization and degradation of which is catalyzed by the naturally occurring endoglycosidase, hyaluronidase. Depolymerization of HA by hyaluronidase has been demonstrated to increase permeability in the SC tissue [428].
This relatively new alternative delivery technology, developed by Halozyme and licensed to several major antibody development companies (Table 7), can reduce delivery of high dose biologics from hours to minutes, as exemplified by the difference between Rituxan (MabThera, in EU) intravenous (IV) dosing, which can take up to 3–4 h, versus Rituxan Hycela® (rituximab coformulated with rHuPH20), which can be dosed in <10 min [410].
rHuPH20 is a 61 kDa glycosylated monomeric protein produced recombinantly by Chinese Hamster Ovary (CHO) cell culture. It catalyzes the depolymerization of HA via hydrolysis of the β-1,4 glycosidic bonds sequentially from the reducing end of HA [429]. By locally degrading HA at the SC injection site, dispersion, bulk flow, and adsorption of the co-administered biologics are increased significantly, allowing for rapid administration of larger volumes of highly concentrated biologics [410, 429]. The effects of locally administered hyaluronidase are fully reversible, and the permeability of the SC tissue is fully restored within 24–48 h [410].
Most high-dose antibodies are formulated to be administered as IV infusions over a period typically ranging from an hour to as much as 12 h. Thus, for years, efforts have been made to reduce the infusion times and improve the patient experience of being administered these drugs. The first use of rHuPH20 to improve SC dosing was with the approval in 2014 of Hyqvia®, a high concentration coformulation of human immunoglobulin (HIg) with rHuHP20 [410, 430] (Table 7). The first mAb approved by the FDA to be coformulated with rHuHP20 for SCD was rituximab, which was approved as Rituxan Hylecta® in 2017 [410, 411] (Table 7). By the end of 2023, four additional rHuHP20-coformulated mAbs had been approved for SCD [410], and in 2024, another three were approved, bringing the total to nine (Table 7). As can be seen, a wide variety of different antibodies, many requiring high doses for treating certain forms of cancer, multiple sclerosis, or autoimmune diseases, have now been coformulated with rHuPH20 for faster, more convenient, SCD [410]. (Table 7).
Summary
In 2024, 15 new antibody-based biologics were approved by the FDA for 16 different indications, representing the greatest number of antibody-based biologics approved by the FDA in a single calendar year. Additionally, the 15 antibodies were amongst a total of 47 drugs approved by the FDA (32%), the highest percentage of antibodies/total drugs approved by the FDA in history. One key factor that stands out is the approval of three new BisAbs, bringing the total of FDA-approved BisAbs to 14. Additionally, the seventh CAR-T, which might be considered a “cellular antibody,” was approved. As this review was being finalized, a nice review of all 53 new drugs approved by the FDA, the European Medicines Agency (EMA), and the Medicines and Healthcare Products Regulatory Agency (MHRA) was published [431]. Considering the large number of antibodies in late-stage and/or registrational clinical trials [1, 7, 8], we should see another large group of novel antibodies approved by the FDA in 2025.
Acknowledgments
None.
Author contributions
William Strohl (Conceptualization [equal], Investigation [equal], Writing—original draft [equal], Writing—review & editing [equal])
Conflict of interest
W.R.S. is the sole owner and employee of BiStro Biotech Consulting LLC, an independent consulting company. W.R.S. also serves as an independent Director for IGM Biosciences and also serves on the Scientific Advisory Boards of two other small biotechnology companies. W.R.S. is an editorial board member of Antibody Therapeutics, but is blinded from reviewing or making decisions for this manuscript.
Funding
None declared.
Data availability
Data supporting the information provided here are derived from the public literature or public websites.
Ethics and consent statement
Consent was not required.
Animal research statement
Not applicable.
References
- 1. Strohl WR. Bistro Biotech Consulting LLC antibody database. (Not published). Updated 3/11/25.
- 2. Ingram I. FDA Revokes EUAs of Four COVID-19 Monoclonal Antibodies. MedPageToday, https://www.medpagetoday.com/infectiousdisease/covid19/113392 January 22 2025, date last accessed. [Google Scholar]
- 3. MacMillan C. FDA Authorizes COVID Drug Pemgarda for High-Risk Patients. Yale Medicine, https://www.yalemedicine.org/news/new-covid-drug-pemgarda January 22 2025, date last accessed. [Google Scholar]
- 4. Novel Drug Approvals for 2024 . U.S. Food and Drug Administration, https://www.fda.gov/drugs/novel-drug-approvals-fda/novel-drug-approvals-2024 January 7 2025, date last accessed. [Google Scholar]
- 5. Advancing Health through Innovation: New Drug Therapy Approvals 2024 . U.S. Food and Drug Administration. Center for Drug Evaluation and Research, https://www.fda.gov/media/184967/download?attachment January 25 2025, date last accessed. [Google Scholar]
- 6. 2024 Biological License Application Approvals . U.S. Food and Drug Administration, https://www.fda.gov/vaccines-blood-biologics/development-approval-process-cber/2024-biological-license-application-approvals January 7 2025, date last accessed. [Google Scholar]
- 7. Crescioli S, Kaplon H, Chenoweth A. et al. Antibodies to watch in 2024. MAbs 2024;16:2297450. 10.1080/19420862.2023.2297450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Crescioli S, Kaplon H, Wang L. et al. Antibodies to watch in 2025. MAbs 2025;17:2443538. 10.1080/19420862.2024.2443538. [DOI] [PubMed] [Google Scholar]
- 9. Strohl WR. Structure and function of therapeutic antibodies approved by the US FDA in 2023. Antib Ther 2024;7:132–56. 10.1093/abt/tbae007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. 2013 Sales of Recombinant Therapeutic Antibodies & Proteins , p. 37. Stuttgart D-70180, Germany: La Merie Publishing, 3/15/14, date last accessed. [Google Scholar]
- 11. 2014 Sales of Recombinant Therapeutic Antibodies & Proteins , p. 39. Stuttgart D-70180, Germany: La Merie Publishing, 3/15/15, date last accessed. [Google Scholar]
- 12. 2015 Sales of Recombinant Therapeutic Antibodies & Proteins , p. 39. Stuttgart D-70180, Germany: La Merie Publishing, 3/13/16, date last accessed. [Google Scholar]
- 13. 2016 Sales of Recombinant Therapeutic Antibodies & Proteins , p. 39. Weikersheim D-97990, Germany: La Merie Publishing, 3/16/17, date last accessed. [Google Scholar]
- 14. 2017 Sales of Recombinant Therapeutic Antibodies & Proteins , p. 53. Weikersheim D-97990, Germany: La Merie Publishing, 3/9/18, date last accessed. [Google Scholar]
- 15. 2018 Sales of Recombinant Therapeutic Antibodies & Proteins , p. 55. Weikersheim D-97990, Germany: La Merie Publishing, 3/31/19, date last accessed. [Google Scholar]
- 16. 2019 Sales of Recombinant Therapeutic Antibodies & Proteins , p. 65. Siges, E-08870, Spain: La Merie Publishing, 3/8/20, date last accessed. [Google Scholar]
- 17. 2020 Sales of Recombinant Therapeutic Antibodies, Proteins, Biosimilars & Other Biologics , p. 88. Ripoll, E-17500, Spain: La Merie Publishing, 3/11/21, date last accessed. [Google Scholar]
- 18. 2021 Sales of Recombinant Therapeutic Antibodies, Proteins, Biosimilars & Other Biologics , p. 66. Ripoll, E-17500, Spain: La Merie Publishing, 3/15/22, date last accessed. [Google Scholar]
- 19. 2022 Sales of Recombinant Therapeutic Antibodies, Proteins, Biosimilars & Other Biologics , p. 71. Ripoll, E-17500, Spain: La Merie Publishing, 3/7/23, date last accessed. [Google Scholar]
- 20. 2023 Sales of Recombinant Therapeutic Antibodies, Proteins, Biosimilars & Other Biologics , p. 77. D-97990 Weikersheim, Germany: La Merie Publishing, 3/7/24, date last accessed. [Google Scholar]
- 21. 2024 Sales of Recombinant Therapeutic Antibodies, Proteins, Biosimilars & Other Biologics , p. 84. D-97990 Weikersheim, Germany: La Merie Publishing, 3/8/25, date last accessed. [Google Scholar]
- 22. Global Antibody Market 2024 to 2034 Growing at a CAGR of 8.1%, the Global Market to Reach USD 581.42 Billion by 2034 . GlobalNewswire, Future Market Insights, Inc, https://www.globenewswire.com/news-release/2024/10/01/2956138/0/en/Global-Antibody-Market-2024-to-2034-Growing-at-a-CAGR-of-8-1-the-Global-Market-to-Reach-USD-581-42-Billion-by-2034-Future-Market-Insights-Inc.html). October 01, 2024 17 January 2025, date last accessed. [Google Scholar]
- 23. Tang D, Kang R. Tarlatamab: the promising immunotherapy on its way from the lab to the clinic. Transl Lung Cancer Res 2023;12:1355–7. 10.21037/tlcr-23-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gerlach J, Odintsov I, Deshiere A. et al. Zenocutuzumab is an effective HER2/HER3 Biclonics® antibody in cancers with NRG1 fusions. In: AACR-NCI-EORTC Virtual International Conference on Molecular Targets and Cancer Therapeutics (October 7 2021, Vol. 20, p. P201 https://merus.nl/wp-content/uploads/2021/10/Gerlach_Jan_Zenocutuzumab-is-an-effective-HER2-HER3-Biclonics-antibody_Final.pdf. 21 February 2025, date last accessed 10.1158/1535-7163.TARG-21-P201. [DOI] [Google Scholar]
- 25. Weisser NE, Wickman G, Abraham L. et al. Abstract 1005: the bispecific antibody zanidatamab’s (ZW25’s) unique mechanisms of action and durable anti-tumor activity in HER2-expressing cancers. Cancer Res 2021;81:1005–5. 10.1158/1538-7445.AM2021-1005. [DOI] [Google Scholar]
- 26. Villanueva J, Wade J, Torres A. et al. Sotatercept: The first FDA-approved activin a receptor IIA inhibitor used in the management of pulmonary arterial hypertension. Am J Cardiovasc Drugs 2025;25:17–24. 10.1007/s40256-024-00694-w. [DOI] [PubMed] [Google Scholar]
- 27. Keam SJ. Nogapendekin alfa Inbakicept: First approval. Drugs. 2024;84:867–74. 10.1007/s40265-024-02060-1. [DOI] [PubMed] [Google Scholar]
- 28. Giffin MJ, Cooke K, Lobenhofer EK. et al. AMG 757, a half-life extended, DLL3-targeted bispecific T-cell engager, shows high potency and sensitivity in preclinical models of small-cell lung cancer. Clin Cancer Res 2021;27:1526–37. 10.1158/1078-0432.CCR-20-2845. [DOI] [PubMed] [Google Scholar]
- 29. Dhillon S. Tarlatamab: first approval. Drugs. 2024;84:995–1003. 10.1007/s40265-024-02070-z. [DOI] [PubMed] [Google Scholar]
- 30. Keam SJ. Zolbetuximab: first approval. Drugs. 2024;84:977–83. 10.1007/s40265-024-02056-x. [DOI] [PubMed] [Google Scholar]
- 31. Nakayama I, Qi C, Chen Y. et al. Claudin 18.2 as a novel therapeutic target. Nat Rev Clin Oncol 2024;21:354–69. 10.1038/s41571-024-00874-2. [DOI] [PubMed] [Google Scholar]
- 32. Olasupo OO, Noronha N, Lowe MS. et al. Non-clotting factor therapies for preventing bleeds in people with congenital hemophilia A or B. Cochrane Database Syst Rev 2024;2024:CD014544. 10.1002/14651858.CD014544.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tislelizumabum . WHO Drug Info, Vol. 32: RL79, p. 174. Geneva, Switzerland: World Health Organization, 2018, https://www.who.int/our-work/access-to-medicines-and-health-products/who-drug-information 2 December 2024, date last accessed. [Google Scholar]
- 34. Tevimbra® Prescribing information . 2024. US License No. 2232. Manufactured and marketed by BeiGene. https://www.beigene.com/PDF/TEVIMBRAUSPI.pdf. 12 September 2024, date last accessed. [Google Scholar]
- 35. TEVIMBRA® Approved in U.S. for First-Line Treatment of Advanced Esophageal Squamous Cell Carcinoma in Combination with Chemotherapy . BeiGene Press Release, 2024, https://ir.beigene.com/news/tevimbra-approved-in-u-s-for-first-line-treatment-of-advanced-esophageal-squamous-cell-carcinoma-in-combination/8379a7c3-35ce-45af-82d3-164c64ecf37c/ 5 December 2024, date last accessed. [Google Scholar]
- 36. Sotaterceptum . WHO Drug Info, Vol. 25: RL65, p. 97. Geneva, Switzerland: World Health Organization, 2011, https://www.who.int/our-work/access-to-medicines-and-health-products/who-drug-information 12 December 2024, date last accessed. [Google Scholar]
- 37. Winrevair® Prescribing information . 2024. US License No. 0002. Manufactured and marketed by Merck. www.accessdata.fda.gov/drugsatfda_docs/label/2024/761363s000lbl.pdf. 12 December 2024, date last accessed. [Google Scholar]
- 38.FDA Approves Merck’s WINREVAIR™ (sotatercept-csrk), a First-in-Class Treatment for Adults with Pulmonary Arterial Hypertension (PAH, WHO* Group 1). 2024. https://www.merck.com/news/fda-approves-mercks-winrevair-sotatercept-csrk-a-first-in-class-treatment-for-adults-with-pulmonary-arterial-hypertension-pah-who-group-1/. 12 December 2024, date last accessed.
- 39. Nogapendekinum alfa . WHO Drug Info, Vol. 34: RL83, p. 61. Geneva, Switzerland: World Health Organization, 2020, https://www.who.int/our-work/access-to-medicines-and-health-products/who-drug-information 23 January 2025, date last accessed. [Google Scholar]
- 40. Inbakicept-pmln . WHO Drug Info, Vol. 33: RL82, p. 644. Geneva, Switzerland: World Health Organization, 2019, https://www.who.int/our-work/access-to-medicines-and-health-products/who-drug-information 23 January 2025, date last accessed. [Google Scholar]
- 41. Anktiva® Prescribing information . 2024. US License No. 2302. Manufactured by Altor BioScience, LLC, an indirect wholly-owned subsidiary of ImmunityBio, Inc. https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761336s000lbl.pdf. 23 January 2025, date last accessed. [Google Scholar]
- 42.FDA approves nogapendekin alfa inbakicept-pmln for BCG-unresponsive non-muscle invasive bladder cancer. 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-nogapendekin-alfa-inbakicept-pmln-bcg-unresponsive-non-muscle-invasive-bladder-cancer. 23 January 2025, date last accessed.
- 43. Tarlatamabum . WHO Drug Info, Vol. 35: RL85, p. 214. Geneva, Switzerland: World Health Organization, 2021, https://www.who.int/our-work/access-to-medicines-and-health-products/who-drug-information. 28 January 2025, date last accessed. [Google Scholar]
- 44. Imdelltra® Prescribing information . 2024. US License No. 1080. Manufactured by Amgen. https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761344s000lbl.pdf. 28 January 2025, date last accessed. [Google Scholar]
- 45. FDA Approves IMDELLTRA™ (Tarlatamab-Dlle), the First and Only T-Cell Engager Therapy for the Treatment of Extensive-Stage Small Cell Lung Cancer . Amgen press release, 2024, https://www.amgen.com/newsroom/press-releases/2024/05/fda-approves-imdelltra-tarlatamabdlle-the-first-and-only-tcell-engager-therapy-for-the-treatment-of-extensivestage-small-cell-lung-cancer. 28 January 2025, date last accessed. [Google Scholar]
- 46. Crovalimabum . WHO Drug Info, Vol. 33: RL81, p. 61. Geneva, Switzerland: World Health Organization, 2019, https://www.who.int/our-work/access-to-medicines-and-health-products/who-drug-information. 23 January 2025, date last accessed. [Google Scholar]
- 47. Piasky® Prescribing information . 2024. US License No. 1048. Manufactured by Genentech. https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761388s000lbl.pdf. 23 January 2025, date last accessed. [Google Scholar]
- 48. Fukuzawa T, Sampei Z, Haraya K. et al. Long lasting neutralization of C5 by SKY59, a novel recycling antibody, is a potential therapy for complement-mediated diseases. Sci Rep 2017;7:1080. 10.1038/s41598-017-01087-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.FDA Approves Crovalimab for Paroxysmal Nocturnal Hemoglobinuria. 2024. https://www.onclive.com/view/fda-approves-crovalimab-for-paroxysmal-nocturnal-hemoglobinuria. 23 January 2025, date last accessed.
- 50. Donanemabum . WHO Drug Info, Vol. 33: RL82, p. 629. Geneva, Switzerland: World Health Organization, 2019, https://www.who.int/our-work/access-to-medicines-and-health-products/who-drug-information. 23 January 2025, date last accessed. [Google Scholar]
- 51. Kisunla® prescribing information . 2024. US License No. 1891. Manufactured by Eli Lilly. https://www.fda.gov/media/180803/download. 2 February 2025, date last accessed. [Google Scholar]
- 52.FDA approves treatment for adults with Alzheimer’s disease. 2024. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-treatment-adults-alzheimers-disease. 2 February 2025, date last accessed.
- 53.Galderma and Chugai Announce Global License Agreement for Nemolizumab, Novel Biotherapy for Skin Diseases. https://www.galderma.com/news/galderma-and-chugai-announce-global-license-agreement-nemolizumab-novel-biotherapy-skin. 27 January 2025, date last accessed.
- 54. Nemolizumabum . WHO Drug Info, Vol. 29: RL74, p. 411. Geneva, Switzerland: World Health Organization, 2015, https://www.who.int/our-work/access-to-medicines-and-health-products/who-drug-information. 27 January 2025, date last accessed. [Google Scholar]
- 55. Nemluvio® Prescribing information . 2024. US License No. 2289. Manufactured by Galderma. https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761390s000lbl.pdf. 27 January 2025, date last accessed. [Google Scholar]
- 56. Galderma receives U.S . FDA Approval for Nemluvio® (Nemolizumab) for Adult Patients Living with Prurigo Nodularis. Galderma Press Release, 2024, https://www.galderma.com/news/galderma-receives-us-fda-approval-nemluvior-nemolizumab-adult-patients-living-prurigo. 27 January 2025, date last accessed. [Google Scholar]
- 57. Axatilimabum . WHO Drug Info, Vol. 34: RL83, p. 12. Geneva, Switzerland: World Health Organization, 2020, https://www.who.int/our-work/access-to-medicines-and-health-products/who-drug-information. 11 February 2025, date last accessed. [Google Scholar]
- 58. Niktimvo® Prescribing information . 2024. US License No. 2228. Manufactured by Incyte Corp. https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761411s000lbl.pdf. 11 February 2025, date last accessed. [Google Scholar]
- 59.FDA approves axatilimab-csfr for chronic graft-versus-host disease. 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-axatilimab-csfr-chronic-graft-versus-host-disease. 11 February 2025, date last accessed.
- 60. Lebrikizumabum . WHO Drug Info, Vol. 24: RL63, p. 63. Geneva, Switzerland: World Health Organization, 2010, https://www.who.int/our-work/access-to-medicines-and-health-products/who-drug-information. 14 February 2025, date last accessed. [Google Scholar]
- 61. Ebglyss® Prescribing information . 2024. US License No. 1891. Manufactured by Eli Lilly. https://pi.lilly.com/us/ebglyss-uspi.pdf. 14 February 2025, date last accessed. [Google Scholar]
- 62.FDA approves Lilly's EBGLYSS™ (lebrikizumab-lbkz) for adults and children 12 Years and older with moderate-to-severe atopic dermatitis. 2024. https://investor.lilly.com/news-releases/news-release-details/fda-approves-lillys-ebglysstm-lebrikizumab-lbkz-adults-and. 14 February 2025, date last accessed.
- 63. Marstacimabum . WHO Drug Info, Vol. 33: RL81, p. 83. Geneva, Switzerland: World Health Organization, 2019, https://www.who.int/our-work/access-to-medicines-and-health-products/who-drug-information. 7 February 2025, date last accessed. [Google Scholar]
- 64. Hympavzi® Prescribing information . 2024. US License No. 1891. Manufactured by Eli Lilly. https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761369s000lbl.pdf. 7 February 2025, date last accessed. [Google Scholar]
- 65.FDA Approves New Treatment for Hemophilia A or B. 2024. https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-hemophilia-or-b. 7 February 2025, date last accessed.
- 66. Zolbetuximabum . WHO Drug Info, Vol. 32: RL79, p. 182. Geneva, Switzerland: World Health Organization, 2018, https://www.who.int/our-work/access-to-medicines-and-health-products/who-drug-information. 17 February 2025, date last accessed. [Google Scholar]
- 67. Vyloy® Prescribing information . 2024. US License No. 2124. Manufactured by Astellas Pharma. https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761365s000lbl.pdf. 17 February 2025, date last accessed. [Google Scholar]
- 68.FDA approves zolbetuximab-clzb with chemotherapy for gastric or gastroesophageal junction adenocarcinoma. 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-zolbetuximab-clzb-chemotherapy-gastric-or-gastroesophageal-junction-adenocarcinoma. 17 February 2025, date last accessed.
- 69. Zanidatamabum . WHO Drug Info, Vol. 34: RL83, p. 112. Geneva, Switzerland: World Health Organization, 2020, https://www.who.int/our-work/access-to-medicines-and-health-products/who-drug-information. 28 January 2025, last date accessed. [Google Scholar]
- 70. Ziihera® Prescribing information . 2024. US License No. 2167. Manufactured by Jazz Pharmaceuticals. https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761416s000lbl.pdf. 17 February 2025, last date accessed. [Google Scholar]
- 71.FDA grants accelerated approval to zanidatamab-hrii for previously treated unresectable or metastatic HER2-positive biliary tract cancer. 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-zanidatamab-hrii-previously-treated-unresectable-or-metastatic-her2. 17 February 2025, last date accessed.
- 72. Zenocutuzumabum . WHO Drug Info, Vol. 32: RL181, p. p174. Geneva, Switzerland: World Health Organization, 2018, https://www.who.int/our-work/access-to-medicines-and-health-products/who-drug-information. 19 February 2025, last date accessed. [Google Scholar]
- 73. Bizengri® Prescribing information . 2024. US License No. 2310. Manufactured by Merus NV. https://www.bizengri.com/pdf/pi.pdf. 19 February 2025, last date accessed. [Google Scholar]
- 74.FDA grants accelerated approval to zenocutuzumab-zbco for non-small cell lung cancer and pancreatic adenocarcinoma. 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-zenocutuzumab-zbco-non-small-cell-lung-cancer-and-pancreatic. 19 February 2025, last date accessed.
- 75. Cosibelimabum . WHO Drug Info, Vol. 34: RL83, p. 22. Geneva, Switzerland: World Health Organization, 2020, https://www.who.int/our-work/access-to-medicines-and-health-products/who-drug-information. 14 February 2025, last date accessed. [Google Scholar]
- 76. Unloxcyt® Prescribing information . 2024. US License No. 2275. Manufactured by Checkpoint Therapeutics. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761297s000lbl.pdf. 14 February 2025, last date accessed. [Google Scholar]
- 77.FDA approves cosibelimab-ipdl for metastatic or locally advanced cutaneous squamous cell carcinoma. 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-cosibelimab-ipdl-metastatic-or-locally-advanced-cutaneous-squamous-cell-carcinoma. 14 February 2025, last date accessed.
- 78. Concizumabum . WHO Drug Info, Vol. 27: RL70, p. 282. Geneva, Switzerland: World Health Organization, 2013, https://www.who.int/our-work/access-to-medicines-and-health-products/who-drug-information. 8 February 2025, last date accessed. [Google Scholar]
- 79. Alhemo® Prescribing information . 2024. US License No. 1261. Manufactured by Novo Nordisk. https://www.novo-pi.com/alhemo.pdf. 8 February 2025, last date accessed. [Google Scholar]
- 80.FDA approves drug to prevent or reduce the frequency of bleeding episodes for patients with hemophilia A with inhibitors or hemophilia B with inhibitors. 2024. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-drug-prevent-or-reduce-frequency-bleeding-episodes-patients-hemophilia-inhibitors-or. 8 February 2025, last date accessed.
- 81. Obecabtagene autoleucel . WHO Drug Info, Vol. 35: RL85, p. 180. Geneva, Switzerland: World Health Organization, 2023, https://www.who.int/our-work/access-to-medicines-and-health-products/who-drug-information. 23 January 2025, last date accessed. [Google Scholar]
- 82. Aucatzyl® Prescribing information . 2024. US License No. 2339. Manufactured by Autolus Ltd. https://www.novo-pi.com/alhemo.pdf. 23 January 2025, last date accessed. [Google Scholar]
- 83.FDA approves obecabtagene autoleucel for adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia. 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-obecabtagene-autoleucel-adults-relapsed-or-refractory-b-cell-precursor-acute. 23 January 2025, last date accessed.
- 84. Dunleavy K. 2024. drug approvals: Small companies loom large with several key FDA nods. Fierce Pharma. https://www.fiercepharma.com/pharma/2024-drug-approvals?utm_medium=email&utm_source=nl&utm_campaign=LS-NL-FiercePharma&oly_enc_id=8564A3052334J4C. 24 January 2025, last date accessed. [Google Scholar]
- 85. Angal S, King DJ, Bodmer MW. et al. A single amino acid substitution abolishes the heterogeneity of chimeric mouse/human (IgG4) antibody. Mol Immunol 1993;30:105–8. 10.1016/0161-5890(93)90432-b. [DOI] [PubMed] [Google Scholar]
- 86. Handlogten MW, Peng L, Christian EA. et al. Prevention of Fab-arm exchange and antibody reduction via stabilization of the IgG4 hinge region. MAbs 2020;12:1779974. 10.1080/19420862.2020.1779974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yang X, Wang F, Zhang Y. et al. Comprehensive analysis of the therapeutic IgG4 antibody pembrolizumab: hinge modification blocks half molecule exchange in vitro and in vivo. J Pharm Sci 2015;104:4002–14. 10.1002/jps.24620. [DOI] [PubMed] [Google Scholar]
- 88. Strohl WR. Optimization of Fc-mediated effector functions of monoclonal antibodies. Curr Opin Biotechnol 2009;20:685–91. 10.1016/j.copbio.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 89. Hale G. Living in LALA land? Forty years of attenuating Fc effector functions. Immunol Rev 2024;328:422–37. 10.1111/imr.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hale G, De Vos J, Davy AD. et al. Systematic analysis of Fc mutations designed to reduce binding to Fc-gamma receptors. MAbs 2024;16:2402701. 10.1080/19420862.2024.2402701. Erratum in: MAbs. 2024; 16: 2416272. 10.1080/19420862.2024.2416272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Jacobsen FW, Stevenson R, Li C. et al. Engineering an IgG scaffold lacking effector function with optimized developability. J Biol Chem 2017;292:1865–75. 10.1074/jbc.M116.748525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wilkinson I, Anderson S, Fry J. et al. Fc-engineered antibodies with immune effector functions completely abolished. PloS One 2021;16:e0260954. 10.1371/journal.pone.0260954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Raje N, Vallet S. Sotatercept, a soluble activin receptor type 2A IgG-Fc fusion protein for the treatment of anemia and bone loss. Curr Opin Mol Ther 2010;12:586–97. [PubMed] [Google Scholar]
- 94. Han KP, Zhu X, Liu B. et al. IL-15:IL-15 receptor alpha superagonist complex: High-level co-expression in recombinant mammalian cells, purification and characterization. Cytokine 2011;56:804–10. 10.1016/j.cyto.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. de Vries Schultink AHM, Doornbos RP, Bakker ABH. et al. Translational PK-PD modeling analysis of MCLA-128, a HER2/HER3 bispecific monoclonal antibody, to predict clinical efficacious exposure and dose. Invest New Drugs 2018;36:1006–15. 10.1007/s10637-018-0593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Roddie C, Dias J, O'Reilly MA. et al. Durable responses and low toxicity after fast off-rate CD19 chimeric antigen receptor-T therapy in adults with relapsed or refractory B-cell acute lymphoblastic leukemia. J Clin Oncol 2021;39:3352–63. 10.1200/JCO.21.00917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Dahl J, Mace M, Kantarjian H. et al. Blinatumomab for the treatment of adult acute lymphoblastic leukemia. Drugs Today (Barc) 2015;51:231–41. 10.1358/dot.2015.51.4.2291051. [DOI] [PubMed] [Google Scholar]
- 98. Sampei Z, Igawa T, Soeda T. et al. Identification and multidimensional optimization of an asymmetric bispecific IgG antibody mimicking the function of factor VIII cofactor activity. PloS One 2013;8:e57479. 10.1371/journal.pone.0057479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Neijssen J, Cardoso RMF, Chevalier KM. et al. Discovery of amivantamab (JNJ-61186372), a bispecific antibody targeting EGFR and MET. J Biol Chem 2021;296:100641. 10.1016/j.jbc.2021.100641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Middleton MR, McAlpine C, Woodcock VK. et al. Tebentafusp, a TCR/anti-CD3 bispecific fusion protein targeting gp100, potently activated antitumor immune responses in patients with metastatic melanoma. Clin Cancer Res 2020;26:5869–78. 10.1158/1078-0432.CCR-20-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sahni J, Patel SS, Dugel PU. et al. Simultaneous inhibition of angiopoietin-2 and vascular endothelial growth factor-a with faricimab in diabetic macular edema: BOULEVARD phase 2 randomized trial. Ophthalmology. 2019;126:1155–70. 10.1016/j.ophtha.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 102. Pillarisetti K, Powers G, Luistro L. et al. Teclistamab is an active T cell-redirecting bispecific antibody against B-cell maturation antigen for multiple myeloma. Blood Adv 2020;4:4538–49. 10.1182/bloodadvances.2020002393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sun LL, Ellerman D, Mathieu M. et al. Anti-CD20/CD3 T cell-dependent bispecific antibody for the treatment of B cell malignancies. Sci Transl Med 2015;7:287ra70. 10.1126/scitranslmed.aaa4802. [DOI] [PubMed] [Google Scholar]
- 104. Engelberts PJ, Hiemstra IH, de Jong B. et al. DuoBody - CD3 x CD20 induces potent T-cell-mediated killing of malignant B cells in preclinical models and provides opportunities for subcutaneous dosing. EBioMedicine. 2020;52:102625. 10.1016/j.ebiom.2019.102625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Cremasco F, Menietti E, Speziale D. et al. Cross-linking of T cell to B cell lymphoma by the T cell bispecific antibody CD20-TCB induces IFNγ/CXCL10-dependent peripheral T cell recruitment in humanized murine model. PloS One 2021;16:e0241091. 10.1371/journal.pone.0241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Pillarisetti K, Edavettal S, Mendonça M. et al. A T-cell-redirecting bispecific G-protein-coupled receptor class 5 member D × CD3 antibody to treat multiple myeloma. Blood. 2020;135:1232–43. 10.1182/blood.2019003342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Grosicki S, Bednarczyk M, Kociszewska K. Elranatamab: a new promising BispAb in multiple myeloma treatment. Expert Rev Anticancer Ther 2023;23:775–82. 10.1080/14737140.2023.2236303. [DOI] [PubMed] [Google Scholar]
- 108. Geuijen C, Rovers E, Nijhuis R. et al. Preclinical activity of MCLA-128, an ADCC enhanced bispecific IgG1 antibody targeting the HER2:HER3 heterodimer. ASCO Ann Meet 2014;32:560. https://ascopubs.org/doi/10.1200/jco.2014.32.15_suppl.560. 28 March 2025, last date accessed. [Google Scholar]
- 109. Edelman GM, Cunningham BA, Gall WE. et al. The covalent structure of an entire gammaG immunoglobulin molecule. Proc Natl Acad Sci U S A 1969;63:78–85. 10.1073/pnas.63.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Merchant AM, Zhu Z, Yuan JQ. et al. An efficient route to human bispecific IgG. Nat Biotechnol 1998;16:677–81. 10.1038/nbt0798-677. [DOI] [PubMed] [Google Scholar]
- 111. Kaplon H, Crescioli S, Chenoweth A. et al. Antibodies to watch in 2023. MAbs. 2023;15:2153410. 10.1080/19420862.2022.2153410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Suurs FV, Lub-de Hooge MN, de Vries EGE. et al. A review of bispecific antibodies and antibody constructs in oncology and clinical challenges. Pharmacol Ther 2019;201:103–19. 10.1016/j.pharmthera.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 113. Ellerman DA. The evolving applications of bispecific antibodies: reaping the harvest of early sowing and planting new seeds. BioDrugs. 2025;39:75–102. 10.1007/s40259-024-00691-0. [DOI] [PubMed] [Google Scholar]
- 114. Driscoll CL, Howarth MR. Matchmaking at the cell surface using bispecifics to put cells on their best behavior. Curr Opin Biotechnol 2025;92:103267. 10.1016/j.copbio.2025.103267. [DOI] [PubMed] [Google Scholar]
- 115. Herrera M, Pretelli G, Desai J. et al. Bispecific antibodies: advancing precision oncology. Trends Cancer 2024;10:893–919. 10.1016/j.trecan.2024.07.002. [DOI] [PubMed] [Google Scholar]
- 116. Oslund RC, Holland PM, Lesley SA. et al. Therapeutic potential of cis-targeting bispecific antibodies. Cell Chem Biol 2024;31:1473–89. 10.1016/j.chembiol.2024.07.004. [DOI] [PubMed] [Google Scholar]
- 117. Goebeler ME, Stuhler G, Bargou R. Bispecific and multispecific antibodies in oncology: opportunities and challenges. Nat Rev Clin Oncol 2024;21:539–60. 10.1038/s41571-024-00905-y. [DOI] [PubMed] [Google Scholar]
- 118. Seymore C. Ivonescimab outperforms pembrolizumab as frontline therapy in PD-L1+ advanced NSCLC. https://www.onclive.com/view/ivonescimab-outperforms-pembrolizumab-as-frontline-therapy-in-pd-l1-advanced-nsclc. 26 January 2025, last date accessed.
- 119.Is today’s pharmaceutical commercial model going extinct? 2019. Charles River Associates. https://media.crai.com/wp-content/uploads/2020/09/16164052/LS-One-Time-Therapies.pdf. 12 March 2025, last date accessed. [Google Scholar]
- 120. Zak KM, Grudnik P, Magiera K. et al. Structural biology of the immune checkpoint receptor PD-1 and its ligands PD-L1/PD-L2. Structure. 2017;25:1163–74. 10.1016/j.str.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 121. Na Z, Yeo SP, Bharath SR. et al. Structural basis for blocking PD-1-mediated immune suppression by therapeutic antibody pembrolizumab. Cell Res 2017;27:147–50. 10.1038/cr.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Tan S, Zhang H, Chai Y. et al. An unexpected N-terminal loop in PD-1 dominates binding by nivolumab. Nat Commun 2017;8:14369. 10.1038/ncomms14369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Jeong TJ, Lee HT, Gu N. et al. The high-resolution structure reveals remarkable similarity in PD-1 binding of cemiplimab and dostarlimab, the FDA-approved antibodies for cancer immunotherapy. Biomedicines. 2022;10:3154. 10.3390/biomedicines10123154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Keytruda® Prescribing information . 2024. US License No. 0002. Manufactured and marketed by Merck. https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf. 6 February 2025, last date accessed. [Google Scholar]
- 125. Opdivo® Prescribing information . 2024. US License No. 1713. Manufactured and marketed by Bristol Myers Squibb. https://packageinserts.bms.com/pi/pi_opdivo.pdf. 6 February 2025, last date accessed. [Google Scholar]
- 126. Lee HT, Lee JY, Lim H. et al. Molecular mechanism of PD-1/PD-L1 blockade via anti-PD-L1 antibodies atezolizumab and durvalumab. Sci Rep 2017;7:5532. 10.1038/s41598-017-06002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Tecentriq® Prescribing information . 2024. US License No. 1048. Manufactured and marketed by Genentech. https://www.gene.com/download/pdf/tecentriq_prescribing.pdf. 6 February 2025, last date accessed. [Google Scholar]
- 128. Bavencio® Prescribing information . 2024. US License No. 1773. Manufactured and marketed by EMD Serono. https://www.emdserono.com/us-en/pi/bavencio-pi.pdf. 6 February 2025, last date accessed. [Google Scholar]
- 129. Imfinzi® Prescribing information . 2024. US License No. 2043. Manufactured and marketed by AstraZeneca. https://den8dhaj6zs0e.cloudfront.net/50fd68b9-106b-4550-b5d0-12b045f8b184/9496217c-08b3-432b-ab4f-538d795820bd/9496217c-08b3-432b-ab4f-538d795820bd_viewable_rendition__v.pdf. 6 February 2025, last date accessed. [Google Scholar]
- 130. Libtayo® Prescribing information . 2024. US License No. 1760. Manufactured and marketed by Regeneron. https://www.regeneron.com/downloads/libtayo_fpi.pdf. 6 February 2025, last date accessed. [Google Scholar]
- 131. Jemperli® Prescribing information . 2024. US License No.1727. Manufactured and marketed by GlaxoSmithKline. https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Information/Jemperli/pdf/JEMPERLI-PI-MG.PDF. 6 February 2025, last date accessed. [Google Scholar]
- 132. Cho SH, Park JM, Lee EH. et al. High-resolution crystal structure of PD-1 in complex with retifanlimab, the FDA-approved immune checkpoint blocking antibody for treating Merkel cell carcinoma. Biochem Biophys Res Commun 2025;742:151106. 10.1016/j.bbrc.2024.151106. [DOI] [PubMed] [Google Scholar]
- 133. Zynyz® Prescribing information . 2024. US License No. 2228. Manufactured and marketed by Incyte Corporation. https://www.zynyz.com/zynyz-prescribing-information. 6 February 2025, last date accessed. [Google Scholar]
- 134. Liu H, Guo L, Zhang J. et al. Glycosylation-independent binding of monoclonal antibody toripalimab to FG loop of PD-1 for tumor immune checkpoint therapy. MAbs. 2019;11:681–90. 10.1080/19420862.2019.1596513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Loqtorzi® Prescribing information . 2024. US License No. 2023. Manufactured and marketed by Coherus. https://loqtorzi.com/pdf/prescribing-information.pdf. 6 February 2025, last date accessed. [Google Scholar]
- 136. Zhang L, Geng Z, Hao B. et al. Tislelizumab: a modified anti-tumor programmed death receptor 1 antibody. Cancer Control 2022;29:10732748221111296. 10.1177/10732748221111296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Gorelik L. Preclinical Characterization of a Novel Fully Human IgG1 Anti-PD-L1 mAb CK-301. Abstract 4606. AACR, 2016, https://checkpointtx.com/wp-content/uploads/2016/05/AACR_CK301_2017_FINAL.pdf. 6 February 2025, last date accessed. [Google Scholar]
- 138.Merck agrees to acquire Acceleron Pharma for $11.5bn. 2021. Pharmaceutical Technology. https://www.pharmaceutical-technology.com/news/merck-acceleron-pharma/?cf-view. 12 March 2025, last date accessed. [Google Scholar]
- 139. Masson G. Drug development cost pharma $2.2B per asset in 2024 as GLP-1s drive financial return: Deloitte. 2025. https://www.fiercebiotech.com/biotech/drug-development-cost-pharma-22b-asset-2024-plus-how-glp-1s-impact-roi-deloitte. 26 March 2025, last date accessed.
- 140. Ishida Y, Agata Y, Shibahara K. et al. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 1992;11:3887–95. https://www.embopress.org/doi/abs/10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Dong H, Zhu G, Tamada K. et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 1999;5:1365–9. 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 142. Freeman GJ, Long AJ, Iwai Y. et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000;192:1027–34. 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Latchman Y, Wood CR, Chernova T. et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2001;2:261–8. 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 144. Brown JA, Dorfman DM, Ma FR. et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol 2003;170:1257–66. 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 145. Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: Implications for tumor immunotherapy. Cancer Immunol Immunother 2005;54:307–14. 10.1007/s00262-004-0593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Berman HM, Westbrook J, Feng Z. et al. The protein data Bank. Nucleic Acids Res 2000;28:235–42. 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Veverka V, Cheng X, Waters LC., et al. Human programmed cell death 1 receptor. 2013. https://www.rcsb.org/structure/2M2D. 1 March 2025, last date accessed. [DOI] [PMC free article] [PubMed]
- 148. Heo YS, Lee HT. PD-L1 in complex with durvalumab. 2017. https://www.rcsb.org/structure/5X8M. 1 March 2025, last date accessed.
- 149. Morgan E, Soerjomataram I, Rumgay H. et al. The global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and projections to 2040: New estimates from GLOBOCAN 2020. Gastroenterology. 2022;163:649–658.e2. 10.1053/j.gastro.2022.05.054. [DOI] [PubMed] [Google Scholar]
- 150. Puhr HC, Prager GW, Ilhan-Mutlu A. How we treat esophageal squamous cell carcinoma. ESMO Open 2023;8:100789. 10.1016/j.esmoop.2023.100789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Wu HX, Pan YQ, He Y. et al. Clinical benefit of first-line programmed death-1 antibody plus chemotherapy in low programmed cell death ligand 1-expressing esophageal squamous cell carcinoma: a post hoc analysis of JUPITER-06 and meta-analysis. J Clin Oncol 2023;41:1735–46. 10.1200/JCO.22.01490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Zhang T, Song X, Xu L. et al. The binding of an anti-PD-1 antibody to FcγRΙ has a profound impact on its biological functions. Cancer Immunol Immunother 2018;67:1079–90. 10.1007/s00262-018-2160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Dahan R, Sega E, Engelhardt J. et al. FcγRs modulate the anti-tumor activity of antibodies targeting the PD-1/PD-L1 Axis. Cancer Cell 2015;28:285–95. 10.1016/j.ccell.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 154. Chen X, Song X, Li K. et al. FcγR-binding is an important functional attribute for immune checkpoint antibodies in cancer immunotherapy. Front Immunol 2019;10:292. 10.3389/fimmu.2019.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Hong Y, Feng Y, Sun H. et al. Tislelizumab uniquely binds to the CC' loop of PD-1 with slow-dissociated rate and complete PD-L1 blockage. FEBS Open Bio 2021;11:782–92. 10.1002/2211-5463.13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Cowles SC, Sheen A, Santollani L. et al. An affinity threshold for maximum efficacy in anti-PD-1 immunotherapy. MAbs 2022;14:2088454. 10.1080/19420862.2022.2088454. Erratum in: MAbs. 2024; 16: 2364972. http://dx.doi.org/10.1080/19420862.2024.2364972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Lee A, Keam SJ. Tislelizumab: First approval. Drugs. 2020;80:617–24. 10.1007/s40265-020-01286-z. [DOI] [PubMed] [Google Scholar]
- 158.BeiGene business wire. https://www.businesswire.com/news/home/20230224005112/en/BeiGene-Receives-10th-Approval-for-PD-1-Inhibitor-Tislelizumab-in-China. 28 February 2025, last date accessed.
- 159. NCT03430843 . A Study of Tislelizumab (BGB-A317) Versus Chemotherapy as Second Line Treatment in Participants with Advanced Esophageal Squamous Cell Carcinoma. BeiGene, https://clinicaltrials.gov/study/NCT03430843?term=NCT03430843&rank=1. 28 February 2025, last date accessed. [Google Scholar]
- 160. Shen L, Kato K, Kim SB. et al. Tislelizumab versus chemotherapy as second-line treatment for advanced or metastatic esophageal squamous cell carcinoma (RATIONALE-302): a randomized phase III study. J Clin Oncol 2022;40:3065–76. 10.1200/JCO.21.01926. Erratum in: J Clin Oncol. 2024; 42: 486. http://dx.doi.org/10.1200/JCO.23.02629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Fabbricatore R. FDA Approves Tislelizumab/Chemo in Gastric/GEJ Adenocarcinoma. 2024. https://www.cancernetwork.com/view/fda-approves-tislelizumab-chemo-in-gastric-gej-adenocarcinoma. 12 March 2025, last date accessed.
- 162. Qiu MZ, Oh DY, Kato K. et al. Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first line treatment for advanced gastric or gastro-oesophageal junction adenocarcinoma: RATIONALE-305 randomised, double blind, phase 3 trial. BMJ. 2024;385:e078876. 10.1136/bmj-2023-078876. [DOI] [PubMed] [Google Scholar]
- 163. Liu C, Liu X, Cao P. et al. Global, regional, national prevalence, mortality, and disability-adjusted life-years of cutaneous squamous cell carcinoma and trend analysis from 1990 to 2021 and prediction to 2045. Front Oncol 2025;15:1523169. 10.3389/fonc.2025.1523169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Checkpoint Therapeutics Announces FDA Approval of UNLOXCYT™ (cosibelimab-ipdl). https://ir.checkpointtx.com/news-events/press-releases/detail/127/checkpoint-therapeutics-announces-fda-approval-of. 10 March 2025, last date accessed.
- 165. Hamilton G, Rath B. Avelumab: combining immune checkpoint inhibition and antibody-dependent cytotoxicity. Expert Opin Biol Ther 2017;17:515–23. 10.1080/14712598.2017.1294156. [DOI] [PubMed] [Google Scholar]
- 166. Martineau R, Susini S, Marabelle A. Fc effector function of immune checkpoint blocking antibodies in oncology. Immunol Rev 2024;328:334–49. 10.1111/imr.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Clingan P, Ladwa R, Brungs D. et al. Efficacy and safety of cosibelimab, an anti-PD-L1 antibody, in metastatic cutaneous squamous cell carcinoma. J Immunother Cancer 2023;11:e007637. 10.1136/jitc-2023-007637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Heldin CH, Moustakas A. Signaling receptors for TGF-β family members. Cold Spring Harb Perspect Biol 2016;8:a022053. 10.1101/cshperspect.a022053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Wu M, Wu S, Chen W. et al. The roles and regulatory mechanisms of TGF-β and BMP signaling in bone and cartilage development, homeostasis and disease. Cell Res 2024;34:101–23. 10.1038/s41422-023-00918-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Martinez-Hackert E, Sundan A, Holien T. Receptor binding competition: a paradigm for regulating TGF-β family action. Cytokine Growth Factor Rev 2021;57:39–54. 10.1016/j.cytogfr.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Maeshima A, Nojima Y, Kojima I. The role of the activin-follistatin system in the developmental and regeneration processes of the kidney. Cytokine Growth Factor Rev 2001;12:289–98. 10.1016/s1359-6101(01)00010-7. [DOI] [PubMed] [Google Scholar]
- 172. Rasheed A, Aslam S, Sadiq H. et al. New and emerging therapeutic drugs for the treatment of pulmonary arterial hypertension: a systematic review. Cureus 2024;16:e68117. 10.7759/cureus.68117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Kovacs G, Bartolome S, Denton CP. et al. Definition, classification and diagnosis of pulmonary hypertension. Eur Respir J 2024;64:2401324. 10.1183/13993003.01324-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Miranda AC, Cornelio CK, Tran BAC. et al. Sotatercept: a first-In-class activin signaling inhibitor for pulmonary arterial hypertension. J Pharm Technol 2025;22:87551225251317957. 10.1177/87551225251317957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Kim KT, Borgstein NG, Yang Y. et al. ACE-011, a soluble activin receptor type IIa IgG-Fc fusion protein, increases hemoglobin and hematocrit levels in postmenopausal healthy women. Blood 2008;112:3866. doi.org/ 10.1182/blood.V112.11.3866.3866. [DOI] [Google Scholar]
- 176. Pearsall RS, Canalis E, Cornwall-Brady M. et al. A soluble activin type IIA receptor induces bone formation and improves skeletal integrity. Proc Natl Acad Sci U S A 2008;105:7082–7. 10.1073/pnas.0711263105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Carrancio S, Markovics J, Wong P. et al. An activin receptor IIA ligand trap promotes erythropoiesis resulting in a rapid induction of red blood cells and haemoglobin. Br J Haematol 2014;165:870–82. 10.1111/bjh.12838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Sherman ML, Borgstein NG, Mook L. et al. Multiple-dose, safety, pharmacokinetic, and pharmacodynamic study of sotatercept (ActRIIA-IgG1), a novel erythropoietic agent, in healthy postmenopausal women. J Clin Pharmacol 2013;53:1121–30. 10.1002/jcph.160. [DOI] [PubMed] [Google Scholar]
- 179. NCT03496207 . A Study of Sotatercept for the Treatment of Pulmonary Arterial Hypertension (PAH) (PULSAR). Acceleron Pharma, https://clinicaltrials.gov/study/NCT03496207?term=NCT03496207&rank=1. 18 January 2025, last date accessed. [Google Scholar]
- 180. NCT04576988 . A Study of Sotatercept for the Treatment of Pulmonary Arterial Hypertension (MK-7962-003/A011–11)(STELLAR). Acceleron Pharma, https://clinicaltrials.gov/study/NCT04576988?term=NCT04576988&rank=1. 18 January 2025, last date accessed. [Google Scholar]
- 181. Gomberg-Maitland M, Badesch DB, Gibbs JSR. et al. Efficacy and safety of sotatercept across ranges of cardiac index in patients with pulmonary arterial hypertension: a pooled analysis of PULSAR and STELLAR. J Heart Lung Transplant 2025;44:609–24. 10.1016/j.healun.2024.11.037. [DOI] [PubMed] [Google Scholar]
- 182. Hoeper MM, Gomberg-Maitland M, Badesch DB. et al. Efficacy and safety of the activin signalling inhibitor, sotatercept, in a pooled analysis of PULSAR and STELLAR studies. Eur Respir J 2025;65:2401424. 10.1183/13993003.01424-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Liao K, Mackenzie H, Ait-Oudhia S. et al. The impact of immunogenicity on the pharmacokinetics, efficacy, and safety of sotatercept in a phase III study of pulmonary arterial hypertension. Clin Pharmacol Ther 2024;115:478–87. 10.1002/cpt.3116. [DOI] [PubMed] [Google Scholar]
- 184. Yang Y, Lundqvist A. Immunomodulatory effects of IL-2 and IL-15; implications for cancer immunotherapy. Cancers (Basel) 2020;12:3586. 10.3390/cancers12123586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Ring AM, Lin JX, Feng D. et al. Mechanistic and structural insight into the functional dichotomy between IL-2 and IL-15. Nat Immunol 2012;13:1187–95. 10.1038/ni.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Ikemizu S, Chirifu M, Davis SJ. IL-2 and IL-15 signaling complexes: different but the same. Nat Immunol 2012;13:1141–2. 10.1038/ni.2472. [DOI] [PubMed] [Google Scholar]
- 187. Wang X, Rickert M, Garcia KC. Structure of the quaternary complex of interleukin-2 with its alpha, beta, and gamma receptors. Science 2005;310:1159–63. 10.1126/science.1117893. [DOI] [PubMed] [Google Scholar]
- 188. Han KP, Zhu X, Liu B. et al. IL-15:IL-15 receptor alpha superagonist complex: high-level co-expression in recombinant mammalian cells, purification and characterization. Cytokine 2011;56:804–10. 10.1016/j.cyto.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Bouchaud G, Garrigue-Antar L, Solé V. et al. The exon-3-encoded domain of IL-15R alpha contributes to IL-15 high-affinity binding and is crucial for the IL-15 antagonistic effect of soluble IL-15R alpha. J Mol Biol 2008;382:1–12. 10.1016/j.jmb.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 190. Alva A, Daniels GA, Wong MK. et al. Contemporary experience with high-dose interleukin-2 therapy and impact on survival in patients with metastatic melanoma and metastatic renal cell carcinoma. Cancer Immunol Immunother 2016;65:1533–44. 10.1007/s00262-016-1910-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Rosenberg SA, Yang JC, Topalian SL. et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA. 1994;271:907–13. 10.1001/jama.1994.03510360033032. [DOI] [PubMed] [Google Scholar]
- 192. Atkins MB, Lotze MT, Dutcher JP. et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 1999;17:2105–16. 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 193. Buchbinder EI, McDermott DF. Interferon, interleukin-2, and other cytokines. Hematol Oncol Clin North Am 2014;28:571–83. 10.1016/j.hoc.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 194. Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood 2006;107:2409–14. 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Karakus U, Sahin D, Mittl PRE. et al. Receptor-gated IL-2 delivery by an anti-human IL-2 antibody activates regulatory T cells in three different species. Sci Transl Med 2020;12:eabb9283. 10.1126/scitranslmed.abb9283. [DOI] [PubMed] [Google Scholar]
- 196. Raeber ME, Sahin D, Karakus U. et al. A systematic review of interleukin-2-based immunotherapies in clinical trials for cancer and autoimmune diseases. EBioMedicine. 2023;90:104539. 10.1016/j.ebiom.2023.104539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Hangasky JA, Fernández RDV, Stellas D. et al. Leveraging long-acting IL-15 agonists for intratumoral delivery and enhanced antimetastatic activity. Front Immunol 2024;15:1458145. 10.3389/fimmu.2024.1458145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Cai M, Huang X, Huang X. et al. Research progress of interleukin-15 in cancer immunotherapy. Front Pharmacol 2023;14:1184703. 10.3389/fphar.2023.1184703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Fujii R, Jochems C, Tritsch SR. et al. An IL-15 superagonist/IL-15Rα fusion complex protects and rescues NK cell-cytotoxic function from TGF-β1-mediated immunosuppression. Cancer Immunol Immunother 2018;67:675–89. 10.1007/s00262-018-2121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Charych DH, Hoch U, Langowski JL. et al. NKTR-214, an engineered cytokine with biased IL-2 receptor binding, increased tumor exposure, and marked efficacy in mouse tumor models. Clin Cancer Res 2016;22:680–90. 10.1158/1078-0432.CCR-15-1631. [DOI] [PubMed] [Google Scholar]
- 201. Park B. Trials investigating bempegaldesleukin in combination with nivolumab discontinued. Medical Professionals Reference. https://www.empr.com/home/news/drugs-in-the-pipeline/trials-investigating-bempegaldesleukin-in-combination-with-nivolumab-discontinued/. Accessed 23 May 2025. [Google Scholar]
- 202. Frentzas S, Ahern ES, Weickhardt AJ. et al. A phase 1/2 study of AU-007, a monoclonal antibody (mAb) that binds to IL-2 and inhibits CD25 binding, in patients with advanced solid tumors: Interim results. Abstract e14507. J Clin Oncol 2023;41:e14507. 10.1200/JCO.2023.41.16_suppl.e14507 21 March 2025, last date accessed. [DOI] [Google Scholar]
- 203. Yoon HY, Heo J, Kim D. et al. A Novel Triple Action and Pre-Clinical Safety Profile of SLC-3010 Predict its Favorable Translation in the Phase I Clinical Study. Abstract 1105. SITC, 2023. 10.1136/jitc-2022-SITC2022.1105 21 March 2025, last date accessed. [DOI] [Google Scholar]
- 204. Atkinson VG, Antras JF, Bedard P. et al. Results from Monotherapy Dose Escalation of MDNA11, a Long-Acting IL-2 Superkine, in a Phase 1/2 Trial Show Evidence of Single-Agent Activity in Advanced Solid Tumors. Abstract CT259, Vol. 84, 2024. 10.1158/1538-7445.AM2024-CT259, 21 March 2025, last date accessed. [DOI] [Google Scholar]
- 205. Ma L, Acuff NV, Joseph IB. et al. A precision engineered Interleukin-2 for bolstering CD8+ T- and NK-cell activity without eosinophilia and vascular leak syndrome in nonhuman primates. Cancer Res Commun 2024;4:2799–814. 10.1158/2767-9764.CRC-24-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Bergamaschi C, Stravokefalou V, Stellas D. et al. Heterodimeric IL-15 in cancer immunotherapy. Cancers 2021;13:837. 10.3390/cancers13040837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Leidner R, Conlon K, McNeel DG. et al. First-in-human phase I/Ib study of NIZ985, a recombinant heterodimer of IL-15 and IL-15Rα, as a single agent and in combination with spartalizumab in patients with advanced and metastatic solid tumors. J Immunother Cancer 2023;11:e007725. 10.1136/jitc-2023-007725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Quijano-Rubio A, Bhuiyan AM, Yang H. et al. A split, conditionally active mimetic of IL-2 reduces the toxicity of systemic cytokine therapy. Nat Biotechnol 2023;41:532–40. 10.1038/s41587-022-01510-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Mortales C, Dutzar B, Chen J. et al. NL-201 upregulates MHC-I expression and intratumoral T-cell receptor diversity, and demonstrates robust antitumor activity as monotherapy and in combination with PD-1 blockade. Cancer Immunol Res 2023;11:1000–10. 10.1158/2326-6066.CIR-22-0304. [DOI] [PubMed] [Google Scholar]
- 210. Kong X, Lin Y, Ouyang C. et al. SHR-1916: a novel PEGylated interleukin-2 analogue with altered cellular selectivity and improved pharmacokinetic profiles for cancer immunotherapy. Drug Des Devel Ther 2025;19:1251–70. 10.2147/DDDT.S493011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Qu F, Darji S, Thompson DH. Recent advances in drug delivery strategies for high-risk BCG-unresponsive non-muscle invasive bladder cancer: a brief review from 2018 to 2024. Pharmaceutics 2024;16:1154. 10.3390/pharmaceutics16091154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.ImmunityBio completes ANKTIVA’s post-approval enrollment of the 100th patient in BCG unresponsive NMIBC CIS Trial and reports a complete response rate of 71% with a durable duration of response ranging up to 54 months. 2024. https://immunitybio.com/immunitybio-completes-anktivas-post-approval-enrollment-of-the-100th-patient-in-bcg-unresponsive-nmibc-cis-trial-and-reports-a-complete-response-rate-of-71-with-a-durable-duration-of-response/. Accessed March 11, 2025.
- 213. NCT03022825 . QUILT-3.032: A Multicenter Clinical Trial of Intravesical Bacillus Calmette-Guerin (BCG) in Combination with ALT-803 (N-803) in Patients with BCG Unresponsive High Grade Non-Muscle Invasive Bladder Cancer. ImmunityBio, https://clinicaltrials.gov/study/NCT03022825?term=NCT03022825&rank=1. 11 March 2025, last date accessed. [Google Scholar]
- 214. Katoh M, Katoh M. Precision medicine for human cancers with notch signaling dysregulation (review). Int J Mol Med 2020;45:279–97. 10.3892/ijmm.2019.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Serrano AG, Rocha P, Freitas Lima C. et al. Delta-like ligand 3 (DLL3) landscape in pulmonary and extra-pulmonary neuroendocrine neoplasms. NPJ Precis Oncol 2024;8:268. 10.1038/s41698-024-00739-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Neuroendocrine Tumors . Cleveland Clinic, https://my.clevelandclinic.org/health/diseases/22006-neuroendocrine-tumors-net. 15 March 2025, last date accessed. [Google Scholar]
- 217. Zhang H, Yang Y, Li X. et al. Targeting the notch signaling pathway and the notch ligand, DLL3, in small cell lung cancer. Biomed Pharmacother 2023;159:114248. 10.1016/j.biopha.2023.114248. [DOI] [PubMed] [Google Scholar]
- 218. Su PL, Chakravarthy K, Furuya N. et al. DLL3-guided therapies in small-cell lung cancer: from antibody-drug conjugate to precision immunotherapy and radioimmunotherapy. Mol Cancer 2024;23:97. 10.1186/s12943-024-02012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Rudin CM, Reck M, Johnson ML. et al. Emerging therapies targeting the delta-like ligand 3 (DLL3) in small cell lung cancer. J Hematol Oncol 2023;16:66. 10.1186/s13045-023-01464-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Saunders LR, Bankovich AJ, Anderson WC. et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci Transl Med 2015;7:302ra136. 10.1126/scitranslmed.aac9459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Uprety D, Remon J, Adjei AA. All that glitters is not gold: the story of rovalpituzumab tesirine in SCLC. J Thorac Oncol 2021;16:1429–33. 10.1016/j.jtho.2021.07.012. [DOI] [PubMed] [Google Scholar]
- 222. Basumallik N, Agarwal M. Small cell lung cancer. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2023, 2025. [PubMed] [Google Scholar]
- 223. Mack M, Riethmüller G, Kufer P. A small bispecific antibody construct expressed as a functional single-chain molecule with high tumor cell cytotoxicity. Proc Natl Acad Sci U S A 1995;92:7021–5. 10.1073/pnas.92.15.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Cheadle EJ. MT-103 micromet/MedImmune. Curr Opin Mol Ther 2006;8:62–8. [PubMed] [Google Scholar]
- 225. Mølhøj M, Crommer S, Brischwein K. et al. CD19-/CD3-bispecific antibody of the BiTE class is far superior to tandem diabody with respect to redirected tumor cell lysis. Mol Immunol 2007;44:1935–43. 10.1016/j.molimm.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 226. Amgen to acquire Micromet . Amgen Press Release. 2012. https://investors.amgen.com/news-releases/news-release-details/amgen-acquire-micromet. Accessed January 15, 2025.
- 227. Sanford M. Blinatumomab: first global approval. Drugs 2015;75:321–7. 10.1007/s40265-015-0356-3. [DOI] [PubMed] [Google Scholar]
- 228. Liu L, Jacobsen FW, Everds N. et al. Biological Characterization of a Stable Effector Functionless (SEFL) Monoclonal Antibody Scaffold in Vitro. J Biol Chem 2017;292:1876–83. 10.1074/jbc.M116.748707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. In H, Park M, Lee H. et al. Immune cell engagers: advancing precision immunotherapy for cancer treatment. Antibodies (Basel) 2025;14:16. 10.3390/antib14010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. NCT05060016 . A Phase 2 Study of Tarlatamab in Patients with Small Cell Lung Cancer (SCLC) (DeLLphi-301). Amgen, https://clinicaltrials.gov/study/NCT05060016?term=NCT05060016&rank=1 15 January 2025, last date accessed. [Google Scholar]
- 231. Ahn MJ, Cho BC, Felip E. et al. Tarlatamab for patients with previously treated small-cell lung cancer. N Engl J Med 2023;389:2063–75. 10.1056/NEJMoa2307980. [DOI] [PubMed] [Google Scholar]
- 232. Ahn MJ, Cho BC, Felip E. et al. Plain language summary: tarlatamab for patients with previously treated small cell lung cancer. Future Oncol 2024;20:3355–64. 10.1080/14796694.2024.2402152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Lai M, Pichardo-Almarza C, Verma M. et al. T-cell engagers: model interrogation as a tool to quantify the interplay of relative affinity and target expression on trimer formation. Front Pharmacol 2024;15:1470595. 10.3389/fphar.2024.1470595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Dowlati A, Hummel HD, Champiat S. et al. Sustained clinical benefit and intracranial activity of tarlatamab in previously treated small cell lung cancer: DeLLphi-300 trial update. J Clin Oncol 2024;42:3392–9. 10.1200/JCO.24.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Kovac MB, Seruga B. Potentially fatal complications of new systemic anticancer therapies: pearls and pitfalls in their initial management. Radiol Oncol 2024;58:170–8. 10.2478/raon-2024-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Pyzik M, Sand KMK, Hubbard JJ. et al. The neonatal Fc receptor (FcRn): a misnomer? Front Immunol 2019;10:1540. 10.3389/fimmu.2019.01540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Minocha M, Thompson CG, Murphy A. et al. Pharmacokinetics of tarlatamab, a delta-like ligand-3 (DLL3) targeted half-life extended bispecific T-cell engager (BiTE®) immunotherapy in adult patients with previously treated small-cell lung cancer: results from DeLLphi-300, a phase I multiple-dose-escalation study. Clin Pharmacokinet 2024;63:1757–68. 10.1007/s40262-024-01451-7. [DOI] [PubMed] [Google Scholar]
- 238. Blincyto® Prescribing information . 2024. US License No. 1080. Manufactured by Amgen. https://www.pi.amgen.com/-/media/Project/Amgen/Repository/pi-amgen-com/Blincyto/blincyto_pi_hcp_english.pdf. 21 February 2025, last date accessed. [Google Scholar]
- 239. Vignesh P, Rawat A, Sharma M. et al. Complement in autoimmune diseases. Clin Chim Acta 2017;465:123–30. 10.1016/j.cca.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 240. Feng Y, Zhao C, Deng Y. et al. Mechanism of activation and biased signaling in complement receptor C5aR1. Cell Res 2023;33:312–24. 10.1038/s41422-023-00779-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Taylor RP, Lindorfer MA. Mechanisms of complement-mediated damage in hematological disorders. Semin Hematol 2018;55:118–23. 10.1053/j.seminhematol.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 242. Chapin J, Terry HS, Kleinert D. et al. The role of complement activation in thrombosis and hemolytic anemias. Transfus Apher Sci 2016;54:191–8. 10.1016/j.transci.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 243. Soliris® Prescribing information . 2007. US License No. 1743. Manufactured by Alexion Pharmaceuticals, Inc. www.accessdata.fda.gov/drugsatfda_docs/label/2007/125166lbl.pdf. 25 January 2024, last date accessed. [Google Scholar]
- 244. Ultomiris® Prescribing information . 2018. US License No. 1743. Manufactured by Alexion Pharmaceuticals, Inc. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761108s000lbl.pdf. 25 January 2024, last date accessed. [Google Scholar]
- 245. Hoy SM. Pozelimab: first approval. Drugs 2023;83:1551–7. 10.1007/s40265-023-01955-9. [DOI] [PubMed] [Google Scholar]
- 246. Veopoz™ Prescribing information . 2023. US License No. 1760. Manufactured by Regeneron Pharmaceuticals, Inc. www.accessdata.fda.gov/drugsatfda_docs/label/2023/761339s000lbl.pdf. 7 January 2024, last date accessed. [Google Scholar]
- 247. Holers VM. Complement and its receptors: new insights into human disease. Ann Rev Immunol 2014;32:433–59. 10.1146/annurev-immunol-032713-120154. [DOI] [PubMed] [Google Scholar]
- 248. Nishimura J, Yamamoto M, Hayashi S. et al. Genetic variants in C5 and poor response to eculizumab. N Engl J Med 2014;370:632–9. 10.1056/NEJMoa1311084. [DOI] [PubMed] [Google Scholar]
- 249. Sampei Z, Haraya K, Gan SW. et al. Beyond recycling antibodies: crovalimab’s molecular design enables four-weekly subcutaneous injections for PNH treatment. Int J Mol Sci 2024;25:11679. 10.3390/ijms252111679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Latuszek A, Liu Y, Olsen O. et al. Inhibition of complement pathway activation with Pozelimab, a fully human antibody to complement component C5. PloS One 2020;15:e0231892. 10.1371/journal.pone.0231892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Igawa T, Ishii S, Tachibana T. et al. Antibody recycling by engineered pH-dependent antigen binding improves the duration of antigen neutralization. Nat Biotechnol 2010;28:1203–7. 10.1038/nbt.1691. [DOI] [PubMed] [Google Scholar]
- 252. NCT04434092 . A Phase III Study Evaluating the Efficacy and Safety of Crovalimab Versus Eculizumab in Participants with Paroxysmal Nocturnal Hemoglobinuria (PNH) Not Previously Treated with Complement Inhibitors. (COMMODORE 2). Hoffmann-La Roche, https://clinicaltrials.gov/study/NCT04434092?term=NCT04434092&rank=1 27 January 2025, last date accessed. [Google Scholar]
- 253. Röth A, He G, Tong H. et al. Phase 3 randomized COMMODORE 2 trial: crovalimab versus eculizumab in patients with paroxysmal nocturnal hemoglobinuria naive to complement inhibition. Am J Hematol 2024;99:1768–77. 10.1002/ajh.27412. [DOI] [PubMed] [Google Scholar]
- 254. NCT04654468 . A Study Evaluating the Efficacy, Safety, Pharmacokinetics and Pharmacodynamics of Crovalimab in Participants with Paroxysmal Nocturnal Hemoglobinuria (PNH) Not Previously Treated with Complement Inhibition (COMMODORE 3). Hoffmann-La Roche, https://clinicaltrials.gov/study/NCT04654468?term=NCT04654468&rank=1 27 January 2025, last date accessed. [Google Scholar]
- 255. Liu H, Xia L, Weng J. et al. Efficacy and safety of the C5 inhibitor crovalimab in complement inhibitor-naive patients with PNH (COMMODORE 3): a multicenter, phase 3, single-arm study. Am J Hematol 2023;98:1407–14. 10.1002/ajh.26998. [DOI] [PubMed] [Google Scholar]
- 256. Sheridan D, Yu ZX, Zhang Y. et al. Design and preclinical characterization of ALXN1210: a novel anti-C5 antibody with extended duration of action. PloS One 2018;13:e0195909. 10.1371/journal.pone.0195909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Muri L, Schubart A, Thorburn C. et al. Inhibition of the different complement pathways has varying impacts on the serum bactericidal activity and opsonophagocytosis against Haemophilus influenzae type b. Front Immunol 2022;13:1020580. 10.3389/fimmu.2022.1020580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Alzheimer’s Association . 2024. Alzheimer’s Disease Facts and Figures. https://www.alz.org/media/documents/alzheimers-facts-and-figures.pdf. 17 November 2024, last date accessed.
- 259. Mohamed Asik R, Suganthy N, Aarifa MA. et al. Alzheimer's disease: a molecular view of β-amyloid induced morbific events. Biomedicines 2021;9:1126. 10.3390/biomedicines9091126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Kametani F, Hasegawa M. Reconsideration of amyloid hypothesis and tau hypothesis in Alzheimer's disease. Front Neurosci 2018;12:25. 10.3389/fnins.2018.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Calero M, Gómez-Ramos A, Calero O. et al. Additional mechanisms conferring genetic susceptibility to Alzheimer's disease. Front Cell Neurosci 2015;9:138. 10.3389/fncel.2015.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Li NM, Liu KF, Qiu YJ. et al. Mutations of beta-amyloid precursor protein alter the consequence of Alzheimer's disease pathogenesis. Neural Regen Res 2019;14:658–65. 10.4103/1673-5374.247469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Kuznetsov AV. Evaluating the combined neurotoxicity of amyloid beta and tau oligomers in Alzheimer's disease: a novel cellular-level criterion. J Biomech Eng 2025;147:041003. 10.1115/1.4067701. [DOI] [PubMed] [Google Scholar]
- 264. Nisticò R, Borg JJ. Aducanumab for Alzheimer's disease: a regulatory perspective. Pharmacol Res 2021;171:105754. 10.1016/j.phrs.2021.105754. [DOI] [PubMed] [Google Scholar]
- 265. Loeffler DA. Antibody-mediated clearance of brain amyloid-β: mechanisms of action, effects of natural and monoclonal anti-aβ antibodies, and downstream effects. J Alzheimers Dis Rep 2023;7:873–99. 10.3233/ADR-230025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Arndt JW, Qian F, Smith BA. et al. Structural and kinetic basis for the selectivity of aducanumab for aggregated forms of amyloid-β. Sci Rep 2018;8:6412. 10.1038/s41598-018-24501-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Heidebrink JL, Paulson HL. Lessons learned from approval of aducanumab for Alzheimer's disease. Annu Rev Med 2024;75:99–111. 10.1146/annurev-med-051022-043645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Dyer O. Aduhelm: Biogen abandons Alzheimer's drug after controversial approval left it unfunded by Medicare. BMJ. 2024;384:q281. 10.1136/bmj.q281. [DOI] [PubMed] [Google Scholar]
- 269. Lannfelt L. A light at the end of the tunnel - from mutation identification to a potential treatment for Alzheimer's disease. Ups J Med Sci 2023;128:128. 10.48101/ujms.v128.10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Söderberg L, Johannesson M, Nygren P. et al. Lecanemab, aducanumab, and gantenerumab - binding profiles to different forms of amyloid-beta might explain efficacy and side effects in clinical trials for Alzheimer's disease. Neurotherapeutics 2023;20:195–206. 10.1007/s13311-022-01308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Noorda K, Noorda K, Sabbagh MN. et al. Amyloid-directed antibodies: past, present, and future. J Alzheimers Dis 2024;101:S3–S22. 10.3233/JAD-240189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Bayer TA. Pyroglutamate aβ cascade as drug target in Alzheimer's disease. Mol Psychiatry 2022;27:1880–5. 10.1038/s41380-021-01409-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Kang C. Donanemab: first approval. Drugs 2024;84:1335–18. 10.1007/s40265-024-02103-7 Erratum in: Drugs. 2024; 84: 1335. 10.1007/s40265-024-02103-7. [DOI] [PubMed] [Google Scholar]
- 274. Jones KE, Aakre JA, Castillo AM. et al. Eligibility for donanemab trial in a population-based study of cognitive aging. J Prev Alzheimers Dis 2025;12:100088. 10.1016/j.tjpad.2025.100088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. NCT04437511 . A Study of Donanemab (LY3002813) in Participants with Early Alzheimer's Disease (TRAILBLAZER-ALZ 2). Eli Lilly, https://clinicaltrials.gov/study/NCT04437511?term=NCT04437511&rank=1 15 February 2025, last date accessed. [Google Scholar]
- 276. Sims JR, Zimmer JA, Evans CD. et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330:512–27. 10.1001/jama.2023.13239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Khartabil N, Awaness A. Targeting amyloid pathology in early Alzheimer's: the promise of donanemab-azbt. Pharmacy (Basel) 2025;13:23. 10.3390/pharmacy13010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Boustani M, Doty EG, Garrison LP Jr. et al. Estimating the economically justifiable price of limited-duration treatment with donanemab for early symptomatic Alzheimer's disease in the United States. Neurol Ther 2024;13:1641–59. 10.1007/s40120-024-00649-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Zimmer JA, Ardayfio P, Wang H. et al. Amyloid-related imaging abnormalities with donanemab in early symptomatic Alzheimer disease: secondary analysis of the TRAILBLAZER-ALZ and ALZ 2 randomized clinical trials. JAMA Neurol 2025;82:461–9. 10.1001/jamaneurol.2025.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Meglio M. Eli Lilly’s Remternetug Demonstrates Significant Amyloid Plaque Removal in Early-Stage Trial. 2023. https://www.neurologylive.com/view/eli-lilly-remternetug-demonstrates-significant-amyloid-plaque-removal-early-stage-trial. 15 February 2025, last date accessed.
- 281. NCT05463731 . A Study of Remternetug (LY3372993) in Participants with Alzheimer's Disease (TRAILRUNNER-ALZ 1). Eli Lilly, https://clinicaltrials.gov/study/NCT05463731?term=NCT05463731&rank=1 15 February 2025, last date accessed. [Google Scholar]
- 282.Prurigo nodularis. Cleveland Clinics. https://my.clevelandclinic.org/health/diseases/25247-prurigo-nodularis. 25 February 2025, last date accessed. [Google Scholar]
- 283. Chovatiya R, Hawkes JE, DiRuggiero D. et al. Type 2 inflammation and its role in dermatologic diseases. Int J Dermatol 2025;64:978–91. 10.1111/ijd.17707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Eczema stats . National Eczema Association. https://nationaleczema.org/research/eczema-facts/. 25 February 2025, last date accessed.
- 285. Kwatra SG, Puelles J, Tran O. et al. Prevalence of prurigo nodularis in the United States. JAAD Int 2024;18:134–6. 10.1016/j.jdin.2023.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Kamata Y, Tominaga M, Takamori K. Mechanisms of itch in atopic dermatitis. Juntendo Med J 2025;71:43–50. 10.14789/ejmj.JMJ24-0036-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Castellani ML, Felaco P, Galzio RJ. et al. IL-31 a Th2 cytokine involved in immunity and inflammation. Int J Immunopathol Pharmacol 2010;23:709–13. 10.1177/039463201002300304. [DOI] [PubMed] [Google Scholar]
- 288. Dillon SR, Sprecher C, Hammond A. et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol 2004;5:752–60. 10.1038/ni1084. Erratum in: Nat Immunol. 2005; 6: 114. [DOI] [PubMed] [Google Scholar]
- 289.What Are the 7 Different Types of Eczema? https://www.healthline.com/health/types-of-eczema. Accessed February 25, 2025.
- 290. Alkon N, Assen FP, Arnoldner T. et al. Single-cell RNA sequencing defines disease-specific differences between chronic nodular prurigo and atopic dermatitis. J Allergy Clin Immunol 2023;152:420–35. 10.1016/j.jaci.2023.04.019. [DOI] [PubMed] [Google Scholar]
- 291. Nemmer JM, Kuchner M, Datsi A. et al. Interleukin-31 signaling bridges the gap between immune cells, the nervous system and epithelial tissues. Front Med (Lausanne) 2021;8:639097. 10.3389/fmed.2021.639097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Hashimoto T, Nattkemper LA, Kim HS. et al. Itch intensity in prurigo nodularis is closely related to dermal interleukin-31, oncostatin M, IL-31 receptor alpha and oncostatin M receptor beta. Exp Dermatol 2021;30:804–10. 10.1111/exd.14279. [DOI] [PubMed] [Google Scholar]
- 293. Sonkoly E, Muller A, Lauerma AI. et al. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol 2006;117:411–7. 10.1016/j.jaci.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 294. Datsi A, Steinhoff M, Ahmad F. et al. Interleukin-31: the “itchy” cytokine in inflammation and therapy. Allergy. 2021;76:2982–97. 10.1111/all.14791. [DOI] [PubMed] [Google Scholar]
- 295. Nakashima C, Otsuka A, Kabashima K. Interleukin-31 and interleukin-31 receptor: new therapeutic targets for atopic dermatitis. Exp Dermatol 2018;27:327–31. 10.1111/exd.13533. [DOI] [PubMed] [Google Scholar]
- 296. Nemoto O, Furue M, Nakagawa H. et al. The first trial of CIM331, a humanized anti-human interleukin-31 receptor a antibody, in healthy volunteers and patients with atopic dermatitis to evaluate safety, tolerability and pharmacokinetics of a single dose in a randomized, double-blind, placebo-controlled study. Br J Dermatol 2016;174:296–304. 10.1111/bjd.14207. [DOI] [PubMed] [Google Scholar]
- 297. Galderma Receives U.S . FDA Approval for Nemluvio® (Nemolizumab) for Patients with Moderate-to-Severe Atopic Dermatitis. 2024. https://www.galderma.com/news/galderma-receives-us-fda-approval-nemluvior-nemolizumab-patients-moderate-severe-atopic. 3 March 2025, last date accessed.
- 298. Keam SJ. Nemolizumab: first approval. Drugs 2022;82:1143–50. 10.1007/s40265-022-01741-z. [DOI] [PubMed] [Google Scholar]
- 299. Galderma’s Nemluvio® (Nemolizumab) Approved in the European Union for Moderate-to-Severe Atopic Dermatitis and Prurigo Nodularis . Galderma Press Release, 2025, https://www.galderma.com/news/galdermas-nemluvior-nemolizumab-approved-european-union-moderate-severe-atopic-dermatitis-and. 3 March 2025, last date accessed. [Google Scholar]
- 300. Vafa O, Gilliland GL, Brezski RJ. et al. An engineered Fc variant of an IgG eliminates all immune effector functions via structural perturbations. Methods 2014;65:114–26. 10.1016/j.ymeth.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 301. Shields RL, Namenuk AK, Hong K. et al. High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improved binding to the Fc gamma R. J Biol Chem 2001;276:6591–604. 10.1074/jbc.M009483200. [DOI] [PubMed] [Google Scholar]
- 302. Medgyesi D, Uray K, Sallai K. et al. Functional mapping of the Fc gamma RII binding site on human IgG1 by synthetic peptides. Eur J Immunol 2004;34:1127–35. 10.1002/eji.200324733. [DOI] [PubMed] [Google Scholar]
- 303. Bruhns P, Iannascoli B, England P. et al. Specificity and affinity of human Fc gamma receptors and their polymorphic variants for human IgG subclasses. Blood 2009;113:3716–25. 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
- 304. Kancko A, Iwayanagi Y, Kitamura H., et al. Pharmaceutical composition for prevention and/or treatment of atopic dermatitis comprising IL-31 antagonist as active ingredient. US patent application 2018/0079817 A1. Filed 13 April 2016.
- 305. Ständer S, Yosipovitch G, Legat FJ. et al. Efficacy and safety of nemolizumab in patients with moderate to severe prurigo nodularis: the OLYMPIA 1 randomized clinical phase 3 trial. JAMA Dermatol 2025;161:147–56. 10.1001/jamadermatol.2024.4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Kwatra SG, Yosipovitch G, Legat FJ. et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med 2023;389:1579–89. 10.1056/NEJMoa2301333. [DOI] [PubMed] [Google Scholar]
- 307. Pelaia C, Heffler E, Crimi C. et al. Interleukins 4 and 13 in asthma: key pathophysiologic cytokines and druggable molecular targets. Front Pharmacol 2022;13:851940. 10.3389/fphar.2022.851940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Teixeira C, Yilmaz O, Bernardo D. et al. IL-13 inhibition in the treatment of atopic dermatitis - new and emerging biologic agents. J Int Med Res 2024;52:3000605241286832. 10.1177/03000605241286832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Junttila IS. Tuning the cytokine responses: an update on interleukin (IL)-4 and IL-13 receptor complexes. Front Immunol 2018;9:888. 10.3389/fimmu.2018.00888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. LaPorte SL, Juo ZS, Vaclavikova J. et al. Molecular and structural basis of cytokine receptor pleiotropy in the interleukin-4/13 system. Cell 2008;132:259–72. 10.1016/j.cell.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Lupardus PJ, Birnbaum ME, Garcia KC. Molecular basis for shared cytokine recognition revealed in the structure of an unusually high affinity complex between IL-13 and IL-13Ralpha2. Structure 2010;18:332–42. 10.1016/j.str.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. McCormick SM, Heller NM. Commentary: IL-4 and IL-13 receptors and signaling. Cytokine 2015;75:38–50. 10.1016/j.cyto.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Ultsch M, Bevers J, Nakamura G. et al. Structural basis of signaling blockade by anti-IL-13 antibody Lebrikizumab. J Mol Biol 2013;425:1330–9. 10.1016/j.jmb.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 314. Okragly AJ, Ryuzoji A, Wulur I. et al. Binding, neutralization and internalization of the interleukin-13 antibody, lebrikizumab. Dermatol Ther (Heidelb) 2023;13:1535–47. 10.1007/s13555-023-00947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. NCT04146363 . Evaluation of the Efficacy and Safety of Lebrikizumab (LY3650150) in Moderate to Severe Atopic Dermatitis (ADvocate1). Eli Lilly, https://clinicaltrials.gov/study/NCT04146363?term=NCT04146363%20&rank=1. 14 February 2025, last date accessed. [Google Scholar]
- 316. NCT04178967 . Evaluation of the Efficacy and Safety of Lebrikizumab (LY3650150) in Moderate to Severe Atopic Dermatitis (ADvocate2). Eli Lilly, https://clinicaltrials.gov/study/NCT04178967?term=NCT04178967&rank=1. 14 February 2025, last date accessed. [Google Scholar]
- 317. NCT04250337 . Safety and Efficacy of Lebrikizumab (LY3650150) in Combination with Topical Corticosteroid in Moderate-to-Severe Atopic Dermatitis. (ADhere). Eli Lilly, https://clinicaltrials.gov/study/NCT04250337?term=NCT04250337&rank=1. 14 February 2025, last date accessed. [Google Scholar]
- 318. Simpson E, Fernández-Peñas P, de Bruin-Weller M. et al. Improvement across dimensions of disease with lebrikizumab use in atopic dermatitis: two phase 3, randomized, double-blind, placebo-controlled monotherapy trials (ADvocate1 and ADvocate2). Adv Ther 2025;42:132–43. 10.1007/s12325-024-02974-y. Erratum in: Adv Ther. 2025; 42: 144-145. https://doi.org/10.1007/s12325-024-03010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Hume DA, MacDonald KP. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood 2012;119:1810–20. 10.1182/blood-2011-09-379214. [DOI] [PubMed] [Google Scholar]
- 320. Stanley ER, Chitu V. CSF-1 receptor signaling in myeloid cells. Cold Spring Harb Perspect Biol 2014;6:a021857. 10.1101/cshperspect.a021857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Ma X, Lin WY, Chen Y. et al. Structural basis for the dual recognition of helical cytokines IL-34 and CSF-1 by CSF-1R. Structure 2012;20:676–87. 10.1016/j.str.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 322. Shang J, Xu Y, Pu S. et al. Role of IL-34 and its receptors in inflammatory diseases. Cytokine 2023;171:156348. 10.1016/j.cyto.2023.156348. [DOI] [PubMed] [Google Scholar]
- 323. Beirão BCB, Raposo TP, Imamura LM. et al. A blocking antibody against canine CSF-1R maturated by limited CDR mutagenesis. Antib Ther 2020;3:193–204. 10.1093/abt/tbaa018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Olivieri A, Mancini G. Current approaches for the prevention and treatment of acute and chronic GVHD. Cells. 2024;13:1524. 10.3390/cells13181524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Ordentlich P, Wolfreys A, Da Costa A. et al. Targeting colony stimulating factor- 1 receptor (CSF-1R) with SNDX-6352, a novel anti-CSF-1R targeted antibody. J Immunother Cancer 2016;4:P402. https://cms.syndax.com/wp-content/uploads/2016/11/SITC_2016_6352_Poster_11.08.16.pdf. [Google Scholar]
- 326. Kitko CL, Arora M, DeFilipp Z. et al. Axatilimab for chronic graft-versus-host disease after failure of at least two prior systemic therapies: results of a phase I/II study. J Clin Oncol 2023;41:1864–75. 10.1200/JCO.22.00958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Wolff D, Cutler C, Lee SJ. et al. Axatilimab in recurrent or refractory chronic graft-versus-host disease. N Engl J Med 2024;391:1002–14. 10.1056/NEJMoa2401537. [DOI] [PubMed] [Google Scholar]
- 328. Troisi R, Balasco N, Autiero I. et al. New insight into the traditional model of the coagulation cascade and its regulation: illustrated review of a three-dimensional view. Res Pract Thromb Haemost 2023;7:102160. 10.1016/j.rpth.2023.102160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Grover SP, Mackman N. Intrinsic pathway of coagulation and thrombosis. Arterioscler Thromb Vasc Biol 2019;39:331–8. 10.1161/ATVBAHA.118.312130. [DOI] [PubMed] [Google Scholar]
- 330. Kitazawa T, Esaki K, Tachibana T. et al. Factor VIIIa-mimetic cofactor activity of a bispecific antibody to factors IX/IXa and X/Xa, emicizumab, depends on its ability to bridge the antigens. Thromb Haemost 2017;117:1348–57. 10.1160/TH17-01-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol 2007;27:1687–93. 10.1161/ATVBAHA.107.141911. [DOI] [PubMed] [Google Scholar]
- 332. Crawley JT, Lane DA. The haemostatic role of tissue factor pathway inhibitor. Arterioscler Thromb Vasc Biol 2008;28:233–42. 10.1161/ATVBAHA.107.141606. [DOI] [PubMed] [Google Scholar]
- 333. Mast AE, Ruf W. Regulation of coagulation by tissue factor pathway inhibitor: implications for hemophilia therapy. J Thromb Haemost 2022;20:1290–300. 10.1111/jth.15697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Castaman G, Matino D. Hemophilia a and B: molecular and clinical similarities and differences. Haematologica. 2019;104:1702–9. 10.3324/haematol.2019.221093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Salen P, Babiker HM. Hemophilia a. [Updated 2023 Jul 17. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2025, https://www.ncbi.nlm.nih.gov/books/NBK470265/. [PubMed] [Google Scholar]
- 336. National Hemophilia Foundation . Hemophilia B. https://www.hemophilia.org/bleeding-disorders-a-z/types/hemophilia-b. Accessed March 16, 2025.
- 337. Franchini M, Veneri D, Lippi G. Inherited factor XI deficiency: a concise review. Hematology. 2006;11:307–9. 10.1080/10245330600921964. [DOI] [PubMed] [Google Scholar]
- 338. Haider MZ, Anwer F. Acquired Hemophilia. [Updated 2022 Dec 13. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2025, https://www.ncbi.nlm.nih.gov/books/NBK560494/. [PubMed] [Google Scholar]
- 339. Meeks SL, Batsuli G. Hemophilia and inhibitors: current treatment options and potential new therapeutic approaches. Hematology Am Soc Hematol Educ Program 2016;2016:657–62. 10.1182/asheducation-2016.1.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Ellsworth P, Ma A. Factor-mimetic and rebalancing therapies in hemophilia a and B: the end of factor concentrates? Hematology Am Soc Hematol Educ Program 2021;2021:219–25. 10.1182/hematology.2021000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Jiménez-Yuste V, Auerswald G, Benson G. et al. Practical considerations for nonfactor-replacement therapies in the treatment of haemophilia with inhibitors. Haemophilia. 2021;27:340–50. 10.1111/hae.14167. [DOI] [PubMed] [Google Scholar]
- 342. Scott LJ, Kim ES. Emicizumab-kxwh: first global approval. Drugs. 2018;78:269–74. 10.1007/s40265-018-0861-2. [DOI] [PubMed] [Google Scholar]
- 343. Hympavzi® (marstasimab-hncq) . GoodRx. https://www.goodrx.com/hympavzi. 31 March 2025, last date accessed.
- 344. Comparing Alhemo® vs Hympavzi® . Drugs.com. https://www.drugs.com/compare/alhemo-vs-hympavzi. 31 March 2025, last date accessed.
- 345. Hemlibra® prescribing information . Genentech. 2023. License 1048. https://www.gene.com/download/pdf/hemlibra_prescribing.pdf. 31 March 2025, last date accessed.
- 346. Hemlibra® prices, coupons, copay cards & patient assistance . Drugs.com. https://www.drugs.com/price-guide/hemlibra.
- 347. Mullard A. FDA approves first anti-TFPI antibody for haemophilia A and B. Nat Rev Drug Discov 2024;23:884. 10.1038/d41573-024-00175-4. [DOI] [PubMed] [Google Scholar]
- 348. NCT03938792 . Study of the Efficacy and Safety PF-06741086 in Adult and Teenage Participants with Severe Hemophilia a or Moderately Severe to Severe Hemophilia B. Pfizer, https://clinicaltrials.gov/study/NCT03938792?term=NCT03938792&rank=1. 31 March 2025, last date accessed. [Google Scholar]
- 349. Keam SJ. Concizumab: first approval. Drugs 2023;83:1053–9. 10.1007/s40265-023-01912-6. Erratum in: Drugs. 2023; 83: 1253-1254. https://doi.org/10.1007/s40265-023-01924-2. [DOI] [PubMed] [Google Scholar]
- 350. NCT04083781 . Research Study to Look at how Well the Drug Concizumab Works in your Body if you Have Haemophilia with Inhibitors (explorer7). Pfizer, https://clinicaltrials.gov/study/NCT04083781?term=NCT04083781&rank=1. 31 March 2025, last date accessed. [Google Scholar]
- 351. Matsushita T, Shapiro A, Abraham A. et al. Phase 3 trial of concizumab in hemophilia with inhibitors. N Engl J Med 2023;389:783–94. 10.1056/NEJMoa2216455. [DOI] [PubMed] [Google Scholar]
- 352. NCT04082429 . Research Study to Look at how Well the Drug Concizumab Works in Your Body if you Have Haemophilia without Inhibitors (explorer8). Pfizer, https://clinicaltrials.gov/study/NCT04082429?term=NCT04082429&rank=1. 31 March 2025, last date accessed. [Google Scholar]
- 353. Chowdary P, Angchaisuksiri P, Apte S. et al. Concizumab prophylaxis in people with haemophilia A or haemophilia B without inhibitors (explorer8): a prospective, multicentre, open-label, randomised, phase 3a trial. Lancet Haematol 2024;11:e891–904. 10.1016/S2352-3026(24)00307-7. Erratum in: Lancet Haematol. 2024; 11: e886. https://doi.org/10.1016/S2352-3026(24)00353-3. [DOI] [PubMed] [Google Scholar]
- 354. Hana C, Thaw Dar NN, Galo Venegas M. et al. Claudins in cancer: a current and future therapeutic target. Int J Mol Sci 2024;25:4634. 10.3390/ijms25094634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Singh AB, Sharma A, Dhawan P. Claudin family of proteins and cancer: An overview. J Oncol 2010;2010:541957. 10.1155/2010/541957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Kim NI, Koo JY, Kim SS. et al. Claudin 18.2 expression profile in primary tumors and their ovarian metastases: implications for targeted therapy. BMC Cancer 2025;25:540. 10.1186/s12885-025-13940-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Katoh M, Katoh M. Claudin 1, 4, 6 and 18 isoform 2 as targets for the treatment of cancer (review). Int J Mol Med 2024;54:100. 10.3892/ijmm.2024.5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Jeremias S. FDA Approves Tislelizumab-jsgr as First-Line Therapy for HER2– Gastric Cancers. 2025. https://www.ajmc.com/view/fda-approves-tislelizumab-jsgr-as-first-line-therapy-for-her2-gastric-cancers. 3 February 2025, last date accessed.
- 359. Menon G, El-Nakeep S, Babiker HM. Gastric cancer. [Updated 2024 Oct 28. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2025, https://www.ncbi.nlm.nih.gov/books/NBK459142/. [PubMed] [Google Scholar]
- 360. Lua WH, Ling WL, Yeo JY. et al. The effects of antibody engineering CH and CL in trastuzumab and pertuzumab recombinant models: impact on antibody production and antigen-binding. Sci Rep 2018;8:718. 10.1038/s41598-017-18892-9. Erratum in: Sci Rep. 2018; 8: 11110. https://doi.org/10.1038/s41598-018-29097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Herceptin® prescribing information . Genentech. 2024. License 1048. https://www.gene.com/download/pdf/herceptin_prescribing.pdf. 26 February 2025, last date accessed.
- 362. Lakayan D, Haselberg R, Gahoual R. et al. Affinity profiling of monoclonal antibody and antibody-drug-conjugate preparations by coupled liquid chromatography-surface plasmon resonance biosensing. Anal Bioanal Chem 2018;410:7837–48. 10.1007/s00216-018-1414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Perjeta® prescribing information . Genentech 2024. License 1048. https://www.gene.com/download/pdf/perjeta_prescribing.pdf. 26 February 2025, last date accessed.
- 364. Kadcyla® prescribing information . Genentech 2024. License 1048. https://www.gene.com/download/pdf/kadcyla_prescribing.pdf. 26 February 2025, last date accessed.
- 365. Enhertu® prescribing information . Daiichi Sankyo 2024. License 2128. https://daiichisankyo.us/prescribing-information-portlet/getPIContent?productName=Enhertu&inline=true. 26 February 2025, last date accessed.
- 366. Ogitani Y, Aida T, Hagihara K. et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res 2016;22:5097–108. 10.1158/1078-0432.CCR-15-2822. [DOI] [PubMed] [Google Scholar]
- 367. Margenza® prescribing information . TerrSera Therapeutics 2024. License 2383. https://documents.tersera.com/margenza-us/prescribing-information.pdf. 26 February 2025, last date accessed.
- 368. Stavenhagen JB, Gorlatov S, Tuaillon N. et al. Fc optimization of therapeutic antibodies enhances their ability to kill tumor cells in vitro and controls tumor expansion in vivo via low-affinity activating Fc-gamma receptors. Cancer Res 2007;67:8882–90. 10.1158/0008-5472.CAN-07-0696. Erratum in: Cancer Res. 2008; 68: 7692. [DOI] [PubMed] [Google Scholar]
- 369. Nordstrom JL, Gorlatov S, Zhang W. et al. Anti-tumor activity and toxicokinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced Fcγ receptor binding properties. Breast Cancer Res 2011;13:R123. 10.1186/bcr3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370. Weisser NE, Sanches M, Escobar-Cabrera E. et al. An anti-HER2 biparatopic antibody that induces unique HER2 clustering and complement-dependent cytotoxicity. Nat Commun 2023;14:1394. 10.1038/s41467-023-37029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Geuijen CAW, De Nardis C, Maussang D. et al. Unbiased combinatorial screening identifies a bispecific IgG1 that potently inhibits HER3 Signaling via HER2-guided ligand blockade. Cancer Cell 2018;33:922–936.e10. 10.1016/j.ccell.2018.04.003. Erratum in: Cancer Cell. 2021; 39: 1163-1164. https://doi.org/10.1016/j.ccell.2021.07.015. [DOI] [PubMed] [Google Scholar]
- 372. De Nardis C, Hendriks LJA, Poirier E. et al. A new approach for generating bispecific antibodies based on a common light chain format and the stable architecture of human immunoglobulin G1. J Biol Chem 2017;292:14706–17. 10.1074/jbc.M117.793497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373. Cruz VL, Souza-Egipsy V, Gion M. et al. Binding affinity of trastuzumab and pertuzumab monoclonal antibodies to extracellular HER2 domain. Int J Mol Sci 2023;24:12031. 10.3390/ijms241512031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Mullard A. FDA approves first claudin-18.2-targeted antibody for gastric cancer. Nat Rev Drug Discov 2024;23:884. 10.1038/d41573-024-00172-7. [DOI] [PubMed] [Google Scholar]
- 375. Zeng Y, Lockhart AC, Jin RU. The preclinical discovery and development of zolbetuximab for the treatment of gastric cancer. Expert Opin Drug Discovery 2024;19:873–86. 10.1080/17460441.2024.2370332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376. Waheed A, Saleem MN, Shah Y. et al. Efficacy and safety of zolbetuximab in gastric cancer. Oncology (Williston Park) 2024;38:472–4. 10.46883/2024.25921031. [DOI] [PubMed] [Google Scholar]
- 377. NCT03504397 . A Phase 3 Efficacy, Safety and Tolerability Study of Zolbetuximab (Experimental Drug) Plus mFOLFOX6 Chemotherapy Compared to Placebo Plus mFOLFOX6 as Treatment for Gastric and Gastroesophageal Junction (GEJ) Cancer (Spotlight). Astellas Pharma, https://clinicaltrials.gov/study/NCT03504397?term=NCT03504397&rank=1. 17 March 2025, last date accessed. [Google Scholar]
- 378. Shitara K, Shah MA, Lordick F. et al. Zolbetuximab plus chemotherapy for locally advanced unresectable or metastatic stomach or gastroesophageal junction cancers: a plain language summary. Future Oncol 2024;20:1861–77. 10.1080/14796694.2024.2342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379. Shitara K, Xu RH, Ajani JA. et al. Global prevalence of claudin 18 isoform 2 in tumors of patients with locally advanced unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma. Gastric Cancer 2024;27:1058–68. 10.1007/s10120-024-01518-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. NCT03653507 . A Study of Zolbetuximab (IMAB362) Plus CAPOX Compared with Placebo Plus CAPOX as First-Line Treatment of Subjects with Claudin (CLDN) 18.2-Positive, HER2-Negative, Locally Advanced Unresectable or Metastatic Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma (GLOW). Astellas Pharma, https://clinicaltrials.gov/study/NCT03653507?term=NCT03653507&rank=1. 17 March 2025, last date accessed. [Google Scholar]
- 381. Shitara K, Shah MA, Lordick F. et al. Zolbetuximab in gastric or gastroesophageal junction adenocarcinoma. N Engl J Med 2024;391:1159–62. 10.1056/NEJMc2409512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382. Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer 2005;5:341–54. 10.1038/nrc1609. Erratum in: Nat Rev Cancer. 2005; 5: 580. [DOI] [PubMed] [Google Scholar]
- 383. Zhao J, Mohan N, Nussinov R. et al. Trastuzumab blocks the receiver function of HER2 leading to the population shifts of HER2-containing homodimers and heterodimers. Antibodies (Basel) 2021;10:7. 10.3390/antib10010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384. Mendell J, Freeman DJ, Feng W. et al. Clinical translation and validation of a predictive biomarker for patritumab, an anti-human epidermal growth factor receptor 3 (HER3) monoclonal antibody, in patients with advanced non-small cell lung cancer. EBioMedicine. 2015;2:264–71. 10.1016/j.ebiom.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385. Garrett JT, Tendler S, Feroz W. et al. Emerging importance of HER3 in tumorigenesis and cancer therapy. Nat Rev Clin Oncol. 2025Online ahead of print;22:348–70. 10.1038/s41571-025-01008-y. [DOI] [PubMed] [Google Scholar]
- 386. Nami B, Maadi H, Wang Z. The effects of pertuzumab and its combination with trastuzumab on HER2 homodimerization and phosphorylation. Cancers (Basel) 2019;11:375. 10.3390/cancers11030375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. Gandullo-Sánchez L, Ocaña A, Pandiella A. HER3 in cancer: from the bench to the bedside. J Exp Clin Cancer Res 2022;41:310. 10.1186/s13046-022-02515-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Bargmann CI, Hung MC, Weinberg RA. The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature 1986;319:226–30. 10.1038/319226a0. [DOI] [PubMed] [Google Scholar]
- 389. Varley JM, Swallow JE, Brammar WJ. et al. Alterations to either c-erbB-2(neu) or c-myc proto-oncogenes in breast carcinomas correlate with poor short-term prognosis. Oncogene 1987;1:423–30. [PubMed] [Google Scholar]
- 390. Slamon DJ, Clark GM, Wong SG. et al. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177–82. 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 391. No author listed . Monoclonal antibody approved for metastatic breast cancer. Oncology (Williston Park) 1998;12:1727. [PubMed] [Google Scholar]
- 392. LaPelusa M, Heumann T, Goff L. et al. Targeted therapies in advanced biliary tract cancers-a narrative review. Chin Clin Oncol 2023;12:14. 10.21037/cco-22-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Jazz Pharmaceuticals and Zymeworks Announce Exclusive License Agreement to Develop and Commercialize Zanidatamab, a HER2-Targeted Bispecific Antibody. 2022. https://investor.jazzpharma.com/news-releases/news-release-details/jazz-pharmaceuticals-and-zymeworks-announce-exclusive-license. 24 February 2025, last date accessed.
- 394. NCT04466891 . A Study of ZW25 (Zanidatamab) in Subjects with Advanced or Metastatic HER2-Amplified Biliary Tract Cancers (HERIZON-BTC-01). Jazz Pharmaceuticals, https://clinicaltrials.gov/study/NCT04466891?term=NCT04466891&rank=1. 24 February 2025, last date accessed. [Google Scholar]
- 395. Harding JJ, Fan J, Oh DY. et al. Zanidatamab for HER2-amplified, unresectable, locally advanced or metastatic biliary tract cancer (HERIZON-BTC-01): a multicentre, single-arm, phase 2b study. Lancet Oncol 2023;24:772–82. 10.1016/S1470-2045(23)00242-5. [DOI] [PubMed] [Google Scholar]
- 396. Harding JJ, Fan J, Oh DY. et al. A plain language summary of the results from the phase 2b HERIZON-BTC-01 study of zanidatamab in participants with HER2-amplified biliary tract cancer. Future Oncol 2024;20:2319–29. 10.1080/14796694.2024.2368952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397. Schram AM, Odintsov I, Espinosa-Cotton M. et al. Zenocutuzumab, a HER2 x HER3 bispecific antibody, is effective therapy for tumors driven by NRG1 gene rearrangements. Cancer Discov 2022;12:1233–47. 10.1158/2159-8290.CD-21-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. Heining C, Horak P, Uhrig S. et al. NRG1 fusions in KRAS wild-type pancreatic cancer. Cancer Discov 2018;8:1087–95. 10.1158/2159-8290.CD-18-0036. [DOI] [PubMed] [Google Scholar]
- 399. Umemoto K, Sunakawa Y. The potential targeted drugs for fusion genes including NRG1 in pancreatic cancer. Crit Rev Oncol Hematol 2021;166:103465. 10.1016/j.critrevonc.2021.103465. [DOI] [PubMed] [Google Scholar]
- 400. Trombetta D, Sparaneo A, Fabrizio FP. et al. NRG1 and NRG2 fusions in non-small cell lung cancer (NSCLC): seven years between lights and shadows. Expert Opin Ther Targets 2021;25:865–75. 10.1080/14728222.2021.1999927. [DOI] [PubMed] [Google Scholar]
- 401. Li H, Xu L, Cao H. et al. Analysis on the pathogenesis and treatment progress of NRG1 fusion-positive non-small cell lung cancer. Front Oncol 2024;14:1405380. 10.3389/fonc.2024.1405380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402. Ptáková N, Martínek P, Holubec L. et al. Identification of tumors with NRG1 rearrangement, including a novel putative pathogenic UNC5D-NRG1 gene fusion in prostate cancer by data-drilling a de-identified tumor database. Genes Chromosomes Cancer 2021;60:474–81. 10.1002/gcc.22942. [DOI] [PubMed] [Google Scholar]
- 403. Laskin J, Liu SV, Tolba K. et al. NRG1 fusion-driven tumors: biology, detection, and the therapeutic role of afatinib and other ErbB-targeting agents. Ann Oncol 2020;31:1693–703. 10.1016/j.annonc.2020.08.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404. Nagasaka M, Ou SI. NRG1 and NRG2 fusion positive solid tumor malignancies: a paradigm of ligand-fusion oncogenesis. Trends Cancer 2022;8:242–58. 10.1016/j.trecan.2021.11.003. [DOI] [PubMed] [Google Scholar]
- 405. Gupta B, Borghaei L, Liu SV. NRG1 fusions: the new kid on the block. Curr Oncol Rep 2025;27:190–4. 10.1007/s11912-025-01640-y. [DOI] [PubMed] [Google Scholar]
- 406. Geuijen C, Rovers E, Gallenne T., et al. Mechanism of Action of MCLA-128, a Humanized Bispecific IgG1 Antibody Targeting the HER2:HER3 Heterodimer. 2015. https://merus.nl/wp-content/uploads/2019/06/MCLA-128-poster-AACR-2015.pdf. 28 March 2025, last date accessed.
- 407. Schram AM, Goto K, Kim DW. et al. Efficacy of zenocutuzumab in NRG1 fusion-positive cancer. N Engl J Med 2025;392:566–76. 10.1056/NEJMoa2405008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408. Huang FL, Liao EC, Li CL. et al. Pathogenesis of pediatric B-cell acute lymphoblastic leukemia: molecular pathways and disease treatments. Oncol Lett 2020;20:448–54. 10.3892/ol.2020.11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Phases of treatment for acute lymphoblastic leukaemia (ALL). https://www.cancerresearchuk.org/about-cancer/acute-lymphoblastic-leukaemia-all/treatment/phases. 23 March 2025, last date accessed.
- 410. Desai M, Kundu A, Hageman M. et al. Monoclonal antibody and protein therapeutic formulations for subcutaneous delivery: high-concentration, low-volume vs. low-concentration, high-volume. MAbs. 2023;15:2285277. 10.1080/19420862.2023.2285277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411. Rituxan Hycela® prescribing information . Manufactured by Genentech. License 1048. https://www.gene.com/download/pdf/rituxan_hycela_prescribing.pdf. 2 April 2025, last date accessed.
- 412. Herceptin Hylecta® prescribing information . Manufactured by Genentech. License 1048. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761106s000lbl.pdf. 2 April 2025, last date accessed.
- 413. Darzalex Faspro® prescribing information . Manufactured by Janssen Biotech. License 1864. https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/DARZALEX+Faspro-pi.pdf. 2 April 2025, last date accessed.
- 414. Phesgo® prescribing information . Manufactured by Genentech. Licence 1048. https://www.gene.com/download/pdf/phesgo_prescribing.pdf. 2 April 2025, last date accessed.
- 415. Vyvgart Hytrulo® prescribing information . Manufactured by Argenx. License 2217. https://argenx.com/content/dam/argenx-corp/products/vyvgart-hytrulo-prescribing-information.pdf. 2 April 2025, last date accessed.
- 416. Tecentriq Hybreza® prescribing information . Genentech. 2024. License 1048. https://www.gene.com/download/pdf/tecentriq_hybreza_prescribing.pdf. 2 April 2025, last date accessed.
- 417. Ocrevus Zunovo® prescribing information . Genentech. 2024. License 1048. https://www.gene.com/download/pdf/ocrevus_zunovo_prescribing.pdf. 2 April 2025, last date accessed.
- 418. Opdivo Qvantig® prescribing information . Bristol-Myers Squibb. 2024. Licenses 1713 and 2187. https://packageinserts.bms.com/pi/pi_opdivo-qvantig.pdf. 2 April 2025, last date accessed.
- 419.FDA approves obecabtagene autoleucel for adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia. 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-obecabtagene-autoleucel-adults-relapsed-or-refractory-b-cell-precursor-acute. 23 March 2025, last date accessed.
- 420. Obecabtagenum autoleucelum . WHO Drug Info, Vol. 35: RL85, p. 180. Geneva, Switzerland: World Health Organization, 2021, https://www.who.int/our-work/access-to-medicines-and-health-products/who-drug-information. 23 March 2025, last date accessed. [Google Scholar]
- 421. Ghorashian S, Kramer AM, Onuoha S. et al. Enhanced CAR T cell expansion and prolonged persistence in pediatric patients with ALL treated with a low-affinity CD19 CAR. Nat Med 2019;25:1408–14. 10.1038/s41591-019-0549-5. [DOI] [PubMed] [Google Scholar]
- 422. Roddie C, Dias J, O'Reilly MA. et al. Durable responses and low toxicity after fast off-rate CD19 chimeric antigen receptor-T therapy in adults with relapsed or refractory B-cell acute lymphoblastic leukemia. J Clin Oncol 2021;39:3352–63. 10.1200/JCO.21.00917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423. Autolus full year 2023. financial results and business updates. https://autolus.gcs-web.com/static-files/ef88aaf3-894b-416d-a602-18707955ad48. 23 March 2025, last date accessed.
- 424. NCT04404660 . A Study of CD19 Targeted CAR T Cell Therapy in Adult Patients with Relapsed or Refractory B Cell Acute Lymphoblastic Leukaemia (ALL). Autolus Ltd, https://clinicaltrials.gov/study/NCT04404660?term=NCT04404660&rank=1. 23 March 2025, last date accessed. [Google Scholar]
- 425. Aucatzyl® prescription information . Manufactured by Autolus. https://www.fda.gov/media/183463/download. 23 March 2025, last date accessed.
- 426. Roddie C, Sandhu KS, Tholouli E. et al. Obecabtagene autoleucel in adults with B-cell acute lymphoblastic leukemia. N Engl J Med 2024;391:2219–30. 10.1056/NEJMoa2406526. [DOI] [PubMed] [Google Scholar]
- 427. Locke KW, Maneval DC, LaBarre MJ. ENHANZE® drug delivery technology: A novel approach to subcutaneous administration using recombinant human hyaluronidase PH20. Drug Deliv 2019;26:98–106. 10.1080/10717544.2018.1551442. Erratum in: Drug Deliv. 2019; 26: 1300. http://dx.doi.org/10.1080/10717544.2019.1594569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428. Garantziotis S, Savani RC. Hyaluronan biology: a complex balancing act of structure, function, location and context. Matrix Biol 2019;78-79:1–10. 10.1016/j.matbio.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429. Pang B, He J, Zhang W. et al. Active expression of human hyaluronidase PH20 and characterization of its hydrolysis pattern. Front Bioeng Biotechnol 2022;10:885888. 10.3389/fbioe.2022.885888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430. Hyqvia® prescribing information . Manufactured by Takeda. License 1898. https://www.shirecontent.com/PI/PDFs/HYQVIA_USA_ENG.pdf#page=13. 2 April 2025, last date accessed.
- 431. Topouzis S, Papapetropoulos A, Alexander SPH. et al. Novel drugs approved by the EMA, the FDA and the MHRA in 2024: a year in review. Br J Pharmacol 2025;182:1416–45. 10.1111/bph.17458. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the information provided here are derived from the public literature or public websites.