Abstract
Objective
To determine the duration of protection from hepatitis B vaccine given in infancy and early childhood.
Design
Cross sectional serological study of hepatitis B virus infection in children of various ages 14 years after the start of a trial of vaccination regimens.
Setting
Two villages in the Gambia.
Participants
Children and adolescents given hepatitis B vaccine in infancy or early childhood: 232 were aged 1-5 years, 225 aged 5-9 years, 220 aged 10-14 years, and 175 aged 15-19 years.
Main outcome measures
Vaccine efficacy against infection and against chronic infection in the different age groups.
Results
Vaccine efficacy against chronic carriage of hepatitis B virus was 94% (95% confidence interval 89% to 97%), which did not vary significantly between the age groups. Efficacy against infection was 80% (76% to 84%). This was significantly lower in the oldest age group (65%, 56 to 73). Of the uninfected participants in this age group, 36% had no detectable hepatitis B virus surface antibody. Time since vaccination and a low peak antibody response were the most powerful risk factors for breakthrough infection (P<0.001 in each case). Low peak antibody response was also a risk factor for chronic carriage (odds ratio 95, 19 to 466).
Conclusions
Children vaccinated in infancy are at increased risk of hepatitis B virus infection in the late teens. The risk of chronic carriage after sexual exposure needs further assessment to determine if booster vaccines are necessary.
What is already known on this topic
An expert panel has declared that booster immunisations are not needed for lifelong immunity to hepatitis B
The evidence for maintenance of immunity in teenagers after vaccination in infancy is slender
The risk of hepatitis B virus infection is increased by sexual exposure
What this study adds
Teenagers vaccinated in infancy have low concentrations of antibody to hepatitis B surface antigen
Even though breakthrough infections are common at this age, protection against chronic infections with hepatitis B virus may be maintained
Introduction
Chronic infection with hepatitis B virus is a leading cause of death from cancer in Africa; a quarter of the 60 million carriers die either of primary hepatocellular carcinoma or cirrhosis of the liver.1,2 However, although hepatitis B vaccination is the simplest and most effective intervention to prevent mortality in adults both globally and in Africa,2 only one country in west Africa and two in southern Africa have a continuing vaccination programme.
The Gambian programme was started in 1986 as part of a 40 year trial to test the efficacy of vaccination against hepatitis B in the prevention of hepatocellular carcinoma.3 Before this trial epidemiological studies were undertaken in the two villages of Manduar and Keneba, where transmission of hepatitis B virus was found to be largely horizontal, from older to younger children, and rates of infection and chronic carriage, which were high, varied markedly between the villages.4,5 Trials of different routes of administration of vaccine were started in these villages in 19846 and vaccination has been continued thereafter.7,8 Nine years later vaccine efficacy against either infection or chronic carriage was 93%.8 Another long term study from Senegal, which was small due to major losses to follow up, reported efficacies 9 to 12 years after vaccination of 63% fir infection and 87% for chronic carriage.9
We investigated vaccine efficacy against infection and protection against chronic carriage after 14 years.
Methods
The demographic and medical background of the villages of Keneba and Manduar, which in 1998 had populations of 1474 and 607 respectively, has been described previously.4 Surveys of hepatitis B virus infections in these villages took place in 1973, 1980,4 1984,5 1989,7 and 19938 and from November 1998 to March 1999. At the time of the third survey in November 1984 all children under the age of 5 years who were seronegative for hepatitis B virus infection were vaccinated. These children were assigned randomly to receive plasma derived vaccine against hepatitis B virus (H-B-Vax, Merck Sharpe & Dohme) according to one of three vaccination regimens: group 1 was given three doses of 2 μg intradermally, group 2 received 2 μg intradermally followed by two 20 μg intramuscular doses, and group 3 received three doses of 20 μg intramuscularly.5 Subsequent vaccination of newborn infants has continued with four doses of various vaccines given intramuscularly: group 4 received 10 μg H-B-Vax, group 5 received recombinant vaccines (5 μg Recombivax, Merck Sharpe & Dohme, and later 10 μg Energix B, SmithKline Beecham), and group 6 received plasma derived vaccine (2.5 μg Hepaccine, Cheil Foods and Chemicals).8 There were no side effects apart from transient tenderness and slight swelling at the site of injection.
Concentrations of antibody to hepatitis B virus surface antigen (anti-HBsAg) were measured two months after vaccination (peak antibody), and this and other tests for hepatitis B virus core antibody (anti-HBc) and hepatitis B virus surface antigen (HBsAg) were carried out at each survey. The laboratory and statistical methods, the definitions of infection, breakthrough infection, chronic carriage, and the full details of the vaccine regimens have been described previously.8 The study and consent procedures were approved by the Gambian government and the Medical Research Council ethics committee.
Results
By the time of the 1998 survey 1041 young people (833 aged 0-14 years, 208 aged ⩾15 years) had been vaccinated in the two villages. We excluded from the study 29 infants who were below 1 year of age and 23 children who had received two or fewer doses of vaccine, leaving 989 children. Of these 989, 856 gave a blood sample, 64 refused to take part, 33 had died, and 36 could not be traced; coverage ranged from 94% for children aged 1-4 years to 81% for those between 15 and 19 years.
Effect of vaccination on pattern of infection
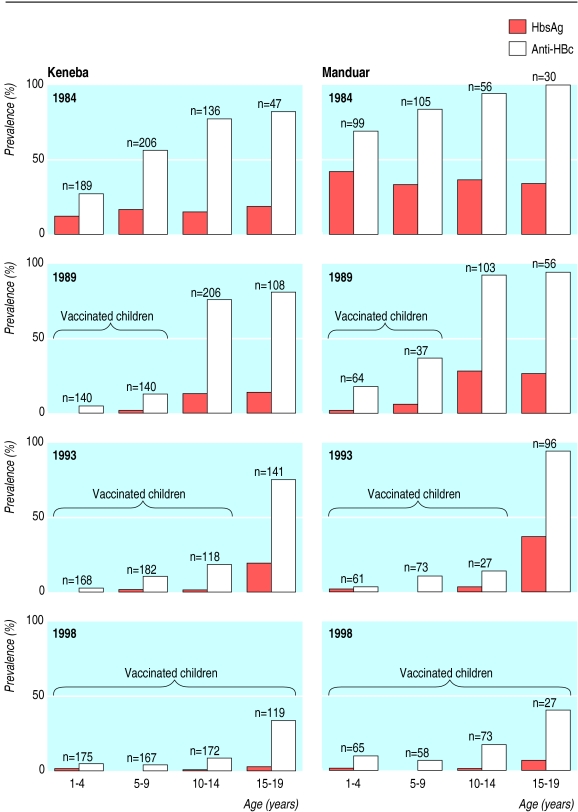
The figure shows that in 1984 at the start of vaccination Manduar had a much higher prevalence of hepatitis B virus infection and HBsAg carriage compared with Keneba. Between 1984 and 1998 vaccination dramatically reduced the prevalence of hepatitis B virus infection in children from 48% (302/620) to 11% (68/624) in Keneba and from 80% (246/309) to 15% (34/223) in Manduar. The corresponding changes in HBsAg carriage rates were from 13% (83/622) to 1% (6/628) in Keneba and from 35% (108/309) to 2% (4/227) in Manduar.
Vaccine efficacy by village and age
Overall, crude vaccine efficacy against HBsAg carriage was 94% (95% confidence interval 89% to 97%), which did not vary significantly between villages or by age group (table 1). Overall crude vaccine efficacy against infection was 80% (76% to 84%), which did not vary between villages but differed according to age group, being significantly lower among those aged ⩾15 years compared with any of the other younger age categories (P<0.001). After we adjusted for age and village, the overall vaccine efficacy against carriage was 94% (89% to 97%) and against infection was 82% (78% to 85%).
Table 1.
Vaccine efficacy in 1998 against chronic carriage of hepatitis B virus and breakthrough infection overall by village and by age group
| Chronic carriage
|
Breakthrough infection
|
||||
|---|---|---|---|---|---|
| No infected in vaccine v control
|
Vaccine efficacy (%) (95% CI)
|
No infected in vaccine v control
|
Vaccine efficacy (%) (95% CI)
|
||
| Overall | 10/855 v 191/931 | 94 (89 to 97) | 102/847 v 564/929 | 80 (76 to 84) | |
| Keneba | 6/628 v 83/622 | 93 (84 to 97) | 68/624 v 302/620 | 78 (72 to 82) | |
| Manduar | 4/227 v 108/309 | 95 (87 to 98) | 34/223 v 246/309 | 81 (74 to 86) | |
| <5 years (group 6) | 3/235 v 68/354 | 93 (79 to 98) | 14/232 v 140/354 | 85 (74 to 91) | |
| 5-9 years (group 5) | 0/225 v 66/309 | 100 | 10/224 v 309/509 | 93 (85 to 96) | |
| 10-14 years (group 4) | 2/220 v 39/191 | 96 (82 to 99) | 33/220 v 156/190 | 82 (75 to 87) | |
| ⩾15 years (groups 1, 2, 3) | 5/175 v 18/77 | 88 (68 to 95) | 53/171 v 68/76 | 65 (56 to 73) | |
Duration of response and breakthrough infections in children immunised in 1984
Table 2 shows the data obtained from groups 1, 2, and 3 at the various surveys. These participants had a median age in 1998 of 16.2 years (range 14.2-21.7 years) and had been followed up for a median of 13.8 (13.5-14.1) years. In each of the groups, which had significantly different peak antibody responses in 1985 (P<0.0001), antibody decayed in a similar and regular exponential manner with time. The proportion of uninfected participants with undetectable antibody concentrations (<10 mIU/ml) differed between the groups (P=0.001) and increased with time (P<0.0001).
Table 2.
Concentrations of antibody to hepatitis B surface antigen (anti-HbsAg) and number infected over time among vaccinated children in groups 1-3
| Year of survey
|
No tested*
|
|
Proportion (%) with anti-HBsAg <10 mIU/ml among those uninfected†
|
Geometric mean (95% CI) anti-HBsAg (mIU/ml)‡ among those uninfected
|
No infected at time of survey
|
No ever infected§
|
|
|---|---|---|---|---|---|---|---|
| Group 1 | |||||||
| 1985 | 53 | 8/47 (17) | 302 (216 to 422) | 7 | 7 | ||
| 1989 | 48 | 9/37 (24) | 60 (37 to 97) | 10 | 11 | ||
| 1993 | 47 | 4/30 (13) | 31 (21 to 46) | 15 | 19 | ||
| 1998 | 52 | 11/30 (37) | 67 (38 to 120) | 21 | 24 | ||
| Group 2 | |||||||
| 1985 | 57 | 3/50 (6) | 579 (421 to 796) | 7 | 7 | ||
| 1989 | 53 | 10/42 (24) | 65 (45 to 95) | 11 | 11 | ||
| 1993 | 49 | 5/37 (14) | 32 (24 to 44) | 10 | 16 | ||
| 1998 | 56 | 12/34 (35) | 60 (30 to 120) | 18 | 23 | ||
| Group 3 | |||||||
| 1985 | 62 | 0/62 (0) | 1045 (758 to 1442) | 2 | 2 | ||
| 1989 | 61 | 3/55 (5) | 134 (83 to 216) | 4 | 6 | ||
| 1993 | 59 | 5/47 (11) | 97 (59 to 162) | 8 | 12 | ||
| 1998 | 63 | 17/47 (36) | 79 (45 to 140) | 14 | 17 | ||
Refers to total number tested for anti-HB core antigen but not necessarily for anti-HBsAg.
Uninfected at time of survey.
Excludes those with anti-HBs <10 mIU/ml.
Includes current and past infections.
The proportion of breakthrough infections and the cumulative proportion of breakthrough infections (consisting of current infections and past infections that were no longer detectable) also increased with time (P<0.0001 in both cases), but neither of these proportions differed significantly between the groups. By 1998, 64 of the 171 (37.4%) vaccinated participants had been infected, and of the 111 uninfected participants, 40 (36%) had undetectable concentrations of antibody.
Vaccine efficacy against infection was 49.2% (27.8% to 64.3%), 36.2% (4.7% to 57.3%), and 92.3% (57.2% to 100%) for groups 1, 2, and 3, respectively (P<0.01 for comparison between group 1 or 2 and 3). In 1998 one of 54 (2%), 4 of 57 (7%), and none of 64 participants in groups 1, 2, and 3 were chronic carriers of HBsAg. Two of the chronic carriers were infected within a year of vaccination; the three others were infected five or more years later. Vaccine efficacy against chronic carriage was 91.0% (36.8% to 98.7%), 65.8% (11.3% to 86.8%), and 100% for groups 1, 2, and 3, respectively.
Breakthrough infections and chronic carriage according to peak antibody responses
Table 3 shows that the number of breakthrough infections was related to vaccination group (P=0.01) and to the peak antibody concentration (P=0.001). Those with an undetectable (equivalent to <10 mIU/ml) response had six times the chance of infection compared with those with high responses (>999 mIU/ml). More importantly, participants whose peak antibody response was <10 mIU/ml were 75 times more likely to become chronic carriers than those with responses ⩾ 10 mIU/ml (P<0.0001). Seven out of the 10 chronic carriers, all of whom had a peak antibody response of less than 10 mIU/ml, were infected before the age of 5 years.
Table 3.
Hepatitis B virus infection and chronic carriage in 1998 in relation to peak antibody concentration. Figures are numbers of breakthrough infections out of total number of children with antibody response
| Group and time of vaccination |
Peak antibody concentration (mIU/ml)
|
||||
|---|---|---|---|---|---|
| <10 | 10-99 | 100-999 | >999 | Total | |
| Group 1 (Nov 84-May 85) | 9*/10 (90) | 5/9 (56) | 4/26 (15) | 3/6 (50) | 21/51 (41) |
| Group 2 (Nov 84-May 85) | 3†/5 (60) | 1/3 (33) | 11/32 (34) | 3*/16 (19) | 18/56 (32) |
| Group 3 (Nov 84-May 85) | 0/0 | 2/2 (100) | 4/22 (18) | 8/39 (21) | 14/63 (22) |
| Group 4 (Oct 85-Sep 89) | 2*/5 (40) | 1/8 (13) | 10*/37 (27) | 11/141 (8) | 24/191 (13) |
| Group 5 (Oct 89-Sep 93) | 0/1 | 0/9 | 4/34 (12) | 5/146 (3) | 9/190 (5) |
| Group 6 (Oct 93-Jul 98) | 5†/18 (28) | 3/27 (11) | 3/73 (4) | 3/101 (3) | 14/219 (6) |
| Overall | 19/39 (49) | 12/58 (21) | 36/224 (16) | 33/449 (7) | 100/770 (13) |
One breakthrough infection resulted in chronic carriage.
Three breakthrough infections resulted in chronic carriage.
Logistic regression analyses of breakthrough infections and chronic carriage
Table 4 shows the independent factors associated with breakthrough infection. Time since vaccination and peak antibody concentrations were strongly associated; sex and village had a significant but lesser effect. Dose (three or four) was not significant; neither was route of administration.
Table 4.
Independent factors associated with core antibody breakthrough infection
| Variable | No (%) of breakthrough infections | Adjusted odds ratio (95% CI) | P value for variable |
|---|---|---|---|
| Vaccination group (median time (years) since vaccination): | |||
| Group 1 (13.6) | 21/52 (40) | 8.9 (3.8 to 20.8) | 0.0001 |
| Group 2 (13.6) | 18/56 (32) | 9.0 (3.8 to 21.1) | |
| Group 3 (13.6) | 14/63 (22) | 7.7 (3.2 to 18.5) | |
| Group 4 (10.7) | 25/220 (11) | 3.3 (1.6 to 7.1) | |
| Group 5 (6.3) | 9/202 (4) | 1.3 (0.5 to 3.3) | |
| Group 6 (2.1) | 15/254 (6) | 1.0 | |
| Sex: | |||
| Male | 60/426 (14) | 1.6 (1.0 to 2.6) | 0.04 |
| Female | 42/421 (10) | 1.0 | |
| Peak antibody (mIU/ml): | |||
| <10 | 19/39 (49) | 11.9 (4.9 to 28.8) | 0.0001 |
| 10-99 | 12/58 (21) | 3.4 (1.5 to 7.8) | |
| 100-999 | 36/224 (16) | 1.8 (1.0 to 3.2) | |
| ⩾1000 | 33/449 (7) | 1.0 | |
| Village: | |||
| Manduar | 34/223 (15) | 1.8 (1.1 to 3.0) | 0.03 |
| Keneba | 68/624 (11) | 1.0 | |
The only factor associated with chronic carriage of hepatitis B virus was a peak response of <10 mIU/ml (8/39 (20.5%) versus 2/731 (0.27%) children with a higher response (odds ratio 95, 95% confidence interval 19 to 466)).
Discussion
We have shown that despite low antibody concentrations, vaccine efficacy against chronic carriage of hepatitis B virus is remarkably well maintained in adolescence. However, vaccine efficacy against infection was also well maintained as 37% of teenagers were infected and 36% of those who were uninfected had undetectable concentrations of anti-HBs.
Natural boosting and immunity
The role of natural boosting in maintaining immunity in highly endemic settings is not clear. In our study antibody concentrations in uninfected older teenagers stabilised over the previous four years, perhaps as a result of increased exposure by the sexual route. In a larger cohort of Gambian people vaccinated in infancy, we have noted transient rises in antibody concentrations, which may be due to transient infections and which probably boost both cellular and humoral immunity.10,11 The role, if any, that sexual exposure plays in maintaining and boosting immunity remains to be defined and at present there are insufficient data to decide if a booster dose would be useful in teenagers in highly endemic areas where 15% of sexual partners may be chronic carriers of hepatitis B virus.4,12 In an area of low endemicity teenagers vaccinated in infancy may lose immunity because of lack of exposure, and a booster dose may be necessary at the onset of sexual maturity. In this setting it may be more sensible to deliver the primary course of vaccine in adolescence.
Risk factors for breakthrough infection
Independent risk factors associated with breakthrough infection were sex, village, time since vaccination, and peak antibody response. Boys and young men had a higher risk, as did people living in Manduar, which before vaccination had a remarkably high rate of infection of 71% in young children over a four year period.5 As age and type of vaccine were directly linked in groups 4, 5, and 6 we were not able to analyse these effects separately for the whole dataset. However, in participants in groups 1, 2, and 3, who were simultaneously given different doses by different routes, time since vaccination seemed to be a major determinant. Breakthrough infections and chronic carriage were clearly and strongly related to peak antibody concentrations. Thus half of the children who failed to produce detectable concentrations of antibody became infected, most within the first five years after vaccination, and of those infected nearly half became chronic carriers. However, as the numbers were small and as vaccines, dose, and routes of administration varied we were not able to assess formally which of these factors were the most important determinants of breakthrough infections resulting in chronic carriage of hepatitis B virus.
Other long term follow up studies
Few of the follow up studies of infants or young children vaccinated against hepatitis B virus have lasted longer than 10 years.9,13–15 The longest was in China, where 52 of the original 477 children (11%) were followed for 15 years: half had detectable concentrations of anti-HBs, and vaccine efficacy was 86.6% for chronic carriage and 86.4% for infection.15 This small study with a large dropout rate formed the basis for a recent consensus statement that no hepatitis B virus booster was required for 15 years after primary vaccination.16 However as the age at first sexual intercourse may be higher in China than in Africa the findings may not be generally applicable to other groups who start sexual activity earlier.17
In conclusion, our long term study of hepatitis B vaccination in infancy in a country where the infection is endemic showed that vaccine efficacy against infection waned with time but efficacy against chronic infection remained high over 14 years. However, the numbers involved are relatively small and a larger study of efficacy during adolescence is necessary before we conclude that a booster dose is not needed before the onset of sexual activity.16 In Africa and elsewhere, the risk of infection and of chronic carriage might be increased by the presence of other sexually transmitted infections, as is the case for HIV-1.18
Figure.
Effect of vaccination against hepatitis B infection on prevalence of hepatitis B virus surface antigen (HBsAg) and hepatitis B virus core antibody (anti-HBc) in two villages in the Gambia
Acknowledgments
We thank Lamin Giana, Joseph Bass, and Adam Jeng for their help in the laboratory and field.
Footnotes
Funding: Medical Research Council (UK) and Merck Sharpe & Dohme (USA).
Competing interests: None declared.
References
- 1.World Health Organization. Prevention of primary liver cell cancer. Report on a meeting of a WHO scientific group. Lancet. 1983;i:463–465. [PubMed] [Google Scholar]
- 2.Kane MA, Clements J, Hu D. Hepatitis B. In: Jamison OT, Mosley WH, Measham AR, Bobadilla J, editors. Disease control priorities in developing countries. A World Bank Book. New York: Oxford University Press; 1993. pp. 321–330. [Google Scholar]
- 3.Gambia Hepatitis Study Group. The Gambia hepatitis intervention study. Cancer Res. 1987;47:5782–5787. [PubMed] [Google Scholar]
- 4.Whittle HC, Bradley AK, McLauchlen K, Ajdukiewicz AB, Howard CR, Zuckerman AJ, et al. Hepatitis B infection in two Gambian villages. Lancet. 1983;i:1203–1206. doi: 10.1016/s0140-6736(83)92477-7. [DOI] [PubMed] [Google Scholar]
- 5.Whittle H, Inskip H, Bradley AK, McLaughlan K, Shenton F, Lamb W, et al. The pattern of childhood hepatitis B infection in two Gambian villages. J Infect Dis. 1990;161:1112–1115. doi: 10.1093/infdis/161.6.1112. [DOI] [PubMed] [Google Scholar]
- 6.Whittle HC, Lamb WH, Ryder RW. Trials of intradermal hepatitis B vaccines in Gambian children. Ann Trop Paediatr. 1987;7:6–9. doi: 10.1080/02724936.1987.11748464. [DOI] [PubMed] [Google Scholar]
- 7.Whittle HC, Inskip H, Hall AJ, Mendy M, Downes R, Hoare S. Vaccination against hepatitis B and protection against chronic viral carriage in the Gambia. Lancet. 1991;337:747–750. doi: 10.1016/0140-6736(91)91367-4. [DOI] [PubMed] [Google Scholar]
- 8.Whittle HC, Maine N, Pilkington J, Mendy M, Fortuin M, Bunn J, et al. Long-term efficacy of continuing hepatitis B vaccination in infancy in two Gambian villages. Lancet. 1995;345:1089–1092. doi: 10.1016/s0140-6736(95)90822-6. [DOI] [PubMed] [Google Scholar]
- 9.Coursaget P, Leboulleux P, Soumare M, le Cann P, Yvonnst B, Chiron JP, et al. Twelve year follow up study of hepatitis B immunization of Senegalese infants. J Hepatol. 1994;21:250–254. doi: 10.1016/s0168-8278(05)80404-0. [DOI] [PubMed] [Google Scholar]
- 10.Gambia Hepatitis Intervention Study. Annual report, 1996. Lyons: International Agency for Research on Cancer; 1996. p. 12. [Google Scholar]
- 11.Jack AD, Hall AJ, Maine M, Mendy M, Whittle HC. What level of hepatitis B antibody is protective? J Infect Dis. 1999;179:489–492. doi: 10.1086/314578. [DOI] [PubMed] [Google Scholar]
- 12.Vall-Mayans M, Hall AJ, Inskip HM, Chotard J, Lindsay SW, Coromina E, et al. Risk factors for transmission of hepatitis B virus to Gambian children. Lancet. 1990;336:1107–1109. doi: 10.1016/0140-6736(90)92580-b. [DOI] [PubMed] [Google Scholar]
- 13.Chen HL, Chang MH, Ni YH, Hsu HY, Lee PI, Lee CY, et al. Sero-epidemiology of hepatitis B virus infection in children: ten years of mass vaccination in Taiwan. JAMA. 1996;276:906–908. [PubMed] [Google Scholar]
- 14.Yuen MF, Lim WL, Cheng LC, Lam SK, Lai CL. Twelve-year follow-up of a prospective randomized trial of hepatitis B recombinant DNA yeast vaccine versus plasma-derived vaccine without booster doses in children. Hepatology. 1999;29:924–927. doi: 10.1002/hep.510290327. [DOI] [PubMed] [Google Scholar]
- 15.Liao SS, Li RC, Li H, Yang JY, Aeng XJ, Gong J, et al. Long-term efficacy of plasma derived vaccine: a 15-year follow-up study among Chinese children. Vaccine. 1999;17:2661–2666. doi: 10.1016/s0264-410x(99)00031-6. [DOI] [PubMed] [Google Scholar]
- 16.European Consensus Group on Hepatitis B Immunity. Are booster immunisations needed for lifelong hepatitis B immunity? Lancet. 2000;355:561–565. [PubMed] [Google Scholar]
- 17.Ratcliffe A, Hill A, Walraven G. Separate lives, different interests: male and female reproduction in the Gambia. Bull World Health Organ. 2000;78:570–579. [PMC free article] [PubMed] [Google Scholar]
- 18.Grosskurth H, Moshu F, Todd J, Mwijarubi E, Klokke A, Senkoro K, et al. Impact of improved treatment of sexually transmitted diseases on HIV infection in rural Tanzania: randomised controlled trial. Lancet. 1995;346:530–536. doi: 10.1016/s0140-6736(95)91380-7. [DOI] [PubMed] [Google Scholar]