Abstract
Objectives: Rosai-Dorfman-Destombes disease (RDD), often associated with autoimmune disease, is rare in the elderly. We report a 79-year-old Japanese man with RDD, who had been treated for non-specific interstitial pneumonia (NSIP) for longer than 6 years. Methods: Detecting RDD lesions was evaluated with imaging studies, including PET-CT, and the RDD diagnosis was based on the pathological findings of a biopsied lymph node. Findings and Clinical course: PET-CT revealed an FDG-avid 5.5 cm-sized mass (SUVmax; 7.12) at the right lung and enlarged lymph nodes at the bilateral supra-clavicular area (SUVmax; 10.12). A supraclavicular lymph node biopsy confirmed the diagnosis of RDD; however, it was characterized by lymph node necrosis, which is rarely noted in the RDD tissue. Three months after the RDD diagnosis, the patient developed cold agglutinin disease (CAD), causing severe anemia, for which packed red blood cell transfusions and sutimlimab® therapy were planned; however, the patient died of presumable NSIP exacerbation. Conclusions: RDD occurs even in the elderly. RDD in this case was associated with autoimmune NSIP and CAD. The presence of necrotic foci in the biopsied lymph node does not contradict the diagnosis of RDD.
Keywords: Rosai-Dorfman-Destombes disease, necrosis, interstitial pneumonitis, cold agglutinin disease, sutimlimab
Introduction
Rosai-Dorfman-Destombes disease (RDD; sinus histiocytosis with massive lymphadenopathy), one type of histiocytosis, affects lymph nodes and extra-nodal sites. Massive bilateral and painless cervical lymphadenopathy is the most characteristic symptom. Besides, the common extra-nodal sites of RDD are noted in the skin, upper respiratory tract, and bone [1,2]. RDD commonly presents in children and young patients as cervical lymphadenopathy; however, an elderly (>75 years) case was also described [2,3]. The diagnosis of RDD is based on the characteristic pathology: the lymph node architecture is expanded by numerous histiocytes filling and distending the sinuses with emperipolesis, in which histiocytes engulf intact lymphocytes, plasma cells, or red blood cells. This phenomenon is crucial for diagnosis. By immunohistochemistry, they express CD68, CD163 (majority), S100, and OCT2 [4]. OCT2 positivity was identified as a novel marker for the monocyte-macrophage phenotype of RDD, expressed in 97% of RDD cases [5]. In pathological findings, lymph node necrosis is generally absent in RDD tissue pathology [6], except for a rare report [7].
The disease belongs to the R group of the 2016 revised histiocytosis classification [8]. RDD is known to be associated with IgG4-related disease, autoimmune disease, and neoplasia [2]. As autoimmune diseases, systemic lupus erythematosus, autoimmune hemolytic anemia (AIHA), ANA-positive or anti-lupus anticoagulant-positive status, etc., have been reported [9,10]. In cases of neoplasia-associated RDD, malignant lymphoma (Hodgkin and non-Hodgkin) or RDD can either precede or follow each other or occur in the same lymph node [2]. Some RDDs could be benign, but there are clonal diseases with RAS-RAF-MAPK pathway abnormalities. When KRAS/NRAS or MAP2K1 mutations are identified, molecular-targeted therapy may be recommended [11]. Thus, therapeutic options include observation, steroids, various immunosuppressive/chemotherapeutic agents, or targeted therapy [2,4,12]. We report here an elderly case of autoimmune disease-associated RDD with a coagulative necrotic area in the lesional lymph node.
Case report
A 79-year-old man, afebrile, was hospitalized for undergoing a bronchoscopic examination to determine the pathology of an underlying pulmonary disease. He had been treated for his non-specific interstitial pneumonia (NSIP) for longer than 6 years, and for a right upper lung mass on CT that had been detected one year ago (Figure 1A). He was suspected of having either autoimmune pneumonia, lung IgG4-related disease, or lung carcinoma. He also had cervical adenopathy without cutaneous lesions. On admission, his laboratory data were WBC 9000/µL, Hb 12.3 g/dL, platelet count 46 K/µL, and CRP 0.52 mg/dL. Lactate dehydrogenase (LDH) 201 U/L, and normal hepatic/renal function. He was ANA-positive (×640). Tumor and other biological markers were CEA 4.82 (<5) ng/mL, Pro-GRP-S 45.2 (<81) pg/mL, CYFRA 2.7 (<3.5) ng/mL, SCC 1.3 (<1.5) ng/mL, MMP-3 55.1 (36.9-121), KL-6 1045 (<499), SP-A 60.1 (<43.5), and SP-D 144 (<110) ng/mL. The serum level of IgG was 1750 mg/dL with IgG4 of 103 mg/dL.
Figure 1.
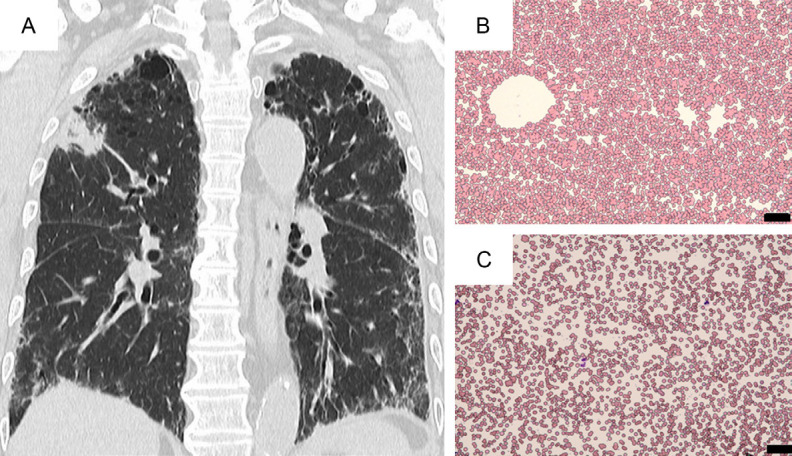
CT of the chest (coronal axis) shows bilateral interstitial pneumonitis with a mass lesion at the right upper lung (A). At the time of CAD development, blood smear at room temperature demonstrated RBC agglutination (B), while after warming of blood at 36ºC, RBC agglutination resolved (C) (May-Giemsa staining; original magnification ×200, scale bar indicates 50 µm).
PET-CT revealed an FDG-avid 5.5 cm-sized mass (SUVmax=7.12) at the right lung, as well as enlarged lymph nodes at the bilateral supra-clavicular area (SUVmax=10.12), mediastinal (SUVmax=11.26), and pulmonary hilar lesions. Also, there were FDG-avid lymph nodes (SUVmax=5.42) at the bilateral cervical area and a mass (SUVmax=5.80) at the right parotid gland (Figure 2). Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) of peri-bronchial lymph nodes revealed no malignancy. The resection of the left supraclavicular lymph node demonstrated the histology of RDD, showing the sinus expansion of large histiocytes with emperipolesis in the abundant pale eosinophilic cytoplasm, associated with coagulative necrotic lesions (Figure 3). Multinucleated giant cells were also noted. IgG4+ cells were scattered, but IgG4-related disease (IgG4-RD) was ruled out. Immunohistochemical staining of histiocytes showed S100 (+), CD68 (+), CD163 (+), OCT2 (+), but CD1a (-), CD3 (-), CD20 (-), MPO (-), AE1/AE3 (-), BRAF V600E(VE1) (-), pERK (-), ALK (-), panTRK (-) and Ki-67 (16%). The diagnosis was made as nodal RDD. However, the respiratory tract lesions were not confirmed to be extra-nodal RDD. After the diagnosis of RDD, we decided to observe the patient without specific therapy, because of his NSIP. However, within 3 months, he developed cold agglutinin disease (CAD) with a titer of ×128. Blood smear at room temperature showed marked RBC aggregates, and after warm-up at 38°C, RBC aggregates resolved (Figure 1B, 1C). Hemolytic anemia was significant (Table 1). A bone marrow smear revealed erythroid hyperplasia without light chain restriction by flow cytometry, and no B-cell lymphoproliferative disease was detected. The patient’s anemia has been treated with packed red blood cells (2 units ×3), and the administration of sutimlimab® (a humanized monoclonal IgG4 antibody that binds and inactivates complement protein C1s; 6.5 g/dose) [13] was planned. However, after a single administration, it was not possible to continue the treatment because the patient’s NSIP worsened. Thus, we could not evaluate the effectiveness of sutimlimab® in CAD. The patient eventually died of respiratory failure.
Figure 2.
PET-CT images. The coronal figure of the torso shows (A) high FDG-avid signals were detected in the right lung, bilateral supraclavicular lymph nodes, and mediastinal lymph nodes. Other minor lesions were also noted. The axial figures show (B) bilateral supraclavicular lesions, and (C) right lung and mediastinal lesions.
Figure 3.

Pathological findings. The biopsied lymph node shows (A) H&E stain; the symbol crosses indicate necrotic areas (original magnification ×12.5, scale bar indicates 500 µm). The inset figure shows an enlarged view of emperipolesis in the sinusoidal histiocytes (original magnification ×100). (B) CD68 stain; a sinusoidal lesion of proliferated CD68-positive histiocytes including large multi-nucleated cells (Touton cells) neighboring with a necrotic area (a symbol cross) (original magnification ×100, scale bar indicates 100 µm).
Table 1.
Clinical data
| Laboratory data (references) | At Dx of RDD | At Dx of CAD |
|---|---|---|
| WBC (3300-8600)/µL | 9000 | 5400 |
| RBC (435-555) 10e4/µL | 451 | 73 |
| Hb (11.6-14.8) g/dL | 12.3 | 7.2 |
| MCV (83.6-98.2) fL | 91.8 | 126 |
| Reticulocyte (3-11) ‰ | NT | 180 |
| Platelet count (158K-348K)/µL | 46K | 236K |
| CRP (<0.14) mg/dL | 0.52 | 6.41 |
| AST (13-30) U/L | 20 | 24 |
| ALT (10-42) U/L | 16 | 15 |
| LDH (124-222) U/L | 201 | 325 |
| Total bilirubin (0.4-1.5) mg/dL | 0.81 | 4.78 |
| Total protein (5.6-8.1) g/dL | 7.3 | 6.1 |
| BUN (8.0-20.0) mg/dL | 18.1 | 25.6 |
| Creatinine (0.46-0.79) mg/dL | 0.91 | 1.04 |
| Haptoglobin (19-170) mg/dL | NT | 5 |
Abbreviations: Dx: diagnosis, RDD: Rosai-Dorfman-Destombes disease, CAD: cold agglutinin disease, WBC: white cell count, RBC: red blood cell count, Hb: hemoglobin, MCV: mean corpuscular volume, CRP: C-reactive protein, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, BUN: blood urea nitrogen, NT: not tested.
Discussion
Regarding the onset ages of RDD, Abla et al. described that the disease has been reported up to age 74 years [2]. Tapia et al.’s case (78-year-old) [3] and our case (79-year-old) were far older. Our case had been treated for NSIP before the diagnosis of RDD, with high values of serum KL-6, SP-A, and SP-D, which help to diagnose interstitial pneumonitis [14]. Hasegawa et al. [15] described a case of IgG4-RDD, presented as diffuse interstitial lung disease overlapping with peri-lymphatic RDD lesions. We diagnosed RDD in the biopsied supraclavicular lymph node, not in the broncoscopic biopsied specimens. The diagnosis of pulmonary RDD is difficult. Al-Maghrabi et al. described a case of a 52-year-old female, in whom multiple bronchoscopic biopsies were not diagnostic, despite having an intrabronchial mass invading the bronchial wall and an extrabronchial lymphadenopathy. RDD in their case was diagnosed with pneumonectomized tissues [16]. The RDD in our case was characteristic of a coagulative necrosis in the lymph node (Figure 3). It was stated that lymph node necrosis is generally absent in RDD tissue pathology [6]. Exceptionally, Hayes et al. reported extensive coagulative necrosis of the intra-sinus histiocytes in a 21-year-old man with generalized lymphadenopathy and arthritis [7]. The cause(s) of rare necrosis in RDD lymph nodes remain unknown.
A correlation between RDD and autoimmune diseases (AID) is well recognized. In Sen et al.’s study, 25 (18%) of 142 cases of RDD had AID, of which 16/25 (64%) were diagnosed with AID before RDD. The most common AIDs were lupus, rheumatoid arthritis, and polymyalgia rheumatica [17]. Previously, RDD complicated by AIHA was described in pediatric and adult cases [18-23]. Of the 7 AIHA cases, including ours, 5 cases were adults aged from 28 to 79 years old. AIHA develops as a warm or a cold type (CAD) [24]. The RDD-associated CAD experienced in our elderly case was also previously described in a pediatric case by Danisious et al. [23]. The abnormal immune response of the host may explain the co-occurrence of RDD and AIDs like NSIP and AIHA.
As mentioned above, therapeutic options in RDD include observation, steroids, various immunosuppressive/chemotherapeutic agents, or targeted therapy [2,4,12]. At the diagnosis of RDD, we decided not to treat RDD, considering her pulmonary problems of NSIP. After the patient developed CAD-triggered anemia, we planned to control it with sutimlimab® [13]. Unfortunately, we could not fully evaluate the efficacy of this drug in CAD, because of the patient’s presumable deterioration of NSIP; however, as mentioned above, we could not rule out other cause(s) of lung disease undetectable by bronchoscopic biopsy.
Regarding the prognosis of patients with RDD, classical nodal sporadic RDD is often self-limited with a good outcome. On the other hand, 5% to 11% of patients may die of their disease [8]. An MEK inhibitor administration could be associated with positive outcomes in KRAS- or MEK-mutated RDD cases [25,26]. In this case, although immunohistochemistry was negative for BRAF V600E, molecular analysis to assess KRAS, NRAS, or MAP2K1 mutations commonly observed in RDD [11] could not be performed; however, it would have helped guide potential targeted therapy.
Conclusions
We report a case of RDD in an elderly male whose pathology in the lymph node was characterized by coagulative necrosis. The patient developed RDD after 6 years of NSIP and, within 3 months after RDD diagnosis, presented with a cold-type AIHA (CAD). Unfortunately, the patient died of presumably deteriorated NSIP.
Acknowledgements
The authors thank Dr. Atsuko Nakazawa, Saitama Children’s Medical Center, for her pathological diagnosis of RDD.
Disclosure of conflict of interest
None.
References
- 1.Rosai J, Dorfman RF. Sinus histiocytosis with massive lymphadenopathy. A newly recognized benign clinicopathological entity. Arch Pathol. 1969;87:63–70. [PubMed] [Google Scholar]
- 2.Abla O, Jacobsen E, Picarsic J, Krenova Z, Jaffe R, Emile JF, Durham BH, Braier J, Charlotte F, Donadieu J, Cohen-Aubart F, Rodriguez-Galindo C, Allen C, Whitlock JA, Weitzman S, McClain KL, Haroche J, Diamond EL. Consensus recommendations for the diagnosis and clinical management of Rosai-Dorfman-Destombes disease. Blood. 2018;131:2877–2890. doi: 10.1182/blood-2018-03-839753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tapia N, Sharma S, Koirala M, Blumenthal D. Rosai-Dorfman disease occurring in an elderly patient with long-standing idiopathic hypertrophic osteoarthropathy. J Clin Rheumatol. 2021;27:S713–S714. doi: 10.1097/RHU.0000000000001561. [DOI] [PubMed] [Google Scholar]
- 4.Ravindran A, Rech KL. How I diagnose Rosai-Dorfman disease. Am J Clin Pathol. 2023;160:1–10. doi: 10.1093/ajcp/aqad047. [DOI] [PubMed] [Google Scholar]
- 5.Ravindran A, Goyal G, Go RS, Rech KL Mayo Clinic Histiocytosis Working Group. Rosai-Dorfman disease displays a unique monocyte-macrophage phenotype characterized by expression of OCT2. Am J Surg Pathol. 2021;45:35–44. doi: 10.1097/PAS.0000000000001617. [DOI] [PubMed] [Google Scholar]
- 6.Rosai J, Dorfman RF. Sinus histiocytosis with massive lymphadenopathy: a pseudolymphomatous benign disorder. Analysis of 34 cases. Cancer. 1972;30:1174–1188. doi: 10.1002/1097-0142(197211)30:5<1174::aid-cncr2820300507>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 7.Hayes MM, Daya H, Uys CJ, Samson RI. Sinus histiocytosis with massive lymphadenopathy. Report of a case associated with necrosis. Arch Pathol Lab Med. 1985;109:151–152. [PubMed] [Google Scholar]
- 8.Emile JF, Abla O, Fraitag S, Horne A, Haroche J, Donadieu J, Requena-Caballero L, Jordan MB, Abdel-Wahab O, Allen CE, Charlotte F, Diamond EL, Egeler RM, Fischer A, Herrera JG, Henter JI, Janku F, Merad M, Picarsic J, Rodriguez-Galindo C, Rollins BJ, Tazi A, Vassallo R, Weiss LM Histiocyte Society. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672–2681. doi: 10.1182/blood-2016-01-690636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaur PP, Birbe RC, DeHoratius RJ. Rosai-Dorfman disease in a patient with systemic lupus erythematosus. J Rheumatol. 2005;32:951–953. [PubMed] [Google Scholar]
- 10.Lopetegui-Lia N, Asad SD, Jafri SI, Harrison JS. Autoimmune diseases and Rosai-Dorfman disease coexist more commonly than expected: two case reports. Am J Case Rep. 2019;20:770–772. doi: 10.12659/AJCR.915627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen Aubart F, Idbaih A, Emile JF, Amoura Z, Abdel-Wahab O, Durham BH, Haroche J, Diamond EL. Histiocytosis and the nervous system: from diagnosis to targeted therapies. Neuro Oncol. 2021;23:1433–1446. doi: 10.1093/neuonc/noab107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salama HA, Jazieh AR, Alhejazi AY, Absi A, Alshieban S, Alzahrani M, Alaskar A, Gmati G, Damlaj M, Abuelgasim KA, Alghamdi A, Alahmari B, Almugairi A, Alzahrani H, Bazarbachi A, Musa MOH, Goyal G. Highlights of the management of adult histiocytic disorders: langerhans cell histiocytosis, erdheim-chester disease, Rosai-Dorfman disease, and hemophagocytic lymphohistiocytosis. Clin Lymphoma Myeloma Leuk. 2021;21:e66–e75. doi: 10.1016/j.clml.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Röth A, Barcellini W, D’Sa S, Miyakawa Y, Broome CM, Michel M, Kuter DJ, Jilma B, Tvedt THA, Fruebis J, Jiang X, Lin S, Reuter C, Morales-Arias J, Hobbs W, Berentsen S. Sutimlimab in cold agglutinin disease. N Engl J Med. 2021;384:1323–1334. doi: 10.1056/NEJMoa2027760. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Zheng P, Huang Z, Huang H, Xue M, Liao C, Sun B, Zhong N. Serum SP-A and KL-6 levels can predict the improvement and deterioration of patients with interstitial pneumonia with autoimmune features. BMC Pulm Med. 2020;20:315. doi: 10.1186/s12890-020-01336-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasegawa M, Sakai F, Okabayashi A, Katsura H, Kamata T, Koh E, Sekine Y, Takemura T, Nakatani Y, Hiroshima K. Rosai-Dorfman disease of the lung overlapping with IgG4-related disease: the difficulty in its differential diagnosis. Intern Med. 2017;56:937–941. doi: 10.2169/internalmedicine.56.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Maghrabi H, Elmahrouk A, Feteih M, Jamjoom A, Al-Maghrabi J. Rosai-Dorfman disease with pulmonary involvement mimicking bronchogenic carcinoma. J Cardiothorac Surg. 2020;15:37. doi: 10.1186/s13019-020-1085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sen M, Ruan GJ, Reynolds SB, Ali H, Yang X, Morlote D, Ravindran A, Shea L, Maron R, Elmets CA, Koster MJ, Abeykoon JP, Salama HA, Cao XX, Ahmed AZ, Go RS, Goyal G. A multi-center study of autoimmunity in Rosai Dorfman disease. 40th annual meeting of Histiocyte Society; 2024. (Abstract) page 39. [Google Scholar]
- 18.Grabczynska SA, Toh CT, Francis N, Costello C, Bunker CB. Rosai-Dorfman disease complicated by autoimmune haemolytic anaemia: case report and review of a multisystem disease with cutaneous infiltrates. Br J Dermatol. 2001;145:323–326. doi: 10.1046/j.1365-2133.2001.04325.x. [DOI] [PubMed] [Google Scholar]
- 19.Sachdeva M, Abdulhaq H. A rare case of Rosai-Dorfman disease in an adult male associated with auto-immune hemolytic anemia. Mediterr J Hematol Infect Dis. 2013;5:e2013022. doi: 10.4084/MJHID.2013.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akria L, Sonkin V, Braester A, Cohen HI, Suriu C, Polliack A. Rare coexistence of Rosai-Dorfman disease and nodal marginal zone lymphoma complicated by severe life-threatening autoimmune hemolytic anemia. Leukemia Lymphoma. 2013;54:1553–1556. doi: 10.3109/10428194.2012.740564. [DOI] [PubMed] [Google Scholar]
- 21.Lardhi AA, Al-Mutairi AK, Al-Qahtani MH, Al-Mutairi AK. Rosai-Dorfman disease complicated by autoimmune hemolytic anemia in a child: a case report and review of the literature. Case Rep Oncol. 2018;11:55–62. doi: 10.1159/000485968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Subhadarshani S, Kumar T, Arava S, Gupta S. Rosai-Dorfman disease with cutaneous plaques and autoimmune haemolytic anemia. BMJ Case Rep. 2019;12:e231927. doi: 10.1136/bcr-2019-231927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danisious T, Hettiarachchi M, Dharmadasa C, Jayaweera H. Rosai-Dorfman disease with renal involvement and associated autoimmune haemolytic anaemia in a 12-year-old girl: a case report. BMC Pediatr. 2020;20:470. doi: 10.1186/s12887-020-02368-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill A, Hill QA. Autoimmune hemolytic anemia. Hematology Am Soc Hematol Educ Program. 2018;2018:382–389. doi: 10.1182/asheducation-2018.1.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moyon Q, Boussouar S, Maksud P, Emile JF, Charlotte F, Aladjidi N, Prévot G, Donadieu J, Amoura Z, Grenier P, Haroche J, Cohen-Aubart F. Lung Involvement in Destombes-Rosai-Dorfman disease: clinical and radiological features and response to the MEK inhibitor cobimetinib. Chest. 2020;157:323–333. doi: 10.1016/j.chest.2019.09.036. [DOI] [PubMed] [Google Scholar]
- 26.Abeykoon JP, Rech KL, Young JR, Ravindran A, Ruan GJ, Dasari S, Morlote DM, King RL, Rummage C, Zanwar S, Acosta-Medina AM, Tobin WO, Shah MV, Bennani NN, Vassallo R, Ryu JH, Koster MJ, Davidge-Pitts CJ, Witzig TE, Goyal G, Go RS Mayo Clinic-University of Alabama at Birmingham Histiocytosis Working Group. Outcomes after treatment with Cobimetinib in patients with Rosai-Dorfman disease based on KRAS and MEK alteration status. JAMA Oncol. 2022;8:1816–1820. doi: 10.1001/jamaoncol.2022.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]



