Abstract
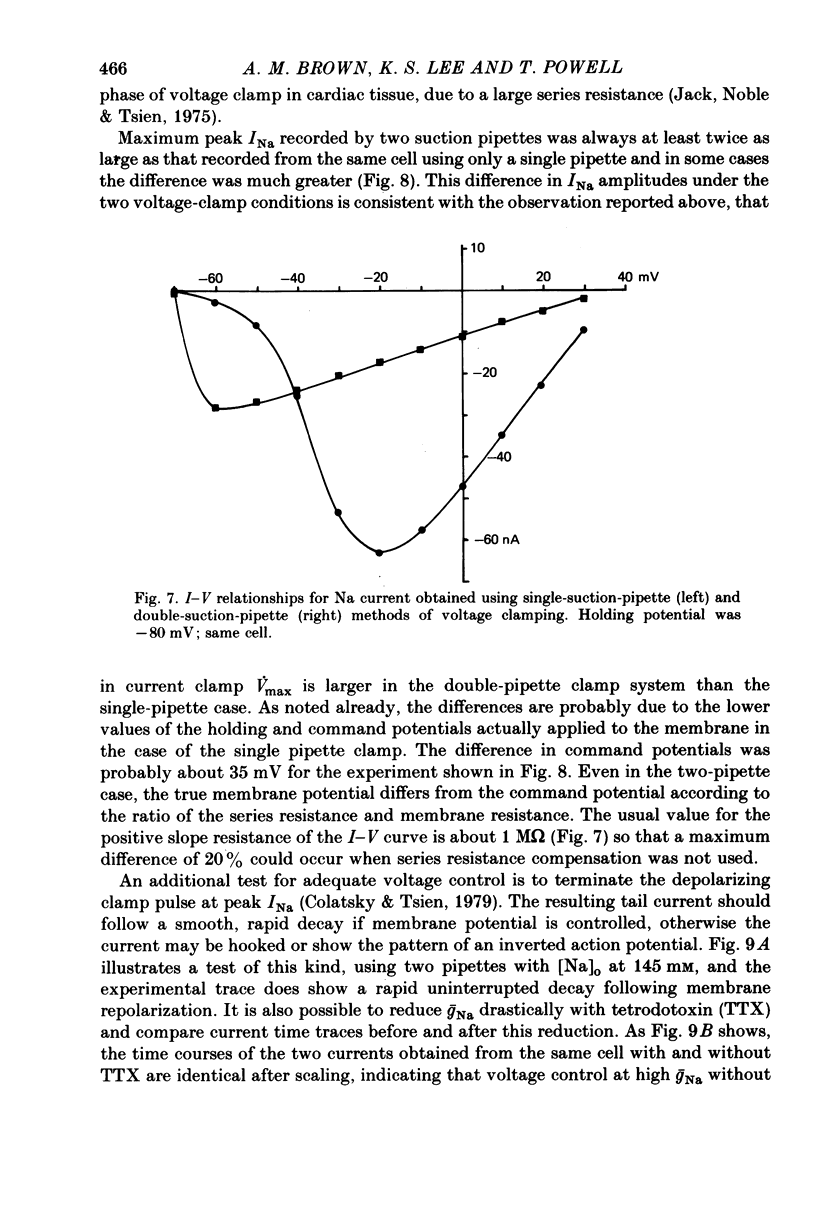
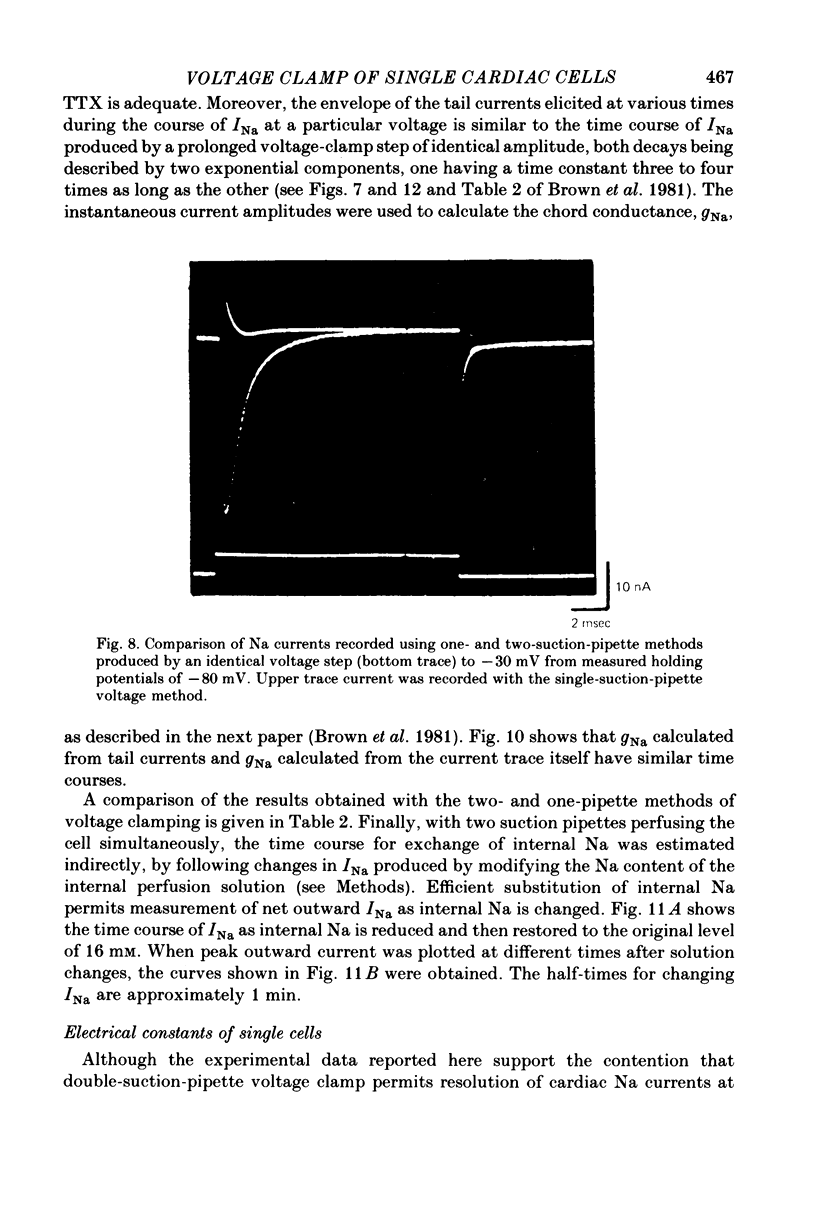
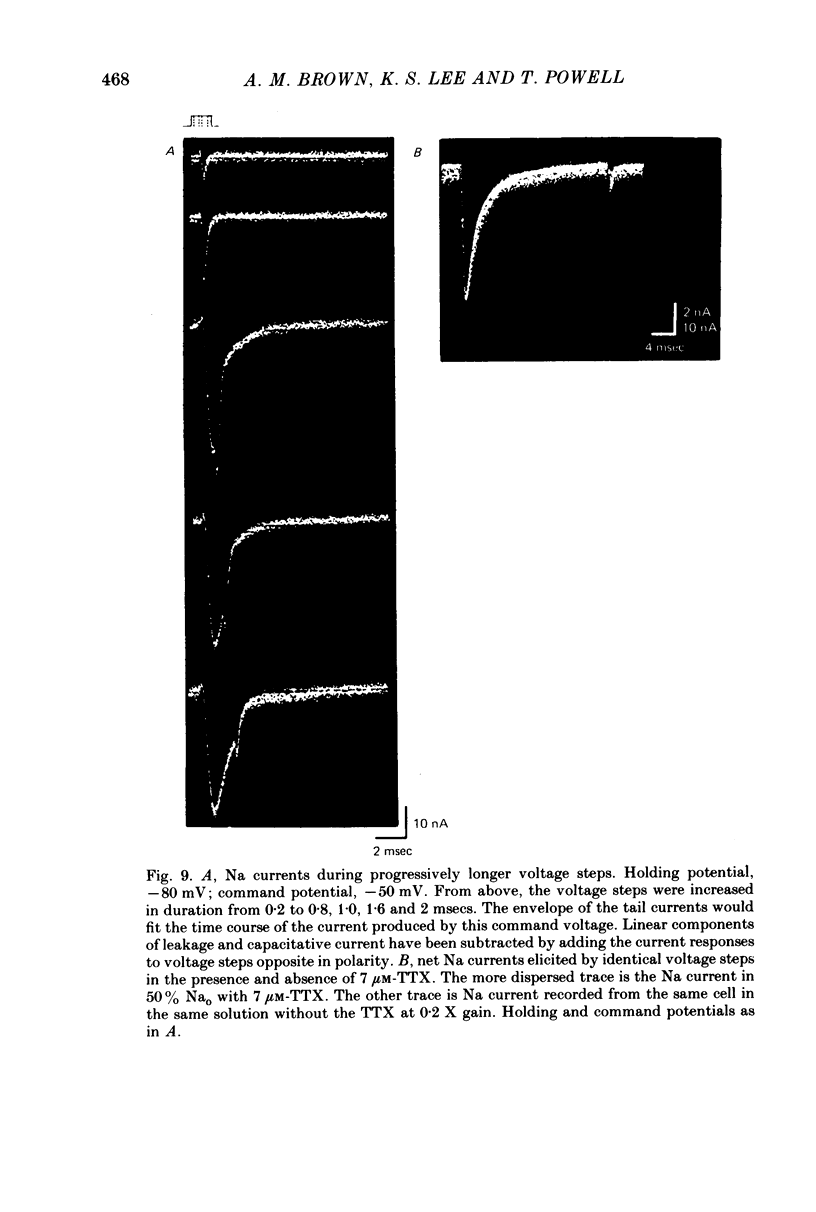
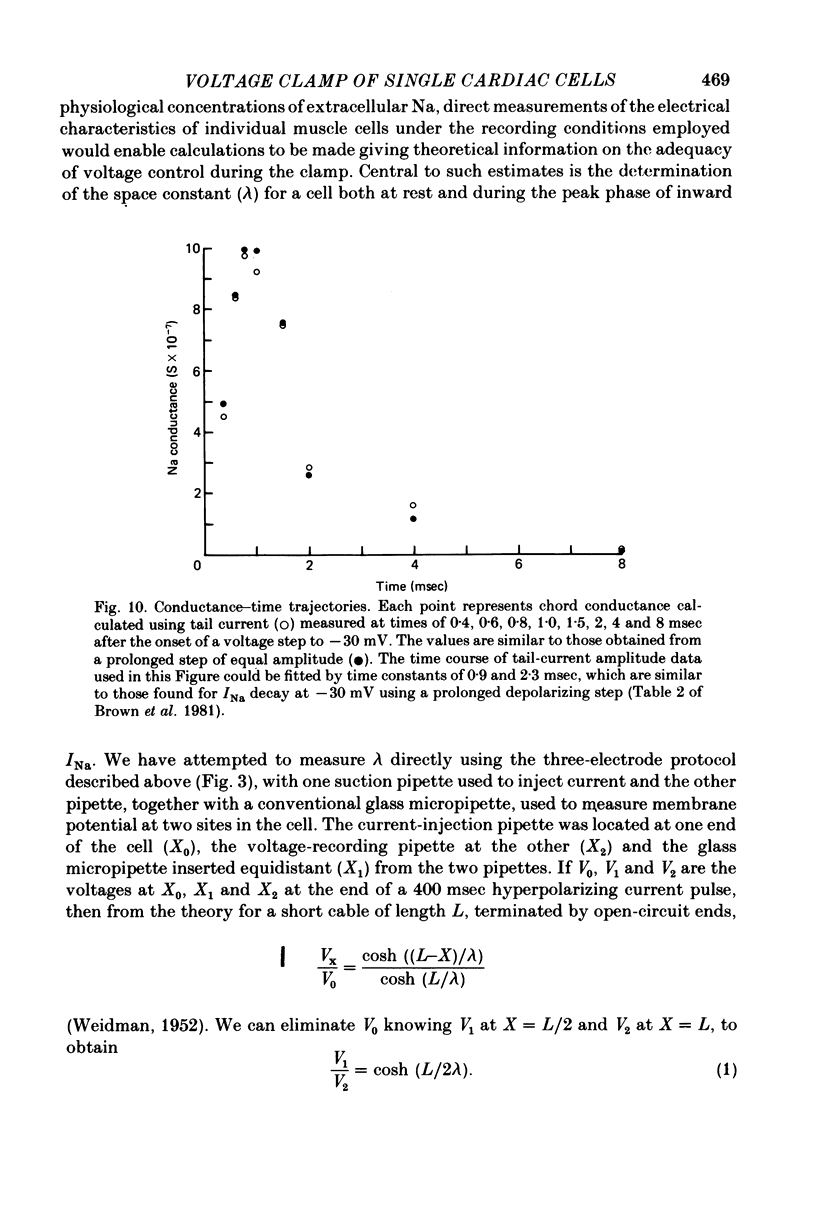
1. Single cells from adult rat ventricle were dispersed using an enzymic dissociation technique. Electrical properties were measured with either suction pipettes or conventional glass micropipettes and the results were compared. 2. Suction pipette and micropipette measurements of resting membrane potentials and action potentials were comparable. Values were similar to those reported previously for both dispersed cardiac myocytes and whole tissue preparations from adult rat ventricle. 3. Voltage clamp with a single suction pipette was used in initial experiments, but the results were not sufficiently accurate. Consequently, voltage clamp of single cells was carried out using two suction pipettes (tip diameters 10-15 micrometers), one for passing current and the other for recording membrane potential. Dialysis of cell contents was performed by each suction pipette. A roving micropipette (tip diameter less than 1 micrometer) was used occasionally to measure membrane potential at selected sites. 4. Using the two-suction-pipette method, voltage-clamp steps rose with time constants of less than 10 microsec and the capacitative current transient decayed with a single time constant of less than 100 microsec. These values are more optimal than those observed in other voltage-clamped cardiac muscle preparations. 5. Single cardiac myocytes had membrane input resistances of 44.5 +/- 4.6 M omega, membrane time constants of 16.2 +/- 0.63 msec and membrane capacitances of 399.7 +/- 42.2 pF. (values are means +/- DS, n = 10-). The length constant, lambda, of a single myocte at ists resting potential and perfused with KH2PO4 was about 500 micrometers. For cells perfused with Cs aspartate solution commonly used in voltage-clamp experiments, the input resistance was approximately quadrupled and lambda was 1100 micrometers. The average length of a myocyte partially aspirated by two suction pipettes was 50 micrometers. At maximum -gNa in 50% extracellular Na, lambda was calculated to be about four times the actual cell length between voltage-recording and current-passing pipettes. 6. The half-time for the disappearance or recovery of outward Na currents, following removal or restitution of intracellular Na with two pipettes, was of the order 1 min, indicating that intracellular ionic composition of the cell could be readily controlled and modified.
Full text
PDF


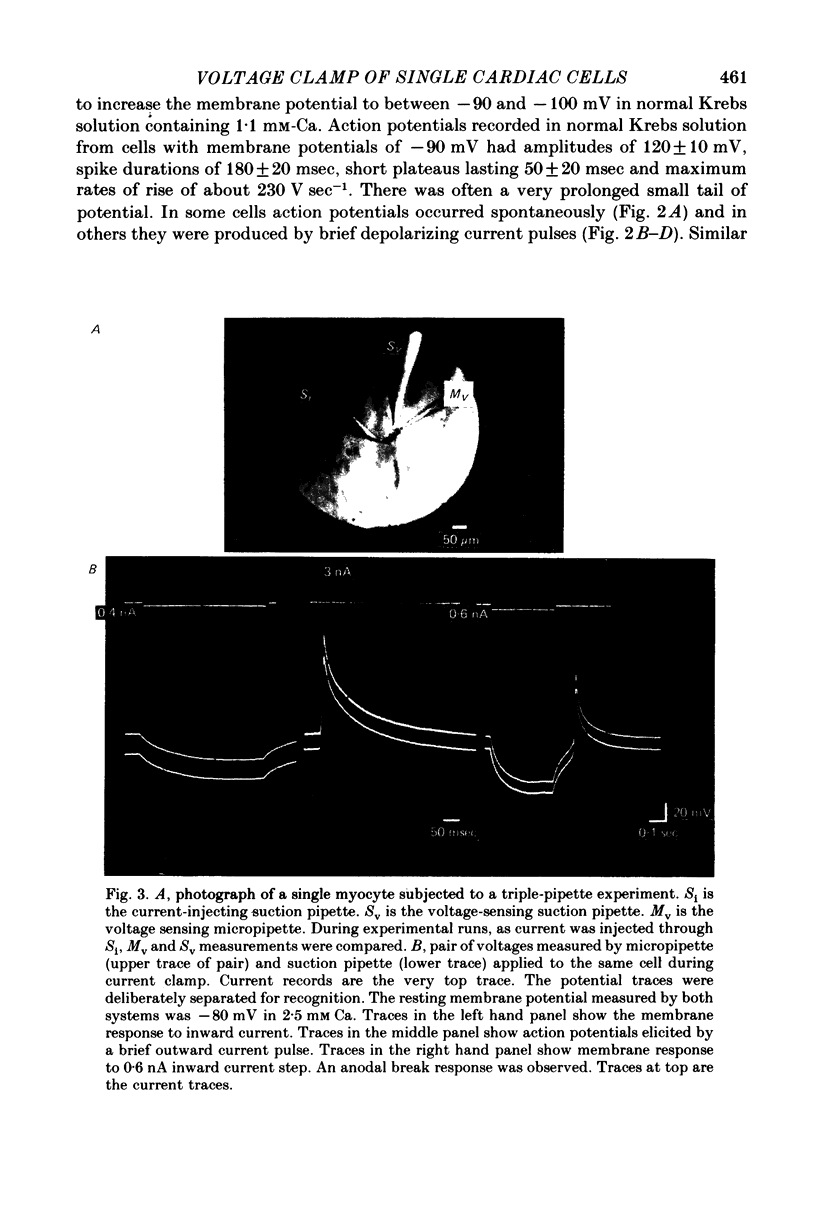
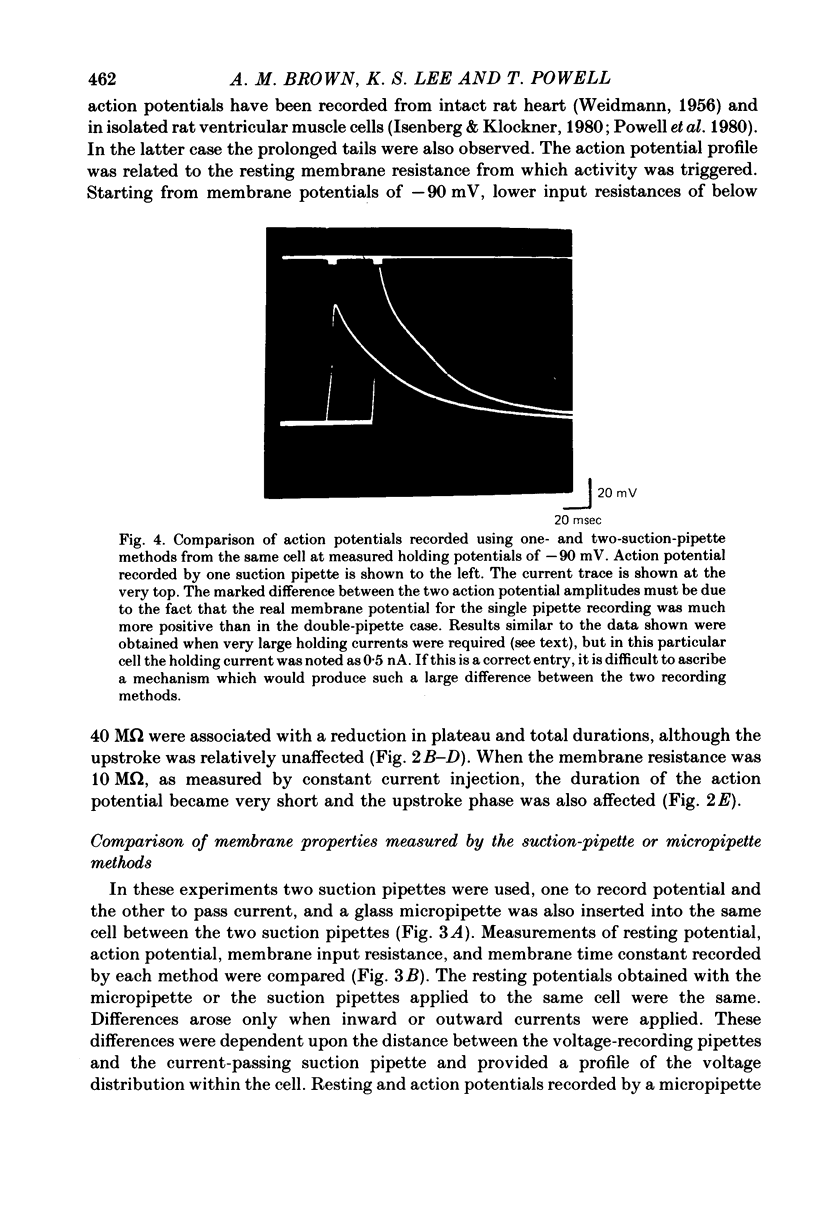

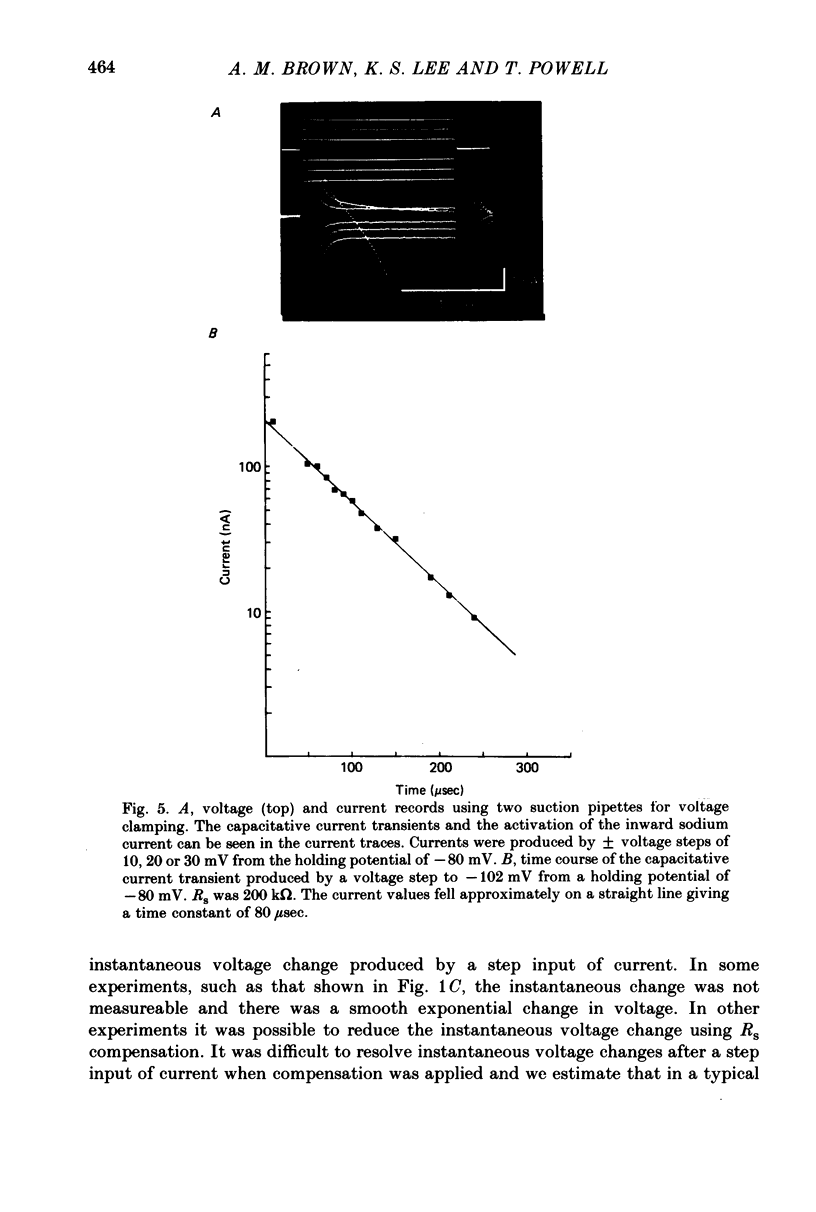
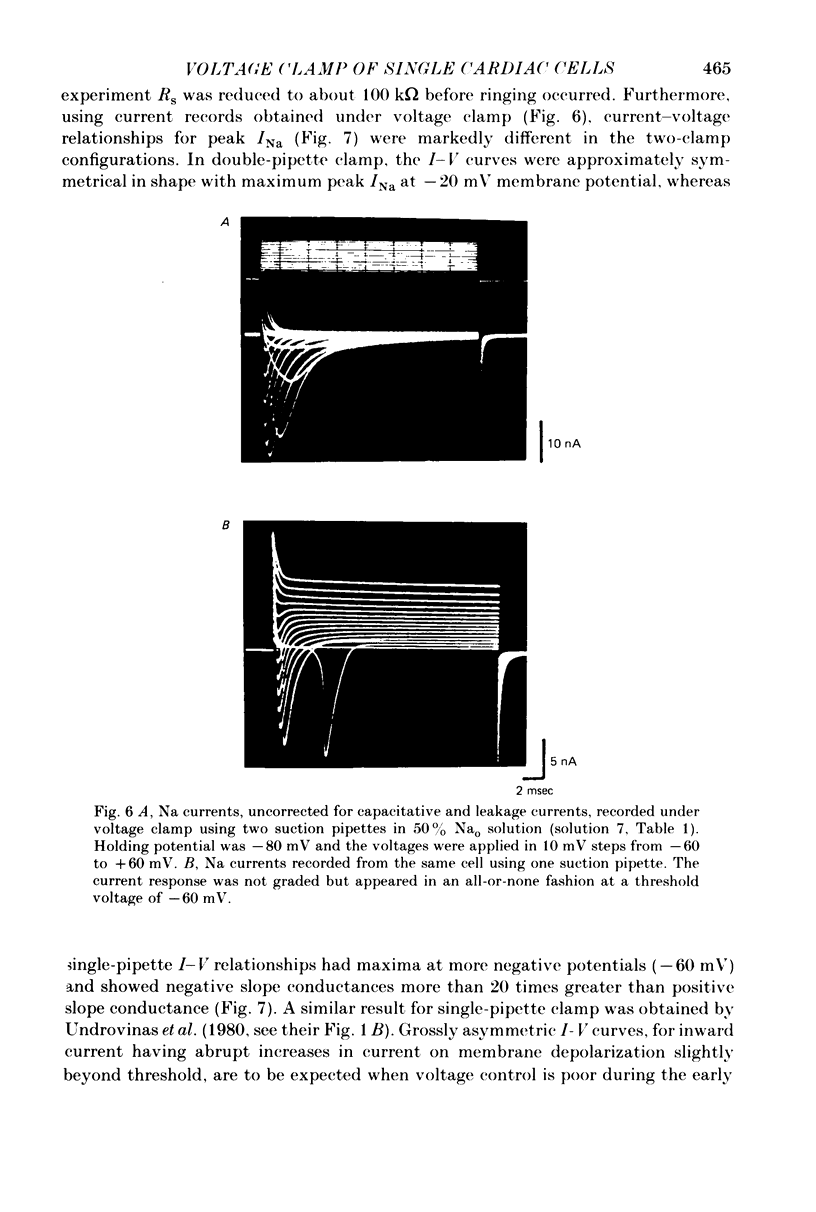
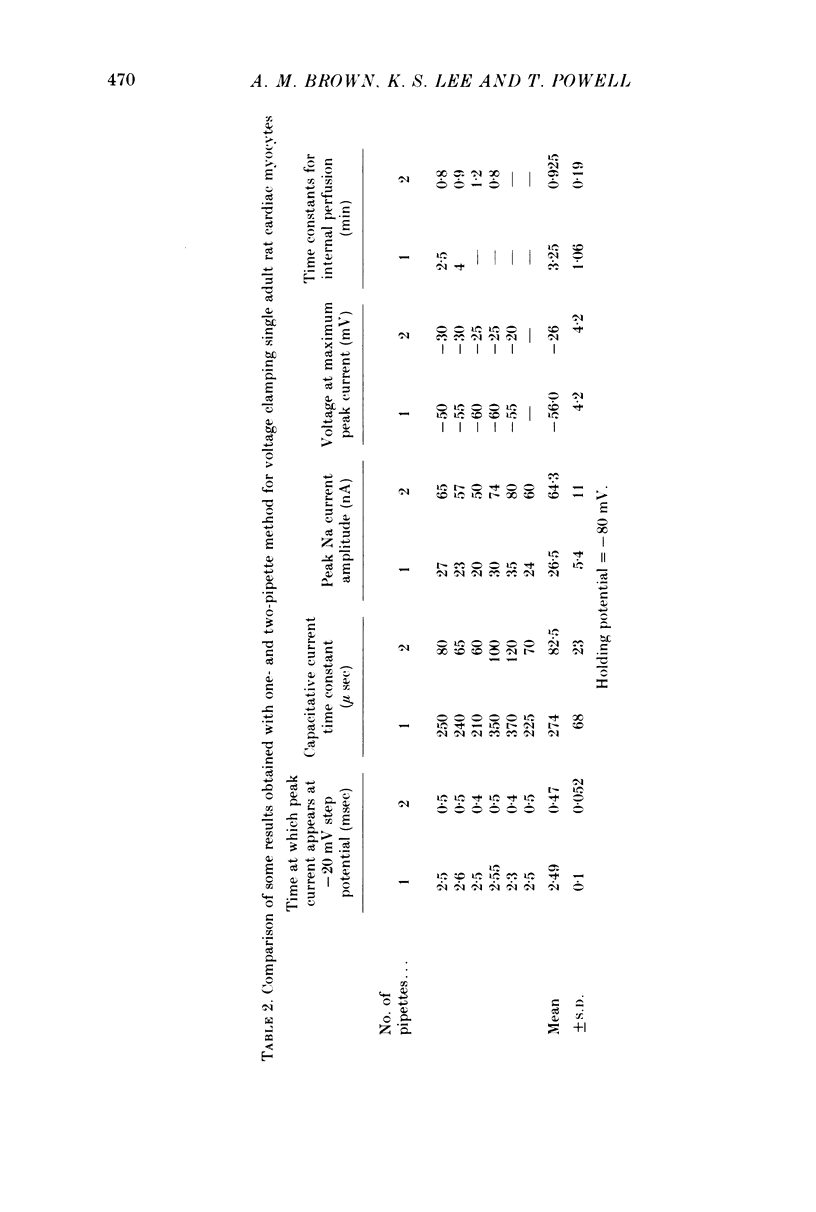
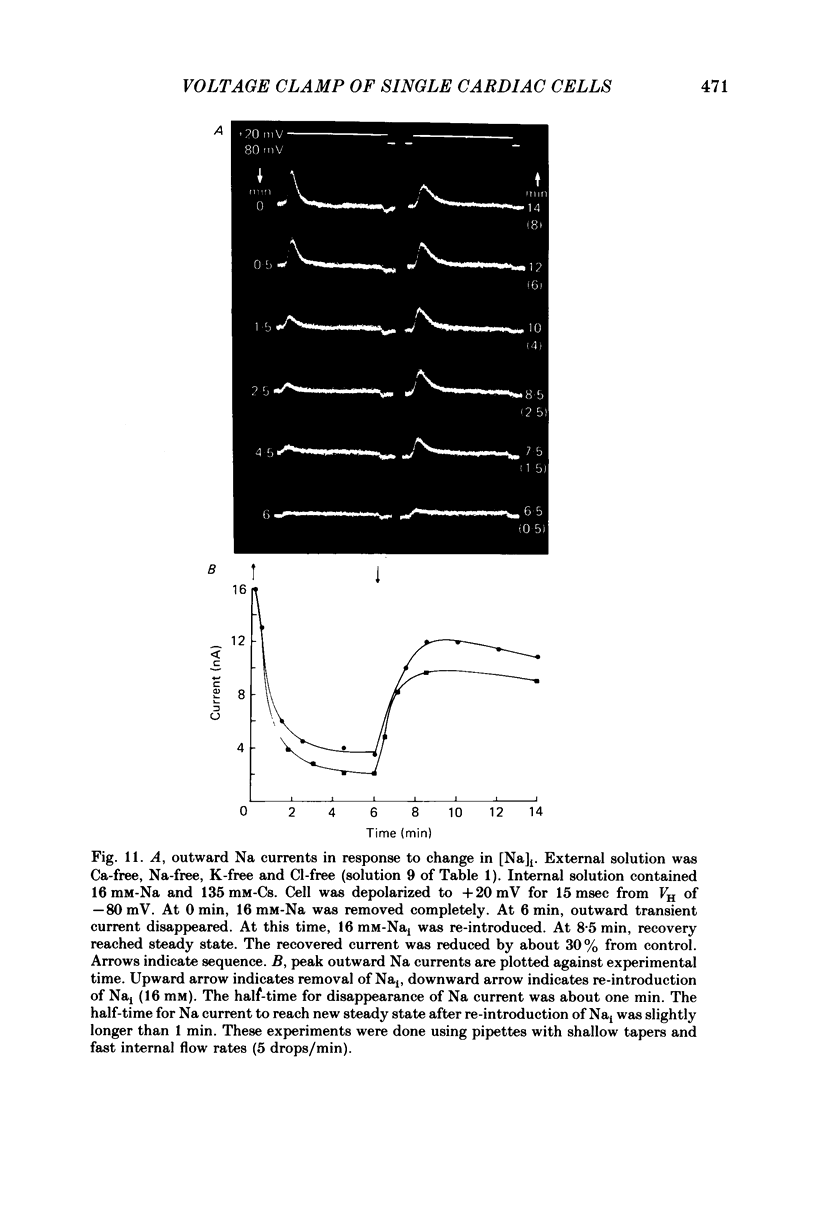
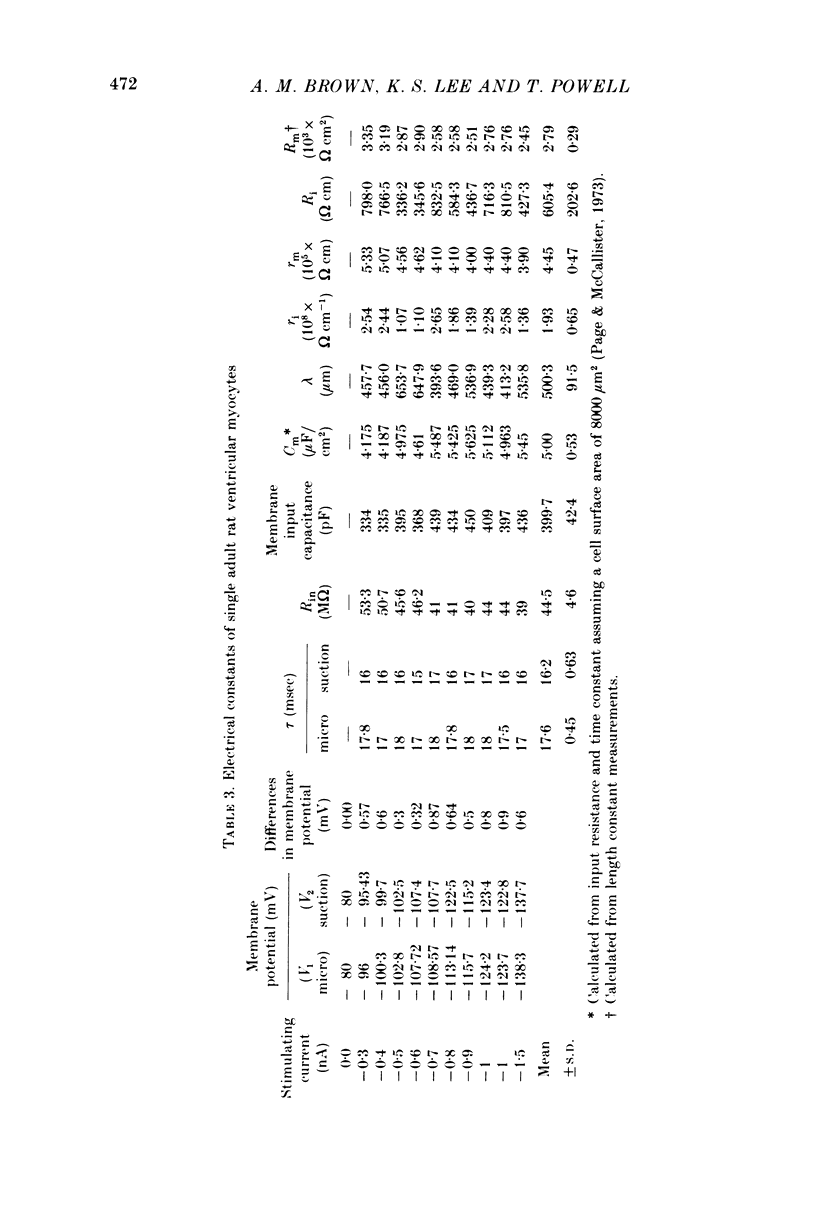
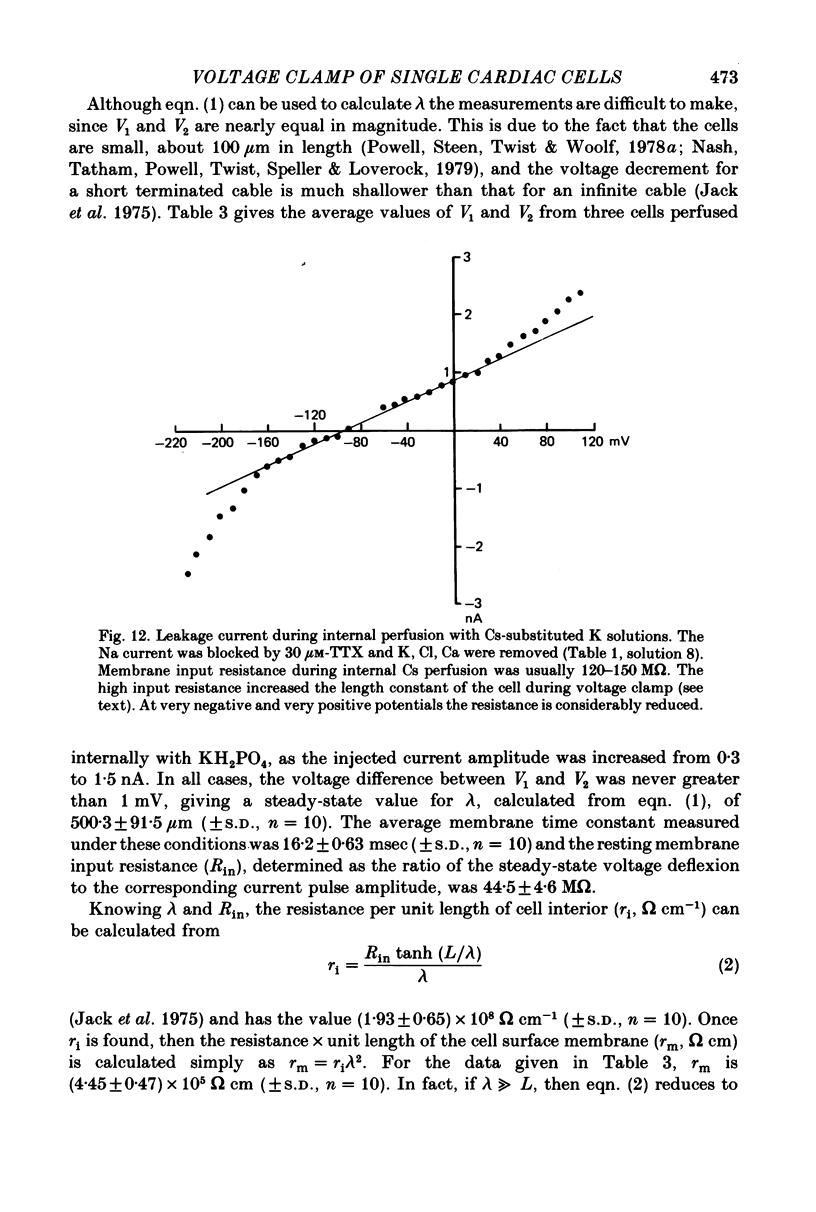




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attwell D., Eisner D., Cohen I. Voltage clamp and tracer flux data: effects of a restricted extra-cellular space. Q Rev Biophys. 1979 Aug;12(3):213–261. doi: 10.1017/s0033583500005448. [DOI] [PubMed] [Google Scholar]
- Beeler G. W., Jr, Reuter H. Membrane calcium current in ventricular myocardial fibres. J Physiol. 1970 Mar;207(1):191–209. doi: 10.1113/jphysiol.1970.sp009056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Lee K. S., Powell T. Sodium current in single rat heart muscle cells. J Physiol. 1981 Sep;318:479–500. doi: 10.1113/jphysiol.1981.sp013879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. F., Giles W., Noble S. J. Membrane currents underlying activity in frog sinus venosus. J Physiol. 1977 Oct;271(3):783–816. doi: 10.1113/jphysiol.1977.sp012026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colatsky J. J., Tsien R. W. Sodium channels in rabbit cardiac Purkinje fibres. Nature. 1979 Mar 15;278(5701):265–268. doi: 10.1038/278265a0. [DOI] [PubMed] [Google Scholar]
- Dudel J., Rüdel R. Voltage and time dependence of excitatory sodium current in cooled sheep Purkinje fibres. Pflugers Arch. 1970;315(2):136–158. doi: 10.1007/BF00586657. [DOI] [PubMed] [Google Scholar]
- Ebihara L., Shigeto N., Lieberman M., Johnson E. A. The initial inward current in spherical clusters of chick embryonic heart cells. J Gen Physiol. 1980 Apr;75(4):437–456. doi: 10.1085/jgp.75.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman Y., Morad M. Measurement of transmembrane potential and current in cardiac muscle: a new voltage clamp method. J Physiol. 1977 Jul;268(3):613–654. doi: 10.1113/jphysiol.1977.sp011875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F., KATZ B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G. Cardiac Purkinje fibres: the slow inward current component under the influence of modified [Ca2+]i. Pflugers Arch. 1977 Oct 19;371(1-2):61–69. doi: 10.1007/BF00580773. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klöckner U. Glycocalyx is not required for show inward calcium current in isolated rat heart myocytes. Nature. 1980 Mar 27;284(5754):358–360. doi: 10.1038/284358a0. [DOI] [PubMed] [Google Scholar]
- Johnson E. A., Lieberman M. Heart: excitation and contraction. Annu Rev Physiol. 1971;33:479–532. doi: 10.1146/annurev.ph.33.030171.002403. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Akaike N., Brown A. M. Properties of internally perfused, voltage-clamped, isolated nerve cell bodies. J Gen Physiol. 1978 May;71(5):489–507. doi: 10.1085/jgp.71.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Akaike N., Brown A. M. The suction pipette method for internal perfusion and voltage clamp of small excitable cells. J Neurosci Methods. 1980 Feb;2(1):51–78. doi: 10.1016/0165-0270(80)90045-x. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Akaike N., Brown A. M. Trypsin inhibits the action of tetrodotoxin on neurones. Nature. 1977 Feb 24;265(5596):751–753. doi: 10.1038/265751a0. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Weeks T. A., Kao R. L., Akaike N., Brown A. M. Sodium current in single heart muscle cells. Nature. 1979 Mar 15;278(5701):269–271. doi: 10.1038/278269a0. [DOI] [PubMed] [Google Scholar]
- Morad M., Trautwein W. The effect of the duration of the action potential on contraction in the mammalian heart muscle. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;299(1):66–82. doi: 10.1007/BF00362542. [DOI] [PubMed] [Google Scholar]
- Nash G. B., Tatham P. E., Powell T., Twist V. W., Speller R. D., Loverock L. T. Size measurements on isolated rat heart cells using Coulter analysis and light scatter flow cytometry. Biochim Biophys Acta. 1979 Sep 20;587(1):99–III. doi: 10.1016/0304-4165(79)90224-1. [DOI] [PubMed] [Google Scholar]
- New W., Trautwein W. Inward membrane currents in mammalian myocardium. Pflugers Arch. 1972;334(1):1–23. doi: 10.1007/BF00585997. [DOI] [PubMed] [Google Scholar]
- Noble D., Tsien R. W. Reconstruction of the repolarization process in cardiac Purkinje fibres based on voltage clamp measurements of membrane current. J Physiol. 1969 Jan;200(1):233–254. doi: 10.1113/jphysiol.1969.sp008690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page E., McCallister L. P. Studies on the intercalated disk of rat left ventricular myocardial cells. J Ultrastruct Res. 1973 Jun;43(5):388–411. doi: 10.1016/s0022-5320(73)90017-8. [DOI] [PubMed] [Google Scholar]
- Powell T., Steen E. M., Twist V. W., Woolf N. Surface characteristics of cells isolated from adult rat myocardium. J Mol Cell Cardiol. 1978 Mar;10(3):287–292. doi: 10.1016/0022-2828(78)90351-6. [DOI] [PubMed] [Google Scholar]
- Powell T., Terrar D. A., Twist V. W. Electrical activity in superfused cells isolated from adult rat ventricular myocardium [proceedings]. J Physiol. 1978 Nov;284:148P–148P. [PubMed] [Google Scholar]
- Powell T., Terrar D. A., Twist V. W. Electrical properties of individual cells isolated from adult rat ventricular myocardium. J Physiol. 1980 May;302:131–153. doi: 10.1113/jphysiol.1980.sp013234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell T., Terrar D. A., Twist V. W. Membrane potentials in muscle cells isolated from adult rat heart [proceedings]. J Physiol. 1978 Sep;282:23P–24P. [PubMed] [Google Scholar]
- Powell T., Twist V. W. A rapid technique for the isolation and purification of adult cardiac muscle cells having respiratory control and a tolerance to calcium. Biochem Biophys Res Commun. 1976 Sep 7;72(1):327–333. doi: 10.1016/0006-291x(76)90997-9. [DOI] [PubMed] [Google Scholar]
- Reuter H., Scholz H. A study of the ion selectivity and the kinetic properties of the calcium dependent slow inward current in mammalian cardiac muscle. J Physiol. 1977 Jan;264(1):17–47. doi: 10.1113/jphysiol.1977.sp011656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougier O., Vassort G., Stämpfli R. Voltage clamp experiments on frog atrial heart muscle fibres with the sucrose gap technique. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;301(2):91–108. doi: 10.1007/BF00362729. [DOI] [PubMed] [Google Scholar]
- Tarr M., Trank J. W. An assessment of the double sucrose-gap voltage clamp technique as applied to frog atrial muscle. Biophys J. 1974 Sep;14(9):627–643. doi: 10.1016/S0006-3495(74)85940-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undrovinas A. I., Yushmanova A. V., Hering S., Rosenshtraukh L. V. Voltage clamp method on single cardiac cells from adult rat heart. Experientia. 1980 May 15;36(5):572–574. doi: 10.1007/BF01965808. [DOI] [PubMed] [Google Scholar]
- WEIDMANN S. Effect of current flow on the membrane potential of cardiac muscle. J Physiol. 1951 Oct 29;115(2):227–236. doi: 10.1113/jphysiol.1951.sp004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDMANN S. Resting and action potentials of cardiac muscle. Ann N Y Acad Sci. 1957 Aug 9;65(6):663–678. doi: 10.1111/j.1749-6632.1957.tb36674.x. [DOI] [PubMed] [Google Scholar]
- WEIDMANN S. The electrical constants of Purkinje fibres. J Physiol. 1952 Nov;118(3):348–360. doi: 10.1113/jphysiol.1952.sp004799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidmann S. Electrical constants of trabecular muscle from mammalian heart. J Physiol. 1970 Nov;210(4):1041–1054. doi: 10.1113/jphysiol.1970.sp009256. [DOI] [PMC free article] [PubMed] [Google Scholar]