Abstract
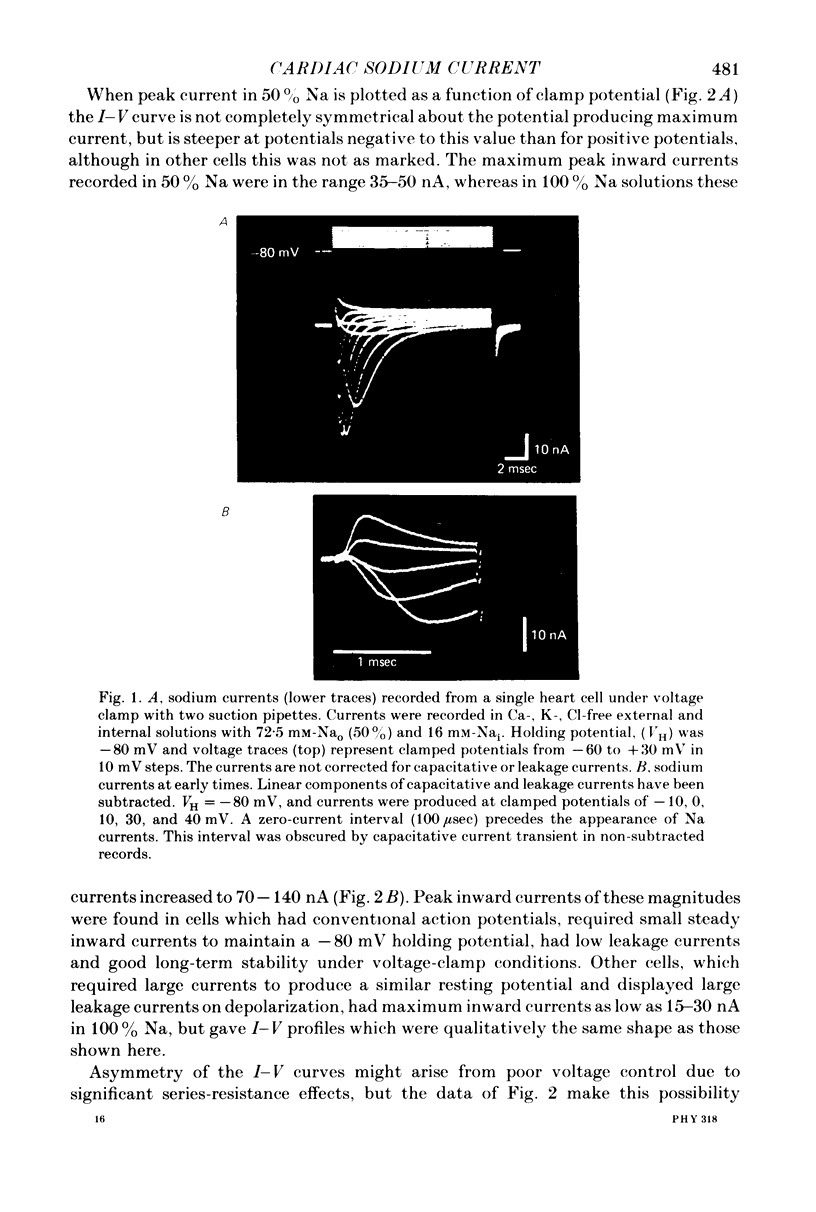
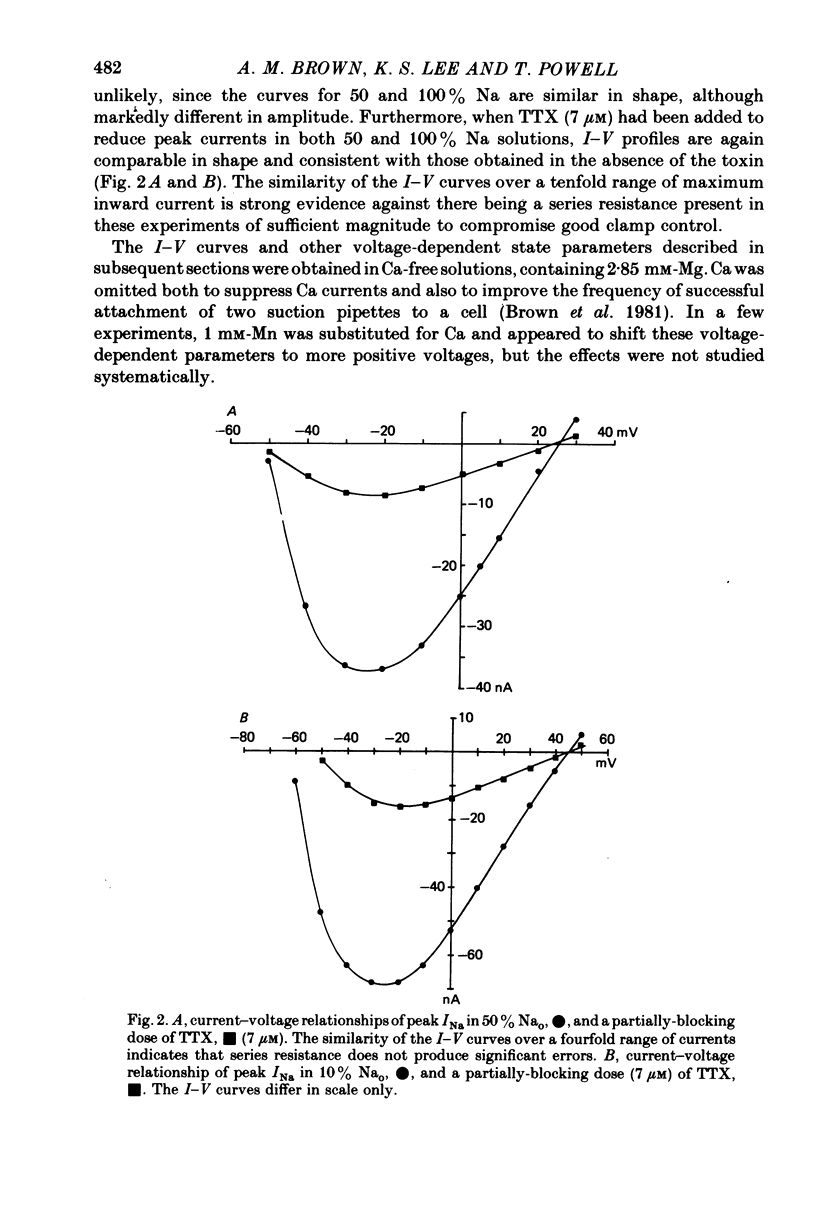
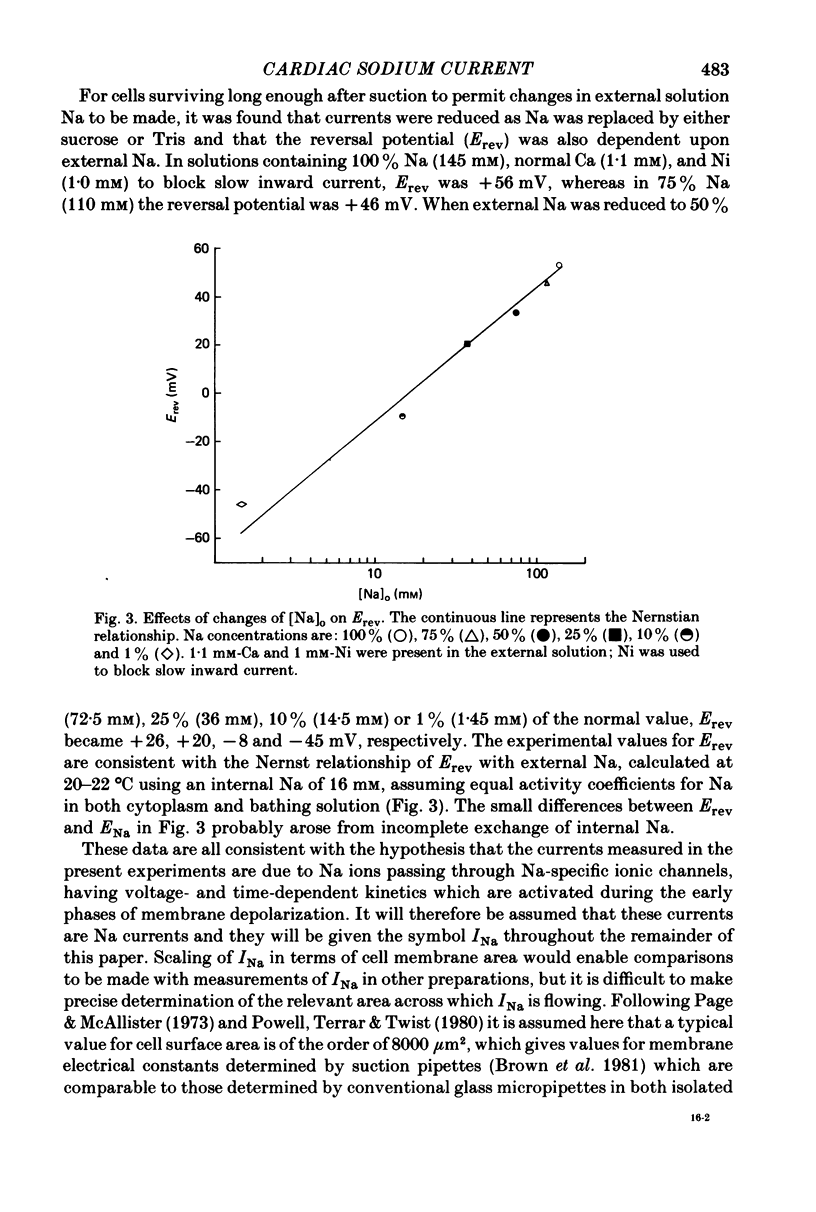
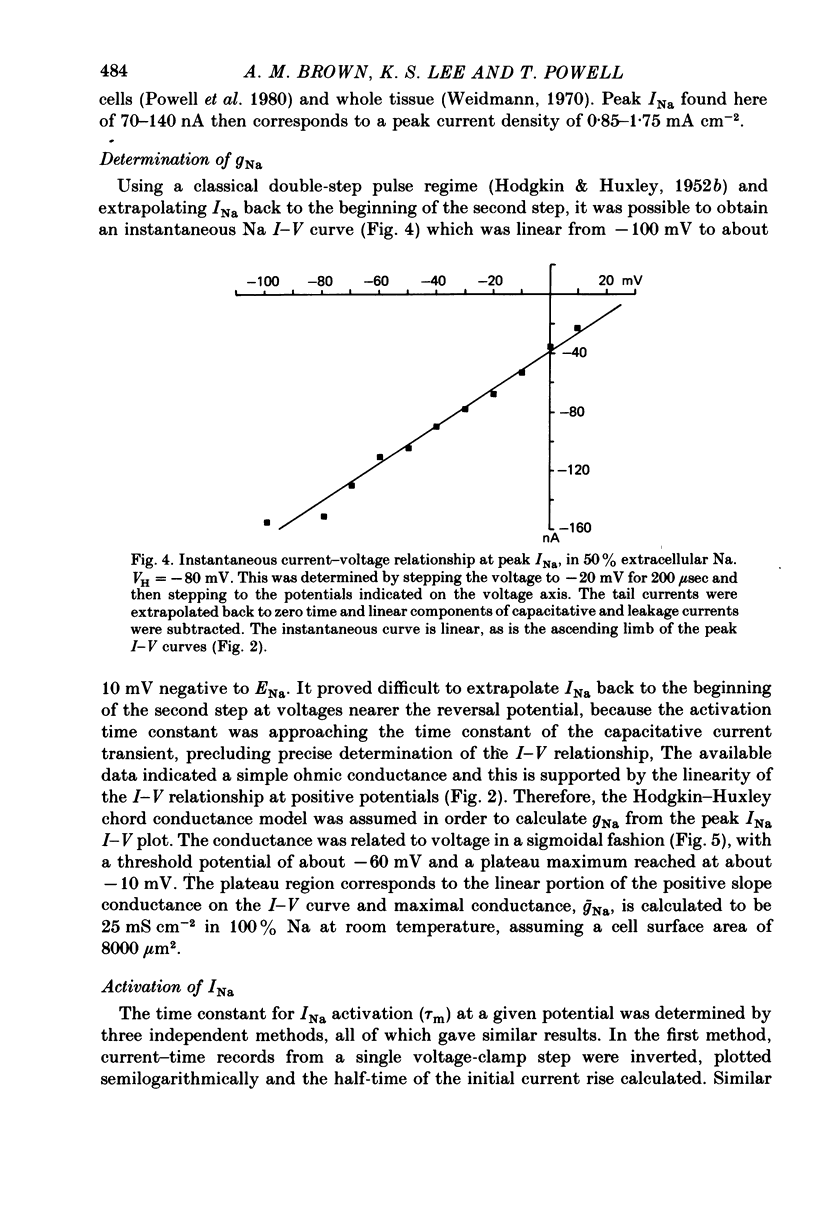
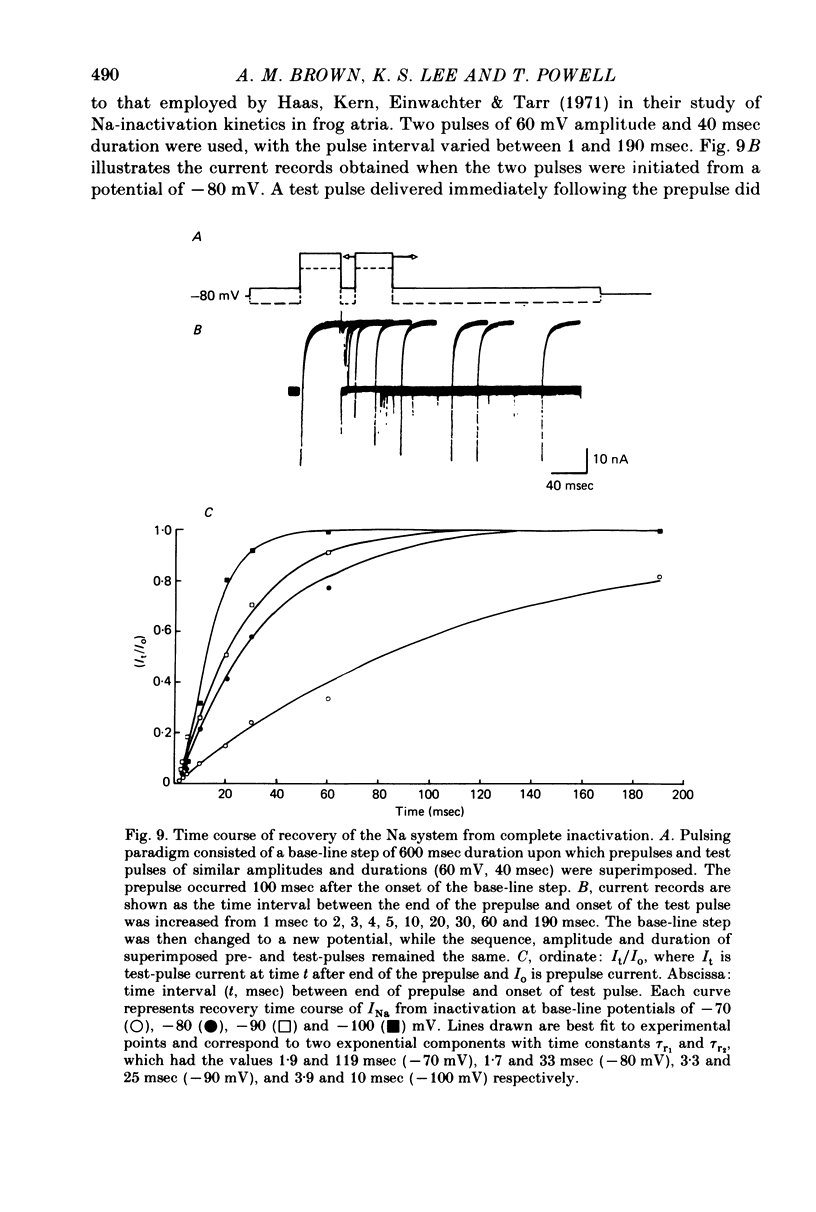
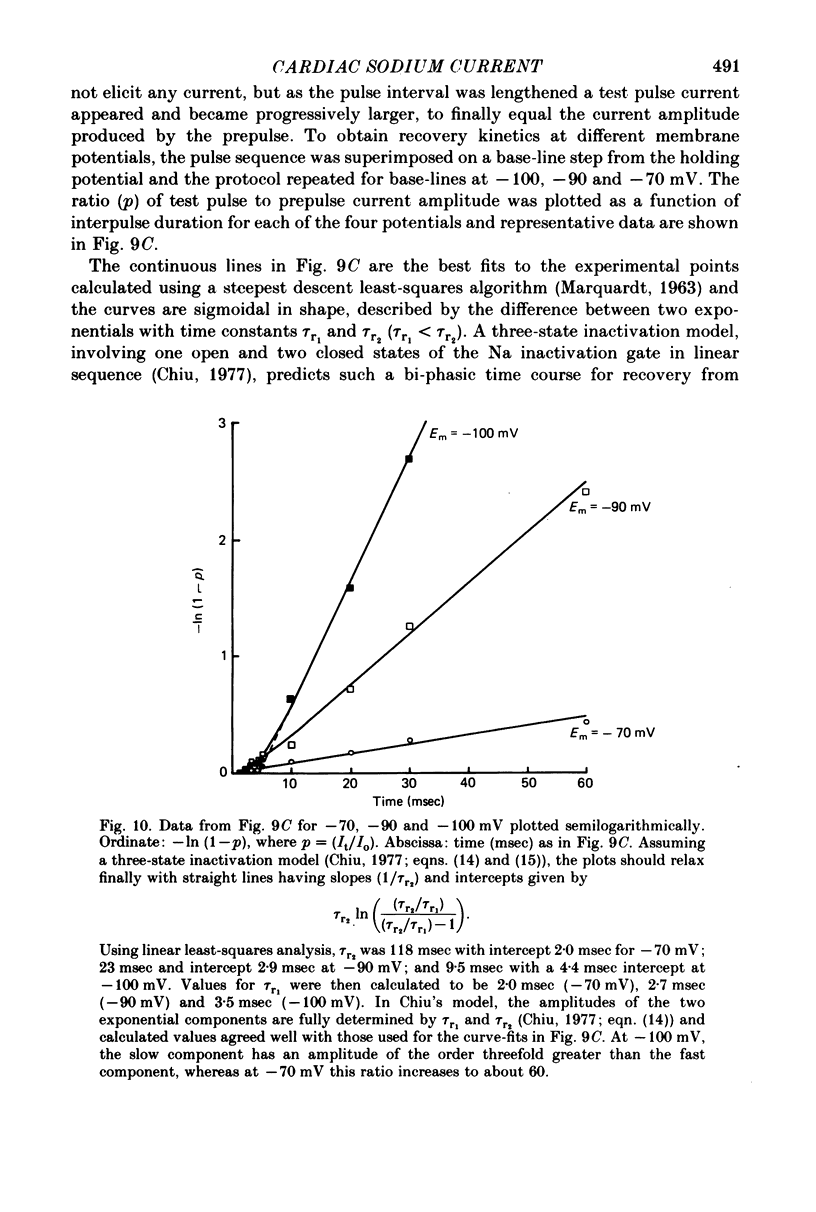
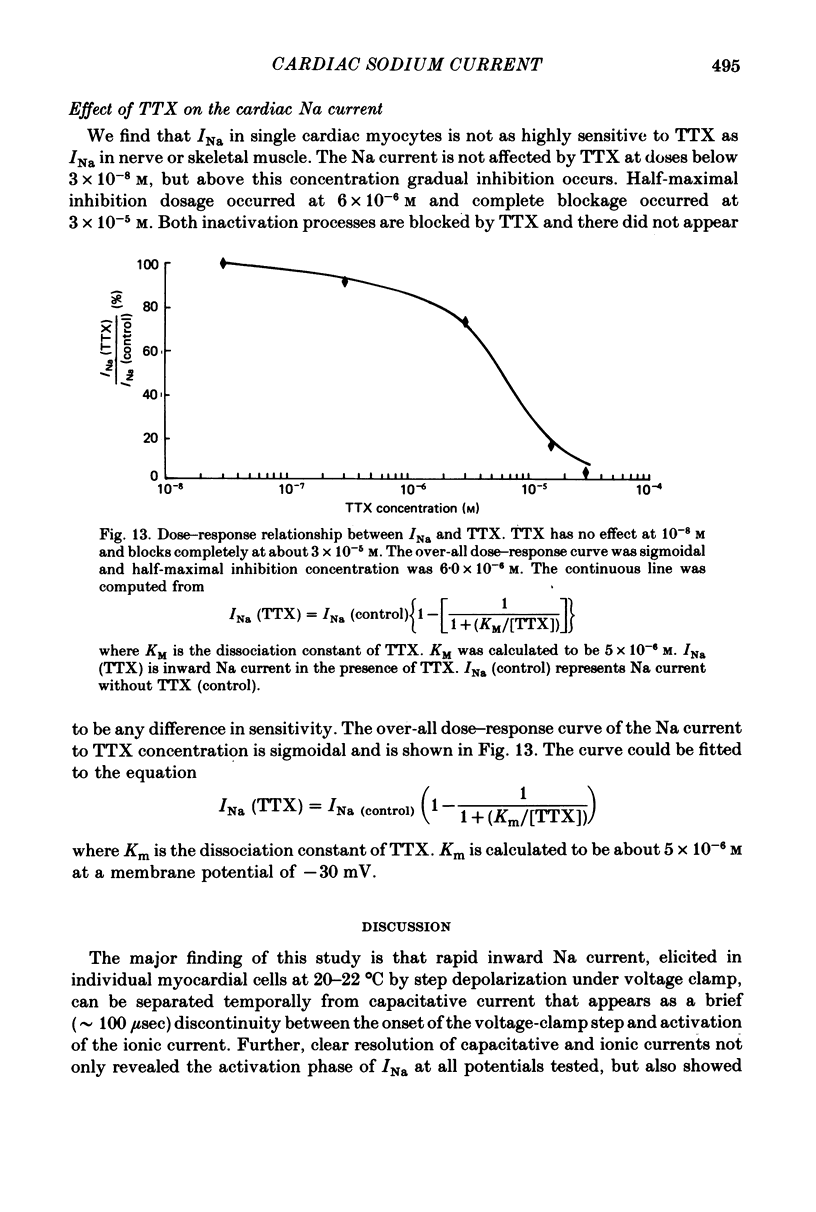
1. Rapid inward Na current (INa) was studied in isolated cells from rat ventricular myocardium by a double-suction-pipette voltage clamp technique. All experiments were carried out at 20-22 degrees C. 2. INa elicited by single depolarizing voltage steps from a holding potential, VH, of -80 mV had a threshold between -70 and -60 mV and was maximal at -30 to -20 mV. Peak currents in Krebs-Ringer solution containing 145 mM Na were of the order 0.9-1.8 mA cm-2, assuming an average cell surface area of 8000 square micrometers. 3. The reversal potential for INa was predicted by the Nernst equation for external Na in the range 1.45-145 mM with 16 mM-Na solution perfusing the interior of the cell. 4. Instantaneous I-V plots were linear for potentials of -100 to + 10 mV. Maximum Na conductance (-gNa) was calculated to be 25 mS cm-2 in 145 mM-Na solutions and gNa was constant for potentials positive to -10 mV. 5. INa activated with a time constant of 0.7 msec at -55 mV, decreasing to 100 microsec on depolarizations positive to + 10 mV. 6. Two time constants (tau h1, tau h2) were required to describe INa inactivation during a maintained depolarization, with tau h2 three to four times as long as tau h1. tau h1 was about 2 msec at -50 mV, decreasing to 0.9 msec at -10 mV. 7. The time course for recovery of INa from inactivation also exhibited two time constants (tau r1, tau r2), with the longer tau r2 having a maximum value of the order 100 msec in the potential range -60 to -80 mV. 8. INa in isolated rat cardiac cells has a low sensitivity to tetrodotoxin, requiring a concentration of 30 micrometers for complete blockade.
Full text
PDF

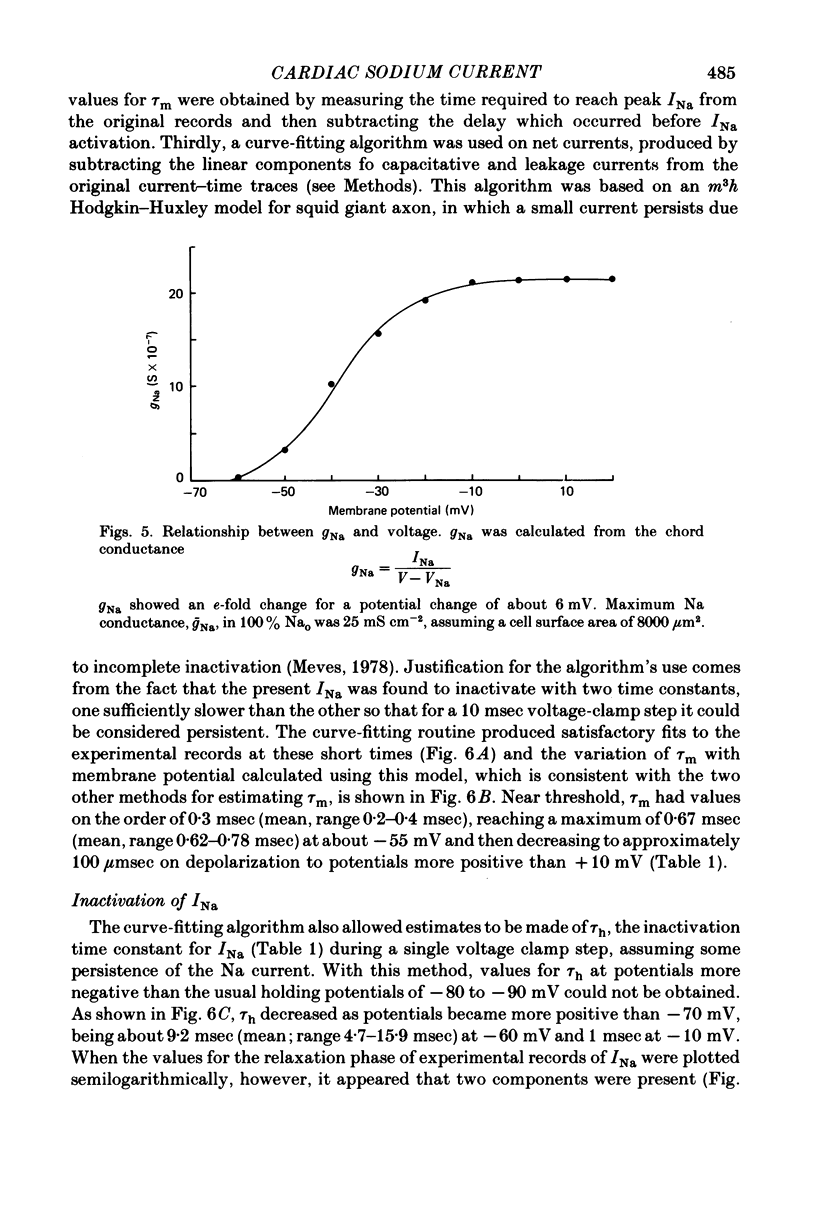
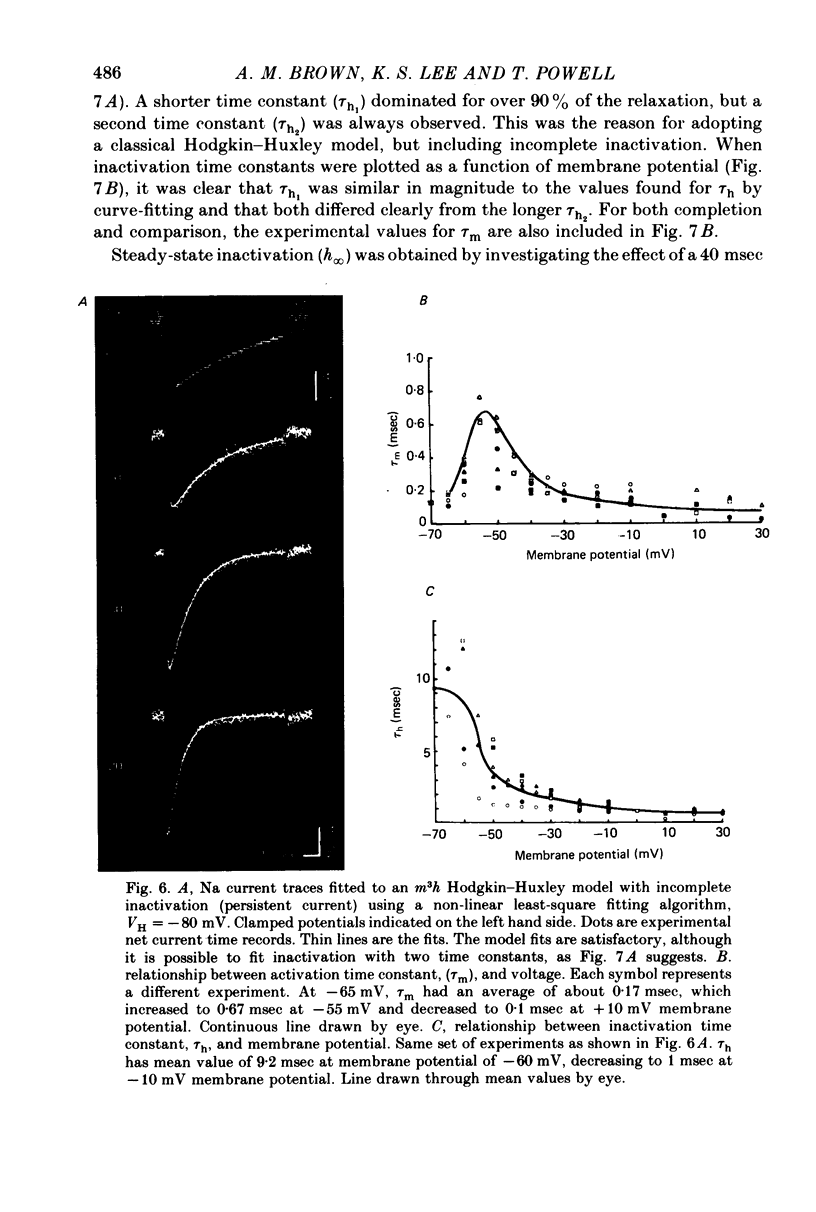

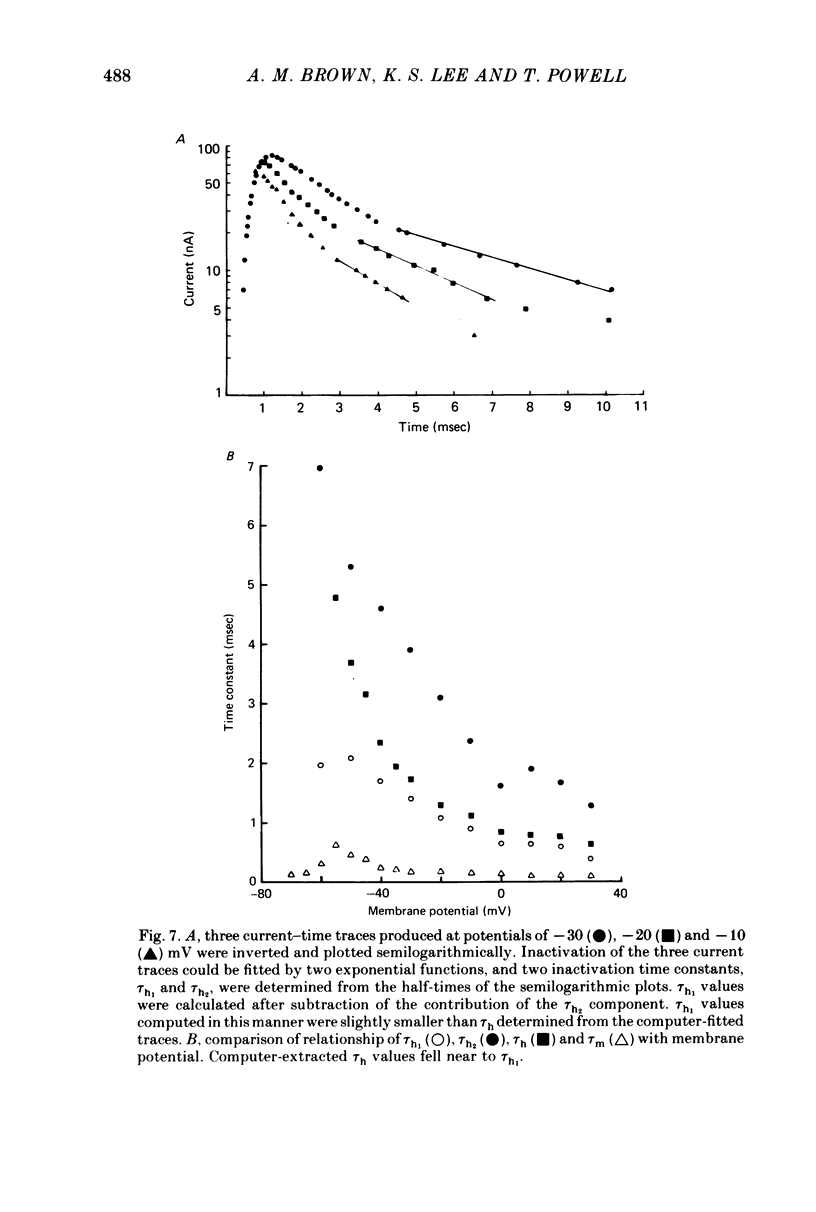
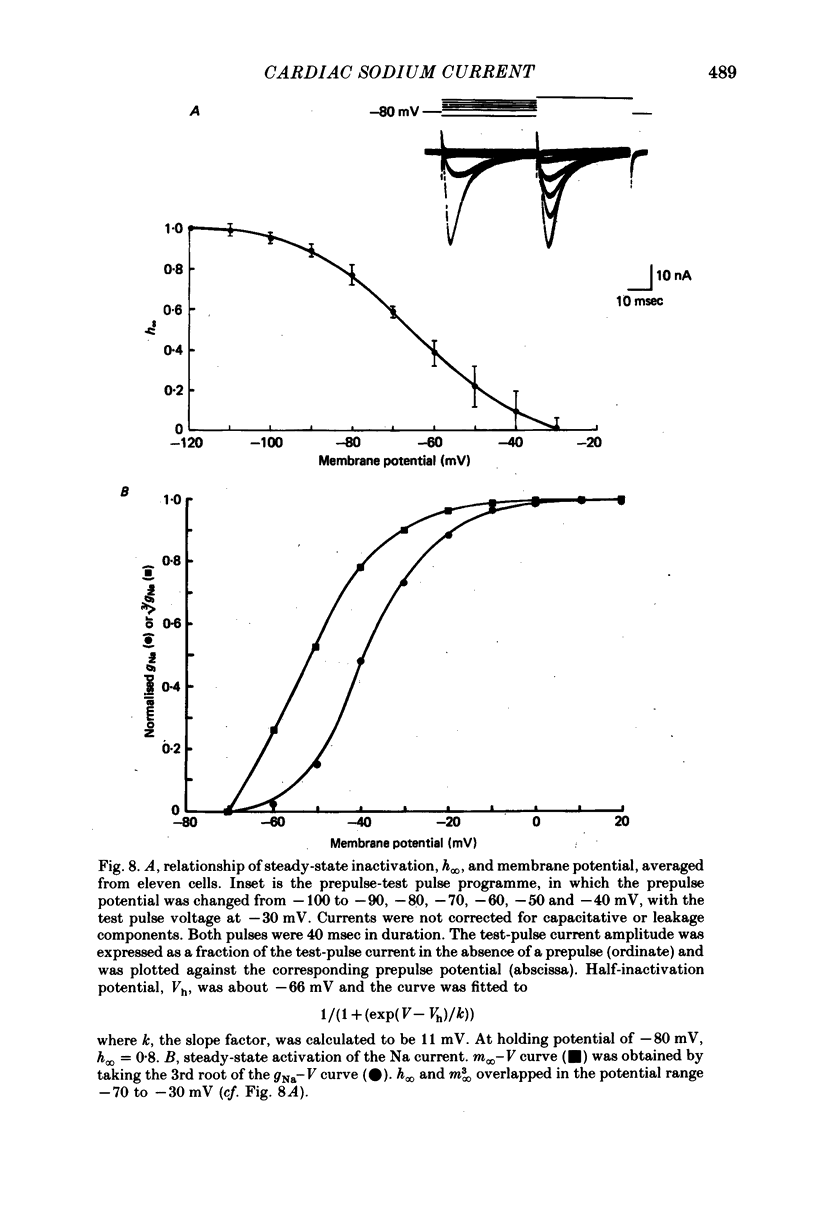






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
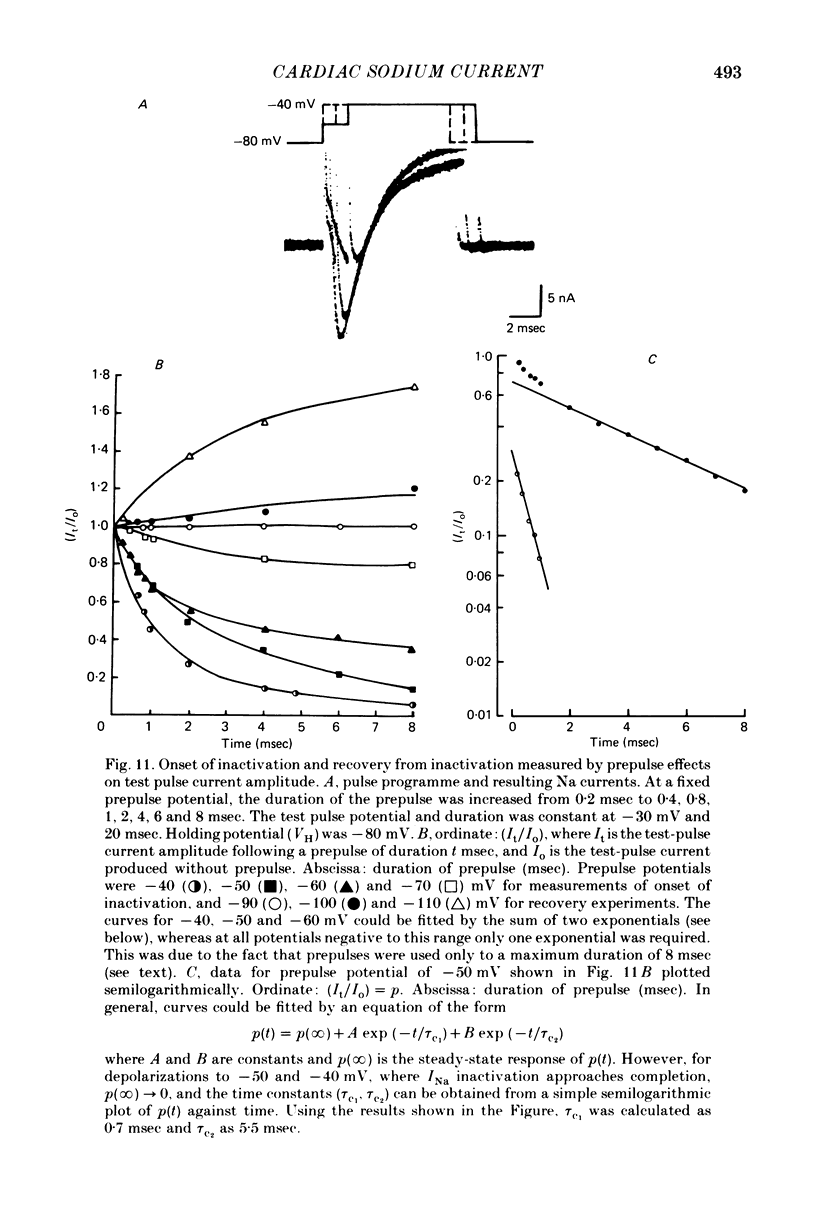
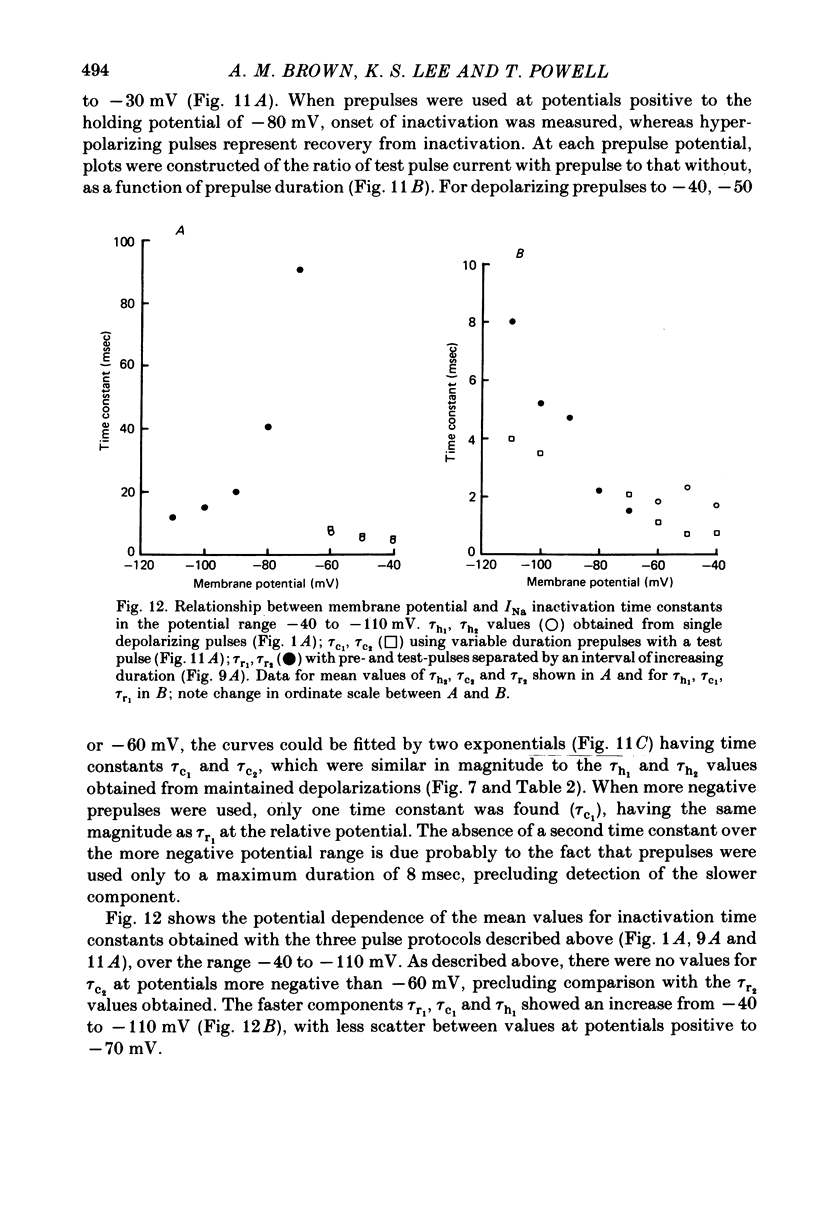
- Attwell D., Cohen I., Eisner D., Ohba M., Ojeda C. The steady state TTX-sensitive ("window") sodium current in cardiac Purkinje fibres. Pflugers Arch. 1979 Mar 16;379(2):137–142. doi: 10.1007/BF00586939. [DOI] [PubMed] [Google Scholar]
- Baer M., Best P. M., Reuter H. Voltage-dependent action of tetrodotoxin in mammalian cardiac muscle. Nature. 1976 Sep 23;263(5575):344–345. doi: 10.1038/263344a0. [DOI] [PubMed] [Google Scholar]
- Beeler G. W., Jr, Reuter H. Voltage clamp experiments on ventricular myocarial fibres. J Physiol. 1970 Mar;207(1):165–190. doi: 10.1113/jphysiol.1970.sp009055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Inactivation of the sodium channel. I. Sodium current experiments. J Gen Physiol. 1977 Nov;70(5):549–566. doi: 10.1085/jgp.70.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Lee K. S., Powell T. Voltage clamp and internal perfusion of single rat heart muscle cells. J Physiol. 1981 Sep;318:455–477. doi: 10.1113/jphysiol.1981.sp013878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. Y. Inactivation of sodium channels: second order kinetics in myelinated nerve. J Physiol. 1977 Dec;273(3):573–596. doi: 10.1113/jphysiol.1977.sp012111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C. J., Colatsky T. J., Tsien R. W. Tetrodotoxin block of cardiac sodium channels during repetitive or steady depolarizations in the rabbit [proceedings]. J Physiol. 1979 Nov;296:70P–71P. [PubMed] [Google Scholar]
- Colatsky J. J., Tsien R. W. Sodium channels in rabbit cardiac Purkinje fibres. Nature. 1979 Mar 15;278(5701):265–268. doi: 10.1038/278265a0. [DOI] [PubMed] [Google Scholar]
- Colatsky T. J. Voltage clamp measurements of sodium channel properties in rabbit cardiac Purkinje fibres. J Physiol. 1980 Aug;305:215–234. doi: 10.1113/jphysiol.1980.sp013359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudel J., Peper K., Rüdel R., Trautwein W. Excitatory membrane current in heart muscle (Purkinje fibers). Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;292(3):255–273. doi: 10.1007/BF00362740. [DOI] [PubMed] [Google Scholar]
- Dudel J., Peper K., Rüdel R., Trautwein W. The effect of tetrodotoxin on the membrane current in cardiac muscle (Purkinje fibers). Pflugers Arch Gesamte Physiol Menschen Tiere. 1967;295(3):213–226. doi: 10.1007/BF01844101. [DOI] [PubMed] [Google Scholar]
- Dudel J., Rüdel R. Voltage and time dependence of excitatory sodium current in cooled sheep Purkinje fibres. Pflugers Arch. 1970;315(2):136–158. doi: 10.1007/BF00586657. [DOI] [PubMed] [Google Scholar]
- Ebihara L., Shigeto N., Lieberman M., Johnson E. A. The initial inward current in spherical clusters of chick embryonic heart cells. J Gen Physiol. 1980 Apr;75(4):437–456. doi: 10.1085/jgp.75.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester D. W., Spence V. A., Walker W. F. The measurement of colonic mucosal-submucosal blood flow in man. J Physiol. 1980 Feb;299:1–11. doi: 10.1113/jphysiol.1980.sp013106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C., Cranefield P. F. Two levels of resting potential in cardiac Purkinje fibers. J Gen Physiol. 1977 Dec;70(6):725–746. doi: 10.1085/jgp.70.6.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gettes L. S., Reuter H. Slow recovery from inactivation of inward currents in mammalian myocardial fibres. J Physiol. 1974 Aug;240(3):703–724. doi: 10.1113/jphysiol.1974.sp010630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L. Kinetics of channel gating in excitable membranes. Q Rev Biophys. 1976 Nov;9(4):491–526. doi: 10.1017/s0033583500002651. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The components of membrane conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):473–496. doi: 10.1113/jphysiol.1952.sp004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas H. G., Kern R., Einwächter H. M., Tarr M. Kinetics of Na inactivation in frog atria. Pflugers Arch. 1971;323(2):141–157. doi: 10.1007/BF00586445. [DOI] [PubMed] [Google Scholar]
- Kenyon J. L., Gibbons W. R. Effects of low-chloride solutions on action potentials of sheep cardiac Purkinje fibers. J Gen Physiol. 1977 Nov;70(5):635–660. doi: 10.1085/jgp.70.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Akaike N., Brown A. M. Properties of internally perfused, voltage-clamped, isolated nerve cell bodies. J Gen Physiol. 1978 May;71(5):489–507. doi: 10.1085/jgp.71.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Akaike N., Brown A. M. Trypsin inhibits the action of tetrodotoxin on neurones. Nature. 1977 Feb 24;265(5596):751–753. doi: 10.1038/265751a0. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Weeks T. A., Kao R. L., Akaike N., Brown A. M. Sodium current in single heart muscle cells. Nature. 1979 Mar 15;278(5701):269–271. doi: 10.1038/278269a0. [DOI] [PubMed] [Google Scholar]
- McAllister R. E., Noble D. The time and voltage dependence of the slow outward current in cardiac Purkinje fibres. J Physiol. 1966 Oct;186(3):632–662. doi: 10.1113/jphysiol.1966.sp008060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves H. Inactivation of the sodium permeability in squid giant nerve fibres. Prog Biophys Mol Biol. 1978;33(2):207–230. doi: 10.1016/0079-6107(79)90029-4. [DOI] [PubMed] [Google Scholar]
- Mobley B. A., Page E. The surface area of sheep cardiac Purkinje fibres. J Physiol. 1972 Feb;220(3):547–563. doi: 10.1113/jphysiol.1972.sp009722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T. Chemicals as tools in the study of excitable membranes. Physiol Rev. 1974 Oct;54(4):813–889. doi: 10.1152/physrev.1974.54.4.813. [DOI] [PubMed] [Google Scholar]
- Powell T., Terrar D. A., Twist V. W. Electrical properties of individual cells isolated from adult rat ventricular myocardium. J Physiol. 1980 May;302:131–153. doi: 10.1113/jphysiol.1980.sp013234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidmann S. Electrical constants of trabecular muscle from mammalian heart. J Physiol. 1970 Nov;210(4):1041–1054. doi: 10.1113/jphysiol.1970.sp009256. [DOI] [PMC free article] [PubMed] [Google Scholar]


