Abstract
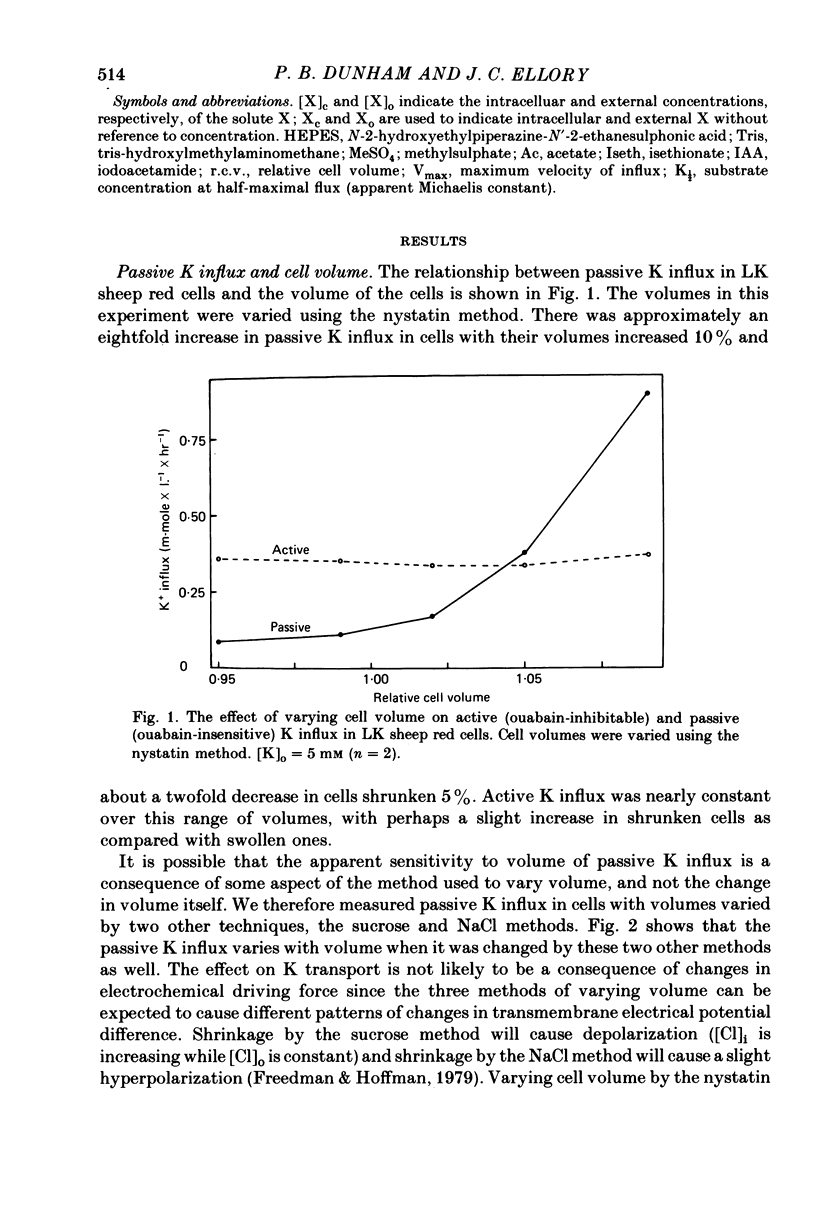
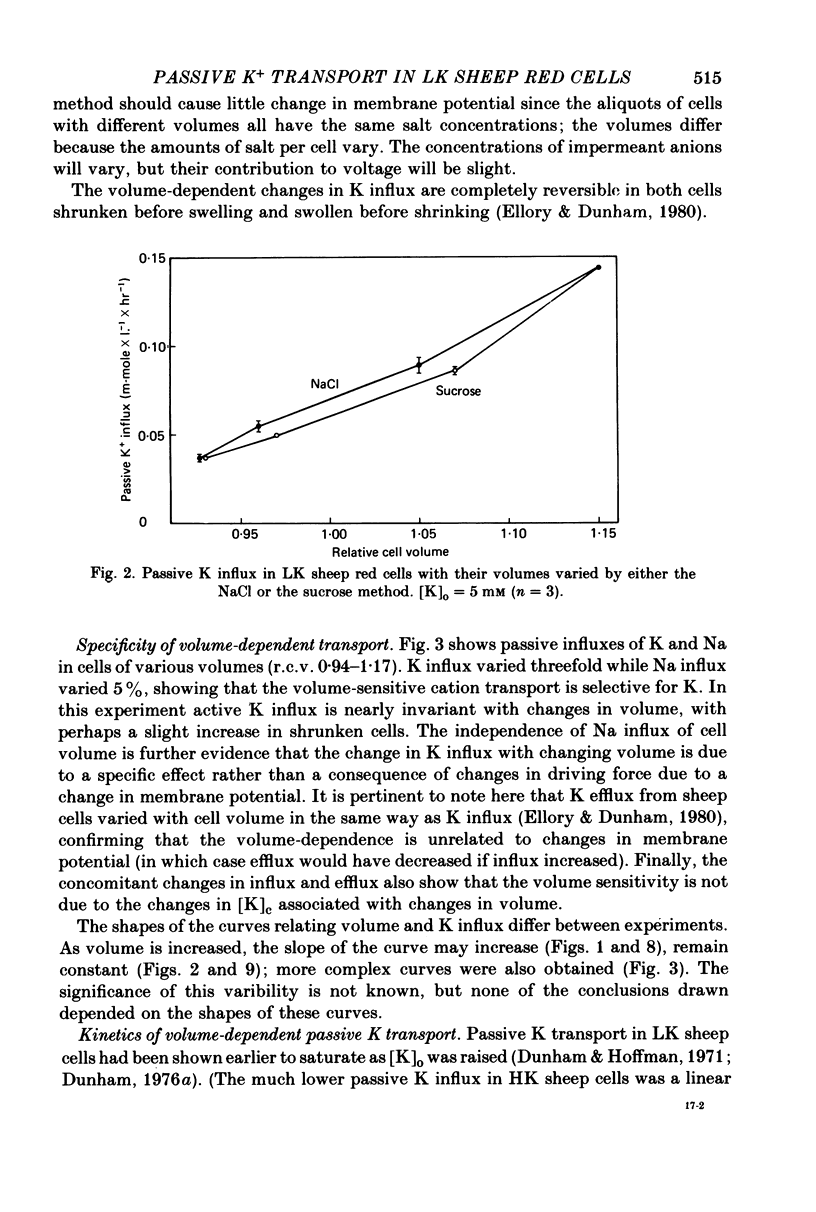
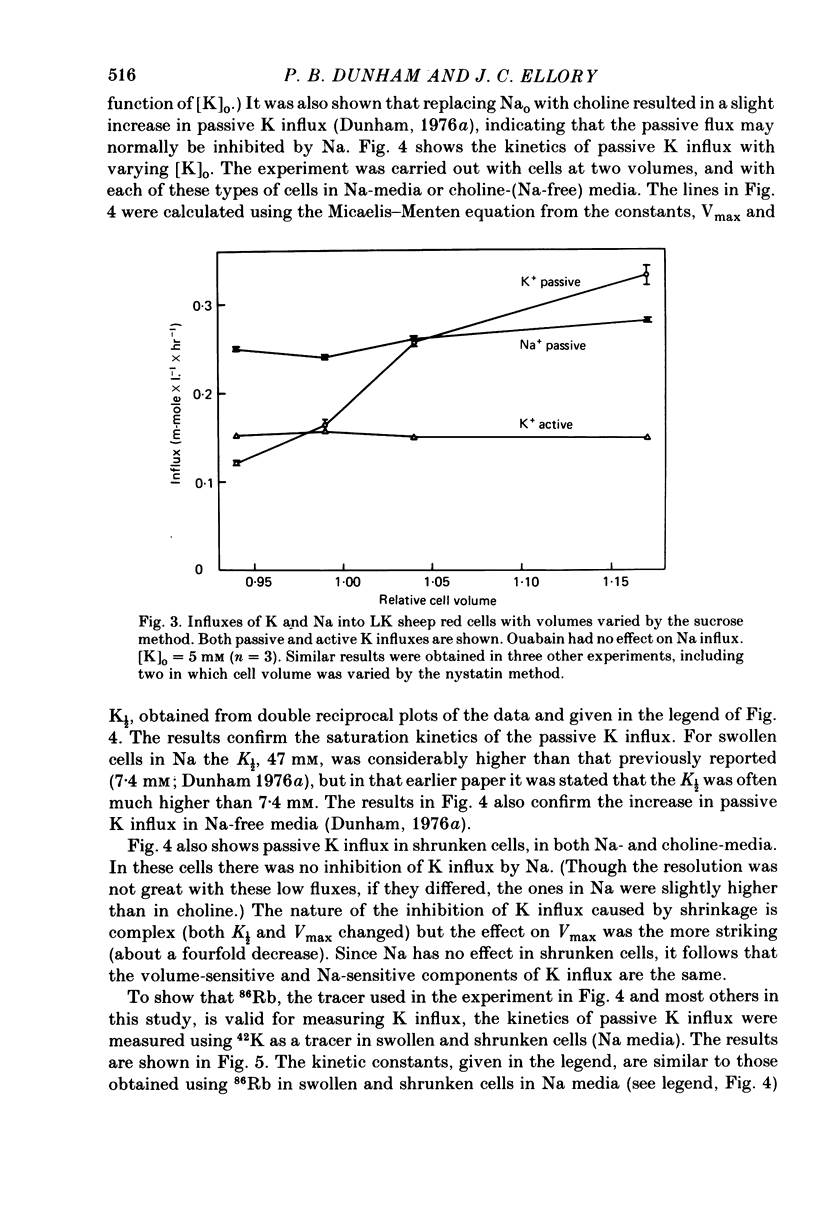
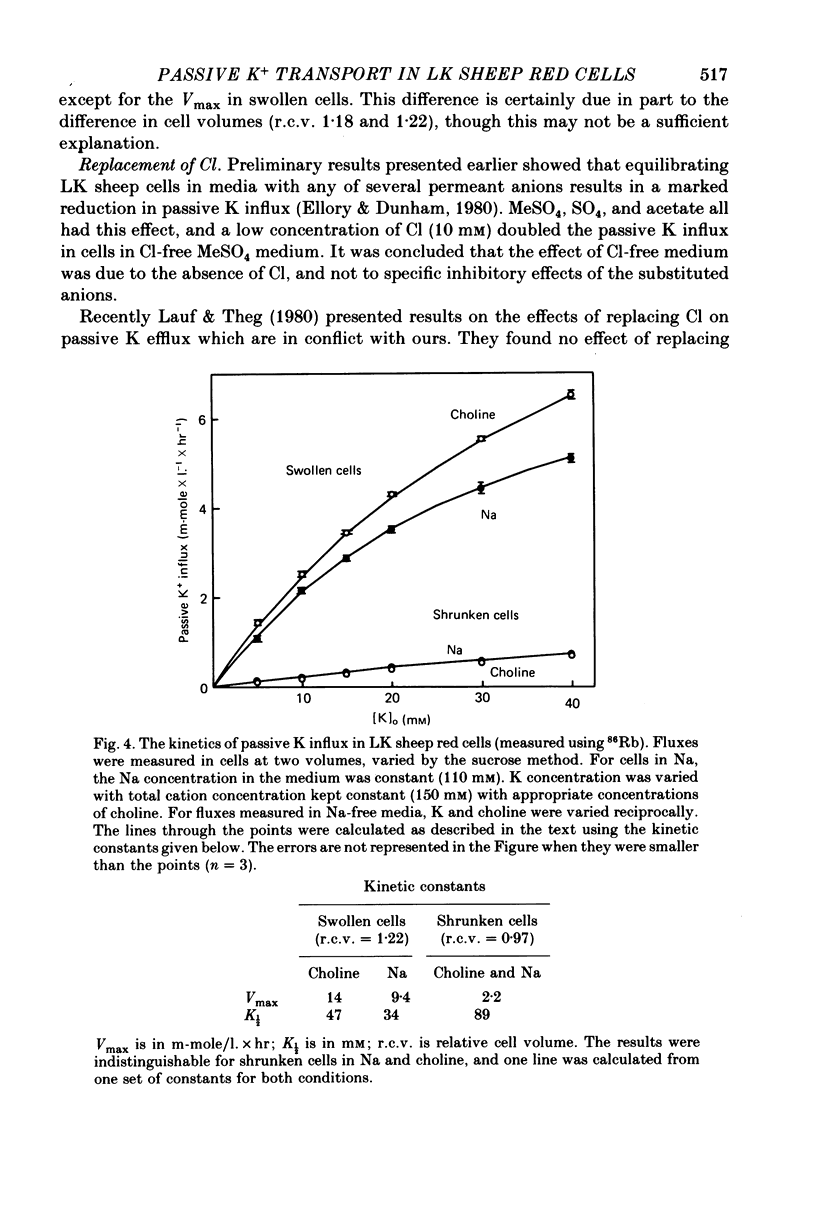
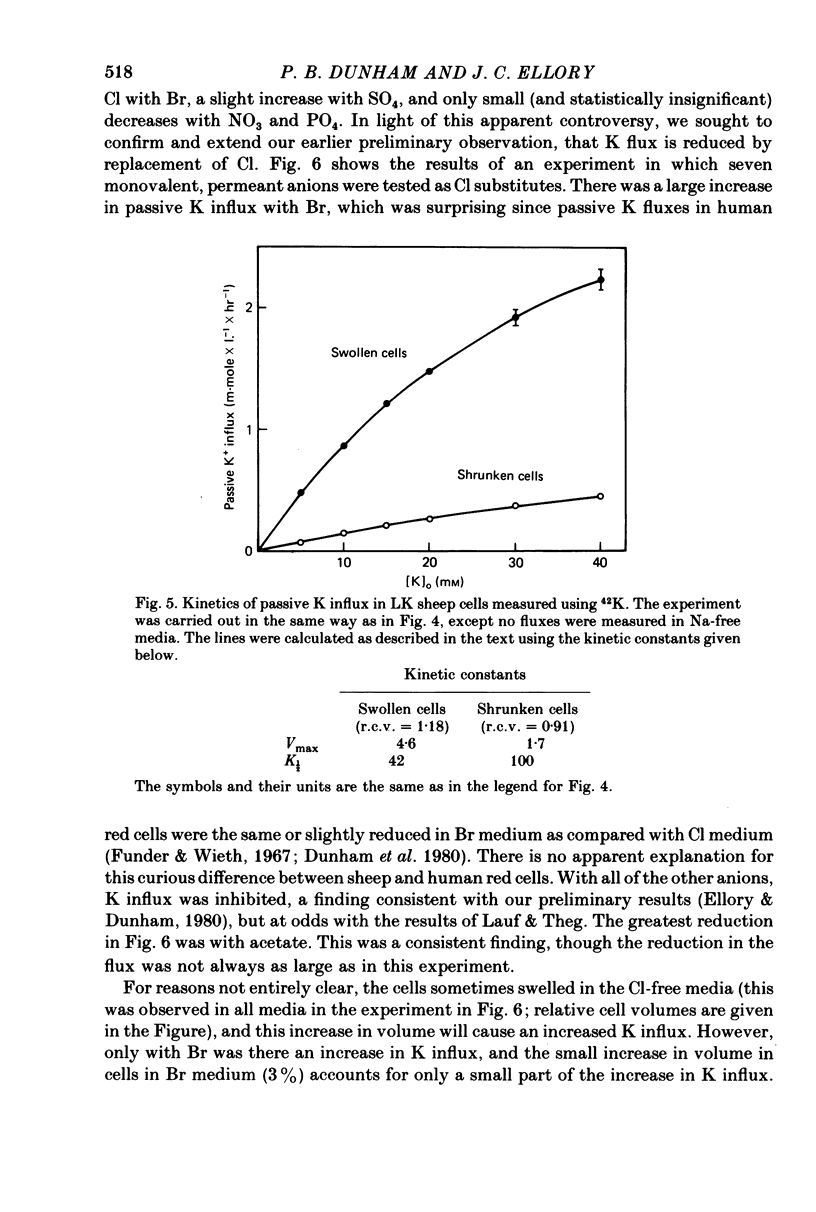
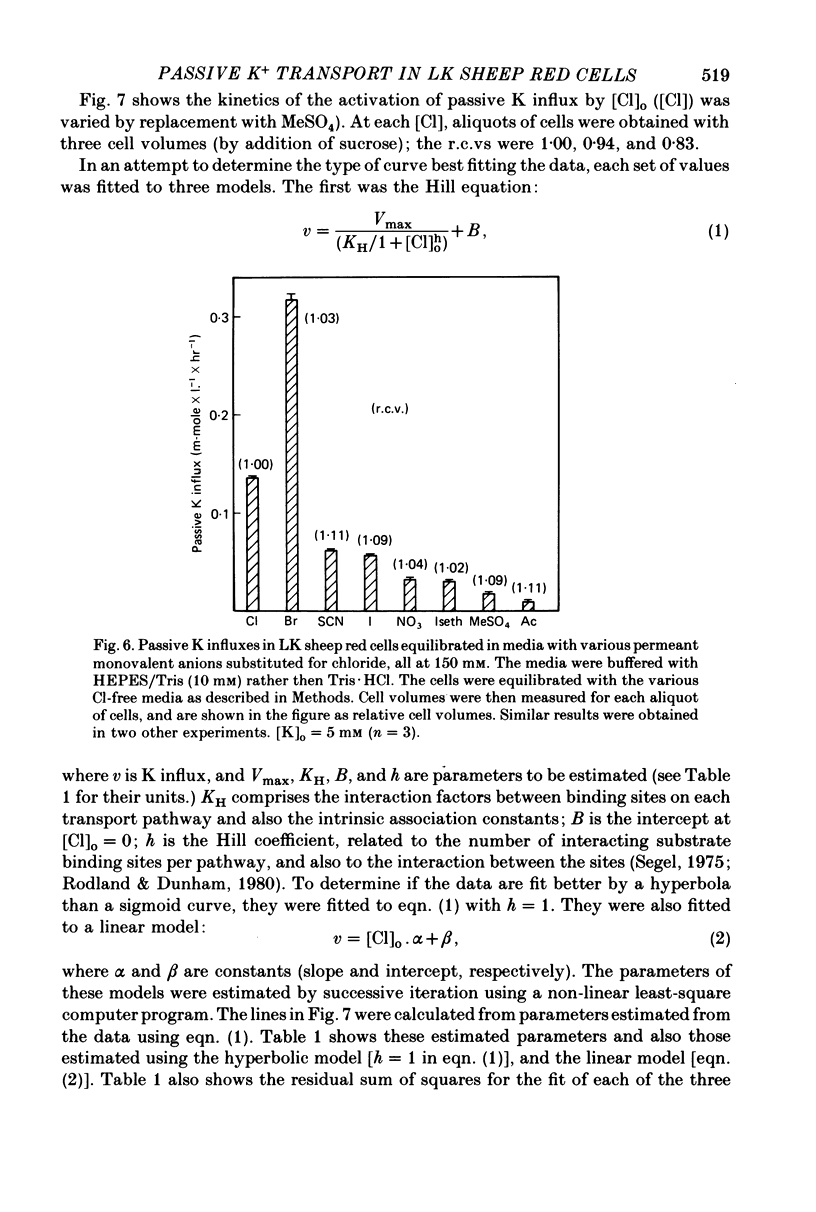
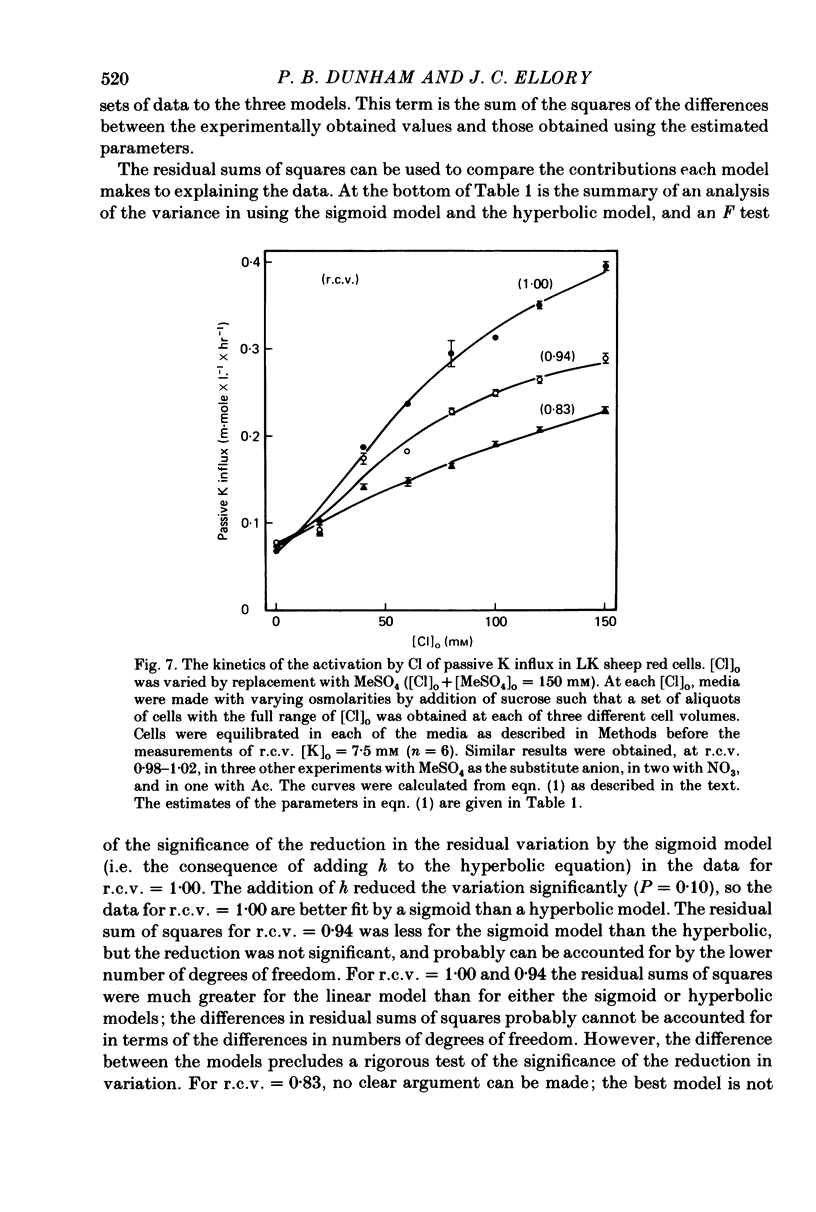
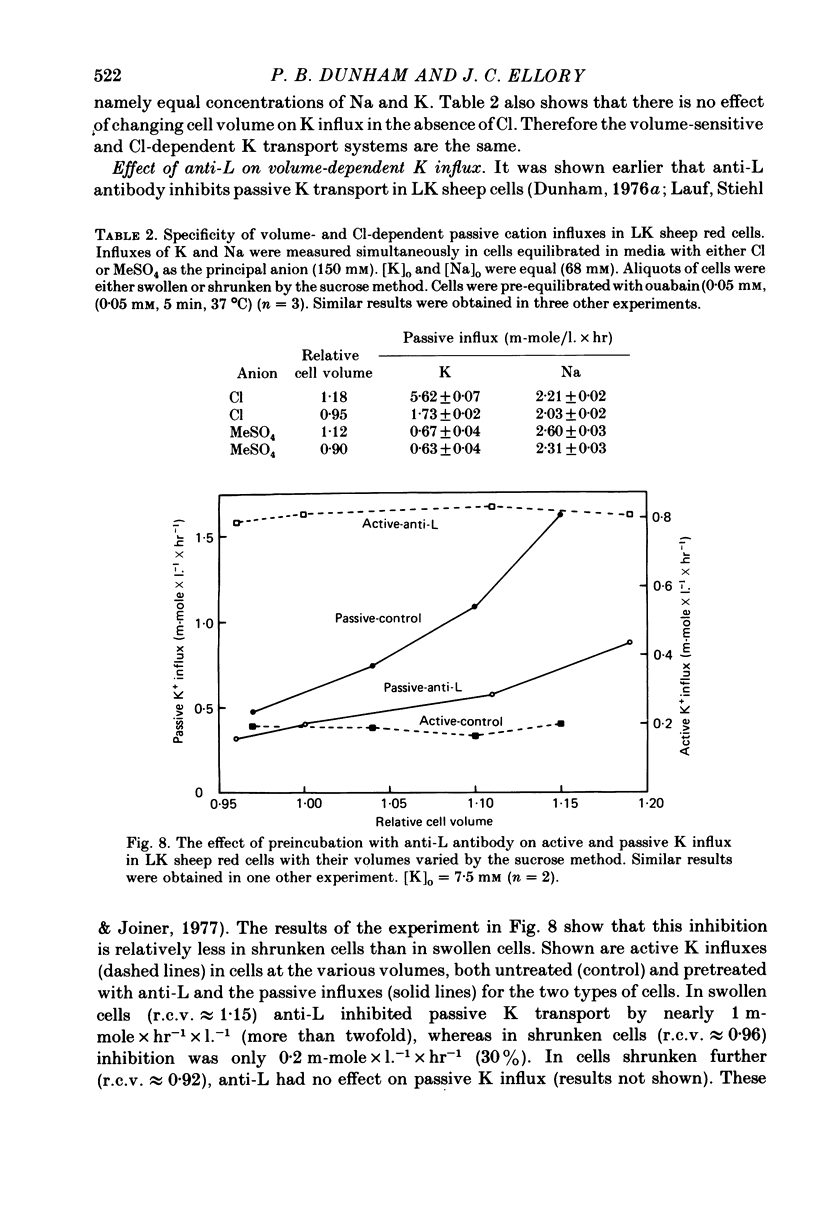
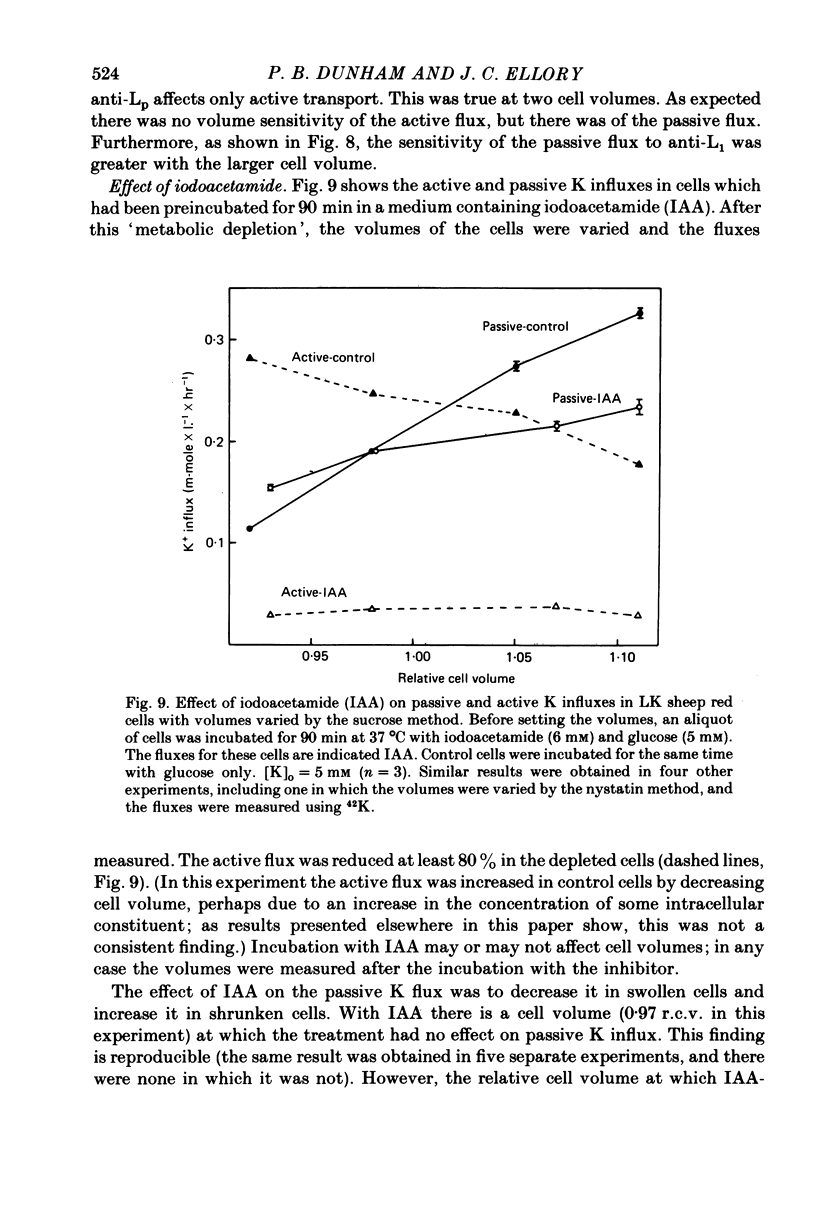
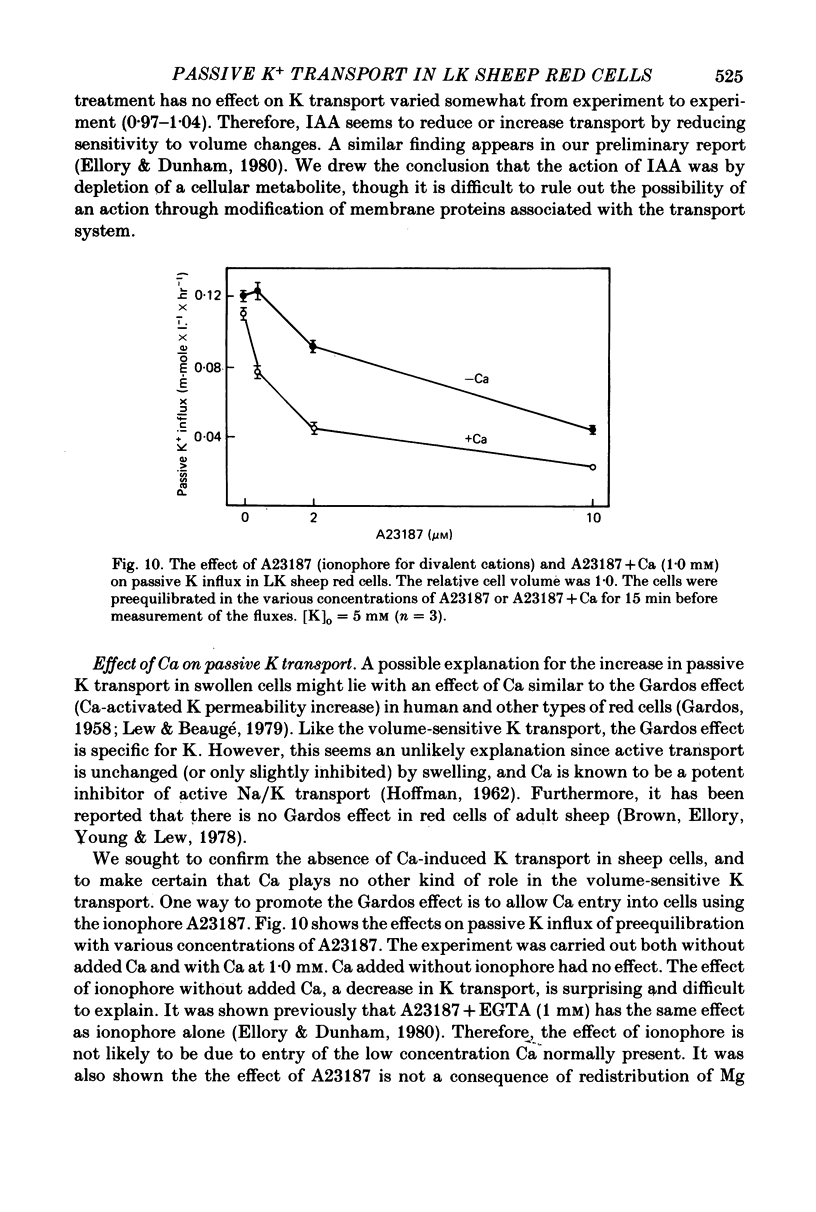
The major pathway of passive K influx (ouabain-insensitive) was characterized in low-K type (LK) red cells of sheep. 1. Passive K transport in these cells was highly sensitive to variations in cell volume; it increased threefold or more in cells swollen osmotically by 10%, and decreased up to twofold in cells shrunken 5-10%. Active K influx was insensitive to changes in cell volume. Three different methods for varying cell volume osmotically all gave similar results. 2. The volume-sensitive pathway was specific for K in that Na influx did not vary with changes in cell volume. 3. The volume-sensitive K influx was a saturable function of external K concentration. It was slightly inhibited by Na, whereas K influx in shrunken cells was unaffected by Na. 4. Passive K influx was dependent on the major anion in the medium in that replacement of Cl with any of six other anions resulted in a reduction of K influx by 50-80% (replacement of Cl by Br caused an increase in K influx). The activation of K influx by Cl followed sigmoid kinetics. 5. Passive K influx is inhibited by anti-L antibody. The antibody affected only that portion of influx which was Cl-dependent and volume-sensitve. Of the subfractions of the antibody, it is anti-L1 which inhibits passive K transport. 6. Pretreatment of cells with iodoacetamide reduced the sensitivity of K influx to cell volume in that the influx was reduced in swollen IAA-treated cells and increased in shrunken IAA-cells. 7. Intracellular Ca has no role in altering passive K transport in LK sheep cells. Therefore, the major pathway of passive K transport in LK sheep red cells is sensitive to changes in cell volume, specific for K, dependent on Cl, and inhibited by anti-L1 antibody, The minor pathway, observed in shrunken cells, has none of these properties.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakker-Grunwald T., Andrew J. S., Neville M. C. K+ influx components in ascites cells: the effects of agents interacting with the (Na+ + K+)-pump. J Membr Biol. 1980;52(2):141–146. doi: 10.1007/BF01869119. [DOI] [PubMed] [Google Scholar]
- Beauge L. A., Adragna N. The kinetics of ouabain inhibition and the partition of rubidium influx in human red blood cells. J Gen Physiol. 1971 May;57(5):576–592. doi: 10.1085/jgp.57.5.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Ellory J. C., Young J. D., Lew V. L. A calcium-activated potassium channel present in foetal red cells of the sheep but absent from reticulocytes and mature red cells. Biochim Biophys Acta. 1978 Aug 4;511(2):163–175. doi: 10.1016/0005-2736(78)90311-5. [DOI] [PubMed] [Google Scholar]
- Carmeliet E., Verdonck F. Reduction of potassium permeability by chloride substitution in cardiac cells. J Physiol. 1977 Feb;265(1):193–206. doi: 10.1113/jphysiol.1977.sp011712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipperfield A. R. An effect of chloride on (Na+K) co-transport in human red blood cells. Nature. 1980 Jul 17;286(5770):281–282. doi: 10.1038/286281a0. [DOI] [PubMed] [Google Scholar]
- Cotterrell D., Whittam R. The influence of the chloride gradient across red cell membranes on sodium and potassium movements. J Physiol. 1971 May;214(3):509–536. doi: 10.1113/jphysiol.1971.sp009446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doljanski F., Ben-Sasson S., Reich M., Grover N. B. Dynamic osmotic behavior of chick blood lymphocytes. J Cell Physiol. 1974 Oct;84(2):215–224. doi: 10.1002/jcp.1040840208. [DOI] [PubMed] [Google Scholar]
- Dunham P. B. Anti-L serum. Two populations of antibodies affecting cation transport in LK erythrocytes of sheep and goats. Biochim Biophys Acta. 1976 Aug 16;443(2):219–226. doi: 10.1016/0005-2736(76)90505-8. [DOI] [PubMed] [Google Scholar]
- Dunham P. B., Blostein R. Active potassium transport in reticulocytes of high-K+ and low-K+ sheep. Biochim Biophys Acta. 1976 Dec 14;455(3):749–758. doi: 10.1016/0005-2736(76)90045-6. [DOI] [PubMed] [Google Scholar]
- Dunham P. B., Ellory J. C. Stimulation of the sodium-potassium pump by trypsin in low potassium type erythrocytes of goats. J Physiol. 1980 Apr;301:25–37. doi: 10.1113/jphysiol.1980.sp013185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham P. B., Hoffman J. F. Active cation transport and ouabain binding in high potassium and low potassium red blood cells of sheep. J Gen Physiol. 1971 Jul;58(1):94–116. doi: 10.1085/jgp.58.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham P. B. Passive potassium transport in LK sheep red cells. Effects of anti-L antibody and intracellular potassium. J Gen Physiol. 1976 Dec;68(6):567–581. doi: 10.1085/jgp.68.6.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham P. B., Stewart G. W., Ellory J. C. Chloride-activated passive potassium transport in human erythrocytes. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1711–1715. doi: 10.1073/pnas.77.3.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellory J. C., Sachs J. R., Dunham P. B., Hoffman J. F. The L antibody and potassium fluxes in LK red cells of sheep and goats. Biomembranes. 1972;3:237–245. doi: 10.1007/978-1-4684-0961-1_16. [DOI] [PubMed] [Google Scholar]
- Ellory J. C., Tucker E. M. Active potassium transport and the L and M antigens of sheep and goat red cells. Biochim Biophys Acta. 1970;219(1):160–168. doi: 10.1016/0005-2736(70)90071-4. [DOI] [PubMed] [Google Scholar]
- Ellory J. C., Tucker E. M. Stimulation of the potassium transport system in low potassium type sheep red cells by a specific antigen antibody reaction. Nature. 1969 May 3;222(5192):477–478. doi: 10.1038/222477a0. [DOI] [PubMed] [Google Scholar]
- Freedman J. C., Hoffman J. F. Ionic and osmotic equilibria of human red blood cells treated with nystatin. J Gen Physiol. 1979 Aug;74(2):157–185. doi: 10.1085/jgp.74.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funder J., Wieth J. O. Effects of some monovalent anions on fluxes of Na and K, and on glucose metabolism of ouabain treated human red cells. Acta Physiol Scand. 1967 Oct-Nov;71(2):168–185. doi: 10.1111/j.1748-1716.1967.tb03723.x. [DOI] [PubMed] [Google Scholar]
- GARDOS G. The function of calcium in the potassium permeability of human erythrocytes. Biochim Biophys Acta. 1958 Dec;30(3):653–654. doi: 10.1016/0006-3002(58)90124-0. [DOI] [PubMed] [Google Scholar]
- Gardner J. D., Aurbach G. D., Spiegel A. M., Brown E. M. Receptor function and ion transport in turkey erythrocytes. Recent Prog Horm Res. 1976;32:567–595. doi: 10.1016/b978-0-12-571132-6.50031-2. [DOI] [PubMed] [Google Scholar]
- Geck P., Pietrzyk C., Burckhardt B. C., Pfeiffer B., Heinz E. Electrically silent cotransport on Na+, K+ and Cl- in Ehrlich cells. Biochim Biophys Acta. 1980 Aug 4;600(2):432–447. doi: 10.1016/0005-2736(80)90446-0. [DOI] [PubMed] [Google Scholar]
- HOFFMAN J. F. Cation transport and structure of the red-cell plasma membrane. Circulation. 1962 Nov;26:1202–1213. doi: 10.1161/01.cir.26.5.1201. [DOI] [PubMed] [Google Scholar]
- Hendil K. B., Hoffmann E. K. Cell volume regulation in Ehrlich ascites tumor cells. J Cell Physiol. 1974 Aug;84(1):115–125. doi: 10.1002/jcp.1040840113. [DOI] [PubMed] [Google Scholar]
- Hoffman J. F., Kregenow F. M. The characterization of new energy dependent cation transport processes in red blood cells. Ann N Y Acad Sci. 1966 Jul 14;137(2):566–576. doi: 10.1111/j.1749-6632.1966.tb50182.x. [DOI] [PubMed] [Google Scholar]
- Kregenow F. M. Functional separation of the Na-K exchange pump from the volume controlling mechanism in enlarged duck red cells. J Gen Physiol. 1974 Oct;64(4):393–412. doi: 10.1085/jgp.64.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauf P. K., Stiehl B. J., Joiner C. H. Active and passive cation transport and L antigen heterogeneity in low potassium sheep red cells: evidence against the concept of leak-pump interconversion. J Gen Physiol. 1977 Aug;70(2):221–242. doi: 10.1085/jgp.70.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauf P. K., Theg B. E. A chloride dependent K+ flux induced by N-ethylmaleimide in genetically low K+ sheep and goat erythrocytes. Biochem Biophys Res Commun. 1980 Feb 27;92(4):1422–1428. doi: 10.1016/0006-291x(80)90445-3. [DOI] [PubMed] [Google Scholar]
- Liu W. C., Slusarchyk D. S., Astle G., Trejo W. H., Brown W. E., Meyers E. Ionomycin, a new polyether antibiotic. J Antibiot (Tokyo) 1978 Sep;31(9):815–819. doi: 10.7164/antibiotics.31.815. [DOI] [PubMed] [Google Scholar]
- Poznansky M., Solomon A. K. Effect of cell volume on potassium transport in human red cells. Biochim Biophys Acta. 1972 Jul 3;274(1):111–118. doi: 10.1016/0005-2736(72)90286-6. [DOI] [PubMed] [Google Scholar]
- Poznansky M., Solomon A. K. Regulation of human red cell volume by linked cation fluxes. J Membr Biol. 1972 Dec 29;10(3):259–266. doi: 10.1007/BF01867859. [DOI] [PubMed] [Google Scholar]
- Rodland K. D., Dunham P. B. Kinetics of lithium efflux through the (Na,K)-pump of human erythrocytes. Biochim Biophys Acta. 1980 Nov 4;602(2):376–388. doi: 10.1016/0005-2736(80)90318-1. [DOI] [PubMed] [Google Scholar]
- Romualdez A., Sha'afi R. I., Lange Y., Solomon A. K. Cation transport in dog red cells. J Gen Physiol. 1972 Jul;60(1):46–57. doi: 10.1085/jgp.60.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roti Roti L. W., Rothstein A. Adaptation of mouse leukemic cells (L5178Y) to anisotonic media. I. Cell volume regulation. Exp Cell Res. 1973 Jun;79(2):295–310. doi: 10.1016/0014-4827(73)90448-5. [DOI] [PubMed] [Google Scholar]
- Sachs J. R. Ouabain-insensitive sodium movements in the human red blood cell. J Gen Physiol. 1971 Mar;57(3):259–282. doi: 10.1085/jgp.57.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W. F., 3rd, McManus T. J. Ouabain-insensitive salt and water movements in duck red cells. I. Kinetics of cation transport under hypertonic conditions. J Gen Physiol. 1977 Jul;70(1):59–79. doi: 10.1085/jgp.70.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W. F., 3rd, McManus T. J. Ouabain-insensitive salt and water movements in duck red cells. III. The role of chloride in the volume response. J Gen Physiol. 1977 Jul;70(1):99–121. doi: 10.1085/jgp.70.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TUCKER E. M. FURTHER OBSERVATIONS ON THE I BLOOD GROUP IN SHEEP. Vox Sang. 1965 Mar-Apr;10:195–205. doi: 10.1111/j.1423-0410.1965.tb04337.x. [DOI] [PubMed] [Google Scholar]
- Wiley J. S., Cooper R. A. A furosemide-sensitive cotransport of sodium plus potassium in the human red cell. J Clin Invest. 1974 Mar;53(3):745–755. doi: 10.1172/JCI107613. [DOI] [PMC free article] [PubMed] [Google Scholar]


