Abstract
1. The effect of burst patterned preganglionic stimulation on the efficacy of transmission at the cat stellate ganglion in vivo was studied to determine whether the increase in acetylcholine (ACh) release that occurs at preganglionic terminals with similar stimulation is synaptically active.
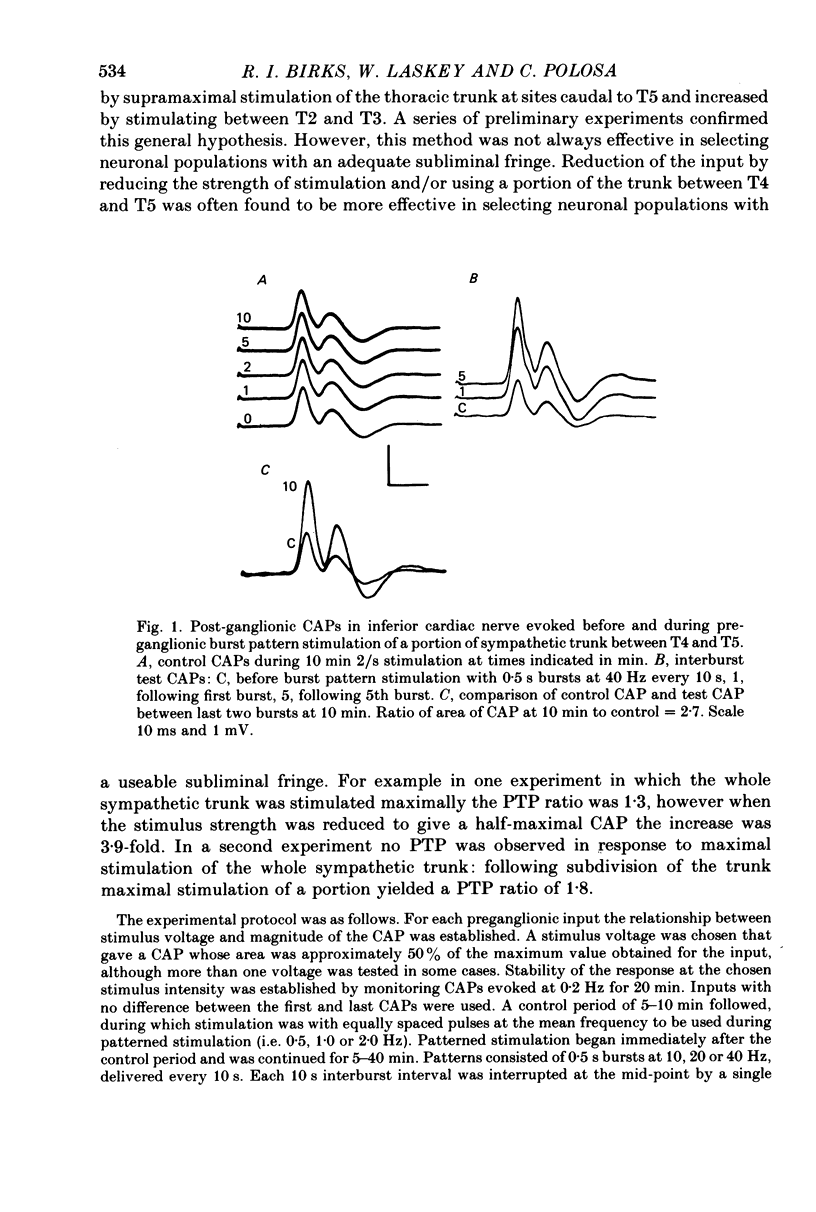
2. Stimulation of the whole or a portion of the thoracic sympathetic trunk between rami T4 and T5 with recording of evoked compound action potentials in the inferior cardiac nerve yielded a preparation with a subliminal fringe with a mean value 2·5 times the size of the discharge zone.
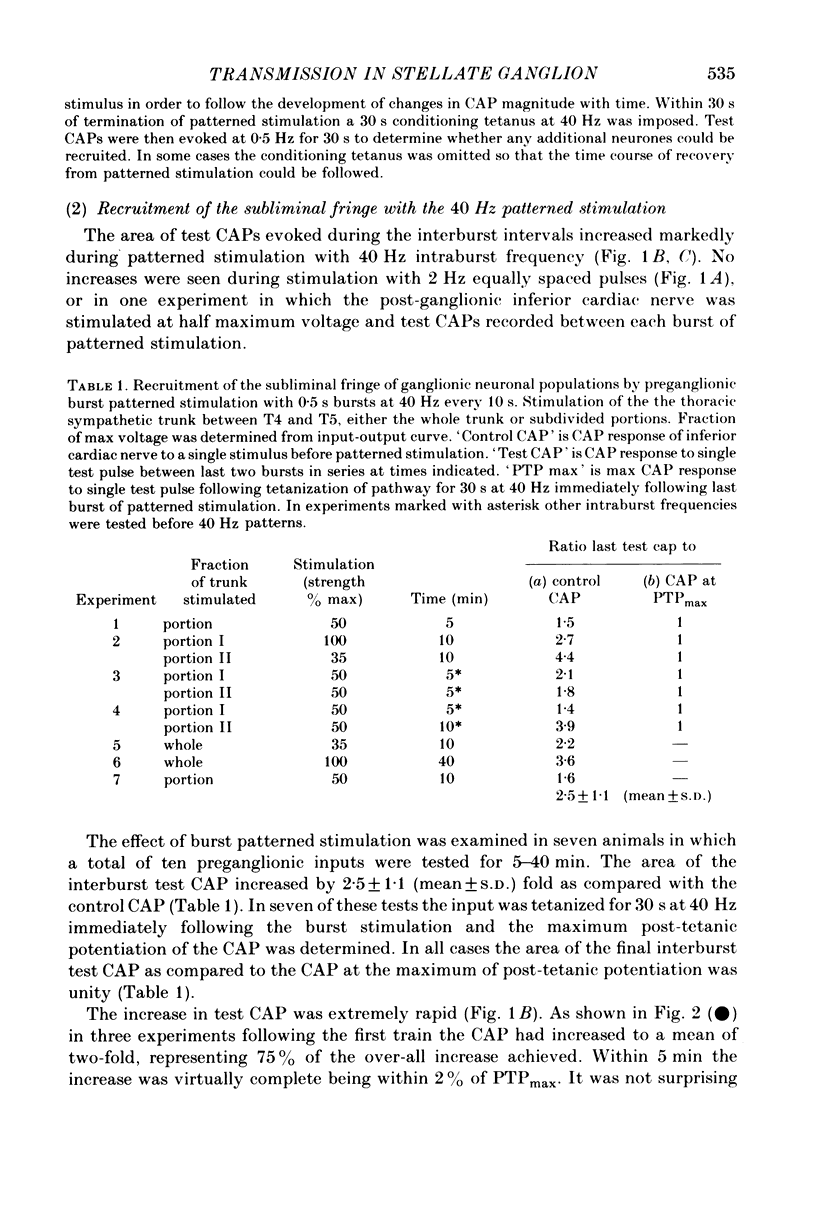
3. Preganglionic stimulation with 0·5 s bursts at 10, 20 and 40 Hz with 10 s interburst intervals caused an increase with time in the area of the compound action potential evoked by a single interburst test stimulus. The increase reached 70-75% of the final value after the first burst, 75-90% after 5 bursts and was virtually complete after 5 min. It was then maintained without attenuation as stimulation was continued.
4. On cessation of burst patterned stimulation test compound action potentials returned to control levels with a half-time of 5 min.
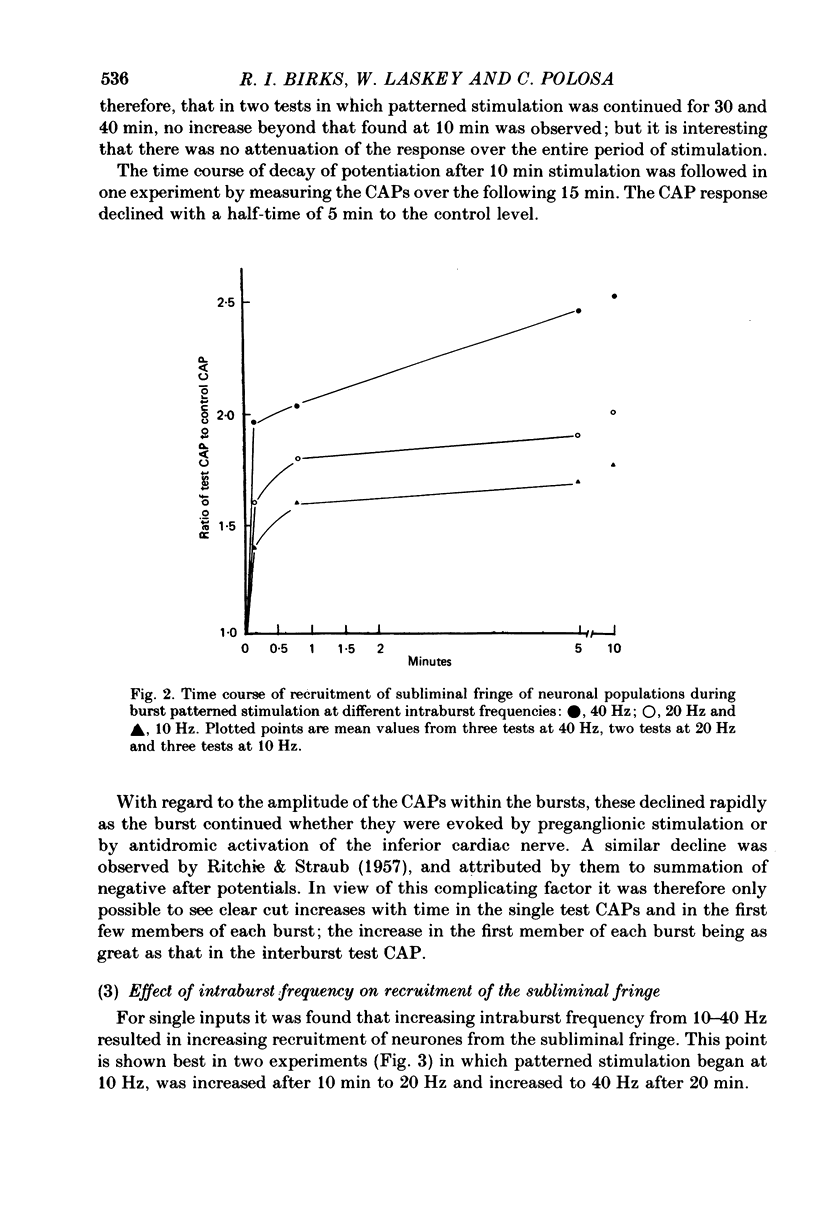
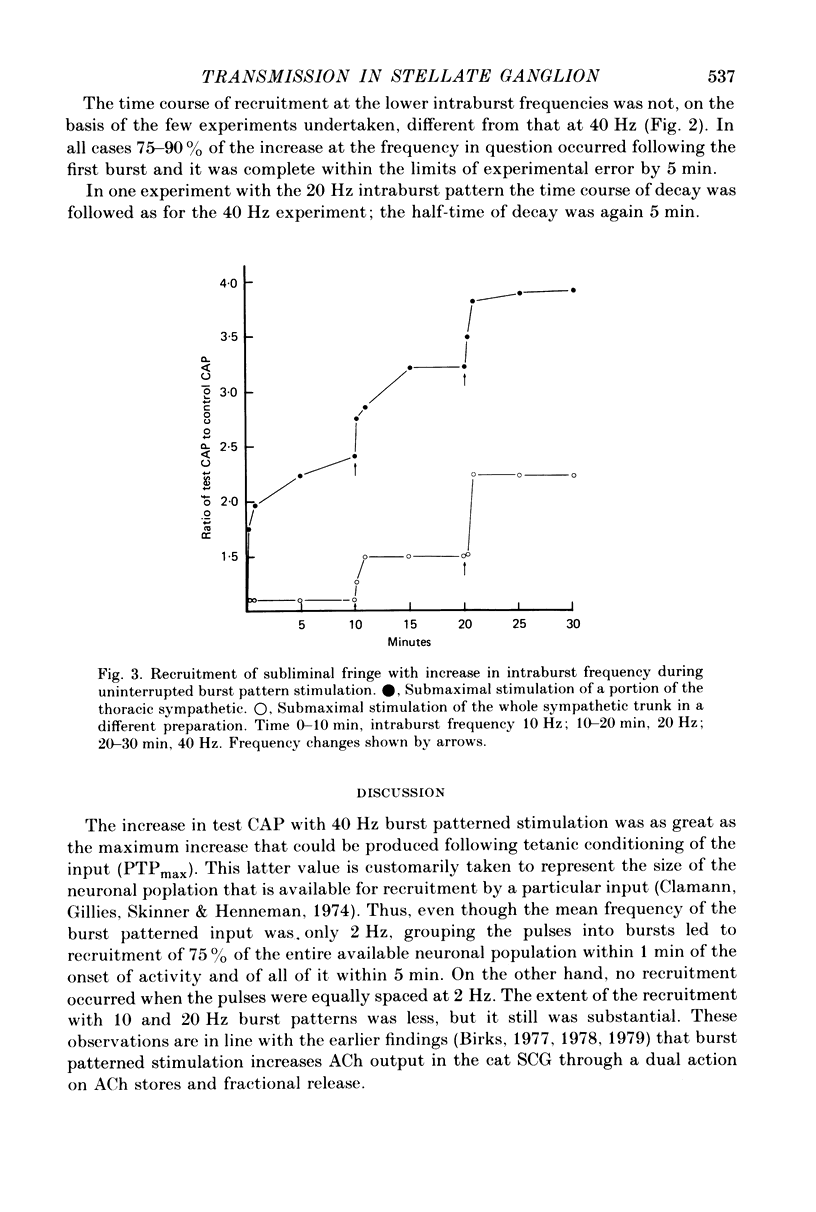
5. The increase in area of test compound action potentials with 40 Hz intraburst frequency was always as great as the increase following tetanization of the pathway. The increases with 20 and 10 Hz bursts were less.
6. There was no increase in area of compound action potentials when equally spaced pulses at 2 Hz or less were applied preganglionically, nor was there any increase when 40 Hz burst patterns were delivered directly to the inferior cardiac nerve.
7. It is concluded that burst patterned preganglionic activity at frequencies similar to those observed in vivo can recruit rapidly into the discharge zone all of the ganglionic neurones that were originally in the subliminal fringe. It is proposed that this effect is related directly to the increase in ACh release at ganglionic synapses that such activity has been shown to induce.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birks R. I. A long-lasting potentiation of transmitter release related to an increase in transmitter stores in a sympathetic ganglion. J Physiol. 1977 Oct;271(3):847–862. doi: 10.1113/jphysiol.1977.sp012028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks R. I. Modulation of transmitter turnover by sodium and the sodium pump [proceedings]. J Physiol. 1979 Oct;295:51P–52P. [PubMed] [Google Scholar]
- Birks R. I. Regulation by patterned preganglionic neural activity of transmitter stores in a sympathetic ganglion. J Physiol. 1978 Jul;280:559–572. doi: 10.1113/jphysiol.1978.sp012401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E. The action of adrenaline on transmission in the superior cervical ganglion. J Physiol. 1944 Jun 15;103(1):55–67. doi: 10.1113/jphysiol.1944.sp004062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groat W. C., Volle R. L. The actions of the catecholamines on transmission in the superior cervical ganglion of the cat. J Pharmacol Exp Ther. 1966 Oct;154(1):1–13. [PubMed] [Google Scholar]
- LIBET B. SLOW SYNAPTIC RESPONSES AND EXCITATORY CHANGES IN SYMPATHETIC GANGLIA. J Physiol. 1964 Oct;174:1–25. doi: 10.1113/jphysiol.1964.sp007471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libet B. Generation of slow inhibitory and excitatory postsynaptic potentials. Fed Proc. 1970 Nov-Dec;29(6):1945–1956. [PubMed] [Google Scholar]
- McCandless D. L., Zablocka-Esplin B., Esplin D. W. Rates of transmitter turnover in the cat superior cervical ganglion estimated by electrophysiological techniques. J Neurophysiol. 1971 Sep;34(5):817–830. doi: 10.1152/jn.1971.34.5.817. [DOI] [PubMed] [Google Scholar]
- Preiss G., Kirchner F., Polosa C. Patterning of sympathetic preganglionic neuron firing by the central respiratory drive. Brain Res. 1975 Apr 11;87(2-3):363–374. doi: 10.1016/0006-8993(75)90434-5. [DOI] [PubMed] [Google Scholar]
- RITCHIE J. M., STRAUB R. W. The after-effects of repetitive stimulation on mammalian non-medullated fibres. J Physiol. 1956 Dec 28;134(3):698–711. doi: 10.1113/jphysiol.1956.sp005676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RITCHIE J. M., STRAUB R. W. The hyperpolarization which follows activity in mammalian non-medullated fibres. J Physiol. 1957 Apr 3;136(1):80–97. doi: 10.1113/jphysiol.1957.sp005744. [DOI] [PMC free article] [PubMed] [Google Scholar]


