Abstract
BACKGROUND
In the last few years, there has been an increased use of theragnostics for the detection of intracranial lesions. The use of 68Ga-DOTATATE has shown beneficial use for meningiomas, but it can pose a challenge when evaluating dura-based metastatic lesions.
OBSERVATIONS
The authors describe the case of a 60-year-old patient with a confirmed diagnosis of renal cell carcinoma (RCC) that presented on brain MRI with two dura-based lesions and an intra-axial mass. All three lesions presented uptake on 68Ga-DOTATATE with different standardized uptake value (SUV) levels.
LESSONS
68Ga-DOTATATE is highly sensitive for detecting benign and malignant CNS tumors, including small lesions. It aids in planning treatment, defining radiosurgical targets, and monitoring response. In RCC, it yields a higher SUV than 18F-fluorodeoxyglucose positron emission tomography, making it a more effective diagnostic tool. In meningiomas, the SUV correlates with tumor growth rate. However, specificity is limited, and interpretation requires correlation with MRI, CT, and clinical findings. As its use in CNS tumors grows, understanding its capabilities and limitations is crucial for proper application.
Keywords: synchronous tumor, brain metastasis, renal cell carcinoma, meningioma, 68Ga-DOTATATE, Gamma Knife radiosurgery
ABBREVIATIONS: FDG-PET = 18F-fluorodeoxyglucose PET, GKRS = Gamma Knife radiosurgery, RCC = renal cell carcinoma, PET = positron emission tomography, PSMA/PET = prostate-specific membrane antigen-targeted molecular imaging with PET, SSTR2 = somatostatin receptor subtype 2, SUV = standardized uptake value, SUVmax = maximum SUV
In the last few decades, the diagnosis of brain tumors has been primarily guided by brain MRI, with the addition of CT scans to evaluate intratumoral calcification and bone invasion. For assessing tumor activity and biology, perfusion sequences and spectroscopy are also used. 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) scans have also been shown to be useful for diagnosing brain tumors, but they can be limited in detecting small metastatic lesions.1
The use of theragnostics has increased in medical practice for the diagnosis and treatment of cancer. They target molecular analogs to receptors present in tumor cells or the endothelium of tumoral vessels, helping identify metastatic implants. The capacity to identify tumors is related to the expression of these markers, which can be present in malignant and benign tumors, creating a diagnostic challenge.
Somatostatin receptor analogs are targeted in 68Ga-DOTATATE, but it is expressed in primary and secondary CNS tumors. We present a case with synchronous meningioma and renal cell carcinoma (RCC) brain metastasis with 68Ga-DOTATATE uptake.
Illustrative Case
A 60-year-old right-handed man presented to an outside emergency department with a 2-week history of left-sided weakness. Initially, he noticed swelling in his knee and suspected a work-related twist injury. His symptoms progressed to left foot dragging and difficulty grasping objects with his left hand. At initial evaluation, he was found to be hypertensive, and a CT angiography study of the head was requested and revealed extensive vasogenic edema in the right frontoparietal region and a hyperdense mass abutting the falx. Further CT imaging of the chest showed multiple lung nodules, and CT imaging of the abdomen and pelvis revealed a heterogeneous suspicious mass in the anterior lower pole of the right kidney and a liver lesion, raising concerns for renal carcinoma. Complementary MRI of the brain revealed a 1.3-cm enhancing intra-axial mass in the right frontal lobe, accompanied by moderate vasogenic edema and a mass effect (Fig. 1A–D). Additionally, two dura-based masses at the right frontal and left infratentorial regions were identified, appearing as meningiomas, but dura-based metastases were also considered in the differential diagnosis (Figs. 2A–C and 3A–C). On neurological examination, he presented with a mild left-sided dysmetria, mild left-sided dysdiadochokinesis, a subtle left-sided pronation, upward drift, and preserved cognition.
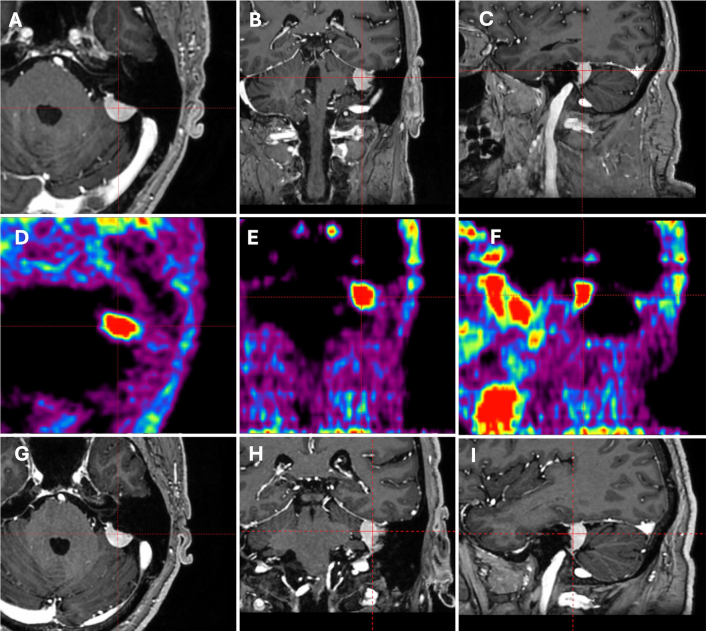
FIG. 1.
A–D: MR images showing a right frontal intra-axial lesion, with perilesional edema well observed on FLAIR imaging (B). E–G: The lesion presented an SUVmax of 2 on 68Ga-DOTATATE. A GKRS treatment was delivered in a single session with 20 Gy at the 87% isodose line, with a delivery time of 8.42 minutes. Dosimetry showed a minimum dose of 18.9 Gy, a maximum dose of 23 Gy, a mean dose of 22.1 Gy, coverage of 99%, a Paddick Conformity Index of 0.62, and a gradient index of 3.64. H–J: Three-month follow-up MR images showing complete lesion control.
FIG. 2.
A–C: MR images showing a right frontal meningioma with a volume of 8.917 cm3. D–F: 68Ga-DOTATATE showed an SUVmax of 11.9. G–I: Three-month follow-up MR images demonstrating an increase in volume to 10.028 cm3.
FIG. 3.
A–C: MR images showing a posterior fossa meningioma. D–F: The SUVmax on 68Ga-DOTATATE was 2.9. G–I: Three-month follow-up MR images showing no volume change.
A CT-guided renal biopsy was performed and showed fragments of necrotic tissue with a small focus of atypical cells with clear cell features, with immunohistochemical expression of PAX8 and CAIX, while CK7 was negative, favoring clear cell RCC.
A 68Ga-DOTATATE positron emission tomography (PET) scan showed uptake in the lesions, with the posterior fossa mass presenting a maximum standardized uptake value (SUVmax) of 2.9 (Fig. 3D–F), the right frontal mass an SUVmax of 11.9 (Fig. 2D–F), and the right intra-axial mass an SUVmax of 2 (Fig. 1E–G).
On multidisciplinary discussion, it was recommended that patient be started on ipilumab and nivolumab and receive Gamma Knife Radiosurgery (GKRS) for the intra-axial lesion. Discussion was held with the patient for the management options of the meningiomas, which included surveillance, resection, and radiosurgery. The patient opted to treat only the metastatic disease and surveil the meningiomas. A masked-based single-session GKRS was delivered to a 0.126-cm3 intra-axial lesion using the Esprit model (Elekta AB) with a prescribed dose of 20 Gy to the 87% isodose line, with coverage of 99%, a Paddick conformity index of 0.62, and a gradient index of 3.64 (Fig. 1).
On 3-month follow-up, the metastatic disease showed complete resolution (Fig. 1H–J). While the posterior fossa tumor had no change in volume (Fig. 3G–I), the right parafalcine presented a volumetric growth of 12.46% (from 8.917 to 10.028 cm3) (Fig. 2G–I). Despite the observed tumor growth, the patient decided to continue with surveillance and will be followed up with serial MRI every 3 months, which will also assess the treated tumor response and screen for possible new metastases.
Informed Consent
The necessary informed consent was obtained in this study.
Discussion
Meningiomas are mostly benign extra-axial tumors that originate from the arachnoid cap cells and correspond to 37.6% of all CNS tumors.2 Meanwhile, metastatic cancer is the main CNS cancer affecting approximately 20% of patients with cancer.3 Synchronous malignant and benign tumors represent a rare entity, with cases of metastatic disease to meningiomas.4,5
The diagnosis of meningiomas mainly relies on MR and CT imaging characteristics. RCC metastasis may be diagnosed late after nephrectomy and appears hypometabolic on FDG-PET scans; therefore, it is not a common imaging modality for this tumor type.6
Currently, there is an increased use of theragnostics for the diagnosis of cranial tumors.7 68Ga-DOTATATE uses the radionuclide gallium-68, which attaches to a somatostatin receptor analog, making it suitable for identifying neuroendocrine tumors, especially in those with somatostatin receptor subtype 2 (SSTR2).6,8,9 The same approach is applied to prostate-specific membrane antigen–targeted molecular imaging with PET (PSMA/PET), where prostate-specific membrane antigen produced in the neovascularization of tumors10 can be targeted for imaging identification. Both of these imaging techniques have demonstrated uptake in benign and malignant CNS tumors, including meningiomas and metastatic RCC, making them valuable tools not only for diagnosis, but also for guiding treatment and response assessment.7,11–19 In RCC, 68Ga-DOTATATE has demonstrated a higher SUV compared with FDG-PET, and better capacity to detect small lesions, as seen in the case presented (0.126 cm3).1,6
Due to the wide variety of tumors detectable by 68Ga-DOTATATE and PSMA/PET, their specificity is reduced, but without loss of sensitivity. Since RCC can also appear as a dura-based lesion, a careful review of the PET scan, along with MR and CT images, can help distinguish between tumor types.13
A critical analysis of the SUV is important in making this differentiation and will be guided by the expression of SSTR2. Meningiomas have been reported to show higher SUVs compared with metastatic breast tumors due to their difference in SSTR2 expression.20 In addition, higher SUVs correlated with the growth rate of meningiomas, explaining the highest SUVmax in the most voluminous meningioma in the presented case, and its growth in 3 months.21 In a postradiation setting, reduction of the SUVmax can be related to a biological response.18
Observations
Both RCC metastasis and meningioma are high expressors of SSTR2, making their differentiation with 68Ga-DOTATATE challenging. In the reported case, the intra-axial lesion with corresponding FLAIR change was easily defined as a brain metastasis. The dura-based lesions presented with dural tail and similar contrast-enhanced patterns. The difference in their volume can be explained by their different SUVs, but not necessarily the difference in their WHO classification.
Limitations
This study is limited by being a single-case report, which is susceptible to bias; long-term follow-up is not available, and histopathological confirmation of the meningiomas is not provided.
Lessons
68Ga-DOTATATE is a highly sensitive tool to diagnose benign and malignant CNS tumors that express SSTR2, including very small lesions. Beyond diagnosis, it can help assist treatment planning by defining the radiosurgical target and assessing the biological response by SUV reduction after the treatment. In RCC, 68Ga-DOTATATE presents higher SUV values when compared with FDG-PET, and in meningiomas, the SUV levels have been correlated with the tumor growth rate.
While the 68Ga-DOTATATE sensitivity is an asset, it has limited specificity, and accurate interpretation requires careful correlation with MRI, CT, and clinical findings. As its use for CNS tumors increases, a thorough understanding of its strengths and limitations is essential for optimal surgical and radiosurgical application.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: all authors. Acquisition of data: Prasad, Goulenko, Madhugiri, Lipinski. Analysis and interpretation of data: Prasad, Goulenko. Drafting the article: Prasad, Goulenko, Madhugiri, Almeida. Critically revising the article: Prasad, Goulenko, Madhugiri, Almeida, Shekher, Lipinski. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Prasad. Statistical analysis: Prasad. Administrative/technical/material support: Prasad. Study supervision: Prasad, Lipinski.
Correspondence
Dheerendra Prasad: Roswell Park Comprehensive Cancer Center, Buffalo, NY. d.prasad@roswellpark.org.
References
- 1.Pope WB.. Brain metastases: neuroimaging. Handb Clin Neurol. 2018;149:89-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostrom QT, Cioffi G, Gittleman H.CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016. Neuro Oncol. 2019;21(suppl 5):v1-v100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sacks P Rahman M.. Epidemiology of brain metastases. Neurosurg Clin N Am. 2020;31(4):481-488. [DOI] [PubMed] [Google Scholar]
- 4.Moody P Murtagh K Piduru S Brem S Murtagh R Rojiani AM.. Tumor-to-tumor metastasis: pathology and neuroimaging considerations. Int J Clin Exp Pathol. 2012;5(4):367-373. [PMC free article] [PubMed] [Google Scholar]
- 5.Sættem M Sundstrøm T Sæle AKM Mahesparan R.. Review of metastasis to meningiomas with case examples. Brain Spine. 2024;4:102862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nadebaum DP Lee ST Nikfarjam M Scott AM.. Metastatic clear cell renal cell carcinoma demonstrating intense uptake on 68Ga-DOTATATE positron emission tomography: three case reports and a review of the literature. World J Nucl Med. 2018;17(3):195-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmisciano P, Watanabe G, Conching A.The role of [68Ga]Ga-DOTA-SSTR PET radiotracers in brain tumors: a systematic review of the literature and ongoing clinical trials. Cancers (Basel). 2022;14(12):2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oronsky B Ma PC Morgensztern D Carter CA.. Nothing but NET: a review of neuroendocrine tumors and carcinomas. Neoplasia. 2017;19(12):991-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LeBlanc RA Oza UD Hayden R Fanous H.. Use of 68Ga DOTATATE, a new molecular imaging agent, for neuroendocrine tumors. Proc (Bayl Univ Med Cent). 2020;33(1):51-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanjore Ramanathan J, Lehtipuro S, Sihto H.Prostate-specific membrane antigen expression in the vasculature of primary lung carcinomas associates with faster metastatic dissemination to the brain. J Cell Mol Med. 2020;24(12):6916-6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Galiza Barbosa F, Queiroz MA, Nunes RF.Nonprostatic diseases on PSMA PET imaging: a spectrum of benign and malignant findings. Cancer Imaging. 2020;20(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filizoglu N Cetin IA Kissa TN Niftaliyeva K Ones T.. 68Ga-PSMA PET/CT to distinguish brain metastasis of renal cell carcinoma from radiation necrosis after stereotactic radiosurgery. Clin Nucl Med. 2021;46(11):913-914. [DOI] [PubMed] [Google Scholar]
- 13.Filizoglu N Oksuzoglu K Ozguven S.. Distinguishing meningioma from metastasis of prostate cancer on 68Ga-PSMA PET/CT. Clin Nucl Med. 2021;46(11):e553-e555. [DOI] [PubMed] [Google Scholar]
- 14.McLaughlin LA, Yildirim O, Rosenblum MK.Identification of incidental brain tumors in prostate cancer patients via PSMA PET/CT. J Neurooncol. 2023;163(2):455-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasikumar A, Joy A, Pillai MR.Diagnostic value of 68Ga PSMA-11 PET/CT imaging of brain tumors-preliminary analysis. Clin Nucl Med. 2017;42(1):e41-e48. [DOI] [PubMed] [Google Scholar]
- 16.Florou VA Halthore A Lucas CG Rivera Lopez CA Pienta KJ.. PSMA PET/CT scan reveals incidental meningioma in a prostate cancer patient. Urol Case Rep. 2025;60:103022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Filizoglu N Ozguven S Ones T Turoglu HT Erdil TY.. Value of 68 Ga-DOTATATE PET/CT in follow-up of renal cell carcinoma with multiple atypical metastatic sites. Clin Nucl Med. 2024;49(12):e674-e676. [DOI] [PubMed] [Google Scholar]
- 18.Barone F, Inserra F, Scalia G.68Ga-DOTATOC PET/CT follow up after single or hypofractionated gamma knife ICON radiosurgery for meningioma patients. Brain Sci. 2021;11(3):375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Umana GE, Ferini G, Harikar MM.Detection of “incidentalomas” on brain and body 68GA-DOTATOC-PET scans: a retrospective study and case illustration. Anticancer Res. 2022;42(12):5867-5873. [DOI] [PubMed] [Google Scholar]
- 20.Unterrainer M, Ruf V, Ilhan H.68Ga-DOTATOC PET/CT differentiates meningioma from dural metastases. Clin Nucl Med. 2019;44(5):412-413. [DOI] [PubMed] [Google Scholar]
- 21.Sommerauer M, Burkhardt JK, Frontzek K.68Gallium-DOTATATE PET in meningioma: a reliable predictor of tumor growth rate? Neuro Oncol. 2016;18(7):1021-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]