Abstract
Breast cancer (BC) is the leading cause of cancer-related death among women worldwide. Due to limited treatment options for patients with advanced BC, preventive and innovative therapeutic strategies are essential to combat this disease. Therefore, finding safe and effective anticancer treatments remains a significant challenge in the 21st century. Plant-derived triterpenoids, widely used for medicinal purposes, exhibit various biological activities. Most triterpenoids are cytotoxic to multiple tumor cells and demonstrate anticancer effects in preclinical animal models. One example is betulinic acid (BA), a natural product mainly extracted from the bark of birch trees. BA is a promising anti-tumor compound with numerous pharmacological properties. However, its poor water solubility limits its optimal therapeutic potential. Additionally, the low BA content in plants hampers large-scale production from these sources. To address these issues, extensive research has focused on producing BA through chemical synthesis and biotransformation. Furthermore, several BA derivatives have been developed through structural modifications, and various delivery systems have been created to improve solubility and enhance therapeutic efficacy. This review discusses recent advances and challenges related to BA and its derivatives in preventing and treating breast tumors, as well as the potential obstacles and future directions for improving delivery systems in BC therapy.
Betulinic acid from plants, biosynthesis, or chemical synthesis can be formulated into BA-nanocarriers, enhancing therapeutic efficacy against breast cancer by improving solubility, bioavailability, stability, delivery, and treatment outcomes.
1. Introduction
1.1. The emergence of natural compounds to overcome breast cancer
Breast cancer (BC) is characterized by the uncontrolled growth of malignant cells in the mammary epithelium, affecting both genders. It is the most common cancer and the leading cause of cancer-related deaths among women worldwide.1,2 The World Health Organization (WHO) reported that 2.3 million women were diagnosed with BC, resulting in 670 000 deaths globally in 2022. This disease develops due to various internal and external factors, such as unhealthy lifestyle choices, environmental influences, and sociopsychological factors.3 Treatments for BC include surgery, chemotherapy, radiotherapy, endocrine therapy, targeted therapy, and immunotherapy.4 Although these treatments have proven effective and have improved over time, there are still significant challenges, such as cost-effectiveness and tumor cell specificity.
Although 70–80% of patients with early BC are cured, late metastasis remains an unresolved challenge. Currently, chemotherapeutic drugs for BC have made significant progress, though their limited effectiveness is a concern. For example, in addition to substantial side effects, the risk of recurrence remains high.5 Therefore, it is essential to explore alternative treatments with fewer severe adverse effects. Ongoing research focuses on novel therapeutics, combination therapies, and biologically inspired drug delivery systems (e.g., nanomedicines and polymer-based drug carriers) to enhance treatment outcomes while reducing side effects. Meanwhile, natural products have been used to combat human diseases for thousands of years due to their diverse biological properties that can be harnessed for medical use.6,7 As a result, extensive research over the past decades has identified numerous phytochemical compounds with chemopreventive potential, which may serve as essential sources of anticancer lead molecules.
Pentacyclic triterpenes are a class of organic compounds widely found in various plant species. They have a broad range of physiological and commercial uses because of their widespread presence and structural diversity.8 An example is betulinic acid (BA), a pentacyclic lupane-type triterpenoid found throughout the plant kingdom. Among lupane-type compounds, BA has garnered significant interest due to its extensive biological activities, including anti-inflammatory, antiviral, and antitumor effects.9 Because of its specific cytotoxicity against several tumor cells, it is considered a promising anti-tumor agent for future development.10
Despite its potential for clinical use, the limited availability of BA in its natural hosts poses a significant hurdle to its commercialization. While birch bark is the primary plant source for extracting BA, the low concentration of BA in its tissues restricts large-scale production for the market.11 Therefore, developing alternative methods to produce this compound is a key research focus. Chemical synthesis, based on betulin (BE), a structurally similar precursor, is widely reported.12 However, it is complicated by several factors, including harsh reaction conditions and high toxicity. Biotransformation has gained increasing interest due to its environmental friendliness and mild reaction conditions. Large-scale BA production is possible through the development of efficient production strains via biotransformation.13 Additionally, many studies suggest that BA has excellent potential as an anticancer agent. However, its limited water solubility, low bioavailability, and potential off-target toxicity restrict its use.
In recent years, nano-drug delivery systems (NDDSs) have gained significant attention as a method to substantially improve low solubility and poor drug bioavailability, enhance targeted drug delivery, and reduce side effects.14,15 A variety of nanocarriers, including nanoparticles, liposomes, and micelles, are combined with drug molecules, resulting in better drug stability, increased bioavailability, and sustained and targeted release.16 Extensive research has focused on leveraging the advantages of NDDSs to overcome the limitations of bioavailability (BA), particularly in improving cellular uptake, increasing drug accumulation in tumor cells, and crossing physiological barriers more effectively.17 A related strategy was employed in developing bone-targeted hyaluronic acid-alendronate bioactive glass nanocomposites for improved osteosarcoma treatment, showing the potential for site-specific drug delivery via ligand-functionalized systems.18
Recent review articles have explored BA's diverse biological activities and its therapeutic potential.9,19,20 For example, Hordyjewska et al.21,22 published comprehensive reviews on BA and its precursor, BE, emphasizing their significant biological and pharmacological properties. These reviews highlighted the strong biological effects of BA and BE, particularly their anticancer properties, and encouraged further research into their mechanisms and therapeutic applications. This also prompted another review, focused explicitly on the anticancer properties of BE and BA.21,22 Likewise, reviews by Simone Fulda23,24 strengthened BA's potential for cancer treatment and prevention, particularly its ability to selectively trigger apoptosis in cancer cells while causing minimal harm to normal cells. These findings underscore the importance of ongoing research and development of BA as a promising treatment for cancer.23,24 Additionally, Hanghang Lou's review provided a detailed look at BA's natural sources, biological traits, and methods of preparation, further supporting its potential as a therapeutic agent due to its broad biological effects.12 Recently, Wang et al. reviewed advances in using nanotechnology to improve the therapeutic effectiveness of BA in cancer treatment, highlighting the promise of BA-loaded nanoformulations for clinical use.25 Despite extensive research into BA's mechanisms, derivatives, methods, and biological effects in BC therapy, several obstacles hinder its translation into clinical practice and market availability. These challenges include pharmacokinetic limitations, formulation problems, regulatory barriers, and a lack of clinical validation. Overcoming these obstacles could speed up BA's progress from an experimental anticancer agent to an approved therapy for BC patients.
This review presents a new perspective on BA by summarizing recent advances in its preparation techniques and anti-tumor uses, while also addressing key obstacles that have limited its clinical use and market availability. It highlights newly discovered sources of BA production and their biological activity, offering insights into how these advances can improve BA's therapeutic effectiveness. The primary focus of this review is on developing new carriers and combining nanotechnology with targeted therapies, which shows great potential for increasing BA's bioavailability and clinical success, thereby reducing the burden of BC. By exploring ongoing progress in nanotechnology, this review highlights its role in bridging research and clinical practice, paving the way for the commercialization of BA and underscoring its essential contribution to future BC treatments.
1.2. Betulinic acid
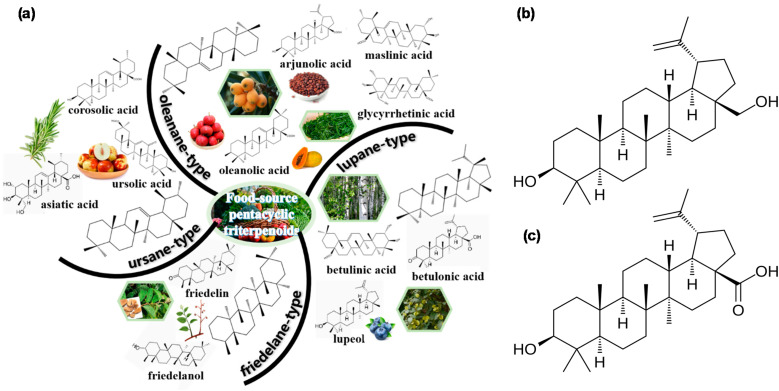
Triterpenes are a group of chemically diverse compounds that are highly relevant to human health. They are divided into four main categories based on their skeletal structures: oleanane, ursane, lupane, and friedelane.26 The related chemical classifications and detailed structures of each group are shown in Fig. 1(a). BA is a naturally occurring pentacyclic lupane-type triterpenoid with a molecular weight of 456.71 g mol−1, widely distributed in the outer bark of various plant species, especially Betula spp. It is usually isolated from medicinal plants and found in multiple species. The compound exists in the plant as either a free aglycon or a glycosylated derivative and can be easily isolated using various solvents, such as ethanolic extraction and dichloromethane.21,27–30Fig. 1(b) and (c) display the representative chemical structures of lupane-type triterpenoids in BE and BA. Compound purification is easily achieved by combining different chromatographic techniques with crystallization, resulting in white powder compounds.31 BA exhibits a wide range of pharmacological effects, with significant research highlighting its antiviral and anticancer properties.27,32,33 It is important to note that the anticancer effects of BA on different types of tumors are more potent at a pH lower than 6.8.34
Fig. 1. (a) Chemical classifications and detailed structures of each group. The chemical structures of (b) Betulin (BE) and (c) Betulinic acid (BA). This figure has been reproduced from ref. 8. Copyright: 2024 by the authors. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
To gain a comprehensive understanding of the research landscape surrounding BA in BC, an analysis was conducted to examine publication trends and research output from 2016 to 2024, as shown in Fig. 2. The systematic investigation followed the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).35,36 The electronic database and search engine Science Direct were used to gather relevant research on the inhibitory effects of BA against BC cells. The selection criteria for included papers were limited to those published in peer-reviewed journals from 2016 onward, with the most recent search conducted in January 2025. This study employed a search strategy that combined the keywords “betulinic acid” and “breast cancer” to retrieve relevant articles. The reviewed literature indicates a steady increase in the number of reviews, research articles, and book chapters published on BA during this period. Notably, from 2016 to 2024, there has been consistent growth in publications, with a significant spike in 2021. This upward trend likely reflects the growing interest in BA's therapeutic potential, particularly in oncology and the management of infectious diseases.10,21 Research articles from this period have explored various aspects of BA, including its synthesis, encapsulation, and antitumor activity.25 Additionally, several studies have reported the successful development of new BA derivatives with enhanced cytotoxicity against a broad range of human cancer cell lines compared to native BA.10 Furthermore, the encapsulation of BA using different delivery systems, such as polymeric nanoparticles (PNPs) and liposomes, has been investigated, providing evidence for an apoptotic mode of cell death.
Fig. 2. The number of articles published (book chapters, reviews, and research articles) on BA in the last nine years (search conducted using keywords “betulinic acid” and “breast cancer” according to the Science Direct database on 07 January 2025, from 2016 to 2024).
Several important biological properties of BA, such as hypoglycemic, anti-inflammatory, antioxidant, and anticancer effects, have been detailed in the literature. Fig. 3 illustrates these health benefits and the related mechanisms of triterpenes.
Fig. 3. Highlights the various health benefits of BA along with their associated mechanisms. This is an original drawing, and the diagram was created using Biorender.
Among the various biological and pharmacological activities linked to BA, its antitumor activity has been the most thoroughly researched. This is because of its specific cytotoxicity against turmeric cells. BA is regarded as a promising anti-tumor compound. However, despite its significant potential, the production of BA from plants remains limited. In particular, the demand for BA for medical and nutritional uses can no longer be satisfied by natural resources. Therefore, alternative methods are needed to produce BA in large quantities.
2. Preparation and isolation of BA
BA is primarily found throughout the plant kingdom, mainly in the outer bark of trees. However, the low concentration of plant-derived BA limits its research and applications. The reported concentration of BA extracted from plants was approximately 5%, and the yield was primarily influenced by factors such as the type of extraction solvent and method. Besides the low yield, the extraction process is unsustainable and environmentally unfriendly.37,38 Another approach is chemical synthesis, where BE, a structurally similar compound, serves as a precursor. However, this method presents challenges, including harsh reaction conditions and high toxicity. Recently, biotransformation has gained importance in producing BA due to its environmental friendliness and mild reaction conditions. By developing efficient production strains, biotransformation can facilitate the large-scale production of BA. Researchers have focused on developing new synthetic methods to produce BA at high concentrations, thereby overcoming these issues. Here, we summarize (Fig. 4) the most advanced techniques for BA preparation and their recent developments, including production from natural sources, chemical synthesis, and microbial biotransformation.
Fig. 4. BA is a plant-derived pentacyclic lupane-type triterpenoid obtained through plant extraction, chemical synthesis, or microbial biotransformation. This is an original drawing, and the diagram was created using Biorender.
2.1. Extraction of BA from various plants
So far, BA has been chiefly found in the outer bark of white birch trees. The amount of BA extracted from the bark was reported to be around 0.002–2%, and the yield varied based on the extraction solvent and method. Mukherjee et al.39 used 70% ethanol to extract BA, yielding 23.76 mg per 10 g of birch bark. Similar results were reported by Kim et al., who obtained a yield of BA of approximately 0.0021% from birch bark via ultrasonic extraction.40 Ethyl acetate has also been used in the maceration extraction of BA, yielding 3.07 mg g−1 of birch bark.41 New environmentally friendly technologies have been developed to address environmental challenges and reduce hazardous waste produced by these BA extraction methods. Optimization could potentially increase the yield to approximately 28.3 mg per 10 g of birch bark.42 BA has also been found in various other plant tissues. The bark of the plane tree was identified as a BA-containing plant in 1948.43 Galgon et al.44 quantified BA in the cork of the plane tree in 1999. The analysis revealed a concentration level of approximately 3.3%. Comparative research showed that using ethanol as a cosolvent with CO2 in supercritical fluid extraction resulted in a high BA extraction yield of 4.34% from dried bark of the plane tree (Platanus acerifolia L.), while also consuming nearly one-third less organic material than methods like solid–liquid extraction (SLE), ultrasound-assisted extraction (UAE), and pressurized liquid extraction (PLE)45 BA has also been identified in the leaves, stems, and bark of Syzygium aromaticum L (SA).46 The botanical materials were initially extracted with a Soxhlet extractor using methanol, and then the extracts were converted into a solid form. The leaf extracts of SA contained more BA than those from the stem and bark, which included 16.9 ± 0.9 μm mL−1, 3.5 ± 0.2 μm mL−1, and 3.8 ± 0.4 μm mL−1, respectively.46 In addition to other plant species, BA was also found in Eucalyptus spp., which is a crucial fiber source for pulp and paper production. BA could be extracted from biomass residues of the pulping industry47,48 and isolated from the Lamiaceae plant family.49 Although BA is mainly isolated from various plants, its natural yield remains low, making large-scale production and pharmaceutical applications challenging.
2.2. Chemical synthesis
The excellent bioactivities of BA boost the high demand for the compound. Although the birch tree is the primary source of BA, the small amount of BA produced in its tissues limits large-scale manufacturing. Therefore, finding alternative methods to produce this compound has become a key research focus, as extraction isn't suitable for mass production. In this context, chemical synthesis of BA using various precursors has been documented. Using BE as a precursor is the most common method. BE is attractive because it is abundant in birch trees, often reaching up to 34% compared to BA; however, its biological activity is less notable. BA is produced through semi-synthesis, involving Jones oxidation of the primary hydroxyl group of BE, followed by reduction of the resulting ketones. A five-step process is typically used to prevent isomerization. First, the primary alcohol is protected, then the secondary alcohol is acetylated, followed by deprotection, oxidation of the primary alcohol, and finally, deacetylation of the secondary alcohol to yield BA.50 Using a biphasic system, significant amounts of BE can be extracted from industrial birch bark byproduct with n-butyl acetate and an aqueous phosphonium hydroxide solution at room temperature. The same biphasic system facilitates the conversion of BE to BA through oxidation with TEMPO and hypervalent iodine(iii) reagents, achieving an 85% yield. Overall, this method proves to be environmentally friendly, cost-effective, reliable, and energy-efficient.
Inspired by the biological importance of BA, its derivatives are currently the focus of extensive research. For example, Debasmita Dutta synthesized 2c, (3S)-3-[2-(4-hydroxymethyl-1H-1,2,3-triazol-1-yl) acetyloxy]-lup-20(29)-en-28-oic acid, a potent inducer of apoptosis in HT-29 human colorectal carcinoma cells. This compound was found to be a highly effective inhibitor of human colorectal carcinoma. When tested with encapsulation using an aptamer to address its insolubility, off-target cytotoxicity, and for tumor-specific targeting, Apt-2cNP induced autophagy, S-phase arrest, and apoptosis, while activating immune cells and exerting a positive anti-tumor effect in the tumor microenvironment. Fig. 5 illustrates the preparation of the BA derivative bound to an aptamer specific for epithelial cell adhesion molecule.51
Fig. 5. Preparation of aptamer-bound BA analogue (Apt-2cNP). This figure has been reproduced from ref. 51. Copyright: 2024 by the authors. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
2.3. Biotechnological biosynthesis techniques
Synthetic biology, also known as genome engineering, presents significant opportunities to accelerate the development of various strains that aim to optimize biosynthetic pathways. Biotechnological biosynthesis techniques are a subset of genome engineering. They involve microbial biotransformation processes that utilize microbes or enzymes to produce biological molecules or compounds such as BA. In this process, lupeol is first synthesized through 2,3-oxidosqualene cyclization, followed by the oxidation of the primary hydroxyl group by cytochrome P450 (CYP) enzymes.52,53 More possibilities for producing BA are constantly being discovered with the identification of genes encoding enzymes critical for these reactions. One of the systems currently used is SCRaMbLE. Fig. 6 provides an overview of the SCRaMbLE system for BA preparation.53 This system is inducible for rearrangement and in vivo deletion of synthetic yeast chromosomes, resulting in strains with improved biosynthetic phenotypes. Gowers and colleagues prepared BA using engineered yeast strains optimized by SCRaMbLE, producing a higher volume of BA per minute compared to natural extraction methods.
Fig. 6. SCRaMbLE of a BA-producing strain, followed by quick screening, creates a diverse library. BA synthesis involves rerouting flux from the natural mevalonate pathway (top left) through three foreign enzymes: AtLUS1, BPLO, and AtATR1. Four genes—AtLUS1, BPLO, tHMG1, and ERG9—are expressed from a URA+ CEN/ARS plasmid, while AtATR1 is inserted into the genome at the HO locus on chromosome IV (right). This figure has been reproduced from ref. 52. Copyright: 2020 by the authors. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Using a gene that encodes a lupeol C-28 oxidase, BA was engineered in yeast platforms with both the WAT11 strain and the CEN.PK strain. The WAT11 strain proved to be a more effective host for the BA pathway compared to CEN.PK strain.54 Thus, the host for BA production greatly influences the amount produced. While using cytochrome P450 enzymes to convert lupeol into BA has been successful, these enzymes are not specific for this biosynthesis and have not demonstrated high yields in metabolic engineering. Alternative methods, such as implementing multiple module biosynthetic pathways using RoCYP01 (CYP716A155)—an enzyme identified from the BA-producing plant Rosmarinus officinalis—have been explored. BA biosynthetic modules using RoCYP01 have also been reported. The first module involves the mevalonate-dependent (MVA) pathway, which produces dimethylallyl diphosphate (DMAPP) and isopentenyl diphosphate (IPP) precursors from the central metabolite acetyl-CoA. The second module is concerned with the production of the triterpenoid precursor 2,3-oxidosqualene (oxSQ). The final module concerns the conversion of lupeol into BA, but the description is incomplete RoCYP01.55
Fig. 7(a) illustrates the biosynthesis of BA using yeast. Saccharomyces cerevisiae is genetically modified to produce BA via the mevalonate pathway and the intermediate molecule squalene 2,3-epoxide. Key enzymes involved in converting acetyl-CoA to squalene include HMG-CoA reductase (HMGR), squalene synthase (ERG9), and bifunctional farnesyl-diphosphate farnesyltransferase. Additionally, the oxidation of nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) to NADP+ is crucial. After this, squalene monooxygenase (ERG1) converts squalene into squalene 2,3-epoxide. The cyclization of this substrate by Arabidopsis lupeol synthase (AtLUP1) leads to lupeol formation. Furthermore, the Catharanthus roseus P450 monooxygenase (CrAO) catalyzes the oxidation of NADPH to NADP+ to transform lupeol into BA.19,56
Fig. 7. (a). Reaction scheme showing the biosynthetic pathway of BA using engineered yeast S. cerevisiae. (b). Overview of the multimodular strategy for producing BA-related triterpenoids in Y. lipolytica. The BA biosynthesis pathway is divided into four modules: the red arrow indicates the heterologous CYP/CPR module (CYP-lupeol C-28 oxidase, CPR-CPR-NADPH-cytochrome P450 reductase). The yellow arrow shows the MVA module, which includes three genes (ERG1, ERG9, and HMG1). The green arrow depicts the redox cofactor supply module, featuring four introduced genes (EMC, EMT, and Rtme – encoding malic enzyme, responsible for NADPH generation, and Gapc – encoding glyceraldehyde-3-phosphate dehydrogenase, responsible for NADH production). The blue arrow represents the acetyl-CoA generation module, with seven endogenous genes overexpressed (ACL1 and ACL2, encoding ATP citrate lyase, to increase acetyl-CoA levels directly; PXA1, MFE1, PEX10, POT1, and TGL3, which participate in the β-oxidation pathway and are responsible for fatty acid catabolism to produce acetyl-CoA). This figure has been reproduced from ref. 57. Copyright: 2019 by the authors. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Jin and colleagues57 also used a multimodular strategy with Y. lipolytica for BA production. Their approach involved four modules: the heterogenous CYP/CPR, MVA, acetyl-CoA generation, and redox cofactor supply. Fig. 7(b) shows the multimodular strategy applied in Y. lipolytica for BA production. First, the best BA-producing strains were identified by screening the cytochrome P450 monooxygenases. Using these modules together, approximately 23.71% BA was obtained.57 The use of plants as hosts for the biosynthesis of BA has also been documented. Therefore, using the Lotus japonicus tree as the host, Suzuki and coworkers58 prepared BA with the help of the Ia bHLHs clade. They later studied the transcription regulation of BA in hydroponically cultivated Fabaceae Lotus japonicus compared to plants grown in soil. Higher levels of secondary aerenchyma (SA) and overexpression of LjbHLH50 and LjbHLH32 were observed in the hydroponically cultivated Fabaceae Lotus japonicus, which enhanced the accumulation of BA.29 The medicinal mushroom Inonotus obliquus was used to investigate the effect of fungal elicitor and oleic acid on the biosynthesis of BA from BE. Fungal elicitor and oleic acid promote the expression of squalene synthase and HMG-CoA reductase, key genes involved in BA production biosynthesis.59
2.4. Critical appraisal of chemical and biotechnological constraints hindering scalable derivatization of BA
While BA is a promising lead compound due to its potent anticancer, anti-inflammatory, and antiviral properties, large-scale chemical derivatization remains a significant challenge. This difficulty largely stems from chemical complexity and biotechnological limitations that hinder its synthetic versatility and industrial scalability. Chemically, the rigid structure of the lupane-type pentacyclic triterpenoid in BA presents significant obstacles to site-selective modifications. Although the molecule has multiple functional groups (e.g., a C-3 hydroxyl and a C-28 carboxylic acid), it lacks activated positions for easy substitution, which restricts chemical diversification without harsh reaction conditions or protection/deprotection steps.60 These issues increase costs, decrease yield, and make the synthesis of analogues more difficult on a commercial scale. Moreover, chemical derivatization often requires toxic solvents, expensive reagents, or catalysts, which may not be environmentally sustainable or aligned with the principles of green chemistry.
Regarding biotechnological constraints, enzymatic or microbial derivatization methods, which could offer greener and regioselective alternatives, are still underdeveloped for the synthesis of BA. The absence of robust biocatalysts or engineered microbial strains capable of selectively converting BA into pharmacologically enhanced derivatives remains a bottleneck. Moreover, low expression levels, substrate specificity, and poor enzyme stability in non-native hosts have hindered the application of such biocatalytic processes to industrial settings.61,62 Additionally, sourcing BA on a large scale presents another challenge. Although it can be extracted from natural sources such as birch bark or obtained through semi-synthesis from BE, these methods are limited by biomass scarcity, low extraction efficiency, and the presence of structurally similar impurities that complicate downstream processing. In summary, while BA's biological activity makes it a valuable pharmacophore, the absence of scalable, efficient, and environmentally friendly chemical or biotechnological derivatization methods remains a significant obstacle. Therefore, addressing this issue requires the development of novel catalytic systems, metabolic engineering strategies, and green extraction technologies to fully realize BA's potential for clinical use.
3. BA cancer mechanisms and treatment
Despite significant advances in cancer treatment, toxic side effects and high mortality rates still pose challenges in cancer therapy. Therefore, the search for an effective anti-tumor treatment with fewer toxic effects has gained momentum over the years. Due to its potential anti-tumor properties, BA has been tested both in vitro and in vivo on various cancer cells, successfully inhibiting tumor growth without causing toxicity. Initially, it was known for its cytotoxic effects against human melanoma cancer cells. Later, it was found to have a wide range of effects against different cancers, including but not limited to gallbladder, gastric, ovarian, breast, bladder, and colorectal cancers, with IC50 values ranging from 1 to 13.0 μg mL−1.22 The effects of BA against cancer cells have shown selectivity, although the mechanisms behind this selectivity are still unknown. Although BA is widely studied across various cancer types, its mechanisms in BC are influenced by different molecular contexts. In ER+ BC, estrogen receptor interaction with mitochondrial apoptotic regulators, such as Bcl-2 family proteins, reduces BA-induced mitochondrial depolarization, requiring higher doses or combination therapy with anti-estrogen or taxane agents. BA treatment has been shown to lower Bcl-2 expression and increase Bax, resulting in the activation of caspase-9 and caspase-3 through the mitochondrial pathway.63 Additionally, BA causes mitochondrial depolarization and apoptosis, which can be partly prevented by Bcl-2 overexpression and cyclosporin A, indicating mitochondrial involvement controlled by Bcl-2 family proteins.64,65 Although these studies do not explicitly mention estrogen receptor crosstalk, they demonstrate how regulation by Bcl-2 family proteins can weaken BA-induced mitochondrial apoptosis, implying the need for higher doses or combined treatments in ER+ cases.
In contrast, TNBC models with mutant p53 remain sensitive to BA through the GRP78-PERK-eIF2α-CHOP-mediated ER stress pathway, revealing a p53-independent apoptotic route that is less common in other cancers. BA directly targets GRP78, triggering ER stress by activating the PERK-eIF2α-CHOP apoptotic cascade, which increases apoptosis and chemosensitivity in BC models, including both MCF-7 and MDA-MB-231 cells.66 A related study also demonstrated GRP78-mediated signaling involving PERK, phosphorylation of eIF2α, and downstream effects on β-catenin/Myc and glycolysis suppression.67 Although p53 status was not always specified in this context, the ER stress-induced apoptotic mechanism mediated by GRP78 appears to be p53-independent, supporting the idea that TNBC models remain sensitive via this pathway.
Moreover, BA's ability to chemosensitize BC cells to taxanes highlights its importance in situations of drug resistance, which are especially challenging in BC. Additionally, BA synergistically makes breast cancer cells more sensitive to taxol, increasing G2/M arrest and apoptosis through GRP78-mediated ER stress.66 Adaptive responses such as SESN2-driven autophagy under hypoxic conditions further point to BA's activity in breast tumors, indicating subtype-specific vulnerabilities and resistance mechanisms. Under hypoxia, BA strongly increases SESN2 expression. Reducing SESN2 levels enhances BA-induced ROS production, DNA damage, and radiosensitivity, while decreasing autophagic flux, indicating that SESN2-mediated autophagy serves as a protective adaptive response.68
Notably, HER2+ models remain underexplored, highlighting a significant gap in future mechanistic research. One study showed that BA inhibits growth and induces apoptosis in HER2+ models, BT474, and MDA-MB-453 by downregulating Sp transcription factors, YY1, and ErbB2 through a cannabinoid receptor-dependent miR-27a-ZBTB10-Sp axis.69 This provides some emerging insights, although the underlying apoptotic or resistance-related mechanisms in HER2+ BC are still less thoroughly explored overall.
Currently, BA is regarded as a mitocan, an anticancer drug that acts on mitochondria. It increases ROS production, decreases the mitochondrial outer membrane potential (MOMP), and elevates the levels of proapoptotic proteins while inhibiting anti-apoptotic ones. This leads to the activation of p38 Mitogen-activated protein kinase (p38 MAPK), the release of cytochrome C, apoptosis-inducing factor (AIF), and a second mitochondria-derived activator of caspase (Smac) into the cytosol, while increasing the levels of Caspase-3 and Caspase-9, ultimately inducing cell apoptosis. p38 MAPK causes ROS generation, leading to direct cell death, while Caspase-3 induces apoptosis or activates poly-ADP-ribose polymerase (PARP) to promote caspase-dependent cell death.28,70,71 BA also induces cell apoptosis by acting on the p53 gene, which promotes gene expression that affects superoxide dismutase 2 (SOD2) in mitochondria, leading to apoptosis. The mitochondria-specific nuclease, endonuclease-G, then moves to the nucleus and cleaves chromatin DNA. Across BC models, BA triggers apoptosis through at least two experimentally supported axes: a direct, mitochondria-centric mechanism that can proceed even with limited BAX/BAK dependence and is modulated by permeability-transition pore inhibitors, and ER-stress/GRP78-PERK-eIF2α-CHOP signaling, which also links to mitochondrial damage via calcium flux. Importantly, ER-stress targeting of GRP78 is supported by target engagement (DARTS) and functional genetics, and BA synergizes with paclitaxel at sub-cytotoxic concentrations. It can also increase autophagic flux; knocking down SESN2 amplifies ROS, DNA damage, and radiosensitization, implying that stress-adaptive autophagy can blunt BA efficacy under hypoxia. Collectively, these data argue that BA's efficacy depends on dose/exposure, subtype/p53 status, hypoxia, and combination partners, and that mechanistic readouts strengthen claims beyond descriptive apoptosis markers.
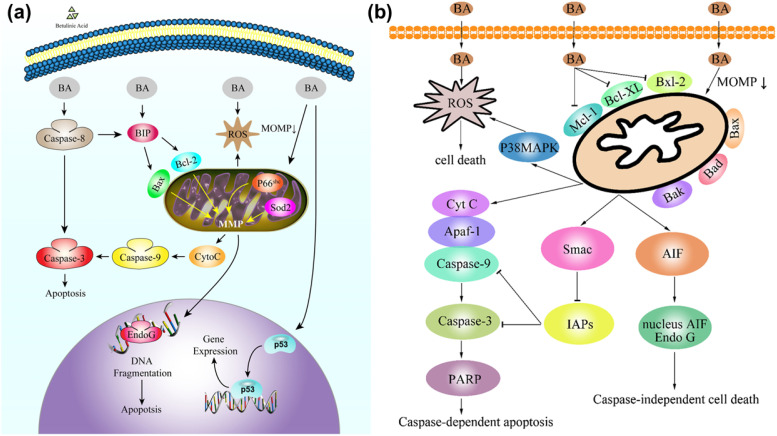
Fig. 8 shows BA's activated mitochondrial pathway in cancer cells.28,72 Combining BA with other anticancer drugs, such as actinomycin D, doxorubicin, and Taxol, induces cancer cell apoptosis through different pathways while also preventing resistance in cancer cells.73 BA also reduces doxorubicin toxicity indicators, which have strong cardiotoxic activity. This includes the generation of ROS, alteration of mitochondrial membrane potential, production of inflammatory cytokines (TNF-α or IL-12), and various histochemical and morphological changes, including apoptosis.74
Fig. 8. Apoptosis induction through the mitochondrial pathway in activated cancer cells by BA, involving (a) p53 gene and (b) Smac and AIF proteins. This figure (a) has been reproduced from ref. 70 with permission from Elsevier Science Ltd, Copyright 2016. This figure (b) has been reproduced from ref. 72. Copyright: 2021 by the authors. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
BA suppresses the signal transducer and activator of transcription (STAT) 3 signaling pathways, which are involved in angiogenesis, apoptosis, proliferation, metabolism, metastasis formation, and differentiation. In BC models, BA attenuates STAT3 signaling, which plays a central role in promoting angiogenesis, invasion, metastasis, and immunosuppression. BA's inhibition of STAT3, as well as FAK, leads to decreased expression of MMPs and elevated TIMP-2, thereby impairing cancer cell migration and invasion. A pivotal study by Zeng et al.75 demonstrated that BA impairs metastasis and modulates immunosuppressive mechanisms in BC models. The authors found that BA inhibits STAT3 activation, along with FAK, leading to systemic BA administration markedly suppressing 4T1 tumor growth and pulmonary metastases, partly through reducing myeloid-derived suppressor cells (MDSC) accumulation in tumors and lungs, thereby overcoming tumor-associated immunosuppressive barriers.75
This compound also impacts the NF-kB signaling pathway, a transcription factor that regulates cytokine production, DNA transcription, and tumor cell survival. BA prevents the activation of IKK via IKKb by reducing the expression of tumor necrosis factor (TNF), a membrane protein on the cell surface, thus inhibiting its interaction with membrane receptors. In MDA-MB-231 TNBC cells, BA suppresses NF-κB activity by reducing levels of phosphorylated p65 and p-IκBα, and by inhibiting nuclear translocation of the NF-κB/p65 complex. This interferes with downstream c-Myc and glycolytic gene expression via the caveolin-1/NF-κB/c-Myc.76
Additionally, BA can directly inhibit phosphorylation of p65, phosphorylation of IkBa, and IKK activation, as well as restrict nuclear translocation of NF-κB/p65, thereby preventing its binding to DNA. This leads to the inhibition of metastasis, angiogenesis, epithelial–mesenchymal transition, invasion, drug resistance, immunosuppression, epigenetic changes, and proliferation by decreasing the levels of proteins such as c-Myc, P21Waf1, and vasodilator-stimulated phosphoprotein (VASP), ultimately causing cancer cell death.70–72Fig. 9 illustrates the NF-kB signaling pathway in turmeric cells. Additionally, BA has demonstrated significant control over cancer growth by inhibiting the epithelial-to-mesenchymal transition (EMT), modulating DNA topoisomerase, and altering the transcription of specificity protein (Sp) transcription factors, as well as inducing autophagy.50 The changes in gene expression and cellular pathways such as PERK, phosphatase and tensin homolog deleted on chromosome ten (PTEN), SUMO1/sentrin-specific peptidase (SENP), and miR-27a in cancer cells are observed in the presence of BA. Sp inhibition reduces cancer gene expression, inhibiting cancer cell growth. It increases cell numbers in the G2/M phase, leading to cell cycle arrest. Additionally, it promotes caspase expression, inducing apoptosis in cancer cells. Furthermore, it causes DNA damage, thereby inhibiting the progression and invasion of cancer cells.72Fig. 10 illustrates the regulation of cancer cell growth by Sp transcription factors.
Fig. 9. NF-kB Inhibition by BA in cancer treatment. This figure has been reproduced from ref. 70 with permission from Elsevier Science Ltd, Copyright 2016.
Fig. 10. Regulation of the growth of the cancer cell via Sp transcription factors. This figure has been reproduced from ref. 72. Copyright: 2021 by the authors. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
3.1. Nanoparticles in BC therapy
Current cancer therapies rely on surgical methods, radiation therapy, and chemotherapy, each carrying the risk of damaging healthy tissues and failing to eliminate cancer cells. Nanotechnology offers a solution to these issues by enabling the precise and targeted delivery of chemotherapy drugs to cancer cells and tumors, thereby aiding in tumor removal and enhancing the effectiveness of radiation and other treatments. This advancement promises to reduce patient risks and increase the chances of successful outcomes survival.77 Further advancements in nanotechnology-based cancer treatment have gone beyond simply improving drug delivery to developing new options made possible by the unique properties of nanomaterials. NPs' large surface area can be customized with ligands, such as antibodies and aptamers.77 These ligands can serve both therapeutic functions and guide the NP carrier within the body. Moreover, their versatile properties enable combination drug delivery, multimodal treatment, and the integration of therapy and diagnosis, known as “theranostic” action.77 Notably, NPs used in cancer therapy include polymeric NPs, micelles, liposomes, and inorganic NPs, among others, as shown in Fig. 11, with each type possessing unique properties that make it suitable for specific applications.
Fig. 11. Some common nanomaterials and carrier types used as controlled release systems for therapeutic applications, including 0D, 1D, and 2D materials. This figure has been reproduced from ref. 78. Copyright: 2024 by the authors. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
3.2. Overview of various BA nanocarrier systems in BC
3.2.1. Polymeric NPs
Polymeric NPs for BA delivery in BC provide enhanced solubility, bioavailability, and targeted release through surface modifications and stimuli-responsive mechanisms. Despite their promise, challenges persist in formulation complexity and clinical translation. For example, Lactoferrin (Lf)-conjugated BA NPs with an average particle size of approximately 147.7 nm, ζ of −28.5 mV, and EE of 75% showed increased cellular uptake in MDA-MB-231 TNBCs. The system utilizes Lf-receptor-mediated targeting to improve selectivity and anticancer effectiveness. However, current data are limited to in vitro studies.79 Moreover, the Pillar[6]arene-based BA delivery system, measuring 124.78 nm with a ζ potential of 25.01 mV, co-loaded with glucose oxidase, enables dual GSH/ROS-triggered release, enhancing tumor selectivity and multimodal anticancer effects.80 Another study reported gelatin-based NPs functionalized with EGCD, TiO2, and RGD peptide, measuring 293.7 nm in size, with ζ = 35.76 mV and EE = 95.37%. These NPs demonstrated improved circulation and dual targeting toward tumor vasculature, although the complexity limits their clinical translation.81 However, their clinical application is limited by formulation challenges and variability in the tumor microenvironment.
3.2.2. Liposomes
Liposomes are biocompatible carriers that improve the solubility and delivery of BA in BC therapy. For example, a study on gold nanoshell-coated BA liposomes with a size of approximately 149 nm showed synergistic chemophotothermal effects, achieving about 83% tumor inhibition in vivo, and outperforming free BA.82 Additionally, folic acid-coated micelle-in-liposome systems, approximately 100 nm in size, enabled the sequential release of BA and celastrol, targeting both cancer-associated fibroblasts and tumors cells.83 Lastly, magnetoliposomes approximately 100–200 nm in size enabled remotely triggered hyperthermia-induced BA release (∼43 °C), thereby enhancing cytotoxicity in MDA-MB-231 cells. These advanced liposomal formulations enhanced therapeutic effectiveness through targeted delivery and stimulus-responsive mechanisms of release.84 However, clinical translation remains limited because of its complex formulation and dependence on external activation systems.
3.3.3. Inorganic NPs
Inorganic NPs for BA delivery in BC offer enhanced ROS generation, mitochondrial targeting, and imaging capabilities. A notable study of ZnO-BA NPs demonstrated strong ROS generation and mitochondrial damage, leading to apoptosis in BC cells.85 Furthermore, Au-BA NPs significantly lowered IC50 values by improving mitochondrial targeting and intracellular delivery accumulation.86 A different study demonstrated a manganese-oleate complex in phytantriol with pluronic F127, enabling dual imaging and therapy with high drug loading and selectivity.87 In addition, PLL-g-PEG/HAuCl4·3H2O/EGCG NPs promoted mitochondrial dysfunction and targeted cancer cells uptake.88 Lastly, BA-CS-MNPs provided magnetic responsiveness and efficient mitochondrial targeting, but their multi-component design might hinder scalability.89 Despite promising efficacy, concerns about long-term toxicity, instability, and complex synthesis restrict their clinical use.
3.3.4. Micelles
Micelles for BA delivery in BC therapy provide improved solubility, redox- or pH-responsive release, and targeted tumor delivery. Their nanoscale size and amphiphilic nature enable deep tumor penetration and controlled drug release. For example, mPEG 2K-SS-BA prodrugs combined with F68-Cypate form redox- and NIR-responsive micelles approximately 122 nm in diameter, allowing synergistic chemo-photothermal therapy through controlled BA release within the tumor's reductive environment.90 Another study shows that BA-Cys-TPGS and HA-Cys-TPGS conjugates form stable micelles approximately 134 nm in size with a high entrapment efficiency of about 98%. These micelles utilize CD44 receptor-mediated targeting through HA to enhance tumor cell uptake. The redox-sensitive disulfide bonds allow glutathione-triggered drug release specifically within the tumor microenvironment. Co-delivery of paclitaxel (PTX) with HA-Cys-TPGS micelles improves the effectiveness of combination therapy against BC.91 These systems demonstrate potent antitumor activity; however, they are limited by their complex multi-component synthesis. In summary, these micellar platforms show promise for targeted, stimuli-responsive BC treatment, warranting further in vivo validation.
3.3.5. Other NPs
Other NPs for BA delivery in BC include advanced hybrid systems that combine chemotherapy with photodynamic or immunotherapy. For example, chitosan oligosaccharide (COS) NPs co-loaded with Ce6 enhance immunotherapy synergy and enable chemotherapeutic dormancy, despite relying on effective light penetration for activation.92 Moreover, porphyrin derivative-based NPs self-assemble into redox-responsive carriers, enabling GSH-triggered dual chemo-photodynamic therapy with synergistic antitumor effects.93 Finally, CuCl2·2H2O NPs co-delivering oleic acid (OA) and Ce6 offer tumor-homing capabilities and multimodal therapy, thereby improving their accumulation and effectiveness. Despite their multifunctionality, these systems face challenges such as complex fabrication, scalability issues, and variability in the tumor microenvironment responsiveness.94 Overall, these innovative platforms show promise for integrating BA with advanced therapies, but they need further in vivo and clinical evaluation.
In summary, polymeric NPs and micelles provide versatile, stimuli-responsive platforms with high drug loading and targeted release, boosting BA's therapeutic effectiveness in BC. Although liposomes excel in co-delivery and combination therapies, they face challenges in complex formulation and scale-up. Inorganic NPs offer potent ROS-mediated cytotoxicity and imaging capabilities but raise concerns about toxicity and biocompatibility. Other hybrid NPs combine multimodal therapy and immunomodulation; however, the complexity of synthesis and tumor heterogeneity limits their application. Overall, each nanocarrier type has unique advantages and translational challenges, emphasizing the need for optimized, scalable systems tailored to tumor microenvironment dynamics. The characteristics of various BA-Loaded NPs are summarized in Table 1.
Table 1. Summary of various BA-Loaded NPs.
| Classification | Materials | Physicochemical properties | Merits | Limitations | Outcomes | Ref. |
|---|---|---|---|---|---|---|
| Polymeric NPs | Lactoferrin (Lf)-conjugated BA NPs | PS: 147.7 nm, ZP: −28.5 mV, EE 75% | TNBC targeting: Rapid, selective uptake | Only in vitro data | Showed selective uptake and apoptosis induction in MDA-MB-231 cells | 79 |
| Pillar[6]arene (co-delivery: Glucose oxidase) | PS: 124.78 nm ZP: −25.01 mV | Synergistic multimodal therapy. Tumor-microenvironment responsiveness | Tumor microenvironment heterogeneity. Complex formulation and potential scalability issues | GSH and ROS dual-responsive drug release. Enhanced combined anti-tumor effects | 80 | |
| EGCD, TiO2 NPs, gelatin | PS: 293.7 nm ZP: 35.76 mV EE: 95.37% DL:/ | Improved solubility & circulation. Dual targeting mechanism | Complexity of fabrication reliance on EPR variability | Improved bioavailability; tumor targeting. Improved anti-tumor effects | 81 | |
| Liposomes | Au nanoshell-coated BA liposomes | PS:149 nm ZP:/ | Combines chemo & photothermal therapy; strong tumor kills | Photothermal equipment required. Scale-up challenges | Combining chemotherapy and photothermal therapy, achieved ∼83% tumor inhibition in mice vs. ∼31% with free BA | 82 |
| FA-coated micelle-in-liposome system | PS: 100 nm | Sequential CAF- and tumor targeting, dual-drug release | Complex formulation and drug attribution | Sequential release enhances CAF targeting and induces robust suppression of apoptosis & metastasis in vivo | 83 | |
| Magnetoliposomes | PS: 100–200 nm | Remote hyperthermia-triggered release, dual-action | Requires an external magnetic field/hyperthermia | Hyperthermia-triggered release at ∼43 °C, enhancing cytotoxicity in MDA-MB-231 under thermal stimulus with minimal effect on normal cells | 84 | |
| Inorganic NPs | ZnO-BA NPs | PS: 40–100 nm EE: 80% | ROS generation, selective cytotoxicity | Long-term toxicity risk | ROS-mediated apoptosis in BC models | 85 |
| Au-BA NPs | PS: <100 nm | Mitochondrial targeting. Low off-target toxicity | Limited scale-up/biocompatibility detail; limited cell lines | Targeted; IC50 ∼13 μM vs. ∼54 μM for free BA. | 86 | |
| Manganese-oleate complex, phytantriol, pluronic F127 | PS: 195 ± 4 nm ZP: −18 ± 3 mV EE: 96 ± 3% DL:/ | Cancer-selective cytotoxicity via ROS generation. Dual imaging and therapeutic functionality | Translational barriers remain. Complex nanostructure fabrication | Enhanced cytotoxicity; selective BC imaging; synergistic anti-tumor activity | 87 | |
| PLL-g-PEG, HAuCl4·3H2O, EGCG | PS: 119.2 ± 3.5 nm ZP: 23.4 ± 0.5 mV EE:/DL: 21.0 ± 0.7% | Induction of mitochondrial dysfunction and apoptosis. Enhanced mitochondrial targeting & potency | Complex multi-component fabrication. Potential nanoparticle toxicity & accumulation | Mitochondrial-targeted delivery; improved cytotoxicity | 88 | |
| BA-CS-MNPs | PS: 23.6 nm ZP: 25.8 mV EE:/DL:/ | Improved dispersion & cellular uptake. Selective cytotoxicity | Scale-up and reproducibility concerns. Limited mechanistic and in vivo data | Enhanced cytotoxicity BA and BA–CS–MNP nanocomposite exhibited cytotoxic- ity in MCF-7 cells in a dose-dependent manner with IC50 values of 2 and 3.6 μg mL−1, respectively | 89 | |
| Micelles | mPEG 2K-SS-BA prodrugs, F68-Cypate | PS: 122.2 nm ZP: 0.2 mV EE:/DL: 7.1% | Pronounced chemo-photothermal synergy & high PTT efficiency. Redox-triggered targeted BA release & NIR photothermal conversion | Complex polymeric prodrug synthesis. Therapeutic efficacy is dependent on tumor redox and irradiation accessibility | Redox/NIR dual-response drug release. Synergistic thermo-chemotherapy anti-tumor efficacy | 90 |
| BA-Cys-TPGS conjugate, HA-Cys-TPGS conjugate (co-delivery: PTX; targeting moiety: HA) | PS: 134.13 ± 2.61 nm ZP: −25.37 ± 1.46 mV EE: 98.09 ± 4.06% DL: 19.41 ± 1.54% | CD44-mediated tumor targeting and enhanced uptake. Synergistic redox-triggered combination therapy | Complex multi-component. Formulation dependence on intratumoral GSH levels | Tumor targeting; redox-responsive drug release. Enhanced synergistic anti-tumor activity | 91 | |
| Other NPs | COS (co-delivery: Ce6) | PS: 182 nm ZP: −5.38 mV EE:/DL:/ | Enhanced immunotherapy synergy. Chemotherapeutic dormancy for controlled release | Complex multi-component synthesis dependency on light penetration | pH-responsiveness; enhanced synergistic anti- tumor activity | 92 |
| Porphyrin derivative | PS: 91.62 nm ZP: −22 mV EE:/DL:/ | Self-assembly into functional NPs. Redox-triggered dual action | Limited evidence for in vivo translation. Dependence on intracellular GSH levels | GSH-responsive drug release; synergistic chemo- and photodynamic therapy | 93 | |
| CuCl2 2H2O (co-delivery: OA, Ce6) | PS: 174.5 ± 5.3 nm ZP: −31.7 mV EE:/DL:/ | Synergistic multi-modal therapy. Tumor-homing & enhanced accumulation | Complex fabrication & scalability challenges. Tumor heterogeneity & redox variability | Tumor targeting, synergistic anti-cancer activity | 94 |
3.3. Stability issues for polymeric NPs and micelles
Polymeric NPs and micelles are extensively studied as DDS due to their customizable size, controlled release capabilities, and ability to encapsulate hydrophobic drugs. These carriers have become promising platforms to address the low solubility and poor bioavailability of BA. However, their stability in physiological conditions remains a key challenge for translation. A major issue is the hydrolytic breakdown of polymers such as poly(lactic-co-glycolic acid) (PLGA) and poly(ε-caprolactone) (PCL). Notably, polymeric NPs, often made from these biodegradable polymers, are vulnerable to this breakdown because they undergo ester bond hydrolysis in aqueous environments, leading to early drug leakage and unpredictable release profiles.95 This degradation not only causes premature drug leakage but also can change particle size and surface charge, which can compromise circulation stability and drug release behavior.95
Similarly, polymeric micelles formed from amphiphilic block copolymers may dissociate upon dilution in blood due to their low critical micelle concentration (CMC), leading to rapid drug release and reduced therapeutic effectiveness.96,97 Additionally, protein adsorption and opsonization can destabilize nanoparticles by causing aggregation, opsonic clearance, and changes in biodistribution.98 Shelf-life stability is also limited because hydrolysis and oxidation during storage can break down polymers and decrease drug encapsulation efficiency. For micelles, freeze-drying or lyophilization is often required, but this process can cause destabilization or incomplete redispersion.99 Furthermore, in vivo physiological stressors such as pH fluctuations, ionic strength, and enzymatic activity can speed up polymer degradation or micelle destabilization, further affecting pharmacokinetics and bioavailability.
Strategies such as PEGylation, crosslinking of micellar cores and shells, and optimizing polymer chemistry have been investigated to enhance circulation stability and shelf life.100 Therefore, although polymeric NPs and micelles show promise, overcoming stability-related issues remains crucial. Comprehensive long-term stability and shelf-life studies of BA-loaded NPs and micelles are limited, highlighting the need for thorough evaluation before successful clinical use. Beyond traditional polymeric NPs and micelles, advanced nanocarriers such as stimuli-responsive micelles and hybrid polymer–lipid systems have been created to tackle drug delivery problems. However, their stability still poses a significant challenge that requires innovative design solutions.101,102
To better understand the stability challenges of polymer-based nanocarriers, it is essential to differentiate between the issues faced by polymeric NPs and polymeric micelles. Although both systems improve the solubility and bioavailability of hydrophobic drugs, such as BA, they each have unique limitations under physiological and storage conditions. A comparison of these stability issues, their causes, and potential solutions is presented in Table 2, providing a clear framework for evaluating the suitability of polymeric and advanced carriers in BC drug delivery.
Table 2. Overview of stability issues, their underlying causes, and potential solutions.
| Carrier system | Material | Stability issues | Causes | Strategies for improvement | Ref. |
|---|---|---|---|---|---|
| Polymeric NPs | PLGA | Hydrolysis → premature drug leakage; reduced shelf-life | Ester bond hydrolysis in aqueous/physiological media; enzymatic degradation | Polymer chemistry tuning; surface PEGylation; controlled crystallinity | 95, 98 and 100 |
| PCL | Aggregation & opsonization | Protein corona formation, ionic strength effects | PEGylation; surface charge optimization; zwitterionic coatings | ||
| PEGylated polymers | Oxidative degradation during storage | Reactive oxygen species (ROS), light/temperature exposure | Antioxidant incorporation; dark/cold storage; lyophilization | ||
| Polymeric micelles | PEG-PLA | Dissociation upon dilution in blood; rapid drug release | Low critical micelle concentration (CMC) | Core/shell crosslinking; higher molecular weight block copolymers | 96, 97 and 99 |
| PEG-PCL | Instability in pH/enzymatic environments | Protonation/deprotonation of functional groups; enzymatic cleavage of polymer backbone | pH-responsive stabilization; covalent crosslinking | ||
| PEG-PPO | Poor redispersion after freeze-drying (lyophilization stress) | Dehydration-induced micelle collapse | Cryoprotectants (e.g., sugars, trehalose); optimized lyophilization protocols | ||
| Stimuli-responsive micelles | Sensitive to premature activation in non-target tissues | Risk of off-target BA release | Use of cleavable linkers (disulfide, hydrazone); tumor-microenvironment triggered release | 101, 103 and 104 | |
| Hybrid polymer-lipids NPs | Limited miscibility between lipid and polymer phases; instability at physiological temperature | Phase separation; inconsistent BA release | Incorporation of stabilizing lipids (DSPC, cholesterol); surface PEGylation | 102, 105 and 106 |
3.4. Properties of BC drugs
Therapeutic drugs for BC available commercially can be categorized as hydrophilic or hydrophobic based on their water solubility. Additionally, they can be classified as either highly charged or neutral based on their electrostatic properties. When designing NPs to carry specific drug types, understanding the drug's behaviors and characteristics is essential to achieve optimal encapsulation and desired release features. Functionalized or tailored NPs are especially promising in drug delivery because of their unique sizes, adjustable surface properties, and controlled release capabilities. Hydrophilic drugs play a vital role in treating BC subtypes and include both large molecules and various small compounds. Most therapeutic drugs used in clinics for BC are hydrophobic, which continues to present challenges for effective delivery to the target.107
Existing BC treatments face several challenges, including a lack of targeted toxicity, which reduces treatment effectiveness and hampers medical diagnosis. These treatments harm healthy tissues, forcing the use of lower doses of anticancer drugs to minimize this toxicity.108 Compared to tumor sites, normal organs often receive 10–20 times more drug deposits. Additionally, most chemotherapy drugs cannot diffuse beyond 40–50 mm from blood vessels, which can lead to multidrug resistance (MDR) and treatment failure. When tumor cells develop MDR after exposure to one anticancer drug, they may become resistant to multiple drugs due to increased expression of drug-removal mechanisms proteins.109 Technological advances in cancer treatment have significantly improved standard therapeutic strategies for BC, leading to lower mortality rates and helping many patients in their battle against cancer recovery.110 However, challenges remain in treating BC with current therapeutic approaches. As a result, there is an urgent need for innovative new therapies to effectively manage BC and address the critical medical needs of patients. Fig. 12 lists the FDA-approved drugs currently available on the market for BC treatment.
Fig. 12. Several FDA-approved drugs are currently available to treat BC. This figure has been reproduced from ref. 78. Copyright: 2024 by the authors. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
3.5. Potential of BA as BC therapy
Several studies have documented the effects of BA as a single-agent treatment against different BC cell lines. These studies have demonstrated that BA can inhibit the growth and spread of BC cells through multiple mechanisms. A notable study by Qi et al.111 enhanced the anticancer activity of BA against BC via a copolymer-based micelles delivery system. As shown in Fig. 13(a), Soluplus micelles exhibited low cytotoxicity toward MDA-MB-231 cells, indicating that they function as non-toxic drug delivery vehicles. Both Soluplus-BA and BA inhibited the proliferation of MDA-MB-231 cells, with IC50 values of 38.81 ± 4.9 mg mL−1 for free BA and 15.45 ± 3.01 mg mL−1 for Soluplus-BA micelles. At the same BA concentration, the inhibitory effect of Soluplus-BA was more prominent, likely due to enhanced cellular uptake of Soluplus-BA micelles compared to BA. The colony formation results further supported this trend, as Soluplus-BA showed a greater reduction in clone formation of MDA-MB-231 cells than BA, as seen in Fig. 13(b). According to the MTT assay results, Soluplus-BA micelles enhanced the inhibitory effect of BA on MDA-MB-231 cells, primarily because of increased levels of ROS and decreased MOMP. As demonstrated in Fig. 13(c), after 5 minutes of treatment, the green fluorescence was more intense compared to cou6, indicating that Soluplus-cou6 micelles were quickly taken up by MDA-MB-231 cells. These results suggest that Soluplus-BA micelles increase ROS production in MDA-MB-231 cells. Additionally, JC-1 assays showed that Soluplus-BA significantly reduced MMP in MDA-MB-231 cells, implying that Soluplus-BA may exert anti-tumor effects through ROS-mediated mitochondrial apoptosis, as shown in Fig. 13(d). Moreover, the in vivo study demonstrated that Soluplus-BA had improved antitumor effects and inhibited angiogenesis, highlighting its potential as an effective anti-BC drug delivery.111
Fig. 13. Soluplus-BA micelles inhibit the proliferation of MDA-MB-231 cells. (a) MDA-MB-231 cells were treated with Soluplus, BA, and Soluplus-BA micelles for 48 hours; cell viability was assessed using the MTT assay. (b) MDA-MB-231 cells were seeded in 6-well plates and treated with BA and Soluplus-BA for 48 hours. After replacing the media with fresh culture medium, the cells were cultured for an additional 10 days and stained with crystal violet. (c) Cell uptake assay. MDA-MB-231 cells were treated with free cou6 and Soluplus-cou6 micelles for 3, 5, and 15 minutes. (d) Soluplus-BA micelles trigger a ROS-mediated mitochondrial apoptosis pathway. MDA-MB-231 cells were treated with Soluplus-BA and BA for 48 hours. After treatment, cells were stained with DCFH-DA for 20 minutes or JC-1 for 30 minutes and photographed under a fluorescence microscope. This figure has been reproduced from ref. 111. Copyright: 2021 by the authors. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Another study reported the encapsulation of BA into PLGA NPs to improve the compound's solubility and therapeutic effectiveness against breast tumor cells. The in vitro results showed increased anti-proliferative effects on MCF-7 BC cells, indicating the potential of this formulation for breast cancer treatment. Further research also revealed that the PLGA-BA formulation exhibited promising anticancer effects against the A549 lung cancer cell line. These findings highlight the significant potential of this approach, and further investigation is needed to understand how PLGA-BA nano-formulation distributes in the human body and the specific mechanisms by which it inhibits tumor cell growth. These efforts provide a crucial theoretical foundation for future advancements and improvements in this vital field study.112 In a different approach, BA was functionalized with various electrophilic groups, producing 23 BA derivatives. Most of these derivatives exhibited improved antiproliferative activity against MCF-7 and MDA-MB-231 cells, as indicated by the MTT assay results. Notably, compound 15b demonstrated excellent activity with an IC50 of 1.09 μM against MCF-7 cells. The study further revealed that compound 15b suppressed the migration and clone formation of MCF-7 cells while inducing apoptosis, autophagy, and cell cycle arrest at the G2/M phase. It also facilitated the generation of intracellular ROS. Lastly, this compound inhibited tumor growth in the BC xenograft mouse model, demonstrating a 36% reduction in tumor size while maintaining the mouse's overall health and weight.113
In the study by Saneja et al.,114 BA-loaded PLGA-mPEG NPs were developed to enhance BA's half-life and therapeutic effectiveness. This formulation had a particle size of less than 200 nm with a spherical-like shape. The formulation demonstrated increased cytotoxicity in the MCF7 and PANC-1 cell lines compared to BA. Additionally, the in vivo test showed that the formulation had superior antitumor efficacy compared to free BA. These loaded NPs can serve as a promising drug carrier for delivering BA. The animals treated with the formulation showed no adverse effects on their blood or metabolic health, indicating its potential as a safe and effective delivery system method.114
Findings from a recent study revealed that BA suppressed aerobic glycolysis in MCF-7 and MDA-MB-231 BC cell lines by reducing lactate production, glucose consumption, and the extracellular acidification rate (ECAR). Additionally, it decreased the expression of proteins associated with aerobic glycolysis, including c-Myc, lactate dehydrogenase A (LDH-A), and phosphorylated/p-PDK1/PDK1 (pyruvate dehydrogenase kinase 1). Mechanistic studies confirmed Caveolin-1 (Cav-1) as a key target of BA in reducing aerobic glycolysis. BA administration increased Cav-1 levels; however, silencing Cav-1 eliminated BA's effect. Further research showed that BA suppresses aerobic glycolysis in BC cells through regulation of the Cav-1/NF-κB/c-Myc pathway. BA inhibited BC growth and glycolytic activity in both the transgenic MMTV-PyVT+/− model and the zebrafish xenotransplantation model without causing adverse effects.115
Another notable study reported that BA derivatives treat human BC using MBA-MD-231 and T47D cells, with 3-O-(E)-p-coumaroylbetulinic acid (CB) resulting in dose- and time-dependent inhibition of cell viability and G0/G1-phase cell cycle arrest. A noticeable decrease in the expression of cyclin D1 and its associated enzyme cyclin-dependent kinase 2 was observed, along with an increase in the cyclin kinase inhibitor p21, active during the G1 phase of the cell cycle. CB treatment induced early apoptosis in BC cells, evidenced by increased levels of cleaved caspase-3, decreased expression of Bcl-2 and survivin, a surge in ROS, and disruption of MOMP.116
A similar study reported on a distinct BA derivative aimed at enhancing the drug's pharmacological properties, with fluorine introduced as a single atom at C-2, resulting in two diastereomers, or in a trifluoromethyl group at C-3. An evaluation of the antiproliferative activity of these groups against five human tumor cell lines was performed. 2-F-BA (compound 6) was significantly more antiproliferative than its diastereomer counterparts. It was also shown that 2 F-BA acts as a dual inhibitor of topoisomerase (Topo) I and IIα in both cell-based and cell-free systems assays.117 These findings highlight the potential of BA derivatives as effective anticancer agents with enhanced pharmacological properties and tumor-suppressing activity. Fig. 14 shows the % cell viability of MCF-7 and MCF-10A in relation to 2-F-BA and free BA.
Fig. 14. Compound 6 inhibited BC cell viability and colony formation. (a) MCF-10A cells were less sensitive to 6 treatments. MCF-7 and MCF-10A cell lines were incubated with specified concentrations of compound 6 for 72 hours. An MTT assay was conducted to assess cell viability. (b) Compound 6 suppressed the colony formation of MCF-7 cells. (c) The data are presented as mean ± SEM from three independent experiments compared to the control. This figure has been reproduced from ref. 117 with permission from Elsevier Science Ltd, Copyright 2019.
While BA exhibits cytotoxic effects on various BC cell lines and has shown tumor-suppressing potential in preclinical studies, it is not currently used as a standalone therapy for the treatment or prevention of BC in clinical settings. The current evidence suggests that BA and its derivatives could be promising as single-agent therapies for BC (Table 3). However, additional research, including clinical trials and improved formulation strategies, is necessary to confirm its potential as an effective treatment option.
Table 3. The inhibitory effects of BA and its derivatives on BC.
| Nanoparticles | Physico-chemical characteristics | Type of study | Cell line/Animal | Outcomes | Ref. |
|---|---|---|---|---|---|
| PVCL-PVA-PEG | PS: 54.77 ± 1.26 nm | In vitro, in vivo | MDA-MB-231 cells, mice | The exposure to BA resulted in a heightened inhibitory impact on MDA-MB-231 cells, primarily caused by the increased accumulation of ROS and the degradation of MMP. Inhibitory effect on angiogenesis | 111 |
| ZP: −1.78 ± 0.78 mV | |||||
| EE: — | |||||
| DL: — | |||||
| PLGA-BA NPs | PS: 196 ± 6.80 nm | In vitro | MCF-7 cells | Enhanced anti-proliferative efficacy against MCF-7 breast cells | 112 |
| ZP: −9.25 ± 0.133 | |||||
| EE: 83 ± 9.24% | |||||
| DL: 7.0 ± 0.4% | |||||
| BA derivative (compound 15b) | — | In vitro, in vivo | MCF-7 and MDA-MB-231 cells | Inhibited tumor growth in BC xenograft mouse | 113 |
| PLGA-mPEG NPs | PS: 147.86 ± 7.68 nm | In vitro, in vivo | MCF7 cells, mice | No hemato- logical and biochemical toxicity. BA NPs exhibited greater antitumor efficacy compared to free BA. | 114 |
| ZP: −5.85 ± 0.51 | |||||
| EE: 79.18 ± 5.76% | |||||
| DL: 9.89 ± 0.72% | |||||
| BA | — | In vitro, in vivo | MCF-7 and MDA-MB-231 cells | The viability of MCF-7 and MDA-MB-231 cells decreased in a time- and dose-dependent manner following BA treatment. BA inhibits aerobic glycolysis activity in BC via modulating the Cav-1/NF-κB/c-Myc pathway | 115 |
| 3-O-(E)-p-Coumaroylbetulinic acid (CB) | — | In vitro | MDA-MB-231, MCF-7 and T47D cells | CB possesses anticancer activity in BC cells and suppresses self-renewal ability in the mammosphere | 116 |
| Fluorinated betulinic acid | — | In vitro | MDA-MB-231 and MCF-7 cells | Compounds 6 (2 F) and 10 (3-CF3) exhibited enhanced antiproliferative effectiveness compared to native BA. | 117 |
3.6. Combination therapy of BA with other anticancer agents in combating BC
Combination therapy using BA and other anticancer agents has become a promising approach to boost treatment effectiveness against BC by harnessing synergistic effects to improve outcomes and potentially lower side effects. Several studies have investigated the combination of BA with other drugs to enhance its efficacy against various cancers. These combinations aim to exploit synergy, increase bioavailability, and overcome resistance. A study by Saikia et al.118 reported on co-encapsulating BA and docetaxel (DTX) into PLGA NPs, offering a novel method to address limitations related to their individual use. The study leverages the complementary actions of both drugs through a single delivery system. Although more in vivo research on these NPs is needed to confirm their therapeutic potential in cancer, this study demonstrated that PLGA-based DTX-BA-NPs, created using a double-emulsion solvent evaporation method, hold promise for improved delivery of DTX and BA in cancer treatment.118
The initial study showing the anti-proliferative effects of BA combined with taxanes was reported by Wróblewska-Łuczka et al.,119 indicating a synergistic interaction between BA and PTX, as well as BA with DTX. Moreover, co-delivering multiple anti-tumor drugs using polymeric NPs enhances synergistic therapeutic effects. Saneja et al.120 used PLGA-mPEG NPs as carriers to co-encapsulate gemcitabine (GEM) and BA, forming combination NPs of GEM and BA. These NPs significantly lowered the IC50 value against PANC-1 cells and showed markedly higher anti-tumor efficacy than the free drugs.120
Specifically, the integration of BA with other anticancer agents has gained increasing attention as a strategy to improve BC treatment, aiming to boost efficacy while reducing toxicity. Compared to traditional chemotherapeutics, BA can selectively target and induce apoptosis in cancer cells, leaving normal cells unaffected, which suggests a potential therapeutic window. Recent advances in nanotechnology-based delivery systems have enabled the simultaneous delivery of multiple chemotherapeutic agents directly to various metabolic pathways within tumor cells, offering innovative methods to enhance both effectiveness and safety. One study examined the synergistic effects of BA and paclitaxel (PTX), a commonly prescribed chemotherapeutic for BC. The researchers developed PTX-BA nanosuspensions, which overcome solubility issues and produce synergistic effects on BC through their respective anti-tumor mechanisms. In vivo studies showed that PTX-BA-NP achieved the most significant tumor suppression among the four formulations tested: PTX-BA-NP, PTX-NP, Taxol®, and BA NP. This approach, combining two chemotherapeutic drugs, indicates that PTX-BA-NP holds great promise for BC therapy.121Fig. 15 shows an image of tumor samples taken at the end of the experiment and demonstrates that each formulation group had a significant reduction in tumor growth compared to the saline control.
Fig. 15. Demonstrates the morphology of the harvested tumors after administration of Taxol®, PTX-NP, BA-NP, PTX-BA-NP, or control (saline) (means ± SDs, n = 3). *p < 0.05, **p < 0.01. This figure has been reproduced from ref. 121 with permission from Elsevier Science Ltd, Copyright 2019.
In a similar study, research was conducted to determine whether particle size influences cellular internalization, tissue distribution, and bioavailability of BA nanosuspensions (BA/NSs), as well as the combined effects of BA/NSs and Taxol® on BC. BA/NSs with varying particle sizes—160, 400, and 700 nm—were developed using a universal green technology. A combination of BA/NS with a particle size of 160 nm and Taxol® was selected to enhance synergistic effects and reduce side effects; therefore, the particle size was adjusted to alter the uptake pathway. The BA/NS with a 160 nm particle size was expected to increase the BA release rate and improve bioavailability compared to larger particles. The combination chemotherapy was found to be safe, indicating that it could improve treatment outcomes while minimizing adverse effects. The potential of combining BA/NSs with Taxol® is promising, and further research on such combination treatments for various cancers is expected to yield therapeutic benefits. This will guide the future optimization of nanoparticle (NP) size and treatment strategies BC.122Fig. 16 shows that combining BA/NSs with Taxol® significantly increased cytotoxicity in 4T1 cells. The cytotoxicity grew as the particle sizes of the BA/NSs decreased. The apoptosis rates for all the formulations were higher than those of free BA and Taxol®, respectively. These results are consistent with the cytotoxicity assay, which indicated that concurrent administration resulted in greater levels of cytotoxicity and apoptosis.
Fig. 16. Cytotoxicity of 4T1 cells treated with various formulations (a) and the percentage of induced apoptosis (b). This figure has been reproduced from ref. 122 with permission from Elsevier Science Ltd, Copyright 2020.
A study by Weber et al.123 examined the combined effects of andrographolide (Andro) and BA against triple-negative breast cancer (TNBC). The combination increased the percentage of cells in G1 and G2 phases, with a higher proportion in the G2 phase, indicating that BA, which has higher activity, may primarily cause G2 arrest rather than Andro at the tested concentrations. The combination of Andro and BA showed strong effectiveness and significant potential in reducing cancer proliferation, inflammation, and angiogenesis.123 The inhibitory effects of BA and other anticancer agents against BC are summarized in Table 4. These findings suggest that combining BA with other anticancer agents may be a promising strategy for treating breast and other cancers, potentially overcoming the limitations of single-agent therapies.
Table 4. The inhibitory effects of BA and other anticancer agents against BC.
| Nanoparticles | Physico-chemical characteristics | Type of study | Cell line/Animal | Outcomes | Ref. |
|---|---|---|---|---|---|
| PTX-BA NPs | PS: 282.54 ± 5.4 nm | In vitro, in vivo | MCF-7 cells, nude mice | PTX-BA-NP achieved the strongest tumor inhibition | 121 |
| ZP: −19.7 ± 0.19 mV | |||||
| EE: — | |||||
| DL: — | |||||
| BA/NS-taxol | PS: 163.7 ± 0.3 nm | In vitro, in vivo | 4T1 cells | The synergistic effects of the combined chemotherapy showed good safety and enhanced treatment with minimal toxicity | 122 |
| ZP: −20.9 ± 0.2 mV | |||||
| EE: — | |||||
| DL: — | |||||
| Andro and BA | — | In vitro | MDA-MB-231 and MDA- MB-468 cells | Increase the percentage of cells in G1 and G2 | 123 |
3.7. Limitations in translational evidence: absence of in vivo pharmacokinetics
BA has shown significant anticancer potential against multiple BC models, such as 4T1, MDA-MB-231, and MCF-7 cell lines, through various mechanisms. However, the lack of comprehensive in vivo pharmacokinetic data remains a major obstacle in translating these findings clinically. Cai et al.124 reported that BA directly targets GRP78, triggering ER stress-mediated apoptosis in BC cells; however, their work was limited to in vitro models, providing little insight into BA's systemic behavior without thorough in vivo pharmacokinetic data. Similarly, Qi et al.111 developed copolymer-based micelles to improve BA delivery, demonstrating enhanced cytotoxicity and stability in vitro. Nonetheless, they did not perform complete pharmacokinetic studies to determine whether these improvements result in increased bioavailability or tumor accumulation in vivo. Additionally, Mertens-Talcott et al.125 employed both in vitro and in vivo approaches, demonstrating that BA suppresses ER-BC growth by modulating Sp transcription factors and the microRNA-27a:ZBTB10 signaling pathway. Their animal studies confirmed tumor growth inhibition; however, they did not analyze detailed pharmacokinetic parameters, such as distribution, clearance, or metabolism. Similarly, Zeng et al.126 provided in vivo evidence that BA reduces metastasis and modulates the tumor immune microenvironment, while Damle et al.127 demonstrated its anticancer activity in MCF-7 xenografts.
Saneja et al.114 reported that long-circulating NPs improved the solubility, stability, and anti-tumor efficacy of BA; however, comprehensive in vivo pharmacokinetic studies on bioavailability, biodistribution, tumor penetration, and systemic safety are still lacking for clinical translation. A summary of preclinical BC models evaluating BA and BA-loaded formulations—including dosing, outcomes, and pharmacokinetic/toxicity notes—is shown in Table 5. Although these studies demonstrate BA's efficacy in animal models, none systematically assess comprehensive pharmacokinetics, such as bioavailability, tumor penetration, or off-target toxicity. This gap emphasizes the disconnect between in vitro promise and in vivo applicability, highlighting the need for rigorous pharmacokinetic and biodistribution studies to determine whether novel BA formulations can overcome their poor solubility and stability to reach clinical relevance. We found no BA BC studies involving rabbits or nonhuman primates. These gaps should temper clinical extrapolations and encourage future work to include co-primary PK endpoints, such as plasma/tumor BA and off-target exposure.
Table 5. Some preclinical in vivo studies of BA in BC models.
| Formulation | Species & model | Dose & administration | Treatment duration | Off-target toxicity reported | PK/tumor penetration measured | Efficacy readouts | Key mechanistic notes | Major gaps | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Free BA ± paclitaxel | Nude mice; breast cancer xenografts | Doses per paper (taxane combo; BA given systemically) | 24 days | Limited | No BA PK; no intratumoral BA | BA chemosensitizes to paclitaxel; ↑ ER-stress (GRP78-PERK axis), ↑ apoptosis; in vivo validation of synergy | Mechanistic target: GRP78; ER-stress pathway | The combo benefit lacks exposure–response. No safety phasing | 124 |
| Unformulated BA (suspension for oral gavage) | Female athymic nude mice; MDA-MB-231 subcutaneous | 20 mg kg−1, oral, every other day, 20 days | 25 days | Not described beyond general observation | No plasma BA, no tumor BA; no biodistribution | ↓ tumor growth; modulation of Sp1/Sp3/Sp4 and miR-27a:ZBTB10 axis in tumors | Links efficacy to the down-regulation of sp TFs in vivo | Lack of exposure data prevents PK/PD linkage; no penetration or metabolism data | 125 |
| Soluplus® polymeric micelles encapsulating BA | Nude mice; MCF-7 | Dose per paper (systemic) | 15 days | Not detailed | No direct BA plasma/tumor levels; rely on efficacy | Greater tumor growth inhibition vs. free BA; anti-angiogenic effect in vivo | Formulation addresses solubility; improves in vivo activity | Missing PK/PD: No C_max/AUC; no tumor distribution; shelf-life/stability not tied to in vivo | 111 |
| Free BA | BALB/c mice; 4T1 orthotopic/metastatic model | 10 mg kg−1, i.p., schedule per text | 11 days | No systemic toxicity panel reported | No plasma/tissue BA; no tumor penetration | ↓ lung metastases; ↓ MDSCs; ↑ CD8+ T cells; improved survival | Anti-metastatic + immune remodeling | No PK; route (i.p.) complicates translation; no off-target exposure assessment | 126 |
| Free BA | Athymic nude mice; MCF-7 xenograft | Dose/route per paper (systemic) | 10–15 days | Not reported | No PK; no penetration | ↓ tumor volume; histologic apoptosis | Confirms ER+ efficacy signal | Lacks exposure, metabolism, and safety panels | 127 |
| PLGA-mPEG- BA NPs | Swiss albino male mice; MCF7 | 10 mg kg−1; intravenous | 13 days | No systemic toxicity observed | NPs formulation prolonged circulation and increased the apparent half-life by ∼7.2-fold relative to free BA; not directly measured | Enhanced tumor growth inhibition in vivo | Mitochondrial pathway activation/MMP collapse | Lack of detailed tumor biodistribution/penetration data | 114 |
4. Challenges in the conventional delivery of BA against BC
Despite its promising pharmacological profile, the clinical translation of BA is significantly limited by challenges related to its conventional delivery methods. One major issue is BA's poor water solubility, which substantially restricts its dissolution and bioavailability in the bloodstream. This pharmacokinetic limitation not only reduces its therapeutic effectiveness but also requires high doses, potentially increasing the risk of systemic toxicity. In addition to solubility problems, low bioavailability and rapid metabolic clearance further hinder BA's therapeutic use. This inherent pharmacokinetic drawback of BA is well documented in the literature, highlighting the urgent need for innovative strategies to address its solubility and bioavailability issues. Several studies have shown that BA's low water solubility (<0.02 mg mL−1) and poor oral absorption are key barriers to reaching therapeutic plasma levels.23,128 Furthermore, its high lipophilicity (log P > 6) leads to poor gastrointestinal dissolution and quick hepatic metabolism, resulting in a short systemic half-life and insufficient tumor targeting. As a result, researchers are actively exploring advanced drug delivery systems, such as NPs, liposomes, and polymeric micelles, to improve solubility, stability, and controlled release BA.129,130
Moreover, following oral administration, BA undergoes extensive first-pass metabolism in the liver, resulting in inactive metabolites and a significant reduction in its half-life. This extensive liver metabolism is a major factor in BA's limited systemic exposure and therapeutic effectiveness after oral intake. Several studies have shown that BA is quickly metabolized by phase I and II enzymes, producing hydroxylated and conjugated derivatives with decreased cytotoxic activity. This rapid biotransformation considerably lowers the bioactive concentration of the compound in the bloodstream, highlighting the need for alternative delivery methods or formulation strategies to bypass the hepatic first-pass effect and extend systemic circulation retention.131 As such, the development of drug delivery systems that enhance lymphatic absorption or protect BA from enzymatic degradation remains a promising avenue to improve its pharmacokinetic profile.
The compound is also subject to efflux by P-glycoprotein transporters, which limits its buildup inside target cancer cells. Another significant issue is the lack of tumor-targeting specificity in traditional formulations. BA, when given in free form, distributes non-selectively throughout the body, decreasing the drug's accumulation at the tumor site and reducing its effectiveness. These factors together hinder the therapeutic potential of BA and pose significant challenges to its clinical use. The role of P-glycoprotein (P-gp) in actively transporting BA out of cancer cells has been shown, contributing to multidrug resistance and further lowering intracellular drug concentration.132,133 Furthermore, the lack of intrinsic tumor-targeting mechanisms in traditional BA formulations results in suboptimal delivery of the drug to cancerous tissues and increased distribution to healthy organs.23 While BA has demonstrated selective toxicity to cancer cells in vitro, its nonspecific biodistribution in vivo remains a challenge for effective treatment.86 As a result, developing advanced delivery systems, such as ligand-functionalized NPs or receptor-mediated targeting platforms, is essential to improve tumor specificity and reduce off-target effects.
Cellular uptake presents another challenge, as the passive diffusion of BA across cellular membranes is inefficient due to its large molecular size and lipophilic nature. This restricts the intracellular delivery of BA to BC cells, which is crucial for triggering apoptosis and promoting anti-tumor activity. These challenges underscore the need for delivery systems that can enable efficient intracellular transport and shield BA from premature degradation within the tumor microenvironment. The poor passive permeability of BA, caused by its bulky pentacyclic triterpenoid structure and high lipophilicity, has been linked to subpar cellular uptake in BC cells.133 Furthermore, the acidic and enzymatically active tumor microenvironment can speed up BA degradation, reducing its pharmacological activity before it reaches intracellular targets. As a result, new delivery strategies such as nanocarriers, pH-responsive systems, and enzyme-inhibiting formulations are being investigated to improve BA's intracellular accumulation, stability, and therapeutic effectiveness in BC treatment.134
From a formulation standpoint, the dose-dependent toxicity and limited versatility of BA in formulations restrict its integration into standard dosage forms. These formulation challenges have hindered BA's clinical development, highlighting the need for more adaptable and safer delivery systems. The high doses required for therapeutic effectiveness not only raise concerns about systemic toxicity but also make it difficult to achieve uniform dispersion in traditional forms such as tablets or injectable solutions.135
Moreover, the physicochemical properties of BA, especially its low water solubility, high lipophilicity, and tendency to form crystals, restrict its compatibility with hydrophilic carriers and excipients commonly used in pharmaceutical formulations.136,137 To overcome these limitations, research has increasingly turned to advanced drug delivery systems such as solid lipid NPs, nanostructured lipid carriers, and polymeric micelles, which can improve solubility, control release, and reduce dose-related side effects.138–140 Overall, these challenges emphasize the need for advanced drug delivery methods to address the limitations of traditional administration routes. Additionally, techniques such as nanoformulations, targeted delivery systems, and prodrug strategies are currently being investigated to enhance the solubility, stability, bioavailability, and tumor-specific delivery of BA in BC treatment.
4.1. Critical challenges hindering the commercialization of BA and possible solutions
Preclinical studies have shown that BA is a promising bioactive compound with potential against cancer. Extensive research in laboratories and using animal models has demonstrated BA's strong anticancer abilities, including its ability to induce programmed cell death, inhibit blood vessel formation, and regulate multiple pathways involved in cancer development. These early findings lay a solid foundation for further investigation into BA's therapeutic potential, serving as an essential step toward future clinical trials and possible applications in human medicine.19 Despite these significant biological benefits, BA still faces challenges due to its poor water solubility, which hampers its effectiveness as a therapeutic agent.141 This issue is also seen with taraxerol, another triterpene, which similarly lacks adequate solubility. The inability to dissolve restricts the drug's maximum therapeutic potential window.29,142 BA also has a short in vivo half-life, which impacts its clinical efficacy.142 A second challenge related to this triterpene, which naturally occurs in many plant species, is the need to assess the reliability of an ongoing supply and the ecological importance of the triterpene's natural sources before beginning drug development. It is essential to ensure the sustainable production of these compounds through chemical synthesis or biosynthetic methods. The complex structural features of these compounds present a significant obstacle to their chemical synthesis, ultimately limiting their progress in the drug development process.143 Although alcohol oxidation to carboxylic acids is the most commonly used method in organic synthesis, BE oxidation to BA acid still faces several challenges that limit the strategies available for this reaction. The presence of the alcohol group at position 3 and the electron-rich double bond at position 20 complicates the oxidation reaction.144 Furthermore, the production of BA through the conversion of BE to BA, whether by chemical synthesis or biosynthesis, is restricted by BE availability and has low conversion efficiency. Although BA's physiological and pharmaceutical significance is recognized, its large-scale synthetic routes are still limited. Therefore, the rapid development of scalable synthetic pathways is critically important for commercialization.
Additionally, suitable formulations are necessary to enable the future commercialization of BA as an active pharmaceutical ingredient (API). Numerous reports suggest that BA-based organic cations and ionic liquids (ILs) could help achieve this goal. As a result, efforts have been made to improve the solubility of existing triterpene compounds and to explore new, more efficient modes of administration, such as liposomes, nanoconstructs, and ILs.6 The work reported by Silva et al.145 focused on the chemical synthesis and in vitro evaluation of the antiproliferative properties of PQ, CQ, and MP betulinates, primaquine (PQ) bisphosphate, chloroquine (CQ) bisphosphate, mepacrine (MP) hydrochloride, proguanil hydrochloride, and sodium artesunate, among other compounds. BA-C6 NPs were synthesized and administered into intracranial xenograft GBM mice via tail vein injection.17 Nanoemulsions have also been used to develop new strategies. Nanoemulsions are water-in-oil or oil-in-water droplets ranging from 20 to 500 nm, which form when a dispersed phase encounters the continuous phase and can be stabilized using surfactants. Therefore, hydrophobic bioactives are encapsulated by oil droplets in an oil-in-water emulsion, representing a drug nanocarrier with high encapsulation efficiency and stability that can enhance drug bioavailability.29,73 Rebouças and coworkers prepared nanoemulsions containing encapsulated BA. The formulation demonstrated colloidal stability and showed no potential cytotoxicity against non-tumor fibroblast cells compared to pure BA.146 Additionally, chitosan, an alkaline polysaccharide, was also used as a BA carrier because of its ability to increase cell membrane permeability.32,91 Tiofack and coworkers prepared CD-MOF-1 as a carrier for BA. The global descriptors, such as electrophilicity, electronegativity, and energy gap, indicated that BA has a small energy gap, which involves greater polarizability and reactivity but less stability. Molecular docking revealed hydrogen bonding between BA and CD-MOF-1 within the hydrophobic cavities of CD-MOF-1. This opened avenues for further research on BA in nanomedicine, offering cost-effective methods and promoting sustainable research.147
In summary, high production costs and regulatory barriers remain obstacles; BA is mainly sourced from plant bark (e.g., Betula species), resulting in low yields. The extraction methods also require large amounts of plant material, making large-scale production inefficient. Metabolic engineering of plant cell cultures or microbes (e.g., engineered E. coli) can boost BA biosynthesis. Another option is to chemically modify BE, which is more abundant in birch bark, to efficiently obtain BA. Green chemistry approaches for sustainable synthesis and environmentally friendly extraction are highly desirable. Additionally, biocatalysis and enzymatic transformation enhance yield efficiency.
BA shows poor selectivity and drug resistance because, in its free form, BA lacks tumor-specific targeting, which can lead to off-target toxicity. Cancer cells develop resistance mechanisms that reduce BA's effectiveness over time. Therefore, targeted therapy approaches such as ligand-functionalized nanoparticles (e.g., HER2-targeted nanocarriers for BC) are used.148 Combination therapy with other anticancer agents (e.g., paclitaxel) is also employed to improve effectiveness and lower resistance. The absence of extensive clinical trials is a significant obstacle because most BA studies are limited to in vitro and in vivo research. This lack of comprehensive pharmacokinetic, toxicological, and clinical data hampers approval by the Food and Drug Administration (FDA) and other regulators. To overcome this, there is an urgent need to expand preclinical and clinical research. Specifically, systematic pharmacokinetic and toxicity studies are crucial for determining safe dosage ranges. In addition, multicenter human clinical trials are necessary to confirm BA's anticancer efficacy. Ultimately, collaboration among pharmaceutical companies, research institutions, and biotech firms is crucial for accelerating clinical translation.
The commercialization of BA faces multiple scientific, economic, and regulatory challenges. However, biotechnological advancements, the integration of nanomedicine, and the expansion of clinical research can facilitate its successful transition into mainstream cancer therapy. By enhancing production methods, improving bioavailability, and developing targeted delivery systems, BA can become a viable commercial anticancer drug in the future.
5. Future perspectives
Despite the growing evidence supporting the anticancer potential of BA, its clinical application for BC treatment is still limited by challenges in drug delivery, formulation issues, and large-scale production concerns. Future research should focus on overcoming these obstacles by developing scalable processes, addressing formulation problems, and tackling pharmacokinetic limitations that currently restrict its therapeutic use. To solve production issues, strategies such as semi-synthetic derivatization, biotransformation with engineered microbes, and green extraction technologies should be explored to ensure consistent, sustainable, and high-yield production. From a formulation perspective, a key area of focus is creating innovative, targeted delivery systems that enhance solubility, prolong systemic circulation, and improve tumor selectivity while minimizing off-target effects. Incorporating stimuli-responsive nanocarriers that release BA in response to tumor microenvironment cues such as pH, enzymes, or redox gradients offers a promising way to improve treatment precision. Simultaneously, ligand-functionalized systems targeting overexpressed receptors such as folate or HER2 on BC cells may increase selective uptake and intracellular drug accumulation. Additionally, combining BA with existing chemotherapeutics, immunomodulators, or sensitizing agents should be further investigated to overcome drug resistance and boost overall treatment effectiveness. Advances in 3D tumor models and organoids are also critical for preclinical testing of BA formulations, providing more physiologically relevant platforms to evaluate efficacy and safety. To bring BA-based therapies closer to clinical use, future efforts should prioritize scaling up feasibility, long-term safety assessments, and regulatory approval. Collaboration among chemists, pharmacologists, biomedical engineers, and clinicians is vital to optimizing delivery systems and designing translational studies. Due to the heterogeneous nature of BC disease, personalized medicine approaches that include genomic profiling and patient-specific delivery strategies could be key to identifying the most effective BA-based therapies for individual patients. In summary, with ongoing innovation in nanotechnology, drug design, and personalized medicine, BA has strong potential to become a valuable therapeutic agent in the prevention and treatment of BC. Lastly, despite the promising in vitro results described, the lack of in vivo pharmacokinetic data remains a significant limitation. Most studies depend heavily on cell-based models, yet they often extrapolate clinical relevance without evidence of improvements in bioavailability, tumor penetration, or reduction of off-target toxicity in animal models. This gap raises concerns about whether the reported stability and activity improvements can genuinely lead to therapeutic benefits within the complexity of a living system. Complete pharmacokinetic evaluations, including assessments of circulation time, biodistribution, clearance, and tissue accumulation, are crucial for confirming the clinical potential of these systems. Therefore, future research must incorporate rigorous in vivo pharmacokinetic analyses to close the gap between preclinical results and meaningful translational outcomes.
6. Conclusion
BA continues to show strong potential as a natural anticancer agent, especially for the prevention and treatment of BC. Its unique ability to induce apoptosis selectively in cancer cells while sparing normal tissues makes it a promising candidate for therapy. However, BA's progress in clinical use is limited by key pharmacokinetic issues, such as poor water solubility, low oral bioavailability, rapid metabolic breakdown, and inefficient tumor targeting with conventional delivery methods. To address these challenges, research has focused on developing advanced delivery systems, including nanocarrier platforms such as polymeric NPs, liposomes, micelles, and PEGylated derivatives, which improve BA's solubility, circulation time, and tumor accumulation. Targeted and stimulus-responsive systems also show promise for enhancing therapeutic accuracy and minimizing systemic toxicity. Despite these advances, challenges persist, including scalability, regulatory hurdles, safety concerns, and differences in BC subtype. Implementing BA-based treatments in clinical practice will require multidisciplinary efforts, thorough preclinical testing, and well-structured clinical trials. Overall, combining innovative delivery technologies with BA's innate anticancer properties offers a promising outlook. Continued development and validation could establish BA as a key player in future BC therapy. Additionally, formulating BA to counteract its low solubility has dramatically enhanced its medicinal use. Developing BA derivatives further enhances their potential, as they offer improved solubility, rapid large-scale production, and potent anticancer effects. Furthermore, integrating pharmacokinetic and pharmacodynamic analyses into preclinical pipelines, along with the development of standardized in vivo evaluation protocols, will be crucial for bridging the gap between laboratory results and real-world applications. These innovations indicate a bright future for the clinical development and commercialization of BA and its derivatives in BC treatment.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
The authors acknowledge financial support from the Department of Science and Innovation (grant number C6A0056).
Data availability
No new data were generated in this work.
References
- Lukong K. E. BBA Clin. 2017;7:64–77. doi: 10.1016/j.bbacli.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akram M. Iqbal M. Daniyal M. Khan A. U. Biol. Res. 2017;50:33. doi: 10.1186/s40659-017-0140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeagu E. I. Obeagu G. U. Medicine. 2024;103:e36905. doi: 10.1097/MD.0000000000036905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Wu S.-G. Breast Cancer: Targets Ther. 2023;15:721–730. doi: 10.2147/BCTT.S432526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrogan B. T. Gilmartin B. Carney D. N. McCann A. BBA-Rev. Cancer. 2008;1785:96–132. doi: 10.1016/j.bbcan.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Oh Y. S. Nutrients. 2016;8:472. [Google Scholar]
- Datta S. Bhattacharya S. Chem. Soc. Rev. 2015;44:5596–5637. doi: 10.1039/c5cs00093a. [DOI] [PubMed] [Google Scholar]
- Teng W. Zhou Z. Cao J. Guo Q. Foods. 2024;13:2226. doi: 10.3390/foods13142226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichewicz R. H. Kouzi S. A. Med. Res. Rev. 2004;24:90–114. doi: 10.1002/med.10053. [DOI] [PubMed] [Google Scholar]
- Aswathy M. Vijayan A. Daimary U. D. Girisa S. Radhakrishnan K. V. Kunnumakkara A. B. J. Biochem. Mol. Toxicol. 2022;36:e23206. doi: 10.1002/jbt.23206. [DOI] [PubMed] [Google Scholar]
- Jäger S. Trojan H. Kopp T. Laszczyk M. N. Scheffler A. Molecules. 2009;14:2016–2031. doi: 10.3390/molecules14062016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou H. Li H. Zhang S. Lu H. Chen Q. Molecules. 2021;26:5583. doi: 10.3390/molecules26185583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. Niu Y. Bakur A. Li H. Chen Q. Molecules. 2017;22:1075. doi: 10.3390/molecules22071075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. Qiang H. Yang W. Xu Y. Feng T. Cai H. Wang S. Liu Z. Zhang Z. Zhang J. J. Contr. Release. 2022;341:31–43. doi: 10.1016/j.jconrel.2021.11.020. [DOI] [PubMed] [Google Scholar]
- Zhang Z. Liang X. Yang X. Liu Y. Zhou X. Li C. ACS Appl. Mater. Interfaces. 2024;16:6689–6708. doi: 10.1021/acsami.3c15686. [DOI] [PubMed] [Google Scholar]
- Saneja A. Kumar R. Singh A. Dubey R. D. Mintoo M. J. Singh G. Mondhe D. M. Panda A. K. Gupta P. N. Int. J. Pharm. 2017;531:153–166. doi: 10.1016/j.ijpharm.2017.08.076. [DOI] [PubMed] [Google Scholar]
- Li Y. Wang Y. Gao L. Tan Y. Cai J. Ye Z. Chen A. T. Xu Y. Zhao L. Tong S. J. Nanobiotechnol. 2022;20:39. doi: 10.1186/s12951-022-01238-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehnath S. Karthikeyan K. Rajan M. Jeyaraj M. Mater. Chem. Phys. 2021;273:125146. [Google Scholar]
- Banerjee S. Banerjee S. Bishayee A. Da Silva M. N. Sukocheva O. A. Tse E. Casarcia N. Bishayee A. Phytomedicine. 2024;132:155858. doi: 10.1016/j.phymed.2024.155858. [DOI] [PubMed] [Google Scholar]
- Sousa J. L. Freire C. S. Silvestre A. J. Silva A. M. Molecules. 2019;24:355. doi: 10.3390/molecules24020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hordyjewska A. Ostapiuk A. Horecka A. Kurzepa J. Phytochem. Rev. 2019;18:929–951. [Google Scholar]
- Hordyjewska A. Ostapiuk A. Horecka A. Pre-Clin J. Clin. Res. 2018;12:72–75. [Google Scholar]
- Fulda S. Int. J. Mol. Sci. 2008;9:1096–1107. doi: 10.3390/ijms9061096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda S. Mol. Nutr. Food Res. 2009;53:140–146. doi: 10.1002/mnfr.200700491. [DOI] [PubMed] [Google Scholar]
- Wang K. Shang J. Tao C. Huang M. Wei D. Yang L. Yang J. Fan Q. Ding Q. Zhou M. Int. J. Nanomed. 2024:14075–14103. doi: 10.2147/IJN.S493489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R. Fazio G. C. Matsuda S. P. Phytochemistry. 2004;65:261–291. doi: 10.1016/j.phytochem.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Gill B. S. Kumar S. Navgeet S. Mol. Biol. Rep. 2016;43:881–896. doi: 10.1007/s11033-016-4032-9. [DOI] [PubMed] [Google Scholar]
- Hamion G. Aucher W. Mercier A. Tewes F. Menard M. Bertaux J. Girardot M. Imbert C. Int. J. Antimicrob. Agents. 2024;63:107166. doi: 10.1016/j.ijantimicag.2024.107166. [DOI] [PubMed] [Google Scholar]
- Moreira Rebouças L. Sousa A. Gramosa N. Araújo T. Oliveira F. D. C. Pessoa C. Araujo R. Santos E. Ricardo N. J. Braz. Chem. Soc. 2022;33:801–1015. [Google Scholar]
- Salau V. F. Erukainure O. L. Aljoundi A. Akintemi E. O. Elamin G. Odewole O. A. SAR QSAR Environ. Res. 2024;35:411–432. doi: 10.1080/1062936X.2024.2352729. [DOI] [PubMed] [Google Scholar]
- Bildziukevich U. Özdemir Z. Wimmer Z. Molecules. 2019;24:3546. doi: 10.3390/molecules24193546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H. Fang X. Lv J. Wang D. MATEC Web Conf. 2016;63:03008. [Google Scholar]
- Ríos J. L. Máñez S. Planta Med. 2018;84:8–19. doi: 10.1055/s-0043-123472. [DOI] [PubMed] [Google Scholar]
- Zhang X. Hu J. Chen Y. Mol. Med. Rep. 2016;14:4489–4495. doi: 10.3892/mmr.2016.5792. [DOI] [PubMed] [Google Scholar]
- Liberati A. Altman D. G. Tetzlaff J. Mulrow C. Gøtzsche P. C. Ioannidis J. P. Clarke M. Devereaux P. J. Kleijnen J. Moher D. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M. J. McKenzie J. E. Bossuyt P. M. Boutron I. Hoffmann T. Mulrow C. D. Shamseer L. Moher D. J. Clin. Epidemiol. 2020;118:60–68. doi: 10.1016/j.jclinepi.2019.11.010. [DOI] [PubMed] [Google Scholar]
- Esievo K. A. N. Balogun E. O. Esievo K. O. Esievo L. O. Esievo E. M. Sani D. Wassagwa J. Uyovbisere E. O. Mumah E. T. J. Drug Deliv. Therapeut. 2024;14:30–42. [Google Scholar]
- Ren H. Omori S. J. Wood Sci. 2012;58:169–173. [Google Scholar]
- Mukherjee D. Kumar N. S. Khatua T. Mukherjee P. K. Phytochem. Anal. 2010;21:556–560. doi: 10.1002/pca.1232. [DOI] [PubMed] [Google Scholar]
- Kim H.-I. Quan F.-S. Kim J.-E. Lee N.-R. Kim H. J. Jo S. J. Lee C.-M. Jang D. S. Inn K.-S. Biochem. Biophys. Res. Commun. 2014;451:282–287. doi: 10.1016/j.bbrc.2014.07.115. [DOI] [PubMed] [Google Scholar]
- Elusiyan C. A. Msagati T. A. Shode F. O. Mamba B. B. Bull. Chem. Soc. Ethiopia. 2011;25:321–332. [Google Scholar]
- Liu J. Chen P. Yao W. Wang J. Wang L. Deng L. He J. Zhang G. Lei J. Ind. Crop Prod. 2015;74:557–565. [Google Scholar]
- Bruckner V. Kovács J. Koczka I. J. Chem. Soc. 1948:948–951. doi: 10.1039/jr9480000948. [DOI] [PubMed] [Google Scholar]
- Galgon T. Höke D. Dräger B. Phytochem. Anal. 1999;10:187–190. [Google Scholar]
- Pinilla J. M. López-Padilla A. Vicente G. Fornari T. Quintela J. Reglero G. J. Supercrit. Fluids. 2014;95:541–545. [Google Scholar]
- Aisha A. F. Abu-Salah K. M. Alrokayan S. A. Siddiqui M. J. Ismail Z. Majid A. M. S. A. Rev. Bras. Farmacogn. 2012;22:335–343. [Google Scholar]
- Domingues R. M. de Melo M. M. Oliveira E. L. Neto C. P. Silvestre A. J. Silva C. M. J. Supercrit. Fluids. 2013;74:105–114. [Google Scholar]
- Domingues R. Sousa G. Freire C. Silvestre A. Neto C. P. Ind. Crop. Prod. 2010;31:65–70. [Google Scholar]
- Machado D. G. Cunha M. P. Neis V. B. Balen G. O. Colla A. Bettio L. E. Oliveira Á. Pazini F. L. Dalmarco J. B. Simionatto E. L. Food Chem. 2013;136:999–1005. doi: 10.1016/j.foodchem.2012.09.028. [DOI] [PubMed] [Google Scholar]
- Fernandes S. Vieira M. Prudêncio C. Ferraz R. Int. J. Mol. Sci. 2024;25:2108. doi: 10.3390/ijms25042108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta D. Al Hoque A. Paul B. Park J. H. Chowdhury C. Quadir M. Banerjee S. Choudhury A. Laha S. Sepay N. J. Biomed. Sci. 2024;31:81. doi: 10.1186/s12929-024-01069-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowers G.-O. Chee S. Bell D. Suckling L. Kern M. Tew D. McClymont D. Ellis T. Nat. Commun. 2020;11:868. doi: 10.1038/s41467-020-14708-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An T. Zha W. Zi J. Appl. Microbiol. Biotechnol. 2020;104:3339–3348. doi: 10.1007/s00253-020-10495-1. [DOI] [PubMed] [Google Scholar]
- Zhou C. Li J. Li C. Zhang Y. BMC Biotechnol. 2016;16:59. doi: 10.1186/s12896-016-0290-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. Zha W. An T. Dong H. Huang Y. Wang D. Yu R. Duan L. Zhang X. Peters R. J. Appl. Microbiol. Biotechnol. 2019;103:7029–7039. doi: 10.1007/s00253-019-10004-z. [DOI] [PubMed] [Google Scholar]
- Li J. Zhang Y. J. Biosci. Bioeng. 2015;119:77–81. doi: 10.1016/j.jbiosc.2014.06.013. [DOI] [PubMed] [Google Scholar]
- Jin C.-C. Zhang J.-L. Song H. Cao Y.-X. Microb. Cell Fact. 2019;18:77. doi: 10.1186/s12934-019-1127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H. Sugano S. S. Muranaka T. Seki H. Plant Biotechnol. 2024;41:319–323. doi: 10.5511/plantbiotechnology.24.0717b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou H. Li H. Wei T. Chen Q. J. Fungi. 2021;7:266. doi: 10.3390/jof7040266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y. Zhu M. Xu T. Ding W. Chen M. Wang Y. Zheng J. Molecules. 2023;28:5715. doi: 10.3390/molecules28155715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wancura J. H. Cipolatti E. P. Manoel E. A. Nitbani F. O. Caicedo-Paz A. V. Mussagy C. U. Abdellatief T. M. Mustafa A. di Bitonto L. Catalysts. 2025;15:600. [Google Scholar]
- O'Connell A. Barry A. Burke A. J. Hutton A. E. Bell E. L. Green A. P. O'Reilly E. Chem. Soc. Rev. 2024;53:2828–2850. doi: 10.1039/d3cs00689a. [DOI] [PubMed] [Google Scholar]
- Sun Y. F. Song C. K. Viernstein H. Unger F. Liang Z. S. Food Chem. 2013;138:1998–2007. doi: 10.1016/j.foodchem.2012.10.079. [DOI] [PubMed] [Google Scholar]
- Potze L. Mullauer F. B. Colak S. Kessler J. H. Medema J. P. Cell Death Dis. 2014;5:e1169. doi: 10.1038/cddis.2014.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullauer F. B. Kessler J. H. Medema J. P. Apoptosis. 2009;14:191–202. doi: 10.1007/s10495-008-0290-x. [DOI] [PubMed] [Google Scholar]
- Cai Y. Zheng Y. Gu J. Wang S. Wang N. Yang B. Zhang F. Wang D. Fu W. Wang Z. Cell Death Dis. 2018;9:636. doi: 10.1038/s41419-018-0669-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y. Liu P. Wang N. Wang S. Yang B. Li M. Chen J. Situ H. Xie M. Lin Y. Wang Z. Oxid. Med. Cell. Longev. 2019;2019:8781690. doi: 10.1155/2019/8781690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güttler A. Weinholdt C. Ruff E. Reidt J. Darnstaedt E. Wildemann A. Petrenko M. Keßler J. Kappler M. Grosse I. Vordermark D. Bache M. Cells. 2023;12:177. doi: 10.3390/cells12010177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. Jutooru I. Lei P. Kim K. Lee S. O. Brents L. K. Prather P. L. Safe S. Mol. Cancer Ther. 2012;11:1421–1431. doi: 10.1158/1535-7163.MCT-12-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo R. Fang D. Chu P. Wu H. Zhang Z. Tang Z. Biomed. Pharmacother. 2016;84:1321–1330. doi: 10.1016/j.biopha.2016.10.018. [DOI] [PubMed] [Google Scholar]
- Morparia S. Metha C. Suvarna V. Nat. Prod. Res. 2025;39:3260–3280. doi: 10.1080/14786419.2024.2412838. [DOI] [PubMed] [Google Scholar]
- Jiang W. Li X. Dong S. Zhou W. Biomed. Pharmacother. 2021;142:111990. doi: 10.1016/j.biopha.2021.111990. [DOI] [PubMed] [Google Scholar]
- Cavazos-Garduño A. Ochoa Flores A. Serrano-Niño J. Beristain C. García H. Rev. Mex. Ing. Quim. 2014;13:689–703. [Google Scholar]
- Ríos J. L. Máñez S. Planta Med. 2018;84:8–19. doi: 10.1055/s-0043-123472. [DOI] [PubMed] [Google Scholar]
- Zeng A. Q. Yu Y. Yao Y. Q. Yang F. F. Liao M. Song L. J. Li Y. L. Yu Y. Li Y. J. Deng Y. L. Yang S. P. Zeng C. J. Liu P. Xie Y. M. Yang J. L. Zhang Y. W. Ye T. H. Wei Y. Q. Oncotarget. 2018;9:3794–3804. doi: 10.18632/oncotarget.23376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anaya-Eugenio G. D. Eggers N. A. Ren Y. Rivera-ChÁvez J. Kinghorn A. D. De Blanco E. J. C. Anticancer Res. 2020;40:6637–6647. doi: 10.21873/anticanres.14688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit S. Sapkota S. Suwal S. Adhikari A. Karki P. Kumar A. J. Contemp. Brachytherapy. 2023;15:229–233. doi: 10.5114/jcb.2023.129016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P. Vedarethinam V. Korsah M. A. Danquah M. K. Jeevanandam J. Appl. Sci. 2024;14:1809. [Google Scholar]
- Halder A. Jethwa M. Mukherjee P. Ghosh S. Das S. Helal Uddin A. Mukherjee A. Chatterji U. Roy P. Artif. Cells Nanomed. Biotechnol. 2020;48:1362–1371. doi: 10.1080/21691401.2020.1850465. [DOI] [PubMed] [Google Scholar]
- Zhu P. Luo W. Qian J. Meng C. Shan W. Xu Z. Zhang W. Liu X. Ling Y. Molecules. 2021;26:5900. doi: 10.3390/molecules26195900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S. Fan X. Wang H. Zhao Y. Zhao W. Li M. Lv R. Wang T. Sun T. ACS Appl. Bio Mater. 2020;3:3518–3525. doi: 10.1021/acsabm.9b01204. [DOI] [PubMed] [Google Scholar]
- Liu Y. Zhang X. Liu Z. Wang L. Luo L. Wang M. Wang Q. Gao D. Nanomed.: Nanotechnol. Bio. Med. 2017;13:1891–1900. doi: 10.1016/j.nano.2017.03.012. [DOI] [PubMed] [Google Scholar]
- Li C. Wang Z. Zhang Y. Zhu Y. Xu M. Lei H. Zhang D. Int. J. Nanomed. 2024;19:1749–1766. doi: 10.2147/IJN.S427783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farcas C. G. Dehelean C. Pinzaru I. A. Mioc M. Socoliuc V. Moaca E.-A. Avram S. Ghiulai R. Coricovac D. Pavel I. Int. J. Nanomed. 2020;15:8175–8200. doi: 10.2147/IJN.S269630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güttler A. Darnstaedt E. Knobloch-Sperlich D. Petrenko M. Kessler J. Grosse I. Vordermark D. Bache M. Antioxidants. 2024;13:1299. doi: 10.3390/antiox13111299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal Y. Asghar S. Hanif R. Amin F. Colloids Surf., A. 2025;721:137170. [Google Scholar]
- Urandur S. Banala V. T. Shukla R. P. Gautam S. Marwaha D. Rai N. Sharma M. Sharma S. Ramarao P. Mishra P. R. Acta Biomater. 2020;113:522–540. doi: 10.1016/j.actbio.2020.06.023. [DOI] [PubMed] [Google Scholar]
- Oladimeji O. Akinyelu J. Daniels A. Singh M. Int. J. Mol. Sci. 2021;22:5072. doi: 10.3390/ijms22105072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein-Al-Ali S. H. Arulselvan P. Fakurazi S. Hussein M. Z. J. Mater. Sci. 2014;49:8171–8182. [Google Scholar]
- Zhang Y. Zhou H. Zhang Z. Zhu Y. Wang T. Yu L. Song H. Colloids Surf., A. 2021;609:125662. [Google Scholar]
- Gautam S. Marwaha D. Singh N. Rai N. Sharma M. Tiwari P. Urandur S. Shukla R. P. Banala V. T. Mishra P. R. Mol. Pharma. 2023;20:1914–1932. doi: 10.1021/acs.molpharmaceut.2c00673. [DOI] [PubMed] [Google Scholar]
- Cheng J. Zhao H. Li B. Zhang H. Zhao Q. Fu S. Han Y. Lu W. Shi J. Yang X. Acta Pharma. Sin. B. 2023;13:879–896. doi: 10.1016/j.apsb.2022.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özdemir Z. Yang M. Kim G. Bildziukevich U. Šaman D. Li X. Yoon J. Wimmer Z. Dyes Pigm. 2021;190:109307. [Google Scholar]
- Fu S. Wang M. Li B. Li X. Cheng J. Zhao H. Zhang H. Dong A. Lu W. Yang X. Biomater. Res. 2023;27:43. doi: 10.1186/s40824-023-00380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danhier F. Ansorena E. Silva J. M. Coco R. Le Breton A. Préat V. J. Contr. Release. 2012;161:505–522. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- Yoo H. S. Park T. G. J. Contr. Release. 2004;96:273–283. doi: 10.1016/j.jconrel.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Torchilin V. P. Pharma. Res. 2007;24:1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- Blanco E. Shen H. Ferrari M. Nat. Biotechnol. 2015;33:941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. Ahn C. H. Park T. G. Macromol. Biosci. 2009;9:336–342. doi: 10.1002/mabi.200800229. [DOI] [PubMed] [Google Scholar]
- Kumari A. Yadav S. K. Yadav S. C. Colloids Surf., B. 2010;75:1–18. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Zhang P. Chen D. Li L. Sun K. J. Nanobiotechnol. 2022;20:31. doi: 10.1186/s12951-021-01221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alawi M. Hilles A. R. Kumar M. Ansari M. D. Mahmood S. Nanomedicine. 2025:1–24. doi: 10.1080/17435889.2025.2523730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Z. Pan S. Gao P. Sheng H. Li L. Shi L. Zhang Y. Cai X. Int. J. Pharm. 2020;575:118841. doi: 10.1016/j.ijpharm.2019.118841. [DOI] [PubMed] [Google Scholar]
- Yuan Y. Y. Mao C. Q. Du X. J. Du J. Z. Wang F. Wang J. Adv. Mater. 2012;24:5476–5480. doi: 10.1002/adma.201202296. [DOI] [PubMed] [Google Scholar]
- Choudhury K. Ghosh S. S. ACS Appl. Nano Mater. 2024;7:3564–3579. [Google Scholar]
- Jain S. Kumar M. Kumar P. Verma J. Rosenholm J. M. Bansal K. K. Vaidya A. J. Funct. Biomater. 2023;14:437. doi: 10.3390/jfb14090437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando I. R. Ferris D. P. Frasconi M. Malin D. Strekalova E. Yilmaz M. D. Ambrogio M. W. Algaradah M. M. Hong M. P. Chen X. Nanoscale. 2015;7:7178–7183. doi: 10.1039/c4nr07443b. [DOI] [PubMed] [Google Scholar]
- Zhong L. Li Y. Xiong L. Wang W. Wu M. Yuan T. Yang W. Tian C. Miao Z. Wang T. Signal Transduct. Target. Ther. 2021;6:201. doi: 10.1038/s41392-021-00572-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan C. Yu M. Xu J. Li B.-Y. Zhao Y. Kankala R. K. Biomed. Pharmacother. 2023;162:114643. doi: 10.1016/j.biopha.2023.114643. [DOI] [PubMed] [Google Scholar]
- Omidi Y. Mobasher M. Castejon A. M. Mahmoudi M. J. Drug Target. 2022;30:687–708. doi: 10.1080/1061186X.2022.2055045. [DOI] [PubMed] [Google Scholar]
- Qi X. Gao C. Yin C. Fan J. Wu X. Guo C. Drug Deliv. 2021;28:1962–1971. doi: 10.1080/10717544.2021.1979125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selepe C. T. Dhlamini K. S. Tshweu L. Kwezi L. Ramalapa B. Ray S. S. Macromol. Mater. Eng. 2025;310:2400283. [Google Scholar]
- Cai S. Guo X. Yang H. Zhao T. Li Y. Deng N. Gao Z. Meng Q. Li X. Wang S. Bioorg. Med. Chem. 2025;119:118062. doi: 10.1016/j.bmc.2025.118062. [DOI] [PubMed] [Google Scholar]
- Saneja A. Sharma L. Dubey R. D. Mintoo M. J. Singh A. Kumar A. Sangwan P. L. Tasaduq S. A. Singh G. Mondhe D. M. Int. J. Pharm. 2017;73:616–626. doi: 10.1016/j.msec.2016.12.109. [DOI] [PubMed] [Google Scholar]
- Jiao L. Wang S. Zheng Y. Wang N. Yang B. Wang D. Yang D. Mei W. Zhao Z. Wang Z. Biochem. Pharma. 2019;161:149–162. doi: 10.1016/j.bcp.2019.01.016. [DOI] [PubMed] [Google Scholar]
- Kushwaha P. P. Singh A. K. Shuaib M. Prajapati K. S. Vardhan P. S. Gupta S. Kumar S. Chem. Biol. Interact. 2020;328:109200. doi: 10.1016/j.cbi.2020.109200. [DOI] [PubMed] [Google Scholar]
- Li J. Chang L.-C. Hsieh K.-Y. Hsu P.-L. Capuzzi S. J. Zhang Y.-C. Li K.-P. Morris-Natschke S. L. Goto M. Lee K.-H. Bioorg.Med. Chem. 2019;27:2871–2882. doi: 10.1016/j.bmc.2019.05.016. [DOI] [PubMed] [Google Scholar]
- Saikia T. Rajak P. Sahu B. P. Patowary L. Drugs Drug-Candidates. 2024;3:566–597. [Google Scholar]
- Wróblewska-Łuczka P. Cabaj J. Bąk W. Bargieł J. Grabarska A. Góralczyk A. Łuszczki J. J. Int. J. Mol. Sci. 2022;23:9641. doi: 10.3390/ijms23179641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saneja A. Kumar R. Mintoo M. J. Dubey R. D. Sangwan P. L. Mondhe D. M. Panda A. K. Gupta P. N. Mater. Sci. Eng.: C. 2019;98:764–771. doi: 10.1016/j.msec.2019.01.026. [DOI] [PubMed] [Google Scholar]
- Wang R. Yang M. Li G. Wang X. Zhang Z. Qiao H. Chen J. Chen Z. Cui X. Li J. Colloids Surf. B Biointerfaces. 2019;174:270–279. doi: 10.1016/j.colsurfb.2018.11.029. [DOI] [PubMed] [Google Scholar]
- Wang R. Wang X. Jia X. Wang H. Li W. Li J. Int. J. Pharm. 2020;588:119799. doi: 10.1016/j.ijpharm.2020.119799. [DOI] [PubMed] [Google Scholar]
- Weber D. Zhang M. Zhuang P. Zhang Y. Wheat J. Currie G. M. CTOIJ. 2017;4:1–10. [Google Scholar]
- Cai Y. Zheng Y. Gu J. Wang S. Wang N. Yang B. Zhang F. Wang D. Fu W. Wang Z. Cell Death Dis. 2018;9:636. doi: 10.1038/s41419-018-0669-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens-Talcott S. U. Noratto G. D. Li X. Angel-Morales G. Bertoldi M. C. Safe S. Mol. Carcinog. 2013;52:591–602. doi: 10.1002/mc.21893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng A.-Q. Yu Y. Yao Y.-Q. Yang F.-F. Liao M. Song L.-J. Li Y.-L. Yu Y. Li Y.-J. Deng Y.-L. Oncotarget. 2017;9:3794. doi: 10.18632/oncotarget.23376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damle A. A. Pawar Y. P. Narkar A. A. Indian J. Exp. Biol. 2013;51:485–491. [PubMed] [Google Scholar]
- Pisha E. Chai H. Lee I.-S. Chagwedera T. E. Farnsworth N. R. Cordell G. A. Beecher C. W. Fong H. H. Kinghorn A. D. Brown D. M. Nat. Med. 1995;1:1046–1051. doi: 10.1038/nm1095-1046. [DOI] [PubMed] [Google Scholar]
- Zhao S. Park C. H. Li X. Kim Y. B. Yang J. Sung G. B. Park N. I. Kim S. Park S. U. J. Agric. Food Chem. 2015;63:8622–8630. doi: 10.1021/acs.jafc.5b03221. [DOI] [PubMed] [Google Scholar]
- Rahman M., Beg S., Kumar V. and Ahmad F. J., Nanomedicine for Bioactives, Springer, 2020, pp. 127–153 [Google Scholar]
- Stielow M. Witczyńska A. Kubryń N. Fijałkowski Ł. Nowaczyk J. Nowaczyk A. Molecules. 2023;28:8038. doi: 10.3390/molecules28248038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z. Wen X. Wang G. Zhou Y. Front. Pharmacol. 2021;12:796745. doi: 10.3389/fphar.2021.796745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistor M. Rugina D. Diaconeasa Z. Socaciu C. Socaciu M. A. Int. J. Mol. Sci. 2023;24:12923. doi: 10.3390/ijms241612923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlSawaftah N. M. Awad N. S. Pitt W. G. Husseini G. A. Polymers. 2022;14:936. doi: 10.3390/polym14050936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. J. Kim M. S. Biomed. Pharmacother. 2025;189:118244. doi: 10.1016/j.biopha.2025.118244. [DOI] [PubMed] [Google Scholar]
- Suresh C. Zhao H. Gumbs A. Chetty C. S. Bose H. S. Bioorg. Med. Chem. Lett. 2012;22:1734–1738. doi: 10.1016/j.bmcl.2011.12.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeu A. Minda D. Boru C. Vlaia L. Vlaia V. Dehelean C. A. Liga S. Puenea G. Muntean D. L. Life. 2025;15:954. doi: 10.3390/life15060954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H. Zhang S. Wang J. Chen Q. Front. Nutr. 2021;8:783831. doi: 10.3389/fnut.2021.783831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezzi M. Pescina S. Padula C. Santi P. Del Favero E. Cantù L. Nicoli S. J. Contr. Release. 2021;332:312–336. doi: 10.1016/j.jconrel.2021.02.031. [DOI] [PubMed] [Google Scholar]
- Viegas C. Patrício A. B. Prata J. M. Nadhman A. Chintamaneni P. K. Fonte P. Pharmaceutics. 2023;15:1593. doi: 10.3390/pharmaceutics15061593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seca A. M. Pinto D. C. Int. J. Mol.Sci. 2018;19:263. [Google Scholar]
- Wu Z. C. Liu X. Y. Liu J. Y. Piao J. S. Piao M. G. Int. J. Nanomed. 2022;17:4195. doi: 10.2147/IJN.S373430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K. Zafar R. Pharmacogn. Rev. 2015;9:19. doi: 10.4103/0973-7847.156317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressmann A. K. Kremsmayr T. Gaertner P. Zirbs R. Bica K. Green Chem. 2017;19:1014–1022. [Google Scholar]
- Silva A. T. Cerqueira M. J. o. Prudêncio C. Fernandes M. H. Costa-Rodrigues J. Teixeira C. t. Gomes P. Ferraz R. ACS Omega. 2019;4:5682–5689. [Google Scholar]
- Choi Y. H. Han H.-K. J. Pharm. Invest. 2018;48:43–60. doi: 10.1007/s40005-017-0370-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiofack S. K. Borzehandani M. Y. Tsobnang P. K. Latif M. A. M. Tejo B. A. Ahmad H. Ngoune J. Rahman M. B. A. Malays. J. Anal. Sci. 2024;28:530–542. [Google Scholar]
- Selepe C. T. Dhlamini K. S. Tshweu L. Moralo M. Kwezi L. Ray S. S. Ramalapa B. Nano Select. 2024;5:2300191. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated in this work.