Abstract
Background and Aims
Tribe Mirbelieae (Fabaceae) represents one of the great species radiations in Australian flora and the largest in the pea-flowered legumes. Traditional amplicon sequencing has failed to resolve relationships within this species-rich and morphologically diverse tribe.
Methods
Target-capture sequencing was used to reconstruct relationships within the core Mirbelioid legumes, which represent a previously hypothesized rapid radiation that dates to the Oligocene and early Miocene epochs.
Key Results
We recovered strongly supported deep nodes resolving relationships between all recognized genera and four novel clades based on 289 low-copy nuclear markers derived from the Angiosperms353 universal probe set. The taxonomically challenging genus Pultenaea was demonstrated to be polyphyletic. Minor changes are required in Aotus, Callistachys, Mirbelia, Oxylobium, Phyllota and Urodon.
Conclusions
Phylogenomic data have robustly resolved relationships in a large legume clade where relationships were long feared irresolvable. This resolution enables the recognition of monophyletic genera within the tribe, with only minimal taxonomic rearrangements. Critically, a new circumscription of Pultenaea supported by both phylogenomic and morphological data has now been achieved.
Keywords: Fabaceae, genomics, phylogenetics, Angiosperms353, Mirbelieae, target capture, next generation sequencing, phylogenomics, legumes, bush peas, Pultenaea, baits
INTRODUCTION
Australia, an island continent, is home to ∼8 % of global flowering plant diversity (Govaerts et al., 2021). Legume diversity is similarly high, and native species occupy almost all terrestrial biomes, representing almost 7 % of the Australian flora and ∼8 % of the global legume flora (Legume Phylogeny Working Group, 2021). Fabaceae tribe Mirbelieae (bush peas and their relatives), sensu Barrett et al. (2021), includes 24 currently accepted genera and 756 recognized species (almost 50 % of Australia’s native pea-flowered legumes), all but one of which are endemic to Australia. Within this tribe, the circumscription of genera in the Pultenaea Group sensu Crisp and Weston (1995; with 18 genera and >540 species recognized) has remained problematic. It has been proposed that difficulties in resolving deep nodes in the Mirbelieae might be attributable to the rapid radiation of this ecologically diverse and species-rich clade (Orthia et al., 2005a, b; Barrett et al., 2021). Relationships between core genera of Mirbelieae have remained unresolved, and several widely accepted genera are not monophyletic in their current circumscription, but a new classification of the tribe will require robust support for relationships (Orthia et al., 2005a, b; Barrett et al., 2021).
Given their high species diversity, the development of a well-resolved phylogenetic tree and robust classification of the tribe Mirbelieae is an important task. The tribe is of ecological and commercial importance through nitrogen fixation, provision of habitat for animals, agronomic potential through biocontrol (as the source of 1080 poison from species of Gastrolobium R.Br.; Peacock et al., 2004), and being a major source of pollen and nectar for native bees and commercial honeybees (Mead et al., 1985; Twigg et al., 1996; Dear et al., 2003; Robinson et al., 2007; Ogilvie et al., 2009; Sprent et al., 2017; Kates et al., 2024). Patterns of diversification within the tribe can also be expected to inform our understanding of the evolution of specific ecological adaptations in response to environmental change, such as drying climates (Byrne et al., 2008; 2011), pollinator shifts (Crisp, 1994; Toon et al., 2014) and biogeographical history of significant disjunctions in the Australian mesic flora (Byrne et al., 2011; Weston and Jordon, 2017). Additionally, investigation of phylodiversity patterns can better guide conservation priorities by quantifying genetic diversity and determining conservation management units (Laity et al., 2015; Broadhurst and Coates, 2017).
The first phylogenetic analyses of the Mirbelieae were based largely on morphological, anatomical and embryological characters and assumed that most of the genera then recognized were monophyletic (Crisp and Weston, 1987, 1995). However, the first phylogenetic tree for Mirbelieae based on molecular data was incongruent with both the earlier morphology-based trees (Crisp and Cook, 2003) and particularly challenged the monophyly of Pultenaea Sm., the largest genus in the Mirbelieae. These results also showed incongruence between plastid and nuclear markers, with a lack of resolution for most deep nodes in their analyses. Crisp and Cook (2003) drew attention to a large, strongly supported clade that showed weak internal resolution, which they called the ‘Mirbelia Group’ [but which Crisp and Weston (1995) had earlier given the informal name the ‘Pultenaea Group’]. Crisp and Cook (2003) suggested that perhaps all genera in this clade should be combined into one large genus, for which the name Pultenaea would have nomenclatural priority.
Orthia et al. (2005a) produced a relatively well-sampled phylogeny of the tribe, also based on trnL-F and ITS, but increased species sampling failed to increase the resolution of phylogenetic relationships. Orthia et al. (2005b) highlighted the incongruence between nuclear and plastid genetic markers as the most likely reason for this. Although many relationships were not strongly supported, Orthia et al. (2005a) did demonstrate that the largest genus in Mirbelieae, Pultenaea Sm., was not supported as monophyletic and that many taxa were geographically clustered, indicating that there is a link between genetic relationships and geography. Barrett et al. (2021) summarized the phylogeny of Mirbelieae using all available trnL-F data and hypothesized that hybridization, genome duplication, incomplete lineage sorting, and recent and rapid radiation might all be contributing to low phylogenetic resolution in the tribe. Renner et al. (2022) also found evidence for hybridization in Pultenaea, because interspecific hybridization was found between two species from eastern Australia, providing the first strong evidence of hybridization in the genus.
Representation of legumes in phylogenomic datasets is rapidly increasing, enabling the resolution of relationships that have confounded taxonomists for centuries (Vatanparast et al., 2018; Koenen et al., 2020a, b; Choi et al., 2022). For example, recent studies have surveyed the entire Fabaceae for genes associated with nitrogen fixation (Folk et al., 2021; Kates et al., 2024) and resolved major clade relationships across the morphologically diverse family (Koenen et al., 2020a; Aecyo et al., 2021; Choi et al., 2022; Ringelberg et al., 2022). Next-generation sequencing has brought plant systematics into the genomic era (Godden et al., 2012; McCormack et al., 2013). Advances in DNA sequencing technologies have aided the development of targeted sequence capture through the enrichment of hundreds of informative markers throughout the genome that are cross-applicable through seed plants, offering a new dimension for understanding the phylogenetic relationship of species (Johnson et al., 2019; Andermann et al., 2020; Breinholt et al., 2021).
The myBaits Angiosperms353 universal probe set has revolutionized our understanding of angiosperm evolution. It has provided unprecedented phylogenetic resolution for many plant groups, including Araliaceae, Cactaceae, Cleomaceae, Combretaceae, Cyperaceae, Gesneriaceae, Nepenthaceae, Gentianales, Myrtales and Poales (Larridon et al., 2020; 2021a, b; Murphy et al., 2020; Shee et al., 2020; Antonelli et al., 2021; Maurin et al., 2021, 2023; Ogutcen et al., 2021; Chincoya and Solórzano, 2022; Elliott et al., 2024; GPWG III, 2024; Saunders et al., 2024). The effectiveness of the Angiosperms353 baits to resolve even close evolutionary relationships has allowed it to become the standard as a universal probe set for angiosperm phylogenomics, and it has now been applied as a phylogenomic platform for exploring the Plant and Fungal Tree of Life (PAFTOL) and Genomics of Australian Plants (GAP) projects (Baker et al., 2022; Zuntini et al., 2024; Simpson et al., 2025). Bioinformatic pipelines and workflows have been developed specifically to work with target-capture datasets (Johnson et al., 2016; McLay et al., 2021; Zhou et al., 2022; Jackson et al., 2023) to aid and improve their analysis, including the removal of paralogues and the detection of interspecific hybrids (Yang and Smith, 2014; Nauheimer et al., 2021).
Here, we aim to create a robust phylogenetic framework for tribe Mirbelieae by applying next generation sequencing and target baits capture using the Angiosperms353 universal probe set. We also aim to test the monophyly of genera within the tribe, whose circumscriptions are, almost without exception, founded on morphological data. This will allow us to propose a durable generic classification for a group that has faced considerable taxonomic instability (Orthia et al., 2005b). We anticipate the need to re-circumscribe some existing genera and the description of new genera to render the currently described genera monophyletic. We have already started this taxonomic process by revising the delimitation of Pultenaea as a clade of >150 species (many still unnamed; Renner et al., 2022; Telford et al., 2022; Barrett et al., 2024a, b), all but one of which are restricted to eastern Australia. We have reinstated the genus Euchilus R.Br. from synonymy under Pultenaea and described three new genera of Western Australian species that were formerly included in Pultenaea (Barrett et al., 2024c). Our new phylogenetic framework will also lay the foundation for a more detailed study into the origins and diversification processes within this uniquely Australian lineage and facilitate the delimitation of additional genera that remain non-monophyletic.
MATERIALS AND METHODS
Sample collection and preparation
We newly sampled 115 accessions of leaf material, mostly from wild populations across Australia, and either silica-dried or stored in CTAB gel (gel used for long term storage of DNA post-CTAB extraction). Fresh leaf samples were also collected from specimens in the living collections of the Australian Botanic Garden, Mount Annan, the Blue Mountains Botanic Garden, Mount Tomah and the Australian National Botanic Gardens, Canberra, with all samples supported by herbarium vouchers. Herbarium specimens collected within the last 30 years (where possible, but ≤120 years for some species) were sampled from the collections of BRI, CANB, MEL and NSW herbaria to fill any critical gaps owing to limitations on fieldwork during the coronavirus 2019 pandemic-related travel restrictions (see Supplementary Data Table S1).
DNA extraction and library preparation
For samples from MEL, DNA extractions were based on a scaled-down version of the CTAB method used by McLay et al. (2022) for Acacia pycnantha Benth. For each sample, ∼100 mg of dried leaf material was placed in a 2 mL SafeLock Microcentrifuge tube (Eppendorf, Germany) along with a tungsten carbide bead and frozen in liquid nitrogen. The tissue was then disrupted using a TissueLyser II (Qiagen, Hilden, Germany). The resultant ground tissue was washed at least once with a sorbitol prewash before lysis for 2 h in a 2 % CTAB buffer with 1.4 m NaCl. After purification with chloroform:isoamyl alcohol, DNA in the resultant aqueous phase was precipitated as described by McLay et al. (2022) but at −20 °C for 1 h and centrifugation for 10 min at 21 300g. After two rounds of washing with 80 % ethanol, the dried DNA pellets were dissolved in 100 μL of 10 mm Tris–HCl pH 8. The concentration of each isolate was determined using a Qubit 3 Fluorometer (v.3.0 BR DNA assay; Invitrogen, Life Technologies, Carlsbad, CA, USA), and the purity of every second isolate was checked with a NanoDrop Lite Spectrophotometer (ThermoFisher Scientific Waltham, MA, USA). For samples from New South Wales, DNA extraction and library preparation for 96 samples was carried out by the Australian Genome Research Facility (AGRF). DNA extraction was carried out using a CTAB protocol (Porebski et al., 1997) to maximize DNA output and yield, because many samples were stored within CTAB gel. Library preparation was completed using an NEB Next Ultra II FS kit (New England Biolabs, MA, USA) by AGRF. Target capture enrichment was completed using the myBaits Angiosperms353 v.1 universal phylogenomics solution for flowering plants developed by Johnson et al. (2019) and carried out by AGRF. Sequencing was then carried out using an Illumina NovaSeq 6000 sequencing platform for 150 bp paired-end reads in the single flow cell, with 300 cycles per sequencing run.
Bioinformatics
Data assembly
Raw Illumina reads from Angiosperms353 sequencing for one species of each genus and outgroup taxa were sourced from the Genomics for Australian Plants project in partnership with Bioplatforms and Australian BioCommons Leadership Share (ABLeS) program Australia (Supplementary Data Table S1; https://australianbiocommons.github.io/ables/acknowledgements/; Simpson et al., 2025). Raw Illumina reads were visualized using Geneious Prime v.2022.0.12 (https://www.geneious.com), then checked for quality and consistency of base quality sores using FastQC v.0.11.9 (Andrews, 2010). Raw Illumina reads were filtered, with reads of <50 bp discarded using FastP v.0.23.1 (Chen et al., 2018). Data were assembled using a modified version of HybPiper v.1.3.1 (Johnson et al., 2016) named HybPiper-nf: containerized and pipelined using Singularity container (Kurtzer et al., 2017) and a Nextflow pipeline (Jackson et al., 2023) using the standard settings without modification. Gene directories were then run within a modified version of the yang-and-smith pipeline (Yang and Smith, 2014), named paragon-nf (Jackson et al., 2023), which contains additional options and bug fixes to detect potential paralogues created during the target enrichment process. The paragone-nf pipeline was used to remove these paralogues to align processed gene directories and produce three outputs using different algorithms: monophyletic outgroups (MO), rooted subtrees (RT) and maximum inclusion (MI), with each method having been described by Jackson et al. (2023). The MO output was used for our study, because the relationships between our outgroups were strongly supported by the much broader study of Zuntini et al. (2024).
Alignment and annotation
From the paragone-nf pipeline, the aligned gene directories from the MO algorithm were selected, because our outgroups were known to be monophyletic. Each gene alignment was visualized and checked using Geneious Prime 2022.0.2 (Biomatters Ltd, Auckland, New Zealand). Whole-gene alignments with significant missing data and evident alignment ambiguities were discarded. The remaining gene alignments were then concatenated using Geneious and exported in the NEXUS file format. Once alignments were concatenated, the matrix was checked for consistency of the alignment, then annotated by marker/gene within Geneious to export partitions for downstream phylogenetic analysis.
Phylogenetics
Maximum likelihood phylogenetic analysis was conducted using IQ-Tree v.2.3.6 (Minh et al., 2020a) using a partition scheme determined by ModelFinder (Kalyaanamoorthy et al., 2017) that was tested for each gene individually and rapid bootstrap analysis with 1000 bootstrap replicates and 1000 Single branch tests (-alrt). However, when a large marker set is used in phylogenetic analysis, it can often yield high support values despite abundant incongruence between gene trees (Minh et al., 2020a). Thus, the site concordance factors (sCF) were calculated using the new algorithms first implemented in IQ-Tree v.2.2.2 to improve accuracy (Mo et al., 2023) and were used to quantify potential discordance, because sCF estimates the concordance level of individual sites (Minh et al., 2020b). The sCF were estimated using both a concatenated tree with 1000 ultrafast bootstraps and an edge-linked fully partitioned model and using 289 single-locus gene trees following the methodology outlined by The Lanfear Lab (http://www.robertlanfear.com/blog/files/concordance_factors.html).
Coalescent-based species trees
Coalescent species trees were inferred using ASTRAL III 5.7.8 (Zhang et al., 2018), by inputting the generated gene tree files that were based on the alignments of each gene. ASTRAL was used to estimate quartet support (-t 1) for the recovered topology at each node of the tree. Then the conflict, concordance and phylogenetic signal were estimated using the PhyParts script (Smith et al., 2015) and visualized using the PhyParts PieCharts script (https://github.com/mossmatters/MJPythonNotebooks/blob/master/PhyParts_PieCharts.ipynb). The PhyParts PieCharts script infers the number of gene trees that support, oppose or provide no information on a particular node with respect to the dominant species tree topology and maps these values onto the tree in a pie chart format.
RESULTS
Sequencing, quality control and target capture
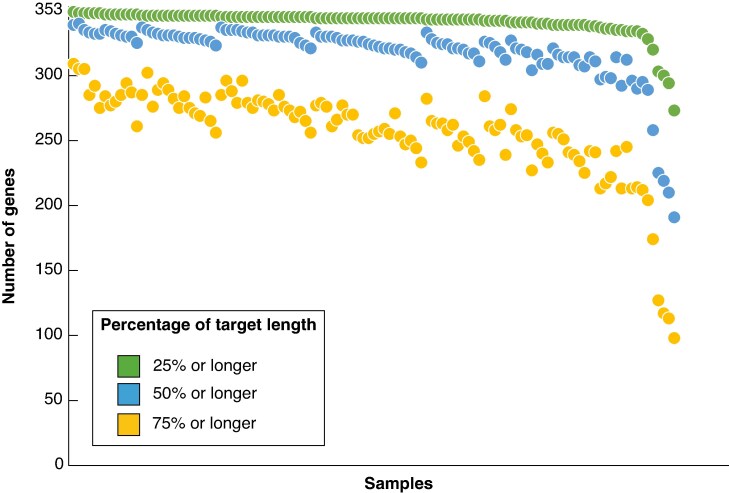
The Illumina NovaSeq 6000 sequencing resulted in reads ranging from 408 021 to 9 596 685 with a mean of 4 678 487 per sample (Table 1). After initial quality control and trimming in the HybPiper-nf pipeline, the total number of paired-end reads ranged from 381 554 to 8 888 664, with a mean of 4 367 361. The mapping of reads to targets in HybPiper was variable and ranged from 3 % (98 868 reads) to 24.9 % (2 508 354 reads) with a mean of 10.07 % (832 533 reads) (Table 1). Read mapping to targets recovered a total of 353 genes (100 %), with 344 being the lowest number of genes recovered per sample, from the 35 000 targets (353 genes), with sequences that were >25 % of the target length ranging from 273 to 349 genes with a mean of 341. Sequences that were >50 % of the target length ranged from 191 to 340 genes with a mean of 318, and sequences at >75 % of the target length ranged from 98 to 309 genes with a mean of 257 (Fig. 1; Table 1), which is exceptional gene recovery (for complete outputs from HybPiper, see Supplementary Data Table S2). Data are available from the SRA (project number PRJNA1270708).
Table 1.
Read filtering and assembly results from HybPiper.
| No. raw reads (paired) | No. trimmed reads (paired) | No. reads mapped to target | No. genes recovered | % target length >25 % | % target length >50 % | % target length >75 % | No. paralogue warnings | |
|---|---|---|---|---|---|---|---|---|
| Minimum | 408 021 | 720 166 | 98 868 (3 %) | 344 | 273 | 191 | 98 | 0 |
| Mean | 4 678 487.112 | 4 367 361.159 | 1 877 200.765 (22 %) | 351 | 341 | 318 | 257 | 4 |
| Median | 4 605 167 | 4 269 683 | 832 533 (10.7 %) | 351 | 344 | 325 | 262 | 4 |
| Maximum | 9 596 685 | 17 516 768 | 2 508 354 (24.9 %) | 353 | 349 | 340 | 309 | 19 |
| Total | – | 955 336 164 | 108 603 450 (11.06 %) | – | – | – | – | – |
No. raw reads (paired) = numbers of RAW paired-end Illumina reads before filtering; No. reads mapped to target = number of reads mapped to baits target sequences; No. genes recovered = number of genes recovered per sample; % target length >25 % = sequences that were >25 % of the target length or greater; % target length >50 % = sequences that were >50 % of the target length or greater; % target length >75 % = sequences that were >25 % of the target length or greater; No. paralogue warnings = number of genes flagged with paralogue warnings.
Fig. 1.
The number of genes recovered per sample was categorized by the percentage of the target length recovered (green = >25 %, blue = >50 % and yellow = >75 %).
Marker selection for phylogenetic inference
From the 353 genes recovered from HybPiper pipeline, the paragone-nf pipeline was used to remove paralogous genes, which resulted in a final set of 299 genes, after filtering, to be used for downstream analysis, with each final gene alignment ranging from 93 to 3302 bp in length (Table 1). Each alignment was visually inspected using Geneious, and 289 genes, which ranged from 103 to 3302 bp in length, were selected based on alignment quality and inclusion of >80 % of species within the alignment. The concatenation of individual gene alignments in the resulting partitioned alignment had 33.3–85.6 % variable sites and 11.4–54.6 % parsimony-informative sites (Table 2).
Table 2.
Phylogenetic tree statistics generated using IQ-Tree.
| Type | Mean | Minimum | Maximum |
|---|---|---|---|
| Number of taxa | 113.66 | 68 | 115 |
| Percentage of taxa | 98.83 | 59.1 | 100 |
| Alignment length | 725.6 | 103 | 3660 |
| Percentage of variable sites | 58.16 | 33.33 | 85.55 |
| Percentage of parsimony-informative sites | 30.24 | 11.42 | 54.57 |
The table shows the total number of taxa in each partition of the matrix, the total percentage of taxa included in the matrix, the length of the alignment for each partition, the percentage of variable sites in each partition and the percentage of sites that were parsimony informative in each partition.
Phylogenetic reconstruction of Mirbelieae
Maximum likelihood
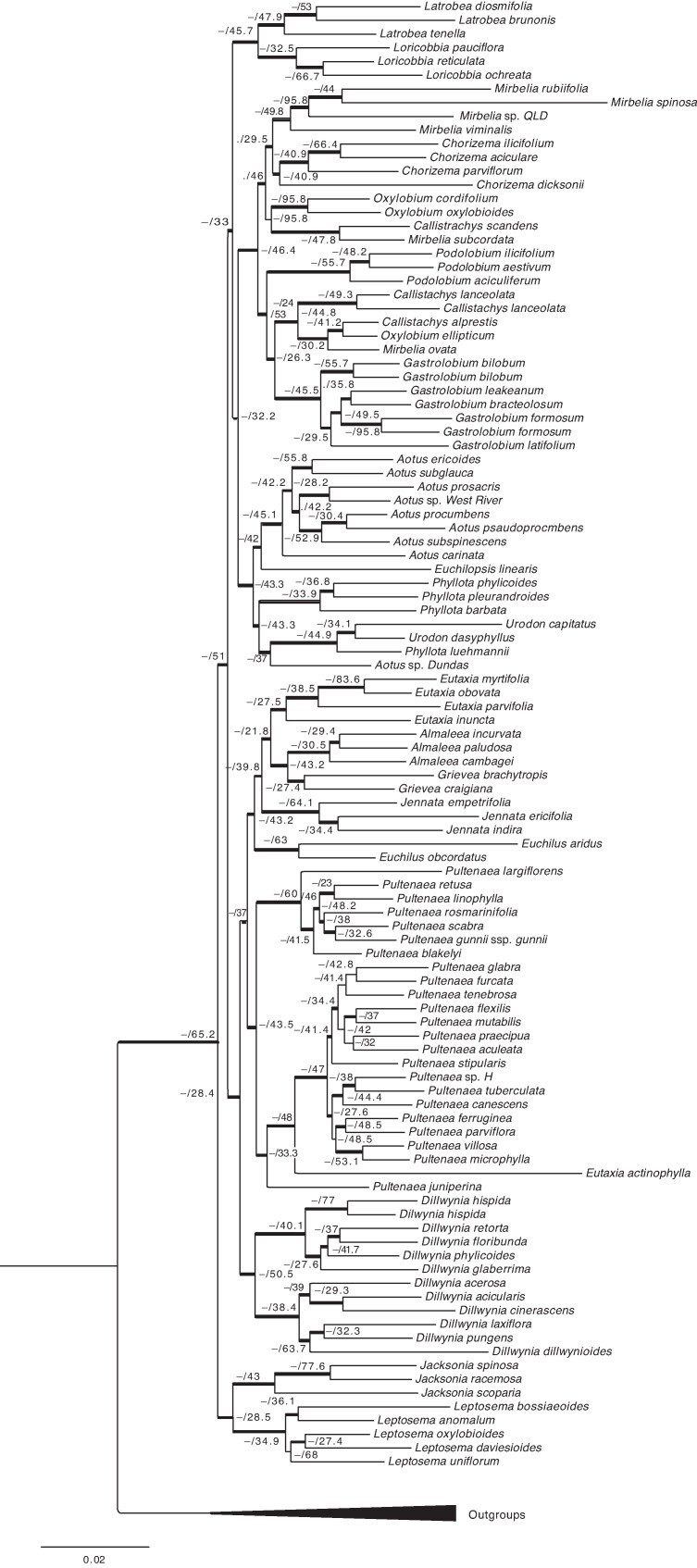
The reconstructed concatenated maximum likelihood phylogenetic tree generated using IQ-Tree and 289 markers/genes (209 271 bases), showed overall good resolution, with all nodes returning bootstrap values of >95 % or higher and sCF ≥ 21.8 % (Fig. 2). Of the 17 traditionally recognized genera included in this study, 11 were found to be monophyletic, with bootstrap support ≥ 95 %: Almaleea Crisp & P.H.Weston, Chorizema Labill., Dillwynia Sm., Euchilopsis F.Muell., Eutaxia R.Br., Gastrolobium, Jacksonia R.Br. ex Sm., Latrobea Meisn., Leptosema Benth., Podolobium R.Br. and Urodon Turcz.). However, Aotus Sm., Callistachys Vent., Mirbelia Sm., Oxylobium Andrews, Phyllota (DC.) DC. ex Benth. and Pultenaea sensu Crisp and Weston (1995) were found to be polyphyletic (Fig. 3). The most densely sampled genus, Pultenaea sensu Crisp and Weston (1995), represented by 33 samples, has representatives in two major clades. The first group comprises 30 taxa from varying geographical ranges, including species that were recently transferred to the new genera Grievea R.L.Barrett, Clugston & Orthia and Jennata R.L.Barrett, Clugston & Orthia, and the reinstated Euchilus R.Br. by Barrett et al. (2024a, b). The group is monophyletic only if Eutaxia and Almaleea are included, rendering the first group of Pultenaea sensu Crisp and Weston (1995) paraphyletic. The second group is small, consisting of a clade of taxa from Western Australia, which Barrett et al. (2024a, b) transferred to the new genus Loricobbia R.L.Barrett, Clugston & Orthia [L. pauciflora (M.B.Scott) R.L.Barrett & T.D.Macfarl., L. reticulata (Sm.) R.L.Barrett & T.D.Macfarl. and L. ochreata (Meisn.) R.L.Barrett & T.D.Macfarl.], which are sister to the sympatric Western Australian genus Latrobea.
Fig. 2.
Maximum likelihood phylogenetic tree showing 115 taxa in Mirbelieae, including outgroups. The numbers at each node indicate the following: (1) SH-like approximate likelihood ratio test (SH-aLRT); (2) UltraFast Bootstrap (UFBoot); and (3) site concordance factors (sCF). The SH-aLRT or/and UFBoot values of >95 % are indicated by a thickened branch and -/ at each node.
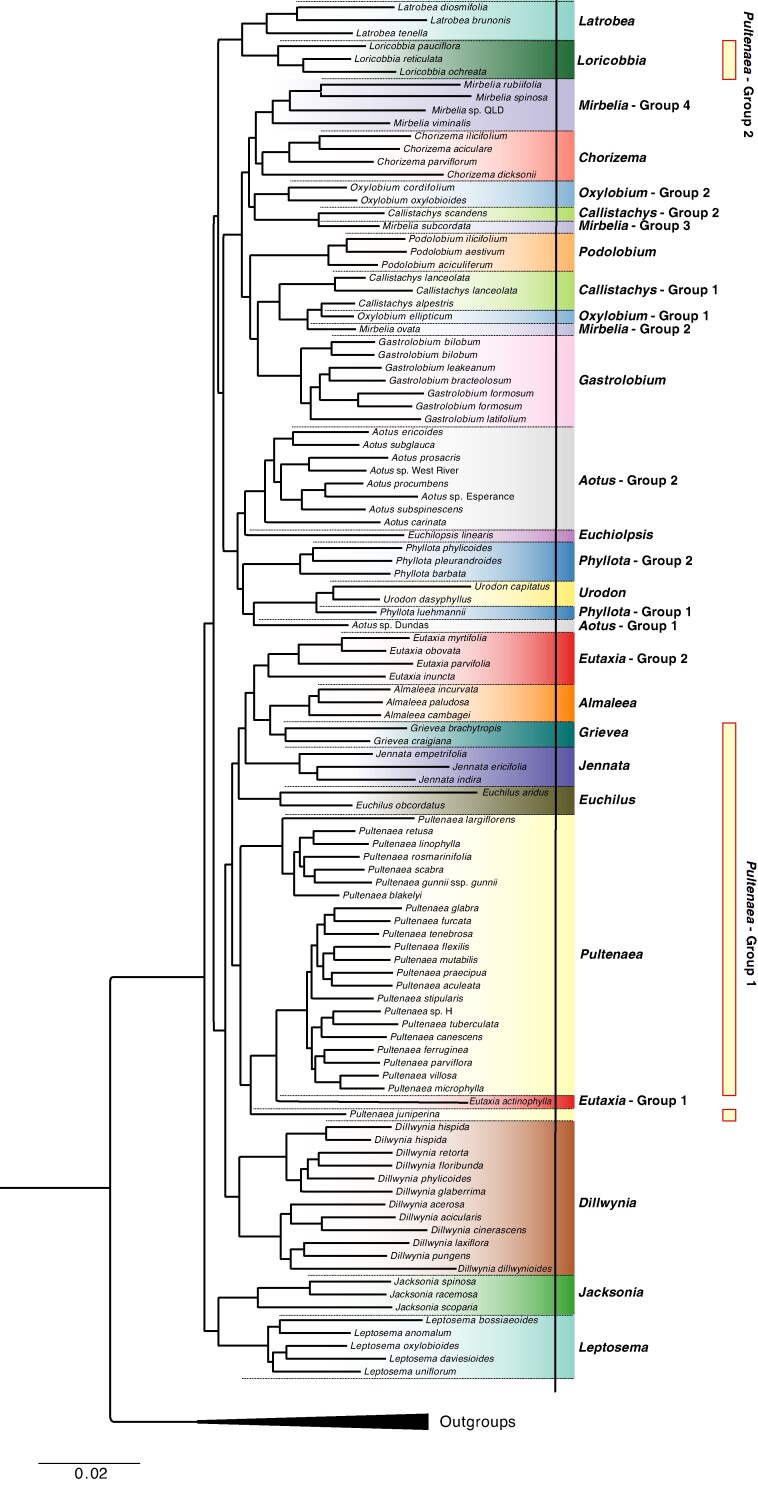
Fig. 3.
Maximum likelihood phylogenetic tree of Mirbelieae with generic groupings. Each separate colour represents a genus based on the current taxonomy (sensu Barrett et al., 2021, 2024c). Each genus with more than a single group is separated, e.g. Aotus Group 1 and Aotus Group 2. The bounded boxes reflect recent taxonomic changes in Pultenaea s.l. to the right of the figure, indicating the Crisp and Weston (1995) concept of Pultenaea and two groups of Pultenaea s.l. recovered.
Coalescent-based species tree
The coalescent-based species tree produced in ASTRAL used the same 289 markers and resulted in a tree topology (Supplementary Data Fig. S1) that was highly congruent with the maximum likelihood-based tree (Fig. 2). Of 106 nodes in each of these two trees, 96 are congruent, including 9 of the 10 most basal nodes in the maximum likelihood tree. The coalescent-based species tree showed some gene tree incongruence, indicated by low quartet support (1–192) values generated by ASTRAL, especially in the most basal nodes (Supplementary Data Fig. S1).
Gene and site concordance
Site concordance factors were mapped on the maximum likelihood-based gene tree (Fig. 2) and ranged from 21.8 to 95.8 % with a mean of 44.62 %, indicating good site concordance support for the reference topology from the gene trees relative to the number of informative sites supporting the grouping of a given node. The sCF were consistent with the quartet support values provided by ASTRAL.
DISCUSSION
The myBaits Angiosperms353 universal baits set has already shown great promise for its use in both the PAFToL and GAP projects, which are large-scale phylogenomics projects that have investigated the phylogenies of angiosperm genera (Baker et al., 2022; Zuntini et al., 2024; Simpson et al., 2025). For Mirbelieae, the Angiosperms353 bait set showed remarkable marker/gene recovery, with the maximum number of 353 genes being recovered across our data set (100 % of target genes; Fig. 1) and a mean of 351 genes recovered per sample, and 289 genes (81.9 %) were found to be free of paralogues and included in the final assembly, after filtering and quality control.
When these results are compared with other studies using the Angiosperms353 bait set, higher numbers of genes were recovered for Mirbelieae (Fabaceae) than for other plant groups (Fig. 1). For example, the analysis of the family Cornaceae recovered a total of 348 genes with a mean of 325 (Thomas et al., 2021), whereas Clarkson et al. (2021) recovered 353 genes with a mean of 335 in Apiaceae, and Chincoya and Solórzano (2022) recovered only 319 genes with a mean of 173 in the Caryophyllids. This indicates that for the tribe Mirbelieae, our results yielded a higher-than-average ratio of gene recovery (Johnson et al., 2019), and our resulting phylogenetic trees resolve the deep nodes of the phylogeny, with all major clades being supported by >95 % (UFBoot; Fig. 2), thereby demonstrating the potential utility of the Angiosperms353 bait set more broadly in legume systematics.
Resolving phylogenetic relationships in tribe Mirbelieae
Our analyses of sequences targeted by the Angiosperms353 universal baits set largely resolved the relationships between major clades in Mirbelieae (Fabaceae). The tree derived by maximum likelihood analysis of concatenated data was highly congruent with that produced by the coalescent-based analysis of ASTRAL (Figs 1 and 2). These trees were broadly congruent with the results of earlier studies that found several genera to be paraphyletic as then circumscribed, based on Sanger-generated sequence data, although these studies lacked bootstrap support for most major clades (Orthia et al., 2005a, b; Barrett et al., 2021). However, for the first time, we have produced a phylogenetic tree with support for most currently recognized genera. A durable genus-level classification for the tribe can now be proposed for several clades that formed polytomies in previous studies.
The topologies of our phylogenetic trees (Figs 1 and 2) show that 11 of the 17 sampled genera (108 taxa excluding outgroup taxa) in the Mirbelieae were monophyletic, including Almaleea (3 species), Chorizema (4), Dillwynia (12), Euchilopsis (1), Eutaxia (5), Gastrolobium (8), Jacksonia (3), Latrobe (3), Leptosema (5), Podolobium (5) and Urodon (2) (Fig. 3). On the contrary, Aotus (9), Callistachys (3), Mirbelia (8), Oxylobium (3), Phyllota (5) and Pultenaea sensu Crisp and Weston (1995) (32) were found to be polyphyletic or paraphyletic. Both Eutaxia and Almaleea sit within Pultenaea Group 1 (Fig. 3), highlighting the problems associated with the generic classification of Pultenaea sensu Crisp and Weston (1995). Given the size of Fabaceae, challenges to circumscription of genera based on morphological data are nothing new, and the ‘resolution of relationships with molecular data is crucial for progress’ (LPWG, 2017).
Although the branch length of Mirbeilia diliata is above average, high data quality scores suggest that this extended branch length is likely to reflects the geographical isolation of this morphologically unique Western Australian species from its nearest relatives in eastern Australia. Its phylogenetic placement aligns with expectations based on morphology. The position of Eutaxia actinophylla Chappell & C.F.Wilkins is anomalous in comparison to other Eutaxa species included in our study. Although marker recovery exceeded 80 % and sequencing alignment quality fell within acceptable parameters, the corresponding long branch lengths of the taxa raise concerns regarding their position in the presented phylogenies (Figs 2, 3). However, owing to limited sampling within the tribe, we are hesitant to discard the sample. The position of Pultenaea juniperina, on a long branch sister to E. actinophylla and a large clade of Pultenaea (Figs 2, 3), would be likely to improve with the addition of more close relatives of P. juniperina, which are currently lacking in our sampling of the genus. We acknowledge that the isolation of this sample might be causing long-branch attraction to the Eutaxia actinophylla sample, and further sampling is required to confirm or reject the monophyly of Eutaxia.
When comparing the topology of our phylogeny (Fig. 2) with earlier studies, neither Crisp and Weston (1987, 1995) nor Crisp and Cook (2003) nor Orthia et al. (2005a, b) were able to resolve relationships among many genera within the tribe. This raised concerns within the botanical community that relationships within the tribe might be irresolvable owing to rapid radiation or other evolutionary processes (Orthia et al., 2005a, b). The most comprehensively sampled phylogeny of core Mirbelieae, which included 200 taxa and all currently recognized genera, was produced by Barrett et al. (2021). However, they used only a single plastid marker (trnL-F), which provided little statistical support for relationships between these genera. Nonetheless, heir results indicated that the largest genus in Mirbelieae, Pultenaea, was polyphyletic, a consistent result in all molecular studies to date.
High bootstrap support values and larger marker datasets
Our resulting phylogenetic tree (Fig. 2) shows good branch support (based on ultrafast bootstrap support). However, in phylogenomic datasets incorporating many markers, high branch support can often be misleading because branch support values, such as bootstrap or posterior probability, are unable to detect discordance between gene trees and species trees (Roycroft et al., 2019; Chan et al., 2020). Discordance between genes can be attributable to many processes, including homoplasy within gene trees, hybridization, gene and genome duplication, horizontal gene transfer and incomplete lineage sorting (ILS) (Léveillé-Bourret et al., 2018).
The use of Angiosperms353 provided strong support for our maximum likelihood phylogenetic tree (Fig. 2), with sCF indicating medium to high support (mean = 44.62 %); the ultrafast bootstrap shows support across most nodes, with 95 % or greater support for all nodes of the tree. Our results compare well with those of other studies based on the Angiosperms353 bait set. For example, Giaretta et al. (2022) used Angiosperms353 to study the genus Eugenia L. (Myrtaceae) and found bootstrap-supported deep nodes, with average sCF (65.4 %) being similar to ours. Using sCF is important because traditional bootstrap may not measure branch support accurately on larger marker set phylogenies (Chan et al., 2020). Our results for the Mirbelieae show that although the sCF is lower than our bootstrap support, sCF could still provide strong support for the tree topology (Fig. 2).
When we compare the maximum likelihood phylogenetic tree (Fig. 2) with the coalescent phylogenetic tree generated in ASTRAL (Supplementary Data Fig. S1), their topologies are largely congruent. However, the concordance factors calculated by ASTRAL are lower than those calculated through IQ-Tree, suggesting some gene conflict from ASTRAL or potentially differential algorithms for calculating support (see Zhang et al., 2018). Although this could indicate that incomplete lineage sorting, hybridization or rapid radiation around the deeper nodes might be playing a role in gene tree conflict in ASTRAL (Reddy et al., 2017), the maximum likelihood tree was mostly unaffected (see discussion by Walker et al., 2017; Carlsen et al., 2018; Stull et al., 2021; Carruthers et al., 2022). Carlsen et al. (2018) found that gene and genome duplication could directly influence gene conflict in the phylogenetic signal, indicating that rapid radiation can result in gene conflict in phylogenies. However, despite our high marker recovery, few paralogues were detected, indicating that gene duplication is not likely to have been a confounding process in Mirbelieae.
The 289 markers used from Angiosperm353 are generally conserved (McDonnell et al., 2021); the markers provided sufficient phylogenetic signal to resolve phylogeny, despite the possibility that Mirbelieae has undergone rapid evolution. The discrepancies in support between our maximum likelihood and coalescence phylogenetic trees (Figs 2 and 3) are likely to be attributable to the differences in the mathematical models selected for the support indices that each method can use. Additionally, the sCF from IQ-Tree was calculated using the new algorithms first introduced in IQ-Tree v.2.2.2, aiming to enhance accuracy in the larger and denser marker set datasets (Mo et al., 2023), which accounts for our strong sCF. Overall, our findings demonstrate robust bootstrap support (Fig. 2) and indicate ample phylogenetic signal in the data to provide valuable insights into relationships within Mirbelieae. This enables us to deduce generic relationships among genera within the group and establishes a solid groundwork for a more detailed investigation into the origins and diversification within the tribe.
Phylogenetic relationships of taxa within Mirbelieae
Although our resulting trees included only ∼20 % of taxa in Mirbelieae, our sampling was undertaken carefully to ensure representation of morphological and biogeographical diversity in the tribe. Significantly, Almaleea, Chorizema, Dillwynia, Euchilopsis, Eutaxia, Gastrolobium, Jacksonia, Latrobea, Leptosema, Podolobium and Urodon were found to be monophyletic and supported as discrete genera (Figs 2, 3). These results demonstrate that the phylogenetic relationships of monophyletic taxa are directly correlated with traditional morphology and taxonomy to a high degree (Barrett et al., 2021, 2024c).
However, Aotus, Callistachys, Mirbelia, Oxylobium, Phyllota and Pultenaea sensu Crisp and Weston (1995) were found to be either polyphyletic or paraphyletic (Figs 2, 3), which indicates homoplasy amongst the morphological characters traditionally used to define these genera. This highlights the importance of careful sampling, which is crucial to improving generic classification within Mirbelieae. Our findings contrast with those of Barrett et al. (2021), because we found Mirbelia and Phyllota to be polyphyletic, possibly owing to differences in taxon sampling (200 versus 115 in this study) or owing to the use of a single genetic marker when compared with 289 in this study. Our results indicate that the morphological characters used to define genera within Mirbelieae are not always consistent with their phylogenetic relationships.
Pultenaea sensu Crisp and Weston (1995) is the largest genus in Mirbelieae, and our results showed that species fell into two main clades within the broader Mirbelieae (Fig. 2): the core Pultenaea (Group 1; with species in eastern and Western Australia) and a small group of species endemic to Western Australia (Group 2; Orthia et al., 2005c). Species in Group 1 do not form a monophyletic group, because Eutaxia and Almaleea are also nested within this clade, and both are defined by distinct morphology (Crisp and Weston, 1991; Wilkins et al., 2010). The morphological distinction of several clades had already been recognized by Orthia et al. (2005c), and their Pultenaea clades D and E (the P. quaerita and P. ericifolia groups) correspond to the clades we recovered as being more closely related to Almaleea and Eutaxia rather than their Pultenaea clades A–C. Their clade F (the P. reticulata group) was likewise recovered as sister to Latrobea.
To rectify the supported polyphyly of Pultenaea reported here, Barrett et al. (2024c) reinstated the genus Euchilus and created three new genera (Grievea, Jennata and Loricobbia), thereby creating a monophyletic concept of Pultenaea. These taxonomic changes were supported by our present results and are strongly congruent with diagnostic morphological features (Orthia et al., 2005c, Barrett et al., 2024c: tables 1 and 2). Congruent with earlier studies based on Sanger sequence data, Zuntini et al. (2024) have confirmed that the monotypic genus Stonesiella Crisp & P.H.Weston also belongs in this clade.
This combined Pultenaea clade 1, plus Almaleea, Eutaxia and Stonesiella, can now be divided into seven subclades. All seven subclades can be recognized readily using morphological characters, and therefore we believe they can be classified readily at generic rank.
Pulteanea sensu stricto, with all but one species (Pultenaea tenuifolia R.Br. ex Sims) endemic to eastern Australia.
The reinstated genus Euchilus (formerly the Pultenaea quaerita group), with all but one species endemic to Western Australia.
The recently named genus Jennata (formerly the Pultenaea ericifolia group), endemic to Western Australia.
The recently named genus Grievea (formerly the Pultenaea brachytropis–P. craigiana group), endemic to Western Australia.
Stonesiella, endemic to eastern Australia.
Almaleea, endemic to eastern Australia.
Eutaxia, with species in both eastern and Western Australia.
Recognition of Euchilus, Grievea and Jennata required the least number of name changes to create monophyletic generic concepts in this clade, in part because many species in the Euchilus clade already had names available in that genus. The three species of Almaleea form a clade that is sister to a small clade consisting of Pultenaea brachytropis Benth. and Pultenaea craigiana C.F.Wilkins, Orthia & Crisp, and, in turn, they are all sister to the four species of the monophyletic Eutaxia (see Wilkins et al., 2009). Morphological examination suggests that P. brachytropis and P. craigiana are dissimilar to Almaleea, justifying their recognition as a distinct genus, Grievea. We acknowledge that Pultenaea adunca Turcz. also belongs in this clade (Barrett et al., 2021), but its relationships to these genera remain uncertain.
Aotus is split into two main groups: Group 1 contains only Aotus sp. Dundas (M.A.Burgman 2835), and Group 2 with nine other species, which is sister to Euchilopsis. The species in Group 2 are monophyletic and can together be recognized by morphological characters. Phyllota is separated into two groups, with the inclusion of Aotus sp. Dundas and two Urodon species in the same clade. The definition of genera in this group has been problematic for some time, with Crisp and Weston (1995) proposing that an additional genus might be needed. An alternative option might be the expansion of Urodon to include Phyllota luehmannii F.Muell. and Aotus sp. Dundas, both of which have morphological affinities to Urodon. An expanded Urodon would form a sister genus to Phyllota s.s. Both Oxylobium and Callistachys are polyphyletic (Fig. 2), but denser sampling is required to infer their detailed phylogenetic relationships.
Generic limits in Mirbelieae
Taking the recent reclassification of Pultenaea into account (Barrett et al., 2024c), our results support the ongoing recognition of 16 monophyletic genera (Almaleea, Chorizema, Dillwynia, Euchilopsis, Euchilus, Eutaxia, Gastrolobium, Grievea, Jacksonia, Jennata, Latrobea, Leptosema, Loricobbia, Podolobium, Pultenaea and Urodon), because this presents the greatest taxonomic and nomenclatural stability, and there are now established morphological concepts for each of these genera. Mirbelia, Oxylobium, Phyllota, Podolobium and Urodon are all likely to require some minor modification to their circumscriptions.
For the genera that were shown to be polyphyletic (Aotus, Callistachys, Eutaxia, Mirbelia, Oxylobium and Phyllota; Fig. 3), each case must be considered based on phylogenetic relationships, morphological distinctiveness and nomenclatural stability. Further sampling is likely to be required for some clades before new classifications can be proposed. Aotus is paraphyletic, because a single taxon, Aotus sp. Dundas, is more closely related to Urodon, a position previously suspected based on morphological affinity. The main Aotus clade recovered here contains eight taxa (44 % of accepted species), and it appears that the genus can be rendered monophyletic simply by the removal of the informally named Aotus sp. Dundas. Phyllota is represented by 5 of the 10 currently accepted species (50 %), and although four of the five taxa group together, Phyllota luehmannii sits as sister to Urodon. This species was somewhat anomalous in Phyllota, hence a close relationship with Urodon is not surprising, and the two genera are closely related in any case.
Barrett et al. (2021) recovered a tree in which Mirbelia was monophyletic based on the samples included, but as our results show, a small number of additional species do not resolve within the main Mirbelia clade. This suggests that the morphological character that currently defines Mirbelia (a dividing septum in the fruit) requires reassessment. With only 7 taxa represented of 25 accepted species (28 %), more detailed sampling, including all taxa of Callistachys (sensu Barrett et al., 2021) and Oxylobium, is required prior to any re-circumscription of Mirbelia. The position of Podolobium (Callistachys) scandens is conflicting between Barrett et al. (2021) and our results, and it is possible that one of these results is erroneous owing to sample contamination.
Pultenaea is the largest and most diverse genus in Mirbelieae, containing >130 named species (Orthia et al., 2005a, b, c; Renner et al., 2022; Telford et al., 2022; Barrett et al., 2024a, b), and our assembly includes only 25.5 % of these (33 species). As outlined above, Pultenaea, as it is traditionally defined, needed to be split into five separate genera to achieve monophyly. A small group represented by three taxa, all endemic to south-west Western Australia, is sister to Latrobea and only distantly related to Pultenaea s.s. This clade is also morphologically distinct from Latrobea, hence it has been named a new genus, Loricobbia. Our results show that the majority of species in the traditional concept of Pultenaea form a single clade that also includes Almaleea and Eutaxia (based on Zuntini et al., 2014; Stonesiella). Most of the species traditionally included in Pultenaea form a clade (based on our limited sampling), and all but one of these species is endemic to eastern Australia, providing clear geographical cohesion. This core Pultenaea clade is also readily definable morphologically (Barrett et al., 2024c). An expanded Pultenaea would have contained significant morphological variability, and a synapomorphy for the broader clade 1 (Pultenaea s.l. plus Almaleea, Eutaxia and Stonesiella) is yet to be identified. Orthia et al. (2005b) suggested that one way to resolve the genetic limits in Mirbelieae would have been to combine all core Mirbelioid genera into a greatly expanded Pultenaea, but this option left much to be desired, because 76 % of taxa in that clade would have changed names, and the resulting mega-genus would have been highly morphologically diverse and difficult to define. Conversely, establishing a small number of additional genera or modest modifications to existing genera will require <8 % of names to change to establish monophyletic genera (Barrett et al., 2021, 2024c), and we are progressively enacting this as the preferable option.
In addition to changes still required at generic rank in Mirbelieae, species diversity in the already large genus Pultenaea remains significantly under-described, with current morphology-based species definitions appearing to be too broad in many instances. This has resulted in multiple species groups being recognized as highly variable morphological entities. For example, Renner et al. (2022) used population genomics to assess genetic differences between different morphotypes of Pultenaea glabra Benth. Their work demonstrated that P. glabra represented a species complex composed of nine genetically and morphologically distinct entities, with eight being new to science. Likewise, Barrett et al. (2024a) found support for 18 species (14 requiring new names) in the Pultenaea setulosa species complex, which could indicate that there are as many if not more undescribed species in other species complexes, e.g. P. polifolia and P. procumbens s.l. Notably, Renner et al. (2022) also found evidence for hybridization between Pultenaea flexilis Sm. and P. glabra. Given this, hybridization can also be expected to occur in other Mirbelieae genera.
To assess putative hybridization in target capture datasets properly, both parental species are needed to determine the hybrid status of an individual (Nauheimer et al., 2021). These results might help to explain incidences of minor incongruence between gene trees and species trees (Fig. 2; Supplementary Data Fig. S1) in the present study (see Twyford and Ennos, 2012). Our current sampling suggests that Pultenaea s.s. has three major clades. If more detailed sampling supports these clades, then it might be justified to recognize them as subgenera to facilitate our understanding of diversity within this large genus.
Conclusion
Core genera in Mirbelieae (Fabaceae/Leguminosae) represent a significant proportion of Australia’s pea-flowered legume diversity (32 %), but until now their phylogenetic relationships have been largely unresolved. However, advances in next generation sequencing and targeted baits capture are finally enabling us to reveal previously obscure relationships. Universal approaches, such as the Angiosperms353 targeted baits sets, are providing unprecedented resolution to resolve phylogenetic relationships within angiosperms (Zuntini et al., 2024).
The paraphyly of Pultenaea sensu Crisp and Weston (1995) has been well demonstrated and is confirmed by our study. However, in contrast to previous studies, we present the first resolved phylogeny of Mirbelieae and demonstrate that 11 of the 17 traditionally recognized genera in core Mirbelieae are monophyletic. With confidence that relationships between Pultenaea and other genera of core Mirbelieae are supported, a new genus-level classification is now both possible and justified for major clades. Specifically, these results have been used to create a monophyletic concept of Pultenaea, the largest genus in the tribe, through the reinstatement of Euchilus and the description of three new genera (Barrett et al., 2024c).
The Angiosperms353 universal bait set has proved to be of great value for legume phylogenetics and understanding plant relationships. For phylogenomic data to inform the taxonomy of some small clades within Mirbelieae, greater taxon sampling is still required. Nevertheless, our results demonstrate that target enrichment sequencing for the generation of phylogenomic data can provide an excellent foundation for resolving and stabilizing taxonomic concepts and providing a new understanding of morphological diversity in the Mirbelieae.
Supplementary Material
ACKNOWLEDGEMENTS
We wish to thank and acknowledge the Australian Biological Resources Study for providing the funding for a postdoctoral research fellowship funded by the National Taxonomy Research Grant Program (NTRGP). The University of Queensland provided funding for sequencing and project outputs. At the Royal Botanic Gardens and Domain Trust, Dr Hervé Sauquet provided guidance and support throughout the project. Andrew Orme, Dr Claire Brandenburger, Kayla Claravall and Scott Yates provided support in the field to collect herbarium and DNA material for the project. Tamera Beath at the Australian National Botanic Garden assisted with sample collection from their living collections. We would like to acknowledge the contribution of the Genomics for Australian Plants Framework Initiative consortium (https://www.genomicsforaustralianplants.com/consortium/) in the generation of data used in this publication. The Initiative is supported by funding from Bioplatforms Australia (enabled by NCRIS), the Ian Potter Foundation, Royal Botanic Gardens Foundation (Victoria), Royal Botanic Gardens Victoria, the Royal Botanic Gardens and Domain Trust, the Council of Heads of Australasian Herbaria, CSIRO, Centre for Australian National Biodiversity Research and the Department of Biodiversity, Conservation and Attractions, Western Australia. Todd McLay and Gareth Holmes (Royal Botanic Gardens Victoria) are thanked for generation of MEL herbarium data used in this publication. The Genomics for Australian Plants Initiative is supported by funding from Bioplatforms Australia (enabled by NCRIS), the Ian Potter Foundation, Royal Botanic Gardens Foundation (Victoria), Royal Botanic Gardens Victoria, the Royal Botanic Gardens and Domain Trust, the Council of Heads of Australasian Herbaria, CSIRO, Centre for Australian National Biodiversity Research and the Department of Biodiversity, Conservation and Attractions, Western Australia. We acknowledge the provision of computing and data resources provided by the Australian BioCommons Leadership Share (ABLeS) program. This program is co-funded by Bioplatforms Australia (enabled by NCRIS), the National Computational Infrastructure and Pawsey Supercomputing Centre.
Contributor Information
James A R Clugston, National Herbarium of New South Wales, Australian Botanic Garden, Mount Annan, NSW 2567, Australia; Montgomery Botanical Center, Coral Gables, FL 33156-4242, USA; Hawkesbury Institute for the Environment, Western Sydney University, Penrith, NSW 2751, Australia.
Russell L Barrett, National Herbarium of New South Wales, Australian Botanic Garden, Mount Annan, NSW 2567, Australia; Evolution and Ecology Research Centre, School of Biological, Earth, and Environmental Sciences, University of New South Wales Sydney, Kensington, NSW 2052, Australia.
Daniel J Murphy, Royal Botanic Gardens Victoria, Melbourne, VIC 3004, Australia.
Matthew A M Renner, National Herbarium of New South Wales, Australian Botanic Garden, Mount Annan, NSW 2567, Australia.
Peter H Weston, National Herbarium of New South Wales, Australian Botanic Garden, Mount Annan, NSW 2567, Australia.
Lyn G Cook, School of Biological Sciences, The University of Queensland, Brisbane, QLD 4072, Australia.
Peter C Jobson, National Herbarium of New South Wales, Australian Botanic Garden, Mount Annan, NSW 2567, Australia.
Brendan J Lepschi, Centre for Australian National Biodiversity Research, Australian National Herbarium, a Joint Venture Between CSIRO and Parks Australia, Canberra, ACT 2601, Australia.
Michael D Crisp, School of Biological Sciences, The University of Queensland, Brisbane, QLD 4072, Australia; Research School of Biology, The Australian National University, Acton, ACT 2601, Australia.
SUPPLEMENTARY DATA
Supplementary data are available at Annals of Botany online and consist of the following. Table S1: table showing all samples. Table S2: table showing the recovery statistics from the Hybpiper output for all samples. Figure S1: ASTRAL phylogenetic tree for tribe Mirbelieae. Node values above represent the number of markers concordant to a given node, and node values below indicate the number of discordant markers. Pie charts represent the proportion of markers that support the topology. Blue, markers supporting the topology; green, markers conflicting with the topology (most commonly a conflicting bipartition); red, markers conflicting with the topology (all other supported conflicting bipartitions); grey, markers with no support for a conflicting bipartition.
FUNDING
Australian Biological Resources Study provided the funding for a postdoctoral research fellowship funded by the DCCEEW Australian Biological Resources Study; National Taxonomy Research Grant Program [Activity Id 4-EHP5TK3].
Genomics for Australian Plants Framework Initiative consortium (AATOL Stage 2: Fabaceae tribe Mirbelieae).
REFERENCES
- Aecyo P, Marques A, Huettel B, et al. 2021. Plastome evolution in the Caesalpinia group (Leguminosae) and its application in phylogenomics and populations genetics. Planta 254: 27. doi: 10.1007/s00425-021-03655-8 [DOI] [PubMed] [Google Scholar]
- Andermann T, Torres Jiménez MF, Matos-Maraví P, et al. 2020. A guide to carrying out a phylogenomic target sequence capture project. Frontiers in Genetics 10: 1407. doi: 10.3389/fgene.2019.01407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews S. 2010. FastQC: a quality control tool for high throughput sequence data. Babraham Bioinformatics. https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (15 January 2025, date last accessed).
- Antonelli A, Clarkson JJ, Kainulainen K, et al. 2021. Settling a family feud: a high-level phylogenomic framework for the Gentianales based on 353 nuclear genes and partial plastomes. American Journal of Botany 108: 1143–1165. doi: 10.1002/ajb2.1697 [DOI] [PubMed] [Google Scholar]
- Baker WJ, Bailey P, Barber V, et al. 2022. A comprehensive phylogenomic platform for exploring the angiosperm tree of life. Systematic Biology 71: 301–319. doi: 10.1093/sysbio/syab035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett RL, Clugston JAR, Albrecht DE, et al. 2024a. Revision of the Pultenaea setulosa species complex (Fabaceae: Mirbelieae) including 14 new species. Australian Systematic Botany 37: 1–105. doi: 10.1071/SB23014 [DOI] [Google Scholar]
- Barrett RL, Clugston JAR, Cook LG, et al. 2021. Understanding diversity and systematics in Australian Fabaceae tribe Mirbelieae. Diversity 13: 391. doi: 10.3390/d13080391 [DOI] [Google Scholar]
- Barrett RL, Clugston JAR, Jobson PC, Rossington P. 2024b. Pultenaea rubescens (Fabaceae: Mirbelieae), a new species from north-east New South Wales. Telopea 27: 203–209. doi: 10.7751/telopea19689 [DOI] [Google Scholar]
- Barrett RL, Clugston JAR, Orthia LA, et al. 2024c. East rarely meets West: a revised delimitation for Pultenaea (Fabaceae: Mirbelieae) with reinstatement of Euchilus and three new genera from south-west Western Australia. Australian Systematic Botany 37: 1–31. doi: 10.1071/SB23029 [DOI] [Google Scholar]
- Breinholt JW, Carey SB, Tiley GP, et al. 2021. A target enrichment probe set for resolving the flagellate land plant tree of life. Applications in Plant Sciences 9: e11406. doi: 10.1002/aps3.11406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadhurst L, Coates D. 2017. Plant conservation in Australia: current directions and future challenges. Plant Diversity 39: 348–356. doi: 10.1016/j.pld.2017.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne M, Steane DA, Joseph L, et al. 2011. Decline of a biome: evolution, contraction, fragmentation, extinction and invasion of the Australian mesic zone biota. Journal of Biogeography 38: 1635–1656. doi: 10.1111/j.1365-2699.2011.02535.x [DOI] [Google Scholar]
- Byrne M, Yeates DK, Joseph L, et al. 2008. Birth of a biome: insights into the assembly and maintenance of the Australian arid zone biota. Molecular Ecology 17: 4398–4417. doi: 10.1111/j.1365-294X.2008.03899.x [DOI] [PubMed] [Google Scholar]
- Carlsen MM, Fér T, Schmickl R, Leong-Škorničková J, Newman M, Kress WJ. 2018. Resolving the rapid plant radiation of early diverging lineages in the tropical Zingiberales: pushing the limits of genomic data. Molecular Phylogenetics and Evolution 128: 55–68. doi: 10.1016/j.ympev.2018.07.020 [DOI] [PubMed] [Google Scholar]
- Carruthers T, Sun M, Baker WJ, Smith SA, de Vos JM, Eiserhardt WL. 2022. The implications of incongruence between gene tree and species tree topologies for divergence time estimation. Systematic Biology 71: 1124–1146. doi: 10.1093/sysbio/syac012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KO, Hutter CR, Wood PL, Grismer LL, Brown RM. 2020. Target-capture phylogenomics provide insights on gene and species tree discordances in Old World treefrogs (Anura: Rhacophoridae). Proceedings: Biological Sciences 287: 20202102. doi: 10.1098/rspb.2020.2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Zhou Y, Chen Y, Gu J. 2018. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34: i884–i890. doi: 10.1093/bioinformatics/bty560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chincoya DA, Solórzano S. 2022. Effectiveness of two universal angiosperm probe sets tested in silico for Caryophyllids taxa with emphasis on cacti species. Genes 13: 570. doi: 10.3390/genes13040570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi I-S, Cardoso D, de Queiroz LP, et al. 2022. Highly resolved papilionoid legume phylogeny based on plastid phylogenomics. Frontiers in Plant Science 13: 823190. doi: 10.3389/fpls.2022.823190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson JJ, Zuntini AR, Maurin O, et al. 2021. A higher-level nuclear phylogenomic study of the carrot family (Apiaceae). American Journal of Botany 108: 1252–1269. doi: 10.1002/ajb2.1701 [DOI] [PubMed] [Google Scholar]
- Crisp MD. 1994. Evolution of bird-pollination in some Australian legumes (Fabaceae). In: Eggelton P, Vane-Wright RI. eds. Phylogenetics and ecology. London: Academic Press, 281–309. [Google Scholar]
- Crisp MD, Cook LG. 2003. Molecular evidence for definition of genera in the Oxylobium group (Fabaceae: Mirbelieae). Systematic Botany 28: 705–713. doi: 10.1043/02-69.1 [DOI] [Google Scholar]
- Crisp MD, Weston PH. 1987. Cladistics and legume systematics, with an analysis of the Bossiaeeae, Brongniartieae and Mirbelieae. In: Stirton CH. ed. Advances in legume systematics part 3. Richmond: Royal Botanic Gardens, Kew, 65–130. [Google Scholar]
- Crisp MD, Weston PH. 1991. Almaleea, a new genus of Fabaceae from south-eastern Australia. Telopea 4: 307–311. doi: 10.7751/telopea19914930 [DOI] [Google Scholar]
- Crisp MD, Weston PH. 1995. Mirbelieae. In: Doyle JJ, Crisp MD. eds. Advances in legume systematics part 7: phylogeny. Richmond: Royal Botanic Gardens, Kew, 245–282. [Google Scholar]
- Dear BS, Moore GA, Hughes SJ. 2003. Adaptation and potential contribution of temperate perennial legumes to the southern Australian wheatbelt: a review. Australian Journal of Experimental Agriculture 43: 1–18. doi: 10.1071/EA01202 [DOI] [Google Scholar]
- Elliott TL, Spalink D, Larridon I, et al. 2024. Global analysis of Poales diversification—parallel evolution in space and time into open and closed habitats. The New Phytologist 242: 727–743. doi: 10.1111/nph.19421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folk RA, Kates HR, LaFrance R, Soltis DE, Soltis PS, Guralnick RP. 2021. High-throughput methods for efficiently building massive phylogenies from natural history collections. Applications in Plant Sciences 9: e11410. doi: 10.1002/aps3.11410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaretta A, Murphy B, Maurin O, Mazine FF, Sano P, Lucas E. 2022. Phylogenetic relationships within the hyper-diverse genus Eugenia (Myrtaceae: Myrteae) based on target enrichment sequencing. Frontiers in Plant Science 12: 759460. doi: 10.3389/fpls.2021.759460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godden GT, Jordon-Thaden IE, Chamala S, et al. 2012. Making next-generation sequencing work for you: approaches and practical considerations for marker development and phylogenetics. Plant Ecology & Diversity 5: 427–450. doi: 10.1080/17550874.2012.745909 [DOI] [Google Scholar]
- Govaerts R, Nic Lughadha E, Black N, Turner R, Paton A. 2021. The world checklist of vascular plants, a continuously updated resource for exploring global plant diversity. Scientific Data 8: 215. doi: 10.1038/s41597-021-00997-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson C, McLay T, Schmidt-Lebuhn AN. 2023. hybpiper-nf and paragone-nf: containerization and additional options for target capture assembly and paralog resolution. Applications in Plant Sciences 11: e11532. doi: 10.1002/aps3.11532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MG, Gardner EM, Liu Y, et al. 2016. HybPiper: extracting coding sequence and introns for phylogenetics from high-throughput sequencing reads using target enrichment. Applications in Plant Sciences 4: apps.1600016. doi: 10.3732/apps.1600016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MG, Pokorny L, Dodsworth S, et al. 2019. A universal probe set for targeted sequencing of 353 nuclear genes from any flowering plant designed using k-medoids clustering. Systematic Biology 68: 594–606. doi: 10.1093/sysbio/syy086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. 2017. ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods 14: 587–589. doi: 10.1038/nmeth.4285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates HR, O’Meara BC, LaFrance R, et al. 2024. Shifts in evolutionary lability underlie independent gains and losses of root-nodule symbiosis in a single clade of plants. Nature Communications 15: 4262. doi: 10.1038/s41467-024-48036-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen EJM, Kidner C, Souza ER, et al. 2020a. Hybrid capture of 964 nuclear genes resolves evolutionary relationships in the mimosoid legumes and reveals the polytomous origins of a large pantropical radiation. American Journal of Botany 107: 1710–1735. doi: 10.1002/ajb2.1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen EJM, Ojeda DI, Steeves R, et al. 2020b. Large-scale genomic sequence data resolve the deepest divergences in the legume phylogeny and support a near-simultaneous evolutionary origin of all six subfamilies. The New Phytologist 225: 1355–1369. doi: 10.1111/nph.16290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzer GM, Sochat V, Bauer MW. 2017. Singularity: scientific containers for mobility of compute. PLoS One 12: e0177459. doi: 10.1371/journal.pone.0177459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laity T, Laffan SW, González-Orozco CE, et al. 2015. Phylodiversity to inform conservation policy: an Australian example. The Science of the Total Environment 534: 131–143. doi: 10.1016/j.scitotenv.2015.04.113 [DOI] [PubMed] [Google Scholar]
- Larridon I, Villaverde T, Zuntini AR, et al. 2020. Tackling rapid radiations with targeted sequencing. Frontiers in Plant Science 10: 1655. doi: 10.3389/fpls.2019.01655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larridon I, Zuntini AR, Barrett RL, et al. 2021a. Resolving the generic limits in Cyperaceae tribe Abildgaardieae using targeted sequencing. Botanical Journal of the Linnean Society 196: 163–187. doi: 10.1093/botlinnean/boaa099 [DOI] [Google Scholar]
- Larridon I, Zuntini AR, Léveillé-Bourret E, et al. 2021b. A new classification of Cyperaceae (Poales) supported by phylogenomic data. Journal of Systematics and Evolution 59: 852–895. doi: 10.1111/jse.12757 [DOI] [Google Scholar]
- Léveillé-Bourret É, Starr JR, Ford BA, Moriarty Lemmon E, Lemmon AR. 2018. Resolving rapid radiations within angiosperm families using anchored phylogenomics. Systematic Biology 67: 94–112. doi: 10.1093/sysbio/syx050 [DOI] [PubMed] [Google Scholar]
- LPWG [Legume Phylogeny Working Group] . 2021. The World Checklist of Vascular Plants (WCVP): Fabaceae, version June 2021, Govaerts R ed. http://sftp.kew.org/pub/data_collaborations/Fabaceae/DwCA/ (22 November 2024, date last accessed).
- LPWG [Legume Phylogeny Working Group] . 2017. A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny. Taxon 66: 44–77. doi: 10.12705/661.3 [DOI] [Google Scholar]
- Maurin O, Anest A, Bellot S, et al. 2021. A nuclear phylogenomic study of the angiosperm order Myrtales, exploring the potential and limitations of the universal Angiosperms353 probe set. American Journal of Botany 108: 1087–1111. doi: 10.1002/ajb2.1699 [DOI] [PubMed] [Google Scholar]
- Maurin O, Anest A, Forest F, et al. 2023. Drift in the tropics: phylogenetics and biogeographical patterns in Combretaceae. Global Ecology and Biogeography 32: 1790–1802. doi: 10.1111/geb.13737 [DOI] [Google Scholar]
- McCormack JE, Hird SM, Zellmer AJ, Carstens BC, Brumfield RT. 2013. Applications of next-generation sequencing to phylogeography and phylogenetics. Molecular Phylogenetics and Evolution 66: 526–538. doi: 10.1016/j.ympev.2011.12.007 [DOI] [PubMed] [Google Scholar]
- McDonnell AJ, Baker WJ, Dodsworth S, et al. 2021. Exploring Angiosperms353: developing and applying a universal toolkit for flowering plant phylogenomics. Applications in Plant Sciences 9: e11443. doi: 10.1002/aps3.11443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLay TGB, Birch JL, Gunn BF, et al. 2021. New targets acquired: improving locus recovery from the Angiosperms353 probe set. Applications in Plant Sciences 9: e11420. doi: 10.1002/aps3.11420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLay TGB, Murphy DJ, Holmes GD, et al. 2022. A genome resource for Acacia, Australia’s largest plant genus. PLoS One 17: e0274267. doi: 10.1371/journal.pone.0274267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead RJ, Oliver AJ, King DR, Hubach PH. 1985. The co-evolutionary role of fluoroacetate in plant-animal interactions in Australia. Oikos 44: 55–60. doi: 10.2307/3544043 [DOI] [Google Scholar]
- Minh BQ, Hahn MW, Lanfear R. 2020b. New methods to calculate concordance factors for phylogenomic datasets. Molecular Biology and Evolution 37: 2727–2733. doi: 10.1093/molbev/msaa106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minh BQ, Schmidt HA, Chernomor O, et al. 2020a. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Molecular Biology and Evolution 37: 1530–1534. doi: 10.1093/molbev/msaa015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo YK, Lanfear R, Hahn MW, Minh BQ. 2023. Updated site concordance factors minimize effects of homoplasy and taxon sampling. Bioinformatics 39: btac741. doi: 10.1093/bioinformatics/btac741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B, Forest F, Barraclough T, et al. 2020. A phylogenomic analysis of Nepenthes (Nepenthaceae). Molecular Phylogenetics and Evolution 144: 106668. doi: 10.1016/j.ympev.2019.106668 [DOI] [PubMed] [Google Scholar]
- Nauheimer L, Weigner N, Joyce E, Crayn D, Clarke C, Nargar K. 2021. HybPhaser: a workflow for the detection and phasing of hybrids in target capture data sets. Applications in Plant Sciences 9: aps3.11441. doi: 10.1002/aps3.11441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie JE, Zalucki JM, Boulter SL. 2009. Pollination biology of the sclerophyllous shrub Pultenaea villosa Willd. (Fabaceae) in southeast Queensland, Australia. Plant Species Biology 24: 11–19. doi: 10.1111/j.1442-1984.2009.00235.x [DOI] [Google Scholar]
- Ogutcen E, Christe C, Nishii K, Salamin N, Möller M, Perret M. 2021. Phylogenomics of Gesneriaceae using targeted capture of nuclear genes. Molecular Phylogenetics and Evolution 157: 107068. doi: 10.1016/j.ympev.2021.107068 [DOI] [PubMed] [Google Scholar]
- Orthia LA, Cook LG, Crisp MD. 2005b. Generic delimitation and phylogenetic uncertainty: an example from a group that has undergone an explosive radiation. Australian Systematic Botany 18: 41–47. doi: 10.1071/SB04016 [DOI] [Google Scholar]
- Orthia LA, Crisp MD, Cook LG, de Kok RPJ. 2005a. Bush peas: a rapid radiation with no support for monophyly of Pultenaea (Fabaceae: Mirbelieae). Australian Systematic Botany 18: 133–147. doi: 10.1071/SB04028 [DOI] [Google Scholar]
- Orthia LA, de Kok RPJ, Crisp MD. 2005c. A revision of Pultenaea (Fabaceae: Mirbelieae). 4. Species occurring in Western Australia. Australian Systematic Botany 18: 149–206. doi: 10.1071/SB04029 [DOI] [Google Scholar]
- Peacock D, Williams B, Spande T, Christensen P. 2004. 1080, Toxic sugars, alkaloids, and a dead cat: the search for a toxicant in Australian Gastrolobium seed. In: Timm RM, Gorenzel WP. eds. Proceedings of the vertebrate pest conference. Davis: University of California, 240–246. [Google Scholar]
- Porebski S, Bailey LG, Baum BR. 1997. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Molecular Biology Reporter 15: 8–15. doi: 10.1007/BF02772108 [DOI] [Google Scholar]
- Reddy S, Kimball RT, Pandey A, et al. 2017. Why do phylogenomic data sets yield conflicting trees? Data type influences the avian tree of life more than taxon sampling. Systematic Biology 66: 857–879. doi: 10.1093/sysbio/syx041 [DOI] [PubMed] [Google Scholar]
- Renner MAM, Barrett RL, Clarke S, Clugston JAR, Wilson TC, Weston PH. 2022. Morphological and molecular evidence refute a broad circumscription for Pultenaea glabra (Fabaceae: Mirbelieae), with implications for taxonomy, biogeography, and conservation. Australian Systematic Botany 35: 225–277. doi: 10.1071/SB21030 [DOI] [Google Scholar]
- Ringelberg JJ, Koenen EJM, Iganci JR, et al. 2022. Phylogenomic analysis of 997 nuclear genes reveals the need for extensive generic redelimitation in Caesalpinioideae (Leguminosae). PhytoKeys 205: 3–58. doi: 10.3897/phytokeys.205.85866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson K, Bell LW, Bennett RG, Henry DA, Tibbett M, Ryan MH. 2007. Perennial legumes native to Australia—a preliminary investigation of nutritive value and response to cutting. Australian Journal of Experimental Agriculture 47: 170–176. doi: 10.1071/EA06043 [DOI] [Google Scholar]
- Roycroft EJ, Moussalli A, Rowe KC. 2019. Phylogenomics uncovers confidence and conflict in the rapid radiation of Australo-Papuan rodents. Systematic Biology 69: 431–444. doi: 10.1093/sysbio/syz044 [DOI] [PubMed] [Google Scholar]
- Saunders TC, Larridon I, Baker WJ, et al. 2024. Tangled webs and spider-flowers: phylogenomics, biogeography, and seed morphology inform the evolutionary history of Cleomaceae. American Journal of Botany 111: e16399. doi: 10.1002/ajb2.16399 [DOI] [PubMed] [Google Scholar]
- Shee ZQ, Frodin DG, Cámara-Leret R, Pokorny L. 2020. Reconstructing the complex evolutionary history of the Papuasian Schefflera radiation through herbariomics. Frontiers in Plant Science 11: 258. doi: 10.3389/fpls.2020.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L, Cantrill DJ, Byrne M, et al. 2025. The genomics for Australian plants (GAP) framework initiative—developing genomic resources for understanding the evolution and conservation of the Australian flora. Australian Systematic Botany 38: 1–19. doi: 10.1071/SB24022 [DOI] [Google Scholar]
- Smith SA, Moore MJ, Brown JW, Yang Y. 2015. Analysis of phylogenomic datasets reveals conflict, concordance, and gene duplications with examples from animals and plants. BMC Evolutionary Biology 15: 150. doi: 10.1186/s12862-015-0423-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent JI, Ardley J, James EK. 2017. Biogeography of nodulated legumes and their nitrogen-fixing symbionts. The New Phytologist 215: 40–56. doi: 10.1111/nph.14474 [DOI] [PubMed] [Google Scholar]
- Stull GW, Qu X-J, Parins-Fukuchi C, et al. 2021. Gene duplications and phylogenomic conflict underlie major pulses of phenotypic evolution in gymnosperms. Nature Plants 7: 1015–1025. doi: 10.1038/s41477-021-00964-4 [DOI] [PubMed] [Google Scholar]
- Telford IIRH, Clugston JAR, Barrett RL. 2022. Pultenaea williamsii (Fabaceae: Mirbelieae), a new species endemic to the New England Tableland Bioregion of New South Wales. Telopea 25: 165–171. doi: 10.7751/telopea15746 [DOI] [Google Scholar]
- Thomas SK, Liu X, Du Z-Y, et al. 2021. Comprehending Cornales: phylogenetic reconstruction of the order using the Angiosperms353 probe set. American Journal of Botany 108: 1112–1121. doi: 10.1002/ajb2.1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toon A, Cook LG, Crisp MD. 2014. Evolutionary consequences of shifts to bird-pollination in the Australian pea-flowered legumes (Mirbelieae and Bossiaeeae). BMC Evolutionary Biology 14: 43. doi: 10.1186/1471-2148-14-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twigg LE, King DR, Bowen LH, Wright GR, Eason CT. 1996. Fluoroacetate content of some species of the toxic Australian plant genus, Gastrolobium, and its environmental persistence. Natural Toxins 4: 122–127. doi: 10.1002/19960403NT4 [DOI] [PubMed] [Google Scholar]
- Twyford AD, Ennos RA. 2012. Next-generation hybridization and introgression. Heredity 108: 179–189. doi: 10.1038/hdy.2011.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatanparast M, Powell A, Doyle JJ, Egan AN. 2018. Targeting legume loci: a comparison of three methods for target enrichment bait design in Leguminosae phylogenomics. Applications in Plant Sciences 6: e1036. doi: 10.1002/aps3.1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JF, Yang Y, Moore MJ, et al. 2017. Widespread paleopolyploidy, gene tree conflict, and recalcitrant relationships among the carnivorous Caryophyllales. American Journal of Botany 104: 858–867. doi: 10.3732/ajb.1700083 [DOI] [PubMed] [Google Scholar]
- Weston P, Jordan G. 2017. Evolutionary biogeography of the Australian flora in the Cenozoic Era. Australian vegetation. [Google Scholar]
- Wilkins CF, Chappill JA, Henderson GR. 2010. An account of Eutaxia (Leguminosae: Mirbelieae) with a focus on the Western Australian species. Nuytsia 20: 109–167. doi: 10.58828/nuy00574 [DOI] [Google Scholar]
- Wilkins CF, Orthia LA, Crisp MD. 2009. A new species of Pultenaea (Mirbelieae: Fabaceae) from Kundip, Western Australia. Nuytsia 19: 191–196. doi: 10.58828/nuy00553 [DOI] [Google Scholar]
- Yang Y, Smith SA. 2014. Orthology inference in nonmodel organisms using transcriptomes and low-coverage genomes: improving accuracy and matrix occupancy for phylogenomics. Molecular Biology and Evolution 31: 3081–3092. doi: 10.1093/molbev/msu245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Rabiee M, Sayyari E, Mirarab S. 2018. ASTRAL-III: polynomial time species tree reconstruction from partially resolved gene trees. BMC Bioinformatics 19: 153. doi: 10.1186/s12859-018-2129- [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Soghigian J, Xiang Q-YJ. 2022. A new pipeline for removing paralogs in target enrichment data. Systematic Biology 71: 410–425. doi: 10.1093/sysbio/syab044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuntini AR, Carruthers T, Maurin O, et al. 2024. Phylogenomics and the rise of the angiosperms. Nature 629: 843–850. doi: 10.1038/s41586-024-07324-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.