Abstract
Background
Disrupted cholesterol homeostasis may accelerate cognitive aging. This study investigated the relationship between serum HDL-C levels and cognitive function, utilizing cross-sectional data and Mendelian randomization (MR) analysis.
Methods
A cross-sectional study was conducted using data from the National Health and Nutrition Examination Survey (NHANES) 2011–2014, including 19,931 participants. Among them, 2,777 individuals aged 60 years and older with complete HDL-C levels and cognitive function data were included. Cognitive function was assessed using tests such as the Consortium to Establish a Registry for Alzheimer’s Disease Immediate and Delayed Recall, the Animal Fluency Test, and the Digit Symbol Substitution Test. Additionally, MR analysis was employed to assess the causal relationship between genetically predicted HDL-C and dementia.
Results
Gender-stratified analyses revealed sex-specific patterns in the relationship between HDL-C and cognitive function. In fully adjusted linear models, men showed consistently positive associations across all cognitive domains, including delayed recall (β = 0.10, 95% CI 0.04–0.17, p < 0.001), immediate recall (β = 0.06, 95% CI 0.00–0.12, p = 0.047), verbal fluency (β = 0.20, 95% CI 0.14–0.26, p < 0.001), processing speed (β = 0.09, 95% CI 0.05–0.14, p < 0.001), and overall composite score (β = 0.45, 95% CI 0.29–0.62, p < 0.001). In women, these associations were attenuated or non-significant for immediate recall, delayed recall, and composite cognition, suggesting non-linearity. Further concentration–response analyses revealed a linear positive association in men and an inverted U-shaped relationship in women. MR analyses indicated a protective association between genetically predicted HDL-C and Alzheimer’s disease risk (OR = 0.51, 95% CI 0.29–0.89, p = 0.019). However, sensitivity analyses revealed attenuation after MR-PRESSO outlier correction (β=-0.013, p = 0.756), and inconsistent estimates across methods, with significant heterogeneity (Q-test p < 0.001) and evidence of pleiotropy. In multivariable analysis, adjusting for LDL-C and TG, IVW (β = 0.290, p = 0.048) and Lasso regression (β = 0.752, p = 0.008) indicated weak positive correlations. However, MR-Egger (β = 0.752, p = 0.008) revealed potential pleiotropic interference (intercept p = 0.050).
Conclusions
Our findings suggest that maintaining optimal serum HDL-C levels may help preserve cognitive function in older adults. Notably, sex-specific associations were observed, warranting further investigation into underlying mechanisms.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13040-025-00484-3.
Keywords: Geriatric health, Cognitive function, Blood lipids, HDL-C
Introduction
Given the lack of effective treatments for cognitive impairment, identifying preventive factors is crucial. Cholesterol is key in maintaining cellular structure, supporting neurotransmission, and regulating neuroinflammation [1]. It is also involved in synaptic function and is essential for the integrity of the blood-brain barrier, which is necessary for normal cognitive processes [2]. Consequently, disturbances in cholesterol metabolism are believed to contribute to the pathogenesis of cognitive decline, and several studies have explored the impact of cholesterol on cognitive function [3, 4]. However, the relationship between cholesterol and cognition remains unclear, with inconsistent findings across studies.
Some studies suggested a strong connection between high total cholesterol levels and an increased risk of cognitive impairment [5]. However, subsequent studies have yielded conflicting results. While some have reported that elevated low-density lipoprotein cholesterol (LDL-C) is associated with cognitive decline [6]others found no significant link between LDL-C and cognitive function [7, 8]. Similarly, the role of high-density lipoprotein cholesterol (HDL-C) in cognitive function appears to be more complex [9]. HDL-C, typically regarded as protective in cardiovascular health, has been associated with better cognitive outcomes in certain studies [10–12]whereas others have found no clear association or reported adverse effects [13]. These inconsistencies may reflect differences in study designs, populations, and the methodologies used to assess cholesterol levels and cognition [14].
Beyond methodological differences, a critical yet underexplored factor is the concentration–response relationship between HDL-C levels and cognitive function. Although some studies have suggested a positive association, the nature of this relationship remains unclear [15]. HDL-C may exert differential effects at various concentration levels, with both beneficial and potentially adverse outcomes observed at the extremes [16]. These variations may result from methodological limitations and reflect the lack of systematic investigation into the nonlinear associations across HDL-C concentration ranges. Clarifying this concentration–response relationship is essential for understanding HDL-C’s role in cognitive decline.
To address this gap, we investigated the relationship between serum HDL-C levels and cognitive function using the National Health and Nutrition Examination Survey (NHANES) data from 2011 to 2014. Additionally, we employed Mendelian randomization (MR) analysis to assess the potential causal relationship between HDL-C and dementia, aiming to provide more robust evidence for the role of HDL-C in cognitive function and to explore its possible concentration-response effects.
Methods
Overall study design
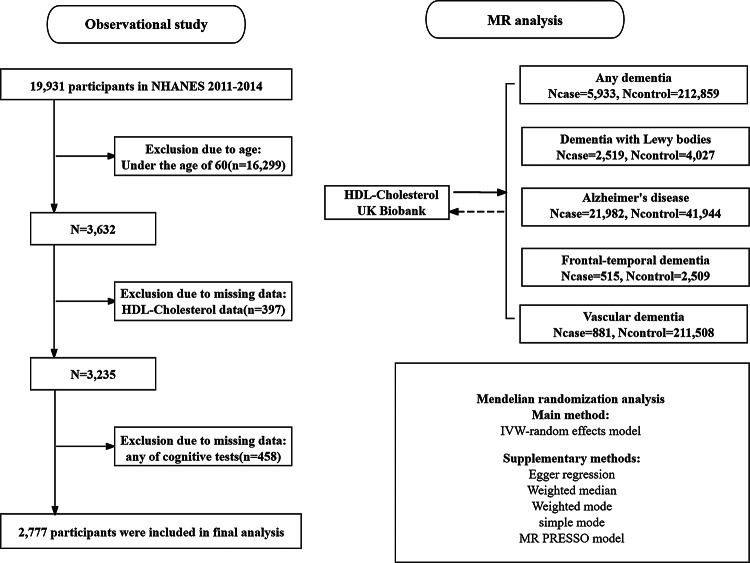
This study consists of two primary components: an observational and an MR analysis, as illustrated in Fig. 1. First, we conducted an observational study using data from the NHANES to evaluate the association between HDL-C levels and cognitive function. Second, we performed an MR analysis to investigate the potential causal relationship between HDL-C levels and five dementia subtypes. To further explore the association between HDL-C and AD, we conducted a multivariable MR (MVMR) analysis to estimate the independent effects of HDL-C, LDL-C, and triglycerides (TG), given their potential interdependence. Additionally, bidirectional MR was employed to assess the possibility of reverse causation between HDL-C levels and cognitive outcomes.
Fig. 1.
Flowchart of NHANES 2011–2014 sample selection and diagram illustrating the MR study design
Observational study
Data collection and study population in NHANES
Data for this study were obtained from the 2011–2012 and 2013–2014 cycles of the NHANES, comprising 19,931 participants. For inclusion in the analysis, participants were required to meet the following criteria: (1) age > 60 years; (2) availability of complete laboratory data for HDL-C levels; and (3) completion of all cognitive function assessments. 2,777 participants met these criteria and were included in the final analysis.
Assessment of HDL-C
HDL-C concentrations were measured using a direct immunoassay technique. In the 2011–2012 cycle, the Roche Modular P chemistry analyzer was employed. In the 2013–2014 cycle, HDL-C was assessed using the Roche Cobas 6000 analyzer and the Roche Modular P system. The distributions of HDL-C were carefully examined, and all values fell within the range of 0.41 to 4.03 mmol/L, which is considered clinically and statistically acceptable. As a result, no outlier trimming or exclusions were performed. For analytical purposes, HDL-C levels were categorized into quartiles: Q1 (≤ 1.10 mmol/L), Q2 (1.10–1.32 mmol/L), Q3 (1.32–1.62 mmol/L), and Q4 (≥ 1.62 mmol/L).
Assessment of cognitive function
Cognitive function was assessed using four standardized tests administered in NHANES: the Consortium to Establish a Registry for Alzheimer’s Disease Word Learning subtests, including Immediate Recall (CERAD-IR) and Delayed Recall (CERAD-DR), as well as the Animal Fluency Test (AFT) and the Digit Symbol Substitution Test (DSST) [17–20]. Each test score was standardized using Z-transformation, and the resulting Z-scores were summed to generate a composite cognitive score, denoted as Sum_Score_Z. Z-scores were calculated using the formula Z = (X-m) / σ, where m is the mean and σ is the standard deviation [21, 22].
Covariates
The covariates in the analysis included age, body mass index (BMI), poverty income ratio (PIR), physical activity, sex, race/ethnicity, marital status, education level, medical history, lipid-lowering drugs use, alcohol use, and smoking status. Age was treated as a continuous variable in years, and BMI was calculated as weight in kilograms divided by height squared in meters. LDL-C, TG, and total cholesterol were measured in mmol/L, while glycated hemoglobin 1c (HbA1c) was expressed as a percentage. PIR indicated socioeconomic status. Physical activity was assessed using standardized questionnaires and divided into four categories: moderate work activity, vigorous work activity, moderate recreational activity, and vigorous recreational activity. Sex was categorized as male or female. Race/ethnicity was classified as Mexican American, Non-Hispanic White, Non-Hispanic Black, or Other. Marital status was classified as living with a partner, married, divorced, single, or living alone. Education level was divided into three groups: ≤ high school, college, and > college. Medical history included the percentage of the population diagnosed with diabetes, congestive heart failure, and coronary heart disease. Lipid-lowering drug use was recorded via self-report. Alcohol use was categorized based on whether participants consumed at least 12 alcoholic drinks per year. Smoking status was categorized as non-smoker (fewer than 100 cigarettes in a lifetime) or current smoker (100 or more cigarettes and currently smoking).
Statistical analysis
The analysis utilized software from X&Y Solutions, Inc. (Beijing, China) in conjunction with R version 3.5.3 (The R Foundation for Statistical Computing, Vienna, Austria). Missing values in covariates were handled using multiple imputation by chained equations (MICE), generating five imputed datasets. Missing values were imputed using a continuous iterative imputation method. Continuous variables were expressed as mean ± standard deviation, while categorical variables were expressed as total counts and percentages. Categorical variables were analyzed using chi-square tests. The normality of continuous variables was first checked. When the normality criteria were met, a one-way analysis of variance was performed, while a Kruskal-Wallis test was conducted when the results were not normally distributed. In this study, after stratification by gender, three sets of progressively adjusted multiple linear regression models were constructed with cognitive scores as the dependent variable: Model 1 was unadjusted; Model 2 adjusted for age, sex, and race/ethnicity; and Model 3 included further adjustments for education level, PIR, BMI, medical history, marital status, alcohol use and smoking status, triglyceride levels, HbA1c levels, and use of lipid-lowering drugs. β indicates the average change in cognitive score for each one mmol/L increase in HDL-C. The 95% CI is the 95% confidence interval, and a value not including zero is considered statistically significant. Additionally, smooth curves were plotted to assess trends to explore the nonlinear relationship between HDL-C and cognitive scores, and then establish a two-segment linear regression model. The maximum likelihood method was used to determine inflection points within the predefined search interval, and the likelihood ratio test compared the goodness-of-fit between the segmented model and the single linear model.
Mendelian randomization
Study design
We conducted a two-sample univariable MR analysis to examine the potential causal effect of HDL-C levels on five dementia outcomes. This approach relies on three core assumptions: (1) the genetic variants used as instruments are strongly associated with HDL-C; (2) they are not related to confounding factors; (3) they affect dementia risk only through HDL-C, not via other pathways. To further rule out the possibility of reverse causality, we also performed a reverse-direction MR analysis, treating dementia as the exposure and HDL-C as the outcome.
Genetic instrument selection and data sources
Using the HG19/GRCh37 reference genome, a large-scale genome-wide association study (GWAS) involving 5,933 cases and 212,859 controls provided insights into the association between genetic polymorphisms and any dementia. The genetic instruments for dementia with Lewy bodies (DLB) were derived from a study conducted by Chia et al. [23]which analyzed sequencing data from 4,027 neurologically healthy controls and 2,591 individuals diagnosed with DLB. Participants were recruited from 44 institutions or consortia, with diagnoses made by established consensus guidelines. Data regarding the correlation between genetic polymorphisms and Alzheimer’s disease (AD) were obtained from a substantial GWAS involving 41,944 control subjects and 21,982 AD patients [24]. Data on the relationship between genetic variants and frontotemporal dementia (FTD) included 515 cases and 2,509 controls from the HG19/GRCh37 reference genome. Genetic instruments for patients with FTD were obtained from a study by Van Deerlin et al. [25]. Details about the relationship between genetic variations and vascular dementia (VD) included 881 cases and 211,508 controls from HG19/GRCh37. The HDL-C data were included in the Nightingale Health Metabolic Biomarkers Phase 1 release, which included 115,078 randomly chosen participants from the UK Biobank cohort. The data were derived from the human metabolite GWAS databases [26]. Among them, the following criteria were included: (1) showed a genome-wide significant association (p < 5 × 10− 8), (2) exhibited independent linkage disequilibrium clumping with r2 < 0.001 and kb < 10,000, and (3) F statistic > 10.
Primary analysis
MR studies were conducted to explore the potential causal connection between HDL-C and five distinct types of dementia. The primary analytical method employed in this investigation was the inverse variance weighted (IVW) approach. Additionally, we used the MR-Egger, weighted median, simple mode, and weighted mode approaches to increase the robustness of our findings.
Bi-directional causality analysis
To analyze the causal associations that exist between HDL-C and each of the five distinct forms of dementia in both directions, the roles of “exposure” and “outcome” were reversed, so that HDL-C was treated as the “exposure” and dementia as the “outcome.” For this purpose, we chose single-nucleotide polymorphisms (SNPs) that demonstrated a significant association with HDL-C, defined by a < 5 × 10− 8 p-value criterion, to serve as instrumental variables.
Sensitivity analysis
To assess the heterogeneity among the SNPs in our investigation, we employed Cochran’s Q test. To evaluate the robustness of our findings, we conducted leave-one-out analyses. Specifically, we systematically excluded each SNP while employing the IVW method to determine the impact of individual genetic variants on our estimates. In addition, MR-PRESSO and MR-Egger regression were used to explore possible horizontal pleiotropy that could influence our findings but is unrelated to the pathway being studied. MR-PRESSO is particularly useful for identifying and adjusting for significant outliers, thereby correcting for horizontal multiplicity effects. This study was reported in agreement with the STROBE guidelines.
Results
Baseline characteristics of participants
Table 1 presents the weighted baseline characteristics of participants by HDL‑C quartile. Significant differences were observed across quartiles (most p < 0.05). Participants in higher HDL‑C quartiles were often female, non‑Hispanic White or non‑Hispanic Black (and less usually Mexican American/Other), and less generally living with a partner/married. They showed higher cognitive test scores (CERAD‑IR/DR, AFT, DSST), greater educational attainment, higher family PIR, greater recreational physical activity (moderate and vigorous), a lower prevalence of current smoking, and lower use of lipid-lowering drugs. In addition, they exhibited a lower prevalence of diabetes, congestive heart failure, and coronary heart disease, together with lower body mass index, triglycerides, and HbA1c concentrations.
Table 1.
Weighted baseline characteristics of included participants
| Characteristics | Quartile of HDL-Cholesterol (mmol/L) | p-value | |||
|---|---|---|---|---|---|
| Q1(<1.10) N = 669 |
Q2 (1.10–1.32) N = 701 |
Q3(1.32–1.62) N = 673 |
Q4 (≥ 1.62) N = 734 |
||
| Age(years) | 68.92 ± 6.72 | 69.09 ± 6.74 | 69.61 ± 6.63 | 70.24 ± 6.95 | 0.001 |
| CERAD immediate recall | 18.93 ± 4.62 | 18.68 ± 4.58 | 19.04 ± 4.65 | 19.64 ± 4.59 | < 0.001 |
| CERAD delayed recall | 0.06 ± 1.00 | 0.13 ± 0.95 | 0.10 ± 1.02 | 0.22 ± 0.98 | 0.010 |
| Animal fluency test | 16.13 ± 5.18 | 16.54 ± 5.56 | 16.73 ± 5.60 | 17.09 ± 4.59 | 0.008 |
| Digit symbol substitution test | 42.50 ± 16.24 | 44.96 ± 16.89 | 47.64 ± 17.50 | 48.50 ± 17.88 | 0.011 |
| BMI (kg/m2) | 30.47 ± 5.67 | 29.82 ± 6.28 | 29.36 ± 6.48 | 26.72 ± 6.13 | < 0.001 |
| LDL-C, mmol/L | 2.68 ± 0.95 | 2.84 ± 0.97 | 2.92 ± 0.94 | 2.93 ± 0.89 | 0.003 |
| Triglycerides, mmol/L | 1.99 ± 1.12 | 1.46 ± 0.66 | 1.27 ± 0.61 | 0.97 ± 0.44 | < 0.001 |
| Total cholesterol, mmol/L | 4.60 ± 1.12 | 4.83 ± 1.17 | 4.98 ± 1.03 | 5.36 ± 1.01 | < 0.001 |
| Family PIR | 2.44 ± 1.57 | 2.48 ± 1.61 | 2.66 ± 1.58 | 2.86 ± 1.60 | < 0.001 |
| HbA1c (%) | 6.39 ± 1.29 | 6.18 ± 1.14 | 5.98 ± 0.95 | 5.75 ± 0.84 | < 0.001 |
| Moderate work activity, n (%) | 28.25 | 28.25 | 26.15 | 27.79 | 0.579 |
| Vigorous work activity, n (%) | 11.81 | 12.55 | 9.96 | 9.31 | 0.190 |
| Moderate recreational activity, n (%) | 34.68 | 36.38 | 40.12 | 43.60 | 0.003 |
| Vigorous recreational activities, n (%) | 5.38 | 7.28 | 11.29 | 12.53 | < 0.001 |
| Sex, n (%) | < 0.001 | ||||
| Male | 71.90 | 58.06 | 38.34 | 29.43 | |
| Female | 28.10 | 41.94 | 61.66 | 70.57 | |
| Race/ethnicity (%) | < 0.001 | ||||
| Mexican American | 10.46 | 10.41 | 8.17 | 6.40 | |
| Non-Hispanic White | 48.88 | 46.66 | 46.66 | 53.00 | |
| Non-Hispanic Black | 19.73 | 19.97 | 25.71 | 25.34 | |
| Other | 20.93 | 22.97 | 19.47 | 15.26 | |
| Marital status (%) | 0.002 | ||||
| Living with partner/married | 62.18 | 61.06 | 55.57 | 53.68 | |
| Divorced/single/living alone | 37.82 | 38.94 | 44.43 | 46.32 | |
| Education level (%) | < 0.001 | ||||
| ≤High school | 55.46 | 50.78 | 46.95 | 42.23 | |
| College | 25.71 | 28.67 | 29.27 | 28.88 | |
| >College | 18.83 | 20.54 | 23.77 | 28.88 | |
| Medical history (%) | |||||
| Diabetes | 33.78 | 27.67 | 20.36 | 11.17 | < 0.001 |
| Congestive heart failure | 10.91 | 7.13 | 5.50 | 4,77 | < 0.001 |
| Coronary heart disease | 15,25 | 10.41 | 5.79 | 5.72 | < 0.001 |
| Lipid-lowering drugs use (%) | < 0.001 | ||||
| Yes | 60.42 | 59.38 | 53.25 | 43.73 | |
| No | 39.58 | 40.72 | 46.75 | 56.26 | |
| Alcohol use (%) | 0.072 | ||||
| Yes | 72.62 | 66.03 | 65.51 | 69.93 | |
| No | 27.38 | 33.97 | 34.34 | 30.07 | |
| Smoking status (%) | < 0.001 | ||||
| Yes | 59.64 | 52.92 | 46.95 | 43.60 | |
| No | 40.36 | 47.08 | 53.05 | 56.13 | |
For continuous variables, data are expressed as mean ± standard deviation and compared using weighted linear regression. Data are expressed as percentages for categorical variables and compared using weighted chi-squared tests
Associations between HDL-C and cognitive function
The results of gender-stratified linear regression indicate that the relationship between HDL-C and cognition exhibits gender specificity (Tables 2 and 3). In the fully adjusted model, male HDL-C was independently positively correlated with all cognitive measures, including delayed recall (β = 0.10, 95% CI 0.04–0.17, p < 0.001), immediate recall (β = 0.06, 95% CI 0.00–0.12, p = 0.047), verbal fluency (β = 0.20, 95% CI 0.14–0.26, p < 0.001), processing speed (β = 0.09, 95% CI 0.05–0.14, p < 0.001), and overall composite score (β = 0.45, 95% CI 0.29–0.62, p < 0.001). Additionally, men in the highest quartile of HDL-C had significantly higher scores on all cognitive function measures than those in the lowest quartile. In contrast, in women, the adjusted positive correlation was attenuated mainly. Continuous HDL-C was no longer significantly associated with immediate or delayed recall (β = 0.03, 95% CI -0.04–0.11, p = 0.401) or composite scores (β = 0.19, 95% CI -0.01–0.39, p = 0.065). However, advantages of verbal fluency and processing speed persisted, primarily in quartile comparisons, suggesting a potential non-linear relationship between HDL and cognitive function in women.
Table 2.
Association of HDL-C with four cognitive test scores in male participants
| Exposure | β (95% CI) | ||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| CERAD immediate recall_Z | |||
| HDL-C, mmol/L | 0.11(0.05, 0.17) | 0.12(0.07, 0.18) | 0.06(0.00, 0.12) |
| HDL-C categories | |||
| ≤ 1.10 mmol/L | 0 [reference] | 0 [reference] | 0 [reference] |
| 1.10 to < 1.32 mmol/L | 0.06(-0.00, 0.13) | 0.04(-0.06, 0.14) | 0.03(-0.07, 0.13) |
| 1.32 to < 1.62 mmol/L | -0.01(-0.08, 0.05) | 0.02(-0.04, 0.08) | -0.06(-0.13, -0.00) |
| ≥ 1.62 mmol/L | 0.14(0.07, 0.20) | 0.16(0.10, 0.22) | 0.07(0.01, 0.13) |
| CERAD delayed recall_Z | |||
| HDL-C, mmol/L | 0.09(0.03, 0.15) | 0.12(0.06, 0.18) | 0.10(0.04, 0.17) |
| HDL-C categories | |||
| ≤ 1.10 mmol/L | 0 [reference] | 0 [reference] | 0 [reference] |
| 1.10 to < 1.32 mmol/L | 0.04(-0.03, 0.10) | 0.07(0.01, 0.13) | 0.05(-0.01, 0.11) |
| 1.32 to < 1.62 mmol/L | 0.07(0.00, 0.13) | 0.11(0.05, 0.18) | 0.05(-0.01, 0.11) |
| ≥ 1.62 mmol/L | 0.12(0.06, 0.19) | 0.16(0.10, 0.22) | 0.12(0.06, 0.19) |
| Animal fluency test_Z | |||
| HDL-C, mmol/L | 0.21(0.15, 0.28) | 0.27(0.21, 0.33) | 0.20(0.14, 0.26) |
| HDL-C categories | |||
| ≤ 1.10 mmol/L | 0 [reference] | 0 [reference] | 0 [reference] |
| 1.10 to < 1.32 mmol/L | 0.05(-0.02, 0.12) | 0.10(0.02, 0.21) | 0.05(-0.01, 0.11) |
| 1.32 to < 1.62 mmol/L | 0.09(0.02, 0.15) | 0.15(0.08, 0.21) | 0.04(-0.02, 0.10) |
| ≥ 1.62 mmol/L | 0.21(0.14, 0.28) | 0.28(0.21, 0.34) | 0.18(0.11, 0.24) |
| Digit symbol substitution test_Z | |||
| HDL-C, mmol/L | 0.19(0.13, 0.25) | 0.23(0.18, 0.28) | 0.09(0.05, 0.14) |
| HDL-C categories | |||
| ≤ 1.10 mmol/L | 0 [reference] | 0 [reference] | 0 [reference] |
| 1.10 to < 1.32 mmol/L | 0.01(-0.05, 0.07) | 0.06(0.00, 0.11) | 0.01(-0.03, 0.06) |
| 1.32 to < 1.62 mmol/L | 0.23(0.17, 0.30) | 0.30(0.25, 0.36) | 0.13(0.09, 0.18) |
| ≥ 1.62 mmol/L | 0.23(0.17,0.29) | 0.27(0.21, 0.32) | 0.10(0.06, 0.15) |
| Sum_Score_Z | |||
| HDL-C, mmol/L | 0.60(0.41, 0.79) | 0.75(0.58, 0.92) | 0.45(0.29, 0.62) |
| HDL-C categories | |||
| ≤ 1.10 mmol/L | 0 [reference] | 0 [reference] | 0 [reference] |
| 1.10 to < 1.32 mmol/L | 0.16(-0.04, 0.36) | 0.31(0.13, 0.49) | 0.18(0.02, 0.34) |
| 1.32 to < 1.62 mmol/L | 0.37(0.17, 0.58) | 0.59(0.41, 0.77) | 0.16(-0.01, 0.33) |
| ≥ 1.62 mmol/L | 0.70(0.50, 0.90) | 0.87(0.69, 1.05) | 0.47(0.30, 0.64) |
The sensitivity analysis converted HDL-C from a continuous to a categorical variable (quartiles). Model 1: Unadjusted. Model 2: Adjusted for age and race/ethnicity. Model 3: Adjusted for age, race/ethnicity, education level, LDL-C, TG, total cholesterol, HbA1c, PIR, BMI, medical history, marital status, physical activity, alcohol use, lipid-lowering drugs use, and smoking status
Table 3.
Association of HDL-C with four cognitive test scores in female participants
| Exposure | β (95% CI) | ||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| CERAD immediate recall_Z | |||
| HDL-C, mmol/L | 0.13(0.08, 0.19) | 0.13(0.08, 0.18) | 0.03(-0.04, 0.11) |
| HDL-C categories | |||
| ≤ 1.10 mmol/L | 0 [reference] | 0 [reference] | 0 [reference] |
| 1.10 to < 1.32 mmol/L | 0.07(0.00, 0.13) | 0.07(0.00, 0.13) | 0.04(-0.03, 0.12) |
| 1.32 to < 1.62 mmol/L | 0.09(0.02, 0.16) | 0.10(0.04, 0.16) | 0.08(0.00, 0.16) |
| ≥ 1.62 mmol/L | 0.20(0.14, 0.27) | 0.22(0.16, 0.29) | 0.07(-0.02, 0.15) |
| CERAD delayed recall_Z | |||
| HDL-C, mmol/L | 0.05(-0.00, 0.10) | 0.06(0.01, 0.11) | 0.03(-0.04, 0.11) |
| HDL-C categories | |||
| ≤ 1.10 mmol/L | 0 [reference] | 0 [reference] | 0 [reference] |
| 1.10 to < 1.32 mmol/L | 0.11(0.04, 0.17) | 0.10(0.04, 0.16) | 0.07(-0.00, 0.14) |
| 1.32 to < 1.62 mmol/L | 0.06(-0.01, 0.12) | 0.08(0.02, 0.14) | 0.07(-0.00, 0.15) |
| ≥ 1.62 mmol/L | 0.14(0.07, 0.20) | 0.17(0.10, 0.23) | 0.06(-0.02, 0.14) |
| Animal fluency test_Z | |||
| HDL-C, mmol/L | 0.12(0.07, 0.17) | 0.27(0.09, 0.19) | 0.06(-0.00, 0.13) |
| HDL-C categories | |||
| ≤ 1.10 mmol/L | 0 [reference] | 0 [reference] | 0 [reference] |
| 1.10 to < 1.32 mmol/L | 0.19(0.12, 0.25) | 0.19(0.13, 0.25) | 0.20(0.13, 0.26) |
| 1.32 to < 1.62 mmol/L | 0.25(0.18, 0.32) | 0.28(0.22, 0.34) | 0.14(0.12, 0.26) |
| ≥ 1.62 mmol/L | 0.20(0.14, 0.27) | 0.23(0.17, 0.29) | 0.19(0.05, 0.20) |
| Digit symbol substitution test_Z | |||
| HDL-C, mmol/L | 0.19(0.13, 0.25) | 0.18(0.13, 0.23) | 0.06(0.00, 0.12) |
| HDL-C categories | |||
| ≤ 1.10 mmol/L | 0 [reference] | 0 [reference] | 0 [reference] |
| 1.10 to < 1.32 mmol/L | 0.15(0.08, 0.22) | 0.15(0.09, 0.21) | 0.09(0.04, 0.15) |
| 1.32 to < 1.62 mmol/L | 0.22(0.15, 0.29) | 0.25(0.19, 0.31) | 0.15(0.09, 0.21) |
| ≥ 1.62 mmol/L | 0.30(0.23,0.37) | 0.30(0.24, 0.36) | 0.10(0.04, 0.17) |
| Sum_Score_Z | |||
| HDL-C, mmol/L | 0.49(0.32, 0.67) | 0.51(0.36, 0.67) | 0.19(-0.01, 0.39) |
| HDL-C categories | |||
| ≤ 1.10 mmol/L | 0 [reference] | 0 [reference] | 0 [reference] |
| 1.10 to < 1.32 mmol/L | 0.51(0.30, 0.73) | 0.51(0.33, 0.69) | 0.40(0.21, 0.60) |
| 1.32 to < 1.62 mmol/L | 0.62(0.40, 0.83) | 0.71(0.52, 0.90) | 0.49(0.28, 0.70) |
| ≥ 1.62 mmol/L | 0.85(0.63, 1.06) | 0.91(0.73, 1.10) | 0.36(0.13, 0.59) |
The sensitivity analysis converted HDL-C from a continuous to a categorical variable (quartiles). Model 1: Unadjusted. Model 2: Adjusted for age and race/ethnicity. Model 3: Adjusted for age, race/ethnicity, education level, LDL-C, TG, total cholesterol, HbA1c, PIR, BMI, medical history, marital status, physical activity, alcohol use, lipid-lowering drugs use, and smoking status
Concentration–response relationship analysis
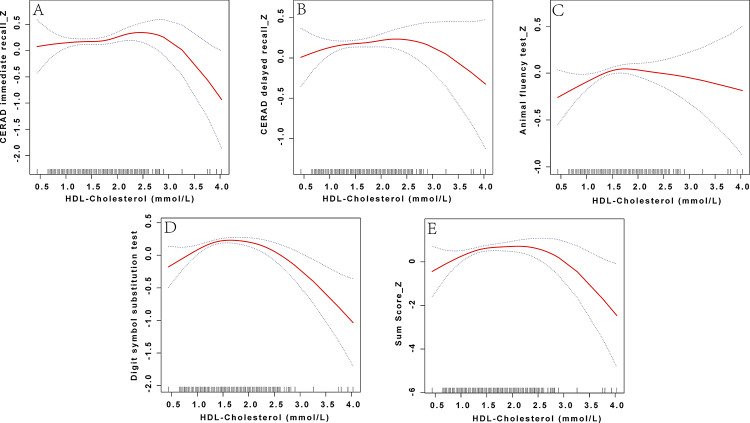
Gender-stratified concentration–Response analyses indicated apparent sex heterogeneity: males showed an approximately linear positive association between HDL-C and cognition, whereas females exhibited a distinct inverted U-shaped pattern with a single inflection point (Figs. 2 and 3). In the male group, the standard linear model showed a significant positive correlation between HDL-C and AFT (β = 0.22, 95% CI 0.07–0.37, p = 0.005) and a marginally significant positive correlation with DSST (β = 0.11, 95% CI 0.00–0.22, p = 0.053), and a positive correlation with Sum_Score_Z (β = 0.42, 95% CI 0.02–0.81, p = 0.038). In contrast, no significant association was observed with CERAD-IR/DR. The two-segment linear model was only marginally better than the linear model in terms of model fit for CERAD-DR (LRT p = 0.018), DSST (LRT p = 0.006), and Sum_Score_Z (LRT p = 0.042), but no clear threshold-driven trend changes were observed (Table 4). In contrast, the linear model was insignificant overall in the female group, while the two-segment linear model showed a significantly better fit. Using a two-step linear regression model, we obtained statistically significant CERAD-IR, AFT, DSST, and total scores with inflection points at 2.28, 1.58, 2.17, and 2.22 mmol/L, respectively. Before the inflection point, AFT (β = 0.37, 95% CI 0.12–0.62, p = 0.004), DSST (β = 0.14, 95% CI 0.02–0.27, p = 0.027), and Sum_Score_Z (β = 0.50, 95% CI 0.08–0.91, p = 0.021) were significantly positively correlated with HDL-C. After the inflection point, CERAD-IR (β = -0.50, 95% CI -0.99−-0.02, p = 0.041), DSST (β = -0.77, 95% CI -1.11−-0.43, p < 0.001), and Sum_Score_Z (β=-1.78, 95% CI -3.02−-0.55, p = 0.005) became negatively correlated (Table 5).
Fig. 2.
Restricted cubic spline curve depicting the relationship between HDL-C and cognitive function in males. HDL-C levels were compared with the Z-scores of four cognitive function tests: CERAD immediate recall (CERAD immediate recall_Z), CERAD delayed recall (CERAD delayed recall_Z), Animal Fluency Test (Animal Fluency Test_Z), and Digit Symbol Substitution Test (Digit Symbol Substitution Test_Z), as well as the composite cognitive score (Sum_Score_Z). Non-linear associations were modeled using restricted cubic splines within generalized additive models, adjusting for age, race/ethnicity, education level, LDL-C, TG, total cholesterol, HbA1c, PIR, BMI, medical history, marital status, physical activity, alcohol use, lipid-lowering drugs use, and smoking status
Fig. 3.
Restricted cubic spline curve depicting the relationship between HDL-C and cognitive function in females. HDL-C levels were compared with the Z-scores of four cognitive function tests: CERAD immediate recall (CERAD immediate recall_Z), CERAD delayed recall (CERAD delayed recall_Z), Animal Fluency Test (Animal Fluency Test_Z), Digit Symbol Substitution Test (Digit Symbol Substitution Test_Z), and the composite cognitive score (Sum_Score_Z). Non-linear associations were modeled using restricted cubic splines within generalized additive models, adjusting for age, race/ethnicity, education level, LDL-C, TG, total cholesterol, HbA1c, PIR, BMI, medical history, marital status, physical activity, alcohol use, lipid-lowering drugs use, and smoking status
Table 4.
Analysis of the concentration‒response relationship between HDL-C and cognitive function in male participants
| CERAD immediate recall_Z | CERAD delayed recall_Z | Animal fluency test_Z | Digit symbol substitution test_Z | Sum_Score_Z | |
|---|---|---|---|---|---|
| Fitting by the standard linear model | 0.04 (-0.11, 0.19) 0.582 | 0.05 (-0.10, 0.20) 0.492 | 0.22 (0.07, 0.37) 0.005 | 0.11(-0.00,0.22)0.053 | 0.42(0.02,0.81)0.038 |
| Fitting by the two-piecewise linear model | |||||
| Inflection point | 1.99 | 0.93 | 0.83 | 1.32 | 1.99 |
| < K segment effect | 0.09 (-0.09, 0.27) 0.319 | 1.26 (0.23, 2.28) 0.016 | -1.02 (-2.77, 0.73) 0.002 | 0.40(0.16,0.64)<0.001 | 0.69(0.21,1.16)0.005 |
| >K segment effect | -0.21(-0.75, 0.32) 0.433 | -0.03(-0.20,0.13)0.705 | 0.25(0.09,0.41)0.034 | -0.07(-0.25,0.10)0.007 | -0.99(-2.42,0.44)0.175 |
| Log likelihood ratio | 0.324 | 0.018 | 0.160 | 0.006 | 0.042 |
Two-segment generalized linear models (GLM) were used and adjusted for age, race/ethnicity, education level, LDL-C, TG, total cholesterol, HbA1c, PIR, BMI, medical history, marital status, physical activity, alcohol use, lipid-lowering drugs use, and smoking status
Table 5.
Analysis of the concentration‒response relationship between HDL-C and cognitive function in female participants
| CERAD immediate recall_Z | CERAD delayed recall_Z | Animal fluency test_Z | Digit symbol substitution test_Z | Sum_Score_Z | |
|---|---|---|---|---|---|
| Fitting by the standard linear model | 0.06 (-0.07, 0.18) 0.397 | 0.03 (-0.09, 0.16) 0.606 | 0.06 (-0.06, 0.18) 0.306 | -0.02(-0.13,0.08)0.658 | 0.13(-0.22,0.48)0.475 |
| Fitting by the two-piecewise linear model | |||||
| Inflection point | 2.28 | 2.25 | 1.58 | 2.17 | 2.22 |
| < K segment effect | 0.15 (-0.00, 0.30) 0.054 | 0.10 (-0.05, 0.26) 0.174 | 0.37 (0.12, 0.62) 0.004 | 0.14(0.02,0.27)0.027 | 0.50(0.08,0.91)0.021 |
| >K segment effect | -0.50(-0.99, -0.02) 0.041 | -0.36(-0.83,0.10)0.128 | -0.16(-0.36,0.04)0.116 | -0.77(-1.11,-0.43)<0.001 | -1.78(-3.02,-0.55) 0.005 |
| Log likelihood ratio | 0.017 | 0.080 | 0.005 | <0.001 | <0.001 |
Two-segment GLM were used and adjusted for age, race/ethnicity, education level, LDL-C, TG, total cholesterol, HbA1c, PIR, BMI, medical history, marital status, physical activity, alcohol use, lipid-lowering drugs use, and smoking status
MR analysis of HDL-C and dementia
This study employed MR to explore the causal relationship between HDL-C and dementia, utilizing large-scale GWAS data (Figure S1). The initial IVW analysis revealed a nominally significant negative association between HDL-C and AD (OR = 0.51, 95% CI 0.29–0.89, p = 0.019), suggesting a potential protective effect of HDL-C. MR-Egger regression did not show significant directional pleiotropy (intercept p = 0.09). After outlier correction using MR-PRESSO, sensitivity analysis demonstrated that the association between HDL-C and AD was no longer significant (β=-0.013, p = 0.756). Furthermore, robust methods, including the weighted median and weighted mode, failed to detect significant associations, and substantial heterogeneity was observed (Q_pval < 0.001). In univariable MR analysis, we found no significant associations between HDL-C and the other four types of dementia. Additionally, bidirectional MR analysis showed no significant correlation between HDL-C and dementia outcomes, suggesting the absence of reverse causality.
In multivariable analysis, adjusting for LDL-C and TG, IVW (β = 0.290, p = 0.048) and Lasso regression (β = 0.752, p = 0.008) indicated weak positive correlations. However, MR-Egger (β = 0.752, p = 0.008) revealed potential pleiotropic interference (intercept p = 0.050).
Overall, these results indicate that the current evidence does not support a robust independent causal effect of HDL-C on AD or other types of dementia. These findings highlight the need for future studies to refine the selection of instrumental variables and consider the complexity of multi-gene regulatory networks.
Discussion
Using large-scale population data, this study identified a significant nonlinear, inverted U-shaped relationship between serum HDL-C levels and cognitive function. Specifically, moderate HDL-C levels were associated with better cognitive function, whereas low and high levels were linked to cognitive decline. This association remained robust after adjusting for confounding factors. These findings challenge the traditional notion that “higher HDL-C is always better,” offering new insights into lipid metabolism in neurodegenerative disease and informing future preventive strategies.
Previous research has predominantly viewed HDL-C as a protective factor for cognitive function, often reporting a linear positive association. For example, Atzmon et al. found significantly better cognitive performance associated with elevated HDL-C in healthy elderly populations. At the same time, Liu et al.’s longitudinal study showed that increased HDL-C was linked to a lower risk of dementia [15, 27]. However, these studies largely overlooked the potential adverse effects of excessively high HDL-C levels and rarely employed nonlinear models to assess concentration–response relationships systematically. By using nonlinear modeling, our study identified critical physiological thresholds, suggesting that the protective effects of HDL-C may reverse at higher concentrations. This advances the current understanding of the complex lipid–cognition relationship.
Mechanistically, HDL-C concentration alone may not fully reflect HDL’s biological functionality. Recent studies have emphasized the importance of HDL’s functional properties, such as cholesterol efflux capacity (CEC), over its concentration in predicting clinical outcomes. Rosenson et al. reported that chronic inflammation or oxidative stress can convert HDL from an anti-inflammatory and antioxidative molecule into a dysfunctional, pro-inflammatory particle [28, 29]. Other studies have shown that CEC predicts cardiovascular events more effectively than HDL-C concentration [30, 31]. Recently, Giacona et al. found that small HDL particles and their CEC were associated with increased gray matter volume, suggesting that HDL subtypes may play a key role in neuroprotection [16]. Although our study lacked functional and subtype-specific HDL data, these findings suggest that dysfunctional HDL may underlie the cognitive risks observed at high HDL-C levels. Future studies should explore the interaction between HDL functionality and the central nervous system.
Sex differences in the association between HDL-C and cognitive function have drawn increasing attention. Some studies have shown that total cholesterol and LDL-C levels are significantly higher in female AD patients carrying the APOE ε4 allele. In contrast, lower cholesterol levels are observed in male AD patients with the APOE ε2 allele, suggesting a complex interplay between genetic background and sex-specific lipid metabolism [32]. Other studies indicate that the association between HDL-C and cognition may be more pronounced in males [4]. These discrepancies may be partly attributed to hormonal influences on lipid-regulating enzymes and sex-specific changes in HDL structure and function. HDL may also influence synaptic plasticity and neuroinflammation through hormone-mediated brain signaling pathways. In our sex-stratified analysis, the association between HDL-C and cognitive function appeared stronger in females than in males. Specifically, cognitive function in females exhibited a more prominent inverted U-shaped (nonlinear) response to HDL-C levels. At the same time, in males, the association was linear and more consistent across HDL-C concentrations. These findings suggest that women may be more sensitive to the cognitive consequences of dysregulated HDL metabolism, potentially due to differences in hormonal regulation or HDL functionality [13, 33]. Further studies are warranted to investigate sex-specific mechanisms in the HDL–cognition relationship and to inform personalized interventions [34].
Clinically, our findings have important implications. Current lipid management guidelines do not define specific HDL-C targets and emphasize overall risk reduction [35]. However, our results suggest the potential need for upper thresholds and careful monitoring in individuals with elevated HDL-C. Functional assays such as CEC and HDL proteomics help identify individuals with dysfunctional HDL profiles who may be at increased cognitive risk. Given the heterogeneity of dementia subtypes, future lipid management strategies should consider more targeted, subtype-specific approaches.
Despite providing deeper insights into HDL-C and cognitive function, our study has several limitations. Firstly, although MR analysis enhanced causal inference, its validity depends on the selection and strength of genetic instruments, and the lack of stringent multiple testing corrections could raise concerns about chance associations [36, 37]. Secondly, the predominantly European and North American populations analyzed limit the generalizability of the findings to other ethnic groups. Thirdly, due to data constraints, we could not stratify by ApoE ε4 genotype, a gene closely linked to lipid metabolism and AD pathogenesis, which might affect the comprehensiveness of our conclusions. Additionally, although we create a comprehensive cognitive score through the Z-score to enhance the reliability and validity of cognitive assessment, the diagnostic criteria for cognitive impairment are defined as a comprehensive score that is one standard deviation below the mean. This method differs from more commonly used screening tools such as MMSE and MoCA, and may limit the applicability of our research results to other populations. Lastly, despite rigorous control of confounding factors and sensitivity analyses, residual confounding from unmeasured variables cannot be excluded. The absence of HDL functional, bioactive, and subtype particle data limits mechanistic exploration. Future research should integrate multi-omics approaches, longitudinal studies, and animal experiments to clarify the mechanisms and causal pathways by which HDL dysfunction influences cognitive impairment.
Conclusion
This study provides evidence that maintaining optimal serum HDL-C levels may play a role in preserving cognitive function in older adults. Gender-stratified analyses reveal distinct sex-specific patterns, with men showing a consistent positive linear association, while women exhibit a nonlinear inverted U-shaped relationship. These findings highlight the complexity of the HDL-C cognition link and suggest that women may be more vulnerable to the cognitive effects of dysregulated HDL metabolism. Further research is needed to explore the underlying mechanisms and guide personalized cognitive health interventions.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Material 2: Figure S1: Forest plot of MR studies investigating the effect of HDL-C levels on various types of dementia. A significant causal relationship was observed between HDL-C levels and AD, but no such relationship was found with any other type of dementia
Acknowledgements
The authors thank the original study’s investigators for sharing the GWAS summary statistics and the National Health and Nutrition Examination Survey.
Abbreviations
- HDL-C
high-density lipoprotein cholesterol
- MR
Mendelian randomization
- NHANES
National Health and Nutrition Examination Survey
- LDL-C
low-density lipoprotein cholesterol
- MVMR
multivariable MR
- TG
triglycerides
- CERAD-IR
Consortium to Establish a Registry for Alzheimer’s Disease- Immediate Recall
- CERAD-DR
Consortium to Establish a Registry for Alzheimer’s Disease- Delayed Recall
- AFT
Animal Fluency Test
- DSST
Digit Symbol Substitution Test
- BMI
Body mass index
- PIR
Poverty income ratio
- HbA1c
Glycated hemoglobin 1c
- MICE
Multiple imputation by chained equations
- GWAS
Genome-wide association study
- DLB
Dementia with lewy bodies
- AD
Alzheimer’s disease
- FTD
Frontotemporal dementia
- VD
Vascular dementia
- IVW
Inverse variance weighted
- SNPs
Single nucleotide polymorphisms
- CEC
Cholesterol efflux capacity
- GLM
Generalized linear models
Author contributions
Corresponding author: Zheyu Zhang. LMF and HTJ have contributed equally to this work and share first authorship. Conceptualisation: all authors. Acquisition, analysis, or interpretation of data: LMF. Drafting of the manuscript: LMF and HTJ. Critical revision of the manuscript for important intellectual content: ZYZ. Statistical analysis: HTJ. Obtained funding: ZYZ. All the authors read and approved the final manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (No.82104967), the Natural Science Foundation of Hunan Province (No.2022JJ40728), and the Scientific Research Launch Project for new employees of the Second Xiangya Hospital of Central South University.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study is a reanalysis of publicly available data. Ethical approval was obtained for the original GWAS and the National Health and Nutrition Examination Survey. In addition, no individual-level data were used in this study. Therefore, no new ethical review board approval was required.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Longmin Fan and Haitao Jiang contributed equally to this work.
References
- 1.Kao Y-C, Ho P-C, Tu Y-K, et al. Lipids and alzheimer’s disease. IJMS. 2020;21:1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brain cell type. -specific cholesterol metabolism and implications for learning and memory. Trends Neurosci. 2022;45:401–14. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Mao Y, Xu T, et al. A cross sectional study of the diabetes mediated GGT to HDL ratio and cognitive function in older adults. Sci Rep. 2025;15:20928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pancani S, Sofi F, Cecchi F, et al. HDL cholesterol is independently associated with cognitive function in males but not in females within a cohort of nonagenarians: the Mugello study. J Nutr Health Aging. 2019;23:552–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillum RF, Obisesan TO. High-density lipoprotein cholesterol, cognitive function and mortality in a U.S. National cohort. Lipids Health Dis. 2011;10:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li L, Zhuang L, Xu Z, et al. U-shaped relationship between non-high-density lipoprotein cholesterol and cognitive impairment in Chinese middle-aged and elderly: a cross-sectional study. BMC Public Health. 2024;24:1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein LB, Toth PP, Dearborn-Tomazos JL, et al. Aggressive LDL-C Lowering and the Brain: Impact on Risk for Dementia and Hemorrhagic Stroke: A Scientific Statement From the American Heart Association. ATVB; 43. Epub ahead of print October 2023. 10.1161/ATV.0000000000000164 [DOI] [PubMed]
- 8.Chou P-S, Chen SC-J, Hsu C-Y, et al. The association between electronegative Low-Density lipoprotein cholesterol L5 and cognitive functions in patients with mild cognitive impairment. JPM. 2023;13:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hottman DA, Chernick D, Cheng S, et al. HDL and cognition in neurodegenerative disorders. Neurobiol Dis 2014; 72 Pt A: 22–36. [DOI] [PMC free article] [PubMed]
- 10.Jin Y, Chifodya K, Han G, et al. High-density lipoprotein in alzheimer’s disease: from potential biomarkers to therapeutics. J Controlled Release. 2021;338:56–70. [DOI] [PubMed] [Google Scholar]
- 11.Poliakova T, Wellington CL. Roles of peripheral lipoproteins and cholesteryl ester transfer protein in the vascular contributions to cognitive impairment and dementia. Mol Neurodegeneration. 2023;18:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Button EB, Gilmour M, Cheema HK, et al. Vasoprotective functions of High-Density lipoproteins relevant to alzheimer’s disease are partially conserved in Apolipoprotein B-Depleted plasma. IJMS. 2019;20:462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bakeberg MC, Gorecki AM, Kenna JE, et al. Elevated HDL levels linked to poorer cognitive ability in females with parkinson’s disease. Front Aging Neurosci. 2021;13:656623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y-H, Chen M-T, He Y-Y, et al. Cognitive impairment and depression precede increased HDL-C levels in middle-aged and older Chinese adults: cross-lagged panel analyses. Lipids Health Dis. 2024;23:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atzmon G, Gabriely I, Greiner W, et al. Plasma HDL levels highly correlate with cognitive function in exceptional longevity. J Gerontol Biol Sci Med Sci. 2002;57:M712–715. [DOI] [PubMed] [Google Scholar]
- 16.Giacona JM, Wang J, Zhang R, et al. Associations between High-Density lipoprotein cholesterol efflux and brain grey matter volume. J Clin Med. 2024;13:6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henry JD, Crawford JR, Phillips LH. Verbal fluency performance in dementia of the alzheimer’s type: a meta-analysis. Neuropsychologia. 2004;42:1212–22. [DOI] [PubMed] [Google Scholar]
- 18.Clark LJ, Gatz M, Zheng L, et al. Longitudinal verbal fluency in normal aging, preclinical, and prevalent alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2009;24:461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canning SJD, Leach L, Stuss D, et al. CME diagnostic utility of abbreviated fluency measures in alzheimer disease and vascular dementia. [DOI] [PubMed]
- 20.Hoyer WJ, Stawski RS, Wasylyshyn C, et al. Adult age and digit symbol substitution performance: A Meta-Analysis. Psychol Aging. 2004;19:211–4. [DOI] [PubMed] [Google Scholar]
- 21.Smagula SF, Zhang G, Gujral S, et al. Association of 24-Hour activity pattern phenotypes with depression symptoms and cognitive performance in aging. JAMA Psychiatry. 2022;79:1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao J, Deng L, Sun C, et al. Association between age at first birth and cognitive function in women 60 years and older: the 2011–2014 cross-sectional National health and nutrition examination survey (NHANES) study. BMC Public Health. 2025;25:1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chia R, Sabir MS, Bandres-Ciga S, et al. Genome sequencing analysis identifies new loci associated with lewy body dementia and provides insights into its genetic architecture. Nat Genet. 2021;53:294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohorts for Heart and Aging Research in Genomic Epidemiology Consortium (CHARGE), et al. Genetic meta-analysis of diagnosed alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat Genet. 2019;51:414–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Deerlin VM, Sleiman PMA, Martinez-Lage M, et al. Common variants at 7p21 are associated with frontotemporal Lobar degeneration with TDP-43 inclusions. Nat Genet. 2010;42:234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richardson TG, Sanderson E, Palmer TM, et al. Evaluating the relationship between Circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: A multivariable Mendelian randomisation analysis. PLoS Med. 2020;17:e1003062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu H, Zou L, Zhou R, et al. Long-Term increase in cholesterol is associated with better cognitive function: evidence from a longitudinal study. Front Aging Neurosci. 2021;13:691423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otocka-Kmiecik A, Mikhailidis DP, Nicholls SJ, et al. Dysfunctional HDL: a novel important diagnostic and therapeutic target in cardiovascular disease? Prog Lipid Res. 2012;51:314–24. [DOI] [PubMed] [Google Scholar]
- 29.Robert SR, Brewer HB, Ansell BJ, et al. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat Rev Cardiol. 2016;13:48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.HDL regulates the. Risk of cardiometabolic and inflammatory-related diseases: focusing on cholesterol efflux capacity. Int Immunopharmacol. 2024;138:112622. [DOI] [PubMed] [Google Scholar]
- 31.Brownell N, Rohatgi A. Modulating cholesterol efflux capacity to improve cardiovascular disease. Curr Opin Lipidol. 2016;27:398–407. [DOI] [PubMed] [Google Scholar]
- 32.Fu J, Huang Y, Bao T, et al. Effects of sex on the relationship between Apolipoprotein E gene and serum lipid profiles in alzheimer’s disease. Front Aging Neurosci. 2022;14:844066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boccardi V, Mancinetti F, Guazzarini AG, et al. Sex-specific associations between serum lipid levels and cognitive performance in older adults: results from a cross-sectional real-world study. Aging Clin Exp Res. 2025;37:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arenaza-Urquijo EM, Boyle R, Casaletto K, et al. Sex and gender differences in cognitive resilience to aging and alzheimer’s disease. Alzheimers Dement. 2024;20:5695–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grundy SM, Stone NJ, Bailey AL, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/ APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary. Circulation. 2019;139:e1082–e1143. [DOI] [PMC free article] [PubMed]
- 36.Zhang Y, Li D, Zhu Z, et al. Evaluating the impact of Metformin targets on the risk of osteoarthritis: a Mendelian randomization study. Osteoarthritis Cartilage. 2022;30:1506–14. [DOI] [PubMed] [Google Scholar]
- 37.Xiang Y, Zhang C, Wang J, et al. Identification of host gene-microbiome associations in colorectal cancer patients using Mendelian randomization. J Transl Med. 2023;21:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 2: Figure S1: Forest plot of MR studies investigating the effect of HDL-C levels on various types of dementia. A significant causal relationship was observed between HDL-C levels and AD, but no such relationship was found with any other type of dementia
Data Availability Statement
No datasets were generated or analysed during the current study.