Abstract
Background
Early identification and treatment of diabetic foot ulcer (DFU) in diabetes mellitus (DM) patients is of great importance for improving life quality. This study aimed to investigate the association between high-density lipoprotein (HDL)-related inflammatory indicators, such as neutrophils-to-HDL ratio (NHR), monocyte-to-HDL ratio (MHR), lymphocyte-to-HDL ratio (LHR), platelet-to-HDL ratio (PHR), and the occurrence of DFU in DM patients.
Methods
This study included 1211 DM patients from the National Health and Nutrition Examination Survey (1999–2004). The relationship between HDL-related inflammatory indicators and DFU was explored with logistic regression models. Using a threshold effects analysis model, the association and inflection points between HDL-related inflammatory indicators and diabetic foot ulcer were investigated. Subgroup analyses were performed to further confirm the relationship in different populations. Mediation analysis was conducted to examine how red blood cell mediates the relationship between HDL-related inflammatory indicators and DFU.
Results
After multivariable adjustment, there is a strongly positive relationship between NHR, MHR, PHR, and DFU, whereas no such associations were found between LHR and DFU. Threshold effect analysis showed an inflection point of 0.29 between MHR and DFU, with a 4.51-fold increase in the prevalence of DFU for each unit rise in MHR when MHR was more than the inflection point. Mediation analysis revealed that red blood cell partially mediates the association between NHR and DFU.
Conclusions
These findings reveal a clear association between NHR, MHR, PHR, and an increased prevalence of DFU, which can be used as potential biomarkers in the prevention and management of DFU.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13098-025-01962-8.
Keywords: Diabetic foot; Lipoproteins, HDL; Neutrophils; Monocytes; Lymphocytes; Blood platelets
Introduction
Diabetes mellitus (DM), with an increase of 630 million patients from 1990, is currently a public health challenge, leading to economic and social burden across countries [1]. It is expected that the prevalence of diabetes will continue to rise due to factors such as obesity, smoking, ageing, and so on [2–4]. As one of the most severe peripheral neuropathy and arterial complications of DM, the underlying mechanism of delayed healing in diabetic foot ulcer (DFU) is often associated with persistent infection, excessive inflammation, and impaired angiogenesis [5, 6]. The overall mortality of DFU can reach nearly 50% mortality within 5 years, while it is also a leading cause of non-traumatic lower-extremity amputations, representing a major clinical challenge to the survival of patients [7–9]. Consequently, early identification and treatment of DFU is of great importance for improving the quality of life for patients.
High-density lipoprotein (HDL), which is often regarded as “good cholesterol” due to its various protective functions, including anti-inflammatory and antioxidant effects, can be associated with the occurrence and progression of a lot of diseases, such as metabolic syndrome and cardiovascular diseases (CVDs) [10]. Specifically, a large-scale population-based study suggests that HDL acts as an independent protective factor against peripheral artery disease (PAD) in patients with DM. This protective effect may, in turn, contribute to a lower risk of developing DFU [11]. Moreover, a machine learning-based study indicates that HDL can be a key predictor for DFU in patients with diabetic peripheral neuropathy [12].
Complete blood count (CBC), consisting of leukocytes, lymphocytes, neutrophils, monocytes, and other blood components, is a common marker for infection, inflammation, and immune function, which can be used to predict the prognosis of diseases [13, 14]. Previous researches demonstrate the relationship between different CBC-derived inflammatory markers and DFU. In a large population study, monocyte-to-lymphocyte ratio (MLR), neutrophil-to-lymphocyte ratio (NLR), neutrophil-monocyte-to-lymphocyte ratio (NMLR), and systemic inflammatory response index (SIRI) showed significant positive correlations with diabetic foot risk [15]. In a matched case-control study, the investigators found that NLR, platelet‒lymphocyte ratio (PLR), and lymphocyte‒white blood cell ratio (LWR) may have value as a predictive marker for amputation in patients with DFU [16]. However, no existing studies were conducted to determine the association between the HDL-related inflammatory indicators, which combined with HDL and CBC, such as neutrophils-to-HDL ratio (NHR), monocyte-to-HDL ratio (MHR), lymphocyte-to-HDL ratio (LHR), platelet-to-HDL ratio (PHR), and occurrence of DFU in individuals with diabetes.
In this study, we aimed to explore the association between NHR, MHR, LHR, PHR, and DFU from data in the National Health and Nutrition Examination Survey (NHANES) for the first time.
Materials and methods
Study design and population
NHANES is a nationwide survey conducted by the National Center for Health Statistics (NCHS), a part of the Centers for Disease Control and Prevention (CDC). The survey involves home health interviews and physical examinations in fully equipped mobile medical centers. Additionally, urine and blood samples are collected from the participants to analyze their health status further. The research protocol was reviewed and approved by the NCHS Ethics Review Board at the CDC, and written informed consent was obtained from all participants.
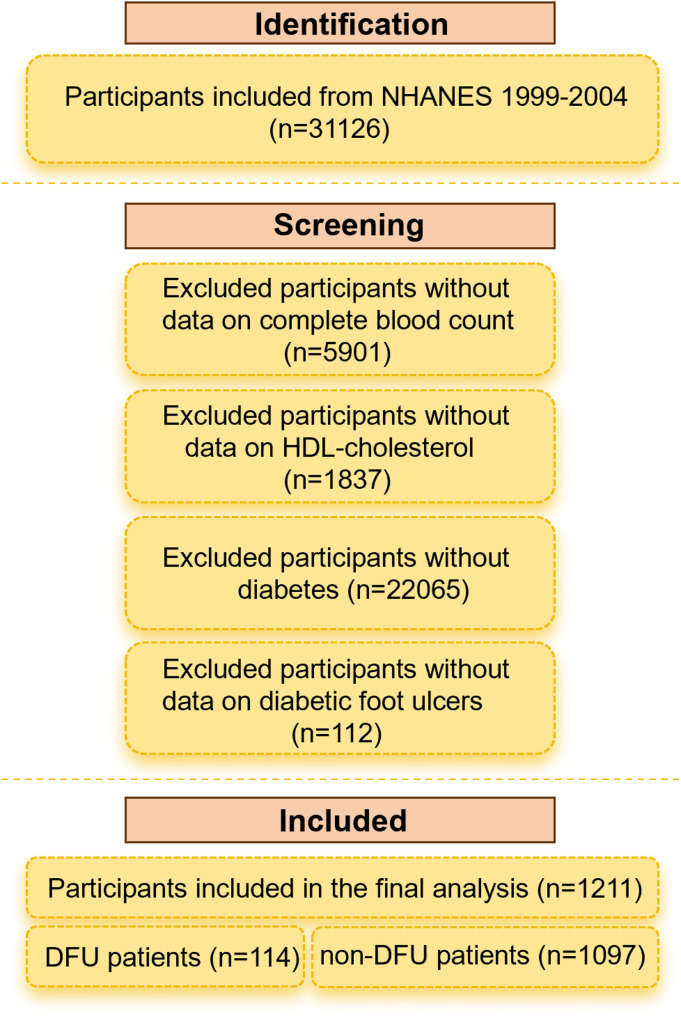
In the study, we excluded participants with missing data on complete blood count (n = 5901) or who did not have data on HDL-cholesterol (n = 1,837), without a diagnosis of DM (n = 22065), or who did not have data on diabetic foot ulcers (n = 112). The final analytic sample included 1211 diabetes patients aged 40–85 years, including 114 DFU patients and 1097 non-DFU patients, as shown in Fig. 1.
Fig. 1.
Flowchart of the selection process for study participants from the National Health and Nutrition Examination Survey (NHANES)
Measurement of HDL-related inflammatory indicators
Blood specimens were measured at the NHANES Mobile Examination (MEC) Centers. The methods used to derive CBC parameters are based on the Beckman Coulter® method of counting and sizing, in combination with an automatic diluting and mixing device for sample processing, and a single beam photometer for hemoglobinometry. The white blood cells (WBC) differential uses VCS technology. Meanwhile, the blood specimens used for HDL measurement were shipped to the Johns Hopkins University Lipoprotein Analytical Laboratory for analysis. The following HDL-related inflammatory indicators were calculated: NHR, MHR, LHR, and PHR using the following formulas: NHR = neutrophil counts/high-density lipoprotein concentration, MHR = monocyte counts/high-density lipoprotein concentration, LHR = lymphocyte counts/high-density lipoprotein concentration, and PHR = platelet counts/high-density lipoprotein concentration.
Definition of diabetes and diabetic foot ulcer
Diabetes was characterized as follows: (1) Having received a medical diagnosis of diabetes by a physician. (2) Fasting blood glucose ≥ 7.0 mmol/L. (3) hemoglobin A1c (HbA1c) ≥ 6.5%. (4) Random blood glucose ≥ 11.1 mmol/L. (5) Administration of antidiabetic drugs. Fulfilling any of these criteria was considered as having diabetes. DFU was identified through survey findings. Specifically, DFU presence was identified through participants’ responses to the question, “Do you have any sores or ulcers on your legs or feet that take more than four weeks to heal?” Participants who answered “Yes” were categorized as having DFU, while those who answered “No” were categorized as not having DFU.
Covariates
Potential confounding factors influencing DFU were carefully considered based on the reference. The covariates considered included age, BMI, gender (male or female), race (White or Non-white), marital status (married or living with a partner, widowed, divorced or separated, never married), family PIR (poverty income ratio), health insurance (Yes or No), CVD (Yes or No), smoking status (every day, some days, not at all), alcohol use, HbA1c, insulin, FPG, CRP, albumin, creatinine and HDL. Supplementary Table S1 offered a specific categorization of the variables.
Statistical analysis
Participants were separated into two groups based on whether they had DFU. Student t-tests and Chi-Square tests tested baseline variable differences. The relationship between HDL-related inflammatory indicators and diabetic foot ulcer was explored with logistic regression models. Model 1, no covariate was adjusted; Model 2 was adjusted for age, gender, and race. Model 3 added to model 2 the marital status and PIR as covariates. Using a threshold effects analysis model, the association and inflection points between HDL-related inflammatory indicators and diabetic foot ulcer were investigated. Subgroup analyses were conducted in terms of age (< 65, ≥ 65), gender (male, female), race(White, Non-white), PIR (< 1.22, 1.22–2.62, and 2.64-5.00), marital status (married or living with a partner, widowed, divorced or separated, never married). Mediation analysis was performed using ‘mediation” in R with 1,000 bootstrap iterations to examine how red blood cell mediates the relationship between HDL-related inflammatory indicators and diabetic foot ulcer. The statistical analyses were conducted using R (Version 4.4.2) and EmpowerStats (version 2.0). A p-value < 0.05 was determined to be significant.
Results
Baseline characteristics of participants
The baseline characteristics of the participants are presented in Table 1. A total of 1,211 adults aged 40–85 years were included in this study, with 114 diagnosed with DFU. The DFU and non-DFU groups displayed some different baseline characteristics, participants with DFU were more likely to have higher BMI and have more CVD events. Among DFU patients, there were significantly higher levels of urine albumin, WBC, neutrophils, basophils, NHR, MHR, PHR, and lower levels of red blood cells (RBCs) and hemoglobin.
Table 1.
Baseline characteristics of the study population
| Characteristics | Total (n = 1211) |
non-DFU (n = 1097) |
DFU (n = 114) |
P value |
|---|---|---|---|---|
| Age (years) | 64.75 ± 11.56 | 64.76 ± 11.55 | 64.61 ± 11.73 | 0.897 |
| BMI (kg/m2) | 30.89 ± 6.56 | 30.79 ± 6.48 | 32.03 ± 7.22 | 0.072 |
| Gender (%) | 0.391 | |||
| Male | 623 (51.45%) | 560 (51.05%) | 63 (55.26%) | |
| Female | 588 (48.55%) | 537 (48.95%) | 51 (44.74%) | |
| Race/Ethnicity (%) | 0.460 | |||
| White | 471 (38.89%) | 423 (38.56%) | 48 (42.11%) | |
| Non-White | 740 (61.11%) | 674 (61.44%) | 66 (57.89%) | |
| Marital status (%) | 0.188 | |||
| Married/Living with a partner | 712 (60.70%) | 653 (61.55%) | 59 (52.68%) | |
| Widowed/Divorced/Separated | 393 (33.50%) | 348 (32.80%) | 45 (40.18%) | |
| Never married | 68 (5.80%) | 60 (5.66%) | 8 (7.14%) | |
| Family PIR | 2.22 ± 1.47 | 2.24 ± 1.48 | 2.06 ± 1.40 | 0.225 |
| Health insurance | 0.771 | |||
| Yes | 1073 (89.49%) | 968 (89.05%) | 105 (92.11%) | |
| No | 126 (10.51%) | 117 (10.76%) | 9 (7.89%) | |
| CVD | 0.017 | |||
| Yes | 371 (30.76%) | 323 (29.55%) | 48 (42.48%) | |
| No | 835 (69.24%) | 770 (70.45%) | 65 (57.52%) | |
| Smoking status | 0.701 | |||
| Every day | 167 (26.05%) | 149 (25.60%) | 18 (30.51%) | |
| Some days | 31 (4.84%) | 28 (4.81%) | 3 (5.08%) | |
| Not at all | 443 (69.11%) | 405 (69.59%) | 38 (64.41%) | |
| Alcohol use (days) | 3.12 ± 21.92 | 3.29 ± 23.00 | 1.51 ± 3.64 | 0.476 |
| HbA1c (%) | 7.47 ± 1.83 | 7.46 ± 1.80 | 7.59 ± 2.11 | 0.479 |
| Insulin, (uU/mL) | 24.80 ± 33.35 | 24.95 ± 34.22 | 23.16 ± 21.76 | 0.725 |
| FPG, (mg/dL) | 166.07 ± 71.77 | 164.67 ± 69.84 | 181.46 ± 89.75 | 0.125 |
| CRP, (mg/dL) | 0.71 ± 1.55 | 0.70 ± 1.57 | 0.85 ± 1.26 | 0.300 |
| Albumin, urine (mg/L) | 260.12 ± 1124.99 | 240.04 ± 1100.98 | 466.15 ± 1335.65 | 0.050 |
| Creatinine, urine (mg/dL) | 110.95 ± 72.96 | 111.21 ± 72.70 | 108.35 ± 75.79 | 0.703 |
| HDL, mg/dL | 1.24 ± 0.36 | 1.24 ± 0.36 | 1.22 ± 0.41 | 0.538 |
| CBC count, 103/µL | ||||
| White blood cell | 7.47 ± 2.18 | 7.43 ± 2.12 | 7.87 ± 2.67 | 0.040 |
| Lymphocyte | 2.14 ± 0.88 | 2.15 ± 0.87 | 2.05 ± 0.95 | 0.268 |
| Monocyte | 0.59 ± 0.20 | 0.58 ± 0.20 | 0.61 ± 0.23 | 0.095 |
| Neutrophils | 4.49 ± 1.66 | 4.44 ± 1.61 | 4.92 ± 2.05 | 0.004 |
| Eosinophils | 0.21 ± 0.15 | 0.21 ± 0.15 | 0.24 ± 0.14 | 0.074 |
| Basophils | 0.04 ± 0.05 | 0.04 ± 0.05 | 0.05 ± 0.06 | 0.047 |
| Red blood cell | 4.60 ± 0.53 | 4.62 ± 0.52 | 4.44 ± 0.52 | < 0.001 |
| Hemoglobin (g/dL) | 13.95 ± 1.59 | 13.99 ± 1.58 | 13.61 ± 1.64 | 0.014 |
| Platelet | 254.80 ± 74.35 | 253.84 ± 73.70 | 263.95 ± 80.07 | 0.167 |
| HDL-related inflammatory indicators | ||||
| NHR | 3.93 ± 1.87 | 3.87 ± 1.79 | 4.51 ± 2.46 | < 0.001 |
| MHR | 0.51 ± 0.23 | 0.51 ± 0.22 | 0.57 ± 0.29 | 0.009 |
| LHR | 1.86 ± 0.95 | 1.86 ± 0.95 | 1.83 ± 0.92 | 0.768 |
| PHR | 219.84 ± 85.76 | 218.14 ± 84.55 | 236.11 ± 95.59 | 0.033 |
BMI, Body Mass Index; PIR, poverty income ratio; CVD, cardiovascular disease; HbA1c, hemoglobin A1c (Glycohemoglobin); FPG, fasting plasma glucose; CRP, C-reactive protein; HDL, high-density lipoprotein; CBC, complete blood cell; NHR, neutrophils-to-HDL ratio; MHR, monocyte-to-HDL ratio; LHR, lymphocyte-to-HDL ratio; PHR, platelet-to-HDL ratio
Association of HDL-related inflammatory indicators and diabetic foot ulcer
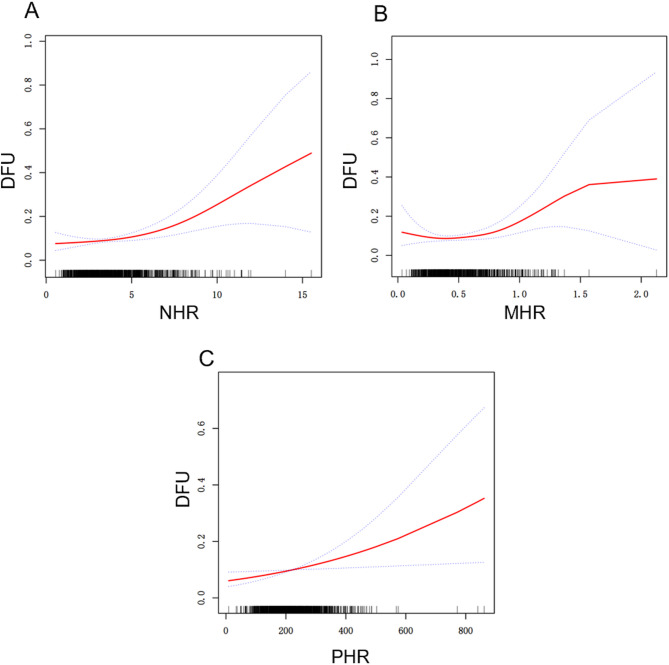
Three models were constructed to examine the relationship between HDL-related inflammatory indicators and diabetic foot ulcer in this study (Table 2). In model 1, positive associations between NHR (OR = 1.17, 95% CI = 1.07–1.28, p = 0.0005), MHR (OR = 2.71, 95% CI = 1.28–5.77, p = 0.0095), PHR (OR = 1.00, 95% CI = 1.00–1.00, p = 0.0341) and DFU were observed. After adjustment for gender, age and race/ethnicity in model 2, the positive associations between NHR (OR = 1.16, 95% CI = 1.06–1.27, p = 0.0019), MHR (OR = 2.44, 95% CI = 1.11–5.35, p = 0.0259), PHR (OR = 1.00, 95% CI = 1.00–1.00, p = 0.0460) and DFU were remained. Furthermore, in model 3 adjusted for gender, age, race/ethnicity, marital status and poverty income ratio, the positive associations between NHR (OR = 1.16, 95% CI = 1.06–1.28, p = 0.0022), MHR (OR = 2.68, 95% CI = 1.20–5.98, p = 0.0161), PHR (OR = 1.00, 95% CI = 1.00–1.00, p = 0.0241) and DFU were still significant. When HDL-related inflammatory indicators was divided into 3 quartiles, with the Q1 group as the reference, the risk of DFU increased with NHR Q3 group (OR = 1.66, 95% CI = 1.03–2.66, p = 0.0364) in model 1 and the risk of DFU increased with PHR Q3 group in three models (Model 1: OR = 1.68, 95% CI = 1.05–2.67, p = 0.0290; Model 2: OR = 1.64, 95% CI = 1.02–2.64, p = 0.0423; Model 3: OR = 1.80, 95% CI = 1.08-2 = 3.00, p = 0.0233). As illustrated in Fig. 2, potential nonlinear association between HDL-related inflammatory indicators and DFU were investigated, NHR (Fig. 2.A), MHR (Fig. 2.B) and PHR (Fig. 2.C) level showed a statistically significant non-linear association with DFU, which indicated that the prevalence of DFU was increased for every unit rise in the above three indicators. Threshold effect analysis showed an inflection point of 0.29 between MHR and DFU, with a 4.51-fold increase in the prevalence of DFU for each unit rise in MHR when MHR was more than the inflection point (Table 3).
Table 2.
The association between high-density lipoprotein-related inflammatory indicators with diabetic foot ulcers
| Exposure | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| OR (95% CI) p-Value | OR (95% CI) p-Value | OR (95% CI) p-Value | |
| NHR | 1.17 (1.07, 1.28) 0.0005 | 1.16 (1.06, 1.27) 0.0019 | 1.16 (1.06, 1.28) 0.0022 |
| NHR tertile | |||
| Q1(0.52–2.97) | 1.0 | 1.0 | 1.0 |
| Q2(2.98–4.37) | 1.10 (0.66, 1.83) 0.7058 | 1.02 (0.61, 1.72) 0.9310 | 1.06 (0.61, 1.84) 0.8291 |
| Q3(4.38–15.52) | 1.66 (1.03, 2.66) 0.0364 | 1.51 (0.93, 2.47) 0.0975 | 1.56 (0.93, 2.61) 0.0928 |
| MHR | 2.71 (1.28, 5.77) 0.0095 | 2.44 (1.11, 5.35) 0.0259 | 2.68 (1.20, 5.98) 0.0161 |
| MHR tertile | |||
| Q1(0.03–0.39) | 1.0 | 1.0 | 1.0 |
| Q2(0.40–0.57) | 1.08 (0.66, 1.77) 0.7670 | 1.02 (0.62, 1.69) 0.9409 | 1.12 (0.65, 1.91) 0.6828 |
| Q3(0.58–2.13) | 1.43 (0.90, 2.29) 0.1339 | 1.32 (0.82, 2.15) 0.2541 | 1.48 (0.89, 2.47) 0.1292 |
| LHR | 0.97 (0.79, 1.20) 0.7681 | 0.95 (0.77, 1.18) 0.6635 | 1.00 (0.79, 1.26) 0.9724 |
| LHR tertile | |||
| Q1(0.31–1.38) | 1.0 | 1.0 | 1.0 |
| Q2(1.39–2.08) | 0.89 (0.55, 1.43) 0.6299 | 0.87 (0.54, 1.41) 0.5766 | 0.99 (0.60, 1.65) 0.9796 |
| Q3(2.09–14.62) | 0.94 (0.59, 1.50) 0.7939 | 0.90 (0.56, 1.46) 0.6816 | 1.02 (0.61, 1.69) 0.9474 |
| PHR | 1.00 (1.00, 1.00) 0.0341 | 1.00 (1.00, 1.00) 0.0460 | 1.00 (1.00, 1.00) 0.0241 |
| PHR tertile | |||
| Q1(9.30-178.57) | 1.0 | 1.0 | 1.0 |
| Q2(178.95-241.18) | 0.97 (0.58, 1.62) 0.9037 | 0.95 (0.57, 1.60) 0.8566 | 1.12 (0.65, 1.94) 0.6847 |
| Q3(241.23–861.40) | 1.68 (1.05, 2.67) 0.0290 | 1.64 (1.02, 2.64) 0.0423 | 1.80 (1.08, 3.00) 0.0233 |
Model 1: unadjusted; Model 2: adjusted for gender, age and race/ethnicity; Model 3: adjusted for gender, age, race/ethnicity, marital status and poverty income ratio. NHR, neutrophils-to-HDL ratio; MHR, monocyte-to-HDL ratio; LHR, lymphocyte-to-HDL ratio; PHR, platelet-to-HDL ratio; OR, odds ratio; 95% CI, 95% confidence interval
Fig. 2.
Adjusted smooth spline of non-linear relationship between high-density lipoprotein-related inflammatory indicators and diabetic foot ulcers. (A) NHR and DFU. (B) MHR and DFU. (C) PHR and DFU. The red solid line indicates the smooth curve fit, while the blue band depicts the 95% confidence interval. The model accounted for gender, age, race, marital status, and PIR. NHR, neutrophils-to-HDL ratio; MHR, monocyte-to-HDL ratio; PHR, platelet-to-HDL ratio; DFU, diabetic foot ulcer
Table 3.
Threshold effect analysis of MHR on diabetic foot ulcers
| Adjusted OR (95% CI), p Value | |
|---|---|
| MHR | |
| Inflection point | 0.29 |
| <0.29 | 0.00 (0.00, 0.22) 0.0134 |
| ≥ 0.29 | 4.51 (1.95, 10.43) 0.0004 |
| P for likelihood test | 0.009 |
Age, race, BMI, marital status and PIR were adjusted. MHR, monocyte-to-HDL ratio; 95% CI, 95% confidence interval; OR, odds ratio
Subgroup analysis
To further investigate the impact of the positive association between NHR, MHR, PHR, and DFU in different populations, subgroup analysis and interaction tests were performed based on age, gender, PIR, race, and marital status. For NHR (Supplementary Table S2), this relationship was significant among participants aged 40–64 (OR = 1.27, 95% CI = 1.11–1.46, p = 0.0007), male (OR = 1.16, 95% CI = 1.02–1.31, p = 0.0187), PIR (1.22–2.62: OR = 1.28, 95% CI = 1.07–1.52, p = 0.0065; 2.64-5.00: OR = 1.38, 95% CI = 1.14–1.67, p = 0.0011), race (White: OR = 1.23, 95% CI = 1.06–1.42, p = 0.0069; Non-White: OR = 1.13, 95% CI = 1.00-1.28, p = 0.0439, p = 0.0439), Married/Living with a partner (OR = 1.27, 95% CI = 1.12–1.44, p = 0.0002). Significant interaction effects were found in PIR and marital status groups. For MHR (Supplementary Table S3), this relationship was significant among participants aged 65–85 (OR = 4.00, 95% CI = 1.25–12.79, p = 0.0192), male (OR = 5.16, 95% CI = 1.77–15.01, p = 0.0026), White (OR = 6.28, 95% CI = 1.78–22.18, p = 0.0043), married/Living with a partner (OR = 5.38, 95% CI = 1.83–15.82, p = 0.0022). Significant interaction effects were found in gender group. For PHR (Supplementary Table S4), this relationship was significant among participants aged 40–64 (OR = 1.00, 95% CI = 1.00-1.01, p = 0.0158), PIR (1.22–2.62: OR = 1.00, 95% CI = 1.00-1.01, p = 0.0412; 2.64-5.00: OR = 1.00, 95% CI = 1.00-1.01, p = 0.0185), Married/Living with a partner (OR = 1.00, 95% CI = 1.00-1.01, p = 0.0067). No significant interaction effects were found among subgroups.
Mediation analysis
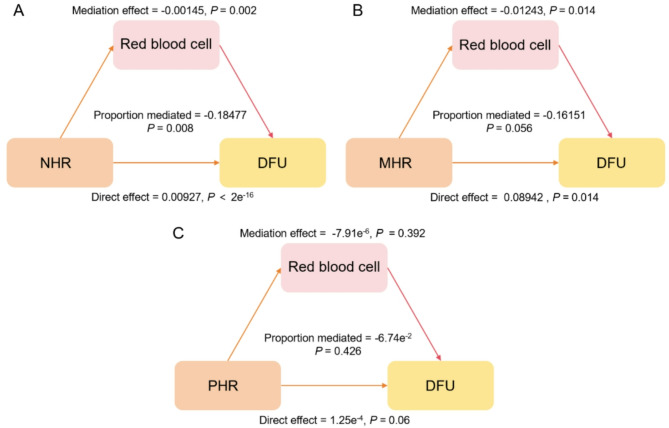
RBCs were the hypothesized mediating variable in our non-parametric bootstrapping (1,000 simulations) mediation analysis, which aimed to elucidate the mechanisms behind the relationship between NHR, MHR, PHR, and DFU. Our findings show that the relationship between NHR and DFU is partially mediated by RBCs (Fig. 3). Both a significant direct effect (average direct effect = 0.00927, p = 2e−16) and a significant indirect effect mediated by red blood cells (average causal mediation effect = − 0.00145, p = 0.002) were included in the significant overall positive association between NHR and DFU (total effect = 0.00783, p = 0.006). Partial mediation was shown by the 18.47% (p = 0.008) of the total impact that was mediated by RBCs.
Fig. 3.
The mediation role of red blood cells in the association between high-density lipoprotein-related inflammatory indicators and DFU. (A) NHR (B) MHR (C) PHR. Adjusted for gender, age, race/ethnicity. NHR, neutrophils-to-HDL ratio; MHR, monocyte-to-HDL ratio; PHR, platelet-to-HDL ratio; DFU, diabetic foot ulcer
Discussion
In the present population-based study, the associations between HDL-related inflammatory indicators and DFU prevalence were explored using data from the NHANES 1999–2004 cycles. To the best of our knowledge, this is the first attempt to assess the relationship between this particular indicator and DFU. The results indicate that there is a positive relationship between NHR, MHR, PHR, and DFU. No such associations were found between LHR and DFU. These findings provide new insights into the prevention and management of complications in people with DM.
HDL-related inflammatory indicators, as novel biomarkers that integrate both lipid metabolism and inflammation status, were discovered the potential clinical value in the context of various diseases and different population groups [17, 18]. In a population-based observational study of 1040 stroke patients and 26,261 healthy controls, per standard deviation (SD) increase in the PHR was associated with an OR of 1.13 (95% CI: 1.03–1.24, P = 0.01) for stroke after full adjustment for established risk factors. Further analysis revealed that when the PHR exceeded 223.684, a significantly stronger association with stroke was observed in the noncancer population. The PHR also remained a significant predictor of CVD mortality after adjusting for demographic characteristics, lifestyle factors, and comorbidities [19]. A retrospective study demonstrated that both LHR and NHR were independently associated with the risk of in-stent restenosis in patients undergoing elective percutaneous coronary intervention (PCI), indicating their potential prognostic utility in PCI outcomes [20]. Similarly, elevated MHR was identified as an independent and clinically useful predictor of in-stent restenosis among patients with non-ST segment elevation acute coronary syndrome following drug-eluting stent implantation [21]. Other studies have highlighted the predictive value of HDL-related inflammatory markers in various conditions, including periodontitis, bipolar disorder, and mental health [22–24]. Our findings further support the relevance of these indices by confirming a nonlinear relationship between HDL-related markers and chronic foot ulceration in patients with DM, underscoring the broad applicability and interpretability of HDL-based inflammatory indicators across clinical contexts. The initiation and progression of DFU involve multifaceted mechanisms, including endocrine dysfunction, chronic inflammation, immune dysregulation, and impaired angiogenesis, all of which collectively impair the wound healing process. Current evidence highlights the roles of various cytokines, lipid metabolites, and immune cells in regulating wound repair and regeneration [25–28]. In this context, our findings, which indicate a positive relationship between NHR, MHR, PHR, and DFU, suggest that HDL-related inflammatory indices may reflect key aspects of these pathological processes. For instance, neutrophils contribute to infection control through phagocytosis but may also delay healing via excessive release of reactive oxygen species and pro-inflammatory cytokines when dysregulated [29]. A higher NHR may thus indicate heightened neutrophil activity coupled with reduced anti-inflammatory capacity of HDL. Similarly, alterations in monocyte subsets and lipid profiles, such as the reduction in non-classical monocytes and vitamin D3 levels observed in DFU patients with peripheral artery disease [30], may be captured by MHR, reinforcing its relevance in immune-metabolic dysregulation. Moreover, platelet activation, implicated in DFU through mechanisms such as modulation of microRNAs (e.g., miR-155) via platelet-rich fibrin [31], may be reflected in PHR, linking platelet-derived inflammation and HDL dysfunction. Although LDL has been identified as a critical biomarker for DFU risk [32], our results emphasize the additional prognostic value of HDL-based ratios. Nonetheless, the precise mechanisms through which NHR, MHR, and PHR influence DFU development warrant further investigation to better understand their roles within this complex pathological network.
The results of our subgroup analysis revealed several noteworthy findings that warrant further discussion. Notably, the association between NHR and DFU was significantly stronger among individuals with higher PIR levels compared to those with lower PIR, suggesting a potential socioeconomic influence on this relationship. Similarly, a more pronounced association between MHR and DFU was observed in White participants compared to non-White individuals, which may reflect ethnic variations in inflammatory or metabolic pathways. In contrast, the association between PHR and DFU remained consistent across all subgroups, indicating that this relationship is robust and independent of the demographic factors examined.
Since RBCs play a part in DFU wound repair, we investigated their potential mediation role in the relationship between DFU and HDL-related parameters. Blood is essential for wound healing, specifically, RBCs continuously provide oxygen, which supports cellular metabolism, encourages the synthesis of angiogenic growth factors and angiogenesis in diabetic wounds, and reduces the expression of proinflammatory cytokines [33, 34]. RBCs were validated as a mediating variable in the association between NHR and DFU by our mediation analysis. A substantial average causal mediation effect was found, suggesting that RBCs have some protective impact against DFU. The entire effect was 18.47% attributable to this mediated route. Crucially, a noteworthy average direct effect was also noted, suggesting that some of the relationship between NHR and DFU occurs via mechanisms other than RBCs.
Our study has several strengths. First of all, it is the first study to investigate the role of RBCs as a mediator and the association between HDL-related inflammatory markers and the incidence of DFU. Second, this study used nationally representative NHANES data, which ensures the results’ external validity and generalizability by covering several years of follow-up. Thirdly, the use of HDL-related indices as an easily accessible and reasonably priced laboratory test adds to the abundance of data available for in-depth research. However, some limitations do exist in this study. The existence of unmeasured variables can nevertheless have an impact on the analytical results even after careful confounding factor modifications. Additionally, the study could not establish a causal association between HDL-related inflammatory indicators and DFU prevalence.
Clinical implications and future directions
The findings from this study carry several potential implications for the clinical management of DFU. The HDL-related inflammatory indices, NHR, MHR, and PHR, may serve as accessible and cost-effective biomarkers for improving risk stratification and early identification of DFU among patients with diabetes. Specifically, these indices could be integrated into routine metabolic panels to enhance screening protocols, allowing clinicians to identify high-risk individuals who may benefit from more intensive foot surveillance, preventive education, or targeted anti-inflammatory interventions.
Moreover, the mediating role of RBCs in the association between NHR and DFU suggests potential avenues for novel therapeutic strategies. Improving erythrocyte function and oxygen delivery, for instance, through nutritional supplementation or medications that enhance microcirculation, may help mitigate ulcer risk in susceptible patients.
Future research should prioritize longitudinal and interventional studies to establish causal relationships between these biomarkers and DFU outcomes. Additionally, exploring the underlying mechanisms could unveil new therapeutic targets. In this context, innovative imaging techniques such as speckle tracking echocardiography could be valuable for non-invasively evaluating the degree of myocardial fibrosis in diabetic patients [35]. Implementing such methodologies in future studies may help elucidate the intricate mechanisms linking systemic inflammation, subclinical cardiac organ damage, and the development of DFU, thereby providing a more comprehensive pathophysiological perspective. Validation in broader and more diverse populations is also essential to refine cutoff values and improve generalizability. Ultimately, these biomarkers may contribute to a more personalized and proactive approach to DFU prevention and management.
Conclusions
Our findings show a clear link between neutrophils/high-density lipoprotein ratio, monocyte/high-density lipoprotein ratio, platelet/high-density lipoprotein ratio, and a higher prevalence of diabetic foot ulcer, with red blood cells mediating the interaction between neutrophils/high-density lipoprotein ratio and diabetic foot ulcer. These findings highlight the importance of monitoring HDL-related inflammatory indicators as biomarkers in the prevention and treatment of diabetic foot ulcer. Future prospective studies are required to determine the causal relationship and potential mechanisms linking HDL-related indices to diabetic foot ulcer development.
Supplementary Information
Acknowledgements
We thank the editor and the reviewers for their useful feedback that improved this manuscript. We also thank the staff at the National Center for Health Statistics of the Centers for Disease Control for designing, collecting, and collating the NHANES data and creating the public database.
Author contributions
RHD and CQY designed the study. RYT and HYL collected and analyzed the data. YQG and MYY contributed to the interpretation of results. RHD and CQY wrote and reviewed the manuscript. All authors contributed to and have approved the final manuscript.
Funding
This study was supported by the Natural Science Foundation Project of Changsha, Hunan Province(kq2403061).
Data availability
All the data are available upon reasonable request.
Declarations
Ethics approval and consent to participate
All participants were required to provide informed consent before participation in the survey. Approval from the ethics board was not required as the data was public.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Collaboration NCDRF. Worldwide trends in diabetes prevalence and treatment from 1990 to 2022: a pooled analysis of 1108 population-representative studies with 141 million participants. Lancet. 2024;404(10467):2077–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orabi M et al. Modeling Adipokine and Insulin-Mediated Crosstalk Between Adipocytes and Beta Cells Using Flow-Enabled Microfluidics. Small, 2025: p. e2504686. [DOI] [PMC free article] [PubMed]
- 3.Paz-Graniel I, et al. Alcohol, smoking and their synergy as risk factors for incident type 2 diabetes. Am J Prev Med. 2025;31;69(5):108011. [DOI] [PubMed]
- 4.Liu C, et al. Trends and comparisons of diabetes burden in China and the world from 1990 to 2021,with forecasts to 2050: a systematic analysis of the global burden of disease study 2021. Diabetol Metab Syndr. 2025;17(1):309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng Z, et al. Comprehensive management of diabetic ulceration: strategies and perspectives. J Control Release. 2025;385:114058. [DOI] [PubMed] [Google Scholar]
- 6.Dawi J, et al. Diabetic foot ulcers: pathophysiology, immune dysregulation, and emerging therapeutic strategies. Biomedicines. 2025;13(5):1076. [DOI] [PMC free article] [PubMed]
- 7.Chen L, et al. Global mortality of diabetic foot ulcer: a systematic review and meta-analysis of observational studies. Diabetes Obes Metab. 2023;25(1):36–45. [DOI] [PubMed] [Google Scholar]
- 8.Chiu CW, et al. Multidisciplinary management improves Re-Admission, major Amputation, and mortality rates in patients with diabetic foot ulcers. Adv Wound Care (New Rochelle); 2025. [DOI] [PubMed]
- 9.Yu J, et al. The severity of diabetes and the risk of diabetic foot amputation: a national cohort study. Endocrinol Metab. 2025;40(4):574–582. [DOI] [PMC free article] [PubMed]
- 10.Sharifi-Zahabi E, et al. High-density lipoprotein particles are associated with the risk of mortality from all-causes and cardiovascular diseases (CVDs) in patients with CVD: a systematic review and meta-analysis study. Lipids Health Dis. 2025;24(1):242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren B, et al. Risk factors for at-risk foot and peripheral artery disease among the population with diabetes: A multicommunity-based cross-sectional study. Diabetes Res Clin Pract. 2023;203:110869. [DOI] [PubMed] [Google Scholar]
- 12.Shi G, et al. Machine learning-based risk prediction model for neuropathic foot ulcers in patients with diabetic peripheral neuropathy. J Diabetes Investig. 2025;16(6):1055–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okada K, et al. Association between baseline white blood cell count and future cardiovascular events in patients with stable coronary artery disease- Sub-analysis of the REAL-CAD trial. Am J Prev Cardiol. 2025;23:101052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, et al. Relationship between neutrophil count and 90-day outcomes and effect of dual antiplatelet therapy in patients with acute ischemic stroke or transient ischemic attack: a post hoc analysis of the INSPIRES trial. J Am Heart Assoc. 2025;14(14):e040492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, et al. Are metabolic abnormalities the missing link between complete blood count-derived inflammatory markers and diabetic foot? Evidence from a large population study. PLoS ONE. 2025;20(6):e0326082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Afshar F, et al. Neutrophil-lymphocyte ratio (NLR); an accurate inflammatory marker to predict diabetic foot ulcer amputation: a matched case-control study. BMC Endocr Disord. 2025;25(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo X, et al. Predictive values of novel high–density lipoprotein–related inflammatory indices in in–stent restenosis among patients undergoing elective percutaneous coronary intervention. Exp Ther Med. 2024;27(2):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marra A, et al. Complete blood count (CBC)-derived inflammation indexes are useful in predicting metabolic syndrome in adults with severe obesity. J Clin Med. 2024;13(5):1353. [DOI] [PMC free article] [PubMed]
- 19.Zhang H, Xu Y, Xu Y. The association of the platelet/high-density lipoprotein cholesterol ratio with self-reported stroke and cardiovascular mortality: a population-based observational study. Lipids Health Dis. 2024;23(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo X, Shen R, Lu P, Ma L. Predictive values of novel high-density lipoprotein-related inflammatory indices in in-stent restenosis among patients undergoing elective percutaneous coronary intervention. Exp Ther Med. 2023;27(2):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nan J, Meng S, Hu H, et al. The predictive value of monocyte count to High-Density lipoprotein cholesterol ratio in restenosis after Drug-Eluting stent implantation. Int J Gen Med. 2020;13:1255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao J, et al. Association between high-density lipoprotein-related inflammation index and periodontitis: insights from NHANES 2009–2014. Lipids Health Dis. 2024;23(1):321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei Y, et al. Gender-specific differences in the association of HDL and HDL-related oxidative stress indicators with the occurrence of major depressive disorder and bipolar disorder: a large-scale study. J Affect Disord. 2025;388:119530. [DOI] [PubMed] [Google Scholar]
- 24.Jingxuan M, et al. Monitoring of mental health in occupational populations: a study on the role and application of HDL-related inflammatory index. Front Public Health. 2025;13:1563742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rembe JD, et al. Immunomarker profiling in human chronic wound swabs reveals IL-1 beta/IL-1RA and CXCL8/CXCL10 ratios as potential biomarkers for wound healing, infection status and regenerative stage. J Transl Med. 2025;23(1):407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu D, et al. Preclinical study of engineering MSCs promoting diabetic wound healing and other inflammatory diseases through M2 polarization. Stem Cell Res Ther. 2025;16(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banerjee D, et al. Uncovering the role of tertiary lymphoid organs in the inflammatory landscape: a novel immunophenotype of diabetic foot ulcers. J Cell Mol Med. 2025;29(7):e70479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geravand F, et al. Association of lipid composition with diabetic foot ulcer size and depth: a cross-sectional study on plant versus animal oils, cholesterol, and omega fatty acids. BMC Nutr. 2025;11(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.R AS, et al. Neutrophil migration is a crucial factor in wound healing and the pathogenesis of diabetic foot ulcers: insights into Pharmacological interventions. Curr Vasc Pharmacol; 2024. [DOI] [PubMed]
- 30.Hammad R, et al. Non-classical monocytes frequency and serum vitamin D(3) levels are linked to diabetic foot ulcer associated with peripheral artery disease. J Diabetes Investig. 2023;14(10):1192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, et al. Platelet-Rich fibrin promotes wound healing by regulating miR-155 levels in diabetic foot ulcer. Wound Repair Regen. 2025;33(4):e70073. [DOI] [PubMed] [Google Scholar]
- 32.Soler-Climent E, et al. Integrative biomarker analysis in diabetic foot: lipids, cellular integrity, and hydration balance. Diabetol Metab Syndr. 2025;17(1):288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang Y, et al. Living hydrogel with blood derived elements for wound healing. Mater Today Bio. 2025;33:102002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guan Y, et al. Sustained oxygenation accelerates diabetic wound healing by promoting epithelialization and angiogenesis and decreasing inflammation. Sci Adv. 2021;7(35):eabj0153. [DOI] [PMC free article] [PubMed]
- 35.Sonaglioni A, Nicolosi GL, Rigamonti E, Lombardo M, La Sala L. Molecular approaches and echocardiographic deformation imaging in detecting myocardial fibrosis. Int J Mol Sci. 2022;23(18):10944. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data are available upon reasonable request.