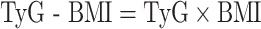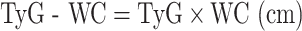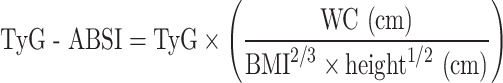Abstract
Background
The triglyceride-glucose (TyG) index, as a measure of insulin resistance, has been confirmed to be associated with adverse clinical outcomes. The new composite indicator, TyG-A body type index (TyG-ABSI), by integrating the TyG index and the A body type index, has demonstrated superior efficacy in predicting the risk of cardiovascular death in the general population compared to traditional indicators. This study aims to deeply explore the association between TyG-ABSI and all-cause mortality and CVD mortality in the population with cardiovascular kidney-metabolic syndrome (CKM) stages 0–3. The analysis will be conducted from multiple dimensions such as the intensity of indicator correlation and potential influencing mechanisms, in order to comprehensively reveal the relationship between the two.
Results
We analyzed data from 13,480 participants in the NHANES cohort (1999–2018) using Cox proportional hazards models and restricted cubic spline functions. The results indicated that elevated TyG-ABSI values were independently associated with a higher risk of all-cause mortality (HR = 1.226, 95% CI 1.104–1.361) and cardiovascular mortality (HR = 1.377, 95% CI 1.149–1.651). Time-dependent receiver operating characteristic (ROC) curves and concordance index evaluations demonstrated that TyG-ABSI yielded more accurate long-term prognostic performance than other TyG-derived metrics. The area under the curve (AUC) of this indicator reached 0.688–0.708 in the prediction of all-cause mortality risk over 5–15 years, and 0.696–0.739 in the prediction of cardiovascular mortality risk. External validation using CHARLS data confirmed the robustness of these findings in predicting all-cause mortality.
Conclusions
Among individuals with CKM stages 0–3, TyG-ABSI demonstrates a stronger association with mortality risk and superior predictive ability compared with other TyG-derived metrics. Its performance suggests a potential role in capturing variations across diverse clinical subgroups, and informing optimal timing for preventive interventions.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-025-02921-3.
Keywords: Triglyceride-glucose index, Insulin resistance, A body shape index, Mortality, Cardiovascular-kidney-metabolic syndrome
Introduction
In 2023, the American Heart Association (AHA) proposed the concept of CKM syndrome to describe multi-system disorders involving interrelated cardiovascular, renal, and metabolic dysfunctions [1, 2]. CKM syndrome increases mortality beyond the sum of its individual components [3], with its development driven by insulin resistance (IR), advanced glycation end-product accumulation, adipose tissue dysfunction, and subsequent oxidative stress and inflammation [4]. A rising trend in the incidence of CKM syndrome has been observed among both males and females over the period from 1988 to 2018. Specifically, the prevalence in women increased from 13.9% to 15.2%, while the increase among men was even more pronounced, increasing from 18.9 to 22.4%, as indicated by recent research [5]. Furthermore, greater CKM severity is associated with higher mortality, underscoring the importance of early prevention [6].
IR is central to CKM pathophysiology, interacting with other molecular and cellular factors to drive disease progression [4, 7]. Characterized by suboptimal responsiveness to insulin stimulation resulting from disruptions in target tissues’ signalling pathways [8], IR—a fundamental pathological basis of metabolic diseases—drives the progression of diabetes, heart disease, obesity, and chronic renal dysfunction through pathways including inflammation, lipotoxicity, and oxidative stress, among others [9–11]. Due to its non-invasive nature, the TyG index has been widely radopted as a surrogate for IR and demonstrates predictive value across a spectrum of diseases. For example, the triglyceride–glucose index demonstrates a non-linear association with incident chronic kidney disease among hypertensive patients with abnormal glucose metabolism, highlighting its capacity to capture broader multisystem risk [12]. It has been demonstrated that TyG-related indices exhibit correlations with IR, secondary hyperinsulinemia, and CVD mortality across diverse health conditions [13–16]. Therefore, the TyG index and its closely associated indices—particularly those reflecting dysfunctional obesity and IR—may provide predictive value for adverse outcomes in CKM syndrome.
Additionally, obesity is characterized as a heterogeneous condition that confers significant cardiovascular health risks [17, 18]. For example, among hypertensive adults with obstructive sleep apnea, the weight-adjusted waist index (WWI) shows a J-shaped association with incident cardiovascular disease, illustrating how central adiposity phenotypes capture risk beyond BMI [19]. CKM syndrome often arises from the accumulation of excessive or impaired fat cells. Hypertrophic visceral and perivascular adipose tissue release inflammatory factors (e.g., IL-6, TNF-α), activating NF-κB, JAK-STAT, and MAPK pathways, reducing nitric oxide (NO) availability, and promoting inflammation [20–22]. Imbalanced adipokines (e.g., increased leptin, decreased adiponectin) enhance sympathetic drive, sodium retention, and vascular dysfunction [22, 23], while lipotoxic intermediates (e.g., ceramides) impair Akt phosphorylation, worsening insulin resistance [24]. Reactive oxygen species (ROS) from perivascular adipose tissue and mitochondria amplify these effects [20]. Kidney-specific mechanisms include PRAT-driven RAAS activation, RSF-related hemodynamic changes, and an adipose-brain-kidney reflex increasing renal sympathetic outflow [23, 25–27].
Fat accumulation in the abdomen and trunk, known as central obesity, significantly increases the risk of cardiovascular diseases and overall mortality [28]. In contrast, peripheral obesity—marked by lipid deposition in the pelvic region and limbs—shows a weaker correlation with increased CVD risk. When body mass index(BMI) is used for evaluating risk and predicting unfavourable outcomes in CVD, an “obesity paradox” often arises. Moreover, the use of BMI for assessing obesity severity may not accurately reflect fat distribution or body metabolism [29]. In contrast to BMI, ABSI incorporates waist circumference into its calculation. This revision overcomes the shortcomings of body mass index (BMI) in characterizing central obesity and introduces a more precise measurement standard. This indicator is in opposition to the obesity paradox in predicting cardiovascular and metabolic diseases, demonstrating a closer correlation with cardiovascular and metabolic health conditions [30].
CKM syndrome stages 0–3 represent a critical preclinical phase, in which early identification of mortality risk factors is essential for timely intervention. The TyG-ABSI, a composite index integrating metabolic and anthropometric parameters, has shown promise in predicting adverse health outcomes in certain populations. However, its association with mortality in individuals with CKM syndrome has not been thoroughly investigated. Accordingly, this study evaluates whether TyG-ABSI is linked to increased mortality risk and whether it provides incremental predictive value over other TyG-related indices in CKM syndrome stages 0–3.
Methods
Study population and design
NHANES is a nationally representative survey that employs a complex, multistage probability sampling design to ensure scientific rigor and broad applicability. This stratified, multilevel sampling approach allows the selected participants to closely reflect the demographic structure of the U.S. civilian, non-institutionalized population. By capturing a cross-sectional snapshot of American society, NHANES provides reliable and generalizable data for health and nutrition research.
CHARLS is a nationally representative baseline survey of Chinese residents aged 45 years and older, employing a multistage, probability-proportional-to-size sampling design across both urban and rural regions. Its primary objective is to systematically collect detailed information on the socioeconomic status, health, and life course of middle-aged and older adults in China [31]. CHARLS collects individual- and household-level data through in-person interviews and comprehensive health assessments, capturing demographic characteristics, health status, socioeconomic conditions, and lifestyle factors. For the present analysis, participants were eligible if they met age criteria and had complete data on key variables; those with missing critical information were excluded to maintain analytical rigor. The CHARLS project received ethical approval from the Biomedical Ethics Review Committee of Peking University (IRB00001052-11015), and all participants provided written informed consent prior to enrollment.
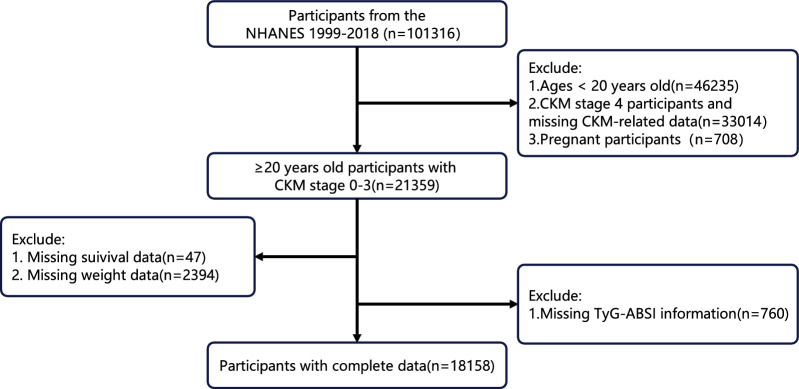
Data from NHANES (1999–2018) were analyzed. Figure 1 outlines the enrollment criteria and exclusions. Participants were excluded if they met any of the following conditions: (1) age < 20 years (n = 46,235); (2) missing CKM syndrome stage data or CKM stage 4 (n = 33,014); (3) pregnancy (n = 708); (4) missing weight data (n = 2,394) or lost to follow-up (n = 47); and (5) missing TyG-ABSI measurements (n = 760). To mitigate bias from incomplete covariate data, random forest imputation (RFI) was employed for data completion, covering variables such as educational level, family income-to-poverty ratio, marital status, smoking and drinking habits, HEI-2015 score, physical activity, HDL-C, LDL-C, HbA1c, uric acid, uACR, eGFR, as well as histories of diabetes mellitus (DM), chronic kidney disease (CKD), liver disease, cancer, hypertension, and use of lipid-lowering, antihypertensive, and hypoglycemic medications. After processing, a total of 18,158 participants were included in the NHANES analysis. Criteria for defining CKM syndrome stages 0–3 are detailed in Table S1.
Fig. 1.
Flow chart for eligible participants. CKM Syndrome, cardiovascular-kidney-metabolic syndrome; TyG, triglyceride-glucose; ABSI, a body shape index; NHANES, National Health and Nutrition Examination Survey
Within the CHARLS cohort, inclusion criteria were: age ≥ 45 years, CKM syndrome stages 0–3, and complete data on key variables (TyG-ABSI, mortality outcomes, and covariates). Participants were excluded for the following reasons: (1) age < 45 years (n = 407); (2) CKM stage 4 or missing CKM staging data (n = 4,800); (3) missing survival status (n = 2,490); (4) absent TyG-ABSI measurements (n = 3,890); and (5) incomplete covariate information (n = 426). Ultimately, 5,695 participants were included in the CHARLS analytic cohort (Fig. S1).
The formulas used to calculate TyG-related indices are as follows [32, 33]:
In NHANES, certified medical staff performed laboratory assessments at mobile testing sites. These fasting plasma glucose and serum lipid levels were analyzed using enzymatic methods at CDC-affiliated laboratories. In CHARLS, venous blood biomarkers are analyzed in a centralized, accredited laboratory, using enzymatic methods accompanied by documented internal quality controls to ensure measurement consistency. Standardized protocols were followed for height, weight, and waist circumference measurements, all performed by health technicians who were uniformly trained and certified, using quality control procedures to minimize measurement errors.
Covariate missingness ranged from 0 to 8.27%. Missing data were imputed using the nonparametric random forest method implemented in the missForest package in R, generating a single imputed dataset [34]. This method is advantageous for its ability to handle mixed data types and complex relationships without distributional assumptions, and has been increasingly applied in epidemiological research [34–36].
Feature selection is crucial to the development of predictive models. This analysis first uses the Boruta algorithm—a supervised feature selection method for categorical data—to identify relevant predictive factors, which are then incorporated into the Cox proportional hazards model to control for confounding variables. Subsequently, Cox regression was used to evaluate the association between TyG-ABSI and all-cause mortality and cardiovascular mortality. The entire process was rich in details and comprehensive in perspective.
Three nested models were constructed for analysis. Model 1 served as an unadjusted baseline, Model 2 incorporates demographic factors such as age, gender and race, Model 3, on this basis, further incorporates a broader range of covariates. It covers lifestyle variables (smoking, drinking, education level, marital status, poverty income ratio, HEI-2015 score and physical activity), clinical comorbidities (hypertension, diabetes, cancer, liver disease and chronic kidney disease), laboratory indicators (HbA1c, uACR, eGFR, LDL-C, HDL-C and serum uric acid), and drug use (lipid-lowering drugs, antihypertensive drugs and hypoglycemic therapy).
To present the differences in mortality rates among the quartiles of TyG-ABSI, the Kaplan–Meier survival curves were plotted, and the survival distributions between groups were compared using the log-rank test (Fig. S2). The multi-hypothesis test was corrected using the Bonferroni method. A two-sided P-value < 0.05 was considered statistically significant.
Definitions of covariates
Standard questionnaires were used to gather data on demographics, health habits, medical background, and medications people were taking. Individuals were classified into three categories based on smoking status: current smokers (people who’d puffed their way through at least 100 cigarettes and were still lighting up), former smokers (those reporting ≥ 100 lifetime cigarettes, now abstinent), and never-smokers (those who had minimal cigarette exposure, smoking fewer than 100 in total). Alcohol intake was categorized as non-drinkers (individuals with a lifetime alcohol consumption of less than 12 drinks), former drinkers (individuals who had at least a dozen drinks in their life or per year in the past, but have given it up in the last 12 months), and current drinkers. Current drinkers were sorted by their typical alcohol consumption amounts: mild drinkers (women ≤ 1 daily, men ≤ 2 daily), moderate drinkers (women 1–3 daily, men 2–4 daily; or binging 2–4 times), and heavy drinkers (women ≥ 4 daily, men ≥ 5 daily; or binging ≥ 5 times). HEI-2015 score reflect U.S. residents’ adherence to dietary recommendations, with higher scores indicating that their daily diets meet healthier standards [37], Table S2 presents the components that make up the HEI-2015 score. Renal function was evaluated via the CKD-EPI equation to determine eGFR [38]. Social Determinants of Health (SDoH) reflect the factors influencing individuals’ health outcomes, and its components are presented in Table S3. Hypertension, diabetes mellitus, cancer, and liver disease were each recorded as “Yes” or “No”. Chronic kidney disease (CKD) is classified into four different levels based on risk: low risk, medium risk, high risk, and extremely high risk [29]. Hypertension, cardiovascular disease, and diabetes history were included as covariates. Their definitions are provided in Table S4.
Study endpoints
The primary endpoints of this analysis were all-cause mortality and CVD mortality. Mortality data were obtained through long-term follow-up of participants using the publicly available NHANES mortality database, which links participant information to the National Death Index through the end of 2019. Cause-specific mortality was classified according to ICD-10 diagnostic codes. Specifically, cardiovascular mortality was categorized under codes I00–I09, I11, I13, I20–I51, and I60–I69 [39].
Statistical analysis
The analysis was conducted using R software (version 4.3.1). To accommodate the intricate multi-level probability sampling scheme and ensure the analysis was nationally representative, the sample weighting methodology provided by NCHS was incorporated for continuous variables. In terms of statistical comparison, for continuous variables, weighted t-tests are used for analysis. For categorical variables, the chi-square test is used for evaluation. For the convenience of in-depth analysis, the TyG-ABSI indicator is converted into a standard score (Z-score). With the aid of the Cox proportional hazards model, this study analyzed the association between the TyG-ABSI index and mortality outcomes. The RCS curve was fitted using the “rcssci” package, with knot placement determined by the minimum AIC criterion, selecting four knots [40]. The research subjects were grouped according to the quartiles of TyG-ABSI to conduct an in-depth analysis of its correlation with mortality. To clarify the advantage of TyG-ABSI in predicting mortality outcomes over other indicators, this study adopted time-dependent receiver operating characteristic (ROC) analysis. Subgroup analyses were conducted to explore potential variations across different groups. Interaction P-values were calculated in accordance with current methodological guidelines [41, 42]. The mediation framework follows the approach proposed by VanderWeele [43], while the E-value logic is based on the method introduced by VanderWeele & Ding [44]. To enhance the reliability of the results, a robustness analysis was also conducted using CHARLS queue data. The incremental predictive value of TyG-ABSI was assessed using the net reclassification improvement (NRI) and integrated discrimination improvement (IDI) indices, with clinical utility evaluated through Decision Curve Analysis (DCA).
In the sensitivity analyses, several complementary approaches were employed to verify the consistency and robustness of the findings across various methodological variations, addressing potential confounders and biases. First, to exclude the potential influence of cancer, early mortality (within 2 or 5 years of baseline), and advanced CKD, individuals with these conditions were excluded (Table S5–S8). This aimed to eliminate the impact of severe comorbidities and early mortality on the association between TyG-ABSI and mortality, ensuring that the findings were not driven by these extreme cases. Second, to evaluate the potential impact of medications, we first excluded lipid-lowering and antidiabetic drugs, and then separately excluded Glucagon-Like Peptide-1 (GLP-1) receptor agonists to assess their specific influence (Table S9, S10). Third, to account for the potential confounding effect of genetic and familial factors, the analysis incorporated a family history of coronary heart disease, heart disease, hypertension, and stroke (Table S11, S12). Fourth, to eliminate the influence of lifestyle factors, sleep duration and depression symptoms were included as covariates (Table S13). This step aimed to account for potential effects of poor sleep or depression, which could confound the relationship between TyG-ABSI and mortality. Fifth, to assess the role of broader social and environmental factors, social determinants of health (SDoH) were included as covariates (Table S14). We also further reduced bias arising from incomplete data by excluding individuals with missing covariate values (Table S15). This helped to determine whether factors such as socioeconomic status, education, and access to healthcare influenced the association between TyG-ABSI and mortality, ensuring that the results were not confounded by these societal factors. To account for competing risks, we included cardiovascular mortality as a competing event in Fine-Gray models (Table S16). This adjustment allowed for a more accurate estimation of the risk of all-cause mortality by considering the potential influence of cardiovascular death as an alternative cause. Additionally, to verify the generalizability of the findings, a sensitivity analysis using the 2011 CHARLS baseline data was conducted (Table S17), tracking patients up to 2020. This analysis, focusing on 5695 individuals at CKM stages 0–3, aimed to confirm the association between TyG-ABSI and all-cause mortality in a different cohort, further reinforcing the robustness of the results (Table S18).
Results
Baseline characteristics
Table 1 presents the baseline characteristics of 18,158 individuals with CKM syndrome stages 0–3, stratified by TyG-ABSI quartiles. Higher TyG-ABSI quartiles were associated with older age, a greater proportion of males, lower educational attainment, a higher poverty-income ratio, and increased prevalence of current smoking and heavy alcohol consumption (all P < 0.05). Biochemically, individuals in higher quartiles exhibited lower HDL-C and eGFR, and higher uACR and HbA1c levels (all P < 0.001). Clinically, higher quartiles were linked to elevated BMI, waist circumference, systolic blood pressure, LDL-C, and uric acid, along with increased prevalence of CKD, liver disease, and cancer. Participants in higher quartiles also reported greater use of lipid-lowering, antihypertensive, and hypoglycemic medications. Notably, both all-cause and cardiovascular mortality rates increased progressively across the quartiles, reaching their highest levels in Q4 (both P < 0.001).
Table 1.
The baseline characteristics stratified by TyG-ABSI index
| Characteristic | Overall, N = 18,158 | Q1, N = 4265 | Q2, N = 4289 | Q3, N = 4493 | Q4, N = 5111 | P-valuea | P-adjustb |
|---|---|---|---|---|---|---|---|
| Age, n(%) | < 0.001 | < 0.001 | |||||
| < 60 | 12,936 (79.426%) | 3875 (93.847%) | 3346 (83.854%) | 3039 (76.415%) | 2,676 (63.584%) | ||
| > = 60 | 5222 (20.574%) | 390 (6.153%) | 943 (16.146%) | 1454 (23.585%) | 2435 (36.416%) | ||
| Gender, n(%) | < 0.001 | < 0.001 | |||||
| Female | 9317 (51.509%) | 2730 (64.343%) | 2322 (53.981%) | 2111 (46.388%) | 2154 (41.321%) | ||
| Male | 8841 (48.491%) | 1535 (35.657%) | 1967 (46.019%) | 2382 (53.612%) | 2957 (58.679%) | ||
| Race, n(%) | < 0.001 | < 0.001 | |||||
| Mexican American | 3386 (8.729%) | 524 (6.689%) | 728 (8.371%) | 968 (10.335%) | 1166 (9.522%) | ||
| Other Hispanic | 1619 (5.655%) | 303 (5.648%) | 407 (5.994%) | 424 (5.392%) | 485 (5.586%) | ||
| Non-Hispanic White | 7894 (67.619%) | 1558 (60.801%) | 1801 (67.056%) | 2022 (69.458%) | 2513 (73.164%) | ||
| Non-Hispanic Black | 3494 (10.884%) | 1426 (19.561%) | 937 (11.534%) | 643 (7.394%) | 488 (5.042%) | ||
| Other/Multiracial | 1765 (7.113%) | 454 (7.301%) | 416 (7.045%) | 436 (7.420%) | 459 (6.686%) | ||
| PIR, n(%) | < 0.001 | < 0.001 | |||||
| < 1.3 | 4818 (18.917%) | 1047 (18.486%) | 1094 (18.681%) | 1147 (17.744%) | 1530 (20.758%) | ||
| 1.3–3..5 | 7874 (40.263%) | 1816 (39.639%) | 1828 (38.866%) | 1932 (39.632%) | 2298 (42.915%) | ||
| > = 3.5 | 5466 (40.820%) | 1402 (41.875%) | 1367 (42.454%) | 1414 (42.625%) | 1283 (36.327%) | ||
| Education Level, n(%) | < 0.001 | < 0.001 | |||||
| Less than 9th grade | 2036 (5.487%) | 206 (3.093%) | 354 (4.149%) | 566 (6.361%) | 910 (8.345%) | ||
| 9-11th Grade | 2573 (10.560%) | 504 (8.511%) | 612 (10.025%) | 650 (11.488%) | 807 (12.219%) | ||
| High school grad/GED | 4130 (23.518%) | 903 (20.549%) | 968 (22.316%) | 1054 (24.949%) | 1205 (26.257%) | ||
| Some college/AA degree | 5243 (31.243%) | 1431 (32.884%) | 1291 (32.220%) | 1243 (30.416%) | 1278 (29.453%) | ||
| College graduate or above | 4176 (29.192%) | 1221 (34.962%) | 1064 (31.290%) | 980 (26.787%) | 911 (23.726%) | ||
| Marital status, n(%) | < 0.001 | < 0.001 | |||||
| Married/Living with a partner | 11,299 (64.776%) | 2316 (58.911%) | 2693 (65.259%) | 2969 (68.122%) | 3321 (66.814%) | ||
| Never married | 3301 (18.711%) | 1359 (29.241%) | 820 (19.549%) | 612 (14.459%) | 510 (11.591%) | ||
| Widowed/Divorced/Separated | 3558 (16.513%) | 590 (11.848%) | 776 (15.192%) | 912 (17.419%) | 1280 (21.594%) | ||
| Smoking status, n(%) | < 0.001 | < 0.001 | |||||
| Never smoker | 10,196 (55.438%) | 2820 (64.959%) | 2521 (58.176%) | 2422 (52.904%) | 2433 (45.709%) | ||
| Former smoker | 4248 (23.826%) | 657 (16.809%) | 863 (20.738%) | 1173 (27.046%) | 1555 (30.713%) | ||
| Current smoker | 3714 (20.736%) | 788 (18.232%) | 905 (21.086%) | 898 (20.049%) | 1123 (23.578%) | ||
| Alcohol Comsumption, n(%) | < 0.001 | < 0.001 | |||||
| Never | 2641 (11.157%) | 603 (11.169%) | 595 (10.309%) | 635 (11.248%) | 808 (11.904%) | ||
| Former | 2665 (12.017%) | 397 (7.761%) | 527 (10.411%) | 712 (13.198%) | 1029 (16.701%) | ||
| Mild | 6238 (37.012%) | 1451 (34.393%) | 1509 (38.254%) | 1573 (37.714%) | 1705 (37.688%) | ||
| Moderate | 2743 (17.489%) | 915 (23.768%) | 683 (17.874%) | 603 (15.720%) | 542 (12.590%) | ||
| Heavy | 3871 (22.325%) | 899 (22.908%) | 975 (23.153%) | 970 (22.121%) | 1027 (21.117%) | ||
| HEI-2015 | 49.989 ± 13.258 | 50.698 ± 13.541 | 49.802 ± 13.255 | 50.084 ± 13.238 | 49.372 ± 12.959 | 0.019 | 0.501 |
| PA, n(%) | < 0.001 | < 0.001 | |||||
| No | 8873 (43.879%) | 1789 (37.213%) | 1983 (42.327%) | 2278 (46.025%) | 2823 (49.951%) | ||
| Yes | 9285 (56.121%) | 2476 (62.787%) | 2306 (57.673%) | 2215 (53.975%) | 2288 (50.049%) | ||
| HDL-C, mg/dl | 54.082 ± 16.040 | 61.791 ± 16.261 | 56.677 ± 15.978 | 51.777 ± 14.248 | 46.079 ± 13.076 | < 0.001 | < 0.001 |
| LDL-C, mg/dl | 116.704 ± 34.643 | 102.383 ± 29.538 | 117.306 ± 32.278 | 123.674 ± 34.220 | 123.460 ± 37.632 | < 0.001 | < 0.001 |
| HbA1C, % | 5.533 ± 0.857 | 5.242 ± 0.389 | 5.354 ± 0.471 | 5.517 ± 0.634 | 6.020 ± 1.345 | < 0.001 | < 0.001 |
| uACR, mg/g | 25.897 ± 223.805 | 14.022 ± 80.938 | 16.909 ± 134.679 | 24.381 ± 235.056 | 48.278 ± 346.018 | < 0.001 | < 0.001 |
| eGFR, ml/min/1.73m2 | 97.091 ± 20.168 | 104.379 ± 18.625 | 98.386 ± 19.008 | 95.210 ± 19.792 | 90.386 ± 20.591 | < 0.001 | < 0.001 |
| Uric Acid, mg/dl | 5.428 ± 1.365 | 4.854 ± 1.205 | 5.282 ± 1.260 | 5.626 ± 1.320 | 5.950 ± 1.417 | < 0.001 | < 0.001 |
| Hypertension, n(%) | < 0.001 | < 0.001 | |||||
| No | 11,303 (66.647%) | 3420 (83.564%) | 2917 (72.521%) | 2666 (63.141%) | 2300 (47.355%) | ||
| Yes | 6855 (33.353%) | 845 (16.436%) | 1372 (27.479%) | 1827 (36.859%) | 2811 (52.645%) | ||
| DM, n(%) | < 0.001 | < 0.001 | |||||
| No | 15,264 (87.822%) | 4134 (97.713%) | 3984 (95.238%) | 3874 (89.196%) | 3272 (69.139%) | ||
| Yes | 2894 (12.178%) | 131 (2.287%) | 305 (4.762%) | 619 (10.804%) | 1839 (30.861%) | ||
| CKD, n(%) | < 0.001 | < 0.001 | |||||
| Low risk | 15596 (89.147%) | 3970 (93.963%) | 3873 (92.091%) | 3878 (89.728%) | 3875 (80.805%) | ||
| Moderate risk | 1920 (8.571%) | 245 (5.307%) | 349 (6.879%) | 457 (8.081%) | 869 (14.019%) | ||
| High risk | 443 (1.669%) | 36 (0.558%) | 41 (0.735%) | 110 (1.608%) | 256 (3.774%) | ||
| Very high risk | 199 (0.613%) | 14 (0.171%) | 26 (0.295%) | 48 (0.583%) | 111 (1.402%) | ||
| Liver disease, n(%) | < 0.001 | < 0.001 | |||||
| No | 17,502 (96.559%) | 4199 (98.757%) | 4148 (96.215%) | 4320 (96.482%) | 4835 (94.781%) | ||
| Yes | 656 (3.441%) | 66 (1.243%) | 141 (3.785%) | 173 (3.518%) | 276 (5.219%) | ||
| Cancer, n(%) | < 0.001 | < 0.001 | |||||
| No | 16,754 (92.001%) | 4089 (95.234%) | 4021 (93.188%) | 4113 (91.644%) | 4531 (87.938%) | ||
| Yes | 1404 (7.999%) | 176 (4.766%) | 268 (6.812%) | 380 (8.356%) | 580 (12.062%) | ||
| Lipid-lowering drug, n(%) | < 0.001 | < 0.001 | |||||
| No | 15,645 (86.966%) | 4114 (96.645%) | 3867 (91.191%) | 3805 (85.259%) | 3859 (74.765%) | ||
| Yes | 2513 (13.034%) | 151 (3.355%) | 422 (8.809%) | 688 (14.741%) | 1252 (25.235%) | ||
| Antihypertensive drug, n(%) | < 0.001 | < 0.001 | |||||
| No | 13,636 (78.012%) | 3804 (91.400%) | 3456 (83.450%) | 3283 (75.499%) | 3093 (61.696%) | ||
| Yes | 4522 (21.988%) | 461 (8.600%) | 833 (16.550%) | 1210 (24.501%) | 2018 (38.304%) | ||
| Hypoglycemic therapy, n(%) | < 0.001 | < 0.001 | |||||
| No | 16,617 (93.489%) | 4201 (99.002%) | 4140 (97.736%) | 4176 (94.335%) | 4100 (82.881%) | ||
| Yes | 1541 (6.511%) | 64 (0.998%) | 149 (2.264%) | 317 (5.665%) | 1011 (17.119%) | ||
| CKM syndrome, n(%) | < 0.001 | < 0.001 | |||||
| CKM 0 | 1866 (12.714%) | 1189 (32.386%) | 504 (14.236%) | 158 (3.934%) | 15 (0.294%) | ||
| CKM 1 | 3841 (23.298%) | 1566 (36.525%) | 1349 (33.872%) | 780 (19.338%) | 146 (3.455%) | ||
| CKM 2 | 11,214 (60.270%) | 1467 (30.613%) | 2300 (50.462%) | 3271 (73.179%) | 4176 (86.835%) | ||
| CKM 3 | 1237 (3.718%) | 43 (0.476%) | 136 (1.431%) | 284 (3.549%) | 774 (9.416%) | ||
| Cardiovascular mortality, n(%) | < 0.001 | < 0.001 | |||||
| No | 17,632 (98.268%) | 4232 (99.660%) | 4220 (99.123%) | 4346 (97.935%) | 4834 (96.353%) | ||
| Yes | 526 (1.732%) | 33 (0.340%) | 69 (0.877%) | 147 (2.065%) | 277 (3.647%) | ||
| All-cause mortality, n(%) | < 0.001 | < 0.001 | |||||
| No | 16,204 (93.043%) | 4126 (98.001%) | 3988 (95.345%) | 4002 (92.496%) | 4088 (86.330%) | ||
| Yes | 1954 (6.957%) | 139 (1.999%) | 301 (4.655%) | 491 (7.504%) | 1023 (13.670%) |
Continuous variables were presented as weighted means ± standard deviation; categorical variables were presented as weighted percentages and unweighted frequencies
CKM Syndrome, cardiovascular-kidney-metabolic syndrome; CVD, cardiovascular disease; HR, hazard ratio; CI, confidence interval; TyG,: triglyceride-glucose; ABSI, a body shape index; uACR, urinary albumin/creatinine ratio; PA, physical activity; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HbA1c, glycosylated hemoglobin; HEI-2015, healthy eating index-2015; PIR, poverty income ratio; DM, diabetes mellitus; HDL, high-density lipoprotein; LDL, low-density lipoprotein
aP values were obtained from survey-weighted linear regression for continuous variables, weighted t-tests are used for analysis. For categorical variables, the chi-square test is used for evaluation. bBonferroni corrections were applied for comparisons among multiple groups
Feature selection
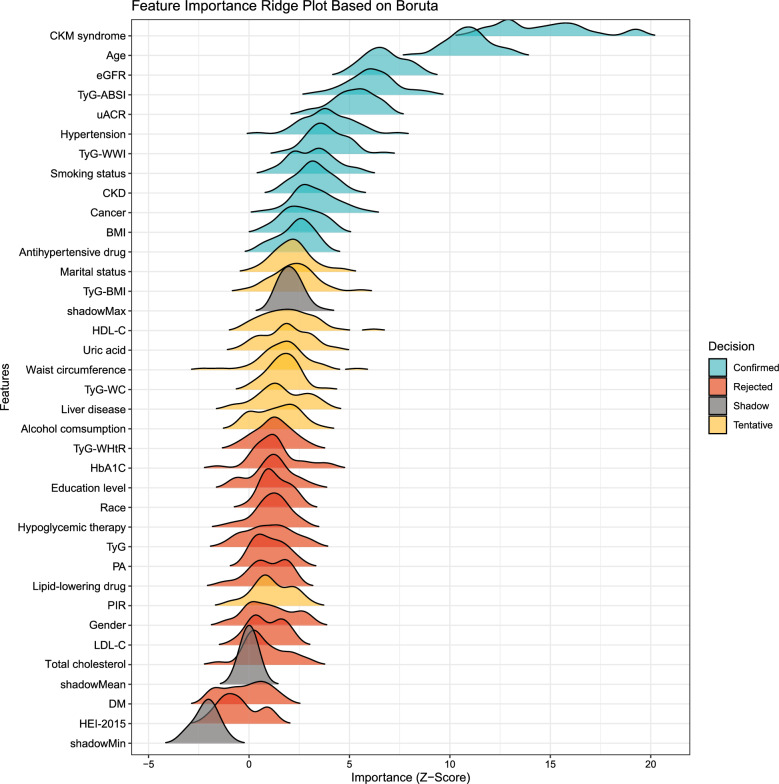
The outcomes of feature selection using Boruta’s algorithm are shown in Fig. 2. After 500 iterations, key predictors, such as eGFR and TyG-ABSI, were identified as the most significant, as indicated by the ridge plot. However, variables including age, gender, smoking habits, and alcohol intake were included in subsequent analyses. Although several of these variables exhibited lower Z-values relative to the top-ranked features in the plot, they were incorporated based on prior studies and clinical experience.
Fig. 2.
Boruta-based feature importance ridge plot for all-cause mortality in CKM Syndrome. CKM Syndrome, cardiovascular-kidney-metabolic syndrome; CVD, cardiovascular disease; HR, hazard ratio; CI, confidence interval; TyG, triglyceride-glucose; ABSI, a body shape index; BMI, body mass index; WWI, weight-adjusted waist index; WHtR, waist-to-height ratio; WC, waist circumference; uACR, urinary albumin/creatinine ratio; PA, physical activity; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HbA1c, glycosylated hemoglobin; HEI-2015, healthy eating index-2015; PIR, poverty income ratio; DM, diabetes mellitus; HDL-C, high-density lipoprotein; LDL-C, low-density lipoprotein
Associations between TyG-ABSI and mortality
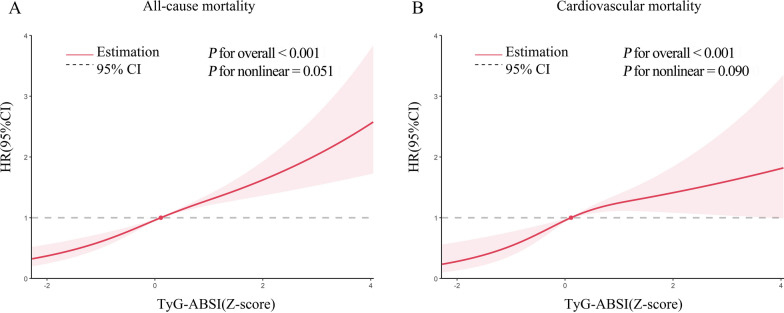
When TyG-ABSI was treated as a continuous variable, the fully adjusted model indicated that each one standard deviation (SD) increase in TyG-ABSI was associated with a significant elevation in all-cause mortality risk (hazard ratio [HR] = 1.226, 95% CI 1.104–1.361). Similarly, the risk of cardiovascular mortality increased substantially (HR = 1.377, 95% CI 1.149–1.651), illustrating a clear quantitative relationship between TyG-ABSI and mortality (Table 2). When TyG-ABSI was stratified into quartiles, a distinct dose–response relationship emerged, with mortality risk rising progressively across successive quartiles (P for trend < 0.001). Notably, individuals in the highest quartile exhibited markedly increased risk: the HR for all-cause mortality was 2.072 (95% CI 1.471–2.919), and the HR for cardiovascular mortality reached 3.643 (95% CI 2.053–6.463), both demonstrating a strong positive association. Kaplan–Meier survival analysis (Fig. S2) further confirmed significant differences in survival across quartiles (all P < 0.001). Moreover, restricted cubic spline (RCS) analysis (Fig. 3) revealed a statistically significant linear relationship between TyG-ABSI and mortality, supporting the robustness of these findings.
Table 2.
Associations between TyG-ABSI and mortality in CKM syndrome stage 0–3
| Characteristic | Model1 | Model2 | Model3 | |||
|---|---|---|---|---|---|---|
| HR(95%CI) | P-value | HR(95%CI) | P-value | HR(95%CI) | P-value | |
| All-cause mortality | ||||||
| Continuous TyG-ABSI | 1.925 (1.795, 2.063) | < 0.001 | 1.515 (1.413, 1.624) | < 0.001 | 1.226 (1.104, 1.361) | < 0.001 |
| Quartile TyG-ABSI | ||||||
| Q1 | Ref | Ref | Ref | |||
| Q2 | 2.238 (1.736, 2.885) | 1.707 (1.306, 2.230) | 1.508 (1.129, 2.013) | |||
| Q3 | 3.797 (2.965, 4.864) | 2.228 (1.688, 2.941) | 1.848 (1.348, 2.534) | |||
| Q4 | 7.209 (5.703, 9.111) | 3.208 (2.443, 4.213) | 2.072 (1.471, 2.919) | |||
| P for trend | < 0.001 | < 0.001 | < 0.001 | |||
| CVD mortality | ||||||
| Continuous TyG-ABSI | 2.086 (1.888, 2.304) | < 0.001 | 1.654 (1.455, 1.879) | < 0.001 | 1.377 (1.149, 1.651) | < 0.001 |
| Quartile TyG-ABSI | ||||||
| Q1 | Ref | Ref | Ref | |||
| Q2 | 3.285 (2.020, 5.342) | 2.447 (1.492, 4.012) | 2.157 (1.249, 3.723) | |||
| Q3 | 6.948 (4.355, 11.085) | 3.971 (2.437, 6.471) | 3.306 (1.893, 5.776) | |||
| Q4 | 13.245 (8.685, 20.199) | 5.610 (3.440, 9.150) | 3.643 (2.053, 6.463) | |||
| P for trend | < 0.001 | < 0.001 | < 0.001 | |||
Model 1: crude model
Model 2 adjusted for age, sex, and ethnicity
Model 3 adjusted for Model 2 plus educational level, marital status, PIR, smoking status, alcohol consumption, HEI-2015, PA, HDL, LDL, HbA1c, eGFR, uACR, uric acid, CKD, liver disease, cancer, hypertension, DM, antihypertensive drug, hypoglycemic therapy, lipid-lowering drug, and CKM syndrome
CKM Syndrome, cardiovascular-kidney-metabolic syndrome; CVD, cardiovascular disease; HR, hazard ratio; CI, confidence interval; TyG, triglyceride-glucose; ABSI, a body shape index; uACR, urinary albumin/creatinine ratio; PA, physical activity; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HbA1c, glycosylated hemoglobin; HEI-2015, healthy eating index-2015; PIR, poverty income ratio; DM, diabetes mellitus; HDL, high-density lipoprotein; LDL, low-density lipoprotein
Fig. 3.
Restricted cubic spline curve for the association between TyG-ABSI and mortality. Adjusted for age, sex, ethnicity, educational level, marital status, PIR, smoking status, alcohol consumption, HEI-2015, PA, HDL, LDL, HbA1c, eGFR, uACR, uric acid, CKD, liver disease, cancer, hypertension, DM, antihypertensive drug, hypoglycemic therapy, lipid-lowering drug, and CKM syndrome. (A) Association between TyG-ABSI index and all-cause in patients with CKM stage 0–3. (B) Association between TyG-ABSI index and cardiovascular disease mortality in patients with CKM stage 0–3. The solid line and red area represent the estimated values and their corresponding 95% CIs. Abbrevaitons: CKM Syndrome, cardiovascular-kidney-metabolic syndrome; CVD, cardiovascular disease; HR, hazard ratio; CI, confidence interval; TyG, triglyceride-glucose; ABSI, a body shape index; uACR, urinary albumin/creatinine ratio; PA, physical activity; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HbA1c, glycosylated hemoglobin; HEI-2015, healthy eating index-2015; PIR, poverty income ratio; DM, diabetes mellitus; HDL, high-density lipoprotein; LDL, low-density lipoprotein
Comparison of TyG-derived indices in mortality prediction
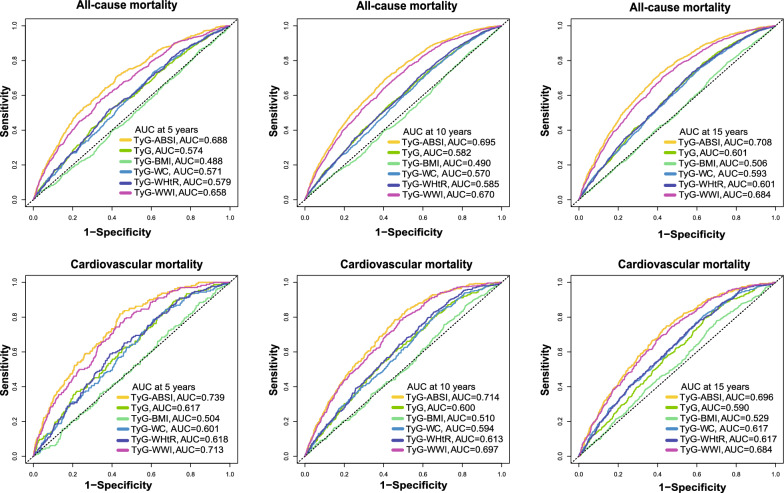
Based on time-dependent ROC curve evaluation, TyG-ABSI exhibited robust capacity for predicting all-cause mortality (AUC: 0.688, 0.695, 0.708) and CVD mortality (AUC: 0.739, 0.714, 0.696) over 5-, 10-, and 15-year intervals (Fig. 4, Tables S19, S20). Notably, at the 5-year interval, the AUC for CVD mortality attained 0.739 (95% CI 0.701–0.770), outperforming other TyG-derived indices (TyG: 0.617; TyG-WWI: 0.713). As the prediction window was extended to 15 years, the AUC of TyG-ABSI remained stable, suggesting that its predictive accuracy was minimally affected by time.
Fig. 4.
Time-dependent Receiver Operating Characteristic analysis of TyG-ABSI in predicting mortality. ROC curves and AUC for the assocaitions of TyG-ABSI, other TyG indices with all-cause mortality in patients with CKM syndrome stage 0–3 at 5-year(A), 10-year(B), and 15-year(C). ROC curves and AUC for the assocaitions of TyG-ABSI, other TyG indices with cardiovascular disease mortality in patients with CKM syndrome stage 0–3 at 5-year(D), 10-year(E), and 15-year(F). Adjusted for age, sex, ethnicity, educational level, marital status, PIR, smoking status, alcohol consumption, HEI-2015, PA, HDL, LDL, HbA1c, eGFR, uACR, uric acid, CKD, liver disease, cancer, hypertension, DM, antihypertensive drug, hypoglycemic therapy, lipid-lowering drug, and CKM syndrome. Abbreviations: ROC: receiver operating characteristic, AUC: area under the curve, CKM Syndrome, cardiovascular-kidney-metabolic syndrome; CVD, cardiovascular disease; HR, hazard ratio; CI, confidence interval; TyG, triglyceride-glucose; ABSI, a body shape index; uACR, urinary albumin/creatinine ratio; PA, physical activity; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HbA1c, glycosylated hemoglobin; HEI-2015, healthy eating index-2015; PIR, poverty income ratio; DM, diabetes mellitus; HDL, high-density lipoprotein; LDL, low-density lipoprotein
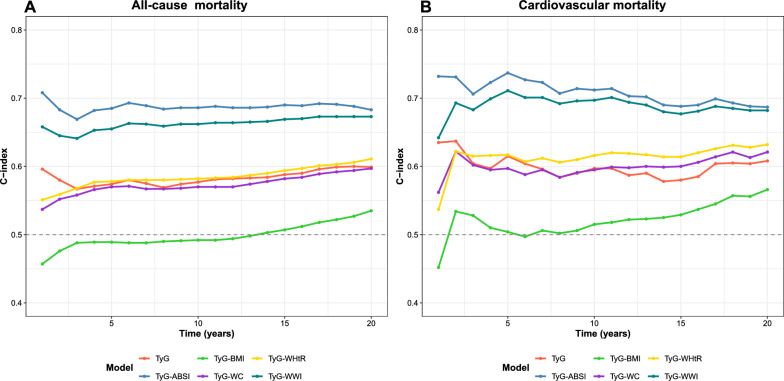
Furthermore, the long-term discriminatory ability of TyG-ABSI was evaluated over a 5- to 15-year predictive timeframe using the C-index (Fig. 5). TyG-ABSI was found to outperform other TyG-based indices in forecasting all-cause and CVD mortality. Specifically, the C-index for TyG-ABSI remained stable at approximately 0.700 throughout the 5- to 15-year window for all mortality outcomes, indicating a robust long-term prognostic capacity (Table S21, S22).
Fig. 5.
C-index evaluation for TyG-ABSI in forecasting mortality. (A) C-index variation of different TyG-derived indices over time for all-cause Mortality. (B) C-index variation of different TyG-derived indices over time for cardiocascular disease mortality. Adjusted for age, sex, ethnicity, educational level, marital status, PIR, smoking status, alcohol consumption, HEI-2015, PA, HDL, LDL, HbA1c, eGFR, uACR, uric acid, CKD, liver disease, cancer, hypertension, DM, antihypertensive drug, hypoglycemic therapy, lipid-lowering drug, and CKM syndrome. Abbreviations: C-index: concordance index, TyG: triglyceride–glucose, ABSI, a body shape index; BMI, body mass index; WWI, weight-adjusted waist index; WHtR, waist-to-height ratio; WC, waist circumference; CKM Syndrome, cardiovascular-kidney-metabolic syndrome; CVD, cardiovascular disease; HR, hazard ratio; CI, confidence interval; TyG, triglyceride-glucose; uACR, urinary albumin/creatinine ratio; PA, physical activity; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HbA1c, glycosylated hemoglobin; HEI-2015, healthy eating index-2015; PIR, poverty income ratio; DM, diabetes mellitus; HDL, high-density lipoprotein; LDL, low-density lipoprotein
Subgroup analyses
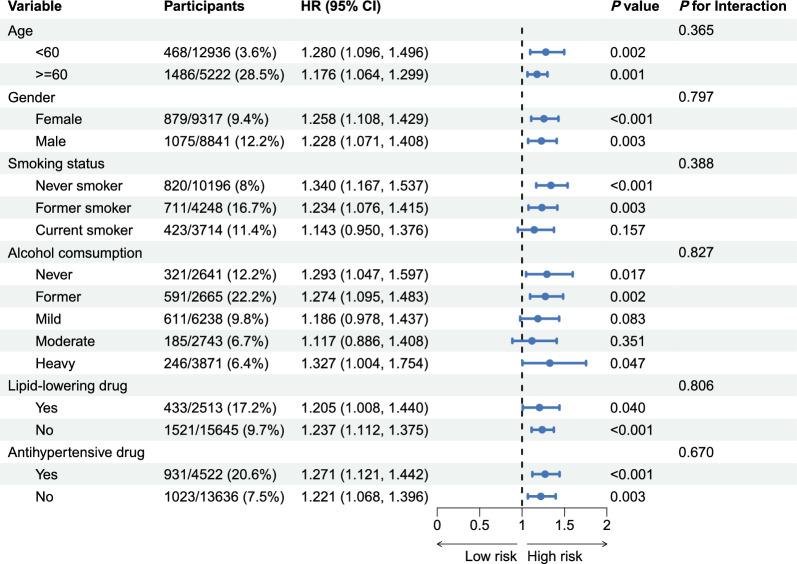
To evaluate the influence of demographic and lifestyle factors on the association between TyG-ABSI and mortality, participants were stratified by age (< 60 years vs. ≥ 60 years), sex (female vs. male), smoking status, alcohol consumption, and use of lipid-lowering or antihypertensive medications, and subgroup analyses were performed. TyG-ABSI was significantly associated with all-cause mortality across all major subgroups (Fig. 6). Specifically, the HR was 1.280 (95% CI 1.096–1.496) for individuals under 60 years and 1.176 (95% CI 1.064–1.299) for those 60 years and older. For females, the HR was 1.258 (95% CI 1.108–1.429), and for males, 1.228 (95% CI 1.071–1.408).
Fig. 6.
Relationships of TyG-ABSI with all-cause mortality varying by subgroup. Adjusted for age, sex, ethnicity, educational level, marital status, PIR, smoking status, alcohol consumption, HEI-2015, PA, HDL, LDL, HbA1c, eGFR, uACR, uric acid, CKD, liver disease, cancer, hypertension, DM, antihypertensive drug, hypoglycemic therapy, lipid-lowering drug, and CKM syndrome. HR, hazard ratio; CI, confidence interval; CKM Syndrome, cardiovascular-kidney-metabolic syndrome; CVD, cardiovascular disease; HR, hazard ratio; CI, confidence interval; TyG, triglyceride-glucose; ABSI, a body shape index; uACR, urinary albumin/creatinine ratio; PA, physical activity; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HbA1c, glycosylated hemoglobin; HEI-2015, healthy eating index-2015; PIR, poverty income ratio; DM, diabetes mellitus; HDL, high-density lipoprotein; LDL, low-density lipoprotein
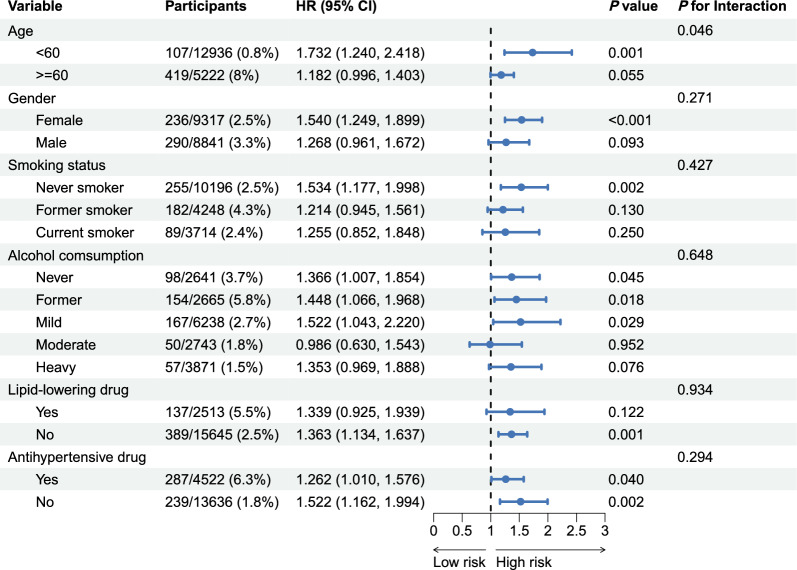
Regarding CVD mortality, subgroup analyses revealed significant interactions with age (P < 0.05; Fig. 7). The association was strongest among participants under 60 years (HR 1.732, 95% CI 1.240–2.418), whereas for those ≥ 60 years, the HR was 1.182 (95% CI 0.996–1.403), reaching only nominal significance. Additionally, a significant association was observed among females (HR 1.540, 95% CI 1.249–1.899), whereas the relationship was not statistically significant in males.
Fig. 7.
Relationships of TyG-ABSI with CVD mortality varying by subgroup. Adjusted for age, sex, ethnicity, educational level, marital status, PIR, smoking status, alcohol consumption, HEI-2015, PA, HDL, LDL, HbA1c, eGFR, uACR, uric acid, CKD, liver disease, cancer, hypertension, DM, antihypertensive drug, hypoglycemic therapy, lipid-lowering drug, and CKM syndrome. Abbrevaitions: HR, hazard ratio; CI, confidence interval; CKM Syndrome, cardiovascular-kidney-metabolic syndrome; CVD, cardiovascular disease; HR, hazard ratio; CI, confidence interval; TyG, triglyceride-glucose; ABSI, a body shape index; uACR, urinary albumin/creatinine ratio; PA, physical activity; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HbA1c, glycosylated hemoglobin; HEI-2015, healthy eating index-2015; PIR, poverty income ratio; DM, diabetes mellitus; HDL, high-density lipoprotein; LDL, low-density lipoprotein
Sensitivity analyses
Sensitivity analyses included excluding individuals with cancer, those who died within 2 or 5 years of baseline, those with advanced CKD, those with missing covariate data, and those receiving lipid-lowering drugs or hypoglycemic therapy; incorporating GLP-1 receptor agonists, social determinants of health (SDoH), depression symptoms, and sleep duration as covariates; accounting for family history of coronary heart disease, heart disease, hypertension, and stroke; and applying Fine-Gray models for competing-risk analysis, all confirming the robustness of the findings. In addition, using the 2011 CHARLS baseline data and tracking it up to 2020, we conducted an analysis of 5,695 patients at CKM stages 0–3 to verify the association between TyG-ABSI and all-cause mortality. Table S18 shows that TyG-ABSI is positively correlated with all-cause mortality. The hazard ratio of Model 3 was 1.375 (95% CI 1.045–1.811). Quartile analysis showed a dose–response trend, with the risk in the highest quartile increasing to 2.492 times (95% CI 1.325–4.688). Restricted cubic spline regression (Fig. S3) confirmed a linear association. Time-dependent ROC analysis (Fig. S4) demonstrated TyG-ABSI’s superior predictive accuracy (AUC = 0.608) compared to other TyG indices (e.g., TyG-WWI, AUC = 0.581). Consistent with NHANES results, these findings reinforce TyG-ABSI’s robustness as a prognostic marker for mortality in CKM stages 0–3.
Mediation analyses
Mediation analyses indicated that ePWV and SIRI partially mediated the relationship, with E-values calculated to assess confounding (Fig. 8, Table S23). For the TyG-ABSI-ePWV pathway, the indirect effects on all-cause and cardiovascular mortality were 1.089 (95% CI 1.078, 1.100) and 1.093 (95% CI 1.077, 1.109), respectively. For the TyG-ABSI-SIRI pathway, these effects were 1.006 (95% CI 1.003, 1.008) and 1.005 (95% CI 1.002, 1.009). Notably, ePWV exhibited higher mediation proportions (from 16.5 to 16.6%) compared to SIRI (from 3.7 to 3.7%) across mortality outcomes.
Fig. 8.
Path diagram of the mediation analysis of ePWV and SIRI. The graphs in (A–D) represented the mediating role in all-cause mortality (via ePWV, SIRI) and CVD mortality (via ePWV, SIRI), respectively. Adjusted for age, sex, ethnicity, educational level, marital status, PIR, smoking status, alcohol consumption, HEI-2015, PA, HDL, LDL, HbA1c, eGFR, uACR, uric acid, CKD, liver disease, cancer, hypertension, DM, antihypertensive drug, hypoglycemic therapy, lipid-lowering drug, and CKM syndrome. Abbrevaitions: HR, hazard ratio. CI, confidence interval. CKM Syndrome, cardiovascular-kidney-metabolic syndrome; CVD, cardiovascular disease; TyG, triglyceride-glucose; ABSI, a body shape index; uACR, urinary albumin/creatinine ratio; PA, physical activity; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HbA1c, glycosylated hemoglobin; HEI-2015, healthy eating index-2015; PIR, poverty income ratio; DM, diabetes mellitus; HDL, high-density lipoprotein; LDL, low-density lipoprotein; ePWV, estimated Pulse Wave Velocity; SIRI, systemic inflammation response index
Incremental value and clinical utility of TyG-ABSI
To further validate the incremental predictive value of TyG-ABSI, net reclassification improvement (NRI), integrated discrimination improvement (IDI) (Table S24), and decision curve analysis (DCA) were evaluated (Fig. S5). Notably, when TyG-ABSI was incorporated into the model comprising core variables from the Framingham risk score, improvements were observed. For all-cause mortality, the NRI was 0.223 (95% CI 0.197–0.240, P < 0.001), with a significant IDI of 0.011 (P = 0.001). For cardiovascular mortality, the NRI was 0.273 (95% CI 0.146–0.285, P < 0.001), accompanied by a significant IDI of 0.006 (P < 0.001). DCA confirmed that TyG-ABSI consistently provided greater net benefit than other TyG-derived indices across 5-, 10-, and 15-year prediction windows for both mortality outcomes, thereby supporting its superior clinical utility in risk stratification.
Discussion
In this study, two large population cohorts, NHANES and CHARLS, were systematically analyzed to examine the relationship between TyG-ABSI and mortality outcomes in patients with CKM syndrome. The main findings are as follows: (1) Each 1 SD increase in TyG-ABSI was associated with a 22.6% higher all-cause mortality and a 37.7% higher cardiovascular mortality; (2) Individuals in the highest TyG-ABSI quartile had 2.07-fold and 3.64-fold higher all-cause and cardiovascular mortality, respectively, compared with those in the lowest quartile; (3) Time-dependent receiver operating characteristic (ROC) curves and C-index analyses demonstrated that TyG-ABSI outperformed other TyG-related indices in predicting long-term mortality; (4) These associations were robust across multiple sensitivity analyses, including external validation of the relationship between TyG-ABSI and all-cause mortality in the CHARLS cohort; and (5) Mediation analyses indicated that both ePWV and SIRI partially mediated the associations, with ePWV accounting for a larger proportion of the effect (16.50–16.60%) compared with SIRI (3.70%).
Central obesity is markedly associated with elevated IR, activation of systemic inflammatory pathways, and increased oxidative stress (22, 45). The interplay of these pathophysiological mechanisms promotes the progression of CKM syndrome, accelerating renal function decline and exacerbating metabolic imbalances, thereby creating a vicious cycle. Consequently, integrating the TyG index with measures of central obesity is essential for evaluating the deleterious effects of CKM syndrome. When combined with obesity-related parameters, the TyG index enhances both the predictive power and diagnostic utility for disease and mortality risk [46–48]. Common anthropometric indicators such as WHtR, WC, and WWI effectively capture abdominal adiposity, and their integration with the TyG index has demonstrated superior predictive ability for mortality [49]. Notably, TyG-derived metrics show significant associations with all-cause mortality (HR: 1.29–1.45, P < 0.01) and cardiovascular mortality (HR 1.45–1.50, P < 0.01) in individuals with metabolic syndrome, while correlations with diabetes-related mortality (HR: 2.53–2.55, both P < 0.001) are even stronger [50]. Furthermore, a study by Yiheng Zhang et al. reported that individuals with metabolism-associated steatotic liver disease (MASLD) who also had pre-diabetes or diabetes exhibited worse outcomes when presenting with high TyG indices, with TyG-WWI emerging as the most effective predictor of adverse events [51, 52]. In Chinese hypertensive adults with obstructive sleep apnea, the composite measure of the triglyceride–glucose index and waist circumference (TyG–WC) independently predicted the first myocardial infarction in a clear dose–response manner, underscoring the value of integrating insulin resistance surrogates with indicators of central adiposity [53].
ABSI has demonstrated superior predictive performance compared with traditional anthropometric measures. Comprehensive analyses indicate that each SD increase in ABSI is associated with a 13% higher risk of hypertension, a 35% higher risk of type 2 diabetes, and a 21% increased risk of cardiovascular disease. Moreover, ABSI has proven to be a better predictor of mortality than waist circumference (WC) or body mass index (BMI) [30]. In adults with diabetes, ABSI exhibited the strongest independent association with mortality, outperforming other anthropometric metrics and achieving the highest predictive accuracy [54]. Although studies on TyG-ABSI remain limited, research by Yong et al. showed that this composite index surpassed other TyG-based markers in forecasting outcomes among patients with hyperuricemia, highlighting its enhanced prognostic value [55]. Recent evidence also indicates that individuals with elevated ABSI and TyG levels face the greatest risk of cardiovascular mortality, regardless of diabetes status, suggesting a synergistic effect that amplifies cardiovascular risk beyond the contribution of each factor alone [33]. Given the complex interplay between insulin resistance and abdominal obesity, further investigation into the association between TyG-ABSI and mortality in CKM syndrome is particularly warranted.
CKM syndrome is a prevalent, multisystem, chronic disorder. Its molecular pathophysiology involves numerous interconnected factors, including insulin resistance (IR), accumulation of advanced glycation end-products, oxidative stress, lipotoxicity, endoplasmic reticulum stress, and chronic inflammation. Among these, IR plays a central role in many metabolic disorders, as impaired insulin signaling disrupts glucose uptake in adipocytes and skeletal muscle cells [56]. Evidence further indicates that IR promotes oxidative stress, impairs insulin signaling pathways, and exacerbates inflammatory responses and cellular injury [57, 58]. In the kidney, Insulin typically boosts nitric oxide generation within endothelial cells, dependent on the phosphatidylinositol 3-kinase (PI3K) route, resulting in vasodilation; however, this mechanism is compromised in insulin-resistant states. In such cases, abnormal mitogen-activated protein kinase pathway signalling causes vasoconstriction, contributing to endothelial dysfunction [59]. These mechanisms work together to promote the development of foam cells, disrupt regular endothelial activity, and trigger excessive smooth muscle cell growth. Moreover, IR can activate the sympathetic nervous system, causing excessive sodium retention, thereby elevating heart strain and ultimately causing structural damage to blood vessel walls and renal hypoplasia [60]. The role of central obesity should not be underestimated when investigating the complex pathophysiology of CKM syndrome, maladaptive expansion of adipose tissue has both local and systemic consequences: locally, it leads to inflammation, hypoxia, dysregulated adipokine secretion, and compromised mitochondrial function [61]. The abundance of pro-inflammatory substances, which are linked to obesity, kickstarts a chain reaction of local tissue and organ inflammation, as well as oxidative stress. This indirect effect can lead to issues like cardiovascular impairment and metabolic disturbances [62, 63]. This worsening IR, in turn, amplifies the clustering of obesity-related cardiovascular risk factors, sustaining a harmful loop of metabolic and cardiovascular disorders [22]. Genetic alterations that impair serum and glucocorticoid kinase 1(SGK-1) activity have been closely linked to metabolic disorders, encompassing hypertension, abnormal glucose processing, and increased adiposity. This key regulatory protein, stimulated by insulin, is vital in controlling sodium transport mechanisms within blood vessels and the kidneys. IR, a central feature of CKM, is mutually reinforcing with both obesity and hypertensive states; moreover, the hyperactivation of SGK-1 may act as a bridge linking these metabolic disorders [62]. Given the severity of this vicious cycle, it is imperative to identify effective indicators capable of recognising high-risk patients.
This study clarified the close association between TyG-ABSI and mortality outcomes. Specifically, for each one SD increase in this indicator, the risk of all-cause mortality rises by 22.6%, and the risk of cardiovascular mortality increases by 37.7%. If the comparison indicators were in the lowest quartile, the all-cause mortality and cardiovascular mortality rates of those in the highest quartile increased to 2.072 times and 3.643 times, respectively. It is worth emphasizing that, compared with other indicators based on TyG, TyG-ABSI has more advantages in predicting the long-term risk of death, and there is a significant linear relationship between its numerical changes and the risk of death. The relatively small separation across the middle quartiles (Q2–Q3) followed by a steeper increase in Q4 is biologically plausible. At moderate levels, subcutaneous adipose tissue can still buffer lipid flux, leading to gradual risk changes; however, at higher levels the adipose ‘expandability’ threshold is exceeded, promoting ectopic lipid deposition and lipotoxic signaling (e.g., ceramides) [64, 65]. These processes can accelerate risk specifically at the extreme range, consistent with a threshold-like amplification despite an overall monotonic association [24].
The larger increase in CKM syndrome prevalence among men (from 18.9% to 22.4%) may be related to the greater rise in adult obesity rates among men, as indicated by recent global analyses [66]. Men are biologically predisposed to earlier and higher accumulation of visceral adipose tissue (VAT) and more pronounced adipose-tissue insulin resistance, which are linked to cardiometabolic risk [67, 68]. Behavioral factors common in working-age men, such as increased sedentary time in desk-based occupations, further amplify central adiposity and metabolic risk [69], with alcohol-related ectopic adiposity potentially adding to this burden [70]. In women, although the increase in CKM syndrome prevalence was smaller (from 13.9 to 15.2%), our subgroup analysis showed a stronger association between elevated TyG-ABSI and cardiovascular mortality. This is consistent with evidence that higher visceral fat and adverse fat distribution confer disproportionate cardiovascular risk in women [71, 72].
Further analyses demonstrated that arterial stiffness, assessed by estimated pulse wave velocity (ePWV), substantially mediated the association between TyG-ABSI and cardiovascular mortality, accounting for 16.5% of the total effect. The Systemic Inflammation Response Index (SIRI) also contributed a smaller but statistically significant mediating effect (3.7%), indicating that both vascular dysfunction and systemic inflammation partially underlie the pathway linking insulin resistance (TyG index) and visceral adiposity (ABSI) to cardiovascular death. Validation in the CHARLS cohort, which differs demographically from NHANES, reinforces the generalizability of these findings.
These results provide clinicians with a practical tool to identify CKM syndrome patients at highest risk. Targeted management of TyG-ABSI in high-risk subgroups could substantially reduce mortality. As TyG-ABSI effectively captures both insulin resistance and persistent central obesity, it has the potential to refine current screening strategies and serve as a more efficient indicator for identifying individuals at elevated risk of adverse outcomes. To our knowledge, this is the first study to examine the relationship between TyG-ABSI and mortality risk in CKM syndrome. Although the precise biological mechanisms linking the TyG index to mortality remain incompletely understood, interdisciplinary research has consistently demonstrated a significant association across diverse clinical settings [73, 74].
Accumulating evidence indicates that both lifestyle modifications—such as adherence to Mediterranean/energy-restricted Mediterranean dietary patterns [75, 76], structured aerobic–resistance exercise [77, 78], smoking cessation [79], and intensive lifestyle programs [80]—and pharmacological interventions including GLP-1 receptor agonists [81], SGLT2 inhibitors [82], and statins can meaningfully reduce central adiposity [83], improve insulin resistance, and favorably modify lipid–glucose metabolism. Given that our results show a disproportionate increase in adverse outcomes among individuals in the highest TyG-ABSI quartile (Q4), such targeted strategies may be particularly impactful in attenuating CKM syndrome progression and reducing long-term cardiovascular risk.
Limitations
Several limitations warrant consideration. First, the observational nature of this study limits the ability to draw causal conclusions; Second, TyG-ABSI was assessed only at baseline, restricting the evaluation of dynamic changes over time and their potential influence on mortality. Third, CHARLS, with its focus on older and rural populations, may introduce survivorship bias due to stricter pre-enrollment survival filtering. Future studies should account for this bias, potentially by incorporating more diverse cohorts with varied age and urban–rural representation. Only all-cause mortality is available in CHARLS, so cross-cohort external validation cannot be conducted for CVD mortality. Moreover, due to the cross-sectional nature of the baseline mediator measurements (ePWV, SIRI), temporal causality between the mediators and the outcome cannot be established.
Conclusion
This study demonstrates that TyG-ABSI is a valuable predictor of both all-cause and cardiovascular-specific mortality in individuals with stage 0–3 CKM syndrome. Compared with other TyG-based indices, TyG-ABSI exhibits superior prognostic performance for mortality risk. Using this composite index helps in identifying populations that may benefit from tailored interventions, including metabolic assessments, individualized lifestyle modifications, and enhanced monitoring, in both clinical and community settings. By targeting these individuals, healthcare providers can more effectively reduce mortality, optimize clinical outcomes, and improve resource allocation in the management of CKM syndrome.
Supplementary Information
Acknowledgements
Thanks to all NHANES and CHARLS participants and contributing researchers. We deeply appreciate the YIWANDOU team for their invaluable contribution to data cleaning and processing. Thanks to Hongyu Kuang for her insightful guidance in revising this manuscript
Abbreviations
- CKM syndrome
Cardiovascular-kidney-metabolic syndrome
- IR
Insulin resistance
- TyG
Triglyceride-glucose index
- CVD
Cardiovascular disease
- WHtR
Waist-to-height ratio
- WC
Waist circumference
- WWI
Weight-adjusted waist index
- BMI
Body mass index
- ABSI
A body shape index
- NHANES
National Health and Nutrition Examination Survey
- eGFR
Estimated glomerular filtration rate
- uACR
Urine albumin-creatinine ratio
- HbA1c
Glycated hemoglobin A1c
- HDL-C
High-density lipoprotein cholesterol
- LDL-C
Low-density lipoprotein cholesterol
- HR
Hazard ratio
- CI
Confidence interval
- SD
Standard deviation
- RCS
Restricted cubic spline
- C-index
Concordance index
- ROC
Receiver operating characteristic
- AUC
Area under the curve
- ePWV
Estimated Pulse Wave Velocity
- SIRI
Systemic inflammation response index
- NRI
Net reclassification improvement
- IDI
Integrated discrimination improvement
- DCA
Decision curve analysis
Author contributions
Mingjie Chen and Jiajie Guo led the design and implementation of the study. Mingjie Chen, Jiajie Guo, Yuwen Shangguan, Zhonghua Sun, and Xueling He were responsible for data selection, organization, and analysis. Qiang Tu contributed to the study design and manuscript writing. Qingkai Yan assisted with cross-checking the findings and exploring their implications. Mingjie Chen and Jiajie Guo drafted the initial version of the paper. Qingkai Yan participated in refining the document, providing expert input. Qingkai Yan also oversaw the research process, ensuring quality control. Each co-author was involved in the final revision, review, and approval of the completed paper.
Funding
The Special Fund for Outstanding Young Talents (Grant BYD02-02).
Data availability
The data included in the work is accessible on https://www.cdc.gov/nchs/nhanes and http://charls.pku.edu.cn.
Declarations
Informed consent
The NHANES study protocol received approval from the Institutional Review Board of the National Center for Health Statistics, and all participants provided written informed consent prior to enrollment.
Consent for publications
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mingjie Chen and Jiajie Guo have contributed equally to this work.
References
- 1.Ndumele CE, Neeland IJ, Tuttle KR, Chow SL, Mathew RO, Khan SS, et al. A synopsis of the evidence for the science and clinical management of cardiovascular-kidney-metabolic (CKM) syndrome: A scientific statement from the American Heart Association. Circulation. 2023;148(20):1636–64. 10.1161/cir.0000000000001186. [DOI] [PubMed] [Google Scholar]
- 2.Claudel SE, Verma A. Cardiovascular-kidney-metabolic syndrome: a step toward multidisciplinary and inclusive care. Cell Metab. 2023;35(12):2104–6. 10.1016/j.cmet.2023.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad FB, Anderson RN. The leading causes of death in the US for 2020. JAMA. 2021;325(18):1829–30. 10.1001/jama.2021.5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sebastian SA, Padda I, Johal G. Cardiovascular-kidney-metabolic (CKM) syndrome: a state-of-the-art review. Curr Probl Cardiol. 2024;49(2):102344. 10.1016/j.cpcardiol.2023.102344. [DOI] [PubMed] [Google Scholar]
- 5.Ji H, Sabanayagam C, Matsushita K, Cheng CY, Rim TH, Sheng B, et al. Sex differences in cardiovascular-kidney-metabolic syndrome: 30-year US trends and mortality risks-brief report. Arterioscler Thromb Vasc Biol. 2025;45(1):157–61. 10.1161/atvbaha.124.321629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Wei X. Association of cardiovascular-kidney-metabolic syndrome with all-cause and cardiovascular mortality: a prospective cohort study. Am J Prev Cardiol. 2025;22:100985. 10.1016/j.ajpc.2025.100985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson PW, D’Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112(20):3066–72. 10.1161/circulationaha.105.539528. [DOI] [PubMed] [Google Scholar]
- 8.Li M, Chi X, Wang Y, Setrerrahmane S, Xie W, Xu H. Trends in insulin resistance: insights into mechanisms and therapeutic strategy. Signal Transduct Target Ther. 2022;7(1):216. 10.1038/s41392-022-01073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu S, Sun H, Liu J, Wang G. Accessing the relationship between six surrogate insulin resistance indexes and the incidence of rapid kidney function decline and the progression to chronic kidney disease among middle-aged and older adults in China: results from the China health and retirement longitudinal study. Diabetes Res Clin Pract. 2024;212:111705. 10.1016/j.diabres.2024.111705. [DOI] [PubMed] [Google Scholar]
- 10.Lee SH, Park SY, Choi CS. Insulin resistance: from mechanisms to therapeutic strategies. Diabetes Metab J. 2022;46(1):15–37. 10.4093/dmj.2021.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silveira Rossi JL, Barbalho SM, Reverete de Araujo R, Bechara MD, Sloan KP, Sloan LA. Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diabetes Metab Res Rev. 2022;38(3):e3502. 10.1002/dmrr.3502. [DOI] [PubMed] [Google Scholar]
- 12.Zhu Q, Chen Y, Cai X, Cai L, Hong J, Luo Q, et al. The non-linear relationship between triglyceride-glucose index and risk of chronic kidney disease in hypertensive patients with abnormal glucose metabolism: a cohort study. Front Med. 2022;9:1018083. 10.3389/fmed.2022.1018083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Z, Gong H, Kan F, Ji N. Association between the triglyceride glucose (TyG) index and the risk of acute kidney injury in critically ill patients with heart failure: analysis of the MIMIC-IV database. Cardiovasc Diabetol. 2023;22(1):232. 10.1186/s12933-023-01971-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y, Gu Y, Zhang B. Associations of triglyceride-glucose (TyG) index with chest pain incidence and mortality among the U.S. population. Cardiovasc Diabetol. 2024;23(1):111. 10.1186/s12933-024-02209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huo RR, Liao Q, Zhai L, You XM, Zuo YL. Interacting and joint effects of triglyceride-glucose index (TyG) and body mass index on stroke risk and the mediating role of TyG in middle-aged and older Chinese adults: a nationwide prospective cohort study. Cardiovasc Diabetol. 2024;23(1):30. 10.1186/s12933-024-02122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang R, Wang Z, Chen J, Bao X, Xu N, Guo S, et al. Prognostic value of triglyceride glucose (TyG) index in patients with acute decompensated heart failure. Cardiovasc Diabetol. 2022;21(1):88. 10.1186/s12933-022-01507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan SS, Ning H, Wilkins JT, Allen N, Carnethon M, Berry JD, et al. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiol. 2018;3(4):280–7. 10.1001/jamacardio.2018.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piché ME, Tchernof A, Després JP. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res. 2020;126(11):1477–500. 10.1161/circresaha.120.316101. [DOI] [PubMed] [Google Scholar]
- 19.Zhao J, Cai X, Hu J, Song S, Zhu Q, Shen D, et al. J-shaped relationship between weight-adjusted-waist index and cardiovascular disease risk in hypertensive patients with obstructive sleep apnea: a cohort study. Diabetes Metab Syndr Obes. 2024;17:2671–81. 10.2147/dmso.S469376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng CK, Ding H, Jiang M, Yin H, Gollasch M, Huang Y. Perivascular adipose tissue: fine-tuner of vascular redox status and inflammation. Redox Biol. 2023;62:102683. 10.1016/j.redox.2023.102683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iacobellis G. Epicardial adipose tissue in contemporary cardiology. Nat Rev Cardiol. 2022;19(9):593–606. 10.1038/s41569-022-00679-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koenen M, Hill MA, Cohen P, Sowers JR. Obesity, adipose tissue and vascular dysfunction. Circ Res. 2021;128(7):951–68. 10.1161/circresaha.121.318093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung MH, Ihm SH. Obesity-related hypertension and chronic kidney disease: from evaluation to management. Kidney Res Clin Pract. 2023;42(4):431–44. 10.23876/j.krcp.23.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zietzer A, Düsing P, Reese L, Nickenig G, Jansen F. Ceramide metabolism in cardiovascular disease: a network with high therapeutic potential. Arterioscler Thromb Vasc Biol. 2022;42(10):1220–8. 10.1161/atvbaha.122.318048. [DOI] [PubMed] [Google Scholar]
- 25.Couch CA, Fowler LA, Goss AM, Gower BA. Associations of renal sinus fat with blood pressure and ectopic fat in a diverse cohort of adults. Int J Cardiol Cardiovasc Risk Prev. 2023;16:200165. 10.1016/j.ijcrp.2022.200165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiu X, Lan X, Li L, Chen H, Zhang N, Zheng X, et al. The role of perirenal adipose tissue deposition in chronic kidney disease progression: mechanisms and therapeutic implications. Life Sci. 2024;352:122866. 10.1016/j.lfs.2024.122866. [DOI] [PubMed] [Google Scholar]
- 27.Li P, Liu B, Wu X, Lu Y, Qiu M, Shen Y, et al. Perirenal adipose afferent nerves sustain pathological high blood pressure in rats. Nat Commun. 2022;13(1):3130. 10.1038/s41467-022-30868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jayedi A, Soltani S, Zargar MS, Khan TA, Shab-Bidar S. Central fatness and risk of all cause mortality: systematic review and dose-response meta-analysis of 72 prospective cohort studies. BMJ. 2020;370:m3324. 10.1136/bmj.m3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothman KJ. BMI-related errors in the measurement of obesity. Int J Obes (Lond). 2008;32(Suppl 3):S56–9. 10.1038/ijo.2008.87. [DOI] [PubMed] [Google Scholar]
- 30.Ji M, Zhang S, An R. Effectiveness of a body shape index (ABSI) in predicting chronic diseases and mortality: a systematic review and meta-analysis. Obes Rev. 2018;19(5):737–59. 10.1111/obr.12666. [DOI] [PubMed] [Google Scholar]
- 31.Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China health and retirement longitudinal study (CHARLS). Int J Epidemiol. 2014;43(1):61–8. 10.1093/ije/dys203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He H-m, Xie Y-y, Chen Q, Li Y-k, Li X-x, Mu Y-k, et al. The additive effect of the triglyceride-glucose index and estimated glucose disposal rate on long-term mortality among individuals with and without diabetes: a population-based study. Cardiovasc Diabetol. 2024;23(1):307. 10.1186/s12933-024-02396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He HM, Xie YY, Chen Q, Li YK, Li XX, Fu SJ, et al. The synergistic effect of the triglyceride-glucose index and a body shape index on cardiovascular mortality: the construction of a novel cardiovascular risk marker. Cardiovasc Diabetol. 2025;24(1):69. 10.1186/s12933-025-02604-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stekhoven DJ, Bühlmann P. Missforest–non-parametric missing value imputation for mixed-type data. Bioinformatics. 2012;28(1):112–8. 10.1093/bioinformatics/btr597. [DOI] [PubMed] [Google Scholar]
- 35.Luo F, Guo J-j, Yuan X-m, Zhou H, Wang Q-y, Chen C-m, et al. Inflammatory markers mediate the association between alternative adiposity indices and mortality in patients with rheumatoid arthritis: data from NHANES 1999–2018. Lipids Health Dis. 2025;24(1):170. 10.1186/s12944-025-02584-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo JJ, Hang QQ, Xu T, Liang WX, Gao JK, Ou HB, et al. Central adiposity indices and inflammatory markers mediate the association between life’s crucial 9 and periodontitis in US adults. Lipids Health Dis. 2025;24(1):199. 10.1186/s12944-025-02619-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Lu C, Li X, Fan Y, Li J, Liu Y, et al. Healthy eating index-2015 and predicted 10-year cardiovascular disease risk, as well as heart age. Front Nutr. 2022;9:888966. 10.3389/fnut.2022.888966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yao H, Wang X, Wu X, Liu Y, Chen Y, Li L, et al. Sex differences in association of healthy eating pattern with all-cause mortality and cardiovascular mortality. BMC Public Health. 2024;24(1):2363. 10.1186/s12889-024-19883-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu H, Chen C, Lu ZX, Nie Z. rcssci: An R package for visualization of restricted cubic spline. Med Res. 2020. 10.1002/mdr2.70015. [Google Scholar]
- 41.Wang X, Piantadosi S, Le-Rademacher J, Mandrekar SJ. Statistical considerations for subgroup analyses. J Thorac Oncol. 2021;16(3):375–80. 10.1016/j.jtho.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Menyhárt O, Győrffy B. Multiplicity corrections in life sciences: challenges and consequences. Int J Epidemiol. 2025. 10.1093/ije/dyaf098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.VanderWeele TJ. Causal mediation analysis with survival data. Epidemiology. 2011;22(4):582–5. 10.1097/EDE.0b013e31821db37e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the e-value. Ann Intern Med. 2017;167(4):268–74. 10.7326/m16-2607. [DOI] [PubMed] [Google Scholar]
- 45.Tong Y, Xu S, Huang L, Chen C. Obesity and insulin resistance: pathophysiology and treatment. Drug Discov Today. 2022;27(3):822–30. 10.1016/j.drudis.2021.11.001. [DOI] [PubMed] [Google Scholar]
- 46.Dang K, Wang X, Hu J, Zhang Y, Cheng L, Qi X, et al. The association between triglyceride-glucose index and its combination with obesity indicators and cardiovascular disease: NHANES 2003–2018. Cardiovasc Diabetol. 2024;23(1):8. 10.1186/s12933-023-02115-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang Y, Zhou Y, Xu Y, Wang X, Zhou Z, Wu K, et al. Inflammatory markers link triglyceride-glucose index and obesity indicators with adverse cardiovascular events in patients with hypertension: insights from three cohorts. Cardiovasc Diabetol. 2025;24(1):11. 10.1186/s12933-024-02571-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li C, Zhang Z, Luo X, Xiao Y, Tu T, Liu C, et al. The triglyceride-glucose index and its obesity-related derivatives as predictors of all-cause and cardiovascular mortality in hypertensive patients: insights from NHANES data with machine learning analysis. Cardiovasc Diabetol. 2025;24(1):47. 10.1186/s12933-025-02591-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Min Y, Wei X, Wei Z, Song G, Zhao X, Lei Y. Prognostic effect of triglyceride glucose-related parameters on all-cause and cardiovascular mortality in the United States adults with metabolic dysfunction-associated steatotic liver disease. Cardiovasc Diabetol. 2024;23(1):188. 10.1186/s12933-024-02287-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei X, Min Y, Song G, Ye X, Liu L. Association between triglyceride-glucose related indices with the all-cause and cause-specific mortality among the population with metabolic syndrome. Cardiovasc Diabetol. 2024;23(1):134. 10.1186/s12933-024-02215-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qiao Y, Wang Y, Chen C, Huang Y, Zhao C. Association between triglyceride-glucose (TyG) related indices and cardiovascular diseases and mortality among individuals with metabolic dysfunction-associated steatotic liver disease: a cohort study of UK Biobank. Cardiovasc Diabetol. 2025;24(1):12. 10.1186/s12933-024-02572-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Wu J, Li T, Qu Y, Wang Y. Association of triglyceride-glucose related indices with mortality among individuals with MASLD combined with prediabetes or diabetes. Cardiovasc Diabetol. 2025;24(1):52. 10.1186/s12933-025-02616-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu J, Cai X, Li N, Zhu Q, Wen W, Hong J, et al. Association between triglyceride glucose index-waist circumference and risk of first myocardial infarction in Chinese hypertensive patients with obstructive sleep apnoea: an observational cohort study. Nat Sci Sleep. 2022;14:969–80. 10.2147/nss.S362101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei S, Jiang W, Zheng H, Zhang J, Yang J, Wang Y, et al. The combined impact of BMI and ABSI on all-cause mortality among American adults with diabetes. Diabetol Metab Syndr. 2025;17(1):48. 10.1186/s13098-025-01614-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang Y, Wei Z, Wang L, Zhang G, Yang G, Yu J, et al. Association of triglyceride-glucose-related obesity indices with all-cause and cardiovascular mortality among individuals with hyperuricemia: a retrospective cohort study. J Am Nutr Assoc. 2025. 10.1080/27697061.2025.2475876. [DOI] [PubMed] [Google Scholar]
- 56.Yaribeygi H, Farrokhi FR, Butler AE, Sahebkar A. Insulin resistance: review of the underlying molecular mechanisms. J Cell Physiol. 2019;234(6):8152–61. 10.1002/jcp.27603. [DOI] [PubMed] [Google Scholar]
- 57.Molina MN, Ferder L, Manucha W. Emerging role of nitric oxide and heat shock proteins in insulin resistance. Curr Hypertens Rep. 2016;18(1):1. 10.1007/s11906-015-0615-4. [DOI] [PubMed] [Google Scholar]
- 58.Henriksen EJ, Diamond-Stanic MK, Marchionne EM. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic Biol Med. 2011;51(5):993–9. 10.1016/j.freeradbiomed.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Artunc F, Schleicher E, Weigert C, Fritsche A, Stefan N, Häring HU. The impact of insulin resistance on the kidney and vasculature. Nat Rev Nephrol. 2016;12(12):721–37. 10.1038/nrneph.2016.145. [DOI] [PubMed] [Google Scholar]
- 60.da Silva AA, do Carmo JM, Li X, Wang Z, Mouton AJ, Hall JE. Role of hyperinsulinemia and insulin resistance in hypertension: metabolic syndrome revisited. Can J Cardiol. 2020;36(5):671–82. 10.1016/j.cjca.2020.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koliaki C, Liatis S, Kokkinos A. Obesity and cardiovascular disease: revisiting an old relationship. Metabolism. 2019;92:98–107. 10.1016/j.metabol.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 62.Hill MA, Yang Y, Zhang L, Sun Z, Jia G, Parrish AR, et al. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism. 2021;119:154766. 10.1016/j.metabol.2021.154766. [DOI] [PubMed] [Google Scholar]
- 63.Szukiewicz D. Molecular mechanisms for the vicious cycle between insulin resistance and the inflammatory response in obesity. Int J Mol Sci. 2023. 10.3390/ijms24129818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sakers A, De Siqueira MK, Seale P, Villanueva CJ. Adipose-tissue plasticity in health and disease. Cell. 2022;185(3):419–46. 10.1016/j.cell.2021.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lopez-Yus M, Hörndler C, Borlan S, Bernal-Monterde V, Arbones-Mainar JM. Unraveling adipose tissue dysfunction: molecular mechanisms, novel biomarkers, and therapeutic targets for liver fat deposition. Cells. 2024. 10.3390/cells13050380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ng M, Dai X, Cogen RM, Abdelmasseh M, Abdollahi A, Abdullahi A, et al. National-level and state-level prevalence of overweight and obesity among children, adolescents, and adults in the USA, 1990–2021, and forecasts up to 2050. Lancet. 2024;404(10469):2278–98. 10.1016/s0140-6736(24)01548-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arner P, Viguerie N, Massier L, Rydén M, Astrup A, Blaak E, et al. Sex differences in adipose insulin resistance are linked to obesity, lipolysis and insulin receptor substrate 1. Int J Obes (Lond). 2024;48(7):934–40. 10.1038/s41366-024-01501-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mitsushio K, Baden MY, Kagisaki T, Kato S, Niki A, Takayama R, et al. Interrelationships among accumulations of intra- and periorgan fats, visceral fat, and subcutaneous fat. Diabetes. 2024;73(7):1122–6. 10.2337/db24-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao W, Sanna M, Chen YH, Tsai MK, Wen CP. Occupational sitting time, leisure physical activity, and all-cause and cardiovascular disease mortality. JAMA Netw Open. 2024;7(1):e2350680. 10.1001/jamanetworkopen.2023.50680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kazibwe R, Chevli PA, Evans JK, Allison M, Michos ED, Wood AC, et al. Association between alcohol consumption and ectopic fat in the multi-ethnic study of atherosclerosis. J Am Heart Assoc. 2023;12(18):e030470. 10.1161/jaha.123.030470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nicklas BJ, Penninx BW, Cesari M, Kritchevsky SB, Newman AB, Kanaya AM, et al. Association of visceral adipose tissue with incident myocardial infarction in older men and women: the health, aging and body composition study. Am J Epidemiol. 2004;160(8):741–9. 10.1093/aje/kwh281. [DOI] [PubMed] [Google Scholar]
- 72.Cesaro A, De Michele G, Fimiani F, Acerbo V, Scherillo G, Signore G, et al. Visceral adipose tissue and residual cardiovascular risk: a pathological link and new therapeutic options. Front Cardiovasc Med. 2023;10:1187735. 10.3389/fcvm.2023.1187735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yao Y, Wang B, Geng T, Chen J, Chen W, Li L. The association between TyG and all-cause/non-cardiovascular mortality in general patients with type 2 diabetes mellitus is modified by age: results from the cohort study of NHANES 1999–2018. Cardiovasc Diabetol. 2024;23(1):43. 10.1186/s12933-024-02120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Q, Xiao S, Jiao X, Shen Y. The triglyceride-glucose index is a predictor for cardiovascular and all-cause mortality in CVD patients with diabetes or pre-diabetes: evidence from NHANES 2001–2018. Cardiovasc Diabetol. 2023;22(1):279. 10.1186/s12933-023-02030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Konieczna J, Ruiz-Canela M, Galmes-Panades AM, Abete I, Babio N, Fiol M, et al. An energy-reduced Mediterranean diet, physical activity, and body composition: an interim subgroup analysis of the PREDIMED-Plus randomized clinical trial. JAMA Netw Open. 2023;6(10):e2337994. 10.1001/jamanetworkopen.2023.37994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zelicha H, Kloting N, Kaplan A, Yaskolka Meir A, Rinott E, Tsaban G, et al. The effect of high-polyphenol Mediterranean diet on visceral adiposity: the DIRECT PLUS randomized controlled trial. BMC Med. 2022;20(1):327. 10.1186/s12916-022-02525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jayedi A, Soltani S, Emadi A, Zargar MS, Najafi A. Aerobic exercise and weight loss in adults: a systematic review and dose-response meta-analysis. JAMA Netw Open. 2024;7(12):e2452185. 10.1001/jamanetworkopen.2024.52185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Batrakoulis A, Jamurtas AZ, Metsios GS, Perivoliotis K, Liguori G, Feito Y, et al. Comparative efficacy of 5 exercise types on cardiometabolic health in overweight and obese adults: a systematic review and network meta-analysis of 81 randomized controlled trials. Circ Cardiovasc Qual Outcomes. 2022;15(6):e008243. 10.1161/circoutcomes.121.008243. [DOI] [PubMed] [Google Scholar]
- 79.Cho JH, Shin SY, Kim H, Kim M, Byeon K, Jung M, et al. Smoking cessation and incident cardiovascular disease. JAMA Netw Open. 2024;7(11):e2442639. 10.1001/jamanetworkopen.2024.42639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Knowler WC, Doherty L, Edelstein SL, Bennett PH, Dabelea D, Hoskin M, et al. Long-term effects and effect heterogeneity of lifestyle and metformin interventions on type 2 diabetes incidence over 21 years in the US Diabetes Prevention Program randomised clinical trial. Lancet Diabetes Endocrinol. 2025;13(6):469–81. 10.1016/s2213-8587(25)00022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lincoff AM, Brown-Frandsen K, Colhoun HM, Deanfield J, Emerson SS, Esbjerg S, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. 2023;389(24):2221–32. 10.1056/NEJMoa2307563. [DOI] [PubMed] [Google Scholar]
- 82.Wang X, Wu N, Sun C, Jin D, Lu H. Effects of SGLT-2 inhibitors on adipose tissue distribution in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Diabetol Metab Syndr. 2023;15(1):113. 10.1186/s13098-023-01085-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xie S, Galimberti F, Olmastroni E, Luscher TF, Carugo S, Catapano AL, et al. Effect of lipid-lowering therapies on C-reactive protein levels: a comprehensive meta-analysis of randomized controlled trials. Cardiovasc Res. 2024;120(4):333–44. 10.1093/cvr/cvae034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data included in the work is accessible on https://www.cdc.gov/nchs/nhanes and http://charls.pku.edu.cn.