Abstract
Two sensitive and selective ion-selective electrodes (ISEs) were developed for the determination of benzydamine hydrochloride (BNZ·HCl): a conventional polyvinyl chloride (PVC) electrode and a coated graphite all solidstate ion-selective electrode (ASS-ISE). Both sensors were constructed using an ion-pair formed between BNZ⁺ and the lipophilic anion tetraphenylborate (TPB⁻), incorporated into the sensing membranes. The sensors exhibited near-Nernstian responses with slopes of 58.09 And 57.88 mV/decade, over a Linear range of 10–5–10–2 M, and detection Limits of 5.81 × 10–8M and 7.41 × 10–8 M, respectively. They demonstrated high accuracy and precision for the determination of BNZ·HCl in pure form, pharmaceutical cream, and biological fluids, with no matrix interference. The method also proved stability-indicating, successfully detecting BNZ·HCl in the presence of its oxidative degradant. Validation was performed according to ICH guidelines, and the method showed strong environmental compatibility based on greenness, whiteness, and blueness assessments, supporting its suitability for sustainable pharmaceutical analysis.
Keywords: Benzydamine hydrochloride, Oxidative degradation, All solidstate ion-selective electrodes, Performance limitations, Sustainability assessment
Introduction
Benzydamine hydrochloride (BNZ·HCl) is a tertiary amine indazole derivative (structure shown in Fig. 1) and a nonsteroidal anti-inflammatory drug (NSAID) with local Anesthetic And analgesic properties. It is widely used in topical formulations, such as 5% (w/w) creams or gels, to treat pain and inflammation of the mouth, throat, and musculoskeletal system [1].
Fig. 1.
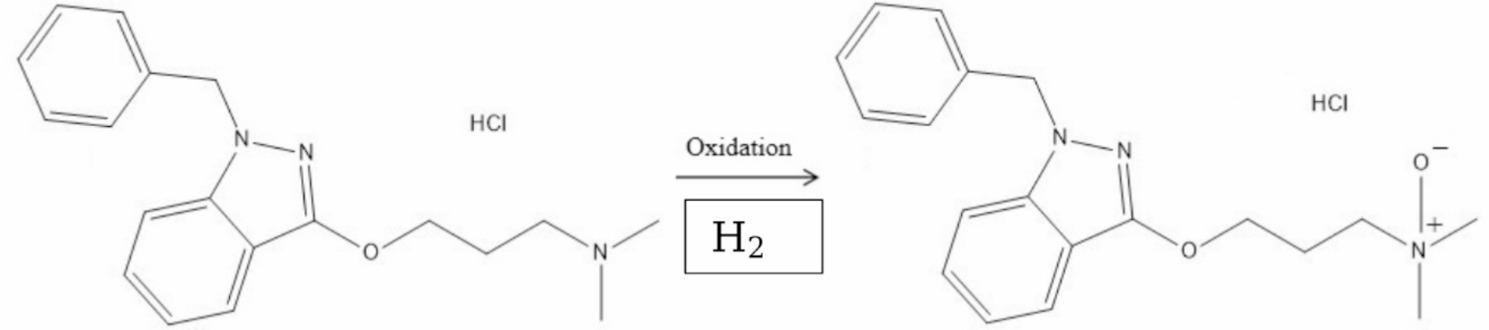
Structural formula of BNZ and its oxidative-degradant [10]
BNZ·HCl exerts its therapeutic effects by suppressing inflammation and inhibiting inflammatory mediators [2]. Although not inherently unstable, photodermatitis has been reported following topical use [3], indicating a potential photosensitive reaction. This may be linked to the phototransformation of BNZ into its N-oxide derivative [4], as shown in Fig. 1. Therefore, assessing the stability of BNZ under light exposure in various formulations is important.
Numerous methods have been developed for the determination of BNZ·HCl. These include reversed-phase high-performance liquid chromatography (RP-HPLC) for topical formulations [5, 6], HPLC with diode array detection for photodegradation studies [7], and fluorometric techniques for plasma monitoring following administration [8]. BNZ·HCl and its impurities have also been simultaneously quantified using HPLC in oral spray and collutory forms [9, 10].
Electrochemical approaches, including amperometric biosensors [11], piezoelectric sensors [12], and various potentiometric methods [13, 14], have gained attention due to their simplicity and cost-effectiveness. A stability-indicating UV spectrophotometric method has also been reported [15].
Ion-selective electrodes (ISEs) measure the potential difference between a selective membrane and a reference electrode, which correlates with the concentration of a target ion [16, 17]. Based on their membrane composition and design, ISEs include liquid, solid-state, glass, coated wire, enzyme-based, and gas-sensing types [18].
Potentiometric ISEs offer several advantages: rapid analysis, minimal sample preparation, low cost, portability, and broad dynamic range. They are also considered among the greenest analytical techniques due to their low environmental impact [19]. PVC-based membranes, the most common type, contain an ion pair complex embedded in a matrix of plasticizer and PVC. Alternatively, liquid membrane ISEs use ion exchangers immobilized in porous matrices to enable selective ion transport [20–22].
While ISEs are easy to use and eco-friendly, they may show limited selectivity compared to voltammetric methods, which provide higher selectivity based on electrochemical profiles but require more complex instrumentation and expertise.
The coated graphite sensor integrates a selective polymer membrane (typically PVC or similar), a plasticizer, and the ion pair complex onto a conductive graphite substrate. This design enhances conductivity, mechanical flexibility, and ease of miniaturization. Key advantages include elimination of internal solution (removing liquid junction errors), fast response, cost-efficiency, and suitability for portable or on-site applications [23].
Despite advancements in ISE technology, no prior study has reported the use of a miniaturized all-solid-state ion-selective electrode (ASS-ISE) for BNZ·HCl determination. This study introduces a novel coated graphite-based ASS-ISE, designed for compatibility with modern solid-state applications.
ISEs have been widely applied for pharmaceutical analysis [24–32], but this study marks the first use of an ASS-ISE for BNZ·HCl, representing a significant innovation in the field.
This study aimed to develop and validate two ion-selective electrodes for the determination of BNZ·HCl: a conventional PVC membrane ISE and a miniaturized coated graphite-based ASS-ISE. The goal was to establish a simple, rapid, and environmentally friendly method for BNZ·HCl analysis in pharmaceutical products, biological fluids, and in the presence of its oxidative degradant.
Both sensors were fabricated, optimized, and validated following IUPAC and ICH guidelines. Performance was assessed in standard and real samples. Additionally, the method was evaluated for environmental sustainability using greenness, whiteness, and blueness metrics, aligning the work with green analytical chemistry principles.
Experimental details
Materials and reagents
Pure standard
A pure standard of BNZ.HCl, certified by the manufacturer to contain 99.46%, was analyzed using the official USP method [33] was provided as a gift standard by EIPICO Company (Cairo, Egypt).
Market sample
Difflam cream 5% (Batch no. D18-2951), procured from the UK market, was labeled to contain 5% w/w of BNZ.HCl and was manufactured by Meda Pharmaceuticals Ltd., Parsonage Road, Skyway House, United Kingdom.
Chemicals and reagents
All chemicals and solvents used throughout the experimental procedures were of pure analytical reagent grade. Bi-distilled water Bi-distilled water was used throughout.
Dioctyl phthalate (DOP), Tetrahydrofuran (THF), and PVC were obtained from Sigma–Aldrich, Germany.
Sodium tetraphenylborate (Na-TPB) (Sigma–Aldrich, Germany), was prepared as a 10−2 M aqueous solution.
Sodium hydroxide (El-Nasr Company, Egypt), was prepared as a 1 M aqueous solution.
Glucose, calcium chloride, potassium chloride, sucrose, glycine, magnesium chloride, nickel chloride, and sodium chloride (El-Nasr Company, Egypt)were each prepared as 10−3 M aqueous solutions.
Hydrochloric acid (El-Nasr Company, Egypt), was prepared as a 1 M aqueous solution.
Monobasic potassium phosphate, boric acid, ethanol, methanol, sodium acetate trihydrate, and glacial acetic acid were also purchased from El-Nasr Company, Egypt. Bi-distilled water was used as a solvent for all solution preparations.
Buffers of varying pH were prepared as prescribed in USP [33]: HCl buffer (pH 2), Acetate buffer (pH 4–5.50), Phosphate buffer (pH 6–8) and Alkaline borate buffer (pH (8–10). Hydrogen peroxide (5% H2O2) was also used.
Instruments
Measurements were carried out using a Jenway 3510 pH meter (USA) equipped with an Ag/AgCl reference electrode (model no. 924017-L03-Q11C).
A Bandelin Sonorex ultrasonic bath (model Rx 510 S) and a magnetic stirrer (Hungarian make) were also employed during the experimental procedures.
Stock and working solutions
A stock solution of BNZ.HCl (10−2 M) was prepared by dissolving 0.346 g of BNZ.HCl in 50 mL of bi-distilled water, shaking thoroughly,, And then diluting to 100 mL with bi-distilled water, Subsequently, various working standard solutions ranging from 10−6 M to 10−2 M were prepared by appropriate dilutions of the BNZ stock solution using bi- distilled water.
Preparation of oxidative degradant
Forced oxidative degradation was carried out according to a previously reported procedure [15]. Specifically, 2 mL of a 10−2 M BNZ.HCl standard stock solution was added to a 100 mL flask containing 10 mL of 5% H2O2. The mixture was allowed to react for 1 h at ambient temperature, then diluted to volume with bi-distilled water. Spectrophotometric Analysis of the resulting solution was performed at 305.6 nm, using the appropriate solvent as a blank. Complete oxidation was confirmed; when the characteristic absorption peak of BNZ.HCl was no longer detectable.
Preparation of BNZ-PVC-membrane sensor (liquid contact electrode)
Ion-pair complex preparation
The BNZ-tetraphenylborate associated complex was prepared by mixing 50 mL of a 10−2 M BNZ.HCl solution, which act as An cation with 50 mL of a 10−2 M sodium tetraphenylborate solution which act as Anion. The resulting solid precipitate was allowed to equilibrate with the supernatant for 6 h. The solid was then collected by filtration, thoroughly washed with bi-distilled water, And air-dried at ambient temperature for 24 h to obtain the powdered ion pair complex.
Sensing membrane preparation
A sensing membrane was prepared by thoroughly mixing 45 mg of dioctyl phthalate (DOP), 45 mg of polyvinyl chloride (PVC), And 10 mg of the BNZ-tetraphenylborate ion pair complex in a 5 cm diameter glass petri dish. The mixture was dissolved in 7 mL THF, and the petri dish was covered with filter paper (Whitman No. 3) to control evaporation.The setup was left undisturbed overnight at room temperature to allow for complete solvent evaporation, yielding a master membrane (sensing layer) with a thickness of approximately 0.1 mm.
PVC electrode assembly and conditioning
An 8-mm diameter disc was cut from the master membrane using a cork borer and affixed to the tip of a PVC electrode using THF as an adhesive. The membrane was attached to an interchangeable PVC tip, which was then clipped into the end of the glassy electrode body.
The assembled sensor was conditioned by immersion in a 10−2 M BNZ solution for 4 h. When not in use, the sensor was stored dry under refrigeration.
Preparation of BNZ-coated graphite sensor (solid contact electrode)
Ion-pair preparation
The same procedure as in 3.3.1 was followed.
Sensing membrane preparation
The sensing membrane was prepared in a 5 cm diameter glass Petri dish by thoroughly mixing 45 mg of DOP, 45 mg of PVC, And 10 mg of the BNZ-tetraphenylborate complex. The resulting mixture was dissolved in 7 mL tetrahydrofuran (THF) and homogenized to ensure uniform distribution. The solvent was then allowed to evaporate slowly at ambient temperature, yielding a concentrated, oily residue suitable for coating the electrode graphite surface.
Coated graphite electrode assembly and conditioning
A graphite sensor was constructed using a commercially available graphite rod (3 mm diameter, 2.5 cm length). One end of the rod served as the electrical contact point, while the opposite end was repeatedly dipped into the electroactive membrane mixture until a uniform coating of the desired thickness was achieved. The coated rod was then allowed to dry completely at ambient temperature. For electrical contact, the coated end of the graphite rod was enclosed within a polytetraethylene (PTEE) tube filled with metallic mercury, into which a copper wire was inserted. Prior to use, the fabricated sensor was pre-conditioned by soaking in a 10−2 M BNZ solution for 6 h. When not in use, the sensor was kept dry under refrigeration.
Measurement conditions for the suggested sensors
The reported procedure in [34] was followed.
Electrochemical configurations of the proposed sensors
-
BNZ-PVC membrane sensor
Internal reference electrode/internal filling solution/PVC membrane/test solution/external Ag/AgCl reference electrode.
-
BNZ-coated graphite sensor:
Graphite electrode/test solution/Ag/AgCl reference electrode.
Operational parameters
Effect of ion-pair percentage
Several membranes with varying ion-pair compositions were tested. The slopes were recorded as the ion-pair percentage increased from 4 to 12% in order to determine the slope closest to the Nernstian value and the highest achievable Nernstian slope.
Effect of pH and temperature
pH values ranging from 2 to 8 were tested to evaluate their effect on the response of both sensors, using 1 × 10⁻² M And 1× 10⁻³ M solutions of BEZ·HCl. The pH was adjusted using 1 M hydrochloric acid And 1 M sodium hydroxide solutions. The results of this investigation were recorded.
The effect of temperature was also studied over the range of 25 °C to 50 °C. The influence on slope values and sensor responses was examined, and the corresponding Nernstian slopes were calculated.
Response time
Response time refers to the duration required for the electrode to reach a stable potential. To evaluate this, three drug concentrations were selected, each ten times higher than the previous one. The results showed that the two constructed electrodes required approximately 40 s for the BNZ-PVC sensor And 50 s for the BNZ-coated graphite sensor to reach a stable potential.
Electrodes reversibility
To evaluate electrode reversibility, the potential was measured by sequentially decreasing the analyte concentration from higher to lower levels. This approach was used to assess the effect of concentration changes on the electrodes’ response behavior.
Lifetime study
The calibration slope for each electrode was measured daily and found to remain practically stable over a period of approximately three months for the PVC sensor and five months for the BNZ-coated graphite sensor.
Soaking time
The time required to achieve maximum And stable response - referred to as soaking time - was found to be 4 h for the BNZ-PVC membrane sensor And 6 h for the BNZ-coated graphite sensor.
Sensors selectivity
The potentiometric selectivity coefficients (KPotAB) of the proposed sensors toward various interfering substances were evaluated according to IUPAC guidelines using the separate solution method [17].
Sensors calibration
The conditioned sensors were calibrated by transferring 25 mL of a 1 × 10⁻² M BEZ solution into 50 mL beakers. A series of BEZ solutions with varying concentrations was prepared by stepwise dilution of the 1 × 10⁻² M stock solution using bidistilled water. The electromotive force (EMF) was continuously measured for each concentration.
The fabricated sensors, in conjunction with an Ag/AgCl reference electrode, were immersed in BNZ solutions with concentrations ranging from 10−6 to 10−2 M. The solutions were gently stirred to ensure proper equilibration, and measurements were recorded once a stable potential was reached. The electromotive force (emf) values were recorded with an accuracy of ± 1 mV. Calibration curves were constructed by plotting the measured emf values against logarithmic of BEZ concentration for both sensors. The resulting calibration plots were used to calculate the NernstainS slopes. Regresian equatins were derived for subsequent measurements of unknown BEZ concentrations using the corresponding sensor.
Preparation of laboratory mixtures
Laboratory-prepared mixtures containing different ratios of BNZ (10−5 M) and its oxidative degradant (ranging from 20 to 80%) were prepared and analyzed. The previously described calibration procedure was applied to determine BNZ recoveries in the presence of its oxidative degradant, thereby assessing the selectivity of the proposed method.
Procedure validation
The proposed procedures were evaluated for linearity, limit of detection (LOD), specificity, precision, and accuracy in accordance with ICH guidelines [35].
Applications
Application to pharmaceutical preparation (cream)
A 10 g sample of Difflam 5% cream was accurately weighed And transferred into a 100 mL beaker. The sample was sonicated with 20 mL of methanol for 10 min, then filtered into a 500 mL volumetric flask. The remaining residue was rinsed three times with 10 mL portions of methanol. The combined filtrate And washings were diluted to volume with bi-disteled water to obtain a cream stock solution theoretically containing 1 mg/mL of BNZ.HCl. Separately, a standard stock solution was prepared by dissolving 0.346 g of BNZ.HCl in a 500 mL volumetric flask And diluting to volume with bi-distilid water to obtain a 10−2 M of BNZ.HCl solution.
The general calibration procedures were then applied using appropriate volumes, covering the working concentration ranges. The BNZ.HCl content in the cream was determined using the respective regression equations derived from the calibration curves.
Additionally, the standard addition method was used by spiking the cream solution with known amounts of BNZ.HCl standard. The percentage recoveries and relative standard deviations (RSD%) were subsequently calculated to assess the accuracy and precision of the method.
Application to biological fluids
The reported procedure in [36] was followed.
Spiked serum
To 0.2 mL of serum, 1.0 mL of ethanol And 0.5 mL of 5% zinc sulfate solution were added. The resulting mixture was centrifuged at 13,000 rpm for 15 min. A clear 1 mL aliquot of the supernatant was then diluted to 14 mL with bistilled water. After deaeration for 5 min, the solution was spiked with 1 mL of a 10−4 M BNZ.HCl solution. The subsequent analytical steps were carried out as previously described.
Spiked urine
A mixture of 1.0 mL urine, 1.0 mL of 5% zinc sulfate solution, And 1.0 mL of ethanol was prepared, And the pH was adjusted to 11 by adding 0.1 mL of 1 M NaOH. The mixture was then centrifuged at 13,000 rpm for 15 min. After centrifugation, a 1 mL aliquot of the clear supernatant was diluted with 14 mL of bidistilled water And subsequently spiked with 1 mL of a 10−4 M BNZ.HCl solution. Subsequent steps of the procedure were carried out as previously described.
Results and discussion
Electrochemical potentiometric techniques are robust and versatile analytical methods known for their high sensitivity, precision, and accuracy. These techniques provide a wide linear dynamic range while utilizing relatively low-cost instrumentation. Ion-selective electrodes (ISEs), a key subgroup of electrochemical sensors, convert the activity of specific ions into electrical signals. The potentiometric approach employed in ISEs enables non-destructive, low-energy analysis of electrode potentials under near-zero current conditions.
ISEs have been widely used for rapid analysis in pharmaceutical, clinical, agricultural, environmental, food, and industrial applications due to their simplicity, affordability, small size, fast response, reliability, and accuracy.
Conventional ISEs typically feature liquid-contact electrodes with an internal filling solution. However, these electrodes are subject to limitations such as membrane leaching and potential contamination. Despite these limitations,, ISEs offer significant advantages including rapid analysis, straightforward fabrication, fast response times, acceptable selectivity toward target ions, a wide linear dynamic range, suitability for online monitoring, safety, portability, and a more environmentally sustainable alternative compared to many other techniques.
Nevertheless, certain challenges remain, particularly in reducing the size of liquid-contact electrodes for sensor miniaturization and handling small sample volumes, as well as improving electrical conductivity without compromising cost-effectiveness.
To overcome these challenges, all-solid-state ion-selective electrodes (ASS-ISEs) have emerged as a promising solution. ASS-ISEs offer ease of operation, rapid measurements, low energy consumption, and enhanced potential for miniaturization. They also contribute to improved reliability, extended operational lifetime, and reduced environmental impact. For optimal function, ASS-ISEs must satisfy three critical criteria: (i) reversible ion-to-electron conduction, (ii) non-polarizable interfaces with high capacitance, and (iii) absence of side reactions [37].
This study focuses on developing two types of ion-selective electrodes (ISEs) for the determination of BNZ.HCl: a PVC membrane sensor and a coated graphite sensor, which also serves as an ASS-ISE. The method exploits the cationic nature of BNZ.HCl (BEZ+), which interacts with an anionic site (TPB-) of large lipophilic additive sodium tetraphenylborate (Na-TPB) to form a water-insoluble ion pair (BEZ–TPB) via precipitation. The resulting precipitate possesses a low solubility product, suitable particle size distribution, and physical compatibility with the electrode matrix. The high selectivity of both sensors enables accurate determination of the drug even in the presence of oxidative degradants, excipients, and biological matrice.
Performance features of the suggested sensors
The performance of the proposed sensors was evaluated following IUPAC recommendations [17]. Calibrations were performed by immersing the electrodes alongside an Ag/AgCl reference electrode in BNZ.HCl solutions over a concentration range of 10−5M–10−2M. Potential measurements obtained from serial analyses of standard BNZ.HCl solutions showed consistent responses both within the same day and over a two-week period, with variations not exceeding ± 1 mV/decade. Key performance characteristics of the proposed sensors are summarized in Table 1.
Table 1.
Key performance characteristics of the proposed sensors
| Parameter | PVC sensor | Coated graphite sensor | |
|---|---|---|---|
|
- Regression equation - Slope (b) - Intercept (a) |
y a = b x b + a 58.09 213.44 |
y a = b x b + a 57.88 255.19 |
|
| Coefficient of determination (r2) | 0.9995 | 0.9996 | |
| Linear range (M) | 10−5 – 10−2 | 10−5 – 10−2 | |
| Working pH range | 2–8 | ||
| Response time | 40 s | 50 s | |
| LOD (M) | 5.81 × 10−8 | 7.41 × 10−8 | |
| Accuracy (%R) c | 98.07 | 98.61 | |
| Precision (% RSD) c | |||
| Repeatability | 0.518 | 0.438 | |
| Intermediate precision | 0.824 | 0.733 | |
aThe recorded sensor potential
bThe molar concentration of the drug
cValues for 3 determinations of 3 different concentrations
Optimization of the proposed sensors
Influence of ion-pair percent
The ion-pair complex is a crucial component in ion-selective electrodes (ISEs), acting as the electroactive material responsible for the selective recognition and detection of the target ion by the sensor. Its percentage composition within the membrane significantly affects the sensor’s performance, including sensitivity, selectivity, and response stability.
BEZ-PVC membrane sensor
The primary constituents of the PVC membrane sensor include PVC, an ion complex, and a plasticizer. Optimal sensor performance depends on maintaining appropriate weight ratios among the ion- pair, plasticizer (DOP), and PVC during membrane preparation. BEZ-tetraphenylborate was synthesized and evaluated as a sensor modifier. The study involved varying the weight% of the ion- pair, while maintaining the PVC-to-plasticizer ratio constant at 1:1, as summarized in Table 2. Notably, the sensor containing 10% (w/w) BEZ-tetraphenylborate exhibited a near-Nernstian slope of 58.09 mV/decade.
Table 2.
Optimization of the membrane composition (w/w%) of the PVC membrane and coated graphite sensors*
| Sensor | Composition % (w/w) | Linear range (M) | Slope (mV/decade) | r2 | ||
|---|---|---|---|---|---|---|
| BNZ-TPB | PVC | DOP | ||||
| PVC membrane sensor | 4 | 48 | 48 | 1 × 10−5 – 1 × 10−2 | 54.31 | 0.9984 |
| 8 | 46 | 46 | 1 × 10−5 – 1 × 10−2 | 55.42 | 0.9991 | |
| 10 | 45 | 45 | 1 × 10−5 – 1 × 10−2 | 58.09 | 0.9995 | |
| 12 | 44 | 44 | 1 × 10−5 – 1 × 10−2 | 56.77 | 0.9995 | |
| Coated graphite sensor | 4 | 48 | 48 | 1 × 10−5 – 1 × 10−2 | 53.68 | 0.9989 |
| 8 | 46 | 46 | 1 × 10−5 – 1 × 10−2 | 55.05 | 0.9993 | |
| 10 | 45 | 45 | 1 × 10−5 – 1 × 10−2 | 57.88 | 0.9996 | |
| 12 | 44 | 44 | 1 × 10−5 – 1 × 10−2 | 56.07 | 0.9994 | |
BNZ-TPB benzydamine-tetraphenylborate
*The bold values reflect the appropriate composition % (w/w) of the ion pair, plasticizer (DOP) and PVC, that exhibited the near-Nernstian slopes of both sensors
BNZ-boated graphite sensor
BNZ-tetraphenylborate, the ion-pair complex, was synthesized and evaluated as a modifying agent for the sensor. The evaluation involved adjusting the weight% of the complex while maintaining a fixed 1:1 ratio between the PVC and plasticizer, as detailed in Table 2. The sensor containing 10% (w/w) BNZ-tetraphenylborate exhibited a slope of 57.88 mV/decade.
Efferct of soaking time
Freshly prepared sensors should be soaked to activate the membrane’s surface toform a thin gel layer where exchange occurs. The proposed sensors were immersed in a 1 × 10−2 M BNZ.HCl solution, And calibration curves were recorded at various time intervals ranging from 0 to 12 h. Measurements continued until significant deviations from the expected Nernstian slopes were observed. As summarized in Table 3, the optimal soaking times were found to be 4 h for the BNZ-PVC sensor And 6 h for the BNZ- coated graphite sensor.
Table 3.
Effect of soaking time on the proposed sensors*
| Soaking time/hour | PVC sensor | Coated graphite sensor |
|---|---|---|
| Slope (mV/decade) | ||
| 0 | 48.19 | 47.89 |
| 2 | 53.62 | 49.14 |
| 4 | 58.09 | 52.26 |
| 6 | 56.13 | 57.88 |
| 8 | 54.38 | 55.43 |
| 10 | 52.11 | 53.77 |
| 12 | 50.87 | 52.06 |
*The bold values reflect the maximum soaking time/hour that exhibited the near-Nernstian slopes of both sensors
Effect of pH and temperature
To determine the optimal working pH range, the stability of potential readings was evaluated across a pH range of 2 to 8. Measurements were performed using 1 × 10−2 M And 1× 10−3 M BNZ.HCl solutions, with potential recorded at each pH value. Representative curves illustrating the effect of pH on both sensors are presented in Fig. 2. Both the BNZ-PVC And BNZ-coated graphite sensors exhibited stable potentials within the pH range of 2 to 8. The electrodes’ slopes remained within the acceptable range (± 10% of the slope) without significant reduction.
Fig. 2.
Effect of pH on the response of BNZ using PVC membrane and coated graphite sensors
The sensor responses And the slopes also remained constant within the temperature range of 25–50 °C, indicating good thermal stability.
Sensors’ response time
For analytical applications, the response time of the prepared sensors is of critical importance. Response time is defined as the average time required to reach a stable potential (response) within ± 1.00 mV of the final equilibrium after sequential immersions in BNZ.HCl solutions with tenfold concentration increments, was evaluated. As detailed in Table 1, the BNZ-PVC membrane sensor exhibited stable responses within 40 s, while the BNZ-coated graphite sensor reached stability within 50 s.
Reversability and lifetime
Reversibility was also investigated, with no change observed in the reversed response, indicating stable sensor performance. The operational Lifetimes were determined to be 3 months for the PVC membrane electrode And 5 months for the coated graphite electrode. This demonstrates that the ASS-ISE offers a long operational lifespan.
Sensors selectivity
The potential interference from related compounds on the sensors’ response to the target drug (BNZ.HCl) was evaluated. Independent potential measurements were carried outusing separate 10−3 M solutions of BNZ.HCL And the potential interfering ions. Selectivity coefficients were then calculated using the following mathematical equation [38]:
 |
In the equation, E1 and E2 represent each electrode response measured for a 10−3 M solution of BNZ.HCl and interferent ion [J+z], respectively, while S denotes the slope of the calibration curve. The tested interfering materials included magnesium chloride, calcium chloride, potassium chloride, nickel chloride, sodium chloride, glucose, sucrose, glycine, urea, and the oxidative degradant of BNZ.HCl. The selectivity coefficients calculated for the developed sensors are presented in Table 4. developed sensors. Table 5 shows the ability of all sensors to accurately determine BNZ.HCL without interference from its oxidative degradant at various ratios.
Table 4.
Selectivity coefficients of the proposed sensors using the separated solution method
| Interferent | − log 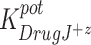 of PVC membrane sensor of PVC membrane sensor |
− log  of coated graphite sensor of coated graphite sensor |
|---|---|---|
| Oxidative-degradant | 1.010 | 1.098 |
| Calcium chloride | 1.004 | 1.059 |
| Magnesium chloride | 1.002 | 1.019 |
| Sodium chloride | 1.007 | 1.091 |
| Nickel chloride | 1.014 | 1.068 |
| Glucose | 1.029 | 1.081 |
| Urea | 1.088 | 1.077 |
| Glycine | 1.062 | 1.039 |
| Sucrose | 1.084 | 1.053 |
Table 5.
Determination of BNZ in the presence of oxidative-degradant by the proposed sensors
| Intact drug (M) | Degradant% | Recovery %a | |
|---|---|---|---|
| PVC sensor | Coated graphite sensor | ||
| ×110−5 M | 80 | 95.59 | 96.01 |
| ×110−4 M | 40 | 97.01 | 98.72 |
| ×110−5 M | 20 | 99.55 | 98.52 |
| ×110−4 M | 10 | 99.97 | 100.07 |
aMean of three determinations
Development and optimization of sability-indicating methods
Given the known photosensitivity of BNZ·HCl, forced oxidative degradation was performed using 5% hydrogen peroxide (H₂O₂) to simulate stress conditions in line with FDA guidelines. Direct exposure to UV light resulted in negligible degradation, confirming prior findings [7, 10, 15]. Complete oxidative degradation was achieved after one hour of H₂O₂ treatment at ambient temperature. The drug was accurately quantified in the presence of 10–80% of its degradant, as summarized in Table 5.
While conventional ISEs with internal filling solutions suffer from limitations such as membrane leaching and contamination, the electrodes developed in this study offer significant advantages. These include fast response times, simple fabrication, a wide linear dynamic range, satisfactory selectivity, portability, and improved environmental compatibility, making them well-suited for practical pharmaceutical analysis and real-time monitoring.
Method validation
Linear range
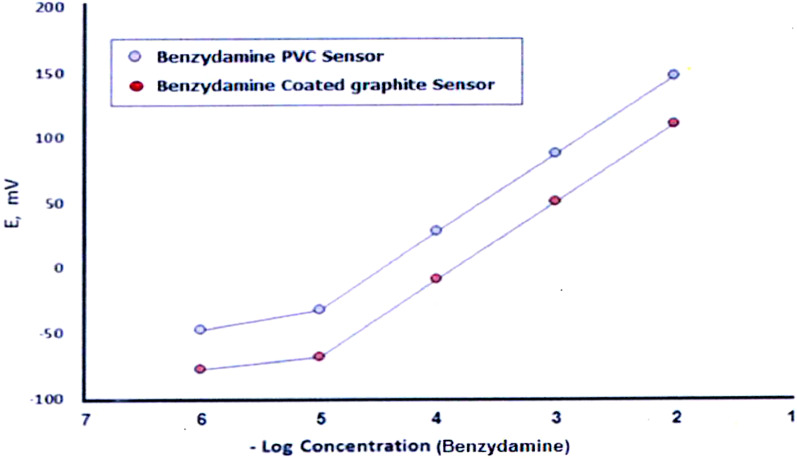
Under the established experimental conditions, calibration curves for each proposed sensor were generated by plotting the measured sensor potentials against the negative logarithm (− Log) of BNZ.HCL concentrations. The resulting regression plots demonstrated Linearity over the concentration range of 10−5M–10−2 M for both sensors, as illustrated in Fig. 3.
Fig. 3.
Profile of the potential in mV/− Log molar concentration of BNZ using PVC membrane and coated graphite sensors
Limit of detection
The limit of detection (LOD) was estimated by identifying the intersection point of the extrapolated linear segments in Fig. 3. The LOD values were found to be 5.81 × 10−8 M for the PVC sensor And 7.41 × 10−8 M for the graphite sensor. These low LOD values indicate a high degree of sensitivity of the proposed sensors. The results are summarized in Table 1.
Accuracy and precision
Accuracy
The accuracy of the developed method, expressed as the mean recovery percentage (R%), was evaluated by performing triplicate analyses at three BNZ.HCL concentration levels within the linear range (10−2 M − 10−5 M). The results, presented in Table 1, demonstrate the acceptable accuracy of the proposed methodology.
Precision
The precision of the developed methodology, expressed as the percentage relative standard deviation (%RSD), was assessed through triplicate analyses at three BNZ concentration levels (10−2 M − 10−5 M) within the linear range. Repeatability was evaluated by performing measurements within a single day, while intermediate precision was assessed over three consecutive days. The low % RSD values, presented in Table 1, confirm the high precision of the proposed method.
Specificity
The specificity of the proposed potentiometric method was demonstrated by its ability to accurately assay the drug in its pharmaceutical formulation without interference from common preservatives or excipients. The high specificity achieved by the developed sensors is summarized in Table 6.
Table 6.
Determination of BNZ in cream formulation; application of standard addition technique
| Cream formulation (B.no. D18-2951) | Founda ± SD | Added BNZ | Recoveryb of added BNZ |
|---|---|---|---|
| 1 × 10−4 M | 98.12 ± 1.446 | 1 × 10−5 M | 99.80 |
| 5 × 10−4 M | 99.01 | ||
| 1 × 10−4 M | 98.34 | ||
| Mean ± RSD % | 99.05 ± 0.74 | ||
aMean of three determinations
bAverage of three replicates experiments
Robustness
The robustness of the proposed method was demonstrated by the stability of the measured potential (mV) despite deliberate minor alterations in experimental conditions. Variables assessed included the time (in seconds) before each measurement (10 s ± 5 s) and the electrode soaking time (4–6 h ± 30 min). These intentional changes had no significant effect on the mV readings for BNZ.HCl, indicating the method’s reliability And consistency under typical operational conditions. Additionally, the effect of pH was studied at different values of 4, 4.5, 5, 5.5, 6.0, 6.5, And 7.0 using a 1.0 × 10⁻3mol L⁻¹ BNZ·HCl solution, with no observable change in electrode response across this pH range.
Statistical comparison with reported method
The analytical performance of the proposed method was benchmarked against a previously reported method [9] through statistical comparison. The absence of significant differences, confirmed by t-test And F-test Analyses at a 95% confidence level, validates the acceptable accuracy and precision of the recommended methodology for quantifying BNZ.HCl in its pharmaceutical formulations (Table 7).
Table 7.
Statistical comparison between the proposed method and the reported method
| Parameters | Sensors | Reported method [19] | |
|---|---|---|---|
| PVC | Coated graphite | ||
| Na | 5 | 5 | |
| Mean | 98.73 | 99.08 | 100.68 |
| Variance | 0.506 | 0.419 | 0.595 |
| %RSD | 0.702 | 0.641 | 0.766 |
| Student’s t-test (2.31)b | 1.481 | 0.977 | – |
| F- value (6.39)b | 1.177 | 1.421 | – |
aNumber of measurements
bThe values in parenthesis are tabulated values of “t” and “F” at (P = 0.05)
Applications
Application to pharmaceutical formulation (cream)
All proposed sensors were tested for selective determination of BNZ.HCl in Difflam 5%. cream The results demonstrated satisfactory performance, closely aligning with the labeled content. Recoveries obtained using the standard addition technique for known amounts of BNZ.HCl spiked into the pharmaceutical formulation (cream) ranged from 98.34 to 99.80%, as presented in Table 6.
Application to biological fluids
The studies confirm that BNZ is systemically absorbed. The results obtained from spiked biological samples—serum and urine samples [39–41]—are presented in Table 8. These findings indicate that the proposed method is suitable for the analysis of BNZ.HCl in biological fluids, with no significant interference from the sample matrices.
Table 8.
Application of the proposed potentiometric method using coated graphite sensor for the determination of bnz.hcl in biological fluid [36]
| Spiked intact drug (M) | Found %a | |
|---|---|---|
| Plasma sample | Urine sample | |
|
1 × 110−5 M 1 × 10−4 M |
96.59 | 95.71 |
| 97.41 | 96.42 | |
| 98.55 | 97.52 | |
| 98.97 | 97 0.07 | |
| Mean ± RSD % | 97.98 ± 1.12 | 96.9 ± 0.94a |
aMean of three determinations
Comparison with published methods
Numerous potentiometric methods using ion-selective electrodes (ISEs) have been reported for the determination of Benzydamine HCl in pure form, pharmaceutical formulations, or saliva samples. However, only our method successfully determines BNZ.HCl in the presence of its oxidative degradant using a fabricated all-solid-state ion-selective electrode (ASS-ISE). This solid, miniaturized, coated graphite sensor offers several advantages: the graphite rod is highly conductive, compact, cost-effective, easy to fabricate, and has a long operational lifetime.
In contrast, other reported methods have notable limitations. For example, the screen-printed ISE [13] is expensive, not widely available, disposable, and has a short lifespan with reduced stability. Similarly, the silver wire-coated electrode [39] is prone to oxidation, requires polishing before each use, and also suffers from limited stability and a short operational lifespan.
Compared to other instrumental methods such as spectrophotometric or chromatographic techniques, which offer high sensitivity and selectivity, potentiometric methods are more cost-effective, require no solvents and utilize simpler instrumentation, and are more environmentally friendly.
Table 9 summarizes the key differences between the proposed method and previously reported UV [15], electrochemical [11, 12, 39], and chromatographic techniques, including HPLC and TLC [6, 7, 10, 42]. It highlights the effect of oxidative degradants on the percent recovery of Benzydamine HCl. The results demonstrate that the coated graphite solid-state sensor offers significant advantages over earlier methods, particularly in terms of accuracy and robustness against degradation.
Table 9.
Comparison of different reported methods and the proposed potentiometric method for BNZ.HCl determination
| Items | UV method [15] | Chromatographic methods | Electrochemical methods | Ion-selective electrodes | |||
|---|---|---|---|---|---|---|---|
| HPLC [6, 7, 10] | TLC [42] | Method [11] | Method [12, 39] | PVC | Coated graphite | ||
| %Recovery | 88% | 98.25 | Identification | Separation and identification | 100.20 | 98.73 | 99.08 |
| Greenness | +VE | −Ve | −Ve | +VE | +VE | +VE | +VE |
| Sustainability | −Ve | −Ve | −Ve | −Ve | −Ve | −VE | +VE |
| Portability | −Ve | −Ve | −Ve | −Ve | −Ve | −VE | +VE |
| Miniaturization | −Ve | −Ve | −Ve | −Ve | −Ve | −VE | +VE |
| Cost-effective | −Ve | −Ve | −Ve | −Ve | −Ve | +VE | +VE |
+VE sign means applicable
− VE sign means not applicable
While potentiometric sensors offer notable advantages such as simplicity, low cost, portability, and real-time monitoring without complex instrumentation [43, 44], they may exhibit lower selectivity under certain analytical conditions compared to voltammetric methods [44, 45].
Limitations
While the developed ion-selective electrodes (ISEs), including PVC membrane and coated graphite electrodes, demonstrated satisfactory performance in terms of sensitivity, selectivity, and robustness, several inherent methodological limitations remain. One key challenge is the potential for electrode contamination, particularly in complex sample matrices. For instance, PVC membranes can experience plasticizer leaching and surface fouling from organic compounds, while coated graphite electrodes may suffer from baseline drift due to surface adsorption and redox interference.
Selectivity is another notable limitation. Although optimized ionophores were used, cross-sensitivity to ions with similar charge or size, as well as matrix effects in high ionic strength samples, can affect accuracy. Additionally, both types of electrodes may exhibit potential drift, limited lifespan due to membrane degradation, and batch-to-batch variability in electrode preparation.
Despite these limitations, the sensors developed in this study were optimized for key parameters such as pH, temperature, response time, and selectivity. These optimizations help mitigate some of the challenges and support the practical utility of ISEs in applications requiring fast, cost-effective, and environmentally friendly analytical methods.
Sustainability assessments (blueness, greenness, and whiteness appraisals)
With the growing demand for eco-friendly, low-waste, and resource-efficient methods, greenness assessment tools have become essential for evaluating the environmental impact of analytical procedures.
Electrochemical techniques inherently align with green chemistry principles by minimizing reagent use, reducing energy consumption, and enabling in situ, real-time analysis—often without extensive sample preparation or hazardous solvents. However, as new sensor technologies and electrode materials emerge, it becomes increasingly important to systematically assess their environmental footprints.
To address this need, various greenness evaluation tools have been developed and adapted for electroanalytical applications. Metrics such as the Analytical Eco-Scale, Green Analytical Procedure Index (GAPI), and AGREE (Analytical GREEnness Metric) offer structured frameworks for assessing ecological and safety aspects of analytical methods. These tools consider multiple criteria, including the toxicity of reagents, waste generation, energy consumption, and procedural complexity. Their application provides a transparent and quantitative means of comparing alternative methods and guiding researchers toward more sustainable analytical practices.
Furthermore, the integration of these greenness metrics into method validation processes encourages the design of environmentally responsible electrochemical sensors and potentiometric devices. By prioritizing non-toxic materials, solvent-free or aqueous systems, and recyclable components, electrochemists contribute not only to scientific advancement but also to the broader goals of environmental stewardship and regulatory compliance.
Furthermore, integrating these greenness metrics into method validation encourages the design of environmentally responsible electrochemical sensors and potentiometric devices. By prioritizing non-toxic materials, solvent-free or aqueous systems, and recyclable components, electrochemists advance both scientific innovation and the broader goals of environmental stewardship and regulatory compliance.
The AGREE open-source program generates a 12-segment circular pictogram designed to evaluate green analytical chemistry (GAC) practices. Each segment corresponds to one of the 12 GAC principles with colors ranging from green for minimal impact to red for significant impact), providing both qualitative and quantitative information about greenness of a method [46]. The overall AGREE score, displayed at the center of the pictogram, summarizes the method’s overall environmental performance. The AGREE pictograms (Fig. 4) and their associated overall scores, both for the method presented here and those previously reported [47], clearly demonstrates the superior greenness of the techniques developed in this study.
Fig. 4.
Greenness profiles of the suggested (a) and reported [9] (b) techniques using the AGREE tool
The proposed and previously reported techniques were evaluated for their “whiteness” using the RGB12 model [46, 47]. This model utilizes twelve Excel based algorithms grouped into blue, green, and red categories, each representing different aspects of method performance. As illustrated in Fig. 5,the proposed technique achieved superior “whiteness” scores compared to the previously reported method [9], underscoring its enhanced overall analytical performance.
Fig. 5.
Whiteness appraisal of the proposed and reported [26] methods using the RGB12 algorithm
The BAGI tool is a novel metric that evaluates the feasibility – or “blueness” or “practicality” - of an analytical technique, providing both visual and numerical assessments. It uses a color-coded scheme, where white indicates no compliance, Light blue indicates low compliance, blue represents medium, And dark blue signifies high compliance. A numerical BAGI score over 60 is considered indicative of a practical method [47]. As illustrated in Fig. 6, the proposed technique achieved a high score of 80, highlighting its strong practical applicability.
Fig. 6.
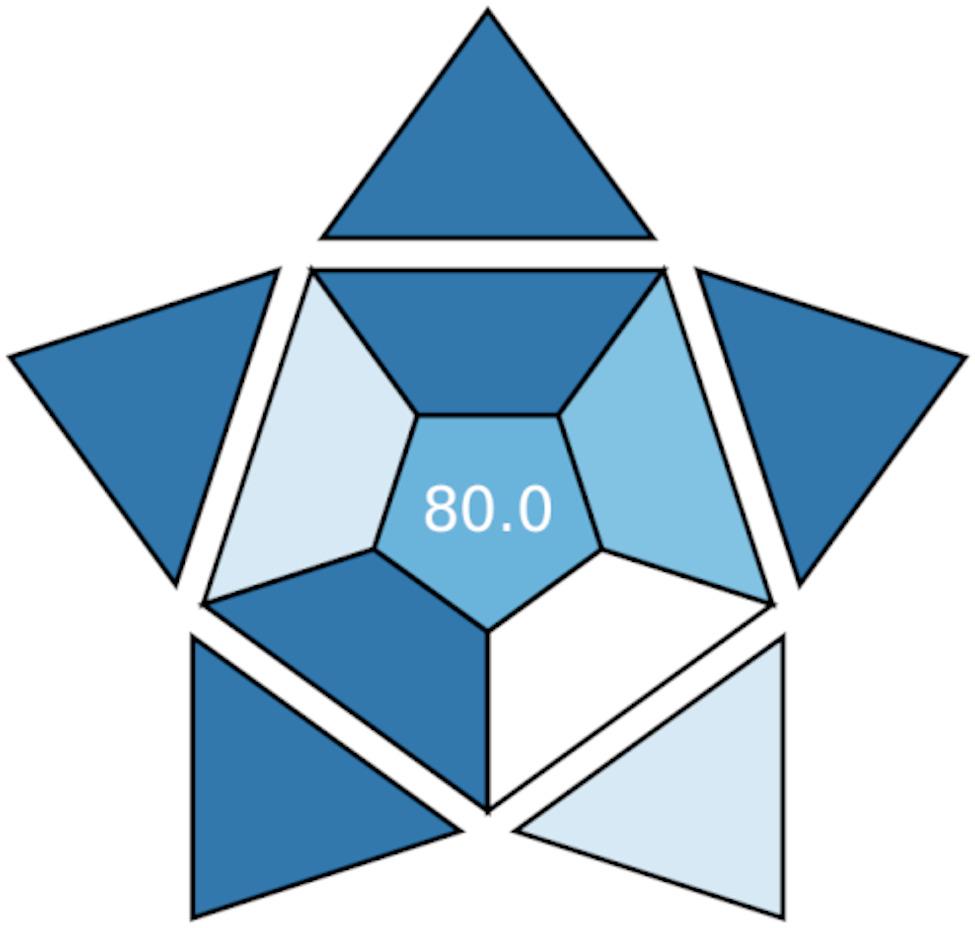
Blueness evaluation of the proposed technique by the BAGI tool
The incorporation of sustainability and greenness assessment tools in electrochemistry is no longer optional but is increasingly recognized as both an ethical responsibility and a scientific imperative. These tools facilitate the development of eco-conscious analytical platforms, encourage sustainable laboratory practices, and align electrochemical research with the global movement toward green innovation and responsible science [48].
Conclusion
A simple, reliable, and cost-effective potentiometric method was developed for the determination of BNZ·HCl using two types of sensors. These sensors were successfully applied to analyze the drug in its pure form, a pharmaceutical cream, and biological fluids, with accurate results and no interference from the sample matrices.
The coated graphite sensor, in particular, stands out as a practical and eco-friendly alternative to traditional methods. Its ease of use, low cost, and portability make it especially suitable for routine quality control, including in resource-limited settings where BNZ·HCl is commonly used.
While some limitations related to sensor durability and selectivity remain, the method offers a strong foundation for broader use in pharmaceutical testing. Continued improvements in sensor design and maintenance can further enhance its reliability in real-world applications.
Acknowledgements
Our Deep acknowledgement to Cairo university for supporting our research through STDF agreement with springer nature.
Author contributions
K.M.K: Conceptualization, Investigation, Methodology, Validation and Editing. R.M.S: Writing original draft, Formal analysis, Methodology and Data creation. M.A.D: Advisor and Data creation. L.M.A. :Data creation and Methodology. A.R.M. :Environmental assessments, revision and editing. All authors have reviewed the manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research did not receive any specifc funds from funding agencies in the public, commercial, or not-for-proft sectors.
Data availability
All data generated or analyzed during this study are included in this article and the raw data is available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All the method was carried out in accordance with relevant guidelines and regulations. Human plasma samples used in this study were obtained from the Holding Company for Vaccines and Biological Products (VACSERA Co.), Giza, Egypt—an authorized national center that adheres to ethical standards for research involving human biological materials.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.National Center for Biotechnology Information. PubChem Compound Summary for CID 12555, Benzydamine. NCBI. 2025. Retrieved July 6, 2025.
- 2.Anand J, Sein, LukasikGlębocka M, Korolkiewicz RP. Recreational abuse with benzydamine hydrochloride (Tantum Rosa). Clin Toxicol. 2007;45(2):198–9. [DOI] [PubMed] [Google Scholar]
- 3.Reed P. Sussex research reveals the addictive potential of a drug legally available in the UK and other countries. University of Sussex; 2017.
- 4.Opaleye ES, Noto AR, Sanchez Z, van der Moura M, Galduróz YG, Carlini JCF. Recreational use of benzydamine as a hallucinogen among street youth in Brazil. Rev Bras Psiquiatr. 2009;31(3):208–13. [DOI] [PubMed] [Google Scholar]
- 5.Hae B, McElNay JC. Highperformance liquid chromatography assay for the measurement of benzydamine hydrochloride in topical pharmaceutical preparations. J Chromatogr. 1987;394:395–9. [DOI] [PubMed] [Google Scholar]
- 6.Carlucci G, Mazzeo P. Highperformance liquid chromatographic determination of 1benzyl-1Hindazol-3ol in benzydamine in pharmaceutical formulations. J Pharm Biomed Anal. 1996;14:655–7. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Moore DE. A study of the photodegradation of benzydamine in pharmaceutical formulations using HPLC with diode array detection. J Pharm Biomed Anal. 1992;73(7):535–40. [DOI] [PubMed] [Google Scholar]
- 8.Catanese B, Lagana A, Marino A, Picollo R, Rotatori M. HPLC determination of benzydamine and its metabolite noxide in plasma following oral administration or topical application in man, using fluorimetric detection. Pharmacol Res Commun. 1986;18(4):385–403. [DOI] [PubMed] [Google Scholar]
- 9.Carlucci G, Iuliano P, Federico LD. Simultaneous determination of benzydamine hydrochloride and five impurities in an oral collutory as a pharmaceutical formulation by highperformance liquid chromatography. J Chromatogr Sci. 2010;48:854–9. [DOI] [PubMed] [Google Scholar]
- 10.Cherniy AV, Gureeva S, Georgiyants V. Development and validation of alternative analytical method for determination of related substances of benzydamine hydrochloride in oral spray by HPLC. J Drug Des Med Chem. 2017;2(6):65–73. [Google Scholar]
- 11.De Jesus DS, Couto CM, Araujo AN, Montenegro MC. Amperometric biosensor based on monoamine oxidase (MAO) immobilized in Solgel film for benzydamine determination in pharmaceuticals. J Pharm Biomed Anal. 2003;33:983–90. [DOI] [PubMed] [Google Scholar]
- 12.Li W, Su X, Zhang H, Nie L, Yao S. Determination of benzydamine hydrochloride in serum and urine by using a benzydamine ionselective piezoelectric sensor. Anal Sci. 1998;14:955–60. [Google Scholar]
- 13.Bakr MA, Abbas SS, Hegazy MA, Badawey AM, ElMosallamy SS. Construction of potentiometric sensors for selective determination of benzydamine hydrochloride in artificial saliva: greenness and whiteness assessment. J Electrochem Soc. 2025;172:017511. 10.1149/1945-7111/ada73b. [Google Scholar]
- 14.Mostafa SM, Farghali AA, Khalil MM. Novel ZnFe ldh/mwcnts and graphene/mwcnts nanocomposites-based potentiometric sensors for benzydamine determination in biological fluids and real water samples. Electroanalysis. 2021;33:1194–204. 10.1002/elan.202060455. [Google Scholar]
- 15.Sharma D, Singh R, Garg R. Development and validation of Stability-Indicating UV spectrophotometric method for the Estimation of benzydamine hydrochloride in bulk and in pharmaceutical dosage form, A novel analytical technique for conducting invitro quality control tests. IJPSR. 2018;9(2):678–86. [Google Scholar]
- 16.Thomas JDR. IonSelective electrode reviews. Elsevier; 2013;5.
- 17.Buck RP, Lindner E. Recommendations for nomenclature of ionselective electrodes (IUPAC recommendations 1994). Pure Appl Chem. 1994;66(12):2527–36. [Google Scholar]
- 18.Thévenot DR, Tóth K, Durst RA, Wilson GS. Electrochemical biosensors: recommended definitions and classification. Biosens Bioelectron. 2001;16(1–2):121–31. [DOI] [PubMed] [Google Scholar]
- 19.Pungor E, Tóth K. Ionselective membrane electrodes. A review. Analyst. 1970;95(1132):625–48. [DOI] [PubMed] [Google Scholar]
- 20.Essam HM, Bassuoni YF, Elzanfaly ES, Zaazaa HES, Kelani KM. Potentiometric sensing platform for selective determination and monitoring of Codeine phosphate in presence of ibuprofen in pharmaceutical and biological matrices. Microchem J. 2020;159:105286. [Google Scholar]
- 21.Abd ElRahman MK, Eid SM, Elghobashy MR, Kelani KM. Inline potentiometric monitoring of butyrylcholinesterase activity based on metabolism of bambuterol at the point of care. Sens Actuators B Chem. 2019;285:216–23. [Google Scholar]
- 22.Ragab MT, Abd ElRahman MK, Ramadan NK, ElRagehy NA, ElZeany BA. Novel potentiometric application for the determination of Pantoprazole sodium and Itopride hydrochloride in their pure and combined dosage form. Talanta. 2015;138:28–35. [DOI] [PubMed] [Google Scholar]
- 23.Freiser H. Coated wire ionselective electrodes. Principles and practice. J Chem Soc Faraday Trans I Phys Chem Condens Phases. 1986;82(4):1217–21. [Google Scholar]
- 24.Frag EYZ, Ali TA, Mohamed GG, Awad YHH. Construction of different types of ionselective electrodes. Characteristic performances and validation for direct potentiometric determination of orphenadrine citrate. Int J Electrochem Sci. 2012;7(5):4443–64. [Google Scholar]
- 25.Mohamed AR, Sayed RA, Shalaby A, Ibrahim H. Application of insilico docking for green electrochemical quantification of Prucalopride succinate in pharmaceutical, urine, and milk matrices. Sci Rep. 2025;15(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lenik J. Application of PVC in construction of ionselective electrodes for pharmaceutical analysis: a review of polymer electrodes for nonsteroidal, antiinflammatory drugs. In: Handbook of polymer pharmaceutical technology. vol. 2; 2015. pp. 212–217.
- 27.Arvand M, Asadollahzadeh SA. Ionselective electrode for aluminum determination in pharmaceutical substances, tea leaves, and water samples. Talanta. 2008;75(4):1046–54. [DOI] [PubMed] [Google Scholar]
- 28.Sakur AA, Nashed D, Noureldin I. Green potentiometric determination of thirdgeneration antihistamines: fexofenadine, desloratadine, and Levocetirizine using carbon paste electrodes. Talanta Open. 2022;5:100116. 10.1016/j.talo.2022.100116. [Google Scholar]
- 29.Kelani KM, Fayez YM, Gad AG, Mahmoud AM. Design of pointofcare electrochemical sensor for therapeutic drug monitoring of Ofloxacin in biological fluids. J Anal Sci Technol. 2024;15(1):41. [Google Scholar]
- 30.Couto RAS, Lima J, Quinaz MB. Recent developments, characteristics and potential applications of screenprinted electrodes in pharmaceutical and biological analysis. Talanta. 2016;146:801–14. [DOI] [PubMed] [Google Scholar]
- 31.Hassan AM, Kelani KM, Hegazy MA, Tantawy MA. Molecular imprinted polymerbased potentiometric approach for the assay of the coformulated Tetracycline hcl, metronidazole and bismuth subcitrate in capsules and spiked human. Anal Chim Acta. 2023;1278:341707. [DOI] [PubMed] [Google Scholar]
- 32.Khalil MM, Issa YM, Mohamed AG. A comparative study of solid and liquid inner contact Paroxetine hydrochloride ionselective electrode membranes. J Iran Chem Soc. 2015;12:1637–48. [Google Scholar]
- 33.United States Pharmacopeial Convention. United States Pharmacopoeia 30 and National Formulary 25. CDROM. 2007.
- 34.Stefan RI, AboulEnein HY. Validation criteria for developing ionselective membrane electrodes for analysis of pharmaceuticals. Validation Chem Meas 2005:73–5.
- 35.International Conference on Harmonization. ICH Q2(R1). 2005.
- 36.Kelani KM, Said RA, ElDosoky MA, Mohamed AR. Innovative voltammetric techniques for Bumadizone analysis in pharmaceutical and biological samples: emphasizing green, white, and blue analytical approaches. Sci Rep. 2024;14(1):19873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park HJ, Jang ST, Choi BG. AllSolidState ionselective electrodes for wearable healthcare devices. Appl Chem Eng. 2024;35(6):513–21. 10.14478/ace.2024.107. [Google Scholar]
- 38.Bakker E, Pretsch E, Bühlmann P. Selectivity of potentiometric ion sensors. Anal Chem. 2000;72(6):1127–33. [DOI] [PubMed] [Google Scholar]
- 39.Khalil MM, Issa YM, Korany MA. Potentiometric determination of benzydamine hydrochloride using PVC membrane and coated wire sensors in pure form, pharmaceutical compounds and biological fluids. Int J Eng Res Gen Sci. 2015;3(1):2091–730. [Google Scholar]
- 40.Metto M, Tesfaye A, Atlabachew M, Abebe A, Fentahun T, Munshea A. A novel Poly(cytosine)Based electrochemical biosensor for sensitive and selective determination of guanine in biological samples. ACS Omega. 2024;9(24):2622–34. 10.1021/acsomega.4c01939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Catanese B, Grasso A, Silverstein B. Studies on the absorption and elimination of benzydamine in the mouse, rat, dog, and man. Arzneimittelforschung. 1966;16(10):1354–7. [PubMed] [Google Scholar]
- 42.Chorna O, Chornyi V, Chubenko A, Hrubnyk I, Mishchenko V, Golik M. Identification of benz y damine and its metabolite in presence of certain antiinflammatory-steroidal drugs. Sci Rise Pharm Sci. 2021. 10.15587/2519-4852.2021.249634. [Google Scholar]
- 43.Özbek O, Ölcenoglu A. The use of bis–thiadiazole and bis–oxadiazol derivatives as ionophores: a novel copper(II)–selective potentiometric electrodes. Microchem J. 2023;190:108679. 10.1016/j.microc.2023.108679. [Google Scholar]
- 44.Berkel C, Özbek O. Green electrochemical sensors, their applications and greenness metrics used: a review. Electroanalysis. 2024;36:e202400286. 10.1002/elan.202400286. [Google Scholar]
- 45.Lyu Y, Gan S, Bao Y, Zhong L, Xu J, Wang W, Liu Z, Ma Y, Yang G, Niu L. Solidcontact ionselective electrodes: response mechanisms, transducer materials and wearable sensors. Membranes. 2020;10:1–24. 10.3390/membranes10060128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Darweish E, Mohamed AR. Sustainable UV approaches supported by greenness and whiteness assessments for estimating a recently fdaapproved combination for managing urologic disorders: tukey’s test. Spectrochim Acta A Mol Biomol Spectrosc. 2024;305:123551. [DOI] [PubMed] [Google Scholar]
- 47.Kelani KM, Said RA, ElDosoky MA, Mohamed AR. A sustainable approach to chromatography: simultaneous determination of paracetamol, methocarbamol, and impurities with focus on environmental and computational metrics. Microchem J. 2025;209:112585. [Google Scholar]
- 48.Helmy MI, El Hamd MA, Obaydo RH, Nashed D, Nessim CK. Bridging pharma and sustainability: green electrochemical analysis of antiparkinsonian drug in pharmaceuticals and plasma, aligned with united nations goals via the NQS index. J Electrochem Soc. 2024;171:077512. 10.1149/1945-7111/ad60f9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article and the raw data is available from the corresponding author on reasonable request.