Abstract
Background
The World Health Organization malaria burden estimates produced from incomplete clinical case reporting and often outdated household asymptomatic parasitaemia surveys in children < 5 years old, are unreliable. Surveillance target groups need to be expanded in line with the epidemiological shift in malaria-eliminating countries towards adults, and particularly men. Furthermore, new tools that can provide granular and timely data, critical to understanding geographic heterogeneity and enabling timely decision-making at the operational level, are needed. This prospective study aimed to demonstrate that blood donor malaria screening could serve as a time-sensitive complementary source of highly detailed malaria surveillance data.
Methods
Consecutive blood donations received from 16 August 2023 to 31 August 2024 at the Ouagadougou and Bobo-Dioulasso Regional Blood Transfusion Centres in Burkina Faso, covering 5 of 13 regions, were screened for malaria using the Sysmex XN-31 automated analyser. XN-31 results, donor age, sex, place of residence, collection date, were analysed using descriptive statistics, chi-squared, and logistic regression tests. Seasonal malaria patterns were compared with publicly available rainfall data.
Results
Donor malaria prevalence was 5.91% (3164/53575) overall. Key predictors of malaria identified were age ≤ 30 years (odds ratio (OR) 2.85, p < 0.001), male sex (OR 1.47, p < 0.001) and rural residency (OR 2.40, p < 0.001), with regional location having a strong influence on the latter. Strong seasonal variability, mirroring that of rainfall with a 3-month lag, was observed with different peak periods and rate of change over time at provincial level. Hot-spots were observed within both Bobo-Dioulasso and Ouagadougou. There were no age or sex-based differences in parasite density or gametocyte carriage, and both measures were directly proportional to malaria prevalence. Only males showed striking seasonal variability in gametocyte carriage (low season 1.39%, 14/1006; high season 4.42%, 66/1494; p < 0.001).
Conclusions
The large data set and spatiotemporal malaria prevalence information, not possible with episodic household malaria surveys, facilitated highly granular analysis and demonstrated the potential to provide dynamic real-time information on the malaria burden using automated XN-31 blood donor malaria screening.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12936-025-05588-z.
Keywords: Malaria surveillance, Asymptomatic blood donors, Burkina Faso, XN-31, Automated malaria detection
Background
High quality surveillance data, which accurately reflects the heterogeneity of malaria prevalence trends within a geospatial framework over time, is an essential component of a comprehensive malaria elimination strategy. The World Health Organization (WHO) has emphasized the importance of surveillance and recommended that it be transformed into a core intervention [1].
Country-specific malaria burden estimates are generally determined using two sources: (1) passive surveillance data derived from clinical case reporting and (2) active surveillance data from periodic community-based surveys where malaria parasitaemia screening is undertaken in randomly selected households [2]. Epidemiological characteristics of asymptomatic infections may differ from those of clinical malaria, thus both forms of surveillance are needed for effective monitoring of malaria trends and intervention planning. The reliability of clinical case reporting as a malaria-metric is dependent on equity in access to healthcare facilities, febrile patient health-seeking behaviour, the sensitivity of the diagnostic method utilized for malaria confirmation, and the completeness of reporting, amongst others [3–6]. Household surveys, such as malaria indicator surveys (MIS) in turn, are limited by their high cost, inherent logistic complexity, and infrequent occurrence. Although malaria screening in MIS is intended to target household members most at risk—namely pregnant women and children under five years of age—all MIS reports published since 2020 have reported malaria prevalence data exclusively for the latter group [7]. Many malaria-endemic countries in sub-Saharan Africa have weak surveillance systems, thus data generated using these traditional means are often incomplete and may not represent the complete malaria burden picture [8, 9]. In this regard, it has been highlighted that complementary data sources, such as the routine screening of pregnant women attending antenatal clinics [10] or school children [11] would fill some of these gaps.
Many individuals in malaria-endemic countries, including children, the primary target of MIS, are parasitaemic but show no clinical signs of infection. Besides being at risk of becoming anaemic, asymptomatic carriers, who mostly outnumber those with clinical malaria in malaria-endemic regions, are a major factor in perpetuating the transmission of malaria [12]. Targeting these populations and being able to rapidly identify changing trends in malaria prevalence is of particular importance, especially in the context of climate change, as well as in situations of declining prevalence when reservoirs of infection become increasingly geographically clustered [13–15]. Consequently, as countries switch their attention from malaria control to possible elimination, surveillance activities would need to be adapted accordingly [16, 17].
Several studies have shown that healthy adult blood donors in malaria-endemic regions commonly harbour malaria parasites [18, 19]. Since blood donations take place continuously throughout the year, and are sourced country wide, blood donors might be considered a suitable population for surveillance. Although blood donors are not fully representative of the general population due to demographic selection biases, they may still be useful for identifying trends within their communities, especially when sampled consistently across time and space. Consequently, blood donors in malaria-endemic countries may be an untapped source for the systematic measurement of the asymptomatic parasite reservoir. A recent study [20] evaluated a high throughput analyser, the Sysmex XN-31 with automated malaria detection functionality at the Malawi Blood Transfusion Service. The findings revealed twice as many malaria-infected blood donors compared to thick smear microscopy. Using the same study data, Kayange et al. [21] subsequently conducted a retrospective in-depth analysis of blood donor demographics, geographic location, and month of collection, which revealed interesting geographical and gender-based trends that largely concurred with those established through traditional MIS. There were however some differences, notably in the absolute prevalence rates, which can be accounted for by temporal (year and month of collection) and test group differences, as well as differing sensitivities of diagnostic tests used for parasitaemia assessment. It was proposed by the authors [21] that the observed differences reflected the rapidly changing malaria dynamics in Malawi, rather than unreliable data, as the most recent MIS [22] that was used for comparison, reflected data that had been obtained three years previously.
The current study addresses the limitations of the study conducted in Malawi [21] by analysing consecutive donor samples over an approximately 12-month period to capture seasonal variation, with the aim of evaluating whether blood donor screening using the XN-31 can provide complementary malaria surveillance data by identifying geographic and demographic trends in parasite positivity, amenable to granular reporting, across two regions of Burkina Faso with different transmission levels.
Methods
Study setting
Burkina Faso is a high malaria burden West African country with malaria transmission occurring all year round, albeit with strong seasonal and regional differences, aligned with the rainy season and other environmental factors. The entire population is at risk of infection throughout the year. The National Blood Transfusion Centre, a public health establishment, is the primary provider of blood transfusion services for the country. This study was conducted at two regional blood transfusion centres (RBTC), namely Bobo-Dioulasso RBTC (BOBO) in the west, and Ouagadougou RBTC (OUA), in the centre of the country. OUA and BOBO are the largest of the RBTCs that historically have collected more than 33,000 and 13,000 blood units, respectively, each year. About 75% of blood units are collected at mobile sites spanning the coverage area of each RBTC (NBTC Annual reports 2018, 2019 and 2020). OUA is in Ouagadougou, the capital of Burkina Faso, with a coverage area (Centre, Centre-Sud, and Plateau-Central regions) of 22,871 km2, comprising 8.4% of the total Burkina Faso land mass (Fig. 1). With approximately 5.6 million inhabitants (23.9% of the total population), it is the most densely populated region [23]. BOBO is in Bobo-Dioulasso, the second largest city, in the Hauts-Bassins administrative region, with its coverage area including the Cascades region, collectively covering 16.1% (43,767 km2) of the country, and having around 3.5 million inhabitants (15%). The figures for both OUA and BOBO are projected to 1 July 2024 from the Population Census 2019 [23].
Fig. 1.
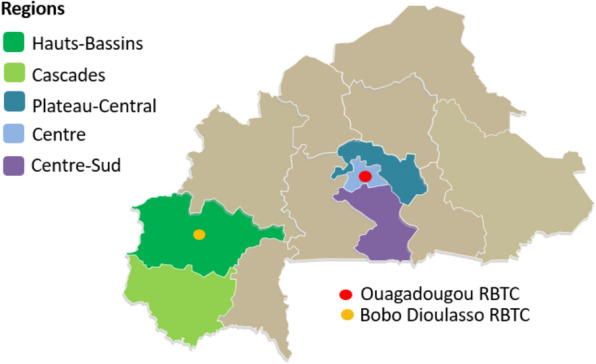
Catchment area of Bobo-Dioulasso and Ouagadougou regional blood transfusion services
Blood donor recruitment criteria
All blood donations collected at the RBTCs in Burkina Faso are from voluntary unpaid donors. To donate blood, individuals must be aged 18–60, weigh ≥ 50 kg, and be in good health. With physician approval, 16–18-year-olds may donate if they weigh ≥ 50 kg, and regular donors may continue until 65. Temporary deferral applies after recent illness, infection, surgery, vaccination, pregnancy, within 6 months postpartum, miscarriage or abortion (past 12 weeks), or invasive procedures. Suspected malaria requires a 15-day deferral post-treatment or symptom resolution. Permanent exclusion applies to those with HIV, hepatitis B/C, genetic blood disorders (e.g. sickle cell), high-risk behaviours (e.g. male-to-male sex, drug use, unprotected sex with multiple or infected partners), and chronic conditions such as cancer, cardiovascular, lung, liver or endocrine disease, epilepsy, or certain mental illnesses. All donors undergo pre-donation screening, including a medical interview, vital signs check, and haemoglobin testing where possible.
Study design
All routinely collected EDTA-anticoagulated venous blood samples obtained from eligible blood donors feeding into the OUA and BOBO RBTCs during 16 August 2023 to 31 August 2024 were screened for malaria using a Sysmex XN-31 automated analyser (Sysmex Corporation, Kobe, Japan), located at each RBTC facility, within 8 h of collection. Although room temperature storage is adequate for blood samples to be tested using XN-31, transportation took place under cold chain conditions, in line with the standard RBTC procedures. The following secondary data on blood donors were collected: sex, age, date and time of blood sample collection, blood processing centre (OUA or BOBO), and place of donor residence (village, town, or city sector). These data were extracted from the routinely used donor enrolment forms and pre-donation health questionnaires completed for each donor at each donation visit.
Automated malaria screening using the Sysmex XN-31 analyser
The XN-31 analyser uses the principle of fluorescence flow cytometry to quantify asexual malaria parasites and gametocytes, providing an absolute and percentage malaria infected red blood cell (MI-RBC) count from a 60 μL venous whole blood sample. The analytical limit of quantification of the MI-RBC count is 20 parasites/μL (p/μL) [20]. Further differentiation of the count into asexual and sexual forms is provided for research purposes only. All species of Plasmodium that cause human malaria are detected. A detailed description of the XN-30, the technically identical predecessor of the XN-31, is provided elsewhere [24]. In this study, EDTA venous whole blood samples, routinely collected for blood typing, underwent standard processing on the XN-31 analyser in sampler mode. The MI-RBC qualitative judgement output (MI-RBC positive/negative), and in malaria positive samples (MI-RBC ≥ 20p/μL), the absolute MI-RBC count and the presence/absence of gametocytes were used for data analysis. Whilst the analyser provides complete blood count information and an indication of malaria species (Plasmodium falciparum or other) by means of a suspect flag, this information was not used in this study.
Analysis of factors influencing prevalence of asymptomatic malaria parasitaemia in blood donors
Donor sex, age and residential location were investigated to determine the primary factors influencing the prevalence of malaria. The XN-31 results were sorted into region, province and urbanization based on the home address provided by the blood donor. Categories for urbanization included urban 1 (cities of Bobo-Dioulasso and Ouagadougou), urban 2 (all other provincial capital cities), urban 3 (all other non-capital towns/cities), peri-urban, and rural. Month of collection was used to assess seasonality of malaria prevalence and compared with publicly available rainfall data for Ouagadougou (Meteostat weather station identifier: 65,503) and Bobo-Dioulasso (Meteostat weather station identifier: 65,510) [25]. For the purposes of data analysis, the Ouagadougou and Bobo-Dioulasso rainfall data were considered representative of the Centre region and Hauts-Bassins region, respectively. For the comparison of donor malaria prevalence trends overall, the average of the monthly rainfall data for Ouagadougou and Bobo-Dioulasso was applied, as no further weather stations with accessible rainfall data, located in the study area, were identified.
Statistical analysis
Data analysis was done using MedCalc® Statistical Software version 19.8 (MedCalc Software Ltd, Ostend, Belgium). Data visualization and graphical representation were performed using RStudio (version 4.4.2). A descriptive summary of donor characteristics was generated using median and interquartile range (IQR) or 95% confidence intervals (CI) for continuous variables while categorical variables were summarized as percentages.
Prevalence estimates, determined by dividing the number of malaria positive samples by the total sample size, were expressed as percentages with the corresponding 95% CI to indicate the precision of the estimate. A minimum of 30 data points were required for prevalence estimates that were mapped to their respective geographical regions, provinces, and urban or rural locations. Seasonal trends were analysed using time series visualization to examine prevalence fluctuations over the months. High malaria season was defined as August to December, and low malaria season as January to July [26]. Chi-squared test was used for comparison of two independent proportions. Adjusted and unadjusted logistic regression models were conducted to assess potential confounding effects and compare predictors of malaria prevalence. Results of the logistic regression model were expressed as odds ratios (OR) with 95% CI. For all statistical tests, p values below 0.05 (two-tailed) were considered statistically significant.
Results
Malaria prevalence and predictors
Malaria screening results
A total of 53,575 blood donations received at OUA and BOBO during the period 16 August 2023 to 31 August 2024 with valid XN-31 results and available demographic and/or location data were included for data analysis. Of these, 3164 tested positive, giving an overall donor malaria parasite prevalence of 5.91% (95% CI: 5.70–6.12). A summary of blood donor characteristics is shown in Table 1.
Table 1.
Summary of blood donor characteristics by region and urban/rural location
| Characteristic | All donors | OUA donors | BOBO donors | ||||
|---|---|---|---|---|---|---|---|
| Number of donors n (%) | 53,575 | 37,202 (69.44) | 16,373 (30.56) | ||||
| Urban | Rural | Not stated | Urban | Rural | Not stated | ||
| 35,442 (95.21) |
1746 (4.69) |
14 (0.04) |
13,993 (85.46) |
2342 (14.30) |
38 (0.23) |
||
|
Median age Years (IQR) |
24 (21–30) |
25 (21–31) |
24 (20–34) |
24.5 (21–34) |
23 (21–29) |
21 (19–25) |
24 (21–31) |
|
Sex—male n (%) |
39,329 (73.41) |
26,129 (73.72) |
1242 (71.13) |
12 (85.71) |
10,286 (73.51) |
1633 (69.73) |
27 (71.05) |
| Sex—female n (%) |
13,493 (25.18) |
9067 (25.58) |
492 (28.18) |
2 (14.29) |
3300 (23.58) |
623 (26.60) |
9 (23.68) |
|
Sex—not stated n (%) |
753 (1.41) |
246 (0.70) |
12 (0.69) |
0 (0.00) |
407 (2.91) |
86 (3.67) |
2 (5.27) |
|
Malaria Prevalence % (95% CI) |
5.91 (5.70–6.12) |
4.86 (4.63–5.09) |
6.30 (5.18–7.59) |
7.69 (0.20–42.86) |
6.75 (6.33–7.20) |
16.35 (14.76–18.08) |
7.90 (1.63–23.07) |
| Median parasitaemia p/μL (IQR) |
90 (40–270) |
100 (40–300) |
100 (40–320) |
470 (n/a) |
90 (40–240) |
90 (40–230) |
590 (200–1210) |
|
Gametocyte carriage % (95%CI) |
0.17 (0.05 −0.44) | 0.17 (0.13–0.22) | 0.11 (0.01- 0.41) | 0.00 |
0.24 (0.16–0.33) |
0.17 (0.05–0.44) |
0.00 |
Data are shown as n (%) for categorical variables, and median (IQR) or proportion (95% CI) for continuous and prevalence variables
IQR = interquartile range; 95% CI = 95% confidence interval; p/μL = parasites/microlitre; n/a = not applicable
Donor demographics
The donors were predominantly males (Table 1) who had a significantly higher malaria parasite prevalence (6.36%; 95% CI: 6.11–6.61; 2500/39329) than female donors (4.40%; 95% CI: 4.06–4.77; 594/13493) (OR = 1.47, p < 0.001).
Most blood donors were 30 years and younger (Table 2). Malaria parasite prevalence decreased progressively as donors got older (Fig. 2) and these differences were statistically significant (Table 2). Within each age group, male donors had consistently higher malaria positivity rates than female donors (Fig. 2, Additional Table S1).
Table 2.
Donor age distribution and asymptomatic malaria prevalence
| Donor age in years | Percentage of donor pool n = 53,575 (number of donors) |
Malaria positivity (95% CI) [number of positive samples] |
Odds ratio (95% CI) |
p value | |
|---|---|---|---|---|---|
| 16–20 |
21.70% (11,625) |
8.98% (8.44–9.54%) [1044] |
4.26 (3.49–5.20) | < 0.001 | |
| 21–30 |
53.13% (28,465) |
6.20% (5.91–6.49%) [1764] |
2.85 (2.35–3.46) | < 0.001 | |
| 31–40 |
15.87% (8505) |
2.85% (2.45–3.23%) [242] |
1.26 (1.01–1.58) | 0.045 | |
| > 40 |
9.09% (4855) |
2.27% (1.86–2.73%) [110] |
Reference category | ||
| Not stated |
0.23% (125) |
3.20% (0.88–7.99%) [4] |
|||
CI = confidence interval. Odds ratios and p values were generated from a logistic regression model assessing the association between age and malaria positivity
Fig. 2.
Asymptomatic malaria prevalence in blood donors by age and sex. The error bars represent the 95% confidence interval of donor malaria prevalence
Urban versus rural location
Location data were available for 99.90% (53,523/53,575) of donors of which 92.35% (49,435/53,523) lived in urban areas, 0.44% (238/53,523) in peri-urban areas, and 7.19% (3850/53523) in a rural location. Rural donors had a malaria prevalence of 12.00% (95% CI: 11.75–14.14; 462/3850) and urban donors 5.39% (95% CI: 5.19–5.60; 2667/49,435). The peri-urban donors were almost exclusively from areas surrounding Bobo-Dioulasso (237/238) with a malaria prevalence of 13.08% (95% CI: 8.89–18.57; 31/237). As there was no significant difference in malaria prevalence between peri-urban and rural donors (p = 0.703), and the sample size was small, for all further analyses, peri-urban donors were included in the rural group. Rural donors overall had a significantly higher malaria prevalence (12.06%; 95% CI: 11.02–13.17; 493/4088) than urban donors (5.39%; 95% CI: 5.19–5.60; 2667/49435) (OR 2.4, p < 0.001).
Malaria prevalence was strongly influenced by geographic location. In the OUA catchment area located in central Burkina Faso, the donor malaria prevalence in both urban (4.86%) and rural (6.30%) areas was lower than that of the BOBO catchment area further west. Here the urban donor malaria prevalence was 6.75% compared with 16.35% for rural donors (Table 1). There was no statistically significant difference between OUA rural (6.30%) and BOBO urban donors (6.75%) (p = 0.478; Tables 1 and 3).
Table 3.
Asymptomatic donor malaria prevalence by urban versus rural location
| All donors n = 53,575 % (95% CI) [pos/total] |
OUA RBTC catchment n = 37,202 % (95% CI) [pos/total] |
BOBO RBTC catchment n = 16,373 % (95% CI) [pos/total] |
OUA versus BOBO p value |
|
|---|---|---|---|---|
| Urban |
5.39% (5.19–5.60) [2667/49435] |
4.86% (4.63–5.09) [1722/35442] |
6.75% (6.33–7.20) [945/13993] |
p < 0.001 |
| Urban 1 |
4.43% (4.23–4.64) [1891/42684] |
3.93% (3.72–4.16) [1231/31303] |
5.80% (5.37–6.26) [660/11381] |
p < 0.001 |
| Urban 2 |
11.26% (10.39–12.18) [622/5526] |
11.37% (10.24–12.59) [370/3253] |
11.09% (9.76–12.54) [252/2273] |
p = 0.746 |
| Urban 3 |
12.60% (10.69–14.76) [154/1225] |
13.66% (11.33–16.32) [121/886] |
9.74% (6.70–13.67) [33/339] |
p = 0.064 |
| Rural |
12.06% (11.02–13.17) [493/4088] |
6.30% (5.18–7.59) [110/1746] |
16.35% (14.76–18.08) [383/2342] |
p < 0.001 |
| Unknown |
7.69% (2.10–19.70) [4/52] |
7.14% (0.18–39.80) [1/14] |
7.90% (1.63–23.07) [3/38] |
p = 0.928 |
Urban 1, Urban 2, and Urban 3 represent sub-groups of the Urban category
OUA RBTC = Ouagadougou Regional Blood Transfusion Centre
BOBO RBTC = Bobo-Dioulasso Regional Blood Transfusion Centre
CI = confidence interval; [pos/total] = number of malaria positive donors/total number of donors
Differences in donor malaria positivity for the regions within the OUA and BOBO RBTC catchment areas are shown in Fig. 3 and Additional Table S2. Urban versus rural variation was also notable at the regional level, with rural prevalence ranging from 4.20% in Centre to 17.80% in Hauts-Bassins (Additional Table S3). Urban prevalence was lowest in Centre and highest in Plateau-Central. Except for Centre-Sud, malaria prevalence by region was higher in rural donors than in urban donors, with the largest difference seen in Hauts-Bassins (Additional Table S3).
Fig. 3.
Donor malaria parasite positivity by region. The error bars represent the 95% confidence interval of donor malaria prevalence
A multivariable logistic regression analysis confirmed that both residence in rural areas and being from regions outside Centre region (OUA) were independently associated with significantly higher odds of malaria positivity after adjusting for age, sex, and malaria season (Additional Table S4).
At provincial level further differences were observed (Additional Table S5). For example, in the province of Houet, malaria prevalence in urban areas differed based on the population density of the towns. The provincial capital Bobo-Dioulasso (urban 1) had a significantly lower malaria prevalence (5.79%) compared to the smaller towns (urban 3) (11.38%, p = 0.002). The same trend was observed in Kadiogo province where Ouagadougou (urban 1) had a significantly lower malaria prevalence (3.93%) than the urban 3 area (9.43%, p = 0.004).
Furthermore, even within a single urban area, such as the cities of Ouagadougou and Bobo Dioulasso, malaria risk was not uniformly distributed. In Ouagadougou (Fig. 4) the central area exhibited the lowest malaria prevalence (3.37%; 95% CI: 3.01–3.77; 316/9367) compared to the peripheral east (3.98%; 95% CI: 3.65–4.33; 535/13437; p = 0.0189) and the peripheral west (4.60%; 95% CI: 4.13–5.11; 345/7497; p < 0.001). Relative to the central areas, donors residing in the peripheral west of Ouagadougou and those in the east had an increased odds ratio for being malaria positive, although it was only statistically significant for the west (1.276, p = 0.003 versus 1.141, p = 0.074). In Bobo-Dioulasso, while the malaria prevalence was 6.80% (95% CI: 6.12–7.53; 368/5411) during the period August to December, at sector level, this ranged from as low as 2.60% (95% CI: 0.84–6.10; 5/192) to a markedly higher 27.14% (95% CI: 16.34–42.40; 19/70).
Fig. 4.
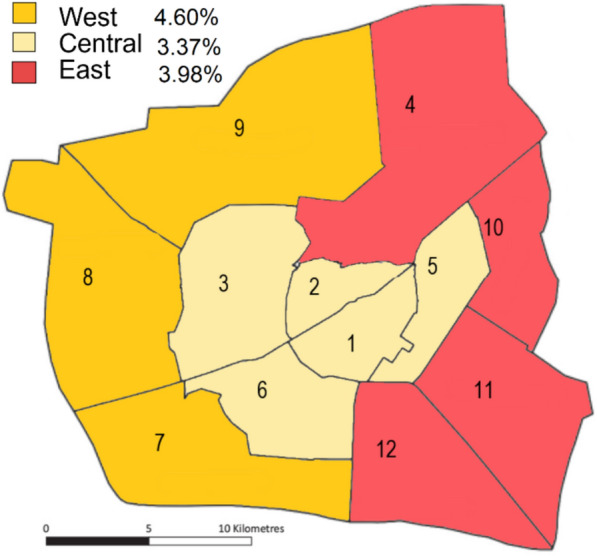
Map of Ouagadougou showing the sector boundaries and the malaria prevalence of the West, East and Central regions of the city
Malaria prevalence trends
Overall malaria prevalence
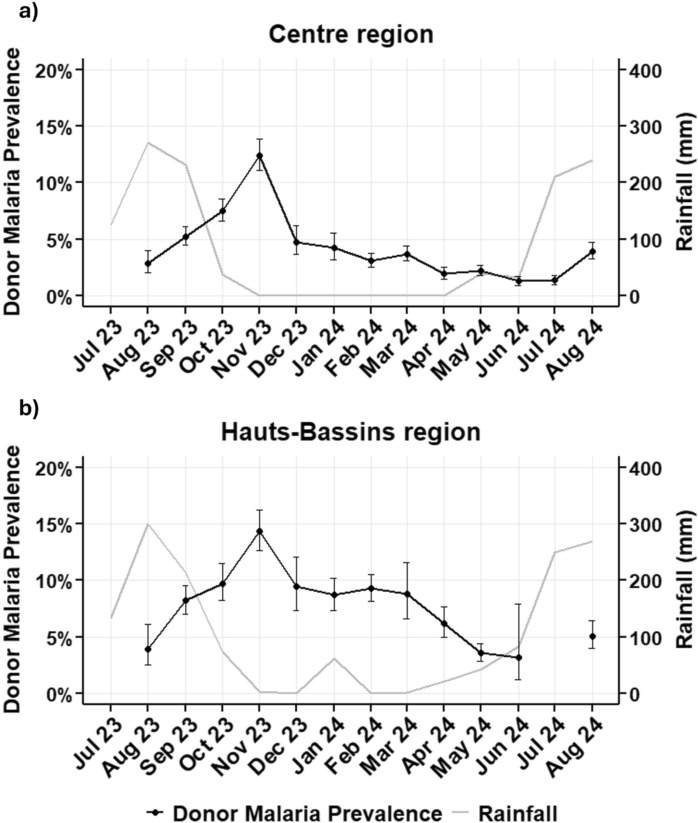
The malaria prevalence for all donors collected between August 2023 and August 2024 by month of donation, and monthly rainfall in millimetres are shown in Fig. 5. A 3-month lag was observed in the peak malaria prevalence (November) compared to the rainfall peak in the preceding August.
Fig. 5.
Donor malaria parasite prevalence and rainfall by month. The error bars represent the 95% confidence interval of donor malaria prevalence
Malaria prevalence trends by sex
The malaria prevalence trend over time was the same for male and female donors, both having the highest positivity rate in November and the lowest in June/July (Fig. 6). Except for August 2023 and April 2024, the malaria prevalence was consistently lower in female donors. The proportion of male to female donors was relatively constant across the period.
Fig. 6.
Asymptomatic malaria prevalence in male and female donors over time. The error bars represent the 95% confidence interval of donor malaria prevalence
Malaria prevalence trends by region
For Hauts-Bassins and Centre, the two regions with consistently sufficient donor numbers over the study period, malaria prevalence was plotted by month (Fig. 7), revealing different patterns. Whilst both regions peaked in November, albeit at different levels, in Centre (Fig. 7a), the prevalence dropped sharply in December, followed by a further gradual decline to the lowest point in July, and then rose again. In contrast, Hauts-Bassins (Fig. 7b), although experiencing a slight decline in December, manifested a longer sustained high transmission until March, and then dropped more sharply to a similarly low prevalence as Centre, in July. These patterns mirrored that of the regional rainfall.
Fig. 7.
Donor asymptomatic malaria prevalence trends and rainfall over time for Centre and Hauts-Bassins regions. a Malaria prevalence and rainfall for Centre region (Ouagadougou weather station identifier 65,503). b Malaria prevalence and rainfall for Hauts-Bassins region (Bobo-Dioulasso weather station identifier 65,510). As there were only 6 samples, the July 24 data point for Hauts-Bassins was omitted
Malaria prevalence trends by province
Whilst all provinces exhibited seasonal malaria prevalence highs and lows, the peaks and rate of change over time were not consistent (Fig. 8, Additional Table S6).
Fig. 8.
Asymptomatic malaria prevalence of donors residing in provinces within the catchment areas of Bobo-Dioulasso and Ouagadougou regional blood transfusion centres over time. Provinces in light grey are outside the RBTC catchment areas. (Bougour = Bougouriba; Ganzour = Ganzourgou Kour = Kourweogo; Oubrit = Oubritenga; Zoundw = Zoundweogo). Created with paintmaps.com
For those provinces where there were sufficient data for analysis across the entire study period (Houet, Kadiogo, Kénédougou, Oubritenga, and Zoundweogo), the peak malaria season from September to November was consistent for all, except Kénédougou province in the Hauts-Bassins region where this was delayed to December to February. While most provinces experienced the lowest donor malaria prevalence in June to August, this was not the case for Zoundweogo in Centre-Sud region, where malaria prevalence rose again in that period. Oubritenga province, in Plateau-Central, experienced a second albeit smaller peak in March to May. Nahouri province, in Centre-Sud region, exhibited a rapid decline, from 17.95% in December to February to just 2.56% in March to May. Elsewhere, the rate of change over time was more gradual. Nahouri also had the biggest seasonal variation with a tenfold difference between the peak level (25.71%) and the lowest level (2.56%). In contrast, Tuy province, showed much less seasonal variation, with a peak prevalence of 26.20% and 14.29% at the lowest level, less than a two-fold difference.
Malaria prevalence trends for urban and rural locations
Overall, although the actual donor malaria prevalence was consistently lower in urban donors over time, the pattern of malaria prevalence was similar for urban and rural areas, both peaking in November. In urban locations, donor malaria prevalence declined steadily from November to July, and then started to rise again in August. In rural areas, whilst July also had the lowest prevalence, there were additional smaller peaks in January and April (Fig. 9, Additional Table S7).
Fig. 9.
Donor asymptomatic malaria prevalence over time by urban and rural locations. The error bars represent the 95% confidence interval of donor malaria prevalence
The trends observed in Fig. 9 were not consistent across the regions. The urban–rural differences were compared for Hauts-Bassins and Centre region, the only two regions with sufficient donors across the study period for trend analysis. Figure 10 shows that the Hauts-Bassins rural donor malaria prevalence mirrors that of the overall donor pool (Fig. 9), with a longer period of sustained higher transmission, whereas in rural Centre the trend was similar to that of urban donors, except for a smaller second peak in April.
Fig. 10.
Donor asymptomatic malaria prevalence over time by urban and rural locations for Centre and Hauts-Bassins regions. The error bars represent the 95% confidence interval of donor malaria prevalence. No prevalence is shown if the sample size was less than 30
Parasite burden and gametocyte carriage
Parasite burden
There was no difference in median MI-RBC count for male (92 p/μL; 95% CI: 86–101.5) and female (98.5 p/μL; 95% CI: 88.6–110.4) infected donors (p = 0.9149). There was also no difference in the median MI-RBC count based on age (Additional Fig. 1) nor were these values different for urban (94 p/μL; 95% CI: 88.4–102) and rural (88 p/μL; 95% CI: 79–109.5) locations (p = 0.4148). Interestingly, the median MI-RBC counts of malaria positive donors showed a similar pattern to the overall prevalence. During the peak malaria prevalence, the median MI-RBC count was highest, and was lowest when malaria prevalence was lowest (Fig. 11).
Fig. 11.
Median Malaria-infected red blood cell (MI-RBC) count by month for all asymptomatic malaria donors. MI-RBC = malaria-infected red blood cells. The error bars represent the 95% confidence interval of the MI-RBC median value
Gametocyte carriage
The overall gametocyte carriage rate in asymptomatic malaria donors was 3.10% (95% CI: 2.52–3.78; 98/3164) of which 9.18% (95% CI: 4.20–17.43; 9/98) had gametocytes only and no asexual parasites. Significant seasonal variation (p < 0.001) was observed with gametocyte carriage increasing to 4.14% (95% CI: 3.27–5.19; 76/1834) in the high season (August to December) and falling to 1.65% (95% CI: 1.04–2.50; 22/1330) in the low season (January to July). The median age of malaria-infected donors was 22 years for gametocyte carriers (95% CI: 21–24) and 24 years for non-carriers (95% CI: 24–24). Gametocyte carriage rates were 3.20% (95% CI: 2.54–3.98; 80/2500) and 2.69% (95% CI: 1.54–4.37; 16/594) for males and females, respectively, which was not statistically significant (p = 0.519). In male donors there was a striking seasonal difference (p < 0.001) but this was not the case for females (p = 0.614) (Table 4).
Table 4.
Percentage of asymptomatic malaria donors with detectable gametocytes by sex and malaria season
| Period | Female donors | Male donors | p value |
|---|---|---|---|
|
Low season Jan–July |
2.36% 95% CI: 0.95–4.86 7/297 |
1.39% 95% CI: 0.76–2.34 14/1006 |
0.244 |
|
High season Aug–Dec |
3.03% 95% CI: 1.39–5.75 9/297 |
4.42% 95% CI: 3.42–5.62 66/1494 |
0.275 |
| p value | 0.614 | < 0.001 |
The peak gametocyte carriage rate (6.59%; 95% CI: 4.75–8.91; 42/637) was observed in November 2023, which coincided with the peak donor malaria prevalence (14.27%; 95% CI: 13.19–15.43; 637/4462), and the trend over time mirrored that of malaria prevalence overall, with the exception of June and July 2024 where the confidence interval was very wide (June—3.23%; 95% CI: 0.39–11.65; 2/62 and July—3.77%; 95% CI: 0.24–7.22; 2/53) (Fig. 12). The median MI-RBC count of 207 p/μL (95% CI: 148–297) for gametocyte carriers was significantly higher (p < 0.001) than that of non-carriers (91 p/μL; 95% CI: 86–98).
Fig. 12.
Donor asymptomatic malaria prevalence and gametocyte carriage trends over time. The error bars repesent the 95% confidence interval of malaria prevalence and gametocyte carriage percentage
Discussion
This study explored the feasibility of using blood donor malaria screening, utilizing the Sysmex XN-31 automated haematology analyser, as a complementary source of malaria surveillance data, at two of the nine RBTCs in Burkina Faso. It included 53,575 donors of both genders, aged 16 years and older, from 5 of the 13 regions (Hauts-Bassins, Cascades, Centre, Plateau-Central and Centre-Sud), spanning an approximately 12-month period (16 August 2023 to 30 August 2024). In comparison, the most recent Demographic and Health Survey (DHS) in Burkina Faso, included 5669 children aged 6–59 months for malaria screening using microscopy and rapid diagnostic tests, between 30 July to 30 November 2021, as a core malaria-metric to inform intervention strategies [27]. Blood donor surveillance and traditional community-based surveys targeting children under 5 years of age are complementary approaches to malaria monitoring. The strength of DHS/MIS surveys lies in their standardized methodology, which enables comparisons across countries and over time. However, in the same regions covered by this study, the 2021 DHS included only 2,117 samples (25 times fewer than in this blood donor screening study) and did not provide temporal data [27]. This highlights the value of using blood donors as a sentinel population: the large sample size and continuous collection allow for real-time temporal analysis of malaria trends.
Predictors of malaria
The overall blood donor asymptomatic malaria prevalence was 5.91% with significant variation based on age, sex, geographic location and time of year. Male donors had a higher malaria prevalence than female donors (6.36% vs 4.40%) with a 1.47 higher risk (p < 0.001). This is in keeping with what was reported in Malawian blood donors [21]. Also, the 2015/2016 Malawian DHS, and a longitudinal household study in rural Uganda, showed that asymptomatic malaria was more common in adult males than adult females [28]. A study in Uganda [29] demonstrated that females cleared asymptomatic infections at a faster rate than males, implicating sex-based host immune response differences as a contributing factor to the male bias in parasitaemic donors. In contrast, studies focussed on data from healthcare facilities in Uganda and Ghana reported a female dominance in clinical malaria cases, which may reflect health-care seeking behaviour and other social factors, rather than biology and environmental exposure to infective vectors [30, 31]. Cotter et al. [17] reported on the striking epidemiological shift in malaria-eliminating countries towards adults, and men in particular, among clinical malaria cases, connected to the increasing importance of ecological and behavioural influences outside the home that lead to increased exposure of these groups to malaria risk. Importantly, these adult men act as reservoirs for infection, as most are asymptomatic and have low density infections, increased gametocytaemia, and often go unnoticed [32]. Including adult men in surveillance would thus be an important component of surveillance activities in support of malaria elimination efforts.
Donor malaria prevalence declined progressively with age (Table 2), independent of other predictors of malaria (Additional Table S4). Notably, this decline was consistent in both sexes, with males retaining a higher malaria risk than females regardless of age (Fig. 2, Additional Table S1). Malaria positivity in the youngest donors (16–20 years) was 8.98% compared with 2.27% in those older than 40 years (p < 0.001). This decline in malaria prevalence from adolescence through adulthood, attributed to the development of partial immunity from repeated exposure to the parasite, has been well-documented in regions with high malaria transmission, where individuals are frequently exposed to P. falciparum, the most common malaria parasite in Africa [33–35].
Unsurprisingly, regional differences were observed. For the study period overall, Plateau-Central had the highest donor malaria prevalence (13.30%), followed by Cascades (10.18%), Centre-Sud (9.51%), Hauts-Bassins (7.90%) and Centre, the lowest (3.96%). These findings are similar to what was reported in the latest Burkina Faso Malaria Profile report, utlizing data from the period July to November 2021 [36].
Although the donor pool was heavily weighted towards urban donors (92.35%), the study was sufficiently powered to conclude that rural locations were associated with a siginificantly higher (p < 0.001) donor malaria prevalence than urban locations (12.06% vs. 5.39%). This is alligned with previous reports from Burkina Faso [27] and other sub-Saharan African countries [37]. However, rural areas were not uniformally at higher risk than urban areas. Regional location seemed to override rural residency. Rural donors in the OUA catchment area (Centre, Plateau-Central and Centre-Sud regions) had a lower malaria positivity rate (6.30%) compared with both urban (6.75%) and rural donors (16.35%) in the BOBO catchment area (Hauts-Bassins and Cascades regions), although this was only significant for comparison between the rural donors in the two catchment areas (p < 0.001) (Table 3). The difference in urban donor malaria prevalence between the RBTC catchment areas was exclusively driven by the cities of Bobo-Dioulasso and Ouagadougou (p < 0.001). Infrastructure differences may be the likely explanation. Bobo-Dioulasso is located in the catchment area of the Houet marsh making it particularly prone to flooding which negatively impacts infrastructure maintenance resulting in poor drainage, further compounded by litter-induced blockage of rainwater drains [38]. Additionally, the urban agglomeration of Bobo-Dioulasso has grown and reached the natural spatial constraints that framed its development, leading to high residential pressure on land and precarious housing [38]. Collectively these factors lead to pockets of stagnating rainwater providing breeding grounds for the Anopheles sp mosquito vector concentrated in areas where infrastructure is poorest. The marked variability of donor malaria prevalence by secteur of residence within Bobo-Dioulasso noted in this study is in keeping with the uneven malaria distribution in geographically distinct regions of Bobo-Dioulasso previously reported [39]. Likewise, the malaria prevalence in peri-urban Bobo-Dioulasso, which at 13.08% was more in keeping with that of rural locations (p = 0.703), can be attributed to these areas being located in challenging terrain with lack of infrastructure, and increasingly subject to uncontrolled occupation, exposing its residents to increased malaria risk [38].
Similarly, within Ouagadougou, the capital of Burkina Faso, asymptomatic malaria prevalence in donors was higher (4.60%) in the west of the city compared with the central area where the prevalence was 3.37% (p < 0.001). Higher malaria prevalence distribution within Ouagadougou has been consistently associated with closer proximity to permanent water bodies [40–43].
Furthermore, differences were also observed within urban areas at provincial level. Large cities, like Bobo-Dioulasso and Ouagadougou, defined as urban 1 in this study, had a lower donor malaria prevalence (4.43%) than other provincial capitals (urban 2, 11.26%) and other smaller towns (urban 3, 12.60%). Whilst this may be influenced by rainfall at a regional level, differences also exist within individual provinces. For example, in Houet province donor-malaria prevalence in Bobo-Dioulasso (5.79%) was significantly lower (p = 0.002) than the smaller less densely populated towns (11.38%) (Additional Table S5).
Although urban areas are generally associated with less conducive malaria breeding conditions, rapid urbanization in general has led to lower housing quality and more standing water associated with higher risk of malaria transmission [44]. At 4.75%, Burkina Faso is currently listed as the country with the fifth highest urbanization growth rate for the period 2020 to 2025 globally [45]. As urbanization continues and vector species adapt, continued monitoring and control of urban malaria in sub-Saharan Africa will become increasingly important [46]. Bassinga et al. [47] reported that spatial distribution of the prevalence of asymptomatic carriage is very heterogeneous across communes in Burkina Faso, both urban and rural, supporting the need for highly granular data to facilitate targeted and localized malaria control interventions.
Malaria trends
The overall donor malaria prevalence observed in this study showed strong seasonality. The rise in malaria prevalence from the start of the study in August, to its peak in November, mirrored that of rainfall recorded with a 3-month lag phase. These findings concur with Bationo et al. [26], who reported a 10-week median time lag between malaria peak onset and rainfall at district level in Burkina Faso. In this study, rainfall dropped sharply after the peak, whereas malaria prevalence dropped more gradually. Interestingly, there was a secondary albeit small rainfall peak in January, and this too was followed by a small secondary peak in malaria prevalence three months later (Fig. 5). Comparison of Hauts-Bassins and Centre, the two regions with the highest donor numbers, revealed that the malaria prevalence patterns were similar but not identical, with differences observed being attributable to differences in rainfall patterns (Fig. 7). Drilling down to provincial level further differences were observed related to the magnitude of malaria prevalence peaks, the timing of such peaks, and the rate of change in prevalence over time (Fig. 8). Although rainfall data were not available at provincial level, according to Bationo et al. [26], this together with other environmental factors would be the most likely influencers on these spatiotemporal patterns of malaria observed in this study.
Parasite burden and gametocyte carriage
The XN-31 analyser provides a percentage and absolute count of MI-RBC and thus permits quantification of parasite burden for infected indivduals. No difference was observed in median MI-RBC counts based on age, sex, and urban or rural locations. MI-RBC counts did however differ based on overall malaria prevalence within the donor population. The median MI-RBC counts of malaria positive donors showed a similar pattern to the overall prevalence (Fig. 11). In a study conducted in children under the age of 5 years, across 36 states of Nigeria, the same trend was reported. Oyibo et al. [48] showed that there was a positive correlation between microscopic asexual parasite density estimates in infected individuals and prevalence of infection in the community. A study conducted in Kenya, in adults and children, showing that sub-microscopic infections (confirmed with quantitative PCR) were proportionately more common in low prevalence areas in adults and children 6 years and older, compared to high prevalence areas, indirectly supports the findings of this study [49].
The XN-31 also detects gametocytes and the overall gametocyte prevalence in asymptomatic malaria donors was 3.10% with significant seasonal variation (p < 0.001), mirroring that of the overall malaria prevalence pattern (Fig. 12). Gametocyte carriage increased to 4.14% in the high season (August to December), peaking at 6.59% in November, which coincided with the malaria prevalence peak, and fell to 1.65% in the low season (January to July). This gametocyte prevalence seasonality is supported by Ouédraogo et al. [50] who showed that P. falciparum gametocyte prevalence was significantly higher at the start and peak of the wet season compared to the dry season.
There was no difference in gametocyte carriage overall between male (3.20%) and female infected donors (2.69%, p = 0.519), in line with what was observed in Malawian blood donors [21] and patients with clinical malaria in Tanzania, Thailand and The Gambia [51]. However, a striking seasonal difference (p < 0.001) was only observed in males (Table 4). Whilst there is no literature to substantiate this, it is feasible that higher intensity of exposure to infected mosquito bites increases the probability of recurrent infections, which together with a slower rate of parasite clearance [29], result in sustained parasitaemia in men during the rainy season. As longer duration infections in immune adults would give parasites more time to develop gametocytes [32], this may account for the substantially higher gametocyte prevalence seen in male donors in this study. Likewise, there is no direct evidence to support the absence of seasonal variation in gametocyte prevalence in females. it is speculated that absence of a significant rise in gametocyte prevalence in females during the rainy season, is linked to the higher rate of parasite clearance [29]. This together with their greater likelihood to seek medical attention in the event of fever, may reduce the likelihood and duration of sustained low grade parasistaemia, and thus fewer gametocytes. Age-based differences in gametocyte prevalence have been reported [32] but this was not observed in this study. The median MI-RBC count of 207 p/μL for gametocyte carriers was significantly higher (p < 0.001) than that of non-carriers (91 p/μL), in line with other findings [21, 49, 52].
Limitations
This study included consecutive blood donors who presented for voluntary blood donation. Blood donors are a heterogeneous group. Occasional or first-time donors tend to be less aware of blood safety, whereas regular donors are often more health-conscious, and may engage in protective behaviours such as sleeping under bed nets in malaria-endemic areas. As a result, malaria prevalence among donors may differ from the general population and the results of this study may have underestimated true community risk. Furthermore, the blood donor population in this study, and as has been observed elsewhere in sub-Saharan Africa [53–55], was biased towards young, urban men. However this did not impact the assessment of predictors of malaria due to the very large study sample size. In some instances, trend analysis was not possible as donations collected in a single month in some rural locations were too few. Furthermore, only demographic data routinely recorded as part the RBTC standard blood donation procedure, such as age, sex and location, were captured. Direct comparison with the most recent Burkina Faso DHS survey [27] was not possible for the following reasons: it was conducted 2 years prior in 2021, did not include temporal data, and only assayed children aged 6 to 59 months. Socioeconomic and behavioural predictors, such as bed net usage, were included in the DHS survey but not recorded in this study.
The scalability of this surveillance approach is currently limited by the need for XN-31 analysers to be available within blood transfusion services. WHO guidelines [56] recommend routine quality testing of blood products, such as platelet count, haematocrit, and haemoglobin, for which haematology analysers are already widely used. Although adherence varies, 22 African countries report legislation on blood safety and quality [57], consequently, analysers for blood product quality control (QC) are present in many facilities. The XN-31, which combines full blood count and malaria detection, is gaining clinical use in endemic regions, particularly in West Africa, where 159 analysers are reportedly in routine use across 12 countries (Prof Yves Traoré, Sysmex Burkina Faso, personal communication). It could therefore serve a dual role in blood donor malaria screening and product QC, and be considered as a replacement option when existing analysers reach end of life. While reagent use would be higher than current QC practice, which only samples a fraction of donations, this added cost could be viewed as an investment in donor health. Asymptomatic parasitaemia is associated with increased anaemia risk [58] and thus identifying and treating parasitaemic donors may help preserve haemoglobin levels and protect the donor pool. Additionally, the XN-31 has been shown to enhance blood safety, as demonstrated by M’baya et al. [20]. Its integration into transfusion services could therefore offer multiple benefits for healthcare systems in malaria-endemic countries.
Finally, the large amount of data collected in this study was not exhaustively analysed. The study aim was to illustrate the potential of XN-31 blood donor malaria screening as a complementary source of malaria surveillance, including the possibilities for granular reporting, but not to provide a detailed surveillance report. Hence only a high level analysis was conducted on the data collected in this study. The full potential of surveillance metrics that this dataset could provide with deeper targeted analyses, in support of generating customized malaria control interventions is being explored.
Conclusions
This study, spanning an approximately 12-month period, screened 53,575 blood donors for malaria across five regions of Burkina Faso and confirmed known risk factors—young age, male sex, and rural location—while also revealing regional and seasonal patterns aligned with rainfall and environmental conditions. The findings highlight the value of blood donors as a consistent, scalable source of real-time malaria surveillance data, particularly in high-burden, transmission-heterogeneous settings like Burkina Faso. Notably, the study identified localized hotspots even within urban centres, challenging assumptions about rural predominance in malaria risk. As malaria prevalence declines and shifts towards adults, particularly men, blood donors represent an increasingly relevant surveillance population. The XN-31 analyser enables high-throughput, accurate detection of malaria parasites, including gametocytes, making it ideal for generating timely, granular data essential for monitoring climate-sensitive diseases and informing targeted control efforts. The reliance on outdated and episodic surveys like DHS may underestimate true burden, underscoring the need for more strategic, dynamic approaches to malaria data collection.
Supplementary Information
Acknowledgements
We would like to thank the following individuals for their invaluable contributions that enabled the successful completion of this study: all the blood donors of Burkina Faso for the gift of their blood in support of saving lives; the RBTC staff for the processing of donor blood samples and capturing the demographic data; Dr Nezien Désire, the head of the National Blood Transfusion Centre in Ouagadougou, Dr Martin Ouedraogo head of the Ouagadougou RBTC, Dr Dieudonné Yonli, the head of the Bobo-Dioulasso RBTC for their support and facilitation towards the practical execution of the study; Prof Eléonore Kafando from the department of laboratory haematology, Joseph KI-ZERBO University for her mentorship and encouragement throughout the study; Professor Yves Traore from Sysmex Burkina Faso for his logistic support and active engagement with the RBTC personnel towards the resolution of logistic problems encountered; and Feyssal Sare of Sysmex Burkina Faso for his technical support of the XN-31 analyser throughout the study.
Abbreviations
- BOBO
Bobo-Dioulasso Regional Blood Transfusion Centre
- CI
Confidence interval
- DHS
Demographic and health survey
- MI-RBC
Malaria-infected red blood cells
- OR
Odds ratio
- OUA
Ouagadougou regional blood transfusion centre
- p/μL
Parasites/microlitre
- RBTC
Regional Blood Transfusion Centre
- WHO
World Health Organization
- XN-31
Sysmex XN-31 haematology analyser
Author contributions
SS, MM, TH, and JS designed the original research; SS, MM, and TH supervised the original research; TH and AP conducted the statistical analysis; MM, TH, JS, and TLC analysed the data; AP, JS, TH, and MM created the figures. All authors contributed to the conceptualization of the core messages; MM wrote the main manuscript text; all authors read and approved the final manuscript. The original research; TH and AP conducted the statistical analysis; MM,TH, JS and TLC analysed the data; AP, JS, TH, and MM created the figures. All authors contributed to the conceptualization of the core messages; MM wrote the manuscript; all authors read and approved the final manuscript.
Funding
Sysmex Europe SE provided the analyser and reagents. Sysmex Corporation Japan covered all other expenses associated with the study.
Availability of data and materials
The datasets used and analysed during this study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Study approval (2023-02-021)) was granted by the Burkina Faso Ethics Board of Health Research on behalf of the Ministry of Health and Public Hygiene, the Ministry of Higher Education, Research and Innovation on 24 February 2023. Informed consent was waived as there was no additional blood draw and consent to donate includes consent to use donated blood samples and biodata for research to improve blood safety and/or in studies of public health importance. Donors are informed that they can withdraw their consent for the use of their donated blood samples for research purposes, inherent in the general consent to donate blood, at any time pre- or post-donation. The malaria screening results were not linked to the individual blood units and thus did not influence the routine RBTC blood product selection and issuing procedures.
Consent for publication
Not applicable.
Competing interests
TH, MM, and JS are full-time employees of Sysmex Europe SE who provided the analysers and reagents for the study. TLC provides consultancy services to Sysmex Europe SE. SS and SCDK received compensation from Sysmex Corporation Japan who funded the study. AP has no competing interests to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. Malaria surveillance, monitoring and evaluation: a reference manual. Geneva: World Health Organization; 2018. [Google Scholar]
- 2.WHO. World Malaria Report. Addressing inequity in the global malaria response. Geneva: World Health Organization; 2024. p. 2024. [Google Scholar]
- 3.Lourenço C, Tatem AJ, Atkinson PM, Cohen JM, Pindolia D, Bhavnani D, et al. Strengthening surveillance systems for malaria elimination: a global landscaping of system performance, 2015–2017. Malar J. 2019;18:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Opoku Afriyie S, Addison TK, Gebre Y, Mutala A-H, Antwi KB, Abbas DA, et al. Accuracy of diagnosis among clinical malaria patients: comparing microscopy, RDT and a highly sensitive quantitative PCR looking at the implications for submicroscopic infections. Malar J. 2023;22:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kizito J, Kayendeke M, Nabirye C, Staedke SG, Chandler CIR. Improving access to health care for malaria in Africa: a review of literature on what attracts patients. Malar J. 2012;11:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mabona M, Zwane T, Raman J, Kuonza L, Mhlongo B, Phafane P. Evaluation of the malaria case surveillance system in KwaZulu-Natal Province, South Africa, 2022: a focus on DHIS2. Malar J. 2024;23:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malaria Indicator Survey (MIS) Overview. https://dhsprogram.com/Methodology/Survey-Types/MIS.cfm. Accessed 14 July 2025.
- 8.Alegana VA, Okiro EA, Snow RW. Routine data for malaria morbidity estimation in Africa: challenges and prospects. BMC Med. 2020;18:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozodiegwu ID, Ambrose M, Battle KE, Bever C, Diallo O, Galatas B, et al. Beyond national indicators: adapting the demographic and health surveys’ sampling strategies and questions to better inform subnational malaria intervention policy. Malar J. 2021;20:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Accrombessi M, Issifou S. Malaria control and elimination in sub-Saharan Africa: data from antenatal care centres. Lancet Glob Health. 2019;7:e1595-6. [DOI] [PubMed] [Google Scholar]
- 11.Gitonga CW, Karanja PN, Kihara J, Mwanje M, Juma E, Snow RW, et al. Implementing school malaria surveys in Kenya: towards a national surveillance system. Malar J. 2010;9:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindblade KA, Steinhardt L, Samuels A, Kachur SP, Slutsker L. The silent threat: asymptomatic parasitemia and malaria transmission. Expert Rev Anti-Infect Ther. 2013;11:623–9. [DOI] [PubMed] [Google Scholar]
- 13.WHO. Malaria elimination: a field manual for low and moderate endemic countries. Geneva: World Health Organization; 2007. [Google Scholar]
- 14.Bousema T, Griffin JT, Sauerwein RW, Smith DL, Churcher TS, Takken W, et al. Hitting hotspots: spatial targeting of malaria for control and elimination. PLoS Med. 2012;9:e1001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamau A, Mogeni P, Okiro EA, Snow RW, Bejon P. A systematic review of changing malaria disease burden in sub-Saharan Africa since 2000: comparing model predictions and empirical observations. BMC Med. 2020;18:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobson JO, Cueto C, Smith JL, Hwang J, Gosling R, Bennett A. Surveillance and response for high-risk populations: what can malaria elimination programmes learn from the experience of HIV? Malar J. 2017;16:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cotter C, Sturrock HJW, Hsiang MS, Liu J, Phillips AA, Hwang J, et al. The changing epidemiology of malaria elimination: new strategies for new challenges. Lancet. 2013;382:900–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmadpour E, Foroutan-Rad M, Majidiani H, Moghaddam SM, Hatam-Nahavandi K, Hosseini SA, et al. Transfusion-transmitted malaria: a systematic review and meta-analysis. Open Forum Infect Dis. 2019;6:ofz283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owusu-Ofori AK, Parry C, Bates I. Transfusion-transmitted malaria in countries where malaria is endemic: a review of the literature from sub-Saharan Africa. Clin Infect Dis. 2010;51:1192–8. [DOI] [PubMed] [Google Scholar]
- 20.M’Baya B, Mfune T, Samon A, Hwandih T, Münster M. Evaluation of the Sysmex XN-31 automated analyser for blood donor malaria screening at Malawi Blood Transfusion Services. Vox Sang. 2021;117:346–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kayange M, M’baya B, Hwandih T, Saker J, Coetzer TL, Münster M. Automated measurement of malaria parasitaemia among asymptomatic blood donors in Malawi using the Sysmex XN-31 analyser: could such data be used to complement national malaria surveillance in real time? Malar J. 2022;21:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chipeta MG, Gorgi E, Mategula D, Macharia PM, Ligomba C, Munyenyembe A, et al. Geostatistical analysis of Malawi’s changing malaria transmission from 2010 to 2017. Wellcome Open Res. 2019;4:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.City Population. https://www.citypopulation.de/en/burkinafaso/admin/. Accessed 21 Sept 2024.
- 24.Pillay E, Khodaiji S, Bezuidenhout BC, Litshie M, Coetzer TL. Evaluation of automated malaria diagnosis using the Sysmex XN-30 analyser in a clinical setting. Malar J. 2019;18:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Weather's Record Keeper. https://meteostat.net/en/. Accessed 12 Feb 2025.
- 26.Bationo C, Cissoko M, Katilé A, Sylla B, Ouédraogo A, Ouedraogo JB, et al. Malaria in Burkina Faso: a comprehensive analysis of spatiotemporal distribution of incidence and environmental drivers, and implications for control strategies. PLoS ONE. 2023;18:e0290233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Institut National de la Statistique et de la Démographie, The DHS Program. Burkina Faso Demographic and Health Survey 2021. Burkina Faso and Rockville, Maryland, USA: INSD and ICF; 2023.
- 28.Newell K, Kiggundu V, Ouma J, Baghendage E, Kiwanuka N, Gray R, et al. Longitudinal household surveillance for malaria in Rakai, Uganda. Malar J. 2016;15:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Briggs J, Teyssier N, Nankabirwa JI, Rek J, Jagannathan P, Arinaitwe E, et al. Sex-based differences in clearance of chronic Plasmodium falciparum infection. Elife. 2020;9:e59872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okiring J, Epstein A, Namuganga JF, Kamya EV, Nabende I, Nassali M, et al. Gender difference in the incidence of malaria diagnosed at public health facilities in Uganda. Malar J. 2022;21:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quaresima V, Agbenyega T, Oppong B, Awunyo J, Adu Adomah P, Enty E, et al. Are malaria risk factors based on gender? A mixed-methods survey in an urban setting in Ghana. Trop Med Infect Dis. 2021;6:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ouédraogo AL, Bousema T, de Vlas SJ, Cuzin-Ouattara N, Verhave J-P, Drakeley C, et al. The plasticity of Plasmodium falciparum gametocytaemia in relation to age in Burkina Faso. Malar J. 2010;9:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamau A, Mtanje G, Mataza C, Mwambingu G, Mturi N, Mohammed S, et al. Malaria infection, disease and mortality among children and adults on the coast of Kenya. Malar J. 2020;19:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mulugeta A, Assefa A, Eshetie A, Asmare B, Birhanie M, Gelaw Y. Six-year trend analysis of malaria prevalence at University of Gondar Specialized Referral Hospital, Northwest Ethiopia, from 2014 to 2019. Sci Rep. 2022;12:1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tairou F, Gaye I, Herrera S, Nawaz S, Sarr L, Cissé B, et al. Malaria prevalence and use of control measures in an area with persistent transmission in Senegal. PLoS ONE. 2024;19:e0303794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Institut National de Santé Publique: Profil du Burkina Faso sur le paludisme. Burkina Faso. 2023.
- 37.Mbishi JV, Chombo S, Luoga P, Omary HJ, Paulo HA, Andrew J, et al. Malaria in under-five children: prevalence and multi-factor analysis of high-risk African countries. BMC Public Health. 2024;24:1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soma A. Living with floods in West African cities: precarity of housing and forms of resilience in the city of Bobo-Dioulasso in Burkina Faso. Curr Urban Stud. 2024;12:774–89. [Google Scholar]
- 39.Soma DD, Kassié D, Sanou S, Karama FB, Ouari A, Mamai W, et al. Uneven malaria transmission in geographically distinct districts of Bobo-Dioulasso, Burkina Faso. Parasit Vectors. 2018;11:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baragatti M, Fournet F, Henry M-C, Assi S, Ouedraogo H, Rogier C, et al. Social and environmental malaria risk factors in urban areas of Ouagadougou, Burkina Faso. Malar J. 2009;8:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ouedraogo B, Inoue Y, Kambiré A, Sallah K, Dieng S, Tine R, et al. Spatio-temporal dynamic of malaria in Ouagadougou, Burkina Faso, 2011–2015. Malar J. 2018;17:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sabatinelli G, Bosman A, Lamizana L, Rossi P. Prevalence of malaria in Ouagadougou and the surrounding rural environment during the period of maximal transmission. Parassitologia. 1986;28(1):17–31. [PubMed] [Google Scholar]
- 43.Wang S-J, Lengeler C, Smith TA, Vounatsou P, Diadie DA, Pritroipa X, et al. Rapid urban malaria appraisal (RUMA) i: epidemiology of urban malaria in Ouagadougou. Malar J. 2005;4:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doumbe-Belisse P, Kopya E, Ngadjeu CS, Sonhafouo-Chiana N, Talipouo A, Djamouko-Djonkam L, et al. Urban malaria in sub-Saharan Africa: dynamic of the vectorial system and the entomological inoculation rate. Malar J. 2021;20:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Urbanization rate by country 2025. https://worldostats.com/urbanization-rate-by-country-2025/. Accessed 4 Feb 2025.
- 46.De Silva PM, Marshall JM. Factors contributing to urban malaria transmission in sub-saharan Africa: a systematic review. J Trop Med. 2012;2012:819563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bassinga H, Ouedraogo M, Cisse K, Yira P, Ouedraogo SC, Nombré A, et al. Prevalence of asymptomatic malaria at the communal level in Burkina Faso: an application of the small area estimation approach. Popul Health Metr. 2024;22:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oyibo W, Latham V, Oladipo O, Ntadom G, Uhomoibhi P, Ogbulafor N, et al. Malaria parasite density and detailed qualitative microscopy enhances large-scale profiling of infection endemicity in Nigeria. Sci Rep. 2023;13:1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salgado C, Ayodo G, Macklin MD, Gould MP, Nallandhighal S, Odhiambo EO, et al. The prevalence and density of asymptomatic Plasmodium falciparum infections among children and adults in three communities of western Kenya. Malar J. 2021;20:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ouédraogo AL, de Vlas SJ, Nébié I, Ilboudo-Sanogo E, Bousema JT, Ouattara AS, et al. Seasonal patterns of Plasmodium falciparum gametocyte prevalence and density in a rural population of Burkina Faso. Acta Trop. 2008;105:28–34. [DOI] [PubMed] [Google Scholar]
- 51.Stepniewska K, Price RN, Sutherland CJ, Drakeley CJ, von Seidlein L, Nosten F, et al. Plasmodium falciparum gametocyte dynamics in areas of different malaria endemicity. Malar J. 2008;7:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baker DA. Malaria gametocytogenesis. Mol Biochem Parasitol. 2010;172:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Osei-Boakye F, Nkansah C, Appiah SK, Abbam G, Derigubah CA, Ukwah BN, et al. Self-reported high-risk behavior among first-time and repeat replacement blood donors; a four-year retrospective study of patterns. PLoS ONE. 2024;19:e0308453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mremi A, Yahaya JJ, Nyindo M, Mollel E. Transfusion-transmitted infections and associated risk factors at the Northern Zone Blood Transfusion Center in Tanzania: a study of blood donors between 2017 and 2019. PLoS ONE. 2021;16:e0249061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.WHO. Global status report on blood safety and availability 2016. Geneva: World Health Organization; 2017.
- 56.WHO. Guidelines for quality assurance programmes in blood transfusion services. Geneva: World Health Organization; 1993.
- 57.WHO. Global status report on blood safety and availability 2021. Geneva: World Health Organization; 2022.
- 58.Hayuma PM, Wang CW, Liheluka E, Baraka V, Madebe RA, Minja DTR, et al. Prevalence of asymptomatic malaria, submicroscopic parasitaemia and anaemia in Korogwe District, north-eastern Tanzania. Malar J. 2021;20:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analysed during this study are available from the corresponding author on reasonable request.