ABSTRACT
The microbiome plays an essential role in the development of the immune system. Both the immune system and microbiome dynamically respond to internal and external cues, and dysregulation of either of these systems can lead to disease pathology. Separate from the adaptive immune system, the innate immune system retains a memory of inflammatory events that determine the quality of future immune responses. The phenomenon is characterized by epigenetic modifications that lead to immunosuppressive or hyperinflammatory cell phenotypes, collectively designated as epigenetic cellular memory. It remains unclear whether and how the microbiome influences epigenetic cellular memory phenotypes to promote immunopathology and chronic disease. Inflammatory signals from the microbiota regulate hematopoiesis and systemic immunity through the production of immunomodulatory ligands and activation of circulating immune cells; however, few studies have directly implicated these mechanisms in the development of epigenetic cellular memory. We posit that a multi-omic systems approach is well-suited to elucidating the complex factors mediating the microbiome’s contribution to this phenomenon. By measuring responses to exogenous influences through multi-omic technologies, it will be possible to identify the regulatory axis that next-generation therapies should target to reverse immunopathology. As chronic inflammatory disorders are on the rise, it is imperative that future therapies leverage both dietary and pharmacological interventions to promote self-reinforcing homeostatic immunity by targeting the mechanisms of epigenetic cellular memory.
KEYWORDS: systems biology, innate immunity, immune memory, microbiome
INTRODUCTION
The mammalian immune system is composed of two branches, and both are essential for protection against infection, the resolution of inflammation, and sustaining homeostasis. Dysregulation of the immune system leads to an inability to calculate an appropriate response to danger- or pathogen-derived signals. Such a response leads to autoimmunity, immune deficiency, or hyperreactivity, and inability to resolve inflammation and return to homeostasis, as in chronic inflammation. The microbiome provides a constant, yet essential, stimulus to the immune system via production of small molecule metabolites, membrane lipids, and proteins (1–4). Diverse microbiota-derived molecules activate pattern recognition receptors (PRRs) expressed primarily on innate immune cells, but also G-protein-coupled receptors (GPCRs) through diverse ligands (5–7). Dysregulation of the microbiome by dietary and environmental factors adversely affects the immune system and leads to chronic inflammation associated with numerous metabolic and inflammatory diseases, from diabetes to neurodegenerative disease and inflammaging (8–11). Importantly, chronic inflammation is thought to persist through epigenetic modifications in stem and progenitor cells, which bias the inflammatory phenotype of effector cells, termed epigenetic cellular memory (12, 13).
In this review, we cover the current perspective on systems approaches for elucidating the mechanisms of innate immune memory and microbiome-mediated inflammation and highlight an underexplored connection between the two. We first provide a brief overview of how the microbiota influences the immune system and is associated with numerous metabolic and inflammatory diseases. Second, we outline the current viewpoints on innate immune memory, from tolerance to trained immunity, and their implication in pathology. Finally, we highlight how a multi-omic systems biology approach can leverage orthogonal technologies, such as proteomics, genomics, and epigenome profiling, to characterize the drivers of epigenetic cellular memory (Fig. 1). We propose that elucidating the complex mechanisms underlying the microbiome-epigenome axis driving host physiology requires cross-discipline investigation and a systems approach.
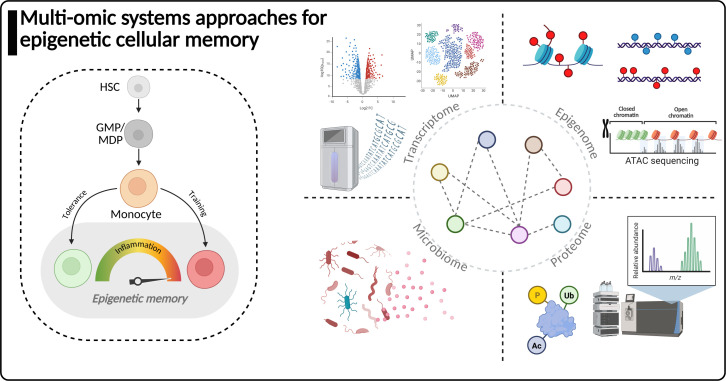
Fig 1.
Systems biology approaches for studying the microbiome-epigenetic cellular memory axis. Left, a schematic representing epigenetic cellular memory induction in monocytes from the hematopoietic system. The epigenetic cellular memory phenotype is induced in circulating cells, such as monocytes, but can also be transmitted back to stem and progenitor cells. Right, different technologies are often leveraged by systems biology approaches. Utilization of transcriptome profiling by bulk or single-cell RNA sequencing reveals the functional status of cells by the genes they express. Epigenome profiling technologies explore methods for analyzing histone post-translational modifications in addition to DNA methylation and chromatin accessibility profiling. Proteomic technologies enabled by mass spectrometry characterize post-translational modifications to proteins (including histones) in addition to profiling of protein abundance and the functional status of a population of cells. Microbiome profiling methods include metagenomic and amplicon sequencing approaches as well as mass spectrometry methods to explore the microbial metabolite landscape. Systems biology approaches must integrate multi-omic data to interpret complex mechanisms of epigenetic cellular memory, which are regulated at multiple levels in cells from the genome, epigenome, transcriptome, proteome, and exogenously by the microbiome.
INFLUENCE OF THE MICROBIOTA ON THE IMMUNE SYSTEM
The multitude of bacteria, viruses, and eukaryotic microorganisms that inhabit different sites of the human body, collectively referred to as the microbiome, contributes substantially to both the maintenance of and deviation from homeostasis. Experimental work with germ-free and gnotobiotic mice has revealed the essential role of the gut microbiota in priming the immune system towards homeostatic immunity. Early work in this area revealed how mice colonized with a defined consortia of Clostridia strains displayed enhanced regulatory T-cell proliferation and activation (14, 15). The microbiota is also known to influence the host antibody repertoire, and seminal work investigating the role of innate immune recognition of bacterial flagellin by TLR5 revealed coordination between the innate and adaptive immune system branches in the production of flagellin-specific immunoglobulins (16). Innate immune cells perceive the microbiota in close contact at such barrier sites as the gastrointestinal tract and the skin, but also in distal tissues where microbes do not normally reside. Microbiota are thought to educate innate immune cells through constitutive low-level escape of microbiome-derived molecules into the circulation, which can activate innate immune cells or reach distal sites such as the brain and other tissues (17–19). The microbiota produces a diverse array of pathogen-associated molecular pattern (PAMP) molecules, which are sensed by PRRs: Toll-like receptors (TLR), NOD-like receptors (NLR), C-type lectin receptors (CLR)—expressed on innate immune cells (7, 20, 21). Diverse PAMPs drive different immune responses suitable for the given pathogen, such as the activation of antiviral pathways after the recognition of viral RNA or increases in the expression of chemoattractant molecules to initiate a localized immune response (6). Education of immune cells in distal tissues has important implications for protection against infection with bacterial or viral pathogens (22, 23). Dysregulation of barrier integrity allows escape of microbiome-derived PAMPs and is associated with heightened systemic inflammation levels in diseases such as inflammatory bowel disease (IBD) (24). Finally, dysregulation of any component has systemic pathological consequences, including metabolic diseases, IBD, allergy, and even responses to cancer immunotherapies (10, 25–28). Thus, the immune system must remain in equilibrium with the microbiota to preserve homeostasis.
FOUNDATIONS OF EPIGENETIC CELLULAR MEMORY
When exposed to a stimulus, immune cells become activated to defend against the present pathogen or danger signals. It is now clear that the innate immune response can be influenced by prior inflammatory signals encountered by a differentiated cell or its immature precursor. Thus, cellular innate immune responses can be broadly categorized into four phenomena: differentiation, priming, and tolerant immune memory or trained immune memory (Table 1), which have been extensively reviewed elsewhere (29, 30). In brief, differentiation is defined as the process by which an immature cell matures into a specialized effector cell and requires the opening of chromatin around lineage-specific gene loci. Priming occurs after transient exposure to a stimulus that causes the upregulation of activation markers or transcription, which does not return to baseline levels before a second exposure to a stimulus. Finally, tolerance and trained innate immune memory represent functional adaptations that are retained after an initial stimulus is removed and transcription returns to baseline levels; however, upon encountering a secondary stimulus, the magnitude of the response is either suppressed (tolerance) or exceeds (training) that of the initial response. Each of these phenomena represents distinct states of a given cell but requires interpretation depending on the biological context.
TABLE 1.
Descriptions of innate immune response phenomena and separation of immune memory from non-memory responses
| Phenomenon | Initial response | Function | Epigenetic status | Example | |
|---|---|---|---|---|---|
| Differentiation | Naïve or immature cells exposed to cytokines or growth factors change morphology and function. | Formation of mature effector cells (e.g., macrophages); cells have constitutively higher levels (above baseline) of inflammatory signaling molecules and lineage- or population-specific markers. | Cells accumulate epigenetic marks and open chromatin near lineage-specific genes. | During myelopoiesis, common myeloid progenitors differentiate into monocytes, which can differentiate into mature macrophages or dendritic cells (31). | |
| Priming | Mature (resting) innate immune cells are exposed to a primary stimulus and maintain a heightened readiness state, which does not return to baseline. | Upon encountering a secondary stimulus, the cell’s response is additive (above an already heightened status) since the initial inflammatory response did not return to baseline. | Cells do not retain epigenetic marks long term, but transcriptional activity is temporarily increased. | LPS stimulation of macrophages temporarily upregulates the expression of inflammasome components, which can then be activated by a secondary trigger (32). | |
| Epigenetic memory | |||||
| Tolerance | Mature innate immune cells exposed to a strong primary stimulus respond strongly at first but decrease or silence inflammatory activity after the removal of the stimulus. | Upon restimulation, the cell cannot mount a response and becomes tolerant to avoid damage from excessive inflammation. | Cells accumulate silencing epigenetic marks around inflammatory gene loci. | Macrophages stimulated with LPS, followed by a rest period, then restimulated with LPS display decreased cytokine production (33). | |
| Training | Mature innate immune cells exposed to a weak or moderate primary stimulus respond strongly at first but decrease or silence inflammatory activity after the removal of the stimulus. | Upon restimulation, the cell exceeds the level of the initial response and maintains an active hyper-inflammatory and metabolic phenotype. | Cells accumulate activating epigenetic marks around inflammatory gene loci. | Macrophages stimulated with β-glucan, followed by a rest period, then stimulated with LPS display increased cytokine production (34). | |
Innate immune memory was first explored in the context of lipopolysaccharide (LPS)-induced immune suppression following exposure to high-dose LPS challenge or sepsis due to bacteremia with a gram-negative pathogen (35, 36). Tolerance to LPS was experimentally characterized by a reduced capacity of human peripheral blood mononuclear cells (PBMCs) and mouse macrophages to produce TNFα and IL-6 cytokines after repeat ex vivo LPS stimulation (34, 36, 37). Innate immune tolerance has also been observed for gram-positive pathogens, which are primarily recognized by peptidoglycan (PGN) structures and associated molecules found in the PGN matrix, such as lipoteichoic acid (LTA). These ligands are sensed extracellularly by immune cells via TLR2 (LTA or di- and tri-acylated membrane lipids) and intracellularly by NOD1 or NOD2 (muramyl dipeptide and other PGN fragments) (38). Pretreatment of mice with PGN before challenge with Staphylococcus aureus was shown to decrease the concentrations of plasma cytokines IFNγ and TNFα, concordant with models of LPS tolerance; nevertheless, the mice had improved survivability and decreased pathogen burden in various tissues (39). Depending on the molecules under study and the cellular pathways activated, the phenotype may not be of tolerance in all respects but a beneficial adaptation to the type of stimulus encountered.
Conversely, trained innate immune memory (referred to herein as trained immunity) was first recognized as the mechanism by which the Bacillus Calmette-Guérin (BCG) vaccine conferred non-specific cross-protection against heterologous pathogen insults such as bacterial and fungal infection (40). While BCG confers only variable efficacy in protecting against pulmonary Mycobacterium tuberculosis due to environmental mycobacteria exposure or previous infection, it remains a prototypical stimulus of innate immune memory (41, 42). In human studies, BCG-induced trained immunity decreased viremia after challenge with the attenuated yellow fever virus while maintaining similar neutralizing antibody responses to non-BCG trained individuals (43). In addition, in a murine Candida albicans infection model, BCG-vaccinated SCID mice lacking T and B cells had lower candidiasis and increased survival following Candida challenge, highlighting the dependence of trained immunity on myeloid cells (40). In vitro studies also explored how stimulation of macrophages with purified ligands such as β-glucan, a fungal cell wall component, followed by a rest period, enhanced cytokine production in response to secondary stimulation with LPS (44). Both innate immune tolerance and training are mediated by myeloid cells such as monocytes and macrophages, with some studies potentiating granulocytes, such as neutrophils, in the process as well (45–47). Mechanistically, innate immune memory (in both training and tolerance contexts) is characterized by epigenetic and metabolic alterations that functionally bias a cell’s response to pathogen insult or PAMP exposure (12, 48). In contrast to adaptive immunity, innate immune memory can persist on the order of weeks to months, versus years, by conferring the trained effect to hematopoietic stem cell (HSC) populations in the bone marrow through cytokines, which direct HSC lineages toward myelopoiesis in an IFNγ and IL-1β-dependent fashion (49).
Models of trained immunity primarily focus on using in vitro or ex vivo stimulation of monocytes or macrophages with molecules known to induce trained immunity, such as β-glucan (34). Classically, the model induces an innate immune response by an initial stimulus, which is followed by a period of rest before the second exposure to a stimulus occurs. The expected phenotype for successful formation of innate immune memory, the process for trained immunity referred to as “training,” is a relative increase in the secretion of cytokines (such as TNFα, IL-1β, or IL-6) after a second stimulation, compared to the level produced following the first stimulation (30). The increased cytokine release is also associated with an increase in the functional capacity of trained macrophages. Macrophages stimulated with β-glucan show enhanced phagocytic clearance of Pseudomonas aeruginosa in vitro and in vivo, whereas LPS-stimulated alveolar macrophages display enhanced efferocytosis of apoptotic neutrophils ex vivo (50, 51). β-glucan, a cell wall component from fungi and a molecule known to induce trained immunity, drives increased aerobic glycolysis by signaling through dectin-1 to activate mTOR and HIF1α (52). Contrary to the immunosuppressive phenotype induced by high-dose LPS challenge, activation of TLRs via stimulation with flagellin, LPS, or Pam3csk4 (a synthetic TLR2 agonist) at minimal effective doses can enhance secondary stimulation cytokine production. Titration of stimulation doses of flagellin, LPS, or Pam3csk4 revealed that IL-6, but not TNFα, concentration increased in the media of trained monocytes after a second stimulation with either LPS or Pam3csk4 (34). Using different PAMPs or molecules to induce training leads to distinct immune phenotypes; therefore, the innate immune memory phenotype must account for the molecule in question and dose as important factors that determine an appropriate immune response (53, 54).
Innate immunity is characterized by a non-specific memory-like capacity which differs from classical adaptive immune memory in that innate immune cells do not possess variable recombination genes for antigen-specific antibody production like T and B lymphocytes. Innate immune memory is characterized by epigenetic mechanisms for maintaining chromatin accessibility at inflammatory or metabolic loci, which allows for rapid transcriptional response to re-stimulation (55). Epigenetic control of chromatin organization ensures that specific regions of the genome are accessible for active transcription and unnecessary regions are silenced or condensed. This epigenetic mechanism also reiterates the difference in longevity of the inflammatory recall response as opposed to the adaptive immune system, in which antigen-specific memory cells remain quiescent for years until encountering their specific antigen (56). Such regions can be identified through post-translational modifications (PTMs) of histone protein tails and DNA methylations, which affect the formation of nucleosomes and their interaction with DNA (57, 58). Histone epigenetic marks associated with trained immunity include histone 3 lysine 4 trimethylation (H3K4me3), which is found at the promoter sites of many genes in the glycolysis pathway and inflammatory cytokines (12). The H3K4me3 modification of histones near target gene loci indicates open chromatin for efficient transcription factor binding and initiation of transcription (59). Other histone markers such as H3K27ac are enriched at important metabolic gene loci, such as Hexokinase 1 (HK1) in trained monocytes (52). The intersection of epigenetics and immunity is fundamental to innate immune memory, as regulation of inflammatory genes by epigenetic marks on histones occurs in parallel with genes in the glycolytic pathway to fuel immune responses. Several interconnected metabolic pathways have been implicated as important outcomes of the induction of trained immunity. A deviation in energy usage to bias glycolysis was recognized early on in monocytes stimulated with β-glucan and was revealed to be controlled by activation of HIF-1α and mTOR (52). Further investigation uncovered the role of the cholesterol metabolism pathway through inhibition of mevalonate, an important precursor for the biosynthesis of cholesterol, via fluvastatin. This intervention decreased TNF cytokine production and lactate accumulation (indicating decreased glycolytic capacity) in β-glucan or BCG-trained monocytes (60). The role of mevalonate in trained immunity was demonstrated to be clinically relevant in patients with hyper IgD syndrome or mevalonate kinase deficiency, where in both cases, individuals accumulate high levels of intracellular mevalonate and display excessive inflammation (60).
Trained immunity is implicated in both protective and pathogenic circumstances, yet the principles of trained immunity could extend beyond previously defined models to potentially explain how the microbiota affects homeostatic immunity and chronic inflammation.
SYSTEMS APPROACHES FOR DECIPHERING THE MICROBIOME-EPIGENETIC CELLULAR MEMORY AXIS
Systems biology seeks to identify causal relationships that exist between different biological or environmental factors and that may explain the underlying mechanisms of pathology (61). Biological complexity is difficult to study with a reductionist approach because multiple layers of regulation exist in a semi-hierarchical structure where one layer may provide feedback into the system, such that control is multidirectional. Large-scale data collection is intrinsic to systems biology approaches aimed at interrogating complex systems. Such data are often collected by next-generation sequencing approaches for genomics, transcriptomics, and microbiome sequencing, or by mass spectrometry- and non-mass spectrometry-based proteomics, metabolomics, and lipidomics. Furthermore, large-scale data inherently generate many possible hypotheses, so the systems biology field has produced numerous machine learning approaches to investigate the data sets generated (62–64).
Employing multi-omic data acquisition schemes can infer whether interrelated pathways are regulated congruently or orthogonally (65, 66). Since epigenetic regulation is a central element of cellular memory induction, assessment of global chromatin accessibility through approaches like the assay for transposase accessible chromatin with sequencing (ATAC-seq) or chromatin immunoprecipitation with sequencing (ChIP-seq) is essential. ATAC-seq is particularly important for trained immunity studies as the approach reveals the genomic landscape of nucleosome-free regions of DNA where active transcription can occur at the time of sampling (67). This tool is particularly useful as the panorama of accessible DNA ultimately defines a cell’s functional capacity by which genes can be expressed and is representative of the cellular memory imprinting. In addition, inferring transcription factor binding motifs can reveal gene regulatory networks that govern functional cellular states (68, 69). Other common technologies include variants of ChIP-seq, which identify loci where specific DNA modifications, transcription factors, or histone PTMs reside (70). Extensions of such assays have been optimized for single-cell-level analysis, which allows characterization of heterogeneous cell populations, an essential tool in surveying the immune system (71, 72). Utilization of modern approaches for multi-omic single-cell data measurements can also provide essential information for how epigenetic cellular memory occurs in innate immune cells (13).
Few studies have explored the proteome and use of proteomics by liquid chromatography coupled with mass spectrometry (LC-MS) in studying trained immunity or innate immune tolerance outside of measuring specific cytokines or proteins by ELISA and western blotting. Previous work characterized the secretome, or collection of secreted proteins, by LC-MS proteomics of LPS-tolerized macrophages and found a decrease in, or no detection of, several cytokines and chemokines such as TNF, CCL2, and IL-1RN, versus LPS-responsive macrophages (37). Moreover, a similar study found that LPS-tolerized macrophages had a reduction in maximal respiration capacity in addition to alterations in several mitochondrial complex I and II proteins (73). In addition, one recent study investigated the response of THP-1 monocytes to an infection challenge with BCG. The authors evaluated the changes in both the global proteome and histone PTM proteome, while integrating an external phosphoproteomics data set of BCG-infected macrophages as well (66). The multi-omic proteome analysis revealed a dynamic interplay between histone-modifying proteins in the total and phosphoproteome found to be associated with functional changes in histone modifications, such as a downregulation of the NuA4 histone acetyltransferase complex and concurrent downregulation of histone H4 acetylation patterns. In addition, the external phosphoproteomics data set revealed downregulation of phosphopeptides from histone modifying proteins. Although this is the first investigation of the proteome by LC-MS in trained innate immune memory, novel insights into proteome-level regulation may improve understanding of the pathways involved in trained innate immune memory beyond transcriptional changes. One unexplored aspect of the proteome is protein turnover and long-lived protein persistence. Although epigenetic mechanisms broadly explain innate immune memory phenotypes in vitro and in vivo, long-lived proteins or proteins with long half-life may also contribute to enhanced cytokine production upon restimulation, particularly in certain signaling proteins or transcription factors as opposed to cytokines, which are known for their short half-life (74–76).
Integrating multi-omics data is essential for investigating complex phenomena where multiple levels of regulation exist, as evidenced in references 13, 66, 77. Network analysis encompasses a suite of methods that leverages the principles of graph theory from the computer science field (78). Biological networks aim to model the underlying regulatory interactions (edges), whether directly physical or indirect, between proteins, genes, metabolites, phenotypes, or other data (nodes), and can be particularly powerful tools for inferring associations between different data types or biological measurements (64, 79). Popular forms of biological networks include protein-protein interaction networks, gene regulatory networks, metabolic reaction networks, and co-expression networks (64, 80–84). Co-expression networks constructed from pairwise correlations seek to infer regulatory interactions that occur due to changes in the expression of a particular feature with other features in multiple samples. The weighted correlation network analysis (WGCNA) and transkingdom network analysis (TkNA) approaches can leverage multi-omic integration of different data sets to find non-parametric correlations between features of different data types (64, 84). The TkNA approach has been utilized in numerous studies by us and others to infer causal relationships from large-scale data, which inform experimentally testable hypotheses (85–87). Correlation networks often reveal hubs of co-expressed genes relating to pathways that change together, as was used in one recent study of transcriptome changes in monocyte progenitor responses to LPS stimulation after high-fat diet exposure (88). Interrogation of epigenetic cellular memory, an inherently complex phenotype, requires assessment of phenotypes across different regulatory levels with epigenetic, transcriptomic, and proteomic analyses. Incorporating epigenetic information into network analyses has yet to become routine practice but serves an important role when predicting the functional roles of driver genes involved in regulating trained immunity, as evidenced in one recent study and new approaches for incorporating epigenome data when deriving gene regulatory networks (77, 89). Network analysis strategies and other machine learning approaches provide alternative means to prioritize important genes, histone marks, or proteins associated with epigenetic cellular memory or inflammatory processes while avoiding the limited interpretability of simple differential expression-based analysis (79, 90).
EPIGENETIC CELLULAR MEMORY AS A DRIVER OF CHRONIC INFLAMMATORY DISEASES: ROOM FOR A SYSTEMS APPROACH TO SUCCEED
To interrogate the molecular mechanisms of epigenetic cellular memory, most research focuses on using highly purified or synthetic ligands with controlled cell culture conditions. Experiments such as those using a known molecule—and receptor with which it engages—have revealed cellular pathways controlling inflammatory or immunosuppressive phenotypes of epigenetic cellular memory. Although defined inputs combined with measurable outcomes greatly increase the reproducibility of results, these experiments do not fully explore the complexity of stimuli that influence the immune system under real-world conditions. Conceptually, epigenetic cellular memory in innate immunity is not limited to acute exposure to purified ligands and has been observed in several adjacent contexts, including diet-induced and brain injury-induced trained immunity (88, 91).
Aside from acute and transient exposure to inflammatory stimuli, chronic inflammation mediated by various sources is well documented to promote disease and affect quality of life (11). Outside established models in the innate immune memory paradigm, chronic exposure to complex stimuli exerts similar phenotypic outcomes on the immune system. Models of a Western-style diet have illustrated profound effects on the immune system (10). The western diet contains high levels of saturated fat and excess simple sugars, which have well-documented roles in causing metabolic diseases such as type 2 diabetes, obesity, and non-alcoholic steatohepatitis in a microbiome-dependent manner (10, 86, 92). Several reports to date have explored how a history of exposure to diet-induced metabolic dysfunction leads to persistent immune system activation and innate immune memory (88, 93–95). These results indicate similar modes of epigenome remodeling by chronic and complex stimuli (i.e., diet), which drive immunity and chronic inflammation. One report indicates this phenomenon also occurs in non-immune cells, such as adipocytes (94). Moreover, recent findings have begun to elucidate the role of past infection history with chronic inflammation and brain atrophy, connecting inflammation to the onset of a hallmark of neurodegeneration (96). A clear role for the microbiome has yet to be established in innate immune memory; however, because the microbiota and diet have been independently identified as modifiers of hematopoiesis, it is likely that there exists some contribution to innate immune memory. For chronic inflammation associated with innate immune memory, mechanistically how the microbiota impacts the development or maintenance of chronic inflammation is unknown, but it is likely to occur through modulation of hematopoiesis via mechanisms implicated in existing studies of central trained immunity (13, 97).
The microbiome plays an essential role in the regulation of homeostatic immunity by functioning as a reservoir of structurally diverse molecules that constitutively activate the immune system (15, 23, 98, 99). Translocation of microbial molecules into central immune organs such as the bone marrow facilitates the expansion of, and bias toward, myelopoiesis and away from lymphopoiesis (100, 101). Stem cell populations are sensitive to inflammatory cytokine cues, such as IL-1β, TNFα, and M-CSF, which bias MPP2 and 3 progenitor expansion toward myeloid lineages—termed emergency myelopoiesis—as part of an adaptation to inflammatory demand (31, 102, 103). Yet, germ-free mice do not display enhanced cytokine production after in vivo or ex vivo challenge with LPS, compared to young or aged SPF mice (100). Long-term exposure to the microbiota enhances education of innate immune cells through alterations in progenitor cell populations. Uptake of bacterial DNA from the microbiota induces TLR activation by CX3CR1+ bone marrow mononuclear phagocytes and is associated with increased expansion of multipotent progenitor cells and myelopoiesis bias (104). Moreover, this study isolated bacterial DNA from CX3CR1+ bone marrow mononuclear phagocytes and other immune cells for 16S rRNA sequencing to characterize the microbial taxa that shed the DNA taken up by bone marrow immune cells. CX3CR1 + cells were the predominant scavenger of bacterial DNA, mainly shed by proteobacteria (104). Multiple lines of evidence now suggest that direct translocation of microbiota into distal tissues, including bone marrow, induces a trained immune memory phenotype that is myeloid-cell dependent. Dextran sodium sulfate (DSS)-induced gut permeabilization increased bacterial cell translocation into bone marrow and induced chromatin accessibility changes in granulocyte monocyte progenitor (GMP) cells, as well as increased TNF production upon ex vivo challenge with LPS or Pam3csk4 (105). Culture-independent and culture-dependent methods identified that Enterococcus faecalis translocates into bone marrow tissue and induces trained immunity in a Mincle (Clec4e)-dependent manner (105). Spatial approaches provide a particularly powerful method for uncovering microbe-host interactions. Recent advancements in spatial microbiome profiling using in situ hybridization probes have been coupled with spatial transcriptomics or spatial proteomics technologies to detect host responses dependent on co-localized bacteria (106, 107). Visualizing the direct association of microbiota with host immune cells may provide a new causal mechanism by which the microbiota promotes immune memory phenotypes in distal tissues through distinct signaling mechanisms than translocation of microbiota-derived molecules.
Although there are limited cases where the microbiome has been directly linked to classical trained immunity, it is imperative to delineate how acute stressors (e.g., infection or vaccination) differ from how chronic stressors (e.g., diet, microbiome, exposome) cause persistent changes in innate immune function. Microbiota may drive innate immune memory phenotypes through direct translocation of cells during gut barrier disruption or constitutive escape of PAMPs, which may explain differences in the strength of responses. Furthermore, transient microbiota perturbation via diet or antibiotics is known to alter immunity and may act as a trigger of inflammatory remodeling of the immune system (108, 109). To better understand the role of the microbiome, studies with synthetic ligands are useful but less accurate when compared to the diverse ligand milieu produced by the microbiota. One study sought to evaluate how diverse gut microbiota activate immune responses to different combinations of TLR ligands produced by human-derived strains and found distinct patterns based on taxonomy (7). Such approaches may better mimic the complex repertoire of immunogenic ligands and inform how such molecules maintain homeostatic immune responses. Nonetheless, revealing the role of the microbiome in this mechanism is an open area of investigation, and a systems approach is well-suited for solving this problem.
CONCLUSION
Herein, we reviewed the relationship between the microbiome and epigenetic cellular memory in chronic inflammation and disease and emphasized the utility of multi-omic systems approaches for studying these phenomena. The microbiome is responsible for guiding the development of a healthy immune system; however, low-grade or acute inflammatory events can contribute to dysregulation of the immune system. Recent findings have revealed epigenetic alterations in hematopoietic stem cells after acute inflammatory challenges, and depending on the stimulus type and dose, these alterations are associated with innate immune tolerance or training; two sides of the same coin (30). Innate immune memory creates a functionally immunosuppressive or hyperinflammatory phenotype bias and contributes to immune dysregulation or the onset of chronic disease. Although we are beginning to understand the mechanisms of cellular memory induction, how the microbiota contributes to this process, from global to individual strain scales, is incompletely understood. When extending the innate immune memory field to encompass chronic disease, it is important not to be confined by established archetypes. Much of the foundational and mechanistic studies on innate immune memory were conducted in vitro using simpler experimental models such as the stimulate-rest-challenge model, as described earlier in the review, which mimics the delay after vaccination and before re-encountering a pathogen. This paradigm does not hold for chronic stimuli since rewiring of immune cell epigenomes occurs in the hematopoietic compartment without any defined rest period, making translation of existing mechanisms challenging. To better inform how the microbiota and/or chronic inflammation mediate innate immune memory, or more simply—rewiring of hematopoietic function toward constitutive inflammation—a systems approach is well-suited to identify the microbial and host drivers of immunopathology. Herein, we sought to connect the host-microbiome and innate immune memory field for an open area of mechanistic insight. Due to the complex nature of such studies, systems biology approaches coupled with multi-omic data can unravel inherently complex interactions governing immune homeostasis.
ACKNOWLEDGMENTS
This research was supported by the Intramural Research Program of the National Institutes of Health (NIH).
The contributions of the NIH authors were made as part of their official duties as NIH federal employees, are in compliance with agency policy requirements, and are considered works of the United States Government. However, the findings and conclusions presented in this paper are those of the author(s) and do not necessarily reflect the views of the NIH or the U.S. Department of Health and Human Services.
Biographies

Jacob W. Pederson is a Predoctoral IRTA Fellow at the National Institute of Allergy and Infectious Diseases, NIH, finishing his doctorate within the Graduate Partnership Program with the University of Oregon. Jacob completed his bachelor’s and master’s degrees at the University of Oregon. In his thesis work, he is using a combination of computational network analysis and systems biology principles, aiming to understand how the underlying mechanisms of specific host-microbe interactions drive or protect against disease. This approach has led him to investigate how dietary fatty acids and microbes stimulate certain macrophage populations via the TLR pathway to promote inflammation in adipose tissue and decrease systemic insulin sensitivity, leading to the onset of metabolic disease.

Dr. Aleksandra Nita-Lazar received her Ph.D. in biochemistry in 2003 from the University of Basel for studies performed at the Friedrich Miescher Institute for Biomedical Research, where she analyzed atypical protein glycosylation using mass spectrometry and protein biochemistry methods. After postdoctoral training at Stony Brook University and Massachusetts Institute of Technology (Ludwig Cancer Foundation Fellow), where she continued to investigate post-translational protein modifications and their influence on cell signaling, she joined the Program in Systems Immunology and Infectious Disease Modeling, now the Laboratory of Immune System Biology, DIR, NIAID, NIH, in April 2009, as an independent investigator and chief of the Cellular Networks Proteomics Unit. Dr. Nita-Lazar was granted tenure in December 2018, and she now continues her work as senior investigator and chief of the Functional Cellular Networks Section. Her main research interests are protein state changes and networks regulating the host-pathogen interactions and macrophage activation.
Contributor Information
Aleksandra Nita-Lazar, Email: nitalazarau@niaid.nih.gov.
Renuka R. Nayak, University of California San Francisco, San Francisco, California, USA
REFERENCES
- 1. Guo C-J, Allen BM, Hiam KJ, Dodd D, Van Treuren W, Higginbottom S, Nagashima K, Fischer CR, Sonnenburg JL, Spitzer MH, Fischbach MA. 2019. Depletion of microbiome-derived molecules in the host using Clostridium genetics. Science 366:eaav1282. doi: 10.1126/science.aav1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bae M, Cassilly CD, Liu X, Park S-M, Tusi BK, Chen X, Kwon J, Filipčík P, Bolze AS, Liu Z, Vlamakis H, Graham DB, Buhrlage SJ, Xavier RJ, Clardy J. 2022. Akkermansia muciniphila phospholipid induces homeostatic immune responses. Nature 608:168–173. doi: 10.1038/s41586-022-04985-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nagashima K, Zhao A, Atabakhsh K, Bae M, Blum JE, Weakley A, Jain S, Meng X, Cheng AG, Wang M, Higginbottom S, Dimas A, Murugkar P, Sattely ES, Moon JJ, Balskus EP, Fischbach MA. 2023. Mapping the T cell repertoire to a complex gut bacterial community. Nature 621:162–170. doi: 10.1038/s41586-023-06431-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim S-M, Park S, Hwang S-H, Lee E-Y, Kim J-H, Lee GS, Lee G, Chang D-H, Lee J-G, Hwang J, et al. 2023. Secreted Akkermansia muciniphila threonyl-tRNA synthetase functions to monitor and modulate immune homeostasis. Cell Host Microbe 31:1021–1037. doi: 10.1016/j.chom.2023.05.007 [DOI] [PubMed] [Google Scholar]
- 5. Chen H, Rosen CE, González-Hernández JA, Song D, Potempa J, Ring AM, Palm NW. 2023. Highly multiplexed bioactivity screening reveals human and microbiota metabolome-GPCRome interactions. Cell 186:3095–3110. doi: 10.1016/j.cell.2023.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Manes NP, Nita-Lazar A. 2021. Molecular mechanisms of the toll-like receptor, STING, MAVS, inflammasome, and interferon pathways. mSystems 6:e00336-21. doi: 10.1128/mSystems.00336-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spindler MP, Siu S, Mogno I, Li Z, Yang C, Mehandru S, Britton GJ, Faith JJ. 2022. Human gut microbiota stimulate defined innate immune responses that vary from phylum to strain. Cell Host Microbe 30:1481–1498. doi: 10.1016/j.chom.2022.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. 2018. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol 14:576–590. doi: 10.1038/s41574-018-0059-4 [DOI] [PubMed] [Google Scholar]
- 9. Loh JS, Mak WQ, Tan LKS, Ng CX, Chan HH, Yeow SH, Foo JB, Ong YS, How CW, Khaw KY. 2024. Microbiota-gut-brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct Target Ther 9:37. doi: 10.1038/s41392-024-01743-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sonnenburg JL, Bäckhed F. 2016. Diet-microbiota interactions as moderators of human metabolism. Nature 535:56–64. doi: 10.1038/nature18846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW, Fasano A, Miller GW, Miller AH, Mantovani A, Weyand CM, Barzilai N, Goronzy JJ, Rando TA, Effros RB, Lucia A, Kleinstreuer N, Slavich GM. 2019. Chronic inflammation in the etiology of disease across the life span. Nat Med 25:1822–1832. doi: 10.1038/s41591-019-0675-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fanucchi S, Domínguez-Andrés J, Joosten LAB, Netea MG, Mhlanga MM. 2021. The intersection of epigenetics and metabolism in trained immunity. Immunity 54:32–43. doi: 10.1016/j.immuni.2020.10.011 [DOI] [PubMed] [Google Scholar]
- 13. Sun SJ, Aguirre-Gamboa R, de Bree LCJ, Sanz J, Dumaine A, van der Velden WJFM, Joosten LAB, Khader S, Divangahi M, Netea MG, Barreiro LB. 2024. BCG vaccination alters the epigenetic landscape of progenitor cells in human bone marrow to influence innate immune responses. Immunity 57:2095–2107. doi: 10.1016/j.immuni.2024.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, Kim S, Fritz JV, Wilmes P, Ueha S, Matsushima K, Ohno H, Olle B, Sakaguchi S, Taniguchi T, Morita H, Hattori M, Honda K. 2013. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500:232–236. doi: 10.1038/nature12331 [DOI] [PubMed] [Google Scholar]
- 15. Honda K, Littman DR. 2016. The microbiota in adaptive immune homeostasis and disease. Nature 535:75–84. doi: 10.1038/nature18848 [DOI] [PubMed] [Google Scholar]
- 16. Cullender TC, Chassaing B, Janzon A, Kumar K, Muller CE, Werner JJ, Angenent LT, Bell ME, Hay AG, Peterson DA, Walter J, Vijay-Kumar M, Gewirtz AT, Ley RE. 2013. Innate and adaptive immunity interact to quench microbiome flagellar motility in the gut. Cell Host Microbe 14:571–581. doi: 10.1016/j.chom.2013.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Caesar R, Tremaroli V, Kovatcheva-Datchary P, Cani PD, Bäckhed F. 2015. Crosstalk between gut microbiota and dietary lipids aggravates WAT inflammation through TLR signaling. Cell Metab 22:658–668. doi: 10.1016/j.cmet.2015.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gutsmann T, Müller M, Carroll SF, MacKenzie RC, Wiese A, Seydel U. 2001. Dual role of lipopolysaccharide (LPS)-binding protein in neutralization of LPS and enhancement of LPS-induced activation of mononuclear cells. Infect Immun 69:6942–6950. doi: 10.1128/IAI.69.11.6942-6950.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vargas-Caraveo A, Sayd A, Maus SR, Caso JR, Madrigal JLM, García-Bueno B, Leza JC. 2017. Lipopolysaccharide enters the rat brain by a lipoprotein-mediated transport mechanism in physiological conditions. Sci Rep 7:13113. doi: 10.1038/s41598-017-13302-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. 2010. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med 16:228–231. doi: 10.1038/nm.2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Y, Spatz M, Da Costa G, Michaudel C, Lapiere A, Danne C, Agus A, Michel M-L, Netea MG, Langella P, Sokol H, Richard ML. 2022. Deletion of both Dectin-1 and Dectin-2 affects the bacterial but not fungal gut microbiota and susceptibility to colitis in mice. Microbiome 10:91. doi: 10.1186/s40168-022-01273-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brown DG, Soto R, Yandamuri S, Stone C, Dickey L, Gomes-Neto JC, Pastuzyn ED, Bell R, Petersen C, Buhrke K, Fujinami RS, O’Connell RM, Stephens WZ, Shepherd JD, Lane TE, Round JL. 2019. The microbiota protects from viral-induced neurologic damage through microglia-intrinsic TLR signaling. Elife 8:e47117. doi: 10.7554/eLife.47117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brown RL, Sequeira RP, Clarke TB. 2017. The microbiota protects against respiratory infection via GM-CSF signaling. Nat Commun 8:1512. doi: 10.1038/s41467-017-01803-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vilardi A, Przyborski S, Mobbs C, Rufini A, Tufarelli C. 2024. Current understanding of the interplay between extracellular matrix remodelling and gut permeability in health and disease. Cell Death Discov 10:258. doi: 10.1038/s41420-024-02015-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baruch EN, Youngster I, Ben-Betzalel G, Ortenberg R, Lahat A, Katz L, Adler K, Dick-Necula D, Raskin S, Bloch N, et al. 2021. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 371:602–609. doi: 10.1126/science.abb5920 [DOI] [PubMed] [Google Scholar]
- 26. Davar D, Dzutsev AK, McCulloch JA, Rodrigues RR, Chauvin J-M, Morrison RM, Deblasio RN, Menna C, Ding Q, Pagliano O, et al. 2021. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 371:595–602. doi: 10.1126/science.abf3363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saavedra PHV, Trzeciak AJ, Lipshutz A, Daman AW, O’Neal AJ, Liu Z-L, Wang Z, Romero-Pichardo JE, Rojas WS, Zago G, van den Brink MRM, Josefowicz SZ, Lucas CD, Anderson CJ, Rudensky AY, Perry JSA. 2024. Broad-spectrum antibiotics disrupt homeostatic efferocytosis. Nat Metab 6:1682–1694. doi: 10.1038/s42255-024-01107-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang Y, Bhosle A, Bae S, McIver LJ, Pishchany G, Accorsi EK, Thompson KN, Arze C, Wang Y, Subramanian A, Kearney SM, Pawluk A, Plichta DR, Rahnavard A, Shafquat A, Xavier RJ, Vlamakis H, Garrett WS, Krueger A, Huttenhower C, Franzosa EA. 2022. Discovery of bioactive microbial gene products in inflammatory bowel disease. Nature 606:754–760. doi: 10.1038/s41586-022-04648-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Netea MG, Domínguez-Andrés J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, Joosten LAB, van der Meer JWM, Mhlanga MM, Mulder WJM, Riksen NP, Schlitzer A, Schultze JL, Stabell Benn C, Sun JC, Xavier RJ, Latz E. 2020. Defining trained immunity and its role in health and disease. Nat Rev Immunol 20:375–388. doi: 10.1038/s41577-020-0285-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Divangahi M, Aaby P, Khader SA, Barreiro LB, Bekkering S, Chavakis T, van Crevel R, Curtis N, DiNardo AR, Dominguez-Andres J, et al. 2021. Trained immunity, tolerance, priming and differentiation: distinct immunological processes. Nat Immunol 22:2–6. doi: 10.1038/s41590-020-00845-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Swann JW, Olson OC, Passegué E. 2024. Made to order: emergency myelopoiesis and demand-adapted innate immune cell production. Nat Rev Immunol 24:596–613. doi: 10.1038/s41577-024-00998-7 [DOI] [PubMed] [Google Scholar]
- 32. Swanson KV, Deng M, Ting JPY. 2019. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol 19:477–489. doi: 10.1038/s41577-019-0165-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Seeley JJ, Ghosh S. 2017. Molecular mechanisms of innate memory and tolerance to LPS. J Leukoc Biol 101:107–119. doi: 10.1189/jlb.3MR0316-118RR [DOI] [PubMed] [Google Scholar]
- 34. Ifrim DC, Quintin J, Joosten LAB, Jacobs C, Jansen T, Jacobs L, Gow NAR, Williams DL, van der Meer JWM, Netea MG. 2014. Trained immunity or tolerance: opposing functional programs induced in human monocytes after engagement of various pattern recognition receptors. Clin Vaccine Immunol 21:534–545. doi: 10.1128/CVI.00688-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cao M, Wang G, Xie J. 2023. Immune dysregulation in sepsis: experiences, lessons and perspectives. Cell Death Discov 9:465. doi: 10.1038/s41420-023-01766-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Foster SL, Hargreaves DC, Medzhitov R. 2007. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature 447:972–978. doi: 10.1038/nature05836 [DOI] [PubMed] [Google Scholar]
- 37. Gillen J, Ondee T, Gurusamy D, Issara-Amphorn J, Manes NP, Yoon SH, Leelahavanichkul A, Nita-Lazar A. 2021. LPS tolerance inhibits cellular respiration and induces global changes in the macrophage secretome. Biomolecules 11:164. doi: 10.3390/biom11020164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Askarian F, Wagner T, Johannessen M, Nizet V. 2018. Staphylococcus aureus modulation of innate immune responses through Toll-like (TLR), (NOD)-like (NLR) and C-type lectin (CLR) receptors. FEMS Microbiol Rev 42:656–671. doi: 10.1093/femsre/fuy025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Murphey ED, Fang G, Sherwood ER. 2008. Pretreatment with the Gram-positive bacterial cell wall molecule peptidoglycan improves bacterial clearance and decreases inflammation and mortality in mice challenged with Staphylococcus aureus. Crit Care Med 36:3067–3073. doi: 10.1097/CCM.0b013e31818c6fb7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kleinnijenhuis J, Quintin J, Preijers F, Joosten LAB, Ifrim DC, Saeed S, Jacobs C, van Loenhout J, de Jong D, Stunnenberg HG, Xavier RJ, van der Meer JWM, van Crevel R, Netea MG. 2012. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci USA 109:17537–17542. doi: 10.1073/pnas.1202870109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Andersen P, Doherty TM. 2005. The success and failure of BCG - implications for a novel tuberculosis vaccine. Nat Rev Microbiol 3:656–662. doi: 10.1038/nrmicro1211 [DOI] [PubMed] [Google Scholar]
- 42. Mangtani P, Abubakar I, Ariti C, Beynon R, Pimpin L, Fine PEM, Rodrigues LC, Smith PG, Lipman M, Whiting PF, Sterne JA. 2014. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis 58:470–480. doi: 10.1093/cid/cit790 [DOI] [PubMed] [Google Scholar]
- 43. Arts RJW, Moorlag SJCFM, Novakovic B, Li Y, Wang S-Y, Oosting M, Kumar V, Xavier RJ, Wijmenga C, Joosten LAB, Reusken CBEM, Benn CS, Aaby P, Koopmans MP, Stunnenberg HG, van Crevel R, Netea MG. 2018. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe 23:89–100. doi: 10.1016/j.chom.2017.12.010 [DOI] [PubMed] [Google Scholar]
- 44. Quintin J, Saeed S, Martens JHA, Giamarellos-Bourboulis EJ, Ifrim DC, Logie C, Jacobs L, Jansen T, Kullberg B-J, Wijmenga C, Joosten LAB, Xavier RJ, van der Meer JWM, Stunnenberg HG, Netea MG. 2012. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 12:223–232. doi: 10.1016/j.chom.2012.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lajqi T, Braun M, Kranig SA, Frommhold D, Pöschl J, Hudalla H. 2021. LPS induces opposing memory-like inflammatory responses in mouse bone marrow neutrophils. Int J Mol Sci 22:9803. doi: 10.3390/ijms22189803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mitroulis I, Ruppova K, Wang B, Chen L-S, Grzybek M, Grinenko T, Eugster A, Troullinaki M, Palladini A, Kourtzelis I, et al. 2018. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell 172:147–161. doi: 10.1016/j.cell.2017.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Parker LC, Jones EC, Prince LR, Dower SK, Whyte MKB, Sabroe I. 2005. Endotoxin tolerance induces selective alterations in neutrophil function. J Leukoc Biol 78:1301–1305. doi: 10.1189/jlb.0405236 [DOI] [PubMed] [Google Scholar]
- 48. Wu D, Shi Y, Zhang H, Miao C. 2023. Epigenetic mechanisms of Immune remodeling in sepsis: targeting histone modification. Cell Death Dis 14:112. doi: 10.1038/s41419-023-05656-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Khan N, Downey J, Sanz J, Kaufmann E, Blankenhaus B, Pacis A, Pernet E, Ahmed E, Cardoso S, Nijnik A, Mazer B, Sassetti C, Behr MA, Soares MP, Barreiro LB, Divangahi M. 2020. M. tuberculosis reprograms hematopoietic stem cells to limit myelopoiesis and impair trained immunity. Cell 183:752–770. doi: 10.1016/j.cell.2020.09.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chakraborty S, Singh A, Wang L, Wang X, Sanborn MA, Ye Z, Maienschein-Cline M, Mukhopadhyay A, Ganesh BB, Malik AB, Rehman J. 2023. Trained immunity of alveolar macrophages enhances injury resolution via KLF4-MERTK-mediated efferocytosis. J Exp Med 220:e20221388. doi: 10.1084/jem.20221388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stothers CL, Burelbach KR, Owen AM, Patil NK, McBride MA, Bohannon JK, Luan L, Hernandez A, Patil TK, Williams DL, Sherwood ER. 2021. β-glucan induces distinct and protective innate immune memory in differentiated macrophages. J Immunol 207:2785–2798. doi: 10.4049/jimmunol.2100107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cheng S-C, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JHA, Rao NA, Aghajanirefah A, et al. 2014. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345:1250684. doi: 10.1126/science.1250684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. O’Farrell A, Niu Z, Li J, Van Eyndhoven LC, Sarma K, Raj A. 2025. Innate immune memory is stimulus specific. bioRxiv:2025.01.22.634275. doi: 10.1101/2025.01.22.634275 [DOI]
- 54. Zhang B, Moorlag SJCFM, Dominguez-Andres J, Bulut Ö, Kilic G, Liu Z, van Crevel R, Xu C-J, Joosten LAB, Netea MG, Li Y. 2022. Single-cell RNA sequencing reveals induction of distinct trained-immunity programs in human monocytes. J Clin Invest 132. doi: 10.1172/JCI147719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ochando J, Mulder WJM, Madsen JC, Netea MG, Duivenvoorden R. 2023. Trained immunity - basic concepts and contributions to immunopathology. Nat Rev Nephrol 19:23–37. doi: 10.1038/s41581-022-00633-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lam N, Lee Y, Farber DL. 2024. A guide to adaptive immune memory. Nat Rev Immunol 24:810–829. doi: 10.1038/s41577-024-01040-6 [DOI] [PubMed] [Google Scholar]
- 57. Bannister AJ, Kouzarides T. 2011. Regulation of chromatin by histone modifications. Cell Res 21:381–395. doi: 10.1038/cr.2011.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lukauskas S, Tvardovskiy A, Nguyen NV, Stadler M, Faull P, Ravnsborg T, Özdemir Aygenli B, Dornauer S, Flynn H, Lindeboom RGH, Barth TK, Brockers K, Hauck SM, Vermeulen M, Snijders AP, Müller CL, DiMaggio PA, Jensen ON, Schneider R, Bartke T. 2024. Decoding chromatin states by proteomic profiling of nucleosome readers. Nature 627:671–679. doi: 10.1038/s41586-024-07141-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Beacon TH, Delcuve GP, López C, Nardocci G, Kovalchuk I, van Wijnen AJ, Davie JR. 2021. The dynamic broad epigenetic (H3K4me3, H3K27ac) domain as a mark of essential genes. Clin Epigenetics 13:138. doi: 10.1186/s13148-021-01126-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bekkering S, Arts RJW, Novakovic B, Kourtzelis I, van der Heijden CDCC, Li Y, Popa CD, Ter Horst R, van Tuijl J, Netea-Maier RT, van de Veerdonk FL, Chavakis T, Joosten LAB, van der Meer JWM, Stunnenberg H, Riksen NP, Netea MG. 2018. Metabolic induction of trained immunity through the mevalonate pathway. Cell 172:135–146. doi: 10.1016/j.cell.2017.11.025 [DOI] [PubMed] [Google Scholar]
- 61. Kitano H. 2002. Systems biology: a brief overview. Science 295:1662–1664. doi: 10.1126/science.1069492 [DOI] [PubMed] [Google Scholar]
- 62. Greener JG, Kandathil SM, Moffat L, Jones DT. 2022. A guide to machine learning for biologists. Nat Rev Mol Cell Biol 23:40–55. doi: 10.1038/s41580-021-00407-0 [DOI] [PubMed] [Google Scholar]
- 63. Mallick H, Rahnavard A, McIver LJ, Ma S, Zhang Y, Nguyen LH, Tickle TL, Weingart G, Ren B, Schwager EH, Chatterjee S, Thompson KN, Wilkinson JE, Subramanian A, Lu Y, Waldron L, Paulson JN, Franzosa EA, Bravo HC, Huttenhower C. 2021. Multivariable association discovery in population-scale meta-omics studies. PLoS Comput Biol 17:e1009442. doi: 10.1371/journal.pcbi.1009442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Newman NK, Macovsky MS, Rodrigues RR, Bruce AM, Pederson JW, Padiadpu J, Shan J, Williams J, Patil SS, Dzutsev AK, Shulzhenko N, Trinchieri G, Brown K, Morgun A. 2024. Transkingdom Network Analysis (TkNA): a systems framework for inferring causal factors underlying host-microbiota and other multi-omic interactions. Nat Protoc 19:1750–1778. doi: 10.1038/s41596-024-00960-w [DOI] [PubMed] [Google Scholar]
- 65. Manes NP, Shulzhenko N, Nuccio AG, Azeem S, Morgun A, Nita-Lazar A. 2017. Multi-omics comparative analysis reveals multiple layers of host signaling pathway regulation by the gut microbiota. mSystems 2:e00107-17 doi: 10.1128/mSystems.00107-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schaefer Z, Iradukunda J, Lumngwena E, Basso K, Blackburn J, Parker I. 2024. Multi-level proteomics reveals epigenetic signatures in BCG-mediated macrophage activation. Mol Cell Proteomics 23:100851. doi: 10.1016/j.mcpro.2024.100851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Grandi FC, Modi H, Kampman L, Corces MR. 2022. Chromatin accessibility profiling by ATAC-seq. Nat Protoc 17:1518–1552. doi: 10.1038/s41596-022-00692-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Alsinet C, Primo MN, Lorenzi V, Bello E, Kelava I, Jones CP, Vilarrasa-Blasi R, Sancho-Serra C, Knights AJ, Park J-E, Wyspianska BS, Trynka G, Tough DF, Bassett A, Gaffney DJ, Alvarez-Errico D, Vento-Tormo R. 2022. Robust temporal map of human in vitro myelopoiesis using single-cell genomics. Nat Commun 13:2885. doi: 10.1038/s41467-022-30557-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bravo González-Blas C, De Winter S, Hulselmans G, Hecker N, Matetovici I, Christiaens V, Poovathingal S, Wouters J, Aibar S, Aerts S. 2023. SCENIC+: single-cell multiomic inference of enhancers and gene regulatory networks. Nat Methods 20:1355–1367. doi: 10.1038/s41592-023-01938-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Furey TS. 2012. ChIP-seq and beyond: new and improved methodologies to detect and characterize protein-DNA interactions. Nat Rev Genet 13:840–852. doi: 10.1038/nrg3306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kaya-Okur HS, Wu SJ, Codomo CA, Pledger ES, Bryson TD, Henikoff JG, Ahmad K, Henikoff S. 2019. CUT&Tag for efficient epigenomic profiling of small samples and single cells. Nat Commun 10:1930. doi: 10.1038/s41467-019-09982-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Xu W, Wen Y, Liang Y, Xu Q, Wang X, Jin W, Chen X. 2021. A plate-based single-cell ATAC-seq workflow for fast and robust profiling of chromatin accessibility. Nat Protoc 16:4084–4107. doi: 10.1038/s41596-021-00583-5 [DOI] [PubMed] [Google Scholar]
- 73. Makjaroen J, Phuengmaung P, Saisorn W, Udomkarnjananun S, Pisitkun T, Leelahavanichkul A. 2023. Lipopolysaccharide tolerance enhances murine norovirus reactivation: an impact of macrophages mainly evaluated by proteomic analysis. Int J Mol Sci 24:1829. doi: 10.3390/ijms24031829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sabatier P, Lechner M, Guzmán UH, Beusch CM, Zeng X, Wang L, Izaguirre F, Seth A, Gritsenko O, Rodin S, Grinnemo K-H, Ye Z, Olsen JV. 2025. Global analysis of protein turnover dynamics in single cells. Cell 188:2433–2450. doi: 10.1016/j.cell.2025.03.002 [DOI] [PubMed] [Google Scholar]
- 75. Rolfs Z, Frey BL, Shi X, Kawai Y, Smith LM, Welham NV. 2021. An atlas of protein turnover rates in mouse tissues. Nat Commun 12:6778. doi: 10.1038/s41467-021-26842-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Liu C, Chu D, Kalantar‐Zadeh K, George J, Young HA, Liu G. 2021. Cytokines: from clinical significance to quantification. Adv Sci (Weinh) 8:e2004433. doi: 10.1002/advs.202004433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Moorlag SJCFM, Folkman L, Ter Horst R, Krausgruber T, Barreca D, Schuster LC, Fife V, Matzaraki V, Li W, Reichl S, Mourits VP, Koeken VACM, de Bree LCJ, Dijkstra H, Lemmers H, van Cranenbroek B, van Rijssen E, Koenen HJPM, Joosten I, Xu C-J, Li Y, Joosten LAB, van Crevel R, Netea MG, Bock C. 2024. Multi-omics analysis of innate and adaptive responses to BCG vaccination reveals epigenetic cell states that predict trained immunity. Immunity 57:171–187. doi: 10.1016/j.immuni.2023.12.005 [DOI] [PubMed] [Google Scholar]
- 78. Pavlopoulos GA, Secrier M, Moschopoulos CN, Soldatos TG, Kossida S, Aerts J, Schneider R, Bagos PG. 2011. Using graph theory to analyze biological networks. BioData Min 4:10. doi: 10.1186/1756-0381-4-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Doncheva NT, Assenov Y, Domingues FS, Albrecht M. 2012. Topological analysis and interactive visualization of biological networks and protein structures. Nat Protoc 7:670–685. doi: 10.1038/nprot.2012.004 [DOI] [PubMed] [Google Scholar]
- 80. Huttlin EL, Bruckner RJ, Navarrete-Perea J, Cannon JR, Baltier K, Gebreab F, Gygi MP, Thornock A, Zarraga G, Tam S, et al. 2021. Dual proteome-scale networks reveal cell-specific remodeling of the human interactome. Cell 184:3022–3040. doi: 10.1016/j.cell.2021.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Badia-I-Mompel P, Wessels L, Müller-Dott S, Trimbour R, Ramirez Flores RO, Argelaguet R, Saez-Rodriguez J. 2023. Gene regulatory network inference in the era of single-cell multi-omics. Nat Rev Genet 24:739–754. doi: 10.1038/s41576-023-00618-5 [DOI] [PubMed] [Google Scholar]
- 82. Okuda S, Yamada T, Hamajima M, Itoh M, Katayama T, Bork P, Goto S, Kanehisa M. 2008. KEGG Atlas mapping for global analysis of metabolic pathways. Nucleic Acids Res 36:W423–6. doi: 10.1093/nar/gkn282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Caspi R, Altman T, Dale JM, Dreher K, Fulcher CA, Gilham F, Kaipa P, Karthikeyan AS, Kothari A, Krummenacker M, Latendresse M, Mueller LA, Paley S, Popescu L, Pujar A, Shearer AG, Zhang P, Karp PD. 2010. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res 38:D473–D479. doi: 10.1093/nar/gkp875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Langfelder P, Horvath S. 2008. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9:559. doi: 10.1186/1471-2105-9-559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lam KC, Araya RE, Huang A, Chen Q, Di Modica M, Rodrigues RR, Lopès A, Johnson SB, Schwarz B, Bohrnsen E, Cogdill AP, Bosio CM, Wargo JA, Lee MP, Goldszmid RS. 2021. Microbiota triggers STING-type I IFN-dependent monocyte reprogramming of the tumor microenvironment. Cell 184:5338–5356. doi: 10.1016/j.cell.2021.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Li Z, Gurung M, Rodrigues RR, Padiadpu J, Newman NK, Manes NP, Pederson JW, Greer RL, Vasquez-Perez S, You H, Hioki KA, Moulton Z, Fel A, De Nardo D, Dzutsev AK, Nita-Lazar A, Trinchieri G, Shulzhenko N, Morgun A. 2022. Microbiota and adipocyte mitochondrial damage in type 2 diabetes are linked by Mmp12+ macrophages. J Exp Med 219:e20220017. doi: 10.1084/jem.20220017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Padiadpu J, Garcia-Jaramillo M, Newman NK, Pederson JW, Rodrigues R, Li Z, Singh S, Monnier P, Trinchieri G, Brown K, Dzutsev AK, Shulzhenko N, Jump DB, Morgun A. 2023. Multi-omic network analysis identified betacellulin as a novel target of omega-3 fatty acid attenuation of western diet-induced nonalcoholic steatohepatitis. EMBO Mol Med 15:e18367. doi: 10.15252/emmm.202318367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Christ A, Günther P, Lauterbach MAR, Duewell P, Biswas D, Pelka K, Scholz CJ, Oosting M, Haendler K, Baßler K, et al. 2018. Western diet triggers NLRP3-dependent innate immune reprogramming. Cell 172:162–175. doi: 10.1016/j.cell.2017.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sonawane AR, DeMeo DL, Quackenbush J, Glass K. 2021. Constructing gene regulatory networks using epigenetic data. NPJ Syst Biol Appl 7:45. doi: 10.1038/s41540-021-00208-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dong X, Yambartsev A, Ramsey SA, Thomas LD, Shulzhenko N, Morgun A. 2015. Reverse enGENEering of regulatory networks from big data: a roadmap for biologists. Bioinform Biol Insights 9:61–74. doi: 10.4137/BBI.S12467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Simats A, Zhang S, Messerer D, Chong F, Beşkardeş S, Chivukula AS, Cao J, Besson-Girard S, Montellano FA, Morbach C, et al. 2024. Innate immune memory after brain injury drives inflammatory cardiac dysfunction. Cell 187:4637–4655. doi: 10.1016/j.cell.2024.06.028 [DOI] [PubMed] [Google Scholar]
- 92. Small L, Brandon AE, Turner N, Cooney GJ. 2018. Modeling insulin resistance in rodents by alterations in diet: what have high-fat and high-calorie diets revealed? Am J Physiol Endocrinol Metab 314:E251–E265. doi: 10.1152/ajpendo.00337.2017 [DOI] [PubMed] [Google Scholar]
- 93. Hata M, Andriessen EMMA, Hata M, Diaz-Marin R, Fournier F, Crespo-Garcia S, Blot G, Juneau R, Pilon F, Dejda A, Guber V, Heckel E, Daneault C, Calderon V, Des Rosiers C, Melichar HJ, Langmann T, Joyal J-S, Wilson AM, Sapieha P. 2023. Past history of obesity triggers persistent epigenetic changes in innate immunity and exacerbates neuroinflammation. Science 379:45–62. doi: 10.1126/science.abj8894 [DOI] [PubMed] [Google Scholar]
- 94. Hinte LC, Castellano-Castillo D, Ghosh A, Melrose K, Gasser E, Noé F, Massier L, Dong H, Sun W, Hoffmann A, Wolfrum C, Rydén M, Mejhert N, Blüher M, Meyenn F. 2024. Adipose tissue retains an epigenetic memory of obesity after weight loss. Nature:1–9. doi: 10.1038/s41586-024-08165-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Seufert AL, Hickman JW, Traxler SK, Peterson RM, Waugh TA, Lashley SJ, Shulzhenko N, Napier RJ, Napier BA. 2022. Enriched dietary saturated fatty acids induce trained immunity via ceramide production that enhances severity of endotoxemia and clearance of infection. Elife 11:e76744. doi: 10.7554/eLife.76744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Duggan MR, Peng Z, Sipilä PN, Lindbohm JV, Chen J, Lu Y, Davatzikos C, Erus G, Hohman TJ, Andrews SJ, Candia J, Tanaka T, Joynes CM, Alvarado CX, Nalls MA, Cordon J, Daya GN, An Y, Lewis A, Moghekar A, Palta P, Coresh J, Ferrucci L, Kivimäki M, Walker KA. 2024. Proteomics identifies potential immunological drivers of postinfection brain atrophy and cognitive decline. Nat Aging 4:1263–1278. doi: 10.1038/s43587-024-00682-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zhang D, Gao X, Li H, Borger DK, Wei Q, Yang E, Xu C, Pinho S, Frenette PS. 2022. The microbiota regulates hematopoietic stem cell fate decisions by controlling iron availability in bone marrow. Cell Stem Cell 29:232–247. doi: 10.1016/j.stem.2021.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Brown EM, Clardy J, Xavier RJ. 2023. Gut microbiome lipid metabolism and its impact on host physiology. Cell Host Microbe 31:173–186. doi: 10.1016/j.chom.2023.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. d’Hennezel E, Abubucker S, Murphy LO, Cullen TW. 2017. Total lipopolysaccharide from the human gut microbiome silences toll-Like receptor signaling. mSystems 2:e00046-17. doi: 10.1128/mSystems.00046-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kovtonyuk LV, Caiado F, Garcia-Martin S, Manz E-M, Helbling P, Takizawa H, Boettcher S, Al-Shahrour F, Nombela-Arrieta C, Slack E, Manz MG. 2022. IL-1 mediates microbiome-induced inflammaging of hematopoietic stem cells in mice. Blood 139:44–58. doi: 10.1182/blood.2021011570 [DOI] [PubMed] [Google Scholar]
- 101. Takizawa H, Regoes RR, Boddupalli CS, Bonhoeffer S, Manz MG. 2011. Dynamic variation in cycling of hematopoietic stem cells in steady state and inflammation. J Exp Med 208:273–284. doi: 10.1084/jem.20101643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Etzrodt M, Ahmed N, Hoppe PS, Loeffler D, Skylaki S, Hilsenbeck O, Kokkaliaris KD, Kaltenbach H-M, Stelling J, Nerlov C, Schroeder T. 2019. Inflammatory signals directly instruct PU.1 in HSCs via TNF. Blood 133:816–819. doi: 10.1182/blood-2018-02-832998 [DOI] [PubMed] [Google Scholar]
- 103. Pietras EM, Reynaud D, Kang Y-A, Carlin D, Calero-Nieto FJ, Leavitt AD, Stuart JM, Göttgens B, Passegué E. 2015. Functionally distinct subsets of lineage-biased multipotent progenitors control blood production in normal and regenerative conditions. Cell Stem Cell 17:35–46. doi: 10.1016/j.stem.2015.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Lee S, Kim H, You G, Kim Y-M, Lee S, Le V-H, Kwon O, Im S-H, Kim Y-M, Kim KS, Sung YC, Kim KH, Surh CD, Park Y, Lee S-W. 2019. Bone marrow CX3CR1+ mononuclear cells relay a systemic microbiota signal to control hematopoietic progenitors in mice. Blood 134:1312–1322. doi: 10.1182/blood.2019000495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Robles-Vera I, Jarit-Cabanillas A, Brandi P, Martínez-López M, Martínez-Cano S, Rodrigo-Tapias M, Femenía-Muiña M, Redondo-Urzainqui A, Nuñez V, González-Correa C, et al. 2025. Microbiota translocation following intestinal barrier disruption promotes mincle-mediated training of myeloid progenitors in the bone marrow. Immunity. doi: 10.1016/j.immuni.2024.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Zhu B, Bai Y, Yeo YY, Lu X, Rovira-Clavé X, Chen H, Yeung J, Nkosi D, Glickman J, Delgado-Gonzalez A, Gerber GK, Angelo M, Shalek AK, Nolan GP, Jiang S. 2025. A multi-omics spatial framework for host-microbiome dissection within the intestinal tissue microenvironment. Nat Commun 16:1230. doi: 10.1038/s41467-025-56237-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lötstedt B, Stražar M, Xavier R, Regev A, Vickovic S. 2024. Spatial host-microbiome sequencing reveals niches in the mouse gut. Nat Biotechnol 42:1394–1403. doi: 10.1038/s41587-023-01988-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Link VM, Subramanian P, Cheung F, Han KL, Stacy A, Chi L, Sellers BA, Koroleva G, Courville AB, Mistry S, Burns A, Apps R, Hall KD, Belkaid Y. 2024. Differential peripheral immune signatures elicited by vegan versus ketogenic diets in humans. Nat Med 30:560–572. doi: 10.1038/s41591-023-02761-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Becattini S, Taur Y, Pamer EG. 2016. Antibiotic-induced changes in the intestinal microbiota and disease. Trends Mol Med 22:458–478. doi: 10.1016/j.molmed.2016.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]