Abstract
The erythroleukemia-inducing Friend spleen focus-forming virus (SFFV) encodes a unique envelope glycoprotein which allows erythroid cells to proliferate and differentiate in the absence of erythropoietin (Epo). In an attempt to understand how the virus causes Epo independence, we have been studying signal transduction pathways activated by Epo to determine if SFFV exerts its biological effects by constitutively activating any of these pathways in the absence of Epo. We previously demonstrated that Stat proteins, the downstream components of the Epo-induced Jak-Stat pathway, are constitutively activated in SFFV-infected cells. In this study, we demonstrate that SFFV also activates Raf-1, MEK and mitogen-activated protein (MAP) kinase, the downstream components of the Raf-1/MAP kinase pathway. This pathway was activated in cells infected with the polycythemia-inducing strain of SFFV, which induces both proliferation and differentiation of erythroid cells in the absence of Epo, as well as in cells infected with the anemia-inducing strain of the virus, which still require Epo for differentiation. Inhibition of Raf-1 by using antisense oligonucleotides led to a partial inhibition of the Epo-independent proliferation of SFFV-infected cells. Expression of the transcription factors c-Jun and JunB, but not c-Fos, was induced in SFFV-infected cells in the absence of Epo, suggesting that constitutive activation of the Raf-1/MAP kinase pathway by the virus may result in deregulation of AP-1 activity. We conclude from our studies that infection of erythroid cells with SFFV leads to the constitutive activation of signal transduction molecules in both the Jak-Stat and Raf-1/MAP kinase pathways and that both of these pathways must be activated to achieve maximum proliferation and differentiation of erythroid cells in the absence of Epo.
Friend spleen focus-forming virus (SFFV) is a replication-defective retrovirus that induces a rapidly occurring erythroleukemia in susceptible strains of adult mice (for a review, see reference 52). Proliferation and differentiation of normal erythroid cells require stimulation with erythropoietin (Epo); however, infection with SFFV induces erythroid cell growth in the absence of Epo (37, 38, 51, 53, 54, 64). Although all strains of the virus cause erythroleukemia, variants of the virus differ in their effects on erythroid cell growth. Infection of mice with the polycythemia-inducing strain of SFFV (SFFVP) induces Epo-independent proliferation and differentiation of erythroid cells (22, 26, 35). In contrast, erythroid cells from mice infected with the anemia-inducing strain of SFFV (SFFVA) proliferate in the absence of Epo but still require Epo for differentiation (22, 31, 61, 65).
Previous studies have shown that the unique glycoprotein encoded by the SFFV envelope gene is responsible for the effects of the virus on erythroid cell growth (9, 33, 68, 69). Differences in the biological effects of SFFVP and SFFVA have been attributed to small sequence differences in the transmembrane region of the envelope glycoproteins of these two viruses (50, 68). Further studies indicated that the SFFV envelope protein associates with the Epo receptor complex at the cell surface and that only cells expressing an Epo receptor capable of transducing a mitogenic signal are rendered factor independent by the virus (3, 12, 25, 29, 70). These results suggest that SFFV may affect erythroid cell growth by activating signal transduction pathways that are normally activated by interaction of Epo with its cell surface receptor.
Studies have shown that binding of Epo to the Epo receptor activates two distinct signal transduction pathways: the Jak-Stat pathway (13, 27, 39) and the Raf-1/mitogen-activated protein kinase (MAPK) pathway (4, 8, 11, 23, 40). Epo activation of the Jak-Stat pathway induces phosphorylation of Jak kinases (27) and activates the DNA-binding activity of Stat family proteins (13, 57). Using the Epo-dependent HCD-57 erythroleukemia cell line, we previously demonstrated that infection with SFFVP abrogates Epo dependence in HCD-57 cells (53) and induces constitutive Stat DNA-binding activity in the absence of Epo (46). In this study, we sought to determine if infection with SFFV also induces constitutive activation of the Raf-1/MAPK signaling pathway.
The Raf-1/MAPK pathway is a growth factor-activated signal transduction pathway that also transduces signals from the cell surface to the nucleus (49). Ligand stimulation of growth factor receptors, including the Epo receptor, induces phosphorylation and activation of the Raf-1 serine/threonine kinase by activating Ras GTP-binding activity (17, 34, 58). Ras-GTP then recruits Raf-1 to the cell membrane, where it is phosphorylated and activated by other kinases (62). Activated Raf-1 phosphorylates and activates MAPK kinase (MEK) (16, 32), which phosphorylates and activates MAPK (10). The targets of activated MAPK include transcription factors that regulate the expression or activity of immediate-early response genes including c-myc (21) and components of the AP-1 transcription factor complex, c-fos (20, 63), c-jun (7, 48), and junB (24).
To determine if SFFV activates the Raf-1/MAPK pathway, we evaluated the effect of SFFV on Raf-1, MEK, and MAPK activity in HCD-57 cells infected with SFFVP or SFFVA. In addition, we analyzed the virus-infected cells for expression of immediate-early response genes whose transcription is induced by Epo or whose activity is regulated by MAPK-mediated posttranslational control. Our results indicate that infection of erythroid cells with SFFV induces constitutive activation of the downstream components of the Raf-1/MAPK pathway and deregulates expression of the Jun component of the AP-1 transcription factor complex. In addition, using c-raf antisense oligonucleotides to inhibit Raf-1 expression, we have also determined that Epo-independent activation of the Raf-1/MAPK pathway alone is not sufficient to induce growth factor-independent proliferation of SFFV-infected erythroid cells.
MATERIALS AND METHODS
Cell lines.
HCD-57 cells are Epo-dependent erythroleukemia cells derived from an NIH Swiss mouse infected with Friend murine leukemia virus at birth (53, 60). HCD-57 cells were maintained in Iscove’s modified Dulbecco minimal essential medium (DMEM) supplemented with 30% fetal calf serum (FCS), 5 × 10−5 M 2-mercaptoethanol, penicillin (100 U/ml), streptomycin (100 μg/ml), l-glutamine, (3 mg/ml) and Epo (0.3 U/ml) in 5% CO2 at 37°C. HCD-57 cells in which Epo dependence had been abrogated by infection with SFFVP or SFFVA (53) were maintained in the same medium without Epo. The Epo used was a tissue culture supernatant from fibroblasts that had been transfected with the human Epo gene as previously described (53).
Cell lysates and immunoprecipitation.
To prepare cell lysates, cells grown to a density of 106 cells/ml in DMEM were washed twice in medium without serum and incubated in medium plus 1.5% serum at 37°C in 5% CO2 overnight (12 to 15 h). The next day, cells were centrifuged at 1,200 × g for 5 min and resuspended in fresh medium plus 1.5% serum and starved for an additional 2 h at 37°C in 5% CO2. Epo was added to the Epo-stimulated cells at a concentration of 0.3 U/ml for 15 min. Cells were then centrifuged at 1,200 × g for 5 min, and the pellet was washed twice with ice-cold phosphate-buffered saline containing 1.0 mM Na3VO4. Cells were resuspended in 1 ml of lysis buffer (20 mM Tris [pH 7.4], 150 mM NaCl, 10% glycerol, 1% Triton X-100, 2 mM EDTA, 2 mM NaPPi, 2 mM NaF, 0.5 mM phenylmethylsulfonyl fluoride, 1 μg each of aprotinin and leupeptin per ml, 1 mM Na3VO4). Insoluble material was removed by centrifugation at 12,000 × g at 4°C for 20 min, and protein concentrations were determined by using a Bio-Rad Laboratories (Hercules, Calif.) protein assay kit. For immunoprecipitation, 1 mg of protein was incubated for 2 h at 4°C with a Raf-1-specific monoclonal antibody (Transduction Laboratories, Lexington, Ky.), anti-MEK purified polyclonal antibody (C-18; Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.), or purified Erk-2 polyclonal antibody (C-14; Santa Cruz Biotechnology). The antigen-antibody complexes were collected with protein A-agarose beads (Gibco-BRL, Gaithersburg, Md.).
Western blot analysis and Raf-1 coupled-kinase assay.
The phosphorylation-induced shift in the molecular weight of Raf-1 was assayed by Western blot analysis. Specifically, immunoprecipitated Raf-1 conjugated to protein A-agarose beads was washed three times with TBST (50 mM Tris-HCl [pH 7.3], 150 mM NaCl, 0.05% Tween 20), resuspended in protein loading buffer, and boiled for 5 min at 95°C. Proteins were resolved by electrophoresis on a sodium dodecyl sulfate (SDS)–7.5% polyacrylamide gel and blotted onto nitrocellulose. Blots were blocked with 5% nonfat milk in TBST plus 2% Tween 20 for 30 min and incubated with anti-Raf antibody at room temperature for 1 h. Blots were then washed three times with TBST plus 2% Tween 20 and incubated with an anti-mouse immunoglobulin G antibody conjugated to horseradish peroxidase (Amersham Corp., Arlington Heights, Ill.) for 30 min at room temperature. Blots were washed three times with TBST plus 2% Tween 20, and protein bands were detected by enhanced chemiluminescence (Amersham).
The coupled-kinase assay was performed as described by Alessi et al. (1), with minor modifications. Briefly, Raf-1 immunoprecipitates were washed as previously described (1) and incubated for 30 min at 30°C in kinase reaction buffer (50 mM Tris-HCl [pH 7.4], 0.03 Brij 35, 0.1 mM EGTA, 0.1% 2-mercaptoethanol, 0.66 μM okadaic acid, 0.27 mM Na3VO4, 13.3 mM magnesium acetate, 0.33 mM ATP) containing 0.4 μg of glutathione S-transferase (GST)–MEK (Upstate Biotechnology, Inc. [UBI], Lake Placid, N.Y.) and 1.5 μg of GST-MAPK (UBI). Two microliters of this reaction mixture was added to a second kinase reaction buffer (25 mM Tris-HCl, pH 7.0, 0.1 mM EGTA, 10 mM magnesium acetate) containing 10 μCi of [γ-32P]ATP and 3.3 mg of myelin basic protein (MBP; Gibco-BRL) and incubated for 10 min at 30°C. Two control reactions were carried out in which either GST-MEK or the Raf-1 immunoprecipitates were replaced with kinase buffer. The reaction was stopped by pipetting 40 μl of the reaction onto phosphocellulose filters. The filters were washed twice with 0.8% phosphoric acid and twice with H2O. The incorporation of 32P into MBP was measured by scintillation counting. To determine the amount of Raf-1 in the reaction, a 20-μl aliquot of the immunoprecipitated protein was taken after the wash steps, resuspended in protein loading buffer, resolved on a 7.5% gel, and detected by Western analysis using the anti-Raf-1 antibody as described above.
Immune complex kinase assays.
Immunoprecipitated proteins conjugated to protein A-agarose beads were washed twice in lysis buffer and twice in kinase buffer (20 mM Tris-HCl [pH 7.4], 20 mM NaCl, 10 mM Mg2Cl, 1 mM dithiothreitol). For Raf-1 kinase assays, immunoprecipitated Raf-1 was resuspended in 35 μl of kinase buffer containing 10 μM ATP, 20 μCi of [Y-32P]ATP, and 2 μg of GST–kinase-inactive (K97A) MEK (MEK−) (UBI). MEK and MAPK activities were assayed in 35 μl of kinase buffer containing 10 μM ATP, 20 μCi of [Y-32P]ATP, 1 μg of leupeptin per ml, and 1 μM okadaic acid. As substrates, 5 μg of GST–kinase-inactive (K71A) MAPK (MAPK−) (UBI) was added to the MEK kinase reaction mixture and 4 μg of MBP was added to the MAPK kinase assay. Reaction mixtures were incubated for 30 min at 30°C. The reactions were stopped with protein loading buffer, and reaction mixtures were boiled for 5 min at 95°C. Proteins were resolved on SDS-polyacrylamide gels and transferred to nitrocellulose. Phosphorylated substrate bands were visualized by autoradiography. Proteins were analyzed by Western blot analysis as described above.
Western blot analysis of MAPK.
For Western blot analysis of MAPK, cells starved and stimulated with Epo as described above were resuspended in 100 μl of SDS sample buffer (62.5 mM Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 50 mM dithiothreitol, 0.1% bromophenol blue) and sonicated for 10 to 15 s to reduce sample viscosity. Samples were then heated at 95°C for 5 min and cooled on ice. Proteins were resolved on a 4 to 20% gel by SDS-polyacrylamide gel electrophoresis and transferred electrophoretically to nitrocellulose membranes. The blots were blocked with 5% nonfat milk in TBST buffer at room temperature for 2 h and then incubated overnight at 4°C with a phosphospecific p42/44 MAPK antibody (New England Biolabs, Inc., Beverly, Mass.). The blot was washed three times with TBST and incubated with anti-mouse immunoglobulin G conjugated to horseradish peroxidase for 1 h at room temperature. The blot was washed three times with TBST, and protein bands were detected by using a Phototope Western detection kit (New England Biolabs). The blot was stripped and reprobed with an antibody that recognizes both phosphorylated and unphosphorylated p42/44 MAPK (New England Biolabs).
Northern analysis.
For Northern analysis of RNA, total RNA was extracted from cells by using RNA STAT-60 (Tel-Test “B,” Friendswood, Tex.) according to the manufacturer’s protocol. Twenty micrograms of RNA was subjected to formalin-agarose gel electrophoresis, transferred to a nitrocellulose filter, and hybridized with probes specific for c-myc (1.5-kb fragment containing exons 2 and 3, from L. Wolff, National Cancer Institute, Bethesda, Md.), c-fos (1.7-kb fragment cloned into pUC18, from D. Blair, National Cancer Institute, Frederick, Md.), c-jun (mouse oligonucleotide ON254; Oncogene Research Products, Cambridge, Mass.), or junB (American Type Culture Collection, Rockville, Md.). Hybridization was carried out at 68°C for 1 h in a hybridization oven, using Stratagene’s Quik Hybe solution. Filters were washed two times at 68°C for 10 min in 2× SSC (1× SSC is 0.15 M NaCl plus 0.15 M sodium citrate) plus 0.1% SDS. The filters were then stripped and reprobed with a β-actin probe (mouse oligonucleotide ON365; Oncogene Research Products) under the same conditions with two additional washes.
Antisense assay.
Cells were treated with a phosphorothioate antisense (5′-TCCCTGTATGTGCTCCAT-3′) or sense (5′-ATGGAGCACATACAGGGA-3′) oligonucleotide synthesized by the phosphoramadite method on an automated synthesizer (Applied Biosystems, Foster City, Calif.), and the effect of each oligonucleotide on cell proliferation was analyzed by using [3H]thymidine incorporation assays as previously described (42, 43). Briefly, SFFV-infected and uninfected HCD-57 cells were washed twice, and 5 × 103 cells/well were seeded in 96-well plates in 100 μl of DMEM plus 10% FCS and incubated for 8 h in the presence of 2.0 μM c-raf antisense or sense oligonucleotide at 37°C in 5% CO2. Cells growing in medium alone or in medium plus Epo were used as controls. After 8 h, a second 2.0 μM aliquot of oligonucleotides was added, and cells were either stimulated with Epo (0.3 U/ml) or grown in the absence of Epo for 42 h at 37°C in 5% CO2. Cells were then pulsed with 1 μCi of [3H]thymidine for 6 h. Cells were harvested onto glass filters, and [3H]thymidine incorporation was measured by scintillation counting.
RESULTS
Infection with SFFV constitutively activates Raf-1 in HCD-57 cells.
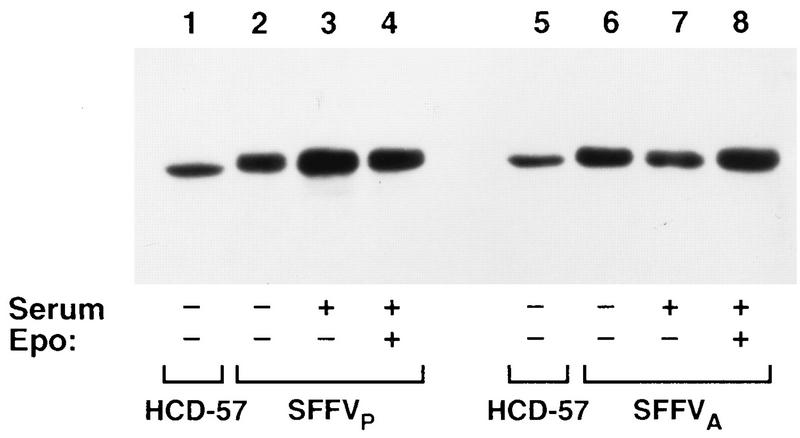
It has been previously demonstrated that Raf-1 is activated by phosphorylation in response to Epo stimulation of HCD-57 cells (8). Phosphorylation of Raf-1 induces a change in its molecular weight which can be detected by a shift in the electrophoretic mobility of the protein (41). To determine if infection with SFFV activates the Raf-1/MAPK pathway, we initially evaluated the effect of the virus on the electrophoretic mobility of Raf-1 purified from HCD-57 cells infected with either SFFVP or SFFVA (53). Uninfected HCD-57 cells and HCD-57 cells infected with either SFFVP or SFFVA were starved for growth factor overnight and then stimulated in complete medium with or without Epo for 15 min. As shown in Fig. 1, the electrophoretic mobility of Raf-1 was shifted in SFFVP- and SFFVA-infected cells after starvation (lanes 2 and 6) or incubation in complete medium without Epo (lanes 3 and 7). Stimulation with Epo did not induce any additional changes in the mobility of Raf-1 (lanes 4 and 8). In comparison, only the unshifted lower-molecular-weight form of Raf-1 was detected in unstimulated HCD-57 cells which did not express the virus (lanes 1 and 5).
FIG. 1.
Infection with SFFV alters the electrophoretic mobility of Raf-1 in HCD-57 cells. Raf-1 was immunoprecipitated from uninfected (lanes 1 and 5), SFFVP-infected (lanes 2 and 4), or SFFVA-infected (lanes 6 and 8) HCD-57 cells after starvation in 1.5% FCS overnight or after incubation of the starved cells for 15 min in complete medium with (lanes 3 and 7) or without (lanes 4 and 8) Epo. The immunoprecipitated proteins were resolved by electrophoresis on an SDS–7.5% polyacrylamide gel and detected by Western blotting using an anti-Raf-1 monoclonal antibody.
To determine if the SFFV-induced shift in Raf-1 mobility was associated with an increase in Raf-1 kinase activity, Raf-1 activation was first evaluated in immune complex kinase assays using GST-MEK− as a substrate. In contrast to Epo-induced activation of Raf-1 in uninfected HCD-57 cells, Raf-1 was constitutively activated in HCD-57 cells infected with either SFFVP or SFFVA (Fig. 2A). The addition of Epo did not result in a reproducible increase in Raf-1 kinase activity in the SFFV-infected cells (Fig. 2A).
FIG. 2.
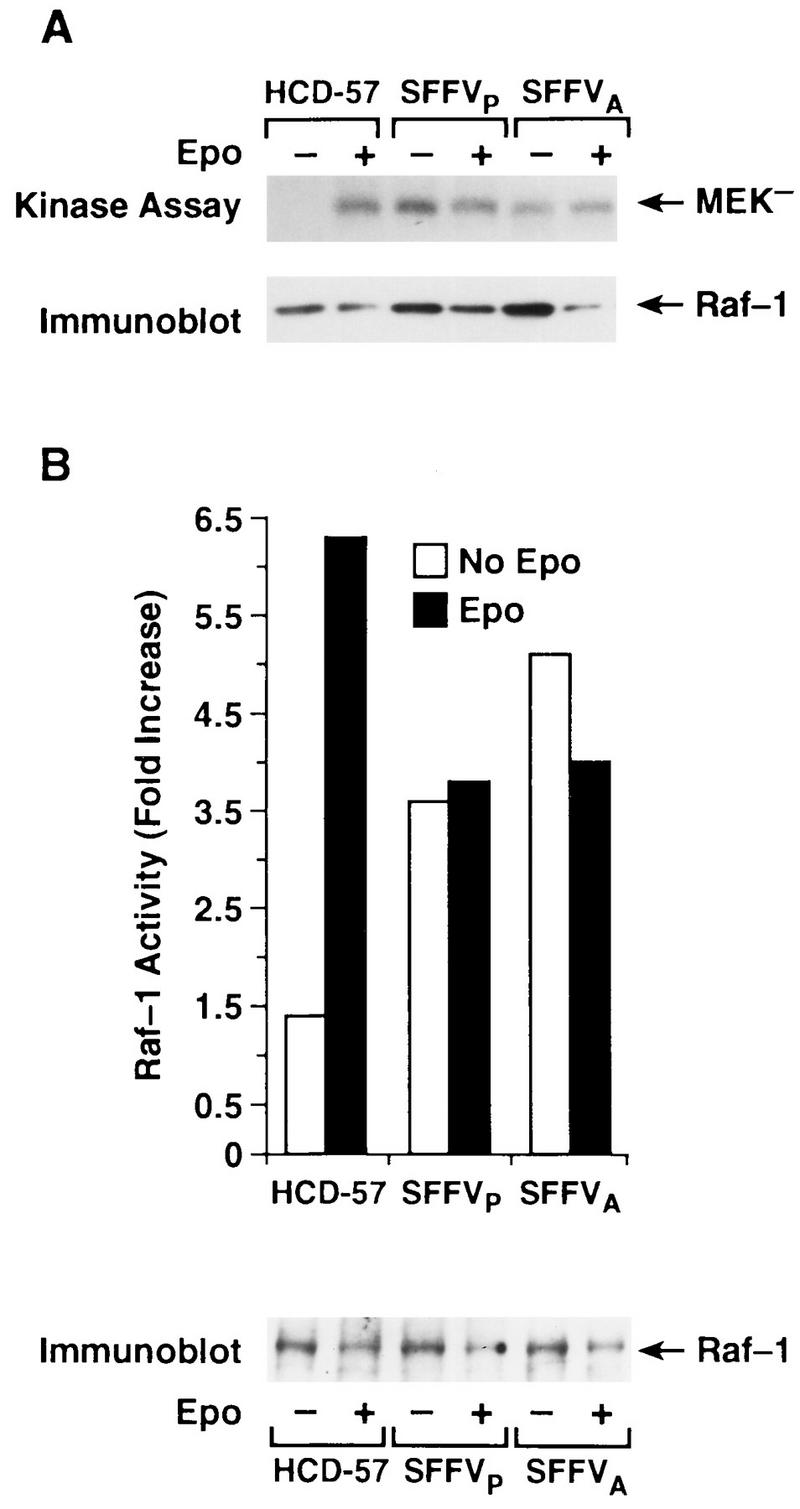
Infection with SFFV leads to constitutive activation of Raf-1 in HCD-57 cells. Raf-1 immunoprecipitates from uninfected and SFFV-infected HCD-57 cells that had been starved overnight in 1.5% FCS and either left unstimulated (−) or stimulated with Epo for 15 min (+) were assayed for Raf-1 kinase activity. (A) Immune complex kinase assay measuring incorporation of 32P into GST-MEK−. Phosphorylation of MEK− was detected by autoradiography. The filter was then probed with an anti-Raf-1 antibody to visualize the total amount of Raf-1 present in the kinase reaction. (B) Coupled-kinase assay measuring Raf-1 activation of MAPK, using exogenous GST-MEK and GST-MAPK as substrates. Activation of GST-MAPK by Raf-1 was assayed by phosphorylation of MBP. Specific incorporation of 32P into MBP was measured by scintillation counting. Results are reported after substraction of values obtained from control reactions in which either the MEK substrate or Raf-1 was not included in the reaction mixture. The amount of Raf-1 in the assay was visualized by Western blot analysis of the immunoprecipitated protein with anti-Raf-1 antibody.
Growth factor-induced activation of Raf-1 initiates a kinase cascade in which Raf-1 phosphorylates and activates MEK (16, 32), which in turn phosphorylates and activates MAPK (10). To determine if the SFFV-induced phosphorylation of MEK observed in the immune complex kinase assays (Fig. 2A) was coupled to activation of downstream subtrates in the kinase cascade, Raf-1 activity was further evaluated in a linked kinase cascade assay measuring phosphorylation of MBP in the presence of GST-MEK and GST-MAPK (Fig. 2B). Consistent with results from the immune complex kinase assays (Fig. 2A), immunoprecipitates of Raf-1 from both unstimulated and Epo-stimulated SFFV-infected cells induced phosphorylation of MBP in the coupled kinase assay, while Raf-1-induced phosphorylation of MBP was observed only in uninfected HCD-57 cells following stimulation with Epo (Fig. 2B). These results indicate that unlike uninfected HCD-57 cells, Raf-1 is constitutively activated in HCD-57 cells infected with SFFV. There was no detectable difference between the effects of SFFVP and SFFVA on Raf-1 activation.
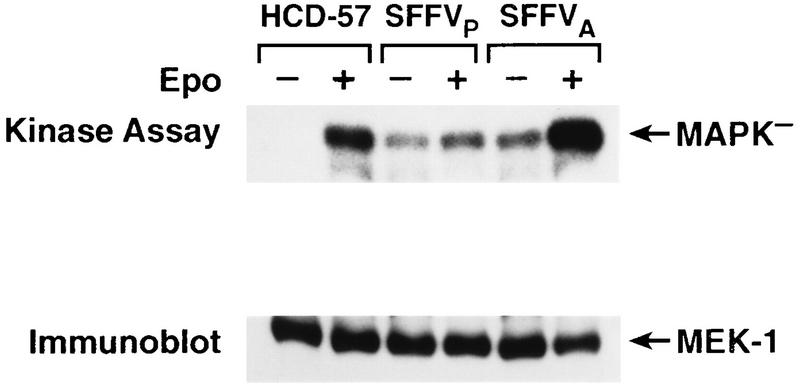
MEK is constitutively activated in SFFV-infected cells.
Although the coupled kinase assay demonstrates that SFFV-induced activation of Raf-1 is sufficient for activation of the downstream substrates in the Raf-1/MAPK pathway in vitro, it does not prove that Raf-1 constitutively activates the downstream substrates in this pathway in SFFV-infected cells. Therefore, we evaluated MEK kinase activity in the virus-infected cell lines in immune complex kinase assays using MAPK− as a substrate (Fig. 3). Consistent with SFFV-induced activation of Raf-1, MEK was constitutively activated in SFFVP- and SFFVA-infected HCD-57 cells, while uninfected HCD-57 cells required Epo to activate MEK (Fig. 3). Stimulation of SFFVA-infected cells with Epo consistently induced a two- to fivefold increase in MEK kinase activity above constitutive levels of activation (Fig. 3), while MEK kinase activity in SFFVP-infected cells was not significantly enhanced by the addition of Epo (Fig. 3).
FIG. 3.
MEK is constitutively activated in SFFV-infected cells. MEK was immunoprecipitated from uninfected and SFFV-infected HCD-57 cells that had been starved overnight in 1.5% FCS and either left unstimulated (−) or stimulated with Epo for 15 min (+). Immunoprecipitates were assayed for MEK kinase activity by immune complex kinase assays measuring incorporation of 32P into GST-MAPK−. Phosphorylation of MAPK− was detected by autoradiography, and the filter was then probed with anti-MEK antibody to visualize the amount of MEK present in the kinase reaction.
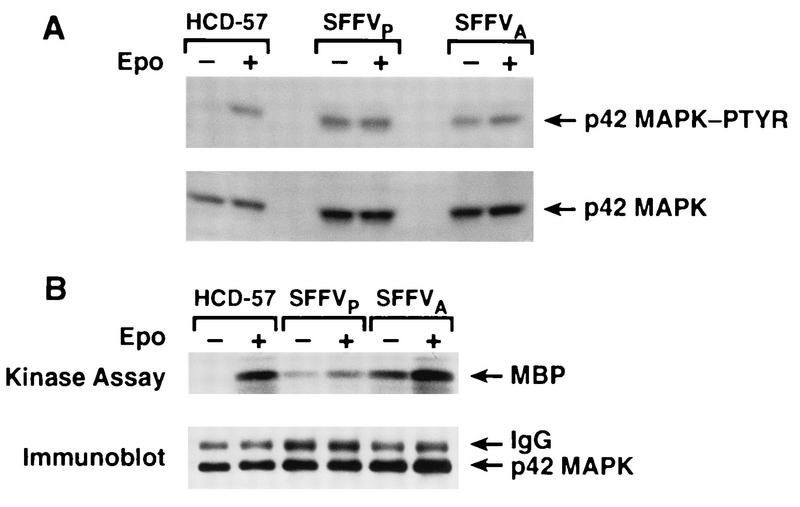
MAPK is constitutively phosphorylated and activated in SFFV-infected cells.
Recent studies have demonstrated that activated Raf-1 induces activation of MAPK through MEK-mediated phosphorylation of both tyrosine and serine/threonine residues (5, 10). Therefore, we evaluated the effect of SFFV infection on phosphorylation and activation of MAPK by using antiphosphotyrosine blotting (Fig. 4A) and immune complex kinase assays (Fig. 4B). A Western blot was probed with an anti-MAPK antibody which specifically recognizes the tyrosine-phosphorylated form of the 42/44-kDa species of MAPK (Fig. 4A). The anti-MAPK-phosphotyrosine antibody detected a tyrosine-phosphorylated 42/44-kDa protein in both unstimulated and Epo-stimulated HCD-57 cells infected with either SFFVP or SFFVA. However, MAPK was detected by this antibody in uninfected HDC-57 cells only when the cells were stimulated with Epo (Fig. 4A). To determine if MAPK was expressed in unstimulated HCD-57 cells, the blot was reprobed with an anti-MAPK antibody which recognizes both phosphorylated and unphosphorylated forms of the protein, and MAPK was readily detected in all of the cell lines (Fig. 4A). Phosphotyrosine-induced activation of MAPK was further evaluated in an immune complex kinase assay using MBP as a substrate (Fig. 4B). MAPK was activated in response to Epo stimulation of uninfected HCD-57 cells; however, MAPK was constitutively activated in the SFFV-infected cell lines (Fig. 4B). Consistent with the enhanced activity of MEK observed when SFFVA-infected cells were stimulated with Epo, a one- to twofold increase in MAPK activity was detected in Epo-stimulated cells infected with SFFVA.
FIG. 4.
MAPK is constitutively phosphorylated and activated in SFFV-infected cells. (A) Uninfected and SFFV-infected HCD-57 cells starved overnight in 1.5% FCS and either left stimulated (−) or stimulated with Epo for 15 min (+) were resuspended in SDS lysis buffer and analyzed by Western blot analysis using a phosphospecific anti-MAPK (p42/44) antibody to detect tyrosine-phosphorylated MAPK (MAPK-PTYR). The blot was then stripped and reprobed with a p42/44-specific anti-MAPK antibody that detects both the phosphorylated and unphosphorylated forms of MAPK. (B) Immunoprecipitated MAPK was assayed in immune complex kinase assays measuring incorporation of 32P into MBP. Phosphorylation of MBP was detected by autoradiography, and the filter was then probed with anti-MAPK antibody to visualize the amount of MAPK present in the kinase reaction. IgG, immunoglobulin G.
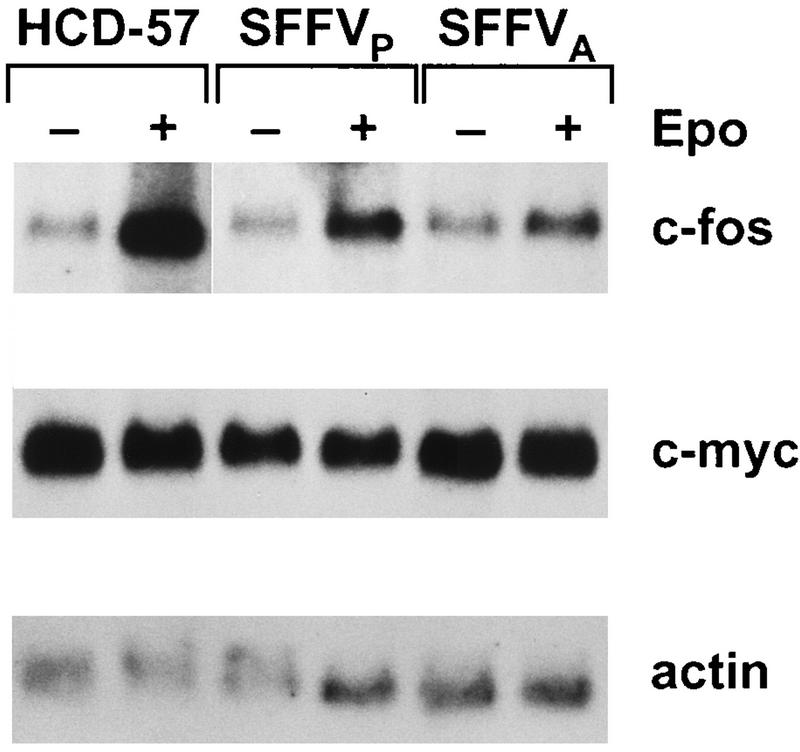
Expression of c-Jun and JunB but not c-Fos and c-Myc is altered by SFFV infection.
Epo-induced early-response genes encoding the transcription factors c-Myc (6, 40, 59) and c-Fos (40, 47, 66) have been identified as targets for receptor tyrosine kinase-mediated regulation by MAPK (21, 28, 56). Therefore, we evaluated the expression of c-myc and c-fos in HCD-57 cells infected with SFFV by Northern analysis (Fig. 5). Transcripts for c-myc were detected in both SFFV-infected and uninfected HCD-57 cells in the absence of Epo (Fig. 5), indicating that c-myc mRNA is constitutively expressed in HCD-57 cells and is not induced by infection with SFFV. In contrast, c-fos was constitutively expressed at low levels in both uninfected and SFFV-infected HCD-57 cells, and stimulation with Epo induced an increase in the level of c-fos mRNA in all three cell lines (Fig. 5). Interestingly, the level of c-fos transcription induced by Epo was significantly less in SFFV-infected cells than in uninfected cells stimulated with Epo (Fig. 5).
FIG. 5.
Infection with SFFV does not alter c-fos or c-myc expression in HCD-57 cells. Uninfected and SFFV-infected HCD-57 cells were starved overnight in 1.5% FCS and either left unstimulated (−) or stimulated for 15 min with Epo (+). RNA was extracted and analyzed by Northern blotting as described in Materials and Methods. The filter was sequentially stripped and reprobed with radiolabeled cDNA probes specific for c-fos, c-myc, or actin mRNA.
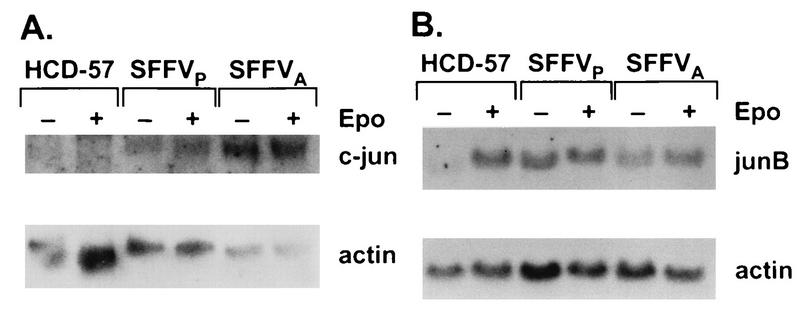
c-Fos is a component of the AP-1 transcription factor complex, which is composed of a variety of Jun and Fos homo- and heterodimers which induce transcription from response elements activated by tetradecanoyl phorbol acetate and other stimuli, including Epo (15, 28, 47). Since an active AP-1 complex requires expression of jun family genes (2, 45, 55), we also evaluated expression of c-jun and junB in virus-infected and uninfected HCD-57 cells. Previous experiments have indicated that expression of c-jun is not induced by Epo (6, 47), while Epo-induced expression of junB has not been evaluated in erythroid cells. Consistent with previous results, c-jun was not expressed in unstimulated HCD-57 cells, and transcription was not induced by Epo (Fig. 6). However, c-jun mRNA was expressed in both unstimulated and Epo-stimulated SFFV-infected cells (Fig. 6). Furthermore, expression of junB mRNA, which was induced by Epo in HCD-57 cells, was constitutive in SFFV-infected cells (Fig. 6). These results demonstrate deregulated expression in SFFV-infected cells of the genes encoding the c-Jun and JunB components of the AP-1 complex.
FIG. 6.
Infection with SFFV activates transcription of c-jun and junB in HCD-57 cells. Uninfected and SFFV-infected HCD-57 cells were starved overnight in 1.5% FCS and either left unstimulated (−) or stimulated with Epo for 15 min (+). RNA was extracted and analyzed by Northern blotting as described in Materials and Methods. The filters were sequentially probed with radiolabeled cDNAs specific for either c-jun and actin (A) or junB and actin (B).
c-raf antisense oligonucleotides inhibit growth factor-independent proliferation of SFFV-infected HCD-57 cells.
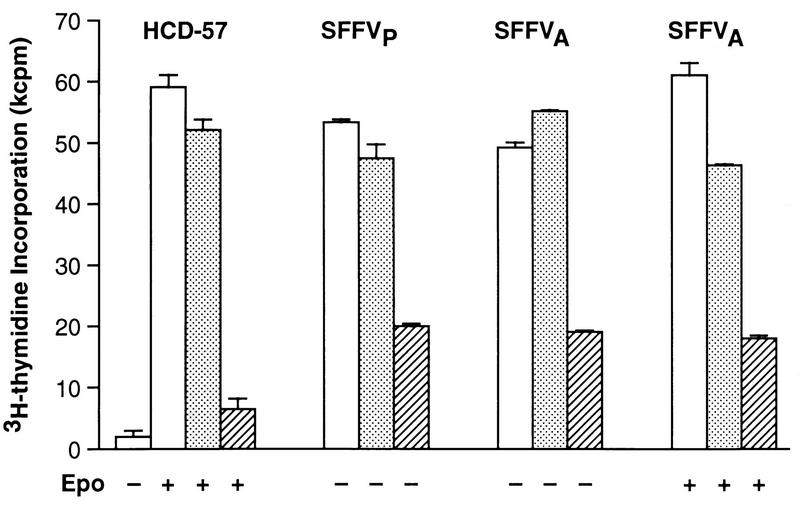
Previous studies using c-raf antisense oligonucleotides to inhibit expression of Raf-1 have demonstrated that Raf-1 is required for growth factor-induced proliferation of factor-dependent cell lines, including HCD-57 cells (8, 43). Therefore, we used previously characterized c-raf antisense oligonucleotides (8, 42, 43) to evaluate the requirement for Raf-1 in SFFV-induced growth factor-independent proliferation of HCD-57 cells (Fig. 7). Identical to the results of our previous study (43), c-raf antisense oligonucleotides inhibited Epo-induced proliferation of uninfected HCD-57 cells by 87%, while sense oligonucleotides had little or no effect on cell growth (Fig. 7). Treatment with c-raf antisense oligonucleotides inhibited the Epo-independent proliferation of HCD-57 cells infected with SFFVP by 58% and inhibited proliferation of HCD-57 cells infected with SFFVA by 65%, while sense oligonucleotides had no significant effect (Fig. 7). In contrast to SFFVP-infected cells, cells infected with SFFVA proliferate better in the presence of Epo (48). Therefore, we also evaluated the effect of c-raf antisense oligonucleotides on Epo-induced proliferation of SFFVA-infected HCD-57 cells. As shown in Fig. 7, c-raf antisense oligonucleotides inhibited the Epo-induced proliferation of SFFVA-infected cells by 60%, similar to their effect on unstimulated cells. These results demonstrate that the requirement for Raf-1 in Epo-induced proliferation of HCD-57 cells is partially abrogated by infection with SFFV.
FIG. 7.
c-raf antisense oligonucleotides inhibit Epo-independent proliferation of HCD-57 cells infected with SFFV. Uninfected and SFFV-infected HCD-57 cells were not treated (□) or treated with 4.0 μM c-raf antisense (▨) or sense (░⃞) oligonucleotide in the presence or absence of Epo and assayed for [3H]thymidine incorporation as described in Materials and Methods.
DISCUSSION
In this study, we have determined that infection of the murine erythroleukemia cell line HCD-57 with SFFV induces constitutive activation of the major downstream components of the Raf-1/MAPK signal transduction pathway. Raf-1 kinase activity, which is activated by Epo in uninfected HCD-57 cells, is constitutively activated in HCD-57 cells infected with either the SFFVP or SFFVA variant of the virus. Consistent with this result, MEK, a direct substrate of Raf-1, and MAPK, the downstream effector molecule in this pathway, were also constitutively activated in both SFFVP- and SFFVA-infected HCD-57 cells.
Although erythroid cells infected with SFFVP and SFFVA differ in the ability to differentiate in the absence of Epo, both viruses were shown in this study to constitutively activate the Raf-1/MAPK pathway in HCD-57 cells, a result that we have recently confirmed in studies using spleen cells from virus-infected mice (46a). This finding suggests that constitutive activation of the Raf-1/MAPK pathway is not sufficient to induce Epo-independent differentiation of erythroid cells. In contrast, constitutive activation of the Jak-Stat pathway by SFFV appears to be required to achieve differentiation of erythroid cells in the absence of Epo (46a).
Since growth factor activation of the Raf-1/MAPK pathway has been linked to activation of the immediate-early gene response, we also evaluated the effect of SFFV infection on expression of several immediate-early genes, including c-myc, c-fos, c-jun, and junB. The c-myc gene was constitutively expressed in both uninfected and SFFV-infected HCD-57 cells; however, only SFFV-infected HCD-57 cells were growth factor independent. These results are consistent with those of previous studies demonstrating that coexpression of v-myc and a constitutively activated v-raf gene renders erythroid cells growth factor independent, while constitutive expression of either gene alone is insufficient for the abrogation of growth factor dependence (30).
The Raf-1/MAPK pathway is thought to regulate c-fos gene expression by inducing MAPK-mediated phosphorylation of Elk-1, a ternary-complex transcription factor that activates the c-fos promoter (20, 63). However, no differences were seen in the level of c-fos expression in uninfected or SFFV-infected HCD-57 cells, despite the fact that the Raf-1/MAPK pathway was constitutively activated in the latter cells. This finding suggests that the Raf-1/MAPK pathway is not involved in regulating c-fos expression in HCD-57 cells. Activation of the Jak-Stat pathway also does not appear to be regulating c-fos expression in these cells since previous data indicated constitutive binding of Stat proteins to the sis-inducible element from the c-fos promoter in HCD-57 cells infected with SFFVP (46). Recent studies have identified several other MAPK-related kinases that are involved in the regulation of growth factor-induced activation of c-fos transcription (28), and these kinases may be activated in SFFV-infected HCD-57 cells.
The active form of the AP-1 transcription factor consists of hetero- or homodimers of Fos and Jun family proteins. Jun family proteins can form homodimers or heterodimers with other Jun family members, but c-Fos alone cannot dimerize and does not bind DNA or activate transcription in the absence of Jun (2). In contrast to uninfected HCD-57 cells, which failed to express c-jun and expressed junB only after Epo stimulation, SFFV-infected cells constitutively expressed both c-jun and junB, suggesting that SFFV may affect AP-1 activity by deregulating expression of jun family genes. Studies are in progress to evaluate this possibility.
Transcriptional activation of c-jun is autoregulated, and a distinct signal transduction pathway has recently been identified which leads to the activation of Jun kinases (JNKs), which in turn phosphorylate and activate c-Jun (32, 36). The pathway leading to JNK activation has been called the stress-activated protein kinase (SAPK) pathway because it is primarily activated by cell stressors such as UV irradiation, which are poor activators of the Raf-1/MAPK pathway (32). However, the JNK/SAPK pathway is also activated by a variety of growth factors, including Epo (15, 18, 44). In addition, constitutively activated MEK can activate JNK and stimulate c-Jun-mediated-transcription (19), suggesting that there is cross talk between the Raf-1/MAPK and the JNK/SAPK signal transduction pathways. Since c-jun and junB are constitutively expressed in SFFV-infected cells, we are currently evaluating the effect of SFFV infection on activation of the JNK/SAPK signal transduction pathway in HCD-57 cells.
The requirement for an intact Raf-1/MAPK pathway in growth factor-induced proliferation has been demonstrated in a number of different experimental systems (14). As previously shown (8, 43), c-raf antisense inhibition of Raf-1 expression in HCD-57 cells almost completely inhibited their proliferation in response to Epo. However, SFFV-infected HCD-57 cells treated with c-raf antisense oligonucleotides could still proliferate in the absence of Epo, although at reduced levels. Our data suggest that Raf-1 is not essential for the Epo-independent proliferation of SFFV-infected HCD-57 cells per se but rather cooperates with other signal transduction pathways to achieve maximum proliferation of SFFV-infected HCD-57 cells in the absence of Epo. One such cooperating pathway is likely to be the Jak-Stat pathway, since our previous studies demonstrated constitutive activation of Stat transcription factors in SFFV-infected erythroid cells (46). Studies are in progress to assess the effects of inhibiting Stat activation on Epo-independent proliferation of SFFV-infected cells.
We conclude from our studies that infection of erythroid cells with SFFV leads to the constitutive activation of the Raf-1/MAPK pathway and that activation of this pathway is required to achieve maximum proliferation of erythroid cells in the absence of Epo. Studies are in progress to examine SFFV-infected erythroid cells for activation of upstream components in the Raf-1/MAPK pathway to better understand how interaction of the SFFV envelope glycoprotein with the Epo receptor leads to activation of the downstream components of this pathway. In addition, we have initiated studies to determine if there is convergence between SFFV-induced activation of the Raf-1/MAPK and Jak-Stat signal transduction pathways.
ACKNOWLEDGMENTS
We thank D. Blair and L. Wolff for kindly providing some of the cDNA probes used in this study, G. Heidecker for the gift of GST-MEK−, and Karen Cannon for helpful assistance in the preparation of the manuscript.
REFERENCES
- 1.Alessi D R, Cohen P, Ashworth A, Cowley S, Leevers S J, Marshall C J. Assay and expression of mitogen-activated protein kinase, MAP kinase kinase, and Raf. Methods Enzymol. 1995;255:279–290. doi: 10.1016/s0076-6879(95)55031-3. [DOI] [PubMed] [Google Scholar]
- 2.Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 3.Barber D L, DeMartino J C, Showers M O, D’Andrea A D. A dominant negative erythropoietin (EPO) receptor inhibits EPO-dependent growth and blocks F-gp55-dependent transformation. Mol Cell Biol. 1994;14:2257–2265. doi: 10.1128/mcb.14.4.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barber D L, Corless C N, Xia K, Roberts T M, D’Andrea A D. Erythropoietin activates Raf1 by an Shc-independent pathway in CTLL-EPO-R cells. Blood. 1997;89:55–64. [PubMed] [Google Scholar]
- 5.Blumer K J, Johnson G L. Diversity in function and regulation of MAP kinase pathways. Trends Biochem Sci. 1994;19:236–240. doi: 10.1016/0968-0004(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 6.Bondurant M C, Yamashita T, Muta K, Krantz S B, Koury M J. c-myc expression affects proliferation but not terminal differentiation or survival of explanted erythroid progenitor cells. J Cell Physiol. 1996;168:255–263. doi: 10.1002/(SICI)1097-4652(199608)168:2<255::AID-JCP4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 7.Cano E, Mahadevan L C. Parallel signal processing among mammalian MAPKs. Trends Biochem Sci. 1995;20:117–122. doi: 10.1016/s0968-0004(00)88978-1. [DOI] [PubMed] [Google Scholar]
- 8.Carroll M P, Spivak J L, McMahon M, Weich N, Rapp U R, May W S. Erythropoietin induces Raf-1 activation and Raf-1 is required for erythropoietin-mediated proliferation. J Biol Chem. 1991;266:14964–14969. [PubMed] [Google Scholar]
- 9.Casadevall N, Lacombe C, Muller O, Gisselbrecht S, Mayeux P. Multimeric structure of the membrane erythropoietin receptor of murine erythroleukemia cells (Friend cells). Cross-linking of erythropoietin with the spleen focus-forming virus envelope protein. J Biol Chem. 1991;266:16015–16020. [PubMed] [Google Scholar]
- 10.Crews C M, Erikson R L. Purification of a murine protein-tyrosine/threonine kinase that phosphorylates and activates the Erk-1 gene product: relationship to the fission yeast byr1 gene product. Proc Natl Acad Sci USA. 1992;89:8205–8209. doi: 10.1073/pnas.89.17.8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damen J E, Liu L, Cutler R L, Krystal G. Erythropoietin stimulates the tyrosine phosphorylation of Shc and its association with Grb2 and a 145-Kd tyrosine phosphorylated protein. Blood. 1993;82:2296–2303. [PubMed] [Google Scholar]
- 12.D’Andrea D A, Yoshimura A, Youssoufian H, Zon L I, Koo J W, Lodish H F. The cytoplasmic region of the erythropoietin receptor contains nonoverlapping positive and negative growth-regulatory domains. Mol Cell Biol. 1991;11:1980–1987. doi: 10.1128/mcb.11.4.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darnell J E, Jr, Kerr I M, Stark G R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 14.Daum G, Eisenmann-Tappe I, Fries H W, Troppmair J, Rapp U R. The ins and outs of Raf kinases. Trends Biochem Sci. 1994;19:474–480. doi: 10.1016/0968-0004(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 15.Davis R J. Transcriptional regulation by MAP kinases. Mol Reprod Dev. 1995;42:459–467. doi: 10.1002/mrd.1080420414. [DOI] [PubMed] [Google Scholar]
- 16.Dent P, Haser W, Haystead T A, Vincent L A, Roberts T M, Sturgill T W. Activation of mitogen-activated protein kinase kinase by v-Raf in NIH 3T3 cells and in vitro. Science. 1992;257:1404–1407. doi: 10.1126/science.1326789. [DOI] [PubMed] [Google Scholar]
- 17.Egan S E, Giddings B W, Brooks M W, Buday L, Sizeland A M, Weinberg R A. Association of Sos Ras exchange protein with Grb2 is implicated in tyrosine kinase signal transduction and transformation. Nature. 1993;363:45–51. doi: 10.1038/363045a0. [DOI] [PubMed] [Google Scholar]
- 18.Foltz I N, Schrader J W. Activation of the stress-activated protein kinases by multiple hematopoietic growth factors with the exception of interleukin-4. Blood. 1997;89:3092–3096. [PubMed] [Google Scholar]
- 19.Franklin C C, Kraft A S. Constitutively active MAP kinase kinase (MEK1) stimulates SAP kinase and c-Jun transcriptional activity in U937 human leukemic cells. Oncogene. 1995;11:2365–2374. [PubMed] [Google Scholar]
- 20.Gille H, Sharrocks A D, Shaw P E. Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature. 1992;358:414–417. doi: 10.1038/358414a0. [DOI] [PubMed] [Google Scholar]
- 21.Gupta S, Davis R J. MAP kinase binds to the NH2-terminal activation domain of c-Myc. FEBS Lett. 1994;353:281–285. doi: 10.1016/0014-5793(94)01052-8. [DOI] [PubMed] [Google Scholar]
- 22.Hankins W D. Increased erythropoietin sensitivity after in vitro transformation of hematopoietic precursors by RNA tumor viruses. J Natl Cancer Inst. 1983;70:725–734. [PubMed] [Google Scholar]
- 23.He T-C, Jiang N, Zhuang H, Wojchowski D M. Erythropoietin-induced recruitment of Shc via a receptor phosphotyrosine-independent, Jak2-associated pathway. J Biol Chem. 1995;270:11055–11061. doi: 10.1074/jbc.270.19.11055. [DOI] [PubMed] [Google Scholar]
- 24.Hipskind R A, Baccarini M, Nordheim A. Transient activation of RAF-1, MEK, and ERK2 coincides kinetically with ternary complex factor phosphorylation and immediate-early gene promoter activity in vivo. Mol Cell Biol. 1994;14:6219–6231. doi: 10.1128/mcb.14.9.6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoatlin M E, Kozak S L, Lilly F, Chakraborti A, Kozak C A, Kabat D. Activation of erythropoietin receptors by Friend viral gp55 and by erythropoietin and down-modulation by the murine Fv-2rresistance gene. Proc Natl Acad Sci USA. 1990;87:9985–9989. doi: 10.1073/pnas.87.24.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horoszewicz J S, Leong S S, Carter W A. Friend leukemia: rapid development of erythropoietin-independent hematopoietic precursors. J Natl Cancer Inst. 1975;54:265–267. doi: 10.1093/jnci/54.1.265. [DOI] [PubMed] [Google Scholar]
- 27.Ihle J N, Witthuhn B A, Quelle F W, Silvennoinen O, Tang B, Yi T. Protein tyrosine phosphorylation in the regulation of hematopoiesis by receptors of the cytokine-receptor superfamily. Blood Cells. 1994;20:65–80. [PubMed] [Google Scholar]
- 28.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 29.Kishi A, Chiba T, Sugiyama M, Machide M, Nagata Y, Amanuma H, Taira H, Katsumata T, Todokoro K. Erythropoietin receptor binds to Friend virus gp55 through other membrane components. Biochem Biophys Res Commun. 1993;192:1131–1138. doi: 10.1006/bbrc.1993.1534. [DOI] [PubMed] [Google Scholar]
- 30.Klinken S P, Rapp U R, Morse H C., III raf/myc-infected erythroid cells are restricted in their ability to terminally differentiate. J Virol. 1989;63:1489–1492. doi: 10.1128/jvi.63.3.1489-1492.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koury M J, Bondurant M C, Duncan D T, Krantz S B, Hankins W D. Specific differentiation events induced by erythropoietin in cells infected in vitro with the anemia strain of Friend virus. Proc Natl Acad Sci USA. 1982;79:635–639. doi: 10.1073/pnas.79.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kyriakis J M, App H, Zhang X F, Banerjee P, Brautigan D L, Rapp U R, Avruch J. Raf-1 activates MAP kinase-kinase. Nature. 1992;358:417–421. doi: 10.1038/358417a0. [DOI] [PubMed] [Google Scholar]
- 33.Li J P, AD D A, Lodish H F, Baltimore D. Activation of cell growth by binding of Friend spleen focus-forming virus gp55 glycoprotein to the erythropoietin receptor. Nature. 1990;343:762–764. doi: 10.1038/343762a0. [DOI] [PubMed] [Google Scholar]
- 34.Li N, Batzer A, Daly R, Yajnik V, Skolnik E, Chardin P, Bar-Sagi D, Margolis B, Schlessinger J. Guanine-nucleotide-releasing factor hSos1 binds to Grb2 and links receptor tyrosine kinases to Ras signaling. Nature. 1993;363:85–88. doi: 10.1038/363085a0. [DOI] [PubMed] [Google Scholar]
- 35.Liao S K, Axelrad A A. Erythropoietin-independent erythroid colony formation in vitro by hemopoietic cells of mice infected with Friend virus. Int J Cancer. 1975;15:467–482. doi: 10.1002/ijc.2910150313. [DOI] [PubMed] [Google Scholar]
- 36.Minden A, Lin A, McMahon M, Lange-Carter C, Derijard B, Davis R J, Johnson G L, Karin M. Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science. 1994;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- 37.Mirand E A. Virus-induced erythropoiesis in hypertransfused-polycythemic mice. Science. 1967;156:832–833. doi: 10.1126/science.156.3776.832. [DOI] [PubMed] [Google Scholar]
- 38.Mirand E A, Steeves R A, Lange R D, Grace J T., Jr Virus-induced polycythemia in mice: erythropoiesis without erythropoietin. Proc Soc Exp Biol Med. 1968;128:844–849. doi: 10.3181/00379727-128-33139. [DOI] [PubMed] [Google Scholar]
- 39.Miura O, Nakamura N, Quelle F W, Witthuhn B A, Ihle J N, Aoki N. Erythropoietin induces association of the JAK2 protein tyrosine kinase with the erythropoietin receptor in vivo. Blood. 1994;84:1501–1507. [PubMed] [Google Scholar]
- 40.Miura Y, Miura O, Ihle J N, Aoki N. Activation of the mitogen-activated protein kinase pathway by the erythropoietin receptor. J Biol Chem. 1994;269:29962–29969. [PubMed] [Google Scholar]
- 41.Morrison D K, Kaplan D R, Rapp U, Roberts T M. Signal transduction from membrane to cytoplasm: growth factors and membrane-bound oncogene products increase Raf-1 phosphorylation and associated protein kinase activity. Proc Natl Acad Sci USA. 1988;85:8855–8859. doi: 10.1073/pnas.85.23.8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muszynski K W, Ruscetti F W, Gooya J M, Linnekin D M, Keller J R. Raf-1 protein is required for growth factor-induced proliferation of primitive hematopoietic progenitors stimulated with synergistic combinations of cytokines. Stem Cells. 1997;15:63–72. doi: 10.1002/stem.150063. [DOI] [PubMed] [Google Scholar]
- 43.Muszynski K W, Ruscetti F W, Heidecker G, Rapp U, Troppmair J, Gooya J M, Keller J R. Raf-1 protein is required for growth factor-induced proliferation of hematopoietic cells. J Exp Med. 1995;181:2189–2199. doi: 10.1084/jem.181.6.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagata Y, Nishida E, Todokoro K. Activation of JNK signaling pathway by erythropoietin, thrombopoietin, and interleukin-3. Blood. 1997;89:2664–2669. [PubMed] [Google Scholar]
- 45.Nakabeppu Y, Ryder K, Nathans D. DNA binding activities of three murine Jun proteins: stimulation by Fos. Cell. 1988;55:907–915. doi: 10.1016/0092-8674(88)90146-8. [DOI] [PubMed] [Google Scholar]
- 46.Ohashi T, Masuda M, Ruscetti S K. Induction of sequence-specific DNA-binding factors by erythropoietin and the spleen focus-forming virus. Blood. 1995;85:1454–1462. [PubMed] [Google Scholar]
- 46a.Ohashi, T., et al. Unpublished data.
- 47.Patel H R, Sytkowski A J. Erythropoietin activation of AP1 (Fos/Jun) Exp Hematol. 1995;23:619–625. [PubMed] [Google Scholar]
- 48.Pulverer B J, Kyriakis J M, Avruch J, Nikolakaki E, Woodgett J R. Phosphorylation of c-jun mediated by MAP kinases. Nature. 1991;353:670–674. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- 49.Rapp U R. Role of Raf-1 serine/threonine protein kinase in growth factor signal transduction. Oncogene. 1991;6:495–500. [PubMed] [Google Scholar]
- 50.Ruscetti S, Wolff L. Biological and biochemical differences between variants of spleen focus-forming virus can be localized to a region containing the 3′ end of the envelope gene. J Virol. 1985;56:717–722. doi: 10.1128/jvi.56.3.717-722.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruscetti S K. Employment of a [3H]thymidine-incorporation assay to distinguish the effects of different Friend erythroleukemia-inducing retroviruses on erythroid cell proliferation. J Natl Cancer Inst. 1986;77:241–245. [PubMed] [Google Scholar]
- 52.Ruscetti S K. Erythroleukaemia induction by the Friend spleen focus-forming virus. Baillieres Clin Haematol. 1995;8:225–247. doi: 10.1016/s0950-3536(05)80239-2. [DOI] [PubMed] [Google Scholar]
- 53.Ruscetti S K, Janesch N J, Chakraborti A, Sawyer S T, Hankins W D. Friend spleen focus-forming virus induces factor independence in an erythropoietin-dependent erythroleukemia cell line. J Virol. 1990;64:1057–1062. doi: 10.1128/jvi.64.3.1057-1062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sassa S, Takaku F, Nakao K. Regulation of erythropoiesis in the Friend leukemia mouse. Blood. 1968;31:758–765. [PubMed] [Google Scholar]
- 55.Sassone-Corsi P, Ransone L J, Lamph W W, Verma I M. Direct interaction between fos and jun nuclear oncoproteins: role of the ‘leucine zipper’ domain. Nature. 1988;336:692–695. doi: 10.1038/336692a0. [DOI] [PubMed] [Google Scholar]
- 56.Seth A, Alvarez E, Gupta S, Davis R J. A phosphorylation site located in the NH2-terminal domain of c-Myc increases transactivation of gene expression. J Biol Chem. 1991;266:23521–23524. [PubMed] [Google Scholar]
- 57.Silvennoinen O, Ihle J N, Schlessinger J, Levy D E. Interferon-induced nuclear signaling by Jak protein tyrosine kinases. Nature. 1993;366:583–585. doi: 10.1038/366583a0. [DOI] [PubMed] [Google Scholar]
- 58.Simon M A, Dodson G S, Rubin G M. An SH3-SH2-SH3 protein is required for p21 Ras1 activation and binds to sevenless and Sos proteins in vitro. Cell. 1993;73:169–177. doi: 10.1016/0092-8674(93)90169-q. [DOI] [PubMed] [Google Scholar]
- 59.Spangler R, Bailey S C, Sytkowski A J. Erythropoietin increases c-myc mRNA by a protein kinase C-dependent pathway. J Biol Chem. 1991;266:681–684. [PubMed] [Google Scholar]
- 60.Spivak J L, Pham T, Isaacs M, Hankins W D. Erythropoietin is both a mitogen and a survival factor. Blood. 1991;77:1228–1233. [PubMed] [Google Scholar]
- 61.Steinheider G, Seidel H J, Kreja L. Comparison of the biological effects of anemia inducing and polycythemia inducing Friend virus complex. Experientia. 1979;35:1173–1175. doi: 10.1007/BF01963269. [DOI] [PubMed] [Google Scholar]
- 62.Stokoe D, Macdonald S G, Cadwallader K, Symons M, Hancock J F. Activation of Raf as a result of recruitment to the plasma membrane. Science. 1994;264:1463–1467. doi: 10.1126/science.7811320. [DOI] [PubMed] [Google Scholar]
- 63.Su B, Karin M. Mitogen-activated protein kinase cascades and regulation of gene expression. Curr Opin Immunol. 1996;8:402–411. doi: 10.1016/s0952-7915(96)80131-2. [DOI] [PubMed] [Google Scholar]
- 64.Tambourin P, Wendling F. Malignant transformation and erythroid differentiation by polycythaemia-inducing Friend virus. Nature New Biol. 1971;234:230–233. doi: 10.1038/newbio234230a0. [DOI] [PubMed] [Google Scholar]
- 65.Tambourin P E, Wendling F, Jasmin C, Smadja-Joffe F. The physiopathology of Friend leukemia. Leuk Res. 1979;3:117–129. doi: 10.1016/0145-2126(79)90009-2. [DOI] [PubMed] [Google Scholar]
- 66.Tsuda H, Aso N, Sawada T, Hata H, Kawakita M, Mori K J, Takatsuki K. Alteration of nuclear proto-oncogene expression by erythropoietin (Epo) in Epo-responsive murine cell lines. Int J Cell Cloning. 1991;9:123–133. doi: 10.1002/stem.5530090203. [DOI] [PubMed] [Google Scholar]
- 67.Wolff L, Ruscetti S. Malignant transformation of erythroid cells in vivo by introduction of a nonreplicating retrovirus vector. Science. 1985;228:1549–1552. doi: 10.1126/science.2990034. [DOI] [PubMed] [Google Scholar]
- 68.Wolff L, Ruscetti S. The spleen focus-forming virus (SFFV) envelope gene, when introduced into mice in the absence of other SFFV genes, induces acute erythroleukemia. J Virol. 1988;62:2158–163. doi: 10.1128/jvi.62.6.2158-2163.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yoshimura A, Longmore G, Lodish H F. Point mutation in the exoplasmic domain of the erythropoietin receptor resulting in hormone-independent activation and tumorigenicity. Nature. 1990;348:647–649. doi: 10.1038/348647a0. [DOI] [PubMed] [Google Scholar]
- 70.Yoshimura A, Zimmers T, Neumann D, Longmore G, Yoshimura Y, Lodish H F. Mutations in the Trp-Ser-X-Trp-Ser motif of the erythropoietin receptor abolish processing, ligand binding, and activation of the receptor. J Biol Chem. 1992;267:11619–11625. [PubMed] [Google Scholar]