Abstract
Respiratory infection of BALB/c mice with the murine gammaherpesvirus 68 (MHV-68) induces the clonal expansion of virus-specific cytotoxic T-lymphocyte (CTL) precursors (CTLp) in the regional, mediastinal lymph nodes (MLN). Some of these CTLps differentiate to become fully functional CTL effectors, which can be detected in both the lymphoid tissue and in the site of pathology in the lung. Though the lymph nodes and spleen harbor substantial populations of latently infected B cells for life, the level of virus-specific CTL activity decreases rapidly in all sites. The CD8+ CTLp numbers fall to background levels in the MLN within several months of the termination of the productive phase of MHV-68 infection in the respiratory epithelium but are maintained at relatively low frequency in the spleen. The continued presence of a gamma interferon-producing, MHV-68-specific CD4+ set can also be demonstrated in cultured spleen cells. The virus-specific immunoglobulin G (IgG) response is slow to develop, with serum neutralizing antibody and enzyme-linked immunosorbent assay titers continuing to rise for several months. The level of total serum IgG increases dramatically within 2 weeks of infection, probably as a consequence of polyclonal B-cell activation, and remains high. The immune response profile is clearly influenced by the persistence of this DNA virus.
Intranasal (i.n.) challenge with the murine gammaherpesvirus 68 (MHV-68) leads to a transient, productive infection of the respiratory epithelium, followed by life-long latency in B lymphocytes (5, 27). The lytic phase in the lung recurs, with ultimately lethal consequences, in mice that lack CD4+ T cells (5). This profile of acute replication in epithelia, followed by persistence in other cell types and periodic (or late-onset) reactivation, is typical for the herpesviruses (HVs). Indeed, the interface between HV and host survival strategies through phylogeny has led to the evolution of a variety of molecular mechanisms designed to achieve a balance between immune control and the need for virus excretion to ensure transmission (3, 20, 29). The nature of the host response to these complex viruses is thus of considerable, general interest.
The recently developed MHV-68 model clearly has considerable potential for illuminating the long-term confrontation between T cells, B cells, and the lymphotrophic gammaherpesviruses, which include Epstein-Barr virus (EBV), the Kaposi’s sarcoma HV, and herpesvirus saimiri (6, 8, 12). Dissection of the immune response to date has relied principally on the in vivo depletion of T-cell subsets with monoclonal antibodies (MAbs) and the use of various genetically disrupted (−/−) mouse strains that lack particular components of the immune system (5, 10, 31, 40). The principal themes so far are that both the acute and persistent phases of MHV-68 infection seem to be controlled by CD8+ T cells, while CD4+ T helper (Th) activity is also required to achieve long-term protection. The present analysis provides the first description of the MHV-68-specific CD8+ cytotoxic T-lymphocyte (CTL) response, together with kinetic studies of both immunoglobulin (Ig) profiles and the CD4+ Th population.
MATERIALS AND METHODS
MHV-68 infection of the mice.
The MHV-68 stocks were grown in owl monkey kidney cells from an isolate originally provided by A. A. Nash (9). The female BALB/cJ mice were purchased from Jackson Laboratory (Bar Harbor, Maine) and, apart from the challenge with MHV-68, kept under specific-pathogen-free conditions in the St. Jude Children’s Research Hospital Animal Resources Centre. Mice were anesthetized with Avertin (2,2,2-tribromoethanol) and infected i.n. with 400 PFU of MHV-68 at 8 to 12 weeks of age (2, 5).
MHV-68-specific CTL assay.
The virus-specific CTL assay for MHV-68 has proven to be difficult to establish, and so the technique is described in some detail. The BALB/c-3T3 cells (ATCC CCL163) were trypsinized, washed once, and infected with MHV-68 at a multiplicity of infection of 10 for 1 to 2 h in Dulbecco modified Eagle medium containing 10% fetal calf serum (FCS) (HyClone, Logan, Utah) at 37°C or left uninfected as controls. The cells were washed once and labeled (0.2 mCi/106 cells) with 51Cr (Amersham Life Sciences, Arlington Heights, Ill.) for 1 h at 37°C. After a further two washes, 5 × 103 targets (per microculture) were incubated with the various lymphoid and inflammatory cell populations in 96-well, flat-bottom plates (Sarstedt, Newton, N.C.) for 4 to 5 h before harvesting of supernatants for γ counting. Threefold effector cell dilutions from effector/target (E:T) ratios of 30:1 were measured in duplicate, while the untreated and Triton X-100-disrupted (total release) controls were assayed in quadruplicate. The percent specific lysis was calculated as 100 × (51Cr release from targets with effectors − 51Cr release from targets alone)/(51Cr release from targets with Triton − 51Cr release from targets alone). The level of 51Cr release from MHV-68-infected targets alone was never >20% of the total release.
Redirected CTL assay.
A measure of the total level of CTL activity is provided by the redirected assay, which utilizes a 4- to 6-h incubation with 51Cr-labeled FcR+ Fas+ P815 target cells coated by a MAb to CD3ε. The virus-specific CD8+ CTLs will be included in this effector population, as will virus-specific CD4+ T cells and other activated CD3ε+ lymphocytes that express the Fas ligand and/or have up-regulated the perforin/granzyme mechanism (23, 28).
Restimulation in bulk culture.
Spleen cells from naive mice were irradiated and infected with MHV-68 at 0.1 PFU/cell for 1 h in RPMI 1640 supplemented with penicillin (60 μg/ml), glutamine (2 mM), 10% FCS, and 30 μM 2-mercaptoethanol (complete medium). These stimulator cells were washed once, irradiated (2,000 rads), and incubated (0.5 × 106/ml) with responder lymphocytes (1.5 × 106/ml) for 5 days in complete medium at 37°C with 5% CO2. Live cells were purified from the cultures by centrifugation on a one-step Ficoll gradient (Fisher Scientific, Pittsburgh, Pa.) and washed twice in complete medium before use.
Limiting dilution analysis (LDA).
The MHV-68-infected stimulators were prepared as for bulk culture (see above), added (6 × 105/well) to twofold dilutions of the responder cells in 96-well plates, and cultured in complete medium for 7 days. Recombinant interleukin-2 (IL-2; 10 U/ml; Boehringer, Indianapolis, Ind.) was provided on day 0 and again on day 5 of culture. After 7 days, 5,000 MHV-68-infected, 51Cr-labeled BALB/c-3T3 cells were added (per microculture) to 20 replicate wells for each dilution of responder cells. Supernatants were harvested for gamma counting after 4 to 5 h. Positive wells were taken as those with levels of specific 51Cr release >3 times the standard deviation above the mean for 20 wells containing only the stimulator and target cell populations. The prevalence of the CTL precursors (CTLp) was determined by a regression plot of log10 (fraction of negative wells) against mean cell number, with the correlation coefficient (r2) being greater than 0.9 in each case. The values were corrected percent for the CD8+ T cells determined by flow cytometric analysis of the starting cell population and are expressed throughout as CD8+ CTLp frequencies.
Flow cytometry.
Lymphocytes recovered directly from infected mice or from in vitro bulk cultures were washed in phosphate-buffered saline (PBS), blocked by incubation with 10% normal mouse serum, and stained (37) with anti-CD8α–fluorescein isothiocyanate (FITC), anti-CD4-FITC, anti-CD62 ligand (CD62L)-phycoerythin, and anti-B220-FITC (Pharmingen, San Diego, Calif.). The cells were washed once after staining and analyzed on a FACScan, using Cellquest software (Becton Dickinson, San Jose, Calif.).
Single-cell cytokine assay.
The numbers of gamma interferon (IFN-γ)-producing cells in lymphocyte populations recovered from bulk cultures were determined by the single-cell enzyme-linked immunosorbent assay (ELISA) spot (ELISPOT) assay as described previously (33).
ELISA for virus-specific antibody.
Virus was concentrated by ultracentrifugation from the supernatant of MHV-68-infected owl monkey kidney cells, disrupted by dilution in PBS with 0.05% Triton X-100, and coated overnight at 4°C onto Nunc Maxisorp immunoplates (Life Technologies, Gaithersburg, Md.). The plates were washed five times with PBS-Tween (0.05%) and then incubated for 1 h at room temperature with PBS-Tween (0.05%)-bovine serum albumin (1%). Fivefold serum dilutions from an initial concentration of 1/20 were incubated for 1 h, followed by a further five washes. Bound antibody was detected with alkaline phosphatase (ALP)-conjugated rabbit anti-mouse IgG (Sigma Chemical Co., St. Louis, Mo.), using n-nitrophenyl phosphate (Sigma) as the ALP substrate and reading the absorbancy at 405 nm. Antibody titers were determined by comparison with standard immune and naive sera included on each plate, with the absorbancy of 1/20 naive mouse serum being characterized arbitrarily as 1 titer unit.
ELISA for total IgG and IgM.
Nunc Maxisorp plates were coated overnight at 4°C with either rabbit anti-mouse IgG (Sigma) or goat anti-mouse IgM (Sigma), each 1 μg/ml in 50 mM NaHCO3 (pH 8.5), and then washed and blocked with PBS-Tween-bovine serum albumin as above. A standard pool of mouse sera was included on each plate for comparison. Fivefold serum dilutions started at 1/1,000 for IgM assays and 1/10,000 for IgG assays. Specific binding was detected with ALP-rabbit anti-mouse IgG (Sigma) or ALP-goat anti-mouse IgM (Sigma). All other steps were performed as described above.
Measuring neutralizing antibody.
Duplicate twofold serum dilutions, starting from an initial concentration of 1/10 in Dulbecco modified Eagle medium containing 10% FCS, were incubated with 30 to 50 PFU of MHV-68 on ice for 1 h in 96-well plates. Freshly trypsinized BALB/c-3T3 cells (2 × 104) were added to each well and allowed to adhere overnight. The cells were then overlaid with minimal essential medium containing 0.75% carboxymethyl cellulose. After 3 to 4 days of culture, the cells were fixed with methanol and stained with Giemsa stain (Sigma). A standard immune serum was included in each experiment to ensure uniformity of results. The neutralization titer was defined as the highest serum dilution giving a greater than 50% reduction in viral plaques. Naive mouse sera had no effect on plaque formation.
RESULTS
CTL effectors in the respiratory tract.
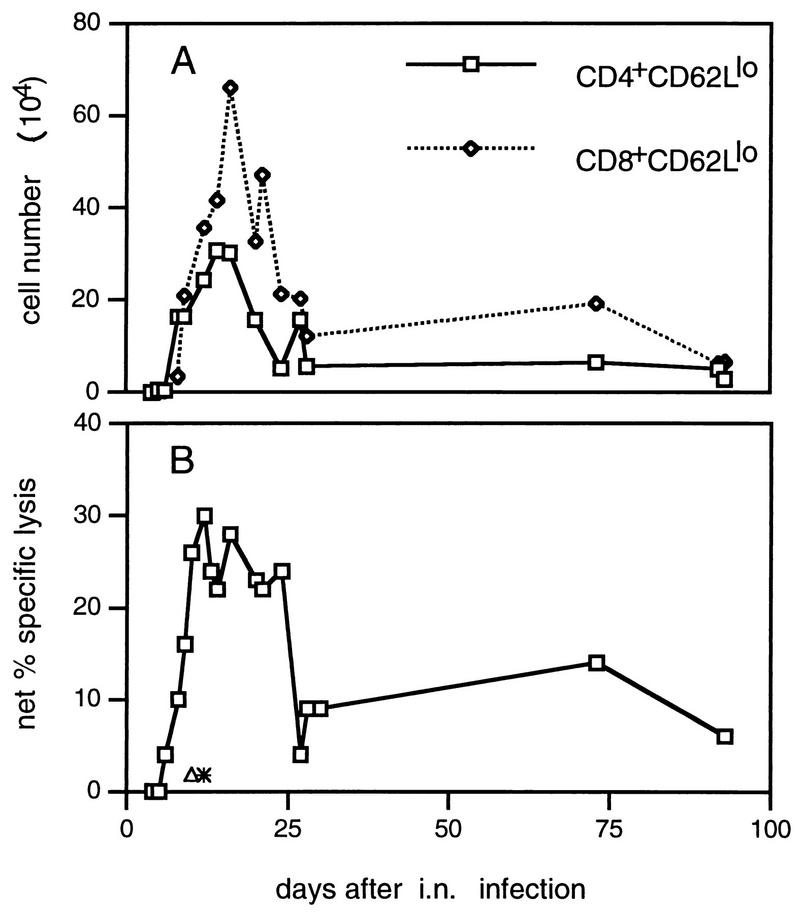
Giving MHV-68 by the i.n. route results in a primary phase of productive infection in respiratory epithelium, which is generally resolved within 12 to 14 days. Mice that are depleted of the majority CD8+ (but not the CD4+) T-cell subset fail to clear the virus and die (10). Activated CD8+ CD62Llo and CD4+ CD62Llo T cells localize to the infected lung, with peak cell counts being recorded for the inflammatory exudate recovered (2) by bronchoalveolar lavage (BAL) at about day 15 after the initial challenge (Fig. 1A). Previous experiments (5) have shown that both the CD4+ and CD8+ BAL populations contain CTLs that mediate CD3ε-dependent lysis of uninfected P815 target cells, an assay that measures the total extent of T-cell activation rather than virus-specific cytotoxicity.
FIG. 1.
Recovery of antiviral CTL from the lung by BAL. Cells were pooled from pairs of mice and adhered to plastic for 1 h at 37°C before assay to remove macrophages. The proportion of activated T cells in each pool was determined by flow cytometric analysis (A). CD8+ CD62 Llo cells typically made up 40% of the total at the peak of the response. Virus-specific cytotoxicity for an E:T ratio of 30:1 (based on the total cells harvested) is shown in panel B. Net specific lysis = % specific lysis of MHV-68-infected targets − % specific lysis of uninfected targets (which did not exceed 5%). Results were pooled from four separate experiments. Each point shows the mean of one to three determinations. ∗, MHV-68-specific cytotoxicity by BAL cells from influenza A/HKx31 virus-infected BALB/c mice. ▵, cytotoxicity for MHV-68-infected BALB/c-3T3 cells mediated by BAL cells from MHV-68-infected C57BL/6 (H-2b) mice.
Virus-specific CTL activity for H-2d-compatible, MHV-68-infected major histocompatibility complex (MHC) class I+ II− BALB/c 3T3 cells peaked in the BAL population at about 10 days after infection and remained at high levels for at least another 10 days (Fig. 1B). The assay is virus specific, as the targets were not lysed by BAL cells from MHV-68-infected H-2b mice or from influenza virus-infected H-2d mice (Fig. 1B). This profile of rapidly emerging CTL effector function coincident with virus clearance is also typical of respiratory infections caused by the negative-strand RNA viruses (7), but the MHV-68-specific T cells do seem to persist for somewhat longer in the infected lung (Fig. 1A).
Lymphocyte numbers and CTLs in lymphoid tissue.
Infection with MHV-68 causes a massive increase in counts for the CD4+ and CD8+ T cells and B220+ B cells (Fig. 2) in the spleen and regional, mediastinal lymph node (MLN) during the acute phase of the disease process. The extent of passive lymphocyte recruitment (38) may be reflected in the much greater numbers of CD4+ and CD8+ T cells with a naive CD62Lhi phenotype, while at least a proportion of the CD4+ CD62Llo and CD8+ CD62Llo sets (Fig. 2) will be comprised of memory T cells specific for unrelated antigens (7). Others in the activated CD62Llo populations will be lymphocytes that have been stimulated in an MHV-68-specific or nonspecific way, the latter being mediated either via a possible viral superantigen or by cytokines (32, 35, 36).
FIG. 2.
Flow cytometric analysis of lymphocyte phenotypes before restimulation. Cells were pooled from two to three mice for each time point, and the mean numbers of each cell type per mouse were derived from the total cell counts and the proportions stained specifically by flow cytometry. The results were pooled from four separate experiments, with each point showing the mean of one to three determinations.
Virus-specific CTL effectors can be detected in freshly isolated MLN and spleen populations during the acute phase of the infectious process (day 13; Fig. 3). The level of 51Cr release for the MHV-68-infected targets was lower than that measured by the antigen-nonspecific redirected protocol and was apparent only at high E:T ratios. Little if any evidence of virus replication is found in homogenates of MLN or spleen at any phase of MHV-68 infection, though latent virus that can be reactivated by culturing viable B cells in contact with susceptible monolayers (infectious center assay) is present for life (4, 34). Perhaps the CTLs that can be assayed with the lytically infected 3T3 targets (day 13; Fig. 3) are stimulated by antigen-presenting dendritic cells that have localized from the respiratory tract (13, 25, 39). Constitutive MHV-68-specific CTL activity was no longer apparent for MLN and spleen cells taken at day 45 after infection, though some effector function was still detectable by the redirected assay (day 45; Fig. 3).
FIG. 3.
Cytotoxic activity of lymphocyte population without prior restimulation. Cells were pooled from three mice per time point and enriched for CD8+ T cells by in vitro depletion with rat anti-mouse I-Ed and rat anti-mouse CD4 MAbs, followed by sheep anti-rat- and sheep anti-mouse-coated magnetic beads (Dynal). The enriched populations contained 70 to 90% CD8+ T cells and <1% CD4+ T cells. The E:T ratios are corrected for to the number of CD8+ T cells in the effector cell populations. □, untreated BALB/c-3T3 cells; ◊, MHV-68-infected BALB/c-3T3 cells; ○, p815 cells plus anti-CD3 MAb.
Restimulation in bulk culture.
Responding CD4+ and CD8+ T cells dominated the lymphocyte populations recovered after 5 days of in vitro culture for the first 30 or so days after primary MHV-68 infection but tended to decrease in relative prevalence at the later time points (Fig. 4A and B). This drop in cell counts was particularly obvious for the CD8+ set in the MLN (Fig. 4A). Somewhat surprisingly, many of these cultured CD4+ and CD8+ T cells expressed the naive CD62Lhi phenotype (Fig. 4A and B). Paralleling the cell counts (Fig. 4A), the level of MHV-68-specific CTL activity was maximal for the MLN populations taken within 30 days of virus challenge but tended to decrease thereafter (Fig. 4C). Both the drop in CD8+ T-cell numbers with time and the concurrent fall in CTL activity was less apparent for the spleen (Fig. 4A and C).
FIG. 4.
Functional analysis of MHV-68-specific T cells after restimulation in bulk culture. Cells were pooled from two to three mice and restimulated in vitro for 5 days with MHV-68-infected irradiated feeder cells (see Materials and Methods). (A and B) Percentages of activated (CD62Llo) and naive (CD62Lhi) T cells after 5 days of culture. The remainder of the cultured cells were almost all B220+ B lymphocytes. (C) Net percent specific lysis (% specific lysis of MHV-68-infected targets − % specific lysis of uninfected targets [which did not exceed 15%]) for an E:T ratio of 30:1 (lymph node cells) or 60:1 (spleen cells). E:T ratios refer to the total number of live cells harvested from day 5 cultures. ⊕, killing of MHV-68-infected H-2-mismatched NIH 3T3 cells. (D) IFN-γ production by cells from the same restimulated populations. This assay detects only responding CD4+ T cells. ◊, CD8-depleted spleen cells; ○, CD4-depleted spleen cells; ⊠, restimulated naive spleen cells. Results were pooled from four separate experiments, and each point shows the mean of one to three determinations.
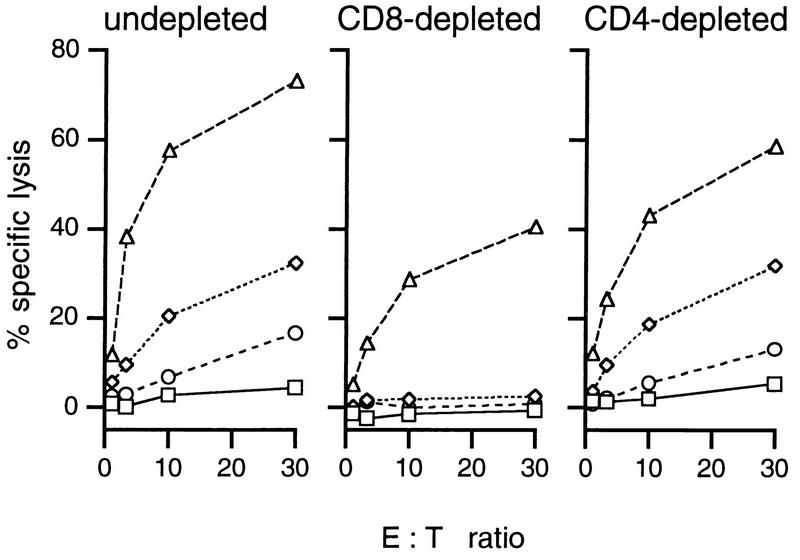
Lymphocyte depletion experiments established that the virus-specific CTL effectors were indeed CD8+ (Fig. 5), while both the CD4+ and CD8+ T-cell subsets contributed to CD3ε-dependent cytotoxicity (CD4 or CD8 depleted; Fig. 5). The numbers of functional CD4+ T cells present after 5 days of in vitro culture were measured independently by the single-cell IFN-γ ELISPOT assay (Fig. 4D). In general, the CD4+ T-cell counts for the MLN cultures did not vary (Fig. 4B) to the extent found for the CD8+ subset (Fig. 4A), and IFN-γ-producing cells remained within a three- to fourfold range for cultured spleen or MLN populations taken from 6 to 110 days after infection (Fig. 4D).
FIG. 5.
CD8 dependence of virus-specific cytotoxicity. Spleen cells from mice immunized i.n. 2 weeks earlier with MHV-68 were restimulated in vitro for 5 days. T-cell subsets were then depleted as indicated, using rat anti-mouse CD4 or rat anti-mouse CD8 MAb followed by sheep anti-rat-coated magnetic beads. The levels of virus-specific and redirected cytotoxicity were then determined as described in Materials and Methods. □, uninfected BALB/c-3T3 cells; ◊, MHV-68-infected BALB/c-3T3 cells; ○, untreated p815 cells; ▵, p815 cells plus anti-CD3 MAb.
Determining CTLp frequencies.
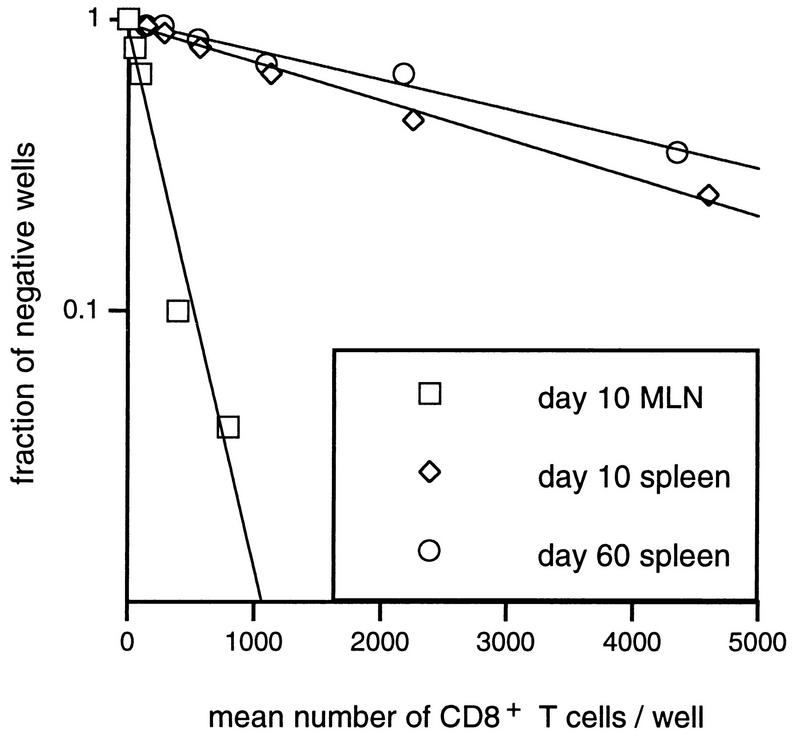
Bulk CTL assays may establish that responding lymphocytes are indeed present in a particular lymphoid organ (Fig. 4C and 5) but provide little insight into the prevalence of the virus-specific T cells. Spleen and MLN populations were thus stimulated for 7 days under LDA conditions to determine CTLp frequencies (Table 1). Typical regression lines are shown in Fig. 6. The results (Table 1; Fig. 6) are expressed relative to the percent CD8+ T cells determined by flow cytometric analysis of the spleen or MLN population used to establish the LDA microcultures.
TABLE 1.
Generation and persistence of the MHV-68-specific CD8+ CTLp responsea
| Day after i.n. infection | Reciprocal CD8+ CTLp
|
|
|---|---|---|
| MLN | Spleen | |
| 10 | 271 | 2,765 |
| 4,909 | ||
| 10 | 219 | 3,817 |
| 13 | 217 | 637 |
| 25 | 2,206 | 3,927 |
| 33 | 4,022 | |
| 60 | 3,441 | |
| 67 | <1/20,000 | 4,880 |
| 92 | 16,437 | |
| 112 | <1/20,000 | 5,998 |
| 134 | 5,437 | |
Cells were pooled from three mice prior to restimulation in each case. The frequencies determined by LDA, corrected for the percentage of CD8+ T cells in the starting population, are expressed as the reciprocal of the number of MHV-68-specific CTLp per total CD8+ T cells. Naive mice had <1/20,000 CTLp in the MLN and <1/100,000 CTLp in the spleen.
FIG. 6.
Regression analysis of virus-specific CTLp frequencies. The plots are typical of the LDA plots used to calculate the data in Table 1. The mean number of CD8+ T cells in the responder cell populations was calculated from the total cell numbers and from flow cytometric staining.
The virus-specific CTLp frequencies for MLN samples from the acute phase of the response were reasonably high (1:200 CD8+ T cells; Table 1), being roughly equivalent to 1:1,000 MLN lymphocytes. However, the CTLp numbers in the MLN tended to decrease dramatically with time (day 67; Table 1). The frequencies in the spleen were lower, but more consistent, from days 10 to 134 after infection (Table 1). In general, the time-related response profiles following bulk culture (Fig. 4C) or LDA (Table 1) show some degree of correlation.
The antibody response.
The level of total serum IgG increased dramatically within 20 days of infection and remained at a consistently high level thereafter (Fig. 7A). This was presumably a consequence of cytokine (or virus)-driven B-cell activation (Fig. 2). CD4+ T cells, which are necessary for the virus-reduced splenomegaly (42), are also likely to have contributed to this polyclonal B-cell response. The net effect may be to diminish the effectiveness of virus-specific humoral immunity. The titers of MHV-68-specific antibody measured by neutralization (Fig. 7B) or ELISA (Fig. 7C) were low during the first 2 weeks or so after infection, though both tended to increase progressively over the subsequent 50 to 70 days (Fig. 7B and C).
FIG. 7.
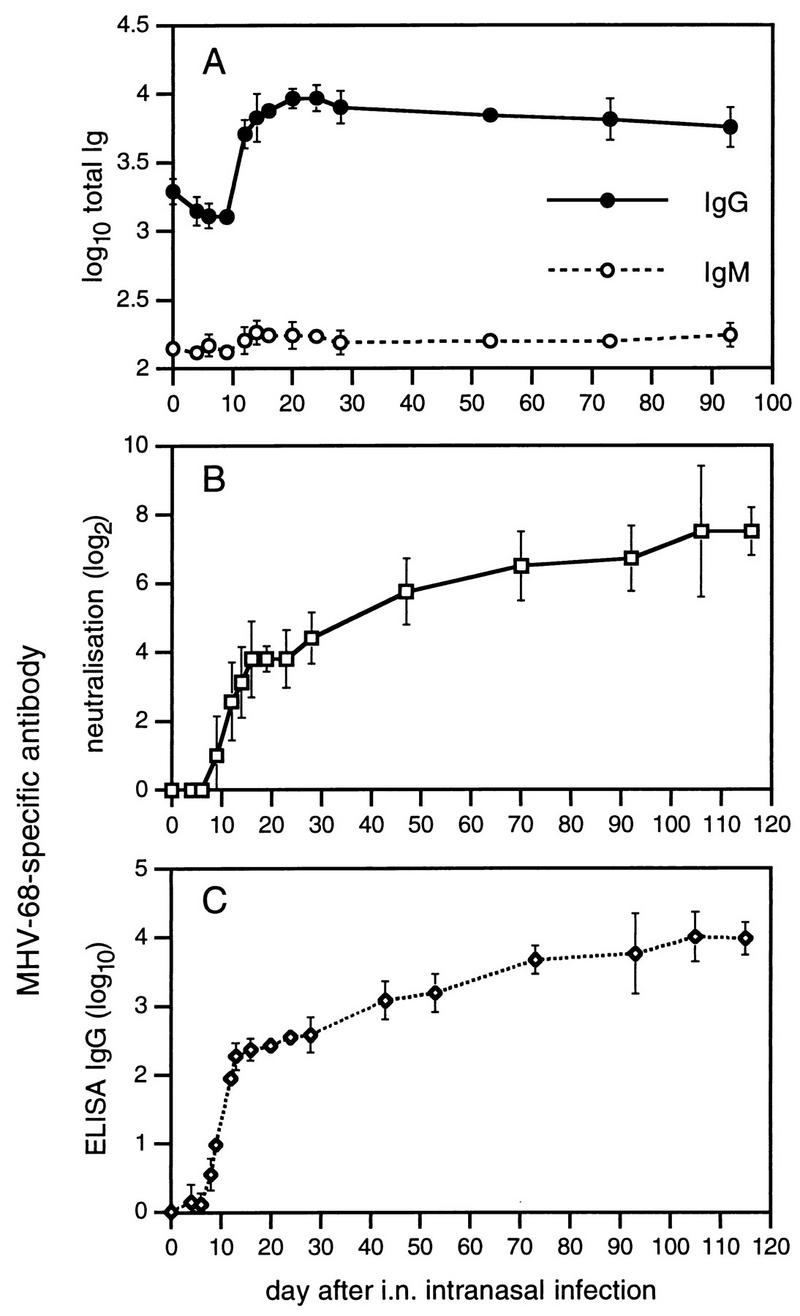
Kinetics of the virus-specific and total serum Ig response in mice infected with MHV-68. Each point shows the mean and standard deviation of results from three to six individual mouse sera pooled from four experiments. The neutralizing antibody titer (B) was determined by plaque inhibition (see Materials and Methods), whereas other antibody titers (A and C) were determined by ELISA. All measurements were made relative to a standard pool of immune sera and are expressed in arbitrary units.
DISCUSSION
This is the first time that we have been able to demonstrate MHV-68-specific CTL activity, though use of the redirected assay with both conventional and perforin knockout (−/−) H-2b-mice (16) indicated that potent CTL effectors mediating target cell death via either the perforin/granzyme or the Fas/Fas ligand pathway are generated during the course of this infection (5). While perforin −/− H-2d mice were not available for these experiments, it is likely from analysis with other models of viral immunity that this MHV-68-specific CTL assay measures only the perforin/granzyme component (16, 17).
Despite the fact that levels of specific 51Cr release as high as 75% were found in these experiments, it is by no means clear that the present CTL assay is optimal. The HVs as a class have developed a variety of mechanisms to minimize MHC class I+ peptide expression (11, 14, 18, 19, 22, 24). All that we know to date about MHV-68 in this regard is that it does not seem to cause any diminution in the level of MHC class I staining (unpublished data). No peptide epitopes, or even source proteins, have yet been identified for this virus, though the search is in progress. Presumably the CTLs that we detect are specific for lytic-phase proteins. The target cells produce infectious virus and soon develop signs of cytopathology. However, it is also possible that the measurable, redirected CTL activity found in the lymphoid tissue in the long-term reflects the presence of effectors specific for epitopes not expressed by the infected 3T3 cells.
Given this reservation about the assay system, the acute phase of the MHV-68-specific CD8+ T-cell response seems to be reasonably similar to that described for other virus infections. Effector CTLs are present in the inflammatory BAL population recovered from the site of virus replication in the lung, and substantial CTLp frequencies are found in the regional MLN. Also, though evidence of productive infection is not normally detected in homogenized lymphoid tissue, there is some virus-specific CTL activity in the MLN and spleen. This may indicate that virus-specific CTL eliminate any of the latently infected B cells reverting to lytic phase.
The CTLp frequencies found for the MLN during the initial stage of virus replication in the respiratory epithelium are about equivalent to those recorded previously for the negative-strand RNA viruses, such as Sendai virus and the influenza A viruses. However, the progressive decrease in MHV-68-specific MLN CTLp frequencies does not occur in mice that have recovered from the transient infections caused by these RNA viruses. Also, the CTLp numbers in the spleen are consistently much lower for the MHV-68 model.
Perhaps continuing reactivation of MHV-68 from the large pool of latently infected B cells leads to progressive immune exhaustion (26). This does not, however, seem to be the case for EBV (29), which has a somewhat similar pathogenesis. Another possibility, in keeping with the high proportion of activated T cells in the MLN at 10 to 25 days after infection (Fig. 2), is that the MHV-68-specific memory CTLp population is maintained in a state of partial activation that leads at least some lymphocytes to undergo apoptosis after further stimulation. Both potent CTL effectors and reasonable CTLp frequencies could be demonstrated in lymphoid tissue before the mononucleosis phase of virus-driven lymphocyte activation (36). Further analysis of CTLp profiles may be more profitably pursued when peptide-pulsed (rather than virus-infected) stimulators can be used in the LDA protocol. A somewhat different picture of the CD8+ T-cell response in the long-term may well emerge when assays are developed to measure CTLp frequencies for epitopes expressed during the latent phase of the infection.
Application of the IFN-γ ELISPOT assay to lymphocyte populations stimulated under bulk culture conditions indicated that MHV-68-specific CD4+ T-cell memory is also sustained in the long term. Earlier studies with CD4-deficient MHC class II −/− H-2b-mice suggested that virus-specific CD4+ Th is required to maintain effective CD8+ T-cell-mediated control of persistent MHV-68 infection. The lytic phase in the lung epithelium reemerged in these MHC class II −/− mice, which died from a progressive wasting disease after about 120 days (5). The Th may also function to promote the production of virus-specific IgG, which could act to limit the spread of the reactivated virus (15).
Analysis of virus-specific Ig (ELISA) and neutralizing serum antibody profiles indeed suggests that there is continuing, MHV-68-specific Th in the lymphoid tissue, contributing to the pattern of a low, delayed IgG class response that increases in magnitude over several months. The fact that significant virus-specific Ig levels are not detected until some 20 days after the initiation of the infection may explain why only CD8+ (and not CD4+) T-cell-mediated effector mechanisms are able to clear MHV-68 from the respiratory tract (10). Perhaps the very extensive B-cell activation (36, 41) that occurs during the first month or more following the initial contact with MHV-68 subverts the specific humoral response. Similar B-cell activation has been described for EBV, but unlike the situation for MHV-68, the increase is predominantly in serum IgM levels (30). Naive B cells produce substantial amounts of IL-6 following in vitro infection with MHV-68 (32). Terminal B-cell differentiation is known to be induced by IL-6 (1). It is also possible that the virus-specific CD8+ effectors eliminate B cells that are both specific for MHV-68 and infected with the virus (21), although any such effect cannot be absolute as there is a progressive increase in neutralizing antibody titers.
This is the first kinetic analysis of immunity to a DNA virus that is maintained as a latent infection in murine lymphoid tissue. The analysis to date indicates that the continued presence of MHV-68 does not tend to increase the magnitude of CD8+ T-cell memory to peptides expressed during the acute phase of the infectious process (7). The memory CD8+ CTLp pool specific for the readily eliminated negative-strand RNA viruses is maintained at a much higher level. Perhaps the MHV-68-specific CTLp population specific for these lytic epitopes is constantly being utilized to provide the effectors that limit further virus production through the persistent phase of the infection (5).
ACKNOWLEDGMENTS
We thank Kristen Branum for technical assistance, Vicki Henderson for assistance with the paper, and Rhonda Cardin for advice on the virology aspects.
This work was supported by Public Health Service grants AI38359 and CA21765 and by the American Lebanese-Syrian Associated Charities. P.G.S. is the recipient of an MRC (UK) traveling fellowship.
REFERENCES
- 1.Akira S, Yoshida K, Tanaka T, Taga T, Kishimoto T. Targeted disruption of the IL-6 related genes: gp130 and NF-IL-6. Immunol Rev. 1995;148:221–253. doi: 10.1111/j.1600-065x.1995.tb00100.x. [DOI] [PubMed] [Google Scholar]
- 2.Allan W, Tabi Z, Cleary A, Doherty P C. Cellular events in the lymph node and lung of mice with influenza. Consequences of depleting CD4+ T cells. J Immunol. 1990;144:3980–3986. [PubMed] [Google Scholar]
- 3.Borysiewicz L K, Sissons J G. Cytotoxic T cells and human herpes virus infections. Curr Top Microbiol Immunol. 1994;189:123–150. doi: 10.1007/978-3-642-78530-6_8. [DOI] [PubMed] [Google Scholar]
- 4.Bowden R J, Simas J P, Davis A J, Efstathiou S. Murine γ-herpesvirus 68 encodes tRNA-like sequences which are expressed during latency. J Gen Virol. 1997;78:1675–1687. doi: 10.1099/0022-1317-78-7-1675. [DOI] [PubMed] [Google Scholar]
- 5.Cardin R D, Brooks J W, Sarawar S R, Doherty P C. Progressive loss of CD8+ T cell-mediated control of a γ-herpesvirus in the absence of CD4+ T cells. J Exp Med. 1996;184:863–871. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang Y, Cesarman E, Pessin M S, Lee F, Culpepper J, Knowles D M, Moore P S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 7.Doherty P C, Topham D J, Tripp R A. Establishment and persistence of virus-specific CD4+ and CD8+ T cell memory. Immunol Rev. 1996;150:23–44. doi: 10.1111/j.1600-065x.1996.tb00694.x. [DOI] [PubMed] [Google Scholar]
- 8.Efstathiou S, Ho Y M, Hall S, Styles C J, Scott S D, Gompels U A. Murine herpesvirus 68 is genetically related to the γ-herpesviruses Epstein-Barr virus and herpesvirus saimiri. J Gen Virol. 1990;71:1365–1372. doi: 10.1099/0022-1317-71-6-1365. [DOI] [PubMed] [Google Scholar]
- 9.Efstathiou S, Ho Y M, Minson A C. Cloning and molecular characterization of the murine herpesvirus 68 genome. J Gen Virol. 1990;71:1355–1364. doi: 10.1099/0022-1317-71-6-1355. [DOI] [PubMed] [Google Scholar]
- 10.Ehtisham S, Sunil-Chandra N P, Nash A A. Pathogenesis of murine gammaherpesvirus infection in mice deficient in CD4 and CD8 T cells. J Virol. 1993;67:5247–5252. doi: 10.1128/jvi.67.9.5247-5252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frisan T, Zhang Q J, Levitskaya J, Coram M, Kurilla M G, Masucci M G. Defective presentation of MHC class I-restricted cytotoxic T-cell epitopes in Burkitt’s lymphoma cells. Int J Cancer. 1996;68:251–258. doi: 10.1002/(SICI)1097-0215(19961009)68:2<251::AID-IJC19>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 12.Ganem D. AIDS. Viruses, cytokines and Kaposi’s sarcoma. Curr Biol. 1995;5:469–471. doi: 10.1016/s0960-9822(95)00093-5. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton-Easton A, Eichelberger M. Virus-specific antigen presentation by different subsets of cells from lung and mediastinal lymph node tissues of influenza virus-infected mice. J Virol. 1995;69:6359–6366. doi: 10.1128/jvi.69.10.6359-6366.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill A, Jugovic P, York I, Russ G, Bennink J, Yewdell J, Ploegh H, Johnson D. Herpes simplex virus turns off the TAP to evade host immunity. Nature. 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 15.Jonjic S, Pavic I, Polic B, Crnkovic I, Lucin P, Koszinowski U H. Antibodies are not essential for the resolution of primary cytomegalovirus infection but limit dissemination of recurrent virus. J Exp Med. 1994;179:1713–1717. doi: 10.1084/jem.179.5.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen K J, Podack E R, Zinkernagel R M, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 17.Kägi D, Hengartner H. Different roles for cytotoxic T cells in the control of infections with cytopathic versus noncytopathic viruses. Curr Opin Immunol. 1996;8:472–477. doi: 10.1016/s0952-7915(96)80033-1. [DOI] [PubMed] [Google Scholar]
- 18.Khanna R, Burrows S R, Moss D J, Silins S L. Peptide transporter (TAP-1 and TAP-2)-independent endogenous processing of Epstein-Barr virus (EBV) latent membrane protein 2A: implications for cytotoxic T-lymphocyte control of EBV-associated malignancies. J Virol. 1996;70:5357–5362. doi: 10.1128/jvi.70.8.5357-5362.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleijnen M F, Huppa J B, Lucin P, Mukherjee S, Farrell H, Campbell A E, Koszinowski U H, Hill A B, Ploegh H L. A mouse cytomegalovirus glycoprotein, gp34, forms a complex with folded class I MHC molecules in the ER which is not retained but is transported to the cell surface. EMBO J. 1997;16:685–694. doi: 10.1093/emboj/16.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein G. Epstein-Barr virus strategy in normal and neoplastic B cells. Cell. 1994;77:791–793. doi: 10.1016/0092-8674(94)90125-2. [DOI] [PubMed] [Google Scholar]
- 21.Kyburz D, Speiser D E, Aebischer T, Hengartner H, Zinkernagel R M. Virus-specific cytotoxic T cell-mediated lysis of lymphocytes in vitro and in vivo. J Immunol. 1993;150:5051–5058. [PubMed] [Google Scholar]
- 22.Levitskaya J, Coram M, Levitsky V, Imreh S, Steigerwald-Mullen P M, Klein G, Kurilla M G, Masucci M G. Inhibition of antigen processing by the internal repeat region of the Epstein-Barr virus nuclear antigen-1. Nature. 1995;375:685–688. doi: 10.1038/375685a0. [DOI] [PubMed] [Google Scholar]
- 23.Lowin B, Hahne M, Mattmann C, Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature. 1994;370:650–652. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- 24.Machold R P, Wiertz E J, Jones T R, Ploegh H L. The HCMV gene products US11 and US2 differ in their ability to attack allelic forms of murine major histocompatibility complex (MHC) class I heavy chains. J Exp Med. 1997;185:363–366. doi: 10.1084/jem.185.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McWilliam A S, Marsh A M, Holt P G. Inflammatory infiltration of the upper airway epithelium during Sendai virus infection: involvement of epithelial dendritic cells. J Virol. 1997;71:226–236. doi: 10.1128/jvi.71.1.226-236.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moskophidis D, Lechner F, Pircher H, Zinkernagel R M. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 27.Nash A A, Sunil-Chandra N P. Interactions of the murine γ-herpesvirus with the immune system. Curr Opin Immunol. 1994;6:560–563. doi: 10.1016/0952-7915(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 28.Podack E R. Functional significance of two cytolytic pathways of cytotoxic T lymphocytes. J Leukocyte Biol. 1995;57:548–552. doi: 10.1002/jlb.57.4.548. [DOI] [PubMed] [Google Scholar]
- 29.Rickinson A B, Moss D J. Human cytotoxic T lymphocyte responses to Epstein-Barr virus infection. Annu Rev Immunol. 1997;15:405–431. doi: 10.1146/annurev.immunol.15.1.405. [DOI] [PubMed] [Google Scholar]
- 30.Rosen A, Gergely P, Jondal M, Klein G, Britton S. Polyclonal Ig production after Epstein-Barr virus infection of human lymphocytes in vitro. Nature. 1977;267:52–54. doi: 10.1038/267052a0. [DOI] [PubMed] [Google Scholar]
- 31.Sarawar S R, Cardin R D, Brooks J W, Mehrpooya M, Hamilton-Easton A M, Mo X Y, Doherty P C. Interferon gamma is not essential for recovery from acute infection with murine gammaherpesvirus 68. J Virol. 1997;71:3916–3921. doi: 10.1128/jvi.71.5.3916-3921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarawar S R, Cardin R D, Brooks J W, Mehrpooya M, Tripp R A, Doherty P C. Cytokine production in the immune response to murine gammaherpesvirus 68. J Virol. 1996;70:3264–3268. doi: 10.1128/jvi.70.5.3264-3268.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarawar S R, Doherty P C. Concurrent production of interleukin-2, interleukin-10, and gammainterferon on in the regional lymph nodes of mice with influenza pneumonia. J Virol. 1994;68:3112–3119. doi: 10.1128/jvi.68.5.3112-3119.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sunil-Chandra N P, Efstathiou S, Nash A A. Murine γ-herpesvirus 68 establishes a latent infection in mouse B lymphocytes in vivo. J Gen Virol. 1992;73:3275–3279. doi: 10.1099/0022-1317-73-12-3275. [DOI] [PubMed] [Google Scholar]
- 35.Tough D F, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 36.Tripp R A, Hamilton-Easton A M, Cardin R D, Nguyen P, Behm F G, Woodland D L, Doherty P C, Blackman M A. Pathogenesis of an infectious mononucleosis-like disease induced by a murine γ-herpesvirus: role for a viral superantigen? J Exp Med. 1997;185:1641–1650. doi: 10.1084/jem.185.9.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tripp R A, Hou S, Doherty P C. Temporal loss of the activated L-selectin-low phenotype for virus-specific CD8+ memory T cells. J Immunol. 1995;154:5870–5875. [PubMed] [Google Scholar]
- 38.Tripp R A, Hou S, McMickle A, Houston J, Doherty P C. Recruitment and proliferation of CD8+ T cells in respiratory virus infections. J Immunol. 1995;154:6013–6021. [PubMed] [Google Scholar]
- 39.Tripp R A, Topham D J, Watson S R, Doherty P C. Bone marrow can function as a lymphoid organ during a primary immune response under conditions of disrupted lymphocyte trafficking. J Immunol. 1997;158:3716–3720. [PubMed] [Google Scholar]
- 40.Usherwood E J, Brooks J W, Sarawar S R, Cardin R D, Young W D, Allen D J, Doherty P C, Nash A A. Immunological control of murine γ-herpesvirus infection is independent of perforin. J Gen Virol. 1997;78:2025–2030. doi: 10.1099/0022-1317-78-8-2025. [DOI] [PubMed] [Google Scholar]
- 41.Usherwood E J, Ross A J, Allen D J, Nash A A. Murine γ-herpesvirus-induced splenomegaly: a critical role for CD4 T cells. J Gen Virol. 1996;77:627–630. doi: 10.1099/0022-1317-77-4-627. [DOI] [PubMed] [Google Scholar]
- 42.Usherwood E J, Stewart J P, Robertson K, Allen D J, Nash A A. Absence of splenic latency in murine γ-herpesvirus 68-infected B cell-deficient mice. J Gen Virol. 1996;77:2819–2825. doi: 10.1099/0022-1317-77-11-2819. [DOI] [PubMed] [Google Scholar]
- 43.Weck K E, Barkon M L, Yoo L I, Speck S H, Virgin H W. Mature B cells are required for acute splenic infection, but not for establishment of latency, by murine gammaherpesvirus 68. J Virol. 1996;70:6775–6780. doi: 10.1128/jvi.70.10.6775-6780.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiertz E J, Jones T R, Sun L, Bogyo M, Geuze H J, Ploegh H L. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell. 1996;84:769–779. doi: 10.1016/s0092-8674(00)81054-5. [DOI] [PubMed] [Google Scholar]