Abstract
Background:
Psychosocial interventions are effective for alcohol use disorder (AUD), but their impact on patients with chronic liver disease and AUD remains unclear. This systematic review and meta-analysis evaluated the effectiveness of psychosocial therapies in preventing return-to-drinking and promoting abstinence among this population.
Methods and Results:
The protocol (CRD42024527757) was registered in Prospero. We included studies involving adults (≥18 y) with chronic liver disease and AUD or harmful alcohol use receiving psychosocial interventions. Databases searched were PubMed, Embase, Cochrane CENTRAL, and APA PsycInfo. Outcomes assessed were return-to-drinking and abstinence. Risk of bias was evaluated using RoB2 for randomized controlled trials (RCTs) and ROBINS-I for non-RCTs. Meta-analysis was performed using random effects (Knap-Hartung adjustment) and fixed effects (inverse variance) models. Funding was provided by the intramural research program at the National Institute of Diabetes and Digestive and Kidney Diseases. Of 2240 studies reviewed, 20 met the inclusion criteria. Meta-analysis of 7 non-RCTs (n=767) demonstrated a 48% reduction in return-to-drinking risk (relative risk [RR]=0.52, 95% CI: 0.28–0.99, p=0.048) with significant heterogeneity (I 2=74%; p=0.006). Subgroup analyses showed greater return-to-drinking reduction in multidisciplinary care (RR=0.40, 95% CI: 0.31–0.52) and recipients of liver transplant (RR=0.40, 95% CI: 0.27–0.58). Four RCTs found no significant return-to-drinking reduction (RR=0.99, 95% CI: 0.45–2.16, p=0.96). Abstinence was not significantly improved in either RCTs or non-RCTs, with moderate- to low-quality evidence.
Conclusions:
Psychosocial therapy reduces return-to-drinking risk in chronic liver disease with AUD, particularly within multidisciplinary teams or transplant populations, but findings are tempered by scarce RCT evidence. Future RCTs should evaluate multidisciplinary interventions in transplant populations.
Keywords: abstinence, digital health, liver transplant, multidisciplinary treatment, return-to-drinking prevention
INTRODUCTION
Alcohol-associated liver disease (ALD) incidence is rising and driving increases in cirrhosis-related mortality, particularly in younger patients. 1 ALD has since replaced HCV as the number one indication for liver transplantation in the United States. 2 Globally, in 2019, alcohol accounted for ~56% of all deaths caused by liver cirrhosis. 3 While over 90% of the patients with heavy drinking will develop steatosis, only 8%–20% will develop cirrhosis. 4
Conversely, alcohol use can also complicate existing chronic liver diseases (CLD), such as viral hepatitis, where concomitant alcohol use disorder (AUD) is common and significantly increases the risk of progression to advanced fibrosis or cirrhosis. 5 Prior HCV cohorts estimated 41% of patients with HCV have moderate to heavy alcohol use, and this proportion increases to 59.7% in those with HCV and i.v. drug use. 5 In metabolic dysfunction–associated steatotic liver disease, up to 30% of patients had positive hair ethyl glucuronide for moderate to excessive alcohol use, suggesting alcohol use is underreported even among the CLD population. 6 Furthermore, concomitant alcohol use can increase mortality in CLD. 7 Alcohol cessation, while beneficial, is challenging, and although there are 4 FDA-approved medications to treat AUD, <10% of eligible patients receive them. 8 More commonly, patients with AUD receive counseling. Currently, common psychosocial interventions for AUD include motivational interviewing (MI), 9 motivational enhancement therapy (MET), 10 cognitive behavioral therapy (CBT), 11 twelve-step facilitation (TSF), 12 and various other group therapy approaches. Despite the promise of psychosocial therapies in patients with CLD and AUD, a minority receive therapy; one study showed only 12% of patients with cirrhosis and AUD had received psychosocial therapy, while another study showed only 34% had received therapy of any kind.13,14 Furthermore, patients with advanced liver disease may have neuropsychiatric complications such as HE or fatigue, which can complicate the efficacy of psychosocial therapy. 15
Prior systematic reviews have evaluated the effectiveness of psychosocial therapy in maintaining abstinence or reducing alcohol drinking in patients with CLD and AUD, but did not have sufficient studies to perform a meta-analysis.16,17 A recent meta-analysis showed the odds of alcohol return-to-drinking appeared lower (OR=0.48) in recipients of liver transplant (LT) when they received addiction care integrated with hepatology compared with hepatology alone. 18 Furthermore, another meta-analysis conducted exclusively in patients with ALD showed that any treatment is associated with a reduction in return-to-drinking, but a majority of the studies focused solely on pharmacotherapy. 19 Only 2 studies in this meta-analysis evaluated psychosocial therapy independently, with 1 study only evaluating 14 patients. Since psychosocial therapy can be time-intensive and needs more resources to deliver, we perform a systematic review and meta-analysis to specifically evaluate whether psychosocial therapy, compared with standard care, improves abstinence or decreases alcohol return-to-drinking in CLD populations with harmful alcohol use or AUD.
METHODS
The methods for this systematic review and meta-analysis were developed in accordance with the PRISMA 2020 guidelines to ensure comprehensive and transparent reporting. 20 Our review protocol was registered with PROSPERO (PROSPERO: CRD42024527757) a priori. The following subsections detail the processes used for study selection, data extraction, risk of bias assessment, and statistical synthesis.
Eligibility criteria
Study selection and participants
We included randomized controlled trials (RCTs) and observational studies (non-RCTs). PICO format (Population, Intervention, Comparator, and Outcome) was used to determine eligibility. Adults 18 years old with CLD and concomitant AUD, alcohol dependence or use were included. Participants with concomitant psychiatric disorders or substance use disorders were included, given the paucity of literature. Reviews, case reports, and animal studies were excluded to maintain a focus on human research. Studies published in any language, provided a full-text English translation was available, until April 30, 2024, were included. The interventions encompassed a variety of psychosocial approaches, including but not limited to MET, CBT, MI, group psychoeducation, TSF, or a combination of approaches. With the guidance and expertise of a psychiatrist (N.D.) and a clinical psychologist (J.W.L.), brief intervention studies were excluded as they were deemed not to provide the full extent of psychosocial therapy. Studies examining only pharmacological therapy were excluded as well. The comparator group consisted of standard care practice without psychosocial therapy, therapeutic nonadherence, or any other interventions considered as comparators by the authors of the respective studies. The primary outcomes were alcohol abstinence and return-to-drinking. Abstinence was defined as the cessation of alcohol drinking after a period of use, while return-to-drinking was defined as drinking after a period of sobriety. Outcomes were detected by alcohol biomarkers or self-reporting and assessed at the longest available follow-up after treatment.
Search and extraction strategy
Search strategy
A librarian (Nancy Terry) provided a search strategy and searched across multiple databases: PubMed, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and APA PsycInfo. Validated search filters were integrated into the search strategy. This strategy underwent testing against a sample of relevant studies and was refined accordingly (Supplemental Table S1, http://links.lww.com/HC9/C121). In addition to electronic searches, additional literature was sourced through citation searches and reference lists of included studies. Clinical trial registries, including ClinicalTrials.gov, were consulted for records published until April 30, 2024. In cases of missing outcomes data or unavailability of full text, study authors were contacted for further information.
Study selection
The search results were imported into EndNote, where duplicate entries were eliminated and subsequently uploaded to Covidence for further review. Eligibility for inclusion into systematic review and meta-analysis was conducted by reviewing title, abstract, and full-text review by 4 reviewers (C.H.S., H.R.C., H.B., and M.R.S.) independently. Any discrepancies were resolved by a fifth independent reviewer (C.C.H.), who was not involved in the initial screening process.
Data extraction and quality assessment
For studies meeting the inclusion criteria, 4 independent reviewers (C.H.S., H.R.C., M.R.S., and J.L.) extracted key participant and intervention details, recording efficacy outcomes using standardized data extraction templates. Data extraction was conducted by 2 reviewers per study, and any discrepancies were resolved through discussion. If necessary, a fifth author (C.C.H.) was involved to facilitate resolution. Data extracted included year and location of study, type of psychosocial therapy, biomarker-confirmed or self-reported alcohol use, etiology of liver disease, duration of psychosocial therapy treatment, noncompliant or standard-of-care comparator groups, and duration of study follow-up. If studies were missing primary outcome information, authors were contacted for missing information. General demographics and clinical characteristics of the cohort—such as sex, ethnicity, concomitant psychiatric disorders, and recreational drug use—were also collected. Quality of evidence for each primary outcome for RCTs and non-RCTs was evaluated independently using the GRADE approach (Grading of Recommendations Assessment, Development, and Evaluation) using GRADEpro software, which is publicly accessible and free, 21 by C.C.H. In short, the quality of evidence starts with RCTs being the highest level of quality, which can be downgraded based on 4 individual factors of risk of bias, inconsistency, indirectness, and imprecision. Each factor is rated according to not serious, serious, and very serious concerns. For example, RCTs can begin as high-quality evidence, which then can be downgraded to moderate quality if there is a high risk of bias or a large degree of heterogeneity resulting in inconsistent results. Observational studies will begin as low-quality evidence studies but subsequently can be upgraded if there is a strong association in effect estimates.
Risk of bias assessment
Two reviewers (J.L. and C.C.H.) independently assessed the risk of bias (ROB) for each included study. Discrepancies were resolved through consensus. Risk of bias reports were constructed using the robvis tool to create graphical representations, such as traffic light and summary plots, of the assessments. 22 The Risk of Bias 2 (RoB2) tool was utilized for RCTs, while the Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-1) was used in non-RCTs.
The RoB2 tool assessed RCTs along 5 domains: bias from the randomization process; intended interventions; missing outcome data; measurement of the outcome; and selection of the reported result. In RoB2, each domain is rated as either low, moderate with some concerns, or high ROB. The ROBINS-1 tool assessed non-RCTs along 7 domains: bias due to confounding; selection of participants; classification of interventions; deviations from intended interventions; missing data; measurement of outcomes; and selection of the reported result. Domains are rated low, moderate, severe, or critical. For both tools, low ROB is assigned if all domains have low ROB, and moderate ROB is assigned if one domain is rated as moderate and there are no high, severe, or critical scores for other domains. In RoB2 for RCTs, high overall ROB is defined as high ROB in at least one domain or moderate ROB across multiple domains. In ROBINS-1 for non-RCTs, severe or critical ROB is assigned if at least one domain had a severe or critical rating, respectively.
Statistical analysis
Effect estimates using relative risk (RR) were calculated using the inverse variance method with Knapp-Hartung adjustments for the random effects model and the inverse variance method for the fixed effects model for both primary outcomes of abstinence and alcohol return-to-drinking. The restricted maximum-likelihood estimator was used to estimate tau-squared. All analyses were performed using RStudio version 4.4.0 employing the “meta” and “metafor” packages. Heterogeneity was assessed visually using forest plots and statistically through a Cochran’s chi-squared (Q test) with a significance level set at p<0.10. The I 2 statistic was used to quantify statistical heterogeneity, with values ≥70% indicating substantial heterogeneity. Both the Baujat plot and the influential analysis were performed. A Baujat plot assessed individual study influence on heterogeneity and effect estimates, and an influential analysis examined the impact of removing each study sequentially, which is recommended by both the Cochrane Handbook (Section 10.10.3) and PRISMA guidelines to ensure conclusions are not influenced by outliers. The funnel plot and statistical tests, such as Harbord’s, were used to evaluate for publication bias. Similarly, subgroup analysis was performed using RStudio employing “meta” and “metafor” packages, and effect estimates were stratified according to RCT and non-RCTs, multidisciplinary team and single provider, LT and non-LT population, and concurrent pharmacological therapy and no pharmacological therapy.
RESULTS
Study selection
After the literature search, 2240 studies were identified following de-duplication (Figure 1). After an abstract and title review, 77 studies were deemed eligible for further full-text review. Fifty-seven were further excluded, with 20 studies remaining for data extraction. Nine were RCTs23–31 and 11 were observational studies (non-RCTs).32–42 The most common reasons for exclusion after full-text review were wrong intervention (n=20) or wrong outcomes described (n=8). Some studies were excluded because they only described a protocol (n=7) without the research results.
FIGURE 1.
PRISMA diagram depicting studies reviewed in full text, excluded, and reasons for excluding.
Study characteristics
The included studies were conducted between 2005 38 and 2024 30 (Table 1). Eleven studies occurred in the United States,23–26,28–31,37,40,42 2 in the United Kingdom,30,32 2 in Italy,39,41 and 1 in China, 27 Germany, 33 Denmark, 34 Canada, 35 Sweden, 38 and Spain. 36 The overall population included 1838 participants, with sample sizes ranging from 14 to 181. The median follow-up was 11 months (IQR: 6, 24). The median age was 52 years old (IQR: 49.3, 55.1), and the proportion of female participants ranged from 0% 29 to 46.2%. 30 The ethnicities were varied, with some cohorts 100% Asian, 27 predominantly Black (63%–89.1%) or all White (100%). 30 Only 4 studies reported concomitant psychiatric disorders,23,26,35,36 while 7 reported on concomitant drug or marijuana use.23,25,26,28,29,35,36 Fourteen studies exclusively examined patients with ALD,25,27,30–40 while most of the remaining studies were of patients with HCV who had harmful or hazardous drinking based on AUDIT or AUDIT-C, or had diagnoses of AUD or alcohol dependence based on DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, 4th Edition) criteria.23,24,26,28,29,42 Two studies, which recruited only patients who were co-infected with HIV/HCV, enrolled patients with any alcohol use24,29; however, of these studies, 1 reported an average intake of 5.42±5.96 drinks per day, 24 while the other study had a mean AUDIT-C score >3, 29 indicating cohorts with moderate to heavy drinking.
TABLE 1.
Summary of included studies
| References | Country | Data collection | Type | Study type | LT vs. NLT | Multidisciplinary team vs. single provider | Liver etiology | Abstinence status at the beginning of the study | Relapse measure | Longest follow-up time (mo) | Treatment duration (mo) | Age (mean, y) | Race | Sex (% male) | Participants (N) | Psychotherapy modality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weinrieb et al 25 | USA | 2000–2004 | Individual | RCT | NLT | Single provider | ALD | Abstinent | Self-reported, biomarker | 6 | 6 | I: 51 | White: 85% Other: 15% |
85 | 46 | MET |
| C: 48 | White: 78% Other: 22% |
82 | 45 | TAU: referral to AA and outpatient programs, but confirmed no MET | ||||||||||||
| North et al 26 | USA | 2008–2010 | Group-Based | RCT | NLT | Single provider | HCV | Mixed abstinence | Biomarker | 6 | 6 | 53 | White: 32% Black: 63% Other: 4% |
61 | 66 | Psychoeducation |
| 48 | General education | |||||||||||||||
| Shen et al 27 | China | 2015–2016 | Individual | RCT | NLT | Multi-D team | ALD | Abstinent | Self-reported | 3 | 3 | I: 44 | Asian: 100% | 80 | 37 | CBT, TSF |
| C: 43 | 64 | 38 | General education | |||||||||||||
| Carrique et al 35 | Canada | 2018–2020 | Individual | Non-RCT | LT | Multi-D team | ALD | Abstinent | Self-reported, biomarker | 11 | 11 | I: 53 | a | 64 | 44 | CBT |
| C: 57 | 76 | 111 | No treatment | |||||||||||||
| López-Pelayo et al 36 | Spain | 1999–2012 | Individual | Non-RCT | NLT | Single provider | ALD | Not described | Self-reported, biomarker | 24 | 24 | 49 | a | 67 | 43 | CBT |
| 77 | Noncompliant | |||||||||||||||
| Björnsson et al 38 | Sweden | 1988–2003 | Group-based | Non-RCT | LT | Multi-D team | ALD | Abstinent | Self-reported | 31 | 12 | 53 | a | 79 | 58 | TSF |
| 40 | No treatment | |||||||||||||||
| Addolorato et al 39 | Italy | 1995–2010 | Individual | Non-RCT | LT | Multi-D team | ALD | Abstinent | Self-reported, biomarker | a | 6 | 49 | a | 89 | 47 | CBT, MET |
| 25 | No treatment | |||||||||||||||
| Peeraphatdit et al 40 | USA | 2013–2017 | Individual | Non-RCT | NLT | Single provider | ALD | Nonabstinent | Biomarker | a | 12 | 50 | White: 86% | 58 | 27 | MET, CBT, group support |
| 108 | No treatment | |||||||||||||||
| Attilia et al 41 | Italy | 2004–2013 | Individual | Non-RCT | LT | Multi-D team | ALD | Abstinent | Self-reported | 60 | 12 | I: 56 | a | 87 | 69 | Other: addiction team-led identification of craving symptoms and risk factors. |
| C: 53 | 78 | 18 | No treatment | |||||||||||||
| Stein et al 24 | USA | 2015–2017 | Individual | RCT | NLT | Single provider | HCV | Nonabstinent | Self-reported, biomarker | 24 | 18 | I: 52 | White: 37% Black: 37% Other: 26% |
83 | 43 | MI |
| C: 51 | White: 40% Black: 40% Other: 21% |
82 | 37 | General education | ||||||||||||
| Dieperink et al 28 | USA | 2008–2011 | Individual | RCT | NLT | Single provider | HCV | Nonabstinent | Self-reported, biomarker | 6 | 3 | I: 56 | White: 69% Black: 29% Other: 3% |
94 | 59 | MET |
| C: 55 | White: 66% Black: 31% Other: 3% |
94 | 61 | General health education | ||||||||||||
| Edelman et al 29 | USA | 2013–2015 | Individual | RCT | NLT | Single provider | HCV | Nonabstinent | Self-reported, biomarker | 12 | 6 | I: 60 | White: 16% Black: 82% Other: 2.0% |
100 | 37 | MET, ISAT (integrated stepped alcohol treatment) |
| C: 62 | White: 7% Black: 89% Other: 4% |
98 | 31 | TAU: annual screening involving health promotion, brief interventions, and referral to addiction treatment as indicated | ||||||||||||
| Dhanda et al 30 | USA, UK | 2021–2022 | Individual | RCT | NLT | Single provider | ALD | Nonabstinent | Self-reported, biomarker | 6 | 6 | I: 49 | White: 100% | 71 | 9 | MI |
| C: 50 | 54 | 11 | TAU: one brief MI-based session | |||||||||||||
| DeMartini et al 31 | USA | 2013 | Individual | RCT | NLT | Multi-D team | ALD | Nonabstinent | Biomarker | 2 | 2 | I: 51 | White: 100% | 73 | 8 | CBT, MET via Digital App |
| C: 52 | White: 86% | 72 | 6 | TAU: evaluated by the liver transplant team and allowed outside treatment | ||||||||||||
| Mehta et al 32 | UK | 2016–2018 | Individual | Non-RCT | NLT | Single provider | ALD | Nonabstinent | Self-reported | 12 | 3 | I: 49 | White: 79% Other: 29% |
79 | 14 | CBT, MET through Digital App |
| C: 46 | White: 78% Other: 22% |
78 | 27 | Noncompliant | ||||||||||||
| Andersen et al 34 | Denmark | 2009–2010 | Individual | Non-RCT | NLT | Single provider | ALD | No description | Self-reported | a | 9 | 55 | a | 63 | 19 | Other: reviewing biomarkers and psychoeducation |
| 79 | 14 | No Information | ||||||||||||||
| Alexandre et al 37 | USA | 2014–2015 | Individual | Non-RCT | NLT | Single provider | ALD | Mixed abstinence | Self-reported | 60 | a | 56 | White: 90% Black: 7% Other: 3% |
67 | 36 | CBT |
| 178 | No treatment | |||||||||||||||
| Dieperink et al 42 | USA | 2003–2004 | Individual | Non-RCT | NLT | Single provider | HCV | Mixed abstinence | Self-reported, biomarker | 22 | a | 51 | White: 62%Black: 19% Other: 19% |
62 | 39 | CBT, MET, Other: 5 patients do not report a specific therapy |
| 8 | No treatment | |||||||||||||||
| Proeschold-Bell et al 23 | USA | 2014–2017 | Individual | RCT | NLT | Multi-D Team | HCV | Mixed abstinence | Self-reported | 12 | 6 | 55 | White: 24% Black: 21% Other: 5% |
72 | 82 | CBT, MET |
| White: 38% Black: 47% Other: 15% |
71 | 72 | TAU: medical provider-delivered screening, brief intervention, and referral to treatment | |||||||||||||
| Erim et al 33 | Germany | a | Group-based | Non-RCT | NLT | Single provider | ALD | Mixed abstinence | Self-reported, biomarker | 6 | 6 | 53 | a | 55 | 42 | CBT |
| 66 | 58 | Noncompliant |
Data not available or not described.
Abbreviations: AA, Alcoholics Anonymous; ALD, alcohol-associated liver disease; C, control; CBT, cognitive behavioral therapy; I, intervention; LT, liver transplant; MET, motivational enhancement therapy; MI, motivational interviewing; Multi-D, multidisciplinary; NLT, non-liver transplant; RCT, randomized controlled trial; T, total cohort; TAU, treatment as usual; TSF, twelve-step facilitation.
Characteristics of interventions
Individual psychosocial therapies included CBT,33,35–37 MET,25,28 MI,24,30 group psychoeducation, 26 and TSF. 38 Some studies used a mix of various therapies, most frequently incorporating CBT and another psychosocial therapy.23,27,31,32,39,40,42 Specifically, CBT and MET were combined in 6 studies,23,31,32,39,40,42 2 of which combined them in a digital platform,31,32 while 1 study employed a combination of CBT and TSF. 27 MET was combined with integrated stepped alcohol treatment in 1 study. 29 The last 2 studies crafted a rehabilitation program based on psychosocial elements.34,41 The effect of these treatments was compared with treatment as usual in 5 cases,23,25,29–31 no treatment in 7 cases,35,37–42 nonpsychosocial education in 4 cases,24,26–28 noncompliance in 3 cases,32,33,36 and undescribed historic controls in 1 study. 34 The duration of these treatments ranged from text messages delivered over the course of 2 months 31 to weekly therapy for 24 months with a median treatment duration of 6 months (IQR: 5, 11.5). 36 Alcohol use was based on self-reporting,23,24,27,32,34,37,38,41 alcohol biomarkers alone,26,31,40 or a combination of both.25,28–30,33,35,36,39,42
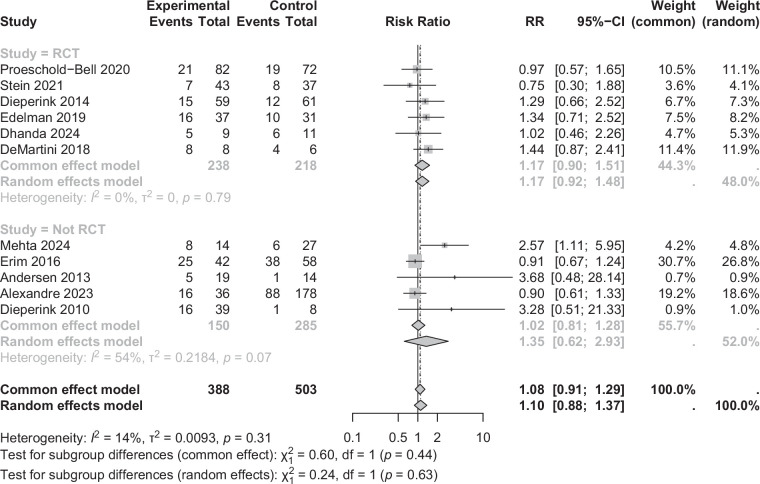
Effects of interventions on alcohol return-to-drinking
Return-to-drinking was considered as a primary outcome in 11 studies (Figure 2), which included a total of 1081 patients.23,25,27,29,33,35,36,38–41 A statistically significant protective effect of psychosocial therapy against return-to-drinking was observed in 4 of the studies,38–41 none of which were RCTs. The first study compared a combined approach of MET, CBT, and group support to no treatment in a cohort of patients with ALD after a recent hospital discharge. 40 This study observed a significant reduction in return-to-drinking by 83% (RR=0.17, 95% CI: 0.04–0.64). The second study compared the effects of a mixed CBT and MI psychosocial technique in a cohort of patients with ALD to no treatment. 39 There was a statistically significant risk reduction by 63% (RR=0.37, 95% CI: 0.18–0.74). A third study noted a statistically significant decrease in risk by 53% (RR=0.47, 95% CI: 0.26–0.84) in patients with ALD receiving MET compared with no treatment. 38 The fourth study showed that a multidisciplinary addiction team, which incorporated psychosocial therapy in patients undergoing LT for ALD, led to a statistically significant decrease in return-to-drinking by 74% (RR=0.26, 95% CI: 0.10–0.71) after transplant compared with a historical cohort of patients with ALD receiving standard of care without the treatment by the addiction team. 41 In total, across the 7 non-RCT studies, psychosocial therapy is associated with a statistically significant 48% (RR= 0.52, 95% CI: 0.28–0.99, p=0.048) and 28% (RR=0.72, 95% CI: 0.57–0.91, p=0.006) risk reduction for return-to-drinking in random effects and fixed effects model, respectively (Figure 2). The non-RCTs demonstrated substantial heterogeneity (I 2=74%), confirmed by a statistically significant Cochran’s Q test (p=0.0009). Influential analysis showed that removing individual studies did not largely reduce the heterogeneity or change the effect estimate (Table 2). Utilizing a Baujat plot (Supplemental Figure S1, http://links.lww.com/HC9/C121), Erim et al 33 and López-Pelayo et al 36 were noted to be the outlier studies that contributed largely to the heterogeneity and to the effect estimate. These 2 studies were also different from other non-RCT studies because the comparator group was a noncompliant group, whereas the other studies used historical controls or control groups that underwent standard of care. Therefore, upon removing these 2 studies, heterogeneity decreased to 0% and showed a relative risk reduction of RR=0.37 (95% CI: 0.24–0.56, p=0.003), which is similar to the RR with all 7 non-RCTs included (Table 2).
FIGURE 2.
Forest plot delineating the relative risk reduction of psychosocial therapies on preventing alcohol return-to-drinking stratified by randomized and nonrandomized controlled trials.
TABLE 2.
Influential analyses using random effects models according to outcomes of preventing alcohol return-to-drinking or promoting abstinence stratified by nonrandomized controlled trials and randomized controlled trials
| Omitted study or studies | RR | 95% CI | p | I 2 (%) |
|---|---|---|---|---|
| Alcohol return to drinking non-RCT influential analysis | ||||
| Erim et al 33 | 0.45 | 0.23–0.87 | 0.026 | 72.9 |
| Carrique et al 35 | 0.53 | 0.25–1.15 | 0.089 | 77.2 |
| López-Pelayo et al 36 | 0.45 | 0.23–0.89 | 0.031 | 67.4 |
| Bjornsson et al 38 | 0.52 | 0.23–1.17 | 0.092 | 75.4 |
| Addolorato et al 39 | 0.55 | 0.25–1.20 | 0.107 | 73.4 |
| Peeraphatdit et al 40 | 0.59 | 0.31–1.13 | 0.09 | 72.4 |
| Attilia et al 41 | 0.58 | 0.28–1.19 | 0.107 | 73.2 |
| Erim et al 33 + Lopez-Pelayo et al 36 | 0.37 | 0.25–0.56 | 0.003 | 0.0 |
| Alcohol return-to-drinking RCT influential analysis | ||||
| Proeschold-Bell et al 23 | 0.89 | 0.25–3.14 | 0.72 | 50.5 |
| Weinrieb et al 25 | 0.94 | 0.19–4.57 | 0.89 | 59.1 |
| North et al 26 | 0.89 | 0.18–4.31 | 0.78 | 52.6 |
| Shen et al 27 | 1.20 | 0.83–1.74 | 0.17 | 0.0 |
| Abstinence non-RCT influential analysis | ||||
| Erim et al 33 | 1.76 | 0.59–5.26 | 0.20 | 59.9 |
| Mehta et al 32 | 0.94 | 0.62–1.44 | 0.69 | 15.3 |
| Andersen et al 34 | 1.24 | 0.49–3.11 | 0.52 | 57.5 |
| Alexandre et al 37 | 1.75 | 0.59–5.20 | 0.20 | 62.7 |
| Dieperink et al 42 | 1.24 | 0.48–3.17 | 0.52 | 57.7 |
| Abstinence RCT influential analysis | ||||
| Proeschold-Bell et al 23 | 1.24 | 0.93–1.6379 | 0.11 | 0.0 |
| Stein et al 24 | 1.21 | 0.96–1.5259 | 0.08 | 0.0 |
| Dieperink et al 28 | 1.15 | 0.85–1.5501 | 0.28 | 0.0 |
| Edelman et al 29 | 1.14 | 0.84–1.5273 | 0.30 | 0.0 |
| Dhanda et al 30 | 1.19 | 0.89–1.5900 | 0.18 | 0.0 |
| DeMartini et al 31 | 1.09 | 0.84–1.4082 | 0.44 | 0.0 |
Abbreviations: RCT, randomized controlled trial; RR, relative risk.
In contrast, the 4 RCTs demonstrated a pooled RR of 0.99 (95% CI: 0.45–2.16, p=0.96) in the random effects model and 1.01 (95% CI: 0.72–1.43, p=0.94) in the fixed effects model, indicating no significant effect on alcohol return-to-drinking. RCTs show low heterogeneity (I 2=39%), confirmed by a statistically insignificant Cochrane’s Q (p=0.18). Interestingly, the Baujat plot (Supplemental Figure S1, http://links.lww.com/HC9/C121) showed that Shen et al 27 contributed significantly to the heterogeneity among the RCTs, and upon removal of this study, heterogeneity decreased to 0%, with an estimated RR of 1.20 (95% CI: 0.83–1.74, p=0.17) (Table 2).
Effects of interventions on abstinence
Abstinence was examined as a primary outcome in 11 studies (Figure 3), which included 891 patients.23,24,28–34,37,42 A statistically significant effect was observed in only 1 non-RCT study. 32 This study examined the effects of a digitally delivered CBT and MET therapy on an ALD cohort, using individuals noncompliant with the treatment as a control. 32 The study showed a 2.6-fold increase in abstinence with psychosocial therapy, when compared with the control group (RR=2.57, 95% CI: 1.11–5.95). In total, across 5 non-RCT studies examining abstinence in a meta-analysis, the pooled RR for abstinence was 1.35 (95% CI: 0.62–2.93, p=0.35) using the random effects model and 1.02 (95% CI: 0.81–1.28, p=0.88) using the fixed effects model, indicating no statistically significant increase in abstinence rates (Figure 3). The non-RCTs demonstrated moderate heterogeneity (I 2=54%) with a statistically significant Cochran’s Q test for heterogeneity (p=0.07). Influential analysis showed that removing Mehta et al 32 reduced heterogeneity to I 2=15%, with effect estimate of RR=0.94 (95% CI: 0.62–1.44, p=0.69), which is similar to earlier results. Similarly, 6 RCTs demonstrated a pooled RR of 1.17 (95% CI: 0.92–1.48, p=0.15) in the random effects model and 1.17 (95% CI: 0.90–1.51, p=0.24) in the fixed effects model with no heterogeneity (I 2=0%), and a Cochran’s Q test (p=0.79) indicating no significant effect on abstinence rates.
FIGURE 3.
Forest plot delineating psychosocial therapy in promoting abstinence stratified by randomized and nonrandomized controlled trials.
Subgroup analysis on studies with return-to-drinking outcomes
When performing subgroup analysis on studies with alcohol return-to-drinking, studies where psychosocial therapy was delivered in a multidisciplinary setting, when compared with a single provider setting, showed statistically significant risk reductions (RR=0.47 [95% CI: 0.27–0.83] when compared to RR=1.05 [95% CI: 0.67–1.65], respectively) (Figure 4). Similarly, when comparing LT and non-LT populations, psychosocial therapy in the LT population showed statistically significant reductions in alcohol return-to-drinking with RR of 0.40 (95% CI: 0.27–0.58) as opposed to RR of 1.01 (95% CI: 0.68–1.49) in the non-LT population (Figure 4). Psychosocial therapy trended toward a larger risk reduction if pharmacological therapy was delivered concurrently (54% vs. 26%), but this was not statistically significant. The relative risk reduction for psychosocial therapy with the use of pharmacological therapy was 0.46 (95% CI: 0.05–4.4) as opposed to an RR of 0.74 (95% CI: 0.44–1.24) for psychosocial therapy without pharmacological therapy. When comparing psychotherapy types, effect sizes were similar between CBT/MET/MI therapy (RR=0.71, 95% CI: 0.39–1.26) and non-CBT/MET/MI therapy (RR=0.57, 95% CI: 0.09–3.66). Subgroup analysis for abstinence outcomes was not feasible due to the limited number of studies: only 1 involved a multidisciplinary team, none included LT populations, and just 2 incorporated concurrent pharmacological therapy. A subgroup analysis showed psychosocial therapy was more effective in preventing return-to-drinking in ALD than HCV (RR=0.57 vs. 1.25), though limited by only 2 HCV studies with wide confidence intervals. No difference was seen in promoting abstinence (Supplemental Figure S2, http://links.lww.com/HC9/C121).
FIGURE 4.
Forest plot evaluating the effect of psychosocial therapy on alcohol return-to-drinking stratified by (A) delivery in a multidisciplinary team versus single provider; (B) liver transplant or non-liver transplant population; (C) concurrent pharmacological therapy; and (D) psychotherapy type.
Risk of bias
The RoB2 tool classified 5 RCT studies as having overall low ROB (Figures 5A, B).25–28,31 Two studies were assigned an overall moderate ROB due to concerns with outcome measurement.24,29 Two studies had high ROB; 1 study had deviations in outcome measurement and delivery of intended intervention, which led to high risk, while another study had concerns across 3 domains: randomization process, outcome measurement, and delivery of intended intervention.23,30
FIGURE 5.
RoB2 and ROBINS-1 assessments by domain in (A) randomized controlled trials and (B) nonrandomized controlled trials. RoB2 and ROBINS-1 assessments with domain distributions in (C) randomized controlled trials and (D) non-randomized controlled trials. Funnel plots evaluating for publication bias in (E) studies with alcohol return-to-drinking and (F) studies with abstinence. Abbreviations: ROB2, Risk of Bias 2; ROBINS-1, Risk of Bias in Non-Randomized Studies.
The ROBINS-1 tool classified 5 non-RCT studies as having overall critical ROB due to having significant uncontrolled confounding variables (Figures 5C, D).34,37,39,41,42 These studies often used historical controls without propensity matching or without adjustment for confounders, and there were concerns with missing data in 1 study. 41 Five studies were assigned an overall serious risk of ROB with concerns across 4 domains: confounding, selection of participants, missing data, and measurement of outcome.32,33,35,36,40 Out of these 5 studies, 2 had serious risk across 1 domain33,35 while the remaining 3 had serious and moderate risk across multiple domains.32,36,40 Only 1 study had a moderate overall ROB because of moderate confounding. 38
Quality of evidence using GRADE
The quality of evidence of the non-RCTs for the outcome of alcohol return-to-drinking was moderate. Although the studies started with low quality of evidence as they were non-RCTs, they were upgraded for a strong association and heterogeneity that can be explained (Table 3). The absolute risk reduction was that for every psychosocial therapy delivered, there would be 179 fewer per 1000 patients who would return to drinking. Eliminating Erim et al 33 and López-Pelayo et al 36 reduced heterogeneity to 0% with similar effect estimates. For RCTs assessing return-to-drinking, the evidence was rated moderate due to imprecision, as estimates had wide confidence intervals on the forest plot (Table 3). For non-RCTs with abstinence outcomes, the quality of evidence was low as there were very serious concerns for risk of bias, and for RCTs, it was downgraded to moderate as there were serious concerns for risk of bias (Table 3).
TABLE 3.
GRADE (Grading of Recommendations Assessment, Development, and Evaluation) approach to evaluate the quality of evidence of studies according to the primary outcomes of preventing alcohol return-to-drinking or promoting abstinence
| Certainty assessment | No. patients | Effect | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Psychosocial therapy | Standard of care | Relative (95% CI) | Absolute (95% CI) | Certainty |
| Alcohol return-to-drinking—nonrandomized studies | |||||||||||
| 7 | Nonrandomized studies | Serious a | Not serious | Not serious | Not serious | Strong association All plausible residual confounding would reduce the demonstrated effect |
73/330 (22.1%) | 163/437 (37.3%) | RR 0.52 (0.28– 0.99) | 179 fewer per 1000 (from 269 fewer to 4 fewer) | ⨁⨁⨁◯ Moderate a |
| Alcohol return-to-drinking—randomized controlled studies | |||||||||||
| 4 | Randomized trials | Not serious | Not serious | Not serious | Serious b | None | 52/168 (31.0%) | 45/146 (30.8%) | RR 0.99 (0.45–2.16) | 3 fewer per 1000 (from 170 fewer to 358 more) | ⨁⨁⨁◯ Moderate b |
| Abstinence—nonrandomized controlled studies | |||||||||||
| 5 | Nonrandomized studies | Very serious a | Not serious | Not serious | Serious b | All plausible residual confounding would reduce the demonstrated effect | 70/150 (46.7%) | 134/285 (47.0%) | RR 1.35 (0.62– 2.93) | 165 more per 1000 (from 179 fewer to 907 more) | ⨁◯◯◯ Very lowa,b |
| Abstinence—randomized controlled trials | |||||||||||
| 6 | Randomized trials | Serious c | Not serious | Not serious | Not serious | None | 71/238 (29.8%) | 59/218 (27.1%) | RR 1.17 (0.92– 1.48) | 46 more per 1000 (from 22 fewer to 130 more) | ⨁⨁⨁◯ Moderate c |
Significant bias as the comparator group often did not adjust for confounding factors.
Effect estimates with broad CIs.
Some trials with high ROB.
Abbreviations: ROB, Risk of Bias; RR, risk ratio.
Evaluation for publication bias
Funnel plots were used to evaluate for publication bias (Figures 5E, F). Upon visualization of the funnel plots, there appeared to be no significant asymmetry that would suggest publication bias. Harbord’s statistical test for publication bias showed insignificant p values of 0.24 for return-to-drinking studies and 0.05 for abstinence studies.
DISCUSSION
The present study provides a systematic review and meta-analysis evaluating the effects of psychosocial therapy on patients with CLD and AUD or harmful alcohol use. We show that the use of psychosocial therapy is correlated with a significant reduction in alcohol return-to-drinking in patients with CLD and AUD or harmful use in non-RCT with 48% risk reduction (RR=0.52, 95% CI: 0.28–0.99, p=0.048). Although heterogeneity was initially high, removing Erim et al 33 and López-Pelayo et al 36 reduced it to 0%, yielding a 63% relative risk reduction (RR=0.37, 95% CI: 0.24–0.56, p=0.003). Erim and colleagues and López-Pelayo were unique from other non-RCTs evaluating return-to-drinking in that noncompliant patients were used as the comparator, while the remaining studies used historical controls, or control groups that underwent standard of care before implementation of psychosocial therapy. Erim and colleagues also assessed alcohol use trajectories through urinary alcohol biomarkers at each follow-up visit, rendering it especially sensitive to nonadherence bias. Erim et al 33 also initially reported higher return-to-drinking rates in the treatment group compared with the noncompliant group; however, the trajectory of urinary alcohol biomarkers indicated a lower overall rate of drinking in the psychosocial group. López-Pelayo et al 36 also differed from other studies as it was exclusively conducted with a population of patients with alcohol-associated hepatitis. Thus, despite initially low-quality evidence from these non-RCTs, the strength of association and the resolution of heterogeneity following the removal of these 2 studies justified upgrading the evidence to moderate quality.
In contrast, the RCTs did not demonstrate a significant reduction in return-to-drinking (RR=0.99, 95% CI: 0.45–2.16, p=0.96), although this analysis was limited to only 4 studies. Shen et al 27 was the only RCT to show a reduction in alcohol return-to-drinking, but also substantially contributed to overall heterogeneity. Conducted in China in an exclusively Asian population, Shen et al 27 differed from the other studies, which predominantly included White and Black participants, reflecting distinct racial and cultural backgrounds. Due to wide confidence intervals, the overall quality of evidence from RCTs was downgraded to moderate. Differences in outcomes between RCTs and non-RCTs may be reflected in variations in patient populations and clinical settings; notably, LT populations and multidisciplinary care delivery were more common in non-RCTs. Nevertheless, this study aligns with prior systematic reviews showing benefits of psychosocial therapy in patients with CLD and AUD, while uniquely quantifying the effect through a meta-analysis.16,17 A recent study by Singal et al 19 shows that any treatment was associated with a 73% reduction (HR=0.27 [95% CI: 0.15–0.46]) in alcohol return-to-drinking specifically in the ALD population, but the majority of the studies in the meta-analysis focused on pharmacotherapy rather than psychosocial therapy. Only 2 studies focused on psychosocial therapy, with 1 study that was notably small with 14 patients. Their pooled effect estimate for the 2 studies was not statistically significant with broad confidence intervals. Our study strengthens the protective effect of psychosocial therapy on alcohol return-to-drinking across a broader spectrum of CLD etiologies, including viral etiologies, which may be more generalizable and even relevant to a MetALD (metabolic dysfunction and alcohol-associated steatotic liver disease) population.
Interestingly, our subgroup analysis found that the effects of psychosocial therapy on preventing return-to-drinking were more pronounced when delivered in a multidisciplinary team setting and associated with a 53% risk reduction (RR=0.47, 95% CI: 0.27–0.83). In this context, a multidisciplinary team was defined as collaboration between a hepatology and addiction specialist, such as a psychologist or psychiatrist, with occasional involvement of a social worker. In contrast, a single provider was defined as psychosocial therapy predominantly delivered by either a psychiatrist or psychologist or hepatologist independently. Although a recent meta-analysis by Singal et al 19 reported no difference between “integrated” and “non-integrated” care, their definition of “integrated” required the addiction team to be embedded within the LT team, whereas our definition of “multidisciplinary” care includes any documented collaboration between addiction and primary teams. Reclassifying their “non-integrated” studies under our criteria yields an effect estimate (HR=0.40) similar to our findings (RR=0.47), suggesting that collaboration, rather than structural embedding, may be the key factor, although further RCTs are needed to confirm this.
Four of the 5 non-RCTs that reported risk reduction were of LT populations, and additional subgroup analysis showed a 60% risk reduction (RR=0.40, 95% CI: 0.27–0.58) in alcohol return-to-drinking among patients undergoing LT when compared with a non-LT population.38,39,41 Only 1 study did not focus on an LT population, but included patients recently discharged from the hospital after excessive alcohol use. 40 These findings suggest that psychosocial interventions may be more effective in patients who have experienced a traumatic medical event related to their drinking behavior, potentially making them more receptive to seeking treatment. This aligns with prior literature on psychosocial therapy for substance use disorders, which indicates that an individual’s motivation is a key determinant of treatment success. 43 However, this can also be confounded by the fact that most studies with LT populations also had psychosocial therapy delivered by a multidisciplinary team, which is another significant factor in preventing alcohol return-to-drinking. Additional subgroup analysis showed that psychosocial therapy, when delivered with pharmacological therapy, had a larger risk reduction of 0.46 (95% CI: 0.05–4.40) than when delivered alone. However, this was not statistically significant due to the broad CI.
There was no significant effect observed for abstinence across RCTs or non-RCTs, although a moderate, insignificant improvement in abstinence was noted across treatment groups. The quality of evidence was low for the non-RCTs and downgraded from high to moderate in RCTs, given concerns for risk of bias. Our findings are similar to prior systematic reviews,16,17 except for 1 study that showed a statistically significant increase in abstinence in those treated with integrated therapy with CBT/MET. 32 While patient population or intervention duration can be inherently different between studies that report abstinence and studies that report alcohol return-to-drinking, motivation may play a significant role. Sixty percent of the studies with abstinence outcomes included cohorts of patients who were nonabstinent at the beginning of the study, implying lower initial motivation to pursue sobriety.23,24,26,28–33,37,40,42 In the single study that reported a statistically significant increase in abstinence following treatment, patients were instructed to use a smartphone app that incorporated psychosocial elements to deliver regular reminders to abstain from drinking. 32 This points to a potential role of method and mode of delivery to affect outcomes, with the promise of routine digital delivery in promoting abstinence.
Therefore, our results support the use of psychosocial therapy to prevent alcohol return-to-drinking, especially in multidisciplinary settings or in LT populations. Importantly, however, the lack of a significant effect on abstinence could suggest that psychosocial interventions are insufficient to initiate or promote abstinence in the CLD population and that structured psychosocial interventions may be more potent at maintaining sobriety than inducing it. This mirrors findings in the AUD population where a meta-analysis shows that psychosocial therapy alone was not associated with higher rates of abstinence but rather combination pharmacotherapy and psychosocial therapy promoted abstinence.44,45 The limited number of RCTs and the large degree of heterogeneity among non-RCTs reveal the need for more RCTs to evaluate the effect of psychosocial therapy, particularly in LT populations and in multidisciplinary settings. It also suggests a need for a more tailored approach to psychosocial therapy, potentially integrating CBT, MET, MI, or group-based components to address the multifaceted nature of addiction and recovery. This is supported by the fact that 4 of the 5 successful studies employed combinations of psychosocial techniques, leading to statistically significant reductions in return-to-drinking39–41 or induction of abstinence. 32 Furthermore, although not statistically significant, it does appear that the effects of psychosocial therapy are more pronounced when used in conjunction with pharmacological therapy. This, alongside the potential of digital platforms to enhance delivery, warrants further investigation.
Despite these conclusions, our study does have several limitations. First, there was a high degree of statistical heterogeneity in the non-RCTs in both the abstinence (I 2=58%) and return-to-drinking (I 2=74%) studies, which limits their generalizability. However, heterogeneity decreased to 0% after removing 2 studies that were different from the others in non-RCTs that had alcohol return-to-drinking as the primary outcome. While this can reflect the methodological consistency of the remaining 5 trials, it can also be from the low power of Cochran’s Q and I 2 statistics to detect modest heterogeneity when only a small number of studies are analyzed. The RCTs had less statistical heterogeneity, which may be secondary to sample size, inherent patient population differences, or delivery setting of the psychosocial therapy. Moreover, several non-RCTs had a high risk of bias, primarily because the comparator group frequently consisted of noncompliant patients or historical controls without adjustment for confounders such as baseline severity of alcohol drinking. In addition, the observed benefits in multidisciplinary programs and among LT populations are based almost entirely on non-RCTs using self-reported alcohol intake, which may be confounded by patients’ heightened motivation to be transplanted and reporting bias. Prospective RCTs with objective drinking measures are therefore needed to validate these findings, particularly in LT populations. Finally, using noncompliant controls may lead to an underestimation of return-to-drinking rates, which can exaggerate the effect size observed in the intervention arm.
CONCLUSIONS
We describe a meta-analysis of 20 studies that showed psychosocial therapy leads to a statistically significant 28% relative risk reduction in alcohol return-to-drinking in patients with CLD and AUD or hazardous alcohol drinking in the non-RCT subgroup. When accounting for the heterogeneity of the studies in a random effects model, there is a 48% return-to-drinking risk reduction in non-RCT trials and a 63% risk reduction after removing 2 trials that used different comparator groups and contributed to a large degree of heterogeneity. These effects were more pronounced when psychosocial therapy was delivered by a multidisciplinary team and in LT populations. The addition of pharmacological therapy also trended toward a larger effect size. However, given that these studies were predominantly non-RCTs, more RCTs are needed before definitive conclusions can be made, especially RCTs evaluating psychosocial therapy utilizing multidisciplinary teams and in LT populations. In the future, multifaceted approaches to treat AUD and CLD that combine psychosocial approaches, digitally delivered therapy, and even pharmacotherapy hold promise and should be considered in RCTs.
Supplementary Material
DATA AVAILABILITY STATEMENT
Data, analytic methods, and study materials can be made available by request to the corresponding author.
AUTHOR CONTRIBUTIONS
Christopher H. Sollenberger, Huai-En R. Chang, and Christine C. Hsu: conceptualization, methodology, drafting of manuscript, visualization, data acquisition, and interpretation of data. Jonathan Lim: drafting of manuscript, data acquisition, visualization, and interpretation of data. Mohammed Rifat Shaik, Hanna Blaney, and Vaishnavi Mathur: data acquisition and critical review of manuscript. Nancy Terry: resources, data curation, and critical review of manuscript. Jeremy W. Luk and Nancy Diazgranados: critical review of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge Dr John Williams, MD, MHS, a Professor Emeritus of Medicine at Duke University Medical Center, for his guidance and expert opinion on systematic reviews and meta-analysis.
FUNDING INFORMATION
This research was supported by the intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases within the National Institutes of Health (NIH). The contributions of the NIH author(s) are considered works of the United States Government. The findings and conclusions presented in this paper are those of the author(s) and do not necessarily reflect the views of the NIH or the U.S. Department of Health and Human Services.
CONFLICTS OF INTEREST
The authors have no conflicts to report.
Footnotes
Abbreviations: ALD, alcohol-associated liver disease; APA, American Psychological Association; AUD, alcohol use disorder; AUDIT, Alcohol Use Disorders Identification Test; AUDIT-C, Alcohol Use Disorders Identification Test – Consumption; CBT, cognitive behavioral therapy; CENTRAL, Cochrane Central Register of Controlled Trials; CLD, chronic liver disease; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; MET, motivational enhancement therapy; MI, motivational interviewing; non-RCT, nonrandomized controlled trial; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PICO, Population, Intervention, Comparator, Outcome; PROSPERO, International Prospective Register of Systematic Reviews; RCT, randomized controlled trial; ROB, Risk of Bias; RoB2, Risk of Bias 2; ROBINS-1, Risk of Bias in Non-Randomized Studies of Interventions; RR, Relative Risk; TSF, twelve-step facilitation.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.hepcommjournal.com.
Contributor Information
Christopher H. Sollenberger, Email: christophersollenberger2023@u.northwestern.edu.
Huai-En R. Chang, Email: rachel.huaien.chang@gmail.com.
Jonathan Lim, Email: jonathan.lim2@nih.gov.
Mohammed Rifat Shaik, Email: mohammedrifat.shaik@nih.gov.
Hanna Blaney, Email: hanna.blaney@medstar.net.
Vaishnavi Mathur, Email: mathurvaishnavi@gmail.com.
Nancy Terry, Email: nancyterrylib@gmail.com.
Jeremy W. Luk, Email: jeremy.luk@nih.gov.
Nancy Diazgranados, Email: nancy.diazgranados@nih.gov.
Christine C. Hsu, Email: christine.hsu@nih.gov.
REFERENCES
- 1. Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: Observational study. Brit Med J. 2018;362:k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cholankeril G, Ahmed A. Alcoholic liver disease replaces hepatitis C virus infection as the leading indication for liver transplantation in the United States. Clin Gastroenterol Hepatol. 2018;16:1356–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu Y, Sun Z, Wang Q, Wu K, Tang Z, Zhang B. Contribution of alcohol use to the global burden of cirrhosis and liver cancer from 1990 to 2019 and projections to 2044. Hepatol Int. 2023;17:1028–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mackowiak B, Fu Y, Maccioni L, Gao B. Alcohol-associated liver disease. J Clin Invest. 2024;134:e176345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825–832. [DOI] [PubMed] [Google Scholar]
- 6. Staufer K, Huber-Schönauer U, Strebinger G, Pimingstorfer P, Suesse S, Scherzer TM, et al. Ethyl glucuronide in hair detects a high rate of harmful alcohol consumption in presumed non-alcoholic fatty liver disease. J Hepatol. 2022;77:918–930. [DOI] [PubMed] [Google Scholar]
- 7. Younossi ZM, Zheng L, Stepanova M, Venkatesan C, Mir HM. Moderate, excessive or heavy alcohol consumption: Each is significantly associated with increased mortality in patients with chronic hepatitis C. Aliment Pharmacol Ther. 2013;37:703–709. [DOI] [PubMed] [Google Scholar]
- 8. Kranzler HR, Soyka M. Diagnosis and pharmacotherapy of alcohol use disorder: A review. JAMA. 2018;320:815–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Whitlock EP, Polen MR, Green CA, Orleans T, Klein J, Force USPST . Behavioral counseling interventions in primary care to reduce risky/harmful alcohol use by adults: A summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2004;140:557–568. [DOI] [PubMed] [Google Scholar]
- 10. Cornelius JR, Douaihy A, Bukstein OG, Daley DC, Wood SD, Kelly TM, et al. Evaluation of cognitive behavioral therapy/motivational enhancement therapy (CBT/MET) in a treatment trial of comorbid MDD/AUD adolescents. Addict Behav. 2011;36:843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McHugh RK, Hearon BA, Otto MW. Cognitive behavioral therapy for substance use disorders. Psychiatr Clin North Am. 2010;33:511–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kelly JF, Abry A, Ferri M, Humphreys K. Alcoholics anonymous and 12-step facilitation treatments for alcohol use disorder: A distillation of a 2020 Cochrane Review for Clinicians and Policy Makers. Alcohol Alcohol. 2020;55:641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rogal S, Youk A, Zhang H, Gellad WF, Fine MJ, Good CB, et al. Impact of alcohol use disorder treatment on clinical outcomes among patients with cirrhosis. Hepatology. 2020;71:2080–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luk JW, Ha NB, Shui AM, Snyder HR, Batki SL, Ostacher MJ, et al. Demographic and clinical characteristics associated with utilization of alcohol use disorder treatment in a multicenter study of patients with alcohol-associated cirrhosis. Alcohol Clin Exp Res (Hoboken). 2025;49:244–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Celik M, Gold MS, Fuehrlein B. A narrative review of current and emerging trends in the treatment of alcohol use disorder. Brain Sci. 2024;14:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khan A, Tansel A, White DL, Kayani WT, Bano S, Lindsay J, et al. Efficacy of psychosocial interventions in inducing and maintaining alcohol abstinence in patients with chronic liver disease: A systematic review. Clin Gastroenterol Hepatol. 2016;14:191–202 e1-4; quiz e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hemrage S, Brobbin E, Deluca P, Drummond C. Efficacy of psychosocial interventions to reduce alcohol use in comorbid alcohol use disorder and alcohol-related liver disease: A systematic review of randomized controlled trials. Alcohol Alcohol. 2023;58:478–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Elfeki MA, Abdallah MA, Leggio L, Singal AK. Simultaneous management of alcohol use disorder and liver disease: A systematic review and meta-analysis. J Addict Med. 2023;17:e119–e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Singal AK, Zhang W, Shetty A, Patel A, Mohammed S, Bhandari P, et al. Treatment of alcohol use disorder in alcohol-associated liver disease: A meta-analysis. Hepatol Commun. 2025;9:e0686.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. [DOI] [PubMed] [Google Scholar]
- 21. Atkins D Best D Briss PA Eccles M Falck-Ytter Y Flottorp S et al.; GRADE Working Group . Grading quality of evidence and strength of recommendations. Brit Med J. 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12:55–61. [DOI] [PubMed] [Google Scholar]
- 23. Proeschold-Bell RJ, Evon DM, Yao J, Niedzwiecki D, Makarushka C, Keefe KA, et al. A randomized controlled trial of an integrated alcohol reduction intervention in patients with hepatitis C infection. Hepatology. 2020;71:1894–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stein MD, Herman DS, Kim HN, Howell A, Lambert A, Madden S, et al. A randomized trial comparing brief advice and motivational interviewing for persons with HIV-HCV co-infection who drink alcohol. AIDS Behav. 2021;25:1013–1025. [DOI] [PubMed] [Google Scholar]
- 25. Weinrieb RM, Van Horn DH, Lynch KG, Lucey MR. A randomized, controlled study of treatment for alcohol dependence in patients awaiting liver transplantation. Liver Transpl. 2011;17:539–547. [DOI] [PubMed] [Google Scholar]
- 26. North CS, Pollio DE, Sims OT, Jain MK, Brown GR, Downs DL, et al. An effectiveness study of group psychoeducation for hepatitis C patients in community clinics. Eur J Gastroenterol Hepatol. 2017;29:679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shen J, Huang F-F, Wang Z-M. Effect of alcoholic anonymous on self-management of drinking behavior in patients with alcoholic liver disease. World Chin J Digestol. 2017;25:904. [Google Scholar]
- 28. Dieperink E, Fuller B, Isenhart C, McMaken K, Lenox R, Pocha C, et al. Efficacy of motivational enhancement therapy on alcohol use disorders in patients with chronic hepatitis C: A randomized controlled trial. Addiction. 2014;109:1869–1877. [DOI] [PubMed] [Google Scholar]
- 29. Edelman EJ, Maisto SA, Hansen NB, Cutter CJ, Dziura J, Deng Y, et al. Integrated stepped alcohol treatment for patients with HIV and liver disease: A randomized trial. J Subst Abuse Treat. 2019;106:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dhanda A, Andrade J, Allende H, Allgar V, Bailey M, Callaghan L, et al. Mental Imagery to Reduce Alcohol-related harm in patients with alcohol use disorder and alcohol-related liver damaGE: The MIRAGE randomised pilot trial results. BMJ Open Gastroenterol. 2024;11:e001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. DeMartini KS, Schilsky ML, Palmer A, Fehon DC, Zimbrean P, O'Malley SS, et al. Text messaging to reduce alcohol relapse in prelisting liver transplant candidates: A pilot feasibility study. Alcohol Clin Exp Res. 2018;42:761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mehta G, Lin S, Nadar A, PV B, Kumar R, Balaji A, et al. AlcoChange: A digital therapeutic for patients with alcohol-related liver disease. JHEP Rep. 2024;6:100993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Erim Y, Böttcher M, Schieber K, Lindner M, Klein C, Paul A, et al. Feasibility and acceptability of an alcohol addiction therapy integrated in a transplant center for patients awaiting liver transplantation. Alcohol Alcohol. 2016;51:40–46. [DOI] [PubMed] [Google Scholar]
- 34. Andersen MM, Aunt S, Jensen NM, Homann C, Manniche J, Svendsen S, et al. Rehabilitation for cirrhotic patients discharged after hepatic encephalopathy improves survival. Dan Med J. 2013;60:A4683. [PubMed] [Google Scholar]
- 35. Carrique L, Quance J, Tan A, Abbey S, Sales I, Lilly L, et al. Results of early transplantation for alcohol-related cirrhosis: Integrated addiction treatment with low rate of relapse. Gastroenterology. 2021;161:1896–1906 e2. [DOI] [PubMed] [Google Scholar]
- 36. López-Pelayo H, Miquel L, Altamirano J, Bataller R, Caballeria J, Ortega L, et al. Treatment retention in a specialized alcohol programme after an episode of alcoholic hepatitis: Impact on alcohol relapse. J Psychosom Res. 2019;116:75–82. [DOI] [PubMed] [Google Scholar]
- 37. Alexandre W, Muhammad H, Agbalajobi O, Zhang G, Gmelin T, Adejumo A, et al. Alcohol treatment discussions and clinical outcomes among patients with alcohol-related cirrhosis. BMC Gastroenterol. 2023;23:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Björnsson E, Olsson J, Rydell A, Fredriksson K, Eriksson C, Sjöberg C, et al. Long-term follow-up of patients with alcoholic liver disease after liver transplantation in Sweden: Impact of structured management on recidivism. Scand J Gastroenterol. 2005;40:206–216. [DOI] [PubMed] [Google Scholar]
- 39. Addolorato G, Mirijello A, Leggio L, Ferrulli A, D'Angelo C, Vassallo G, et al. Liver transplantation in alcoholic patients: Impact of an alcohol addiction unit within a liver transplant center. Alcohol Clin Exp Res. 2013;37:1601–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Peeraphatdit T, Kamath PS, Karpyak VM, Davis B, Desai V, Liangpunsakul S, et al. Alcohol rehabilitation within 30 days of hospital discharge is associated with reduced readmission, relapse, and death in patients with alcoholic hepatitis. Clin Gastroenterol Hepatol. 2020;18:477–485.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Attilia ML, Lattanzi B, Ledda R, Galli AM, Farcomeni A, Rotondo C, et al. The multidisciplinary support in preventing alcohol relapse after liver transplantation: A single-center experience. Clin Transplant. 2018;32:e13243. [DOI] [PubMed] [Google Scholar]
- 42. Dieperink E, Ho SB, Heit S, Durfee JM, Thuras P, Willenbring ML. Significant reductions in drinking following brief alcohol treatment provided in a hepatitis C clinic. Psychosomatics. 2010;51:149–156. [DOI] [PubMed] [Google Scholar]
- 43. Jhanjee S. Evidence based psychosocial interventions in substance use. Indian J Psychol Med. 2014;36:112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gao J, Cao J, Guo T, Xiao Y. Association between alcoholic interventions and abstinence rates for alcohol use disorders: A meta-analysis. Medicine (Baltimore). 2018;97:e13566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ghosh A, Morgan N, Calvey T, Scheibein F, Angelakis I, Panagioti M, et al. Effectiveness of psychosocial interventions for alcohol use disorder: A systematic review and meta-analysis update. Am J Drug Alcohol Abuse. 2024;50:442–454. [DOI] [PubMed] [Google Scholar]