Abstract
1. The soleus, extensor digitorum longus and peroneus tertius muscles of the hind leg were paralysed by botulinum toxin injection in neonatal rats varying in age from 8 to 31 days. The soleus muscle was similarly paralysed in adult rats.
2. Muscles were excised after different periods of block, neuromuscular transmission was assessed in vitro, and the nerve terminal size and amount of terminal sprouting and multiple innervation determined histologically using silver (Ag) and zinc iodide-osmium (ZIO) tetroxide stains.
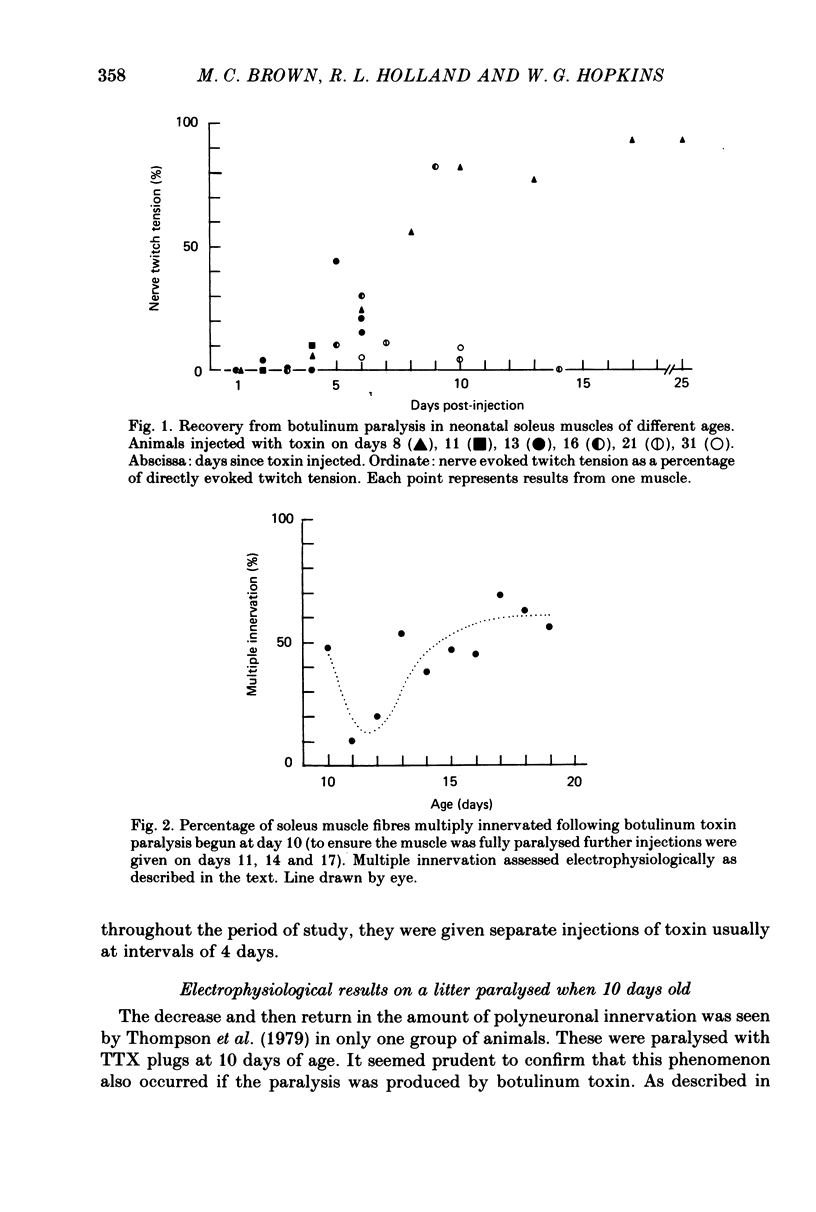
3. Recovery from the block induced by botulinum toxin was more rapid in immature multiply-innervated muscles than in muscles of older rats in which all multiple innervation had been eliminated.
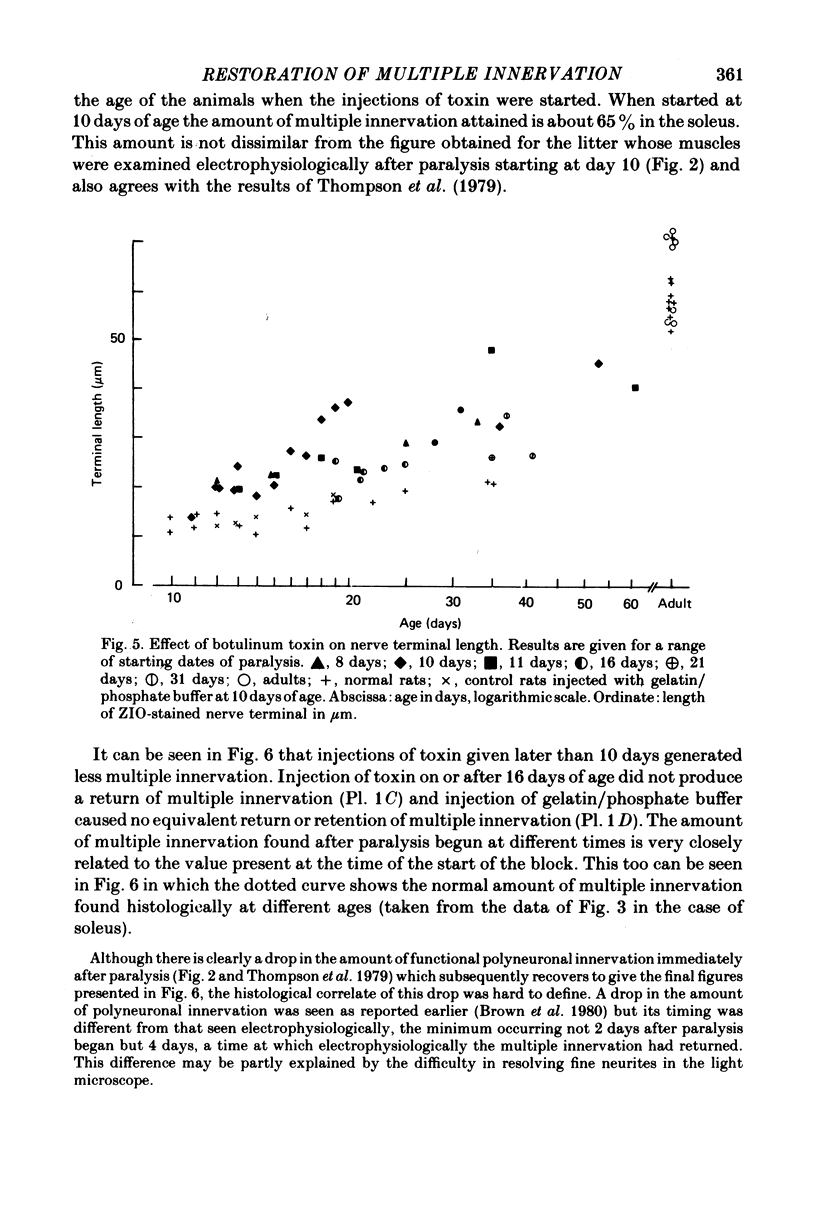
4. Terminals in muscles blocked during the first month after birth grew rapidly in size and developed a characteristic granulose morphology in the first few days following the block. This change did not occur in fully adult rat solei.
5. Sprouts growing from the terminals were infrequent in muscles paralysed before day 16. Terminal sprouts were more frequent in muscles paralysed between 16 and 31 days of age, but were very infrequent in adult solei.
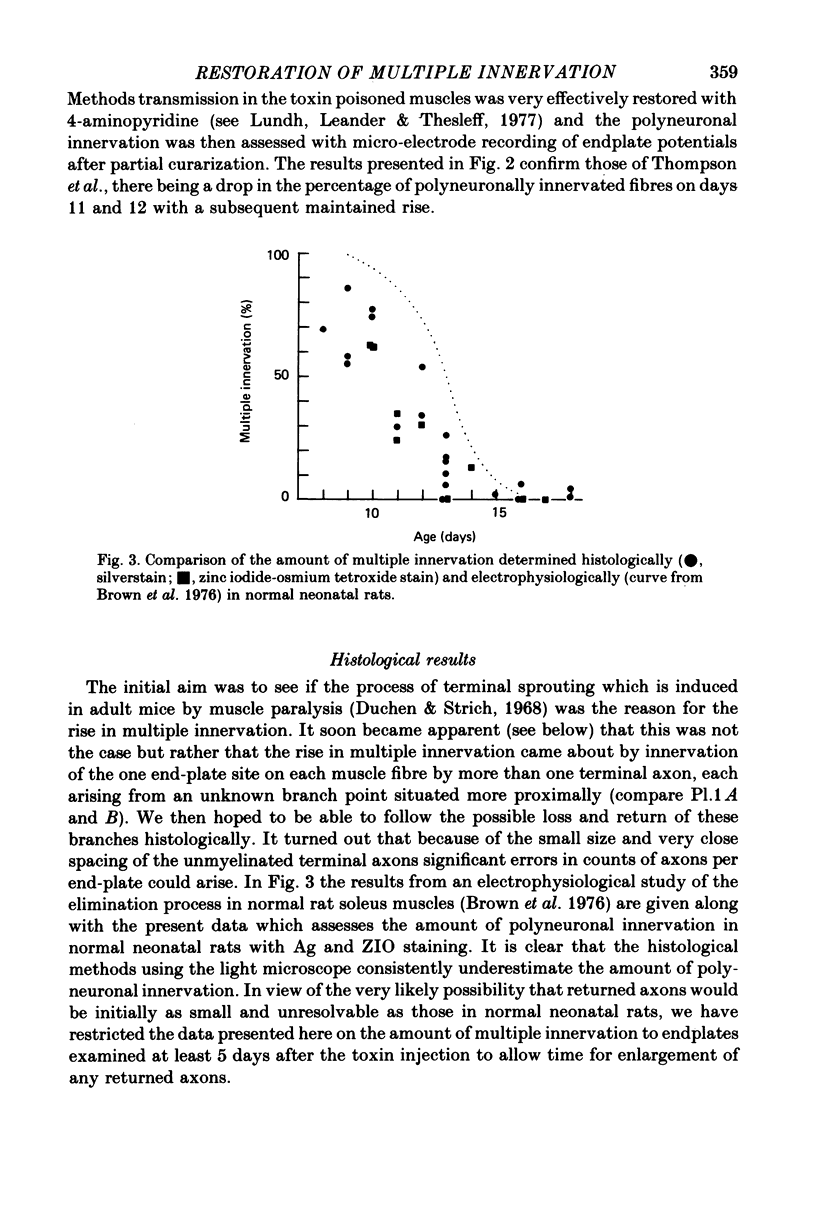
6. In confirmation of Thompson, Kuffler & Jansen (1979) paralysis begun at 10 days was followed initially by a continued fall in multiple innervation detected electrophysiologically. After 2 days the percentage of muscle fibres with more than one input rose. The extra inputs did not come from terminal sprouts. They innervated the single synaptic site on each muscle fibre as they do during normal synaptogenesis.
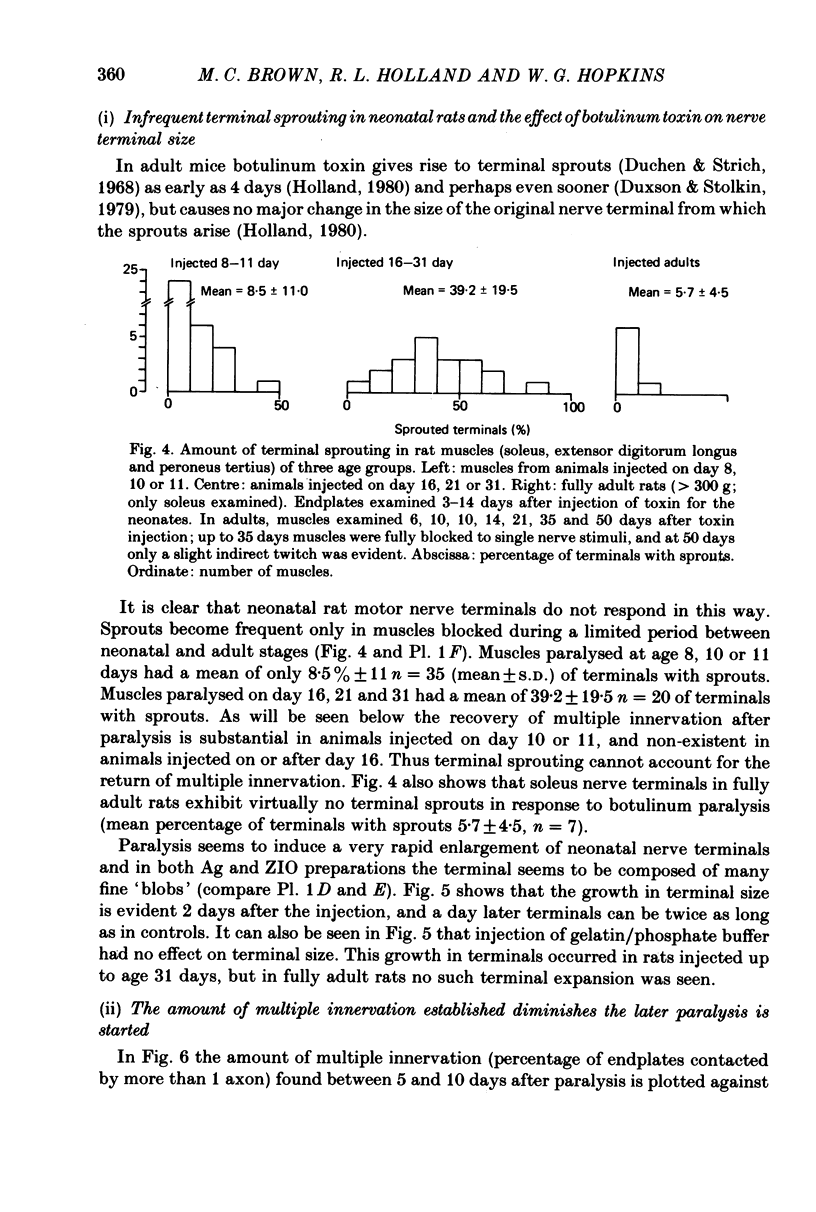
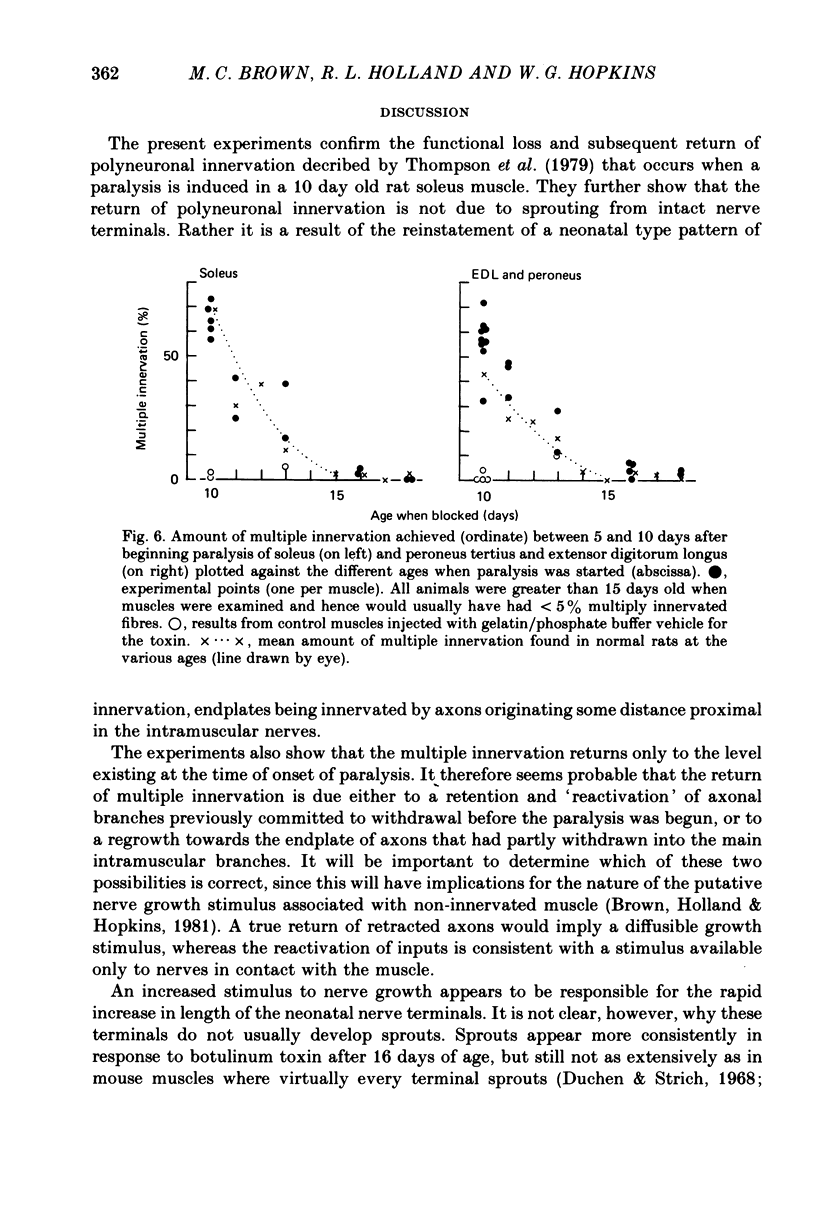
7. The amount of multiple innervation regained 5-10 days after the start of paralysis became progressively less the later paralysis started and was approximately equal to the level existing at the time the block was begun. Thus paralysis starting on or after day 16 (when all excess inputs have normally withdrawn) caused no return of multiple innervation.
8. The return of multiple inputs and the swelling of the nerve terminals are presumably responses of the motoneurone to growth stimuli from inactive muscle. It is not clear whether the return of multiple innervation in the 10-15 day old rats is due to reactivation of inputs still in close contact with the end-plate before withdrawal or to regrowth of partly retracted nerve branches.
9. A parallel is drawn between the limited period when inactivity can reinstate multiple innervation, and the critical period in the developing visual cortex.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagust J., Lewis D. M., Westerman R. A. Polyneuronal innervation of kitten skeletal muscle. J Physiol. 1973 Feb;229(1):241–255. doi: 10.1113/jphysiol.1973.sp010136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Pettigrew A. G. The formation of synapses in amphibian striated muscle during development. J Physiol. 1975 Oct;252(1):203–239. doi: 10.1113/jphysiol.1975.sp011141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Pettigrew A. G. The formation of synapses in striated muscle during development. J Physiol. 1974 Sep;241(2):515–545. doi: 10.1113/jphysiol.1974.sp010670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit P., Changeux J. P. Consequences of tenotomy on the evolution of multiinnervation in developing rat soleus muscle. Brain Res. 1975 Dec 5;99(2):354–358. doi: 10.1016/0006-8993(75)90036-0. [DOI] [PubMed] [Google Scholar]
- Bixby J. L., van Essen D. C. Regional differences in the timing of synapse elimination in skeletal muscles of the neonatal rabbit. Brain Res. 1979 Jun 22;169(2):275–286. doi: 10.1016/0006-8993(79)91030-8. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Holland R. L., Hopkins W. G. Motor nerve sprouting. Annu Rev Neurosci. 1981;4:17–42. doi: 10.1146/annurev.ne.04.030181.000313. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Holland R. L., Ironton R. Nodal and terminal sprouting from motor nerves in fast and slow muscles of the mouse. J Physiol. 1980 Sep;306:493–510. doi: 10.1113/jphysiol.1980.sp013410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Ironton R. Motor neurone sprouting induced by prolonged tetrodotoxin block of nerve action potentials. Nature. 1977 Feb 3;265(5593):459–461. doi: 10.1038/265459a0. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Ironton R. Sprouting and regression of neuromuscular synapses in partially denervated mammalian muscles. J Physiol. 1978 May;278:325–348. doi: 10.1113/jphysiol.1978.sp012307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Jansen J. K., Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976 Oct;261(2):387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepel F., Mariani J., Delhaye-Bouchaud N. Evidence for a multiple innervation of Purkinje cells by climbing fibers in the immature rat cerebellum. J Neurobiol. 1976 Nov;7(6):567–578. doi: 10.1002/neu.480070609. [DOI] [PubMed] [Google Scholar]
- Duchen L. W., Strich S. J. The effects of botulinum toxin on the pattern of innervation of skeletal muscle in the mouse. Q J Exp Physiol Cogn Med Sci. 1968 Jan;53(1):84–89. doi: 10.1113/expphysiol.1968.sp001948. [DOI] [PubMed] [Google Scholar]
- Duxson M. J., Stolkin C. Early structural changes in the motor nerve terminals of rat skeletal muscle after local injection of botulinum toxin [proceedings]. J Physiol. 1979 Nov;296:12P–12P. [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970 Feb;206(2):419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman J. W., Purves D. The elimination of redundant preganglionic innervation to hamster sympathetic ganglion cells in early post-natal life. J Physiol. 1980 Apr;301:213–228. doi: 10.1113/jphysiol.1980.sp013200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman J. W. The reorganization of synaptic connexions in the rat submandibular ganglion during post-natal development. J Physiol. 1977 Dec;273(1):155–177. doi: 10.1113/jphysiol.1977.sp012087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundh H., Leander S., Thesleff S. Antagonism of the paralysis produced by botulinum toxin in the rat. The effects of tetraethylammonium, guanidine and 4-aminopyridine. J Neurol Sci. 1977 May;32(1):29–43. doi: 10.1016/0022-510x(77)90037-5. [DOI] [PubMed] [Google Scholar]
- MAILLET M. [Modification of the Champy osmium tetroxide-potassium iodide technic. Results of its application to the study of nerve fibers]. C R Seances Soc Biol Fil. 1959;153:939–940. [PubMed] [Google Scholar]
- Miyata Y., Yoshioka K. Selective elimination of motor nerve terminals in the rat soleus muscle during development. J Physiol. 1980 Dec;309:631–646. doi: 10.1113/jphysiol.1980.sp013531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba T., Nakamura T., Grob D. Staining for nerve fiber and cholinesterase activity in fresh frozen sections. Am J Clin Pathol. 1967 Jan;47(1):74–77. doi: 10.1093/ajcp/47.1.74. [DOI] [PubMed] [Google Scholar]
- O'Brien R. A., Ostberg A. J., Vrbová G. Observations on the elimination of polyneuronal innervation in developing mammalian skeletal muscle. J Physiol. 1978 Sep;282:571–582. doi: 10.1113/jphysiol.1978.sp012482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestronk A., Drachman D. B. Motor nerve sprouting and acetylcholine receptors. Science. 1978 Mar 17;199(4334):1223–1225. doi: 10.1126/science.204007. [DOI] [PubMed] [Google Scholar]
- Redfern P. A. Neuromuscular transmission in new-born rats. J Physiol. 1970 Aug;209(3):701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley D. A. Tenotomy delays the postnatal development of the motor innervation of the rat soleus. Brain Res. 1978 Mar 17;143(1):162–167. doi: 10.1016/0006-8993(78)90760-6. [DOI] [PubMed] [Google Scholar]
- Srihari T., Vrbová G. The role of muscle activity in the differentiation of neuromuscular junctions in slow and fast chick muscles. J Neurocytol. 1978 Oct;7(5):529–540. doi: 10.1007/BF01260887. [DOI] [PubMed] [Google Scholar]
- Thompson W., Kuffler D. P., Jansen J. K. The effect of prolonged, reversible block of nerve impulses on the elimination of polyneuronal innervation of new-born rat skeletal muscle fibers. Neuroscience. 1979;4(2):271–281. doi: 10.1016/0306-4522(79)90088-5. [DOI] [PubMed] [Google Scholar]



