Abstract
1. Experiments on the frog olfactory bulb have been performed in vitro in order to determine whether primary afferent transmission is modified by presynaptic inhibition.
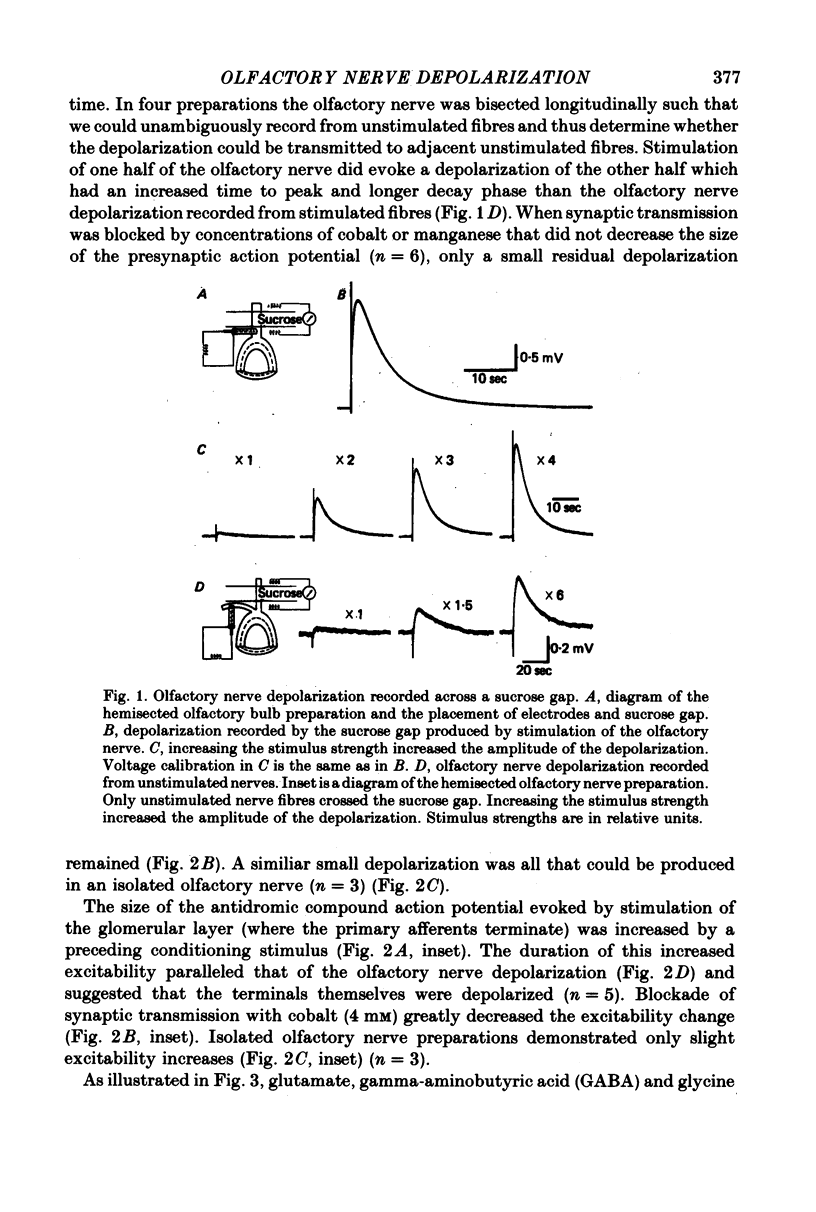
2. Stimulation of the olfactory nerve resulted in a prolonged depolarization of the olfactory nerve as recorded across a sucrose gap. Unstimulated olfactory nerve fibres adjacent to the stimulated fibres were also depolarized.
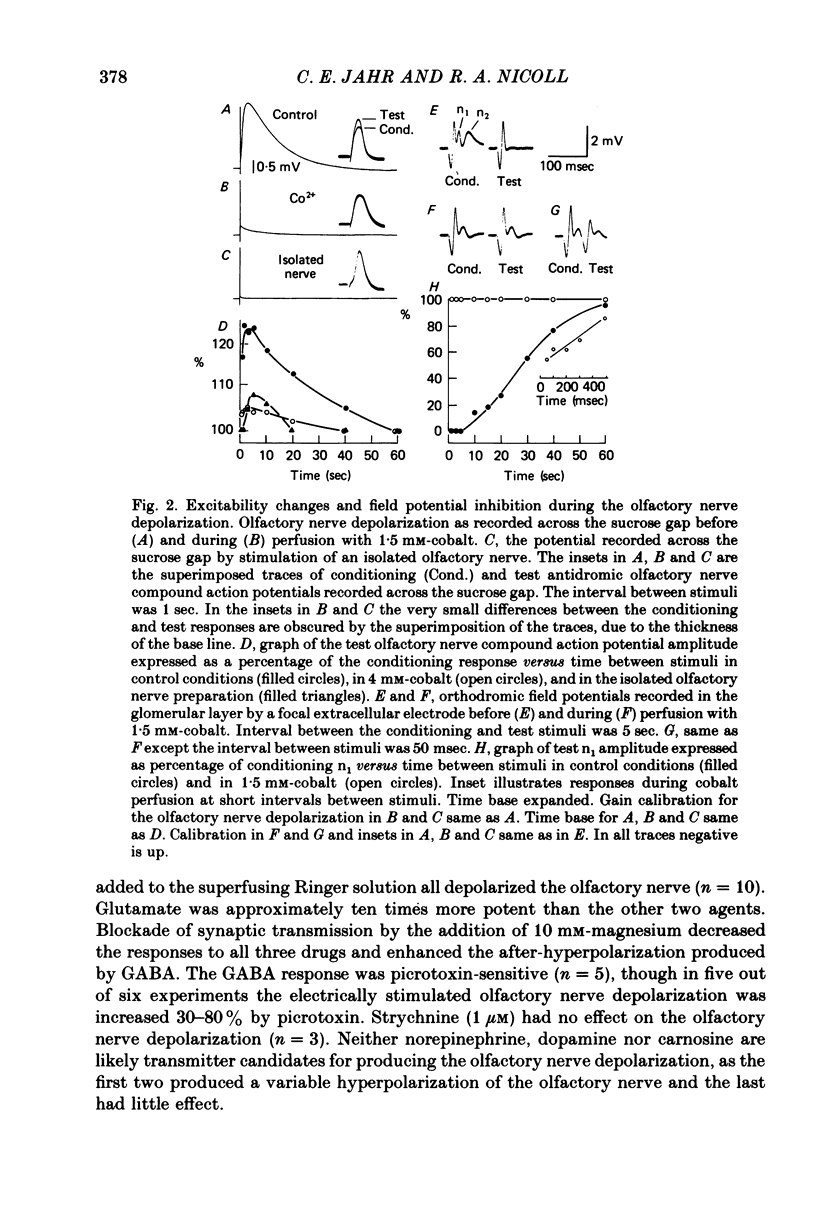
3. An excitability increase of the olfactory nerve terminals was found that lasted the entire duration of the olfactory nerve depolarization, indicating that the terminals themselves were depolarized. Both the olfactory nerve depolarization and the excitability increase were blocked by cobalt and manganese ions.
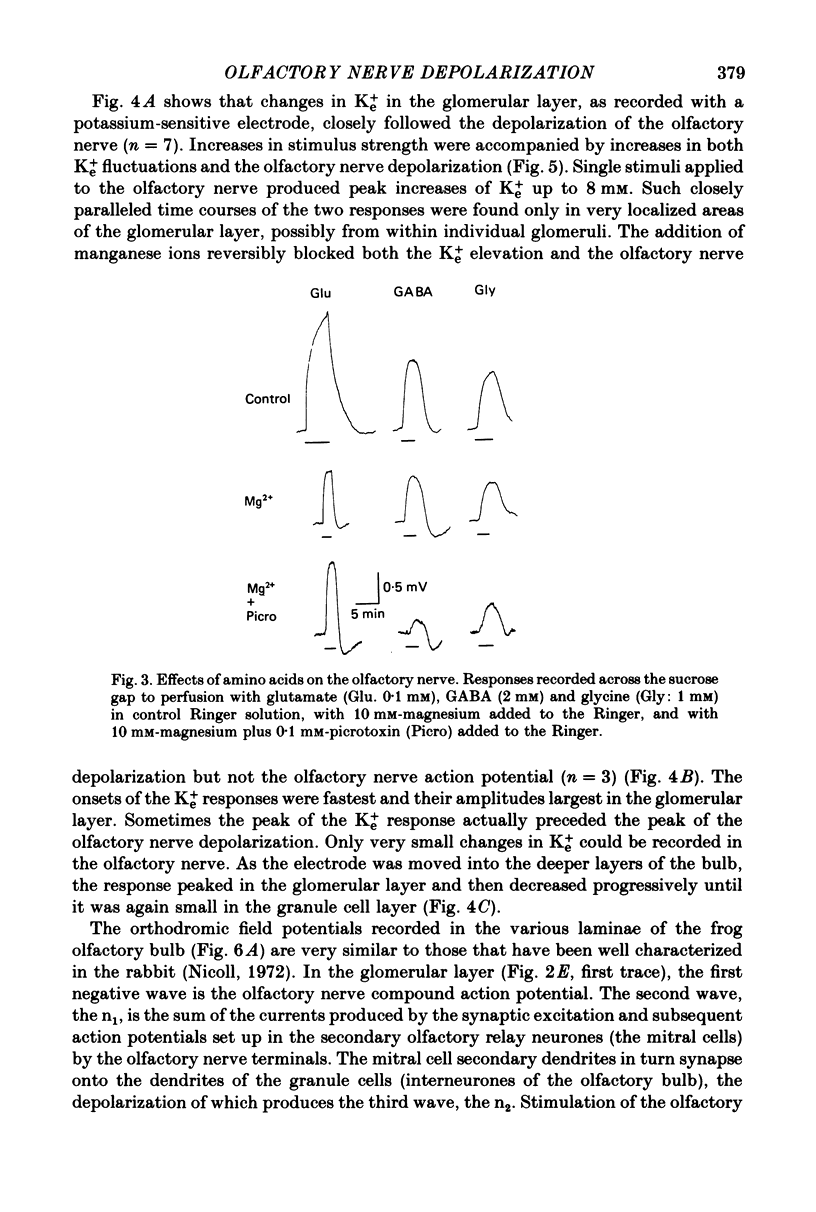
4. Low concentrations of glutamate were found to produce a substantial depolarization of the olfactory nerve. Although gamma-aminobutyric acid (GABA) also elicited a depolarization of the olfactory nerve, picrotoxin, a GABA antagonist, did not reduce the stimulus-evoked olfactory nerve depolarization.
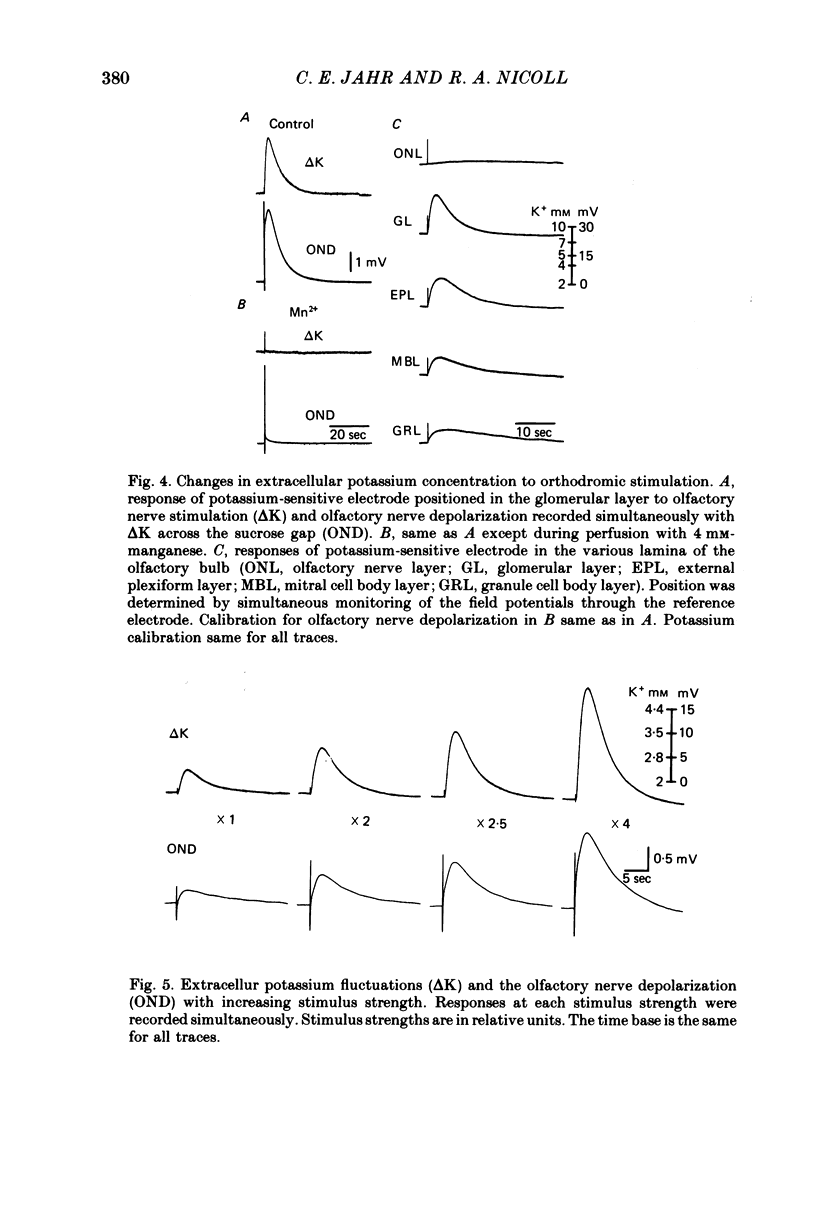
5. Recording with potassium-sensitive electrodes in the olfactory nerve terminal region demonstrated an increase in extracellular potassium with the same rise time and duration as the olfactory nerve depolarization. Cobalt and manganese blocked the potassium increase and the olfactory nerve depolarization without affecting the presynaptic action potential.
6. The focally recorded extracellular current resulting from orthodromic synaptic excitation of the secondary olfactory relay neurones was blocked at short intervals by paired stimulation and decreased for the duration of the olfactory nerve depolarization. This suggests a decreased release of transmitter from the olfactory nerve terminals.
7. The possible role of potassium and/or a neurotransmitter in generating the olfactory nerve depolarization and inhibition is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen M. W. The contribution by glial cells to surface recordings from the optic nerve of an amphibian. J Physiol. 1970 Oct;210(3):565–580. doi: 10.1113/jphysiol.1970.sp009227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDEL J., KUFFLER S. W. Presynaptic inhibition at the crayfish neuromuscular junction. J Physiol. 1961 Mar;155:543–562. doi: 10.1113/jphysiol.1961.sp006646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erulkar S. D., Weight F. F. Extracellular potassium and trasmitter release at the giant synapse of squid. J Physiol. 1977 Apr;266(2):209–218. doi: 10.1113/jphysiol.1977.sp011764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman W. J. Relation of glomerular neuronal activity to glomerular transmission attenuation. Brain Res. 1974 Jan 4;65(1):91–107. doi: 10.1016/0006-8993(74)90338-2. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Alger B. E. Presynaptic inhibition: transmitter and ionic mechanisms. Int Rev Neurobiol. 1979;21:217–258. doi: 10.1016/s0074-7742(08)60639-x. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. Dorsal root potentials and changes in extracellular potassium in the spinal cord of the frog. J Physiol. 1979 May;290(2):113–127. doi: 10.1113/jphysiol.1979.sp012763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A. Olfactory nerves and their excitatory action in the olfactory bulb. Exp Brain Res. 1972;14(2):185–197. doi: 10.1007/BF00234798. [DOI] [PubMed] [Google Scholar]
- OTTOSON D. Olfactory bulb potentials induced by electrical stimulation of the nasal mucosa in the frog. Acta Physiol Scand. 1959 Nov 15;47:160–172. doi: 10.1111/j.1748-1716.1960.tb00066.x. [DOI] [PubMed] [Google Scholar]
- Pickles H. G., Simmonds M. A. Possible presynaptic inhibition in rat olfactory cortex. J Physiol. 1976 Sep;260(2):475–486. doi: 10.1113/jphysiol.1976.sp011526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinching A. J., Powell T. P. The neuropil of the glomeruli of the olfactory bulb. J Cell Sci. 1971 Sep;9(2):347–377. doi: 10.1242/jcs.9.2.347. [DOI] [PubMed] [Google Scholar]
- Schmidt R. F. Presynaptic inhibition in the vertebrate central nervous system. Ergeb Physiol. 1971;63:20–101. doi: 10.1007/BFb0047741. [DOI] [PubMed] [Google Scholar]
- Somjen G. G. Extracellular potassium in the mammalian central nervous system. Annu Rev Physiol. 1979;41:159–177. doi: 10.1146/annurev.ph.41.030179.001111. [DOI] [PubMed] [Google Scholar]
- WALL P. D. Excitability changes in afferent fibre terminations and their relation to slow potentials. J Physiol. 1958 Jun 18;142(1):1–21. doi: 10.1113/jphysiol.1958.sp005997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westrum L. E. Electron microscopy of degeneration in the lateral olfactory tract and plexiform layer of the prepyriform cortex of the rat. Z Zellforsch Mikrosk Anat. 1969;98(2):157–187. doi: 10.1007/BF00338323. [DOI] [PubMed] [Google Scholar]


