Summary
ARID1A, a subunit of the BAF chromatin remodeler, is frequently mutated in cancer. Predicting how ARID1A loss impacts cancer therapy response is challenging because it influences many cellular pathways. G quadruplex (G4) binding ligands, such as pyridostatin, show anticancer effects, but the genetic determinants influencing the response to G4 ligands are not fully understood. Here, we show that ARID1A-deficient cells are selectively sensitive to pyridostatin compared to isogenic controls. This was apparent in ovarian and colorectal cancer cell line models, and in vivo studies suggest that G4 ligands hold promise for ARID1A-deficient cancers. While ARID1A modulates pyridostatin-induced transcriptional responses, we show that toxicity in ARID1A-deficient cells arises from the defective repair of topoisomerase-induced breaks. Notably, these cells fail to efficiently accumulate non-homologous end joining proteins on chromatin following pyridostatin exposure. These data uncover a role for ARID1A in the cellular response to G4 ligands, linking remodeling to G4 ligand-induced responses.
Keywords: ARID1A, G quadruplex, pyridostatin, SWI/SNF, non-homologous end joining, DNA repair
Graphical abstract

Highlights
-
•
ARID1A loss sensitizes cells to the G4 ligands pyridostatin and CX-5461
-
•
ARID1A modulates the transcriptional response to pyridostatin (PDS)
-
•
ARID1A promotes PDS-dependent non-homologous end joining chromatin association
The ARID1A subunit of the BAF chromatin remodeling complex is frequently mutated in cancer. Simões-Sousa et al. demonstrate that ARID1A promotes the assembly of repair factors on chromatin and promotes cellular survival following exposure to G quadruplex ligands, which hold promise for cancer treatment.
Introduction
The SWI/SNF family of chromatin remodelers comprises three complexes: BAF, PBAF, and GBAF, which are defined by their subunit composition.1 These complexes share a number of core subunits and can have either SMARCA4 (BRG1) or SMARCA2 (hBRM) as the catalytic subunit. ARID1A (also called BAF250A) is a subunit that is found only in the BAF complex (Figure 1A).
Figure 1.
Generation of isogenic ARID1A cell line models
(A) Schematic of the mammalian SWI/SNF chromatin remodeling complexes BAF and PBAF, showing core subunits (green) and complex specific components (purple, BAF; pink, PBAF). ARID1A, a component of BAF complex, is highlighted in red.
(B) Summary of isogenic cell lines used in this study. TOV21G, an ARID1A-deficient ovarian clear cell carcinoma cell line, was used to engineer two clones with ARID1A re-expression (TOV.C1 and TOV.C2) and to generate an empty vector control (TOV.EV). HCT116, an ARID1A-proficient colon carcinoma cell line, was engineered to generate an ARID1A knockout (HCT.KO).
(C) Flowchart of TOV21G isogenic cell line generation.
(D and E) Immunoblot analysis of whole-cell extracts showing ARID1A expression in TOV21G cell lines and derivatives (D) and HCT116 parental and KO cell lines (E). α-tubulin was used as a loading control.
(F–H) Quantitative proteomic analysis of the log2FC (log2 fold change) of SWI/SNF subunits in TOV21G with reconstituted ARID1A cell lines TOV.C1 (F) and TOV.C2 (G) compared with parental TOV21G and in HCT116 ARID1A-KO (HCT.KO) compared with HCT116 parental cell line (H). Points correspond to individual biological replicates (n = 2); bar shows mean of replicates.
(I) Volcano plots showing differential protein expression in ARID1A-rescued (TOV.C1 and TOV.C2) versus parental TOV21G cell lines and ARID1A knockout HCT116 (HCT.KO) versus parental cell line.
(J) Principal-component analysis (PCA) of whole-proteome data comparing all cell line models used in this study. ARID1A status is indicated by color (red, deficient; black, proficient); different cell lines are distinguished by shape (circle, TOV21G; square, HCT116).
See also Figure S1.
ARID1A is involved in multiple pathways that protect genome stability.1 It plays a role in both major DNA double-strand break (DSB) repair pathways: non-homologous end joining (NHEJ) and homologous recombination (HR). In addition, ARID1A has been implicated in the response to replication stress.2,3 The ARID1A gene is mutated in a wide range of cancer types, including up to 50% of ovarian clear cell cancers (OCCCs) and around 10% of colorectal cancers.1 It is therefore critical to understand when and how each of the functions of ARID1A comes into play in response to genotoxic agents, particularly those with therapeutic potential.
G quadruplex (G4) structures are secondary structures that can form in guanine-rich single-stranded DNA sequences by virtue of the ability of guanines to form G-tetrads that are stabilized by Hoogsteen hydrogen bonding.4 They contribute to the regulation of gene expression, and G4s are frequently located in regulatory regions of genes, such as promoters and enhancers.5 G4 binding ligands, compounds that can selectively bind to G4 structures, have been shown to impair the proliferation or survival of some cancer cells. One way this occurs is through interfering with the expression of oncogenes or transcripts arising from other key genes that contain G4s in their regulatory regions, thus leading to impaired fitness or viability.6
Importantly, another route to impaired cancer cell fitness in response to G4 ligand exposure is through DNA damage. Ligand-stabilized G4 structures lead to DNA DSBs in either a transcription- or replication-dependent manner,7 and cellular survival following G4 ligand-induced DSBs can be influenced by several cellular pathways.8,9,10 For instance, stabilized G4s can create barriers for the replication machinery, leading to stalled or collapsed replication forks. This outcome can be avoided through the activity of helicases that resolve G4s, such as BLM.11 When replication conflicts are not avoided, BRCA1- and BRCA2-dependent HR activity has been shown to promote survival following exposure to G4 ligands.9,12 Outside of S phase, stabilized G4s have been shown to trap TOP2 molecules on chromatin in a transcription-dependent manner.10,13 This results in lesions that rely on TDP2-dependent processing followed by repair via NHEJ.10 The clinical exploration of potential therapeutic indications for G4 binding ligands is underway, with a focus on HR-deficient cancers. However, due to the importance of NHEJ in repairing lesions created by TOP2 trapping, it is possible that cancers with NHEJ deficiencies represent an important therapeutic setting. Therefore, gaining a deeper understanding of the genetic alterations in cancer cells that make them vulnerable to G4 ligands will enhance their targeted and effective application.
Here, we generated isogenic cell lines to study ARID1A and found that deficiency leads to increased cellular sensitivity to G4 binding ligands, including pyridostatin (PDS). Sensitivity to G4 ligands was apparent in both OCCC and colorectal cancer cell line models, and in vivo studies suggest that G4 ligands could hold promise for treating ARID1A-deficient cancers. We show that the transcriptional response to PDS is influenced by ARID1A but that the major impact of ARID1A loss on viability following PDS treatment is through its effect on DSB repair. Specifically, we find that NHEJ activity is important for the ARID1A-dependent sensitivity to PDS, and we show that ARID1A-deficient cells are unable to efficiently assemble NHEJ proteins on chromatin following exposure to PDS. These data show that ARID1A is an important determinant for cellular responses to G4 ligands due to its ability to modulate NHEJ repair activities.
Results
Generation of isogenic ARID1A cell lines
In order to gain deeper insights into ARID1A biology, we set out to create cell lines with genetic modulation of the ARID1A subunit of BAF (Figure 1A). Because of the prevalence of ARID1A mutations in OCCCs, we began by re-expressing ARID1A in the TOV21G cell line, which is an OCCC cell line that has a loss-of-function mutation in ARID1A alleles, leading to a lack of protein expression. We generated two independently derived clonal cell lines in which we introduced an ARID1A expression construct, referred to hereafter as TOV.C1 and TOV.C2 (Figures 1B and 1C). ARID1A expression was apparent by western blot analysis (Figure 1D) and immunofluorescence (Figure S1A). As an additional control, we introduced the empty vector (EV) into the TOV21G cell line (TOV.EV; Figures 1B–1D and S1A). The ARID1A-expressing lines show a modest decrease in proliferation, which is also evident in the EV control, suggesting that it is not solely due to ARID1A activity, and a slight increase in S and G2 phase relative to parental cells (Figures S1B and S1D).
ARID1A is also frequently misregulated in colon cancer, so we made use of a CRISPR-Cas9 knockout (KO) mutation of ARID1A in the HCT116 colon cancer cell line (Figure 1B). We confirmed the absence of expression with western blotting and immunofluorescence (Figures 1E and S1A). Here, in contrast to the TOV21G-derived cells, we find that the absence of ARID1A leads to a modest decrease in proliferation and a small increase in G2-phase cells relative to parental cells (Figures S1C and S1E).
To further characterize both cell line models, we performed proteomic analysis using quantitative mass spectrometry. As expected, we found increased ARID1A expression in the TOV.C1 and TOV.C2 cell lines (Figures 1F and 1G). In addition, the levels of several other BAF complex subunits, most notably SMARCA2, were also modestly higher in the ARID1A-expressing lines, likely reflecting increased BAF complex stability (Figures 1F and 1G). In the HCT116 cells, we found that the ARID1A KO cells displayed loss of ARID1A protein expression as well as most other BAF subunits, again consistent with an impact on BAF complex stability when ARID1A is deficient (Figure 1H). Unsurprisingly, ARID1A status impacted the proteome, with substantial up- and downregulation of a subset of proteins (Figure 1L). Principal-component analysis (PCA) of the proteomic data from all cell lines suggests that while cell line background accounted for the greatest variability between samples, the loss of ARID1A had a substantial impact in both cell lines (Figure 1J).
ARID1A-deficient cells show selective sensitivity to G4 binding ligands
Treatment of cells with the G4 binding ligand PDS often leads to impaired fitness.6 To test whether this effect is modulated by the presence of ARID1A, we treated our panel of cell lines with PDS and monitored survival. We found that in both cell line models, survival of ARID1A-proficient cells was significantly higher than that of ARID1A-deficient cells (Figures 2A and 2B). To ensure that the differential vulnerability to PDS was due to ARID1A deficiency, we used small interfering RNA (siRNA) to deplete ARID1A (Figure S2A) and found that reduced ARID1A expression in either reconstituted TOV.C1 cells or HCT116 parental cells leads to PDS hypersensitivity (Figures 2C and 2D). Using an assay to directly monitor cell death, we found that the ARID1A-deficient cells have increased levels of cell death following exposure to PDS when compared with the ARID1A-proficient cells (Figures 2E and 2F), together suggesting that ARID1A loss leads to increased sensitivity to PDS.
Figure 2.
ARID1A-deficient cells show selective sensitivity to G4 binding ligands
(A and B) Bar graphs showing percentage of survival of isogenic ARID1A cell lines upon treatment with 10 μM PDS for 6 days in (A) TOV21G and (B) HCT116. Viability is expressed as a percentage of viability in untreated cells. Points correspond to independent biological replicates, n = 3, mean ± SEM, data were analyzed by ordinary one-way ANOVA with Tukey’s test (TOV21G) or n = 2, mean ± SEM, data were analyzed by paired t tests (HCT116); ∗∗p < 0.01 and ∗∗∗p < 0.001.
(C and D) Bar graph showing survival upon treatment with PDS in siRNA-mediated ARID1A-depleted cells by sulforhodamine B (SRB) assay. Percentage of survival of PDS-treated versus untreated is plotted following depletion of ARID1A in TOV.C1 (C) and HCT116 (D) cell lines upon 6 days of treatment with 10 or 20 μM PDS, respectively. Points correspond to independent biological replicates (TOV.C1 n = 4, HCT116 n = 2), mean ± SEM, data were analyzed by paired t tests; ∗∗p < 0.01.
(E and F) Quantification of dead cells by propidium iodide staining in TOV21G-derived cell lines following treatment with 10 μM PDS (E) and in HCT.KO versus HCT116 parental following treatment with 20 μM PDS (F) for 5 days. Points correspond to independent biological replicates, n = 3, mean ± SEM, data were analyzed by ordinary one-way ANOVA with Tukey’s test (TOV21G) or paired t tests (HCT116); ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
(G and H) Bar graphs showing percentage of survival following 6 days of treatment with 8 μM CX-5461 in TOV21G-derived cell lines (G) and following 6 days of treatment with 10 μM CX-5461 in HCT116 isogenic cell lines (H). Points correspond to independent biological replicates, n = 3, mean ± SEM, data were analyzed by ordinary one-way ANOVA with Tukey’s test (TOV21G) or paired t tests (HCT116); ∗∗p < 0.01 and ∗∗∗∗p < 0.0001.
(I) Schematic of the in vivo assay. TOV.C1 and TOV.C2 (ARID1A-proficient) or TOV21G (ARID1A-deficient) cells were injected subcutaneously into mice, and tumors were allowed to form. ARID1A-reconstituted cell lines did not form tumors. Mice bearing tumors derived from TOV21G parental cells were treated with either CX-5461 or vehicle (VEH; regimen 1: 50 mg/kg, twice weekly; regimen 2: 75 mg/kg, three times weekly). Figure was generated using BioRender.
(J) Tumor growth curve of mice bearing TOV21G xenografts treated with VEH or CX-5461 under treatment regimen 1 (see schematic in I). n = 5 mice for VEH condition, and n = 6 for CX-5461-treated condition. Points represent the mean ± SEM at each time point.
(K) Area under the curve (AUC) analysis of tumor volume over time from (J). Points correspond to individual mice, with a line at the median. Data were analyzed by unpaired t tests (ns, not significant).
See also Figure S2.
ARID1A is found specifically in the BAF complex but not in the other two highly related SWI/SNF complexes, PBAF and GBAF. To see whether this phenotype is shared by other SWI/SNF complexes, we used cell lines with CRISPR-Cas9-mediated KO of PBRM1,14,15 which is found specifically in the PBAF complex (Figure S2B). These were tested in cell growth assays alongside the isogenic parental RPE1-hTERT cell line. In contrast to our data with ARID1A KO cells, we find no reproducible or significant sensitivity when cells are exposed to PDS (Figure S2C), suggesting that not all SWI/SNF complexes are involved in the cellular response to G4 binding ligands.
CX-5461 is a G4 binding ligand that is undergoing clinical evaluation.16 To see whether the vulnerability of ARID1A-deficient cells to G4 ligands has clinical utility, we looked at the response to CX-5461. Like PDS, we find that CX-5461 treatment differentially impacts ARID1A-deficient cells (Figures 2G and 2H). Next, we investigated the response to CX-5461 in mouse models using the ARID1A-deficient ovarian cancer line TOV21G in parallel with the ARID1A-reconstituted cells. We found that ARID1A re-expression resulted in a failure to establish tumors in mice (Figure 2I), consistent with the role of ARID1A as a tumor suppressor.
Mice bearing tumors established from ARID1A-deficient TOV21G cells were treated with vehicle or 50 mg/kg CX-5461 twice weekly (Figure 2I). While there was no statistically significant difference in tumor volume or survival between the two groups, there was a trend toward reduced tumor volume in the CX-5461-treated cohort compared with those treated with vehicle alone (Figures 2J and 2K). We therefore tested a higher dose of CX-5461 using 75 mg/kg three times weekly. Again, we found a non-significant trend toward reduced tumor volume and increased survival in the CX-5461-treated cohort when compared with vehicle alone (Figures S2D and S2E). These data suggest that there may be an impact of G4 ligands on ARID1A-deficient tumors, but further work is required to determine whether there is clinical value.
ARID1A loss impacts PDS-dependent transcriptional responses
We next investigated whether the loss of ARID1A influences the transcriptional response to PDS. We performed RNA sequencing (RNA-seq) to analyze the gene expression profiles of ARID1A-proficient and ARID1A-deficient TOV cell lines, either without treatment or following 4 h exposure to 10 μM PDS (Figure S3A).
Strikingly, we find that ARID1A has a profound influence on the magnitude of gene expression changes following PDS exposure (Figure 3A). In the presence of ARID1A, there are 1,222 genes that are significantly downregulated and 355 that are significantly upregulated in response to PDS (adjusted p value [padj] < 0.05). When ARID1A is deficient, these numbers rise to 3,954 and 1,478, respectively. We find that almost all of the genes whose expression is altered in the ARID1A-proficient cells are also significantly changed in the ARID1A-deficient cells (Figure 3B), suggesting that the differences between the samples reflect the magnitude of response rather than different pathways.
Figure 3.
ARID1A loss impacts PDS-dependent transcriptional responses
(A) Volcano plots showing differentially expressed genes in ARID1A-proficient and ARID1A-deficient cells treated with PDS (10 μM, 4 h) compared to untreated (UT) control. Significantly upregulated and downregulated genes are represented in red and blue colors, respectively (adjusted p value [padj] < 0.05).
(B) Venn diagram showing overlap of significantly changed genes (padj <0.05) upon PDS treatment (10 μM, 4 h) in ARID1A-deficient and ARID1A-proficient backgrounds.
(C) Quantification of γH2AX foci formation in TOV21G isogenic cell lines UT or treated with PDS (10 μM, 4 h) (n = 1).
(D) Heatmap showing expression levels of DNA damage-induced genes from RNA-seq data of ARID1A-proficient and ARID1A-deficient TOV21G isogenic cell lines, UT or treated with PDS (10 μM, 4 h).
(E) Venn diagram showing the number of significantly altered genes following PDS treatment (10 μM, 4 h) with potential G4-forming sequences (PQSs) at their promoters. Table summarizes the total number of differentially expressed genes, the number of genes with a PQS at their promoter, and the respective percentage for upregulated and downregulated genes in ARID1A-proficient and ARID1A-deficient backgrounds.
(F) Volcano plots displaying log2FC of PDS versus UT RNA-seq against the −log10(padj) values in ARID1A-proficient and ARID1A-deficient backgrounds. Significant genes (padj < 0.05) are highlighted in red, and ARID1A is highlighted with an arrow.
(G) Immunoblot showing ARID1A protein expression response to PDS (10 μM, 6 days) in ARID1A-proficient and ARID1A-deficient cells. β-actin was used as a loading control.
See also Figure S3.
PDS exposure leads to DNA DSBs and, at later time points, has been shown to induce inflammatory and p53-dependent transcriptional responses.17 Because ARID1A contributes to DSB repair, one potential explanation for these data is that the ARID1A-deficient cells have a greater burden of DNA damage, leading to a heightened damage-induced transcriptional response. We therefore tested whether the ARID1A-deficient cells have greater numbers of DNA breaks by looking at γH2AX foci as a readout of DNA DSBs. We used similar conditions to those in the RNA-seq analysis (10 μM PDS for 6 h) and found that, as expected, PDS treatment leads to increased γH2AX foci (Figure 3C). Importantly, however, there is no difference between the cell lines (Figure 3C), suggesting that the number of DNA breaks at this time point is relatively similar in the ARID1A-proficient and ARID1A-deficient cell lines. Furthermore, we interrogated the genes with significant PDS-induced changes and found relatively few differential effects on DNA damage-responsive genes in any of the datasets (Figures 3D and S3A). Together, these data suggest that differences in DNA damage are not driving the differential transcriptional response to PDS in ARID1A-deficient versus -proficient cells.
Since PDS is not influencing gene expression indirectly via DNA damage, these results suggest it is working through stabilization of G4-forming sequences that are enriched in gene-regulatory elements. If correct, most of the genes whose expression is influenced by PDS will contain G4-forming sequences in their promoters or enhancers. Indeed, we find that almost 80% of the significantly up- or downregulated genes have predicted G4-forming sequences (PQSs) in their promoters (Figures 3E and S3B), which is more than expected by chance since it has been estimated that just over 42% of promoters contain G4 sequences.18 This is true in both ARID1A-proficient and ARID1A-deficient cells (Figure 3E), which suggests that the differential effect of ARID1A is on the magnitude of PDS effects on G4-dependent gene expression of genes with G4s in their promoters. This is likely to be an indirect effect since ARID1A is primarily enriched at enhancers rather than promoters,19,20 and the expression of most of these genes is unaffected by ARID1A in untreated cells (Figure S3C).
While the ARID1A protein is not stably expressed in TOV21G cells due to truncating mutations (p.Y551LfsTer72/p.Q758RfsTer75), the transcript is generated and detectable in the RNA-seq dataset. We noted that ARID1A itself is one of the genes that is upregulated in response to PDS (Figure 3F). Interestingly, the ARID1A gene has a high density of PQSs in its regulatory and coding sequences (Figure S3D), and mutation of one of these sequences was found to reduce ARID1A gene expression.21 The G4-forming sequences in the coding region are present in the expression construct used to rescue ARID1A expression (Figure S3D), and we find that increased protein expression was evident in the ARID1A-proficient lines following PDS treatment (Figure 3G). These data suggest that at early time points, PDS treatment leads to changes in gene expression, including ARID1A expression, through modulating G4s in gene-regulatory elements and that ARID1A antagonizes this response, perhaps indicative of a feedback response.
ARID1A-dependent survival following PDS treatment is related to DNA damage responses
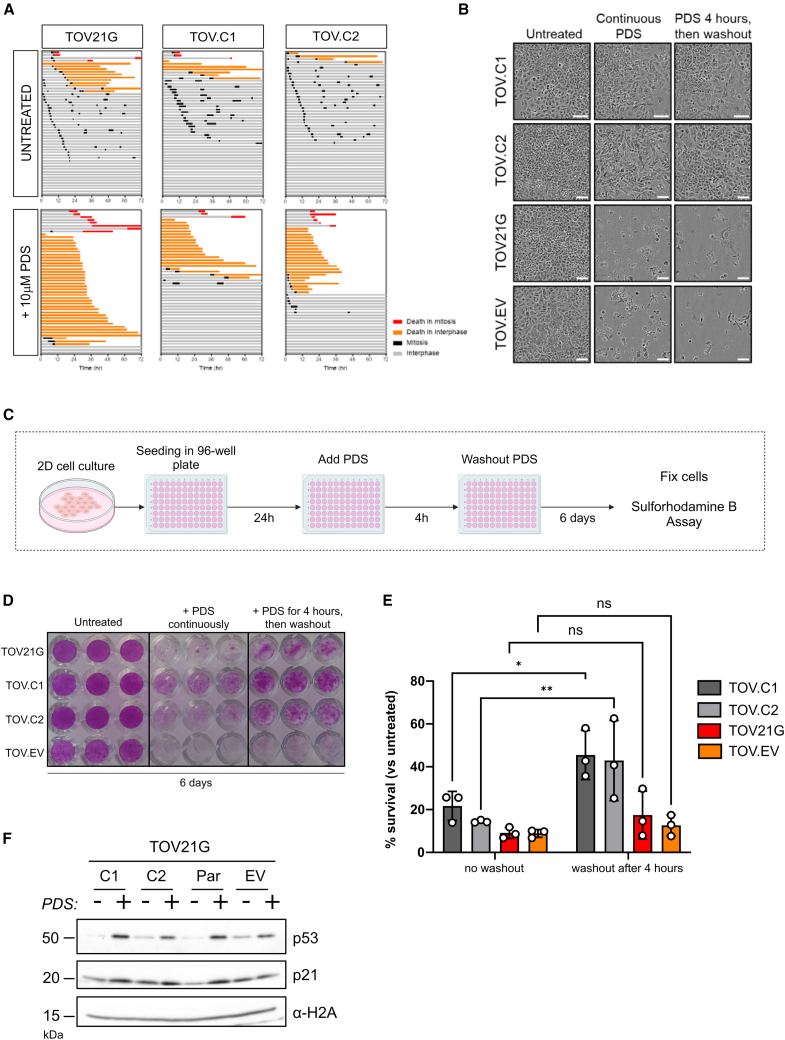
The cytotoxicity of PDS is related to DNA damage,10,13 and ARID1A is known to influence DNA damage responses.1 To explore this, we monitored survival over time using live-cell imaging. ARID1A-deficient TOV21G cells and the two ARID1A-expressing clones were grown in the presence or absence of 10 μM PDS, and cell fate was monitored over a period of 72 h. We found that a greater number of the ARID1A-deficient cells undergo cell death when compared with the ARID1A-expressing lines when treated with PDS (Figures 4A, 4B, and S4A). It was evident that while many ARID1A-expressing cells did not undergo cell death following PDS treatment during this time frame, they also did not undergo cell division, suggesting that these cells arrested cell cycle progression in response to PDS (Figure 4A). To determine whether the PDS-treated ARID1A-expressing cells were viable and able to re-enter the cell cycle, we performed an experiment where PDS was washed out from the media after 4 h and compared survival with cells treated continuously with PDS (Figure 4C). The survival of the ARID1A-deficient cells was modestly but not significantly different between these treatment conditions (Figures 4B–4E). Notably, however, when ARID1A is expressed, survival is significantly greater when cells are transiently treated with PDS compared with continuous treatment (Figures 4B–4E).
Figure 4.
ARID1A-dependent survival following PDS treatment is related to DNA damage responses
(A) Cell fate profiles comparing ARID1A-deficient TOV21G and ARID1A-proficient (TOV.C1 and TOV.C2) following treatment with 10 μM PDS for 72 h. Tracks represent individual cells, with different colors representing cell cycle events.
(B) Representative phase-contrast images of TOV21G-derived cell lines, either untreated or treated with 10 μM PDS or exposed to 10 μM PDS for 4 h followed by drug washout. Images are from 6 days after PDS addition. Scale bars represent 100 μm.
(C) Schematic of the experimental design used in the viability assays with and without drug washout (in D and E). Figure was generated using BioRender.
(D) Representative sulforhodamine B (SRB)-stained plate showing growth of TOV21G isogenic cell lines across treatment conditions (described in C). Plate was fixed at day 6 after treatment initiation.
(E) Quantification of cell survival from SRB assays, normalized to untreated control. Cell survival is shown after days, with cells treated for either 4 h or undergoing continuous treatment with 10 μM PDS. Values show mean ± SEM from three biological replicates, representing three independent experiments. Points correspond to independent biological replicates, n = 3, mean ± SEM, data were analyzed by two-way ANOVA with Šídák’s test; ∗p < 0.05 and ∗∗p < 0.01.
(F) Immunoblot analysis of p53 and p21 in TOV21G-derived cell lines with or without treatment with 10 μM PDS for 4 h. α-H2A was used as a loading control.
See also Figure S4.
These data suggest that ARID1A-proficient cells undergo a transient cell-cycle arrest in response to damage caused by PDS. In support of this, we observe a shift in the cell cycle population following PDS treatment (Figure S4B). To look at this more directly, we monitored the levels of p53, which is stabilized in response to DNA damage, and its DNA damage-upregulated target, p21. We found upregulation of both proteins in response to PDS treatment in these cell lines (Figure 4F). This is consistent with previous reports showing a DNA damage checkpoint response to PDS7,9 and suggests that in the absence of ARID1A, PDS leads to DNA damage-induced apoptosis, whereas in the presence of ARID1A, cells respond to PDS-induced DNA damage by transiently arresting cell cycle progression.
ARID1A-deficient cells fail to efficiently assemble NHEJ proteins on chromatin after PDS exposure
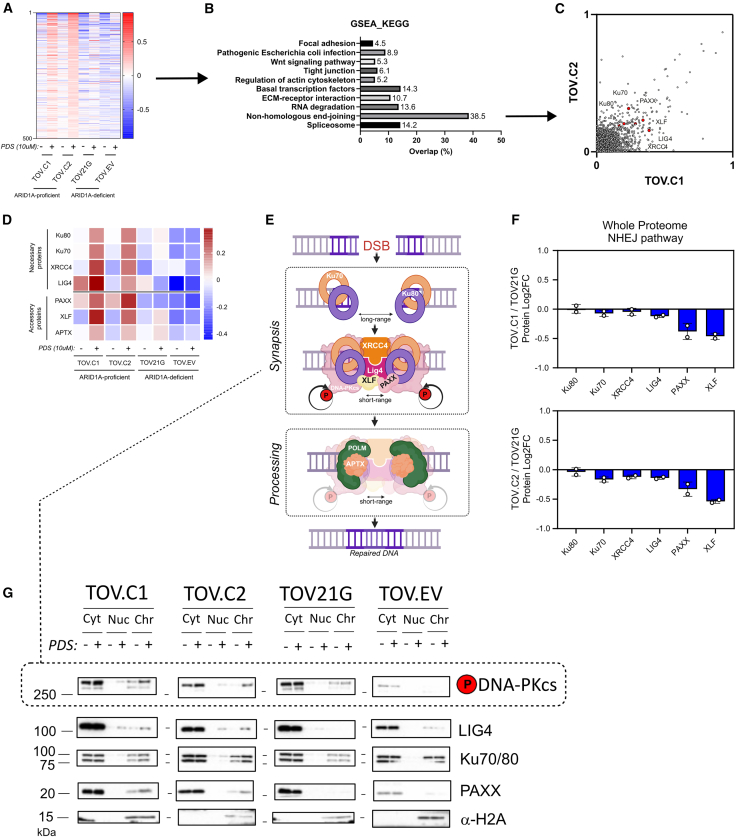
The survival and DNA damage response data suggest that ARID1A-expressing cells are ultimately able to repair the PDS-induced DNA damage and re-enter the cell cycle, while ARID1A-deficient cells are not. Both NHEJ and HR activities have been implicated in mediating survival following PDS exposure.9,10 To shed light on the repair pathways that might be altered in the absence of ARID1A, we analyzed the proteins associated with the chromatin fraction (chromatome) in the TOV21G-derived cell line panel before and after PDS treatment (10 μM, 4 h) by mass spectroscopy (Figure S5A).
We identified proteins that showed increased abundance in the chromatin fraction in the ARID1A-proficient cells upon PDS treatment. Strikingly, many of these proteins were not enriched in the PDS-treated, ARID1A-deficient chromatin (Figure 5A). When pathway analysis was performed on this group of proteins, we found that the NHEJ pathway was a top hit (Figure 5B). There was very good concordance between the two independently derived ARID1A-re-expressing clones (C1 and C2), with NHEJ proteins associating with chromatin post-PDS in both (Figures 5C and S5B). In contrast, these proteins were not substantially enriched in the chromatin fraction of PDS-treated, ARID1A-deficient TOV21G or EV cells (Figure 5D). The NHEJ factors enriched in chromatin in the ARID1A-proficient cells included Ku70 and Ku80, XRCC4, XLF, and PAXX (Figure 5E).
Figure 5.
ARID1A-deficient cells fail to efficiently assembly non-homologous end joining proteins on chromatin after PDS exposure
(A) Heatmap of chromatin-associated proteins ordered according to the top 500 most enriched proteins in chromatin in the ARID1A-proficient cell lines (TOV.C1 and TOV.C2) following treatment with 10 μM PDS for 4 h.
(B) KEGG pathway enrichment analysis of the 500 proteins represented in (A). Enrichment analysis was done using gene set enrichment analysis (GSEA), and the percentage of proteins in the heatmap (in A) that were in each gene set is plotted.
(C) Correlation plot highlighting chromatin-bound levels of a subset of non-homologous end joining (NHEJ) proteins in the ARID1A-reconstituted cell lines.
(D) Heatmap showing NHEJ factors on chromatin either in untreated conditions or following treatment with PDS (10 μM, 4 h).
(E) Schematic of the NHEJ pathway of double-strand break (DSB) repair. DSB ends are quickly bound by Ku70 and Ku80 proteins, resulting in a long-range synapsis. DNA-PKcs is recruited by Ku proteins, with XRCC4 and LIG4, generating a stable complex (short-range synapsis) that allows the DNA ends to be efficiently re-ligated. Depending on the nature of the broken ends, additional factors (such as POLM, APTX, XLF, and PAXX) contribute to NHEJ repair. Figure was generated using BioRender.
(F) Bar graph representing quantitative whole-proteome analysis of NHEJ pathway proteins in TOV.C1 and TOV.C2, relative to TOV21G parental cell line. Points correspond to independent biological replicates, n = 2, mean ± SEM.
(G) Immunoblots of subcellular fractions (cytoplasmic, nucleoplasmic, and chromatin) prepared from TOV21G-derived cell lines, either untreated or treated with 10 μM PDS for 4 h. α-H2A was used as loading control for the chromatin fraction.
See also Figure S5.
We performed a similar analysis in the HCT116 cell line background and found that, consistent with the results above, the ARID1A KO cells have lower levels of core NHEJ proteins associated with chromatin in PDS-treated samples (Figure S5C). Analysis of the proteomes of both TOV21G- and HCT116-derived cell lines showed no substantial or consistent difference in the total abundance of NHEJ factors when ARID1A is deficient (Figures 5F and S5D), suggesting that the differential association with chromatin is due to impaired chromatin association, either recruitment or retention, in the absence of ARID1A rather than a deficiency of NHEJ factors.
To investigate this using an orthogonal approach, we performed western blot analysis of cytoplasmic, nucleoplasmic, and chromatin-bound fractions prepared from the TOV21G cell line panel before and after PDS treatment. We find that there is a detectable increase in chromatin-bound NHEJ proteins after PDS treatment in the two clones with ARID1A re-expression, but this is less apparent in the parental and EV controls (Figure 5G). Using an antibody against phospho-S2056, we similarly find increased phospho-DNA-PK in the chromatin of ARID1A-proficient cells (Figure 5G). Similar patterns were observed when we performed the same fractionation analysis in HCT116 parental and ARID1A KO cells (Figure S5E).
NHEJ activity is important for the repair of DSBs
Together, these data support the notion that there is greater NHEJ activity taking place on chromatin in ARID1A-proficient cells. To test whether NHEJ is functionally important for viability following PDS exposure in these cell lines, we depleted components of the pathway (DNA-PKcs, XLF, and PAXX) and monitored survival. We found that with depletion of these factors, the ARID1A-proficient TOV.C1 cells show reduced viability following PDS treatment compared with non-targeting control-depleted cells (Figures 6A, 6B, and S6A). In contrast, there was no further reduction in survival when NHEJ factors were depleted in ARID1A-deficient TOV21G cells (Figures 6A, 6B, and S6A), suggesting that a major function of ARID1A in promoting survival after PDS is via NHEJ activity.
Figure 6.
NHEJ activity is important for the repair of PDS-induced DNA damage
(A) Bar graph showing relative survival of PDS-treated ARID1A-proficient (TOV.C1) and ARID1A-deficient (TOV21G) cell lines following siRNA-mediated depletion of core NHEJ components (DNA-PKcs, XLF, and PAXX). Percentage of survival is plotted for PDS-treated (10 μM, 4 h) versus untreated cells. Non-targeting siRNA (siNT) was used as a control. Points correspond to independent biological replicates, n = 3, mean ± SEM, data were analyzed by ordinary two-way ANOVA with Dunnett’s test; ∗∗∗∗p < 0.0001 and ns, not significant.
(B) Immunoblots of siRNA depletion of NHEJ components in cells used in (A). α-tubulin was used as loading control.
(C) Bar graph showing relative survival of PDS-treated TOV.C1 and TOV21G parental cells following depletion of 53BP1. Percentage of survival is plotted for PDS-treated (10 μM, 4 h) versus untreated cells. siNT was used as control. Points correspond to independent biological replicates, n = 3, mean ± SEM, data were analyzed by ordinary two-way ANOVA with Fisher’s test; ∗p < 0.05 and ns, not significant.
(D) Immunoblots of siRNA depletion of 53BP1 in cells used in (C). α-tubulin was used as loading control.
(E) Heatmap of phosphopeptides enriched in chromatin-bound NHEJ proteins in ARID1A-proficient and -deficient cell lines, untreated or upon treatment with 10 μM PDS for 4 h.
(F) Bar graph showing relative survival of PDS-treated TOV.C1 and TOV21G parental cells following depletion of TDP2. Percentage of survival is plotted for PDS-treated (10 μM, 4 h) versus untreated cells. siNT was used as control. Each point corresponds to an independent biological replicate, representing three independent experiments. Points correspond to independent biological replicates, n = 3, mean ± SEM, data were analyzed by ordinary two-way ANOVA with Fisher’s test; ∗∗∗∗p < 0.0001 and ns, not significant.
(G) Immunoblot of siRNA depletion of TDP2 in cells used in (F). α-tubulin was used as loading control.
(H) Schematic model. Following TOP2 trapping at PDS-bound G4s, TDP2 facilitates TOP2 removal, leaving a DSB. ARID1A facilitates NHEJ assembly on chromatin, potentially through stabilizing short-range synapsis, and enabling effective DSB repair. ARID1A loss impairs NHEJ chromatin assembly or stabilization, leading to defective DNA repair and ultimately cell death. Figure was generated using BioRender.
All siRNAs were used in the same experiment in each of the three biological replicates; hence, the siNT controls are the same for each set of siRNAs represented in (A), (C), and (F). See also Figure S6.
53BP1 is an upstream regulator of DNA repair pathway choice and promotes NHEJ.22,23 We tested the impact of 53BP1 depletion on viability and found very similar patterns as loss of other NHEJ factors (Figures 6C, 6D, and S6B), suggesting that 53BP1 is particularly important for PDS responses in ARID1A-proficient cells. One possible explanation for these results is that ARID1A interacts with either 53BP1 or NHEJ factors to promote their recruitment or retention on chromatin following PDS treatment. Indeed, ARID1A was recently reported to interact directly with DNA-PKcs.24 To test this possibility, we immunoprecipitated ARID1A from extracts prepared from both untreated and PDS-treated cells. We were unable to detect DNA-PKcs, but we did find a weak but reproducible interaction with 53BP1 (Figure S6C). This was not increased by PDS treatment but nevertheless raised the possibility that ARID1A promotes NHEJ by recruiting 53BP1 to chromatin. However, in contrast to NHEJ factors, 53BP1 associated with chromatin did not show a consistent pattern in response to PDS, and its chromatin association was not regulated by ARID1A status (Figure S6D). Moreover, we found no difference in 53BP1 foci formation following PDS treatment in ARID1A-proficient versus -deficient TOV cells (Figure S6E). In HCT116 cells, 53BP1 chromatin association was also unaffected by either PDS or ARID1A (Figure S6F). In addition, the HCT116 ARID1A KO cells have significantly more 53BP1 foci than the ARID1A-proficient cells following PDS treatment (Figure S6G). Together, these data indicate that ARID1A is not required for 53BP1 foci formation in response to PDS exposure and suggest that ARID1A functions downstream of 53BP1 to facilitate NHEJ activity.
During NHEJ, synapsis follows end binding, thus tethering the two broken ends together to promote repair. Recent work demonstrated that long-range, weakly tethered synapsis is followed by structural remodeling into a more stable, short-range synaptic complex25 (Figure 5E). This transition requires DNA-PKcs kinase activity,25,26 and we found that there was a stronger phospho-S2056 DNA-PK signal in chromatin from ARID1A-proficient cells following PDS treatment (Figure 5G). By interrogating the phosphorylation events in the chromatome data, we determined that there was a pronounced enrichment of multiple phosphorylation events of NHEJ factors in the ARID1A-proficient, but not the ARID1A-deficient, cells following PDS treatment (Figure 6E). These include known DNA damage-induced events, such as the well-characterized autophosphorylation of DNA-PKcs on T2647.27,28,29 It is possible, therefore, that ARID1A promotes stable, short-range synaptic complex formation, thus stabilizing NHEJ factors on chromatin at DNA DSBs.
PDS cytotoxicity has been reported to arise through trapping TOP2 intermediates on chromatin.10,13 TOP2 cleavage complexes are removed by TDP2, which allows their repair by NHEJ.30 Consequently, if ARID1A promotes survival through NHEJ, loss of TDP2 would prevent ARID1A from doing this. We therefore depleted TDP2 from ARID1A-proficient TOV.C1 cells and found that survival is substantially reduced following PDS treatment, while it has no significant impact on the survival of ARID1A-deficient cells (Figures 6F, 6G, and S6H). These data are consistent with a model in which PDS-trapped TOP2 must first be removed by TDP2, after which ARID1A promotes NHEJ activity at the DNA breaks (Figure 6H). The absence of ARID1A impairs efficient NHEJ and, consequently, leads to reduced survival following PDS treatment.
Discussion
Here, we show that ARID1A is a key determinant of the cellular response to G4 binding ligands such as PDS. We demonstrate that ARID1A influences the transcriptional response of genes with G4-containing sequences to PDS. This includes the ARID1A gene, which is upregulated in response to PDS. However, we find that impaired NHEJ activity is the primary reason for altered viability in ARID1A-deficient cells. Interestingly, we can see decreased NHEJ, but not 53BP1, association with chromatin when ARID1A is absent, suggesting that ARID1A functions downstream of 53BP1 to promote NHEJ recruitment or stability on chromatin following DSB formation via TDP2 processing (Figure 6H). The weak interaction we detect between ARID1A and 53BP1 might indicate that 53BP1 helps to recruit or stabilize ARID1A at sites of PDS-induced damage.
Once localized to PDS-induced DSBs, ARID1A might promote NHEJ indirectly by creating a permissive chromatin environment for NHEJ assembly at DSBs. Given the reported interaction with DNA-PKcs,24 it is also possible that ARID1A binds and recruits NHEJ factors to chromatin in the vicinity of PDS-dependent DSBs. Alternatively, or additionally, ARID1A-dependent remodeling activity might promote the transition from long-range to short-range synapsis during NHEJ. Since this transition leads to a more stable association of NHEJ factors with chromatin, a role for ARID1A in promoting this transition would result in an ARID1A-dependent increase in NHEJ factors in the chromatin fraction of PDS-treated cells, in line with what we observe. Finally, it is possible that the failure of NHEJ factors to assemble on chromatin following PDS in ARID1A-deficient cells is an indirect effect. For example, ARID1A could influence global chromatin architecture or RNA processing factors that alter the ability of NHEJ factors to efficiently assemble onto chromatin.
Loss of the PBAF-specific PBRM1 subunit does not show the same vulnerability to PDS treatment (Figure S2), consistent with the fact that PBAF shows little impact on NHEJ when compared with BAF (for review, see Harrod et al.1). It is possible that cell line background plays a role here, but it is likely that the BAF complex, and not PBAF, is important for this activity. In that regard, ARID1B is an ortholog of ARID1A that can be incorporated into the BAF complex instead of ARID1A and has both overlapping and independent cellular roles.31 Whether ARID1B-containing BAF complexes contribute to the cellular response to PDS is not yet clear. Unlike findings in other cell line models (for example, Trizzino et al.32), we do not see ARID1B upregulation when ARID1A is deficient in these cell lines (Figure 1), but the ARID1B present in these cells could still promote survival following PDS-induced damage.
One striking finding from this study was the increased magnitude of PDS-induced gene expression changes when ARID1A is absent (Figure 3). There are several potential explanations for this, such as more G4 structures in ARID1A-deficient cells or less-efficient TOP2 trapping by PDS when ARID1A is present. However, if correct, these possibilities would result in increased DNA DSBs in ARID1A-deficient cells, yet we see no substantial differences in γH2AX in this time frame. Of note, we also found that proteins involved in RNA metabolism, including splicing and degradation, fail to mobilize onto chromatin in the absence of ARID1A following PDS treatment (Figure 5B). Therefore, one potential way by which ARID1A influences gene expression is through degradation, stabilization, or processing of PDS-regulated transcripts, leading to an apparently muted transcriptional response relative to cells lacking ARID1A. Another, perhaps more appealing, possibility is that ARID1A, by virtue of binding to enhancer elements, influences the topological organization of chromosomes, which might in turn influence the ability of PDS-stabilized G4s to regulate the magnitude of gene expression.
Because G4 ligands are being developed and utilized in the clinic, it is important to understand how common genetic alterations in cancer cells impact the cellular response to these molecules. Using isogenic cell line models, our findings establish ARID1A deficiency as a vulnerability to the G4 binding ligands PDS and CX-5461, raising the possibility that ARID1A or NHEJ deficiency could be considered alongside HR deficiency for clinical utility. Given the prevalence of ARID1A mutations in cancers such as ovarian clear cell carcinoma33 and colorectal cancer, these results provide a good rationale for further evaluation of G4 ligands in these settings.
Limitations of the study
While we demonstrate that impaired NHEJ underlies much of the reduced viability in ARID1A-deficient cells following PDS treatment, it is possible that this is not the sole reason for the differential sensitivity. ARID1A has been implicated in HR repair of DNA DSBs,34,35 and HR deficiency also sensitizes cells to these G4 ligands.9,12 Moreover, the altered gene expression dynamics could also contribute to the impaired fitness of the ARID1A-deficient cells. Furthermore, as highlighted above, it is not clear whether a direct interaction between ARID1A and NHEJ factors is required for the repair of PDS-induced damage or if the impact of ARID1A on NHEJ chromatin association is mediated indirectly through ARID1A-dependent changes in chromatin accessibility. Finally, as the study used only female mice, the in vivo findings are not generalizable, as there may be sex-based differences.
Resource availability
Lead contact
Further information and requests for resources and reagents should be direct to and will be fulfilled by the lead contact, Jessica A. Downs (jessica.downs@icr.ac.uk).
Materials availability
All reagents and materials are listed in the key resources table. Cell lines are available at CancerTools.org. All other materials generated in this study are available from the lead contact upon request.
Data and code availability
-
•
Proteomic datasets have been deposited to PRIDE under accession number PRIDE: PXD063556, and transcriptome datasets have been deposited to GEO under accession number GEO: GSE295547. Raw data were deposited on Mendeley at https://data.mendeley.com/datasets/ys34fgz4cx/2.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Acknowledgments
We thank the Institute of Cancer Research Flow Cytometry Facility, Light Microscopy Facility, and Biological Services Unit for support, and we thank the ICR Scientific Computing Team for HPC services. We thank all members of the Downs lab for helpful discussions. The Stucki lab is supported by project grants from the Swiss National Science Foundation (31003A_163141 and 310030_189141) and a grant from the Helmut Horten Foundation. Work in the K.J.H. lab is supported by Cancer Research UK (DRCRPG-Nov22/100008 and RRCOER-Jun24/100006). J.S.C. and M. Pardo acknowledge funding from the Wellcome Trust (223745/Z/21/Z), BBSRC (BB/Y004477/1), and ICR Core Funding. This work was further supported by Cancer Research UK (DRCRPG-Jun24/100004; J.A.D.) and the Medical Research Council (MR/W001276/1; J.A.D.).
Author contributions
S.S.-S., N.A., K.A.L., A.H., and J.A.D. conceived the study and designed experiments; S.S.-S., N.A., K.A.L., A.H., M. Pardo, M. Pedersen, C.R.-E., Z.K., K.A.G.B., and A.R. performed and analyzed experiments, analyzed data, created models, and provided resources; S.B., M.S., K.J.H., J.S.C., and J.A.D. acquired funding and supervised; J.A.D. wrote the manuscript with input from S.S.-S., N.A., K.A.L., and A.H.; and all authors contributed to review and editing.
Declaration of interests
S.S.S., N.A., and J.A.D. hold a patent on the use of SWI/SNF deficiency as a biomarker for G4 ligand treatment (PCT/GB2024/051658).
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| ARID1A | Merck | HPA005456; RRID: AB_1078205 |
| γH2AX (Ser139) (20E3) | Cell Signaling | 92845; RRID: AB_2118009 |
| 53BP1 | Merck | 05–726; RRID: AB_309940 |
| Phospho-DNA-PKcs/Phospho-PRKDC (Ser2056) | Cell Signaling | 68716S; RRID: AB_2939025 |
| DNA Ligase IV/LIG4 (D5N5N) | Cell Signaling | 14649S; RRID: AB_2750871 |
| Ku70 (D10A7) | Cell Signaling | 4588S; RRID: AB_11179211 |
| Ku80 (C48E7) | Cell Signaling | 2180S; RRID: AB_2218736 |
| PAXX/C9orf142 | Abcam | ab126353; RRID: AB_11131160 |
| XLF/NHEJ1 | Abcam | ab189917 |
| TDP2 | Fisher Scientific | 69s87; RRID: AB_10715453 |
| p53 | Santa Cruz Technology | sc-126; RRID: AB_628082 |
| p21 | Santa Cruz Technology | sc-6246: RRID: AB_628073 |
| α-H2A | Abcam | ab13923; RRID: AB_300750 |
| α-tubulin | Abcam | ab7291; RRID: AB_2241126 |
| β-actin | Merck | A5060; RRID: AB_476738 |
| Goat Anti-rabbit IgG HRP-conjugated | Agilent | P0448; RRID: AB_2617138 |
| Rabbit Anti-mouse IgG HRP-conjugated | Agilent | P0260; RRID: AB_2636929 |
| Alexa Fluor 488 goat Anti-rabbit | Invitrogen | A11008; RRID: AB_143165 |
| Alexa Fluor 647 goat Anti-mouse | Invitrogen | A21235; RRID: AB_2535804 |
| Bacterial and virus strains | ||
| XL1-Blue Competent Cells | Agilent | 200130 |
| Chemicals, peptides, and recombinant proteins | ||
| Pyridostatin hydrochloride | Merck | SML2690 |
| CX-5461 | MedChemExpress | HY-13323 |
| Hoechst 33342 trihydrochloride, trihydrate | Merck | H1399 |
| DAPI | Merck | D9542 |
| Deposited data | ||
| PRIDE | https://www.ebi.ac.uk/pride/ | PXD063556 |
| RNA-sequencing | https://www.ncbi.nlm.nih.gov/geo/ | GSE295547 |
| Mendeley dataset | https://data.mendeley.com/datasets/ys34fgz4cx/2 | N/A |
| Experimental models: Cell lines | ||
| TOV21G | ATCC | CRL-3577 |
| TOV.C1 (ARID1A re-expression) | This study | N/A |
| TOV.C2 (ARID1A re-expression) | This study | N/A |
| HCT116 | Horizon Discovery | ATCC: CCL-247 |
| HCT.KO (HCT116 ARID1A KO) | Horizon Discovery | 8289 |
| HEK293TN | Systems Biosciences | LV900A-1 |
| HEK293 KO1 (PBRM1 KO) | Lane et al.15 | N/A |
| HEK293 KO2 (PBRM1 KO) | Lane et al.15 | N/A |
| HEK293 KO3 (PBRM1 KO) | Lane et al.15 | N/A |
| RPE1 | ATCC | CRL-4000 |
| RPE1 KO1 (PBRM1 KO1) | Feng et al.14 | N/A |
| RPE1 KO2 (PBRM1 KO2) | Feng et al.14 | N/A |
| 786-O | ATCC | CRL-1932 |
| 786-O KO1 (PBRM1 KO) | Lane et al.15 | N/A |
| 786-O KO2 (PBRM1 KO) | Lane et al.15 | N/A |
| 786-O KO3 (PBRM1 KO) | Lane et al.15 | N/A |
| 786-O KO4 (PBRM1 KO) | Lane et al.15 | N/A |
| Experimental models: Organisms/strains | ||
| CD1 | Charles River Laboratories | Crl:CD1(ICR) |
| Recombinant DNA | ||
| pLentipuro-ARID1A | Addgene | #39478 |
| pLentipuro-EV | Addgene | #39481 |
| psPAX2 | Addgene | #12260 |
| MD2G | Addgene | #12259 |
| Software and algorithms | ||
| GraphPad Prism | GraphPad | v10.4.2 |
| InkScape | InkScape | v1.4 |
| Incucyte software | Sartorius | v2023A Rev2 |
| SlideBook | 3i | v2024.2 |
| ImageJ | N/A | v1.5.3 or v1.5.4 |
| GNU Image Manipulation Program (GIMP) | N/A | v3.0.2 |
| MetaXpress | Molecular Devices | v6.7.2.290 |
| FlowJo | FlowJo | v10.10.0 |
| R | N/A | v4.3.2 |
| R Studio | N/A | v2024.12.1 |
| ShinyGo | https://bioinformatics.sdstate.edu/go/ | v0.82 |
| BioRender | biorender.com | N/A |
| Proteome Discoverer | Thermo Scientific | v2.4 or v3.0 |
| dplyr | https://dplyr.tidyverse.org/ | v1.1.4 |
| FactoMineR | Lê et al.36 | v2.11 |
| FastQC | http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ | v0.11.9 |
| TrimGalore | http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ | v0.6.6 |
| Ribodetector | Deng et al.37 | v0.2.7 |
| RSeQC | Wang et al.38 | v4.0.0 |
| STAR | Dobin et al.39 | v2.7.6a |
| HTSeqCount | Anders et al.40 | N/A |
| DESeq2 | Love et al.41 | v1.38.3 |
| eulrr | https://github.com/jolars/eulerr | v7.9.2 |
| ComplexHeatmap | Gu et al.42 | v2.20.0 |
| pqsfinder | Hon et al.43 | v2.24.0 |
| bedtools | Quinlan et al.44 | v2.29.2 |
| Integrative Genomics Viewer (IGV) | Robinson et al.45 | v2.16.0 |
Experimental model and study participant details
Human cell lines
Cell lines were authenticated, routinely tested for mycoplasma and cultured at 37°C in a 5% CO2 humidified incubator. TOV21G (ATCC), and derived cell lines with reconstituted ARID1A or empty vector, were cultured in base medium consisting of 1:1 mixture of MCDB 105 Medium (Merck) made up in sterile water for cell culture (Merck) supplemented with a final concentration of 1.5g/L sodium bicarbonate (Gibco) and Medium 199 (Merck) supplemented with a final concentration of 2.2g/L sodium bicarbonate, supplemented with 15% FBS (Gibco). HCT116 and the ARID1A KO derivative were cultured in McCoys 5A Modified Media (Gibco) supplemented with 10% FBS, and 1% penicillin/streptomycin (Gibco). HEK293TN (Systems Biosciences) were maintained in Dulbecco Modified Minimal Essential Medium (DMEM)(Merck) supplemented with 10% FBS (Gibco), 200μM glutamax (Gibco), and 1% penicillin/streptomycin (P/S) (Merck). hTERT-RPE1 (RPE1, ATCC) cells were maintained in DMEM/F-12 (Merck) supplemented with 10% FBS, 200μM glutamax (Gibco), 0.26% sodium bicarbonate (Gibco), and 1% penicillin/streptomycin (P/S). 786-O cells (ATCC) were maintained in RPMI 1640 medium (Merck) supplemented with 10% FBS and 1% P/S. PBRM1 knockouts were generated and described in.15
In vivo studies
For this study, 9-12-week-old female CD1 nude mice were used (Charles River Laboratories, strain code #086). All procedures involving animals were approved by the Animal Welfare and Ethical Review Board at the Institute of Cancer Research in accordance with National Home Office Regulations under the Animals (Scientific Procedure) Act 1986.
Method details
Plasmids
ARID1A re-expression and empty-vector control in TOV21G cell line was performed using the plasmids pLenti-puro-ARID1A (ARID1A) and pLenti-puro-EV (EV) plasmids (gift from the Le-Ming Sheh lab, Addgene plasmids #39478 and #39481, respectively).
ARID1A re-expression
Plasmids were midiprepped using the Qiagen Plasmid Plus kit as per manufacturer’s protocol and stored at −20°C. They were used to generate viral particles for transfection into ARID1A-deficient OCCC cell line, TOV21G. For lentivirus production, 10 μg of ARID1A or EV plasmids were transfected in combination with the lentiviral packaging and envelope plasmids, pMD.2G and psPAX2 using Lipofectamine 3000 according to the manufacturer’s protocol (Thermo Fisher Scientific). Lentiviral production was carried out in HEK293TN. Viral particle-containing medium was harvested at 48 h and 72 h post transfection, combined and filtered through 0.45 μm filters, then stored at −80°C. For transduction, viral particle-containing medium was added to parental TOV21G in 10cm2 dishes to reach a Multiplicity of Infection (MOI) of 1 with 6 μg/mL polybrene (Merck). Cells were incubated for 24 h then selected with 1.5 mg/mL G418 (Merck) for seven days. Protein lysates from the resulting cellular pool were screened for ARID1A expression via Western blot analysis. Positive clones were single cell sorted into 96-well plates by BD FACSAria III sorter (BD Biosciences) then sub-cloned. Positive clones were screened again by Western blot analysis and verified via immunofluorescence and mass spectroscopy.
Immunoblotting
Total protein extraction
Total cell protein was extracted by boiling cell pellets in sample buffer (0.35 M Tris pH 6.8, 0.1 g/mL sodium dodecyl sulfate, 93 mg/mL dithiothreitol, 30% glycerol, 50 μg/mL bromophenol blue) to denature and resolved by SDS-PAGE.
Chromatin fractionation
For analysis of proteins in different cell compartments, cells were separated into cytoplasm, nucleoplasm and chromatin fractions. For nuclear isolation, cell pellets were incubated on ice with NIB-250 buffer (15mM Tris-HCl [pH = 7.5], 60mM KCl, 15mM NaCl, 5mM MgCl2, 1mM CaCl2, 250mM sucrose) with 1mM DTT, 1x cOmplete EDTA-free protease inhibitor cocktail (Roche), 1x PhosSTOP phosphatase inhibitor cocktail (Roche), and 0.3% NP-40 for 5 min. Nuclei were isolated by centrifugation at 500 RCF for 5 min at 4°C and supernatant (cytoplasmic fraction) was transferred to a new tube and stored at −20°C. Nuclear pellet was washed with NIB-250, centrifuged at 500 RCF for 5 min at 4°C and resuspended in hypotonic buffer (10mM HEPES [pH = 7.9], 3mM EDTA, 0.2mM EGTA) containing 1mM DTT, 1x cOmplete EDTA-free protease inhibitor cocktail, and 1x PhosSTOP phosphatase inhibitor cocktail, then incubated for 30 min on ice, vortexing every 5–10 min. Insoluble chromatin fraction was collected by centrifugation for 4 min at 1,700 RCF and supernatant (nucleoplasmic fraction) transferred to a fresh tube and stored at −20°C. The chromatin pellet was resuspended in the appropriate volume of NuPage 4x LSB (Laemmli Sample Buffer)(Invitrogen) containing 5% β-mercaptoethanol and sonicated using the Bioruptor Pico (Diagenode)(2 × 30 s, 30 s gap).
Western blotting
Proteins were boiled to denature at 95°C for 5 min in 4x LSB containing 5% β-mercaptoethanol, resolved by SDS-PAGE in a Novex 4%–20% Tris-Glycine gel (Thermo Fisher Scientific), alongside a Precision Plus Protein Standards (Bio-Rad) for size marker, then electroblotted onto a 0.45 μm nitrocellulose membrane (GE Healthcare Life Sciences).
Membranes were blocked in 5% dried skimmed milk (Marvel) dissolved in TBS buffer containing 0.1% Tween 20 (TBST), and membranes were incubated with primary antibodies dissolved in 5% milk-TBST overnight at 4°C. The following day, membranes were washed three times for 10 min with TBST then incubated in HRP-conjugated secondary antibodies diluted in 5% milk-TBST for 1 h at room temperature. Following three 10-min washes with TBST, proteins were detected using chemiluminescence enhanced Luminata Forte Western HRP Substrate (Merck) and imaged on an iBright 750 gel imaging system (Invitrogen). Details of the primary and secondary antibodies used for Western blotting in this study are detailed in the key resources table. Western blots were processed for publication using GIMP software (v3.0.2).
Immunofluorescence
Cells were seeded at appropriate pre-determined densities on 22mm coverslips 48 h prior to treatment. Cells underwent treatment and were fixed in 4% paraformaldehyde (Electron Microscopy Sciences) in PBS, for 10 min at room temperature. After two 4-min washes with PBS, the paraformaldehyde-fixed cells were permeabilised with 0.5% Triton X- in PBS for 5 min at room temperature. Cells were then washed three times for four minutes each with PBS prior to immunostaining. Fixed cells were blocked with 1% BSA-PBS for 1 h at room temperature, then incubated with primary antibodies diluted in 1% BSA-PBS at the working concentrations, overnight at 4°C. Coverslips were washed three times for four minutes each with PBS, then incubated with secondary antibody and DNA stain 1μg/mL DAPI (Merck) or Hoechst (Merck), indicated in the relevant figures, at the appropriate working concentrations in 1% BSA-PBS, for 1 h at room temperature and protected from light. After a final three four-minute PBS washes, the slips were mounted onto poly-L-lysine glass slides (Fisher Scientific) with Vectashield mounting medium (Vector Laboratories). The cells were imaged with the Advanced Spinning Disc Confocal Microscope using Slidebook 6 software (3i). All images were obtained with 63x oil lens with z-stacks at relevant intervals. Images were exported as maximum intensity projections for analysis.
Cell growth analysis
Cells were seeded at 1 x 103 cells/well density in clear bottom black 96-well plates (Greiner Bio-One) and placed in the Incucyte SX5 (Sartorius) equipped with a 10× objective and maintained at 37°C in a humidified 5% CO2 atmosphere, then imaged every four hours, for a total of 72–96 h. Confluence (% of area covered by cells) was used as a proxy for cell growth. Data was exported and transferred to GraphPad Prism for analysis and presentation.
Drug treatments
For SRB assays, cell death assays and time-lapse imaging, TOV21G and HCT116 parental and derivative cell lines were treated with 10 μM PDS and 20 μM PDS, respectively, or with 8 μM CX-5461. PDS was dissolved in water, CX-5461 was dissolved in DMSO. Treatment was carried out for 6 days or, for drug washout experiments, for 4 h then medium containing drug was removed, replaced by normal media and cells were let grow for up to 6 days. PBRM1 KO and matching parental cell lines were treated with a range concentration of PDS (0–50 μM) for 5 days. For cell cycle analysis, microscopy, RNA-seq and proteomics, TOV21G and HCT116 cells lines were treated with 10 μM or 20 μM PDS, respectively, for 4 h then harvested and analyzed. For in vivo experiments, CX-5461 was dissolved in vehicle of 50 mM NaH2PO4.
Quantitative mass spectrometry and proteomics data analysis
Chromatin enrichment
Flash frozen cell pellets were thawed on ice and resuspended in nuclear extraction buffer (15 mM Tris-HCl pH7.5, 60 mM KCl, 15 mM NaCl, 5 mM MgCl2, 1 mM CaCl2, 250 mM sucrose, 0.3% NP-40, freshly supplemented with 1 mM DTT and Halt Protease and Phosphatase inhibitor (Thermo Scientific) and incubated on ice for 5 min. Nuclei were collected by centrifugation (600 rcf, 5 min at 4°C), washed once with nuclear extraction buffer without NP-40 pelleted again, then re-suspended in pre-chilled hypotonic buffer (3 mM EDTA, 0.2 mM EGTA and freshly supplemented with 1 mM DTT and Halt Protease and Phosphatase inhibitor) and incubated on ice for 30 min to release chromatin. Chromatin was pelleted for 5 min at 1,700 rcf at 4°C cooled centrifuge and subsequently washed twice with hypotonic buffer.
MS sample preparation and TMT labeling
Whole cell or chromatin pellets were solubilised using probe sonication in lysis buffer 100 mM triethylammonium bicarbonate (TEAB), 1% sodium deoxycholate (SDC), 10% isopropanol, 50 mM NaCl, 1:1,000 Pierce Universal Nuclease (Thermo Scientific) supplemented with Halt Protease and Phosphatase inhibitor. Protein concentration was measured with the Quick Start Bradford protein assay (Bio-Rad) following the manufacturer’s protocol. Protein with an equal contribution from each individual sample were reduced with 5 mM tris-2-carboxyethyl phosphine (TCEP) for 1 h, followed by alkylation with 10 mM iodoacetamide (IAA) for 30 min, then digested by adding trypsin (Pierce) at final concentration 75 ng/μL to each sample and incubating the samples for 18 h at RT. For each sample, 100 μg of protein digest was used for phospho-peptides enrichment, while 30 μg was allocated for chromatin and whole-cell proteomics. Phospho-enriched peptides were labeled with TMT-10plex or TMTpro multiplexing reagents (Thermo Scientific) according to the manufacturer’s protocol. Once the samples were combined, SDC was precipitated by adding formic acid (FA) to a final concentration of 2% (v/v), followed by centrifugation at 10,000 rpm for 5 min. The supernatant containing TMT-labelled peptides was dried using a centrifugal vacuum concentrator.
High-pH reversed-phase peptide fractionation and liquid chromatography–mass spectrometry analysis
Prior to MS, TMT-labelled peptides were fractionated with high-pH reversed-phase (RP) chromatography using the Waters XBridge C18 column (2.1 × 150 mm, 3.5 μm) on a Dionex UltiMate 3000 high-performance liquid chromatography (HPLC) system. Mobile phase A was 0.1% ammonium hydroxide (v/v), mobile phase B was 100% acetonitrile and 0.1% ammonium hydroxide (v/v). Peptide separation was performed with a gradient elution at 200 μL/min with the following steps: isocratic for 5 min at 5% phase B, gradient for 40 min to 35% phase B, gradient to 80% phase B in 5 min, isocratic for 5 min, and re-equilibrated to 5% phase B. Fractions were collected in a 96-well plate every 42 s to a total of 65 fractions, then concatenated into 12 fractions, dried and reconstituted in 0.1% TFA.
Phosphopeptides enrichment
Fractionated peptides were reconstituted in 10 μL of binding solution containing 20% isopropanol and 0.5% formic acid, then loaded onto 10 μL of phospho-peptides enrichment resin (High-Select Fe-NTA Magnetic Phosphopeptide Enrichment Kit, Thermo) pre-washed and conditioned with binding solution in custom-made filter tips fitted onto Eppendorf tubes. Binding was performed at room temperature for 30 min, followed by three washes with 40 μL of binding solution. Washes were performed by centrifugation at 300g, and the flow-through fractions were collected for total proteome analysis. Phosphopeptides were eluted in three steps using 30 μL of 40% acetonitrile and 400 mM ammonium hydroxide. Both eluted phosphopeptides and flow-through samples were vacuum-dried and stored at −20°C until LC-MS analysis.
LC-MS analysis
LC-MS analysis was performed on the Dionex UltiMate 3000 UHPLC system coupled with the Orbitrap Fusion Lumos or Orbitrap Ascend mass spectrometers (Thermo Scientific). Samples were analyzed with the EASY-Spray C18 capillary column (75 μm × 50 cm, 2 μm) at 50°C. Mobile phase A was 0.1% formic acid and mobile phase B was 80% acetonitrile, 0.1% formic acid. The gradient separation method was as follows: 150 min gradient up to 38% B, for 10 min up to 95% B, for 5 min isocratic at 95% B, re-equilibration to 5% B in 10 min, for 10 min isocratic at 5% B. Precursors between 375 and 1500 m/z were selected with a mass resolution of 120 000, automatic gain control (AGC) of 4 × 105, and IT (injection time) of 50 ms, with the top speed mode in 3 s for high collision dissociation (HCD) fragmentation with a quadrupole isolation width of 0.7 Th (Thomson unit). The collision energy was set at 35% (TMTpro) or 38% (TMT-10plex), with AGC at 1 × 105 and IT at 86 ms. The samples were analyzed on an Orbitrap Fusion Lumos had following settings: A Dionex Ultimate 3000 system and mass spectrometer (Thermo Scientific) were used for data acquisition. From each fraction 10 μL was injected onto a C18 trapping column (Acclaim PepMap 100, 100 μm × 2 cm, 5 μm, 100 Å) at a 10 μL/min flow rate. The samples were subjected to a low-pH gradient elution on a nanocapillary reversed phase column (Acclaim PepMap C18, 75 μm × 50 cm, 2 μm, 100 Å) at 45°C. Mobile phases A and B were 0.1% formic acid and 80% acetonitrile, 0.1% formic acid respectively. The separation was performed at 300 nL/min flow rate and 90 min gradient from 5% to 38% phase B followed by 10 min up to 95% phase B, isocratic for 5 min at 95% B, re-equilibrated to 5% phase B in 5 min, and isocratic for 10 min at 5% phase B. MS1 scans were collected with mass resolution of 120,000, automatic gain control of 4 × 105, and injection time of 50 ms. Precursor ions (Top Speed mode, 3 s) were fragmented with collision-induced dissociation (CID) with a quadrupole isolation width of 0.7 Th (Thomson unit). Collision energy was set at 35%, with AGC at 1 × 104 and IT at 50 ms. Quantification was obtained at the MS3 level with higher-energy collisional dissociation (HCD) fragmentation of the top 7 most abundant CID fragments isolated with synchronous precursor selection (SPS). Quadrupole isolation width was set at 0.7 Th, collision energy was applied at 65%, and the AGC setting was at 1 × 105 with IT at 105 ms. The HCD MS3 spectra were acquired for the range 100–500 m/z with a resolution of 50,000. Targeted precursors were dynamically excluded for further fragmentation for 45 s with 7 ppm mass tolerance. Phosphopeptide samples were analyzed with an HCD method at the MS2 level with CE 38%, AGC 1 × 105 and max IT 105 ms. The samples analyzed on Orbitrap Ascend had following settings: MS1 scans were performed over a mass range of m/z 400–1600 at 120,000 resolution in the Orbitrap, with standard AGC settings and automatic injection times. Ions with charge states +2 to +6 were included. Dynamic exclusion was set to 45 s with a repeat count of 1, a ±10 ppm mass tolerance, and isotopes were excluded from further analysis.
MS2 spectra were acquired in the ion trap using a Turbo scan rate, with 32% HCD collision energy and a maximum injection time of 35 ms. Real-time database searching against Homo sapiens (canonical and isoforms) was conducted using the Comet search engine, considering tryptic peptides with a maximum of 1 missed cleavage. Static modifications were set for carbamidomethylation of C (+57.0215 Da) and TMTpro labeling on K and N-termini (+304.207 Da). Variable modifications included deamidation of N/Q (+0.984 Da) and oxidation of M (+15.9949 Da), allowing up to 2 variable modifications per peptide. A close-out feature was enabled, limiting to 4 peptides per protein. SPS10-MS3 scans were performed on selected precursors using the Orbitrap at 45,000 resolution with 55% HCD collision energy, a 200% normalized AGC target, and a 200 ms maximum injection time. Data were collected in centroid mode with single micro-scan acquisition.
Database search and protein quantification
The SequestHT and Comet search engines were used to analyze the acquired spectra in Proteome Discoverer 2.4 and 3.0 (Thermo Scientific) for protein identification and quantification. For chromatin and whole cell proteomes, the precursor mass was set to 20 ppm and fragment mass tolerance was 0.5 Da. Spectra were searched for fully tryptic peptides with maximum 2 missed-cleavages. TMTpro at N-term/Lys and Carbamidomethyl at Cys were defined as static modifications. Dynamic modifications included oxidation of Met and Deamidation of Asn/Gln. For phospho-enriched peptides, the precursor mass was set to 10 ppm and fragment mass tolerance was 0.02 Da. TMT-10plex or TMTpro at N-term and Carbamidomethyl at Cys were defined as static modifications, while dynamic modifications included oxidation of Met, Deamidation of Asn/Gln, TMTpro or Acetyl at Lys residue. Peptide confidence was estimated with the Percolator node. Peptide FDR was set at 1% and validation was based on q-value and target-decoy database search. Spectra were searched against reviewed UniProt human protein entries. The reporter ion quantifier node included a TMT quantification method with an integration window tolerance of 15 ppm and integration method based on the most confident centroid peak at the MS3 or MS2 level. Only unique peptides were used for quantification, considering protein groups for peptide uniqueness. Peptides with average reporter signal-to-noise >3 were used for quantification. The data was normalised to total loading at the proteome level, while for the respective phospho-proteome the data was corrected for loading for phosphorylated peptides only. Relative abundances were calculated by dividing normalised protein/peptide abundances by the average abundance of all TMT channels per biological replicate. PCA plot was generated in R by combining relative normalised abundances HCT116 and TOV21G datasets into a single dataset using dplyr (v1.1.4)(Wickham H, François R, Henry L, Müller K, Vaughan D (2023). _dplyr: A Grammar of Data Manipulation) and correlations were calculated and plotted using FactoMineR (v2.11).
Cell cycle analysis
Cells were trypsinised, washed with PBS and fixed in 70% ice-cold ethanol 96 h after treatment with or without 10 μM PDS (Merck). Samples were stored overnight at −20°C. Fixed cell pellets were washed once in PBS then incubated in PBS containing 5 μg/mL propidium iodide (Merck) and 100 μg/mL RNAse A (Merck) for 30 min at 37°C and protected from light. Analysis of the stained cells were carried out on the BD LSR II flow cytometer or the BD FACSymphony A5 flow cytometer with 20,000 single cell events recorded per sample after gating. Data analysis was performed using FlowJo v10.10.0 software.
Sulforhodamine B (SRB) assays
Cells were seeded in 96-well plates at the appropriate densities to reach 70–80% confluence after 5–6 days in the absence of treatment. This was at 15,000 cells/mL (1,500 cells/well) for TOV21G cell lines, 10,000 cells/ml (1,000 cells/well) for the HCT116, RPE1, HEK293, and 786-O cell lines. Cells were allowed to adhere overnight before addition of drug. Cells were allowed to grow for 5–6 days, after which they were fixed by adding 100 μL 10% TCA (Merck) and incubated at 4°C for 24 h. Plates were then washed five times with water and allowed to completely dry before staining with 100 μL 0.057% SRB (Merck) in 1% acetic acid (Fisher Scientific) and incubated for 1 h room temperature. Unbound dye was removed by washing with 1% acetic acid, and the plate was allowed to dry completely. Protein-bound dye was solubilised in 100 μL of 10mM Tris-HCl (pH 9.5) for 10 min at room temperature. The optical density measurements at 564 nm were quantitated using the SpectraMax Microplate Absorbance Reader (PerkinElmer). Cell growth was expressed as a percentage of the absorbance measured in the treated wells compared with the control wells.
Cell death assay (celigo)
Cells were seeded in 96-well plates at the appropriate densities to reach 70–80% confluence after 6 days in the absence of treatment. Cells were allowed to adhere overnight before treatment with PDS and incubated for 5 days. 30 min prior to analysis on the Celigo (Nexelcom Bioscience), 20 μL media containing propidium iodide (Merck) and Hoechst to a final concentration of 2 μg/mL each was added to well. The proportion of dead cells was defined as the number of PI-positive cells as a percentage of total (Hoechst-positive) cells.
RNA-seq
For RNA-sequencing, pellets were harvested by scraping cells in ice-cold PBS following by centrifugation at 7,500 x g for 10 min. Three independent biological replicates were used for TOV21G parental cells (n = 3), and two independent biological replicates of each ARID1A complemented cell line (TOV.C1 and TOV.C2), which were combined for n = 4 in total for analysis purposes. Pellets were resuspended in 500μL TRIzol reagent (Ambion, Thermo Fisher Scientific) and total RNA was extracted using a Direct-zol RNA miniprep kit (Zymo Research) according to the manufacturer’s protocol. RNA concentration and quality was confirmed with the High Sensitivity RNA ScreenTape (Agilent), using the Agilent 4150 Tapestation. Library preparation and sequencing was performed by Novogene Corporation Ltd. Novogene NGS Stranded RNA Library Prep Set was used to generate 250-300bp insert strand specific libraries, and ribosomal RNA was removed using TruSeq Stranded Total RNA Library Prep. 50 million 150bp paired-end reads were sequenced on an Illumina NovaSeq 6000. Fastq reads were checked using FastQC (v0.11.9) (Andrews, S. (2010). FastQC: A Quality Control Tool for High Throughput Sequence Data [Online]. http://www.bioinformatics.babraham.ac.uk/projects/fastqc/) and trimmed using TrimGalore (v0.6.6) (Krueger F, Trimgalore (2023), GitHub repository, https://github.com/FelixKrueger/TrimGalore). Residual ribosomal RNA reads were removed using Ribodetector with -e norRNA setting (v0.2.7)37 and strandedness was detected using RSeQC (v4.0.0).38 Reads were aligned to the T2T-CHM13v2 genome using STAR alignment software (v2.7.6a)39 and reads mapping to genes were quantified using HTSeqCount.40 Differential analysis of gene expression was calculated in R using DESeq2 (v1.38.3).41 Area-proportional venn diagrams with ellipses were generated using eulerr package v7.0.2 (eulrr Larsson J (2024). eulerr: Area-Proportional Euler and Venn Diagrams with Ellipses). Heatmaps displaying scaled regularised log transformed read counts for each RNA-seq replicate were plotted using ComplexHeatmap v2.20.0,42 with columns split by condition and rows split by k-means clustering. Gene IDs for each cluster were extracted using dplyr (v1.1.4) for pathway analysis. Pathway enrichment was calculated using Hallmark signature gene sets46 on ShinyGo.47 Potential quadruplex-forming sequences (PQS) in the T2T-CHM13v2 genome were identified using pqsfinder (v2.24.0)43 using default settings. Promoter coordinates (hsEPDnew version 00648) were lifted over from hg38 to T2T coordinates using the UCSC liftOver command line tool. Genes with PQS at promoters were defined using the bedtools (v2.29.2) window tool as genes with promoters within 100bp of a PQS. Screenshots of genomic regions were generated using Integrative Genomics Viewer (IGV, v2.16.0).
Tumor growth and in vivo treatments
For experiments involving tumor growth, TOV21G cells were expanded, then trypsinised and filtered through 40μm cell strainers. 5 × 106 cells were mixed with Corning Matrigel matrix basement membrane (Fisher Scientific) at a 2:1 ratio (cells:Matrigel) in a total volume of 100μL, and were injected subcutaneously in the right flank of 9-12-week-old female CD1 nude mice (Charles River Laboratories). Tumors were allowed to grow for 3–4 weeks and thereafter mice were randomised based on tumor volume (∼100mm3) before start of treatment. The number of mice per treatment group is indicated in the figure legends. Mice were treated with CX-5461 or vehicle solution by oral gavage at the indicated times and doses detailed in figure legends. Tumors were measured using calipers twice weekly and tumor volumes calculated using the formula length x width x height (mm) x 0.5236 and data plotted in GraphPad Prism. Data from tumor growth are plotted as mean ± SEM and area under curve (A.U.C) was calculated from start of inoculation. Mouse body weights were measured twice weekly.
High-throughput fluorescence microscopy
TOV21G and HCT116 parental and derivative cell lines were seeded in 96-well plates at the density of 10,000 cells per well. 24 h later, cells were treated with 10 μM and 20 μM PDS, respectively, for 4 h, then fixed with 4% paraformaldehyde (Electron Microscopy Sciences) for 10 min at room temperature. After three rounds of 4-min washes with 1xPBS, cells were permeabilised with 0.2% Triton-PBS, for 10 min at room temperature. After additional three rounds of 4-min washes with 1xPBS, cells were blocked with 1% BSA at room temperature for 30 min then incubated overnight at 4°C, in the relevant primary antibodies, either ƴH2AX or 53BP1, both diluted 1:500 in 1% BSA. Cells then underwent three 4-min washes with 1xPBS and were subsequently incubated with the relevant secondary antibodies (anti-rabbit green 488 for ƴH2AX and anti-mouse red for 53BP1). After an hour incubation in the secondary antibodies at room temperature, cells were washed three times for 4 min in 1xPBS. 200 μL PBS was added to each well and cells were analyzed using an ImageXpress Microconfocal Spinning Disk system (Molecular Devices) to detect ƴH2AX and 53BP1 foci, and foci number were quantified using MetaXpress software.
Cell fate profiling
To measure proliferation, apoptosis induction, and to perform cell fate profiling, 1 × 104 cells were seeded per well in μclear 96 well plates (Greiner Bio-One) and IncuCyte Kinetic Caspase-3/7 Apoptosis Assay Reagent (Sartorius) added at 1:2000 concentration. Cells were then imaged using an IncuCyte SX5 (Sartorius) equipped with a 10× objective and maintained at 37°C in a humidified 5% CO2 atmosphere. Phase contrast and fluorescence images (3 images per well) were collected every 10 min, using confluence and fluorescence as proxies for proliferation and apoptosis, respectively. Apoptosis was quantitated by measuring green fluorescence object count in 3 images per well. Image sequences were then exported in MPEG-4 format and individual cells were analyzed manually to generate cell fate profiles. Timing data were imported into GraphPad Prism for statistical analysis and presentation. Note that 0 h on the fate profiles represents when imaging started.
RNA interference
For RNAi-mediated inhibition, a cell suspension of 250,000 cell/ml suspension was prepared in media without antibiotics and mixed with transfection reagent consisting in 1:1 mixture of 120nM siRNA diluted in Opti-MEM media (Life-Technologies) and 100nM Lipofectamine RNAiMAX Transfection Reagent (Thermo Fisher Scientific) diluted in Opti-MEM media. Each cells/transfection reagent mixture was transferred to a 6cm dish and incubated at 37°C in a 5% CO2 humidified incubator for 24h. Knock-down was confirmed by immunoblotting. ON-TARGETplus siRNA pools targeting ARID1A, DNA-PKcs, XLF, PAXX, 53BP1 and TDP2 were obtained from Dharmacon. Non-targeting control was a mixture of Mission siRNA Universal Negative control #2 (Sigma) and siRNA Negative control (Eurogentec).
Co-immunoprecipitation (CO-IP)
Cell pellets of TOV21G-proficient cell line (TOV.C1) or HCT116 cells (untreated and treated with 10 or 20μM pyridostatin, respectively, for 4 h) were lysed in 50 mM Tris-HCl pH 8, 300 mM NaCl, 0.2% Igepal, 1 mM EDTA, containing 1 mM DTT and Halt protease and phosphatase inhibitors (Pierce). Lysates were then diluted to 50 mM Tris-HCl pH 8, 150 mM NaCl, 0.1% Igepal, 1 mM EDTA. Anti-53BP1 (Milipore 05–726) and normal mouse IgG (Millipore) (2 μg) were coupled to 25 μL of Protein G Dynabeads (Thermo) according to manufacturer’s instructions. Antibody-coupled beads were incubated with cell lysates containing 0.5–1 mg of protein for 1–2 h at 4°C. Beads were then washed three times with 10 mM Tris-ClH pH 8, 150 mM NaCl, 0.1% Igepal. Immunoprecipitates were eluted in 1x LDS (Life Technologies) at 70°C for 10 min. Proteins were separated in 3–8% NuPAGE Tris-Acetate gels (Life Technologies) and transferred to nitrocellulose membranes. Immunodetection was performed according to standard procedures using anti-53BP1 (Abcam ab21083, 1:2000) and anti-ARID1A (Sigma HPA005456, 1:1000) antibodies, followed by incubation with HRP-labelled Protein A (GE Healthcare). Membranes were developed with ECL Prime (Cytiva) or Immobilon ECL Ultra Western HRP Substrate (Millipore) and chemiluminescence was captured with an iBright imager (Invitrogen) or on X-ray film (Hyperfilm ECL, Cytiva).
Quantification and statistical analysis
The number (n) of biological repeats, or individual mice in the case of in vivo studies (Figures 2 and S2), and data represented in figures, are indicated in the Fig. legend for each experiment. Depending on the experiment and the appropriate statistical test, ordinary 1-way ANOVA with Tukey’s test, paired or unpaired t test, or two-way ANOVA with Šídák’s, Dunnett’s, or Fisher’s tests were used, and the analysis used is indicated in the corresponding Fig. legend for each experiment. Graphs were generated and statistical analyses performed using GraphPad Prism (v10.4.2). Microscopy images were analyzed using ImageJ (v1.5.3 or 1.5.4), CellProfiler (v4.0.7) or MetaXpress software (v6.7.2.290), and visualized using GraphPad Prism. Omics data were visualised using the indicated packages in R (v4.3.2) and R Studio (v2024.12.1). Figures were generated using Inkscape Software. Some graphics were generated using BioRender (biorender.com), indicated in figure legends.
Published: September 11, 2025
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2025.116277.
Supplemental information
References
- 1.Harrod A., Lane K.A., Downs J.A. The role of the SWI/SNF chromatin remodelling complex in the response to DNA double strand breaks. DNA Repair. 2020;93 doi: 10.1016/j.dnarep.2020.102919. [DOI] [PubMed] [Google Scholar]
- 2.Bayona-Feliu A., Barroso S., Muñoz S., Aguilera A. The SWI/SNF chromatin remodeling complex helps resolve R-loop-mediated transcription-replication conflicts. Nat. Genet. 2021;53:1050–1063. doi: 10.1038/s41588-021-00867-2. [DOI] [PubMed] [Google Scholar]
- 3.Tsai S., Fournier L.A., Chang E.Y.C., Wells J.P., Minaker S.W., Zhu Y.D., Wang A.Y.H., Wang Y., Huntsman D.G., Stirling P.C. ARID1A regulates R-loop associated DNA replication stress. PLoS Genet. 2021;17 doi: 10.1371/journal.pgen.1009238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spiegel J., Adhikari S., Balasubramanian S. The Structure and Function of DNA G-Quadruplexes. Trends Chem. 2020;2:123–136. doi: 10.1016/j.trechm.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansel-Hertsch R., Beraldi D., Lensing S.V., Marsico G., Zyner K., Parry A., Di Antonio M., Pike J., Kimura H., Narita M., et al. G-quadruplex structures mark human regulatory chromatin. Nat. Genet. 2016;48:1267–1272. doi: 10.1038/ng.3662. [DOI] [PubMed] [Google Scholar]
- 6.Kosiol N., Juranek S., Brossart P., Heine A., Paeschke K. G-quadruplexes: a promising target for cancer therapy. Mol. Cancer. 2021;20:40. doi: 10.1186/s12943-021-01328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez R., Miller K.M., Forment J.V., Bradshaw C.R., Nikan M., Britton S., Oelschlaegel T., Xhemalce B., Balasubramanian S., Jackson S.P. Small-molecule-induced DNA damage identifies alternative DNA structures in human genes. Nat. Chem. Biol. 2012;8:301–310. doi: 10.1038/nchembio.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masud T., Soong C., Xu H., Biele J., Bjornson S., McKinney S., Aparicio S. Ubiquitin-mediated DNA damage response is synthetic lethal with G-quadruplex stabilizer CX-5461. Sci. Rep. 2021;11:9812. doi: 10.1038/s41598-021-88988-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmer J., Tacconi E.M.C., Folio C., Badie S., Porru M., Klare K., Tumiati M., Markkanen E., Halder S., Ryan A., et al. Targeting BRCA1 and BRCA2 Deficiencies with G-Quadruplex-Interacting Compounds. Mol. Cell. 2016;61:449–460. doi: 10.1016/j.molcel.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olivieri M., Cho T., Alvarez-Quilon A., Li K., Schellenberg M.J., Zimmermann M., Hustedt N., Rossi S.E., Adam S., Melo H., et al. A Genetic Map of the Response to DNA Damage in Human Cells. Cell. 2020;182:481–496.e421. doi: 10.1016/j.cell.2020.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lerner L.K., Sale J.E. Replication of G Quadruplex DNA. Genes. 2019;10 doi: 10.3390/genes10020095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groelly F.J., Porru M., Zimmer J., Benainous H., De Visser Y., Kosova A.A., Di Vito S., Serra V., Ryan A., Leonetti C., et al. Anti-tumoural activity of the G-quadruplex ligand pyridostatin against BRCA1/2-deficient tumours. EMBO Mol. Med. 2022;14 doi: 10.15252/emmm.202114501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bossaert M., Pipier A., Riou J.F., Noirot C., Nguyên L.T., Serre R.F., Bouchez O., Defrancq E., Calsou P., Britton S., Gomez D. Transcription-associated topoisomerase 2alpha (TOP2A) activity is a major effector of cytotoxicity induced by G-quadruplex ligands. eLife. 2021;10 doi: 10.7554/eLife.65184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng H., Lane K.A., Roumeliotis T.I., Jeggo P.A., Somaiah N., Choudhary J.S., Downs J.A. PBAF loss leads to DNA damage-induced inflammatory signaling through defective G2/M checkpoint maintenance. Genes Dev. 2022;36:790–806. doi: 10.1101/gad.349249.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lane K.A., Harrod A., Wu L., Roumeliotis T.I., Feng H., Foo S., Begg K.A.G., Schiavoni F., Amin N., Zenke F.T., et al. PBRM1 directs PBAF to pericentromeres and protects centromere integrity. Nat. Commun. 2025;16:1980. doi: 10.1038/s41467-025-57277-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hilton J., Gelmon K., Bedard P.L., Tu D., Xu H., Tinker A.V., Goodwin R., Laurie S.A., Jonker D., Hansen A.R., et al. Results of the phase I CCTG IND.231 trial of CX-5461 in patients with advanced solid tumors enriched for DNA-repair deficiencies. Nat. Commun. 2022;13:3607. doi: 10.1038/s41467-022-31199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Escarcega R.D., Patil A.A., Moruno-Manchon J.F., Urayama A., Marrelli S.P., Kim N., Monchaud D., McCullough L.D., Tsvetkov A.S. Pirh2-dependent DNA damage in neurons induced by the G-quadruplex ligand pyridostatin. J. Biol. Chem. 2023;299 doi: 10.1016/j.jbc.2023.105157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huppert J.L., Balasubramanian S. G-quadruplexes in promoters throughout the human genome. Nucleic Acids Res. 2007;35:406–413. doi: 10.1093/nar/gkl1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carcamo S., Nguyen C.B., Grossi E., Filipescu D., Alpsoy A., Dhiman A., Sun D., Narang S., Imig J., Martin T.C., et al. Altered BAF occupancy and transcription factor dynamics in PBAF-deficient melanoma. Cell Rep. 2022;39 doi: 10.1016/j.celrep.2022.110637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park Y.K., Lee J.E., Yan Z., McKernan K., O'Haren T., Wang W., Peng W., Ge K. Interplay of BAF and MLL4 promotes cell type-specific enhancer activation. Nat. Commun. 2021;12:1630. doi: 10.1038/s41467-021-21893-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan T., Zhao B., Wu Q., Wang W., Shi J., Li D., Stovall D.B., Sui G. Characterization of G-quadruplex formation in the ARID1A promoter. Int. J. Biol. Macromol. 2020;147:750–761. doi: 10.1016/j.ijbiomac.2020.01.210. [DOI] [PubMed] [Google Scholar]
- 22.Tarsounas M., Sung P. The antitumorigenic roles of BRCA1-BARD1 in DNA repair and replication. Nat. Rev. Mol. Cell Biol. 2020;21:284–299. doi: 10.1038/s41580-020-0218-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cejka P., Symington L.S. DNA End Resection: Mechanism and Control. Annu. Rev. Genet. 2021;55:285–307. doi: 10.1146/annurev-genet-071719-020312. [DOI] [PubMed] [Google Scholar]
- 24.Kanno S.I., Kobayashi T., Watanabe R., Kurimasa A., Tanaka K., Yasui A., Ui A. Armadillo domain of ARID1A directly interacts with DNA-PKcs to couple chromatin remodeling with nonhomologous end joining (NHEJ) pathway. Nucleic Acids Res. 2025;53 doi: 10.1093/nar/gkaf150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loparo J.J. Holding it together: DNA end synapsis during non-homologous end joining. DNA Repair. 2023;130 doi: 10.1016/j.dnarep.2023.103553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stinson B.M., Loparo J.J. Repair of DNA Double-Strand Breaks by the Nonhomologous End Joining Pathway. Annu. Rev. Biochem. 2021;90:137–164. doi: 10.1146/annurev-biochem-080320-110356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amiri Moghani A.R., Sharma M.K., Matsumoto Y. In cellulo phosphorylation of DNA double-strand break repair protein XRCC4 on Ser260 by DNA-PK. J. Radiat. Res. 2018;59:700–708. doi: 10.1093/jrr/rry072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Douglas P., Sapkota G.P., Morrice N., Yu Y., Goodarzi A.A., Merkle D., Meek K., Alessi D.R., Lees-Miller S.P. Identification of in vitro and in vivo phosphorylation sites in the catalytic subunit of the DNA-dependent protein kinase. Biochem. J. 2002;368:243–251. doi: 10.1042/BJ20020973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beli P., Lukashchuk N., Wagner S.A., Weinert B.T., Olsen J.V., Baskcomb L., Mann M., Jackson S.P., Choudhary C. Proteomic investigations reveal a role for RNA processing factor THRAP3 in the DNA damage response. Mol. Cell. 2012;46:212–225. doi: 10.1016/j.molcel.2012.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomez-Herreros F., Romero-Granados R., Zeng Z., Alvarez-Quilon A., Quintero C., Ju L., Umans L., Vermeire L., Huylebroeck D., Caldecott K.W., Cortes-Ledesma F. TDP2-dependent non-homologous end-joining protects against topoisomerase II-induced DNA breaks and genome instability in cells and in vivo. PLoS Genet. 2013;9 doi: 10.1371/journal.pgen.1003226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pagliaroli L., Trizzino M. The Evolutionary Conserved SWI/SNF Subunits ARID1A and ARID1B Are Key Modulators of Pluripotency and Cell-Fate Determination. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.643361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trizzino M., Barbieri E., Petracovici A., Wu S., Welsh S.A., Owens T.A., Licciulli S., Zhang R., Gardini A. The Tumor Suppressor ARID1A Controls Global Transcription via Pausing of RNA Polymerase II. Cell Rep. 2018;23:3933–3945. doi: 10.1016/j.celrep.2018.05.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stewart J., Cunningham N., Banerjee S. New therapies for clear cell ovarian carcinoma. Int. J. Gynecol. Cancer. 2023;33:385–393. doi: 10.1136/ijgc-2022-003704. [DOI] [PubMed] [Google Scholar]
- 34.Davo-Martinez C., Helfricht A., Ribeiro-Silva C., Raams A., Tresini M., Uruci S., van Cappellen W.A., Taneja N., Demmers J.A.A., Pines A., et al. Different SWI/SNF complexes coordinately promote R-loop- and RAD52-dependent transcription-coupled homologous recombination. Nucleic Acids Res. 2023;51:9055–9074. doi: 10.1093/nar/gkad609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bakr A., Della Corte G., Veselinov O., Kelekçi S., Chen M.J.M., Lin Y.Y., Sigismondo G., Iacovone M., Cross A., Syed R., et al. ARID1A regulates DNA repair through chromatin organization and its deficiency triggers DNA damage-mediated anti-tumor immune response. Nucleic Acids Res. 2024;52:5698–5719. doi: 10.1093/nar/gkae233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le S., Josse J., Husson F. FactoMineR: An R package for multivariate analysis. J. Stat. Software. 2008;25:1–18. doi: 10.18637/jss.v025.i01. [DOI] [Google Scholar]
- 37.Deng Z.L., Münch P.C., Mreches R., McHardy A.C. Rapid and accurate identification of ribosomal RNA sequences via deep learning. Nucleic Acids Res. 2022;50 doi: 10.1093/nar/gkac112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L., Wang S., Li W. RSeQC: quality control of RNA-seq experiments. Bioinformatics. 2012;28:2184–2185. doi: 10.1093/bioinformatics/bts356. [DOI] [PubMed] [Google Scholar]
- 39.Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T.R. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anders S., Pyl P.T., Huber W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gu Z., Eils R., Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics. 2016;32:2847–2849. doi: 10.1093/bioinformatics/btw313. [DOI] [PubMed] [Google Scholar]
- 43.Hon J., Martínek T., Zendulka J., Lexa M. pqsfinder: an exhaustive and imperfection-tolerant search tool for potential quadruplex-forming sequences in R. Bioinformatics. 2017;33:3373–3379. doi: 10.1093/bioinformatics/btx413. [DOI] [PubMed] [Google Scholar]
- 44.Quinlan A.R., Hall I.M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robinson J.T., Thorvaldsdóttir H., Winckler W., Guttman M., Lander E.S., Getz G., Mesirov J.P. Integrative genomics viewer. Nat. Biotechnol. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liberzon A., Birger C., Thorvaldsdóttir H., Ghandi M., Mesirov J.P., Tamayo P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015;1:417–425. doi: 10.1016/j.cels.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ge S.X., Jung D., Yao R. ShinyGO: a graphical gene-set enrichment tool for animals and plants. Bioinformatics. 2020;36:2628–2629. doi: 10.1093/bioinformatics/btz931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dreos R., Ambrosini G., Périer R.C., Bucher P. The Eukaryotic Promoter Database: expansion of EPDnew and new promoter analysis tools. Nucleic Acids Res. 2015;43:D92–D96. doi: 10.1093/nar/gku1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Proteomic datasets have been deposited to PRIDE under accession number PRIDE: PXD063556, and transcriptome datasets have been deposited to GEO under accession number GEO: GSE295547. Raw data were deposited on Mendeley at https://data.mendeley.com/datasets/ys34fgz4cx/2.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.