Abstract
Sarcopenia, a progressive and systemic skeletal muscle disorder marked by the accelerated deterioration of both muscle function and mass, is highly prevalent among the elderly population, significantly contributing to an elevated risk of adverse outcomes, including falls, fractures, and muscle weakness. Clinical investigations have identified a strong correlation between sarcopenia and several prevalent degenerative skeletal muscle disorders. This correlation is attributed to imbalances in joint mechanics resulting from localized muscle atrophy and the influence of musculoskeletal secretory factors. In this review, we discuss the broader implications of sarcopenia and critically evaluate the currently established assessment methods. Furthermore, the clinical significance of prevalent musculoskeletal disorders (including osteoporosis, osteoarthritis, and spinal pathologies) in relation to sarcopenia, alongside the underlying mechanisms influencing this relationship, is summarized. Additionally, the effects of sarcopenia on the therapeutic efficacy of medications and surgical interventions for musculoskeletal conditions are reviewed. Sarcopenia is intricately linked to the onset, progression, and prognosis of musculoskeletal disorders. Future research should prioritize elucidating the potential mechanisms that connect muscle loss with skeletal muscle diseases, and investigating whether mitigating sarcopenia symptoms could decelerate the progression of these disorders, thereby paving new pathways for therapeutic interventions.
Subject terms: Bone quality and biomechanics, Metabolic disorders
Introduction
Sarcopenia is a progressive and generalized skeletal muscle disorder, characterized by a rapid deterioration in both muscle function and mass, which contributes to a heightened risk of adverse outcomes, including impaired physical function, increased falls, fractures, muscle weakness, and even mortality.1 Firxst introduced in the 1980s, sarcopenia was described as “an age-related decline in lean body”, with significant implications for mobility, nutritional status, and personal autonomy.2 The World European Working Group in Older People 2 (EWGSOP2) defines sarcopenia as a “progressive and generalized loss of skeletal muscle mass and strength,” highlighting the critical role of muscle strength deterioration.1 They further recommend that muscle strength be regarded as the most reliable measure of muscle function. Epidemiological data indicate that sarcopenia affects ~10%–16% of the global elderly population, with a notably higher prevalence (18%–66%) among those with underlying medical conditions such as diabetes, degenerative lumbar spine disease, cancer, or critical illness.3 Estimates of sarcopenia prevalence among community-dwelling older adults globally vary between 10% and 27%, with prevalence ranging from 8% to 36% in individuals under 60, and 10% to 27% in those over 60, according to sarcopenia working groups in Europe, Asia, and the United States, based on regional statistics.4 The observed heterogeneity in sarcopenia prevalence rates likely reflects inconsistencies in operational definitions and diagnostic approaches among different countries or regions.
Sarcopenia can be classified into primary (age-related) and secondary forms, depending on whether aging is the sole contributing factor or if additional pathological or lifestyle-related factors are involved.5 Secondary sarcopenia arises from comorbidities (e.g., cachexia, malnutrition, obesity) that adversely affect muscle mass and function, as well as from disuse atrophy due to chronic pain, musculoskeletal disorders, or prolonged sedentary behavior.6 The pathophysiology of sarcopenia involves multiple interrelated mechanisms, including chronic low-grade inflammation, vascular dysfunction, mitochondrial impairment, reduced satellite cell (myogenic progenitor) activity, disrupted muscle protein homeostasis, anabolic resistance, and neuromuscular degeneration.5,7–9 Age-related skeletal muscle remodeling is characterized by a shift from fast-twitch (type II) to slow-twitch (type I) fiber composition, accompanied by progressive intramuscular and intermuscular fat infiltration (myosteatosis) and a reduction in type II fiber-associated satellite cell populations.10 In addition, Insulin resistance secondary to metabolic dysregulation (e.g., diabetes and dyslipidemia) compromises skeletal muscle glucose metabolism while enhancing ectopic lipid deposition through increased cellular uptake of triglycerides and free fatty acids.11,12 Notably, several pathogenic mechanisms contributing to sarcopenia share common features with degenerative musculoskeletal disorders, including chronic low-grade inflammation, mitochondrial dysfunction, ectopic fat deposition, and metabolic dysregulation.
The human musculoskeletal system, comprising bones, cartilage, ligaments, tendons, joints, muscles, connective tissues, and other associated structures, plays a critical role in ensuring the normal functioning of life processes, movement, and various bodily activities. With advancing age, localized and systemic degenerative lesions of the musculoskeletal system, often resulting from mechanical injury and metabolic dysfunctions, significantly impact the quality of daily life and contribute to the increased risk of adverse outcomes in the elderly population. Clinical studies have revealed that a substantial proportion of patients with musculoskeletal disorders experience varying degrees of muscle loss, which is closely linked to musculoskeletal pain, low back pain, and other associated symptoms.13–18 As research on sarcopenia advances, growing evidence highlights shared pathological mechanisms between sarcopenia and musculoskeletal disorders, driving increased investigation into their common etiological factors and bidirectional interactions. This review summarizes the correlations between sarcopenia and prevalent musculoskeletal disorders (Fig. 1), focusing on clinical prevalence, underlying pathogenesis, treatment strategies, and prognosis. Investigating the influence of sarcopenia on musculoskeletal diseases may unlock novel avenues for mitigating the progression of these disorders.
Fig. 1.
The association of sarcopenia with musculoskeletal disorders (Created in BioRender. Mao, X. (2024) https://BioRender.com/l97t655)
Diagnosis, evaluation, and management of sarcopenia
The variability in outcomes from sarcopenia assessments and diagnostics can be attributed to the diverse definitions of sarcopenia and diagnostic tools employed in studies across different regions and research settings. The EWGSOP2 definition of sarcopenia, which remains the most widely recognized standard, utilizes muscle strength and muscle mass assessments as key diagnostic indicators.1 The EWGSOP 2018 operational definition of sarcopenia is as follows: (1) Reduced muscle strength; (2) Decreased muscle mass or impaired muscle quality; (3) Diminished physical performance. If criterion 1 is met, sarcopenia should be suspected. Meeting criterion 2 confirms a diagnosis of sarcopenia. Severe sarcopenia is diagnosed when all three criteria (1, 2, and 3) are fulfilled. A comprehensive review of 283 studies addressing sarcopenia definitions up until 2015 revealed that 264 studies (93.3%) relied on muscle mass measurements to define sarcopenia, while 198 studies (70%) employed low muscle mass (LMM) as the sole diagnostic criterion.19 Among these studies, 43.6% employed dual energy X-ray absorptiometry (DEXA) as the primary technique for muscle mass measurement.19 Other commonly utilized methods for muscle measurement included bioelectrical impedance analysis (19.3%) and the L3 psoas major cross-sectional index (calculated as the total bilateral area of the psoas major muscles divided by the square of body height, as captured on a computed tomography (CT) axial slice at the L3 vertebral level) (13.6%).19
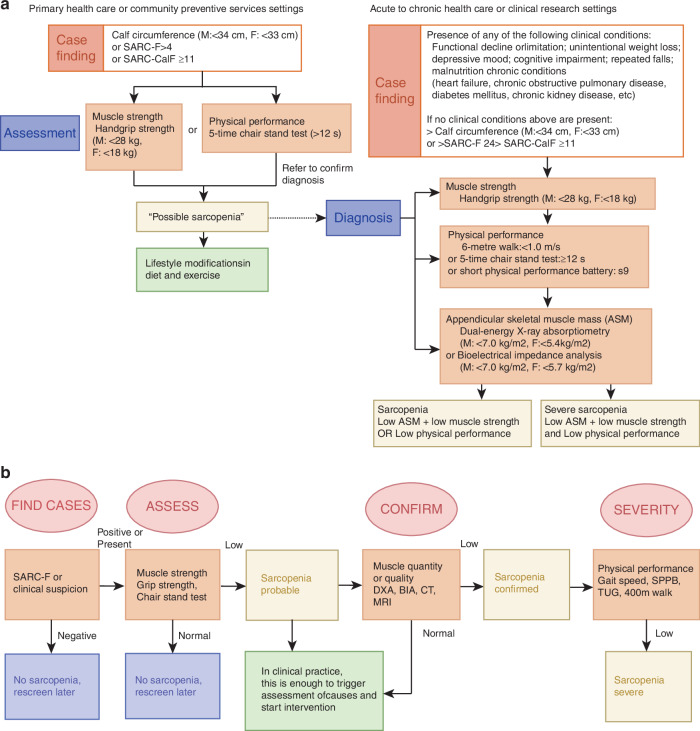
We evaluated the diagnostic algorithms for sarcopenia established by four major consensus groups: the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), the International Working Group on Sarcopenia (IWGS), the Foundation for the National Institutes of Health (FNIH), and the Asian Working Group for Sarcopenia (AWGS).1,20–22 The International Working Group on Sarcopenia provided an initial framework for clinical assessment. In contrast, the Foundation for the National Institutes of Health (FNIH) guidelines emphasize the importance of differential diagnosis, requiring exclusion of weakness attributable to other medical conditions. More recently, the updated European Working Group on Sarcopenia in Older People 2 (EWGSOP2) and Asian Working Group for Sarcopenia (AWGS) consensus statements have introduced more comprehensive and detailed diagnostic algorithms for sarcopenia evaluation. The EWGSOP2 proposes a stepwise diagnostic approach incorporating muscle strength, mass, and physical performance measures with severity grading. In contrast, the AWGS recommends a two-tiered assessment framework: (1) an initial screening tool for community or clinical settings, and (2) standardized diagnostic criteria for research applications and definitive diagnosis (Fig. 2).
Fig. 2.
Diagnostic Algorithms for Sarcopenia from AWGS 2019 and EWGSOP2 Consensus Guidelines. a Diagnostic algorithm for sarcopenia recommended by the Asian Working Group for Sarcopenia (AWGS 2019 consensus guidelines). b Diagnostic algorithm for sarcopenia from the revised European Working Group on Sarcopenia in Older People consensus (EWGSOP2)
Recommendations for Sarcopenia Assessment: (1) Diagnostic Framework: We endorse the EWGSOP2 or AWGS criteria for early clinical screening and diagnosis of sarcopenia. (2) Community Screening: For population-based preventive screening, grip strength and physical performance measures alone serve as adequate preliminary indicators. (3) Diagnostic Confirmation: Muscle mass quantification remains the cornerstone for definitive sarcopenia diagnosis. Standardized measurement techniques should be employed to ensure data accuracy for both clinical diagnosis and research enrollment.
The evaluation of muscle strength
The primary parameters for the evaluation of sarcopenia encompass both muscle strength and muscle mass, each of which serves as a critical diagnostic criterion. The European Working Group 2 on Sarcopenia in Older People (EWGSOP2) recommends a structured, stepwise diagnostic framework to effectively determine the presence of sarcopenia in patients.1
To assess muscle strength, the measurement of grip strength is often considered a straightforward and convenient methodology. Nevertheless, the reliability of grip strength measurements can be significantly affected by variations in the equipment used and the specific measurement protocols employed. It is strongly recommended that a standardized testing protocol be adopted, incorporating the use of calibrated handheld dynamometers under rigorously defined conditions, supplemented by reference data from a representative population to ensure the precision and accuracy of the results.23 This evaluation requires participants to rise from a seated position in a chair and return to a seated posture without using their arms to assist. The time taken to complete five consecutive sit-to-stand cycles should be measured, or alternatively, the number of sit-to-stand repetitions performed within a 30-second interval can be recorded.1,24 This test has been demonstrated to yield valid, reliable, and effective indicators of lower limb strength, making it a valuable tool in sarcopenia assessment.25
The evaluation of muscle mass
The evaluation of muscle mass is predominantly performed using imaging techniques, with Dual-energy X-ray absorptiometry (DXA) being the most frequently utilized method. Magnetic resonance imaging (MRI) and computed tomography (CT) are widely regarded as the gold standards for non-invasive assessments of both qualitative and quantitative changes in body composition, including muscle mass and muscle quality.24,26 Muscle mass may be expressed as skeletal muscle mass (SMM), appendicular skeletal mass (ASM), or the cross-sectional area of specific muscle groups or anatomical regions. Nonetheless, the widespread application of these methods is constrained by challenges related to accessibility, high costs, non-portability of devices, and the need for highly specialized personnel, thus limiting their feasibility in primary healthcare settings.24
We systematically reviewed the assessment criteria and diagnostic cut-off values endorsed by four major consensus groups (EWGSOP2, IWGS, FNIH, and AWGS).1,20–22 Additionally, we evaluated the strengths and limitations of these commonly used methods (Table 1). The diagnostic cut-off variations between working groups primarily reflect two factors: the temporal evolution of evidence (with earlier consensus like IWGS/FNIH relying on less comprehensive data) and population heterogeneity due to geographic, ethnic, and sociocultural influences in sampled cohorts.
Table 1.
Assessment methods for sarcopenia and standardization indicators, advantages and disadvantages
| Examination | Evaluation of indicators | Evaluation criteria | Advantages | Disadvantages | |
|---|---|---|---|---|---|
| Sift | ARC-F | / |
A score of 4 or higher is predictive of sarcopenia and associated adverse outcomes. |
For community screening of people at early risk for sarcopenia |
Some subjectivity; less precision |
| Poll | |||||
|
Muscular Strength |
Grip strength | / |
Male <27 kg;female <16 kg (EWGSOP2) |
Simple and easy to operate; reliable indicators available |
Less accurate; susceptible to other diseases |
|
Male <28 kg;female<18 kg (AWGS) | |||||
| Male <26 kg;female <16 kg | |||||
| (FNIH) | |||||
|
Repeat chair stand test |
5 chair stand tests | >15 s (EWGSOP2) | |||
| ≥12 s (AWGS) | |||||
|
Muscle mass |
DEXA | ASMI = ASM/height2 | Male < 7.0 kg/m2 |
Relatively low cost, low radiation, more accurate and stable differentiation of LM, FM and other indicators |
There is a degree of underestimation of sarcopenia compared to CT |
| Female < 5.5 kg/m2 | |||||
| (EWGSOP2) | |||||
| Male <7.0 kg/m2; | |||||
| Female <5.4 kg/m2 (AWGS) | |||||
|
Male <7.23 kg/m2; female <5.67 kg/m2 (IWCS) | |||||
|
ASM adjusted for BMI (ASMBMI) |
Male <0.789 | ||||
| Female <0.512 | |||||
| BIA | ASMI |
Male <7.0 kg/m2 female <5.7 kg/m2 (AWGS) |
Simple to operate; affordable equipment; widely |
There is a degree of error in the conversion equation; the raw measurements do not form a normalized standard |
|
| Available and portable | |||||
| CT |
Cross-sectional area CSA of mid-thigh muscles |
Male <84 cm2 |
The gold standard for non-invasive assessment of qualitative and quantitative changes in body composition and muscle quantity/mass |
Radioactive; high cost of access, lack of portable equipment; need for highly specialized personnel |
|
| Female <84 cm2 | |||||
|
Cross-sectional area of the psoas muscle |
SMI male 52–55 cm2/m2 | ||||
| Female 39–41 cm2/m2 | |||||
| SMI male 52.4 cm2/m2 | |||||
| Female 38.5 cm2/m2 | |||||
| MRI |
Evaluation site similar to CT |
/ |
Same as CT; non-radioactive; superior for assessment of muscle mass abnormalities such as muscle destruction, abnormal edema, fatty tissue infiltration, etc. |
High cost of access; lack of portable equipment; need for highly specialized personnel; no standardized reference data and thresholds yet established |
|
| US | MV, MT, CSA, etc. | / |
Low cost, easy to perform, repeatable measurements; non-invasive |
Standardized reference data and thresholds not yet established |
|
|
Physical function |
Gait speed | / |
Single cut-off speed ≤0.8 m/s (EWGSOP2, FNIH) |
Fast test; no special equipment or training required; better suited as a routine medical screening test |
Less accurate; susceptible to other diseases |
|
6 m walking speed <1.0 m/s (AWGS) | |||||
|
4-m walking speed <1.0 m/s (IWGS) | |||||
| SPPB | / |
≤8 points (EWGSOP2) ≤9 points (AWGS) |
|||
| TUG | / | ≥20 s |
DEXA Dual-energy X-ray absorptiometry, CT computed tomography, BIA Bioelectrical impedance analysis, MRI Magnetic resonance imaging, ASM Appendicular Skeletal Muscle Mass, SPPB Simple Physical Performance Battery, TUG Timing and advancement test
Dual-energy X-ray absorptiometry (DEXA)
Dual-energy X-ray absorptiometry (DXA) is currently recognized as the most widely used technique for assessing whole-body muscle mass. DXA facilitates the evaluation of muscle mass at both the whole-body and regional levels, making it a valuable tool in the clinical assessment of sarcopenia. The technique involves a whole-body scan utilizing an X-ray source emitting at two distinct energy levels (40 and 70 keV).27 The radiation dose from a full-body scan is typically around 5 μSv, rendering DXA a particularly safe and viable option for repeated body composition analyses.27 Among various imaging modalities, DXA stands out for being relatively cost-effective, offering low radiation exposure, and providing highly accurate and consistent differentiation between lean body mass (LBM), fat mass (FM), and bone mineral content (BMC) at both the regional and systemic levels.26,28 In clinical practice, dual-energy X-ray absorptiometry (DXA) typically measures appendicular skeletal muscle mass (ASM), calculated as the sum of lean muscle mass in both upper and lower extremities. To account for interindividual variability, ASM is commonly normalized through three adjustment methods: 1) height-squared adjustment (ASM/height2), 2) weight adjustment (ASM/weight), or 3) BMI adjustment (ASM/BMI).29 Among these, the height-squared adjusted index (ASMI = ASM/height2) has emerged as the standard reference parameter for DXA-based sarcopenia assessment. The revised EWGSOP2 guidelines recommend ASMI thresholds of <6 kg/m2 for women and <7.0 kg/m2 for men as diagnostic cut-offs for low muscle mass in European populations, as part of the updated criteria for hypomuscular dysfunction.1 For the Asian population, the Asian Working Group for Sarcopenia (AWGS) defines the ASMI thresholds similarly, with values of <6 kg/m2 for women and <7.0 kg/m2 for men.22 Numerous studies have demonstrated strong correlations between DXA-derived lower extremity skeletal muscle mass and measurements obtained via CT and MRI. However, it is important to note that DXA tends to slightly underestimate sarcopenia prevalence compared to these more advanced imaging techniques.30,31
CT
Computed tomography (CT) is extensively utilized for tumor staging and follow-up in various diseases, allowing for sarcopenia assessment in both prospective and retrospective analyses without necessitating additional scans.27 CT-based evaluation of muscle mass typically involves measuring the cross-sectional area of muscle from a single CT slice, differentiating between tissues based on X-ray attenuation values, with muscle tissue identified through a standardized range of attenuation (−29 to +150 HU).32–34 There are two common methods for muscle mass assessment: one measures the combined cross-sectional area of the paraspinal, psoas major, and abdominal muscles in a single abdominal CT slice at the L3 level, while the other assesses the bilateral psoas major muscles at the L3 and L4 vertebral levels.34 CT provides precise visualization of muscle density, and reduced muscle density is commonly associated with increased fat infiltration.27 Consequently, CT serves as a valuable diagnostic tool for routine evaluation of both muscle mass and volume.
The cross-sectional area of the psoas muscle at the L3 vertebral level and the mid-thigh muscle area are robust indicators of whole-body skeletal muscle mass. CT scans of specific lumbar vertebrae, particularly L3, show strong correlations with whole-body muscle mass and are frequently used to identify sarcopenia. In their systematic review, Behrang Amini and colleagues concluded that the most frequently measured muscle group in CT assessments is the abdominal wall musculature (142 of 330 participants).35 The most commonly used anatomical landmark for muscle mass measurements in CT scans is the L3 vertebra (123 of 142 participants).35 The skeletal muscle index (SMI) is the most frequently utilized metric for quantifying abdominal wall muscle mass (114 of 142 participants).35 The study reported that the critical range for SMI at the L3 level, based on abdominal CT scans, aligns closely with the findings of Daly et al.36 Combining the results, the typical SMI thresholds for sarcopenia were 52–55 cm2/m2 in men and 39–41 cm2/m2 in women.35 However, given variations in the precise anatomical definitions across different studies, further research is warranted to evaluate the impact of measurement site variations on muscle mass indices (SMI) and indicators of myopathy, such as muscle area (MA), intermuscular adipose tissue (IMAT), and low-density lean tissue. Notably, muscle mass measurements at L3 have been found to differ significantly from those at other vertebral levels. Therefore, the L3-based sarcopenia thresholds are applicable primarily to patients undergoing abdominal CT scans, excluding those receiving only thoracic scans (e.g., for lung cancer screening) or pelvic-only imaging (e.g., for hip fractures).37 Additionally, Brian A. Derstine and colleagues evaluated skeletal muscle mass across vertebral levels from T10 to L5.37 When L3 is unavailable, they recommend using L2, L4, L5, L1, T12, T11, or T10 in that order of preference.
Mid-thigh CT scans can be used to measure skeletal muscle (SM), intermuscular adipose tissue (IMAT), and subcutaneous adipose tissue (SAT) areas.38,39 CT imaging of these regions offers a more comprehensive assessment of muscle mass loss, muscle weakness, and overall muscle function. However, the precise anatomical localization of these measurement sites can vary. For instance, the L3 vertebral level can refer to the upper, middle, or lower portion of the vertebra. Similarly, definitions for mid-thigh CT cross-sections can vary, including measurements taken at the midpoint between the greater trochanter and the intercondylar fossa,40 the femur midpoint,41 the distal 20 cm of the greater trochanter.42 Or the midpoint between the femur and lateral condyle,43 etc.35 Further research is needed to assess how variations in measurement site localization affect cross-sectional area (CSA), muscle area (MA), intermuscular adipose tissue (IMAT), and other parameters related to muscle mass and fat infiltration.
MRI
Magnetic resonance imaging (MRI) operates by detecting the absorption and emission of radiofrequency energy by hydrogen nuclei. The absence of ionizing radiation is a key advantage of MRI, making it particularly well-suited for long-term monitoring of disease progression and treatment outcomes. MRI techniques are primarily employed to evaluate adipose tissue distribution and quantity, and to a lesser extent, skeletal muscle mass.44 It is widely regarded as the most advanced imaging modality for characterizing muscle mass loss. MRI allows for a comprehensive evaluation of body composition, revealing abnormalities such as muscle disruption, abnormal edema, fatty infiltration, and fibrous connective tissue formation (myofibrosis).45,46 One of the key strengths of MRI is its ability to detect both compositional and structural changes in skeletal muscle associated with aging and disease progression, including decreased muscle contractility accompanied by increased intramuscular adipose (IMAT) and connective tissues (IMCT).26,45 The anatomical sites assessed by MRI are similar to those evaluated by CT, with common focus areas being the psoas and mid-thigh muscles.47 Studies have indicated that the lumbar L3 vertebral level is optimal for assessing total tissue volume of skeletal muscle (SM), visceral adipose tissue (VAT), and subcutaneous adipose tissue (SAT), whereas mid-thigh muscle measurements show a stronger correlation with total body muscle volume than lumbar muscle measurements from L1 to L5.48
The Dixon sequence is highly effective at separating fat from water, providing an accurate quantitative assessment of sarcopenia. Chemical shift-based water/fat separation imaging and dual- or multipoint Dixon sequences are commonly employed techniques for quantifying muscle fat fraction. These methods exploit the differences in resonance frequencies between fat and water protons, enabling precise differentiation and quantification of fat content through careful adjustment of echo time.32 In addition to these, various MRI sequences, including T1-weighted imaging, Dixon sequences, T2 mapping (T2 relaxation times), diffusion-weighted imaging (DWI), and non-proton MRI, can be applied for a multifaceted evaluation of muscle mass.29,49 However, the current use of these advanced sequences remains predominantly in the realm of research.
Despite the clear advantages, MRI, alongside CT, is regarded as the gold standard for non-invasive body composition and muscle mass evaluation.24,26 Nevertheless, the widespread use of MRI in primary care is hindered by high equipment costs, lack of portability, and the requirement for highly specialized operators.24 As a result, MRI remains largely confined to research applications. The absence of standardized imaging protocols, normal reference data, and consistent thresholds, along with ambiguities in image segmentation and analysis, continues to limit its clinical utility.29,49
Ultrasound (US)
Ultrasound (US) is a non-ionizing imaging technique that offers detailed visualization of muscle morphology and size, as well as surrounding structures, allowing for dynamic assessment of soft tissue in real time.50 The primary parameters measured by US include muscle volume (MV), muscle thickness (MT), pennation angle (PA), fascicle length (Lf), echo-intensity (EI), and cross-sectional area (CSA).50,51 Studies have demonstrated that the use of US to assess muscle thickness and volume yields results that are not significantly different from those obtained via MRI.34,52,53 Ultrasound is cost-effective, easy to perform, and highly reproducible, making it an ideal screening tool for sarcopenia, especially in community settings or hospitals. Moreover, three predictive equation models, based on US measurements, have been proposed by two studies and have shown efficacy in assessing muscle mass.53,54 The accuracy of these models has been confirmed through comparisons with DEXA measurements, showing no significant statistical difference (r2 = 0.96; r2 = 0.929, standard error of estimate = 2.5 kg; r2 = 0.955, standard error of estimate = 2.0 kg). However, the validity of these ultrasound-based predictive equations requires further verification, given the limited experimental data currently available.
As a relatively recent method for muscle mass assessment, ultrasound has demonstrated considerable promise in providing accurate measurements of muscle mass. It holds potential to become a more precise and accessible diagnostic tool for sarcopenia. Efforts are underway to standardize ultrasound techniques for assessing muscle mass.51 Unfortunately, a global consensus on the use of ultrasound for this purpose has yet to be established.
Bioelectrical impedance analysis
Bioelectrical impedance analysis (BIA) utilizes equations that calculate differences in electrical conductivity between various tissues to evaluate body composition, including muscle, fat, and bone. The accuracy of BIA is highly dependent on proper tissue hydration.55,56 BIA is simple, affordable, widely accessible, and portable, and has been endorsed by both Asian and European guidelines as an effective tool for assessing muscle mass in sarcopenia.1,57 Due to these advantages, BIA is increasingly being considered a practical alternative to dual-energy X-ray absorptiometry (DEXA). Multi-frequency BIA, which employs both low and high frequencies to calculate intercellular, extracellular, and total body water, has demonstrated superior accuracy over single-frequency BIA, especially in populations with obesity or underweight conditions.56 BIA relies on conversion equations to estimate muscle mass, a process influenced by factors such as age, ethnicity, and the type of measurement equipment used.58–60 Recent studies have begun to investigate the development of age-independent conversion equations.60 However, the impact of ethnic variability on these equations remains unresolved.Certain equations, like the Sergi equation for adult Australians (Caucasians) and the Kyle equation for male muscle mass assessment, have proven effective in specific populations.58 Further research is necessary to validate these prediction equations across diverse populations. It has been observed that BIA tends to overestimate skeletal muscle mass compared to DEXA measurements, particularly in older adults.61–63 This discrepancy may be attributed to the fact that the study population was primarily elderly or that the selected BIA methods or equations were suboptimal.64
To mitigate errors from BIA prediction equations, researchers have shifted focus to the raw bioelectrical parameters measured by BIA, such as resistance (R), reactance (Xc), and phase angle (PhA)These fundamental bioelectrical measures, particularly PhA, have shown promise in predicting disease prognosis, mortality, and other clinical outcomes.65,66 PhA has been found to negatively correlate with muscle mass and strength in older adults, suggesting it could serve as a valuable bioelectrical marker for assessing sarcopenia risk. Nevertheless, further exploration is needed to clarify the relationship between PhA’s predictive capabilities and the diagnosis of sarcopenia.67–69
The evaluation of physical functioning
Physical function, as defined by the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO),70 refers to “an objectively quantified measure of whole-body performance pertaining to mobility”. This concept encompasses multiple dimensions, particularly focusing on both muscular and neural function. In the absence of comorbid conditions that precipitate declines in physical functionality, such as dementia, gait instability, or balance impairments, physical function is typically assessed through various objective measures, including gait speed, the Short Physical Performance Battery (SPPB), physical functioning scales, and the Timed-Up and Go (TUG) test.1
Gait speed serves as a highly reliable and sensitive indicator of daily physical activity, and it exhibits a strong correlation with frailty and survival outcomes in elderly populations.71 It is widely regarded as a rapid, safe, and highly reliable diagnostic tool for sarcopenia, frequently implemented in clinical practice.1 Gait speed is extensively utilized in evaluating physical function within community-dwelling older adult populations and has demonstrated predictive value for adverse outcomes linked to sarcopenia, including falls, frailty, cognitive decline, disability, and mortality.72,73 The Short Physical Performance Battery (SPPB) provides an objective and comprehensive assessment of lower extremity function in older adults, incorporating evaluations of gait speed, balance, and the ability to perform chair stands.74 The Timed-Up and Go (TUG) test, another widely used tool for assessing physical function in community-dwelling older adults, has been validated as a reliable and effective measure of functional mobility.75,76 This test can be seamlessly integrated into routine physical examinations; it is expedient and requires neither specialized equipment nor extensive training.75
Screening methods for sarcopenia within community settings must be simple, rapid, and user-friendly. Specifically, the SARC-F questionnaire is recommended in numerous guidelines as a preferred tool for early sarcopenia screening and for identifying individuals at elevated risk.1,22,77 Moreover, the 2019 Asian Working Group for Sarcopenia (AWGS) guidelines recommend the use of calf circumference (CC) as a screening metric for sarcopenia, setting reference thresholds at <34 cm for men and <33 cm for women.22
Drugs for sarcopenia
Non-pharmacological treatment
Insufficient physical activity results in muscular atrophy, functional deterioration, and increased adiposity. Both lean body mass and fat mass influence bone mineral density, highlighting the critical role of physical activity in preventing bone demineralization. Resistance training, either alone or in combination with aerobic and balance exercises, is considered the most effective intervention for enhancing the quality of life in individuals with sarcopenia.78 Studies have demonstrated that resistance exercise stimulates muscle protein synthesis, thereby promoting hypertrophy, increasing muscle strength, and enhancing physical performance.79,80 Furthermore, maintaining sufficient physical activity can prevent or mitigate muscle loss by preserving insulin sensitivity and augmenting mitochondrial function.81 Endurance exercise promotes mitochondrial biogenesis by upregulating peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α), thereby improving skeletal muscle adaptability and overall function.82–84 A 5-year longitudinal study involving 1 863 older adults (aged 70–79) in the United States revealed that sustaining moderate physical activity—defined as at least 150 min of moderate-intensity exercise per week, such as brisk walking—significantly lowers the risk of developing or exacerbating metabolic syndrome.85
Nutritional supplementation
Nutritional supplementation is believed to enhance various physical function outcomes in older adults and those suffering from conditions like frailty or muscle wasting, particularly when incorporating multiple nutrients.86 The key nutritional components associated with sarcopenia and frailty pathogenesis include: (1) protein/amino acids, (2) vitamin D, (3) polyphenols (e.g., catechins and isoflavones), (4) antioxidant nutrients (carotenoids, selenium, vitamins E and C), (5) ursolic acid, and (6) long-chain polyunsaturated fatty acids.87 Dietary protein provides essential amino acids necessary for muscle protein synthesis. Notably, leucine—a branched-chain amino acid (BCAA)—plays a critical regulatory role by enhancing mitochondrial biogenesis and function in skeletal myocytes.88 This mechanism may help mitigate age-related muscle degeneration in both physiological aging and pathological conditions. The Asian consensus guidelines on sarcopenia recommend oral nutritional supplementation (ONS) with high-quality protein, specific amino acids (including leucine and L-carnitine), or β-Hydroxy-β-methylbutyrate (HMB) - a bioactive leucine metabolite.89 This leucine metabolite enhances muscle protein synthesis through mTOR activation and anti-apoptotic effects.90 While proven effective for maintaining muscle mass in aging adults, HMB’s benefits for strength and physical function require further validation.91 Omega-3 polyunsaturated fatty acids (PUFAs) enhance muscle protein synthesis in older adults through mTOR pathway activation and exhibit anti-inflammatory properties.92 However, clinical trial evidence has not demonstrated significant efficacy in attenuating age-related muscle loss. Preclinical evidence indicates isoflavones both reduce skeletal muscle adiposity93 and prevent denervation-induced atrophy94 in male mice. Clinically, their phytoestrogenic properties may offer protection against postmenopausal sarcopenia in older women.95,96
In older adults with sarcopenia, high- to moderate-quality evidence suggests that combining nutritional interventions with exercise exerts a more pronounced effect on grip strength and multiple physical function metrics compared to exercise alone.78 The concurrent implementation of resistance training combined with nutritional supplementation is anticipated to yield superior therapeutic outcomes.97 In a randomized double-blind controlled trial (n = 62 male pairs), catechin supplementation alone or resistance training alone demonstrated modest improvements in muscle mass and strength versus placebo.98 Notably, the combined intervention (catechin + resistance exercise) showed significantly greater benefits, including: (1) Increased appendicular muscle mass index. (2) Improved performance on the timed up-and-go test. (3) Enhanced levels of muscle growth factors. Compared to monotherapies, combination therapy with HMB and low-magnitude high-frequency vibration (LMHFV) synergistically increased muscle mass while reducing both total adiposity and intramuscular lipid deposition.99 The observed effects are principally regulated by activation of the canonical Wnt/β-catenin signaling cascade.
Pharmacotherapy
Currently, no pharmacological treatments have been specifically approved for the management of sarcopenia. Medications commonly recommended for potential use include growth hormone (GH), anabolic-androgenic steroids, selective androgen receptor modulators (SARMs), estrogens, protein anabolics, vitamin D, appetite stimulants, myostatin inhibitors, activin II receptor antagonists, beta-blockers, angiotensin-converting enzyme inhibitors (ACE inhibitors), angiotensin receptor blockers (ARBs), and troponin activators, among others.100,101
Vitamin D plays a critical role in regulating bone metabolism, particularly in maintaining phosphorus and calcium homeostasis. In the elderly population, approximately 50% exhibit reduced vitamin D levels, primarily due to an age-related decline in vitamin D receptor expression in skeletal muscle. Observational studies have demonstrated a positive correlation between lower vitamin D levels and decreased muscle strength and mass in older adults.102 However, a recent systematic review of randomized controlled trials (RCTs) on vitamin D monotherapy for sarcopenia found no significant beneficial effects of supplementation on muscle mass or strength in older adults aged 50 years and above.103 Compared with placebo, vitamin D monotherapy demonstrated no significant improvement in grip strength (HGS), gait speed (assessed by timed up-and-go test, TUG), appendicular lean mass (ALM), overall muscle strength, or physical performance in older adults. Notably, vitamin D supplementation was associated with a significant reduction in Short Physical Performance Battery (SPPB) scores.
Myostatin (MSTN), a transforming growth factor-β (TGF-β) superfamily member, negatively regulates skeletal muscle growth and induces atrophy through Smad2/3-dependent signaling pathways.104 The humanized monoclonal antibody LY2495655 (LY) specifically targets and neutralizes myostatin, demonstrating efficacy in increasing lean body mass while potentially enhancing muscle strength and physical function.105 In clinical trials involving elderly participants, 24-week LY treatment significantly improved key functional outcomes, including stair-climbing capacity, chair-stand performance, and walking speed. Se-Jin Lee comprehensively reviewed current clinical applications of myostatin (MSTN) inhibitors, categorizing them into two classes: (1) relatively specific MSTN inhibitors (including MYO-029, domagrozumab, LY2495655, REGN1033, AMG-745/PINTA-745, BMS-986089/RO7239361, and SRK-015), and (2) broader-spectrum agents targeting MSTN, GDF-11, and activin A (including bimagrumab and ACE-031/083).106 Clinical trial evidence demonstrates that these inhibitors (particularly bimagrumab and ACE-031) significantly increase thigh muscle volume and show clinically meaningful effects on overall muscle mass.107 Most clinical trials observed that while MSTN inhibitors increased muscle mass, these gains did not translate proportionally to measurable improvements in muscle strength or physical function. This differential response may reflect the high prevalence of comorbidities in elderly populations. Importantly, therapeutic efficacy appears particularly limited in patients with inflammatory myopathies, malignancies, chronic obstructive pulmonary disease (COPD), or end-stage renal disease (ESRD), who showed minimal clinical response to MSTN inhibition. Emerging clinical trial evidence demonstrates that MSTN inhibitors exert dual metabolic benefits, significantly reducing adipose tissue mass while improving glucose homeostasis.108 These pleiotropic effects suggest broader therapeutic potential, particularly for managing complex metabolic-muscle disorders such as sarcopenic obesity or multi-morbidity-associated sarcopenia. Future investigations should systematically evaluate these multidimensional therapeutic applications through targeted clinical studies.109
Clinical evidence demonstrates that sterol supplementation effectively attenuates age-related declines in muscle mass and grip strength while increasing lean leg mass and limb strength in community-dwelling elderly males.110 However, these therapeutic benefits must be weighed against significant adverse effect profiles, including dermatological manifestations (acne, seborrhea), obstructive sleep apnea, thromboembolic events, and potential oncological risks (particularly prostate cancer).111
Selective androgen receptor modulators (SARMs), including enobosarm (GSK2881078), demonstrate tissue-specific anabolic activity with favorable pharmacokinetic profiles.112 These agents effectively promote muscle hypertrophy while mitigating the adverse effects associated with conventional steroidal therapies.112 Preliminary data suggest that oxymetholone increases fat-free mass, grip strength, and physical performance metrics, along with enhancing type I muscle fiber cross-sectional area. However, its clinical application remains limited by hepatotoxicity risks, particularly in hemodialysis-dependent patients.113
Current pharmacotherapies for sarcopenia demonstrate limited efficacy in improving physical function and activities of daily living, with variable responses across patient populations. Furthermore, many of these interventions are associated with clinically significant adverse effects. These limitations, coupled with the paucity of large-scale clinical trials, have hindered the widespread clinical adoption of pharmacological interventions for sarcopenia management. Yves Rolland et al.101 have systematically reviewed the clinical efficacy of these pharmacological agents, which may have ameliorative effects on muscle loss. Overall, the currently available pharmacotherapies for sarcopenia demonstrate efficacy in enhancing muscle mass and/or strength, yet fail to produce clinically meaningful improvements in physical performance.101 Notably, mitochondrial-targeted therapies are gaining increasing research attention. As a representative example, the nicotinic acid derivative acipimox enhances mitochondrial function in human skeletal muscle cells through NAD+ biosynthesis upregulation.114 The Mitochondria-Targeting Agent MitoQ demonstrates therapeutic potential in murine cancer cachexia models, where it enhances muscle strength and mass, stimulates β-oxidation, and induces a metabolic shift from glycolytic to oxidative fiber types.115 However, current evidence remains limited to preclinical studies utilizing animal and cellular models.
Sarcopenia and osteoporosis
Clinical relevance
Muscle and bone loss are prevalent among older adults, with the World Health Organization defining osteoporosis as a skeletal disorder marked by compromised bone strength and heightened fracture risk.116,117 Recently, the term “Osteosarcopenia” has been introduced to describe the concurrent manifestation of osteopenia or osteoporosis alongside sarcopenia.118 Osteoporosis deteriorates bone microarchitecture and diminishes bone strength, whereas sarcopenia is typified by a progressive decline in muscle mass and functionality throughout the body.119 These two conditions share several common risk factors—including age, gender, genetic predisposition, metabolic factors, and mechanical stimuli—and are strongly linked to adverse outcomes such as frailty, falls, fractures, hospitalizations, and mortality, significantly increasing the healthcare burden.120,121
A multitude of clinical studies have confirmed a robust association between sarcopenia and osteoporosis. A study involving 288 elderly individuals in Belgium revealed a fourfold increase in the risk of comorbid osteoporosis in sarcopenic patients compared to their non-sarcopenic counterparts (OR = 4.18; 95% CI: 1.92–9.12).122 An Australian study further suggests that individuals with concurrent sarcopenia and osteoporosis are at a greater risk for falls and fractures than those with either condition alone.123 A Korean study of 324 patients with hip fractures found that 93 (28.7%) were diagnosed with osteomuscular decompensation, and this group exhibited a significantly higher 1-year mortality rate (15.1%) compared to those with osteoporosis alone (5.1%) or sarcopenia alone (10.3%).124
Chen and colleagues conducted a comprehensive summary of the global epidemiological landscape of sarcopenia, reporting an overall prevalence of 18.5% based on data from 63 369 subjects across 63 studies.125 The estimated prevalence of sarcopenia exhibits considerable variation due to heterogeneity in population characteristics, geographic regions, age, sex, study design, diagnostic criteria, and clinical settings. Regionally, sarcopenia prevalence was notably higher in Oceania (22.9%), Africa (21.6%), and South America (20.8%) compared to Europe (10.7%).125
Mechanisms of occurrence
Mechanical stimulus
Mechanical stimulation occurs when muscles attach to bones to generate movement, providing the necessary strain to maintain optimal bone health.120 Increased muscle mass induces stretching of the periosteum and collagen fibers when mechanical forces exceed a certain threshold, thereby promoting bone growth.119,126 During human growth and development, muscles, which precede skeletal growth, help shape the contours of the skeleton and drive periosteal bone expansion, adjusting skeletal density to support the necessary load-bearing capacity.127–129 Numerous studies have demonstrated a strong association between the loss of muscle and bone mass and the aging process.130 A 4-year longitudinal study of an elderly Japanese population reported a high co-prevalence of osteoporosis (57.8%) among individuals with sarcopenia (n = 1 099, prevalence of 8.2%).131 The study found that individuals with sarcopenia exhibited reduced bone density, while those with osteoporosis had diminished muscle mass and function.131 The study concluded that osteoporosis (OP) may elevate the short-term risk of sarcopenia (SP), suggesting that osteoporosis could be a predictor of future sarcopenia risk.
Musculoskeletal crosstalk
The relationship between bone and muscle extends beyond mechanical coupling. Notably, muscle mass in distal extremities correlates with cortical bone thickness, suggesting the existence of systemic paracrine/endocrine signaling between these tissues.132 Emerging evidence has identified multiple molecular pathways mediating this biochemical crosstalk within the musculoskeletal unit.
Bones and muscles are interconnected not solely through mechanical stimulation. Muscle mass in distal extremities is also correlated with cortical bone thickness, indicating potential paracrine or endocrine crosstalk that further links muscle and bone. The musculoskeletal system has been shown to communicate through autocrine, paracrine, and endocrine signaling, with multiple pathways identified in this integrated system.133–135 Bone can receive anabolic signals from muscle, with several myokines—such as myostatin (muscle growth inhibitor), insulin-like growth factor-1 (IGF-1), interleukin-6 (IL-6), IL-15, irisin, fibroblast growth factor 2 (FGF2), and matrix metalloproteinase 2 (MMP2)—being upregulated during muscle contraction and contributing to both bone formation and resorption.135–138 Conversely, prostaglandin E2 (PGE2) and Wnt3a, secreted by osteoblasts, along with osteocalcin (OCN) and IGF-1 from osteoclasts, and sclerostin from both cell types, may regulate skeletal muscle cells.135 Hormones critical in the development of osteomalacia include growth hormone/insulin-like growth factor-1 (GH/IGF-1) and sex steroids.119,129 These hormones are also integral to the regulation of both bone and muscle development.129
The receptor activator of nuclear factor kappa-B ligand (RANKL) plays a pivotal role in physiological bone remodeling and osteoclast formation, as well as in the activation of osteoclasts under pathological conditions.139 This process is mediated through its interaction with the nuclear factor kappa-B (RANK) receptor. Osteoprotegerin (OPG), a decoy receptor for RANKL, protects bone from excessive resorption by competitively binding to RANKL, thereby inhibiting the RANKL/RANK signaling pathway.140 Given its critical function in regulating bone metabolism, the RANKL/RANK/OPG axis is indispensable for maintaining bone homeostasis, and dysregulation of this system can contribute to bone-related disorders, including osteoporosis. Evidence suggests that the RANK signaling pathway significantly influences skeletal muscle physiology, with effects that parallel those observed in osteoporosis.141 Activation of RANK has been shown to exacerbate muscle atrophy, increase fatigue susceptibility, and promote a shift toward fast-twitch fiber predominance. RANK signaling modulates skeletal muscle function by impairing the activity of the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA), a key regulator of intracellular Ca2⁺ homeostasis. Pharmacological inhibition of RANKL enhances limb strength and muscle mass while concurrently improving metabolic function, as evidenced by increased insulin sensitivity and muscle glucose uptake.142 Additionally, RANKL blockade downregulates the expression of negative regulators of myogenesis (e.g., myostatin) and pro-inflammatory mediators (e.g., protein tyrosine phosphatase receptor-γ, PTPRγ) in skeletal muscle.
The Wnt/β-catenin signaling pathway serves as a critical regulator of bone development and homeostasis.143 Emerging evidence demonstrates that Wnt-mediated signaling plays an essential role in modulating osteoblast metabolism, including the stimulation of aerobic glycolysis, glutamine catabolism, and fatty acid oxidation.144 Key cytoplasmic components of the Wnt pathway—such as GSK3β, Dishevelled (Dvl), and adenomatous polyposis coli (APC)—along with their regulatory partners (e.g., microtubule-actin crosslinking factor 1, MACF1), represent promising therapeutic targets for the treatment of diverse bone disorders.145 In addition, The Wnt/β-catenin signaling pathway plays a crucial role in skeletal muscle development, growth, and regeneration, while simultaneously suppressing intramuscular adipogenesis.146,147 Lin et al. comprehensively reviewed the regulatory potential of Wnt/β-catenin signaling in skeletal muscle-bone interactions.148 Current evidence clearly establishes the involvement of Wnt/β-catenin signaling, particularly through the Wnt3a-mediated pathway, in musculoskeletal crosstalk. While Wnt4 and Wnt10b have been implicated in muscle-bone communication, their specific roles in age-related musculoskeletal changes require further investigation.
Heredity
Muscle and bone originate from somites and share a common mesenchymal progenitor.149 Despite their independent development, muscle and bone form secondary relationships through mutual attachment and exhibit a high degree of reciprocal regulation.126 Studies have demonstrated that sarcopenia and osteoporosis are governed by distinct genetic factors, and that muscle strength, muscle mass, bone geometry, and bone mass are all significantly influenced by genetic regulation.149 Similarly, extensive experimental studies in animals and human cell models have provided evidence of shared genetic pathways regulating both bone and muscle mass. Genetic polymorphisms in the IGF1 gene150,155, myostatin (muscle growth inhibitor)151, low-density lipoprotein receptor-related protein 5 (LRP5)152, and the vitamin D receptor (VDR)153,154 have been linked to both bone and muscle loss.149,155
Insulin-like growth factor-1 (IGF-1) plays a fundamental role in promoting the proliferation and differentiation of activated satellite cells, while serving as a critical regulator of growth across multiple tissues, particularly in skeletal muscle and bone. These biological effects are mediated through IGF-1 receptor (IGF-1R) phosphorylation, which subsequently activates two key downstream signaling cascades: the Ras/Raf/MEK/ERK pathway and the PI3K/Akt/mTOR pathway.156 While the liver serves as the primary source of circulating IGF-1 in humans, skeletal muscle and bone tissues contribute to local IGF-1 availability through autocrine/paracrine secretion.157,158 IGF-1 exerts potent mitogenic and differentiation-promoting effects on both osteoblasts and myocytes, with particularly significant actions on muscle satellite cells.159 These mechanisms underlie IGF-1’s direct anabolic effects on skeletal muscle and bone tissue.
As a coreceptor in the Wnt signaling pathway, LRP5 plays a pivotal role in mechanotransduction by converting mechanical loading into osteogenic responses through Wnt pathway activation.160 Meanwhile, LRP6 serves as a critical regulator of bone homeostasis, modulating both bone formation and resorption processes. Emerging evidence suggests that LRP5 and LRP6 participate in skeletal muscle myogenesis through Wnt pathway regulation, though they exhibit distinct expression patterns of myogenic and synaptic markers.161 Notably, LRP5 has been shown to enhance cardiomyocyte proliferation via activation of the AKT/P21 signaling cascade.162 However, the current understanding of LRP5/LRP6-mediated muscle regulation remains limited, and their precise molecular mechanisms require further experimental validation.
Genome-wide association studies (GWAS) have progressively identified multiple genes with the potential to co-regulate both bone and muscle. For example, the Mettl21c gene regulates myogenesis and calcium homeostasis in muscle cells, as well as bone cell viability and resistance to apoptosis, through the NF-κB signaling pathway.163 The identified dual roles for the GLYAT gene in both bone development and muscle growth, linked to its regulation of glucose and energy metabolism.164 Medina-Gomez employed bivariate GWAS to analyze the polymorphic effects of eight loci on total-body lean mass (TB-LM) and total-body less head bone mineral density (TBLH-BMD) in children, including seven loci previously associated with bone mineral density: WNT4, GALNT3, MEPE, CPED1/WNT16, TNFSF11, RIN3, and PPP6R3/LRP5131.165 And a newly discovered locus, 17p11.2, has shown a strong association with total body lean mass (TB-LM), potentially due to the influence of the TOM1L2/SREBF1 gene.165 The effects of active SREBP-1 and SREBF1 products on osteoblast and myoblast differentiation are well-established.166,167 miRNAs expressed by TOM1L2 are implicated in osteogenic differentiation and skeletal muscle development,168,169 although the specific role of this gene in the musculoskeletal system remains to be clarified.
The discovery of these polymorphic genes suggests that shared genetic factors between bone and muscle may contribute to the co-occurrence of osteoporosis and sarcopenia in individuals, highlighting the need for systemic treatments targeting both tissues.
Treatment of osteomyopenia
Non-pharmacological treatment
Reduced or insufficient physical activity can result in muscle wasting, functional decline, and increased adipose tissue accumulation. Furthermore, both lean body mass and adiposity are known to influence bone density. Therefore, maintaining regular physical activity is crucial for preventing both muscle and bone loss. Weight-bearing aerobic exercises (e.g., walking, jogging, tai chi) have been shown to attenuate age-related bone loss through mechanical loading effects. In contrast, resistance training (e.g., weightlifting, swimming, cycling) promotes muscle hypertrophy and increases bone mineral density (BMD), with these effects being primarily localized to the specific musculoskeletal regions engaged during exercise.170 Resistance exercise (RE) is considered one of the most effective strategies for mitigating osteomuscular degeneration. Clinical studies have shown that progressive resistance exercise promotes osteoclastogenesis and muscle protein synthesis, leading to improvements in bone microarchitecture, muscle mass, strength, and functional capacity in older adults with osteoporosis and sarcopenia.121 Mechanistic studies reveal that resistance training specifically elevates serum levels of procollagen type 1 N-terminal peptide (P1NP), a biomarker of bone formation, while simultaneously increasing osteoblast proliferation without corresponding elevation in bone resorption markers.171 This dual mechanism demonstrates that aerobic exercise exerts comprehensive skeletal benefits by both suppressing bone catabolism and promoting anabolic activity. Whole-body vibration (WBV) is a therapeutic modality that delivers high-frequency mechanical stimulation via a vibrating platform, targeting bone mechanoreceptors to promote osteogenesis. Clinical evidence indicates that whole-body vibration (WBV) therapy demonstrates significant benefits in improving key physical performance measures in elderly populations, including muscular strength, postural balance, mobility, and gait function.172 However, the therapeutic efficacy of WBV for enhancing muscle mass and bone mineral density (BMD) remains inconclusive, with existing studies reporting conflicting outcomes.173
Nutritional supplementation has proven equally effective in managing osteomyopenia. Multinutrient supplementation has been shown to improve various physical functioning outcomes in older adults, as well as in individuals affected by specific medical conditions or frailty-related muscle loss.86 As previously discussed, low serum vitamin D levels show a significant positive correlation with decreased muscle strength and mass in older adults. While current evidence fails to demonstrate substantial benefits of vitamin D supplementation for sarcopenia management, its critical role in bone health maintenance is well-established. Research indicates that vitamin D supplementation improves bone mineral density (BMD) exclusively in individuals with 25-hydroxyvitamin D [25(OH)D] levels below 30 nmol/L.174 Notably, high-dose supplementation (>4 000 IU/day) may paradoxically increase fall and fracture risks.175 Consequently, current guidelines do not recommend routine calcium or vitamin D supplementation for osteoporosis prevention in healthy community-dwelling adults without documented deficiencies.176 The European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis, and Musculoskeletal Diseases (ESCEO)177 advises a daily intake of 800 IU of vitamin D for postmenopausal women, aiming to maintain 25(OH)D levels above 50 nmol/L.
Pharmacotherapy
Growth hormone (GH) is a critical molecule that facilitates both bone and muscle development throughout human growth. Its physiological effects are primarily mediated via the GH/IGF-I axis.178 The GH/IGF-I axis governs muscle cell proliferation, modulates myofibril size and fiber type, promotes osteoblast proliferation and differentiation, inhibits osteoclast activity, and regulates renal 1α-hydroxylase activity (which activates 25-OH-vitamin D) and phosphate reabsorption.129 Despite its potential, recombinant human growth hormone (rhGH) therapy for age-related muscle, bone loss, and adiposity alterations remains a subject of ongoing debate. In a pivotal study involving 12 elderly males treated with rhGH for 6 months, Rudman et al.179 observed an 8.8% increase in lean body mass, a 1.6% improvement in lumbar spine bone density, and a 14.4% reduction in adiposity, while femoral neck bone density remained largely unchanged. A 7-year longitudinal study in osteoporotic patients revealed that rhGH replacement therapy demonstrated greater efficacy in the initial 4 years, marked by a substantial increase in lumbar spine BMD, followed by a diminished effect over the subsequent 3 years.180
Selective androgen receptor modulators (SARMs) were developed to elicit anabolic effects in muscle and bone, while circumventing the dose-limiting androgenic effects commonly associated with testosterone, such as prostate growth, acne, and oily skin.129 Andarine has been characterized as an ideal SARM due to its once-daily administration, complete oral bioavailability, and substantial preclinical evidence documenting its anabolic effects on muscle and bone.181 Intervention with the selective androgen receptor modulator (SARM) ostarine in ovariectomized female rats resulted in increased gastrocnemius muscle weight, improved bone biomechanical properties, and enhanced bone mineral density.182 Clinical trials have reported improvements in skeletal muscle mass with SARM treatment; however, its effects on muscle strength and function, as well as its long-term efficacy, require further investigation.183,184 The U.S. Food and Drug Administration (FDA) has not approved SARM for the treatment of sarcopenia. The U.S. Food and Drug Administration (FDA) has not approved SARMs for the treatment of sarcopenia. However, clinical data on their efficacy and safety continue to emerge, suggesting potential therapeutic roles as anabolic and functional agents in various musculoskeletal disorders.185
Emerging evidence suggests that select anabolic agents, including myostatin inhibitors, can simultaneously enhance muscle and bone mass.186 Notably, the soluble activin type IIB receptor (ActRIIB-Fc) demonstrated significant efficacy in a murine muscular atrophy model, increasing both limb/axial bone mass and improving long bone biomechanics.187 Mechanistic analysis revealed that the osteogenic effects of ActRIIB-Fc were activity-independent, with no correlation observed between bone mass changes and potential alterations in murine locomotor behavior.188 These findings position myostatin modulation as a promising therapeutic strategy for musculoskeletal disorders, particularly in patients with limited mobility. However, further preclinical and clinical research is required to establish the long-term safety and therapeutic potential of these compounds.
Sarcopenia and osteoarthritis
Osteoarthritis is the most common chronic joint disease, which can affect any joint. However, it most frequently targets the knees, hips, hands, facet joints, and feet, involving both cartilage and the surrounding tissues.189 Osteoarthritis is primarily characterized by the degeneration and loss of articular cartilage, along with intra-articular bone remodeling, osteophyte formation, ligament laxity, muscle weakness around the joints, and synovitis, etc.190 It is mainly manifested as joint pain, stiffness, and limited movement. Several specific risk factors for osteoarthritis have been identified, including obesity, metabolic diseases, age, gender, ethnicity, genetics, nutrition, joint overload or abnormalities, previous injuries, bone density, and muscle function.190–192
The correlation between sarcopenia and osteoarthritis
Mechanical load is undoubtedly a key risk factor in the development of osteoarthritis and is regarded as the only critical factor in its progression.193 The risk factors for osteoarthritis are heightened by increased mechanical stress on the joints (such as obesity, excessive joint load, or joint misalignment) or by reducing the joints’ mechanical protection (such as through injury or muscle loss). This weakens the joints’ ability to withstand normal loads, accelerating the onset of osteoarthritis.193
Growing evidence suggests that reduced lower limb muscle strength is commonly observed in patients with knee or hip osteoarthritis at various stages. Lower limb muscle weakness is a known predictor for the onset of knee osteoarthritis, though there is conflicting evidence regarding its role in the progression of the disease.194 The prevalence of sarcopenia (45.2%) among 7 495 knee osteoarthritis patients (average age 68.5 years, with 72.4% being female) in Asia and Europe is more than twice that of the control group (31.2%), with an odds ratio (OR) of 2.07.195 However, obesity continues to play a significant role. In a large sample screening of 11 456 adults in the United States, sarcopenia—defined by BMI-adjusted SMI—was found to be associated with osteoarthritis, with the correlation being stronger in the smoking population.196
However, it is worth noting that some studies have not demonstrated a clear correlation between sarcopenia or thigh muscle weakness and osteoarthritis. For example, a study analyzing a population of 1 653 participants with or at risk for knee osteoarthritis found that muscle wasting alone was not associated with an increased risk of knee osteoarthritis, whereas a combination of obesity and muscle wasting was linked to a higher risk.197 Alternatively, it only demonstrates a correlation between knee osteoarthritis and high fat mass combined with low lower limb muscle mass in female patients, with no such evidence found in male OA patients.198 The discrepancies in the analysis of these clinical data may arise from varying definitions of sarcopenia and the influence of other confounding factors that can contribute to both sarcopenia and osteoarthritis. This highlights the need for more precise definitions of patient subgroups and careful selection of endpoints to better clarify the link between the two conditions.
The relationship between osteoarthritis and muscle loss can be studied longitudinally using animal models in which muscle weakness or osteoarthritis is induced.By using rat anterior cruciate ligament transection (ACLT) to simulate human knee osteoarthritis (KOA)-like changes, it was found that the increased expression of MuRF-1 and atrogin-1, the key signaling molecules of muscle atrophy, may be linked to alterations in the neuromuscular junction (NMJ).199 ACLT-induced KOA promotes neuromuscular junction (NMJ) remodeling and atrophy in the quadriceps and tibialis anterior (TA) muscles, which is associated with signs of inflammation and alterations in muscle gene and protein expression.199
Due to the current lack of specific biomarkers that can fully validate cartilage degeneration and muscle weakness, along with the absence of unified and effective diagnostic indicators for muscle atrophy, verifying the relationship and underlying mechanisms between muscle atrophy and osteoarthritis has become increasingly complex. Clinical studies have identified muscle weakness as a potential key risk factor for knee osteoarthritis. Patients with osteoarthritis, particularly those with pain symptoms, show lower quadriceps muscle strength compared to the general population, as indicated by imaging evidence.200 Unlike typical muscle atrophy, which is often characterized by type II fiber atrophy, the muscle atrophy in osteoarthritis patients shows more uniform fiber involvement. This is primarily associated with the functional impairments caused by the disease.201 Further research is needed to explore potential factors such as muscle growth inhibitors, systemic or local inflammatory mediators, cytokines, and inflammatory transcription factors.
The impact of sarcopenia on the therapeutic effect of osteoarthritis
Sarcopenia and osteoarthritis share many similarities in both physical and pathological manifestations, with correlations ranging from traditional mechanical interactions to endocrine and biochemical signaling.Therefore, exploring common treatment approaches for both conditions, or adjusting the treatment of one to improve the other, is currently a key focus in clinical research.
Currently, the primary drug treatments for osteoarthritis include nonsteroidal anti-inflammatory drugs (such as celecoxib), central analgesics (such as duloxetine), opioid agonists (like tramadol), and intra-articular injections of hyaluronic acid.192 The treatment methods mainly aimed at relieving pain in this part are not highly correlated with the treatment of sarcopenia. Non-pharmacological treatments for osteoarthritis, such as weight loss (for overweight individuals) and strengthening exercises, are similar to the interventions used for sarcopenia. Muscle mass and strength serve as crucial links between sarcopenia and osteoarthritis in clinical treatment. Structured exercise interventions targeting lower limb muscle strengthening can help alleviate pain and improve functional status.Clinical studies have shown that combining diet with exercise can significantly reduce weight, alleviate pain, improve functional status, and lower inflammatory markers more effectively than exercise alone.202 Liao et al.203 found that resistance training combined with protein supplementation can effectively improve muscle mass, strength, and function, enhance joint stability and body balance in osteoarthritis patients at risk of muscle atrophy, thereby reducing pain and improving mobility.
The impact of sarcopenia on osteoarthritis surgery and prognosis
OA patients with persistent pain, functional loss, and late stage imaging changes may choose to undergo total hip arthroplasty (THA) or total knee arthroplasty (TKA). TKA and THA surgeries positively impact muscle strength and performance in osteoarthritis patients, promoting joint mobility and aiding in recovery. Sarcopenia has been found to affect postoperative rehabilitation outcomes following total joint arthroplasty (TJA). A follow-up study of 90 438 osteoarthritis patients revealed that those with muscle atrophy experienced longer hospital stays, higher risks of medical complications and reoperation within 90 days, and an increased rate of prosthesis failure within 2 years.204 A follow-up study of 90 438 osteoarthritis patients found that those with muscle atrophy had longer hospital stays, increased risks of medical complications and reoperation within 90 days, and a higher rate of prosthesis failure within 2 years.205 Overall, patients with advanced osteoarthritis, whether or not they have sarcopenia, have shown significant clinical improvements in muscle mass, strength, function, pain, and daily living abilities following TKA surgery.206 In addition, TKA surgery improves the muscle strength of the quadriceps and hamstrings in patients with knee arthritis, with gradual recovery approaching the strength of the healthy side at 1, 3, and 6 months post-surgery.163
In addition, sarcopenia may increase the risk of prosthetic infection after joint replacement surgery. Babu et al.165 used the psoas lumbar vertebral index (PLVI), a marker of central muscle atrophy, to analyze patients with and without prosthetic infections following THA and TKA. They found a significant difference in PLVI between the two groups, with infected patients having significantly lower PLVI. Multivariate logistic regression analysis further indicated that PLVI is an important predictor of postoperative prosthetic infection.
Sarcopenia and spinal degenerative diseases
Sarcopenia and scoliosis
Adult degenerative scoliosis (ADS) is a common spinal condition characterized by abnormal curvature of the spine, often resulting in pain, stiffness, and loss of function. The pathogenesis of ADS is primarily attributed to asymmetric degeneration of intervertebral discs and facet joints at various levels.166 This asymmetrical pathological change creates an imbalance in spinal load, ultimately leading to the formation of abnormal spinal curvature, which progressively worsens and compromises the spine’s functional integrity. Clinical studies have shown that ~50% of patients with degenerative lumbar scoliosis (DLS) also experience muscle atrophy.15,167 Yawara et al.168 included 971 women (average age 70.4 years) in their study and found a comorbidity rate of 59.8% between degenerative lumbar scoliosis (DLS) and sarcopenia. Logistic regression analysis identified a reduction in trunk muscle mass as an age-related risk factor for DLS.168 Unfortunately, there is currently limited clinical data and research on the comorbidity of muscle wasting in patients with scoliosis. More studies with larger sample sizes are needed to better evaluate the correlation between degenerative scoliosis and muscle wasting.
The onset of degenerative scoliosis is linked to factors such as asymmetric degeneration of intervertebral discs and facet joints, reduction of the muscles surrounding the spine, uneven distribution of mechanical loads, and the degradation of inflammatory factors and the extracellular matrix (ECM).166 Sarcopenia may influence scoliosis by disrupting the balance between the spine’s extensor and flexor muscles. Clinical studies have found that the difference in the paraspinal and lumbar muscle area in patients with degenerative scoliosis is significantly greater on the convex side compared to the concave side. This may be due to fat infiltration on both sides and muscle hypertrophy on the convex side.169 Another study found that in patients with degenerative lumbar scoliosis (DLS), the multifidus muscle on the concave side exhibited a reduction in muscle fiber size and a decrease in the number of cell nuclei, suggesting that degenerative scoliosis is associated with muscle degeneration on the concave side.74
The impact of sarcopenia on scoliosis may also be linked to certain secreted factors. The pathogenesis of sarcopenia involves oxidative stress and chronic inflammation. Oxidative stress, driven by the accumulation of reactive oxygen species, along with inflammatory factors such as IL-6 and TNF-α, can promote mitochondrial dysfunction and further induce muscle cell apoptosis.182 High levels of mitochondrial DNA (mtDNA) and mtDNA deletions have been found in the paraspinal muscles of patients with scoliosis, indicating a potential link to muscle atrophy.183 Pentosidine, a potential biomarker of sarcopenia and an advanced glycation end product (AGE), has been found in higher serum concentrations in elderly women with degenerative lumbar scoliosis (DLS). It is also associated with the severity of coronary artery disease and sagittal displacement in these patients.167,184 This suggests that elevated levels of AGEs could serve as potential biomarkers for the progression of lumbar scoliosis and kyphotic deformities.
Sarcopenia and intervertebral disc degeneration
Intervertebral disc degeneration (IVDD) is one of the most common clinical health issues, and the progressive decline in paraspinal muscle mass, strength, and function due to sarcopenia (SP) has been identified as a significant factor contributing to IVDD.185,207 Qi et al.208 explored the causal relationship between sarcopenia-related traits—such as appendicular lean mass (ALM), grip strength (GS), and walking speed (WP)—and intervertebral disc degeneration (IVDD) using a two-sample Mendelian randomization approach. They concluded that severe sarcopenia is a significant risk factor for intervertebral disc degeneration (IVDD). Clinical studies have shown that among 120 IVDD patients, 28.3% also had concurrent muscular dystrophy.209
A cross-sectional study has shown a correlation between lumbar disc herniation and paraspinal muscle degeneration, but found no association with muscle asymmetry.210 The paraspinal muscles, including the multifidus and erector spinae, are situated on both sides of the spine and play a vital role in maintaining spinal stability and function. These muscles have been shown to be closely associated with the development of various spinal conditions, including lumbar disc herniation, lumbar spinal stenosis, and paraspinal kyphosis.211–215 Multifidus muscle biopsies from individuals with lumbar spine lesions have revealed increased levels of muscle degeneration, inflammation, and reduced vascularization.216 A retrospective study involving 132 patients with intervertebral disc degeneration (IVDD) and healthy controls identified a potential bidirectional relationship between multifidus muscle degeneration and the progression of IVDD, suggesting mutual influence and interaction between these conditions.217 Guangming Xu et al.218 conducted a quantitative analysis of the effects of erector spinae and multifidus muscle atrophy on spinal tissue using a human-based finite element (FE) spinal model. Their findings indicated that muscle atrophy primarily increases the risk of damage to the L4-L5 intervertebral discs, L1 vertebrae, and L3-S1 joint capsules, as evidenced by significant stress and strain differences in these areas. Additionally, the intervertebral vacuum phenomenon (IVP) is recognized as one of the imaging markers associated with intervertebral disc degeneration. Camino Willhuber, Gaston et al.219 found that patients who underwent lumbar decompression surgery exhibited a higher degree of intervertebral vacuum phenomenon (IVP). The severity of IVP was positively correlated with fat infiltration in the multifidus and erector spinae muscles, with a stronger correlation observed in the multifidus. Adipose degeneration of paraspinal muscles is believed to influence the recovery of patients with lumbar disc degeneration (LDD) undergoing open microdiscectomy, particularly at 1 and 6 months post-surgery. This degeneration may negatively affect the overall recovery process and the success of the surgical intervention.220 Additionally, animal studies have been employed to investigate the relationship between intervertebral disc degeneration and paraspinal muscle atrophy. Hey HWD et al.221 successfully simulated human paraspinal muscle atrophy using TSC1 gene knockout mice, and their findings revealed that paraspinal muscle atrophy significantly accelerated intervertebral disc degeneration and height loss in the mice.A study using a ram model of intervertebral disc degeneration (IVDD) suggests that back muscle injury resulting from IVDD is characterized by structural remodeling of muscles, fat, and connective tissue, rather than being limited to muscle atrophy alone.222
Segmental mechanical dysfunction resulting from defects in the structure and activation of the multifidus muscle may lead to cumulative damage to the annular fibers of the intervertebral discs, a process that has been shown to contribute to intervertebral disc degeneration.223 Sven Hoppe et al.224 conducted a 3D analysis of paraspinal lumbar muscle fat infiltration using MRI T2-weighted images and identified a positive correlation between the degree of fat infiltration in the paraspinal muscles and the Pfirrmann grade of intervertebral disc degeneration.Clinical studies have shown that the multifidus muscles of patients with disc herniation contain a significant proportion of fibroblast adipogenic progenitor cells (FAPs) and satellite cells (SCs). These cells are believed to be the primary contributors to muscle fibrosis and fat infiltration in these patients.225
At the molecular level, signal transduction pathways involving fibroblast growth factor (FGF) have been found to be associated with various musculoskeletal disorders, including intervertebral disc degeneration (IVDD), sarcopenia, osteoarthritis (OA), and osteoporosis (OP).226 Numerous fibroblast growth factor (FGF) family members have been shown to regulate the synthesis, catabolism, and ossification of cartilage tissue, while also influencing the regeneration and differentiation of muscle stem cells, myoblasts, and muscle tissue. A study utilizing 3T magnetic resonance imaging found a significant positive correlation between the proton density fat fraction (PDFF muscle) of paraspinal muscle fat content and an increased risk of intervertebral disc degeneration (IVDD) in individuals with impaired blood glucose levels.207 This suggests that the relationship between paraspinal muscle atrophy, fat infiltration, and IVDD may be driven, at least in part, by dysregulated blood glucose metabolism in these patients. Increasing evidence suggests that adipose tissue acts as a significant source of pro-inflammatory cytokines. Animal studies have shown that the multifidus adipose tissue in sheep with intervertebral disc degeneration (IVDD) contains a higher proportion of M1 macrophages and elevated expression of pro-inflammatory cytokines, such as TNF. In line with these findings, researchers observed fat infiltration in the multifidus muscle of patients who had undergone surgery for intervertebral disc herniation. They discovered that TNF expression and markers of pro-inflammatory M1 macrophages were elevated in multifidus muscles with high-fat infiltration, confirming that intervertebral disc degeneration is linked to the dysregulation of local inflammatory responses, particularly multifidus myositis.227 Additionally, data from 21 patients with lumbar disc herniation who underwent microdiscectomy in Australia showed that impaired regenerative capacity of the multifidus muscle was linked to adverse outcomes post-surgery.228 This study also suggested that inflammatory imbalances in subcutaneous fat in the back area may be a contributing factor to these poor outcomes.
Unfortunately, current research on the correlation between IVDD and sarcopenia is mainly based on clinical sample observations, and further exploration of the specific mechanisms is urgently needed.
Sarcopenia and lumbar spondylolisthesis
Degenerative lumbar spondylolisthesis (DLS) refers to the forward or backward displacement of a vertebral body relative to the adjacent vertebra, caused by degenerative changes, without the presence of a corresponding vertebral ring fracture or defect.227 DLS results from the combined influence of multiple factors, including pelvic tilt and misalignment, the strength of the iliac neck ligaments, and degeneration of adjacent intervertebral discs or facet joints. These factors contribute to the instability and displacement of the vertebral body.229 Among them, the reduction of paraspinal muscles and fat infiltration are also one of the main reasons.230,231 The paraspinal muscles play a critical role in providing functional support and maintaining spinal stability. For example, the multifidus muscle is essential for spinal rotation and stability, while the erector spinae muscle assists with spinal flexion and extension.231–233 At the molecular level, factors such as fat infiltration into muscle tissue, insulin resistance, and mitochondrial dysfunction can contribute to muscle atrophy and reduced muscle strength. This decline in muscle function compromises spinal stability, which can ultimately lead to the development of degenerative lumbar spondylolisthesis (DLS).234
Cao et al.233 observed the fat infiltration rate (FIR) of paraspinal muscles (PM) in patients with pure L4 vertebral body degenerative lumbar spondylolisthesis (DLS) and found that FIR of the paraspinal muscles is an independent factor associated with asymptomatic adult L4 DLS. This finding suggests that FIR has a high predictive value for identifying L4 DLS in these patients. Duan et al.230 retrospectively analyzed preoperative MRI scans of 23 patients with spinal spondylolisthesis who underwent single-level L4-5 transforaminal lumbar interbody fusion (TLIF) surgery. They found that the degree of fat infiltration in the multifidus muscle, particularly at the L3 level, was significantly associated with adjacent segment degeneration (ASD) following surgery for degenerative spondylolisthesis. Paul Köhli et al.235 examined the functional cross-sectional area (FCSA) and degree of fat infiltration in the psoas major, erector spinae (ES), and multifidus (MF) muscles in patients undergoing surgery for L4/5 level degenerative lumbar spondylolisthesis (DLS). They found a significant correlation between the condition of the paraspinal muscles and the severity of DLS slippage. Specifically, greater degeneration in the ES and MF muscles was associated with a higher degree of slippage, whereas the psoas muscle exhibited an inverse relationship. Another study on the cross-sectional area (CSA) and fat degeneration of lumbar paraspinal muscles in adult DLS patients with chronic nerve root disease found that these patients had smaller MF FCSA and higher fat infiltration in the MF, indicating segmental atrophy of the MF muscles.231 This atrophy may be compensated for by ES muscle hypertrophy.236 James C McKenzie et al.237 compared outcomes between sarcopenia and non-sarcopenia patients who underwent lumbar fusion surgery and found no significant differences in clinical results, suggesting that sarcopenia does not negatively impact the success of lumbar fusion surgery in treating degenerative spondylolisthesis. In another clinical study, preoperative and postoperative MRI data from 95 patients with lumbar spinal stenosis who underwent microsurgical decompression (MSD) were analyzed. The study found that a decrease in lumbar muscle volume, as indicated by the psoas muscle index (PMI), was significantly correlated with the progression of postoperative spondylolisthesis. However, there was no statistically significant correlation between multifidus muscle volume and the progression of postoperative slippage.238
Sarcopenia and thoracolumbar disease
Emerging evidence demonstrates an association between paravertebral muscle degeneration and various spinal pathologies. However, current research has predominantly focused on lumbar spine disorders, with limited investigation into paraspinal muscle changes in cervical and thoracic spine conditions. A critical unanswered question is whether cervical paravertebral muscle evaluation could serve as a reliable surrogate marker for sarcopenia assessment, which would potentially eliminate the need for additional lumbar CT scans and reduce both healthcare costs and patient radiation exposure. In this context, Furkan Ufuk et al. conducted a pioneering study examining the correlation between cervical paraspinal muscle measurements from CT scans and the established L3 muscle index (L3MI), providing preliminary reference values for clinical assessment.239 The size of the deep extensor muscles, muscle asymmetry, and the ratio of functional cross-sectional area to total cross-sectional area have been found to influence surgical outcomes in patients with degenerative cervical spondylotic myelopathy (DCM).240 Patients with DCM who display increased paraspinal fat infiltration and asymmetry in the deep cervical muscles tend to experience more severe spinal canal damage, as indicated by maximum canal compromise (MCC). Notably, conflicting evidence exists regarding the clinical relevance of cervical paravertebral muscle degeneration. Some studies have failed to demonstrate significant correlations between cervical paraspinal muscle atrophy and fatty infiltration in patients undergoing anterior cervical discectomy and fusion (ACDF).241,242
Existing investigations into thoracic spine pathologies and sarcopenia have primarily examined their association with vertebral fractures and osteoporosis. Emerging evidence indicates that the skeletal muscle index (SMI) derived from T12-level CT imaging serves as both a reliable diagnostic marker for osteoporosis and a predictive tool for fracture risk.243 Epidemiological data from community-dwelling older adults reveal that sarcopenia constitutes a significant risk factor for moderate-to-severe thoracic fragility fractures, with fracture severity correlating with the degree of sarcopenia.244 Biomechanical modeling using the AnyBody simulation system demonstrates maximal spinal loading in older adults occurs at the thoracolumbar junction (T9/T10–L1/L2).245 The loss of paravertebral muscle mass increases compressive and shear stresses on osteoporotic vertebrae, likely explaining the elevated fracture risk observed in sarcopenic populations.246
Discussion
In this review, we discuss current assessment methods for sarcopenia and its correlation with common musculoskeletal disorders, particularly age-related degenerative diseases. Currently, standardized diagnostic criteria for sarcopenia remain lacking in clinical practice. Based on existing evidence, we recommend adopting the comprehensive evaluation frameworks proposed by EWGSOP2 and AWGS for sarcopenia diagnosis. The assessment approach should be tailored to specific clinical contexts: For screening purposes, simplified evaluations focusing on muscle strength and physical performance may be employed. However, in research settings or when comorbid conditions may confound functional assessments, imaging modalities remain the gold standard for precise muscle mass quantification. Selection of anatomical regions for evaluation should be guided by clinical objectives. For instance, when investigating lumbar spine disorders with concurrent sarcopenia, the lumbar dorsal muscle group at the L3 vertebral level represents the preferred measurement site, as it provides standardized and reproducible assessment. However, despite the relative accuracy and ease of use of clinical methods like ultrasound and bioelectrical impedance analysis, these techniques still have limitations and lack a standardized evaluation protocol.
Muscle atrophy, a key feature of sarcopenia, is most prevalent in the elderly population and is closely associated with various adverse health outcomes in this group. It has been found to be highly correlated with conditions such as osteoporosis, osteoarthritis, and various spinal disorders.Further exploration into the mechanisms linking sarcopenia and musculoskeletal diseases, as well as the search for common pathogenic factors, may uncover new therapeutic targets for both conditions. Future research should also focus on developing interventions that address both sarcopenia and musculoskeletal disorders. In clinical practice, alongside the treatment of common skeletal and muscular conditions, greater emphasis should be placed on assessing patients’ muscle loss to improve treatment efficacy, restore physical function, and enhance quality of life.
Our work still has certain limitations. Firstly, we selected only a subset of common musculoskeletal disorders, particularly those with a strong correlation to muscle atrophy, and did not include diseases that have received less research attention. Secondly, some of the studies we reviewed may have differing definitions and assessment criteria for muscle atrophy, and sample sizes in these studies could be insufficient. Therefore, the correlation between muscle atrophy and skeletal muscle diseases requires further investigation with larger sample sizes and standardized evaluation methods.
Acknowledgements
This work was supported by The National Natural Science Foundation of China (82405429); The Medical and Health Science and Technology Program of Hangzhou (ZD20250272); Key Discipline of Traditional Chinese Medicine in Zhejiang Province (2024-XK-57) and The Construction Fund of Key Medical Discipline of Hangzhou (2025HZZD16).
Author contributions
Dong Wang and Hongting Jin: Study conceptualization and design. Hao Pan and Dong Wang: Conceptual framework development. Xinning Mao, Dong Wang: Literature research and methodology design. Xinning Mao, Ke Lv, Weihui Qi: Writing—original draft preparation. Tenghui Li, Dong Wang: Supervision, manuscript revision, and critical editing. Hao Pan, Yueli Sun: Manuscript review and validation.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Xinning Mao, Ke Lv, Weihui Qi
Contributor Information
Hongting Jin, Email: hongtingjin@163.com.
Hao Pan, Email: harper1966@alu.zcmu.edu.cn.
Dong Wang, Email: hz-wdong@alu.zcmu.edu.cn.
References
- 1.Cruz-Jentoft, A. J. et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing48, 601 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg, I. H. Sarcopenia: origins and clinical relevance. J. Nutr.127, 990s–991s (1997). [DOI] [PubMed] [Google Scholar]
- 3.Yuan, S. & Larsson, S. C. Epidemiology of sarcopenia: prevalence, risk factors, and consequences. Metabolism144, 155533 (2023). [DOI] [PubMed] [Google Scholar]
- 4.Petermann-Rocha, F. et al. Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle13, 86–99 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jimenez-Gutierrez, G. E. et al. Molecular mechanisms of inflammation in sarcopenia: diagnosis and therapeutic update. Cells11, 2359 (2022). [DOI] [PMC free article] [PubMed]
- 6.Du, Y. et al. Associations of physical activity with sarcopenia and sarcopenic obesity in middle-aged and older adults: the Louisiana osteoporosis study. BMC Public Health22, 896 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larsson, L. et al. Sarcopenia: aging-related loss of muscle mass and function. Physiol. Rev.99, 427–511 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Safdar, A. et al. Aberrant mitochondrial homeostasis in the skeletal muscle of sedentary older adults. PLoS One.5, e10778 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joseph, A. M. et al. The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high- and low-functioning elderly individuals. Aging Cell11, 801–809 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cruz-Jentoft, A. J. & Sayer, A. A. Sarcopenia. Lancet393, 2636–2646 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Kaido, T. Proposal of definition and diagnostic criteria for sarcopenic obesity by ESPEN and EASO. Hepatobiliary Surg. Nutr.12, 431–434 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lisco, G. et al. Sarcopenia and diabetes: a detrimental liaison of advancing age. Nutrients16, 63 (2023). [DOI] [PMC free article] [PubMed]
- 13.Lin, T. et al. Prevalence of sarcopenia in pain patients and correlation between the two conditions: a systematic review and meta-analysis. J. Am. Med. Dir. Assoc.23, 902.e901–902.e920 (2022). [DOI] [PubMed] [Google Scholar]
- 14.Park, S. et al. The prevalence and impact of sarcopenia on degenerative lumbar spinal stenosis. Bone Jt. J.98-b, 1093–1098 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Eguchi, Y. et al. Associations between sarcopenia and degenerative lumbar scoliosis in older women. Scoliosis Spinal Disord.12, 9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veronese, N. et al. Pain increases the risk for sarcopenia in community-dwelling adults: results from the English longitudinal study of ageing. J. Gerontol. A Biol. Sci. Med. Sci.78, 1013–1019 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuo, S. et al. Clinical features of sarcopenia in patients with lumbar spinal stenosis. Spine45, E1105–e1110 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Chua, M. et al. Gender differences in multifidus fatty infiltration, sarcopenia and association with preoperative pain and functional disability in patients with lumbar spinal stenosis. Spine J.22, 58–63 (2022). [DOI] [PubMed] [Google Scholar]
- 19.Han, A., Bokshan, S. L., Marcaccio, S. E., DePasse, J. M. & Daniels, A. H. Diagnostic criteria and clinical outcomes in sarcopenia research: a literature review. J. Clin. Med.7, 70 (2018). [DOI] [PMC free article] [PubMed]
- 20.Fielding, R. A. et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med Dir. Assoc.12, 249–256 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Studenski, S. A. et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J. Gerontol. A Biol. Sci. Med. Sci.69, 547–558 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen, L. K. et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc.21, 300–307.e302 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Roberts, H. C. et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing40, 423–429 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Beaudart, C. et al. Sarcopenia in daily practice: assessment and management. BMC Geriatrics16, 170 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones, C. J., Rikli, R. E. & Beam, W. C. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res. Q. Exerc. Sport70, 113–119 (1999). [DOI] [PubMed] [Google Scholar]
- 26.Guerri, S. et al. Quantitative imaging techniques for the assessment of osteoporosis and sarcopenia. Quant. Imaging Med. Surg.8, 60–85 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chianca, V. et al. Sarcopenia: imaging assessment and clinical application. Abdom. Radiol.47, 3205–3216 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shepherd, J. A., Ng, B. K., Sommer, M. J. & Heymsfield, S. B. Body composition by DXA. Bone104, 101–105 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tagliafico, A. S., Bignotti, B., Torri, L. & Rossi, F. Sarcopenia: how to measure, when and why. Radio. Med.127, 228–237 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maden-Wilkinson, T. M., Degens, H., Jones, D. A. & McPhee, J. S. Comparison of MRI and DXA to measure muscle size and age-related atrophy in thigh muscles. J. Musculoskelet. Neuronal Interact.13, 320–328 (2013). [PubMed] [Google Scholar]
- 31.Levine, J. A. et al. Measuring leg muscle and fat mass in humans: comparison of CT and dual-energy X-ray absorptiometry. J. Appl. Physiol.88, 452–456 (2000). [DOI] [PubMed] [Google Scholar]
- 32.Giraudo, C., Cavaliere, A., Lupi, A., Guglielmi, G. & Quaia, E. Established paths and new avenues: a review of the main radiological techniques for investigating sarcopenia. Quant. imaging Med. Surg.10, 1602–1613 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boutin, R. D., Yao, L., Canter, R. J. & Lenchik, L. Sarcopenia: current concepts and imaging implications. Ajr. Am. J. Roentgenol.205, W255–266 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Albano, D., Messina, C., Vitale, J. & Sconfienza, L. M. Imaging of sarcopenia: old evidence and new insights. Eur. Radiol.30, 2199–2208 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Amini, B., Boyle, S. P., Boutin, R. D. & Lenchik, L. Approaches to assessment of muscle mass and myosteatosis on computed tomography: a systematic review. J. Gerontol. Ser. A Biol. Sci. Med. Sci.74, 1671–1678 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daly, L. E., Prado, C. M. & Ryan, A. M. A window beneath the skin: how computed tomography assessment of body composition can assist in the identification of hidden wasting conditions in oncology that profoundly impact outcomes. Proc. Nutr. Soc.77, 135–151 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Derstine, B. A. et al. Skeletal muscle cutoff values for sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci. Rep.8, 11369 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuriyama, K. et al. Relationship between sarcopenia classification and thigh muscle mass, fat area, muscle CT value and osteoporosis in middle-aged and older Japanese adults. Bone163, 116487 (2022). [DOI] [PubMed] [Google Scholar]
- 39.Mizuno, T. et al. Relationship between quadriceps muscle computed tomography measurement and motor function, muscle mass, and sarcopenia diagnosis. Front. Endocrinol.14, 1259350 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodpaster, B. H. et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J. Appl. Physiol.90, 2157–2165 (2001). [DOI] [PubMed] [Google Scholar]
- 41.Brooks, N. et al. Resistance training and timed essential amino acids protect against the loss of muscle mass and strength during 28 days of bed rest and energy deficit. J. Appl. Physiol.105, 241–248 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cauza, E. et al. Effects of progressive strength training on muscle mass in type 2 diabetes mellitus patients determined by computed tomography. Wien. Medizinische Wochenschr.159, 141–147 (2009). [DOI] [PubMed] [Google Scholar]
- 43.Breda, A. P. et al. Skeletal muscle abnormalities in pulmonary arterial hypertension. PloS One.9, e114101 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prado, C. M. & Heymsfield, S. B. Lean tissue imaging: a new era for nutritional assessment and intervention. J. Parenter. Enter. Nutr.38, 940–953 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Csapo, R., Malis, V., Sinha, U., Du, J. & Sinha, S. Age-associated differences in triceps surae muscle composition and strength - an MRI-based cross-sectional comparison of contractile, adipose and connective tissue. BMC Musculoskelet. Disord.15, 209 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fischer, M. A., Pfirrmann, C. W., Espinosa, N., Raptis, D. A. & Buck, F. M. Dixon-based MRI for assessment of muscle-fat content in phantoms, healthy volunteers and patients with achillodynia: comparison to visual assessment of calf muscle quality. Eur. Radiol.24, 1366–1375 (2014). [DOI] [PubMed] [Google Scholar]
- 47.Tosato, M. et al. Measurement of muscle mass in sarcopenia: from imaging to biochemical markers. Aging Clin. Exp. Res.29, 19–27 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Schweitzer, L. et al. What is the best reference site for a single MRI slice to assess whole-body skeletal muscle and adipose tissue volumes in healthy adults? Am. J. Clin. Nutr.102, 58–65 (2015). [DOI] [PubMed] [Google Scholar]
- 49.Engelke, K. et al. Magnetic resonance imaging techniques for the quantitative analysis of skeletal muscle: state of the art. J. Orthop. Transl.42, 57–72 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giovannini, S. et al. Sarcopenia: diagnosis and management, state of the art and contribution of ultrasound. J. Clin. Med.10, 5552 (2021). [DOI] [PMC free article] [PubMed]
- 51.Perkisas, S. et al. Application of ultrasound for muscle assessment in sarcopenia: 2020 SARCUS update. Eur. Geriatr. Med.12, 45–59 (2021). [DOI] [PubMed] [Google Scholar]
- 52.Scott, J. M. et al. Reliability and validity of panoramic ultrasound for muscle quantification. Ultrasound Med. Biol.38, 1656–1661 (2012). [DOI] [PubMed] [Google Scholar]
- 53.Takai, Y. et al. Validity of ultrasound muscle thickness measurements for predicting leg skeletal muscle mass in healthy Japanese middle-aged and older individuals. J. Physiological Anthropol.32, 12 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takai, Y. et al. Applicability of ultrasound muscle thickness measurements for predicting fat-free mass in elderly population. J. Nutr. Health Aging18, 579–585 (2014). [DOI] [PubMed] [Google Scholar]
- 55.Gába, A., Kapuš, O., Cuberek, R. & Botek, M. Comparison of multi- and single-frequency bioelectrical impedance analysis with dual-energy X-ray absorptiometry for assessment of body composition in post-menopausal women: effects of body mass index and accelerometer-determined physical activity. J. Hum. Nutr. Dietetics28, 390–400 (2015). [DOI] [PubMed] [Google Scholar]
- 56.Aleixo, G. F. P. et al. Bioelectrical impedance analysis for the assessment of sarcopenia in patients with cancer: a systematic review. Oncologist25, 170–182 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen, L. K. et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc.15, 95–101 (2014). [DOI] [PubMed] [Google Scholar]
- 58.Yu, S. C., Powell, A., Khow, K. S. & Visvanathan, R. The performance of five bioelectrical impedance analysis prediction equations against dual X-ray absorptiometry in estimating appendicular skeletal muscle mass in an adult Australian population. Nutrients8, 189 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sergi, G. et al. Assessing appendicular skeletal muscle mass with bioelectrical impedance analysis in free-living Caucasian older adults. Clin. Nutr.34, 667–673 (2015). [DOI] [PubMed] [Google Scholar]
- 60.Yamada, Y. et al. Developing and validating an age-independent equation using multi-frequency bioelectrical impedance analysis for estimation of appendicular skeletal muscle mass and establishing a cutoff for sarcopenia. Int. J. Environ. Res. Public Health14, 809 (2017). [DOI] [PMC free article] [PubMed]
- 61.Bosaeus, I., Wilcox, G., Rothenberg, E. & Strauss, B. J. Skeletal muscle mass in hospitalized elderly patients: comparison of measurements by single-frequency BIA and DXA. Clin. Nutr.33, 426–431 (2014). [DOI] [PubMed] [Google Scholar]
- 62.Cheng, K. Y. et al. Diagnosis of sarcopenia by evaluating skeletal muscle mass by adjusted bioimpedance analysis validated with dual-energy X-ray absorptiometry. J. Cachexia Sarcopenia Muscle12, 2163–2173 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shafiee, G. et al. Prevalence of sarcopenia in the world: a systematic review and meta- analysis of general population studies. J. Diab. Metab. Disord.16, 21 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reiss, J. et al. Case finding for sarcopenia in geriatric inpatients: performance of bioimpedance analysis in comparison to dual X-ray absorptiometry. BMC Geriatrics16, 52 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garlini, L. M. et al. Phase angle and mortality: a systematic review. Eur. J. Clin. Nutr.73, 495–508 (2019). [DOI] [PubMed] [Google Scholar]
- 66.Di Vincenzo, O., Marra, M., Di Gregorio, A., Pasanisi, F. & Scalfi, L. Bioelectrical impedance analysis (BIA) -derived phase angle in sarcopenia: a systematic review. Clin. Nutr.40, 3052–3061 (2021). [DOI] [PubMed] [Google Scholar]
- 67.Basile, C. et al. Phase angle as bioelectrical marker to identify elderly patients at risk of sarcopenia. Exp. Gerontol.58, 43–46 (2014). [DOI] [PubMed] [Google Scholar]
- 68.Santana, N. M., Pinho, C. P. S., da Silva, C. P., Dos Santos, N. F. & Mendes, R. M. L. Phase angle as a sarcopenia marker in hospitalized elderly patients. Nutr. Clin. Pract.33, 232–237 (2018). [DOI] [PubMed] [Google Scholar]
- 69.Bellido, D., García-García, C., Talluri, A., Lukaski, H. C. & García-Almeida, J. M. Future lines of research on phase angle: strengths and limitations. Rev. Endocr. Metab. Disord.24, 563–583 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beaudart, C. et al. Assessment of muscle function and physical performance in daily clinical practice : a position paper endorsed by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Calcif. tissue Int.105, 1–14 (2019). [DOI] [PubMed] [Google Scholar]
- 71.Soltani, A. et al. Real-world gait speed estimation, frailty and handgrip strength: a cohort-based study. Sci. Rep.11, 18966 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chou, M. Y. et al. Role of gait speed and grip strength in predicting 10-year cognitive decline among community-dwelling older people. BMC Geriatrics19, 186 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Binotto, M. A., Lenardt, M. H. & Rodríguez-Martínez, M. D. C. Physical frailty and gait speed in community elderly: a systematic review. Rev. da Esc. de. Enferm. da U S P52, e03392 (2018). [DOI] [PubMed] [Google Scholar]
- 74.Shafaq, N. et al. Asymmetric degeneration of paravertebral muscles in patients with degenerative lumbar scoliosis. Spine37, 1398–1406 (2012). [DOI] [PubMed] [Google Scholar]
- 75.Podsiadlo, D. & Richardson, S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J. Am. Geriatrics Soc.39, 142–148 (1991). [DOI] [PubMed] [Google Scholar]
- 76.Steffen, T. M., Hacker, T. A. & Mollinger, L. Age- and gender-related test performance in community-dwelling elderly people: Six-Minute Walk Test, Berg Balance Scale, Timed Up & Go Test, and gait speeds. Phys. Ther82, 128–137 (2002). [DOI] [PubMed] [Google Scholar]
- 77.Dent, E. et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): screening, diagnosis and management. J. Nutr. Health Aging22, 1148–1161 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shen, Y. et al. Exercise for sarcopenia in older people: a systematic review and network meta-analysis. J. Cachexia Sarcopenia Muscle14, 1199–121 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Da Boit, M. et al. Sex differences in the response to resistance exercise training in older people. Physiological Rep.4, e12834 (2016). [DOI] [PMC free article] [PubMed]
- 80.Vikberg, S. et al. Effects of resistance training on functional strength and muscle mass in 70-year-old individuals with pre-sarcopenia: a randomized controlled trial. J. Am. Med. Dir. Assoc.20, 28–34 (2019). [DOI] [PubMed] [Google Scholar]
- 81.Perry, C. G. et al. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J. Physiol.588, 4795–4810 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Geng, T. et al. PGC-1alpha plays a functional role in exercise-induced mitochondrial biogenesis and angiogenesis but not fiber-type transformation in mouse skeletal muscle. Am. J. Physiol. Cell Physiol.298, C572–579 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dubé, J. J. et al. Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete’s paradox revisited. Am. J. Physiol. Endocrinol. Metab.294, E882–888 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Biensø, R. S. et al. Effects of exercise training on regulation of skeletal muscle glucose metabolism in elderly men. J. Gerontol. Ser. A Biol. Sci. Med. Sci.70, 866–872 (2015). [DOI] [PubMed] [Google Scholar]
- 85.Peterson, M. J. et al. Walking in old age and development of metabolic syndrome: the health, aging, and body composition study. Metab. Syndr. Relat. Disord.8, 317–322 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Veronese, N. et al. Effect of nutritional supplementations on physical performance and muscle strength parameters in older people: a systematic review and meta-analysis. Ageing Res. Rev.51, 48–54 (2019). [DOI] [PubMed] [Google Scholar]
- 87.Robinson, S. M. et al. Does nutrition play a role in the prevention and management of sarcopenia? Clin. Nutr.37, 1121–1132 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ruocco, C., Segala, A., Valerio, A. & Nisoli, E. Essential amino acid formulations to prevent mitochondrial dysfunction and oxidative stress. Curr. Opin. Clin. Nutr. Metab. Care24, 88–95 (2021). [DOI] [PubMed] [Google Scholar]
- 89.Chen, L. K. et al. Roles of nutrition in muscle health of community-dwelling older adults: evidence-based expert consensus from Asian Working Group for Sarcopenia. J. Cachexia Sarcopenia Muscle13, 1653–1672 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.He, X. et al. β-Hydroxy-β-methylbutyrate, mitochondrial biogenesis, and skeletal muscle health. Amino Acids48, 653–664 (2016). [DOI] [PubMed] [Google Scholar]
- 91.Wu, H. et al. Effect of beta-hydroxy-beta-methylbutyrate supplementation on muscle loss in older adults: a systematic review and meta-analysis. Arch. Gerontol. Geriatr.61, 168–175 (2015). [DOI] [PubMed] [Google Scholar]
- 92.Smith, G. I. et al. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am. J. Clin. Nutr.93, 402–412 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kurrat, A. et al. Lifelong exposure to dietary isoflavones reduces risk of obesity in ovariectomized Wistar rats. Mol. Nutr. Food Res.59, 2407–2418 (2015). [DOI] [PubMed] [Google Scholar]
- 94.Tabata, S. et al. The influence of isoflavone for denervation-induced muscle atrophy. Eur. J. Nutr.58, 291–300 (2019). [DOI] [PubMed] [Google Scholar]
- 95.Glisic, M. et al. Phytoestrogen supplementation and body composition in postmenopausal women: a systematic review and meta-analysis of randomized controlled trials. Maturitas.115, 74–83 (2018). [DOI] [PubMed] [Google Scholar]
- 96.Aubertin-Leheudre, M., Lord, C., Khalil, A. & Dionne, I. J. Six months of isoflavone supplement increases fat-free mass in obese-sarcopenic postmenopausal women: a randomized double-blind controlled trial. Eur. J. Clin. Nutr.61, 1442–1444 (2007). [DOI] [PubMed] [Google Scholar]
- 97.Cermak, N. M., Res, P. T., de Groot, L. C., Saris, W. H. & van Loon, L. J. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am. J. Clin. Nutr.96, 1454–1464 (2012). [DOI] [PubMed] [Google Scholar]
- 98.Mafi, F., Biglari, S., Ghardashi Afousi, A. & Gaeini, A. A. Improvement in skeletal muscle strength and plasma levels of follistatin and myostatin induced by an 8-week resistance training and epicatechin supplementation in sarcopenic older adults. J. Aging Phys. Act.27, 384–391 (2019). [DOI] [PubMed] [Google Scholar]
- 99.Wang, J. et al. Vibration and β-hydroxy-β-methylbutyrate treatment suppresses intramuscular fat infiltration and adipogenic differentiation in sarcopenic mice. J. Cachexia Sarcopenia Muscle11, 564–577 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cho, M. R., Lee, S. & Song, S. K. A review of sarcopenia pathophysiology, diagnosis, treatment and future direction. J. Korean Med. Sci.37, e146 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rolland, Y., Dray, C., Vellas, B. & Barreto, P. S. Current and investigational medications for the treatment of sarcopenia. Metab. Clin. Exp.149, 155597 (2023). [DOI] [PubMed] [Google Scholar]
- 102.Dhaliwal, R. & Aloia, J. F. Effect of vitamin D on falls and physical performance. Endocrinol. Metab. Clin. North Am.46, 919–933 (2017). [DOI] [PubMed] [Google Scholar]
- 103.Prokopidis, K. et al. Effect of vitamin D monotherapy on indices of sarcopenia in community-dwelling older adults: a systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle13, 1642–1652 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lan, X. Q. et al. The role of TGF-β signaling in muscle atrophy, sarcopenia and cancer cachexia. Gen. Comp. Endocrinol.353, 114513 (2024). [DOI] [PubMed] [Google Scholar]
- 105.Becker, C. et al. Myostatin antibody (LY2495655) in older weak fallers: a proof-of-concept, randomised, phase 2 trial. Lancet Diab. Endocrinol.3, 948–957 (2015). [DOI] [PubMed] [Google Scholar]
- 106.Lee, S. J. Targeting the myostatin signaling pathway to treat muscle loss and metabolic dysfunction. J. Clin. Invest.131, e148372 (2021). [DOI] [PMC free article] [PubMed]
- 107.Rooks, D. et al. Safety and pharmacokinetics of bimagrumab in healthy older and obese adults with body composition changes in the older cohort. J. Cachexia Sarcopenia Muscle11, 1525–1534 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Garito, T. et al. Bimagrumab improves body composition and insulin sensitivity in insulin-resistant individuals. Diab. Obes. Metab.20, 94–102 (2018). [DOI] [PubMed] [Google Scholar]
- 109.Lee, S. J. et al. Functional redundancy of type I and type II receptors in the regulation of skeletal muscle growth by myostatin and activin A. Proc. Natl. Acad. Sci. USA117, 30907–30917 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sinha-Hikim, I., Cornford, M., Gaytan, H., Lee, M. L. & Bhasin, S. Effects of testosterone supplementation on skeletal muscle fiber hypertrophy and satellite cells in community-dwelling older men. J. Clin. Endocrinol. Metab.91, 3024–3033 (2006). [DOI] [PubMed] [Google Scholar]
- 111.Mudali, S. & Dobs, A. S. Effects of testosterone on body composition of the aging male. Mech. Ageing Dev.125, 297–304 (2004). [DOI] [PubMed] [Google Scholar]
- 112.Neil, D. et al. GSK2881078, a SARM, produces dose-dependent increases in lean mass in healthy older men and women. J. Clin. Endocrinol. Metab.103, 3215–3224 (2018). [DOI] [PubMed] [Google Scholar]
- 113.Supasyndh, O. et al. Effect of oral anabolic steroid on muscle strength and muscle growth in hemodialysis patients. Clin. J. Am. Soc. Nephrol.8, 271–279 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.van de Weijer, T. et al. Evidence for a direct effect of the NAD+ precursor acipimox on muscle mitochondrial function in humans. Diabetes64, 1193–1201 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pin, F., Huot, J. R. & Bonetto, A. The mitochondria-targeting agent MitoQ improves muscle atrophy, weakness and oxidative metabolism in C26 tumor-bearing mice. Front. Cell Dev. Biol.10, 861622 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Genant, H. K. et al. Interim report and recommendations of the World Health Organization Task-Force for Osteoporosis. Osteoporos. Int. Found. USA10, 259–264 (1999). [DOI] [PubMed] [Google Scholar]
- 117.Lane, N. E. Epidemiology, etiology, and diagnosis of osteoporosis. Am. J. Obstet. Gynecol.194, S3–11 (2006). [DOI] [PubMed] [Google Scholar]
- 118.Polito, A., Barnaba, L., Ciarapica, D. & Azzini, E. Osteosarcopenia: a narrative review on clinical studies. Int. Jo. Mol. Sci.23, 5591 (2022). [DOI] [PMC free article] [PubMed]
- 119.Clynes, M. A., Gregson, C. L., Bruyère, O., Cooper, C. & Dennison, E. M. Osteosarcopenia: where osteoporosis and sarcopenia collide. Rheumatology60, 529–537 (2021). [DOI] [PubMed] [Google Scholar]
- 120.Kirk, B., Al Saedi, A. & Duque, G. Osteosarcopenia: a case of geroscience. Aging Med.2, 147–156 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kirk, B., Zanker, J. & Duque, G. Osteosarcopenia: epidemiology, diagnosis, and treatment-facts and numbers. J. Cachexia sarcopenia Muscle11, 609–618 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Locquet, M. et al. Bone health assessment in older people with or without muscle health impairment. Osteoporos. Int.29, 1057–1067 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Huo, Y. R. et al. Phenotype of osteosarcopenia in older individuals with a history of falling. J. Am. Med. Dir. Assoc.16, 290–295 (2015). [DOI] [PubMed] [Google Scholar]
- 124.Yoo, J. I., Kim, H., Ha, Y. C., Kwon, H. B. & Koo, K. H. Osteosarcopenia in patients with hip fracture is related with high mortality. J. Korean Med. Sci.33, e27 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen, S. et al. Global epidemiological features and impact of osteosarcopenia: a comprehensive meta-analysis and systematic review. J. Cachexia Sarcopenia Muscle15, 8–20 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Karasik, D. & Kiel, D. P. Evidence for pleiotropic factors in genetics of the musculoskeletal system. Bone.46, 1226–1237 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sharir, A., Stern, T., Rot, C., Shahar, R. & Zelzer, E. Muscle force regulates bone shaping for optimal load-bearing capacity during embryogenesis. Development138, 3247–3259 (2011). [DOI] [PubMed] [Google Scholar]
- 128.Nowlan, N. C. et al. Developing bones are differentially affected by compromised skeletal muscle formation. Bone.46, 1275–1285 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Girgis, C. M., Mokbel, N. & Digirolamo, D. J. Therapies for musculoskeletal disease: can we treat two birds with one stone? Curr. Osteoporos. Rep.12, 142–153 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Locquet, M., Beaudart, C., Durieux, N., Reginster, J. Y. & Bruyère, O. Relationship between the changes over time of bone mass and muscle health in children and adults: a systematic review and meta-analysis. BMC Musculoskelet. Disord.20, 429 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yoshimura, N. et al. Is osteoporosis a predictor for future sarcopenia or vice versa? Four-year observations between the second and third ROAD study surveys. Osteoporos. Int.28, 189–199 (2017). [DOI] [PubMed] [Google Scholar]
- 132.Hirschfeld, H. P., Kinsella, R. & Duque, G. Osteosarcopenia: where bone, muscle, and fat collide. Osteoporos. Int.28, 2781–2790 (2017). [DOI] [PubMed] [Google Scholar]
- 133.Kaji, H. Linkage between muscle and bone: common catabolic signals resulting in osteoporosis and sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care16, 272–277 (2013). [DOI] [PubMed] [Google Scholar]
- 134.Kawao, N. & Kaji, H. Interactions between muscle tissues and bone metabolism. J. Cell. Biochem.116, 687–695 (2015). [DOI] [PubMed] [Google Scholar]
- 135.Tagliaferri, C., Wittrant, Y., Davicco, M. J., Walrand, S. & Coxam, V. Muscle and bone, two interconnected tissues. Ageing Res. Rev.21, 55–70 (2015). [DOI] [PubMed] [Google Scholar]
- 136.Elkasrawy, M. N. & Hamrick, M. W. Myostatin (GDF-8) as a key factor linking muscle mass and bone structure. J. Musculoskelet. Neuronal Interact.10, 56–63 (2010). [PMC free article] [PubMed] [Google Scholar]
- 137.Pedersen, B. K. & Febbraio, M. A. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat. Rev. Endocrinol.8, 457–465 (2012). [DOI] [PubMed] [Google Scholar]
- 138.Cianferotti, L. & Brandi, M. L. Muscle-bone interactions: basic and clinical aspects. Endocrine45, 165–177 (2014). [DOI] [PubMed] [Google Scholar]
- 139.Ono, T., Hayashi, M., Sasaki, F. & Nakashima, T. RANKL biology: bone metabolism, the immune system, and beyond. Inflamm. Regen.40, 2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Boyce, B. F. & Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys.473, 139–146 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dufresne, S. S. et al. Muscle RANK is a key regulator of Ca2+ storage, SERCA activity, and function of fast-twitch skeletal muscles. Am. J. Physiol. Cell Physiol.310, C663–672 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bonnet, N., Bourgoin, L., Biver, E., Douni, E. & Ferrari, S. RANKL inhibition improves muscle strength and insulin sensitivity and restores bone mass. J. Clin. Invest.133, 3214-3223 (2023). [DOI] [PMC free article] [PubMed]
- 143.Marini, F., Giusti, F., Palmini, G. & Brandi, M. L. Role of Wnt signaling and sclerostin in bone and as therapeutic targets in skeletal disorders. Osteoporos. Int.34, 213–238 (2023). [DOI] [PubMed] [Google Scholar]
- 144.Karner, C. M. & Long, F. Wnt signaling and cellular metabolism in osteoblasts. Cell Mol. Life Sci.74, 1649–1657 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hu, L., Chen, W., Qian, A. & Li, Y. P. Wnt/β-catenin signaling components and mechanisms in bone formation, homeostasis, and disease. Bone Res.12, 39 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang, X. et al. CLIC5 promotes myoblast differentiation and skeletal muscle regeneration via the BGN-mediated canonical Wnt/β-catenin signaling pathway. Sci. Adv.10, eadq6795 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang, J. et al. GSK3 regulation Wnt/β-catenin signaling affects adipogenesis in bovine skeletal muscle fibro/adipogenic progenitors. Int. J. Biol. Macromol.275, 133639 (2024). [DOI] [PubMed] [Google Scholar]
- 148.Lin, W. et al. Wnt/β-catenin signaling pathway as an important mediator in muscle and bone crosstalk: a systematic review. J. Orthop. Transl.47, 63–73 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Karasik, D. & Kiel, D. P. Genetics of the musculoskeletal system: a pleiotropic approach. J. Bone Miner. Res.23, 788–802 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rosen, C. J. et al. Congenic mice with low serum IGF-I have increased body fat, reduced bone mineral density, and an altered osteoblast differentiation program. Bone35, 1046–1058 (2004). [DOI] [PubMed] [Google Scholar]
- 151.Steelman, C. A., Recknor, J. C., Nettleton, D. & Reecy, J. M. Transcriptional profiling of myostatin-knockout mice implicates Wnt signaling in postnatal skeletal muscle growth and hypertrophy. FASEB J.20, 580–582 (2006). [DOI] [PubMed] [Google Scholar]
- 152.Ferrari, S. L. et al. Polymorphisms in the low-density lipoprotein receptor-related protein 5 (LRP5) gene are associated with variation in vertebral bone mass, vertebral bone size, and stature in whites. Am. J. Hum. Genet.74, 866–875 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bischoff-Ferrari, H. A. et al. Vitamin D receptor expression in human muscle tissue decreases with age. J. Bone Miner. Res.19, 265–269 (2004). [DOI] [PubMed] [Google Scholar]
- 154.Uitterlinden, A. G. et al. The association between common vitamin D receptor gene variations and osteoporosis: a participant-level meta-analysis. Ann. Intern. Med.145, 255–264 (2006). [DOI] [PubMed] [Google Scholar]
- 155.Langlois, J. A. et al. Association between insulin-like growth factor I and bone mineral density in older women and men: the Framingham Heart Study. J. Clin. Endocrinol. Metab.83, 4257–4262 (1998). [DOI] [PubMed] [Google Scholar]
- 156.Kok, H. J. & Barton, E. R. Actions and interactions of IGF-I and MMPs during muscle regeneration. Semin Cell Dev. Biol.119, 11–22 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhao, Z., Yan, K., Guan, Q., Guo, Q. & Zhao, C. Mechanism and physical activities in bone-skeletal muscle crosstalk. Front. Endocrinol.14, 1287972 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yakar, S., Werner, H. & Rosen, C. J. Insulin-like growth factors: actions on the skeleton. J. Mol. Endocrinol.61, T115–t137 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bikle, D. D. et al. Role of IGF-I signaling in muscle bone interactions. Bone.80, 79–88 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kang, K. S. & Robling, A. G. New Insights into Wnt-Lrp5/6-β-Catenin Signaling in Mechanotransduction. Front. Endocrinol.5, 246 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gessler, L., Kurtek, C., Merholz, M., Jian, Y. & Hashemolhosseini, S. In adult skeletal muscles, the co-receptors of canonical Wnt signaling, Lrp5 and Lrp6, determine the distribution and size of fiber types, and structure and function of neuromuscular junctions. Cells11, 3968 (2022). [DOI] [PMC free article] [PubMed]
- 162.Zhou, H. et al. LRP5 regulates cardiomyocyte proliferation and neonatal heart regeneration by the AKT/P21 pathway. J. Cell Mol. Med.26, 2981–2994 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang, X. F., Ma, Z. H. & Teng, X. R. Isokinetic strength test of muscle strength and motor function in total knee arthroplasty. Orthop. Surg.12, 878–889 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Guo, Y. F. et al. Suggestion of GLYAT gene underlying variation of bone size and body lean mass as revealed by a bivariate genome-wide association study. Hum. Genet.132, 189–199 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Babu, J. M. et al. Sarcopenia as a risk factor for prosthetic infection after total hip or knee arthroplasty. J. Arthroplast.34, 116–122 (2019). [DOI] [PubMed] [Google Scholar]
- 166.Petrosyan, E., Fares, J., Lesniak, M. S., Koski, T. R. & El Tecle, N. E. Biological principles of adult degenerative scoliosis. Trends Mol. Med.29, 740–752 (2023). [DOI] [PubMed] [Google Scholar]
- 167.Eguchi, Y. et al. Pentosidine concentration is associated with degenerative lumbar scoliosis in older women: preliminary results. Eur. Spine J.27, 597–606 (2018). [DOI] [PubMed] [Google Scholar]
- 168.Eguchi, Y. et al. Analysis of skeletal muscle mass in women over 40 with degenerative lumbar scoliosis. Eur. Spine J.28, 1618–1625 (2019). [DOI] [PubMed] [Google Scholar]
- 169.Kim, H. et al. Asymmetry of the cross-sectional area of paravertebral and psoas muscle in patients with degenerative scoliosis. Eur. Spine J.22, 1332–1338 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Benedetti, M. G., Furlini, G., Zati, A. & Letizia Mauro, G. The effectiveness of physical exercise on bone density in osteoporotic patients. Biomed. Res. Int.2018, 4840531 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pasqualini, L. et al. Effects of a 3-month weight-bearing and resistance exercise training on circulating osteogenic cells and bone formation markers in postmenopausal women with low bone mass. Osteoporos. Int.30, 797–806 (2019). [DOI] [PubMed] [Google Scholar]
- 172.Sañudo, B., Reverte-Pagola, G., Seixas, A. & Masud, T. Whole-body vibration to improve physical function parameters in nursing home residents older than 80 years: a systematic review with meta-analysis. Phys. Ther.104, pzae025 (2024). [DOI] [PMC free article] [PubMed]
- 173.Reis-Silva, A. et al. Evidence of whole-body vibration exercises on body composition changes in older individuals: a systematic review and meta-analysis. Front. Physiol.14, 1202613 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Macdonald, H. M. et al. 25-hydroxyvitamin D threshold for the effects of vitamin D supplements on bone density: secondary analysis of a randomized controlled trial. J. Bone Min. Res.33, 1464–1469 (2018). [DOI] [PubMed] [Google Scholar]
- 175.Burt, L. A. et al. Effect of high-dose vitamin D supplementation on volumetric bone density and bone strength: a randomized clinical trial. JAMA322, 736–745 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Reid, I. R. & Bolland, M. J. Calcium and/or vitamin D supplementation for the prevention of fragility fractures: who needs it? Nutrients12, 1011 (2020). [DOI] [PMC free article] [PubMed]
- 177.Curtis, E., Litwic, A., Cooper, C. & Dennison, E. Determinants of muscle and bone aging. J. Cell. Physiol.230, 2618–2625 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Giustina, A., Mazziotti, G. & Canalis, E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr. Rev.29, 535–559 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rudman, D. et al. Effects of human growth hormone in men over 60 years old. N. Engl. J. Med.323, 1–6 (1990). [DOI] [PubMed] [Google Scholar]
- 180.Biermasz, N. R., Hamdy, N. A., Pereira, A. M., Romijn, J. A. & Roelfsema, F. Long-term skeletal effects of recombinant human growth hormone (rhGH) alone and rhGH combined with alendronate in GH-deficient adults: a seven-year follow-up study. Clin. Endocrinol.60, 568–575 (2004). [DOI] [PubMed] [Google Scholar]
- 181.Mohler, M. L. et al. Nonsteroidal selective androgen receptor modulators (SARMs): dissociating the anabolic and androgenic activities of the androgen receptor for therapeutic benefit. J. Med. Chem.52, 3597–3617 (2009). [DOI] [PubMed] [Google Scholar]
- 182.Meng, S. J. & Yu, L. J. Oxidative stress, molecular inflammation and sarcopenia. Int. J. Mol. Sci.11, 1509–1526 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Campbell, G. R. et al. Mitochondrial DNA deletions and depletion within paraspinal muscles. Neuropathol. Appl. Neurobiol.39, 377–389 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Eguchi, Y. et al. Advanced glycation end products are associated with sarcopenia in older women: aging marker dynamics. J. women aging33, 328–340 (2021). [DOI] [PubMed] [Google Scholar]
- 185.Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet392, 1789–1858 (2018). [DOI] [PMC free article] [PubMed]
- 186.Li, G. et al. Muscle-bone crosstalk and potential therapies for sarco-osteoporosis. J. Cell Biochem.120, 14262–14273 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bialek, P. et al. A myostatin and activin decoy receptor enhances bone formation in mice. Bone60, 162–171 (2014). [DOI] [PubMed] [Google Scholar]
- 188.Puolakkainen, T. et al. Treatment with soluble activin type IIB-receptor improves bone mass and strength in a mouse model of Duchenne muscular dystrophy. BMC Musculoskelet. Disord.18, 20 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bijlsma, J. W., Berenbaum, F. & Lafeber, F. P. Osteoarthritis: an update with relevance for clinical practice. Lancet377, 2115–2126 (2011). [DOI] [PubMed] [Google Scholar]
- 190.Litwic, A., Edwards, M. H., Dennison, E. M. & Cooper, C. Epidemiology and burden of osteoarthritis. Br. Med. Bull.105, 185–199 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Buckwalter, J. A. & Martin, J. A. Osteoarthritis. Adv. drug Deliv. Rev.58, 150–167 (2006). [DOI] [PubMed] [Google Scholar]
- 192.Katz, J. N., Arant, K. R. & Loeser, R. F. Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA325, 568–578 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Vincent, T. L. Mechanoflammation in osteoarthritis pathogenesis. Semin. Arthritis Rheumatism49, S36–s38 (2019). [DOI] [PubMed] [Google Scholar]
- 194.Roos, E. M., Herzog, W., Block, J. A. & Bennell, K. L. Muscle weakness, afferent sensory dysfunction and exercise in knee osteoarthritis. Nat. Rev. Rheumatol.7, 57–63 (2011). [DOI] [PubMed] [Google Scholar]
- 195.Pegreffi, F. et al. Prevalence of sarcopenia in knee osteoarthritis: a systematic review and meta-analysis. J. Clin. Med.12, 1532 (2023). [DOI] [PMC free article] [PubMed]
- 196.Peng, P. et al. Association between sarcopenia and osteoarthritis among the US adults: a cross-sectional study. Sci. Rep.14, 296 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Misra, D. et al. Risk of knee osteoarthritis with obesity, sarcopenic obesity, and sarcopenia. Arthritis Rheumatol.71, 232–237 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Suh, D. H. et al. Body composition is more closely related to the development of knee osteoarthritis in women than men: a cross-sectional study using the Fifth Korea National Health and Nutrition Examination Survey (KNHANES V-1, 2). Osteoarthr. Cartil.24, 605–611 (2016). [DOI] [PubMed] [Google Scholar]
- 199.Cunha, J. E. et al. Knee osteoarthritis induces atrophy and neuromuscular junction remodeling in the quadriceps and tibialis anterior muscles of rats. Sci. Rep.9, 6366 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Slemenda, C. et al. Quadriceps weakness and osteoarthritis of the knee. Ann. Intern. Med.127, 97–104 (1997). [DOI] [PubMed] [Google Scholar]
- 201.Terracciano, C. et al. Differential features of muscle fiber atrophy in osteoporosis and osteoarthritis. Osteoporos. Int.24, 1095–1100 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Messier, S. P. et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA.310, 1263–1273 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Liao, C. D. et al. Effects of protein-rich nutritional composition supplementation on sarcopenia indices and physical activity during resistance exercise training in older women with knee osteoarthritis. Nutrients13, 2487 (2021). [DOI] [PMC free article] [PubMed]
- 204.Ardeljan, A. D., Polisetty, T. S., Palmer, J., Vakharia, R. M. & Roche, M. W. Comparative analysis on the effects of sarcopenia following primary total knee arthroplasty: a retrospective matched-control analysis. J. knee Surg.35, 128–134 (2022). [DOI] [PubMed] [Google Scholar]
- 205.Tzartza, C. L. et al. Comparative analysis on the effect of sarcopenia in patients with knee osteoarthritis before and after total knee arthroplasty. Diseases11, 36 (2023). [DOI] [PMC free article] [PubMed]
- 206.Ho, K. K. et al. End-stage knee osteoarthritis with and without sarcopenia and the effect of knee arthroplasty - a prospective cohort study. BMC Geriatrics21, 2 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Diallo, T. D. et al. Associations of myosteatosis with disc degeneration: A 3T magnetic resonance imaging study in individuals with impaired glycaemia. J. Cachexia Sarcopenia Muscle14, 1249–1258 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Qi, W. et al. Causal associations between sarcopenia-related traits and intervertebral disc degeneration: a two-sample mendelian randomization analysis. Eur. Spine J.33, 2430–2438 (2024). [DOI] [PubMed] [Google Scholar]
- 209.Yuan, H. et al. A comparison of interferential current efficacy in elderly intervertebral disc degeneration patients with or without sarcopenia: a retrospective study. BMC Musculoskelet. Disord.25, 214 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yazici, A. & Yerlikaya, T. The relationship between the degeneration and asymmetry of the lumbar multifidus and erector spinae muscles in patients with lumbar disc herniation with and without root compression. J. Orthop. Surg. Res.17, 541 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Suo, M. et al. The association between morphological characteristics of paraspinal muscle and spinal disorders. Ann. Med.55, 2258922 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Jiang, J. et al. Multifidus degeneration, a new risk factor for lumbar spinal stenosis: a case-control study. World Neurosurg.99, 226–231 (2017). [DOI] [PubMed] [Google Scholar]
- 213.Zhao, W. P., Kawaguchi, Y., Matsui, H., Kanamori, M. & Kimura, T. Histochemistry and morphology of the multifidus muscle in lumbar disc herniation: comparative study between diseased and normal sides. Spine25, 2191–2199 (2000). [DOI] [PubMed] [Google Scholar]
- 214.Delisle, M. B., Laroche, M., Dupont, H., Rochaix, P. & Rumeau, J. L. Morphological analyses of paraspinal muscles: comparison of progressive lumbar kyphosis (camptocormia) and narrowing of lumbar canal by disc protrusions. Neuromuscul. Disord. NMD.3, 579–582 (1993). [DOI] [PubMed] [Google Scholar]
- 215.Chen, Y. Y., Pao, J. L., Liaw, C. K., Hsu, W. L. & Yang, R. S. Image changes of paraspinal muscles and clinical correlations in patients with unilateral lumbar spinal stenosis. Eur. Spine J.23, 999–1006 (2014). [DOI] [PubMed] [Google Scholar]
- 216.Shahidi, B. et al. Lumbar multifidus muscle degenerates in individuals with chronic degenerative lumbar spine pathology. J. Orthop. Res.35, 2700–2706 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Liu, C. et al. Is there a correlation between upper lumbar disc herniation and multifidus muscle degeneration? A retrospective study of MRI morphology. BMC Musculoskelet. Disord.22, 92 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Xu, G. et al. Development of a finite element full spine model with active muscles for quantitatively analyzing sarcopenia effects on lumbar load. Comput. Methods Prog. Biomed.240, 107709 (2023). [DOI] [PubMed] [Google Scholar]
- 219.Camino-Willhuber, G. et al. Association between lumbar intervertebral vacuum phenomenon severity and posterior paraspinal muscle atrophy in patients undergoing spine surgery. Eur. Spine J.33, 1013–1020 (2024). [DOI] [PubMed] [Google Scholar]
- 220.Stanuszek, A. et al. Preoperative paraspinal and psoas major muscle atrophy and paraspinal muscle fatty degeneration as factors influencing the results of surgical treatment of lumbar disc disease. Arch. Orthop. Trauma Surg.142, 1375–1384 (2022). [DOI] [PubMed] [Google Scholar]
- 221.Hey, H. W. D. et al. Paraspinal myopathy-induced intervertebral disc degeneration and thoracolumbar kyphosis in TSC1mKO mice model-a preliminary study. Spine J.22, 483–494 (2022). [DOI] [PubMed] [Google Scholar]
- 222.Hodges, P. W. et al. Multifidus muscle changes after back injury are characterized by structural remodeling of muscle, adipose and connective tissue, but not muscle atrophy: molecular and morphological evidence. Spine40, 1057–1071 (2015). [DOI] [PubMed] [Google Scholar]
- 223.Maas, H., Noort, W., Hodges, P. W. & van Dieën, J. Effects of intervertebral disc lesion and multifidus muscle resection on the structure of the lumbar intervertebral discs and paraspinal musculature of the rat. J. Biomech.70, 228–234 (2018). [DOI] [PubMed] [Google Scholar]
- 224.Hoppe, S. et al. 3D analysis of fatty infiltration of the paravertebral lumbar muscles using T2 images-a new approach. Eur. Spine J.30, 2570–2576 (2021). [DOI] [PubMed] [Google Scholar]
- 225.Agha, O. et al. Intervertebral disc herniation effects on multifidus muscle composition and resident stem cell populations. JOR Spine3, e1091 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Li, H. Z. et al. Role of signaling pathways in age-related orthopedic diseases: focus on the fibroblast growth factor family. Mil. Med. Res.11, 40 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.James, G., Chen, X., Diwan, A. & Hodges, P. W. Fat infiltration in the multifidus muscle is related to inflammatory cytokine expression in the muscle and epidural adipose tissue in individuals undergoing surgery for intervertebral disc herniation. Eur. Spine J.30, 837–845 (2021). [DOI] [PubMed] [Google Scholar]
- 228.Chen, X., Hodges, P. W., James, G. & Diwan, A. D. Do markers of inflammation and/or muscle regeneration in lumbar multifidus muscle and fat differ between individuals with good or poor outcome following microdiscectomy for lumbar disc herniation? Spine46, 678–686 (2021). [DOI] [PubMed] [Google Scholar]
- 229.Fitzgerald, J. A. & Newman, P. H. Degenerative spondylolisthesis. J. Bone Jt. Surg. Br. Vol.58, 184–192 (1976). [DOI] [PubMed] [Google Scholar]
- 230.Duan, P. G. et al. Is the Goutallier grade of multifidus fat infiltration associated with adjacent-segment degeneration after lumbar spinal fusion? J. Neurosurg. Spine34, 190–195 (2021). [DOI] [PubMed] [Google Scholar]
- 231.Lee, E. T. et al. Association of lumbar paraspinal muscle morphometry with degenerative spondylolisthesis. Int. J. Environ. Res. Public Health18, 4037 (2021). [DOI] [PMC free article] [PubMed]
- 232.Lee, H. J. et al. The relationship between cross sectional area and strength of back muscles in patients with chronic low back pain. Ann. Rehabilit. Med.36, 173–181 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Cao, B. et al. Correlation between fat infiltration of paraspinal muscle and L4 degenerative lumbar spondylolisthesis in asymptomatic adults. Asian J. Surg.46, 834–840 (2023). [DOI] [PubMed] [Google Scholar]
- 234.Correa-de-Araujo, R. et al. Myosteatosis in the context of skeletal muscle function deficit: an interdisciplinary workshop at the National Institute on Aging. Front. Physiol.11, 963 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Köhli, P. et al. The relationship between paraspinal muscle atrophy and degenerative lumbar spondylolisthesis at the L4/5 level. Spine J.24, 1396–1406 (2024). [DOI] [PubMed] [Google Scholar]
- 236.Thakar, S. et al. Lumbar paraspinal muscle morphometry and its correlations with demographic and radiological factors in adult isthmic spondylolisthesis: a retrospective review of 120 surgically managed cases. J. Neurosurg. Spine24, 679–685 (2016). [DOI] [PubMed] [Google Scholar]
- 237.McKenzie, J. C. et al. Sarcopenia does not affect clinical outcomes following lumbar fusion. J. Clin. Neurosci.64, 150–154 (2019). [DOI] [PubMed] [Google Scholar]
- 238.Ikeda, N. et al. Factors influencing slippage after microsurgical single level lumbar spinal decompression surgery - are the psoas and multifidus muscles involved? Acta Neurochirurgica166, 26 (2024). [DOI] [PubMed] [Google Scholar]
- 239.Ufuk, F., Herek, D. & Yüksel, D. Diagnosis of sarcopenia in head and neck computed tomography: cervical muscle mass as a strong indicator of sarcopenia. Clin. Exp. Otorhinolaryngol.12, 317–324 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Naghdi, N., Elliott, J. M., Weber, M. H., Fehlings, M. G. & Fortin, M. Morphological changes of deep extensor neck muscles in relation to the maximum level of cord compression and canal compromise in patients with degenerative cervical myelopathy. Glob. Spine J.14, 1184–1192 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ma, Y. et al. Study on the consistency between CT hounsfield units and MRI evaluation of preoperative cervical paraspinal muscular fat infiltration in patients undergoing ACDF. J. Orthop. Surg. Res.19, 435 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Pinter, Z. W. et al. Cervical paraspinal muscle fatty degeneration is not associated with muscle cross-sectional area: qualitative assessment is preferable for cervical sarcopenia. Clin. Orthop. Relat. Res.479, 726–732 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Hu, J. S., Jin, Y. P., Wu, J. K. & Ni, J. G. Skeletal muscle index based on CT at the 12th thoracic spine level can predict osteoporosis and fracture risk: a propensity score-matched cohort study. Front. Med.11, 1387807 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Chiu, C. M. et al. Dose-dependent association between sarcopenia and moderate-to-severe thoracic vertebral fragility fracture in older adults. Gerontology69, 533–540 (2023). [DOI] [PubMed] [Google Scholar]
- 245.Ignasiak, D., Rüeger, A., Sperr, R. & Ferguson, S. J. Thoracolumbar spine loading associated with kinematics of the young and the elderly during activities of daily living. J. Biomech.70, 175–184 (2018). [DOI] [PubMed] [Google Scholar]
- 246.Ignasiak, D., Valenzuela, W., Reyes, M. & Ferguson, S. J. The effect of muscle ageing and sarcopenia on spinal segmental loads. Eur. Spine J.27, 2650–2659 (2018). [DOI] [PubMed] [Google Scholar]