Abstract
Fukumi Fiber, a new six-rowed hull-less barley cultivar, has an extremely high β-glucan content; this is the world’s first cultivar with two genes (wax and amo1) boosting the content of β-glucan and one gene (ant28.2131) suppressing the browning reaction after cooking, to our knowledge. The β-glucan content of pearled barley is 13.2% in Fukumi Fiber, and is approximately three times higher than that of the standard barley cultivar Ichibanboshi and approximately two times higher than that of the waxy cultivars Daishimochi and Kirari-mochi. Fukumi Fiber has a standard plump grain percentage required for a six-rowed hull-less barley cultivars. The yield is over 10% higher than that of Ichibanboshi. Fukumi Fiber is suitable for cultivation in the plains of central and western Japan and was released in 2018. It can be used for cooked pearled barley and various purposes such as confectionery, noodles, and bread. The spread of this cultivar is expected to lead to a stable supply and the expansion of high-value-added domestic waxy barley.
Keywords: hull-less barley, food, β-glucan, waxy, polyphenol, proanthocyanidin-free, discoloration
Introduction
Barley grain is richer in 1-3,1-4 β-glucan (β-glucan), a water-soluble dietary fiber, than other grains such as rice and wheat. It suppresses elevated blood glucose levels (Yokoyama et al. 1997), reduces blood cholesterol (Ikegami et al. 1996, Oda et al. 1993), and improves immune function (Tada et al. 2008, Vetvicka et al. 1996). To label health claims on the products, food companies must prepare products that ensure a certain amount of β-glucan, and agricultural producers must produce barley raw materials that meet this requirement. Therefore, cultivars with high β-glucan content are important and in demand by consumers and producers.
Waxy barley has a sticky texture, a desirable trait for Japanese cooked pearled barley (Yanagisawa et al. 2011, Yoshikawa et al. 1998), and has higher β-glucan content than the normal barley (Ullrich et al. 1986). Barley has been used in Japanese food culture since ancient times, and various waxy cultivars have been selected and bred in Japan. Six-rowed hull-less cultivar Daishimochi, was bred by introducing low-amylose wax gene from waxy landrace Mochimugi D (Doi et al. 1999). The two-rowed hull-less cultivar Kirari-mochi is proanthocyanidin-free (ant28.494) as well as amylose-free with wax gene from Shikoku hadaka 97, and achieved higher β-glucan content and lower levels of discoloration of cooked pearled barley after incubation at higher temperatures than Ichibanboshi (a standard cultivar of hull-less barley) (Yanagisawa et al. 2011). β-glucan content became further higher by the six-rowed hulled cultivar Kihadamochi bred by introducing the amylose-free wax gene from Azhul, which has higher β-glucan content than other amylose-free cultivars such as Kirari-mochi (Tonooka et al. 2022). Incidentally, the alleles of the wax gene are known to be different among Mochimugi D, Shikoku hadaka 97, and Azhul (Hu et al. 2014). In these waxy cultivars Daishimochi, Kirari-mochi, and Kihadamochi, the β-glucan content of pearled barley is approximately 6%–8% (Doi et al. 1999, Tonooka et al. 2022, Yanagisawa et al. 2011). A much higher content (12.8%) of β-glucan was achieved by the two-rowed hulless cultivar Waxy Fiber, which has both lys5h and amylose-free (wax) (Yanagisawa et al. 2016). However, in the lys5h line, starch synthesis is considerably reduced because of the reduced ability of the amyloplast to incorporate the substrate ADP-glucose, which is the site of starch synthesis (Patron et al. 2004). Therefore, Waxy Fiber has shrunken grains, lower yields, poor plump grain percentage, and poor pearling quality (Yanagisawa et al. 2016). For such a reason, the cultivars bred and marketed to date have a β-glucan content of 6%–8%, and those with higher content have poor grain quality and low yields. Therefore, higher β-glucan cultivars that combine high yield, superior grain quality and stability are requisite.
To our knowledge, Fukumi Fiber is the world’s first cultivar having triple recessive alleles, amo1 (high amylose), wax, and ant28.2131. Amylose and β-glucan content are known to be high in the amo1 genotype (Fastnaught et al. 1996, Merritt 1967, Schondelmaier et al. 1992). Lines with both amo1 and wax have even higher β-glucan content (Fujita et al. 1999). The β-glucan content of pearled barley is 13.2% in Fukumi Fiber. It is approximately three times as much as the standard cultivar Ichibanboshi and approximately twice as much as Daishimochi and Kirari-mochi.
Fukumi Fiber has the proanthocyanidin-free allele ant28.2131, which is resistant to browning after cooking like Kirari-mochi. The average yield is over 10% higher than that of Ichibanboshi. Boosting the production of this cultivar is expected to lead to a stable supply and the expansion of high-value-added domestic waxy barley.
Materials and Methods
Breeding process
Fukumi Fiber was derived from a cross between Yon R Kei 3755 (amo1, wax) and Yon Kei 9814 (ant28.2131), and selected by pedigree method of breeding (Fig. 1). The amo1 phenotype was identified by its high grain hardness. It was later verified using the starch synthase IIIa (ssIIIa) marker which was tightly linked to amo1 locus (Li et al. 2011). Yon R Kei 3755 was crossed with Yon Kei 9814 as a male parent in 2009. Twenty-eight F1 plants were grown in a greenhouse, and 467 F2 plants were grown in the field in 2009. Sixteen F3 individuals with proanthocyanidin-free, waxy and high level of grain hardness were selected in 2011. Yield trials were initiated at the Western Region Agricultural Research Center, National Agriculture and Food Research Organization (WARC/NARO) in 2011. Local adaptability tests were conducted at two different stations in 2014. Performance tests for recommended cultivars were conducted at a total of eight different prefectural stations from 2016 to 2017, with some locations tested in multiple years. Fukumi Fiber was released in Japan in 2018.
Fig. 1.
Pedigree of Fukumi Fiber.
Plant material for yield trials
All the cultivars were sown in late November at Zentsuji, Kagawa, and grown using standard yield trial methods at WARC/NARO (Table 1). Ichibanboshi (Ito et al. 1995) was a standard six-rowed, uzu-type, hull-less cultivar in Japan. Daishimochi (Doi et al. 1999), Kirari-mochi (Yanagisawa et al. 2011), and Waxy Fiber (Yanagisawa et al. 2016) were used as reference cultivars of waxy barley. The percentage of grains greater than 2.2 mm for the two-row barley and 2.0 mm for the six-row barley was used as the plump grain percentage. Harvested grains had no sprouting damage.
Table 1. Agronomic characteristics of Fukumi Fiber (Means of 2012–2017 growing years).
| Cultivar | Row type | Semi-dwarf type | Waxy type | amo1 type | Proantho-cyanidin-free type | Degree of spring habita | Heading date | Maturity date | Culm length (cm) | Spike length (cm) | Number of spikes (/m2) | Grain yield | Volume weight (g/l) | 1000-grain weight (g) | Plump grain (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (kg/a) | (%) | |||||||||||||||
| Fukumi Fiber | six | uzu | amylose-free | amo1 | ant28.2131 | V | 5-Apr ab | 18-May a | 84 a | 5.6 b | 354 a | 56.2 a | 114 | 798 b | 32.8 bc | 95.9 b |
| Ichibanboshi | six | uzu | – | – | – | V | 4-Apr a | 15-May a | 82 a | 5.2 b | 333 a | 49.4 a | 100 | 827 a | 35.0 b | 99.2 a |
| Daishimochi | six | uzu | Low-amylose | – | – | V | 6-Apr a | 17-May a | 78 a | 4.6 a | 370 a | 44.4 a | 90 | 827 a | 31.4 c | 99.4 a |
| Kirari-mochi | two | –c | amylose-free | – | ant28.494 | I | 4-Apr a | 17-May a | 75 a | 7.0 c | 412 a | 47.2 a | 96 | 821 ab | 41.4 a | 98.8 a |
Basel dressing N–P–K = 0.6–0.6–0.6 kg/a; top dressing N–K = 0.3–0.3 kg/a. The plot design was 4.95 m2 with two replications.
a –: I: Spring type; V: Winter type.
b The same letters in the same column are not significantly different (P < 0.05), as revealed by Tukey’s test.
c –: Normal type.
PCR amplification and restriction enzyme digestion of the ssIIIa gene
To identify ssIIIa variations, we performed Cleaved Amplified Polymorphic Sequence (CAPS) analysis (Li et al. 2011). PCR was performed using total DNA from leaves or seeds on a TaKaRa PCR Thermal Cycler Dice (Takara TP600), using the specific primer sets SSIIIa-P5F and SSIIIa-P5R designed by Li et al. (2011).
Measurement of β-glucan content and dietary fiber
Total β-glucan content in pearled barley was analyzed according to McCleary and Glennie-Holmes (1985) using the β-Glucan assay kit (Megazyme Ltd., Ireland) and expressed on a dry weight basis. Dietary fiber content was determined using the modified Prosky method (Enzymatic - LC method) by Japan Food Research Laboratories.
Measurement of pearling quality parameters
The grain hardness index (HI) of 100 whole grains was measured using the single-kernel characterization system 4100 (SKCS, Perten Instruments). The pearling time was measured as the time required to grind 200 g of grains to obtain a 60% pearl yield using the TM-05 grain-testing mill (Satake CO.). The pearling machine of the same type was replaced in 2015, therefore the pearling time values differ between the 2012–2014 and 2015–2021 growing years. In addition, pearled barley was autoclaved (105°C × 10 min), steamed for 40 min while reducing pressure, and measured the hue color immediately after cooking using a spectrophotometer (Minolta CM-3500d). After measurement, the cooked pearled barley was incubated at 70°C for 18 h and measured the hue color in the same manner. Discoloration values reflected the change in pearled barley color after incubation.
Results
Agronomic characteristics
The agronomic characteristics of Fukumi Fiber are shown in Table 1. Fukumi Fiber has a slightly longer culm, slightly longer spike, and more spikes than Ichibanboshi, though the difference is statistically insignificant (Table 1). Fukumi Fiber headed one day after Ichibanboshi and matured three days later. The thousand-grain weight was lower than that of Ichibanboshi but higher than that of Daishimochi, although the difference was statistically insignificant. The plump grain percentage was above 95%, although it was lower than that of Ichibanboshi. The yield was higher by 13.7%, 26.7%, and 19.0% compared with Ichibanboshi, Dishimochi, and Kirari-mochi, respectively.
Fukumi Fiber was resistant to Barley yellow mosaic virus (BaYMV) and Japanese soil-borne wheat mosaic virus (JSBWMV), and its resistance to powdery mildew and scab was moderate, the same as that of Ichibanboshi (Table 2). Fukumi Fiber was more susceptible to pre-harvest sprouting than Ichibanboshi and Daishimochi but was similar to Kirari-mochi, which is also proanthocyanidin-free.
Table 2. Evaluation data of resistance and tolerance.
| Cultivar | Disease resistance | Lodging resistance (culm breaking)e | Pre-harvest sprouting tolerance | Qsd1 typeg | Qsd2 typeh | ||||
|---|---|---|---|---|---|---|---|---|---|
| BaYMV (types I–III)a | JSBWMVb | Powdery mildewc | Scabd | Judgmente | Germination rate (%)f | ||||
| Fukumi Fiber | R | R | M | M | R | S | 73.5 ai | D | D |
| Ichibanboshi | R | R | M | M | MR | R | 5.8 b | D | D |
| Daishimochi | Mj | n.tl | MSj | Mj | R | R | 5.8 b | n.t. | n.t. |
| Kirari-mochi | RRk | n.t. | RRk | MRk | R | S | 80.8 a | D | D |
RR: highly resistant, R: resistant, MR: moderately resistant, M: moderate, MS: moderately susceptible, S: susceptible, SS: highly susceptible.
a Estimated in 2014–2017 growing years, tested in Tsukuba and Tsukubamirai, Ibaraki; Otsu, Kumamoto; and Tochigi, Tochigi.
b Estimated in 2015–2016 growing years, tested in Tsukubamirai, Ibaraki.
c Estimated in 2014, 2016, and 2017 growing years, tested in Tsukuba, Ibaraki.
d Estimated in 2014–2017 growing years, tested in Tsukuba, Ibaraki; Tikugo and Tikushino, Fukuoka.
e Estimated in 2012–2017 growing years, tested in Zuntsuji, Kagawa.
f Means of 2012–2017 growing years. Germination ratio of 100 seeds of 18℃ on the 7th day of incubation, tested in Zuntsuji, Kagawa.
g Determined using a marker based on the SNP in Exon 9 reported by Sato et al. (2016), with marker primers dCAPS-QSD1E9-TaqI-F: CCGTACAATCTTAGCGAGGATGGTGATTGGGGGCTTGAGATTTTGGAAGT and dCAPS-QSD1E9-TaqI-R: CTTTCCGACAAAATTCTACTATCTCCTCCT and restriction enzyme TaqI. D: dormancy allele.
h Determined using a marker based on the SNP in Exon 7 reported by Nakamura et al. (2016), with marker primers CAPS-QSD2E7-2733RsaI-F: TGCATGAGGCAAGACACCTA and CAPS-QSD2E7-3158RsaI-R: TCACCTGCAGCATGAGATTG and restriction enzyme RsaI. D: dormancy allele.
i The same letters in the same column are not significantly different (P < 0.05), as revealed by Tukey’s test.
l n.t.: Not tested.
The amo1 genotype was first estimated by grain hardness and later verified by PCR analysis of the ssIIIa marker (Fig. 2). Genetic mapping studies have indicated that ssIIIa is very tightly linked to the amo1 locus (Li et al. 2011). Lines with amo1 can be selected using the ssIIIa marker (Li et al. 2011). Glacier AC38 is a mutant with a high amylose (amo1) gene derived from Glacier (Merritt 1967). Experimental line A (EL-A) with the wild-type Wax allele (normal amylose) and amo1 genes was derived from the Ichibanboshi/Yon R Kei 3102 (amo1) cross. When examined using CAPS analysis of the ssIIIa marker, the cultivars with wild-type alleles of ssIIIa, Ichibanboshi, Daishimochi, and Kirari-mochi, showed a fragment of 464 bp. In contrast, the cultivars/lines with amo1, Fukumi Fiber, EL-A, and GlacierAC38 showed DNA fragments of 303 and 161 bp, respectively. The starch granules of Fukumi Fiber were smaller and more cracked than those of Ichibanboshi (Fig. 3).
Fig. 2.

CAPS analysis of ssIIIa marker to estimate amo1 genotype. M; molecular marker, Lane A; Fukumi Fiber (amo1), Lane B; EL-A (amo1), Lane C; Glacier AC38 (amo1), Lane D; Ichibanboshi, Lane E; Daishimochi, Lane F; Kirari-mochi.
Fig. 3.
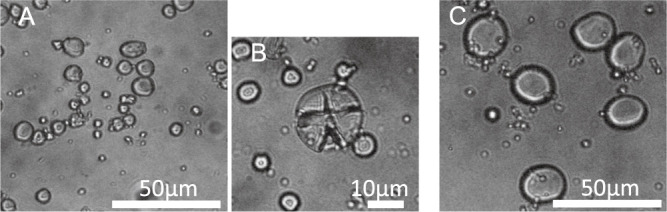
Small and cracked starch granules of Fukumi Fiber. A and B; Fukumi Fiber, C; Ichibanboshi. Images of starch granules were obtained with a Carl Zeiss Axiophot optical microscope at magnifications of 20 (A, C) and 40 (B).
Pearling characteristics and β-glucan contents
Quality parameters, β-glucan content, and dietary fiber of pearled barley are listed in Table 3. Fukumi Fiber had a higher SKCS HI and a longer milling time than other cultivars. The broken kernel rate of Fukumi Fiber was significantly lower than that of Ichibanboshi, and was also lower than that of Daishimochi and Kirari-mochi, although the difference was not significant. The β-glucan content of the pearled barley of Fukumi Fiber is approximately three times higher than that of Ichibanboshi and approximately two times higher than that of Daishimochi and Kirari-mochi. The soluble and insoluble fiber contents of pearled barley in Fukumi Fiber were higher than in other cultivars.
Table 3. Pearling quality parameters of Fukumi Fiber.
| Cultivar | SKCS Hardness indexa | Pearling timea (s) | Whiteness of pearled graina (%) | Broken kernel ratea (%) | β-glucanb (%) | Dietary fiberc | Cooked pearled barleyb | Discoloration of the cooked pearled barley after incubatingb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soluble | Insoluble | Total | L* | a* | b* | L* | a* | b* | ||||||||
| Fukumi Fiber | 103 ad | 1149 a | 41.1 b | 0.7 b | 13.2 a | 10.6 | 10.1 | 20.7 | 74.4 a | 1.3 ab | 17.6 a | –2.9 a | +0.9 b | –0.7 b | ||
| Ichibanboshi | 51 c | 422 b | 45.3 a | 28.1 a | 4.3 c | 3.9 | 4.4 | 8.3 | 74.4 a | 1.1 ab | 11.6 b | –7.7 b | +3.8 a | +3.2 a | ||
| Daishimochi | 71 b | 635 b | 44.1 ab | 4.1 b | 6.3 b | n.t.e | n.t. | n.t. | 71.4 a | 1.7 a | 12.3 b | –8.1 b | +3.7 a | +3.5 a | ||
| Kirari-mochi | 68 b | 652 b | 45.9 a | 6.7 b | 6.6 b | 6.7 | 2.9 | 9.5 | 73.8 a | 0.8 b | 16.3 a | –2.0 a | +1.0 b | –0.0 b | ||
Basel dressing N–P–K = 0.6–0.6–0.6 kg/a; top dressing N–K = 0.3–0.3 kg/a. The plot design was 4.95 m2 with two replications.
a Means of 2012–2017 growing years.
b Means of 2013–2017 growing years.
c Grew in 2014.
d The same letters in the same column are not significantly different (P < 0.05), as revealed by Tukey’s test.
The color parameters of the cooked pearled barley of Fukumi Fiber are shown in Table 3. The L* value of cooked pearled Fukumi Fiber grains was as high as that of Ichibanboshi and Kirari-mochi. Discoloration in L* and a* of cooked pearled barley after incubation at 70°C was suppressed in Fukumi Fiber compared to those in Ichibanboshi and Daishimochi. Like Kirari-mochi, Fukumi Fiber pearled barley hardly turned brown even after cooking and incubation at 70°C (Fig. 4).
Fig. 4.

Cooked pearled barley incubated at 70°C for 18 h. A; Fukumi Fiber, B; Kirari-mochi, C; Ichibanboshi, D; Daishimochi.
Comparison between Fukumi Fiber and other high β-glucan cultivar
The agronomic characteristics and pearling quality parameters are listed in Tables 4 and 5. Fukumi Fiber had the highest β-glucan content of the pearled barley among the cultivars examined, being similar to Waxy Fiber with both lys5h and wax. The yield of Fukumi Fiber was 32.1% higher than that of Waxy Fiber. The percentage of plump grain of Fukumi Fiber was not significantly different from that of the standard cultivar, but that of Waxy Fiber with lys5h was significantly lower, with the shrunk grains (Figs. 5, 6, Table 4). Fukumi Fiber has less seed plumpness than Ichibanboshi, but more than Waxy Fiber (Fig. 6). Fukumi Fiber had the highest SKCS HI among the cultivars examined; it also had a longer pearling time, and fewer broken kernels than the other cultivars. The L* values of cooked pearled barley were higher than those of Waxy Fiber. The discoloration evaluated by changes in L* and a* of cooked pearled barley was limited in Fukumi Fiber compared to those in Waxy Fiber.
Table 4. Agronomic characteristics of high β-glucan cultivars/lines (Means of 2019–2021 growing years).
| Cultivar | Heading date | Maturity date | Culm length (cm) | Spike length (cm) | Number of spikes (/m2) | Grain yield | Volume weight (g/l) | 1000-grain weight (g) | Plump grain (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| (kg/a) | (%) | |||||||||
| Fukumi Fiber | 27-Mar aa | 13-May a | 92 a | 5.4 b | 517 a | 68.6 a | 111 | 796 b | 31.4 c | 94.1 a |
| Waxy Fiber | 25-Mar a | 8-May a | 85 a | 7.4 a | 568 a | 51.9 a | 84 | 747 c | 35.6 b | 51.0 b |
| Ichibanboshi | 26-Mar a | 8-May a | 86 a | 5.1 b | 511 a | 61.6 a | 100 | 828 ab | 33.8 bc | 99.3 a |
| Daishimochi | 29-Mar a | 12-May a | 84 a | 4.4 b | 465 a | 64.2 a | 104 | 838 a | 32.2 c | 99.6 a |
| Kirari-mochi | 25-Mar a | 12-May a | 79 a | 6.9 a | 634 a | 71.3 a | 116 | 813 ab | 40.1 a | 98.6 a |
Basel dressing N–P–K = 0.6–0.6–0.6 kg/a; top dressing N–K = 0.3–0.3 kg/a. The plot design was 3.15 m2 with two replications.
a The same letters in the same column are not significantly different (P < 0.05), as revealed by Tukey’s test.
Table 5. Pearling quality parameters of high β-glucan cultivars/lines (Means of 2019–2021 growing years).
| Cultivar | Row type | Semi-dwarf type | Waxy type | amo1 type | lys5h type | Proantho-cyanidin-free type | SKCS Hardness index | Pearling time (s) | Whiteness of pearled grain (%) | Broken kernel rate (%) | β-glucan (%) | Cooked pearled barley | Discoloration of the cooked pearled barley after incubating | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | |||||||||||||
| Fukumi Fiber | six | uzu | amylose-free | amo1 | – | ant28.2131 | 110 aa | 732 a | 35.0 c | 0.4 b | 15.1 a | 74.1 b | 1.4 c | 19.7 a | –3.7 a | +0.8 b | –1.2 b | |
| Waxy Fiber | two | –b | amylose-free | – | lys5h | – | 84 b | 523 b | 35.8 bc | 1.0 b | 14.3 a | 67.6 c | 2.8 a | 18.5 a | –9.6 b | +3.8 a | +0.6 ab | |
| Ichibanboshi | six | uzu | – | – | – | – | 56 c | 302 c | 42.5 a | 24.1 a | 4.3 c | 76.7 a | 1.0 c | 12.0 b | –7.4 b | +3.4 a | +2.3 a | |
| Daishimochi | six | uzu | Low-amylose | – | – | – | 79 b | 473 b | 40.6 ab | 2.5 b | 6.8 b | 71.5 b | 2.1 b | 14.0 b | –7.9 b | +3.3 a | +2.3 a | |
| Kirari-mochi | two | – | amylose-free | – | – | ant28.494 | 72 b | 426 bc | 41.7 a | 5.4 b | 6.8 b | 72.8 b | 1.1 c | 17.5 a | –3.0 a | +0.8 b | –1.1 b | |
Basel dressing N–P–K = 0.6–0.6–0.6 kg/a; top dressing N–K = 0.3–0.3 kg/a. The plot design was 3.15 m2 with two replications.
a The same letters in the same column are not significantly different (P < 0.05), as revealed by Tukey’s test.
b –: Normal type.
Fig. 5.

Grains. Fukumi Fiber grains do not have a shrunken surface unlike Waxy Fiber. From left, Fukumi Fiber, Ichibanboshi, and Waxy Fiber.
Fig. 6.

Cross section of grains. Grain plumpness of Fukumi Fiber is intermediate between Ichibanboshi and Waxy Fiber with lys5h. From above, Fukumi Fiber, Ichibanboshi, and Waxy Fiber.
Discussion
Agronomic characteristics
Previous studies have shown that two genes wax and amo1 interact and further increase the β-glucan content (Fujita et al. 1999). Grains of lines with wax and amo1 do not shrink unlike Waxy Fiber, which is a high β-glucan cultivar having wax and lys5h. However, the grain weight is often inferior due to low starch content and poor filling in the lines with wax and amo1. Of such lines, Yon kei 9519 has an inferior yield and a low percentage of plump grain (Tonooka et al. 2018). Fukumi Fiber is a cultivar that has been selected to address these shortcomings. Its amo1 genotype was verified using DNA marker analysis (Fig. 2) and cracks of starch granules (Fig. 3). The starch from the amo1 endosperm has a high amylose content and exhibits a prominent degree of cracking at the surface of the granules (Boren et al. 2008). This cultivar has no major drawbacks in yield and grain quality compared to the standard barley cultivars (Tables 1–5). The yield of Fukumi Fiber was over 10% higher than that of Ichibanboshi (Tables 1, 4). Although there was no significant difference in yield-related traits, Fukumi Fiber has a slightly longer culm, slightly longer spike, and more spikes than Ichibanboshi, suggesting its higher yield potential. The plump grain percentage of Fukumi Fiber was above 94% (Tables 1, 4) and met the Grading Standards for Agricultural Products. It was lower than that of the standard cultivar Ichibanboshi, but higher than that of the high-β-glucan-content cultivar Waxy Fiber. Fukumi Fiber has a grain-filling period 2–4 days longer than Ichibanboshi and 2–3 days longer than Daishimochi (Tables 1, 4). Because of the long grain-filling period, it is considered that the grains were full even though the rate of starch synthesis was slow. If ultra-high β-glucan and plump grains are required, lengthening the grain-filling period and increasing photosynthetic capacity are conceivable.
Quality characteristics
Fukumi Fiber had the highest β-glucan content of the pearled barley among the cultivars examined; it also had the highest SKCS HI. Henry and Cowe (1990) reported a positive relationship between the β-glucan content and grain hardness. We examined the correlation between β-glucan content and pearling characteristics in the cultivars tested. For the cultivars shown in Table 3, the correlation coefficients between β-glucan content and SKCS HI and pearling time were 0.986 and 0.997, respectively, both statistically significant at the 5% and 1% levels. In contrast, the correlation with the broken kernel rate was not significant but showed a coefficient of –0.703. Furthermore, no correlations were significant for the cultivars shown in Table 5. However, the coefficients for SKCS HI, pearling time, and broken kernel rate were relatively high at 0.869, 0.877, and –0.706, respectively. Therefore, it suggested that the higher β-glucan content of Fukumi Fiber resulted in a higher SKCS HI and longer pearling time.
The browning of cooked pearled barley grains is significantly correlated with the content of flavanol, which is the major factor for the browning (Kohyama et al. 2010). The main flavanols quantified in the analyzed barley varieties were catechin, two dimers (procyanidin B3 and prodelphinidin B3), and four trimers (Holtekjølen et al. 2006). Fukumi Fiber is proanthocyanidin-free, resulting in a brighter color and less browning of cooked pearled barley. Proanthocyanidin-free cultivars such as Kirari-mochi show poor tolerance to pre-harvest sprouting (Nagamine et al. 2006, Yanagisawa et al. 2011). Himi et al. (2012) reported that ant28 encodes Hvmyb10, an R2R3 MYB domain protein that regulates proanthocyanidin accumulation in developing grains. The ant28-494 allele contains a nucleotide change predicted to create a stop codon, while the ant28-2131 allele has a change which likely leads to the loss of five amino acids (Himi et al. 2012). The germination rate was slightly lower in Fukumi Fiber with ant28-2131 than in Kirari-mochi with ant28-494; however, no significant difference was observed (Table 2). In addition, genes such as Qsd1 and Qsd2 are known to be involved in pre-harvest sprouting tolerance (Nakamura et al. 2016, Sato et al. 2016). Taira et al. (2021) suggested that the improvement in pre-harvest sprouting tolerance of Shirayuri Nijo, compared to Shiratae Nijo, in ant28-494 can be attributed to the dormancy-type variation of Qsd1 and the introduction of the genetic background of Haruka Nijo, which has strong dormancy. Fukumi Fiber had the dormancy-type Qsd1 and Qsd2 alleles (Table 2). Therefore, further improvement of pre-harvest sprouting tolerance in Fukumi Fiber may require the introduction of an ant gene with less impact on pre-harvest sprouting than ant28 or introduction of novel sprouting tolerance factors that are distinct from Qsd1 and Qsd2.
Fukumi Fiber is already grown commercially in western Japan and is used in cooked pearled barley and other products. Fukumi Fiber, with its extremely high β-glucan and good cooked pearled barley, has the potential to be used for conventional cooked pearled barley with rice, as well as for various other applications, such as for bread, noodles, cereal, and confectionery when ground into barley flour. The spread of this cultivar is expected to lead to a stable supply and the expansion of high-value-added domestic waxy barley.
Author Contribution Statement
A.T., T.Yanagisawa and T.N. designed this study, and A.T., T.Yanagisawa, T.Yoshioka, T.N. and T.S. were involved in the breeding process, and A.T. and T.S. performed DNA marker identification, and A.T. wrote the paper.
Acknowledgments
The authors thank the supporting staff at WARC/NARO. We sincerely appreciate the valuable support from the relevant breeding stations in conducting the performance tests for recommended cultivars, local adaptability tests, and disease resistance tests. We also extend our gratitude to H. Aoki (CARC/NARO, currently Hokkaido Agricultural Research Center, National Agriculture and Food Research Organization: HARC/NARO) for developing the Qsd1 marker sequence and conducting genotype identification using the Qsd1 and Qsd2 markers. This work was partially supported by a grant from the Ministry of Agriculture, Forestry, and Fisheries.
Literature Cited
- Boren, M., Glaring M.A., Ghebremedhin H., Olsson H., Blennow A. and Jansson C. (2008) Molecular and physicochemical characterization of the high-amylose barley mutant Amo1. J Cereal Sci 47: 79–89. [Google Scholar]
- Doi, Y., Ito M., Fujita M., Domon E., Ishikawa N., Katayama T. and Kamio M. (1999) Breeding of a new naked barley waxy cultivar “Daishimochi”. Bulletin of the Shikoku National Agricultural Experiment Station 64: 21–36 (in Japanese with English summary). [Google Scholar]
- Fastnaught, C.E., Berglund P.T., Holm E.T. and Fox G.T. (1996) Genetic and environmental variation in β-glucan content and quality parameters of barley for food. Crop Sci 36: 941–946. [Google Scholar]
- Fujita, M., Domon E. and Doi Y. (1999) Grain and starch characteristics of the double recessive lines for amylose-free and high amylose gene in barley. Breed Sci 49: 217–219 (in Japanese with English summary). [Google Scholar]
- Henry, R.J. and Cowe I.A. (1990) Factors influencing the hardness (milling energy) and malting quality of barley. J Inst Brew 96: 135–136. [Google Scholar]
- Himi, E., Yamashita Y., Haruyama N., Yanagisawa T., Maekawa M. and Taketa S. (2012) Ant28 gene for proanthocyanidin synthesis encoding the R2R3 MYB domain protein (Hvmyb10) highly affects grain dormancy in barley. Euphytica 188: 141–151. [Google Scholar]
- Holtekjølen, A.K., Kinitz C. and Knutsen S.H. (2006) Flavanol and bound phenolic acid contents in different barley varieties. J Agric Food Chem 54: 2253–2260. [DOI] [PubMed] [Google Scholar]
- Hu, G., Burton C.S. and Hong Z. (2014) Molecular and chemical characterization of a new waxy allele in barley (Hordeum vulgare L.). Cereal Chem 91: 438–444. [Google Scholar]
- Ikegami, S., Tomita M., Honda S., Yamaguchi M., Mizukawa R., Suzuki Y., Ishii K., Ohsawa S., Kiyooka N., Higuchi M.et al. (1996) Effect of boiled barley-rice-feeding in hypercholesterolemic and normolipemic subjects. Plant Foods Human Nutr 49: 317–328. [DOI] [PubMed] [Google Scholar]
- Ito, M., Ishikawa N., Domon E., Doi Y., Katayama T., Kamio T., Kato M., Yoshikawa R. and Tsutsumi T. (1995) ‘Ichibanboshi’. A newly released naked barley cultivar. Bulletin of the Shikoku National Agricultural Experiment Station 59: 109–121 (in Japanese with English summary). [Google Scholar]
- Kohyama, N., Nagamine T. and Murata M. (2010) Relationship between browning and changes in flavanols of cooked barley after incubation. Nippon Shokuhin Kagaku Kogaku Kaishi 57: 372–379 (in Japanese with English summary). [Google Scholar]
- Li, Z., Li D., Du X., Wang H., Larroque O., Jenkins C.L.D., Jobling S.A. and Morell M.K. (2011) The barley amo1 locus is tightly linked to the starch synthase IIIa gene and negatively regulates expression of granule-bound starch synthetic genes. J Exp Bot 62: 5217–5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleary, B.V. and Glennie-Holmes M. (1985) Enzymatic quantification of (1-3, 1-4)-β-D-glucan in barley and malt. J Inst Brew 91: 285–295. [Google Scholar]
- Merritt, N.R. (1967) A new strain of barley with starch of high amylose content. J Inst Brew 73: 583–585. [Google Scholar]
- Nagamine, T., Yamaguchi E., Ozeki M., Sekiwa T., Watanabe N., Watanabe H., Ohno K., Kumekawa N., Mochizuki T., Kawada N.et al. (2006) Quality and agronomic characteristics of promised malting barley lines with proanthocyanidin-free allele. Bulletin of the Tochigi Agricultural Experiment Station 58: 79–86 (in Japanese with English summary). [Google Scholar]
- Nakamura, S., Pourkheirandish M., Morishige H., Kubo Y., Nakamura M., Ichimura K., Seo S., Kanamori H., Wu J., Ando T.et al. (2016) Mitogen-activated protein kinase kinase 3 regulates seed dormancy in barley. Curr Biol 26: 775–781. [DOI] [PubMed] [Google Scholar]
- Oda, T., Aoe S., Sanada H. and Ayano Y. (1993) Effects of soluble and insoluble fiber preparations isolated from oat, barley, and wheat on liver cholesterol accumulation in cholesterol-fed rats. J Nutr Sci Vitanimol (Tokyo) 39: 73–79. [DOI] [PubMed] [Google Scholar]
- Patron, N.J., Greber B., Fahy B.F., Laurie D.A., Parker M.L. and Denyer K. (2004) The lys5 mutations of barley reveal the nature and importance of plastidial ADP-Glc transporters for starch synthesis in cereal endosperm. Plant Physiol 135: 2088–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, K., Yamane M., Yamaji N., Kanamori H., Tagiri A., Schwerdt J.G., Fincher G.B., Matsumoto T., Takeda K. and Komatsuda T. (2016) Alanine aminotransferase controls seed dormancy in barley. Nat Commun 7: 11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schondelmaier, J., Jacobi A., Fischbeck G. and Jahoor A. (1992) Genetical studies on the mode of inheritance and localization of the amo1 (high amylose) gene in barley. Plant Breed 109: 274–280. [Google Scholar]
- Tada, R., Adachi Y., Ishibashi K., Tsubaki K. and Ohno N. (2008) Binding capacity of a barley β-d-glucan to the β-glucan recognition molecule dectin-1. J Agric Food Chem 56: 1442–1450. [DOI] [PubMed] [Google Scholar]
- Taira, M., Yanaka M., Nakamura K., Sakai T., Matsunaka H., Sugita T., Tonooka T., Nishio Z., Kawada N., Araki H.et al. (2021) “Shirayuri Nijo”, a new two-rowed hulled barley cultivar for food with proanthocyanidin-free gene, moderate tolerance to pre-harvest sprouting, and high yield. Breed Res 23: 109–115 (in Japanese with English summary). [Google Scholar]
- Tonooka, T., Kawada N. and Araki H. (2018) Screening and evaluation of genetic resources containing high level of β-glucan for breeding of barley cultivars for functional foods. Breed Res 20: 144–150 (in Japanese with English summary). [Google Scholar]
- Tonooka, T., Yanagisawa T., Aoki E., Taira M. and Yoshioka T. (2022) Breeding of a new six-rowed waxy barley cultivar “Kihadamochi” exhibiting high levels of yield and β-glucan content. Breed Res 24: 146–152 (in Japanese with English summary). [Google Scholar]
- Ullrich, S.E., Clamcy J.A., Eslick R.F. and Lance R.C.M. (1986) β-glucan content and viscosity of extracts from waxy barley. J Cereal Sci 4: 279–285. [Google Scholar]
- Vetvicka, V., Thornton B.P. and Ross G.D. (1996) Soluble beta-glucan polysaccharide binding to the lectin site of neutrophil or natural killer cell complement receptor type 3 (CD11b/CD18) generates a primed state of the receptor capable of mediating cytotoxicity of iC3b-opsonized target cells. J Clin Invest 98: 50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa, T., Nagamine T., Takahashi A., Takayama T., Doi Y., Matsunaka H. and Fujita M. (2011) Breeding of Kirari-mochi: A new two-rowed waxy hull-less barley cultivar with superior quality characteristics. Breed Sci 61: 307–310. [Google Scholar]
- Yanagisawa, T., Aoki E. and Taira M. (2016) Breeding of a new waxy barley cultivar ‘Waxy Fiber’ with high beta-glucan content. Beibaku Kairyo (Rice, Wheat and Barley Improvement), 2016, 9–12 (in Japanese). [Google Scholar]
- Yokoyama, W.H., Hudson C.A., Knuckles B.E., Chiu M.M., Sayre R.N., Turnlund J.R. and Schneeman B.O. (1997) Effect of barley β-glucan in durum wheat pasta on human glycemic response. Cereal Chem 74: 293–296. [Google Scholar]
- Yoshikawa, R., Nakamura K., Hatta K. and Nakamura H. (1998) Varietal differences of pearling characteristics, amylose content, gelatinization, properties and boiling characteristics in barley. Tohoku Agricultural Reserch 51: 97–98 (in Japanese with English summary). [Google Scholar]



