Abstract
Purpose
Huge (≥10 cm) hepatocellular carcinoma (HCC) poses significant treatment and prognosis challenges. This study aimed to determine whether preoperative transarterial chemoembolization (TACE) for huge HCC is necessary.
Methods
This single-center, retrospective cohort study evaluated 435 patients with huge HCC who underwent upfront hepatectomy or hepatectomy after preoperative TACE from January 2009 to December 2018. TACE’s impact on survival and prognostic factors, including microvascular invasion (MVI) and satellite nodules (SNs), was analyzed.
Results
The preoperative TACE group (n = 33) had a lower incidence of MVI (P = 0.009) and higher postoperative morbidity (P = 0.001), particularly pleural effusion (P = 0.004) and Clavien-Dindo class III–IV complications (P = 0.033), compared with the upfront hepatectomy group (n = 402). Short-term mortality (P = 0.828) and recurrence within 6 months (P = 0.654) were comparable between groups. The 1-, 3-, and 5-year survival curves showed no significant between-group differences in recurrence-free survival (RFS) (P = 0.172) and overall survival (OS) (P = 0.450). Local regional therapy for intrahepatic recurrences and surgical resection for extrahepatic recurrences were associated with better OS. MVI, SN, and hepatic vein tumor thrombosis were identified as significant risk factors for poorer RFS and OS. In patients without SN, preoperative TACE improved RFS (P = 0.039) but not OS.
Conclusion
Preoperative TACE for huge HCC was associated with reduced MVI but did not improve RFS and OS. Survival outcomes were more significantly influenced by SN, suggesting that upfront hepatectomy without TACE should be prioritized.
Keywords: Hepatectomy, Hepatocellular carcinoma, Microvascular invasion, Satellite nodule, Transarterial chemoembolization
INTRODUCTION
Hepatocellular carcinoma (HCC) accounts for 75%–85% of primary liver cancer, which is the sixth most commonly diagnosed cancer and the third leading cause of cancer-related death [1]. Huge HCCs (≥ 10 cm) have a poor prognosis, with early recurrence and distant metastasis, due to a higher risk of major vascular invasion, microvascular invasion (MVI), and satellite nodules (SNs) [2,3,4].
According to the Barcelona Clinic Liver Cancer (BCLC) guidelines, intermediate (BCLC B) patients are typically recommended for transarterial chemoembolization (TACE) [5]. However, a recent meta-analysis has demonstrated that surgical resection offers better survival compared with TACE alone in patients with huge HCC [6]. In addition, recent studies suggest that hepatectomy after preoperative TACE for huge HCC significantly contributes to improved survival rates [7]. However, some reports showed that preoperative TACE significantly prolonged operative time due to more challenging dissections involving inflammatory pedicles and perihepatic adhesion and showed no association between preoperative TACE and improvement of survival outcomes [8,9,10,11]. As a result, debate concerning the feasibility of preoperative TACE is still ongoing.
This study aimed to compare the perioperative and survival outcomes of hepatectomy for huge HCC according to preoperative TACE and analyze prognostic factors related to the survival outcomes.
METHODS
Ethics statement
The Institutional Review Board of Asan Medical Center (No. S2024-1423-0001) approved the study protocol and waived the need for informed consent due to the retrospective study design.
Study design and patient selection
This retrospective cohort study was conducted at a single center and included patients with Child-Pugh class A or B liver function diagnosed with huge HCC (≥ 10 cm) who underwent hepatectomy between January 2009 and December 2018. No patient had distant metastasis at the time of diagnosis, and all were confirmed as having a single HCC pathologically. The exclusion criteria were patients who (1) were pathologically diagnosed with primary liver cancer other than HCC, (2) had other organ cancer during pre- or postoperative follow-up, (3) underwent non-curative resection, and (4) underwent liver transplantation during the follow-up period (Fig. 1). Preoperative imaging evaluations were reviewed, and if there was a strong indication of extrahepatic (EH) metastasis on initial imaging that corresponded with the recurrence site on postoperative follow-up imaging, the case was classified as a non-curative resection. Patients’ medical records were retrospectively reviewed.
Fig. 1. Flowchart of the patient selection process. HCC, hepatocellular carcinoma; LN, lymph node; TACE, transarterial chemoembolization.
Preoperative evaluation, procedure, and follow-up protocol
Preoperative evaluations included dynamic abdomen and pelvic CT, chest CT, liver MRI, bone scans, or PET CT, as well as upper gastrointestinal endoscopy, liver function tests, hepatitis B and C serology, α-FP level measurement, and Child-Pugh classification to assess surgical readiness. The extent of hepatic resection was primarily based on future remnant volume, while also considering a tumor-free margin. If the future remnant volume appeared insufficient, the ratio of the future remnant liver to the total functional liver volume, excluding the tumor volume, was calculated using CT volumetry. Preoperative TACE was performed in cases of patients’ refusal or personal reasons for delaying surgery, or prolonged preoperative evaluation due to comorbidities.
The surgical procedure has been described in detail in a previous publication [4]. Patients in the upfront hepatectomy group had not previously received neoadjuvant therapy. Anatomical liver resection was performed according to standard anatomical liver resection techniques, ensuring negative margins (R0 resection). Partial hepatectomy was performed as a nonanatomical resection because huge HCCs usually occupy more than 1 hepatic segment. Portal vein tumor thrombosis (PVTT), portal vein invasion (Vp), was classified into 4 stages based on the classification system established by the Japanese Liver Cancer Study Group [12]. For cases with Vp3–4, hepatic vein tumor thrombus (HVTT) and inferior vena cava (IVC) tumor thrombosis were combined, a tumor thrombectomy was performed, and the affected vessel wall was resected and reconstructed when necessary.
For patients who underwent TACE prior to hepatectomy, the procedure involved lipiodol and a cisplatin dose of 2 mg/kg. A catheter was used to infuse a 1:1 emulsion of lipiodol (up to 20 mL) and cisplatin into the feeding artery. This was followed by embolization with gelfoam until arterial flow stasis was achieved at the terminal feeding artery level. All huge HCCs were treated in a single TACE session. Initial follow-up examinations (laboratory tests and CT) were performed 1 month after TACE.
Patients were followed up 1 month after surgery, every 3 months during the first year, and then every 6 months thereafter, with the follow-up period extending beyond 60 months until December 2023. Follow-up was based on dynamic abdomen and pelvic CT, and tumor markers, such as α-FP, were also monitored. When HCC recurrence was suspected, liver MRI was performed as needed, and additional imaging studies, such as chest CT, bone scanning, and PET-CT, were also conducted to assess the extent of HCC recurrence.
Outcomes
The perioperative and short-term outcomes included postoperative morbidity, in-hospital mortality, and recurrence and mortality within 6 months. The postoperative morbidities were classified by Clavien-Dindo (CD) classification, and post-hepatectomy liver failure (PHLF) was defined according to the definition and grading system by the International Study Group of Liver Surgery [13,14]. In-hospital mortality was defined as death during the hospital stay for hepatectomy for huge HCC.
During the follow-up period, when the first recurrence with or without an increase in serum α-FP levels was suspected, thorough imaging studies were conducted. Based on the initial recurrence site, cases were classified as intrahepatic (IH) if confined to the liver, EH if present outside the liver, and simultaneous IH and EH if detected in both the liver and other regions. We considered the pattern of recurrence, residual liver function, and performance status for treatment decisions. The patients with IH initial recurrence were primarily managed with TACE. For patients who did not respond adequately to repeat TACE, alternative locoregional treatment (LRT), such as radiofrequency ablation, radiotherapy, and percutaneous ethanol injection, was administered. Surgical resection was performed when feasible, considering the patient’s condition. Systemic chemotherapy was added or substituted when complete remission was not achieved with LRT, or when the lesions were multiple. Overall survival (OS) and recurrence-free survival (RFS) were defined as the time from surgery to death or recurrence during the follow-up period, respectively.
Statistical analysis
For continuous variables, parametric tests (Student t-test) were used when data followed a normal distribution, and nonparametric tests (Mann-Whitney U-test) were applied when the normality assumption was violated. Data with a normal distribution are presented as the mean and standard deviation, whereas data without a normal distribution are reported as the median and interquartile range. Categorical variables were compared using the chi-square or Fisher exact test when expected frequencies were low. Survival analysis was performed using the Kaplan-Meier method, and comparisons between groups were made using the log-rank test. Multivariate analysis to identify risk factors was conducted using Cox proportional hazards models, adjusted for confounding variables. The hazard ratio (HR) and 95% confidence interval (CI) for each risk factor were calculated in the model. All statistical analyses were performed using IBM SPSS Statistics ver. 25.0 (IBM Corp.), with a P-value of <0.05 considered statistically significant.
RESULTS
Baseline characteristics and pathological findings
A total of 435 patients were included, with 402 in the upfront hepatectomy group and 33 in the preoperative TACE group. The baseline characteristics and pathological findings of these 2 groups are summarized in Table 1. The preoperative TACE group was significantly older than the upfront group (P = 0.031), with lower preoperative total bilirubin levels (P = 0.046) and a lower incidence of MVI (P = 0.009).
Table 1. Baseline characteristics and pathological findings of patients.
Values are presented as number only, mean ± standard deviation, or number (%).
TACE, transarterial chemoembolization; NBNC, non-hepatitis B and non-hepatitis C; INR, international normalized ratio; ES grade, Edmondson-Steiner differentiation grade.
a)Thrombosis involving the right, left, or middle hepatic vein, or inferior vena cava. b)Thrombosis involving the first-order branch (Vp3) or main portal vein (Vp4). c)Lymph node dissection was conducted on imaging-suspected metastatic nodes in hepatoduodenal and portocaval levels. d)Indicating involved resection margin with cancer cells, not abutting.
*P < 0.05, statistically significant.
Perioperative and short-term outcomes
A comparison of the perioperative and short-term outcomes between the 2 groups is shown in Table 2. Right hemihepatectomy w ith or w ithout c audate lobectomy (n = 199, 45.7%) was the most common anatomical resection. Combined resection of an adjacent organ was performed only in 11 patients in the upfront hepatectomy group (2.7%) with suggestive HCC direct invasion. Specifically, combined resections included the diaphragm (n = 5), right adrenal gland (n = 3), lung (n = 1), stomach (n = 1), and transverse colon (n = 1). Of these patients, all (n = 8) pathologically showed positive HCC direct invasion, except 2 with diaphragm involvement and 1 with right adrenal gland involvement.
Table 2. Perioperative and short-term outcomes of patients.
Values are presented as mean (range), mean ± standard deivation, or number (%).
TACE, transarterial chemoembolization; PVTT, portal vein tumor thrombectomy; Vp, portal vein invasion; IVC, inferior vena cava; NA, not available; CD class, Clavien-Dindo classification; PHLF, post-hepatectomy liver failure; HCC, hepatocellular carcinoma.
a)Due to local invasion to adjacent organs (transverse colon, stomach, IVC, right adrenal gland, lung, diaphragm). b)Bile leakage, bile duct stricture, and common bile duct injury requiring endoscopic, radiologic, or surgical interventions
*P < 0.05, statistically significant.
IVC tumor thrombectomy was significantly more frequent in the preoperative TACE group. However, in the upfront hepatectomy group, thrombectomy involved opening the pericardium with IVC-right atrium clamping due to HVTT extending to the supradiaphragmatic level of the IVC. In contrast, in the preoperative TACE group, IVC tumor thrombectomy was performed for tumor thrombus extending to the infradiaphragmatic level of the IVC, and the thrombus was removed through the hepatic vein stump after hepatectomy.
TACE-related complications included bleeding due to tumor rupture and abscess due to tumor necrosis, which were managed with embolization and percutaneous drainage for 66 and 40 days from preoperative TACE to hepatectomy, respectively. The preoperative TACE group had significantly higher postoperative morbidity (P = 0.001), particularly an increased incidence of pleural effusion (P = 0.004) and a higher rate of CD class III–IV complications (P = 0.033). None of the patients in the preoperative TACE group died during the hospital stay, and 3 cases of in-hospital mortality occurred in the upfront hepatectomy group. Of these patients, 1 underwent right trisectionectomy and 1 underwent central bisectionectomy; both underwent thrombectomy for HVTT extended to the IVC above the supradiaphragmatic level. These patients died on postoperative days 3 and 39 due to heart failure and cardiac tamponade, respectively. The remaining 1 patient died from PHLF at 2.6 months postoperatively. Most cases of mortality within 6 months (n = 29, 6.7%) were attributed to early HCC recurrence (n = 24, 82.7%). HCC recurrence within 6 months (n = 154, 35.4%) developed in IH (n = 93, 60.3%), EH (n = 37, 24.0%), and simultaneous IH and EH (n = 24, 15.6%) sites. There were no statistically significant differences in the incidence of mortality and recurrence within 6 months between t he 2 g roups (P = 0.828 and P = 0.654, respectively).
Survival outcomes according to preoperative transarterial chemoembolization
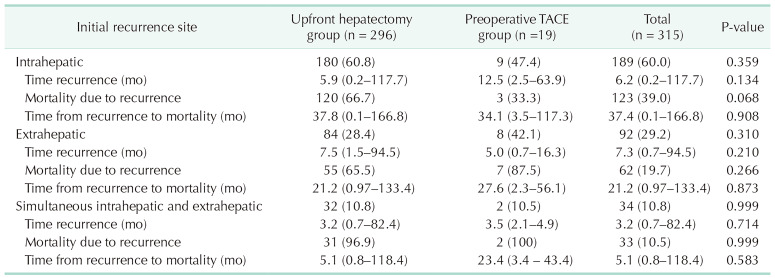
When comparing the 2 groups by each initial recurrence site, shown in Table 3, there were no significant differences in the time to first recurrence after surgery, recurrence-related mortality, or the time from first recurrence to death. The distribution of initial recurrence sites across the 2 groups is shown in Supplementary Fig. 1. The median follow-up time of the entire cohort was 57.9 months (range, 0.1–172.8 months). There were no significant differences in HCC recurrence rates between the upfront hepatectomy group (n = 296, 73.6%) and the preoperative TACE group (n = 19, 57.6%) (P = 0.075), or i n mortality rates (n = 217 [ 54.0%] a nd n = 13 [ 39.4%], respectively) (P = 0.152). The 1-, 3-, and 5-year rates of R FS and OS for the entire cohort were 47.6%, 31.7%, and 26.9% and 82.5%, 64.6%, and 55.5%, with a median survival time (MST) of 10.1 and 77.3 months, respectively. The 1-, 3-, and 5-year RFS rates in the upfront hepatectomy group were 46.8%, 31.7%, and 25.9%, respectively, compared with 57.6%, 40.7%, and 40.7% in the preoperative TACE group, with an MST of 9.4 and 18.9 months, respectively. The RFS curves between the 2 groups showed no significant difference (P = 0.172) (Fig. 2A). The 1-, 3-, and 5-year OS rates of the upfront hepatectomy group were 82.6%, 64.6%, and 55.5%, respectively, compared with 78.0%, 74.9%, and 61.3% in the preoperative TACE group. The MST of the upfront hepatectomy group was 76.0 months, whereas the MST was not reached for the preoperative TACE group within the study period. The OS curves between the 2 groups were not significantly different (P = 0.450) (Fig. 2B).
Table 3. The clinical patterns of initial hepatocellular carcinoma recurrence.
Values are presented as number (%) or median (range).
TACE, transarterial chemoembolization.
Fig. 2. Survival outcomes of all patients with huge hepatocellular carcinoma. Recurrence-free survival (A) and overall survival (B) according to preoperative transarterial chemoembolization (TACE).
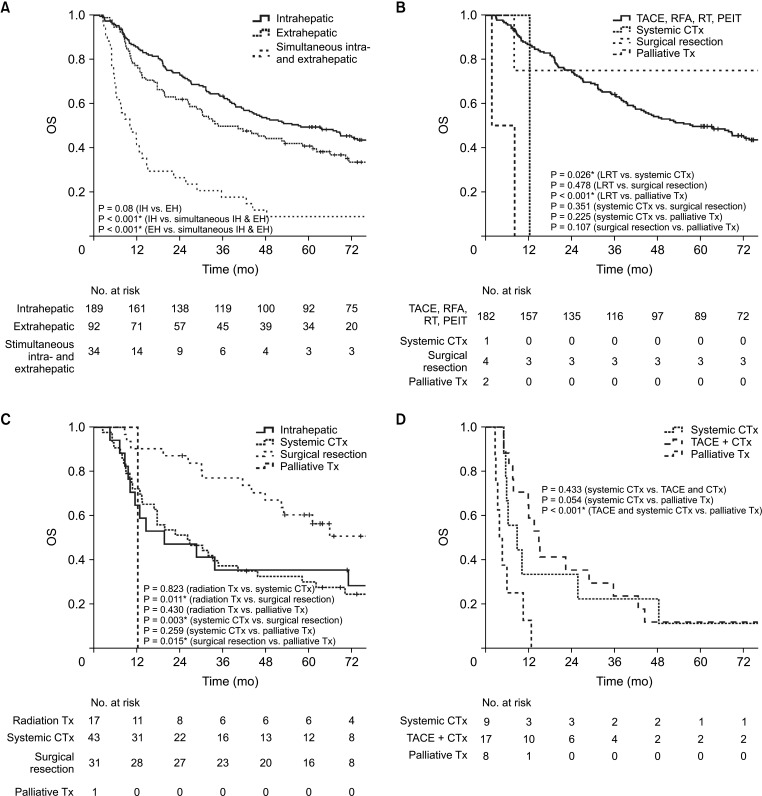
Survival outcomes after hepatocellular carcinoma recurrence
The initial recurrence pattern and the corresponding treatments for each group were shown in Supplementary Table 1. The 1-, 3-, 5-year OS rates of entire patients who experienced HCC recurrence during the follow-up period were 96.5%, 54.4%, and 42.5%, respectively. The OS of patients with IH or EH initial recurrences was significantly higher than that of patients with simultaneous IH and EH initial recurrence (P < 0.001) (Fig. 3A). The OS analysis of patients with IH showed that LRT was superior to systemic chemotherapy and palliative treatment (P = 0.026 and P < 0.001, respectively) (Fig. 3B). For patients with EH, surgical resection for HCC recurrence was associated with significantly higher OS rates than radiotherapy, chemotherapy, or palliative treatment (P = 0.011, P = 0.003, and P = 0.015, respectively) (Fig. 3C). For patients with simultaneous IH and EH, TACE with chemotherapy was associated with significantly better OS than palliative treatment (P < 0.001) (Fig. 3D).
Fig. 3. Survival outcomes of patients with recurrent hepatocellular carcinoma (HCC). (A) Overall survival (OS) of patients with HCC recurrence according to the initial recurrence site. (B) OS of patients with intrahepatic initial HCC recurrence according to management. (C) OS of patients with extrahepatic initial HCC recurrence according to management. (D) OS of patients with simultaneous intra- and extrahepatic initial HCC recurrence according to management. TACE, transarterial chemoembolization; RFA, radiofrequency ablation; RT, radiotherapy; PEIT, percutaneous ethanol injection therapy; CTx, chemotherapy; Tx, treatment. *P < 0.05, statistically significant.
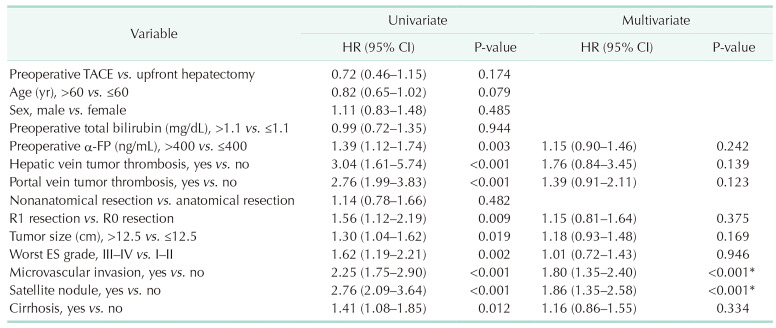
Prognostic factors for recurrence-free survival and overall survival
Univariate and multivariate analyses identified significant prognostic factors for RFS, as shown in Table 4. MVI (HR, 1.80; 95% CI, 1.35–2.40; P < 0.001) and SN (HR, 1.86; 95% CI, 1.35–2.58; P < 0.001) were significant unfavorable factors for RFS. Univariate and multivariate analyses also identified significant prognostic factors for OS, as shown in Table 5. HVTT (HR, 3.88; 95% CI, 2.10–7.16; P < 0.001), MVI (HR, 1.99; 95% CI, 1.39–2.85; P < 0.001), and SN (HR, 1.9; 95% CI, 1.35–2.58; P < 0.001) were significant unfavorable factors for OS. However, preoperative TACE was not a significant prognostic factor for RFS or OS.
Table 4. Univariate and multivariate analyses of prognostic factors for recurrence-free survival.
HR, hazard ratio; CI, confidence interval; TACE, transarterial chemoembolization; ES grade, Edmondson-Steiner differentiation grade.
*P < 0.05, statistically significant.
Table 5. Univariate and multivariate analyses of prognostic factors for overall survival.
HR, hazard ratio; CI, confidence interval; HR, hazard ratio; TACE, transarterial chemoembolization; ES grade, Edmondson-Steiner differentiation grade.
*P < 0.05, statistically significant.
To identify the factors associated with SN, logistic regression analysis was performed, revealing that PVTT (odds ratio [OR], 5.15; 95% CI, 2.57–10.42; P < 0.001) and MVI (OR, 6.02; 95% CI, 2.49–18.02; P < 0.001) were independently associated with the presence of SN (Supplementary Table 2).
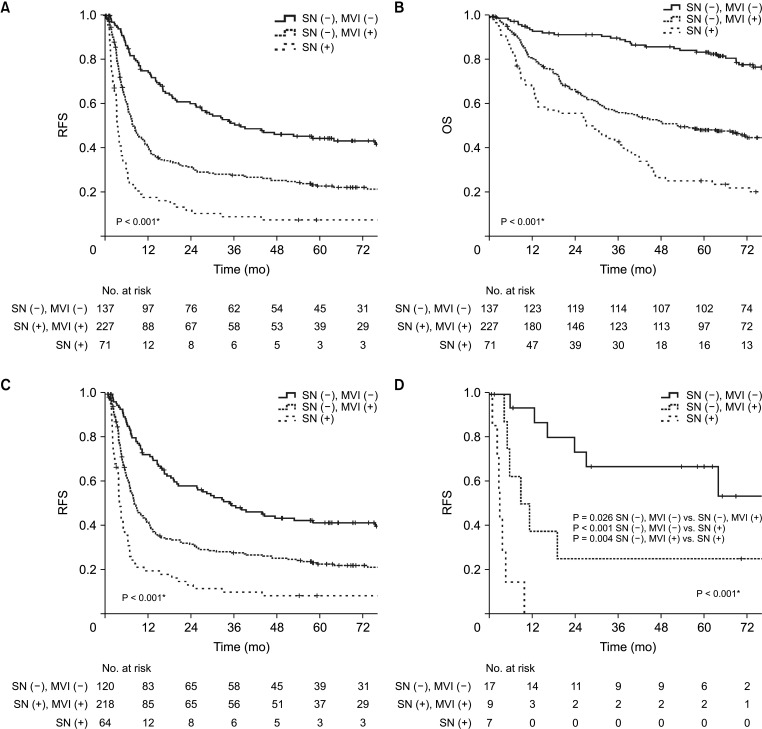
Subgroup analysis of recurrence-free survival based on satellite nodule and microvascular invasion presence
To better understand the prognostic impact of SN and MVI, subgroup analyses of RFS were conducted according to these factors. However, due to the extremely small number of patients with SN (+) and MVI (−) (n = 4 [0.9%] in the upfront hepatectomy group and n = 1 [3.0%] in the preoperative TACE group), meaningful survival analysis for this subgroup was not feasible. Therefore, patients were stratified into 3 groups—SN (−) and MVI (−), SN (−) and MVI (+), and SN (+). The patients with SN (−) and MVI (−) had the most favorable RFS, showing significantly higher survival rates compared with the other groups (Fig. 4A). Specifically, the RFS rate was significantly lower for patients who had either SN or MVI, or both. Similar to RFS, the patients with SN (−) and MVI (−) had the most favorable OS (Fig. 4B). In the upfront hepatectomy group, RFS was markedly reduced in patients with SN (+), with survival curves showing a steep decline within the first 12 months post-hepatectomy (Fig. 4C). Similarly, the preoperative TACE group showed that patients with SN (+) had a substantially lower RFS (Fig. 4D).
Fig. 4. Subgroup analysis of survival outcomes by satellite nodules (SN) and microvascular invasion (MVI). (A) Recurrence-free survival (RFS) of the entire cohort. (B) Overall survival (OS) of the entire cohort. (C) RFS of the upfront hepatectomy group. (D) RFS of the preoperative transarterial chemoembolization group. *P < 0.05, statistically significant.
Survival outcomes of patients without satellite nodules according to preoperative transarterial chemoembolization
To evaluate the effect of preoperative TACE in the absence of SN, survival outcomes were analyzed in patients without SN. Among these patients, those in the preoperative TACE group demonstrated significantly better RFS compared with the upfront hepatectomy group (P = 0.039) (Fig. 5A). Among patients without SN, the 1-, 3-, and 5-year RFS rates for the upfront hepatectomy group were 52.0%, 35.3%, and 29.3%, respectively, compared with 74.6%, 52.6%, and 52.6% in the preoperative TACE group. The median RFS times were 12.7 and 63.9 months, respectively. However, there was no significant difference in the OS rate between the 2 groups (P = 0.127) (Fig. 5B).
Fig. 5. Survival outcomes of patients without satellite nodules (SN) according to preoperative transarterial chemoembolization (TACE). (A) Recurrence-free survival of patients without SN according to preoperative TACE. (B) Overall survival of patients without SN according to preoperative TACE. *P < 0.05, statistically significant.
DISCUSSION
In this retrospective study involving a cohort of 435 patients with huge HCC, preoperative TACE for huge HCC was found to be significantly associated with increased postoperative morbidity and a lower incidence of MVI. However, preoperative TACE did not show a significant difference in postoperative RFS and OS when compared with upfront hepatectomy. Additionally, favorable survival outcomes for huge HCC were strongly associated with the absence of MVI and SN.
The time to surgery after TACE was significantly prolonged due to the treatment protocol, with additional delays caused by complications, such as hemorrhage and abscess formation. These findings underscore a potential limitation of preoperative TACE, as extended intervals between TACE and surgery may affect patient outcomes and complicate the overall treatment strategy. Additionally, there was a higher incidence of postoperative morbidity associated with preoperative TACE, primarily due to an increased occurrence of pleural effusion. This complication necessitated the insertion of percutaneous drains, resulting in a higher proportion of CD class III–IV complications. While pleural effusion can also occur after hepatectomy, it is more likely to occur after TACE due to chemical injury resulting from the migration of embolic agents, such as lipiodol, to the lung vasculature [15]. Additionally, embolization of the inferior phrenic artery may cause pleural irritation, further increasing the risk of pleural complications [15].
Preoperative TACE for HCC has been considered to enhance the detection of latent IH metastasis, reduce tumor size to increase resectability, and ultimately improve postoperative RFS and OS [16,17]. However, the effectiveness of preoperative TACE in reducing recurrence and extending survival remains controversial [11,18,19]. Yang et al. [19] concluded that preoperative TACE for resectable huge HCC should be avoided due to its failure to achieve complete necrosis, leading to surgical delays and complicating the treatment of recurrent lesions, without offering any clear benefits. A previous study also found that even when preoperative TACE achieved ≥90% necrosis, it did not correlate with reduced recurrence or improved survival [20]. Similarly, a randomized controlled trial for large HCC (mean size approximately 9 cm) reported that preoperative TACE did not improve survival outcomes and, in some cases, led to patients becoming ineligible for surgery due to disease progression or liver failure [11]. These outcomes may be due to tumor necrosis promoting metastasis by reducing adhesive strength and TACE primarily targeting well-differentiated HCC while sparing poorly differentiated tumors [21,22]. In addition, if incomplete tumor necrosis occurs, the remaining viable tumor cells are less firmly attached and thus more likely to be dislodged into the bloodstream during surgical manipulation [23]. In the current study, complete necrosis was achieved in only 9% of cases, and some cases of huge HCC required combined resection due to invasion into adjacent organs and bile ducts. Therefore, it is impractical to delay surgery with the expectation of achieving complete necrosis through TACE. Given these limitations, we suggest that, rather than relying on TACE, upfront hepatectomy should be prioritized as the initial treatment strategy for huge HCC.
Our study found that MVI was significantly lower in the preoperative TACE group. Considering MVI is a crucial factor in the prognosis of patients with HCC, those with MVI generally have poorer survival outcomes than those without MVI [24]. Despite the significant reduction in MVI rates, there was no corresponding improvement in RFS. This suggests that while preoperative TACE may lower MVI, it does not necessarily lead to enhanced RFS. Although the reduction of MVI can positively impact prognosis, other risk factors, such as SN, may exert a greater influence on RFS in cases of huge HCC. Our findings indicated that SN, apart from MVI, was another significant risk factor for RFS, with no significant difference in SN frequency between the groups. This suggests that while TACE effectively targets the primary tumor and reduces MVI, it may have limited efficacy in inducing necrosis in SN. Consequently, residual SN may have contributed more significantly to recurrence and metastasis, potentially offsetting the positive effects of TACE on survival outcomes.
Interestingly, the present study found that cases in which SN was present without MVI were extremely rare in both the upfront hepatectomy and preoperative TACE groups, with 93% of SN-positive cases also exhibiting MVI. This strong co-occurrence suggests that MVI plays a critical role in the metastatic process leading to SN formation. SNs are believed to result from tumor cell invasion into the portal venous system, which is more commonly observed in larger HCCs, especially in huge HCC [25,26,27]. Additionally, severe MVI, defined as invasion into more than 5 vessels or more than 50 tumor cells, has been significantly associated with SN, further underscoring its prognostic importance [28]. These findings highlight the strong association between SN and MVI, suggesting that MVI is not only closely linked to SN but may also play a pivotal role in the mechanism of SN formation.
Following these observations, we analyzed survival outcomes based on the presence of SN and MVI to further understand their prognostic implications. Across the entire cohort, our results demonstrated that patients without SN and MVI had significantly better RFS and OS compared with those with either or both. This trend was also consistent in both the upfront hepatectomy and preoperative TACE groups. Specifically, the presence of SN was associated with markedly poorer survival outcomes, demonstrating that SN are an independent prognostic factor playing a critical role in determining recurrence and survival. This finding is consistent with that of previous reports, indicating that SNs contribute to a more aggressive tumor phenotype, potentially facilitating local and distant spread, which may lead to earlier and more frequent recurrence [4,29]. This could explain why, despite the reduction of MVI through preoperative TACE, significant improvements in survival outcomes were still not observed; the presence of SN independently contributes to poor prognosis. Furthermore, the steep decline observed in RFS curves compared to the more gradual decline in OS curves suggests that, even in the presence of SN or MVI, aggressive treatment of recurrence can still provide an OS benefit. This indicates that while SN and MVI are strong predictors of recurrence, their impact on OS can be mitigated through timely and effective management of recurrence. For IH recurrences, LRT is recommended where feasible, whereas aggressive surgical resection should be considered for EH recurrences to potentially improve OS.
Interestingly, in the current study, for patients with huge HCC without SN, preoperative TACE was associated with significantly better RFS. This improvement may be attributed to the lower incidence of MVI. While there is an association between reduced MVI and preoperative TACE, it is important to note that a definite causal relationship has not been established. Nonetheless, these findings are consistent with those of previous studies. Zhou et al. [16] reported that preoperative TACE was effective in reducing MVI rates compared to liver resection alone, likely due to its potent cytotoxic effects and the tumor necrosis induced by blocking tumor vessels prior to surgery. Similarly, Li et al. [7] suggested that the reduced incidence of MVI in the TACE group was due to the effects of TACE. In a recent nationwide propensity score matching study by Hong et al. [30], approximately 30% of huge HCC cases were reported to not involve MVI. Consistent with this, our upfront hepatectomy group showed a similar pattern. However, about half of the cases in the preoperative TACE group exhibited no MVI, suggesting a potential effect of TACE. Taken together, these findings suggest that preoperative TACE could serve as a viable strategy for managing huge HCC in the absence of SN. By reducing the incidence of MVI, TACE may improve RFS, particularly in selected cases without the presence of SN.
This study has several limitations. First, this retrospective study is subject to selection biases and confounding factors, which could influence the observed relationship between preoperative TACE and reduced MVI incidence. The lack of definitive pre-surgical MVI status means that the apparent reduction in MVI may reflect differences between groups rather than a direct effect of TACE, emphasizing the need for cautious interpretation and further research. Second, the small sample size may have limited the detection of significant differences in survival outcomes. It was recognized that patients with huge HCC should proceed directly to surgery without preoperative TACE, unless there are valid reasons for delaying the operation, such as the need to manage comorbidities and personal reasons. As a result, the number of patients in the TACE group was inevitably small. Finally, the short-term follow-up period for some patients might not fully reflect long-term survival outcomes, especially regarding late recurrences. However, given the short MST for patients with huge HCC, assessing the 5-year survival rate may provide meaningful insights.
In conclusion, preoperative TACE was not shown to significantly improve RFS or OS in patients with huge HCC, despite being associated with reduced MVI. The presence of SN, as a significant independent risk factor, can limit the impact of preoperative TACE on survival outcomes. Upfront hepatectomy should be suggested as the primary approach for most cases of huge HCC, particularly in patients where SN is anticipated.
Footnotes
Fund/Grant Support: None.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization, Data curation, Project administration, Resources, Software: All authors.
- Formal analysis: BGN, SHK, SH.
- Investigation: BGN.
- Methodology: CSA, KHK.
- Supervision: SGL.
- Validation: GWS, DHJ, GCP.
- Visualization: DBM, TYH.
- Writing – Original Draft: BGN, SH.
- Writing – Review & Editing: All authors.
SUPPLEMENTARY MATERIALS
Supplementary Fig. 1 and Supplementary Tables 1, 2 can be found via https://doi.org/10.4174/astr.2025.109.3.194.
The pattern of the initial recurrence according to preoperative TACE
Univariate and multivariate logistic regression analyses for satellite nodule
The proportions of initial recurrence sites. TACE, transarterial chemoembolization; IH, intrahepatic; EF, extrahepatic.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IO, Ikai I, et al. Tumor size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11:1086–1092. doi: 10.1002/lt.20472. [DOI] [PubMed] [Google Scholar]
- 3.Eguchi S, Takatsuki M, Hidaka M, Soyama A, Tomonaga T, Muraoka I, et al. Predictor for histological microvascular invasion of hepatocellular carcinoma: a lesson from 229 consecutive cases of curative liver resection. World J Surg. 2010;34:1034–1038. doi: 10.1007/s00268-010-0424-5. [DOI] [PubMed] [Google Scholar]
- 4.Hwang S, Lee YJ, Kim KH, Ahn CS, Moon DB, Ha TY, et al. Long-term outcome after resection of huge hepatocellular carcinoma ≥ 10 cm: single-institution experience with 471 patients. World J Surg. 2015;39:2519–2528. doi: 10.1007/s00268-015-3129-y. [DOI] [PubMed] [Google Scholar]
- 5.Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang L, Xing H, Zhang H, Zhong J, Li C, Lau WY, et al. Surgical resection versus transarterial chemoembolization for BCLC intermediate stage hepatocellular carcinoma: a systematic review and meta-analysis. HPB (Oxford) 2018;20:110–119. doi: 10.1016/j.hpb.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Li C, Wang MD, Lu L, Wu H, Yu JJ, Zhang WG, et al. Preoperative transcatheter arterial chemoembolization for surgical resection of huge hepatocellular carcinoma (≥ 10 cm): a multicenter propensity matching analysis. Hepatol Int. 2019;13:736–747. doi: 10.1007/s12072-019-09981-0. [DOI] [PubMed] [Google Scholar]
- 8.Paye F, Jagot P, Vilgrain V, Farges O, Borie D, Belghiti J, et al. Preoperative chemoembolization of hepatocellular carcinoma: a comparative study. Arch Surg. 1998;133:767–772. doi: 10.1001/archsurg.133.7.767. [DOI] [PubMed] [Google Scholar]
- 9.Bogdanovic A, Bulajic P, Masulovic D, Bidzic N, Zivanovic M, Galun D, et al. Liver resection versus transarterial chemoembolization for huge hepatocellular carcinoma: a propensity score matched analysis. Sci Rep. 2021;11:4493. doi: 10.1038/s41598-021-83868-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao ZH, Bai DS, Jiang GQ, Jin SJ. Review of preoperative transarterial chemoembolization for resectable hepatocellular carcinoma. World J Hepatol. 2015;7:40–43. doi: 10.4254/wjh.v7.i1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou WP, Lai EC, Li AJ, Fu SY, Zhou JP, Pan ZY, et al. A prospective, randomized, controlled trial of preoperative transarterial chemoembolization for resectable large hepatocellular carcinoma. Ann Surg. 2009;249:195–202. doi: 10.1097/SLA.0b013e3181961c16. [DOI] [PubMed] [Google Scholar]
- 12.Liver Cancer Study Group of Japan. The general rules for the clinical and pathological study of primary liver cancer. Jpn J Surg. 1989;19:98–129. doi: 10.1007/BF02471576. [DOI] [PubMed] [Google Scholar]
- 13.Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011;149:713–724. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark TW. Complications of hepatic chemoembolization. Semin Intervent Radiol. 2006;23:119–125. doi: 10.1055/s-2006-941442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Q, Tuo F, Li R, Wang X, Wang J, Huang Z, et al. Transarterial chemoembolization combined with hepatectomy for the treatment of intermediate-stage hepatocellular carcinoma. Front Oncol. 2020;10:578763. doi: 10.3389/fonc.2020.578763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mo A, Zhang Q, Xia F, Huang Z, Peng S, Cao W, et al. Preoperative transcatheter arterial chemoembolization and prognosis of patients with solitary large hepatocellular carcinomas (≥5 cm): multicenter retrospective study. Cancer Med. 2023;12:7734–7747. doi: 10.1002/cam4.5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng X, Sun P, Hu QG, Song ZF, Xiong J, Zheng QC, et al. Transarterial (chemo) embolization for curative resection of hepatocellular carcinoma: a systematic review and meta-analyses. J Cancer Res Clin Oncol. 2014;140:1159–1170. doi: 10.1007/s00432-014-1677-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y, Lin K, Liu L, Qian Y, Yang Y, Yuan S, et al. Impact of preoperative TACE on incidences of microvascular invasion and long-term post-hepatectomy survival in hepatocellular carcinoma patients: a propensity score matching analysis. Cancer Med. 2021;10:2100–2111. doi: 10.1002/cam4.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SG, Hwang S, Jung JP, Lee YJ, Kim KH, Ahn CS, et al. Outcome of patients with huge hepatocellular carcinoma after primary resection and treatment of recurrent lesions. Br J Surg. 2007;94:320–326. doi: 10.1002/bjs.5622. [DOI] [PubMed] [Google Scholar]
- 21.Bonfil RD, Bustuoabad OD, Ruggiero RA, Meiss RP, Pasqualini CD. Tumor necrosis can facilitate the appearance of metastases. Clin Exp Metastasis. 1988;6:121–129. doi: 10.1007/BF01784843. [DOI] [PubMed] [Google Scholar]
- 22.Bruix J, Llovet JM, Castells A, Montañá X, Brú C, Ayuso MC, et al. Transarterial embolization versus symptomatic treatment in patients with advanced hepatocellular carcinoma: results of a randomized, controlled trial in a single institution. Hepatology. 1998;27:1578–1583. doi: 10.1002/hep.510270617. [DOI] [PubMed] [Google Scholar]
- 23.Adachi E, Matsumata T, Nishizaki T, Hashimoto H, Tsuneyoshi M, Sugimachi K, et al. Effects of preoperative transcatheter hepatic arterial chemoembolization for hepatocellular carcinoma: the relationship between postoperative course and tumor necrosis. Cancer. 1993;72:3593–3598. doi: 10.1002/1097-0142(19931215)72:12<3593::aid-cncr2820721208>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 24.Lim KC, Chow PK, Allen JC, Chia GS, Lim M, Cheow PC, et al. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg. 2011;254:108–113. doi: 10.1097/SLA.0b013e31821ad884. [DOI] [PubMed] [Google Scholar]
- 25.Okusaka T, Okada S, Ueno H, Ikeda M, Shimada K, Yamamoto J, et al. Satellite lesions in patients with small hepatocellular carcinoma with reference to clinicopathologic features. Cancer. 2002;95:1931–1937. doi: 10.1002/cncr.10892. [DOI] [PubMed] [Google Scholar]
- 26.Liu F, Yan T, Cui D, Jiang J. Identification and validation of a prognostic model based on four genes related to satellite nodules in hepatocellular carcinoma. Sci Rep. 2024;14:15633. doi: 10.1038/s41598-024-66610-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pandey D, Lee KH, Wai CT, Wagholikar G, Tan KC. Long term outcome and prognostic factors for large hepatocellular carcinoma (10 cm or more) after surgical resection. Ann Surg Oncol. 2007;14:2817–2823. doi: 10.1245/s10434-007-9518-1. [DOI] [PubMed] [Google Scholar]
- 28.Hwang YJ, Bae JS, Lee Y, Hur BY, Lee DH, Kim H, et al. Classification of microvascular invasion of hepatocellular carcinoma: correlation with prognosis and magnetic resonance imaging. Clin Mol Hepatol. 2023;29:733–746. doi: 10.3350/cmh.2023.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwang S, Kim KH, Moon DB, Ahn CS, Ha TY, Song GW, et al. Prediction of post-resection prognosis using the ADV score for huge hepatocellular carcinomas ≥13 cm. J Liver Cancer. 2021;21:45–57. doi: 10.17998/jlc.21.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong SK, Lee KW, Lee S, Hong SY, Suh S, Han ES, et al. Impact of tumor size on hepatectomy outcomes in hepatocellular carcinoma: a nationwide propensity score matching analysis. Ann Surg Treat Res. 2022;102:193–204. doi: 10.4174/astr.2022.102.4.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The pattern of the initial recurrence according to preoperative TACE
Univariate and multivariate logistic regression analyses for satellite nodule
The proportions of initial recurrence sites. TACE, transarterial chemoembolization; IH, intrahepatic; EF, extrahepatic.