Abstract
Post-translational modification (PTM) plays a key role in the regulation of liquid–liquid phase separation (LLPS), which participates in cell behaviors and pathological processes. Quantitative analysis of PTM-regulated LLPS is therefore essential for understanding cellular processes and discovering new disease targets. However, the membrane-free structure, dynamic characteristics, and distinct chemical microenvironment of biomolecular condensates pose challenges to traditional methods. In this contribution, an electrochemical method is proposed to analyze PTM-regulated LLPS for the first time by exploring its effect on electrochemical species. By taking dephosphorylation as a model, we demonstrate that the degree of LLPS initiated by dephosphorylation can be quantitatively assessed. Importantly, this label-free method does not require probe immobilization or signal tagging, making it easily extendable to various PTM-involved LLPS processes. Overall, this work provides a highly sensitive, simplified, and universal approach for the quantitative study of LLPS, with broad potential applications in physiological and pathological research studies.
Keywords: biomolecular condensates, phase separation, post-translational modification, electrochemistry, bioanalysis
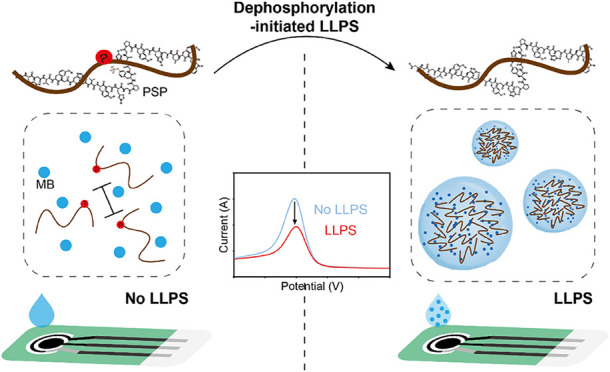

Introduction
Biomolecular condensates, formed through a dynamic process known as liquid–liquid phase separation (LLPS), play essential roles in cellular functions such as transcription, stress response, and disease progression. − Post-translational modifications (PTMs) such as phosphorylation, acetylation, and ubiquitination dynamically modulate the physicochemical properties of biomolecules (e.g., charge, hydrophobicity, and interaction networks). , These alterations directly influence their propensity to undergo LLPS, thereby enabling precise spatiotemporal regulation of condensate formation and disassembly, and consequently modulating cellular processes. , As the understanding of LLPS and condensates in biological systems deepens, especially regarding their potential as novel targets for disease diagnosis and therapy, , there is an urgent need to advance quantitative methods for analyzing PTM-regulated LLPS.
Conventional methods for studying PTM-involved LLPS include fluorescence assays, mass spectrometry, and immunoassays. , Although these techniques are well-established, they face significant challenges. For instance, due to the dynamic nature of condensates and the lack of clear membrane boundaries, their weak interactions are prone to interference from exogenous fluorescent tags and are difficult to be effectively preserved with immune affinity-based approaches. , Additionally, the distinct microenvironment within condensates causes fluctuations in the quantum yield of fluorescent reagents. The low abundance and transient nature of PTM hinder the sensitivity of mass spectrometry. , Electrochemical technology, with the merits of high sensitivity, portability, and rapid response, offers a promising potential solution. , However, its use in LLPS research has remained less explored.
In this work, an electrochemical approach is introduced for the first time to study PTM-regulated LLPS. As illustrated in Scheme , a phosphate-related process is selected as an example, representing a well-studied and functionally significant PTM for regulating LLPS. A peptide fragment containing a phosphate group is designed and named PSP. Upon dephosphorylation, PSP can spontaneously initiate LLPS, leading to condensate formation. We first investigate how these condensates influence the redox signal of methylene blue (MB), a typical redox indicator. Since this influence exhibits a quantitative correlation with the degree of LLPS, it is further developed into a method for quantitative profiling of PTM-regulated LLPS. Remarkably, dephosphorylation-induced LLPS, even caused by trace alkaline phosphatase (ALP), can be reliably detected, demonstrating the high sensitivity of the electrochemical system. Meanwhile, this approach eliminates the need for labeling the PTM enzyme or modification of phase-separating molecules, thereby minimizing interferences with the interaction process. The exclusion of electrode modification or tag-labeling steps makes it adaptable for studying LLPS involving different types of PTM. This work offers a simple, sensitive, and versatile “plug-and-play” modular strategy for facile analysis of LLPS and related biological processes.
1. Illustration Depicting the Electrochemical Analysis of Dephosphorylation-Modulated LLPS. Bar with Lines at Both Ends Means Electrostatic Repulsion.
Results and Discussion
Redox Signal of MB Changes upon Interaction with Biomolecular Condensates
To investigate the influence of biomolecular condensate on the electrochemical signal of MB, a phase-separating peptide (named PS) with self-coacervation property is introduced. The formation of peptide condensates is confirmed through increased solution turbidity (Figure a) and the fusion process recorded by microscopic imaging (Figures b, S1, and S2) as well as increased viscosity (Figure S3). , Next, MB is mixed with PS, and the turbidity of the solution is found to be increased (Figure c). Microscopic images reveal intact condensate structures with encapsulated MB (Figure d). Subsequently, electrochemical measurements using square wave voltammetry (SWV) are performed with a compact device (Figure S4). As shown in Figure e, the signal of MB sharply decreases after incubation with PS. In contrast, treatments with another peptide (a phosphorylated peptide named PSP) or bovine serum albumin (BSA) barely induce any changes (Figure f). These observations clearly demonstrate that the electrochemical signal of MB undergoes significant changes upon interaction with biomolecular condensates.
1.
(a) UV–vis absorbance spectra of PBS solutions with and without PS. (b) The microscopic image of the condensates in the presence of PS. (c) UV–vis absorbance spectra of MB with and without PS. (d) The microscopic image of the PS condensates after incubation with MB. (e) SWV responses and (f) statistical results of MB incubated separately with PS, PSP, and BSA. An error bar means the standard deviation of three independent replicates.
Electrochemical Signal Does not Change under Non-LLPS Conditions
We employ chymotrypsin to cleave the PS sequence. The cleaved PS induces a sharp increase in the electrochemical signal of MB, which nearly matches the case of free MB. The response is also significantly higher than signals from PS treated with denatured chymotrypsin or BSA as controls (Figure a,b). These results verify that the observed signal changes are not solely due to simple interactions with amino acids. Next, we investigate whether PS induces signal changes by forming phase-separated condensates. The pH condition is adjusted to regulate the charge of the peptide and control the formation of condensates. UV–vis absorption spectra show that the mixtures of MB and PS exhibit different turbidity levels and peak values at various pH values, indicating that condensates form with pH above 6 and reach a maximum around pH 8 (Figure S5). Correspondingly, alterations in the electrochemical signal of MB only occur at pH levels above 6 (Figure c,d). Compared with pH 5, the electrochemical signal decreases significantly at pH 8 (Figure S6). Furthermore, under non-phase separation conditions (pH 5), there are barely any differences among the effects of PS, PSP, and BSA on the electrochemical signal of MB (Figure S7). These findings underscore that condensate formation through LLPS is essential for the observed signal changes, as the degree of LLPS varies with pH conditions.
2.
(a) SWV responses of MB and (b) statistical results obtained when PS is differently treated. (c) SWV responses of MB and (d) statistical results obtained under different pH conditions.
Electrochemical Changes are Related to Condensate Encapsulation
To directly elucidate how PS condensates influence the electrochemical responses of MB, we removed the peptide condensates from mixtures through centrifugation. Under non-phase separation conditions (pH 5), the presence or absence of PS has nearly no effect on MB content in the supernatant after centrifugation. In contrast, under phase separation conditions (pH 8, 9), the MB contents in the supernatants of the mixtures sharply decrease (Figure S8). The result indicates that a significant portion of MB is encapsulated within PS condensates and separated into the precipitate, supporting the conclusion that electrochemical changes are primarily related to MB encapsulation by the condensate structures. This effect likely originates from the spatial confinement within condensates, promotes concentration-dependent aggregation and thereby facilitates short-range π–π stacking of aromatic conjugated planes and conformational alignment. , Together with the spatial hindrance of condensates on the electrode surface, these factors collectively impede the electron transfer. Meanwhile, we explore the effects of the chemical microenvironment within biomolecular condensates (particularly in terms of polarity, viscosity, and pH) on the electrochemical responses separately. , It is found that the peak current first rises and then drops as the pH and viscosity increase (Figures S9 and S10), while it keeps rising with increased polarity (Figure S11). Given that condensates tend to be highly viscous, acidic, and nonpolar internally, these factors collectively verify that the electrochemical signal changes of MB are closely related to the unique chemical microenvironment within the condensates. Therefore, it is concluded that the electrochemical responses of MB are closely related to the encapsulation inside condensates, driven by both spatial confinement and hindrance as well as chemical environment.
Analysis of PTM-Modulated LLPS
With condensation-modulated electrochemical signals of MB elucidated, it is reasonable to explore whether this change can be used for the quantitative study of LLPS and the condensate. First, the quantitative relationship between the electrochemical changes and the concentration of PS, the component unit of condensates, is explored. As shown in Figure , under phase separation conditions (pH 8 and 9), increasing PS concentration causes a gradual decrease in the electrochemical signal of MB, accompanied by a lightening of the solution color (Figure a–d). The extent of current variation increases gradually (Figure e).
3.
Color changes of MB with the increasing concentration of PS (a) at pH 8 and (b) pH 9. SWV responses of MB with different concentrations of PS (c) at pH 8 and (d) pH 9. (e) Statistical results of the current changes. An error bar means the standard deviation of three independent replicates.
An electrochemical method is further developed for the quantitative analysis of PTM-regulated LLPS, using dephosphorylation-involved LLPS as an example. As illustrated in Figure a, a phosphorylated peptide, PSP, is designed by introducing a phosphate group to a tyrosine residue on PS. PSP exhibits a very weak ability to undergo LLPS and form condensates (Figure S12). Accordingly, PSP causes almost no change in the electrochemical signal. Upon addition of ALP, a substantial number of condensates are formed, enriching MB inside, which is proved by increased turbidity (Figure b) and direct microscopic image (Figure c). These results indicate that the dephosphorylation of PS successfully triggers LLPS. Consequently, the formed condensates lead to a significant decrease in the intensity of the electrochemical signal (Figure d,e). As a comparison, thermally denatured ALP cannot induce a significant decrease in electrochemical signal, consistent with the weak formation of condensates (Figure f).
4.
(a) Schematic illustration of dephosphorylation-regulated LLPS and electrochemical analysis. (b) UV–vis absorption spectra of MB with differently treated PSP. (c) Microscopic image of dephosphorylation-initiated LLPS. The inset shows the particle size distribution. (d) SWV responses and (e) statistical results of MB with differently treated PSP. (f) Microscopic images of MB with PSP treated by denatured ALP. Inset: particle size distribution.
Additionally, using transglutaminase 2 (TG2, another PTM enzyme) and BSA as controls, only ALP causes an increase in solution turbidity and a significant reduction in electrochemical signals (Figure a–c). Under non-phase separation conditions (pH 5), there is no significant difference between ALP, BSA, and TG2 in either turbidity or electrochemical responses (Figure d–f). The comparison confirms that the electrochemical changes specifically depend on the PTM activity of ALP. The feasibility of electrochemical analysis of PTM-regulated LLPS is demonstrated.
5.
(a) UV–vis absorption spectra and (b) SWV responses obtained when PSP was treated with different proteins at pH 8. (c) Statistical results. (d) UV–vis absorption spectra and (e) SWV responses obtained when treating PSP with different proteins at pH 5. (f) Statistical results.
Quantitative Analysis of ALP-Modulated LLPS
After optimizing the key parameters, including pH, concentration of Mg2+, and concentration of MB (Figures S13–S15), we apply the electrochemical method for quantitative analysis of ALP-regulated LLPS. With the increase of ALP concentration, the electrochemical signal decreases gradually, indicating enhanced LLPS driven by ALP-catalyzed dephosphorylation. Simultaneously, microscopic observation and particle size analysis confirm the progressive formation of condensates (Figures and S16), demonstrating the excellent performance of our method. Furthermore, a linear correlation is observed between the electrochemical responses and ALP concentration over 3 orders of magnitude (from 1 × 10–8 to 2 × 10–4 g/mL). The regression equation is fitted as follows:
where y means the peak current and x represents the logarithm of ALP concentration (Figure S17a,b). This indicates that our electrochemical strategy can also be extended as a simple, sensitive biosensing platform for protein detection, with a minimum detectable concentration as low as 0.01 μg/mL. In comparison, a commercial assay kit for ALP typically detects concentrations from 1.125 to 13.125 μg/mL, while requiring a minimum detectable concentration around 1.125 μg/mL (Figure S17c), highlighting the superior sensitivity of the proposed electrochemical method. It is expected to develop point-of-care testing (POCT) devices or diagnostic platforms through quick and sensitive electrochemical analysis of disease-related LLPS. Moreover, it can also be employed for rapid drug screening by directly monitoring LLPS changes.
6.
(a) SWV signals and (b–h) microscopic images recording different degrees of dephosphorylation-regulated LLPS caused by ALP (0.01, 0.1, 1, 10, 20, 100, and 200 μg/mL).
Conclusion
In summary, this work introduces the first electrochemical approach for the quantitative analysis of PTM-regulated LLPS by uncovering that biomolecular condensates modulate the properties of electrochemical species. This method avoids chemical modification of exogenous tags, thereby minimizing interference with condensate interactions and enhancing the accuracy. Leveraging the inherent advantages of electrochemical technology, the method exhibits high sensitivity, which is suitable for analyzing low-abundance PTM. Remarkably, dephosphorylation-induced LLPS, even caused by trace ALP (0.01 μg/mL), can be reliably detected. Furthermore, since this method does not require electrode modification or tag labeling, it can be broadly applied to analyze LLPS involving various types of PTM, possessing high versatility. Overall, this work provides a label-free, universal, and highly sensitive “plug-and-play” strategy for LLPS quantification.
Experimental Section
Materials and Chemicals
The peptide PS (GHGVYGHGVYGHGPYGHGPYGHGLYW) and PSP (GHGVYGHGVYGHGP{pTYR}GHGPYGHGLYW) were synthesized by GenScript Biotech (Nanjing, China). MB, BSA, TG2, and chymotrypsin were obtained from Merck Co., Ltd. ALP was obtained from Sangon Biotech Co., Ltd. (Shanghai, China). 1,4-Dioxane was purchased from Macklin Biochemical Technology Co., Ltd. (Shanghai, China). The screen-printed electrode (SPE) that included a carbon working electrode (2 mm diameter), a carbon counter electrode, and a silver/silver chloride reference electrode was purchased from Poten Technology Co., Ltd. (Weihai, China). Ultrapure water was prepared using a Millipore purification system. All other chemical reagents used in this study were of analytical grade or higher quality and were not subjected to further purification.
Construction of PS Condensations and Interaction with MB
To form peptide condensates, 2 μL of PS (3.75 mM) was introduced into a 96-well plate, followed by the addition of 48 μL of PBS (200 mM). For the interaction with MB, 2 μL of PS (3.75 mM) was mixed with 48 μL of PBS (200 mM) containing MB with a concentration of 10 μg/mL. After thorough blending, the solutions were used for further study. The UV–vis absorbance spectra of the solutions were monitored using a Synergy HT multifunction microplate reader (BioTek Instruments, Inc., USA) or directly observed under a microscope (IX73, Olympus).
Chymotrypsin Treatment
Chymotrypsin (10 μg/mL) prepared in PBS (200 mM, pH = 8) containing 2 mM CaCl2 was mixed with an equal amount of peptide (PS or PSP) with a concentration of 3.75 mM. After reacting at 37 °C for 30 min, 4 μL of the solution was mixed with 46 μL of PBS (200 mM) with or without MB. For deactivation experiments, the enzyme was heated to 100 °C for 30 min before being employed to treat the peptides. For specificity experiments, peptides were treated with BSA.
Centrifugal Assay
Two microliters of PS (3.75 mM) or ultrapure water was mixed with 48 μL of PBS (200 mM) containing MB. After thorough mixing, the solutions were centrifuged at 15,000 rpm for 5 min. The supernatants were then collected for analysis.
Electrochemical Analysis
Twenty-five microliters of solution was dropped onto the SPE. The electrochemical signals were recorded by SWV. The experimental parameters were set as follows: the initial and final potentials were 0 and −0.5 V, the step potential was 1 mV, the amplitude was 25 mV, the frequency was 60 Hz, and the quiet time was 2 s.
Sensitivity Determination of MB to Polarity, pH, and Viscosity
To determine the polarity sensitivity, MB with a concentration of 10 μg/mL was added to the H2O-1,4-dioxane mixture with ratios ranging from 0 to 100%. The electrochemical signals of the mixtures were then determined. The trends of peak current and potential with increasing polarity were analyzed. For pH sensitivity investigation, MB was added to PBS with varying pH values, and the absorption spectra were measured. For viscosity sensitivity investigation, MB was added to glycerin with different dilutions ranging from 0 to 50%, and the absorption spectra were measured.
Analysis of PTM-Modulated LLPS
For electrochemical analysis of dephosphorylation-regulated phase separation, an ALP solution with the concentration of 10 μg/mL was prepared in PBS (200 mM pH = 8) containing 2 mM MgCl2. PSP (3.75 mM) was mixed with an equal amount of ALP or PBS and allowed to react at 37 °C for 30 min. 4 μL of the reaction solution was then mixed with 46 μL of PBS (200 mM) with or without MB. After thorough mixing, the solutions were used for further analysis. For deactivation experiments, ALP was heated at 100 °C for 30 min before being utilized to treat the peptides. For selectivity experiments, peptides were separately treated with TG2 and BSA.
Supplementary Material
Acknowledgments
This work was supported by the Innovative Key Project of Suzhou Institute of Biomedical Engineering and Technology, Chinese Academy of Sciences (Grant No. CX202501006), the China Postdoctoral Science Foundation (Grant No. 2025M772613), and the National Natural Science Foundation of Shandong Province (Grant No. ZR2024QH169).
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacsau.5c00865.
Microscopic images of the PBS solution, MB with PSP, and dynamic fusion processes of condensate droplets; photographs recording seepage flow distances and the electrochemical analysis equipment; UV–vis absorption spectra under different pH values; electrochemical signals measured at different conditions and statistical results; the sensitivity of MB to pH, viscosity, and the polarity; SWV responses of PS condensates; SWV responses of different concentrations of ALP; particle size distribution of condensates resulted by ALP. (PDF)
CRediT: Yiwei Han conceptualization, investigation, writing - original draft; Jianyang Lu investigation; Zhenzhen Guo investigation; Peng Miao conceptualization, project administration, supervision, writing - review & editing.
The authors declare no competing financial interest.
References
- Lyon A. S., Peeples W. B., Rosen M. K.. A Framework for Understanding the Functions of Biomolecular Condensates across Scales. Nat. Rev. Mol. Cell Biol. 2021;22(3):215–235. doi: 10.1038/s41580-020-00303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei G. F., Lyons H., Li P. L., Sabari B. R.. Transcription Regulation by Biomolecular Condensates. Nat. Rev. Mol. Cell Biol. 2025;26(3):213–236. doi: 10.1038/s41580-024-00789-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti S., Hyman A. A.. Biomolecular Condensates at the Nexus of Cellular Stress, Protein Aggregation Disease and Ageing. Nat. Rev. Mol. Cell Biol. 2021;22(3):196–213. doi: 10.1038/s41580-020-00326-6. [DOI] [PubMed] [Google Scholar]
- Kim T. H., Tsang B., Vernon R. M., Sonenberg N., Kay L. E., Forman-Kay J. D.. Phospho-dependent Phase Separation of FMRP and CAPRIN1 Recapitulates Regulation of Translation and Deadenylation. Science. 2019;365(6455):825–829. doi: 10.1126/science.aax4240. [DOI] [PubMed] [Google Scholar]
- Yasuda S., Tsuchiya H., Kaiho A., Guo Q., Ikeuchi K., Endo A., Arai N., Ohtake F., Murata S., Inada T., Baumeister W., Fernández-Busnadiego R., Tanaka K., Saeki Y.. Stress- and Ubiquitylation-dependent Phase Separation of the Proteasome. Nature. 2020;578(7794):296–300. doi: 10.1038/s41586-020-1982-9. [DOI] [PubMed] [Google Scholar]
- Rhine K., Odeh H. M., Shorter J., Myong S.. Regulation of Biomolecular Condensates by Poly(ADP-ribose) Chem. Rev. 2023;123(14):9065–9093. doi: 10.1021/acs.chemrev.2c00851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Wan R., Zou Z., Lao L., Shao G., Zheng Y., Tang L., Yuan Y., Ge Y., He C., Lin S.. O-GlcNAcylation Determines the Translational Regulation and Phase Separation of YTHDF proteins. Nat. Cell Biol. 2023;25(11):1676–1690. doi: 10.1038/s41556-023-01258-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G., Xie J., Kong W., Xie J., Li Y., Du L., Zheng Q., Sun L., Guan M., Li H., Zhu T., He H., Liu Z., Xia X., Kan C., Tao Y., Shen H. C., Li D., Wang S., Yu Y., Yu Z.-H., Zhang Z.-Y., Liu C., Zhu J.. Phase Separation of Disease-Associated SHP2Mutants Underlies MAPK Hyperactivation. Cell. 2020;183(2):490–502. doi: 10.1016/j.cell.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J., He H., Kong W., Li Z., Gao Z., Xie D., Sun L., Fan X., Jiang X., Zheng Q., Li G., Zhu J., Zhu G.. Targeting Androgen Receptor Phase Separation to Overcome Antiandrogen Resistance. Nat. Chem. Biol. 2022;18(12):1341–1350. doi: 10.1038/s41589-022-01151-y. [DOI] [PubMed] [Google Scholar]
- Alberti S., Gladfelter A., Mittag T.. Considerations and Challenges in Studying Liquid-Liquid Phase Separation and Biomolecular Condensates. Cell. 2019;176(3):419–434. doi: 10.1016/j.cell.2018.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y. F., Li X., Li P. L., Lin Y.. A Brief Guideline for Studies of Phase-separated Biomolecular Condensates. Nat. Chem. Biol. 2022;18(12):1307–1318. doi: 10.1038/s41589-022-01204-2. [DOI] [PubMed] [Google Scholar]
- Kilgore H. R., Young R. A.. Learning the Chemical Grammar of Biomolecular Condensates. Nat. Chem. Biol. 2022;18(12):1298–1306. doi: 10.1038/s41589-022-01046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabarics M., Lettow M., Kirschbaum C., Greis K., Manz C., Pagel K.. Mass Spectrometry-Based Techniques to Elucidate the Sugar Code. Chem. Rev. 2022;122(8):7840–7908. doi: 10.1021/acs.chemrev.1c00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Xie X., Gao R., Chen Z., Yang M., Wen Z., Weng Y., Fan X., Zhang G., Liu L., Zeng X., Han Y., Cao M., Wang X., Li J., Yang Z., Li T., Chen P. R.. Spatiotemporal Protein Interactome Profiling through Condensation-Enhanced Photocrosslinking. Nat. Chem. 2025;17(1):111–123. doi: 10.1038/s41557-024-01663-1. [DOI] [PubMed] [Google Scholar]
- Labib M., Sargent E. H., Kelley S. O.. Electrochemical Methods for the Analysis of Clinically Relevant Biomolecules. Chem. Rev. 2016;116(16):9001–9090. doi: 10.1021/acs.chemrev.6b00220. [DOI] [PubMed] [Google Scholar]
- Cardoso A. G., Viltres H., Ortega G. A., Phung V., Grewal R., Mozaffari H., Ahmed S. R., Rajabzadeh A. R., Srinivasan S.. Electrochemical Sensing of Analytes in Saliva: Challenges, Progress, and Perspectives. TrAC, Trends Anal. Chem. 2023;160:116965. doi: 10.1016/j.trac.2023.116965. [DOI] [Google Scholar]
- González-Fernández E., Avlonitis N., Murray A. F., Mount A. R., Bradley M.. Methylene Blue not Ferrocene: Optimal Reporters for Electrochemical Detection of Protease Activity. Biosens. Bioelectron. 2016;84:82–88. doi: 10.1016/j.bios.2015.11.088. [DOI] [PubMed] [Google Scholar]
- Gabryelczyk B., Cai H., Shi X. Y., Sun Y., Swinkels P. J. M., Salentinig S., Pervushin K., Miserez A.. Hydrogen Bond Guidance and Aromatic Stacking Drive Liquid-Liquid Phase Separation of Intrinsically Disordered Histidine-rich Peptides. Nat. Commun. 2019;10:5465. doi: 10.1038/s41467-019-13469-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekhi S., Garcia C. G., Barai M., Rizuan A., Schuster B. S., Kiick K. L., Mittal J.. Expanding the Molecular Language of Protein Liquid-Liquid Phase Separation. Nat. Chem. 2024;16(7):1113–1124. doi: 10.1038/s41557-024-01489-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C. Y., Guo J. R., Qu X. L., Sun H. X., Chai H., Miao P.. Distance-Based Visual miRNA Biosensor with Strand Displacement Amplification-Mediated DNA Hydrogel Assembly. ACS Mater. Lett. 2024;6(6):2111–2117. doi: 10.1021/acsmaterialslett.4c00650. [DOI] [Google Scholar]
- Lim Z. W., Ping Y., Miserez A.. Glucose-Responsive Peptide Coacervates with High Encapsulation Efficiency for Controlled Release of Insulin. Bioconjugate Chem. 2018;29(7):2176–2180. doi: 10.1021/acs.bioconjchem.8b00369. [DOI] [PubMed] [Google Scholar]
- King M. R., Petry S.. Phase Separation of TPX2 Enhances and Spatially Coordinates Microtubule Nucleation. Nat. Commun. 2020;11(1):270. doi: 10.1038/s41467-019-14087-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Wu H.-C., Zhu C. X., Ehrlich A., Shaw L., Nikolka M., Wang S. H., Molina-Lopez F., Gu X. D., Luo S. C., Zhou D. S., Kim Y.-H., Wang G.-J. N., Gu K., Feig V. R., Chen S. C., Kim Y., Katsumata T., Zheng Y. Q., Yan H., Chung J. W., Lopez J., Murmann B., Bao Z. N.. Multi-scale Ordering in Highly Stretchable Polymer Semiconducting Films. Nat. Mater. 2019;18(6):594–601. doi: 10.1038/s41563-019-0340-5. [DOI] [PubMed] [Google Scholar]
- Liu T. F., Yang J. J., Geyer F., Conrad-Burton F. S., Sánchez R. H., Li H. X., Zhu X. Y., Nuckolls C. P., Steigerwald M. L., Xiao S. X.. Stringing the Perylene Diimide Bow. Angew. Chem., Int. Ed. 2020;59(34):14303–14307. doi: 10.1002/anie.202004989. [DOI] [PubMed] [Google Scholar]
- Dai Y. F., Chamberlayne C. F., Messina M. S., Chang C. J., Zare R. N., You L. C., Chilkoti A.. Interface of biomolecular condensates modulates redox reactions. Chem. 2023;9(6):1594–1609. doi: 10.1016/j.chempr.2023.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgore H. R., Mikhael P. G., Overholt K. J., Boija A., Hannett N. M., Van Dongen C., Lee T. I., Chang Y. T., Barzilay R., Young R. A.. Distinct Chemical Environments in Biomolecular Condensates. Nat. Cell Biol. 2024;20(3):291–301. doi: 10.1038/s41589-023-01432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M. R., Ruff K. M., Lin A. Z., Pant A., Farag M., Lalmansingh J. M., Wu T., Fossat M. J., Ouyang W., Lew M. D., Lundberg E., Vahey M. D., Pappu R. V.. Macromolecular Condensation Organizes Nucleolar Sub-phases to Set up a pH Gradient. Cell. 2024;187(8):1889–1906. doi: 10.1016/j.cell.2024.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxner M., Trang A., Mehta J., Forsyth C., Swanson B., Keshavarzian A., Bhushan A.. The Versatility and Diagnostic Potential of VOC Profiling for Noninfectious Diseases. BME Front. 2023;4:0002. doi: 10.34133/bmef.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan W. C. W.. Principles of Nanoparticle Delivery to Solid Tumors. BME Front. 2023;4:0016. doi: 10.34133/bmef.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.









