Abstract
The emerging of ultrasmall gold nanoparticles (nanoclusters) with atomic precision provides opportunities for precisely studying crystalline–amorphous heterostructures, despite the construction of such structures being challenging. In this work, we developed an acid-induction method and synthesized a Au52(TBBT)30 (TBBTH = 4-tert-butylbenzenelthiol) nanocluster with the kernel composed of two parts: the amorphous Au22 part and the fcc Au21 part, which represents the first construction of fcc-amorphous homometal heterojunction with ∼1 nm size. Density function theory (DFT) revealed that the HOMO–LUMO majorly distributed in the amorphous part and the HOMO–LUMO gap was dominated by the amorphous part, indicating the redox activity of the amorphous Au22 part in contrast to the fcc Au21 part, which was experimentally confirmed by differential pulse voltammetry, antioxidation test and anti-Galvanic reaction. But for electro-catalyzing reduction of CO2 to CO, the crystalline surface sites were revealed to be more catalytically active than the amorphous surface sites in catalyzing the reduction of CO2 to CO, and the most active sites were assigned to the cosurface sites of amorphous Au22 and fcc Au21, which is also responsible for the high performance of Au52(TBBT)30 relative to the pure fcc-structured Au52(TBBT)32 (the highest CO FE: 96.7% at −0.67 V vs 73.3% at −0.57 V; CO partial current density at the corresponding potential: −7.3 vs −2.7 mA cm–2).
Keywords: atomic precision, Au52(TBBT)30 , fcc-amorphous homometal heterojunction, electrocatalytic reduction, active location probing

Since the discovery of amorphous structures over 60 years ago, , amorphous-forming mechanisms and practical applications have been actively studied. − Amorphous phases possess a large amount of bonds, which are randomly oriented with unsaturated electronic configurations relative to their crystalline counterparts. , Moreover, the superior elastic strain of the local structure in the amorphous phase can accelerate the charge transfer between the active center and the reaction intermediate. These advantages endow amorphous materials with good flexibility and abundant defect sites on the surface, potentially active for catalysis. , However, every coin has two sides: the inherent low conductivity of the amorphous phase does not facilitate catalysis. In contrast, the crystalline phases usually improve the catalytic performance through their contribution to the high conductivity of the materials. , Hence, crystalline–amorphous combined heterostructures have recently received extensive attention because such materials are expected to shed the separated disadvantages of crystalline and amorphous components, and have combined or synergetic merits of the two components. ,, However, it is difficult to characterize such materials in bulk or large nanosize with atomic precision, which retards the in-depth structure (composition)-property correlation. , The emergence of metal nanoclusters − in recent years provides opportunities for precise study of such materials at ∼1 nm scale despite the construction and characterization of them being challenging. Over a dozen fcc-structured metal nanoclusters − have been known, and recently an amorphous Au–Cd nanocluster was also revealed; however, metal nanoclusters composed of coexistent fcc and amorphous parts have not been reported thus far, to the best of our knowledge, which stimulates our research enthusiasm. Fortunately we synthesized such a nanocluster by developing an acid-induction method, and probed its various active locations for electrocatalytic reduction of CO2 to CO, which will be shown below.
The synthesis details are described in the Supporting Information. Briefly, tetraoctylammonium bromide (TOAB) was first dissolved into THF in a trineck flask; then, HAuCl4·4H2O was added. After the mixed solution was stirred with 600 rpm for 10 min, 4-tert-butylbenzenelthiol (TBBTH) was added. When the color of the solution changed to yellow from deep red, a cold-water solution with NaBH4 was immediately added. In rapid sequence, dilute nitric acid (50%, v/v) was added into the reaction mixture. Without the addition of an adequate amount of nitric solution, the target nanocluster cannot be obtained after trying various reaction conditions, which indicates the critical role of nitric acid in this reaction. Hydrogen ions from nitric acid exhibit at least two effects: On one hand, nitric acid can accelerate the reaction by enhancing the hydrolysis of NaBH4; on the other hand, it can reduce the etching ability of the thiolate by interacting with thiol group, thus influencing the reaction kinetics and thermodynamics. − The growth process of gold nanoclusters was maintained for 5 h. Preparative thin-layer chromatography (PTLC) was applied to purify the crude product, which was collected and thoroughly washed with methanol. Dark crystals grew out of mixed toluene and acetonitrile under 4 °C after 1 week. The as-obtained crystals dissolved in dichloromethane were characterized with the UV/vis/NIR spectrometer, which reveals two absorption peaks at 415 and 533 nm (Figure a). Electrospray ionization mass spectrometry (ESI-MS) was employed to determine the nanocluster’s composition. − Cesium acetate (Cs(OAc)2) was added to assist the nanocluster ionization. As shown in Figure b, two intense peaks were observed at 5200.36 and 7733.40 M/Z, which correspond to the compositions [Au52(TBBT)30Cs3]3+ (calculated: 5200.41 M/Z, deviation: 0.05 M/Z) and [Au52(TBBT)30Cs2]2+ (calculated: 7733.49 M/Z, deviation: 0.09 M/Z), respectively. The experimental isotopic patterns were in accordance with the simulated isotopic pattern (see Figure c and d). The charge of the nanocluster should be neutral, since the adduct charge number is equal to the adducted Cs+ number, and no sound signals were found in the mass spectra without the addition of Cs(OAC)2. Therefore, the nanocluster should be Au52(TBBT)30, which was further confirmed by X-ray photoelectron spectroscopy (XPS) and single-crystal X-ray crystallography (SCXC). As shown in Figure S1, Au, S and C elements are observed in the survey spectrum and high-resolution spectra of Au52(TBBT)30. The peaks of Au 4f7/2 and 4f5/2 appear at 84.28 and 87.98 eV (Figure S1b), respectively, and the peak-spliting and fitting (Figure S2) clearly demonstrate the presence of Au (0). Note that the newly obtained Au52(TBBT)30 and the reported Au52(PET)32 (PET = phenylethanethiolate) and Au52(TBBT)32 are heterocomposition-homosized nanoclusters, and they have various compositions/structures, indicating the diversity in composition/structure of metal nanoclusters. However, the non-fcc Au52(TBBT)30 nanocluster cannot be produced from fcc Au52(TBBT)32 by reacting it with nitric acid (Figure S3).
1.
UV/vis/NIR absorption (a) and ESI-MS (b) spectra of Au52(TBBT)30; high-resolution mass spectra for [Au52(TBBT)30Cs3]3+ (c) and [Au52(TBBT)30Cs2]2+ (d) in constrast with the simulated one.
The crystals adopt the triclinic P1̅ space group. Figure S4 shows the total structure of Au52(TBBT)30 nanocluster revealed by SCXC, which is composed of a crystalline–amorphous Au38 kernel and an exterior shell with four -SR-Au-SR-Au-SR- staples, six -SR-Au-SR- staples and one TBBT thiolate. As shown in Figure , the Au38 kernel of Au52(TBBT)30 could be divided into two parts.
2.
Anatomy of the crystalline–amorphous structure of the Au52(TBBT)30 nanocluster: (a) archimedean antiprism capped with two half-octahedra; (b) quasi-icosahedral 12-atom unit; (c) Au16 unit; (d) octahedron Au6 unit; (e) Au19 unit; (f) three amorphous atoms; (g) amorphous structure of Au22 unit; (h) fcc-Au21 unit; (i) crystalline–amorphous Au38 kernel. All atoms are Au.
The first part is composed of 22 gold atoms (Figure ), which can be considered as an amorphous structure. To better understand this amorphous structure, more detailed analyses are given as follows. An archimedean antiprism is capped with two half-octahedra is formed by ten Au atoms (Figures a and S5), which is wrapped by a quasi-icosahedron composed of 12 atoms (Figure b) with sharing six Au atoms (Figure c). Meanwhile, an octahedron (Figure d) is connected to the Archimedean antiprism capped with two half-octahedra by sharing an Au3 facet (Figure e). There are also three amorphous atoms (Figure f), which are connected between the quasi-icosahedral Au12 and the archimedean antiprism capped with two half-octahedrons and between quasi-icosahedral Au12 and octahedron (Figure g), respectively. Note that the archimedean antiprism capped with two half-octahedra, an icosahedron (quasi-icosahedron), and an octahedron which are the basic structural units of an amorphous model structure. Therefore, the Au22 part is regarded as the amorphous structure. The second part consists of twenty-one gold atoms packed in the fcc mode, and its top and bottom are enclosed by two (100) faces (Figure h). The amorphous part connected the bottom (100) face and a Au3 facet of the fcc part by sharing five Au atoms (Figure c and g). The five shared atoms (atoms in the red frame) form the novel crystalline–amorphous interface (Figure i), which was first found in gold nanoclusters.
For the protective layer, there are six monomeric staples (-RS-Au-SR-) (Figure S6b), four dimeric staples (-RS-Au-SR-Au-SR-) (Figure S6d) and six bridging thiolates (Figure S6f): one monomeric staple, one dimeric staple and five bridging thiolates adsorb on the fcc-structured Au21 (Figure S6c, e, and g); two monomeric staples, two dimeric staples and one bridging thiolate are anchored to the Au22 amorphous part (Figure S6e and g). Three monomeric staples and one dimeric staple bridge the fcc-structured Au21 and the Au22 amorphous part (Figures S6 and c). Compared with pure fcc structure, the amorphous structure needs more monomeric staples (-RS-Au-SR-) for protection compared with dimeric staples (-RS-Au-SR-Au-SR-) as shown in Table S1, probably due to the relative unstability of the amorphous structure. Thus, obtaining an amorphous structure might require the conditions where the formation of a monomeric staple is preferred. For example, acid can break the AuSR polymers and benefit the synthesis of the amorphous structure, which is evidenced by this work and the previous work.
The Au–Au bond lengths in the Au38 kernel are 2.680–3.742 Å, and the average bond length is 2.920 Å, which is similar to the Au–Au distance (2.883 Å) in bulk Au. The Au–S bond lengths of the staples are 2.228–2.381 Å, and the averaged bond length of 2.301 Å is slightly shorter than the Au–S bond length of the bridging thiolates (2.348 Å). The S–Au–S bond angles of the staples are 165.96–176.55° with an averaged value of 171.28°, while the Au–S–Au angles of the staples are 89.54–105.48° with an averaged value of 98.83°.
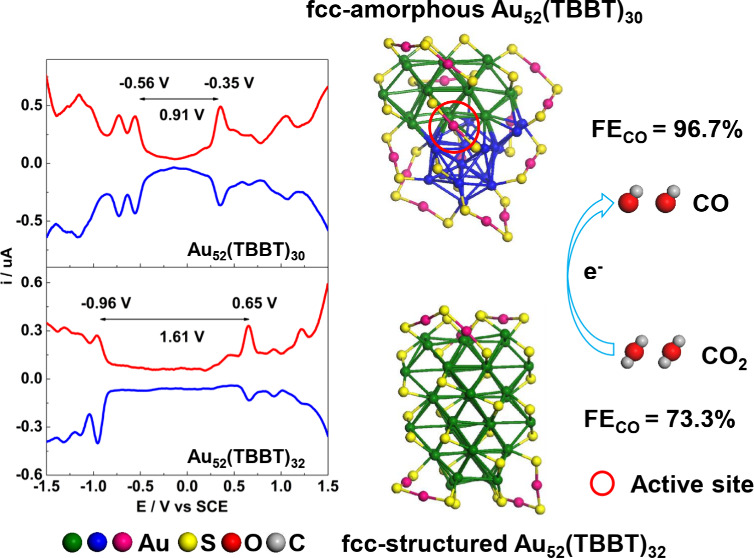
An advantage of metal nanocluster research is that their electronic structure can be obtained by computing in the same manner as that molecular compounds are treated. − Since many physicochemical processes, especially the redox process, is related to the HOMO–LUMO, it is conceived that the HOMO–LUMO information might provide clues for searching the redox active location of gold nanoclusters. − To test the concept, theoritical calculations (see Supporting Information for the details) were conducted on the novel Au52(TBBT)30 nanocluster and some other existing nanoclusters. As shown in Figures and S7, the HOMO and LUMO are majorly distributed on the Au22 amorphous part in the kernel of Au52(SR)30, which is also quantitatively supported by the Hirshfeld method in the Multiwfn program (for the average contribution of every gold atom in various locations, see Table S2). The HOMO–LUMO gap differences between two pairs of nanoclusters with close sizes but different structures (Au36(SR)24 vs Au38(SR)24‑T, Au52(SR)32 vs Au52(SR)30) are essentially the same, and their values are 0.76 and 0.75 eV, respectively, indicating that the non-fcc part (icosahedral Au13 or amorphous part) dominates the HOMO–LUMO gap (functional group-like), which is also supported by the fact that the HOMO–LUMO gap difference between Au44(SR)28 and Au49(2,4-DMBT)27 is 0.52 eV (deviated from the above-mentioned HOMO–LUMO gap difference of 0.76 or 0.75 eV) when icosahedral Au13 or the amorphous part is not contained in the dual-packed-kernelled Au49(2,4-DMBT)27. These facts indicate that the amorphous part is the redox active location in the kernel of Au52(SR)30, which is experimentally supported by the electrochemical results. As shown in Figure S8, differential pulse voltammetry (DPV) reveals that fcc-structured gold nanoclusters have larger electrochemical gaps (EG) than those of icosahedral Au13-based gold nanoclusters with close sizes. For examples, fcc-structured Au28 has a larger EG than that of icosahedral Au13-based Au25 , (1.96 vs 1.60 V), and fcc-structured Au36 has a larger EG than that of bi-icosahedral Au38 (2.00 vs 1.18 V). For crystalline–amorphous Au52(TBBT)30, the EG of DPV is 0.91 V, far smaller than the 1.61 V EG for Au52(TBBT)32, indicating that the Au22 amorphous part dominates the EG of DPV in Au52(TBBT)30, which provides experimental support for the speculation that the amorphous Au22 part is the active location (functional group) in Au52(TBBT)30 for the redox reaction. To find more evidence, we examined the reactivity of Au52(TBBT)30 and found that crystalline–amorphous Au52(TBBT)30 can be turned to pure-fcc Au52(TBBT)32 and Au36(TBBT)24 when oxidized by oxygen and Cd(NO3)2, respectively (Figures S9 and S10). When 2 mg of NaBH4 (3 equivs) is added into the Au52(TBBT)30 solution, Au52(TBBT)30 also can be transformed into the pure-fcc structured Au36(TBBT)24 (Figure S11). When Au52(TBBT)32 is exposed to air for 2 days, its structure remains unchanged (Figure S12). Similarly, no structural transformation occurs when Au36(TBBT)24 reacts with Cd2+ (Figure S13). Given the structure differences between Au52(TBBT)30 and Au52(TBBT)32 (or Au36(TBBT)24 ), it can be concluded that the fcc-Au21 unit in the kernel of Au52(TBBT)30 does not change, but the amorphous Au22 unit turns to a fcc structure in the transformation from Au52(TBBT)30 to Au52(TBBT)32 (or Au36(TBBT)24) (Figure S14), indicating the activity of the amorphous Au22 unit in the kernel of Au52(TBBT)30.
3.

HOMO and LUMO of three groups of gold nanoclusters with comparable sizes but different structures (Au36(SR)24, Au38(SR)24-T, Au38(SR)24-Q; Au44(SR)28 and Au49(SR)27; Au52(SR)32 and Au52(SR)30).
To investigate the difference between the amorphous and crystalline units in catalysis, the electrocatalytic reduction of CO2 was used as a model reaction due to its significance and popularity. ,− For comparison, the pure-fcc structured Au52(TBBT)32 was also investigated. As shown in the LSV curves of Figure a, both Au52(TBBT)30 and Au52(TBBT)32 nanoclusters show higher current density in CO2-saturated 0.5 M KHCO3 solution than that in the Ar-saturated electrolyte, illustrating the occurrence of CO2 reduction on these two Au nanoclusters. The observed much higher current density on Au52(TBBT)30 than that on Au52(TBBT)32 in CO2-saturated electrolyte suggests that Au52(TBBT)30 is more active for the reduction of CO2 molecules than Au52(TBBT)32. As clearly observed in Figure b, Au52(TBBT)30 shows higher CO FE than Au52(TBBT)32 in the whole selected potential range. Au52(TBBT)30 exhibits the highest CO FE of 96.7% at −0.67 V, while Au52(TBBT)32 displays the highest CO FE of 73.3% at −0.57 V. The higher CO FE results in larger CO partial current density, as seen in Figure c. Specifically, the CO partial current density on Au52(TBBT)30 at −0.67 V reaches −7.3 mA cm–2, which is much larger than −2.7 mA cm–2 on Au52(TBBT)32 at −0.57 V. Besides, the mass activty of Au52(TBBT)30 at −0.67 V reaches −281.1 mA mg–1, which is 2.5 times that of Au52(TBBT)32 (−112.9 mA mg–1) at −0.67 V (Figure S15a). The higher CO FE and the relevant CO current density and mass activity of Au52(TBBT)30 compared with Au52(TBBT)32 indicate that the crystalline–amorphous structure can more efficiently catalyze the reduction of CO2 to CO than the pure crystalline structure. The high catalytic selectivity of Au52(TBBT)30 for CO production also suppresses the competitive hydrogen evolution reaction, and the corrresponding H2 FE of Au52(TBBT)30 at −0.67 V is only 3.1% (Figure S15b). Moreover, the nearly unchanged current density and CO FE on Au52(TBBT)30 (Figure d) during electrolysis for 20 h verify its favorable electrocatalytic durability for CO2 reduction. Both TEM (Figure S16) and XRD (Figure S17) experiments further verify the structure stability of Au52(TBBT)30 during the CO2 reduction process.
4.
Linear sweep voltammetry (LSV) curves of Au52(TBBT)30 and Au52(TBBT)32 in Ar-saturated (dotted line) and CO2-starated (solid line) 0.5 M KHCO3 solution with a scan rate of 10 mV s–1 (a); CO Faradaic efficiencies (FE) of these two nanoclusters at different applied potentials (b); corresponding CO partial current density (c); electrocatalytic stability of Au52(TBBT)30 nanocluster for the reduction of CO2 to CO at −0.67 V for 20 h (d).
In situ surface enhanced infrared absorption spectroscopy in attenuated total reflectance mode (ATR-SEIRAS) was employed to investigate the adsorption behavior of the crucial intermediates on theses two nanoclusters. As shown in Figure a and b, the appreciable bands centered at 1925 cm–1 for Au52(TBBT)30 and centered at 1905 cm–1 for Au52(TBBT)32 should be attributed to the bridge-bonded CO* on their surfaces, and the relatively positive shift of the adsorbed band for Au52(TBBT)30 indicates that the binding energy of CO on Au52(TBBT)30 is weaker than that on Au52(TBBT)32. , Moreover, the density functional theory (DFT) calculations were performed to survey the efficient active sites on these two nunoclusters, and to gain insight into the mechanism of the reduction of CO2 on these sites. The given thiolates were removed to expose the active sites for the adsorption of CO2 molecules. − For Au52(TBBT)30, the bridging thiolates in six -Au-SCH3-Au- motifs on the FCC unit surface, the amorphous unit surface and the cosurface of the amorphous and fcc units (Figure S18) were removed to expose Au52s−S1 and Au52s−S2 (Figure S19a, d), Au52s−S3 and Au52s−S4 (Figure S20a, d), and Au52s−S5 and Au52s−S6 (Figure S21a, d) sites, respectively. For the Au52(TBBT)32 nanocluster, two bridging thiolates in two -Au-SCH3-Au- motifs on the FCC unit surface (Figure S22) were removed to expose Au52–S1 and Au52–S2 sites (Figure S23a, d). The free energies of every elementary reaction in CO2 reduction on Au52s–S1-S6 and Au52–S1-S2 are shown in Figures S24 and S25, respectively. It is clear that the formation of COOH*, one key intermediate usually hindered by the weak adsorption, is facile on the investigated sites of the two clusters (Figures S19b and e, S20b and e, S21b and e, S23b and e, S24, and 25). However, the desorption of CO*, another important intermediate, from these investigated sites shows an increase of free energy (Figure S19c and f, S20c and f, S21c and f, S23c and f, S24, and S25), indicating the liberation of CO should be a rate-determining step. For the Au52(TBBT)30 nanocluster, Au52s−S6 shows the smallest increase of free energy for the desorption of CO* on these potential catalysis sites (Figure S24), indicating that Au52s−S6 is the most active site on Au52(TBBT)30 for catalyzing the reduction of CO2 to CO; i.e., the cosurface of the crystalline and amorphous units are more catalytically active than the single crystalline (amorphous) unit surface, which was attributed to the synergy of the crystalline and amorphous units. The increase of free energy for the desorption of CO* on Au52s−S2 is smaller than that on Au52s−S3 (Figure S24), indicating that the crystalline surface sites are more catalytically active than the amorphous surface sites. For Au52(TBBT)32, Au52–S2 shows a smaller increase of free energy in the desorption of CO* than Au52–S1 (Figure S25), indicating that Au52–S2 is more active than Au52–S1. Notably, the increase of free energy for the desorption of CO* on Au52s−S6 is smaller than that on Au52–S2 (Figures c, S21f, and S23f), accounting for the better electrocatalytic activity of Au52(TBBT)30 than that of Au52(TBBT)32. As shown in Figure e and f, Au52s−S6 shows higher affinity than Au52–S2, indicating the weaker Au-CO* bond on Au52s−S6 than that on Au52–S2, in accordance with the above-mentioned free energy results. Besides, the density of states (DOS) calculation also provides evidence: the d-band center of Au52–S2 is more upshifted than that of Au52s−S6 (Figure S26), indicating the relatively stronger interaction between the active gold centers and the adsorbates (CO*) for Au52–S2, which retards the liberation of CO and thus inhibits the catalysis on Au52(TBBT)32, which is also consistent with the ATR-SEIRAS result. In addtion, the larger resistance for the desorption of H* on Au52s−S6 in comparison with that on Au52–S2 (Figures d and S27) further supports that Au52(TBBT)30 can more efficiently catalyze the reduction of CO2 to CO, demonstrating the importance of the active site on the joint surface in metal nanoclusters.
5.
In situ electrocatalytic ATR-SEIRAS spectra for the reduction of CO2 on Au52(TBBT)30 (a) and Au52(TBBT)32 (b) in CO2 saturated 0.5 M KHCO3 solution at various applied potentials (the potential interval between every two adjacent spectra is 0.1 V). Free energy diagram for the reduction of CO2 to CO (c) and HER (d) on Au52s−S6 of Au52(TBBT)30 and Au52–S2 of Au52(TBBT)32. Charge density differences for CO* adsorption states and the corresponding charge transforming of Au52–S2(e) and Au52s−S6 (f).
In summary, a novel crystalline–amorphous heterojunction gold nanocluster Au52(TBBT)30 was synthesized by developing an acid-induction method and structurally resolved by single-crystal X-ray crystallography, which reveals its unique kernel consisting of two parts: the Au22 amorphous part and the fcc-structured Au21 part, demonstrating the first construction of an ∼1 nm fcc-amorphous heterojunction with atomic precision. The amorphous Au22 and fcc Au21 parts show various reactivity, indicated by DFT calculations, which reveals that the HOMO–LUMO majorly distributes on the Au22 amorphous part in the kernel and the HOMO–LUMO gap is dominated by the Au22 amorphous part. The high redox activity of the amorphous Au22 part compared to the fcc Au21 part was experimentally supported by differential pulse voltammetry, antioxidation test, and AGR reaction. However, the crystalline surface sites are more catalytically active than the amorphous surface sites in catalyzing the reduction of CO2 to CO, and the most active sites are found to locate on the cosurface of the amorphous Au22 and fcc Au21 parts which is attributed to the synergy of the two parts. This is also supported by the fact that fcc-amorphous heterojunctioned Au52(TBBT)30 can far more efficiently catalyze CO2RR than the pure fcc-structured Au52(TBBT)32 (CO FE: 96.7% vs 73.3%; CO partial current density: 7.3 vs 3.5 mA cm–2). Thus, our work represents an important advance in atomically precise heterojunction synthesis, characterization and structure–property correlation, having important implications for nanoparticle tuning and active location probing, and is expected to stimulate more work in related fields.
Supplementary Material
Acknowledgments
The authors would like to thank National Natural Science Foundation of China (Nos. 22373082, 22471275, 21905284, 21829501, 21925303, 22075290, 22075291, 92475105, U24A20480, 22272179, 21771186, 21528303, 21222301, 21171170, 22403096), Science and Technology Innovation Program of Hunan Province (2023RC1055), Hefei Institute of Physical Science, Chinese Academy of Sciences (BR-E44BGGBR12B), CASHIPS Director’s Fund (BJPY2019A02), Key Program of 13th five-year plan, CASHIPS (KP-2017-16), Collaborative Innovation Program of Hefei Center for Physical Science and Technology (2020HSC-CIP005, 2022HSC-CIP018) and the State Key Laboratory of Mesoscience and Engineering, Institute of Process Engineering, Chinese Academy of Sciences (MESO-24-A01) for financial support.
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/prechem.5c00006.
¶.
S.Z., D.C., and P.W. contributed equally. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
References
- Bernal J. D.. Geometry of the structure of monatomic liquids. Nature. 1960;185(4706):68–70. doi: 10.1038/185068a0. [DOI] [Google Scholar]
- Bernal J. D., Mason J.. Packing of Spheres: Co-ordination of Randomly Packed Spheres. Nature. 1960;188(4754):910–911. doi: 10.1038/188910a0. [DOI] [Google Scholar]
- Miracle D. B.. A structural model for metallic glasses. Nat. Mater. 2004;3(10):697–702. doi: 10.1038/nmat1219. [DOI] [PubMed] [Google Scholar]
- Sheng H. W., Luo W. K., Alamgir F. M., Bai J. M., Ma E.. Atomic packing and short-to-medium-range order in metallic glasses. Nature. 2006;439(7075):419–25. doi: 10.1038/nature04421. [DOI] [PubMed] [Google Scholar]
- Yang Y., Zhou J., Zhu F., Yuan Y., Chang D. J., Kim D. S., Pham M., Rana A., Tian X., Yao Y., Osher S. J., Schmid A. K., Hu L., Ercius P., Miao J.. Determining the three-dimensional atomic structure of an amorphous solid. Nature. 2021;592(7852):60–64. doi: 10.1038/s41586-021-03354-0. [DOI] [PubMed] [Google Scholar]
- Tan M., Huang B., Su L., Jiao X., Feng F., Gao Y., Huang Q., Huang Z., Ge Y.. Amorphous Nanomaterials: Emerging Catalysts for Electrochemical Carbon Dioxide Reduction. Adv. Energy Mater. 2024;14(40):2402424. doi: 10.1002/aenm.202402424. [DOI] [Google Scholar]
- Han H., Choi H., Mhin S., Hong Y.-R., Kim K. M., Kwon J., Ali G., Chung K. Y., Je M., Umh H. N., Lim D.-H., Davey K., Qiao S.-Z., Paik U., Song T.. Advantageous crystalline–amorphous phase boundary for enhanced electrochemical water oxidation. Energy Environ. Sci. 2019;12(8):2443–2454. doi: 10.1039/C9EE00950G. [DOI] [Google Scholar]
- Danilovic N., Subbaraman R., Chang K.-C., Chang S. H., Kang Y. J., Snyder J., Paulikas A. P., Strmcnik D., Kim Y.-T., Myers D., Stamenkovic V. R., Markovic N. M.. Activity–Stability Trends for the Oxygen Evolution Reaction on Monometallic Oxides in Acidic Environments. J. Phys. Chem. Lett. 2014;5(14):2474–2478. doi: 10.1021/jz501061n. [DOI] [PubMed] [Google Scholar]
- Liu J., Ji Y., Nai J., Niu X., Luo Y., Guo L., Yang S.. Ultrathin amorphous cobalt–vanadium hydr(oxy)oxide catalysts for the oxygen evolution reaction. Energy Environ. Sci. 2018;11(7):1736–1741. doi: 10.1039/C8EE00611C. [DOI] [Google Scholar]
- Xiao G., Zeng Y., Jiang Y., Ning J., Zheng W., Liu B., Chen X., Zou G., Zou B.. Controlled Synthesis of Hollow Cu2‑xTe Nanocrystals Based on the Kirkendall Effect and Their Enhanced CO Gas-Sensing Properties. Small. 2013;9(5):793–799. doi: 10.1002/smll.201202083. [DOI] [PubMed] [Google Scholar]
- Yu L., Zhang L., Wu H. B., Lou X. W.. Formation of NiCo3–S4 Hollow Nanoprisms with Enhanced Pseudocapacitive Properties. Angew. Chem., Int. Ed. 2014;53(14):3711–3714. doi: 10.1002/anie.201400226. [DOI] [PubMed] [Google Scholar]
- Zhang L. Y., Ouyang Y., Wang S., Wu D., Jiang M., Wang F., Yuan W., Li C. M.. Perforated Pd Nanosheets with Crystalline/Amorphous Heterostructures as a Highly Active Robust Catalyst toward Formic Acid Oxidation. Small. 2019;15(47):1904245. doi: 10.1002/smll.201904245. [DOI] [PubMed] [Google Scholar]
- Li F.-M., Xia C., Fang W., Chen Y., Xia B. Y.. RhCuBi Trimetallenes with Composition Segregation Coupled Crystalline-Amorphous Heterostructure Toward Ethanol Electrooxidation. Adv. Energy Mater. 2024;14(21):2400112. doi: 10.1002/aenm.202400112. [DOI] [Google Scholar]
- Ren H., Zhou J., Zhang A., Wu Z., Cai J., Fu X., Zhou J., Wan Z., Zhou B., Huang Y., Duan X.. Precision Control of Amphoteric Doping in CuxBi2Se3 Nanoplates. Precision Chemistry. 2024;2(8):421–427. doi: 10.1021/prechem.4c00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Y., Wan L., Qin X., Hu W., Yang J.. Proposed Quantum Twisting Scanning Probe Microscope over Twisted Bilayer Graphene. Nano Lett. 2024;24(15):4433–4438. doi: 10.1021/acs.nanolett.4c00205. [DOI] [PubMed] [Google Scholar]
- Jin R., Zeng C., Zhou M., Chen Y.. Atomically Precise Colloidal Metal Nanoclusters and Nanoparticles: Fundamentals and Opportunities. Chem. Rev. 2016;116(18):10346–10413. doi: 10.1021/acs.chemrev.5b00703. [DOI] [PubMed] [Google Scholar]
- Chakraborty I., Pradeep T.. Atomically Precise Clusters of Noble Metals: Emerging Link between Atoms and Nanoparticles. Chem. Rev. 2017;117(12):8208–8271. doi: 10.1021/acs.chemrev.6b00769. [DOI] [PubMed] [Google Scholar]
- Ghosh A., Mohammed O. F., Bakr O. M.. Atomic-Level Doping of Metal Clusters. Acc. Chem. Res. 2018;51(12):3094–3103. doi: 10.1021/acs.accounts.8b00412. [DOI] [PubMed] [Google Scholar]
- Qian J., Yang Z., Lyu J., Yao Q., Xie J.. Molecular Interactions in Atomically Precise Metal Nanoclusters. Precis. Chem. 2024;2(10):495–517. doi: 10.1021/prechem.4c00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C., Qian H., Li T., Li G., Rosi N. L., Yoon B., Barnett R. N., Whetten R. L., Landman U., Jin R.. Total Structure and Electronic Properties of the Gold Nanocrystal Au36(SR)24 . Angew. Chem., Int. Ed. 2012;51(52):13114–13118. doi: 10.1002/anie.201207098. [DOI] [PubMed] [Google Scholar]
- Zeng C., Chen Y., Iida K., Nobusada K., Kirschbaum K., Lambright K. J., Jin R.. Gold Quantum Boxes: On the Periodicities and the Quantum Confinement in the Au28, Au36, Au44, and Au52 Magic Series. J. Am. Chem. Soc. 2016;138(12):3950–3953. doi: 10.1021/jacs.5b12747. [DOI] [PubMed] [Google Scholar]
- Liao L., Wang C., Zhuang S., Yan N., Zhao Y., Yang Y., Li J., Deng H., Wu Z.. An Unprecedented Kernel Growth Mode and Layer-Number-Odevity-Dependent Properties in Gold Nanoclusters. Angew. Chem., Int. Ed. 2020;59(2):731–734. doi: 10.1002/anie.201912090. [DOI] [PubMed] [Google Scholar]
- Li S., Du X., Liu Z., Li Y., Shao Y., Jin R.. Size Effects of Atomically Precise Gold Nanoclusters in Catalysis. Precis. Chem. 2023;1(1):14–28. doi: 10.1021/prechem.3c00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang S., Chen D., Ng W.-P., Liu L.-J., Sun M.-Y., Liu D., Nawaz T., Xia Q., Wu X., Huang Y.-L., Lee S., Yang J., Yang J., He J.. Phosphine-Triggered Structural Defects in Au44 Homologues Boost Electrocatalytic CO2 Reduction. Angew. Chem., Int. Ed. 2023;62(33):e202306696. doi: 10.1002/anie.202306696. [DOI] [PubMed] [Google Scholar]
- Zhuang S., Chen D., Liao L., Zhao Y., Xia N., Zhang W., Wang C., Yang J., Wu Z.. Hard-Sphere Random Close-Packed Au47Cd2(TBBT)31 Nanoclusters with a Faradaic Efficiency of Up to 96% for Electrocatalytic CO2 Reduction to CO. Angew. Chem., Int. Ed. 2020;59(8):3073–3077. doi: 10.1002/anie.201912845. [DOI] [PubMed] [Google Scholar]
- Zhuang S., Liao L., Li M.-B., Yao C., Zhao Y., Dong H., Li J., Deng H., Li L., Wu Z.. The fcc structure isomerization in gold nanoclusters. Nanoscale. 2017;9(39):14809–14813. doi: 10.1039/C7NR05239A. [DOI] [PubMed] [Google Scholar]
- Zhuang S., Liao L., Zhao Y., Yuan J., Yao C., Liu X., Li J., Deng H., Yang J., Wu Z.. Is the kernel-staples match a key-lock match? Chem. Sci. 2018;9(9):2437–2442. doi: 10.1039/C7SC05019D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang S., Liao L., Yuan J., Wang C., Zhao Y., Xia N., Gan Z., Gu W., Li J., Deng H., Yang J., Wu Z.. Kernel Homology in Gold Nanoclusters. Angew. Chem., Int. Ed. 2018;57(47):15450–15454. doi: 10.1002/anie.201808997. [DOI] [PubMed] [Google Scholar]
- Negishi Y., Nobusada K., Tsukuda T.. Glutathione-protected gold clusters revisited: Bridging the gap between gold(I)-thiolate complexes and thiolate-protected gold nanocrystals. J. Am. Chem. Soc. 2005;127(14):5261–5270. doi: 10.1021/ja042218h. [DOI] [PubMed] [Google Scholar]
- Desireddy A., Conn B. E., Guo J., Yoon B., Barnett R. N., Monahan B. M., Kirschbaum K., Griffith W. P., Whetten R. L., Landman U., Bigioni T. P.. Ultrastable silver nanoparticles. Nature. 2013;501(7467):399–402. doi: 10.1038/nature12523. [DOI] [PubMed] [Google Scholar]
- Negishi Y., Nakazaki T., Malola S., Takano S., Niihori Y., Kurashige W., Yamazoe S., Tsukuda T., Häkkinen H.. A Critical Size for Emergence of Nonbulk Electronic and Geometric Structures in Dodecanethiolate-Protected Au Clusters. J. Am. Chem. Soc. 2015;137(3):1206–1212. doi: 10.1021/ja5109968. [DOI] [PubMed] [Google Scholar]
- Wan X.-K., Yuan S.-F., Tang Q., Jiang D.-e., Wang Q.-M.. Alkynyl-Protected Au23 Nanocluster: A 12-Electron System. Angew. Chem., Int. Ed. 2015;54(20):5977–5980. doi: 10.1002/anie.201500590. [DOI] [PubMed] [Google Scholar]
- Bootharaju M. S., Joshi C. P., Alhilaly M. J., Bakr O. M.. Switching a Nanocluster Core from Hollow to Nonhollow. Chem. Mater. 2016;28(10):3292–3297. doi: 10.1021/acs.chemmater.5b05008. [DOI] [Google Scholar]
- Yao Q., Yuan X., Fung V., Yu Y., Leong D. T., Jiang D.-e., Xie J.. Understanding seed-mediated growth of gold nanoclusters at molecular level. Nat. Commun. 2017;8:927. doi: 10.1038/s41467-017-00970-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia T., Guan Z.-J., Zhang C., Zhu X.-Z., Chen Y.-X., Zhang Q., Yang Y., Sun D.. Eight-Electron Superatomic Cu31 Nanocluster with Chiral Kernel and NIR-II Emission. J. Am. Chem. Soc. 2023;145(18):10355–10363. doi: 10.1021/jacs.3c02215. [DOI] [PubMed] [Google Scholar]
- Li S., Wu Q., You X., Ren X., Du P., Li F., Zheng N., Shen H.. Anchoring Frustrated Lewis Pair Active Sites on Copper Nanoclusters for Regioselective Hydrogenation. J. Am. Chem. Soc. 2024;146(40):27852–27860. doi: 10.1021/jacs.4c10251. [DOI] [PubMed] [Google Scholar]
- Sun Y., Liu X., Xiao K., Zhu Y., Chen M.. Active-Site Tailoring of Gold Cluster Catalysts for Electrochemical CO2 Reduction. ACS Catal. 2021;11(18):11551–11560. doi: 10.1021/acscatal.1c02193. [DOI] [Google Scholar]
- Zeng C., Chen Y., Liu C., Nobusada K., Rosi N. L., Jin R.. Gold tetrahedra coil up: Kekule-like and double helical superstructures. Sci. Adv. 2015;1(9):e1500425. doi: 10.1126/sciadv.1500425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C., Li T., Das A., Rosi N. L., Jin R.. Chiral Structure of Thiolate-Protected 28-Gold-Atom Nanocluster Determined by X-ray Crystallography. J. Am. Chem. Soc. 2013;135(27):10011–10013. doi: 10.1021/ja404058q. [DOI] [PubMed] [Google Scholar]
- Zhuang S., Chen D., Fan W., Yuan J., Liao L., Zhao Y., Li J., Deng H., Yang J., Yang J., Wu Z.. Single-Atom-Kernelled Nanocluster Catalyst. Nano Lett. 2022;22(17):7144–7150. doi: 10.1021/acs.nanolett.2c02290. [DOI] [PubMed] [Google Scholar]
- Zhang C., Guan S., Li H. Y., Dong X. Y., Zang S. Q.. Metal Clusters Confined in Chiral Zeolitic Imidazolate Framework for Circularly Polarized-Luminescence Inks. Nano Lett. 2024;24(6):2048–2056. doi: 10.1021/acs.nanolett.3c04698. [DOI] [PubMed] [Google Scholar]
- Liu C., Ren X., Lin F., Fu X., Lin X., Li T., Sun K., Huang J.. Structure of the Au23–xAgx(S-Adm)15 Nanocluster and Its Application for Photocatalytic Degradation of Organic Pollutants. Angew. Chem., Int. Ed. 2019;58(33):11335–11339. doi: 10.1002/anie.201904612. [DOI] [PubMed] [Google Scholar]
- Shen H., Deng G., Kaappa S., Tan T., Han Y.-Z., Malola S., Lin S.-C., Teo B. K., Häkkinen H., Zheng N.. Highly Robust but Surface-Active: An N-Heterocyclic Carbene-Stabilized Au25 Nanocluster. Angew. Chem., Int. Ed. 2019;58(49):17731–17735. doi: 10.1002/anie.201908983. [DOI] [PubMed] [Google Scholar]
- Chen S. W., Ingram R. S., Hostetler M. J., Pietron J. J., Murray R. W., Schaaff T. G., Khoury J. T., Alvarez M. M., Whetten R. L.. Gold nanoelectrodes of varied size: Transition to molecule-like charging. Science. 1998;280(5372):2098–2101. doi: 10.1126/science.280.5372.2098. [DOI] [PubMed] [Google Scholar]
- Lee D., Donkers R. L., Wang G. L., Harper A. S., Murray R. W.. Electrochemistry and optical absorbance and luminescence of molecule-like Au38 nanoparticles. J. Am. Chem. Soc. 2004;126(19):6193–6199. doi: 10.1021/ja049605b. [DOI] [PubMed] [Google Scholar]
- Jin R.. Quantum sized, thiolate-protected gold nanoclusters. Nanoscale. 2010;2(3):343–362. doi: 10.1039/B9NR00160C. [DOI] [PubMed] [Google Scholar]
- Liao L., Zhou S., Dai Y., Liu L., Yao C., Fu C., Yang J., Wu Z.. Mono-Mercury Doping of Au25 and the HOMO/LUMO Energies Evaluation Employing Differential Pulse Voltammetry. J. Am. Chem. Soc. 2015;137(30):9511–9514. doi: 10.1021/jacs.5b03483. [DOI] [PubMed] [Google Scholar]
- Kwak K., Choi W., Tang Q., Kim M., Lee Y., Jiang D. E., Lee D.. A molecule-like PtAu24(SC6H13)18 nanocluster as an electrocatalyst for hydrogen production. Nat. Commun. 2017;8:14723. doi: 10.1038/ncomms14723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X. S., Luan X., Su H. F., Li J. J., Yuan S. F., Lei Z., Pei Y., Wang Q. M.. Structure Determination of Alkynyl-Protected Gold Nanocluster Au22(tBuC≡C)18 and Its Thermochromic Luminescence. Angew. Chem., Int. Ed. 2020;59(6):2309–2312. doi: 10.1002/anie.201912984. [DOI] [PubMed] [Google Scholar]
- Zhu W., Zhang S., Fan W., Yang Y., Zhao H., Fei W., Bi H., He J., Li M.-B., Wu Z.. Atomically Precise Metal Nanoclusters as Single Electron Transferers for Hydroborylation. Precis. Chem. 2023;1(3):175–182. doi: 10.1021/prechem.3c00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S. B., Li Y. Z., Li M. B., Yuan J. Y., Yang J. L., Wu Z. K., Jin R. C.. Structural isomerism in gold nanoparticles revealed by X-ray crystallography. Nat. Commun. 2015;6:8667. doi: 10.1038/ncomms9667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao L., Zhuang S., Wang P., Xu Y., Yan N., Dong H., Wang C., Zhao Y., Xia N., Li J., Deng H., Pei Y., Tian S.-K., Wu Z.. Quasi-Dual-Packed-Kerneled Au49(2,4-DMBT)27 Nanoclusters and the Influence of Kernel Packing on the Electrochemical Gap. Angew. Chem., Int. Ed. 2017;56(41):12644–12648. doi: 10.1002/anie.201707582. [DOI] [PubMed] [Google Scholar]
- Zhu M., Aikens C. M., Hollander F. J., Schatz G. C., Jin R.. Correlating the crystal structure of A thiol-protected Au25 cluster and optical properties. J. Am. Chem. Soc. 2008;130(18):5883–5884. doi: 10.1021/ja801173r. [DOI] [PubMed] [Google Scholar]
- Heaven M. W., Dass A., White P. S., Holt K. M., Murray R. W.. Crystal structure of the gold nanoparticle [N(C8H17)4][Au25(SCH2CH2Ph)18] J. Am. Chem. Soc. 2008;130(12):3754–3755. doi: 10.1021/ja800561b. [DOI] [PubMed] [Google Scholar]
- Qian H., Eckenhoff W. T., Zhu Y., Pintauer T., Jin R.. Total Structure Determination of Thiolate-Protected Au38 Nanoparticles. J. Am. Chem. Soc. 2010;132(24):8280–8281. doi: 10.1021/ja103592z. [DOI] [PubMed] [Google Scholar]
- Zhu W., Michalsky R., Metin O., Lv H., Guo S., Wright C. J., Sun X., Peterson A. A., Sun S.. Monodisperse Au Nanoparticles for Selective Electrocatalytic Reduction of CO2 to CO. J. Am. Chem. Soc. 2013;135(45):16833–16836. doi: 10.1021/ja409445p. [DOI] [PubMed] [Google Scholar]
- Kim D., Resasco J., Yu Y., Asiri A. M., Yang P.. Synergistic geometric and electronic effects for electrochemical reduction of carbon dioxide using gold–copper bimetallic nanoparticles. Nat. Commun. 2014;5:4948. doi: 10.1038/ncomms5948. [DOI] [PubMed] [Google Scholar]
- Yang H. B., Hung S.-F., Liu S., Yuan K., Miao S., Zhang L., Huang X., Wang H.-Y., Cai W., Chen R., Gao J., Yang X., Chen W., Huang Y., Chen H. M., Li C. M., Zhang T., Liu B.. Atomically dispersed Ni(i) as the active site for electrochemical CO2 reduction. Nat. Energy. 2018;3(2):140–147. doi: 10.1038/s41560-017-0078-8. [DOI] [Google Scholar]
- Chang C.-J., Lin S.-C., Chen H.-C., Wang J., Zheng K. J., Zhu Y., Chen H. M.. Dynamic Reoxidation/Reduction-Driven Atomic Interdiffusion for Highly Selective CO2 Reduction toward Methane. J. Am. Chem. Soc. 2020;142(28):12119–12132. doi: 10.1021/jacs.0c01859. [DOI] [PubMed] [Google Scholar]
- Deng G., Kim J., Bootharaju M. S., Sun F., Lee K., Tang Q., Hwang Y. J., Hyeon T.. Body-Centered-Cubic-Kernelled Ag15Cu6 Nanocluster with Alkynyl Protection: Synthesis, Total Structure, and CO2 Electroreduction. J. Am. Chem. Soc. 2023;145(6):3401–3407. doi: 10.1021/jacs.2c10338. [DOI] [PubMed] [Google Scholar]
- Ye C., Raaijman S. J., Chen X., Koper M. T. M.. Enhanced Electrochemical CO2 Reduction to Formate on Poly(4-vinylpyridine)-Modified Copper and Gold Electrodes. ACS Appl. Mater. Interfaces. 2022;14(40):45263–45271. doi: 10.1021/acsami.2c10452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Z., Pearce A. J., Mayer J. M., Wang H.. Bridge Sites of Au Surfaces Are Active for Electrocatalytic CO2 Reduction. J. Am. Chem. Soc. 2022;144(19):8641–8648. doi: 10.1021/jacs.2c01098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso D. R., Kauffman D., Matranga C.. Active sites of ligand-protected Au25 nanoparticle catalysts for CO2 electroreduction to CO. J. Chem. Phys. 2016;144(18):184705. doi: 10.1063/1.4948792. [DOI] [PubMed] [Google Scholar]
- Zhao S., Austin N., Li M., Song Y., House S. D., Bernhard S., Yang J. C., Mpourmpakis G., Jin R.. Influence of Atomic-Level Morphology on Catalysis: The Case of Sphere and Rod-Like Gold Nanoclusters for CO2 Electroreduction. ACS Catal. 2018;8(6):4996–5001. doi: 10.1021/acscatal.8b00365. [DOI] [Google Scholar]
- Wang W., Chen D., Fung V., Zhuang S., Zhou Y., Wang C., Bian G., Zhao Y., Xia N., Li J., Deng H., Liao L., Yang J., Jiang D.-e., Wu Z.. Gapped and Rotated Grain Boundary Revealed in Ultra-Small Au Nanoparticles for Enhancing Electrochemical CO2 Reduction. Angew. Chem., Int. Ed. 2025;64:e202410109. doi: 10.1002/anie.202410109. [DOI] [PubMed] [Google Scholar]
- You Q., Jiang X.-L., Fan W., Cui Y.-S., Zhao Y., Zhuang S., Gu W., Liao L., Xu C.-Q., Li J., Wu Z.. Pd8 Nanocluster with Nonmetal-to-Metal- Ring Coordination and Promising Photothermal Conversion Efficiency. Angew. Chem., Int. Ed. 2024;63(3):e202313491. doi: 10.1002/anie.202313491. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Chen D., Gu W., Fan W., Wang R., Fang L., You Q., Zhuang S., Bian G., Liao L., Zhou Z., Xia N., Yang J., Wu Z.. Chemical Synthesis of ∼ 1 nm Multilevel Capacitor-like Particles with Atomic Precision. Angew. Chem., Int. Ed. 2025;64:e202420931. doi: 10.1002/anie.202420931. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






