Abstract
Obesity is a global health problem that increases the risk of type 2 diabetes, cardiovascular diseases, fatty liver disease, and cancer. The pathological outcomes of obesity and the responses to weight loss interventions vary significantly among individuals. The use of noninvasive biomarkers is critical for the early risk prediction of diseases associated with obesity and monitoring disease progression. MicroRNAs (miRNAs) are small noncoding RNAs that play pivotal roles in biological processes of adipose development, inflammation, and function. Dysregulation of numerous miRNAs has been implicated in the pathogenesis of obesity and associated diseases. In addition to exerting their function in the cytoplasm, mature miRNAs can be packaged into vesicles, released into extracellular space and body fluids, and act as paracrine and endocrine factors mediating intercellular and interorgan crosstalk. Encapsulation of miRNAs in extracellular vesicles (EVs) protects them from degradation and enhances their stability in body fluids. Moreover, the unique EV-miRNA signature reflects the state of the origin cells and is functionally related to disease pathology, supporting their potential as sensitive and specific biomarkers for clinical diagnostics. Adipose tissue is the main source of circulating EV-miRNAs in Obesity. Here we highlight the implication of adipose tissue-derived EV-miRNAs in metabolic disorders associated with obesity. Current understanding of the molecular mechanisms governing the sorting of miRNAs into EVs and recent advancements in relevant techniques are reviewed. In addition, limitations and future perspectives in this field are discussed.
Keywords: miRNA, extracellular vesicle, obesity, metabolic diseases, biomarkers, RNA binding protein, EV-miRNA sorting, miRNA therapeutics


Introduction
Obesity is a major public health problem impacting more than 40% of adults and nearly 20% of children in the United States, and it is projected that obesity will affect nearly half of the US population by 2030. , Obesity induces chronic inflammation and insulin resistance, contributing directly and indirectly to the development of chronic diseases, including type 2 Diabetes (T2D), nonalcoholic fatty liver disease (NAFLD), cardiovascular diseases (CVD), and certain cancers. Obesity is linked to 30–53% of new diabetes cases in the US yearly, , and 50–90% of individuals with obesity have NAFLD. Alarmingly, the prevalence of obesity has increased significantly in young children, accelerating the onset of the associated complications including cirrhosis, liver failure, and cancer at younger age. NAFLD impacts over one-third of children with obesity and 20%–50% of these children have already progressed to steatohepatitis (NASH) at the time of diagnosis. Early diagnosis of obesity-related alterations is critical for predicting complications of obesity and for ensuring proper disease management. Despite recent advances in obesity treatment, it is noteworthy that the pathophysiologic processes of obesity are complicated and therapeutic outcomes vary significantly among individuals. Within the population of individuals affected by obesity, there exists a subset who are metabolically healthy, exhibiting normal glucose and lipid metabolism and do not develop hepatic steatosis. Specific and precise biomarkers for individual disease risk are of paramount importance for guiding personalized treatment strategies.
MicroRNAs (miRNAs) are short noncoding RNAs (∼22 nucleotides) that regulate gene expression post-transcriptionally via binding to the 3′ untranslated region (3′UTR) of the specific target mRNA, thereby impacting mRNA stability and protein translation. , Endogenous miRNAs are transcribed by RNA polymerase II from the miRNA genes, which are typically found in intergenic regions or introns of protein-coding genes. In the nucleus of mammalian cells (Figure ), miRNA genes are transcribed into primary miRNA transcript (Pri-miRNA) which is more than 1 kb in length and contains a stem-loop structure. , The Pri-miRNA is cleaved to produce the ∼70nt precursor miRNA (Pre-miRNA) by the enzyme Drosha and the DGCR8 complex. The Pre-miRNA is exported by exportin 5 into the cytoplasm, where it is further cleaved into ∼22 nt mature miRNA by Dicer and loaded onto the Argonaute (Ago) proteins to form the RNA Induced Silencing Complex (RISC) where miRNA binds to specific mRNA targets for degradation or translational repression. Each miRNA pairs complementarily with the 3′ untranslated region of numerous mRNA targets, primarily mediated by the seed sequence of the miRNA. One mRNA can be targeted by multiple miRNAs, reflecting the complex regulatory network of miRNAs.
1.
EV-miRNA biogenesis and secretion. In the cell nucleus, miRNAs are transcribed from miRNA genes to Pri-miRNA and processed to Pre-miRNA, followed by transportation into the cytoplasm and generation of mature miRNAs. The single strain mature miRNA is loaded into an RNA-induced silencing complex (RISC) to bind to target mRNA for posttranscriptional inhibition. miRNAs can be packaged and sorted into extracellular vesicles: (1) Microvesicles formed by outward budding; (2) Exosomes formed by incorporating the early endosome with the intraluminal vesicle (ILs) containing miRNAs to form multivesicular bodies (MVB), followed by fusion of MVB with cell membrane to release exosomes. Created in BioRender. Jiang, S. (2025) https://BioRender.com/a9k1zqv.
In addition to being important intracellular regulators of gene expression, miRNAs can be released into the extracellular space and circulation via packaging in extracellular vesicles (EVs) or binding to proteins such as AGO2. EVs are lipid bilayer membrane particles that are generally classified into microvesicles (MVs) and exosomes, which differ in size, cargos, and biogenesis pathways. , Microvesicles (generally 200 to 2000 nm in size) arise from outward budding of the plasma membrane that are excised and shed into the extracellular space (Figure ). Exosomes (range from 30 to 150/200 nm in size), also referred to as small Extracellular vesicles (sEVs), originate from the inward budding of the plasma membrane to form the early endosome that incorporate the intraluminal vesicle (ILs) containing miRNAs to form multivesicular bodies (MVBs). After the fusion of the MVBs with the cell membrane, the ILs are released outside the cells as exosomes. miRNAs in EVs can be delivered to neighboring cells as well as distal organs, mediating intercellular and interorgan communication upon uptake by recipient cells. miRNAs in EVs are protected from endogenous RNases degradation and EV-miRNAs can regulate gene expression and impact function of the recipient cells. Due to their stability and functional activity, EV-miRNAs have potential to be specific and accessible diagnostic biomarkers relevant to disease pathophysiology.
The temporal and spatial expression of numerous miRNAs have been reported to be dysregulated in obesity and associated diseases, including T2DM, cardiovascular diseases, fatty liver diseases, and cancer. − Obesity-associated miRNAs play pivotal roles in regulating biological processes, including adipocyte development, inflammation, lipid metabolism, insulin signaling, and β-cell apoptosis in a cell type-specific and cooperative manner. Several human and animal studies have identified dysregulated miRNAs in EVs or in circulation in metabolic diseases associated with obesity. ,, However, the origin cells of the EV-miRNAs and their specific actions on targeted tissues have not been insufficiently elucidated. Obesity is characterized by the excessive expansion of white adipose tissues. Using a mouse model with fat-specific knockout of the miRNA-processing enzyme Dicer, CR Kahn’s group have revealed adipose tissue as the main source of EV-miRNAs in obesity. , A comprehensive understanding of how and to what extent adipose tissue derived EV-miRNAs impact intercellular and interorgan communication is critical for discovering and validating specific biomarkers and developing new therapeutics. The present review centered on adipose tissues and highlighted the implication of adipose tissue-derived EV-miRNAs in metabolic disorders associated with obesity. An overview of the current understanding of EV-miRNA sorting mechanisms and available techniques for EV-miRNA analysis is provided. Finally, how adipose-EV-miRNAs contributes to precision diagnostics and future perspectives are discussed.
Adipose Tissue-Derived EV-miRNAs in Mediating Intercellular Communication and Interorgan Crosstalk in Obesity
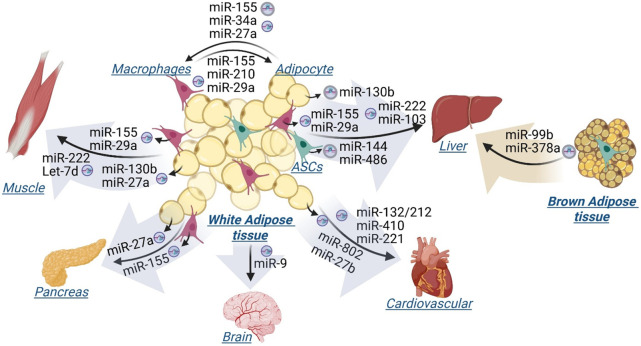
In obesity, adipose tissues undergo significant remodeling and secrete EV-miRNAs, particularly from adipocytes and macrophages, which impact the function and metabolism of adipose tissues as well as distal organs. , There are generally two types of adipose tissues, white and brown, which differ in their distribution and function. White adipose tissue (WAT), particularly visceral adipose tissues, is associated with increased risk of obesity-related complications, such as T2DM and cardiovascular diseases. In contrast, brown adipose tissue (BAT) is the main site of adaptive thermogenesis and is associated with protection against obesity and metabolic diseases. This review highlighted the latest studies which demonstrated the role of specific EV-miRNAs originating from adipose tissues in mediating the adipose tissue intercellular and interorgan communication in obesity-associated metabolic disorders (Figure ). Using in vivo and/or in vitro approaches, these studies determined that the specific miRNAs in EVs are derived from adipose tissue and are subsequently transported into cells of major metabolic tissues, including adipose tissue, liver, skeletal muscle, pancreas, and brain, where they impact metabolic functions.
2.
Adipose tissue-derived EV-miRNAs mediate intercellular and interorgan communication. miRNAs originate from white adipose tissues, including adipocytes, adipose tissue macrophages, and adipose tissue stem cells (ASCs), and brown adipose tissues are transported by Extracellular Vesicles (EVs) and taken up by indicated target cells and tissues. Created in BioRender. Jiang, S. (2025) https://BioRender.com/s6u4job.
EV-miRNAs Mediated Intercellular Communication within White Adipose Tissue
Adipose tissues are highly heterogeneous, composed of adipose progenitors, adipocytes, and immune cells, which are spatially organized and interact to determine tissue functions. Obesity is associated with an increased infiltration of adipose tissue macrophages (ATMs) into adipose tissue. Multiple studies have demonstrated that EV-miRNAs mediate adipocyte-macrophage crosstalk within white adipose tissue. Using a mouse model of obesity, high fat diet induces increased miR-155 in adipocytes and microvesicles (MV) derived from adipocytes, which is transferred to ATMs, inducing M1 proinflammatory phenotype. Also, miR-34a expression is increased in adipocytes of visceral adipose tissues in obese mice and exosomes secreted from mature adipocytes deliver miR-34a into macrophages, suppressing M2 polarization via downregulating Kruppel-like factor 4 (KLF4). Another microRNA, miR-27a, was also shown to be released from adipocytes and impact macrophage polarization. Conversely, ATMs can release exosomes that transport miRNAs to insulin target cells. For example, expression of miR-155 is increased in obese ATMs and obese ATM-derived exosomes. Exosomal miR-155 secreted by ATMs in obese mice can be taken up by adipocytes, impairing insulin sensitivity via targeting the nuclear receptor PPARγ and subsequently decreasing downstream insulin signaling. miR-29a expression increases in obese ATMs and ATM exosomes, and miR-29a is transferred into adipocytes causing insulin resistance. A recent study also identified exosomal miR-210 which is derived from ATMs and attenuates insulin sensitivity in adipocytes by silencing GLUT4.
Impact of Adipose Tissue-Derived EV-miRNAs on the Skeletal Muscle
Adipocyte-derived exosomal miR-27a induces insulin resistance in skeletal muscle by repressing PPARγ expression. Additionally, miR-130b is upregulated in adipocytes, ATMs, as well as circulation of individuals with obesity, playing an important role in regulating beige adipogenesis. , Adipocyte-derived circulating miR-130b mediates metabolic crosstalk between adipose tissue and muscle in overweight/obesity. Similarly, ATM-derived exosomal miR-155 and miR-29a from obese mice have been shown to impair insulin sensitivity in skeletal muscle cells. , miR-222 is another miRNA identified in white adipose tissue-derived exosomes that promotes obesity-associated insulin resistance in skeletal muscle. Also, adipose tissue-derived exosomes containing Let-7d inhibit the proliferation of muscular progenitor cells by suppressing the expression of the transcription factor HMGA2.
Impact of Adipose Tissue-Derived EV-miRNAs on the Liver
Similar to their effects on muscle, miR-130b, miR-155, miR-29a, and miR-222, delivered by EVs, have been shown to activate hepatic inflammation and impair hepatic insulin sensitivity, respectively. ,,, The expression of miR-103 is increased in the adipose tissue-derived exosomes, adipose tissue, and liver of mice with Nonalcoholic Steatohepatitis (NASH), and adipose tissue-derived exosomal miR-103 aggravates NASH, likely by interacting with PTEN and inhibiting autophagy. EVs from adipose tissue stem cells (ASCs) deliver miR-144 and miR-486 into liver, promoting hepatocyte proliferation by suppressing expression of Txnip, a protein of the α-arrestin family that regulates the cellular redox homeostasis.
Impact of Adipose Tissue-Derived EV-miRNAs on the Pancreas
Adipose tissue macrophages have been reported to mediate obesity-induced β-cell dysfunction through releasing extracellular vesicles containing miR-155. Additionally, a recent study showed that miR-27a-5p, elevated in adipocytes and islets in HFD-fed mice and delivered by visceral adipocyte-derived EVs, impairs pancreatic insulin secretion in obesity-associated type 2 diabetes mellitus.
Impact of Adipose Tissue-Derived EV-miRNAs on the Brain
Adipose tissue is also recognized to communicate with the central nervous system via EVs. Wang et al. demonstrated that miR-9–3p in adipose tissue-derived EVs induces synaptic damage and cognitive impairment in obesity-associated insulin resistance.
Impact of Adipose Tissue-Derived EV-miRNAs on the Cardiovascular System
Guo et al. demonstrated that adipose tissue-derived exosomal miR-132/212 from diet-induced obesity mice exacerbates atherosclerosis progression by promoting endothelial apoptosis and vascular smooth muscle cell proliferation. miR-410–5p, upregulated in the expression in the renal and adipose tissues in response to high fat diet, is secreted into the bloodstream in exosomes and transferred to the heart, where it induces cardiac fibrosis. Other studies revealed that hypertrophic adipocyte-derived exosomal miR-802–5p contributes to insulin resistance in cardiac myocytes through targeting HSP60, and perivascular adipose tissue-derived EV miR-221–3p mediates vascular remodeling by enhancing vascular smooth muscle cells proliferation and migration. Adipocyte-derived exosomal miR-27b is also reported to be delivered into endothelial cells to induce inflammation and atherogenesis in obesity by inhibiting PPARα protein expression.
Impact of Brown Adipose Tissue-Derived EV-miRNAs on the Liver
EVs released from brown adipose tissue have recently been recognized to exert beneficial effects. , Exosomal miR-99b derived from brown fat tissue has been identified to regulate expression of fibroblast growth factor-21 (FGF21) in the liver, which is associated with improved glucose tolerance. Another study demonstrated that cold-activated brown fat-derived EV-miR-378a-3p is delivered to the liver, where it stimulates hepatic gluconeogenesis by targeting P110a.
EV-miRNAs Derived from Other Tissues
While adipose tissue is known as a major source of EV-miRNAs in general, other tissues, such as liver and skeletal muscle, have also been demonstrated to release certain EV-miRNAs which exert metabolic functions on distant organs. For example, miR-29b-containing exosomes released from atrophied muscle can be delivered to neuronal cells, leading to inhibition of neuronal differentiation. miR-122 is highly enriched in hepatocytes and hepatocyte-derived exosomes deliver miR-122 to alveolar macrophages, initiating lung inflammation. In another study, hepatocytes release miR-3075-enriched EVs during early onset of obesity, which attenuate insulin resistance via action on adipose tissue and skeletal muscle. These studies suggest a complex interaction among metabolic organs mediated by heterogeneous population of miRNA-containing EVs that originate from different tissues.
EV Biogenesis and Mechanisms of EV-miRNA Sorting
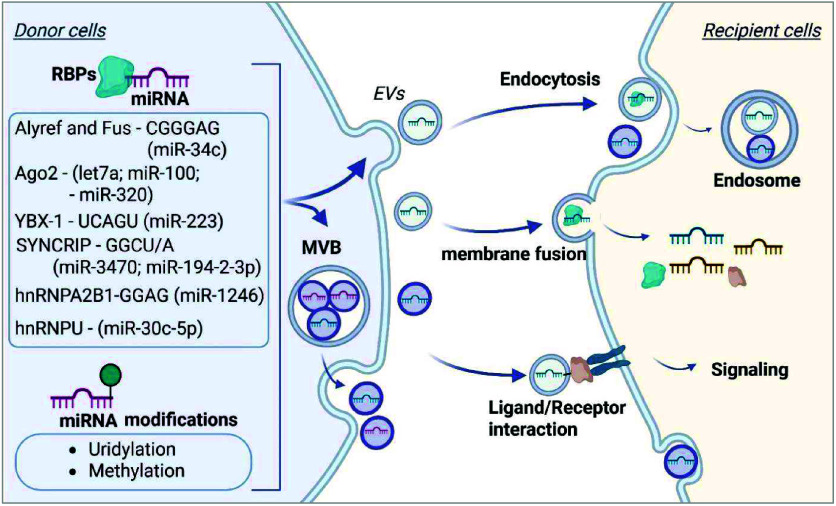
Cell-cell interaction mediated by EV-miRNAs can be influenced by various factors, including donor cell miRNA abundance, the machinery of EV-miRNA secretion from donor cells, and uptake by recipient cells. The miRNA content in EVs differs from that in the donor cells and there is significant heterogeneity in the cargo composition of different EVs, suggesting that miRNAs are selectively sorted into specific EVs. A deeper understanding of the mechanisms behind EV-miRNA sorting and disease specificity is essential for leveraging their diagnostic and therapeutic potential. Although the detailed mechanisms guiding the selection of miRNAs remain largely unclear, analysis of distinct cargo content and cell biology studies have begun to uncover the molecular pathways involved in the processes of miRNA sorting into EVs. ,, Several miRNA recognition and sorting mechanisms have been identified, including specific RNA-binding proteins (RBPs), miRNA-binding motifs, and miRNA sequence modifications (Figure ).
3.
EV-miRNA sorting and uptake pathways. In donor cells, binding of several RNA-binding proteins (RBPs) to specific miRNA motifs and modifications of miRNA sequences impact EV sorting and secretion. Secreted EVs are internalized by the target cells via Endocytosis, or membrane fusion, or act on target cells via Ligand/Receptor interaction. Created in BioRender. Jiang, S. (2025) https://BioRender.com/chuz2q9.
RNA-Binding Proteins (RBPs) and Specific Sequence Motifs of miRNAs
Multiple RBPs have been reported to bind specific RNA molecules, assisting with RNA sorting into EVs. Proteins such as Argonaute 2 (Ago2), hnRNPA2B1, and Y-box binding protein 1 (YBX1) recognize sequence or structural motifs in miRNAs and mediate their packaging into EVs. Specifically, Ago2 has been demonstrated to exert specific control over the sorting of Let7a, miR-100, and miR-320. Specific sequence motifs or secondary structures in miRNAs influence their enrichment in EVs. Research has identified that miRNAs enriched in small extracellular vesicles (sEVs) often possess higher GC content and specific sequence motifs, termed ″EXOmotifs″, which are associated with enhanced miRNA sorting into sEVs compared to those retained in cells. Particularly, introducing EXOmotifs CGGGAG into miR-34c-5p in brown adipocytes results in EV-enriched distribution, and the RNA binding proteins Alyref and Fus are identified to bind to miR-34c-CGGGAG and facilitate sorting. In 293T cells, YBX1 facilitates the sorting of miR-223 into exosomes through binding to the miR-223 sequence motif UCAGU, whereas mitochondrial localized RNA-binding protein YBAP1 acts as a negative regulator of miR-223 enrichment in exosomes. , The RNA binding protein SYNCRIP (Synaptotagmin-binding Cytoplasmic RNA-interacting Protein), also known as hnRNP-Q, has been identified to associate with specific miRNAs enriched in exosomes that contain a common extra-seed sequence (hEXO motif GGCU/A), such as miR-3470a and miR-194–2–3p. Villarroya-Beltri et al. reported that the protein heterogeneous nuclear ribonucleoprotein A2B1 (HNRNPA2B1), after sumoylation, binds miRNAs through recognizing the EXOmotif GGAG and loads miRNAs into exosomes. A recent study identified that the base excision repair enzyme Apurinic/apyrimidinic endonuclease 1 (APE1) cooperates with hnRNPA2B1 for the EV-sorting of a subset of miRNAs, including miR-1246, through direct binding to GGAG stretches. In addition to exosomes, miRNAs can also be selectively sorted into large EVs. In endothelial cells, heterogeneous Nuclear Ribonucleoprotein U (hnRNPU) has been shown to regulate the sorting of miR-30c-5p into large EVs. hnRNPU binds to specific miRNA sequences, retaining them intracellularly and preventing their export. Knockdown of hnRNPU results in increased levels of miR-30c-5p in EVs, indicating its role in miRNA retention and selective export.
Post-transcriptional Modifications
Emerging evidence indicates that post-transcriptional modifications of miRNAs, such as uridylation and methylation, can affect miRNA stability, localization, and interactions with RNA-binding proteins, thereby significantly influencing their selective enrichment into EVs. For instance, miRNAs with uridylation at the 3′ end were preferentially sorted into exosomes, while 3′ end adenylated miRNAs tend to remain within the parent cells. Zhang et al. reported that miRNA 3′-end uridylation mediates the packaging of miR-223/142 into MVs in response to lipopolysaccharide-induced acute lung injury. Recent studies have demonstrated the impact of the methylation status of miRNAs and their functional significance. Garbo et al. showed that N6-methyladenosine (m6A) methylation of miRNAs can inhibit their intracellular function by weakening their coupling to AGO2, while enhance their packaging into EVs by interacting with the RNA-binding protein hnRNPA2B1. In addition, like other RNAs, miRNAs undergo adenosine-to-inosine (A-to-I) editing, which can alter their target specificity and interactions with RNA-binding proteins, affecting the sorting mechanisms of miRNAs into EVs.
EV-miRNA Uptake by Recipient Cells
After being released into the extracellular space, EVs can deliver the cargo into neighboring or distant cells and exert biological functions. In general, recipient cells interact and take up EVs via several pathways, including endocytosis, membrane fusion, and receptor–ligand interaction (Figure ). EVs are typically taken up via endocytosis, including macropinocytosis, Clathrin-mediated endocytosis, and phagocytosis. The process of endocytosis involves the cell’s plasma membrane invaginating to form endosome encapsulating the EVs and cargo release. While less common than endocytosis, EV contents can be directly released into the recipient cell’s cytoplasm via membrane fusion of EVs with the recipient cells. Besides, EVs interact with target cells via receptor–ligand interactions on their surface and trigger downstream signaling pathways. The roles of lipid rafts and a range of specific receptors and protein interactions in EV uptake have been reported and extensively summarized in previous reviews. , In the context of obesity, numerous studies have provided evidence that adipose-derived EVs carry miRNAs that are taken up by a range of cell types, including macrophages, hepatocytes, myocytes, pancreatic cells, etc. (Figure ). It was reported that exosomes derived from brown fat preferentially target liver after intravenous injection and promote energy expenditure in hepatocytes. In vivo assessment of the biodistribution kinetics of white adipocytes-derived EVs demonstrated accumulation of adipose-derived EVs in several target organs, with highest intensities found in the pancreas. These studies support that EV-mediated organ crosstalk might be tissue-specific. , Despite advances in understanding the processes of EV secretion and uptake, the precise mechanisms governing EV targeting specificity across various cell types remain largely unclear. The uptake of miRNA-containing EVs can be influenced by various factors, including the size, contents, surface compounds of EVs, as well as recipient cell-related factors including the recipient cell type, cell state, and cell surface compounds. ,, Adipose-derived EVs carry unique markers such as adiponectin and FABP4. , Identifying the specific signature of miRNA-containing EVs under physiological and pathological conditions may provide insight into the EV targeting specificity.
Technological Advancements in EV-miRNA Analysis
Liquid biopsies, particularly for detecting circulating miRNAs, provide notable advantages in metabolic disorders due to their minimally invasive nature, ease of collection, and suitability for longitudinal monitoring without requiring surgical procedures The high heterogeneous population of EVs that originate from different tissues, contain different miRNAs, and exert different functions represents a challenge for the application of EVs of bodily fluids as specific biomarkers and therapeutic targets. Identifying the cells of origin and specific target tissues of EV-miRNAs and the underlying mechanisms is essential for understanding their physiological functions and disease relevance. Recent technological advancements have significantly improved the analysis of Adipose-derived EV-miRNAs, further enhancing their potential for both research and clinical applications.
Isolation of Adipose-Derived EVs
Isolating EVs is a necessary yet challenging step for downstream applications in EV-miRNA research and clinical diagnostics. , Adipose-derived EVs can be isolated directly from different types of adipose tissues (white and brown fat) or specific cell types of adipose tissues, including adipocytes, adipose tissue macrophages, and adipose stem cells. Advanced isolation methods have been pivotal in improving the purity and specificity of EV isolation. Ultracentrifugation remains the most widely used method for EV isolation. Size exclusion chromatography (SEC) has emerged as a gentle and reproducible technique that separates EVs from contaminants based on the particle size while preserving vesicle integrity. , Polymer-based precipitation methods, such as the commercial ExoQuick kit, offer simplicity and scalability, making them suitable for clinical and high-throughput applications. Immunoaffinity-based techniques further enhance specificity by selectively capturing EV subpopulations using antibody-coated surfaces. Antibodies targeting EV surface markers such as CD9, CD63, and CD81 are employed to specifically isolate vesicles of interest. In parallel with these technologies, a simple and rapid PCR-free integrated microfluidic platform has been developed for the absolute quantification of both free-floating miRNAs and EV-miRNAs in plasma. This platform achieves a detection sensitivity of 1 pM with less than 10% uncertainty and requires only 20 μL of sample, completing the assay within 30 min. Unlike traditional RT-qPCR methods, this technology eliminates the need for EV extraction, RNA purification, reverse transcription, and amplification. These advancements collectively enhance the robustness and reliability of EV isolation and analysis, laying the foundation for precise EV-miRNA analysis and the exploration of their potential as biomarkers in diagnostics and therapeutics.
EV Characterization
Characterizing EVs is essential to understanding their properties, biological functions, and potential applications. Advanced analytical tools have been developed to provide comprehensive insights into EV size, concentration and morphology. Nanoparticle tracking analysis (NTA) is one of the most commonly used methods in EV research, offering quantitative data on particle size and concentration. Nanosight instruments further enhance the capability by incorporating a laser and camera for detecting fluorescent particles. This feature allows researchers to phenotype vesicle subpopulations by labeling them with specific antibodies or fluorescent markers. Electron microscopy (EM) provides high-resolution imaging, offering detailed views of EV morphology and confirming their vesicular structure. Immune-labeling in EM can also identify EV-associated proteins, aiding in the functional analysis of these vesicles. Moreover, flow cytometry enables the detection and analysis of individual EVs, often using fluorescently labeled antibodies against surface markers such as CD9, CD63, and CD81, which are commonly enriched on EVs. One of the great advantages of this method is the ability to analyze multiple labels on individual particles, allowing for the identification of various EV types and subtypes. These advanced characterization techniques collectively enhance our understanding of EVs, paving the way for their application in diagnostics, therapeutics, and broader biological research.
EV-miRNA Profiling Analysis
Next-Generation Sequencing (NGS) technologies have revolutionized miRNA profiling by enabling the high-throughput and highly accurate analysis of miRNA sequences. NGS circumvents issues like cross-hybridization and background noise common to other methods, providing comprehensive profiling of all miRNAs within a sample, including novel and low-abundance miRNAs. The application of NGS in minimally invasive samples, such as serum and plasma, has been particularly valuable for studying circulating miRNAs in metabolic diseases and other conditions. NGS technology for miRNA analysis at single-cell and spatial resolutions has rapidly evolved, helping to uncover complex cell–cell interactions and regulatory mechanisms.
Post-transcriptional Modifications of miRNAs in EVs
Recent advances in sequencing and biomedical techniques have enabled the detection of miRNA post-transcriptional modifications, which influence miRNA stability, localization, and sorting into EVs. For example, platforms such as TAIL-seq and PAL-seq are optimized for analyzing 3′-end modifications in small RNAs. Although mass spectrometry remains technically challenging due to the small size and low abundance of miRNAs, improved enrichment and purification strategies have enhanced its application in PTM validation. Additionally, antibody-based immunoprecipitation methods using antimodified nucleotide antibodies (e.g., anti-m6A or anti-m5C) have been adapted to isolate and characterize chemically modified miRNAs in EV fractions.
Identification of RNA-Binding Proteins in miRNA Sorting into EVs
As discussed previously, several RNA-binding proteins (RBPs) have been reported to recognize and guide miRNAs into EVs. , Techniques such as RNA immunoprecipitation followed by high-throughput sequencing (RIP-seq) are commonly used to detect miRNA and RBP interactions and identify miRNAs selectively enriched in EVs. A study described the artificially barcoded, exosome microRNAs (bEXOmiRs) to monitor EV release quantitatively using deep sequencing. Another high-throughput assay platform, CRISPR-assisted individually barcoded sEV-based release regulator (CIBER) screening for identifying key players in sEV release, enabling the EV biogenesis and release regulator analysis. Integration of lipidomic and proteomic data sets could help uncover specific lipid environments that preferentially bind and sort certain miRNAs.
Identifying the Cellular Origin and Target Tissues of EV-miRNAs
Florescent labeling has become a powerful technique for tracking EV-miRNAs, enabling researchers to study their dynamics, localization, and biological roles. This method involves labeling either the EVs themselves or the miRNAs within them using fluorescent dyes, RNA-specific probes, or tagged molecules. Fluorescent dyes such as PKH26, DiI, and DiO are commonly used to label the lipid bilayer of EVs. These dyes allow researchers to visualize EV uptake by recipient cells and monitor their intracellular trafficking using a fluorescence microscope or flow cytometry. For labeling miRNAs within EVs, molecular beacons or fluorescent RNA-binding probes are commonly employed. These tools hybridize to target miRNA sequences, enabling the direct visualization of specific miRNAs. These advanced labeling techniques offer critical tools for exploring EV-miRNA biology, facilitating a deeper understanding of the mechanisms of miRNA transfer and EV-mediated intercellular and interorgan communication in adipose tissues.
Single-particle techniques, such as high-resolution imaging flow cytometry and nanoflow cytometry, enable the tracing of tissue or cell originated EVs by detecting cell surface markers and other biomolecules through fluorescent labeling. When integrated with computational strategies, such as single-vesicle RNA profiling technologies, these approaches allow for the analysis of the EV RNA content at the single-vesicle level. Enrichment analysis can further identify distinct EV subpopulations and provide insights into their cell-of-origin of the adipose tissues. Additionally, transgenic mouse models expressing fluorescent reporters under cell type specific promoters have been utilized to label EVs originating from defined cell populations. When combined with fluorescence microscopy, these models facilitate the in vivo tracking of EVs from specific cellular sources. In addition, tissue specific miRNA knockout mice, such as using adipocyte specific Adiponectin-cre mice, can be an invaluable tool to pinpoint the miRNA expression in adipose tissue and its interaction with other tissues. Furthermore, adipose tissue transplantation provides useful approaches to track adipose-derived EV-miRNAs.
Therapeutic Targets for Obesity-Related Diseases
MicroRNAs are emerging as attractive therapeutic targets for obesity and other disorders due to their actions as potent intracellular regulators of gene expression and mediators of intercellular communication. The leading challenge of miRNA therapeutics is the difficulty of delivering a specific target. It is crucial to define mechanisms by which EV-miRNAs mediate interaction among metabolic organs, thus providing the basis for guiding Adipose-derived EVs as a potential stable and specific delivery route for miRNA therapeutics. With the knowledge of cellular source of EVs and their functions on target tissues, adipose-derived EVs can be engineered and loaded with specific miRNAs or miRNA antisense inhibitors for the treatment of obesity and obesity-associated metabolic diseases, such as Diabetes and fatty liver diseases.
Summary and Future Perspectives
In summary, Adipose tissue-derived EV-miRNAs hold significant potential as invaluable tools for enhancing precision diagnostics and personalized treatment strategies in obesity-related metabolic disorders. This potential stems from the following unique properties of EV-miRNAs from adipose tissue: 1) Link of EV-miRNA signature to adipose dysfunction: Obesity is associated with dysregulation of adipose tissue miRNA expression and secretion, impacting adipose tissue function and metabolism. The unique EV-miRNA signature links to the source tissue and allows for early detection of adipose tissue dysfunction without direct biopsy of adipose tissue; 2) Pathological relevance to obesity complications: Adipose tissue-derived EV-miRNAs are taken up by specific recipient cells in main metabolic organs and influence numerous biological processes, such as inflammation, insulin action, and cell proliferation, leading to development of metabolic diseases, including diabetes, fatty liver diseases, and cardiovascular diseases etc. The specific sorting of miRNAs into EVs and their actions in specific recipient cells enhance the pathological relevance of EV-miRNAs for precise disease detection and personalized therapeutic strategies; 3) Stability in body fluids: miRNAs are remarkably stable in EVs in body fluids, ensuring sample integrity and accurate analysis results; 4) Noninvasive disease monitoring: The analysis of EV-miRNAs in body fluids offers a straightforward and noninvasive method for monitoring disease progression and evaluating therapeutic responses over time; 5) Insights into disease mechanisms: Advancement in detection and analysis techniques allows for sensitive and specific measurement of miRNAs in EVs. Also, combining EV-miRNA analysis with other omics data, such as proteomics and metabolomics, can provide a comprehensive understanding of disease mechanisms and enhance diagnostic precision.
Despite their potential, several challenges hinder the application of EV-miRNAs in clinical diagnostics and other applications. 1) Current understanding of EV-miRNA sorting and uptake mechanisms remains limited. While multiple adipose-derived EV-miRNAs have been linked to obesity-related pathophysiology (Figure ), the exact molecular pathways governing the sorting of EV-miRNAs from adipose tissue and their uptake by recipient cells in the context of obesity remain largely unclear. 2) It has not been fully elucidated to what extent each specific EV-miRNA in body fluid originates from adipose tissue compared to other tissues/organs. 3) EVs are highly heterogeneous and differ in biogenesis pathways, sizes, cargos, and function. How the tissue/cell types and disease states influence the heterogeneity of EVs and the underlying regulatory mechanisms require further investigation. 4) Reliable methods to isolate different subtypes of EVs and accurately identify the origin cell types of EVs are still underdeveloped. 5) Many human studies have examined circulating miRNAs from whole blood or serum/plasma without the step of EV isolation. The relative clinical potential of these circulating miRNAs, as compared to EV-miRNAs, in obesity diagnostics warrants further exploration. 6) While miRNAs are the most extensively studied small noncoding RNAs, increased evidence suggest that other small RNAs, including tRNA-derived sRNAs and rRNA-derived sRNA, are also present in biological fluids and have potential to be biomarkers and therapeutic targets. Studying their biogenesis, traffic, and biological functions and how they interact with miRNAs may lead to new insight in the field of small RNA research.
Overall, the unique biological and functional properties of EVs and their miRNA cargoes enable their potential for advancing diagnostic precision. A deeper understanding of the mechanisms governing the origin and destination of EV-carried miRNAs, alongside the development of standardized protocols and advanced techniques for EV isolation and characterization, is essential for achieving more precise, early, and personalized diagnostics and ensuring proper disease management.
Acknowledgments
This work is supported by the National Institutes of Health (R01DK138986), Yale department of Pathology, and Yale Liver center.
Glossary
Vocabulary Section
- Extracellular Vesicles (EVs)
EVs are membrane-enclosed nanoparticles released by cells into the extracellular space, involved in intercellular communication.
- MicroRNAs (miRNAs)
miRNAs are small noncoding RNA molecules that regulate gene expression post-transcriptionally by binding to target mRNAs.
- Adipose Tissue
Adipose tissue is a type of connective tissue that stores fat and functions as an endocrine organ by secreting hormones and bioactive molecules. It is broadly categorized into white adipose tissue and brown adipose tissue.
- Obesity
Obesity is a chronic metabolic condition characterized by excessive fat accumulation, which is associated with altered EV-miRNA profiles that contribute to systemic inflammation and metabolic dysregulation.
- Intercellular Communication
the ways by which cells in a multicellular organism communicate with neighboring or distant cells to coordinate their activities.
- EV Profiling
Experimental and computational techniques used to characterize EV populations.
The authors declare no competing financial interest.
Published as part of Precision Chemistry special issue “Precision Diagnostics”.
References
- Hales C. M., Carroll M. D., Fryar C. D., Ogden C. L.. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. NCHS Data Brief. 2020;360:1–8. [PubMed] [Google Scholar]
- Ward Z. J., Bleich S. N., Cradock A. L., Barrett J. L., Giles C. M., Flax C., Long M. W., Gortmaker S. L.. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N Engl J. Med. 2019;381(25):2440–2450. doi: 10.1056/NEJMsa1909301. [DOI] [PubMed] [Google Scholar]
- Collaborators G. B. D. O., Afshin A., Forouzanfar M. H., Reitsma M. B., Sur P., Estep K., Lee A., Marczak L., Mokdad A. H., Moradi-Lakeh M.. et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J. Med. 2017;377(1):13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]; Field A. E., Coakley E. H., Must A., Spadano J. L., Laird N., Dietz W. H., Rimm E., Colditz G. A.. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med. 2001;161(13):1581–1586. doi: 10.1001/archinte.161.13.1581. [DOI] [PubMed] [Google Scholar]
- Cameron N. A., Petito L. C., McCabe M., Allen N. B., O’Brien M. J., Carnethon M. R., Khan S. S.. Quantifying the Sex-Race/Ethnicity-Specific Burden of Obesity on Incident Diabetes Mellitus in the United States, 2001 to 2016: MESA and NHANES. J. Am. Heart Assoc. 2021;10(4):e018799. doi: 10.1161/JAHA.120.018799. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ogden C. L., Carroll M. D., Curtin L. R., McDowell M. A., Tabak C. J., Flegal K. M.. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Cholankeril G., Perumpail R. B., Pham E. A., Ahmed A., Harrison S. A.. Nonalcoholic Fatty Liver Disease: Epidemiology, Natural History, and Diagnostic Challenges. Hepatology. 2016;64(3):954. doi: 10.1002/hep.28719. [DOI] [PubMed] [Google Scholar]; Milic S., Lulic D., Stimac D.. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World J. Gastroenterol. 2014;20(28):9330–9337. doi: 10.3748/wjg.v20.i28.9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A., Reddy S., Goel P.. Causes, Consequences, and Preventive Strategies for Childhood Obesity: A Narrative Review. Cureus. 2024;16(7):e64985. doi: 10.7759/cureus.64985. [DOI] [PMC free article] [PubMed] [Google Scholar]; Viswanathan S., McNelis K., Makker K., Calhoun D., Woo J. G., Balagopal B.. Childhood obesity and adverse cardiometabolic risk in large for gestational age infants and potential early preventive strategies: a narrative review. Pediatr. Res. 2022;92(3):653–661. doi: 10.1038/s41390-021-01904-w. [DOI] [PubMed] [Google Scholar]
- Anderson E. L., Howe L. D., Jones H. E., Higgins J. P., Lawlor D. A., Fraser A.. The Prevalence of Non-Alcoholic Fatty Liver Disease in Children and Adolescents: A Systematic Review and Meta-Analysis. PLoS One. 2015;10(10):e0140908. doi: 10.1371/journal.pone.0140908. [DOI] [PMC free article] [PubMed] [Google Scholar]; Li J., Ha A., Rui F., Zou B., Yang H., Xue Q., Hu X., Xu Y., Henry L., Barakat M.. et al. Meta-analysis: global prevalence, trend and forecasting of non-alcoholic fatty liver disease in children and adolescents, 2000–2021. Aliment Pharmacol Ther. 2022;56(3):396–406. doi: 10.1111/apt.17096. [DOI] [PubMed] [Google Scholar]; Liu J., Mu C., Li K., Luo H., Liu Y., Li Z.. Estimating Global Prevalence of Metabolic Dysfunction-Associated Fatty Liver Disease in Overweight or Obese Children and Adolescents: Systematic Review and Meta-Analysis. Int. J. Public Health. 2021;66:1604371. doi: 10.3389/ijph.2021.1604371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal N. P., Schwimmer J. B.. The progression and natural history of pediatric nonalcoholic fatty liver disease. Clin. Liver Dis. 2016;20(2):325–338. doi: 10.1016/j.cld.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; Brumbaugh D. E., Friedman J. E.. Developmental origins of nonalcoholic fatty liver disease. Pediatr. Res. 2014;75(1–2):140–147. doi: 10.1038/pr.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]; Castillo-Leon E., Cioffi C. E., Vos M. B.. Perspectives on youth-onset nonalcoholic fatty liver disease. Endocrinol Diabetes Metab. 2020;3(4):e00184. doi: 10.1002/edm2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluher M.. Metabolically Healthy Obesity. Endocr Rev. 2020;41:3. doi: 10.1210/endrev/bnaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cheng Q., Yuan X., Lin S., Zhao Y., Wang H., Zhu F., Wang Y., Xu T., Wu J., Wang K.. et al. Serum proteome profiling reveals differentially expressed proteins between subjects with metabolically healthy obesity and nonalcoholic fatty liver disease. J. Proteomics. 2022;260:104556. doi: 10.1016/j.jprot.2022.104556. [DOI] [PubMed] [Google Scholar]
- Krol J., Loedige I., Filipowicz W.. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010;11(9):597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- Bartel D. P.. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- O’Brien J., Hayder H., Zayed Y., Peng C.. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front Endocrinol (Lausanne) 2018;9:402. doi: 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim V. N., Han J., Siomi M. C.. Biogenesis of small RNAs in animals. Nat. Rev. Mol. Cell Biol. 2009;10(2):126–139. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- Bartel D. P.. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J. A., Goberdhan D. C. I., O’Driscoll L., Buzas E. I., Blenkiron C., Bussolati B., Cai H., Di Vizio D., Driedonks T. A. P., Erdbrugger U.. et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell Vesicles. 2024;13(2):e12404. doi: 10.1002/jev2.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrado C., Barreca M. M., Zichittella C., Alessandro R., Conigliaro A.. Molecular Mediators of RNA Loading into Extracellular Vesicles. Cells. 2021;10(12):3355. doi: 10.3390/cells10123355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janas T., Janas M. M., Sapon K., Janas T.. Mechanisms of RNA loading into exosomes. FEBS Lett. 2015;589(13):1391–1398. doi: 10.1016/j.febslet.2015.04.036. [DOI] [PubMed] [Google Scholar]
- Colombo M., Raposo G., Thery C.. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- Nedaeinia R., Manian M., Jazayeri M. H., Ranjbar M., Salehi R., Sharifi M., Mohaghegh F., Goli M., Jahednia S. H., Avan A.. et al. Circulating exosomes and exosomal microRNAs as biomarkers in gastrointestinal cancer. Cancer Gene Ther. 2017;24(2):48–56. doi: 10.1038/cgt.2016.77. [DOI] [PubMed] [Google Scholar]; Jiang H., Qian Y., Shen Z., Liu Y., He Y., Gao R., Shen M., Chen S., Fu Q., Yang T.. Circulating microRNA-135a-3p in serum extracellular vesicles as a potential biological marker of non-alcoholic fatty liver disease. Mol. Med. Rep. 2021;24(1):498. doi: 10.3892/mmr.2021.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryggestad J. B., Teague A. M., Sparling D. P., Jiang S., Chernausek S. D.. Macrophage-Derived microRNA-155 Increases in Obesity and Influences Adipocyte Metabolism by Targeting Peroxisome Proliferator-Activated Receptor Gamma. Obesity (Silver Spring) 2019;27(11):1856–1864. doi: 10.1002/oby.22616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji C., Guo X.. The clinical potential of circulating microRNAs in obesity. Nat. Rev. Endocrinol. 2019;15(12):731–743. doi: 10.1038/s41574-019-0260-0. [DOI] [PubMed] [Google Scholar]
- Benavides-Aguilar J. A., Torres-Copado A., Isidoro-Sanchez J., Pathak S., Duttaroy A. K., Banerjee A., Paul S.. The Regulatory Role of MicroRNAs in Obesity and Obesity-Derived Ailments. Genes (Basel) 2023;14(11):2070. doi: 10.3390/genes14112070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Huang B., Qin Y., Wang D., Jin Y., Su L., Wang Q., Pan Y., Zhang Y., Shen Y.. Adipocyte microRNA-802 promotes adipose tissue inflammation and insulin resistance by modulating macrophages in obesity. Elife. 2024;13:e99162. doi: 10.7554/eLife.99162. [DOI] [PMC free article] [PubMed] [Google Scholar]; Agbu P., Carthew R. W.. MicroRNA-mediated regulation of glucose and lipid metabolism. Nat. Rev. Mol. Cell Biol. 2021;22(6):425–438. doi: 10.1038/s41580-021-00354-w. [DOI] [PMC free article] [PubMed] [Google Scholar]; Matz A. J., Qu L., Karlinsey K., Zhou B.. MicroRNA-regulated B cells in obesity. Immunometabolism (Cobham) 2022;4(3):e00005. doi: 10.1097/IN9.0000000000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lee S., Cho Y. K., Kim H., Choi C., Kim S., Lee Y. H.. miR-10a regulates cell death and inflammation in adipose tissue of male mice with diet-induced obesity. Mol. Metab. 2024;90:102039. doi: 10.1016/j.molmet.2024.102039. [DOI] [PMC free article] [PubMed] [Google Scholar]; Brosnan C. A., Palmer A. J., Zuryn S.. Cell-type-specific profiling of loaded miRNAs from Caenorhabditis elegans reveals spatial and temporal flexibility in Argonaute loading. Nat. Commun. 2021;12(1):2194. doi: 10.1038/s41467-021-22503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Sun H., Zheng L., Zhang J., Su H., Li B., Wu Q., Liu Y., Xu Y., Song X.. et al. Adipose-derived miRNAs as potential biomarkers for predicting adulthood obesity and its complications: A systematic review and bioinformatic analysis. Obes Rev. 2024;25(7):e13748. doi: 10.1111/obr.13748. [DOI] [PubMed] [Google Scholar]; Vonhogen I. G. C., Mohseni Z., Winkens B., Xiao K., Thum T., Calore M., da Costa Martins P. A., de Windt L. J., Spaanderman M. E. A., Ghossein-Doha C.. Circulating miR-216a as a biomarker of metabolic alterations and obesity in women. Noncoding RNA Res. 2020;5(3):144–152. doi: 10.1016/j.ncrna.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S., Kumar A., Kim S., Su Y., Singh S., Sharma M., Almousa S., Rather H. A., Jain H., Lee J.. et al. A Liquid Biopsy-Based Approach to Isolate and Characterize Adipose Tissue-Derived Extracellular Vesicles from Blood. ACS Nano. 2023;17(11):10252–10268. doi: 10.1021/acsnano.3c00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandao B. B., Lino M., Kahn C. R.. Extracellular miRNAs as mediators of obesity-associated disease. J. Physiol. 2022;600(5):1155–1169. doi: 10.1113/JP280910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomou T., Mori M. A., Dreyfuss J. M., Konishi M., Sakaguchi M., Wolfrum C., Rao T. N., Winnay J. N., Garcia-Martin R., Grinspoon S. K.. et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature. 2017;542(7642):450–455. doi: 10.1038/nature21365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raheem J., Sliz E., Shin J., Holmes M. V., Pike G. B., Richer L., Gaudet D., Paus T., Pausova Z.. Visceral adiposity is associated with metabolic profiles predictive of type 2 diabetes and myocardial infarction. Commun. Med. (Lond) 2022;2:81. doi: 10.1038/s43856-022-00140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavalda-Navarro A., Villarroya J., Cereijo R., Giralt M., Villarroya F.. The endocrine role of brown adipose tissue: An update on actors and actions. Rev. Endocr Metab Disord. 2022;23(1):31–41. doi: 10.1007/s11154-021-09640-6. [DOI] [PubMed] [Google Scholar]
- Massier L., Jalkanen J., Elmastas M., Zhong J., Wang T., Nono Nankam P. A., Frendo-Cumbo S., Backdahl J., Subramanian N., Sekine T.. et al. An integrated single cell and spatial transcriptomic map of human white adipose tissue. Nat. Commun. 2023;14(1):1438. doi: 10.1038/s41467-023-36983-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; Backdahl J., Franzen L., Massier L., Li Q., Jalkanen J., Gao H., Andersson A., Bhalla N., Thorell A., Ryden M.. et al. Spatial mapping reveals human adipocyte subpopulations with distinct sensitivities to insulin. Cell Metab. 2021;33(11):2301. doi: 10.1016/j.cmet.2021.10.012. [DOI] [PubMed] [Google Scholar]; Langin D.. Adipocyte heterogeneity revealed by spatial transcriptomics of human adipose tissue: Painting and more. Cell Metab. 2021;33(9):1721–1722. doi: 10.1016/j.cmet.2021.08.011. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Mei H., Chang X., Chen F., Zhu Y., Han X.. Adipocyte-derived microvesicles from obese mice induce M1 macrophage phenotype through secreted miR-155. J. Mol. Cell Biol. 2016;8(6):505–517. doi: 10.1093/jmcb/mjw040. [DOI] [PubMed] [Google Scholar]
- Pan Y., Hui X., Hoo R. L. C., Ye D., Chan C. Y. C., Feng T., Wang Y., Lam K. S. L., Xu A.. Adipocyte-secreted exosomal microRNA-34a inhibits M2 macrophage polarization to promote obesity-induced adipose inflammation. J. Clin Invest. 2019;129(2):834–849. doi: 10.1172/JCI123069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao F., Yu Y., Feng L., Li J., Zhang M., Lan X., Yan X., Liu Y., Guan F., Zhang M.. et al. Adipogenic miR-27a in adipose tissue upregulates macrophage activation via inhibiting PPARgamma of insulin resistance induced by high-fat diet-associated obesity. Exp. Cell Res. 2017;355(2):105–112. doi: 10.1016/j.yexcr.2017.03.060. [DOI] [PubMed] [Google Scholar]
- Ying W., Riopel M., Bandyopadhyay G., Dong Y., Birmingham A., Seo J. B., Ofrecio J. M., Wollam J., Hernandez-Carretero A., Fu W.. Adipose Tissue Macrophage-Derived Exosomal miRNAs Can Modulate In Vivo and In Vitro Insulin Sensitivity. Cell. 2017;171(2):372–384.e312. doi: 10.1016/j.cell.2017.08.035. [DOI] [PubMed] [Google Scholar]
- Liu T., Sun Y. C., Cheng P., Shao H. G.. Adipose tissue macrophage-derived exosomal miR-29a regulates obesity-associated insulin resistance. Biochem. Biophys. Res. Commun. 2019;515(2):352–358. doi: 10.1016/j.bbrc.2019.05.113. [DOI] [PubMed] [Google Scholar]
- Patra D., Ramprasad P., Sharma S., Dey U., Kumar V., Singh S., Dasgupta S., Kumar A., Tikoo K., Pal D.. Adipose tissue macrophage-derived microRNA-210–3p disrupts systemic insulin sensitivity by silencing GLUT4 in obesity. J. Biol. Chem. 2024;300(6):107328. doi: 10.1016/j.jbc.2024.107328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Du H., Wei S., Feng L., Li J., Yao F., Zhang M., Hatch G. M., Chen L.. Adipocyte-Derived Exosomal MiR-27a Induces Insulin Resistance in Skeletal Muscle Through Repression of PPARgamma. Theranostics. 2018;8(8):2171–2188. doi: 10.7150/thno.22565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prats-Puig A., Ortega F. J., Mercader J. M., Moreno-Navarrete J. M., Moreno M., Bonet N., Ricart W., Lopez-Bermejo A., Fernandez-Real J. M.. Changes in circulating microRNAs are associated with childhood obesity. J. Clin Endocrinol Metab. 2013;98(10):E1655–1660. doi: 10.1210/jc.2013-1496. [DOI] [PubMed] [Google Scholar]; Luo W., Kim Y., Jensen M. E., Herlea-Pana O., Wang W., Rudolph M. C., Friedman J. E., Chernausek S. D., Jiang S.. miR-130b/301b Is a Negative Regulator of Beige Adipogenesis and Energy Metabolism In Vitro and In Vivo. Diabetes. 2022;71(11):2360–2371. doi: 10.2337/db22-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. C., Li Y., Wang X. Y., Zhang D., Zhang H., Wu Q., He Y. Q., Wang J. Y., Zhang L., Xia H.. et al. Circulating miR-130b mediates metabolic crosstalk between fat and muscle in overweight/obesity. Diabetologia. 2013;56(10):2275–2285. doi: 10.1007/s00125-013-2996-8. [DOI] [PubMed] [Google Scholar]
- Li D., Song H., Shuo L., Wang L., Xie P., Li W., Liu J., Tong Y., Zhang C. Y., Jiang X.. et al. Gonadal white adipose tissue-derived exosomal MiR-222 promotes obesity-associated insulin resistance. Aging (Albany NY) 2020;12(22):22719–22743. doi: 10.18632/aging.103891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itokazu M., Onodera Y., Mori T., Inoue S., Yamagishi K., Moritake A., Iwawaki N., Shigi K., Takehara T., Higashimoto Y.. et al. Adipose-derived exosomes block muscular stem cell proliferation in aged mouse by delivering miRNA Let-7d-3p that targets transcription factor HMGA2. J. Biol. Chem. 2022;298(7):102098. doi: 10.1016/j.jbc.2022.102098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z., Wang L., Xu S., Zhang H., Cheng J., Pan S.. Microvesicle-Shuttled microRNA-130b Activates the Hepatic Inflammation by Inhibiting Glucocorticoid-Receptor-Mediated Immunosuppression in High-Fat Diet-Induced Obese Mice. Vet Sci. 2024;11(11):565. doi: 10.3390/vetsci11110565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M. M., Ren Y., Zhou Y. W., Xu L. L., Zhang M. M., Ding L. P., Cheng W. X., Jin X.. Antagonizing adipose tissue-derived exosome miR-103-hepatocyte phosphatase and tensin homolog pathway alleviates autophagy in non-alcoholic steatohepatitis: A trans-cellular crosstalk. World J. Gastroenterol. 2023;29(29):4528–4541. doi: 10.3748/wjg.v29.i29.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niitsu Y., Komiya C., Takeuchi A., Hara K., Horino M., Aoki J., Okazaki R., Murakami M., Tsujimoto K., Ikeda K.. et al. Increased serum extracellular vesicle miR-144–3p and miR-486a-3p in a mouse model of adipose tissue regeneration promote hepatocyte proliferation by targeting Txnip. PLoS One. 2023;18(5):e0284989. doi: 10.1371/journal.pone.0284989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H., Luo Z., Jin Z., Ji Y., Ying W.. Adipose Tissue Macrophages Modulate Obesity-Associated beta Cell Adaptations through Secreted miRNA-Containing Extracellular Vesicles. Cells. 2021;10(9):2451. doi: 10.3390/cells10092451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Qian B., Yang Y., Niu F., Lin C., Yuan H., Wang J., Wu T., Shao Y., Shao S.. et al. Visceral Adipocyte-Derived Extracellular Vesicle miR-27a-5p Elicits Glucose Intolerance by Inhibiting Pancreatic beta-Cell Insulin Secretion. Diabetes. 2024;73(11):1832–1847. doi: 10.2337/db24-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Li L., Zhang Z., Zhang X., Zhu Y., Zhang C., Bi Y.. Extracellular vesicles mediate the communication of adipose tissue with brain and promote cognitive impairment associated with insulin resistance. Cell Metab. 2022;34(9):1264–1279.e1268. doi: 10.1016/j.cmet.2022.08.004. [DOI] [PubMed] [Google Scholar]
- Guo B., Zhuang T. T., Li C. C., Li F., Shan S. K., Zheng M. H., Xu Q. S., Wang Y., Lei L. M., Tang K. X.. et al. MiRNA-132/212 encapsulated by adipose tissue-derived exosomes worsen atherosclerosis progression. Cardiovasc Diabetol. 2024;23(1):331. doi: 10.1186/s12933-024-02404-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou T., Zhu M., Ma Y. C., Xiao F., Yu X., Xu L., Ma L. Q., Yang J., Dong J. Z.. MicroRNA-410–5p exacerbates high-fat diet-induced cardiac remodeling in mice in an endocrine fashion. Sci. Rep. 2018;8(1):8780. doi: 10.1038/s41598-018-26646-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z., Li J., Fu Y., Zheng Y., Ma M., Wang C.. Hypertrophic Adipocyte-Derived Exosomal miR-802–5p Contributes to Insulin Resistance in Cardiac Myocytes Through Targeting HSP60. Obesity (Silver Spring) 2020;28(10):1932–1940. doi: 10.1002/oby.22932. [DOI] [PubMed] [Google Scholar]
- Li X., Ballantyne L. L., Yu Y., Funk C. D.. Perivascular adipose tissue-derived extracellular vesicle miR-221–3p mediates vascular remodeling. FASEB J. 2019;33(11):12704–12722. doi: 10.1096/fj.201901548R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Yang L. J., Liu H., Song Y. J., Yang Q. Q., Liu Y., Qian S. W., Tang Q. Q.. Exosomal miR-27b-3p secreted by visceral adipocytes contributes to endothelial inflammation and atherogenesis. Cell Rep. 2023;42(1):111948. doi: 10.1016/j.celrep.2022.111948. [DOI] [PubMed] [Google Scholar]
- Camino T., Lago-Baameiro N., Sueiro A., Bravo S. B., Couto I., Santos F. F., Baltar J., Casanueva F. F., Pardo M.. Brown Adipose Tissue Sheds Extracellular Vesicles That Carry Potential Biomarkers of Metabolic and Thermogenesis Activity Which Are Affected by High Fat Diet Intervention. Int. J. Mol. Sci. 2022;23(18):10826. doi: 10.3390/ijms231810826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Cui L., Wang J., Zheng S., Zhang H., Ke S., Cao X., Shi Y., Li J., Zen K.. et al. Cold-activated brown fat-derived extracellular vesicle-miR-378a-3p stimulates hepatic gluconeogenesis in male mice. Nat. Commun. 2023;14(1):5480. doi: 10.1038/s41467-023-41160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J., Wang L., Zhou Y., Zhang P., Chen X.. Muscle-derived extracellular vesicles mediate crosstalk between skeletal muscle and other organs. Front Physiol. 2025;15:1501957. doi: 10.3389/fphys.2024.1501957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. P., Yang W. S., Wong Y. H., Wang K. H., Teng Y. C., Chang M. H., Liao K. H., Nian F. S., Chao C. C., Tsai J. W.. et al. Muscle atrophy-related myotube-derived exosomal microRNA in neuronal dysfunction: Targeting both coding and long noncoding RNAs. Aging Cell. 2020;19(5):e13107. doi: 10.1111/acel.13107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu J., Sheng M., Cao Y., Jia L., Zhang C., Weng Y., Yu W.. Ischemia and reperfusion-injured liver-derived exosomes elicit acute lung injury through miR-122–5p regulated alveolar macrophage polarization. Int. Immunopharmacol. 2024;131:111853. doi: 10.1016/j.intimp.2024.111853. [DOI] [PubMed] [Google Scholar]
- Ji Y., Luo Z., Gao H., Dos Reis F. C. G., Bandyopadhyay G., Jin Z., Manda K. A., Isaac R., Yang M., Fu W.. et al. Hepatocyte-derived exosomes from early onset obese mice promote insulin sensitivity through miR-3075. Nat. Metab. 2021;3(9):1163–1174. doi: 10.1038/s42255-021-00444-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Groot M., Pinilla-Vera M., Fredenburgh L. E., Jin Y.. Identification of miRNA-rich vesicles in bronchoalveolar lavage fluid: Insights into the function and heterogeneity of extracellular vesicles. J. Controlled Release. 2019;294:43–52. doi: 10.1016/j.jconrel.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nolte-’t Hoen E. N., Buermans H. P., Waasdorp M., Stoorvogel W., Wauben M. H., t Hoen P. A.. Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions. Nucleic Acids Res. 2012;40(18):9272–9285. doi: 10.1093/nar/gks658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixson A. C., Dawson T. R., Di Vizio D., Weaver A. M.. Context-specific regulation of extracellular vesicle biogenesis and cargo selection. Nat. Rev. Mol. Cell Biol. 2023;24(7):454–476. doi: 10.1038/s41580-023-00576-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot M., Lee H.. Sorting Mechanisms for MicroRNAs into Extracellular Vesicles and Their Associated Diseases. Cells. 2020;9(4):1044. doi: 10.3390/cells9041044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie A. J., Hoshino D., Hong N. H., Cha D. J., Franklin J. L., Coffey R. J., Patton J. G., Weaver A. M.. KRAS-MEK Signaling Controls Ago2 Sorting into Exosomes. Cell Rep. 2016;15(5):978–987. doi: 10.1016/j.celrep.2016.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Martin R., Wang G., Brandao B. B., Zanotto T. M., Shah S., Kumar Patel S., Schilling B., Kahn C. R.. MicroRNA sequence codes for small extracellular vesicle release and cellular retention. Nature. 2022;601(7893):446–451. doi: 10.1038/s41586-021-04234-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Singh J., Schekman R.. Two RNA-binding proteins mediate the sorting of miR223 from mitochondria into exosomes. Elife. 2023;12:e85878. doi: 10.7554/eLife.85878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurtleff M. J., Temoche-Diaz M. M., Karfilis K. V., Ri S., Schekman R.. Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. Elife. 2016;5:e19276. doi: 10.7554/eLife.19276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santangelo L., Giurato G., Cicchini C., Montaldo C., Mancone C., Tarallo R., Battistelli C., Alonzi T., Weisz A., Tripodi M.. The RNA-Binding Protein SYNCRIP Is a Component of the Hepatocyte Exosomal Machinery Controlling MicroRNA Sorting. Cell Rep. 2016;17(3):799–808. doi: 10.1016/j.celrep.2016.09.031. [DOI] [PubMed] [Google Scholar]; Hobor F., Dallmann A., Ball N. J., Cicchini C., Battistelli C., Ogrodowicz R. W., Christodoulou E., Martin S. R., Castello A., Tripodi M.. et al. A cryptic RNA-binding domain mediates Syncrip recognition and exosomal partitioning of miRNA targets. Nat. Commun. 2018;9(1):831. doi: 10.1038/s41467-018-03182-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroya-Beltri C., Gutierrez-Vazquez C., Sanchez-Cabo F., Perez-Hernandez D., Vazquez J., Martin-Cofreces N., Martinez-Herrera D. J., Pascual-Montano A., Mittelbrunn M., Sanchez-Madrid F.. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat. Commun. 2013;4:2980. doi: 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiapane G., Notarangelo M., Canarutto G., Fabbiano F., Dalla E., Degrassi M., Antoniali G., Gualandi N., De Sanctis V., Piazza S.. et al. The DNA-repair protein APE1 participates with hnRNPA2B1 to motif-enriched and prognostic miRNA secretion. Oncogene. 2024;43(24):1861–1876. doi: 10.1038/s41388-024-03039-8. [DOI] [PubMed] [Google Scholar]
- Zietzer A., Hosen M. R., Wang H., Goody P. R., Sylvester M., Latz E., Nickenig G., Werner N., Jansen F.. The RNA-binding protein hnRNPU regulates the sorting of microRNA-30c-5p into large extracellular vesicles. J. Extracell Vesicles. 2020;9(1):1786967. doi: 10.1080/20013078.2020.1786967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppers-Lalic D., Hackenberg M., Bijnsdorp I. V., van Eijndhoven M. A. J., Sadek P., Sie D., Zini N., Middeldorp J. M., Ylstra B., de Menezes R. X.. et al. Nontemplated nucleotide additions distinguish the small RNA composition in cells from exosomes. Cell Rep. 2014;8(6):1649–1658. doi: 10.1016/j.celrep.2014.08.027. [DOI] [PubMed] [Google Scholar]
- Zhang D., Lee H., Wang X., Groot M., Sharma L., Dela Cruz C. S., Jin Y.. A potential role of microvesicle-containing miR-223/142 in lung inflammation. Thorax. 2019;74(9):865–874. doi: 10.1136/thoraxjnl-2018-212994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbo S., D’Andrea D., Colantoni A., Fiorentino F., Mai A., Ramos A., Tartaglia G. G., Tancredi A., Tripodi M., Battistelli C.. m6A modification inhibits miRNAs’ intracellular function, favoring their extracellular export for intercellular communication. Cell Rep. 2024;43(6):114369. doi: 10.1016/j.celrep.2024.114369. [DOI] [PubMed] [Google Scholar]
- Kwok Z. H., Wang C., Jin Y.. Extracellular Vesicle Transportation and Uptake by Recipient Cells: A Critical Process to Regulate Human Diseases. Processes (Basel) 2021;9(2):273. doi: 10.3390/pr9020273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy L. A., Pink R. C., Carter D. R.. Routes and mechanisms of extracellular vesicle uptake. J. Extracell Vesicles. 2014:3. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. J., Wang C.. A review of the regulatory mechanisms of extracellular vesicles-mediated intercellular communication. Cell Commun. Signal. 2023;21(1):77. doi: 10.1186/s12964-023-01103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Li Z., Qi M., Zhao P., Duan Y., Yang G., Yuan L.. Brown adipose tissue-derived exosomes mitigate the metabolic syndrome in high fat diet mice. Theranostics. 2020;10(18):8197–8210. doi: 10.7150/thno.43968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulaj K., Harger A., Bauer M., Caliskan O. S., Gupta T. K., Chiang D. M., Milbank E., Reber J., Karlas A., Kotzbeck P.. et al. Adipocyte-derived extracellular vesicles increase insulin secretion through transport of insulinotropic protein cargo. Nat. Commun. 2023;14(1):709. doi: 10.1038/s41467-023-36148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissanayake K., Godakumara K., Muhandiram S., Kodithuwakku S., Fazeli A.. Do extracellular vesicles have specific target cells?; Extracellular vesicle mediated embryo maternal communication. Front Mol. Biosci. 2024;11:1415909. doi: 10.3389/fmolb.2024.1415909. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mathieu M., Martin-Jaular L., Lavieu G., Thery C.. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019;21(1):9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- Yanez-Mo M., Siljander P. R., Andreu Z., Zavec A. B., Borras F. E., Buzas E. I., Buzas K., Casal E., Cappello F., Carvalho J.. et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbar N., Pinnick K. E., Paget D., Choudhury R. P.. Isolation and Characterization of Human Adipocyte-Derived Extracellular Vesicles using Filtration and Ultracentrifugation. J. Vis Exp. 2021;(170):e61979. doi: 10.3791/61979. [DOI] [PubMed] [Google Scholar]
- Di K., Fan B., Gu X., Huang R., Khan A., Liu C., Shen H., Li Z.. Highly efficient and automated isolation technology for extracellular vesicles microRNA. Front Bioeng Biotechnol. 2022;10:948757. doi: 10.3389/fbioe.2022.948757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercadal M., Herrero C., Lopez-Rodrigo O., Castells M., de la Fuente A., Vigues F., Bassas L., Larriba S.. Impact of Extracellular Vesicle Isolation Methods on Downstream Mirna Analysis in Semen: A Comparative Study. Int. J. Mol. Sci. 2020;21(17):5949. doi: 10.3390/ijms21175949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoorvogel W.. Functional transfer of microRNA by exosomes. Blood. 2012;119(3):646–648. doi: 10.1182/blood-2011-11-389478. [DOI] [PubMed] [Google Scholar]
- Boing A. N., van der Pol E., Grootemaat A. E., Coumans F. A., Sturk A., Nieuwland R.. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J. Extracell Vesicles. 2014;3:3. doi: 10.3402/jev.v3.23430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D. J., Di K. L., Fan B. Y., Wu J., Gu X. R., Sun Y. F., Khan A., Li P., Li Z. Y.. MicroRNAs in extracellular vesicles: Sorting mechanisms, diagnostic value, isolation, and detection technology. Front Bioeng Biotech. 2022;10:948959. doi: 10.3389/fbioe.2022.948959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider M. A., Hurwitz S. N., Meckes D. G. Jr.. ExtraPEG: A Polyethylene Glycol-Based Method for Enrichment of Extracellular Vesicles. Sci. Rep. 2016;6:23978. doi: 10.1038/srep23978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi E. S., Faruque H. A., Kim J. H., Kim K. J., Choi J. E., Kim B. A., Kim B., Kim Y. J., Woo M. H., Park J. Y.. CD5L as an Extracellular Vesicle-Derived Biomarker for Liquid Biopsy of Lung Cancer. Diagnostics (Basel) 2021;11(4):620. doi: 10.3390/diagnostics11040620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauro B. J., Greening D. W., Mathias R. A., Ji H., Mathivanan S., Scott A. M., Simpson R. J.. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods. 2012;56(2):293–304. doi: 10.1016/j.ymeth.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Ramshani Z., Zhang C., Richards K., Chen L., Xu G., Stiles B. L., Hill R., Senapati S., Go D. B., Chang H. C.. Extracellular vesicle microRNA quantification from plasma using an integrated microfluidic device. Commun. Biol. 2019;2:189. doi: 10.1038/s42003-019-0435-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sousa K. P., Rossi I., Abdullahi M., Ramirez M. I., Stratton D., Inal J. M.. Isolation and characterization of extracellular vesicles and future directions in diagnosis and therapy. Wiley Interdiscip Rev. Nanomed Nanobiotechnol. 2023;15(1):e1835. doi: 10.1002/wnan.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crescitelli R., Lasser C., Jang S. C., Cvjetkovic A., Malmhall C., Karimi N., Hoog J. L., Johansson I., Fuchs J., Thorsell A.. et al. Subpopulations of extracellular vesicles from human metastatic melanoma tissue identified by quantitative proteomics after optimized isolation. J. Extracell Vesicles. 2020;9(1):1722433. doi: 10.1080/20013078.2020.1722433. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gavinho B., Sabatke B., Feijoli V., Rossi I. V., da Silva J. M., Evans-Osses I., Palmisano G., Lange S., Ramirez M. I.. Peptidylarginine Deiminase Inhibition Abolishes the Production of Large Extracellular Vesicles From Giardia intestinalis, Affecting Host-Pathogen Interactions by Hindering Adhesion to Host Cells. Front Cell Infect Microbiol. 2020;10:417. doi: 10.3389/fcimb.2020.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgeorges A., Hollerweger J., Lassacher T., Rohde E., Helmbrecht C., Gimona M.. Differential fluorescence nanoparticle tracking analysis for enumeration of the extracellular vesicle content in mixed particulate solutions. Methods. 2020;177:67–73. doi: 10.1016/j.ymeth.2020.02.006. [DOI] [PubMed] [Google Scholar]
- Sorrells J. E., Martin E. M., Aksamitiene E., Mukherjee P., Alex A., Chaney E. J., Marjanovic M., Boppart S. A.. Label-free characterization of single extracellular vesicles using two-photon fluorescence lifetime imaging microscopy of NAD(P)H. Sci. Rep. 2021;11(1):3308. doi: 10.1038/s41598-020-80813-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C., Ulke-Lemee A., Deng J., Batulan Z., O’Brien E. R.. Characterization of heat shock protein 27 in extracellular vesicles: a potential anti-inflammatory therapy. FASEB J. 2019;33(2):1617–1630. doi: 10.1096/fj.201800987R. [DOI] [PubMed] [Google Scholar]
- Friedlander M. R., Chen W., Adamidi C., Maaskola J., Einspanier R., Knespel S., Rajewsky N.. Discovering microRNAs from deep sequencing data using miRDeep. Nat. Biotechnol. 2008;26(4):407–415. doi: 10.1038/nbt1394. [DOI] [PubMed] [Google Scholar]
- Diehl P., Fricke A., Sander L., Stamm J., Bassler N., Htun N., Ziemann M., Helbing T., El-Osta A., Jowett J. B.. et al. Microparticles: major transport vehicles for distinct microRNAs in circulation. Cardiovasc. Res. 2012;93(4):633–644. doi: 10.1093/cvr/cvs007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Wang X., Na X., Zhang Y., Li Z., Chen X., Cai L., Song J., Xu R., Yang C.. Highly Multiplexed, Efficient, and Automated Single-Cell MicroRNA Sequencing with Digital Microfluidics. Small Methods. 2024;8(3):e2301250. doi: 10.1002/smtd.202301250. [DOI] [PubMed] [Google Scholar]; Wang N., Zheng J., Chen Z., Liu Y., Dura B., Kwak M., Xavier-Ferrucio J., Lu Y. C., Zhang M., Roden C.. et al. Single-cell microRNA-mRNA co-sequencing reveals non-genetic heterogeneity and mechanisms of microRNA regulation. Nat. Commun. 2019;10(1):95. doi: 10.1038/s41467-018-07981-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bai Z., Zhang D., Gao Y., Tao B., Zhang D., Bao S., Enninful A., Wang Y., Li H., Su G.. et al. Spatially exploring RNA biology in archival formalin-fixed paraffin-embedded tissues. Cell. 2024;187(23):6760–6779.e6724. doi: 10.1016/j.cell.2024.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Nie H., Lu F.. Dynamic RNA 3′ Uridylation and Guanylation during Mitosis. iScience. 2020;23(8):101402. doi: 10.1016/j.isci.2020.101402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H. D., Min J. E., Choi M., Jeong S. Y., Moon K. S., Lee J. H., Eom H. Y.. LC-MS-Based Direct Quantification of MicroRNAs in Rat Blood. ACS Omega. 2023;8(44):41728–41736. doi: 10.1021/acsomega.3c06045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa S., Wakata Y., Ebi F., Isobe M., Kurosawa N.. Development and validation of monoclonal antibodies against N6-methyladenosine for the detection of RNA modifications. PLoS One. 2019;14(10):e0223197. doi: 10.1371/journal.pone.0223197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannigan M. M., Zagore L. L., Licatalosi D. D.. Mapping transcriptome-wide protein-RNA interactions to elucidate RNA regulatory programs. Quant Biol. 2018;6(3):228–238. doi: 10.1007/s40484-018-0145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee P., Raghava Kurup R., Hundley H. A.. RNA immunoprecipitation to identify in vivo targets of RNA editing and modifying enzymes. Methods Enzymol. 2021;658:137–160. doi: 10.1016/bs.mie.2021.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A., Wawro P., Morgens D. W., Portela F., Bassik M. C., Pfeffer S. R.. Genome-wide interrogation of extracellular vesicle biology using barcoded miRNAs. Elife. 2018;7:e41460. doi: 10.7554/eLife.41460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunitake K., Mizuno T., Hattori K., Oneyama C., Kamiya M., Ota S., Urano Y., Kojima R.. Barcoding of small extracellular vesicles with CRISPR-gRNA enables comprehensive, subpopulation-specific analysis of their biogenesis and release regulators. Nat. Commun. 2024;15(1):9777. doi: 10.1038/s41467-024-53736-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Di K., Fan B., Wu J., Gu X., Sun Y., Khan A., Li P., Li Z.. MicroRNAs in extracellular vesicles: Sorting mechanisms, diagnostic value, isolation, and detection technology. Front Bioeng Biotechnol. 2022;10:948959. doi: 10.3389/fbioe.2022.948959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzar Dominkus P., Stenovec M., Sitar S., Lasic E., Zorec R., Plemenitas A., Zagar E., Kreft M., Lenassi M.. PKH26 labeling of extracellular vesicles: Characterization and cellular internalization of contaminating PKH26 nanoparticles. Biochim Biophys Acta Biomembr. 2018;1860(6):1350–1361. doi: 10.1016/j.bbamem.2018.03.013. [DOI] [PubMed] [Google Scholar]
- Riches A., Campbell E., Borger E., Powis S.. Regulation of exosome release from mammary epithelial and breast cancer cells - a new regulatory pathway. Eur. J. Cancer. 2014;50(5):1025–1034. doi: 10.1016/j.ejca.2013.12.019. [DOI] [PubMed] [Google Scholar]
- Cha M., Jeong S. H., Bae S., Park J. H., Baeg Y., Han D. W., Kim S. S., Shin J., Park J. E., Oh S. W.. et al. Efficient Labeling of Vesicles with Lipophilic Fluorescent Dyes via the Salt-Change Method. Anal. Chem. 2023;95(14):5843–5849. doi: 10.1021/acs.analchem.2c05166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautaniemi K., Zini J., Lofman E., Saari H., Haapalehto I., Laukka J., Vesamaki S., Efimov A., Yliperttula M., Laaksonen T.. et al. Addressing challenges in the removal of unbound dye from passively labelled extracellular vesicles. Nanoscale Adv. 2021;4(1):226–240. doi: 10.1039/D1NA00755F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rond L., Libregts S., Rikkert L. G., Hau C. M., van der Pol E., Nieuwland R., van Leeuwen T. G., Coumans F. A. W.. Refractive index to evaluate staining specificity of extracellular vesicles by flow cytometry. J. Extracell Vesicles. 2019;8(1):1643671. doi: 10.1080/20013078.2019.1643671. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shang M., Ji J. S., Song C., Gao B. J., Jin J. G., Kuo W. P., Kang H.. Extracellular Vesicles: A Brief Overview and Its Role in Precision Medicine. Methods Mol. Biol. 2017;1660:1–14. doi: 10.1007/978-1-4939-7253-1_1. [DOI] [PubMed] [Google Scholar]
- Luo T., Chen S. Y., Qiu Z. X., Miao Y. R., Ding Y., Pan X. Y., Li Y., Lei Q., Guo A. Y.. Transcriptomic Features in a Single Extracellular Vesicle via Single-Cell RNA Sequencing. Small Methods. 2022;6(11):e2200881. doi: 10.1002/smtd.202200881. [DOI] [PubMed] [Google Scholar]; Pultar M., Oesterreicher J., Hartmann J., Weigl M., Diendorfer A., Schimek K., Schadl B., Heuser T., Brandstetter M., Grillari J.. et al. Analysis of extracellular vesicle microRNA profiles reveals distinct blood and lymphatic endothelial cell origins. J. Extracell Biol. 2024;3(1):e134. doi: 10.1002/jex2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Wang J., Yin X., Shi H., Sun B., Ji M., Song H., Liu J., Dou Y., Xu C.. et al. Construction of a mouse model that can be used for tissue-specific EV screening and tracing in vivo. Front Cell Dev Biol. 2022;10:1015841. doi: 10.3389/fcell.2022.1015841. [DOI] [PMC free article] [PubMed] [Google Scholar]; Luo W., Dai Y., Chen Z., Yue X., Andrade-Powell K. C., Chang J.. Spatial and temporal tracking of cardiac exosomes in mouse using a nano-luciferase-CD63 fusion protein. Commun. Biol. 2020;3(1):114. doi: 10.1038/s42003-020-0830-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R. M., Zhao S., Ramirez Solano M. A., Zhu W., Michell D. L., Wang Y., Shyr Y., Sethupathy P., Linton M. F., Graf G. A.. et al. Bioinformatic analysis of endogenous and exogenous small RNAs on lipoproteins. J. Extracell Vesicles. 2018;7(1):1506198. doi: 10.1080/20013078.2018.1506198. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kuhle B., Chen Q., Schimmel P.. tRNA renovatio: Rebirth through fragmentation. Mol. Cell. 2023;83(22):3953–3971. doi: 10.1016/j.molcel.2023.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]