Abstract
Brain capillaries contribute to neurovascular coupling (NVC) by sensing neural activity and coordinating upstream arteriole dilation. However, the mechanisms underlying conducted vasodilation remain incompletely understood. Recent findings (PNAS, 2024) identify a novel process, “electrocalcium coupling,” in which hyperpolarizing signals from K+ channels drive long-range Ca2+ signaling in capillaries, revealing new insights into the integration of vasodilatory signals in the brain.
Keywords: Neurovascular coupling, cerebral blood flow, Ca2+ signaling, brain capillaries, membrane potential, K+ channels, TRP channels
Neurovascular coupling (NVC) ensures that cerebral blood flow is rapidly redirected to the brain’s most active regions [1]. This process relies on the brain’s extensive capillary network, which functions as a sensory web to detect neural activity and coordinate upstream arteriole dilation, thereby increasing local blood flow [2]. Capillary endothelial cells express multiple sensors of localized neuronal activity that orchestrate conductive vasodilation through distinct mechanisms. Reactive oxygen species generate substances that activate TRPA1 (transient receptor potential ankyrin 1) cation channels in brain capillary endothelial cells, inducing intercellular Ca2+ waves that are converted to electrical signals within a specialized vascular segment known as the arteriole-capillary transition (ACT) zone [3]. Prostacyclin triggers slowly propagating, Ca2+-dependent vasodilator signals through pathways linked to capillary endothelial cell EP1 Gq-protein coupled receptors (GqPCRs) [4]. In contrast, stimulation of inwardly rectifying K+ (KIR) channels on capillary endothelial cells with K+ ions generates hyperpolarizing signals that rapidly propagate through the vascular network by electrical conduction [2]. Initially, it was proposed that conducted vasodilation instigated by KIR channels was purely electrical and independent of Ca2+ [2]. This assumption was challenged by a study revealing that brain capillaries exhibit diverse, neuronal activity-driven Ca2+ signaling patterns in vivo [5]. This activity includes long-lasting, high-amplitude events resulting from Ca2+ release mediated by inositol triphosphate (IP3) receptors on the endoplasmic reticulum (ER) [5]. Intriguingly, this study also reported that blocking KIR channels with Ba2+ suppressed Ca2+ signaling activity [5], suggesting that these events depend on plasma membrane hyperpolarization.
Building on this foundation, Nelson and colleagues recently investigated whether electrical and Ca2+ signals in brain capillaries operate in distinct spatiotemporal domains or are functionally linked [6]. Their findings, published in the December 2024 Proceedings of the National Academy of Sciences, provide revolutionary insight into vasodilator signal integration during NVC (Figure 1).
Figure 1. Proposed mechanism of electrocalcium coupling in brain capillaries.
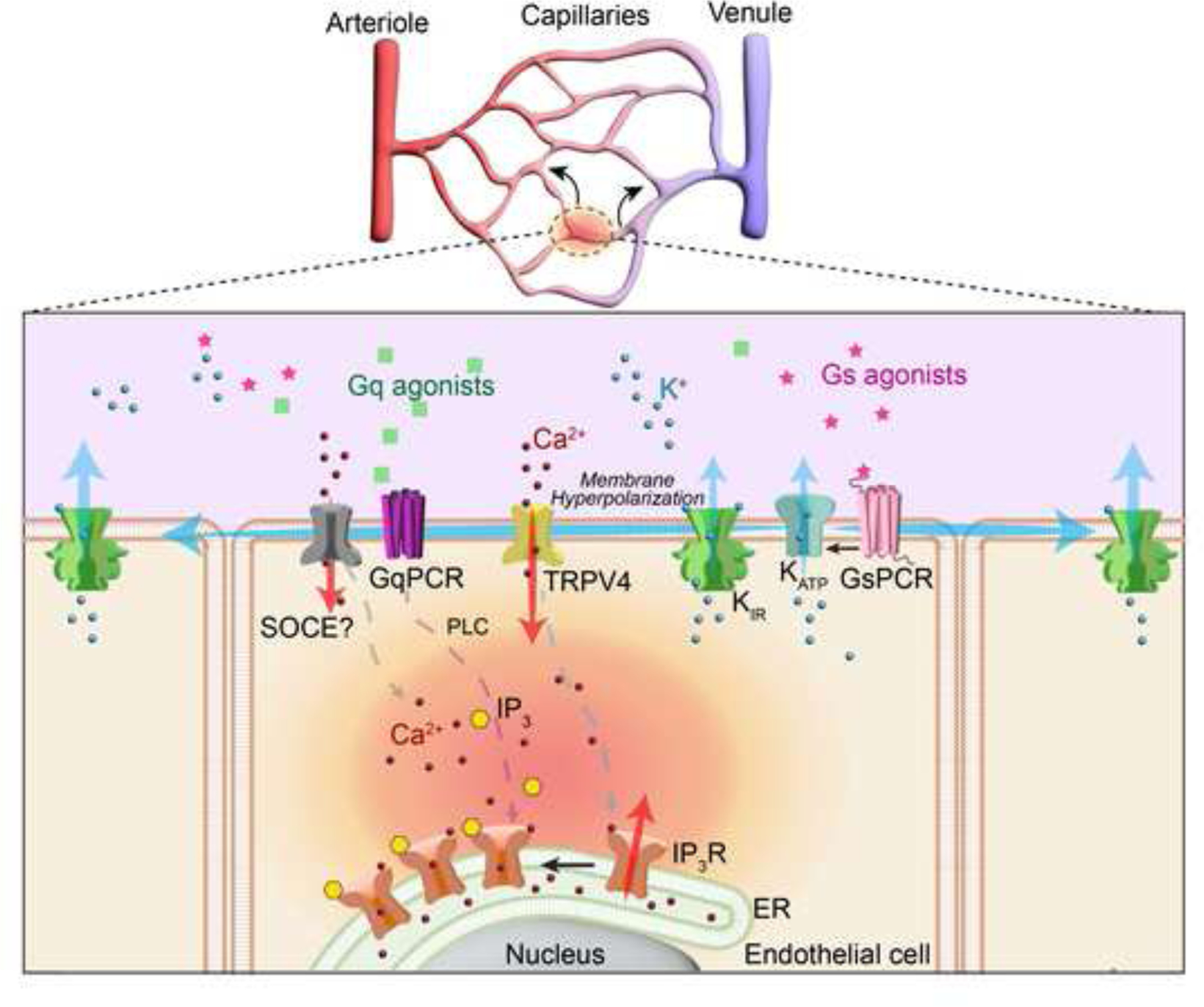
Neuronally driven increases in extracellular K+ activating KIR2.1 channel and/or locally released or circulating GsPCR agonists activating KATP channels hyperpolarize the endothelial cell plasma membrane, enhancing the electrochemical driving force for Ca2+ influx through TRPV4 channels. This initial Ca2+ signal is amplified by Ca2+-induced Ca2+ release via IP₃ receptors (IP₃Rs), requiring IP₃ generated by GqPCR/phospholipase C (PLC) signaling. Depletion of ER Ca2+ stores may also activate store-operated Ca2+ entry (SOCE). Bidirectional conduction of hyperpolarization stimulates KIR2.1 activity, regenerating the electrical signal to “pseudopropagate” Ca2+ signaling throughout the capillary network.
Mughal et al. used in vivo imaging and computational modeling to demonstrate that electrical signals generated by KIR2.1 channels drive dynamic, long-distance Ca2+ signaling in brain capillaries by a process they call “electrocalcium coupling”. Ca2+ signaling activity in capillaries within the brain’s barrel cortex, measured using 2-photon microscopy, was 70–80% lower in endothelial cell-specific KIR2.1 knockout mice compared with controls, confirming the effects of Ba2+ block [5]. Activation of KIR2.1 by applying a modest concentration of KCl (10 mM) directly to a single ACT zone-adjacent capillary increased Ca2+ signaling activity throughout the field of view, which roughly encompasses the brain area perfused by a single parenchymal arteriole. These data suggest that hyperpolarization of the endothelial cell plasma membrane creates a propagating signal that enhances Ca2+ dynamics throughout the local capillary network. Further analysis showed that induced Ca2+ signals were evenly distributed on the arteriole and venule sides of the capillary beds, suggesting that electrical signal propagation is bidirectional. The authors propose that hyperpolarization-induced increases in the electrochemical driving force for Ca2+ influx, coupled with the unique properties of KIR2.1 channels - activation by modest increases in extracellular K+ and membrane hyperpolarization [7] - allow rapid, long-range electrical signals to spread through the network, with hyperpolarization of endothelial cells at distal sites driving Ca2+ influx. The authors call this process “pseudopropagation” of Ca2+ signalings to distinguish it from ATP-mediated intercellular Ca2+ waves that travel through the brain capillary network [3].
Electrocalcium coupling in brain capillaries was further explored by inhibiting ATP-sensitive K+ (KATP) channels with the selective antagonist glibenclamide. This treatment suppressed baseline Ca2+ signals in brain capillary ECs, providing evidence that tonic KATP channel activity contributes to maintaining a hyperpolarized membrane potential. Inhibition of adenylate cyclase with SQ22536 also suppressed baseline Ca2+ signaling. The authors argue that this effect is due to the suppression of protein-kinase A-dependent activation of KATP channels downstream of an unidentified Gs-PCR (GsPCR). However, it is also possible that increased ATP levels following adenylate cyclase block account for decreased channel activity. Applying a bolus of the selective KATP channel activator pinacidil to a single capillary bidirectionally increased Ca2+ signal activity throughout the field of view, similar to the effects of KIR2.1 channel activation. Pinacidil did not impact capillary Ca2+ dynamics in endothelial cell KIR2.1 knockout mice, suggesting that membrane hyperpolarization induced by KATP channel activity is propagated by KIR2.1 channels to produce long-distance Ca2+ signaling. Interestingly, pharmacological inhibition of TRPV4 (TRP vanilloid 4) eliminated increases in Ca2+ signaling activity in response to activators of KATP and KIR2.1 channels, suggesting that membrane hyperpolarization drives Ca2+ influx through tonically activated TRPV4 channels.
Mughal et al. further identified GqPCR signaling and ER Ca2+ stores as essential components of electrocalcium coupling. They show endothelial cell-specific deletion of Gαq/11 expression reduces baseline capillary endothelial cell Ca2+ signals and prevents the induction of Ca2+ events in response to focal stimulation of KATP and KIR2.1 channels. In addition, the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) pump inhibitor cyclopiazonic acid (CPA) also significantly reduced baseline and hyperpolarization-evoked Ca2+ signals, underscoring the importance of intracellular Ca2+ stores. The authors propose that hyperpolarization-driven Ca2+ signals are sustained by ER Ca2+ release, likely mediated by IP3 generated downstream of GqPCRs. Computational modeling studies bolstered these findings, showing that Ca2+ influx and ER store recharge are necessary to sustain electrocalcium coupling, suggesting a potential role for store-operated Ca2+ entry in this process.
The work of Mughal et al. provides compelling evidence that hyperpolarizing electrical signals generated by K+ channel activity are converted into Ca2+ signals that “pseudopropagate” over large areas of the cerebral capillary network (Figure 1). Interestingly, these findings parallel a previous study showing that intercellular Ca2+ signals slowly propagating through capillaries are converted into fast electrical signals within the ACT zone [3]. The dynamic interplay of electrical and Ca2+ signaling brain capillaries suggests a tightly regulated “exchange rate” for vasodilator mechanisms, with implications for NVC. Future research should explore the contribution of bidirectional electrocalcium coupling to CBF regulation and other critical processes, such as glymphatic clearance [8]. Understanding how electrocalcium coupling is altered in pathologies affecting NVC, such as aging, Alzheimer’s disease [9], and cerebral small vessel diseases, [10] will be crucial for identifying novel therapeutic targets.
Grants
This study was supported by grants from NIH/NHLBI (R35HL155008) and NIH/NINDS (R33NS115132) to SE.
Footnotes
Declaration of Interest Statement
The authors declare no conflicts of interest, financial or otherwise.
CRediT authorship contribution statement
Boris Lavanderos: Writing – Original Draft. Writing – review & editing María Paz Saldias: Visualization.
Scott Earley: Supervision, Writing – Original Draft. Writing – review & editing. Funding acquisition.
REFERENCES CITED
- [1].Schaeffer S, Iadecola C, Revisiting the neurovascular unit, Nat Neurosci, 24 (2021) 1198–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Longden TA, Dabertrand F, Koide M, Gonzales AL, Tykocki NR, Brayden JE, Hill-Eubanks D, Nelson MT, Capillary K(+)-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow, Nat Neurosci, 20 (2017) 717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Thakore P, Alvarado MG, Ali S, Mughal A, Pires PW, Yamasaki E, Pritchard HA, Isakson BE, Tran CHT, Earley S, Brain endothelial cell TRPA1 channels initiate neurovascular coupling, Elife, 10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rosehart AC, Longden TA, Weir N, Fontaine JT, Joutel A, Dabertrand F, Prostaglandin E(2) Dilates Intracerebral Arterioles When Applied to Capillaries: Implications for Small Vessel Diseases, Front Aging Neurosci, 13 (2021) 695965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Longden TA, Mughal A, Hennig GW, Harraz OF, Shui B, Lee FK, Lee JC, Reining S, Kotlikoff MI, Konig GM, Kostenis E, Hill-Eubanks D, Nelson MT, Local IP(3) receptor-mediated Ca(2+) signals compound to direct blood flow in brain capillaries, Sci Adv, 7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mughal A, Hennig GW, Heppner T, Tsoukias NM, Hill-Eubanks D, Nelson MT, Electrocalcium coupling in brain capillaries: Rapidly traveling electrical signals ignite local calcium signals, Proc Natl Acad Sci U S A, 121 (2024) e2415047121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Longden TA, Nelson MT, Vascular inward rectifier K+ channels as external K+ sensors in the control of cerebral blood flow, Microcirculation, 22 (2015) 183–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mestre H, Mori Y, Nedergaard M, The Brain’s Glymphatic System: Current Controversies, Trends Neurosci, 43 (2020) 458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mughal A, Harraz OF, Gonzales AL, Hill-Eubanks D, Nelson MT, PIP(2) Improves Cerebral Blood Flow in a Mouse Model of Alzheimer’s Disease, Function (Oxf), 2 (2021) zqab010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Thakore P, Yamasaki E, Ali S, Sanchez Solano A, Labelle-Dumais C, Gao X, Chaumeil MM, Gould DB, Earley S, PI3K block restores age-dependent neurovascular coupling defects associated with cerebral small vessel disease, Proc Natl Acad Sci U S A, 120 (2023) e2306479120. [DOI] [PMC free article] [PubMed] [Google Scholar]


