Abstract
The human immunodeficiency virus type 1 (HIV-1) vif gene is conserved among most lentiviruses, suggesting that vif is important for natural infection. To determine whether an intact vif gene is positively selected during mother-to-infant transmission, we analyzed vif sequences from five infected mother-infant pairs following perinatal transmission. The coding potential of the vif open reading frame directly derived from uncultured peripheral blood mononuclear cell DNA was maintained in most of the 78,912 bp sequenced. We found that 123 of the 137 clones analyzed showed an 89.8% frequency of intact vif open reading frames. There was a low degree of heterogeneity of vif genes within mothers, within infants, and between epidemiologically linked mother-infant pairs. The distances between vif sequences were greater in epidemiologically unlinked individuals than in epidemiologically linked mother-infant pairs. Furthermore, the epidemiologically linked mother-infant pair vif sequences displayed similar patterns that were not seen in vif sequences from epidemiologically unlinked individuals. The functional domains, including the two cysteines at positions 114 and 133, a serine phosphorylation site at position 144, and the C-terminal basic amino acids essential for vif protein function, were highly conserved in most of the sequences. Phylogenetic analyses of 137 mother-infant pair vif sequences and 187 other available vif sequences from HIV-1 databases revealed distinct clusters for vif sequences from each mother-infant pair and for other vif sequences. Taken together, these findings suggest that vif plays an important role in HIV-1 infection and replication in mothers and their perinatally infected infants.
The majority of AIDS cases in children occur as a result of mother-to-infant transmission of human immunodeficiency virus type 1 (HIV-1), at an estimated rate of more than 30% (1, 8, 12, 13, 24, 29, 37, 38, 50, 54, 61, 65). However, the molecular mechanisms and the factors involved in perinatal transmission are not known, making it difficult to develop strategies for the prevention and treatment of HIV-1 infection in children. Several maternal factors, including the advanced clinical stage of the mother, low CD4+ cell counts, maternal immune response to HIV-1 antigenemia, recent infection, high levels of circulating HIV-1, and maternal disease progression, have been implicated in an increased risk of mother-to-infant transmission of HIV-1 (1, 5, 7, 8, 13, 20, 22, 49, 50). Furthermore, the possibility of viral determinants associated with maternal transmission cannot be discounted, since more than half of the children born to HIV-1-infected mothers are uninfected.
In addition to the usual retroviral gag, pol, and env genes, HIV-1 has several regulatory and accessory genes, including tat, rev, nef, vif, vpu, and vpr. Genetic variability in HIV-1 has been observed in several regions of the genome but mainly in the variable regions of the envelope gene within infected individuals (41). Little sequence information is available on HIV-1 accessory and regulatory genes within and among infected individuals (41). HIV-1 variants arise during retroviral replication by errors made during reverse transcription (11, 47, 48) because of immunologic pressure for change, alteration in cell tropism, and replication efficiency (23, 34, 55, 57). Several studies have shown a correlation between viral dynamics and HIV-1 disease progression (19, 25, 31, 70).
To understand the molecular mechanisms involved in mother-to-infant transmission of HIV-1, we (2) and others (39, 40, 53, 69) have shown that the minor HIV-1 genotypes of infected mothers are transmitted to their infants. This conclusion was based on an analysis of HIV-1 env sequences from mother-infant pairs following perinatal transmission. The sequences from mothers were found to be more heterogeneous than the sequences from infants. Initially, the minor genotypes in the infants predominated as a homogeneous sequence but became more diverse as the infants grew older (2, 39). Recently, greater HIV-1 genetic distances relative to the time of infection were shown for infected children with low virion-associated RNA levels and slow disease progression relative to children with high virion-associated RNA levels and rapid disease progression (19). In addition, selective transmission was demonstrated for sexual transmission of HIV-1 from transmitters to recipients, including a homogeneous sequence population present in the recipients (10, 35, 45, 68, 72–74).
Genetic analysis of HIV-1 sequences in other regions of the genome, in addition to the variable regions of env, from mother-infant pairs following perinatal transmission has been very limited. The possibility exists, however, that several other regions or motifs in the HIV-1 genome are involved in mother-to-infant transmission and are critical determinants of perinatal transmission. Since the accessory gene vif is highly conserved and functional during natural infection (60, 66), there should be a high prevalence of intact vif open reading frames in HIV-1 maternal-fetal isolates that are involved in perinatal transmission. Therefore, we sought to examine vif sequences from mother-infant pairs following perinatal transmission.
The vif open reading frame is conserved among most lentiviruses (42) and facilitates HIV-1 infection and cytopathogenicity (15, 17, 36, 51, 58, 59, 62). In addition, vif is required for HIV-1 replication in primary lymphocytes and macrophages (15, 17, 18, 51, 59, 64). These studies suggest that an intact vif gene may be required during natural HIV-1 infection. Sova et al. (60) performed sequence analysis of the HIV-1 vif gene in DNA from infected patient peripheral blood mononuclear cells (PBMC) and found limited sequence variability and a high level of conservation of vif during natural infection. However, an analysis of vif sequences following HIV-1 mother-to-infant transmission has not been performed. Since HIV-1 in sexual (73) and vertical (33) transmissions seems to be macrophage tropic and since vif is required for viral replication in macrophages, vif should have a role in HIV-1 infection and replication in mothers and infants and in perinatal transmission.
In this study, we analyzed vif sequences from five mother-infant pairs following perinatal transmission. We show that the vif open reading frame was conserved in most of the mother-infant pair sequences. The functional domains required for vif function in terms of viral infectivity and replication were also present in most of the mother-infant pair sequences. The data presented here support the notion that an intact vif open reading frame is necessary for HIV-1 infection and replication in maternal-fetal isolates that are involved in perinatal transmission.
MATERIALS AND METHODS
Patient population and sample collection.
This study was approved by the Human Subjects Committee of the University of Arizona, Tucson, and the Institutional Review Board of the Children’s Hospital Medical Center, Cincinnati, Ohio. Written informed consent was obtained for participation in the study. We studied five HIV-1-infected mother-infant pairs. Blood samples were collected from mother-infant pairs; the infants’ ages at the time of specimen collection were 6 weeks (infant A), 4.75 months (infant B), 14 months (infant C), 28 months (infant D), and 34 months (infant E). The demographic, clinical, and laboratory findings for the HIV-1-infected mother-infant pairs are summarized in Table 1.
TABLE 1.
Demographic, clinical, and laboratory findings for HIV-1-infected mother-infant pairs
| Patient | Age | Sexa | Raceb | CD4+ lymphocytes/mm3 (%) | Antiretroviral drugc | Clinical evaluationd |
|---|---|---|---|---|---|---|
| Mothers | ||||||
| A | 36 yr | B | 706 | None | Asymptomatic | |
| B | 28 yr | B | 509 | None | Asymptomatic | |
| C | 23 yr | W | 818 | None | Asymptomatic | |
| D | 31 yr | W | 480 | None | Asymptomatic | |
| E | 26 yr | B | 395 | ZDV | Symptomatic AIDS | |
| Infants | ||||||
| A | 6 wk | F | B | 2,994 (53) | None | Asymptomatic, P1A |
| B | 4.75 mo | M | B | 1,942 (42) | None | Asymptomatic, P1A |
| C | 14 mo | F | W | 772 (26) | ZDV | Symptomatic AIDS, P-2A, D1, 3, F |
| D | 28 mo | M | W | 46 (8) | ddC | Symptomatic AIDS, P-2A, B, F, failed to respond to ZDV therapy |
| E | 34 mo | M | B | 588 (34) | ZDV | Symptomatic AIDS, P-2A, B |
F, female; M, male.
B, black; W, white.
ZDV, zidovudine; ddC, zalcitibine.
Evaluation for infants was based on the criteria in reference 9.
Isolation of DNA from PBMC.
PBMC were isolated by a single-step Ficoll-Paque procedure (Pharmacia-LKB) from the whole blood of HIV-1-positive mothers and their infants. DNA was isolated according to a modification of the procedure described by Oram et al. (44). Approximately 106 PBMC were centrifuged at 12,000 rpm (Eppendorf model 5417C centrifuge) for 2 min, and the cell pellet was resuspended in 0.5 ml of TNE buffer (0.5 M Tris-HCl [pH 7.5], 0.1 M NaCl, 1 mM EDTA). The suspension was treated with 0.5% sodium dodecyl sulfate and 10 μg of proteinase K (Boehringer Mannheim Biochemicals) per ml at 60°C for 3 h, followed by several extractions with phenol and chloroform. The DNA was precipitated with ethanol, dissolved in 50 to 100 μl of TE buffer (10 mM Tris-HCl [pH 7.5], 1 mM EDTA), and sheared by repeated pipetting.
PCR amplification.
A two-step PCR amplification, first with outer primers and then with nested or inner primers, was performed to detect the presence of HIV-1 in infected patient PBMC (2). An equal amount of PBMC DNA was used for each HIV-1-infected patient, as determined by end-point dilution (16). For pairs A and B, we used DNA oligonucleotide primers VF4937 (5′-GGACCAGCAAAGCTCCTCTGGAAAGT, nucleotides [nt] 4937 to 4962, sense), VF5710 (5′-CAGTGCAAAAAATTCCCCTCCACAATT, nt 5710 to 5735, antisense), VF52 (5′-GAGAAGCTTTAATACAAGATAATAGTGACAT, nt 4979 to 4999, sense), and VF32 (5′-CTCGGATCCCATAAGTTTCATAGATATGTTG, nt 5988 to 5707, antisense), provided by David Volsky, St. Luke’s-Roosevelt Hospital Center (60). The nucleotides are numbered as in HXB-2 (41). PCRs were performed according to the procedure of Ahmad et al. (2–4) with a 25-μl reaction mixture containing 2.5 μl of 10× PCR buffer (100 mM Tris-HCl [pH 8.3], 100 mM KCl, 0.02% Tween 20), 2.5 mM MgCl2, 40 μM (each) dATP, dCTP, dGTP, and dTTP, a 0.2 to 1.0 μM concentration of each outer primer pair, and 2.5 U of ULTma DNA polymerase (Perkin-Elmer Cetus, Norwalk, Conn.). The reactions were carried out at 94°C for 30 s, 45°C for 45 s, and 72°C for 1 min for 35 cycles. In an attempt to amplify a larger fragment encompassing other regions in addition to vif, for pairs C, D, and E, we used DNA oligonucleotide primers VIF5 (5′-TGGCAGCAATTTCACCGGTACTA, nt 4580 to 4602, sense) and VPR1 (5′-CAACTTGGCAATGAAAGCAACAC, nt 5916 to 5939, antisense) as outer primers and VIF6 (5′-TCAAGCAGGAATTTGGAATTCCC, nt 4633 to 4655, sense) and VPR2 (5′-GGTACAAGCAGTTTAGGCTGACT, nt 5875 to 5898, antisense) as inner primers; these primers were synthesized according to the published HIV-1 NL4-3 sequence (41). PCRs were performed as described above with a 25-μl reaction mixture containing 2.5 μl of 10× buffer (25 mM tris-(hydroxymethyl)-methylaminopropanesulfonic acid, sodium salt [TAPS] [pH 9.3]), 50 mM KCl, 2 mM MgCl2, 1 mM 2-mercaptoethanol, 400 μM (each) dATP, dCTP, dGTP, and TTP, a 0.2 μM concentration of each outer primer, and 2.5 U of TaKaRa LA Taq polymerase (TaKaRa Biomedicals, Shiga, Japan). The reactions were carried out at 95°C for 30 s, 50°C for 45 s, and 72°C for 3 min for 35 cycles. The amplified DNA products were analyzed by electrophoresis on a 1.2% agarose gel. Negative controls consisting of DNA from PBMC of seronegative individuals were included in each set of reactions and were negative in all the assays. After the first round of PCR, 1 μl of the product was amplified for 25 cycles with the corresponding inner primers at 95°C for 30 s, 55°C for 45 s, and 72°C for 1 min (for inner primers VF52 and VF32) and 95°C for 30 s, 55°C for 45 s, and 72°C for 3 min (for inner primers VIF6 and VPR2). The PCR products were analyzed by electrophoresis on a 1.2% agarose gel. To avoid contamination, all the samples, reagents, and first- and second-round PCR products were kept separately and dispensed in a separate room free from all laboratory DNAs. We also included the known HIV-1 NL4-3 sequence for PCR amplifications as a control to assess errors generated by ULTma DNA polymerase and TaKaRa LA Taq polymerase.
Cloning and DNA sequencing.
The PCR products amplified by inner primer pairs VF52-VF32 and VIF6-VPR2 were blunt ended with DNA polymerase I (Gibco-BRL, Gaithersburg, Md.), treated with T4 polynucleotide kinase (Gibco-BRL), and cloned into the SmaI site of the pGem 3Zf (+) vector (Promega Corp., Madison, Wis.). Individual bacterial colonies were screened for the presence of recombinants by restriction enzyme analysis of plasmid DNA. The clones with the correct sizes of inserts were selected and propagated for DNA preparation, followed by nucleotide sequencing of 10 to 19 clones for each patient according to the Sequenase protocol (U.S. Biochemical Corp., Cleveland, Ohio) as reported before (2, 4).
Computer alignment and analysis of HIV-1 sequences.
The nucleotide sequences of the vif genes (576 bp) from the five mother-infant pairs were translated to corresponding amino acid sequences (192 amino acids). Alignments were performed manually, as no positions contained gaps. Pairwise distances, defined as the percentages of mismatches between two aligned nucleotide sequences, were used to study the extent of genetic variability within an individual and between mother and infant. For intraindividual variability (within mother and infant sequence sets), pairwise distances were calculated for all possible comparisons of pairs of sequences within the sets. For interindividual variability (between related mother-infant sets and between epidemiologically unlinked individual sets), each sequence from one set was compared with each sequence from the other set. The selection pressure was calculated as the ratio of nonsynonymous to synonymous substitutions (42) by comparison of all possible pairs of sequences between related mother-infant sets. The phylogenetic analysis was performed with PHYLIP version 3.5 software (14). The tree was built from a distance matrix (function DNADIST) by use of the neighbor-joining method (function NEIGHBOR). The robustness of the neighbor-joining tree was assessed by bootstrap resampling of the multiple alignments (function SEQBOOT). One tree was generated for the 137 sequences of the five mother-infant pairs and the reference HIV-1 NL4-3 sequence (GenBank accession no. U26942), used as a root for the tree display. Another tree contained the sequences of the five mother-infant pairs and 187 outgroup sequences extracted from genetic databases, including 152 vif sequences published in three recent studies of the variability of the vif gene (60, 63, 66). These outgroup sequences were extracted with Entrez version 6.04 (56), a program for retrieving data from databases, and Sequin version 2.20 (26), a program for managing the data. Entrez allowed us to retrieve and align all the sequences encompassing the vif gene present in the genetic databases. Sequin was used to merge the alignment of our sequences with the alignment of the outgroup sequences and to save them in a format compatible with PHYLIP.
Nucleotide sequence accession numbers.
The sequences have been submitted to GenBank with accession numbers AF019419 to AF019555.
RESULTS
PCR amplification of the HIV-1 vif gene from mother-infant pair PBMC DNA.
PCR amplification of the vif gene was performed as a two-step procedure as described by Ahmad et al. (2). The first round of amplification was performed with primer pairs VF4937-VF5710 and VIF5-VPR1, followed by nested PCR amplification with primer pairs VF52-VF32 (54) and VIF6-VPR2. The inner primer pairs yielded 728- and 1,246-bp fragments from PBMC DNA of infected mother-infant pairs (data not shown). HIV-1 was not detected in PBMC DNA from a normal donor. An equal amount of PBMC DNA from each HIV-infected patient was used in PCR amplifications, as determined by end-point dilution (16). In addition, we confirmed the PCR results with another set of primers from the gag (6) and env (2) regions, and the PCR results from the gag and env regions correlated with those from the vif region. To determine errors generated by ULTma DNA polymerase and TaKaRa LA Taq polymerase, we included a known HIV-1 sequence (NL4-3) for PCR amplification and sequencing.
Coding potential of the vif open reading frame in maternal-fetal isolates.
The multiple alignments of the deduced amino acid sequences (192 amino acids) of the vif gene of HIV-1 from PBMC DNA of the five mother-infant pairs are shown in Fig. 1A to E. The amino acid sequences were deduced from the nucleotide sequences of 137 different vif sequences and were aligned with reference to the subtype B consensus sequence (Fig. 1A to E). The coding potential of the vif open reading frame was maintained in most of the 78,912 bp sequenced. We analyzed 137 different vif clones, and 123 clones contained an intact vif open reading frame, an 89.8% frequency of conservation of the intact vif open reading frame. The frequency of defective vif genes in our five mother-infant pair sequences was 10.2%. A total of 13 clones contained stop codons, and 1 clone, in mother C (CM09), had a 150-bp (50-amino-acid) deletion at the 3′ end. In addition, three clones (BM10, EM01, and EM03) that contained stop codons also lacked initiation codons. These data demonstrated that vif sequences directly derived from the five HIV-1-infected mother-infant pairs following perinatal transmission were conserved. The high frequency of intact vif open reading frames observed here is in agreement with the data of Sova et al. (60) and Wieland et al. (66), who found intact vif in 87 and 90% of clones analyzed, respectively. It is interesting to note that vif-encoded amino acid sequences from each mother-infant pair displayed a pattern that was not seen in epidemiologically unlinked pairs (Fig. 1). No common signature sequence was seen in all mother-infant pair sequences. These data suggested that an intact vif open reading frame is conserved in maternal-fetal isolates following perinatal transmission.
FIG. 1.
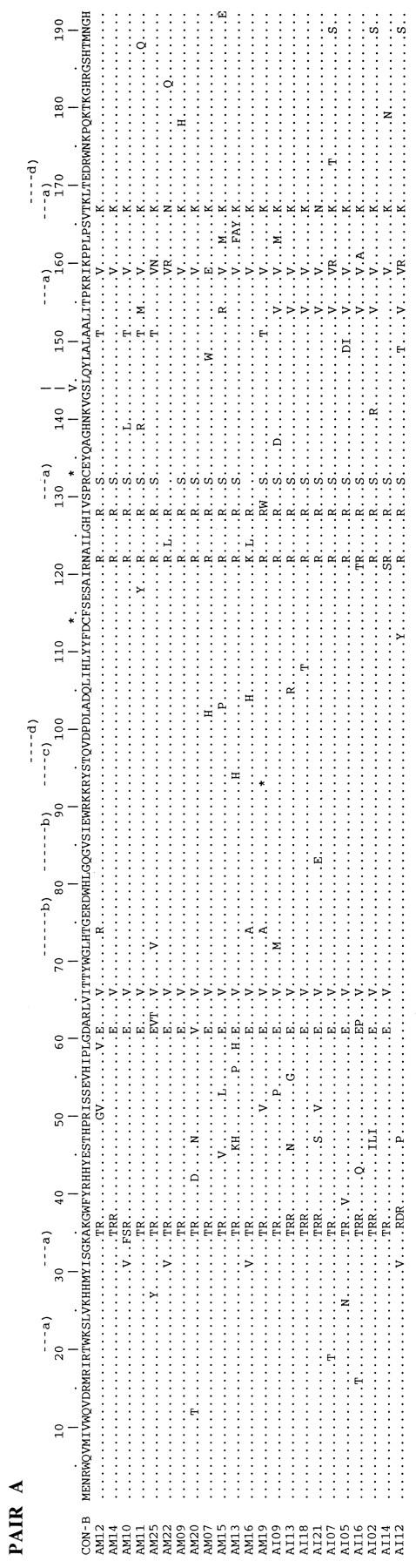
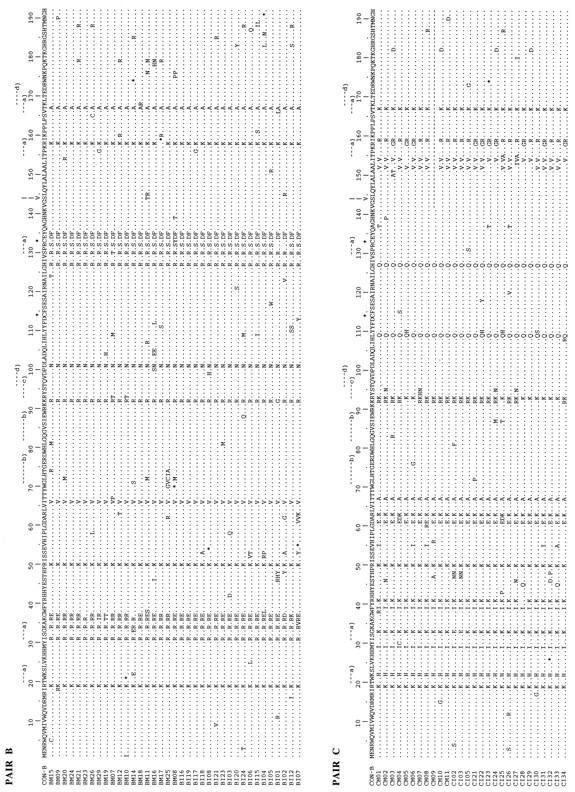

Multiple alignments of deduced amino acid sequences for the vif gene of HIV-1 from five mother-infant pairs following perinatal transmission. In the five mother-infant pair sequences, A, B, C, D, and E correspond to mother-infant pairs A, B, C, D, and E, respectively. In each mother-infant pair sequence, M represents mother and I represents infant. In the alignment, the top sequence (CON-B) is the consensus sequence of the clade or subtype B, as defined elsewhere (41, 60). Dots indicate amino acids identical to those in the CON-B sequence, dashes represent gaps, and asterisks represent stop codons. Above each alignment, asterisks locate the two cysteine residues critical for Vif function; a, protein kinase C phosphorylation site; b, N myristoylation site; c, cyclic-AMP- and GMP-dependent protein kinase phosphorylation sites; and d, casein kinase II phosphorylation site. In addition, above each alignment, a vertical arrow at position 144 indicates a serine of the most highly conserved motif SLQXLA (positions 144 to 149) among all lentivirus Vif proteins (43); the serine at position 144 is critical for Vif function (71). The basic amino acid (lysine and arginine) motifs at positions 157 to 160 and 173 to 184, important for Vif activity (18), are conserved.
Comparison of vif sequences from epidemiologically linked maternal-fetal isolates.
To determine the degree of variability of the vif gene from five mother-infant pairs, we analyzed variations in nucleotide and amino acid sequences as shown in Table 2. The nucleotide sequences of the vif genes in the mother sets (mothers A, B, C, D, and E) differed by 2.6, 2.1, 1.6, 0.9, and 1.8% (median values), respectively (range, 0.2 to 4.5%). The variability in the infant set (infants A, B, C, D, and E) was similar to that in the mother set: 2.5, 1.8, 1.9, 1.2, and 2.4% (median values), respectively (range, 0.2 to 4.3%). Interestingly, the variability between mother and infant sets (epidemiologically linked pairs A, B, C, D, and E) was also on the same order, 3.1, 2.3, 1.8, 1.1, and 2.7% (median values), respectively (range, 0 to 5%). The median values of amino acid sequence variability for vif products for mothers A, B, C, D, and E were 4.6, 3.7, 3.0, 1.7, and 3.6%, respectively; within infants, respective values were 4.7, 3.3, 3.5, 2.4, and 4.7%; and between epidemiologically linked mother-infant pairs, respective values were 5.2, 3.7, 3.2, 3.7, and 5.8%. There was no difference in the variability of vif sequences with increasing infant age. However, the variability in general was greater between mother-infant pairs than within mothers or within infants. These results indicated that the sequence variability within and among vif clones obtained from mother-infant pairs in our study is on the same order as reported before for vif (60, 63) and gag (35, 52, 72) genes from infected individuals. We also determined whether the low variability of the vif gene from maternal-fetal isolates was due to errors generated by ULTma DNA polymerase or TaKaRa LA Taq polymerase. We rarely found any errors generated by ULTma DNA polymerase or TaKaRa LA Taq polymerase when using the known sequence of HIV-1 NL4-3 for PCR amplifications and DNA sequencing of the vif gene. Despite the low variability in the deduced amino acid sequences for vif from the five mother-infant pairs, the sequences from the infants displayed amino acid patterns similar to those of the sequences from their mothers.
TABLE 2.
Distances in the vif sequences within mother sets, within infant sets, and between mother and infant sets
| Sequence | Paira | % Distancesb
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Within mother set
|
Within infant set
|
Between mother and infant sets
|
||||||||
| Min | Median | Max | Min | Median | Max | Min | Median | Max | ||
| Nucleotide | A | 1.2 | 2.6 | 4.5 | 1.0 | 2.5 | 3.8 | 1.2 | 3.1 | 5.0 |
| B | 0.3 | 2.1 | 4.5 | 0.2 | 1.8 | 4.3 | 0.5 | 2.3 | 4.7 | |
| C | 0.5 | 1.6 | 2.6 | 0.3 | 1.9 | 3.1 | 0.3 | 1.8 | 3.5 | |
| D | 0.2 | 0.9 | 1.6 | 0.3 | 1.2 | 2.1 | 0 | 1.1 | 2.1 | |
| E | 0.3 | 1.8 | 3.3 | 0.9 | 2.4 | 4.0 | 0.9 | 2.7 | 4.9 | |
| Total | 0.2 | 1.8 | 4.5 | 0.2 | 2.0 | 4.3 | 0 | 2.2 | 5.0 | |
| Amino acid | A | 1.0 | 4.6 | 7.3 | 2.1 | 4.7 | 8.3 | 1.0 | 5.2 | 9.9 |
| B | 0.5 | 3.7 | 7.3 | 0 | 3.3 | 7.3 | 5.2 | 3.7 | 7.3 | |
| C | 1.4 | 3.0 | 4.7 | 1.0 | 3.5 | 6.3 | 0.5 | 3.2 | 6.8 | |
| D | 0 | 1.7 | 3.6 | 0.5 | 2.4 | 4.2 | 0.5 | 3.7 | 7.3 | |
| E | 0.5 | 3.6 | 6.8 | 1.6 | 4.7 | 7.8 | 1.6 | 5.8 | 9.4 | |
| Total | 0 | 3.3 | 7.3 | 0 | 3.7 | 8.3 | 0.5 | 4.3 | 9.9 | |
Totals were calculated for all pairs together.
Min, minimum; max, maximum.
Comparison of vif sequences from epidemiologically unlinked maternal-fetal isolates.
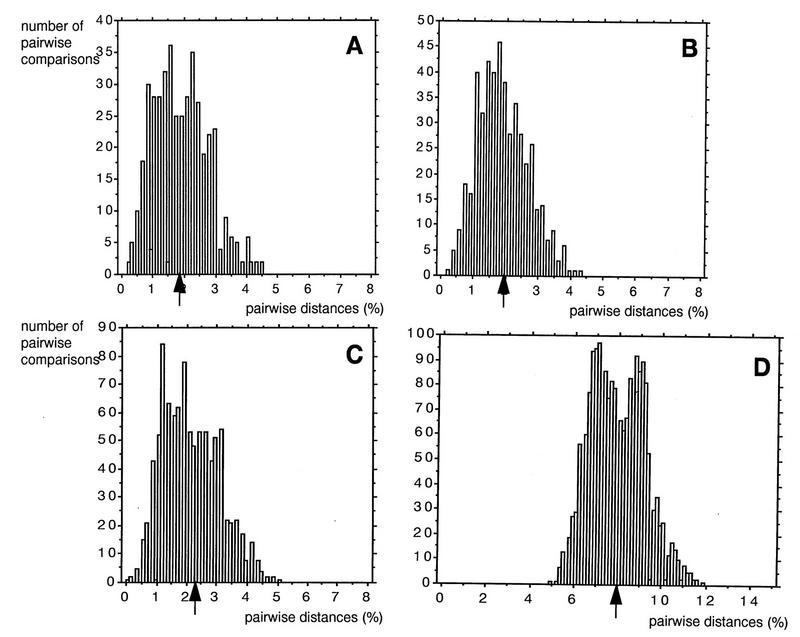
Figure 2 shows histograms of the distributions of the vif nucleotide sequence distances within mothers, within infants, between epidemiologically linked mother-infant pairs, and between two epidemiologically unlinked mothers; the medians of the distributions were 1.9, 1.9, 2.1, and 8.0%, respectively. The data suggested that vif sequences from epidemiologically linked mother-infant pairs were closer than those from epidemiologically unlinked individuals, keeping in mind the fact that a low degree of variability of the vif gene was observed in sequences from our five mother-infant pairs and other infected individuals (60, 66). By using the sequence distances for a conserved region such as vif, we were able to easily differentiate the epidemiologically unlinked individuals from the epidemiologically linked mother-infant pairs (Fig. 2D). Interestingly, the vif sequences from older infants (28 and 34 months old) were closer to those from their mothers (pairs D and E) than to those from epidemiologically unlinked individuals, suggesting that epidemiologically linked viral sequences can be identified even in older infants.
FIG. 2.
Distribution of the vif gene nucleotide distances between epidemiologically linked and unlinked mother-infant pairs. The percentages of mismatches were calculated between nucleotide sequences within mother sets (A), within infant sets (B), between epidemiologically linked mother-infant pair sequences (C), and between epidemiologically unlinked mother sequences (D). The distance percentages were rounded off to the nearest decimal. The numbers on the y axes represent the total numbers of pairwise comparisons that yielded the corresponding percent nucleotide distances. The median values of the distributions (indicated by the arrows) were 1.9% (A), 1.9% (B), 2.1% (C), and 8.0% (D).
Rates of accumulation of synonymous and nonsynonymous substitutions.
The selective pressure on mother-infant pair vif sequences was determined by calculating the ratios of nonsynonymous to synonymous substitutions (42). Several HIV-1 sequence analyses have suggested that nonsynonymous/synonymous substitution ratios of more than 1 indicate positive selective pressures by immune responses selecting for escape variants (2, 25, 69, 70, 72). The selective pressure on the vif gene, quantified as the ratio of nonsynonymous to synonymous substitutions, showed no evidence for positive selection pressure for change. Comparisons of infant sequences with mother sequences from pairs A, B, C, D, and E gave ratios of nonsynonymous to synonymous substitutions of 0.4, 0.5, 0.2, 0.9, and 0.5, respectively. Thus, there was very little selection pressure (a ratio of <1) on vif sequences to change. These values are comparable to (although greater than) those found for the gag gene (72).
Phylogenetic tree analysis of vif sequences of maternal-fetal isolates.
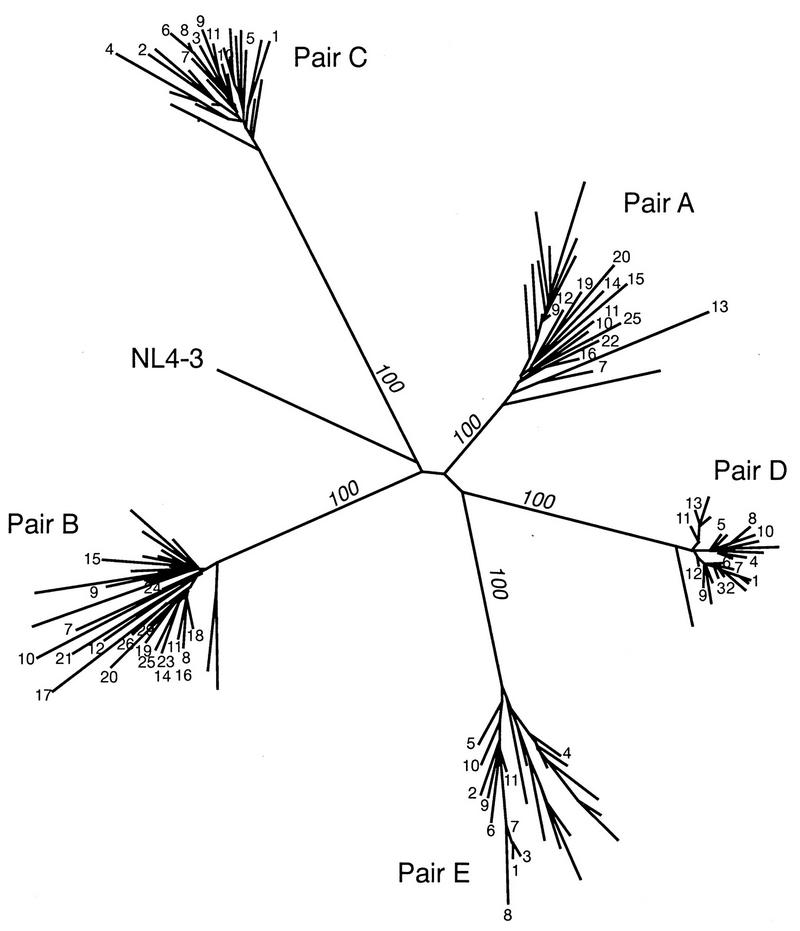
The similarity relationships among the 137 vif sequences from the five mother-infant pairs and 187 other vif sequences from infected individuals (in HIV-1 databases) were traced and are shown in Fig. 3 and 4. The phylogenetic tree analysis performed as described in Materials and Methods for the 137 vif sequences from the five mother-infant pairs revealed that the five mother-infant pairs were well discriminated, separated, and confined within subtrees (Fig. 3), indicating the absence of PCR product cross-contamination (27, 28). These subtrees were equidistant from each other. The root of the tree was the reference HIV-1 NL4-3 sequence. High bootstrap values further emphasized the separation of the subtrees into distinct clusters. Bootstrap analysis performed by resampling the data sets 100 times formed the same clusters of the sequences all 100 times for the five mother-infant pairs. Furthermore, the five subtrees showed homogeneous vif sequences in which some mother and infant sequences were intermingled.
FIG. 3.
Phylogenetic tree of 137 vif sequences from five mother-infant pairs (A, B, C, D, and E). The distances were calculated between the nucleotide sequences from the five mother-infant pairs. Each leaf of the tree represents one vif sequence. The mother sequences in each pair are labeled with the number of the clones (Fig. 1), whereas the infant sequences are unlabeled. The root of the tree was the reference HIV-1 NL4-3 sequence (41). The numbers at branch points indicate the numbers of occurrences of branches over 100 bootstrap resamplings of the data sets. The mother-infant pairs formed a distinct cluster and were discriminated, separated, and confined within subtrees, indicating the absence of PCR product cross-contamination (27, 28).
FIG. 4.
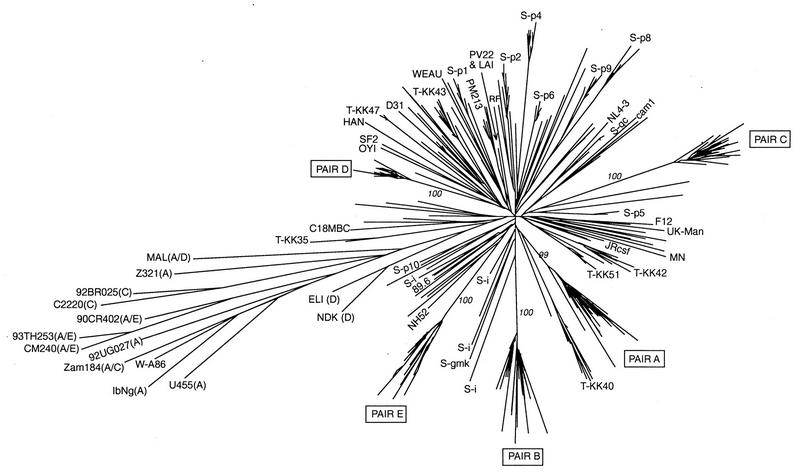
Phylogenetic tree of 137 mother-infant pair (A, B, C, D, and E) sequences and 187 other vif sequences from HIV-1 databases. The sequences from Sova et al. (60) are labeled S-p1 to S-p10, S-gc, S-i, and S-gmk (p, gc, i, and gmk represent clusters of sequences from patients); the sequences from Wieland et al. (66) are unlabeled, except for W-A86 (GenBank no. Z30637); and the sequences from Tominaga et al. (63) are labeled T. The other 35 sequences from the HIV-1 databases belong to the following HIV-1 subtypes: B, LAI (GenBank accession no. K02013), MN (M17449), CAM1 (D10112), Jrcsf (M38429), Jrfl (U63632), NL4-3 (U26942), NY5 (M19921), NH52 (L07424), RF (M17451), B clade (U26546), MCK1 (D86068), PM213 (D86069), PV22 (K02083), OYI (M26727), SF2 (K02007), HAN (U43141), D31 (U43096), WEAU (U21135), UK-Manchester (U23487), F12 (Z11530), 89.6 (U39362), and C18MBC (U37270); A, U455 (M62320), Z321 (U76035), IbNg (L39106), and 92UG027 (U51190); C, 92BR025 (U52953) and C2220 (U46016); A/C (recombinant), Zam184 (U86780); A/D (recombinant), MAL (X04415); A/E (recombinant), CM240 (U54771), 93TH253 (U51189), and 90CR402 (U51188); and D, ELI (K03454) and NDK (M27323). The values in italics are the numbers of occurrences of the corresponding branches over 100 bootstrap resamplings of the data sets. The tree grouped the 324 vif sequences, and the largest, starlike cluster contained all subtype B sequences. Subtypes other than B are indicated in parentheses after the isolate names. The five mother-infant pair sequences clustered with subtype B sequences.
In the second phylogenetic tree analysis (Fig. 4), the 137 vif sequences from the five mother-infant pairs and 187 other available vif sequences were included. These 187 additional vif sequences were the result of three independent analyses of the vif gene from infected individuals (60, 63, 66) and included 35 sequences belonging to subtypes B, A, A/E, C, and D (in HIV-1 databases). The tree grouped the 324 vif sequences in a manner similar to that for the env and gag genes (2, 35, 41, 69, 70, 72). The largest, starlike cluster contained all subtype B sequences. The subtype A, A/E, and C sequences diverged in a common lineage. Subtype D sequences (ELI and NDK) diverged independently from the center of the tree. In the subtype B cluster, the vif sequences from each individual and from each mother-infant pair were grouped closely together in subtrees. The average genetic distance between two sequences of subtype B (intra- and interindividual distances included) was 6.9% (range, 0 to 11.8%). The average pairwise distance within the cluster containing sequences of subtypes A and C and recombinants was 11% (range, 2.8 to 15.1%). The average distance between sequences of subtype B and sequences of the cluster of subtypes A and C and recombinants was 12.8% (range, 8.3 to 16.3%). These values did not change when the two sequences of subtype D were included in the subtype B cluster. This analysis further suggested that the vif sequences from the five mother-infant pairs were more closely related to subtype B than to any other subtype. The vif sequences from our five mother-infant pairs were distinct from each other and from vif sequences from other infected individuals (in HIV-1 databases, including HIV-1 NL4-3, which is used in our laboratory).
Conservation of functional domains required for Vif protein function in maternal-fetal isolates.
We next examined the presence of functional domains essential for Vif function in deduced Vif amino acid sequences from five mother-infant pairs. Ma et al. (32) showed that cysteines present at positions 114 and 133 (HXB2 clone numbering) are essential for viral infectivity. Both cysteines were present in 134 of the 137 Vif sequences (Fig. 1A to E). In one clone from mother B (BM08) and in two clones from mother-infant pair E (EM02 and EI07), cysteine was replaced with tyrosine and arginine, respectively. These data indicated a strong selection for the cysteine residues that are critical for vif-mediated viral infectivity in maternal-fetal isolates during perinatal transmission. We also identified protein kinase C phosphorylation, N myristoylation, and cyclic AMP- and cyclic GMP-dependent protein kinase phosphorylation sites in the Vif sequences (Fig. 1A to E). The 10 potential phosphorylation sites identified in the subtype B consensus Vif sequence were conserved in most of the mother-infant pair Vif sequences (Fig. 1A to E). Yang et al. (71) showed that phosphorylation of Vif by a serine/threonine protein kinase(s) plays an important role in regulating HIV-1 replication and infectivity. Serine at position 144 is present in the motif SLQXLA (positions 144 to 149), which is the most highly conserved sequence among all lentivirus Vif proteins (43). Mutation of serine to alanine at position 144 resulted in 90% inhibition of HIV-1 replication (71). The SLQXLA motif at positions 144 to 149 and the serine at position 144 were examined in Vif amino acid sequences and found to be highly conserved in 135 of the 137 clones (Fig. 1A to E). The other important domain essential for Vif function, for membrane localization during HIV-1 replication, requires basic amino acids at the C terminus (21). The C terminus of Vif contains a high density of basic amino acids, such as lysine and arginine, clustered at positions 157 to 160 and 173 to 184 (21, 41). Mutations of these basic amino acids to alanine impaired Vif function and HIV-1 replication (21). We investigated the presence of these basic amino acid domains in the C termini of the deduced amino acid sequences for the mother-infant pair vif sequences (Fig. 1A to E). The basic amino acids (lysine and arginine) at positions 157 to 160 and 173 to 184 in the C termini were highly conserved (Fig. 1A to E), supporting the significance of these essential domains for Vif function (21) during mother-to-infant transmission. The data on the conservation of the functional domains required for Vif function suggested that functional Vif is essential for HIV-1 infection and replication in maternal-fetal isolates.
Importance of vif in mother-infant HIV-1 infection.
The vif open reading frame was generally conserved in sequences from five mother-infant pairs following perinatal transmission (Fig. 1A to E). We found that 123 of the 137 clones analyzed had a conserved intact vif open reading frame. The frequency of an intact vif open reading frame in the five mother-infant pair sequences analyzed was 89.8%. The functional domains required for Vif function (in terms of viral infectivity and replication) including the two cysteines at positions 114 and 133, a serine phosphorylation site at position 144, and the basic amino acids at the C terminus, were highly conserved in mother and infant sequences (Fig. 1). The variability of the vif gene within mothers, within infants, and between mother-infant pairs was low, but there was a distinction between epidemiologically linked and unlinked individuals (Fig. 2). Our data showed that there was no difference in vif sequence variability with increasing age of the infants (Tables 1 and 2), contrary to the situation for V3 region sequences (2). There was no obvious correlation between vif intactness and the disease status of the mothers or infants (Table 1 and Fig. 1). Considering the complete sequence analysis of the vif gene from five mother-infant pairs, an intact vif gene with functional sites conserved is important for HIV-1 infection and replication in maternal-fetal isolates that are involved in perinatal transmission, as shown before for natural HIV-1 infection (60, 66).
DISCUSSION
We have performed a complete analysis of HIV-1 vif sequences from five mother-infant pairs following perinatal transmission. The vif sequences directly derived from the DNA of uncultured PBMC, the closest in vivo situation, revealed an 89.8% frequency of conserved intact vif open reading frames in maternal-fetal isolates. The two cysteines at positions 114 and 133 (32), the serine phosphorylation site at position 144 (71), and the C-terminal basic amino acid domains (21), required for vif-dependent viral infectivity and replication, were highly conserved in most of the mother-infant pair Vif sequences. Our results also showed a low degree of variability of vif sequences following mother-to-infant transmission. However, the epidemiologically linked mother-infant pair vif sequences were easily distinguishable from those of the epidemiologically unlinked individuals. These findings indicate that an intact vif open reading frame is required for HIV-1 infection and replication in mothers and infants and suggest a role in perinatal transmission. Our results are consistent with the earlier published analysis of vif sequences from HIV-1-infected individuals (60, 66), which suggested that vif plays a critical role in natural HIV-1 infection.
The coding potential of the vif gene was maintained in most of the sequences (78,912 bp were sequenced), except for 13 sequences containing stop codons and 1 having a deletion (Fig. 1A to E). A total of 89.8% of the vif clones obtained from uncultured PBMC DNA from five mother-infant pairs contained intact vif open reading frames. Similar observations of 87% (60) and 90% (66) conservation of intact vif open reading frames in infected individuals and 83% (60) conservation in short-term virus cultures have been reported. The frequency of inactive vif genes in isolates from our five mother-infant pairs was 10.2%, on the same order as the 13% (60) and 10% (66) frequencies observed for uncultured PBMC DNA from HIV-1-infected individuals. In contrast, a frequency of 31% defective vif genes in infected individuals has been reported (63). These differences could be attributed to the time of sampling, clinical stage, or geographic origin. The frequency of inactivating mutations found in our five mother-infant pair vif sequences was higher than those observed for gag (1.5%) (30, 52) and nef (3.3%) (46) genes. However, the possibility exists that the inactive vif genes that persisted in the mothers may have been transmitted to their infants at a very low rate.
Phylogenetic analysis performed on the five mother-infant pair vif sequences and 187 other vif sequences from HIV-1 databases clearly demonstrated that the five pairs were well discriminated, separated, and confined within subtrees (Fig. 4), indicating the absence of PCR product cross-contamination (27, 28). In addition, our five mother-infant pair vif sequences formed clusters distinct from those of all the other sequences, including HIV-1 NL4-3, which is used in our laboratory (Fig. 3). The variability of the vif gene within mothers, within infants, between mother-infant pairs, and between epidemiologically unlinked mothers was 1.9, 1.9, 2.1, and 8.0%, respectively, suggesting that the epidemiologically linked sequences were closer than the epidemiologically unlinked sequences. Our data also suggested that the low variability of vif sequences was not due to errors generated by ULTma or TaKaRa polymerases. Therefore, the low variability of vif sequences observed in mother-infant pairs persisted in vivo and was in agreement with those reported for infected individuals (60, 66).
The data presented here do not provide evidence for positive selection pressure for change in vif sequences. This finding is in contrast to the situation for env V3 region sequences from maternal-fetal isolates, for which positive selection pressure for change was observed (2, 39, 53, 69). The V3 region sequence population is known to be variable and to contain several variants or genotypes (2, 41, 69, 70). In a V3 region sequence analysis for mother-infant pairs, it was shown that HIV-1 minor genotypes were transmitted from mothers to infants (2, 39, 40, 53, 69). The minor genotypes predominated initially as a homogeneous virus population in the infants and then became heterogeneous as the infants grew older (2, 39), supporting the notion that there was strong pressure on the V3 region sequences to change. On the contrary, vif (like gag [72]) evolves with little selection, and variants from mothers persist during transmission. Evidence for this conclusion is provided by the low variability of and little selection pressure for the five mother-infant pair vif sequences.
The most interesting observation was the high conservation of the functional domains essential for Vif function in mother-infant pair sequences. The two cysteines at positions 114 and 133 were present in 134 of the 137 clones (Fig. 1A to E), confirming the finding of Ma et al. (32), who demonstrated that the two cysteines in HIV-1 Vif are critical for Vif-mediated viral infectivity. Phosphorylation of Vif by serine protein kinases at position 144 plays an important role in regulating HIV-1 replication and infectivity (71). Mutation of serine to alanine at position 144 in the motif SLQXLA (positions 144 to 149) (43) results in a loss of Vif activity and 90% inhibition of HIV-1 replication (71). The mother-infant pair Vif sequences contained the motif SLQXLA and the serine residue at position 144, supporting the preservation of these elements in the Vif protein (43, 71). In addition, Vif sequences contained the basic amino acids (lysine and arginine) at positions 157 to 160 and 173 to 184 of the C terminus (Fig. 1A to E) that are required for membrane localization-dependent Vif activity and HIV-1 replication (21). Our data also supported the importance of these basic amino acids at the C terminus (21) during maternal-fetal transmission of HIV-1. These motifs were also preserved in vif sequences from HIV-1-infected individuals (60, 63, 66, 67), consistent with our results. The conservation or selection of functional domains, such as cysteines, serine phosphorylation sites, and motifs of basic amino acids at the C terminus that are required for Vif function in maternal-fetal isolates suggests that a functional vif gene is required for HIV-1 replication in maternal-fetal isolates.
The molecular mechanisms of maternal transmission of HIV-1 are not known. The demonstration of selective transmission of HIV-1 minor genotypes or variants from mothers to infants was a significant first step in understanding the molecular mechanisms involved in perinatal transmission of HIV-1 (2, 39, 40, 53, 69). In addition, the elucidation of viral factors or determinants influencing maternal transmission may provide useful information for the development of strategies for the prevention and treatment of HIV-1 infection in children. Since the vif gene is conserved among most lentiviruses (41, 43) and is required for viral replication in primary lymphocytes and macrophages (15, 17, 18, 51, 59, 64), its role in HIV-1 transmission seems logical. The data presented here, showing a high frequency (89.8%) of intact vif open reading frames and conserved functional domains for Vif function, suggest that vif is involved in instituting HIV-1 infection and replication in mothers and their perinatally infected infants and may be one of the viral determinants of mother-to-infant transmission. This information may be helpful in the development of strategies for the prevention of HIV-1 mother-to-infant transmission by means of perinatal interventions.
ACKNOWLEDGMENTS
We thank John J. Marcahlonis, Department of Microbiology and Immunology, College of Medicine, The University of Arizona, for support, encouragement, and review of the manuscript. We also thank Raymond C. Baker, Division of General Pediatrics, Children’s Hospital Medical Center, Cincinnati, Ohio, for providing HIV-1-infected mother-infant pair blood samples; Scott Martin for technical help; Tobias Hahn and Mohammad Husain, Department of Microbiology and Immunology, The University of Arizona, for reviewing the manuscript; and the AIDS Research and Reference Reagent Program for providing HIV-1 NL4-3 (contributed by Malcolm Martin).
This work was supported by grants to N.A. from the National Institutes of Health (AI 40378) and the Arizona Disease Control Research Commission (9601).
REFERENCES
- 1.Ahmad N. Maternal-fetal transmission of human immunodeficiency virus. J Biomed Sci. 1996;2:238–250. doi: 10.1007/BF02253703. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad N, Baroudy B M, Baker R C, Chappey C. Genetic analysis of human immunodeficiency virus type 1 envelope V3 region isolates from mothers and infants after perinatal transmission. J Virol. 1995;69:1001–1012. doi: 10.1128/jvi.69.2.1001-1012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad N, Schiff G M, Baroudy B M. Detection of viremia by a one step polymerase chain reaction method in hepatitis C virus infection. Virus Res. 1993;30:303–315. doi: 10.1016/0168-1702(93)90098-8. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad N, Kuramoto I K, Baroudy B M. A ribonuclease protection assay for the direct detection and quantitation of hepatitis C virus RNA. Clin Diagn Virol. 1993;1:233–244. doi: 10.1016/0928-0197(93)90005-p. [DOI] [PubMed] [Google Scholar]
- 5.Albert J, Gaines H, Sonnerborg A, Nystrom G, Pehrson P O, Chiodi F, Sydow M V, Moberg L, Lidman K, Christensson B, Asjo B, Fenyo E M. Isolation of human immunodeficiency virus (HIV) from plasma during primary HIV infection. J Med Virol. 1987;23:67–73. doi: 10.1002/jmv.1890230108. [DOI] [PubMed] [Google Scholar]
- 6.Albert J, Fenyo E M. Simple, sensitive, and specific detection of human immunodeficiency virus type 1 in clinical specimens by polymerase chain reaction with nested primers. J Clin Microbiol. 1990;28:1560–1564. doi: 10.1128/jcm.28.7.1560-1564.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson R M, Medley G F. Epidemiology of HIV infection and AIDS: incubation and infectious periods, survival and vertical transmission. AIDS. 1989;2:S57–S63. [PubMed] [Google Scholar]
- 8.Blanche S, Rouzious C, Guihard Moscato M-I, Veber F, Mayaux M-J, Jacomet J, Crepy A, Douard D, Robin M, Courpotin C, Ciraru-Vigeneran N, Deist F, Griscelli C the French Collaborative Group. A prospective study of infants born to women seropositive for human immunodeficiency virus type 1. N Engl J Med. 1989;320:1643–1648. doi: 10.1056/NEJM198906223202502. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control. Classification system for HIV in children under 13 years of age. Morbid Mortal Weekly Rep. 1987;36:225–236. [PubMed] [Google Scholar]
- 10.Cichutek K, Merget H, Norley S, Linde R, Kreuz W, Gahr M, Kurth R. Development of quasispecies of human immunodeficiency virus type 1 in vivo. Proc Natl Acad Sci USA. 1992;89:7365–7369. doi: 10.1073/pnas.89.16.7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dougherty J, Temin H. Determination of the rate of base-pair substitution and insertion mutations in retrovirus replication. J Virol. 1988;62:2817–2822. doi: 10.1128/jvi.62.8.2817-2822.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehrnst A, Lindergren S, Dictor M, Johanon B, Sonnerborg A, Czajkowski J, Sundin G, Bohlin A-E. HIV in pregnant women and their offsprings: evidence for late transmission. Lancet. 1991;338:203–207. doi: 10.1016/0140-6736(91)90347-r. [DOI] [PubMed] [Google Scholar]
- 13.European Collaborative Study. Mother to child transmission of HIV-1. Lancet. 1988;ii:1039–1042. [PubMed] [Google Scholar]
- 14.Felsenstein J. Phylip phylogenetic inference package. Cladiatice. 1989;5:164–166. [Google Scholar]
- 15.Fisher A G, Ensoli B, Ivanoff L, Chamberlain M, Petteway S, Ratner L, Gallo R C, Wong-Staal F. The sor gene of HIV-1 is required for efficient virus transmission in vitro. Science. 1987;237:888–892. doi: 10.1126/science.3497453. [DOI] [PubMed] [Google Scholar]
- 16.Furtado M R, Kingsley L A, Wolinsky S M. Changes in the viral mRNA expression pattern correlate with a rapid rate of CD4+ T-cell number decline in human immunodeficiency virus type 1-infected individuals. J Virol. 1995;69:2092–2100. doi: 10.1128/jvi.69.4.2092-2100.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gabuzda D H, Lawrence K, Langhoff E, Terwilliger E, Dorfman T, Haseltine W A, Sodroski J. Role of vif in replication of human immunodeficiency virus type 1 CD4+ T lymphocytes. J Virol. 1992;66:6489–6495. doi: 10.1128/jvi.66.11.6489-6495.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabuzda D H, Li H, Lawrence K, Vasir B S, Crawford K, Langhoff E. Essential role of vif in establishing productive HIV-1 infection in peripheral blood T lymphocytes and monocytes/macrophages. J Acquired Immune Defic Syndr. 1994;7:908–915. [PubMed] [Google Scholar]
- 19.Ganeshan S, Dickover R E, Korber B T M, Bryson Y J, Wolinsky S M. Human immunodeficiency virus type 1 genetic evolution in children with divergent rates of development of disease. J Virol. 1997;71:663–677. doi: 10.1128/jvi.71.1.663-677.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goedert J J, Duliege A M, Amos C I, Felton S, Biggar R J the International Registry of HIV-Exposed Twins. High risk of HIV-1 infection for first born twins. Lancet. 1991;338:1471–1475. doi: 10.1016/0140-6736(91)92297-f. [DOI] [PubMed] [Google Scholar]
- 21.Gonclaves J, Shi B, Yang X, Gabuzda D. Biological activity of human immunodeficiency virus type 1 Vif requires membrane targeting by C-terminal basic domains. J Virol. 1995;69:7196–7204. doi: 10.1128/jvi.69.11.7196-7204.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hira S, Kamanga J, Bhat G J, Mwale C, Tembo G, Luo N, Perine P L. Perinatal transmission of HIV-1 in Zambia. Br Med J. 1989;299:1250–1252. doi: 10.1136/bmj.299.6710.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang S S, Boyle T J, Lyerly H K, Cullen B R. Identification of the envelope V3 loop as the primary determinant of cell tropism. Science. 1991;253:71–74. doi: 10.1126/science.1905842. [DOI] [PubMed] [Google Scholar]
- 24.Italian Multiculture Study. Epidemiology, clinical features and prognostic factors of pediatric HIV infection. Lancet. 1988;ii:1043–1046. [PubMed] [Google Scholar]
- 25.Iversen A K, Shpaer E G, Rodrigo A G, Hirsch M S, Walker B D, Sheppard H W, Merigan T C, Mullins J I. Persistence of attenuated rev genes in human immunodeficiency virus type 1-infected asymptomatic individuals. J Virol. 1995;69:5743–5753. doi: 10.1128/jvi.69.9.5743-5753.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kans J A. Conference Proceedings of the Cold Spring Harbor Laboratory Genome Mapping and Sequencing Meeting. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1996. Sequin; p. 114. [Google Scholar]
- 27.Korber B T M, Learn G, Mullins J I, Hahn B H, Wolinsky S M. Protecting HIV databases. Nature. 1995;378:242–243. doi: 10.1038/378242a0. [DOI] [PubMed] [Google Scholar]
- 28.Learn G H, Korber B T M, Foley B, Hahn B H, Wolinsky S M, Mullins J I. Maintaining the integrity of human immunodeficiency virus sequence databases. J Virol. 1996;70:5720–5730. doi: 10.1128/jvi.70.8.5720-5730.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lepage P, Vande Perre P, Carael M, Nsengumuremyi F, Nkurunziza J, Butzler J-P, Sprecher S. Postnatal transmission of HIV from mother to child. Lancet. 1987;i:400. doi: 10.1016/s0140-6736(87)92423-8. [DOI] [PubMed] [Google Scholar]
- 30.Louwagie J, McCurchan F E, Peeters M, Brennan T P, Sanders-Buell E, Eddy E, van der Groen G, Fransen K, Gershy-Damet G-M, Deleys R, Burke D S. Phylogenetic analysis of gag genes from 70 international HIV-1 isolates provides evidence for multiple genotypes. AIDS. 1993;7:769–780. doi: 10.1097/00002030-199306000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Lu S-L, Schacker T, Musey L, Shriner D, McElrath M J, Corey L, Mullins J I. Divergent patterns of progression to AIDS after infection from the same source: human immunodeficiency virus type 1 evolution and antiviral responses. J Virol. 1997;71:4284–4295. doi: 10.1128/jvi.71.6.4284-4295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma X, Sova P, Volsky D J. Cysteine residues in the Vif protein of human immunodeficiency virus type 1 are essential for viral infectivity. J Virol. 1994;68:1714–1720. doi: 10.1128/jvi.68.3.1714-1720.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matala, E., V. R. K. Yedavalli, and N. Ahmad. Unpublished data.
- 34.McNearny T, Westervelt P, Thielan B J, Trowbridge D B, Garcia J, Whittler R, Ratner L. Limited sequence heterogeneity among biologically distinct human immunodeficiency virus type 1 isolates from individuals involved in a clustered infectious outbreak. Proc Natl Acad Sci USA. 1990;87:1917–1922. doi: 10.1073/pnas.87.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McNearny T, Hornickora Z, Markham R, Birdnell A, Arnes M, Saah A, Ratner L. Relationship of human immunodeficiency virus type 1 sequence heterogeneity to stage of disease. Proc Natl Acad Sci USA. 1992;89:10247–10251. doi: 10.1073/pnas.89.21.10247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michaels F, Hattori N, Gallo R, Franchini G. The HIV-1 Vif protein is located in the cytoplasm of infected cells and its effect on virus replication is equivalent in HIV-2. AIDS Res Hum Retroviruses. 1993;9:1025–1029. doi: 10.1089/aid.1993.9.1025. [DOI] [PubMed] [Google Scholar]
- 37.Mok J Q, Giaquinto C, DeRossi A, Gruch-Worner I, Ades A E, Pekham C S. Infants born to mothers seropositive for human immunodeficiency virus—preliminary findings from a multiculture European study. Lancet. 1987;i:1164–1168. doi: 10.1016/s0140-6736(87)92142-8. [DOI] [PubMed] [Google Scholar]
- 38.Muary W, Potts B, Rabson A B. HIV-1 infection of first trimester and term human placental tissue, a possible mode of maternal-fetal transmission. J Infect Dis. 1989;460:583–588. doi: 10.1093/infdis/160.4.583. [DOI] [PubMed] [Google Scholar]
- 39.Mulder-Kampinga G A, Kuiken C, Dekker J, Scherpbier H J, Boer K, Goudsmit J. Genomic human immunodeficiency virus type 1 RNA variation in mother and child following intrauterine virus transmission. J Gen Virol. 1993;74:1747–1756. doi: 10.1099/0022-1317-74-9-1747. [DOI] [PubMed] [Google Scholar]
- 40.Mulder-Kampinga G A, Simonon A, Kuiken C L, Dekker J, Scherpbier H J, van de Perre P, Boer K, Goudsmit J. Similarity in env and gag genes between genomic RNAs of human immunodeficiency virus type 1 (HIV-1) from mother and infant is unrelated to time of HIV-1 RNA positivity in the child. J Virol. 1995;69:2285–2296. doi: 10.1128/jvi.69.4.2285-2296.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Myers G, Korber B, Hahn B H, Jeang K-T, Mellors J W, McCutchan F E, Henderson L E, Pavlakis G N. Human retroviruses and AIDS database. Los Alamos, N.Mex: Los Alamos National Laboratory; 1995. [Google Scholar]
- 42.Nei M, Gojobori T. Simple methods for estimating the number of synonymous and nonsynonymous nucleotide substitutions. Mol Biol Evol. 1986;3:418–426. doi: 10.1093/oxfordjournals.molbev.a040410. [DOI] [PubMed] [Google Scholar]
- 43.Oberste M S, Gonda M A. Conservation of amino acid sequence motifs in lentivirus Vif proteins. Virus Genes. 1992;6:95–102. doi: 10.1007/BF01703760. [DOI] [PubMed] [Google Scholar]
- 44.Oram J D, Downing R G, Roff M, Sernankambo N, Clegg J C S, Featherstone A-S R, Booth J C. Sequence analysis of the V3 loop regions of the env genes of Ugandian human immunodeficiency proviruses. AIDS Res Hum Retroviruses. 1991;7:605–614. doi: 10.1089/aid.1991.7.605. [DOI] [PubMed] [Google Scholar]
- 45.Pang S, Shlesinger Y, Darr E S, Moudgh T, Ho D D. Rapid generation of sequence variation during primary HIV-1 infection. AIDS. 1992;6:453–460. doi: 10.1097/00002030-199205000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Pearson W R, Lipman D J. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peterson B D, Poresz B J, Loeb J A. Fidelity of HIV-1 reverse transcriptase. Science. 1988;242:1168–1171. doi: 10.1126/science.2460924. [DOI] [PubMed] [Google Scholar]
- 48.Roberts J D, Preston B D, Johnston L A, Soni A, Loeb L A, Kunkel T A. Fidelity of two retroviral reverse transcriptases during DNA-dependent DNA synthesis in vitro. Mol Cell Biol. 1989;9:469–476. doi: 10.1128/mcb.9.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rossi P, Moschese V P A, Broliden C, Fundaro I, Quinti A, Plebani C, Giaquinto P, Tovo K, Ljunggren K, Rosen J. Presence of maternal antibodies to human immunodeficiency virus 1 envelope glycoprotein gp120 epitopes correlates with uninfected status of children born to seropositive mothers. Proc Natl Acad Sci USA. 1989;86:8055–8058. doi: 10.1073/pnas.86.20.8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ryder R W, Nsa W, Hassig S E, Behets F, Rayfield M, Kungola E, Nelson A, Mulenda U, Francis H, Mwandagalirwa K, Davachi F, Rogers M, Nzilambi N, Greenberg A, Mann J, Quinn T C, Piot P, Curran J W. Perinatal transmission of human immunodeficiency virus type 1 to infants of seropositive women in Zaire. N Engl J Med. 1988;320:1637–1642. doi: 10.1056/NEJM198906223202501. [DOI] [PubMed] [Google Scholar]
- 51.Sakai K, Dewhurst S, Ma X, Volsky D J. Differences in cytopathogenicity and host cell range among infectious molecular clones of human immunodeficiency virus type 1 simultaneously isolated from an individual. J Virol. 1988;62:4078–4085. doi: 10.1128/jvi.62.11.4078-4085.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salmien M, Nykanen A, Brummer-Korvenkontio H, Kantenen M L, Liitsola K, Leinikki P. Molecular epidemiology of HIV-1 based on phylogenetic analysis of in vivo gag p7/p9 direct sequences. Virology. 1993;195:185–194. doi: 10.1006/viro.1993.1359. [DOI] [PubMed] [Google Scholar]
- 53.Scarlatti G, Leitner T, Hapi E, Wahlberg J, Marchisi P, Clerici-Schoeller M A, Wigzell H, Fenyo E M, Albert J, Uhlen M, Rossi P. Comparison of variable region 3 sequences of human immunodeficiency virus type 1 from infected children with RNA/DNA sequences of virus populations of their mothers. Proc Natl Acad Sci USA. 1993;90:1721–1725. doi: 10.1073/pnas.90.5.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scott G B, Fischl M A, Khmas N, Fletcher M A, Dickinson G M, Levine R S, Parks W P. Mothers of infants with acquired immunodeficiency syndrome: evidence for both symptomatic and asymptomatic carriers. JAMA. 1985;253:363–366. [PubMed] [Google Scholar]
- 55.Shioda T, Levy J A, Cheng-Mayer C. Macrophage and T cell line tropism of HIV-1 is determined by specific regions of the envelope gp120 gene. Nature. 1991;349:167–169. doi: 10.1038/349167a0. [DOI] [PubMed] [Google Scholar]
- 56.Shuler G D, Epstein J A, Ohkawa H, Kans J A. Entrez: molecular biology database and retrieval system. Methods Enzymol. 1996;266:141–162. doi: 10.1016/s0076-6879(96)66012-1. [DOI] [PubMed] [Google Scholar]
- 57.Siliciano R F, Lawton T, Knall R W, Karr R W, Berman P, Gregory T, Reinherz E L. Analysis of host-virus interactions in AIDS with anti-gp120 T cell clones: effect of HIV sequence variation and a mechanism for CD4+ cell depletion. Cell. 1988;54:561–575. doi: 10.1016/0092-8674(88)90078-5. [DOI] [PubMed] [Google Scholar]
- 58.Sodroski J, Goh W C, Rosen C, Tartar A, Portetella D, Bumy A, Haseltine W A. Replicative and cytopathic potential of HTLV III/LAV with sor gene deletions. Science. 1986;231:1549–1553. doi: 10.1126/science.3006244. [DOI] [PubMed] [Google Scholar]
- 59.Sova P, Volsky D J. Efficiency of viral DNA synthesis during virus infection of permissive and nonpermissive cells with vif-negative human immunodeficiency virus type 1. J Virol. 1993;67:6322–6326. doi: 10.1128/jvi.67.10.6322-6326.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sova P, van Rannst M, Gupta P, Balachandran R, Chao W, Itescu S, McKinley G, Volsky D J. Conservation of an intact human immunodeficiency virus type 1 vif gene in vitro and in vivo. J Virol. 1995;69:2557–2564. doi: 10.1128/jvi.69.4.2557-2564.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sprecher S, Soumenkoff G, Puissant F, Degueldre M. Vertical transmission of HIV in 15 week fetus. Lancet. 1986;ii:288–289. doi: 10.1016/s0140-6736(86)92110-0. [DOI] [PubMed] [Google Scholar]
- 62.Strebel K, Daughtery D, Clouse K, Cohen D, Folks T, Martin M A. The HIV ‘A’ (sor) gene product is essential for virus infectivity. Science. 1987;328:728–730. doi: 10.1038/328728a0. [DOI] [PubMed] [Google Scholar]
- 63.Tominaga K, Kato S, Negishi M, Takano T. A high frequency of defective vif genes in peripheral blood mononuclear cells from HIV type 1-infected individuals. AIDS Res Hum Retroviruses. 1996;12:1543–1549. doi: 10.1089/aid.1996.12.1543. [DOI] [PubMed] [Google Scholar]
- 64.von Schwedler U, Song J, Aiken C, Trono D. vif is critical for human immunodeficiency virus type 1 proviral DNA synthesis in infected cells. J Virol. 1993;67:4945–4955. doi: 10.1128/jvi.67.8.4945-4955.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weinbreck P, Loustand V, Denis F, Vidal B, Muvnier M, DeLumley I. Postnatal transmission of HIV infection. Lancet. 1988;i:482. [Google Scholar]
- 66.Wieland U, Hartman J, Suhr H, Salzberger B, Eggers H J, Kuhn J E. In vivo genetic variability of the HIV-1 vif gene. Virology. 1994;203:43–45. doi: 10.1006/viro.1994.1453. [DOI] [PubMed] [Google Scholar]
- 67.Wieland U, Seelhoff A, Hofmann A, Kuhn J E, Eggers H J, Mugyeny P, Schwander S. Diversity of the vif gene of human immunodeficiency virus type 1 in Uganda. J Gen Virol. 1997;78:393–400. doi: 10.1099/0022-1317-78-2-393. [DOI] [PubMed] [Google Scholar]
- 68.Wolfs T F W, Zwart G, Bakker M, Goudsmit J. HIV-1 genomic RNA diversification following sexual and parental virus transmission. Virology. 1992;189:103–110. doi: 10.1016/0042-6822(92)90685-i. [DOI] [PubMed] [Google Scholar]
- 69.Wolinsky S M, Wike C M, Korber B T M, Hutto C, Parks W P, Rosenblum L L, Kuntsman K J, Furtado M R, Munoz I L. Selective transmission of human immunodeficiency virus type 1 variants from mother to infants. Science. 1992;255:1134–1137. doi: 10.1126/science.1546316. [DOI] [PubMed] [Google Scholar]
- 70.Wolinsky S M, Korber B T M, Neumann A U, Daniels M, Kunstman K J, Whetsell A J, Furtado M R, Cao Y, Ho D D, Safrit J T, Koup R A. Adaptive evolution of human immunodeficiency virus-type 1 during the natural course of infection. Science. 1996;272:537–542. doi: 10.1126/science.272.5261.537. [DOI] [PubMed] [Google Scholar]
- 71.Yang X, Goncalves J, Gabuzda D. Phosphorylation of Vif and its role in HIV-1 replication. J Biol Chem. 1996;271:10121–10129. doi: 10.1074/jbc.271.17.10121. [DOI] [PubMed] [Google Scholar]
- 72.Zhang J, MacKenzie L Q, Cleland A, Holms E C, Leigh Brown A J, Simmonds P. Selection for specific sequences in the external envelope protein of human immunodeficiency virus type 1 upon primary infection. J Virol. 1993;67:3345–3356. doi: 10.1128/jvi.67.6.3345-3356.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu T, Mo H, Wang N, Nam D S, Cao Y, Koup R A, Ho D D. Genotypic and phenotypic characterization of HIV-1 in patients with primary infection. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- 74.Zhu T, Wang N, Carr A, Nam S, Moor-Jankowski R, Cooper D, Ho D D. Genetic characterization of human immunodeficiency virus type 1 in blood and genital secretions: evidence for viral compartmentalization and selection during sexual transmission. J Virol. 1996;70:3098–3107. doi: 10.1128/jvi.70.5.3098-3107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]