Abstract
Human immunodeficiency virus type 1 (HIV-1) requires the presence of specific chemokine receptors in addition to CD4 to enter target cells. The chemokine receptor CCR5 is used by the macrophage-tropic strains of HIV-1 that predominate during the asymptomatic stages of infection. Here we identify a small tyrosine-rich region of CCR5 proximal to the N-terminal cysteine that is critical for entry of macrophage-tropic and dual-tropic variants of HIV-1. HIV-1 infection of cells expressing CCR5 mutants with changes in this region was substantially reduced compared with the infection of cells bearing wild-type CCR5. Simian immunodeficiency virus (SIVmac239) entry was also ablated on a subset of these mutants but enhanced on others. These differences in virus entry were correlated with the relative ability of soluble, monomeric HIV-1 and SIVmac239 gp120 glycoproteins to bind the CCR5 mutants. These results identify a region of CCR5 that is necessary for the physical association of the gp120 envelope glycoprotein with CCR5 and for HIV-1 infection.
Human immunodeficiency virus (HIV-1) is the etiologic agent of AIDS, which results from the destruction of CD4-positive lymphocytes in infected individuals (6, 22, 24). The related virus simian immunodeficiency virus (SIVmac) can cause an AIDS-like disease in macaques (26, 27). The entry of HIV-1 into target cells is mediated by the viral envelope glycoproteins, gp120 and gp41, which are assembled into an oligomeric structure on the viral membrane (18, 19). The HIV-1 exterior glycoprotein, gp120, binds to the cellular receptor CD4 (12, 31). CD4 expression on target cells is not sufficient for viral entry, however, and the chemokine receptors CXCR4, CCR5, CCR3, and CCR2b, as well as the orphan receptor STRL33, can function as necessary coreceptors for HIV-1 (2, 8, 14, 16, 17, 23, 29). Among these coreceptors, CCR5 is thought to be especially important because primary viruses that infect T cells and macrophages efficiently use CCR5 (11). Furthermore, individuals who fail to express CCR5 appear to be largely protected from HIV-1 infection (13, 30, 34). SIVmac also uses CCR5, as well as the orphan receptors STRL33, gpr15, and gpr1, as a coreceptor (15, 20). Soluble HIV-1 or SIVmac gp120 glycoproteins incubated with soluble CD4 (sCD4) can bind CCR5 and compete with the binding of the natural chemokine ligands of CCR5, which include MIP-1α, MIP-1β, and RANTES (37, 38). This binding is dependent on the presence of the third variable (V3) loop of HIV-1 gp120, and the sequence of the V3 loop to a large extent determines which coreceptor can be used by HIV-1 (8, 9, 38). Binding of the envelope glycoproteins to the chemokine receptors is thought to trigger additional conformational changes in the gp41 transmembrane glycoprotein, leading to the fusion of the viral and target cell membranes.
Several studies have examined HIV-1 entry into cells expressing chimeras constructed between human CCR5 and either human CCR2b or murine CCR5 (5, 7, 21, 33). These studies in general have not been able to identify discrete domains that are required for HIV-1 entry. Rather, they collectively indicate that all or most of the external domains of CCR5 participate in supporting HIV-1 entry. Interpretation of these studies, however, should include the caveat that the various external domains are likely to interact quite closely, and thus indirect effects of the exchange of relatively large domains on the observed phenotypes cannot be excluded.
In this study, we used a panel of CCR5 alanine substitution mutants to explore the interaction of CCR5 exterior domains with HIV-1 and SIV envelope glycoproteins. We show that a region of the N terminus proximal to the first cysteine of CCR5 plays an important role in the association of the gp120 glycoprotein with CCR5 and in HIV-1 and SIV entry.
MATERIALS AND METHODS
Plasmids.
Plasmids pHXBH10ΔenvCAT and pSVIIIenv, used to produce recombinant HIV-1 virions containing the envelope glycoproteins from the primary HIV-1 isolates YU2 and 89.6, or the SIVmac239 envelope glycoproteins, have been described previously (8, 25, 35). Plasmid pCD4, used to express full-length CD4 in CF2Th cells, has been described elsewhere (36). For expression of CCR5, cDNA was cloned in a pcDNA3 vector. To create plasmids expressing the CCR5 alanine substitution mutants, mutagenesis of this pcDNA3 vector was performed by the QuikChange method as specified by the manufacturer (Stratagene, Inc.).
Cell lines.
CF2Th canine thymocytes (ATCC CRL 1430) and HEK293T cells were obtained from the American Type Culture Collection. Cells were maintained as described previously (8).
env complementation assay.
A single round of HIV-1 infection was assayed by using a previously described env complementation assay (8). Briefly, recombinant HIV-1 with the nef gene replaced by a gene encoding chloramphenicol acetyltransferase (CAT) was used to infect CF2Th cells transfected by the calcium phosphate method with 10 μg of plasmid encoding CD4 and 5 to 20 μg of plasmid encoding wild-type or mutant CCR5. For these assays, 10,000 cpm of reverse transcriptase activity of the recombinant viruses containing the YU2, 89.6, or SIVmac239 envelope glycoproteins was used, and cells were incubated with virus for 1 h at 37°C before washing. Cells were lysed after infection, and CAT activity was measured, indicating the level of infection (25). Normalized values for entry on mutant CCR5 receptors are calculated by expressing CAT activity of the mutant receptor as a percentage of the expected activity of wild-type CCR5 with the same mean fluorescence. This latter value is determined by extrapolating a line between the two wild-type CCR5 values, obtained by transfecting with various amounts of plasmid DNA, whose expression most closely bounds that of the mutant receptor. Mutant receptors whose mean fluorescence was greater than that of the highest wild-type CCR5 value, or lower than the lowest wild-type CCR5 value, were excluded from analysis.
Antibodies.
A cocktail composed of equal parts of the anti-CCR5 antibodies 5C7, 2C4, 3A9, 3D8, 10G11, 5H11, and 1G4 (39) was used to measure surface expression of the CCR5 mutant proteins. The use of this antibody cocktail minimizes the chance that antibody recognition of the mutant CCR5 molecules will be disrupted by the introduced amino acid changes. For some experiments, the 5C7 antibody, whose epitope maps to the N terminus of CCR5, and the 2D7 antibody, whose epitope maps to the second CCR5 exterior loop (37a), were used individually, to confirm the efficiency of recognition of individual CCR5 mutants by this cocktail.
Binding assay.
HEK293T cells were transfected by the calcium phosphate method with 30 μg of plasmid DNA encoding wild-type or mutant CCR5 receptors. Fluorescence-activated cell sorting (FACS) analysis using the 5C7 and 2D7 antibodies was used to confirm comparable expression on transfected cells. Cells were resuspended in binding buffer (50 mM HEPES [pH 7.5], 1 mM CaCl2, 5 mM MgCl2, 0.5% bovine serum albumin). Approximately 106 cells were mixed with 0.1 nM 125I-labeled MIP-1α (DuPont NEN) or 0.5 nM 125I-labeled YU2 or SIVmac239 soluble gp120 glycoprotein (38) and competed with the indicated concentrations of unlabeled MIP-1α or YU2 or SIVmac239 soluble envelope glycoprotein, respectively. Assays for gp120 envelope glycoprotein binding also included 100 nM sCD4. Cells were incubated for 30 min at 37°C in a total volume of 0.1 ml, centrifuged, resuspended in 0.6 ml of the same buffer containing 500 mM NaCl, and recentrifuged. Bound ligand was quantitated by liquid scintillation counting. Nonspecific binding was determined in the presence of 100 nM unlabeled competitor and subtracted from each value for bound ligand.
RESULTS
Effect of CCR5 amino acid changes on CCR5 expression and HIV-1 entry.
A panel of CCR5 mutants in which alanine was substituted for most of the charged and aromatic residues in the exterior domains was created (Fig. 1). Cell surface expression of these mutants was examined following transfection of CF2Th cells with plasmids encoding human CD4 and the mutated CCR5 proteins. In parallel, CF2Th cells were transfected with the CD4-expressing plasmid and different amounts of the plasmid expressing the wild-type CCR5 protein. FACS analysis was performed 48 h after transfection with an aliquot of the transfected cells, using a cocktail of anti-CCR5 monoclonal antibodies. Eleven of the mutant proteins exhibited levels of surface expression (legend to Fig. 1) below the lowest value detectable with wild-type CCR5, and these mutants were excluded from further analysis.
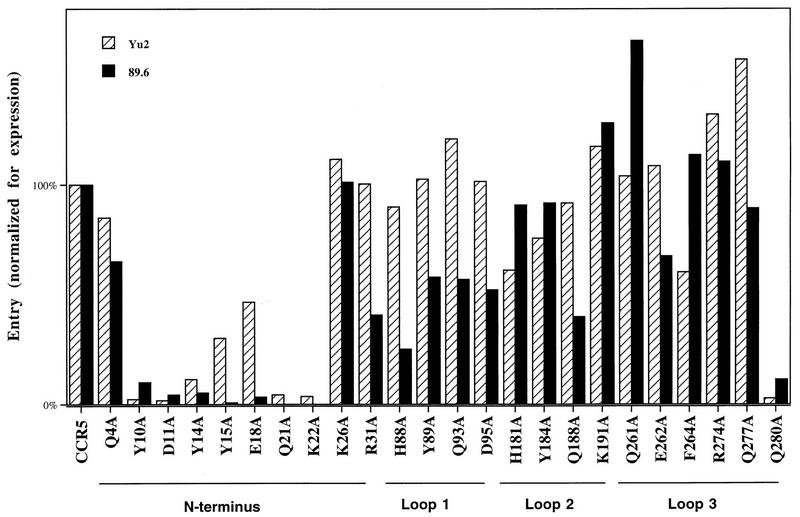
FIG. 1.
Relative entry of YU2 and 89.6 viruses into CF2Th cells expressing mutant CCR5 proteins. Entry is expressed as the percentage of expected CAT conversion on wild-type CCR5 at the same level of surface expression determined by FACS, as described in Materials and Methods. Data represent averages of values from two to five independent experiments. For all values, variation of normalized values was less than 25% of the value indicated. Surface expression of mutants Y10A, D11A, Q21A, D95A, K191A, and Q280A was consistently lower than that of wild-type CCR5 when the same amount of plasmid DNA was transfected. Mutants D2A, Y3A, C20A, Q27A, R168A, K171A, R172A, Y176A, C178A, S270A, and D276A failed to express at levels above which detectable entry could be measured in cells expressing wild-type CCR5 (data not shown).
The remainder of the transfected CF2Th cells were incubated with recombinant viruses containing envelope glycoproteins from two primary HIV-1 isolates, YU2 and 89.6. The YU2 isolate is macrophage tropic, while the 89.6 isolate is dual tropic (10, 28). A linear relationship between wild-type CCR5 cell surface expression and the efficiency of entry of the HIV-1 recombinant was observed in multiple experiments (e.g., Fig. 2). Figure 1 shows the efficiency of infection by the recombinant virus of CF2Th cells expressing CD4 and the CCR5 mutants. These values represent the ratio of infection that was actually observed for the mutant CCR5 proteins to that expected for the wild-type CCR5 with same cell surface expression level. The latter value was extrapolated from the infection levels observed for wild-type CCR5 with the higher and lower expression values closest to those of the mutant (Fig. 2). Mutants Y10A, D11A, Y14A, Y15A, E18A, K21A, Q22A, and Q280A were substantially less efficient at supporting the entry of viruses with YU2 and 89.6 envelope glycoproteins than was wild-type CCR5 protein expressed at comparable levels (Fig. 1). Most of these mutants demonstrated a lower than wild-type expression level for the same quantity of DNA transfected, with the exception of mutants Y15A and E18A, which typically expressed at wild-type or higher levels (Fig. 2). Also of note is the greater sensitivity of viruses with the 89.6 envelope glycoproteins to the E18A change (Fig. 1). This glutamic acid is common to CXCR4 and CCR5, and the specific contribution of this amino acid to 89.6 entry may help explain the ability of this virus to use both coreceptors. We conclude that the CCR5 region between but not including glutamine 4 and lysine 26 plays an important role in the entry of primary HIV-1.
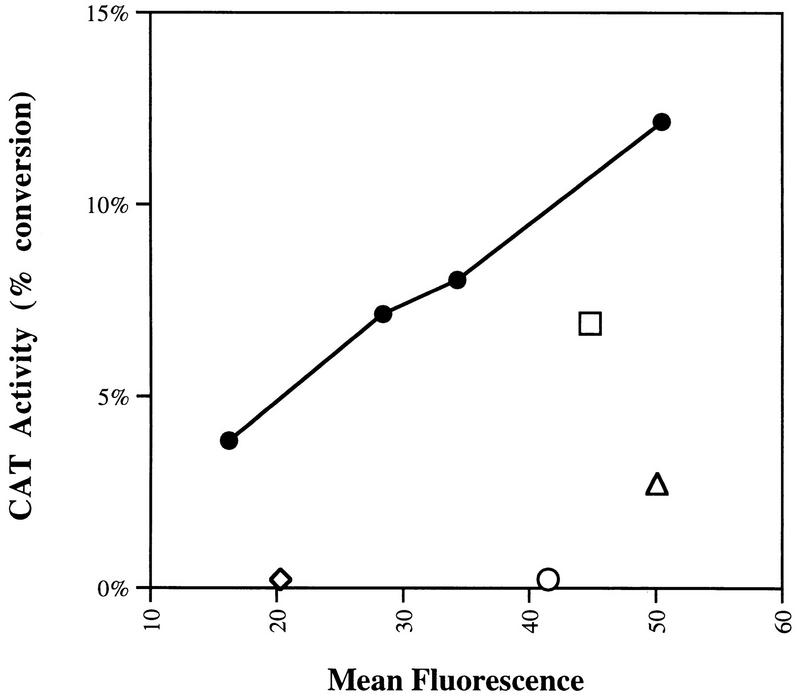
FIG. 2.
Relationship of wild-type and mutant CCR5 surface levels and infectability. A representative experiment of the kind used to assemble Fig. 1 is shown. CAT activity (percent conversion of chloramphenicol) in cells transfected with 5, 10, 15, or 20 μg of plasmid DNA expressing wild-type CCR5 protein (•) or 15 μg of plasmid expressing mutant CCR5 proteins Y10A (◊), Y14A (○), Y15A (▵), or E18A (□) after incubation with recombinant YU2 viruses, is shown. The level of wild-type or mutant CCR5 protein detected on the cell surface by FACS is plotted on the x axis.
Effect of CCR5 amino acid changes on SIVmac entry.
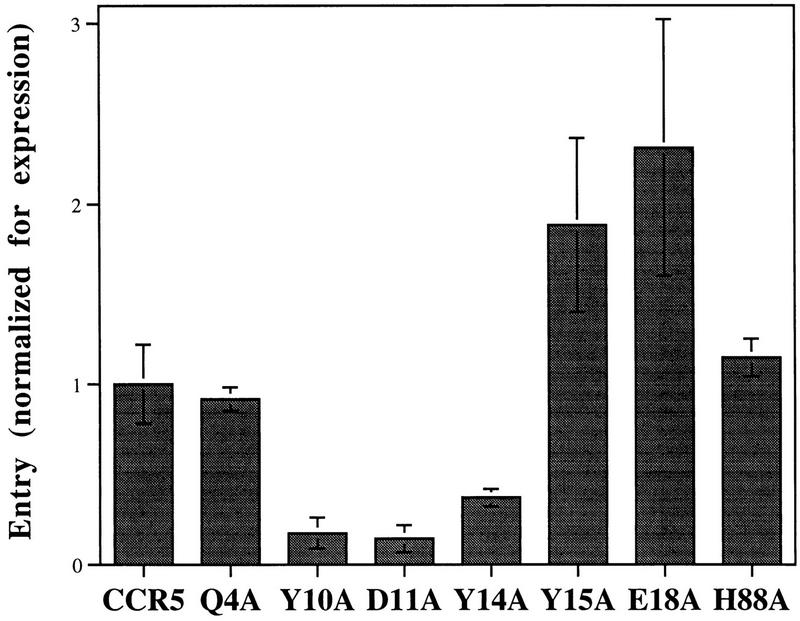
We then investigated the effect of changes in the N-terminal CCR5 residues on the entry of an HIV-1 recombinant pseudotyped with the SIVmac239 envelope glycoproteins. This was of interest both because the envelope glycoproteins of SIVmac239 and HIV-1 are relatively divergent and because additional SIVmac coreceptors that exhibit sequence similarity to CCR5 in the N-terminal region have been identified (15, 20). These receptors retain the tyrosines located at CCR5 positions 10, 14, and 15. Figure 3 shows that relative to cells expressing the wild-type CCR5 protein, SIVmac239 entry into cells expressing mutants Y10A, D11A, and Y14A was decreased. Surprisingly, cells expressing mutants Y15A and E18A supported SIVmac239 infection better than cells expressing comparable levels of the wild-type CCR5 protein (Fig. 3). In these experiments, normalized values for SIVmac239 infection of cells expressing mutants K21A and Q22A could not be determined, because SIV entry was not detectable on cells expressing wild-type CCR5 whose surface expression was comparable to that of these two poorly expressing mutants.
FIG. 3.
Relative entry of SIVmac239 recombinants into CF2Th cells expressing N-terminal CCR5 mutants. Entry is expressed as the ratio of observed chloramphenicol conversion to that expected for wild-type CCR5 at the same level of surface expression, as in Fig. 1. Data represent averages of values from two or three independent experiments. Error bars represent the range of observed values.
Effect of CCR5 amino-terminal residue changes on binding of MIP-1α and gp120-sCD4 complexes.
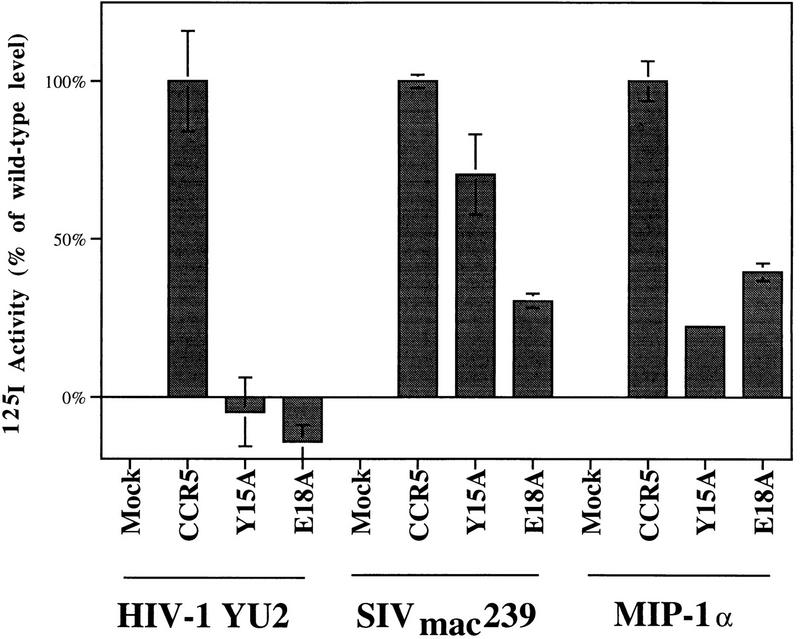
The ability of mutants Y15A and E18A expressed on the surface of HEK293T cells to bind YU2 or SIVmac239 gp120 envelope glycoproteins in the presence of sCD4 was examined. Mutants Y15A and E18A were both expressed on the cell surface at somewhat higher levels than wild-type CCR5 when comparable amounts of plasmid DNA were transfected (Fig. 2). Both CCR5 mutants demonstrated markedly lower affinities for YU2 gp120-sCD4 complexes compared to wild-type CCR5 (Fig. 4 and 5a). These affinities correspond to the relative ability of the recombinant YU2 virus to infect cells expressing these mutants. The binding of SIVmac239 gp120-sCD4 complexes exhibited some sensitivity to the Y15A and E18A changes as well (Fig. 4 and 5b), although the change of affinity was much less dramatic than in the case of HIV-1 YU2-sCD4 complexes. The dissociation constants for the SIVmac239 gp120-sCD4 complexes were 17.2 nM, 21.2, and 11.7 nM for Y15A, E18A, and wild-type CCR5, respectively. MIP-1α, a natural ligand for CCR5, also demonstrated a reduced affinity for mutants Y15A and E18A (Fig. 4). It is likely that the lower efficiency of infection by viruses containing the YU2 envelope glycoproteins of cells expressing mutants Y15A and E18A is due, at least in part, to a substantially lower affinity of the envelope glycoprotein-CD4 complex for these mutants.
FIG. 4.
Specific association of 125I-labeled YU2 or SIVmac239 gp120 envelope glycoproteins or 125I-labeled MIP-1α with cells expressing wild-type or mutant CCR5 receptors. HEK293T cells transfected with plasmids expressing wild-type CCR5, Y15A, or E18A were incubated with 0.5 nM 125I-labeled YU2 or SIVmac239 gp120 glycoprotein and 100 nM unlabeled CD4 or 0.5 nM 125I-labeled MIP-1α for 30 min at 37°C, washed, and counted. Radioactive counts measured on mock-transfected cells were considered background and subtracted from all values. Values shown are normalized to the wild-type CCR5 values measured for each ligand. Expression levels in this experiment, as measured by FACS with the anti-CCR5 antibody 2D7, were 79 for cells expressing wild-type CCR5, 75 for cells expressing Y15A, 66 for cells expressing E18A, and 10 for mock-transfected cells.
FIG. 5.
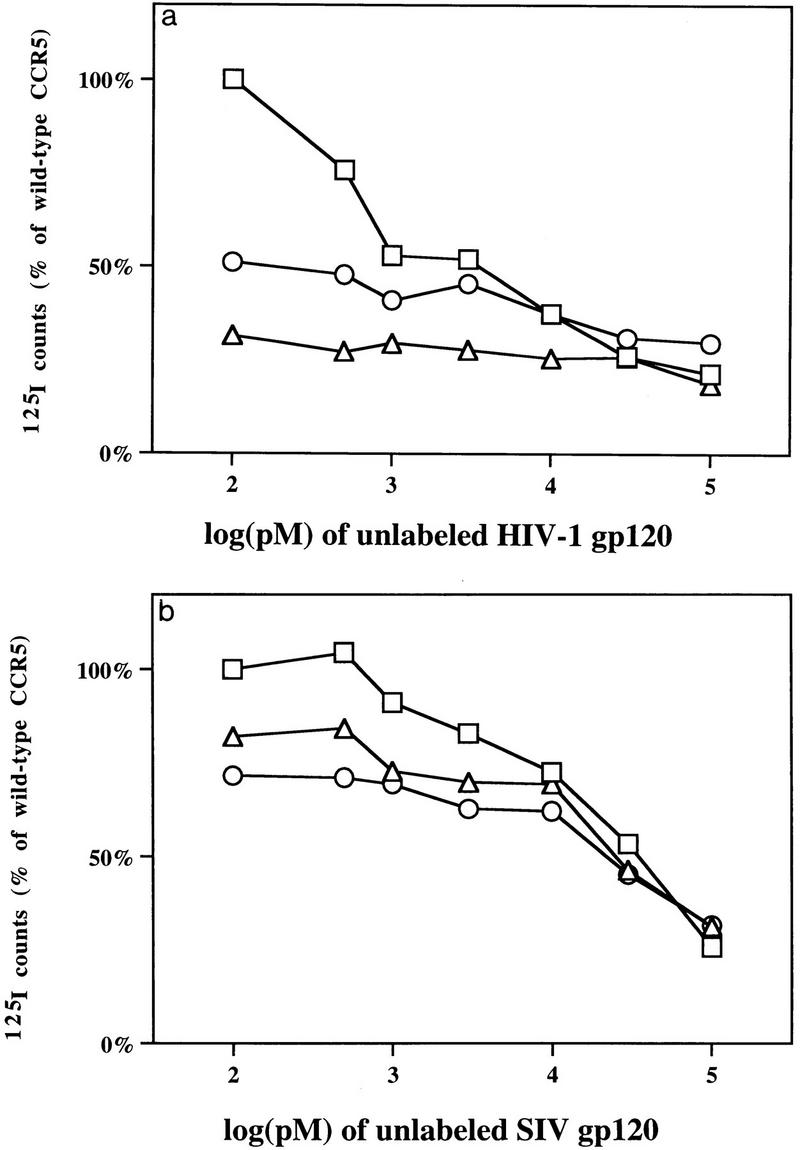
(a) Binding of HIV-1 YU2 gp120 glycoprotein to cells expressing CCR5 mutants. HEK293T cells transfected with wild-type CCR5 (□), Y15A (○), or E18A (▵) were incubated with 0.5 nM 125I-labeled HIV-1 YU2 gp120, 100 nM unlabeled soluble CD4, and the indicated amounts of unlabeled HIV-1 YU2 gp120 for 30 min at 37°C, and washed, and counted. Counts were normalized to the level observed for wild-type CCR5 in the absence of unlabeled competitor. (b) Binding of SIVmac239 gp120 glycoprotein to cells expressing CCR5 mutants. The binding experiment is identical to that in panel a except that 0.5 nM 125I-labeled SIVmac239 gp120 glycoprotein was incubated with 100 nM sCD4 and the indicated amounts of unlabeled SIVmac239 gp120 glycoprotein.
DISCUSSION
We have shown that several changes in a region proximal to the N-terminal cysteine of CCR5 result in substantial reductions in the entry of the macrophage-tropic HIV-1 variant YU2 and the dual-tropic HIV-1 variant 89.6. It is notable that three of the residues that appear to be important for HIV-1 entry are tyrosines. All of the known coreceptors for primate immunodeficiency viruses, but only a fraction of receptors with homology to chemokine receptors, have an N terminus that is rich in tyrosines as well as acidic amino acids. There is ample precedent for a high-affinity binding site to be composed of an aromatic residue surrounded by charged or polar residues (1, 40). For example, the binding site on CD4 for gp120 is composed of phenylalanine 43 and several positively charged residues (3, 4). In the case of CCR5 binding to the gp120-CD4 complex, more than one tyrosine may be necessary for a high-affinity association. Supporting this conclusion are the observations that alteration of with any one of three tyrosines affects viral entry and that other coreceptors that support macrophage-tropic HIV-1 or SIV entry (gpr15, gpr1, STRL33, and CCR3) have similarly arranged tyrosines (15, 20, 29).
The relative entry of HIV-1 into cells expressing the Y15A, E18A, and wild-type CCR5 proteins correlates with the ability of each of these CCR5 molecules to bind soluble complexes of the gp120 and CD4 glycoproteins. This finding suggests that the major effect on virus entry of the amino acid changes in this CCR5 region is due to a change in ability of these CCR5 mutants to bind envelope glycoprotein-CD4 complexes. A direct association of CD4 with this region of CCR5 cannot be ruled out by these studies, although the differential affinity of the SIV and HIV-1 envelope-sCD4 complexes for the CCR5 mutants suggests that the envelope glycoprotein is the major determinant of the strength of this interaction. The reduced affinity of MIP-1α for E18A and Y15A mutants suggests that these or nearby residues may constitute a portion of a common binding site for HIV-1 gp120 and the natural ligands of CCR5.
SIV entry also demonstrated sensitivity to changes in the amino-terminal CCR5 motif, implying that this portion of the N terminus of CCR5 may be generally important for viruses that use CCR5 as a coreceptor. The enhanced entry of SIVmac239 on cells expressing Y15A or E18A is possibly the result of an enhanced accessibility to or flexibility of the actual virus binding site, which probably includes residues immediately in the vicinity of Y15 and E18. The observation that a change in asparagine 13 of CCR5 allows the SIVmac239 gp120 envelope glycoprotein to bind CCR5 in an sCD4-independent manner supports this conclusion (32). The enhanced entry of SIVmac239 on cells expressing Y15A or E18A does not appear to correlate with the slightly lower affinity of these mutants for soluble monomeric gp120-sCD4 complexes. This discrepancy may be accounted for by the sensitivity of the virus entry assay, but not the binding assay, to an enhanced on rate, or by differences in the properties of monomeric and oligomeric envelope glycoproteins in the contexts of these different assays. The decreased sensitivity of SIVmac239, compared to HIV-1, to changes in tyrosine 15 and glutamic acid 18 may help explain why STRL33, which has a glycine and serine, respectively, at these positions, functions as a more efficient coreceptor for SIVmac239 than for HIV-1 (15, 29).
The identification of a region on CCR5 that is important for the entry of diverse viruses which use CCR5 may imply that this region associates with a relatively conserved structure on the HIV-1 envelope glycoproteins. Further characterization of this interaction may prove useful in efforts to understand the role of chemokine receptors in viral fusion and perhaps in efforts to block this interaction pharmacologically.
ACKNOWLEDGMENTS
The first two authors contributed equally to this work.
This work was supported by NIH grant AI 41581, by the Rubenstein/Cable Fund, and by the Mathers Charitable Foundation.
REFERENCES
- 1.Albritton L M, Kim J W, Tseng L, Cunningham J M. Envelope-binding domain in the cationic amino acid transporter determines the host range of ecotropic murine retroviruses. J Virol. 1993;67:2091–2096. doi: 10.1128/jvi.67.4.2091-2096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 3.Arthos J, Deen K C, Chaikin M A, Fornwald J A, Sathe G, Sattentau Q J, Clapham P R, Weiss R A, McDougal J S, Pietropaolo C, et al. Identification of the residues in human CD4 critical for the binding of HIV. Cell. 1989;57:469–481. doi: 10.1016/0092-8674(89)90922-7. [DOI] [PubMed] [Google Scholar]
- 4.Ashkenazi A, Presta L G, Marsters S A, Camerato T R, Rosenthal K A, Fendly B M, Capon D J. Mapping the CD4 binding site for human immunodeficiency virus by alanine-scanning mutagenesis. Proc Natl Acad Sci USA. 1990;87:7150–7154. doi: 10.1073/pnas.87.18.7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atchison R E, Gosling J, Monteclaro F S, Franci C, Digilio L, Charo I F, Goldsmith M A. Multiple extracellular elements of CCR5 and HIV-1 entry: dissociation from response to chemokines. Science. 1996;274:1924–1926. doi: 10.1126/science.274.5294.1924. [DOI] [PubMed] [Google Scholar]
- 6.Barre-Sinoussi F, Chermann J C, Rey F, Nugeyre M T, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vezinet-Brun F, Rouzioux C, Rozenbaum W, Montagnier L. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 7.Bieniasz P D, Fridell R A, Aramori I, Ferguson S S, Caron M G, Cullen B R. HIV-1-induced cell fusion is mediated by multiple regions within both the viral envelope and the CCR-5 co-receptor. EMBO J. 1997;16:2599–2609. doi: 10.1093/emboj/16.10.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 9.Cocchi F, DeVico A L, Garzino-Demo A, Cara A, Gallo R C, Lusso P. The V3 domain of the HIV-1 gp120 envelope glycoprotein is critical for chemokine-mediated blockade of infection. Nat Med. 1996;2:1244–1247. doi: 10.1038/nm1196-1244. [DOI] [PubMed] [Google Scholar]
- 10.Collman R, Balliet J W, Gregory S A, Friedman H, Kolson D L, Nathanson N, Srinivasan A. An infectious molecular clone of an unusual macrophage-tropic and highly cytopathic strain of human immunodeficiency virus type 1. J Virol. 1992;66:7517–7521. doi: 10.1128/jvi.66.12.7517-7521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalgleish A G, Beverley P C, Clapham P R, Crawford D H, Greaves M F, Weiss R A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 13.Dean M, Carrington M, Winkler C, Huttley G A, Smith M W, Allikmets R, Goedert J J, Buchbinder S P, Vittinghoff E, Gomperts E, Donfield S, Vlahov D, Kaslow R, Saah A, Rinaldo C, Detels R, O’Brien S J. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 14.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 15.Deng H, Unatmaz, K. V. D, Littman D. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 16.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 17.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 18.Earl P L, Doms R W, Moss B. Oligomeric structure of the human immunodeficiency virus type 1 envelope glycoprotein. Proc Natl Acad Sci USA. 1990;87:648–652. doi: 10.1073/pnas.87.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Earl P L, Moss B, Doms R W. Folding, interaction with GRP78-BiP, assembly, and transport of the human immunodeficiency virus type 1 envelope protein. J Virol. 1991;65:2047–2055. doi: 10.1128/jvi.65.4.2047-2055.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farzan M, Choe H, Martin K, Marcon L, Hofmann W, Karlsson G, Sun Y, Barrett P, Marchand N, Sullivan N, Gerard N, Gerard C, Sodroski J. Two orphan seven-transmembrane segment receptors which are expressed in CD4-positive cells support simian immunodeficiency virus infection. J Exp Med. 1997;186:405–411. doi: 10.1084/jem.186.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farzan M, Choe H, Martin K A, Sun Y, Sidelko M, Mackay C R, Gerard N P, Sodroski J, Gerard C. HIV-1 entry and macrophage inflammatory protein-1beta-mediated signaling are independent functions of the chemokine receptor CCR5. J Biol Chem. 1997;272:6854–6857. doi: 10.1074/jbc.272.11.6854. [DOI] [PubMed] [Google Scholar]
- 22.Fauci A S, Macher A M, Longo D L, Lane H C, Rook A H, Masur H, Gelmann E P. NIH conference. Acquired immunodeficiency syndrome: epidemiologic, clinical, immunologic, and therapeutic considerations. Ann Intern Med. 1984;100:92–106. doi: 10.7326/0003-4819-100-1-92. [DOI] [PubMed] [Google Scholar]
- 23.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 24.Gallo R C, Salahuddin S Z, Popovic M, Shearer G M, Kaplan M, Haynes B F, Palker T J, Redfield R, Oleske J, Safai B, et al. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984;224:500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- 25.Helseth E, Kowalski M, Gabuzda D, Olshevsky U, Haseltine W, Sodroski J. Rapid complementation assays measuring replicative potential of human immunodeficiency virus type 1 envelope glycoprotein mutants. J Virol. 1990;64:2416–2420. doi: 10.1128/jvi.64.5.2416-2420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanki P J, McLane M F, King N W, Jr, Letvin N L, Hunt R D, Sehgal P, Daniel M D, Desrosiers R C, Essex M. Serologic identification and characterization of a macaque T-lymphotropic retrovirus closely related to HTLV-III. Science. 1985;228:1199–1201. doi: 10.1126/science.3873705. [DOI] [PubMed] [Google Scholar]
- 27.Letvin N L, Daniel M D, Sehgal P K, Desrosiers R C, Hunt R D, Waldron L M, MacKey J J, Schmidt D K, Chalifoux L V, King N W. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science. 1985;230:71–73. doi: 10.1126/science.2412295. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Hui H, Burgess C J, Price R W, Sharp P M, Hahn B H, Shaw G M. Complete nucleotide sequence, genome organization, and biological properties of human immunodeficiency virus type 1 in vivo: evidence for limited defectiveness and complementation. J Virol. 1992;66:6587–6600. doi: 10.1128/jvi.66.11.6587-6600.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao F, Alkhatib G, Peden K W, Sharma G, Berger E A, Farber J M. STRL33, a novel chemokine receptor-like protein, functions as a fusion cofactor for both macrophage-tropic and T cell line-tropic HIV-1. J Exp Med. 1997;185:2015–2023. doi: 10.1084/jem.185.11.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu R, Paxton W A, Choe S, Ceradini D, Martin S R, Horuk R, MacDonald M E, Stuhlmann H, Koup R A, Landau N R. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 31.Maddon P J, Dalgleish A G, McDougal J S, Clapham P R, Weiss R A, Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986;47:333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- 32.Martin, K., R. Wyatt, M. Farzan, H. Choe, L. Marcon, E. Desjardins, J. Robinson, J. Sodroski, C. Gerard, and N. Gerard. CD4-independent binding of SIV GP120 to rhesus CCR5. Science, in press. [DOI] [PubMed]
- 33.Rucker J, Samson M, Doranz B J, Libert F, Berson J F, Yi Y, Smyth R J, Collman R G, Broder C C, Vassart G, Doms R W, Parmentier M. Regions in beta-chemokine receptors CCR5 and CCR2b that determine HIV-1 cofactor specificity. Cell. 1996;87:437–446. doi: 10.1016/s0092-8674(00)81364-1. [DOI] [PubMed] [Google Scholar]
- 34.Samson M, Libert F, Doranz B J, Rucker J, Liesnard C, Farber C M, Saragosti S, Lapoumeroulie C, Cognaux J, Forceille C, Muyldermans G, Verhofstede C, Burtonboy G, Georges M, Imai T, Rana S, Yi Y, Smyth R J, Collman R G, Doms R W, Vassart G, Parmentier M. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan N, Sun Y, Li J, Hofmann W, Sodroski J. Replicative function and neutralization sensitivity of envelope glycoproteins from primary and T-cell line-passaged human immunodeficiency virus type 1 isolates. J Virol. 1995;69:4413–4422. doi: 10.1128/jvi.69.7.4413-4422.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thali M, Bukovsky A, Kondo E, Rosenwirth B, Walsh C T, Sodroski J, Gottlinger H G. Functional association of cyclophilin A with HIV-1 virions. Nature. 1994;372:363–365. doi: 10.1038/372363a0. [DOI] [PubMed] [Google Scholar]
- 37.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Maddon P J, Moore J P. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature. 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 37a.Wu, L. Unpublished observations.
- 38.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardosa A A, Desjardin E, Newman W, Gerard C, Sodroski J. CD4-induced interactions of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;184:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 39.Wu L, Paxton W A, Kassam N, Ruffing N, Rottman J B, Sullivan N, Choe H, Sodroski J, Newman W, Koup R A, Mackay C R. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med. 1997;185:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zingler K, Young J A. Residue Trp-48 of Tva is critical for viral entry but not for high-affinity binding to the SU glycoprotein of subgroup A avian leukosis and sarcoma viruses. J Virol. 1996;70:7510–7516. doi: 10.1128/jvi.70.11.7510-7516.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]