Abstract
This study aims to investigate the relationship between the metabolic score for visceral fat (METS-VF) and the risk of asthma incidence, and to assess its role in predictive models using machine learning methods. Data from the National Health and Nutrition Examination Survey database from 2001 to 2018 were used, with a total of 13,695 participants included after excluding those with missing values. The Boruta algorithm was employed to screen variables, which were then randomly divided into training and validation sets at a 7:3 ratio and incorporated into machine learning predictive models for analysis. SHapley Additive exPlanations visualization analysis was used to evaluate the importance of each variable. The Boruta algorithm identified 14 variables, including age, gender, education level, race, marital status, smoking history, alcohol consumption, poverty-income ratio, body mass index, METS-VF, presence of hypertension, diabetes, cancer, and cardiovascular disease. Among the various machine learning models evaluated, the CatBoost model demonstrated the highest area under the receiver operating characteristic curve, with a value of 0.640 (95% CI: 0.617–0.664). This finding underscores its superiority as the optimal predictive model in this context. SHapley Additive exPlanations visualization analysis revealed that body mass index was the most significant variable associated with asthma incidence risk, followed by gender, race, marital status, and smoking history. There is a certain association between the METS-VF and the risk of asthma incidence. CatBoost delivers high predictive accuracy alongside transparent interpretability, positioning it as an effective tool for asthma risk screening.
Keywords: asthma, Boruta algorithm, machine learning, METS-VF, NHANES
1. Introduction
Asthma is a chronic respiratory disease that poses a significant threat to human health. Data from the Global Burden of Disease study indicate that the incidence and prevalence of asthma vary across different regions and time periods. From 1990 to 2019, the global incidence and mortality rates of asthma exhibited an overall downward trend, with the incidence rate decreasing from 601.20 to 477.92 cases per 100,000 population, and the mortality rate declining from 8.60 to 5.96 deaths per 100,000 population.[1] However, despite the reduction in incidence, the overall burden of asthma remains substantial, particularly in regions with a high Socio-Demographic Index, where the prevalence and incidence of asthma are higher.[2] During the COVID-19 pandemic from 2020 to 2021, the incidence and prevalence of asthma increased globally, especially in high Socio-Demographic Index regions and among children under the age of 5.[3]
The metabolic score for visceral fat (METS-VF) is a reliable indicator for assessing visceral fat content. Elevated visceral fat is closely associated with various metabolic disorders and diseases, which may exacerbate inflammatory states and thereby influence disease onset. Studies have shown that increased METS-VF is significantly correlated with the incidence of diseases across multiple systems, including diabetes,[4] osteoarthritis,[5] gallstones,[6] cataracts,[7] and stroke.[8] A study utilizing data from the National Health and Nutrition Examination Survey (NHANES) between 2001 and 2018 revealed a positive correlation between METS-VF and asthma prevalence. After adjusting for all confounding factors, the positive correlation between elevated METS-VF and asthma prevalence became more pronounced, particularly when METS-VF exceeded 5.24, at which point the risk of asthma increased significantly.[9] Another study supported this finding, indicating that an increase in visceral adipose tissue (VAT) is associated with a higher risk of asthma. The study demonstrated that for every 200-gram increase in VAT, the risk of asthma increased by 10.4%, 20.8%, and 20.3%, respectively. This association was more significant among women and individuals aged 40 years or older.[10]
As a representative national health survey, the NHANES database encompasses comprehensive demographic, laboratory, examination, and questionnaire data, providing reliable support for the holistic assessment of the health status of the study population. Therefore, this study fully leverages the unique strengths of the NHANES database, employing the Boruta algorithm and machine learning predictive models to systematically investigate the association between the METS-VF and the risk of asthma onset, thereby revealing its potential clinical significance.
2. Methods
2.1. Data sources
The data for this study were derived from the NHANES. This survey is a significant public health investigation that focuses on systematically assessing the health and nutritional status of the US population and strictly adheres to the STROBE guidelines for reporting observational studies. The study protocol of NHANES has been approved by the Ethics Review Board of the National Center for Health Statistics, and all participants provided written informed consent.
2.2. Definitions of METS-VF and asthma
The metabolic score for insulin resistance (METS-IR) is calculated as follows: METS-IR = ln (2 * fasting blood glucose + triglycerides) * body mass index (BMI)/[ln(high-density lipoprotein cholesterol)]. The waist-to-height ratio is defined as the waist circumference divided by height. The METS-VF is computed using the formula: METS-VF = 4.466 + 0.011 * [ln(METS-IR)]3 + 3.239 * [ln(WHtR)]3 + 0.319 * sex + 0.594 * ln(age), where sex is coded as 1 for males and 0 for females.
Asthma diagnosis was determined based on 2 questions: “Has a doctor or other health professional ever told you that you have asthma?” and “Do you still have asthma?” Participants were classified as having asthma if they answered “yes” to either question.
2.3. Study variables
Participants aged 18 years or older were included in the study. Data were collected on demographics (age, sex, race, education level, poverty-to-income ratio [PIR], and marital status), physical examination data (BMI), and questionnaire data (smoking habits, alcohol consumption frequency, hypertension, diabetes, asthma, cancer, and cardiovascular disease [CVD]).
2.4. Statistical analysis
All analyses were performed using R 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria) with the following packages: “Boruta,” “corrplot,” “caret,” “shapviz,” “fastshap,” “shapper,” and “DALEX” packages. After excluding cases with missing values, the dataset was randomly divided into a training cohort (70%) and a validation cohort (30%). In the validation cohort, potential risk factors associated with asthma were identified using the Boruta algorithm. The Boruta algorithm is a feature selection method based on random forests, designed to identify the most relevant predictors in a dataset by comparing the importance of original features with their randomly permuted counterparts. In this study, we employed the Boruta algorithm with 1500 trees to ensure reliable feature selection and incorporated the selected variables into machine learning models. Ten different machine learning algorithms were included: logistic regression, support vector machine, gradient boosting machine, neural network, random forest, Xgboost, K-nearest neighbors, Adaboost, light gradient boosting machine, and CatBoost. The performance of each machine learning model was evaluated using the area under the curve (AUC) as well as accuracy, sensitivity, specificity, precision, and F1 score. We used repeated 10-fold cross validation to reduce overfitting and improve robustness. These metrics helped further delineate the strengths and limitations of each model in predicting asthma risk.
To elucidate the relative importance of each feature in the best-performing machine learning model, we employed SHapley Additive exPlanations (SHAP) visualization analysis. SHAP values, derived from game theory, provide a fair attribution of each feature’s contribution to model predictions. By decomposing model outputs into contributions from individual features, SHAP analysis offers insights into which variables most significantly drive the model’s predictive power. This approach not only enhances model interpretability but also provides valuable information on the potential mechanisms linking the selected predictors to asthma risk. In summary, integrating Boruta-selected variables into multiple machine learning models, combined with rigorous performance evaluation and SHAP-based feature importance analysis, enabled us to identify the most effective asthma risk prediction model and elucidate the key factors influencing its performance.
3. Results
3.1. Boruta algorithm analysis
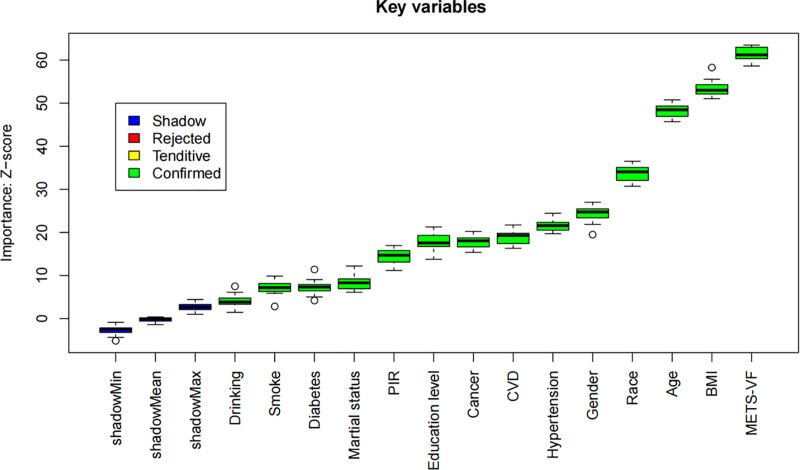
The Boruta algorithm identified 14 significant variables associated with asthma risk: alcohol consumption, sex, race, smoking status, hypertension, diabetes, cancer, PIR, BMI, CVD, age, METS-VF, education, and marital status. The results of the feature selection process are illustrated in Figure 1, where variables in the green zone are designated as important features. Figure 2 displays the score evolution plot generated by the Boruta algorithm. The x-axis represents the number of iterations, while the y-axis indicates the feature importance score (Z-score). Variables in blue denote shadow features, which are features that have not yet been classified as important or unimportant and require further analysis. The importance of green variables is significantly higher than that of their corresponding shadow features, thus they are labeled as “important.” Variables in red are considered unimportant.
Figure 1.
Boruta algorithm feature selection results. Green bars denote confirmed important variables (n = 14) associated with asthma risk, including age, sex, race, education, marital status, smoking, alcohol use, PIR, BMI, METS-VF, hypertension, diabetes, cancer, and CVD. Red bars indicate unimportant features, while blue bars represent tentative features. BMI = body mass index, CVD = cardiovascular disease, METS-VF = metabolic score for visceral fat, PIR = poverty-to-income ratio.
Figure 2.
Boruta algorithm score evolution plot. Z-scores (y-axis) of original features (green) and shadow features (blue/red) across 1500 iterations (x-axis). Variables stabilized in the green zone (e.g., BMI, METS-VF) are confirmed as significant predictors. BMI = body mass index, METS-VF = metabolic score for visceral fat.
3.2. Feature selection in ML model development and validation
The correlation heatmap of the study variables is shown in Figure 3, highlighting the significant relationships among the correlated variables. In this study, we found significant positive correlations between BMI and METS-VF, as well as between METS-VF and age.
Figure 3.
Correlation heatmap of study variables. Spearman correlation coefficients between 14 Boruta-selected variables. Darker red indicates stronger positive correlations (e.g., BMI-METS-VF: R > 0.6), while darker blue indicates negative correlations. White squares denote weak or no correlation. BMI = body mass index, METS-VF = metabolic score for visceral fat.
The performance evaluation of various machine learning models for predicting asthma is presented in Table 1, highlighting key metrics such as accuracy, sensitivity, specificity, precision, and F1 score.
Table 1.
Assessment of performance metrics for 10 mL models.
| Model | Accuracy | Sensitivity | Specificity | Precision | F1 |
|---|---|---|---|---|---|
| Logistic | 0.565 | 0.647 | 0.551 | 0.195 | 0.299 |
| SVM | 0.451 | 0.481 | 0.446 | 0.127 | 0.201 |
| GBM | 0.565 | 0.656 | 0.55 | 0.196 | 0.302 |
| NeuralNetwork | 0.63 | 0.563 | 0.641 | 0.208 | 0.304 |
| RandomForest | 0.508 | 0.671 | 0.481 | 0.178 | 0.282 |
| Xgboost | 0.63 | 0.559 | 0.642 | 0.208 | 0.303 |
| KNN | 0.769 | 0.175 | 0.869 | 0.183 | 0.179 |
| Adaboost | 0.741 | 0.322 | 0.811 | 0.222 | 0.263 |
| LightGBM | 0.557 | 0.651 | 0.541 | 0.192 | 0.297 |
| CatBoost | 0.529 | 0.731 | 0.495 | 0.195 | 0.308 |
GBM = gradient boosting machine, KNN = K-nearest neighbors, LightGBM = light gradient boosting machine, SVM = support vector machine.
In the evaluation of machine learning models, the area under the receiver operating characteristic curve is a key indicator for assessing the model’s ability to distinguish between positive and negative cases. A higher AUC value, especially one approaching 1.0, indicates superior performance. Among the machine learning models in the validation cohort, the CatBoost model achieved the highest AUC value, demonstrating its superior predictive capability for the target variable compared with other models. This suggests that the CatBoost model can more effectively identify positive and negative samples, thereby reducing the likelihood of misclassification. Although the CatBoost model did not have the highest values for other metrics such as accuracy, specificity, and precision, its overall performance in terms of discrimination ability was the most robust (Table 1 and Fig. 4). Therefore, the CatBoost model was identified as the most suitable model for the research context of this study.
Figure 4.
ROC curves for 10 machine learning models. Receiver operating characteristic curves comparing the discriminative performance of models in the validation cohort. CatBoost (AUC = 0.640, 95% CI: 0.617–0.664) outperforms others, as indicated by the highest curve. AUC = area under the curve, ROC = receiver operating characteristic.
Decision curve analysis was employed to compare the clinical utility of different predictive models. The decision curve analysis curve provides the net benefit of each model across a range of probability thresholds, thereby facilitating a comprehensive assessment of their practical applicability in clinical settings (Fig. 5).
Figure 5.
Decision curve analysis (DCA) for 10 models. Net benefit (y-axis) plotted against threshold probabilities (x-axis). CatBoost demonstrates the highest net benefit across most probability thresholds, suggesting superior clinical utility.
3.3. SHAP value interpretation
In this study, SHAP visualization analysis was employed to elucidate the contribution of individual feature variables to the predictions of the optimal machine learning model, CatBoost. The bar plot (Fig. 6) illustrates the importance of each feature variable, ranked in descending order according to their mean SHAP values. This visualization highlights the relative contribution of each feature to the model’s predictive power. The beeswarm plot (Fig. 7) provides a comprehensive overview of the global distribution of feature variables, sorted by their mean SHAP values. Each point in the figure represents the SHAP value of a specific feature within the sample, with color indicating the magnitude of the feature value (red for high values and blue for low values). This visualization elucidates the complex relationships among feature values and their impact on predictions, including both monotonic and nonlinear patterns. Among the evaluated features, BMI emerged as the most important feature variable for asthma risk, exhibiting the highest SHAP value, followed by sex, race, marital status, and smoking status.
Figure 6.
SHAP bar plot for CatBoost Model. Mean absolute SHAP values ranked by importance. BMI is the dominant predictor, followed by sex, race, marital status, and smoking history. Error bars represent 95% confidence intervals. BMI = body mass index, SHAP = SHapley Additive exPlanations.
Figure 7.
SHAP Beeswarm plot. Global distribution of SHAP values for all 14 features. Each dot represents 1 participant; red (high feature values) and blue (low feature values) illustrate non-linear relationships with asthma risk (e.g., high BMI increases risk). BMI = body mass index, SHAP = SHapley Additive exPlanations.
To further explore the contribution of feature variables to individual predictions, this study generated waterfall plots (Fig. 8) and force plots (Fig. 9). The waterfall plot clearly displays the hierarchical order and magnitude of contributions from different features to individual asthma predictions, with yellow arrows indicating positive contributions and brown arrows indicating negative contributions. This visualization provides a clear understanding of how each feature influences the final prediction. The force plot presents the contributions of feature variables in a different format, with arrow color and length representing the direction and magnitude of the contributions, respectively. This visualization generates a predictive output value for each individual, thereby providing a detailed breakdown of how each feature impacts model predictions. In summary, SHAP analysis offers valuable insights into the relative importance and contribution of each feature variable in the CatBoost model, thereby enhancing the interpretability and transparency of the predictive model.
Figure 8.
SHAP waterfall plot for individual prediction. Example of a single participant’s prediction breakdown. Positive contributions (yellow arrows) from BMI (SHAP = +0.12) and smoking (SHAP = +0.08) increase predicted risk, while marital status (SHAP = −0.05) reduces it. Final probability: 0.68. BMI = body mass index, SHAP = SHapley Additive exPlanations.
Figure 9.
SHAP force plot for individual prediction. Alternative visualization of Figure 8. Base probability (0.10) is pushed higher by BMI (red arrow) and lower by marital status (blue arrow), yielding a final output (f(x) = 0.68). BMI = body mass index, SHAP = SHapley Additive exPlanations.
4. Discussion
This study, based on data from the NHANES from 2001 to 2018, utilized machine learning methods to explore the relationship between the METS-VF and the risk of asthma onset. Additionally, SHAP visualization analysis was employed to assess the importance of related factors. The results indicated a positive correlation between METS-VF and the risk of asthma onset. Furthermore, through the analysis of machine learning models, relevant factors associated with asthma onset were effectively identified. These findings provide a novel perspective for understanding the potential role of visceral fat in the pathogenesis of asthma and offer valuable references for future research and clinical practice.
VAT functions as an active endocrine organ, secreting a variety of bioactive substances, including cytokines, hormones, and adipokines. These substances play important roles in systemic inflammatory responses and immune regulation, thereby potentially influencing the risk of asthma onset.[11,12] Studies have shown that adipokines and pro-inflammatory factors secreted by VAT are closely associated with various metabolic and cardiovascular diseases. These metabolic disorders may exacerbate inflammatory conditions, thereby affecting the occurrence of respiratory diseases such as asthma.[10,13] The functional characteristics of VAT differ from those of subcutaneous adipose tissue; VAT releases a large amount of free fatty acids and hormones/cytokines. These substances are transported to the liver via the portal vein, where they interact with hepatocytes and various immune cells, thereby affecting systemic metabolic balance and immune responses.[11,12] Moreover, adipokines secreted by VAT, such as adiponectin and leptin, have been proven to play important roles in regulating the immune system and inflammatory responses. Dysregulation of these factors may lead to chronic low-grade inflammation, thereby increasing the risk of asthma.[14,15] The inflammatory characteristics of VAT are also related to its secretion of cytokines, including tumor necrosis factor and interleukins (such as IL-6). The increased secretion of these cytokines in obese states may lead to enhanced systemic inflammatory responses, thereby affecting the onset of asthma.[15,16] Studies have also indicated that abnormal function of VAT may further increase the risk of inflammatory diseases such as asthma by affecting vascular function and immune regulation.[15,16] In summary, VAT plays an important role in systemic inflammatory responses and immune regulation through its secretion of various bioactive substances, and these mechanisms may be important factors influencing the risk of asthma onset.
In this study, METS-VF, as an indicator for assessing visceral fat distribution, was incorporated into machine learning models for analysis. The results showed a certain correlation between METS-VF and the risk of asthma onset, suggesting that the accumulation of visceral fat may be one of the potential relevant factors for asthma. This finding is consistent with previous studies on the correlation between obesity and asthma, further emphasizing the important role of visceral fat in the onset of asthma.[9,10]
In this study, machine learning methods were employed to construct predictive models for evaluating the relationships between multiple variables and the risk of asthma onset. A total of 14 variables were selected using the Boruta algorithm, including age, sex, education level, race, marital status, smoking history, alcohol consumption, PIR, BMI, METS-VF, comorbid hypertension, diabetes, cancer, and CVD. These variables encompassed sociodemographic characteristics, lifestyle factors, and comorbid conditions. Machine learning models are capable of handling complex non-linear relationships and interactions among variables, thereby more accurately identifying key factors associated with the risk of asthma onset. Among various machine learning models, the CatBoost model achieved the highest AUC value (AUC = 0.640, 95% CI: 0.617–0.664), indicating moderate discriminative ability in predicting asthma risk. These results suggest that the application of machine learning methods in medical predictive modeling holds great promise and can provide strong support for the early diagnosis and prevention of diseases.
Despite the comprehensive approach employed in this study, the AUC values achieved by the machine learning models were moderate, with the highest AUC reaching only 0.64. This level of discriminative ability indicates that while the models have some capacity to distinguish between individuals at higher and lower risk of asthma, their predictive power is not sufficiently robust for high-confidence clinical decision-making. Several factors may contribute to this limitation. First, asthma is a highly heterogeneous disease influenced by a complex interplay of genetic, environmental, and lifestyle factors. The variables included in our models, while clinically relevant, may not fully capture the multifactorial nature of asthma risk. The absence of key genetic markers, detailed environmental exposure data (such as air pollution or allergen exposure), and other emerging biomarkers may have limited the models’ ability to achieve higher discriminative accuracy. Second, the data used in this study were derived from NHANES, a cross-sectional survey designed for population health assessment rather than detailed clinical investigation. The inherent limitations of survey data, such as potential measurement errors, recall bias, and the lack of longitudinal follow-up, may have introduced noise and reduced the precision of the models. Additionally, the relatively low prevalence of asthma in the general population may have further challenged the models’ ability to accurately identify high-risk individuals. Third, while machine learning models can capture complex nonlinear relationships between predictors and outcomes, they are also susceptible to overfitting, especially when the dataset is not sufficiently large or diverse. Despite efforts to mitigate overfitting through techniques such as cross-validation and feature selection using the Boruta algorithm, the models may still have struggled to generalize well beyond the training data. Finally, the AUC value is a measure of overall discriminative ability and does not fully capture other aspects of model performance, such as calibration or clinical utility. While the AUC values were moderate, the models may still provide valuable insights when used in conjunction with other clinical tools and expert judgment. Future research should focus on incorporating additional relevant variables, exploring more advanced modeling techniques (e.g., SMOTE, ensemble learning, cost-sensitive learning), and validating the models in larger and more diverse cohorts to improve their predictive accuracy and clinical applicability.
SHAP visualization analysis revealed that BMI was the most strongly associated variable with asthma risk, followed by sex, race, marital status, and smoking history. This finding indicates that BMI, as an important indicator of individual obesity, may play a key role in the onset of asthma. Additionally, sociodemographic factors (such as sex and race) and lifestyle factors (such as smoking history) also hold significant positions in asthma risk. Through SHAP visualization analysis, researchers can gain a clearer understanding of the mechanisms by which each variable contributes to the model, providing targeted evidence for subsequent intervention measures.
Obesity-asthma interactions are largely mediated by chronic low-grade inflammation. Systemic elevations of interleukin-13 and CCL17 in obese individuals potentiate airway inflammation and immune dysregulation, thereby impairing asthma control.[17] Moreover, increased airway neutrophilic inflammation (particularly pronounced in obese female patients) supports the notion that obesity exacerbates asthma through inflammatory pathways.[18] Beyond systemic inflammation, obesity alters airway mechanics and suppresses lymphocyte function, further aggravating asthmatic symptoms.[19] Importantly, the magnitude of this association varies by age and sex, with distinct phenotypic profiles observed across different demographic strata.[20,21] Chronic low-grade inflammation linked to obesity also contributes to comorbidities such as diabetes and CVD by promoting insulin resistance and metabolic dysfunction.[22,23] Finally, this pro-inflammatory milieu continues to amplify airway immune responses, intensifying asthma severity.[24,25]
Despite the achievements of this study in exploring the relationship between the METS-VF and the risk of asthma onset, several limitations should be acknowledged. First, the cross-sectional design of the NHANES database precludes the establishment of causality. Second, although machine learning models are capable of handling complex variable relationships, their interpretability remains limited, and the underlying mechanisms of some variables require further in-depth investigation. Third, this study did not differentiate between asthma subtypes, which may have distinct pathogenic mechanisms and risk factors, potentially influencing the study results. Fourth, the predictive power of the CatBoost model was only moderate, and its performance was insufficient to provide highly accurate predictions.
5. Conclusions
This study employed machine learning methods to elucidate the association between the METS-VF and the risk of asthma onset, identifying key factors such as BMI, sex, and race. These findings provide novel insights into the role of visceral fat in asthma pathogenesis and offer a theoretical basis for future intervention strategies.
Acknowledgments
Relevant data were obtained from the NHANES database. The authors thank the patients who contributed to this study. The authors extend their gratitude to all contributing investigators for their invaluable data contributions.
Author contributions
Conceptualization: Chao Li, Fen Wu, Yunpeng Wang.
Data curation: Chao Li, Fen Wu, Jijing Zhao, Yunpeng Wang.
Formal analysis: Chao Li, Fen Wu, Jijing Zhao, Yunpeng Wang.
Funding acquisition: Chao Li, Mingjun Ying, Fen Wu, Jijing Zhao, Yunpeng Wang.
Investigation: Chao Li, Mingjun Ying, Fen Wu, Yunpeng Wang.
Methodology: Chao Li, Mingjun Ying, Fen Wu, Yunpeng Wang.
Project administration: Chao Li, Mingjun Ying, Yunpeng Wang.
Resources: Chao Li, Mingjun Ying, Jijing Zhao, Yunpeng Wang.
Software: Chao Li, Mingjun Ying, Jijing Zhao, Yunpeng Wang.
Supervision: Chao Li, Mingjun Ying, Jijing Zhao, Yunpeng Wang.
Validation: Chao Li, Mingjun Ying, Jijing Zhao, Yunpeng Wang.
Visualization: Chao Li, Mingjun Ying, Jijing Zhao, Yunpeng Wang.
Writing – original draft: Chao Li, Mingjun Ying, Fen Wu, Jijing Zhao, Yunpeng Wang.
Writing – review & editing: Chao Li, Mingjun Ying, Fen Wu, Jijing Zhao, Yunpeng Wang.
Abbreviations:
- AUC
- area under the curve
- BMI
- body mass index
- CVD
- cardiovascular disease
- METS-IR
- metabolic score for insulin resistance
- METS-VF
- metabolic score for visceral fat
- NHANES
- National Health and Nutrition Examination Survey
- PIR
- poverty-to-income ratio
- SHAP
- SHapley Additive exPlanations
- VAT
- visceral adipose tissue
The NHANES study protocol was approved by the NCHS Research Ethics Review Board and informed written consent was obtained from all participants in the study.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
How to cite this article: Li C, Ying M, Wu F, Zhao J, Wang Y. The association between metabolic score for visceral fat and asthma incidence risk: A machine learning analysis based on the NHANES database. Medicine 2025;104:38(e44640).
References
- [1].Cao Y, Chen S, Chen X, et al. Global trends in the incidence and mortality of asthma from 1990 to 2019: an age-period-cohort analysis using the Global Burden of Disease study 2019. Front Public Health. 2022;10:1036674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wang Z, Li Y, Gao Y, et al. Global, regional, and national burden of asthma and its attributable risk factors from 1990 to 2019: a systematic analysis for the Global Burden of Disease Study 2019. Respir Res. 2023;24:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fu X, Fang D, Ge M, Chen Q, Huang H, Liu R. The global burden and trends of asthma from 1990 to 2021, and its changes during the COVID-19 pandemic: an observational study. Public Health. 2025;241:47–54. [DOI] [PubMed] [Google Scholar]
- [4].Tripathi H, Singh A, Farheen, et al. The metabolic score for visceral fat (METS-VF) as a predictor of diabetes mellitus: evidence from the 2011–2018 NHANES study. PLoS One. 2025;20:e0317913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Xue H, Zhang L, Xu J, et al. Association of the visceral fat metabolic score with osteoarthritis risk: a cross-sectional study from NHANES 2009–2018. BMC Public Health. 2024;24:2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lin H, Shi K, Luo S, Ye W, Cai X. Elevated metabolic score for visceral fat was associated with increased prevalence of gallstones in American adults: a cross-sectional study. Front Med (Lausanne). 2024;11:1474368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Guo W, Xue H, Li Q, et al. Association between visceral fat metabolism score and cataract risk in US adults: National Health and Nutrition Examination Survey 1999 to 2008. Am J Ophthalmol. 2025;274:184–95. [DOI] [PubMed] [Google Scholar]
- [8].Cao Y, Wen W, Zhang H, Li W, Huang G, Huang Y. The association between visceral fat metabolic score and stroke: mediation by declining kidney function. Diabetol Metab Syndr. 2025;17:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liu Q, Han X, Chen Y, Gao Y, Yang W, Huang L. Asthma prevalence is increased in patients with high metabolism scores for visceral fat: study reports from the US. Front Endocrinol (Lausanne). 2023;14:1162158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lin T, Mao H, Huang S, Xie Z, Xu Z. Association between asthma and visceral adipose tissue in adults, a cross-sectional study from NHANES 2011–2018. Sci Rep. 2024;14:23217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Girard J, Lafontan M. Impact of visceral adipose tissue on liver metabolism and insulin resistance. Part II: visceral adipose tissue production and liver metabolism. Diabetes Metab. 2008;34:439–45. [DOI] [PubMed] [Google Scholar]
- [12].Lafontan M, Girard J. Impact of visceral adipose tissue on liver metabolism. Part I: heterogeneity of adipose tissue and functional properties of visceral adipose tissue. Diabetes Metab. 2008;34(4 Pt 1):317–27. [DOI] [PubMed] [Google Scholar]
- [13].Saxton SN, Clark BJ, Withers SB, Eringa EC, Heagerty AM. Mechanistic links between obesity, diabetes, and blood pressure: role of perivascular adipose tissue. Physiol Rev. 2019;99:1701–63. [DOI] [PubMed] [Google Scholar]
- [14].Ragino YI, Stakhneva EM, Polonskaya YV, Kashtanova EV. The role of secretory activity molecules of visceral adipocytes in abdominal obesity in the development of cardiovascular disease: a review. Biomolecules. 2020;10:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tanaka K, Sata M. Roles of perivascular adipose tissue in the pathogenesis of atherosclerosis. Front Physiol. 2018;9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Michailidou Z, Gomez-Salazar M, Alexaki VI. Innate immune cells in the adipose tissue in health and metabolic disease. J Innate Immun. 2022;14:4–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhang X, Zheng J, Zhang L, et al. Systemic inflammation mediates the detrimental effects of obesity on asthma control. Allergy Asthma Proc. 2018;39:43–50. [DOI] [PubMed] [Google Scholar]
- [18].Scott HA, Gibson PG, Garg ML, Wood LG. Airway inflammation is augmented by obesity and fatty acids in asthma. Eur Respir J. 2011;38:594–602. [DOI] [PubMed] [Google Scholar]
- [19].Sideleva O, Black K, Dixon AE. Effects of obesity and weight loss on airway physiology and inflammation in asthma. Pulm Pharmacol Ther. 2013;26:455–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wadden D, Allwood Newhook LA, Twells L, Farrell J, Gao Z. Sex-specific association between childhood BMI trajectories and asthma phenotypes. Int J Pediatr. 2018;2018:9057435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Holguin F, Bleecker ER, Busse WW, et al. Obesity and asthma: an association modified by age of asthma onset. J Allergy Clin Immunol. 2011;127:1486–93.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Asghar A, Sheikh N. Role of immune cells in obesity induced low grade inflammation and insulin resistance. Cell Immunol. 2017;315:18–26. [DOI] [PubMed] [Google Scholar]
- [23].Weijie Z, Meng Z, Chunxiao W, et al. Obesity-induced chronic low-grade inflammation in adipose tissue: a pathway to Alzheimer’s disease. Ageing Res Rev. 2024;99:102402. [DOI] [PubMed] [Google Scholar]
- [24].Khan UI, Rastogi D, Isasi CR, Coupey SM. Independent and synergistic associations of asthma and obesity with systemic inflammation in adolescents. J Asthma. 2012;49:1044–50. [DOI] [PubMed] [Google Scholar]
- [25].Adair D, Bagheri A, Yosef M, et al. High interleukin (IL)-6 is associated with lower lung function and increased likelihood of metabolic dysfunction in asthma. Pulm Ther. 2025;11:41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]